Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Assembly and Study Selection
2.2. Hydrogen Production Normalization
2.3. Statistical Analysis
2.3.1. Descriptive Statistics and Analysis of Variance (ANOVA)
2.3.2. Principal Component Analysis (PCA) for Microbial Communities, Pearson Correlation Analysis for VFAs, and t-Test
2.4. Heatmap Visualization of Microbial Contributions and Methanogen Suppression Experiment
3. Result and Discussion
3.1. H2 Production and Substrate Impact
3.2. Volatile Fatty Acids and Their Impact on H2 Production
3.3. Microbial Consortia Toward H2 Production
3.4. Suppression of Methanogenesis and Its Influence on H2 Production
3.5. Temperature and pH Optimization
4. Significance for Industrial Processes and Research Prospects
| Spices Name | Role | Pathway | Examples | Conditions | References |
|---|---|---|---|---|---|
| Clostridium spp. | One of the most essential genera is involved in hydrogen production, especially in dark fermentation processes. | Clostridium species use the butyrate and acetate pathways during fermentation. They break down complex carbohydrates into simpler compounds like acetate, butyrate, and hydrogen. | Clostridium butyricum Clostridium thermocellum Clostridium acetobutylicum | These species thrive in low pH (typically between 5.0 and 6.0) and anaerobic environments, making them suitable for hydrogen production in mesophilic and thermophilic conditions. | [97,110] |
| Thermotoga spp. | They are known for producing hydrogen under thermophilic conditions (55–80 °C). They are hyperthermophilic bacteria that excel at breaking sugars and starches into hydrogen and acetate. | They use a fermentation pathway to convert glucose into hydrogen, carbon dioxide, and organic acids. | Thermotoga maritima Thermotoga neapolitana | Optimal hydrogen production occurs at high temperatures (around 70 °C), suppressing hydrogen-consuming methanogens. | [71,77] |
| Enterobacter spp. | These facultative anaerobic bacteria can produce hydrogen in dark fermentation, primarily when grown with organic substrates like glucose or starch. | Hydrogen production occurs through a mixed-acid fermentation pathway where organic acids like acetate, butyrate, and ethanol are produced alongside hydrogen. | Enterobacter cloacae Enterobacter aerogenes | Enterobacter species are more tolerant to pH variations and can operate under aerobic and anaerobic conditions, though hydrogen production is higher under anaerobic conditions. | [23] |
| Ruminococcus spp. | These microbes, which originate from the gut microbiome of ruminant animals, break down complex carbohydrates like cellulose and produce hydrogen by enzymatic means. | Like Clostridium, Ruminococcus species ferment complex polysaccharides into hydrogen, acetate, and butyrate. | Ruminococcus albus Ruminococcus flavefaciens | These species thrive in anaerobic environments, producing optimal hydrogen at neutral pH. | [46] |
| Bacillus spp. | Bacillus can produce hydrogen from carbohydrates and organic wastes, particularly under anaerobic and thermophilic conditions. | They ferment sugars and organic acids to produce hydrogen, primarily through the butyrate pathway. | Bacillus licheniformis Bacillus cereus | Bacillus species tolerate various environmental conditions, including pH and temperature variations. | [3,81] |
| Ethanoligenens spp. | This genus is involved in the dark fermentation of organic materials into hydrogen. | These bacteria use simple sugars and produce hydrogen, ethanol, and acetate. | Ethanoligenens harbinense | Anaerobic conditions with an acidic to neutral pH (around 5.5 to 7) are optimal for hydrogen production. | [11,70] |
| Caldicellulosiruptor spp. | These thermophilic bacteria are capable of degrading complex lignocellulosic biomass into hydrogen. | They efficiently convert cellulose and other biomass into hydrogen through fermentation. | Caldicellulosiruptor saccharolyticus Caldicellulosiruptor bescii | Optimal hydrogen production occurs at temperatures between 65 °C and 75 °C, and they are highly effective in converting plant biomass into hydrogen. | [77,97] |
