Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Qualitative Preliminary Phytochemical Screening
2.3. Synthesis of Lanthanum Oxide Nanoparticles Using Eco-Friendly Leaf Extract as a Reducing Agent
2.4. Microplate Assay for Biofilm Inhibition
2.5. Photocatalytic Dye Degradation
2.6. Characterization
3. Results
3.1. Gas Chromatography-Mass Spectrum Study (GC-MS)
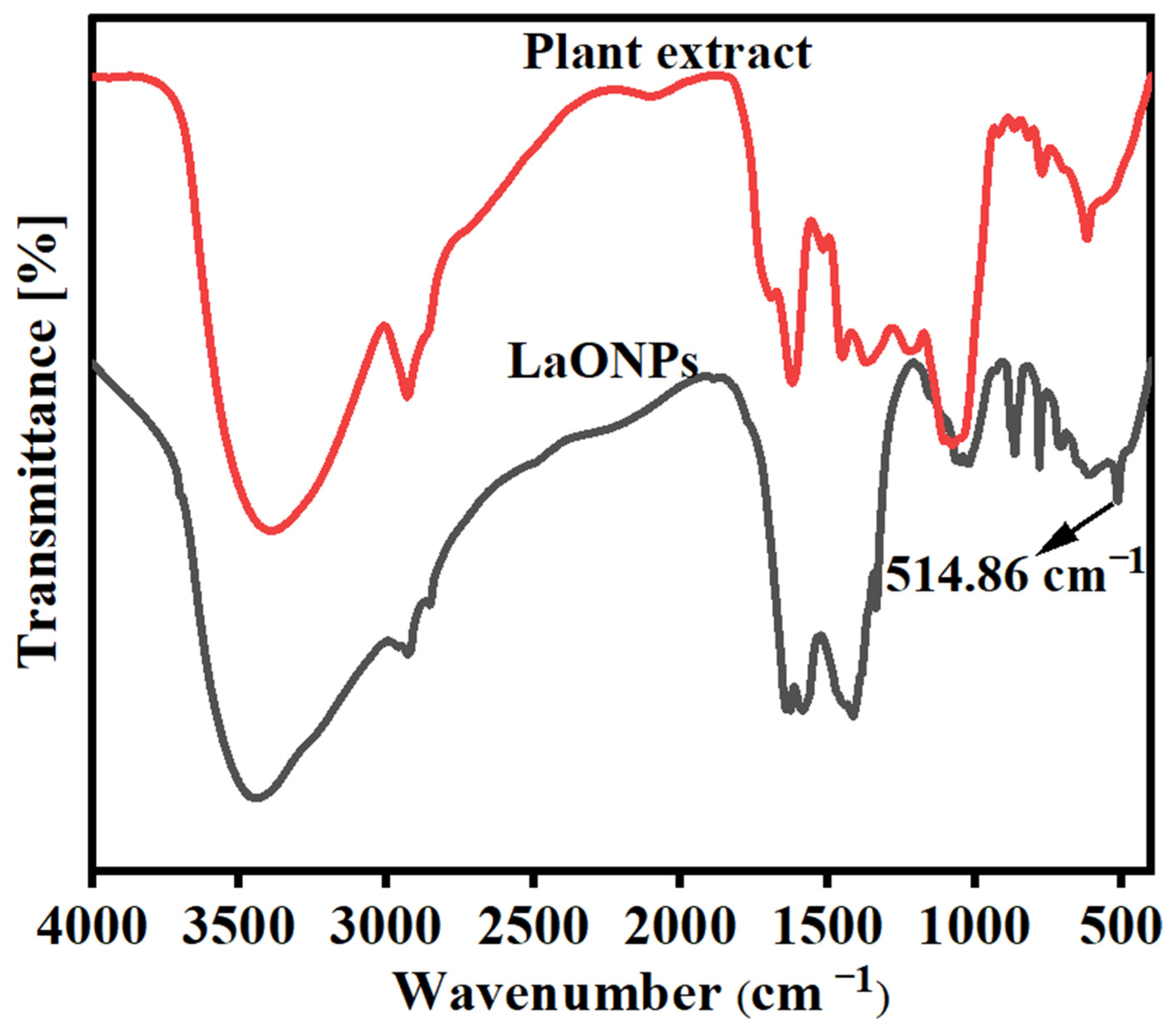
3.2. FT-IR
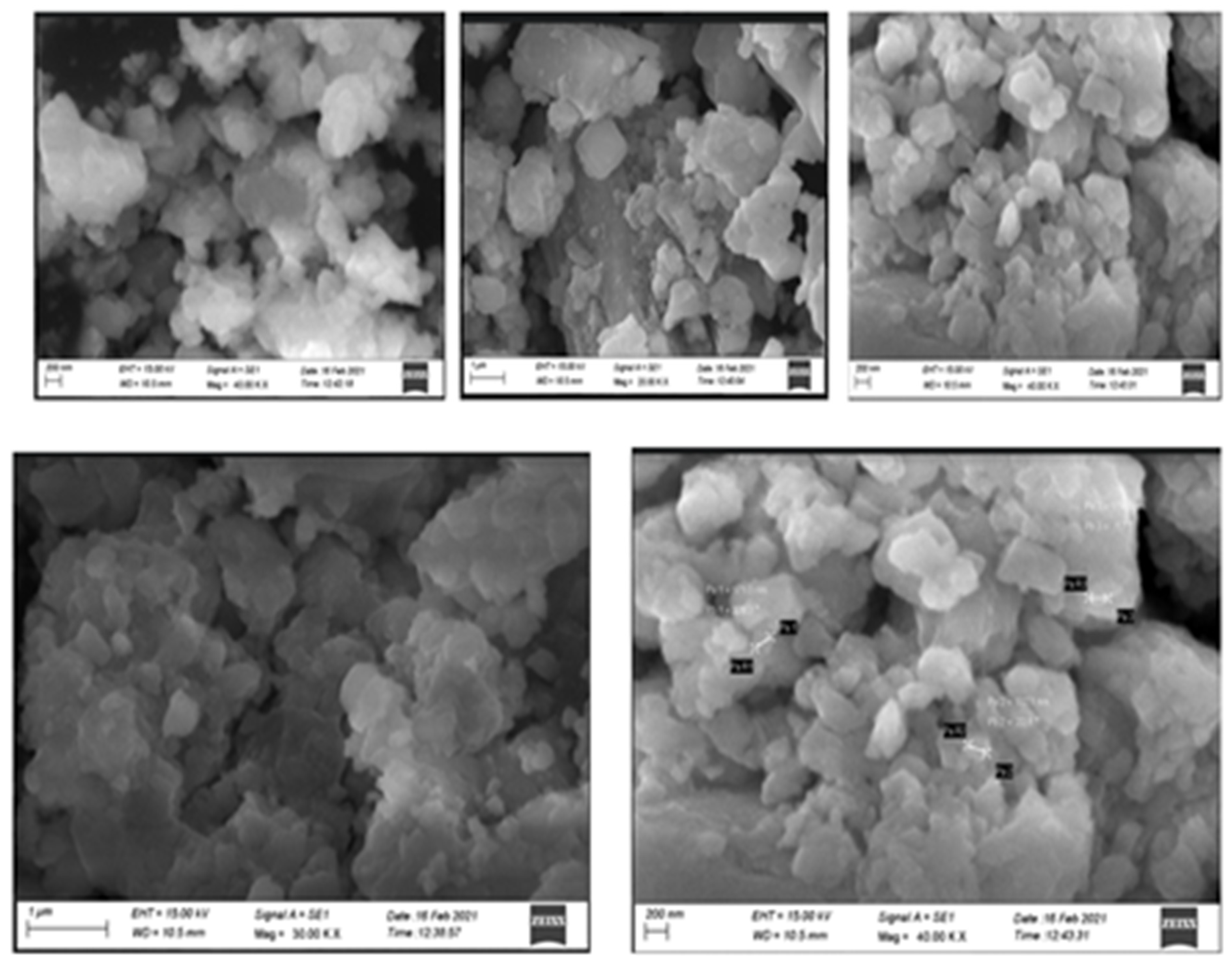
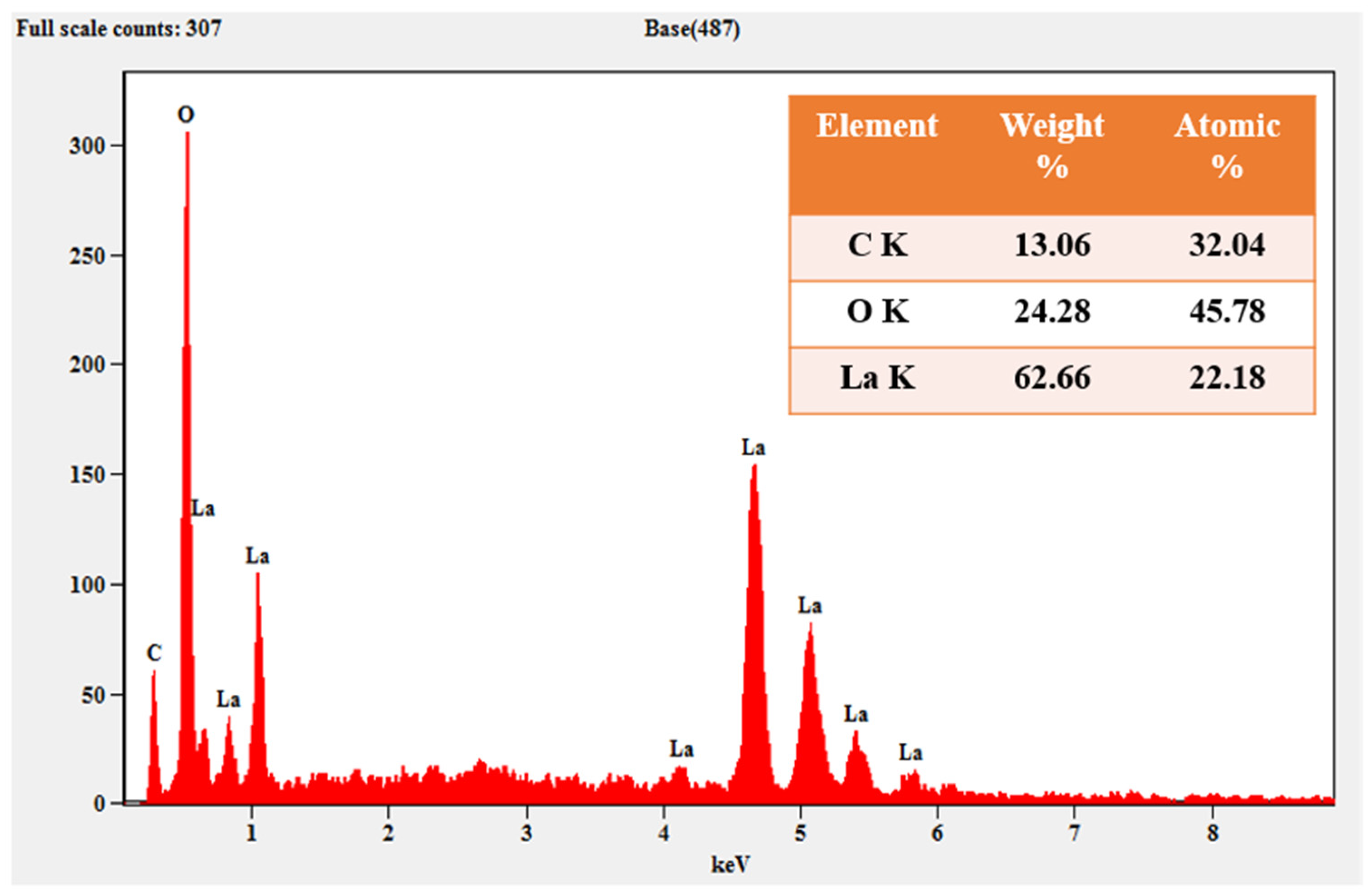
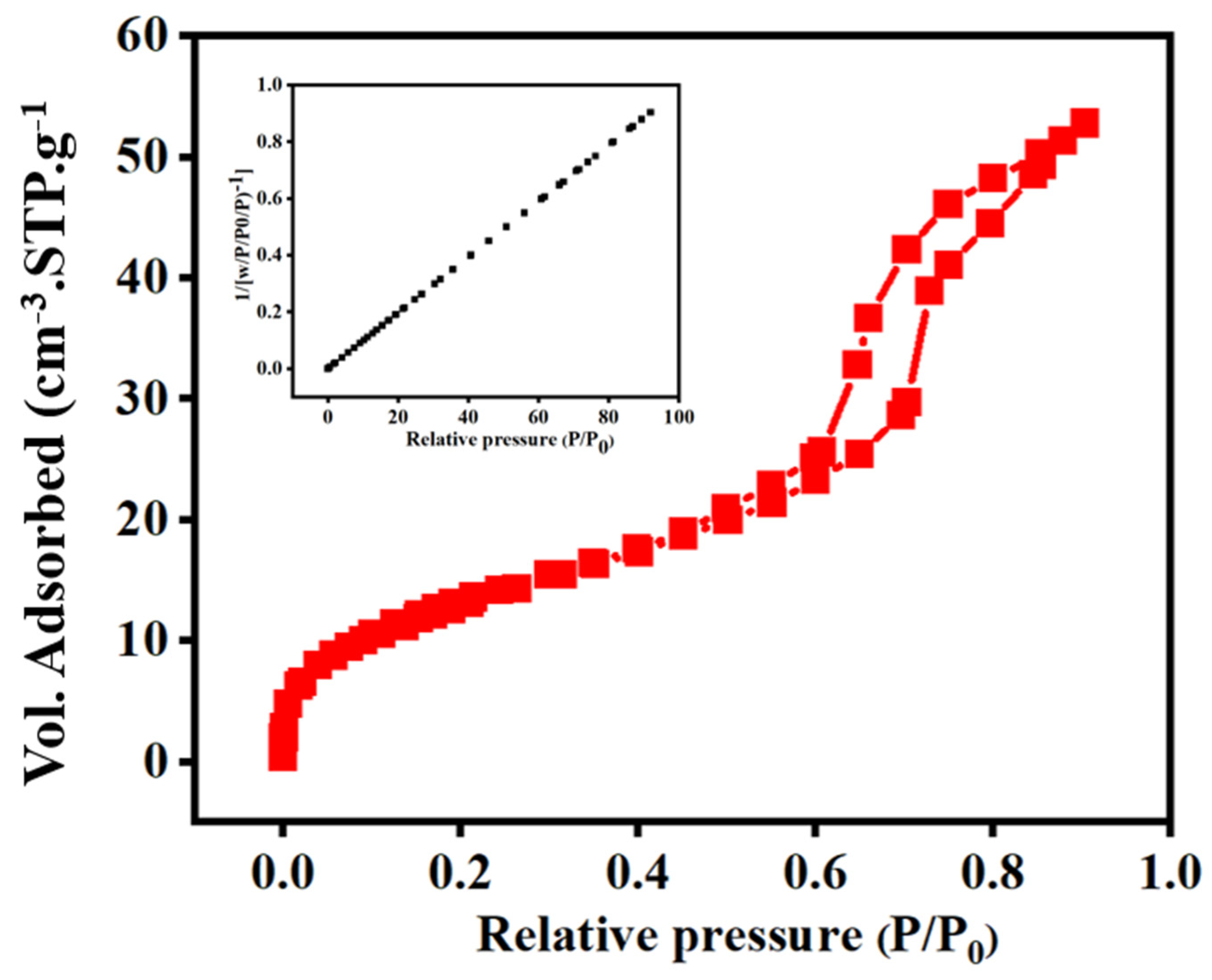
3.3. XRD Analysis and EDAX Analysis
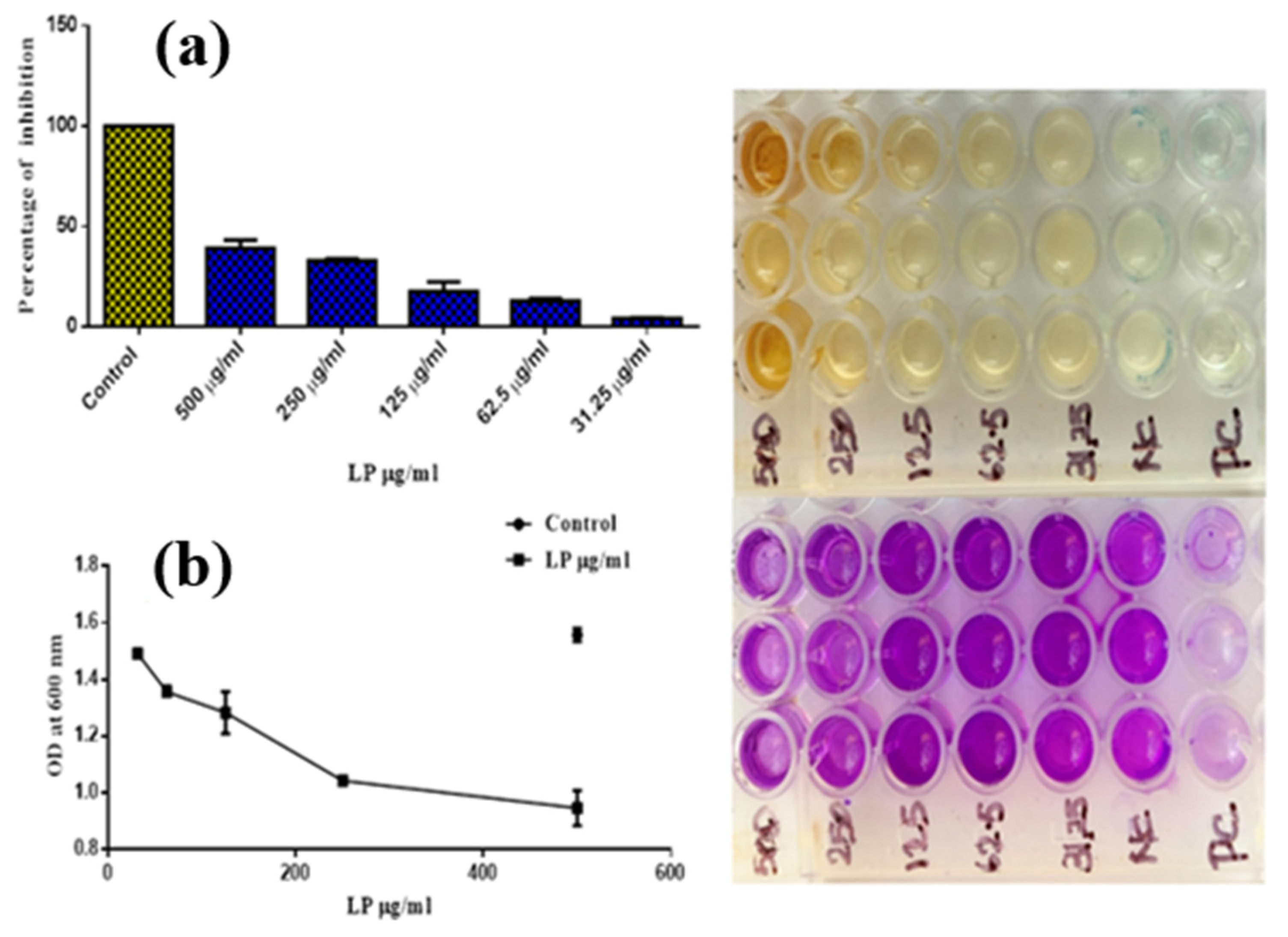
3.4. Biofilm-Inhibition Activities
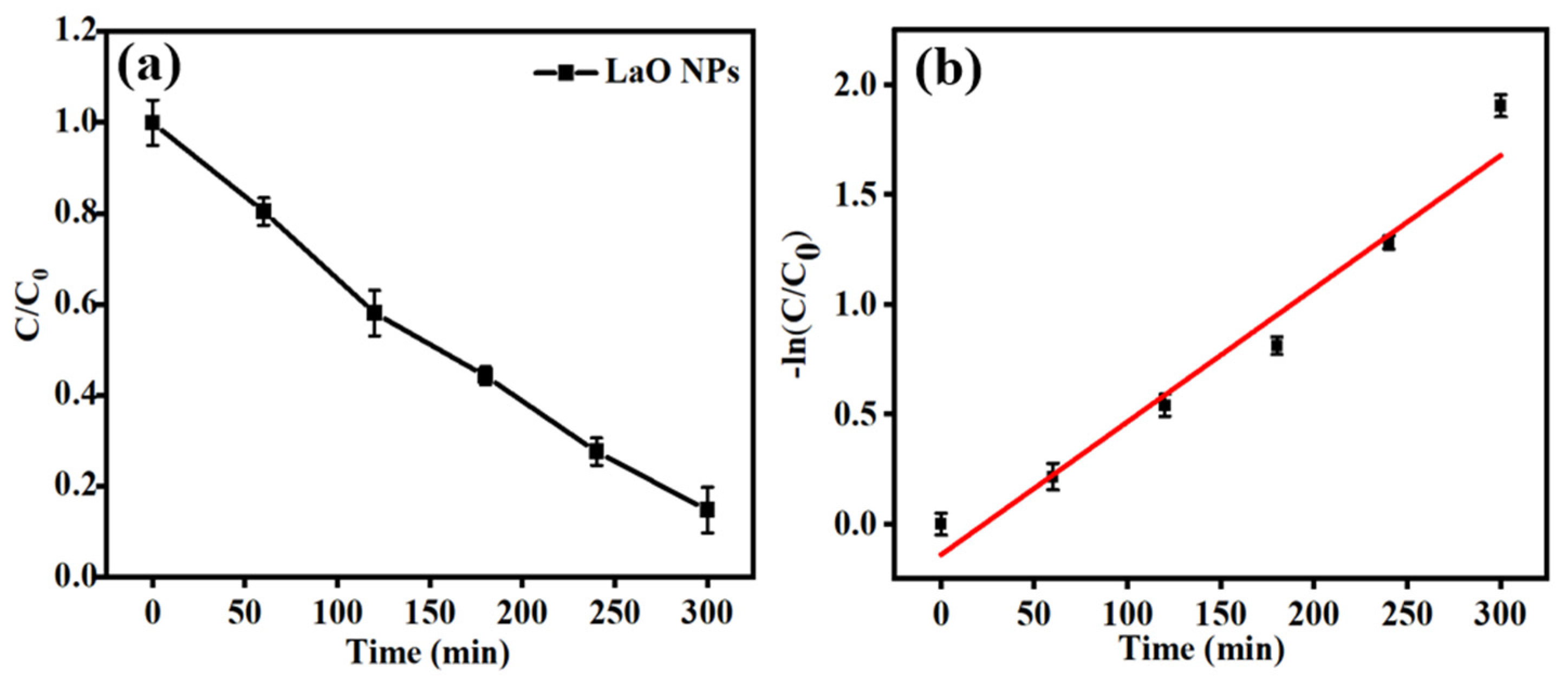
3.5. Photocatalytic Dye-Degradation Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonatto, C.C.; Silva, L.P. Higher Temperatures Speed up the Growth and Control the Size and Optoelectrical Properties of Silver Nanoparticles Greenly Synthesized by Cashew Nutshells. Ind. Crops Prod. 2014, 58, 46–54. [Google Scholar] [CrossRef]
- Mishra, A.N.; Bhadauria, S.; Gaur, M.S.; Pasricha, R.; Kushwah, B.S. Synthesis of Gold Nanoparticles by Leaves of Zero-Calorie Sweetener Herb (Stevia rebaudiana) and Their Nanoscopic Characterization by Spectroscopy and Microscopy. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, P118–P124. [Google Scholar] [CrossRef]
- Yilmaz, M.; Turkdemir, H.; Kilic, M.A.; Bayram, E.; Cicek, A.; Mete, A.; Ulug, B. Biosynthesis of Silver Nanoparticles Using Leaves of Stevia rebaudiana. Mater. Chem. Phys. 2011, 130, 1195–1202. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green Synthesis of Silver Nanoparticles Using Artocarpus Heterophyllus Lam. Seed Extract and Its Antibacterial Activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Worakitjaroenphon, S.; Shanmugam, P.; Boonyuen, S.; Smith, S.M.; Chookamnerd, K. Green Synthesis of Silver and Gold Nanoparticles Using Oroxylum Indicum Plant Extract for Catalytic and Antimicrobial Activity. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Shanmugam, P.; Boonyuen, S.; Tangjaideborisu, Y.; Na Nakorn, P.; Tantayanon, S.; Pothu, R.; Boddula, R. Anthocyanin Rich-Berry Extracts Coated Magnetic Fe3O4 Bionanocomposites and Their Antibacterial Activity. Inorg. Chem. Commun. 2023, 156, 111291. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and Characterization of ZrO2 Nanoparticles-Antimicrobial Activity and Their Prospective Role in Dental Care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Niu, F. Green Synthesis of Gallic Acid-Coated Silver Nanoparticles with High Antimicrobial Activity and Low Cytotoxicity to Normal Cells. Process Biochem. 