Biohydrogen Produced via Dark Fermentation: A Review
Abstract
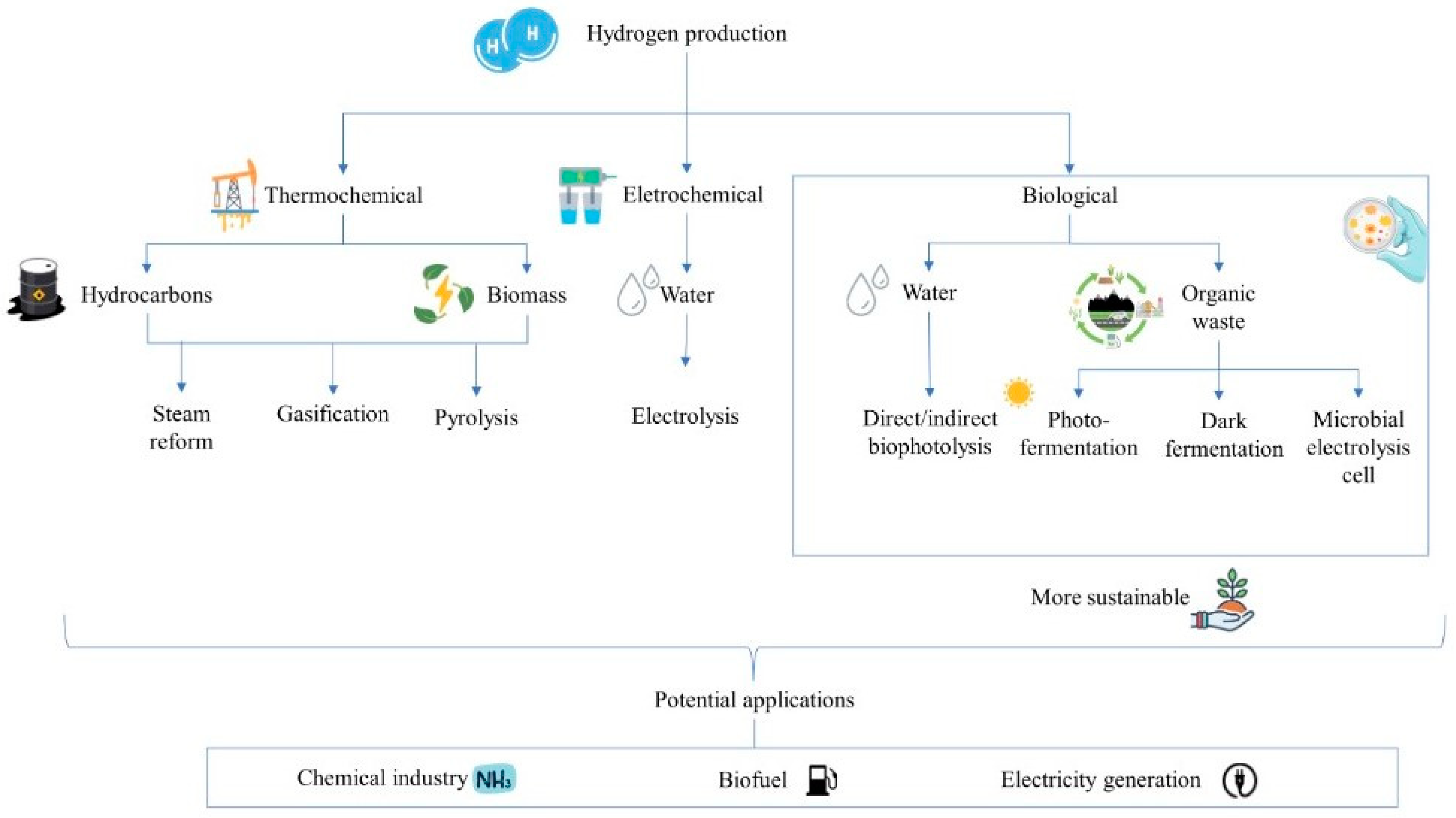
1. Introduction
2. The Dark Fermentation Metabolic Pathway
3. Parameters Affecting Dark Fermentation
3.1. Inoculum and Pretreatments
3.2. Substrates Used in Dark Fermentation
3.2.1. Lignocellulosic Biomass
3.2.2. Industrial Processing Residues
3.2.3. Food Waste
3.2.4. Algal Biomass
3.2.5. Manure from Animal Farming
3.3. Temperature
3.4. pH
3.5. Hydraulic Retention Time (HRT)
3.6. Partial bioH2 Pressure
3.7. Organic Loading Rate (OLR)
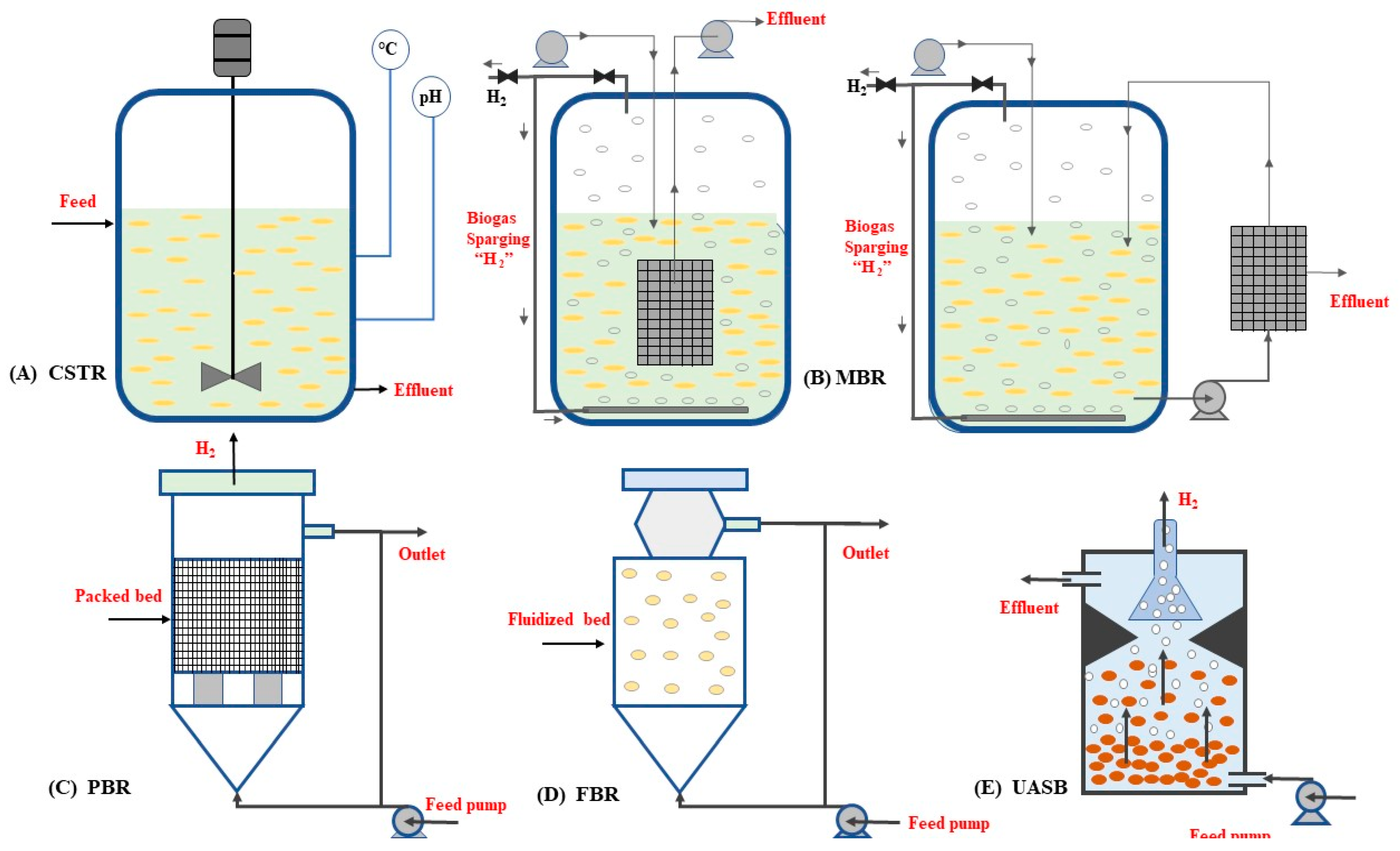
3.8. Bioreactors Used for bioH2 Production
3.8.1. Continuous Stirred Tank Reactors (CSTRs)
3.8.2. Membrane Bioreactors (MBRs)
3.8.3. Packed Bed Reactors (PBRs)
3.8.4. Anaerobic Fluidized Bed Reactors (AFBRs)
3.8.5. Upflow Anaerobic Sludge Blanket Reactors (UASBs)
4. Technologies to Increase bioH2 Production
4.1. Integrated Production Strategies
4.1.1. Dark Fermentation—Photofermentation
4.1.2. Dark Fermentation—Microbial Electrolysis Cells
4.2. Nanoparticles (NPs)
4.3. Genetic and Metabolic Engineering
5. Mathematical Modeling for Biohydrogen Production
6. Advantages and Disadvantages of Dark Fermentation and Other Methods of Renewable Hydrogen Production
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. World Energy Outlook 2023; IEA: Paris, France, 2023. [Google Scholar]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T.; Kamarudin, S.K.; Daud, W.R.W. Overview Biohydrogen Technologies and Application in Fuel Cell Technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Sinharoy, A.; Pakshirajan, K. A Novel Application of Biologically Synthesized Nanoparticles for Enhanced Biohydrogen Production and Carbon Monoxide Bioconversion. Renew. Energy 2020, 147, 864–873. [Google Scholar] [CrossRef]
- Kumar, G.; Park, J.H.; Sivagurunathan, P.; Lee, S.H.; Park, H.D.; Kim, S.H. Microbial Responses to Various Process Disturbances in a Continuous Hydrogen Reactor Fed with Galactose. J. Biosci. Bioeng. 2017, 123, 216–222. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A Brief Overview on Its Sources, Production and Environmental Impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen Production by Dark Fermentation of Wheat Powder Solution: Effects of C/N and C/P Ratio on Hydrogen Yield and Formation Rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Grimes, C.A.; Varghese, O.K.; Ranjan, S. Light, Water, Hydrogen The Solar Generation of Hydrogen by Water Photoelectrolysis; Springer: New York, NY, USA, 2008. [Google Scholar]
- IEA. Global Hydrogen Review 2023; IEA: Paris, France, 2023. [Google Scholar]
- Balat, M. Potential Importance of Hydrogen as a Future Solution to Environmental and Transportation Problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. Current Status of Hydrogen Energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review; IEA: Paris, France, 2021. [Google Scholar]
- Manish, S.; Banerjee, R. Comparison of Biohydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Masihi, F.; Rezaeitavabe, F.; Karimi-Jashni, A.; Riefler, G. Optimization and Enhancement of Biohydrogen Production in a Single-Stage Hybrid (Dark/Photo) Fermentation Reactor Using Fe3O4 and TiO2 Nanoparticles. Int. J. Hydrogen Energy 2024, 52, 295–305. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Pérez-Bernal, M.F.; Bernet, N.; Trably, E. Sequential Dark Fermentation and Microbial Electrolysis Cells for Hydrogen Production: Volatile Fatty Acids Influence and Energy Considerations. Bioresour. Technol. 2023, 374, 128803. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-Hydrogen Production through Dark Fermentation: An Overview. Biomass Convers. Biorefin. 2022, 14, 12699–12724. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S. Biohydrogen Production Through Dark Fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of Pre-Treatment and Hydraulic Retention Time on Biohydrogen Production from Organic Wastes. Int. J. Hydrogen Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Microbial Technologies in Advanced Biofuels Production; Springer: New York, NY, USA, 2012; Volume 9781461412083, ISBN 9781461412083. [Google Scholar]
- Policastro, G.; Lamboglia, R.; Fabbricino, M.; Pirozzi, F. Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy. Fermentation 2022, 8, 706. [Google Scholar] [CrossRef]
- Khanna, N.; Das, D. Biohydrogen Production by Dark Fermentation. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 401–421. [Google Scholar] [CrossRef]
- Nirmala, N.; Praveen, G.; AmitKumar, S.; SundarRajan, P.S.; Baskaran, A.; Priyadharsini, P.; SanjayKumar, S.P.; Dawn, S.S.; Pavithra, K.G.; Arun, J.; et al. A Review on Biological Biohydrogen Production: Outlook on Genetic Strain Enhancements, Reactor Model and Techno-Economics Analysis. Sci. Total Environ. 2023, 896, 165143. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen Production from Biomass and Wastes via Dark Fermentation: A Review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the Potential of Bio-Hydrogen Production by Fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Sparling, R.; Islam, R.; Cicek, N.; Carere, C.; Chow, H.; Levin, D.B. Formate Synthesis by Clostridium thermocellum during Anaerobic Fermentation. Can. J. Microbiol. 2006, 52, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Benemann, J.R. Biological Hydrogen Production; Fundamentals and Limiting Processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Chang, J.S. Bioreactor and Process Design for Biohydrogen Production. Bioresour. Technol. 2011, 102, 8524–8533. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Arriaga, S.; Alatriste-Mondragón, F.; De León-Rodríguez, A.; Rosales-Colunga, L.M.; Razo-Flores, E. Fermentative Biohydrogen Production: Trends and Perspectives. Rev. Environ. Sci. Biotechnol. 2008, 7, 27–45. [Google Scholar] [CrossRef]