| Desulfovibrio spp. | Although primarily known for their sulfate-reducing capabilities, some Desulfovibrio species can produce hydrogen under specific conditions. | These bacteria reduce protons to form hydrogen as a by-product of sulfate reduction in environments lacking sulfate. | Desulfovibrio vulgaris | Desulfovibrio can operate in anaerobic environments with a wide range of pH and temperatures, often found in microbial electrolysis cells (MECs). | [19,55] |
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tariq, M.; Liu, Y.; Rizwan, A.; Shoukat, C.A.; Aftab, Q.; Lu, J.; Zhang, Y. Impact of Elevated CO2 on Soil Microbiota: A Meta-Analytical Review of Carbon and Nitrogen Metabolism. Sci. Total Environ. 2024, 950, 175354. [Google Scholar] [CrossRef] [PubMed]
- Qazi, A.; Hussain, F.; Rahim, N.A.B.D.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Otlewska, A. Biohydrogen Production from Fruit and Vegetable Waste, Sugar Beet Pulp and Corn Silage via Dark Fermentation. Renew. Energy 2020, 153, 1226–1237. [Google Scholar] [CrossRef]
- Koul, Y.; Devda, V.; Varjani, S.; Guo, W.; Ngo, H.H.; Taherzadeh, M.J.; Chang, J.-S.; Wong, J.W.C.; Bilal, M.; Kim, S.-H.; et al. Microbial Electrolysis: A Promising Approach for Treatment and Resource Recovery from Industrial Wastewater. Bioengineered 2022, 13, 8115–8134. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, J.L.; Veerubhotla, R.; Pandit, S.; Das, D. Biohydrogen Production Using Microbial Electrolysis Cell. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 843–869. [Google Scholar]
- Nabgan, W.; Tuan Abdullah, T.A.; Nabgan, B.; Jalil, A.A.; Nordin, A.H.; Ul-Hamid, A.; Hassan, N.S.; Hussain, I.; Coelho, A.; Amin, A.; et al. Catalytic Biohydrogen Production from Organic Waste Materials: A Literature Review and Bibliometric Analysis. Int. J. Hydrog. Energy 2021, 46, 30903–30925. [Google Scholar] [CrossRef]
- Lee, D.-J.; Lee, S.-Y.; Bae, J.-S.; Kang, J.-G.; Kim, K.-H.; Rhee, S.-S.; Park, J.-H.; Cho, J.-S.; Chung, J.; Seo, D.-C. Effect of Volatile Fatty Acid Concentration on Anaerobic Degradation Rate from Field Anaerobic Digestion Facilities Treating Food Waste Leachate in South Korea. J. Chem. 2015, 2015, 640717. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic Bioconversion of Food Waste into Energy: A Critical Review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies 2021, 14, 1831. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H.; Pandey, A. Dark Fermentation: Production and Utilization of Volatile Fatty Acid from Different Wastes—A Review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Wang, B.; Zhou, D. Effects of the Applied Voltage on Electroactive Microbial Biofilm Viability and Hydrogen Production in a Recalcitrant Organic Waste-Fed Single-Chamber Membrane-Free Microbial Electrolysis Cell Performance. Chem. Eng. J. 2023, 469, 144002. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial Electrohydrogenesis Cell and Dark Fermentation Integrated System Enhances Biohydrogen Production from Lignocellulosic Agricultural Wastes: Substrate Pretreatment towards Optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Yan, X.; Wang, B.; Liang, H.; Yang, J.; Zhao, J.; Ndayisenga, F.; Zhang, H.; Yu, Z.; Qian, Z. Enhanced Straw Fermentation Process Based on Microbial Electrolysis Cell Coupled Anaerobic Digestion. Chin. J. Chem. Eng. 2022, 44, 239–245. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Abd Rahman, N. Food Waste and Food Processing Waste for Biohydrogen Production: A Review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santiago, V.; Valdez-Vazquez, I.; Vital-Jácome, M.; Zavala-Méndez, M.; Razo-Flores, E.; Carrillo-Reyes, J. Carbohydrates/Acid Ratios Drives Microbial Communities and Metabolic Pathways during Biohydrogen Production from Fermented Agro-Industrial Wastewater. J. Environ. Chem. Eng. 2023, 11, 110302. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of Bacteria and Archaea Participating in the Bioconversion of Organic Waste for Methane Production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef]
