Abstract
This review surveys recent advances and emerging prospects in phase-transfer catalysis (PTC) for fuel desulfurization. In response to increasingly stringent environmental regulations, the removal of sulfur from transportation fuels has become imperative for curbing SOx emissions. Conventional hydrodesulfurization (HDS) operates under severe temperature–pressure conditions and displays limited efficacy toward sterically hindered thiophenic compounds, motivating the exploration of non-hydrogen routes such as oxidative desulfurization (ODS). Within ODS, PTC offers distinctive benefits by shuttling reactants across immiscible phases, thereby enhancing reaction rates and selectivity. In particular, PTC enables efficient migration of organosulfur substrates from the hydrocarbon matrix into an aqueous phase where they are oxidized and subsequently extracted. The review first summarizes the deployment of classic PTC systems—quaternary ammonium salts, crown ethers, and related agents—in ODS operations and then delineates the underlying phase-transfer mechanisms, encompassing reaction-controlled, thermally triggered, photo-responsive, and pH-sensitive cycles. Attention is next directed to a new generation of catalysts, including quaternary-ammonium polyoxometalates, imidazolium-substituted polyoxometalates, and ionic-liquid-based hybrids. Their tailored architectures, catalytic performance, and mechanistic attributes are analyzed comprehensively. By incorporating multifunctional supports or rational structural modifications, these systems deliver superior desulfurization efficiency, product selectivity, and recyclability. Despite such progress, commercial deployment is hindered by the following outstanding issues: long-term catalyst durability, continuous-flow reactor design, and full life-cycle cost optimization. Future research should, therefore, focus on elucidating structure–performance relationships, translating batch protocols into robust continuous processes, and performing rigorous environmental and techno-economic assessments to accelerate the industrial adoption of PTC-enabled desulfurization.
1. Introduction
Society and economy are continuously developing. Consequently, the global energy demand is rising steadily []. Renewable energy is widely regarded as a vital alternative to address future resource shortages and combat climate change. However, its current R & D level cannot meet the practical needs of modern economic and technological development independently []. Consequently, the three major fossil energy sources, namely, coal, oil, and natural gas, remain the primary power sources for the development of modern society []. Among them, oil accounts for over 30% of the total energy mix. As a vital category of petroleum products, the consumption of transportation fuels, such as gasoline, diesel, and aviation kerosene, has been increasing daily []. With the continuous consumption of these fuels, the proportion of poor-quality, high-sulfur crude oils is expected to grow []. Simultaneously, increasing fuel consumption means a rise in pollutant emissions, which pose multiple threats to the atmosphere and human health [].
1.1. Sulfur-Containing Compounds in Fuels and Their Hazards
Fuel oil, a significant product of the petroleum refining industry, is the most widely used oil product globally []. It is prepared from crude oil through processes such as atmospheric and vacuum distillation, thermal cracking, catalytic cracking, and hydrocracking []. Fuel oils have a complex composition. In the liquid phase, branched and straight-chain alkane compounds predominate, followed by aromatic compounds such as benzene, toluene, xylene, and their alkyl derivatives []. Sulfur-containing compounds, including thiols, sulfides, disulfides, and thiophene-type compounds, like thiophene (TH), benzothiophene (BT), and dibenzothiophene (DBT), and their alkyl derivatives, are key impurities in fuels [,]. Previous studies have shown that among the abovementioned sulfides, thiophene and its derivatives account for over 90% of the sulfides in FCC gasoline. In diesel fuel, thiophene accounts for more than 80% of the total sulfides, while benzothiophene and dibenzothiophene account for over 70% of the thiophene-type sulfides.
The combustion of sulfur-containing compounds in fuel oil gives rise to gaseous sulfur oxides (SOx). As a significant contributor to air pollution, excessive emissions of SOx precipitate the formation of acid rain. This phenomenon directly inflicts damage and corrosion on outdoor industrial equipment and buildings, impairs plant foliage, and causes soil acidification. Consequently, it leads to vegetation degradation and a reduction in crop yields []. Moreover, acid rain can contaminate lakes and groundwater, resulting in decreased fish and shrimp production and severe contamination of drinking water sources. Secondly, the presence of sulfides in fuel oil is intricately associated with hazy weather, which has drawn extensive public attention []. On the one hand, when harmful gases such as SOx and NOx are directly emitted into the atmosphere and cannot be promptly dispersed with the atmospheric flow, they undergo a series of chemical reactions, yielding sol-gel particles such as sulfates and nitrates []. These particles constitute the major components of respirable particulate matter (PM). On the other hand, the sulfide content in fuel is directly proportional to the amount of particulate matter emitted from vehicle exhausts []. Research indicates that particulate matter in exhausts primarily consists of carbon soot, soluble organic matter, and sulfates. Among these, the sulfate content is approximately 10%, and the sulfides in fuel serve as the direct reactants for the formation of sulfate particles []. Hence, the presence of sulfides in fuel is one of the key factors contributing to the atmospheric particulate pollutant PM. As the primary cause of hazy weather, PM can carry various harmful substances, remaining suspended in the atmosphere for extended periods and being transported over long distances. This situation induces numerous human respiratory and cardiovascular diseases and significantly elevates the incidence of cancer in humans, thus posing a grave threat to human health [].
1.2. Fuel Oil Sulfur Content Control Standards
Over the past few decades, despite the global endeavor to develop renewable and clean energy sources, it is an undeniable fact that fossil fuels, particularly oil and oil-derived fuels, remain a robust impetus for economic growth in contemporary societies, especially in the developing world []. However, air pollution stemming from the utilization of fossil fuels represents a critical and inescapable issue. Research by the World Health Organization has demonstrated that 800,000 individuals die prematurely each year as a consequence of urban air pollution, with vehicle emissions being one of the most substantial contributors to air quality degradation []. Against this backdrop, governments have successively introduced increasingly stringent emission standards regarding sulfur content. For instance, in the Fourth Edition of the World Fuel Code, the sulfur content in gasoline is capped at 10 ppmw []. The European Union (EU), acting as a “pioneer” in promoting cleaner fuel standards, has established EU standards that serve as a crucial reference for other countries to either directly adopt or formulate their own emission regulations. As early as 2009, the maximum allowable sulfur content in gasoline standards decreased from 50 ppmw in Euro IV to 10 ppmw in Euro V. In the Euro VI standard, introduced in 2013, the maximum allowable impurity content, specifically sulfur, in gasoline remained at 10 ppmw. Compared to developed countries in Europe and the United States, China’s gasoline standards were relatively tardy in their formulation and implementation. Nevertheless, with the rapid development of China’s economy and the growing national concern over environmental issues, the sulfur content in gasoline was restricted to 10 ppmw in the national V standard implemented in 2017. Similarly, in the most recent diesel standards implemented across the country, the sulfur content is also limited to 10 ppmw. In the Asia–Pacific region, the demand for cleaner fuels with a sulfur content of less than 10 ppm is projected to increase from 61.7% to 81.7% between 2020 and 2030 []. Evidently, regardless of the development level of countries and regions, the utilization of clean fuels with ultra-low sulfur content represents a general trend. In the future, in the context where electric vehicles cannot completely supplant traditional energy-powered vehicles, countries will continue to upgrade their fuel quality indices and impose more stringent requirements on the sulfur content in fuels [].