2015, 50, 357–366. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Khan, A.; Chen, Y.; Shah, N.S.; Muhammad, N.; Khan, A.U.; Tahir, K.; Khan, F.U.; Murtaza, B.; Hassan, S.U.; et al. Biomedical Applications of Green Synthesized Nobel Metal Nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 173, 150–164. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and Characterization of Silver Nanoparticles Using Aqueous Extract of Seaweed Enteromorpha compressa and Its Biomedical Properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Sivakami, K.U.; Vaideeswaran, S.; Venis, A.R.; Juliet Helina, J.K.A.; Balaganesh, M. Impregnation, Silver Activating Capability and Biological Applications of Ionic Liquids. Mater. Lett. X 2022, 13, 100134. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using Chromolaena Odorata Root Extract for Smart Nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Natesh Kumar, B.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bio-Inspired Green Synthesis of Fe3O4 Spherical Magnetic Nanoparticles Using Syzygium cumini Seed Extract. Phys. B Condens. Matter 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Mokhtary, M.; Torabi, M. Nano Magnetite (Fe3O4), an Efficient and Robust Catalyst for the One-Pot Synthesis of 1-(Aryl(Piperidin-1-Yl)Methyl)Naphthalene-2-Ol and 1-(α-Amido Alkyl)-2-Naphthol under Ultrasound Irradiation. J. Saudi Chem. Soc. 2017, 21, S299–S304. [Google Scholar] [CrossRef]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.S.M.; Venckatesh, R. Biosynthesis and Characterization of Phyto Mediated Zinc Oxide Nanoparticles: A Green Chemistry Approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Sarmah, A.K.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Plant-Mediated Synthesis and Applications of Iron Nanoparticles. Mol. Biotechnol. 2018, 60, 154–168. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. One-Step Green Synthesis of Bimetallic Fe/Pd Nanoparticles Used to Degrade Orange II. J. Hazard. Mater. 2016, 303, 145–153. [Google Scholar] [CrossRef]
- Sareethammanuwat, M.; Boonyuen, S.; Arpornmaeklong, P. Effects of Beta-Tricalcium Phosphate Nanoparticles on the Properties of a Thermosensitive Chitosan/Collagen Hydrogel and Controlled Release of Quercetin. J. Biomed. Mater. Res. Part A 2021, 109, 1147–1159. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.L.T.; Boonyuen, S.; Ohno, T.; Andou, Y. Accessing Effects of Aliphatic Dicarboxylic Acid towards the Physical and Chemical Changes in Low Temperature Hydrothermally Reduced Graphene Hydrogel. J Porous Mater. 2021, 28, 1291–1300. [Google Scholar] [CrossRef]