- Dessì, P.; Porca, E.; Waters, N.R.; Lakaniemi, A.-M.; Collins, G.; Lens, P.N.L. Thermophilic versus Mesophilic Dark Fermentation in Xylose-Fed Fluidised Bed Reactors: Biohydrogen Production and Active Microbial Community. Int. J. Hydrog. Energy 2018, 43, 5473–5485. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Yoro, K.O.; Bodunrin, M.O.; Ayeni, A.O.; Daramola, M.O. Integrated System Approach to Dark Fermentative Biohydrogen Production for Enhanced Yield, Energy Efficiency and Substrate Recovery. Rev. Environ. Sci. Biotechnol. 2018, 17, 501–529. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Wang, B.; Wu, G.; Zhang, H.; Phulpoto, I.A.; Zhao, J.; Yang, J. Thermophilic-Operating Environment Promotes Hydrogen-Producing Microbial Growth in a Lignocellulose-Fed DF-MEC System for Enhanced Biohydrogen Evolution. Process Saf. Environ. Prot. 2022, 167, 213–224. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.-h.; Wu, G.; Zhan, X. Impact of Total Solids Content on Anaerobic Co-Digestion of Pig Manure and Food Waste: Insights into Shifting of the Methanogenic Pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef]
- Danko, A.S.; Pinheiro, F.; Abreu, Â.A.; Alves, M.M. Effect of Methanogenic Inhibitors, Inocula Type, and Temperature on Biohydrogen Production from Food Components. Environ. Eng. Manag. J. 2008, 7, 531–536. [Google Scholar]
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of Antibiotics on Anaerobic Digestion of Sewage Sludge: Performance of Anaerobic Digestion and Structure of the Microbial Community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef] [PubMed]
- Ndayisenga, F.; Yu, Z.; Wang, B.; Yang, J.; Wu, G.; Zhang, H. Using Bioelectrohydrogenesis Left-over Residues as a Future Potential Fertilizer for Soil Amendment. Sci. Rep. 2022, 12, 17779. [Google Scholar] [CrossRef] [PubMed]
- Gallipoli, A.; Braguglia, C.M.; Gianico, A.; Montecchio, D.; Pagliaccia, P. Kitchen Waste Valorization through a Mild-Temperature Pretreatment to Enhance Biogas Production and Fermentability: Kinetics Study in Mesophilic and Thermophilic Regimen. J. Environ. Sci. 2020, 89, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Law, A.W.S.; Rubio Rincón, F.; van de Vossenberg, J.; Al Saffar, Z.; Welles, L.; Rene, E.R.; Lopez Vazquez, C. Volatile Fatty Acid Production from Food Waste: The Effect of Retention Time and Lipid Content. Bioresour. Technol. 2023, 367, 128298. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Xu, K.-Q.; Kobayashi, T.; Li, Y.-Y.; Inamori, Y. Effect of Organic Loading Rate on Continuous Hydrogen Production from Food Waste in Submerged Anaerobic Membrane Bioreactor. Int. J. Hydrog. Energy 2014, 39, 16863–16871. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Berrocal-Bravo, M.J.; Valdez-Vazquez, I. Biohydrogen Production from Food Waste and Waste Activated Sludge in Codigestion: Influence of Organic Loading Rate and Changes in Microbial Community. J. Chem. Technol. Biotechnol. 2023, 98, 230–237. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal Component Analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Increasing Power Generation for Scaling up Single-Chamber Air Cathode Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, M. Microbial Fuel Cells—Challenges and Applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 9781119113478. [Google Scholar]
- Hahs-Vaughn, D.L.; Lomax, R.G. An Introduction to Statistical Concepts; Routledge: London, UK, 2020; ISBN 9781315624358. [Google Scholar]
- Jerrold, H.Z. Confidence Limits for a Population Proportion. In Biostatistical Analysis, 5th ed.; Pearson Education, Inc.: Hoboken, NJ, USA, 2010; pp. 543–548. [Google Scholar]