It is evident that the presence of sulfur compounds in fuel oil exerts a profoundly negative impact on human health, the ecological environment, and the national economy []. Efficient and practical fuel desulfurization technologies have, thus, emerged as an effective means to address these pollution issues and comply with environmental regulations []. Presently, hydrodesulfurization is the prevalently adopted desulfurization technology in the petroleum refining industry []. However, due to its stringent operating conditions, which demand high temperature, high pressure, and hydrogen, as well as the relatively poor removal efficiency of phenyl sulfide with higher content in fuel oil, influenced by the steric hindrance effect, the cost investment required to produce clean fuel oil meeting the latest national standards (sulfur content < 10 ppm) for ultra-deep desulfurization is substantial []. To surmount this challenge, a variety of non-hydrodesulfurization (NHD) techniques have been investigated and developed [], including extractive desulfurization (EDS) [], biological desulfurization (BDS) [], adsorptive desulfurization (ADS) [], and oxidative desulfurization (ODS) []. Among these desulfurization methods, oxidative desulfurization is most likely to involve phase-transfer catalysis processes [], whereas adsorptive desulfurization, biodesulfurization, and extractive desulfurization typically do not rely on PTC mechanisms [].
1.3. Phase-Transfer Catalysis
In the realm of fuel desulfurization, sulfurous entities—most notably thiophenic compounds [], which exhibit significant resistance to removal via conventional techniques—can undergo efficient oxidation to form sulfone derivatives, followed by their elimination through phase-transfer catalysis (PTC). As a specialized catalytic strategy [], PTC relies on catalysts capable of mediating the transport of reactants across immiscible phases, such as liquid-liquid or solid–liquid systems. These catalysts facilitate or even enable reactions between species dissolved in distinct solvents by shuttling reactive intermediates across phase boundaries, thereby promoting chemical transformations. In a two-phase reaction system, the introduction of a trace amount of PTC allows a reactant to migrate from one phase to another, enabling interaction with a co-reactant in the target phase. This process converts heterogeneous reactions into pseudo-homogeneous ones, leading to a substantial acceleration of reaction kinetics. Phase-transfer catalysts typically feature hydrophilic ionic moieties and hydrophobic segments; even when the catalyst itself is not fully soluble in pure water, it can transport anions into the organic phase via ion-pair formation [].
As an effective catalytic paradigm, phase-transfer catalysis presents innovative solutions to challenges in fuel desulfurization by optimizing interphase mass transfer of reactants, thereby enhancing both reaction efficiency and selectivity. By improving the contact between oxidants and sulfur-containing substrates, PTC systems maximize oxidant utilization and minimize resource waste. Concurrently, these catalysts reduce the requirement for extractants, lowering operational costs and mitigating environmental toxicity, thus precluding secondary contamination of petroleum products [].
In summary, the application of phase-transfer catalysts in fuel desulfurization can not only enhance the desulfurization efficiency and decrease the cost but also mitigate environmental pollution, thereby holding significant environmental and economic implications. Through continuous optimization and enhancement of the performance of phase-transfer catalysts, along with the development of novel phase-transfer catalysts and the improvement of the associated processes, it is anticipated that the industrial application of fuel desulfurization technology will be realized, thus providing robust technical support for the production of clean fuels and environmental protection.
2. Mechanism of Phase-Transfer Catalysis
In conventional Makosza-type phase-transfer catalysis [], the catalyst continuously shuttles reactive anions from the aqueous phase into the organic phase as lipophilic ion pairs, where they engage with the organic substrate. In other words, the active anion is “carried” into the oil phase for reaction, a mechanism characterized by the free and continuous supply of anions within the organic medium. The overall substitution process is shown in Scheme 1.
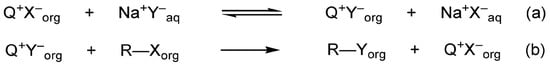
Scheme 1.
The equilibrium of the ion exchange between aqueous and organic phases determines the PT-catalyzed nucleophilic substitution by inorganic anions: (a) ion exchange between the aqueous and organic phases; (b) nucleophilic substitution in the organic phase. Reprinted with permission from MDPI [].
By contrast, in oxidative desulfurization of fuels, phase-transfer catalysis facilitates the migration of organosulfur compounds from the oil phase into the aqueous phase (or, alternatively, transports active oxidizing complexes from the aqueous phase into the organic phase). After oxidation in the aqueous layer to form hydrophilic products, these species are returned to the oil phase for extraction. This pathway more closely resembles a cooperative oxidation process rather than a simple anion shuttle. The phase-transfer catalyst in this context is amphiphilic, bridging substrates and oxidants between the two immiscible phases. For example, Noyori and co-workers [] demonstrated highly efficient sulfide-to-sulfoxide/sulfone conversion using a ternary catalytic system comprising sodium tungstate, phenylphosphonic acid, and methyltrioctylammonium hydrogen sulfate (Na2WO4-C6H5PO3H2-[CH3(n-C8H17)3N]HSO4). At its core, methyltrioctylammonium hydrogen sulfate serves as the phase-transfer carrier, translocating the activated peroxytungstate anion [W(O)(O2)2]2− from the aqueous phase to the hydrophobic sulfide interface. This strategy markedly enhances the oxidation efficiency of hydrogen peroxide toward sulfides, obviates the need for organic solvents, and achieves an exceptional catalyst turnover number (TON) of 122,000.
Conventional homogeneous catalysts deliver exceptional activity and chemoselectivity, yet their practical deployment is often hindered by labor-intensive product separation and limited recyclability []. Heterogeneous systems, in contrast, can be isolated by straightforward solid–liquid separation, but their intrinsic performance is frequently constrained by diffusional resistance, preventing them from matching the kinetic efficiency of their homogeneous counterparts []. Phase-transfer catalysts (PTCs) resolve this longstanding dichotomy through a stimulus-responsive solubility paradigm. During the reaction, a PTC remains molecularly dispersed within the bulk medium, exploiting the advantages of a homogeneous environment, namely, rapid mass transfer and unrestricted access to catalytically active sites. Once the transformation reaches completion, a programmed external cue such as a temperature or pH shift induces spontaneous phase segregation, converting the catalyst into an insoluble state that is readily recovered by simple filtration or decantation []. Functionally, PTCs mediate the stoichiometric migration of reactants across otherwise immiscible domains, thereby enabling transformations that are kinetically or thermodynamically disfavored under conventional homogeneous conditions []. A rigorous understanding of the molecular features that govern this switchable behavior is essential. Accordingly, contemporary PTCs are commonly classified according to the principal driving force that triggers their phase transition, providing a rational framework for the design of next-generation catalysts with both high efficiency and facile recyclability.
2.1. Reaction-Controlled Phase-Transfer Catalysts
Reaction-controlled phase-transfer catalysis introduces a reactant-triggered dynamic solubility cycle that synchronizes catalytic activity with an instantaneous change in phase behavior []. At the outset, the catalyst persists as an insoluble solid dispersed in the reaction medium []. Contact with a designated reactant—hereinafter the phase-change inducer—generates a soluble, catalytically competent intermediate, thereby establishing a transient homogeneous domain in which mass transport is rapid and the turnover frequency is maximized []. This active species subsequently engages a second substrate to furnish the desired product. Once the inducer is depleted, the coordination environment of the catalytic center reorganizes spontaneously; solubility is lost, the original insoluble form re-emerges, and the catalyst precipitates directly from the medium []. The “dissolve-during-reaction/precipitate-on-completion” loop enables near-quantitative catalyst recovery by simple filtration or decantation, marrying the intrinsic efficiency of homogeneous catalysis with the operational economy typifying heterogeneous processes [].