- Aswini, R.; Hartati, S.; Jothimani, K.; Pothu, R.; Shanmugam, P.; Lee, Y.-Y.; Masimukku, S.; Boddula, R.; Selvaraj, M.; Al-Qahtani, N. Revolutionizing Microorganism Inactivation: Magnetic Nanomaterials in Sustainable Photocatalytic Disinfection. J. Environ. Manag. 2024, 370, 122738. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green Approaches for the Synthesis of Metal and Metal Oxide Nanoparticles Using Microbial and Plant Extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef]
- Rigopoulos, N.; Gkaliouri, C.M.; Sakavitsi, V.; Gournis, D. Full Factorial Design Synthesis of Silver Nanoparticles Using Origanum Vulgare. Reactions 2023, 4, 505–517. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Zhang, J.; Ding, X.; Qi, S.; Tang, C. Preparation and Characterization of Lanthanum Borate Nanowires. Mater. Lett. 2007, 61, 1596–1600. [Google Scholar] [CrossRef]
- Bu, W.; Zhang, L.; Hua, Z.; Chen, H.; Shi, J. Synthesis and Characterization of Uniform Spindle-Shaped Microarchitectures Self-Assembled from Aligned Single-Crystalline Nanowires of Lanthanum Phosphates. Cryst. Growth Des. 2007, 7, 2305–2309. [Google Scholar] [CrossRef]
- Parameswaran, S.; Bakkiyaraj, R.; Shanmugam, P.; Boonyuen, S.; Venugopal, T. Investigation of Biological Efficacy Assessment of Cobalt-Doped Cerium Oxide Nanocomposites against Pathogenic Bacteria, Fungi, and Lung Cancer Cells. Mater. Chem. Phys. 2024, 321, 129496. [Google Scholar] [CrossRef]
- Talluri, B.; Yoo, K.; Kim, J. Lanthanum Oxide Rods as a Novel and Efficient Bifunctional Hydrogen and Oxygen Evolution Electrocatalyst for Overall Water Splitting. Ceram. Int. 2022, 48, 18645–18650. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Gao, C.; Wang, X.; Gao, C.; Liu, X. Copper Doped Lanthanum Hydroxide Nanorods as a Low Temperature Processable Hole Transport Material for Perovskite Solar Cells. J. Power Sources 2024, 590, 233797. [Google Scholar] [CrossRef]
- Aswini, R.; Jothimani, K.; Kannan, K.; Pothu, R.; Shanmugam, P.; Boddula, R.; Radwan, A.B.; Periyasami, G.; Karthikeyan, P.; Al-Qahtani, N. Carica Papaya Leaf-Infused Metal Oxide Nanocomposite: A Green Approach towards Water Treatment and Antibacterial Applications. Environ. Geochem. Health 2024, 46, 334. [Google Scholar] [CrossRef]