- Singh, N.; Rai, P.; Pandey, A.; Pandey, A. Exploring the Potential of Bacillus Licheniformis AP1 for Fermentive Biohydrogen Production Using Starch Substrate: BBD Based Process Parameter Optimization. Fuel 2022, 319, 123668. [Google Scholar] [CrossRef]
- Cai, G.; Jin, B.; Monis, P.; Saint, C. Metabolic Flux Network and Analysis of Fermentative Hydrogen Production. Biotechnol. Adv. 2011, 29, 375–387. [Google Scholar] [CrossRef]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.H.; Chu, C.Y.; Leu, H.J.; Marone, A.; et al. Biohydrogen Production by Dark Fermentation: Scaling-up and Technologies Integration for a Sustainable System. Rev. Environ. Sci. Biotechnol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark Fermentation Biorefinery in the Present and Future (Bio)Chemical Industry. Rev. Environ. Sci. Biotechnol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous Dark Fermentative Hydrogen Production by Mesophilic Microflora: Principles and Progress. Int. J. Hydrogen Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fundamentals of the Fermentative Production of Hydrogen. Water Sci. Technol. 2005, 52, 21–29. [Google Scholar] [CrossRef]
- Zhao, Z.T.; Ding, J.; Wang, B.Y.; Bao, M.Y.; Liu, B.F.; Pang, J.W.; Ren, N.Q.; Yang, S.S. Advances in the Biomass Valorization in Dark Fermentation Systems: A Sustainable Approach for Biohydrogen Production. Chem. Eng. J. 2024, 481, 148444. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L.; Hussy, I. Sustainable Fermentative Hydrogen Production: Challenges for Process Optimisation. Int. J. Hydrogen Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial Production of Hydrogen: An Overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Seol, E.H.; Lee, E.Y.; Park, S. Fermentative Hydrogen Production by a New Chemoheterotrophic Bacterium Rhodopseudomonas palustris P4. Int. J. Hydrogen Energy 2002, 27, 1373–1379. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Rojas, S.M.; Lynn, R.; Ventura, G.; Rey, F.; Nayve, P.; Lantican, N.B. Potential for Biohydrogen Production from Organic Wastes with Focus on Sequential Dark- and Photofermentation: The Philippine Setting. Biomass Convers. Biorefinery 2021, 13, 8535–8548. [Google Scholar] [CrossRef]
- Do Carmo Lamaison, F.; De Andrade, P.A.M.; Bigaton, A.D.; Andreote, F.D.; Antônio, R.V.; Reginatto, V. Long-Term Effect of Acid and Heat Pretreatment of Sludge from a Sugarcane Vinasse Treatment Plant on the Microbial Community and on Thermophilic Biohydrogen Production. Int. J. Hydrogen Energy 2015, 40, 14124–14133. [Google Scholar] [CrossRef]
- Taifor, A.F.; Zakaria, M.R.; Mohd Yusoff, M.Z.; Toshinari, M.; Hassan, M.A.; Shirai, Y. Elucidating Substrate Utilization in Biohydrogen Production from Palm Oil Mill Effluent by Escherichia Coli. Int. J. Hydrogen Energy 2017, 42, 5812–5819. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, S.; Li, Y.; Ding, P.; Zhang, Y.; Zhao, P. Green-Synthesized Nickel Oxide Nanoparticles Enhances Biohydrogen Production of Klebsiella Sp. WL1316 Using Lignocellulosic Hydrolysate and Its Regulatory Mechanism. Fuel 2021, 305, 121585. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Muthukumar, K. Biohydrogen Production from Enzymatically Digested Cotton Stalks Using Citrobacter Freundii. J. Inst. Eng. (India) Ser. E 2023, 104, 11–18. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Molina, D.; Soccol, C.R. Hydrogen Production by Dark Fermentation Using a New Low-Cost Culture Medium Composed of Corn Steep Liquor and Cassava Processing Water: Process Optimization and Scale-Up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef]
- Song, Z.X.; Li, X.H.; Li, W.W.; Bai, Y.X.; Fan, Y.T.; Hou, H.W. Direct Bioconversion of Raw Corn Stalk to Hydrogen by a New Strain Clostridium Sp. FS3. Bioresour. Technol. 2014, 157, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, Z.; Fan, Y.; Hou, H. Biohydrogen Production from Dairy Manures with Acidification Pretreatment by Anaerobic Fermentation. Environ. Sci. Pollut. Res. 2010, 17, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.N.; Li, Y.H.; Zheng, H.Q.; Fan, Y.T.; Hou, H.W. Direct Degradation of Cellulosic Biomass to Bio-Hydrogen from a Newly Isolated Strain Clostridium sartagoforme FZ11. Bioresour. Technol. 2015, 192, 60–67. [Google Scholar] [CrossRef]
- Rabelo, C.A.B.S.; Soares, L.A.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Optimization of Hydrogen and Organic Acids Productions with Autochthonous and Allochthonous Bacteria from Sugarcane Bagasse in Batch Reactors. J. Environ. Manag. 2018, 223, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Bouchareb, E.M.; Derbal, K.; Bedri, R.; Slimani, K.; Menas, S.; Lazreg, H.; Maaref, F.; Ouabdelkader, S.; Saheb, A.; Bouaita, R.; et al. Improving Biohydrogen Production by Dark Fermentation of Milk Processing Wastewater by Physicochemical and Enzymatic Pretreatments. Appl. Biochem. Biotechnol. 2023, 196, 2741–2756. [Google Scholar] [CrossRef]
- Meier, T.R.W.; Cremonez, P.A.; Maniglia, T.C.; Sampaio, S.C.; Teleken, J.G.; da Silva, E.A. Production of Biohydrogen by an Anaerobic Digestion Process Using the Residual Glycerol from Biodiesel Production as Additive to Cassava Wastewater. J. Clean. Prod. 2020, 258, 120833. [Google Scholar] [CrossRef]
- Ramu, S.M.; Dinesh, G.H.; Thulasinathan, B.; Thondi Rajan, A.S.; Ponnuchamy, K.; Pugazhendhi, A.; Alagarsamy, A. Dark Fermentative Biohydrogen Production from Rice Mill Wastewater. Int. J. Energy Res. 2021, 45, 17233–17243. [Google Scholar] [CrossRef]