Wu et al. [] exemplified this concept with an amphiphilic peroxopolytungstate, [C16H33(CH3)2NOH]3[PO4{WO(O2)2}2]. During the reaction, the peroxotungstate active species generated by the interaction of hydrogen peroxide with insoluble polymetallic oxoacid salts migrate into the organic phase to form a microemulsion, effecting the efficient epoxidation of cyclooctene (>99% yield). Upon depletion of hydrogen peroxide, the active species relinquish their peroxo ligands and reorganize into hydrophobic polymeric precipitates that return to the solid phase, achieving a recovery of >85%. Spectroscopic analysis identified the active entity as the mononuclear peroxotungstate [H2PO4WO(O2)2]−, while reversible W–O–O–W bridge reconstitution proved crucial to the phase transition. The same switchable platform shows considerable promise for oxidative desulfurization. In a complementary study, another group [] leveraged the pronounced solubility swing of CPC-PW4O16 ([π-C5H5NC16H33]3[PO4(WO3)4]). H2O2 converts the precursor into the amphiphilic species CPC-PW4O20, emulsifying an otherwise biphasic system and driving the epoxidation of polybutadiene at the interface. Once the oxidant is consumed, the catalyst precipitates autonomously, enabling a 92.7 wt% recovery without measurable loss of activity. By obviating the energy-intensive separations that plague classical catalytic regimes, RC-PTCs provide a compelling blueprint for the next generation of high-performance, low-waste catalytic technologies (as shown in Figure 1).
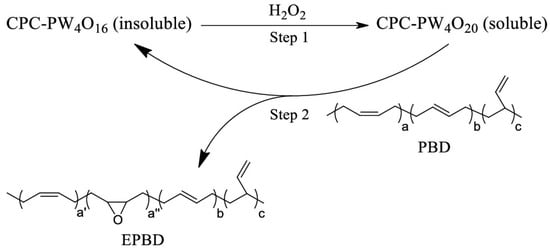
Figure 1.
Catalytic mechanism of CPC-PW4O16 for epoxidation of PBD. Reprinted with permission from American Chemical Society [].
2.2. Thermally Controlled Phase-Transfer Catalysis
Thermally controlled phase-transfer catalysis (TC-PTC) exploits temperature-dependent solubility to switch a catalyst reversibly between homogeneous and heterogeneous states, thereby combining high intrinsic activity with effortless post-reaction recovery []. The strategy relies on catalysts engineered with thermoresponsive ligands or supports whose solubility profile changes sharply over a narrow temperature window []. Xue et al. [] illustrated this concept using [(C18H37)2(CH3)2N]7PW11O39, a hybrid system comprising bulky dialkyl-dimethyl-ammonium cations and a lacunary Keggin anion [PW11O39]7−. In an aqueous H2O2/1,4-dioxane medium, the material is completely insoluble at ambient temperature, permitting easy handling and dosing. Upon heating to 333 K, however, the catalyst dissolves, generating a truly homogeneous environment that maximizes mass-transfer rates and catalytic turnover. During the catalytic process, upon heating, hydrogen peroxide reacts with the lacunary polyoxometalate anion [PW11O39]7− to generate peroxytungstophosphate active species, which dissolve and migrate into the organic phase as temperature rises to effect homogeneous catalytic oxidation; after the reaction and cooling, these active species reorganize via hydrophobic interactions into an insoluble solid that precipitates back into the solid phase, facilitating facile recovery and reuse (as shown in Figure 2).
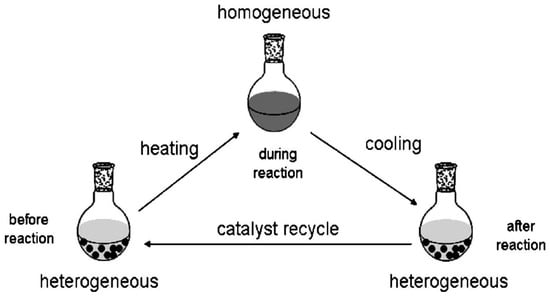
Figure 2.
Representation of the temperature-controlled phase-transfer catalytic system. Reprinted with permission from Elsevier [].
2.3. Photo-Responsive Phase-Transfer Catalysis
Photo-responsive phase-transfer catalysis (PR-PTC) represents a cutting-edge approach that combines the principles of photochemistry with traditional phase-transfer catalysis. By incorporating light-sensitive moieties—such as azobenzene or spiropyran—into the molecular framework of the catalyst, it becomes possible to reversibly modulate its hydrophilicity, lipophilicity, or ion-pairing capacity through light irradiation. This enables precise, reversible, and spatiotemporally controlled switching of catalytic activity and phase behavior in multiphase reaction systems. Yang and co-workers [] developed a representative example in the form of a polyoxometalate-based catalyst coated with a photo-responsive surfactant, denoted Azo-SEP. The innovation lies in the azobenzene functional groups attached to the catalyst’s periphery, which undergo reversible photoisomerization upon exposure to UV or visible light. This photoinduced conformational change dynamically alters the amphiphilicity of the complex, enabling light-controlled phase shuttling between immiscible solvent systems such as toluene and water/dimethylformamide (DMF) mixtures. In practice, the catalyst exhibits high activity for the oxidation of benzothiazine in toluene under visible light, where it remains in a hydrophobic, homogeneous form. Upon completion of the reaction, UV irradiation triggers a conformational change in the azobenzene units, increasing the catalyst’s affinity for the aqueous phase and inducing its migration into the polar medium, thereby achieving spontaneous, in situ separation of the active species from the product phase. Subsequent exposure to visible light restores the catalyst’s original hydrophobic configuration, allowing it to re-enter the organic phase and enabling efficient catalytic recycling. This light-controlled switching mechanism offers a novel and energy-efficient strategy for reversible catalyst deployment and recovery, paving the way for more sustainable and programmable catalytic processes (as shown in Figure 3).
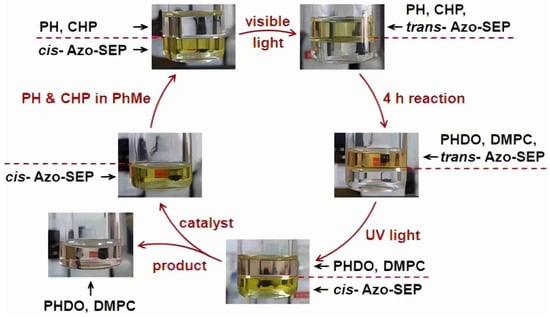
Figure 3.
Schematic catalytic and phase-transfer recycle of photo-responsive catalyst Azo-SEP, driven by alternate UV and visible light irradiations (reduced oxidant DMPC = dimethylphenylcarbinol). Reprinted with permission from American Chemical Society [].