- Alphonsa Juliet Helina, J.K.; Aswin Kumar, I.; Viswanathan, N. Fabrication and Analyzing of Drypetes sepiaria Encapsulated Chitosan Hybrid Beads as Anticorrosion Agent. Mater. Today Proc. 2021, 47, 1929–1936. [Google Scholar] [CrossRef]
- Ismail, W.; Belal, A.; Abdo, W.; El-Shaer, A. Investigating the Physical and Electrical Properties of La2O3 via Annealing of La(OH)3. Sci. Rep. 2024, 14, 7716. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Zhang, Y.; Wang, D.-C.; Su, Y.-M.; Wu, J.; Liu, J.-Q.; Yang, L.-L.; Jin, Z. Hydrothermal Synthesis of Lanthanum Oxide Nanoparticles Modified Pumice: High Lanthanum Oxide Loading Ratio and Efficiency Phosphate Removal. J. Environ. Chem. Eng. 2024, 12, 111587. [Google Scholar] [CrossRef]
- Udomkun, P.; Boonupara, T.; Smith, S.M.; Kajitvichyanukul, P. Green Ag/AgCl as an Effective Plasmonic Photocatalyst for Degradation and Mineralization of Methylthioninium Chloride. Separations 2022, 9, 191. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Ramar, K.; Handayani, M.; Kasilingam, T.; Gnanamoorthy, G.; Shaik, M.R.; Shaik, B.; Guru, A. Hydrothermal Green Synthesis of Aloe Vera Gel-Biotemplated Iron Oxide Nanoparticles for Robust Photocatalytic Degradation of Methylene Blue, Chromium (VI) Reduction, and Antibacterial Efficacy. Water Air Soil Pollut. 2024, 235, 309. [Google Scholar] [CrossRef]
- Awad, T.S.; Asker, D.; Hatton, B.D. Food-Safe Modification of Stainless Steel Food-Processing Surfaces to Reduce Bacterial Biofilms. ACS Appl. Mater. Interfaces 2018, 10, 22902–22912. [Google Scholar] [CrossRef]
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Zhang, X.; Gao, H.; Mou, L.; Wu, M.; Zhang, W.; Xin, F.; Jiang, M. Biofilm Application in the Microbial Biochemicals Production Process. Biotechnol. Adv. 2021, 48, 107724. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Li, M.; Samia, A.; Yu, X. (Bill) Study on the Behaviors of Fungi-Concrete Surface Interactions and Theoretical Assessment of Its Potentials for Durable Concrete with Fungal-Mediated Self-Healing. J. Clean. Prod. 2021, 292, 125870. [Google Scholar] [CrossRef]
- Keerthana, A.K.; Ashraf, P.M. Carbon Nanodots Synthesized from Chitosan and Its Application as a Corrosion Inhibitor in Boat-Building Carbon Steel BIS2062. Appln Nanoscin 2020, 10, 1061–1071. [Google Scholar] [CrossRef]