- Jolliffe, I. Principal Component Analysis. In Encyclopedia of Statistics in Behavioral Science; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lee Rodgers, J.; Nicewander, W.A. Thirteen Ways to Look at the Correlation Coefficient. Am. Stat. 1988, 42, 59–66. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-F.; Li, Y.-Y.; Xu, K.-Q.; Ebie, Y.; Inamori, Y.; Kong, H.-N. A PH- and Temperature-Phased Two-Stage Process for Hydrogen and Methane Production from Food Waste. Int. J. Hydrog. Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Bio, P.; Daripada, H.; Makanan, S.; Anaerobik, F. Bio-Hydrogen Production from Food Waste through Anaerobic Fermentation. Sains Malays. 2014, 43, 1927–1936. [Google Scholar]
- Al-Haddad, S.; Okoro-Shekwaga, C.K.; Fletcher, L.; Ross, A.; Camargo-Valero, M.A. Assessing Different Inoculum Treatments for Improved Production of Hydrogen through Dark Fermentation. Energies 2023, 16, 1233. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Lee, S.-M.; Cha, J.; Lee, H.S.; Kang, S.G. Biohydrogen Production from Food Waste Using Glucose-Adapted Hyperthermophilic Archaeon. Waste Biomass Valorization 2023, 14, 2923–2930. [Google Scholar] [CrossRef]
- Liu, Z. A Review on the Emerging Conversion Technology of Cellulose, Starch, Lignin, Protein and Other Organics from Vegetable-Fruit-Based Waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, L.; Nithya, K.; Loh, K.-C.; Dai, Y.; He, Y.; Wah Tong, Y. Optimizing Mixing Strategy to Improve the Performance of an Anaerobic Digestion Waste-to-Energy System for Energy Recovery from Food Waste. Appl. Energy 2019, 249, 28–36. [Google Scholar] [CrossRef]
- Ilakovac, B.; Voca, N.; Pezo, L.; Cerjak, M. Quantification and Determination of Household Food Waste and Its Relation to Sociodemographic Characteristics in Croatia. Waste Manag. 2020, 102, 231–240. [Google Scholar] [CrossRef]
- Chu, C.-F.; Ebie, Y.; Xu, K.-Q.; Li, Y.-Y.; Inamori, Y. Characterization of Microbial Community in the Two-Stage Process for Hydrogen and Methane Production from Food Waste. Int. J. Hydrog. Energy 2010, 35, 8253–8261. [Google Scholar] [CrossRef]
- Laothanachareon, T.; Kanchanasuta, S.; Mhuanthong, W.; Phalakornkule, C.; Pisutpaisal, N.; Champreda, V. Analysis of Microbial Community Adaptation in Mesophilic Hydrogen Fermentation from Food Waste by Tagged 16S RRNA Gene Pyrosequencing. J. Environ. Manag. 2014, 144, 143–151. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Wang, J.; Li, Y.; Ma, L.; Chen, W.; Duan, Y.; Zhai, Y.; Li, D. Microbial Community Dynamics and Volatile Fatty Acid Production during Anaerobic Digestion of Microaerated Food Waste under Different Organic Loadings. Bioresour. Technol. Rep. 2024, 27, 101949. [Google Scholar] [CrossRef]
- Li, Y.; Su, D.; Feng, H.; Yan, F.; Liu, H.; Feng, L.; Liu, G. Anaerobic Acidogenic Fermentation of Food Waste for Mixed-Acid Production. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 631–635. [Google Scholar] [CrossRef]
- Schroder, C.; Selig, M.; Schonheit, P. Glucose Fermentation to Acetate, CO2 and H2 in the Anaerobic Hyperthermophilic Eubacterium Thermotoga Maritima: Involvement of the Embden-Meyerhof Pathway. Arch. Microbiol. 1994, 161, 460–470. [Google Scholar] [CrossRef]
- Peters, D. Carbohydrates for Fermentation. Biotechnol. J. 2006, 1, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Litti, Y.V.; Khuraseva, N.D.; Vishnyakova, A.V.; Zhuravleva, E.A.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.A.; Parshina, S.N. Comparative Study on Biohydrogen Production by Newly Isolated Clostridium Butyricum SP4 and Clostridium Beijerinckii SP6. Int. J. Hydrog. Energy 2023, 48, 27540–27556. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of Initial Uncontrolled PH on Acidogenic Fermentation of Brewery Spent Grains to Biohydrogen and Volatile Fatty Acids Production: Optimization and Scale-Up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef]