- Sydney, E.B.; Novak, A.C.; Rosa, D.; Pedroni Medeiros, A.B.; Brar, S.K.; Larroche, C.; Soccol, C.R. Screening and Bioprospecting of Anaerobic Consortia for Biohydrogen and Volatile Fatty Acid Production in a Vinasse Based Medium through Dark Fermentation. Process Biochem. 2018, 67, 1–7. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Biohythane Production from Food Waste in a Two-Stage Process: Assessing the Energy Recovery Potential. Environ. Technol. 2022, 43, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Chou, Y.P.; Wu, S.Y.; Liu, C.M. Pretreatment Conditions of Rice Straw for Simultaneous Hydrogen and Ethanol Fermentation by Mixed Culture. Int. J. Hydrogen Energy 2016, 41, 4421–4428. [Google Scholar] [CrossRef]
- Cruz-López, A.; Cruz-Méndez, A.; Suárez-Vázquez, S.I.; Reyna-Gómez, L.M.; Pecina-Chacón, D.E.; de León Gómez, H. Effect of Hydraulic Retention Time on Continuous Biohydrogen Production by the Codigestion of Brewery Wastewater and Cheese Whey. Bioenergy Res. 2022, 17, 1155–1166. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Soccol, V.T.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Biohydrogen Production in Cassava Processing Wastewater Using Microbial Consortia: Process Optimization and Kinetic Analysis of the Microbial Community. Bioresour. Technol. 2020, 309, 123331. [Google Scholar] [CrossRef]
- Arisht, S.N.; Mahmod, S.S.; Abdul, P.M.; Indera Lutfi, A.A.; Takriff, M.S.; Lay, C.H.; Silvamany, H.; Sittijunda, S.; Jahim, J.M. Enhancing Biohydrogen Gas Production in Anaerobic System via Comparative Chemical Pre-Treatment on Palm Oil Mill Effluent (POME). J. Environ. Manag. 2022, 321, 115892. [Google Scholar] [CrossRef]
- Jung, J.H.; Sim, Y.B.; Baik, J.H.; Park, J.H.; Kim, S.M.; Yang, J.; Kim, S.H. Effect of Genus Clostridium abundance on Mixed-Culture Fermentation Converting Food Waste into Biohydrogen. Bioresour. Technol. 2021, 342, 125942. [Google Scholar] [CrossRef]
- Luo, L.; Sriram, S.; Johnravindar, D.; Louis Philippe Martin, T.; Wong, J.W.C.; Pradhan, N. Effect of Inoculum Pretreatment on the Microbial and Metabolic Dynamics of Food Waste Dark Fermentation. Bioresour. Technol. 2022, 358, 127404. [Google Scholar] [CrossRef]
- Kim, J.K.; Nhat, L.; Chun, Y.N.; Kim, S.W. Hydrogen Production Conditions from Food Waste by Dark Fermentation with Clostridium beijerinckii KCTC 1785. Biotechnol. Bioprocess Eng. 2008, 13, 499–504. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and Combined Effect of Various Pretreatment Methods for Biohydrogen Production from Food Waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third Generation Biohydrogen Production by Clostridium butyricum and Adapted Mixed Cultures from Scenedesmus Obliquus Microalga Biomass. Fuel 2015, 153, 128–134. [Google Scholar] [CrossRef]
- Stanislaus, M.S.; Zhang, N.; Yuan, Y.; Zheng, H.; Zhao, C.; Hu, X.; Zhu, Q.; Yang, Y. Improvement of Biohydrogen Production by Optimization of Pretreatment Method and Substrate to Inoculum Ratio from Microalgal Biomass and Digested Sludge. Renew. Energy 2018, 127, 670–677. [Google Scholar] [CrossRef]
- Chen, S.; Qu, D.; Xiao, X.; Miao, X. Biohydrogen Production with Lipid-Extracted Dunaliella Biomass and a New Strain of Hyper-Thermophilic Archaeon Thermococcus Eurythermalis A501. Int. J. Hydrogen Energy 2020, 45, 12721–12730. [Google Scholar] [CrossRef]
- Batista, A.P.; Moura, P.; Marques, P.A.S.S.; Ortigueira, J.; Alves, L.; Gouveia, L. Scenedesmus Obliquus as Feedstock for Biohydrogen Production by Enterobacter Aerogenes and Clostridium butyricum. Fuel 2014, 117, 537–543. [Google Scholar] [CrossRef]
- Hangri, S.; Derbal, K.; Policastro, G.; Panico, A.; Contestabile, P.; Pontoni, L.; Race, M.; Fabbricino, M. Combining Pretreatments and Co-Fermentation as Successful Approach to Improve Biohydrogen Production from Dairy Cow Manure. Environ. Res. 2024, 246, 118118. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Wang, H.; Zhou, Y.; Xiao, B.; Zhou, L.; Yu, G.; Yang, M.; Xiong, Y.; Wu, S. Dark Co-Fermentation of Rice Straw and Pig Manure for Biohydrogen Production: Effects of Different Inoculum Pretreatments and Substrate Mixing Ratio. Environ. Technol. 2021, 42, 4539–4549. [Google Scholar] [CrossRef]
- García-Depraect, O.; Gómez-Romero, J.; León-Becerril, E.; López-López, A. A Novel Biohydrogen Production Process: Co-Digestion of Vinasse and Nejayote as Complex Raw Substrates Using a Robust Inoculum. Int. J. Hydrogen Energy 2017, 42, 5820–5831. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.P. Predictive and Explicative Models of Fermentative Hydrogen Production from Solid Organic Waste: Role of Butyrate and Lactate Pathways. Int. J. Hydrogen Energy 2014, 39, 7476–7485. [Google Scholar] [CrossRef]
- Guo, Y.P.; Kim, S.H.; Sung, S.H.; Lee, P.H. Effect of Ultrasonic Treatment of Digestion Sludge on Bio-Hydrogen Production from Sucrose by Anaerobic Fermentation. Int. J. Hydrogen Energy 2010, 35, 3450–3455. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Naina Mohammed, S. Biohydrogen Production from Sugar Industry Effluents Using Nickel Based Electrode Materials in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2019, 44, 17381–17388. [Google Scholar] [CrossRef]
- Sampath, P.; Brijesh; Reddy, K.R.; Reddy, C.V.; Shetti, N.P.; Kulkarni, R.V.; Raghu, A.V. Biohydrogen Production from Organic Waste—A Review. Chem. Eng. Technol. 2020, 43, 1240–1248. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark Fermentation of Complex Waste Biomass for Biohydrogen Production by Pretreated Thermophilic Anaerobic Digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Structure of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–12. [Google Scholar]