2.4. pH-Tunable Phase-Transfer Catalysis
The proton activity of a reaction medium dictates the speciation, solubility, and coordination properties of substrates, intermediates, and catalysts. By deliberately adjusting pH, one can tailor the ionization state of the reacting partners, their affinity for the catalyst, and, crucially, the hydrophilic–lipophilic balance that underpins phase-transfer efficiency. A pH-controlled PTC therefore relies on a molecular framework whose polarity changes sharply upon protonation or deprotonation—most commonly through pendant amino, carboxyl, or sulfonamide groups. A recent advance [] features a pH-responsive, water-soluble polymeric ruthenium complex designed for asymmetric transfer hydrogenation (ATH). The material is obtained by copolymerising N,N-dimethylaminopropyl acrylamide (DMAPA) with N-styrylsulfonyl-1,2-diphenylethylenediamine (V-TsDPEN) and subsequently coordinating Ru, affording P(DMAPA1.2-TsDPEN)-Ru0.74. In aqueous solution, this catalyst exhibits a highly attractive pH-induced phase-separation behavior: below pH 6.5, the protonated polymer-supported Ru catalyst is water-soluble and operates as a homogeneous catalyst for the asymmetric transfer hydrogenation of ketones, concomitantly generating OH− and raising the solution pH; when the pH exceeds 8.5, deprotonation triggers catalyst precipitation and recovery, thereby enabling a pH-driven, reversible phase-transfer cycle (as shown in Figure 4). Across a broad substrate scope—including electronically diverse aryl ketones bearing multiple functional groups—the catalyst maintains excellent activity and stereocontrol. Even after eight consecutive cycles in water, conversions remain above 95% and enantiomeric excess consistently exceeds 93%, underscoring the robustness of this self-partitioning strategy and its promise for sustainable, high-precision catalytic processes.
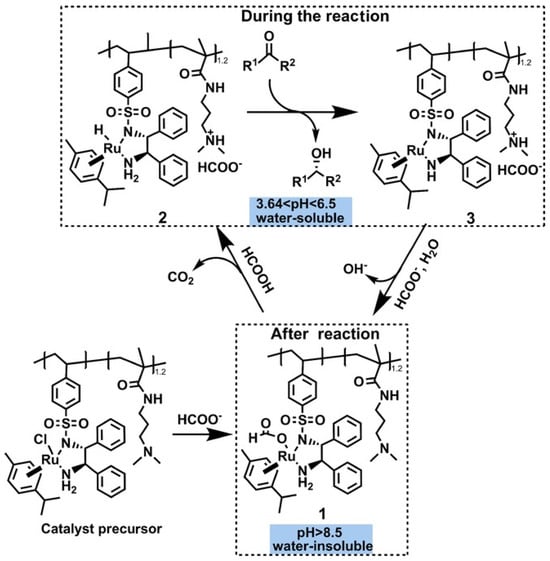
Figure 4.
Proposed catalytic cycle for the reduction of ketones by a pH-responsive Ru catalyst. Reprinted with permission from Wiley [].
3. Conventional Phase-Transfer Catalysts
Traditional phase-transfer catalysts (PTCs) are compounds that facilitate mass transfer between two-phase systems—typically aqueous and organic phases. These catalysts primarily include quaternary ammonium salts, crown ethers, and polyethers. Experimental data from 1970 demonstrated [] that quaternary ammonium salts exhibit remarkable efficiency as catalysts in facilitating substitution reactions and are capable of significantly increasing the reaction rate. Additionally, the catalytic effects manifested by quaternary ammonium and phosphonium salts in a wide array of reactions, including their stability, selectivity, and influence on reaction rates, have been discussed in meticulous detail. These discussions further underscore that phase-transfer catalysis is not confined to the transfer of inorganic anions; it can also transfer chemicals such as inorganic cations, acids, hydrogen peroxide, and ammonia into the organic phase through suitable phase-transfer catalysts to initiate the reaction. At the theoretical level, the success of phase-transfer catalysis hinges on two core elements: firstly, the phase-transfer catalyst must possess the ability to transfer one reactant to the phase where the other reactant resides, and secondly, the transferred reactant must be in an appropriate environment to undergo the chemical reaction. Furthermore, it has been explored in the literature that in nonpolar media, quaternary ammonium salts form ion pairs with anions that may serve as active reaction intermediates. The formation of such ion pairs may diminish the interaction energy between the cation and anion, thereby augmenting the reactivity of the anion. Cetyltrimethylammonium bromide (CTAB) was selected as a phase-transfer catalyst in another study designed to promote the reaction between oxidizing agents (e.g., hydrogen peroxide) and sulfur-containing compounds (e.g., sulfides in FCC gasoline). In this study, the role of CTAB is to transfer the peroxyacids generated from hydrogen peroxide from the aqueous phase to the oil phase, which subsequently reacts with the sulfur-containing compounds in the oil phase. The experimental results indicated that the reaction temperature had a substantial impact on the desulfurization efficiency, which decreased at elevated temperatures, while the addition of CTAB significantly enhanced the desulfurization efficiency at room temperature. Moreover, the desulfurization efficiency of FCC gasoline increased with an increase in the CTAB dosage. Theoretically, the mechanism of CTAB as a phase-transfer catalyst involves the formation of ion pairs with the oxidant in the aqueous phase, and these ion pairs are transferred to the oil phase under agitation, circumventing the solvation of the reactants in the proton solvent, thereby generating more active anions to drive the oxidative desulfurization reaction. This catalytic mechanism not only simplifies the otherwise intricate chemical conversion process but also improves the reaction efficiency [].
Despite the demonstrated utility of conventional phase-transfer catalysts (PTCs) in oxidative desulfurization (ODS), their performance is still constrained by interfacial mass transfer limitations and suboptimal reaction conditions. Traditionally, PTCs—such as quaternary ammonium salts, crown ethers, and polyethers—are employed to facilitate the migration of reactants between immiscible phases, typically aqueous and organic media. However, the inherent phase-boundary resistance often hampers catalytic efficiency. In recent years, the integration of auxiliary techniques such as ultrasonic irradiation or high-shear mixing has emerged as a promising strategy to enhance catalyst–substrate interactions, thereby generating synergistic effects that significantly improve reaction performance. For instance, Biswajit Saha and colleagues [] systematically investigated the ultrasound-assisted oxidative removal of thiophene using a variety of PTCs, including tetrabutylammonium bromide, phenyltrimethylammonium bromide, ethyltriphenylphosphonium bromide, benzyltrimethylammonium bromide, tetrapropylammonium bromide, tetrabutylammonium hydrogen sulfate, and 18-crown-6 ether. Among these, benzyltrimethylammonium bromide delivered the highest thiophene conversion rate—up to 82.59%—under conditions of 1200 rpm stirring and 30 kHz ultrasonic frequency. This finding highlights the strong synergistic enhancement achieved by coupling ultrasound with appropriately selected PTCs. The cavitation effect generated by ultrasound enhances catalyst dispersion and interfacial area, thereby accelerating reaction kinetics and offering a compelling approach to improve desulfurization processes. Further parametric analysis revealed that several operational factors critically influence ODS efficiency. As the stirring speed increased from 300 to 1500 rpm, thiophene conversion initially rose, plateauing around 1200–1500 rpm, making 1200 rpm the optimal condition for subsequent experiments. With respect to ultrasound intensity, introducing sonication improved conversion rates, but an increase in frequency from 30 kHz to 40 kHz led to a significant drop in efficiency—from 83% to 66%—indicating that there exists an optimal frequency range for maximum catalytic performance, with 30 kHz being more favorable. Catalyst dosage also showed a positive correlation with conversion, as increasing the concentration from 0.49 to 4.92 g/L enhanced thiophene removal. Similarly, raising the oxidant-to-sulfur molar ratio from 5 to 20 led to improved conversions. Temperature played a substantial role as well; elevating the reaction temperature from 40 °C to 60 °C boosted conversion from 34% to 88%. Collectively, these findings underscore the critical importance of reaction condition optimization in maximizing the effectiveness of oxidative desulfurization systems and demonstrate that integrating phase-transfer catalysis with auxiliary intensification techniques such as ultrasound holds considerable promise for next-generation fuel purification technologies.