- Gaspari, M.; Treu, L.; Centurion, V.B.; Kotsopoulos, T.A.; Campanaro, S.; Kougias, P.G. Impacts of Long Chain Fatty Acids Injection on Biogas Reactors Performance Stability and Microbial Community Structure and Function. J. Clean. Prod. 2023, 418, 138048. [Google Scholar] [CrossRef]
- Silva, S.A.; Cavaleiro, A.J.; Pereira, M.A.; Stams, A.J.M.; Alves, M.M.; Sousa, D.Z. Long-Term Acclimation of Anaerobic Sludges for High-Rate Methanogenesis from LCFA. Biomass Bioenergy 2014, 67, 297–303. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Croese, E.; Pereira, M.A.; Euverink, G.-J.W.; Stams, A.J.M.; Geelhoed, J.S. Analysis of the Microbial Community of the Biocathode of a Hydrogen-Producing Microbial Electrolysis Cell. Appl. Microbiol. Biotechnol. 2011, 92, 1083–1093. [Google Scholar] [CrossRef]
- Jodhani, S.; Sebastian, J.; Lee, J.; Venkiteshwaran, K.; Lee, H.-S.; Singh, V.; Ormeci, B.; Hussain, A. Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment. Fermentation 2024, 10, 162. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Acidogenic Fermentation of Food Waste for Production of Volatile Fatty Acids: Bacterial Community Analysis and Semi-Continuous Operation. Waste Manag. 2020, 109, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, N. The Effects of Volatile Fatty Acids on the Performance of The Effects of Volatile Fatty Acids on the Performance of Microbial Electrolysis Cells. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2015. [Google Scholar]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Dinopoulou, G.; Sterritt, R.M.; Lester, J.N. Anaerobic Acidogenesis of a Complex Wastewater: II. Kinetics of Growth, Inhibition, and Product Formation. Biotechnol. Bioeng. 1988, 31, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, C.B.; Mendez-Acosta, H.O.; García-Sandoval, J.P.; Leal-Ascencio, T.; Hernandez-Martinez, E. A Simple Unstructured Kinetic Model for Anaerobic Treatment of a Class of Agro-industrial Waste. J. Chem. Technol. Biotechnol. 2023, 98, 257–268. [Google Scholar] [CrossRef]
- Veeken, A.; Kalyuzhnyi, S.; Scharff, H.; Hamelers, B. Effect of PH and VFA on Hydrolysis of Organic Solid Waste. J. Environ. Eng. 2000, 126, 1076–1081. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Short-Chain Fatty Acids and Hydrogen Production in One Single Anaerobic Fermentation Stage Using Carbohydrate-Rich Food Waste. J. Clean. Prod. 2021, 284, 124727. [Google Scholar] [CrossRef]
- Dahiya, S.; Mohan, S.V. Selective Control of Volatile Fatty Acids Production from Food Waste by Regulating Biosystem Buffering: A Comprehensive Study. Chem. Eng. J. 2019, 357, 787–801. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Girón-Bol, L.M.; Gómez-Muñoz, D.F.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P. Thermal Pre-Treatment as a Tool for Energy Recovery from Food Waste through Anaerobic Digestion. Effect on Kinetic and Physicochemical Characteristics of the Substrate. Environ. Technol. Innov. 2021, 21, 101262. [Google Scholar] [CrossRef]
- García-Depraect, O.; León-Becerril, E. Fermentative Biohydrogen Production from Tequila Vinasse via the Lactate-Acetate Pathway: Operational Performance, Kinetic Analysis and Microbial Ecology. Fuel 2018, 234, 151–160. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Luo, L.; Liu, H. Startup Performance of Microbial Electrolysis Cell Assisted Anaerobic Digester (MEC-AD) with Pre-Acclimated Activated Carbon. Bioresour. Technol. Rep. 2019, 5, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jariyaboon, R.; Hayeeyunu, S.; Usmanbaha, N.; Bin Ismail, S.; O-Thong, S.; Mamimin, C.; Kongjan, P. Thermophilic Dark Fermentation for Simultaneous Mixed Volatile Fatty Acids and Biohydrogen Production from Food Waste. Fermentation 2023, 9, 636. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Wang, B.; Wu, G.; Zhang, H. Synergistic Application of Thermally-Pretreated-Biocatalyst and Dark-Fermentative Process Coupled with Bioelectrohydrogenesis Promotes Biohydrogen Production from Agricultural Straw Wastes. Energy Convers. Manag. X 2024, 22, 100541. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Rupani, P.F.; Krishnan, S.; Ahmad, F.; Rezania, S.; Rashid, M.A.; Sha, C.; Md Din, M.F. Biohydrogen Production via Thermophilic Fermentation: A Prospective Application of Thermotoga Species. Energy 2020, 197, 117199. [Google Scholar] [CrossRef]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and Applications of Clostridium Co-Culture Systems in Biotechnology. Front. Microbiol. 2020, 11, 560223. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Trably, E.; Hamelin, J.; Latrille, E.; Meynial-Salles, I.; Benomar, S.; Giudici-Orticoni, M.-T.; Steyer, J.-P. Sub-Dominant Bacteria as Keystone Species in Microbial Communities Producing Bio-Hydrogen. Int. J. Hydrog. Energy 2013, 38, 4975–4985. [Google Scholar] [CrossRef]
- Zuroff, T.R.; Xiques, S.B.; Curtis, W.R. Consortia-Mediated Bioprocessing of Cellulose to Ethanol with a Symbiotic Clostridium Phytofermentans/Yeast Co-Culture. Biotechnol. Biofuels 2013, 6, 59. [Google Scholar] [CrossRef]
- Habashy, M.M.; Ong, E.S.; Abdeldayem, O.M.; Al-Sakkari, E.G.; Rene, E.R. Food Waste: A Promising Source of Sustainable Biohydrogen Fuel. Trends Biotechnol. 2021, 39, 1274–1288. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Wang, Y.; Liu, H. Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste. Fermentation 2024, 10, 179. [Google Scholar] [CrossRef]
- Thong, S.; Mamimin, C.; Kongjan, P.; Reungsang, A. Thermophilic Fermentation for Enhanced Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–139. [Google Scholar]
- Catal, T.; Lesnik, K.L.; Liu, H. Suppression of Methanogenesis for Hydrogen Production in Single-Chamber Microbial Electrolysis Cells Using Various Antibiotics. Bioresour. Technol. 2015, 187, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-G.; Rhee, C.; Shin, S.G.; Shin, J.; Mohamed, H.O.; Choi, Y.-J.; Chae, K.-J. Methanogenesis Stimulation and Inhibition for the Production of Different Target Electrobiofuels in Microbial Electrolysis Cells through an On-Demand Control Strategy Using the Coenzyme M and 2-Bromoethanesulfonate. Environ. Int. 2019, 131, 105006. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Lee, M. 2-Bromoethanesulfonate Affects Bacteria in a Trichloroethene-Dechlorinating Culture. Appl. Environ. Microbiol. 2001, 67, 2371–2374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karthikeyan, R.; Cheng, K.Y.; Selvam, A.; Bose, A.; Wong, J.W.C. Bioelectrohydrogenesis and Inhibition of Methanogenic Activity in Microbial Electrolysis Cells—A Review. Biotechnol. Adv. 2017, 35, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Park, W.; Kim, C.-W.; Kim, I.S. Methanogenesis Control by Employing Various Environmental Stress Conditions in Two-Chambered Microbial Fuel Cells. Bioresour. Technol. 2010, 101, 5350–5357. [Google Scholar] [CrossRef]
- Khan, S.; Deng, Z.; Phulpoto, I.A.; Jalil, A.; Wang, B.; Yu, Z. Synergistic Impacts of Wheat Straw on Coal Bio-Methanation: Insights into Microbial Community Dynamics Toward Nontargeted Metabolomics. Int. J. Energy Res. 2024, 2024. [Google Scholar] [CrossRef]
- Martinez-Fernandez, G.; Denman, S.E.; Yang, C.; Cheung, J.; Mitsumori, M.; McSweeney, C.S. Methane Inhibition Alters the Microbial Community, Hydrogen Flow, and Fermentation Response in the Rumen of Cattle. Front. Microbiol. 2016, 7, 01122. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological Insights into Anaerobic Digestion for Biogas, Hydrogen or Volatile Fatty Acids (VFAs): A Review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Su, X.; Zhao, W.; Xia, D. The Diversity of Hydrogen-Producing Bacteria and Methanogens within an in situ Coal Seam. Biotechnol. Biofuels 2018, 11, 245. [Google Scholar] [CrossRef]