- Pérez-Barragán, J.; García-Depraect, O.; Maya-Yescas, R.; Vallejo-Rodríguez, R.; Palacios-Hinestroza, H.; Coca, M.; Castro-Muñoz, R.; León-Becerril, E. Solid and Liquid Fractionation of Sugarcane and Agave Bagasse during Ozonolysis and Enzymatic Hydrolysis: Impact on Biohydrogen and Biogas Production. Ind. Crops Prod. 2024, 210, 118175. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving Enzymatic Hydrolysis of Lignocellulosic Biomass by Bio-Coordinated Physicochemical Pretreatment—A Review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent Developments in Pretreatment Technologies on Lignocellulosic Biomass: Effect of Key Parameters, Technological Improvements, and Challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass—An Overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of Fungal and Physicochemical Processes for Lignocellulosic Biomass Pretreatment—A Review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Thanigaivelan, A.; Das, D.B.; Show, P.L.; Banat, F. Augmented Biohydrogen Production from Rice Mill Wastewater through Nano-Metal Oxides Assisted Dark Fermentation. Bioresour. Technol. 2021, 319, 124243. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Junior, J.R.; Zevallos Torres, L.A.; Medeiros, A.B.P.; Woiciechowski, A.L.; Martinez-Burgos, W.J.; Soccol, C.R. Enhancement of Biohydrogen Production in Industrial Wastewaters with Vinasse Pond Consortium Using Lignin-Mediated Iron Nanoparticles. Int. J. Hydrogen Energy 2021, 46, 27431–27443. [Google Scholar] [CrossRef]
- Li, W.; Cheng, C.; Cao, G.; Ren, N. Enhanced Biohydrogen Production from Sugarcane Molasses by Adding Ginkgo Biloba Leaves. Bioresour. Technol. 2020, 298, 122523. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A Review of Sustainable Hydrogen Production Using Seed Sludge via Dark Fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- UNEP. Think Eat Save Tracking Progress to Halve Global Food Waste; UNEP: Nairobi, Kenya, 2024; ISBN 9789280741391. [Google Scholar]
- Regueira-Marcos, L.; García-Depraect, O.; Muñoz, R. Elucidating the Role of PH and Total Solids Content in the Co-Production of Biohydrogen and Carboxylic Acids from Food Waste via Lactate-Driven Dark Fermentation. Fuel 2023, 338, 127238. [Google Scholar] [CrossRef]
- Singh, D.; Tembhare, M.; Machhirake, N.; Kumar, S. Biogas Generation Potential of Discarded Food Waste Residue from Ultra-Processing Activities at Food Manufacturing and Packaging Industry. Energy 2023, 263, 126138. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Capela, I.; Tarelho, L.A.C. Dark Fermentative Hydrogen Production from Food Waste: Effect of Biomass Ash Supplementation. Int. J. Hydrogen Energy 2019, 44, 26213–26225. [Google Scholar] [CrossRef]
- Alian, M.; Saadat, S.; Rezaeitavabe, F. An Investigation on the Dose-Dependent Effect of Iron Shaving on Bio-Hydrogen Production from Food Waste. Int. J. Hydrogen Energy 2021, 46, 19886–19896. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Li, H.; Zhang, Z. Continuous Co-Generation of Biohydrogen and Biomethane through Two-Stage Anaerobic Digestion of Hydrothermally Pretreated Food Waste. Energy Convers. Manag. 2022, 268, 116000. [Google Scholar] [CrossRef]
- Jung, J.H.; Sim, Y.B.; Ko, J.; Park, S.Y.; Kim, G.B.; Kim, S.H. Biohydrogen and Biomethane Production from Food Waste Using a Two-Stage Dynamic Membrane Bioreactor (DMBR) System. Bioresour. Technol. 2022, 352, 127094. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid Profiling of Five Microalgae Species from Large-Scale Production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Allewaert, C.C.; Vanormelingen, P.; Daveloose, I.; Verstraete, T.; Vyverman, W. Intraspecific Trait Variation Affecting Astaxanthin Productivity in Two Haematococcus (Chlorophyceae) Species. Algal Res. 2017, 21, 191–202. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The Future of Anaerobic Digestion and Biogas Utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Duran, M. Biohydrogen Production from Cattle Manure and Its Mixtures with Renewable Feedstock by Hyperthermophilic Caldicellulosiruptor bescii. J. Clean. Prod. 2021, 292, 125969. [Google Scholar] [CrossRef]
- Woon, J.M.; Khoo, K.S.; AL-Zahrani, A.A.; Alanazi, M.M.; Lim, J.W.; Cheng, C.K.; Sahrin, N.T.; Ardo, F.M.; Yi-Ming, S.; Lin, K.S.; et al. Epitomizing Biohydrogen Production from Microbes: Critical Challenges vs Opportunities. Environ. Res. 2023, 227, 115780. [Google Scholar] [CrossRef]
- Shin, H.S.; Youn, J.H.; Kim, S.H. Hydrogen Production from Food Waste in Anaerobic Mesophilic and Thermophilic Acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. A Critical Review on Issues and Overcoming Strategies for the Enhancement of Dark Fermentative Hydrogen Production in Continuous Systems. Int. J. Hydrogen Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Kim, S.H.; Shin, H.S. Bioreactor Design for Continuous Dark Fermentative Hydrogen Production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef]
- Karadag, D.; Puhakka, J.A. Effect of Changing Temperature on Anaerobic Hydrogen Production and Microbial Community Composition in an Open-Mixed Culture Bioreactor. Int. J. Hydrogen Energy 2010, 35, 10954–10959. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of PH on Hydrogen Production from Glucose by a Mixed Culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Martínez, V.L.; Salierno, G.L.; García, R.E.; Lavorante, M.J.; Galvagno, M.A.; Cassanello, M.C. Biological Hydrogen Production by Dark Fermentation in a Stirred Tank Reactor and Its Correlation with the PH Time Evolution. Catalysts 2022, 12, 1366. [Google Scholar] [CrossRef]
- Pattra, S.; Lay, C.H.; Lin, C.Y.; O-Thong, S.; Reungsang, A. Performance and Population Analysis of Hydrogen Production from Sugarcane Juice by Non-Sterile Continuous Stirred Tank Reactor Augmented with Clostridium butyricum. Int. J. Hydrogen Energy 2011, 36, 8697–8703. [Google Scholar] [CrossRef]