- Uthaman, S.; Vishwakarma, V.; George, R.P.; Ramachandran, D.; Kumari, K.; Preetha, R.; Premila, M.; Rajaraman, R.; Mudali, U.K.; Amarendra, G. Enhancement of Strength and Durability of Fly Ash Concrete in Seawater Environments: Synergistic Effect of Nanoparticles. Constr. Build. Mater. 2018, 187, 448–459. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tang, M.; Wang, C.; Gao, D.; Zhou, Y. Developing a Z-Scheme Ag2CO3/ZIF-8 Heterojunction for the Surface Decoration of Cotton Fabric toward Repeatable Photocatalytic Dye Degradation. Appl. Surf. Sci. 2023, 610, 155605. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective Polymeric Films for Industrial Substrates: A Critical Review on Past and Recent Applications with Conducting Polymers and Polymer Composites/Nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Aruljothi, C.; Balaji, P.; Vaishnavi, E.; Pazhanivel, T.; Vasuki, T. Magnetic Recyclable CuFe2O4/rGO Nanocomposite for the Degradation of Tetracycline under Sunlight Irradiation. J. Chem. Technol. Biotechnol. 2023, 98, 1908–1917. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Fuziki, M.E.K.; Tusset, A.M.; dos Santos, O.A.A.; Lenzi, G.G. Chlorophyll Sensitization of TiO2: A Mini-Review. Reactions 2023, 4, 766–778. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Rashmi, B.N.; Harlapur, S.F.; Gurushantha, K.; Ravikumar, C.R.; Kumar, M.R.A.; Santosh, M.S.; Kumar, V.G.D.; Kumar, A.N.; Azad, A.K.; Ananda Murthy, H.C. Facile Green Synthesis of Lanthanum Oxide Nanoparticles Using Centella asiatica and Tridax Plants: Photocatalytic, Electrochemical Sensor and Antimicrobial Studies. Appl. Surf. Sci. Adv. 2022, 7, 100210. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Prabhakar, U.P.S.; Shanmugam, P.; Boonyuen, S.; Chandrasekar, L.P.; Pothu, R.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Non-Covalent Functionalization of Surfactant-Assisted Graphene Oxide with Silver Nanocomposites for Highly Efficient Photocatalysis and Anti-Biofilm Applications. Mater. Sci. Energy Technol. 2024, 7, 205–215. [Google Scholar] [CrossRef]
- Boonyuen, S.; Smith, S.M.; Luengnaruemitchai, A.; Nakorn, P.N.; Tangjaideborisu, Y.; Shanmugam, P. Amplified Photocatalytic Decay of Organic Dyes and Antibiotic Pollutants Using Hexagonal Honeycomb Mesoporous Silica (SBA-15)/CeO2 Nanocomposites under Visible-Light Exposure. Inorg. Chem. Commun. 2025, 173, 113862. [Google Scholar] [CrossRef]
- Cheng, Z.; Meng, D.; Gao, S.; Wang, X.; Gao, D.; Guo, Q.; Hu, X.; Wang, L.; Song, J. In-Situ Synthesis of La2O3/Sepiolite Composite with Promoted Visible-Light Driven Photocatalytic Decomposition for Tetracycline. J. Water Process Eng. 2025, 69, 106709. [Google Scholar] [CrossRef]