- Indugu, N.; Narayan, K.; Stefenoni, H.A.; Hennessy, M.L.; Vecchiarelli, B.; Bender, J.S.; Shah, R.; Dai, G.; Garapati, S.; Yarish, C.; et al. Microbiome-Informed Study of the Mechanistic Basis of Methane Inhibition by Asparagopsis taxiformis in Dairy Cattle. mBio 2024, 15, e00782-24. [Google Scholar] [CrossRef]
- Castro-Villalobos, M.C.; García-Morales, J.L.; Fernández, F.J. By-Products Inhibition Effects on Bio-Hydrogen Production. Int. J. Hydrog. Energy 2012, 37, 7077–7083. [Google Scholar] [CrossRef]
- Vargas, J.V.C.; Kava, V.; Balmant, W.; Mariano, A.B.; Ordonez, J.C. Modeling Microalgae Derived Hydrogen Production Enhancement via Genetic Modification. Int. J. Hydrog. Energy 2016, 41, 8101–8110. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Pan, J.; Liu, Y.; Duan, C.-H.; Li, M. Genomic and Transcriptomic Insights into Methanogenesis Potential of Novel Methanogens from Mangrove Sediments. Microbiome 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Morra, S.; Arizzi, M.; Allegra, P.; La Licata, B.; Sagnelli, F.; Zitella, P.; Gilardi, G.; Valetti, F. Expression of Different Types of [FeFe]-Hydrogenase Genes in Bacteria Isolated from a Population of a Bio-Hydrogen Pilot-Scale Plant. Int. J. Hydrog. Energy 2014, 39, 9018–9027. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef]
- Xiong, Y.; Harb, M.; Hong, P.-Y. Performance and Microbial Community Variations of Anaerobic Digesters under Increasing Tetracycline Concentrations. Appl. Microbiol. Biotechnol. 2017, 101, 5505–5517. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Ye, Y.; Bui, X.T.; Hoang, N.B. Advanced Strategies for Enhancing Dark Fermentative Biohydrogen Production from Biowaste towards Sustainable Environment. Bioresour. Technol. 2022, 351, 127045. [Google Scholar] [CrossRef]
- Khetkorn, W.; Khanna, N.; Incharoensakdi, A.; Lindblad, P. Metabolic and Genetic Engineering of Cyanobacteria for Enhanced Hydrogen Production. Biofuels 2013, 4, 535–561. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Pengadeth, D. Mixed Culture Biotechnology and Its Versatility in Dark Fermentative Hydrogen Production. Bioresour. Technol. 2024, 394, 130286. [Google Scholar] [CrossRef]
- Liu, I.-C.; Whang, L.-M.; Ren, W.-J.; Lin, P.-Y. The Effect of PH on the Production of Biohydrogen by Clostridia: Thermodynamic and Metabolic Considerations. Int. J. Hydrog. Energy 2011, 36, 439–449. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Tu, W.; Xia, J.; Zou, Y.; Gong, X.; Yu, P.; Huang, W.E.; Wang, H. Deep Insight into Oriented Propionate Production from Food Waste: Microbiological Interpretation and Design Practice. Water Res. 2023, 243, 120399. [Google Scholar] [CrossRef] [PubMed]
- Santiago, S.G.; Morgan-Sagastume, J.M.; Monroy, O.; Moreno-Andrade, I. Biohydrogen Production from Organic Solid Waste in a Sequencing Batch Reactor: An Optimization of the Hydraulic and Solids Retention Time. Int. J. Hydrog. Energy 2020, 45, 25681–25688. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial Fuel Cell Hybrid Systems for Wastewater Treatment and Bioenergy Production: Synergistic Effects, Mechanisms and Challenges. Renew. Sustain. Energy Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. The Effects of PH on the Production of Volatile Fatty Acids and Microbial Dynamics in Long-Term Reactor Operation. J. Environ. Manag. 2022, 319, 115700. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, B.H.; Voorthuyzen, H.; Bergveld, P. The PH-Static Enzyme Sensor: Design of the PH Control System. Sens. Actuators B Chem. 1990, 1, 546–549. [Google Scholar] [CrossRef]
- Comer, J.E.A.; Hibbert, C. PH Electrode Performance under Automated Management Conditions. J. Anal. Methods Chem. 1997, 19, 213–224. [Google Scholar] [CrossRef]
- Dahiya, S.; Venkata Mohan, S. Synergy of Selective Buffering, Intermittent PH Control and Bioreactor Configuration on Acidogenic Volatile Fatty Acid Production from Food Waste. Chemosphere 2022, 302, 134755. [Google Scholar] [CrossRef]