- Mohan, S.V. Waste to Renewable Energy: A Sustainable and Green Approach towards Production of Biohydrogen by Acidogenic Fermentation. In Sustainable Biotechnology: Sources of Renewable Energy; Springer: Dordrecht, The Netherlands, 2010; pp. 129–164. ISBN 9789048132942. [Google Scholar]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M.; Ghafari, S.; Vikineswary, S.; Zinatizadeh, A.A. Influences of Environmental and Operational Factors on Dark Fermentative Hydrogen Production: A Review. Clean 2012, 40, 1297–1305. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H.; Sen, B.; Chu, C.Y.; Kumar, G.; Chen, C.C.; Chang, J.S. Fermentative Hydrogen Production from Wastewaters: A Review and Prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Lin, C.N.; Wu, S.Y.; Chang, J.S.; Chang, J.S. Biohydrogen Production in a Three-Phase Fluidized Bed Bioreactor Using Sewage Sludge Immobilized by Ethylene-Vinyl Acetate Copolymer. Bioresour. Technol. 2009, 100, 3298–3301. [Google Scholar] [CrossRef]
- Hafez, H.; Nakhla, G.; El. Naggar, M.H.; Elbeshbishy, E.; Baghchehsaraee, B. Effect of Organic Loading on a Novel Hydrogen Bioreactor. Int. J. Hydrogen Energy 2010, 35, 81–92. [Google Scholar] [CrossRef]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.U. Strategies for Improvement of Biohydrogen Production from Organic-Rich Wastewater: A Review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- García-Depraect, O.; Castro-Muñoz, R.; Muñoz, R.; Rene, E.R.; León-Becerril, E.; Valdez-Vazquez, I.; Kumar, G.; Reyes-Alvarado, L.C.; Martínez-Mendoza, L.J.; Carrillo-Reyes, J.; et al. A Review on the Factors Influencing Biohydrogen Production from Lactate: The Key to Unlocking Enhanced Dark Fermentative Processes. Bioresour. Technol. 2021, 324, 124595. [Google Scholar] [CrossRef]
- Sinharoy, A.; Kumar, M.; Pakshirajan, K. An Overview of Bioreactor Configurations and Operational Strategies for Dark Fermentative Biohydrogen Production. In Bioreactors: Sustainable Design and Industrial Applications in Mitigation of GHG Emissions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–288. ISBN 9780128212646. [Google Scholar]
- Qyyum, M.A.; Ihsanullah, I.; Ahmad, R.; Ismail, S.; Khan, A.; Nizami, A.S.; Tawfik, A. Biohydrogen Production from Real Industrial Wastewater: Potential Bioreactors, Challenges in Commercialization and Future Directions. Int. J. Hydrogen Energy 2022, 47, 37154–37170. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Martinez-Burgos, W.J.; De Bona Sartor, G.; Letti, L.A.J.; De Carvalho, J.C.; Soccol, C.R.; Medeiros, A.B.P. Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent. Fermentation 2024, 10, 141. [Google Scholar] [CrossRef]
- Kiani Deh Kiani, M.; Parsaee, M.; Safieddin Ardebili, S.M.; Reyes, I.P.; Fuess, L.T.; Karimi, K. Different Bioreactor Configurations for Biogas Production from Sugarcane Vinasse: A Comprehensive Review. Biomass Bioenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Biohydrogen Production from Crude Glycerol by Immobilized Klebsiella Sp. TR17 in a UASB Reactor and Bacterial Quantification under Non-Sterile Conditions. Int. J. Hydrogen Energy 2014, 39, 9580–9587. [Google Scholar] [CrossRef]
- Elmoutez, S.; Abushaban, A.; Necibi, M.C.; Sillanpää, M.; Liu, J.; Dhiba, D.; Chehbouni, A.; Taky, M. Design and Operational Aspects of Anaerobic Membrane Bioreactor for Efficient Wastewater Treatment and Biogas Production. Environ. Chall. 2023, 10, 100671. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, M.; Sachdeva, M.; Yadav, M.; Tiwari, A. Thermophilic Biohydrogen Production for Commercial Application: The Whole Picture. Int. J. Energy Res. 2016, 40, 127–145. [Google Scholar] [CrossRef]
- Andersson, J.; Björnsson, L. Evaluation of Straw as a Biofilm Carrier in the Methanogenic Stage of Two-Stage Anaerobic Digestion of Crop Residues. Bioresour. Technol. 2002, 85, 51–56. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.S. Biohydrogen Production From Renewable Biomass Resources. In Biomass, Biofuels, Biochemicals: Biohydrogen, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. ISBN 9780444642035. [Google Scholar]
- Sivaranjani, R.; Veerathai, S.; Jeoly Jenifer, K.; Sowmiya, K.; Rupesh, K.J.; Sudalai, S.; Arumugam, A. A Comprehensive Review on Biohydrogen Production Pilot Scale Reactor Technologies: Sustainable Development and Future Prospects. Int. J. Hydrogen Energy 2023, 48, 23785–23820. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Celis, L.B.; Alatriste-Mondragón, F.; Razo-Flores, E. Different Start-up Strategies to Enhance Biohydrogen Production from Cheese Whey in UASB Reactors. Int. J. Hydrogen Energy 2012, 37, 5591–5601. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Shin, H.S. A Simple Method to Reduce the Start-up Period in a H2-Producing UASB Reactor. Int. J. Hydrogen Energy 2011, 36, 1466–1473. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P. Integrated Dark- and Photo-Fermentation: Recent Advances and Provisions for Improvement. Int. J. Hydrogen Energy 2016, 41, 19957–19971. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Zampol Lazaro, C.; Hallenbeck, P.C. Increased Hydrogen Yield and COD Removal from Starch/Glucose Based Medium by Sequential Dark and Photo-Fermentation Using Clostridium butyricum and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2017, 42, 18832–18843. [Google Scholar] [CrossRef]
- Rezaeitavabe, F.; Saadat, S.; Talebbeydokhti, N.; Sartaj, M.; Tabatabaei, M. Enhancing Bio-Hydrogen Production from Food Waste in Single-Stage Hybrid Dark-Photo Fermentation by Addition of Two Waste Materials (Exhausted Resin and Biochar). Biomass Bioenergy 2020, 143, 105846. [Google Scholar] [CrossRef]