- Parasuraman, B.; Shanmugam, P.; Govindasamy, P.; Nangan, S.; Gnanasekaran, L.; Thangavelu, P. Photocatalytic Degradation of Tetracycline Contaminated Wastewater over Bi2S3/BiWO6/rGO Ternary Nanocomposite under Visible Light Irradiation. J. Taiwan Inst. Chem. Eng. 2023, 166, 105249. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Kamaraj, C.; Ranjith, R.; Sivakumar, S.; Dewiani, R.Y.; Muqoyyanah; Thammasak, R.; Periyasami, G.; Murni, H. Synthesis of Multifunctional rGO/g-C3N4/FeTiO3 Ternary Nanocomposites for Photocatalyst, Antibacterial, and Ecotoxicity Assessment of Zebrafish Embryo Model. J. Alloys Compd. 2024, 1007, 176256. [Google Scholar] [CrossRef]
- Bhagya, N.P.; Prashanth, P.A.; Raveendra, R.S.; Sathyanarayani, S.; Ananda, S.; Nagabhushana, B.M.; Nagabhushana, H. Adsorption of Hazardous Cationic Dye onto the Combustion Derived SrTiO3 Nanoparticles: Kinetic and Isotherm Studies. J. Asian Ceram. Soc. 2016, 4, 68–74. [Google Scholar] [CrossRef]
- Abumousa, R.A. MgO@ZrO2@g-C3N4 Composite for Efficient Photodegradation of Alizarin Red Dye. Inorg. Chem. Commun. 2023, 155, 111086. [Google Scholar] [CrossRef]
- Aaga, G.F.; Anshebo, S.T. Green Synthesis of Highly Efficient and Stable Copper Oxide Nanoparticles Using an Aqueous Seed Extract of Moringa Stenopetala for Sunlight-Assisted Catalytic Degradation of Congo Red and Alizarin Red s. Heliyon 2023, 9, e16067. [Google Scholar] [CrossRef]
- Padmaja, B.; Dhanapandian, S.; Suthakaran, S.; Ashokkumar, K.; Krishnakumar, N. Hydrothermally Developed SnO2 Nanoparticles and Its Photocatalytic Degradation of Alizarin Red S, Brilliant Green and Methyl Orange Dyes and Electrochemical Performances. Inorg. Chem. Commun. 2023, 149, 110363. [Google Scholar] [CrossRef]
- Rao, Y.; Zhang, Y.; Li, A.; Zhang, T.; Jiao, T. Photocatalytic Activity of G-TiO2@Fe3O4 with Persulfate for Degradation of Alizarin Red S under Visible Light. Chemosphere 2021, 266, 129236. [Google Scholar] [CrossRef]
- Akshatha, S.; Sreenivasa, S.; Parashuram, L.; Udaya Kumar, V.; Sharma, S.C.; Nagabhushana, H.; Kuar, S.; Maiyalagan, T. Synergistic effect of hybrid Ce3+/Ce4+ doped Bi2O3 nano-sphere photocatalyst for enhanced photocatalytic degradation of alizarin red S dye and its NUV excited photoluminescence studies. J. Environ. Chem. Eng. 2019, 7, 103053. [Google Scholar] [CrossRef]