Quaternary ammonium salts, as prototypical phase-transfer catalysts (PTCs), facilitate interfacial mass transport by electrostatically binding aqueous-phase anionic oxidants (e.g., H2O2− and FeO42−) via their positively charged head groups (Q+), while simultaneously leveraging their hydrophobic alkyl chains to solubilize and shuttle these oxidants into the organic phase. This dual functionality effectively overcomes the mass transfer resistance at the aqueous–organic interface, a key bottleneck in biphasic oxidative desulfurization (ODS) processes. Bhasarkar and co-workers [] investigated this mechanism using a model fuel system composed of dibenzothiophene (DBT) dissolved in n-decane. A series of comparative experiments incorporating ultrasonic irradiation and conventional magnetic stirring were conducted, with reaction progress and product profiles systematically monitored via high-performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FT-IR), and gas chromatography–mass spectrometry (GC-MS) analyses. Initial optimization studies established the optimal loading of the phase-transfer agent (PTA), tetrabutylammonium bromide (TBAB), at 60 mg/L, with a suitable dosage of phosphotungstic acid (PWA) set at 0.4 g per 20 mL of reaction mixture. The optimal operating temperatures were determined to be 50 °C under ultrasonic conditions and 60 °C under magnetic stirring. Distinct desulfurization behaviors were observed across different experimental conditions. In the ultrasound-only system (Type A), the process predominantly followed a radical-based mechanism. Although ultrasonication enhanced emulsification and interfacial contact, the inherent instability and short lifetimes of the generated radicals limited their effective reaction with DBT, resulting in modest kinetics and low overall desulfurization efficiency. In contrast, the addition of TBAB (Type B) significantly amplified oxidative activity. When performic acid was used as the oxidant, the DBT removal efficiency reached a peak of 74.22%, clearly demonstrating the critical role of the PTA in facilitating oxidant transport across phase boundaries and enhancing reaction rates. In the magnetically stirred system with TBAB (Type C), the catalyst also improved DBT oxidation, although the overall performance was inferior to that of the ultrasound-assisted system. Interestingly, in the elevated static pressure ultrasound experiments (Type D), the DBT removal rate declined to 58.55%. This was attributed to suppressed microconvection and diminished cavitational intensity, which undermined the physical enhancements typically conferred by ultrasonication. Although direct comparison with prior studies is complicated by variations in experimental setups, the results consistently highlight the efficacy of quaternary ammonium salts as interfacial transport enhancers. Notably, the findings suggest that under magnetic stirring, the contribution of PTA to DBT oxidation is more pronounced than under certain ultrasonic conditions, likely due to differences in oxidant stability and the mechanistic pathway of activation.
Mark Daniel G. de Luna and colleagues [] systematically evaluated how operating parameters influence dibenzothiophene (DBT) removal in a methanol-assisted oxidative desulfurization (MAOD) system. Phosphotungstic acid (HPW), identified by FT-IR, X-ray diffraction (XRD), and scanning electron microscopy (SEM) as possessing an intact Keggin structure, an average crystallite diameter of 82.39 nm, and a fragmented morphology, served as the primary catalyst. Increasing the impeller speed from 5000 to 10,000 rpm enhanced sulfur conversion by intensifying shear, generating finer droplets, and enlarging the interfacial area; DBT removal rose from 41.02% to 49.58% after 10 min and from 92.0% to 99.01% after 30 min. Inverting the phase-transfer agent (PTA) dosage produced the opposite trend. A PTA/HPW molar ratio of 1:1 proved optimal, achieving complete desulfurization within 10 min, whereas higher ratios diminished performance. Under 10,000 rpm stirring at 40 °C with this 1:1 ratio, the apparent rate constant reached 0.1528 min−1 and sulfur conversion was quantitative. The team [] next intensified mass transfer through high-shear mixing and investigated the effect of calcining HPW at 200, 300, and 400 °C. Progressive calcination removed lattice water, weakened O–H vibrational bands, dispersed the cubic phase, lowered crystallinity, and reduced particle size. With a PTA:HPW ratio of 1:2, every calcined catalyst attained 100% sulfur removal within 30 min. The sample calcined at 400 °C was the most active, delivering complete desulfurization in 30 min with only 0.10 g catalyst. Although uncalcined HPW performs well at lower temperatures in other studies, these results show that HPW treated at 400 °C can also realize full DBT conversion with a favorable kinetic constant under MAOD conditions, highlighting its strong potential for practical fuel upgrading.
Researchers have also undertaken two complementary investigations that systematically evaluate the oxidative reactivity of sludge-derived ferrate(VI) and delineate process-optimization strategies across contrasting sulfur-compound matrices. In the first study [], dibenzothiophene (DBT) served as the target substrate and tetrabutylammonium bromide (TOAB) was employed as the phase-transfer agent. A previously unrecognized nonlinear correlation between Fe(VI) concentration and oxidative performance was uncovered, as follows: at 537 ppm the formation of monoprotonated Fe(VI) complexes drives DBT conversion in the model fuel to nearly quantitative levels, yet further increases in ferrate dosage shift the medium toward alkalinity, depress the redox potential, and sharply curtail sulfur removal. Under high-shear mixing at 8157 rpm and a moderate temperature of 41.7 °C, the system achieved a 53.2% sulfur-removal efficiency from pyrolysis oil, confirming the superior affinity of Fe(VI) for the rigid, planar DBT framework (as shown in Figure 5). The follow-up investigation [] pivoted to benzothiophene (BT), whose greater steric encumbrance imposes additional kinetic constraints. Elevating the Fe(VI) concentration to 600 ppm reduced BT conversion by 21.6% relative to the DBT system, owing to accelerated ferrate self-decomposition and the formation of brominated side products. Temperature exerted an inhibitory influence here; raising the reaction temperature from 40 °C to 60 °C lowered BT conversion by 51.6%, in stark contrast to the thermally promoted behavior observed for DBT. By coupling an extreme shear rate of 10,800 rpm with low-temperature operation at 40 °C, the investigators attained 88.1% BT conversion in the model fuel and boosted the desulfurization efficiency of pyrolysis oil to 54.7%. Side-by-side analysis indicates that DBT’s planar geometry readily forms stable charge-transfer complexes with Fe(VI), whereas the steric hindrance around BT’s aromatic ring renders its oxidation far more sensitive to mass-transfer efficiency. In practical fuel tests, the parameters set optimized for BT—low temperature and high shear—enhanced overall sulfur removal by 2.8% compared with the DBT-tuned protocol, underscoring the need to tailor operating conditions to the specific sulfur speciation present in real-world feedstocks.
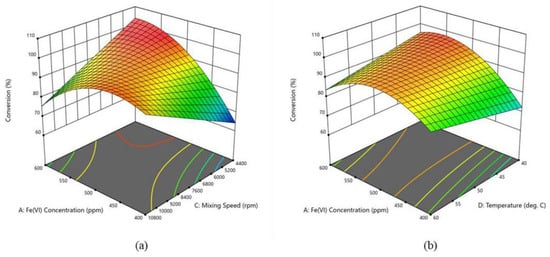
Figure 5.
Model graphs of interacting variables for an MAOD of a DBT model fuel: (a) Fe(VI) concentration & agitation speed on a 3D surface; (b) Fe(VI) concentration & temperature on a 3D surface. Reprinted with permission from Elsevier [].