- Das, S.R.; Basak, N. Optimization of Process Parameters for Enhanced Biohydrogen Production Using Potato Waste as Substrate by Combined Dark and Photo Fermentation. Biomass Convers. Biorefin 2022, 14, 4791–4811. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Chowdhury, R. Ameliorated Hydrogen Production through Integrated Dark-Photo Fermentation in a Flat Plate Photobioreactor: Mathematical Modelling and Optimization of Energy Efficiency. Energy Convers. Manag. 2020, 226, 113549. [Google Scholar] [CrossRef]
- Niño-Navarro, C.; Chairez, I.; Christen, P.; Canul-Chan, M.; García-Peña, E.I. Enhanced Hydrogen Production by a Sequential Dark and Photo Fermentation Process: Effects of Initial Feedstock Composition, Dilution and Microbial Population. Renew. Energy 2020, 147, 924–936. [Google Scholar] [CrossRef]
- Eroglu, E.; Melis, A. Photobiological Hydrogen Production: Recent Advances and State of the Art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Singh, S.P.; Asthana, R.K.; Singh, S. Biohydrogen Production from Sugarcane Bagasse by Integrating Dark- and Photo-Fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.H.; Bhatia, S.K.; Brar, S.K. An Overview on Progress, Advances, and Future Outlook for Biohydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent Advances and Emerging Challenges in Microbial Electrolysis Cells (MECs) for Microbial Production of Hydrogen and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Chandrasekhar, K.; Ismail, M.; Kalil, M.S. Hydrogen Gas Production with an Electroformed Ni Mesh Cathode Catalysts in a Single-Chamber Microbial Electrolysis Cell (MEC). Int. J. Hydrogen Energy 2015, 40, 14095–14103. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Cardeña, R.; Cortón, E.; Buitrón, G. Hydrogen Production in Two-Chamber MEC Using a Low-Cost and Biodegradable Poly(Vinyl) Alcohol/Chitosan Membrane. Bioresour. Technol. 2021, 319, 124168. [Google Scholar] [CrossRef]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.H.; Wang, X. Organic Waste to Biohydrogen: A Critical Review from Technological Development and Environmental Impact Analysis Perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- Hasibar, B.; Ergal, İ.; Moser, S.; Bochmann, G.; Rittmann, S.K.M.R.; Fuchs, W. Increasing Biohydrogen Production with the Use of a Co-Culture inside a Microbial Electrolysis Cell. Biochem. Eng. J. 2020, 164, 107802. [Google Scholar] [CrossRef]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.L.; Bergel, A. Microbial Electrolysis Cell (MEC): Strengths, Weaknesses and Research Needs from Electrochemical Engineering Standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Md Jahim, J.; Ismail, M.; Lim, S.S. Biocathode in Microbial Electrolysis Cell; Present Status and Future Prospects. Renew. Sustain. Energy Rev. 2015, 47, 23–33. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial Electrolysis Cell with a Microbial Biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Kim, K.Y.; Ajayi, F.F.; Chang, I.S.; Kim, I.S. Selective Inhibition of Methanogens for the Improvement of Biohydrogen Production in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 13379–13386. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Lee, H.S. Hydrogen Production from Sugar Beet Juice Using an Integrated Biohydrogen Process of Dark Fermentation and Microbial Electrolysis Cell. Bioresour. Technol. 2015, 198, 223–230. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling Dark Fermentation and Microbial Electrolysis Cells for Higher Hydrogen Yield: Technological Competitiveness and Challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Li, X.H.; Liang, D.W.; Bai, Y.X.; Fan, Y.T.; Hou, H.W. Enhanced H2 Production from Corn Stalk by Integrating Dark Fermentation and Single Chamber Microbial Electrolysis Cells with Double Anode Arrangement. Int. J. Hydrogen Energy 2014, 39, 8977–8982. [Google Scholar] [CrossRef]
- Khongkliang, P.; Kongjan, P.; Utarapichat, B.; Reungsang, A.; O-Thong, S. Continuous Hydrogen Production from Cassava Starch Processing Wastewater by Two-Stage Thermophilic Dark Fermentation and Microbial Electrolysis. Int. J. Hydrogen Energy 2017, 42, 27584–27592. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martín-Marroquín, J.M.; Corona, F. The Role of Magnetic Nanoparticles in Dark Fermentation. Biomass Convers. Biorefin 2023, 13, 16299–16320. [Google Scholar] [CrossRef]
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K. Application of Nanotechnology in the Production of Biohydrogen: A Review. Chem. Eng. Technol. 2023, 46, 218–233. [Google Scholar] [CrossRef]
- Maroušek, J. Review: Nanoparticles Can Change (Bio)Hydrogen Competitiveness. Fuel 2022, 328, 125318. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Improving Mechanisms of Biohydrogen Production from Grass Using Zero-Valent Iron Nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef]
- Shanmugam, S.; Hari, A.; Pandey, A.; Mathimani, T.; Felix, L.O.; Pugazhendhi, A. Comprehensive Review on the Application of Inorganic and Organic Nanoparticles for Enhancing Biohydrogen Production. Fuel 2020, 270, 117453. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Nanoparticles in Biological Hydrogen Production: An Overview. Indian. J. Microbiol. 2018, 58, 8–18. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced Anaerobic Digestion of Waste Activated Sludge Digestion by the Addition of Zero Valent Iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Yang, G.; Sun, Z. Optimization of Biohydrogen Production Using Acid Pretreated Corn Stover Hydrolysate Followed by Nickel Nanoparticle Addition. Int. J. Energy Res. 2020, 44, 1843–1857. [Google Scholar] [CrossRef]
- Sarma, S.; Ortega, D.; Minton, N.P.; Dubey, V.K.; Moholkar, V.S. Homologous Overexpression of Hydrogenase and Glycerol Dehydrogenase in Clostridium pasteurianum to Enhance Hydrogen Production from Crude Glycerol. Bioresour. Technol. 2019, 284, 168–177. [Google Scholar] [CrossRef]