- Begum, S.; Ahmaruzzaman, M. Geen Synthesis of SnO2 Nanoparticles loaded on Activated Carbon and its Application as Photocalayst in the Degradation of Alizarin Red S Dye. Mater. Today Proc. 2018, 5, 2314–2320. [Google Scholar] [CrossRef]
- Jabeen, U.; Shah, S.M.; Khan, S.U. Photo Catalytic Degradation of Alizarin Red S Using ZnS and Cadmium Doped ZnS Nanoparticles under Unfiltered Sunlight. Surf. Interfaces 2017, 6, 40–49. [Google Scholar] [CrossRef]








| Phyto-Constituents | Inference | Phyto-Constituents | Inference |
|---|---|---|---|
| Carbohydrates | + | Anthraquinone Glycosides | + |
| Reducing Sugar | + | Saponin Glycosides | + |
| Hexose Sugar | + | Cyanogenic Glycosides | − |
| Non-Reducing Sugar (Starch) | + | Alkaloids | + |
| Proteins | − | Tannins | + |
| Amino Acids | − | Phenolic Compounds | − |
| Tyrosine | − | Flavonoids | − |
| Steroids | + | Terpenoids | + |
| Glycosides | + | Saponins | + |
| RT | Name of the Compound | Molecular Formula | Molecular Weight | Peak Area % |
|---|---|---|---|---|
| 5.811 | 1,1-diethoxy-3-methyl- butane | C9H20O2 | 160 | 1.06 |
| 5.843 | 1,1-diethoxy- pentane | C9H20O2 | 160 | 1.36 |
| 6.025 | 3,3-diethoxy-2-butanone | C8H16O3 | 160 | 1.00 |
| 7.840 | 1,1,3-triethoxy- propane | C9H20O3 | 176 | 35.82 |
| 16.590 | 2,5-dimethyl-5-hexen-3-ol | C8H16O | 128 | 1.22 |
| 18.739 | 5-cyclohexyl- undecane | C17H34 | 238 | 1.50 |
| 19.758 | diundecyl ester 1,2-benzenedicarboxylic acid | C30H50O4 | 474 | 1.32 |
| 19.889 | ethyl ester hexadecanoic acid | C18H36O2 | 284 | 5.99 |
| 20.851 | 1-hexadecanol | C16H34O | 242 | 6.18 |
| 21.075 | 1-cyclopentylethyl ester pentanoic acid | C12H22O2 | 198 | 1.01 |
| 21.194 | 3,7-dimethyl-6-octen-1-ol | C10H20O | 156 | 3.58 |
| 21.778 | 1,10-decanediol | C10H22O2 | 174 | 1.73 |
| 23.164 | Hexyl(hexyloxy)methanethioate | C13H26O2S | 246 | 1.19 |
| 23.824 | cis-3-hexenyl-.alpha.-methylbutyrate | C11H20O2 | 184 | 1.65 |
| 25.867 | tetrahydro-2h-pyran-2-one | C8H14O2 | 142 | 2.50 |
| 26.158 | 2-(2-propenyl)-1,4-butanediol | C7H14O2 | 130 | 2.45 |
| 26.322 | alpha-tocopheryl acetate | C31H52O3 | 472 | 10.72 |
| 26.648 | (2e,6e)-1,1-dideutero-3,7,11-trimethyl-2,6,10-dodecatrien-1-ol | C15H24D2O | 224 | 14.38 |
| 28.284 | 10-methyl-6-trimethylsilyl-5(z),9-undecadien-1-otms | C18H38OSi2 | 326 | 1.76 |
| 28.757 | dinonyl ester 1,2-benzenedicarboxylic acid | C26H42O4 | 418 | 3.57 |
| Tested Sample Concentration (μg/mL) | OD Value at 600 nm (in Triplicate) | ||
|---|---|---|---|
| Control | 1.577 | 1.532 | 1.561 |
| 500 µg/mL | 0.978 | 0.874 | 0.987 |