Quaternary phosphonium salts comprise a central phosphorus atom bonded to four alkyl or aryl groups in a stable tetrahedral configuration. Compared to quaternary ammonium salts, they often exhibit superior thermal and chemical stability, enabling excellent catalytic activity under harsher conditions. Due to these advantages, butyltriphenylphosphonium bromide ([BTPP]Br) has been investigated as a phase-transfer catalyst (PTC) in ultrasonic-assisted oxidative desulfurization (UAODS) []. The desulfurization reaction employed a one-pot methodology within a hydrogen peroxide–acetic acid system, utilizing [BTPP]Br which acts as both the PTC and extractant, eliminating the need for an additional solvent. Systematic evaluation of key parameters revealed their impact on desulfurization efficiency. Extending the ultrasonic oxidation time from 5 min to 30 min significantly increased efficiency from 11.6% to 75.2%. This enhancement is attributed to prolonged interfacial contact, facilitating the transfer of oxidized dibenzothiophene (DBT sulfone) from the organic to the aqueous phase, and allowing for increased generation of oxidizing species (e.g., hydroxyl radicals). The H2O2:CH3COOH volume ratio critically influenced performance, with an optimal ratio of 1:3 yielding 75.2% efficiency (as shown in Figure 6). Deviations from this ratio reduced efficiency, likely due to suboptimal oxidant concentration or detrimental side reactions at higher acid or peroxide concentrations. Increasing the [BTPP]Br loading also improved efficiency; raising the catalyst-to-fuel mass ratio from 1:10 to 1:1 increased desulfurization from 10.8% to 75.2%.
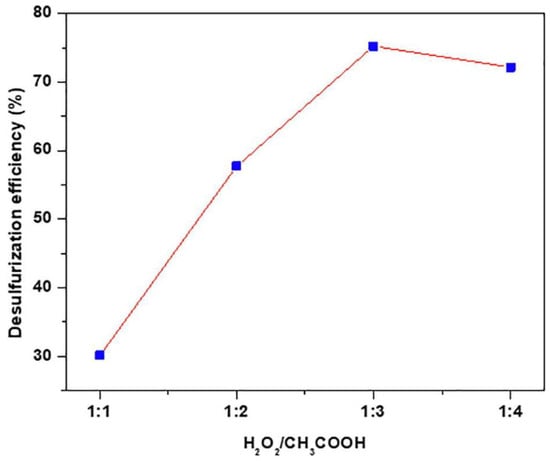
Figure 6.
Effect of H2O2/CH3COOH on desulfurization efficiency. Reprinted with permission from Elsevier [].
4. Design and Innovation of Novel Phase-Transfer Catalysis Systems
Novel phase-transfer catalysts (PTCs) represent an advanced class of catalysts developed by incorporating innovative chemical structures, functional groups, or composite materials into traditional PTC frameworks []. These novel catalysts exhibit significant advantages in oxidative desulfurization, organic synthesis, and other chemical reactions [].
4.1. Quaternary Ammonium Salt-Modified Polyoxometalate Catalysts
Quaternary ammonium polyoxometalate phase-transfer catalysts represent advanced bifunctional materials integrating phase-transfer capabilities with polyoxometalate (POMs) oxidation activity, achieving synergistic enhancement in sulfur compound oxidation []. Structurally, these catalysts form stable ion-pair complexes through hydrophobic long-chain quaternary ammonium cations (e.g., tetraoctylammonium) supporting nanoscale polyoxometalate anions (e.g., phosphomolybdate, silicotungstate). Functionally, they combine “phase-transfer-oxidation” dual active sites to synchronously complete sulfur compound enrichment from oil phase to deep oxidation. Performance-wise, they eliminate traditional dependencies on external phase-transfer agents (e.g., TOAB) and oxidants (e.g., Fe(VI)), significantly reducing side reaction risks and process complexity. For example, Rezvani’s research team constructed efficient Keggin-type polyoxometalate (POMs) catalytic systems through carrier engineering and interfacial synergy design. The core innovation involved integrating iron-substituted phosphotungstate (PW11Fe) with functionalized supports, leveraging the amphiphilic properties of tetrabutylammonium cations (TBA+) to enhance interphase mass transfer. Early work [] the researchers employed a thermal decomposition method to generate a porous, rough surface, subsequently loading it with a cavity-structured (TBA)4PW11Fe@PbO catalyst, which exhibited a crystallite size of 62 nm. By tailoring the porous structure of PbO nanoparticles, this catalyst achieved 97% total sulfur removal from diesel after 2 h at 60 °C. Notably, PbO supports not only provided structural stability but also enhanced reactive oxygen species mobility through surface oxygen vacancies. Building on this [], the research team further expanded the carrier design strategy by employing a sol-gel method to deposit the catalytically active component onto the surface of a TiO2@PVA core–shell structure, yielding spherical nanoparticles with an average crystallite size of 33.20 nm. In this hybrid system, TiO2’s semiconducting properties promoted photogenerated carrier separation, while PVA’s hydroxyl network improved catalyst dispersion in reaction media. Under identical conditions, this system achieved 99% DBT removal from model fuels (as shown in Figure 7), validating the synergistic mechanism of organic–inorganic hybrid supports.
Figure 7.
Effect of reaction temperature and reaction time on the ODS process of (a) DBT and (b) sulfur content of gas oil. Reprinted with permission from Elsevier [].
In their latest study [], the team innovatively developed NiO@BNT composite supports. Here, NiO nanoparticles served as strong oxidation centers, while bentonite (BNT) lamellar structures provided abundant sulfur adsorption sites. The synergistic effect of this bifunctional support enabled 98% total sulfur removal from gasoline within 1 h at 35 °C—representing a 41.7% reduction in reaction temperature and 50% shortening of treatment time compared to earlier work—significantly improving process economy (as shown in Figure 8).
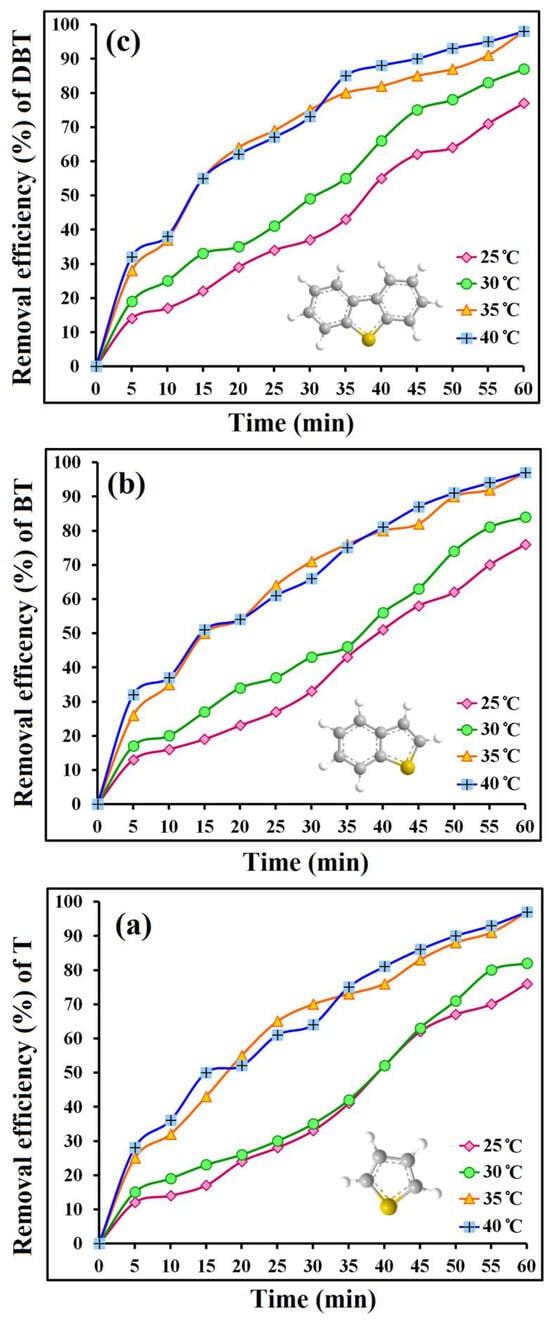
Figure 8.