- Son, Y.S.; Jeon, J.M.; Kim, D.H.; Yang, Y.H.; Jin, Y.S.; Cho, B.K.; Kim, S.H.; Kumar, S.; Lee, B.D.; Yoon, J.J. Improved Bio-Hydrogen Production by Overexpression of Glucose-6-Phosphate Dehydrogenase and FeFe Hydrogenase in Clostridium acetobutylicum. Int. J. Hydrogen Energy 2021, 46, 36687–36695. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, Q.; Xu, Q.; Zhu, L.; Huang, H. Fermentative Hydrogen Production from Jerusalem Artichoke by Clostridium tyrobutyricum Expressing Exo-Inulinase Gene. Sci. Rep. 2017, 7, 7940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, G.; Jin, B.; Monis, P.; Saint, C. A Genetic and Metabolic Approach to Redirection of Biochemical Pathways of Clostridium butyricum for Enhancing Hydrogen Production. Biotechnol. Bioeng. 2013, 110, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Chung, D.; Elkins, J.G.; Guss, A.M.; Westpheling, J. Metabolic Engineering of Caldicellulosiruptor Bescii Yields Increased Hydrogen Production from Lignocellulosic Biomass. Biotechnol. Biofuels 2013, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, E.; Lim, S.W.; Yeo, W.S.; Nandong, J. A Review on Process Modeling and Design of Biohydrogen. Int. J. Hydrogen Energy 2022, 47, 30404–30427. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bakonyi, P.; Rózsenberszki, T.; Kumar, G.; Koók, L.; Kelemen, G.; Kim, S.H.; Bélafi-Bakó, K. Assessment via the Modified Gompertz-Model Reveals New Insights Concerning the Effects of Ionic Liquids on Biohydrogen Production. Int. J. Hydrogen Energy 2018, 43, 18918–18924. [Google Scholar] [CrossRef]
- Karthic, P.; Joseph, S.; Arun, N.; Kumaravel, S. Optimization of Biohydrogen Production by Enterobacter species Using Artificial Neural Network and Response Surface Methodology. J. Renew. Sustain. Energy 2013, 5, 033104. [Google Scholar] [CrossRef]
- Sydney, E.B.; Duarte, E.R.; Martinez Burgos, W.J.; de Carvalho, J.C.; Larroche, C.; Soccol, C.R. Development of Short Chain Fatty Acid-Based Artificial Neuron Network Tools Applied to Biohydrogen Production. Int. J. Hydrogen Energy 2020, 45, 5175–5181. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Sanz, R.; Marugán, J. Perovskite Materials for Hydrogen Production by Thermochemical Water Splitting. Int. J. Hydrogen Energy 2016, 41, 19329–19338. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; do Nascimento Junior, J.R.; Medeiros, A.B.P.; Herrmann, L.W.; Sydney, E.B.; Soccol, C.R. Biohydrogen Production from Agro-Industrial Wastes Using Clostridium beijerinckii and Isolated Bacteria as Inoculum. Bioenergy Res. 2022, 15, 987–997. [Google Scholar] [CrossRef]
- Rosa, D.; Medeiros, A.B.P.; Martinez-Burgos, W.J.; do Nascimento, J.R.; de Carvalho, J.C.; Sydney, E.B.; Soccol, C.R. Biological Hydrogen Production from Palm Oil Mill Effluent (POME) by Anaerobic Consortia and Clostridium beijerinckii. J. Biotechnol. 2020, 323, 17–23. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Biohydrogen Production through Photo-Fermentation of Lignocellulosic Biomass. Biomass Convers. Biorefin 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Kondo, A.; Chang, J.S. Recent Insights into Biohydrogen Production by Microalgae—From Biophotolysis to Dark Fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]

| Substrate | Inoculum | Inoculum Pretreatment | Predominant Microorganisms | pH | Temperature | bioH2 | Reference |
|---|---|---|---|---|---|---|---|
| Corn stalk | Cow Manure | Microwave for 1.5 min | Clostridium sartagoforme | 6.47 | 35 °C | 87.2 mL bioH2/g of corn stalk | [52] |
| Rice straw | Sludge | Thermal, 95–100 °C for 1 h | Mixed culture | 5.5 | 37 °C | 0.77 L bioH2/L culture medium | [59] |
| Sugarcane bagasse | Anaerobic bioreactor sludge | - | Clostridium bifermentans (62.69% relative abundance), Bacillus coagulans (31.67%) and Enterobacter aerogenes (2.72%) | 7.2 | 37 °C | 23.10 mmoL bioH2/L culture medium | [53] |
| Corn stalk | Cattle manure | Microwave (no description of conditions) | Clostridium butyricum | Without pH adjustment | 36° C | 92.9 mL bioH2/g corn stalk | [50] |
| Brewery wastewater and cheese whey | Anaerobic reactor sludge | Thermal, 100 °C for 40 min | Bacillus spp. (25%), Firmicutes Clostridia (20%), Firmicutes bacilli (8%), (<5%) Lactococcus lactis, (<5%) Alcaligenes spp. and (<5%) Paracoccus solventevorans | 5.5 | 35 °C | 6.22 mmol bioH2/g DQO | [60] |
| Glycerol and wastewater from cassava processing | Anaerobic reactor sludge | Thermal, 100 °C for 30 min | Brevundimonas and Bacillus | - | 38.5 °C | 0.86 L bioH2/L culture medium | [55] |
| Corn steep liquor and cassava processing water | Vinasse effluent | Thermal, 95 ± 2 °C for 15 min | Porphyromonadaceae 16%, Clostridiaceae 31%, Ruminococcaceae 0.85%, Enterococcaceae 51%, others 1.5% | 6 | 37 °C | 107 mL bioH2/g DQO removed | [49,61] |
| Corn steep liquor and cassava processing water | Chicken manure | Thermal, 95 ± 2 °C for 15 min | Porphyromonadaceae 75%, Clostridiaceae 15%, Ruminococcaceae 6%, Enterococcaceae 3%, others 1% | 6 | 37 °C | 83.1 mL bioH2/g DQO removed | [49,61] |
| Rice mill wastewater | Rice mill wastewater | Thermal, 100 °C for 15 min | Bacillus thuringiensis | 5.5 | 37 °C | 1.63 ± 0.14 mol bioH2/mol glucose | [56] |
| Dairy processing wastewater | Anaerobic reactor sludge | Thermal, 90 °C for 30 min | Mixed culture | 5.5 | 55 °C | 254 mL of cumulative bioH2 | [54] |
| Palm oil mill effluent | Anaerobic reactor sludge | Thermal, 85 °C for 60 min | Clostridia, Bacilli, Bacteroidia, Thermoanaerobacteria and Gammaproteobacteria | 5.5 | 60 °C | 2.25 mol of bioH2/mol of total soluble carbohydrates | [62] |