| 250 µg/mL | 1.032 | 1.036 | 1.060 |
| 125 µg/mL | 1.198 | 1.335 | 1.315 |
| 62.5 µg/mL | 1.363 | 1.372 | 1.335 |
| 31.25 µg/mL | 1.495 | 1.486 | 1.494 |
| Tested Sample Concentration (μg/mL) | Percentage of Inhibition (in Triplicate) | Mean Value (%) | ||
|---|---|---|---|---|
| Control | 100 | 100 | 100 | 100 |
| 500 µg/mL | 37.14 | 43.83 | 36.56 | 39.18 |
| 250 µg/mL | 33.67 | 33.41 | 31.87 | 32.99 |
| 125 µg/mL | 23.00 | 14.20 | 15.48 | 17.56 |
| 62.5 µg/mL | 12.40 | 11.82 | 14.20 | 12.81 |
| 31.25 µg/mL | 3.92 | 4.49 | 3.98 | 4.13 |
| Samples | Quantity of ARS (mg/L) | Dosage of Photocatalyst (mg) | Photocatalytic Duration | Light Source | Degradation Efficiency (%) | References |
|---|---|---|---|---|---|---|
| MgO@ ZrO2@g-C3N4 | 25 | 50 | 60 | OSRAM lamp 58 IM/W | 92% | [57] |
| CuO NPs | 40 | 100 | 120 | Solar light | 95.4 | [58] |
| SnO2 | 3.5 | 5 | 120 | Sun light | 39 | [59] |
| G-TiO2@Fe3O4/PS/Vis | 100 | 250 | 60 | Visible light | 100 | [60] |
| Ce3+/Ce4+/Bi2O3/Vis | 20 | 1000 | 120 | Visible light | 78 | [61] |
| SnO2-NP-AC | 35 | 75 | 60 | Sun light | 99 | [62] |
| Cd–ZnS | 4 | 500 | 200 | Sunlight | 96.7 | [63] |
| La2O3 | 10 | 50 | 300 | 150 W Xe | 90.12 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakami, K.U.; Vaideeswaran, S.; Rosevenis, A.; Boddula, R.; Shenbagam, K.; Balaganesh, M.; Prabhakar, U.P.S.; Shanmugam, P.; Barakat, F.M.; Boonyuen, S.; et al. Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles. Reactions 2025, 6, 32. https://doi.org/10.3390/reactions6020032
Sivakami KU, Vaideeswaran S, Rosevenis A, Boddula R, Shenbagam K, Balaganesh M, Prabhakar UPS, Shanmugam P, Barakat FM, Boonyuen S, et al. Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles. Reactions. 2025; 6(2):32. https://doi.org/10.3390/reactions6020032
Chicago/Turabian StyleSivakami, Krishnamoorthy Uma, Sundararajan Vaideeswaran, Ambrose Rosevenis, Rajender Boddula, Kanagarajan Shenbagam, Muniraj Balaganesh, Usan Pathinathan Saleth Prabhakar, Paramasivam Shanmugam, Fatemah M. Barakat, Supakorn Boonyuen, and et al. 2025. "Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles" Reactions 6, no. 2: 32. https://doi.org/10.3390/reactions6020032
APA StyleSivakami, K. U., Vaideeswaran, S., Rosevenis, A., Boddula, R., Shenbagam, K., Balaganesh, M., Prabhakar, U. P. S., Shanmugam, P., Barakat, F. M., Boonyuen, S., & Pothu, R. (2025). Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles. Reactions, 6(2), 32. https://doi.org/10.3390/reactions6020032