- NGUYEN, T.; PYOKIM, J.; SUNKIM, M.; KWANOH, Y.; SIM, S. Optimization of Hydrogen Production by Hyperthermophilic Eubacteria, Thermotoga Maritima and Thermotoga Neapolitana in Batch Fermentation. Int. J. Hydrog. Energy 2008, 33, 1483–1488. [Google Scholar] [CrossRef]
- Abo-Hashesh, M.; Wang, R.; Hallenbeck, P.C. Metabolic Engineering in Dark Fermentative Hydrogen Production; Theory and Practice. Bioresour. Technol. 2011, 102, 8414–8422. [Google Scholar] [CrossRef]
- Wang, S.; Tang, H.; Peng, F.; Yu, X.; Su, H.; Xu, P.; Tan, T. Metabolite-Based Mutualism Enhances Hydrogen Production in a Two-Species Microbial Consortium. Commun. Biol. 2019, 2, 82. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.-Z. Co-Culture of Clostridium Thermocellum and Clostridium Thermosaccharolyticum for Enhancing Hydrogen Production via Thermophilic Fermentation of Cornstalk Waste. Int. J. Hydrog. Energy 2012, 37, 10648–10654. [Google Scholar] [CrossRef]
- Beschkov, V.; Parvanova-Mancheva, T.; Vasileva, E. Experimental Study of Bio-Hydrogen Production by Clostridium Beijerinckii from Different Substrates. Energies 2023, 16, 2747. [Google Scholar] [CrossRef]
- Sim, Y.-B.; Yang, J.; Kim, S.M.; Joo, H.-H.; Jung, J.-H.; Kim, D.-H.; Kim, S.-H. Effect of Bioaugmentation Using Clostridium Butyricum on the Start-up and the Performance of Continuous Biohydrogen Production. Bioresour. Technol. 2022, 366, 128181. [Google Scholar] [CrossRef] [PubMed]
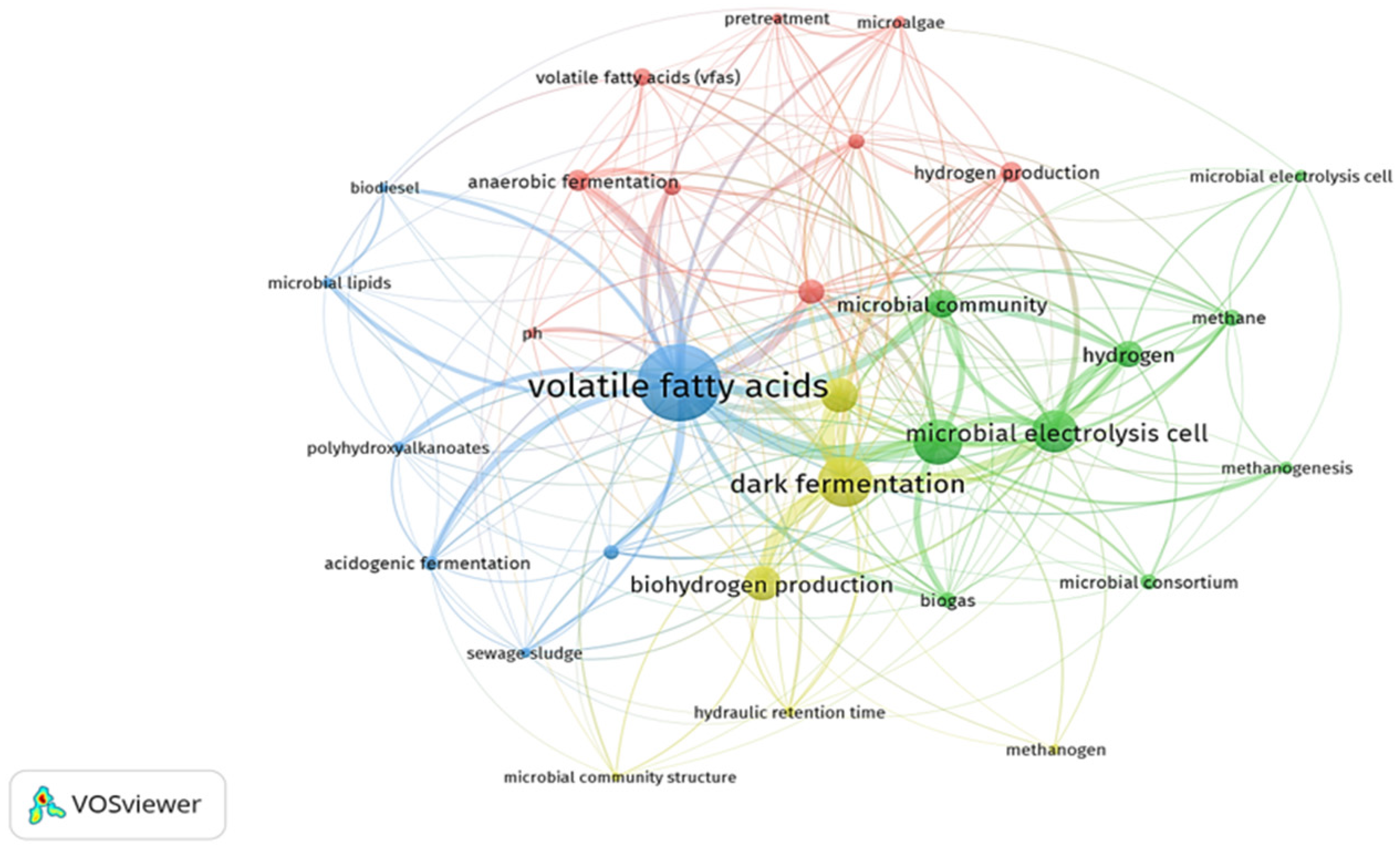
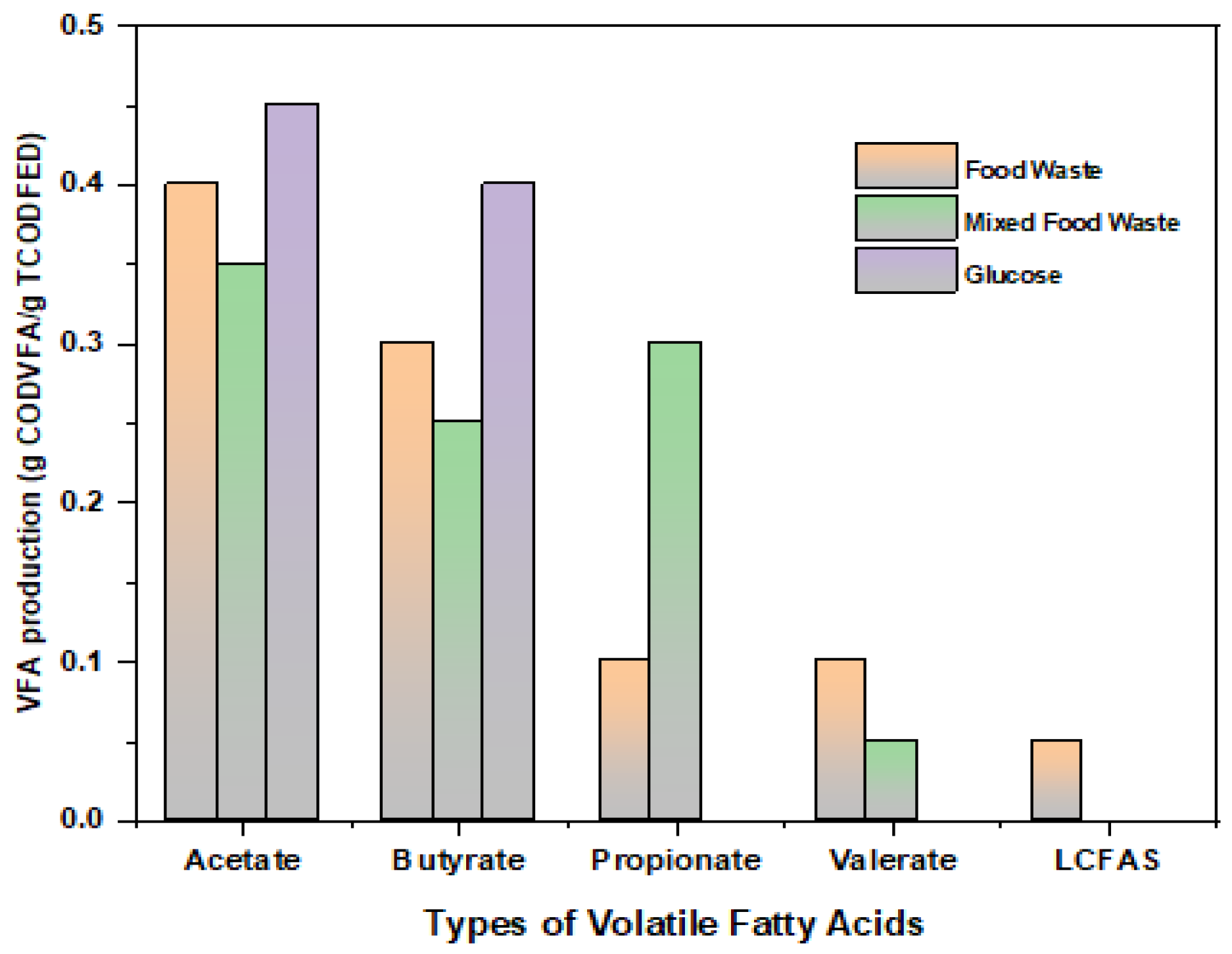
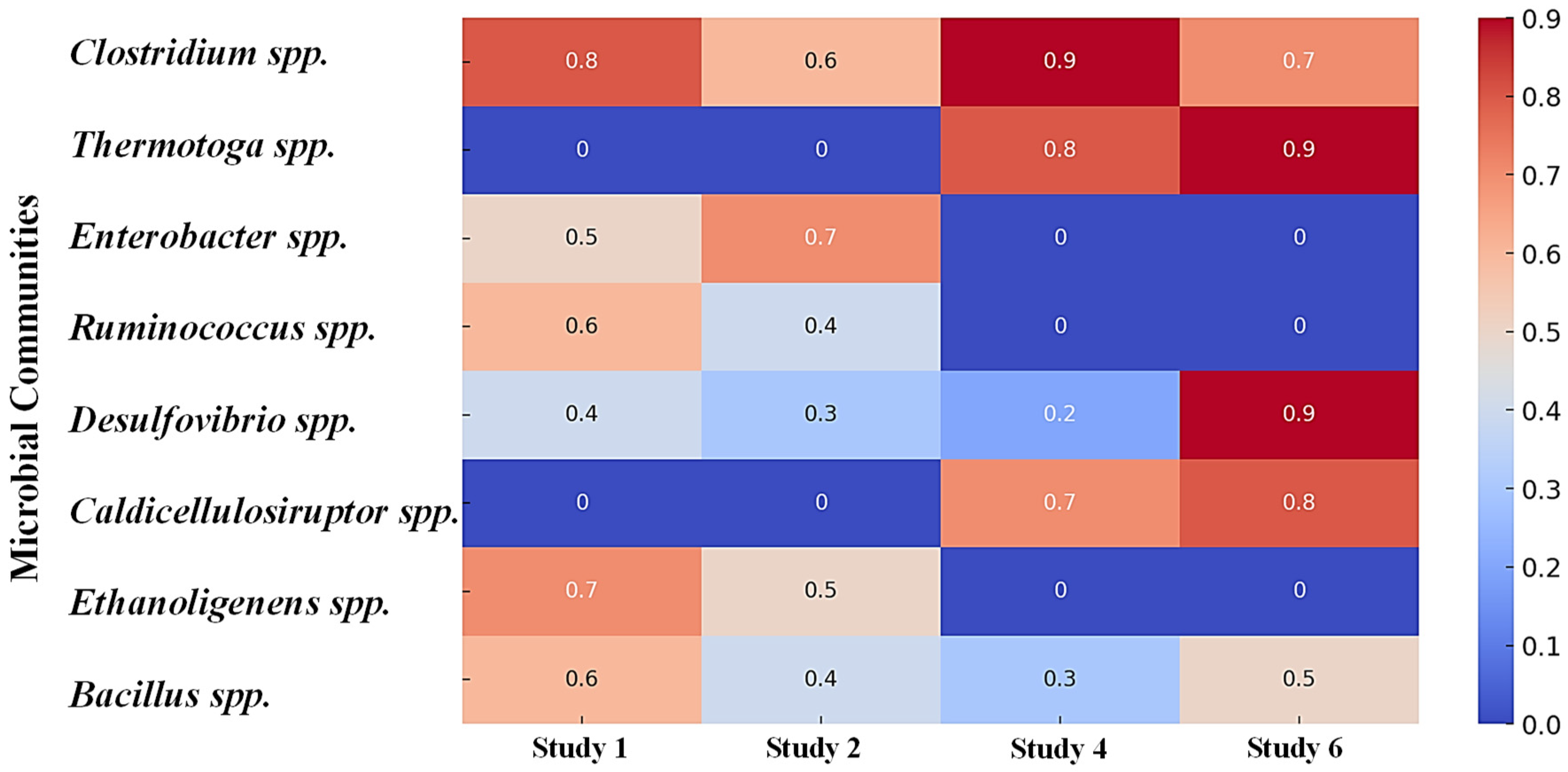
| Parameters | t-Test | p-Value | r Value | CI (95%) | Average | S.D. | References |
|---|---|---|---|---|---|---|---|
| Hydrogen production | 4.32 | 0.02 | 0.85 | 120, 220 | 168.57 | ±52.09 | [37,39] |
| Microbial community | 3.11 | 0.05 | 0.78 | 0.15, 0.80 | 0.65 | ±0.12 | [38,55] |
| VFA production | 2.89 | 0.03 | 0.70 | 0.20, 0.75 | 0.55 | ±0.10 | [47,56] |
| Temperature | 3.45 | 0.01 | 0.82 | 30, 70 | 55 °C | ±10 °C | [37,55] |
| pH | 4.05 | 0.02 | 0.77 | 5.0, 7.5 | 5.8 | ±0.5 | [38,39] |
| Parameters | Study 1 [37] | Study 2 [38] | Study 4 [39] | Study 6 [55] | Average | 95% Confidence Interval (CI) | Statistical Insight |
|---|---|---|---|---|---|---|---|
| Hydrogen production (mL H2/g substrate) | 205 mL/H2 VS added | 108.90 mL/H2 | 191.8 mL H2/g glucose added | 0.63 m3 H2/m3 cathode liquid volume per day | 168.57 mL H2/g | [120, 220] | t = 432. p = 002. r = 0.8, a significant difference in hydrogen yield between the substrate. |
| Microbial community | Clostridium sp., methanogenic bacteria | Mixed anaerobic culture | Clostridium spp. (Dominant) | Desulfovibrio vulgaris, Firmicutes | 0.65 | [0.15, 0.80] | t = 3.11. p = 005. r = 0.78, a strong correlation between specific microbial species and H2 production. |
| VFA production | Acetate, butyrate, valerate, ethanol | - | Butyrate, acetate | - | 0.55 | [0.20, 0.75] | t = 2.89. p = 003. r = 0.70, moderate correlation between VFA concentration and H-production. |
| Temperature | 55 °C | - | 55 °C | - | 55 °C | [30, 70] | t = 3.45. p = 001. r = 0.82, significant impact of temperature on H2 production with optimal results at 55 °C. |
| pH | 5.5 | 5.5 | 7 | pH maintained at the cathode | 5.8 | [5.0, 7.5] | t = 4.05. p = 002. r = 0.77, pH is critical in optimizing hydrogen production, with pH 5.5 flavoring H2 production and pH 7 suppressing methanogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, A.; Yu, Z. Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainability 2024, 16, 10755. https://doi.org/10.3390/su162310755
Jalil A, Yu Z. Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainability. 2024; 16(23):10755. https://doi.org/10.3390/su162310755
Chicago/Turabian StyleJalil, Anam, and Zhisheng Yu. 2024. "Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis" Sustainability 16, no. 23: 10755. https://doi.org/10.3390/su162310755
APA StyleJalil, A., & Yu, Z. (2024). Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainability, 16(23), 10755. https://doi.org/10.3390/su162310755