Effect of reaction temperature and time on the CODS process of (a) T, (b) BT, and (c) DBT. Reprinted with permission from Elsevier [].
From single metal oxides (PbO) to core–shell structures (TiO2@PVA), and further to multifunctional composite supports (NiO@BNT), this series of studies demonstrates a progressive optimization path for carrier functionalities. This design strategy not only addresses the conflicting requirements of catalyst dispersibility, stability, and activity but also achieves milder reaction conditions through inter-carrier synergy. Similarly, Aghbolagh et al. [] employed a sol-gel method to anchor copper-substituted phosphomolybdic acid onto the CuO surface, thereby constructing a bifunctional TBAPMo11Cu@CuO nanocomposite with a distinctive “withered-like” porous morphology and an average crystallite size of 28.1 nm. Experiments showed efficient removal of thiophene (Th), dibenzothiophene (DBT), pyridine (Py), and carbazole (CBZ) from fuels at 35 °C within 1 h, achieving total sulfur and nitrogen removal rates of 97% and 99%, respectively. Notably, the team introduced polyethylene glycol (PEG-200) as a green extractant into the phase-transfer catalytic system. Compared to acetonitrile used in previous studies, PEG-200 forms stable hydrogen-bonded complexes with oxidation products, significantly improving extraction efficiency while avoiding toxicity issues associated with traditional organic solvents. Furthermore, the team systematically optimized reaction parameters using chemometric methods (sum of ranking differences, SRD) []. The results indicated that under optimal conditions (catalyst dosage of 10 mg, O/S of 6, and temperature of 35 °C), thiophene removal increased to 98% and DBT to 99%, with only a 5% efficiency decline after five catalyst cycles. SRD analysis revealed nonlinear relationships between catalyst structure and reaction conditions: exceeding the CuO loading threshold caused active site overcoverage, leading to reduced efficiency—a finding that provides quantitative guidance for carrier design.
In another study, Zhang et al. [] successfully synthesized a reaction-controlled phase-transfer catalyst [C7H7(CH3)3N]9PW9O34 (abbreviated as Q9PW9) with a three-vacant Keggin structure, characterized by powder XRD, FT-IR, and thermogravimetric analysis (TGA). Oxidative desulfurization experiments evaluated its activity toward DBT, thiophene (TH), benzothiophene (BT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT), revealing a removal efficiency order of DBT > 4,6-DMDBT > BT > TH. Under optimal conditions, 100% DBT conversion was achieved. Testing with real diesel in an extraction-oxidation system demonstrated Q9PW9’s superior performance, reducing sulfur content from 150 mg·L−1 to 4.7 mg·L−1 (96.9% desulfurization efficiency). A separate team [] immobilized sandwich-type silicotungstate polyoxometalate ((n-C4H9)4N)7H5Si2W18Mn4O68 (TBA-SiWMn) onto polyvinyl alcohol (PVA) via sol-gel methods under 65 °C oil bath conditions. Comprehensive characterization using elemental analysis, 113Mn phosphorus nuclear magnetic resonance (113Mn NMR), 29Si NMR, XRD, SEM, FT-IR, and ultraviolet–visible spectroscopy (UV-vis) revealed the structural integrity. This organic–inorganic hybrid nanocatalyst, with an average crystallite size of 33 nm, exhibits a unique conical–lamellar porous aggregated morphology. In gasoline catalytic oxidative desulfurization (CODS) with CH3COOH/H2O2 oxidant, TBA-SiWMn@PVA demonstrated outstanding activity and recyclability. Under optimized conditions, total sulfur in real gasoline decreased from 0.4582 wt% to 0.0141 wt%, and the mercaptan content dropped from 88 ppm to 3 ppm. Kinetic studies indicated pseudo-first-order behavior, with apparent activation energies of 51.652 kJ·mol−1 for BT and 52.465 kJ·mol−1 for Th oxidation.
4.2. Imidazolium-Functionalized Polyoxometalate Catalysts
Imidazolium polyoxometalate phase-transfer catalysts represent efficient materials combining the properties of imidazolium cations and polyoxometalates (POMs). These catalysts utilize the amphiphilic nature of imidazolium cations [] to facilitate oxidant and sulfide transfer between phases, while leveraging POMs’ high redox activity to accelerate oxidation reactions. Rezvani et al. [] successfully prepared a novel phase-transfer nanocatalyst (IMID@PMA@CS) based on chitosan (CTS) and modified heteropoly anions (KTHPA). This lamellar porous nanocomposite retained the Keggin structure of phosphomolybdic acid while providing abundant active sites through chitosan’s porous surface, significantly enhancing amphiphilicity and catalytic performance. Under mild conditions, the catalyst achieved >97% oxidative desulfurization efficiency for gasoline sulfides (e.g., thiophene, benzothiophene, and dibenzothiophene). After five cycles, activity remained nearly unchanged, demonstrating excellent reusability. Impressive performance was also observed in real gasoline, reducing total sulfur from 0.498 wt% (4980 ppm) to 0.018 wt% (180 ppm) and confirming practical application potential. Building on this, the team further explored modification effects by synthesizing IMID-KTHPA@CTS nanocatalysts []. These composites incorporated modified heteropolyacid (H3PW12O40) and imidazole (C3H4N2) immobilized on chitosan, characterized comprehensively via FT-IR, 31P NMR, XRD, and SEM. This organic–inorganic hybrid nanocatalyst, with a crystallite size of approximately 61 nm, exhibits a morphology of entwined, flattened granules and possesses a pronounced porous architecture. Under mild conditions (35 °C, 1 h), they exhibited high removal rates for real gasoline sulfur compounds and thiophene model fuels, significantly reducing total sulfur content. Both studies highlight that immobilizing modified POMs on biocompatible materials like chitosan enhances desulfurization efficiency and recyclability, with negligible activity loss over cycles.
In another study, Yue’s research team [] synthesized target catalysts [Rmim]4Mo8O26 by reacting 1-bromoalkanes with 1-methylimidazole to form 1-alkyl-3-methylimidazolium bromides, followed by reaction with ammonium molybdate solutions. Here, Rmim represents imidazolium cations with varying alkyl chain lengths. The long alkyl chains endow amphiphilicity, enabling H2O2-responsive phase transfer from organic to acetonitrile (MeCN) phases, thereby enhancing desulfurization efficiency. Under optimal conditions (50 °C, O/S molar ratio of 5, Mo/S molar ratio of 1:1, MeCN/diesel volume ratio of 1:5), [Omim]4Mo8O26 achieved 99.8% DBT removal. This design integrates imidazolium lipophilicity with polyoxomolybdate oxidation activity, enabling efficient catalyst utilization and rapid mass transfer via phase-transfer processes.