| Food waste | Sludge from a hydrogen-producing reactor | Centrifugation at 5000 rpm for 5 min, freezing for two months, and thermal pretreatment at 90 °C for 30 min | Clostridium, Romboutsia, Sporolactobacillus, Streptococcus, Terrisporobacter and others in smaller fractions | 8.2 | 37 °C | 1.12 ± 0.02 mol bioH2/mol glucose | [63] |
| Food waste | Anaerobic reactor sludge | Alkaline, pH 10 using 5 M NaOH | Clostridium, Paraclostridium, Streptococcus, Lactococcus, Enterococcus and Prevotella | 7.5 | 35 °C | 157.25 ± 7.62 mL of bioH2 g/VS | [64] |
| Food waste | Strain bank | - | Clostridium beijerinckii | 5.5 | 40 °C | 128 mL bioH2/g DQO removed | [65] |
| Food waste | Microorganisms present in the substrate | - | - | 5.5 | 37 °C | 118 mL bioH2/g VS | [66] |
| Algal biomass (Scenedesmus obliquus) | Anaerobic reactor sludge | - | Clostridium butyricum | - | 37 °C | 116.3 mL bioH2/g VS | [67] |
| Algal biomass (Chlorella vulgaris) | Anaerobic reactor sludge | Thermal, 90 °C for 60 min | Mixed culture | 5.5 | 35 °C | 190.9 mL bioH2/g VS | [68] |
| Algal biomass (Dunaliella primolecta) | - | - | Thermococcus eurythermalis | - | 85 °C | 192.35 mL bioH2/g VS | [69] |
| Algal biomass (Dunaliella tertiolecta) | - | - | Thermococcus eurythermalis | - | 85 °C | 183.02 mL bioH2/g VS | [69] |
| Algal biomass (Scenedesmus obliquus) | Strain bank | - | Clostridium butyricum | - | 37 °C | 113.1 mL bioH2/g VS | [70] |
| Algal biomass (Scenedesmus obliquus) | Strain bank | - | Enterobacter aerogenes | - | 30 °C | 57.6 mL bioH2/g VS | [70] |
| Cattle manure and cheese whey | Digestate | Thermal, 105 °C for 1.5 h | Mixed culture | 6–7 | 35 °C | 0.33 L bioH2/L culture medium | [71] |
| Cattle manure | Cattle manure | Infrared radiation for 2 h | Mixed culture | 5.0 | 36 °C | 31.5 mL bioH2/g VS | [51] |
| Cattle manure | Anaerobic reactor sludge | Acid, pH 2.0 using 6 M HCL | Mixed culture | - | - | 44.59 mL bioH2/g VS | [72] |
| Vinasse and Nejayote | Digestate from mesophilic anaerobic digester treating food waste | Light heat-shock, (30 to 60 °C) for 20–30 min followed by micro aeration | Acetobacter orientalis, 42.94% | 5.5 | 35 °C | 115 NmL H2/g VS | [73] |
| Process | Methods | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Water splitting | Electrolysis | Fragmentation of the water molecule using electric current and some electrolytes such as bases, acids and salts | Easily scale-up Water is used as the main raw material Process carried out at ambient temperatures and pressures Can achieve efficiencies of 60% | Short life of electrodes due to corrosion | [171] |
| Thermolysis | Fragmentation of the water molecule using high pressures and temperatures (1800–5000 °C) | Water is used as the main raw material Solar energy and different types of biomass can be used as energy sources | Large amounts of energy are required in the process High pressures and temperatures are required Low efficiency, maximum 40% | [49,172] | |
| Photolysis | Fragmentation of the water molecule using photons of light | Solar energy can be used | Extremely low process efficiency, between 0.1–1.0% Highly expensive TiO2, IrO2, or RuO2 electrodes must be used | [49] | |
| Biological | Dark Fermentatio | Biological catabolic process carried out by bacteria in which one of the main gaseous bioproducts is hydrogen | Solid or liquid waste is used as substrates The process can be carried out at ambient pressures and temperatures Relatively fast process compared to other biological methods of hydrogen production | Low yield, maximum 4 moles of H2 per mole of glucose Other metabolites are generated during the process that affect the process yield Complex purification processes are required Slow process compared to electrolysis | [173,174] |
| Photofermentation | Biological reaction for the production of hydrogen, carried out in two stages. The first stage takes place in the absence of light and the second in the presence of light, the latter being carried out by purple bacteria | Waste can be used as substrate Higher yields can be obtained than dark fermentation Process can be carried out at ambient temperatures and pressures | Light-dependent process Two-step process More time-consuming process Slower process | [175] | |
| Bio-photolyses | Fractionation of the water molecule by sunlight and catalyzed by photosynthetic microorganisms such as microalgae and cyanobacteria | Waste can be used as substrates The process can be carried out at ambient pressure and temperature conditions | Light-dependent process A process carried out in two stages, in the first stage biomass is produced and hydrogen is obtained in the second stage A slower process than photofermentation | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, M.M.; Sartor, G.d.B.; Martinez-Burgos, W.J.; Scapini, T.; Edwiges, T.; Soccol, C.R.; Medeiros, A.B.P. Biohydrogen Produced via Dark Fermentation: A Review. Methane 2024, 3, 500-532. https://doi.org/10.3390/methane3030029
Albuquerque MM, Sartor GdB, Martinez-Burgos WJ, Scapini T, Edwiges T, Soccol CR, Medeiros ABP. Biohydrogen Produced via Dark Fermentation: A Review. Methane. 2024; 3(3):500-532. https://doi.org/10.3390/methane3030029
Chicago/Turabian StyleAlbuquerque, Marcela Moreira, Gabriela de Bona Sartor, Walter Jose Martinez-Burgos, Thamarys Scapini, Thiago Edwiges, Carlos Ricardo Soccol, and Adriane Bianchi Pedroni Medeiros. 2024. "Biohydrogen Produced via Dark Fermentation: A Review" Methane 3, no. 3: 500-532. https://doi.org/10.3390/methane3030029
APA StyleAlbuquerque, M. M., Sartor, G. d. B., Martinez-Burgos, W. J., Scapini, T., Edwiges, T., Soccol, C. R., & Medeiros, A. B. P. (2024). Biohydrogen Produced via Dark Fermentation: A Review. Methane, 3(3), 500-532. https://doi.org/10.3390/methane3030029