4.3. Other Catalyst Types
Beyond functionalized polyoxometalates, novel phase-transfer catalysts also encompass innovative systems such as ionic liquids and nanomaterials. Ionic liquids (ILs) [] have found extensive applications in phase-transfer catalysis due to their unique physicochemical properties, including negligible vapor pressure, high solubility, and thermal stability. For example, Desai et al. [] synthesized a novel ionic liquid-based phase-transfer catalyst [TPMP][Tos] and applied it in ultrasonic-assisted oxidative desulfurization (UAODS). Prepared via specific reaction steps using methyl toluenesulfonate (MT) and triphenylphosphine (TPP), this catalyst achieved up to 99.4% desulfurization efficiency under optimized conditions (70 °C, mass ratio of IL to model fuel (IL/MF) of 0.8, and O/S molar ratio of 10) for DBT-containing model fuels (The calculated residual sulfur content was determined to be 3 ppm). The [TPMP][Tos] catalyst not only functions as a phase-transfer agent but also acts as an extractant. Its structure contains multiple aromatic rings that facilitate sulfide extraction and transfer through π–π interactions with sulfur compounds. UAODS with [TPMP][Tos] demonstrated superior efficiency compared to traditional oxidative desulfurization, highlighting the synergistic benefits of ultrasonic irradiation and catalyst performance. Vafaee’s research team [] successfully synthesized a nickel ferrite (NiFe2O4)-polyethylene glycol (PEG) nanocomposite (NiFe2O4–PEG) via hydrothermal methods by varying the PEG concentration and synthesis time. Characterized using XRD, FTIR, Brunauer–Emmett–Teller (BET), SEM, vibrating sample magnetometry (VSM), and transmission electron microscopy (TEM), the results reveal that the catalyst is a magnetic mesoporous composite composed of 20–100 nm spherical particles (TEM), featuring a 53 nm spinel NiFe2O4 core uniformly encapsulated by crystalline PEG. It exhibits a BET surface area of 2.50 m2/g and a micropore diameter of 1.21 nm, where the presence of PEG contributes to enhanced dispersion and stability of the catalyst. Central composite design (CCD) optimization identified optimal conditions (0.149 g catalyst, 15 mL oxidant, 11.96 min sonication, and 70 MHz ultrasonic power), achieving 85.5% desulfurization efficiency (the optimal residual sulfur content is 29 ppm). After three cycles, DBT conversion decreased from 85% to 78%, indicating promising application potential despite slight activity loss (Table 1).

Table 1.
Performance summary of selected phase-transfer catalysts.
Notably, Ghahremani et al. [] investigated UAOD for high-sulfur Iranian heavy crude oil (38,638 ppm S) using CuSO4 as a phase-transfer catalyst. Key parameters, including ultrasonic power, reaction time, and catalyst dosage, positively correlated with desulfurization efficiency within certain ranges, while optimal PTA dosage and preheating temperature were identified. Under optimized conditions (70 °C preheating, 4.8 wt% acetic acid, 2.92 wt% H2O2, 10 min reaction time, 1 wt% CuSO4, and 7 W/mL ultrasonic power), 48.68 wt% sulfur removal was achieved. CuSO4 outperformed other PTAs (FeSO4, FeCl2, and FeCl3), and sequential extraction and UAOD stages increased desulfurization to 52.15 wt% and 53.55 wt%, respectively. UAOD achieved threefold higher sulfur removal in 10 min compared to traditional ODS over 90 min.
5. Conclusions and Perspectives
Fuel desulfurization is critical for environmental protection and compliance with stringent sulfur emission standards. Phase-transfer catalysis (PTC) offers unique advantages in fuel desulfurization by facilitating reactant transfer between phases, thereby enhancing reaction efficiency and selectivity. The role of PTC in fuel desulfurization generally involves the following three key aspects:
(i) Facilitation of Sulfur Compound Transfer
Phase-transfer catalysts have the capability to transfer sulfur compounds present in fuel oil from the oil phase to the aqueous phase, thereby achieving the objective of desulfurization. Specifically, the hydrophilic moiety of the catalyst interacts with the aqueous phase, while the lipophilic part interacts with the oil phase, encapsulating the sulfur compounds in ionic pairs. Their transfer between the two phases is facilitated through means such as stirring.
(ii) Enhancement of Reaction Activity
Phase-transfer catalysts can activate sulfur compounds, rendering them more amenable to reaction with the desulfurizing agent. For instance, under alkaline conditions, the phase-transfer catalyst can accelerate the hydrolysis reaction of sulfur compounds, converting them into water-soluble sulfates or sulfites, thus fulfilling the desulfurization objective.
(iii) Improvement in Reaction Selectivity
Phase-transfer catalysis can enhance the selectivity of the desulfurization reaction and prevent the simultaneous removal of other impurities, thereby improving the quality of the desulfurized product. Selective removal of different types of sulfur compounds can be accomplished by selecting appropriate catalysts and reaction conditions.
Oxidative desulfurization (ODS) is widely regarded as the most promising industrial-scale alternative to hydrodesulfurization (HDS) owing to its mild operating conditions, low capital and energy requirements, and outstanding efficacy toward thiophenic sulfur compounds that are notoriously resistant to HDS. Polyoxometalate- and ionic-liquid-based catalysts accelerate the aerobic or peroxy oxidation of sulfides and, by functioning as phase-transfer agents, shuttle the sulfur species from the hydrocarbon matrix into a polar phase where they are selectively oxidized and removed. Despite these advantages, several practical hurdles—including catalyst selectivity and recyclability, oxidant storage and delivery, and the possible impact of ODS on fuel quality—remain to be overcome. Future efforts should, therefore, concentrate on optimizing phase-transfer catalysts (PTCs), discovering new catalytic formulations, and refining process configurations to deliver higher desulfurization efficiencies at lower operating costs. Key research priorities are outlined below:
(i) Transition from Batch to Continuous Processing
Laboratory studies are almost exclusively conducted in batch reactors, a format that cannot meet refinery demands for high throughput, steady-state control, and reproducibility. Continuous-flow reactors—such as jet-mix, recirculating ultrasonic, dual-channel, or membrane-emulsification devices—should be developed and supported by CFD-guided optimization of residence time and interfacial renewal frequency. Integrated solutions that combine corrosion-resistant construction materials with precise thermal management and in-line separation modules are essential for scale-up.
(ii) Maximizing Desulfurization Yield via Enhanced Phase Transfer
Although sulfur removal rates above 90% are common, the isolated yields of oxidized products remain unsatisfactory, reflecting inefficient use of oxidant, unfavorable product partitioning, and side reactions. Strategies to raise phase-transfer efficiency include tailoring the lipophilicity/hydrophilicity balance of the PTC cation, introducing multifunctional amphiphiles, or coupling the catalyst with emulsifiers to enrich reactive oxygen species in the organic phase and thereby improve both the reaction rate and selectivity.
(iii) Balancing Fuel Retention with Sulfur Removal
Severe oxidative or acidic conditions can trigger side reactions of aromatics and light alkanes or cause co-extraction of valuable hydrocarbons, diminishing overall fuel yield. Comprehensive mapping of reaction variables on fuel composition is needed to establish operating windows that minimize product loss. A quantitative “desulfurization-efficiency versus fuel-loss” model, grounded in rigorous mass balances, will offer a rational basis for optimizing temperature, oxidant dosage, and reaction time.
(iv) Catalyst Durability and Regeneration
Industrial viability hinges on the ability to recycle PTCs without significant deactivation. While several polyoxometalate-derived systems retain activity over a few cycles, prolonged exposure leads to structural degradation and fouling. Systematic longevity tests are required to catalogue deactivation pathways and to develop gentle regeneration protocols such as solvent washing or mild redox treatment. Parallel efforts should target intrinsically robust PTC architectures that resist hydrolysis and oxidative breakdown.
(v) Comprehensive Environmental and Economic Assessments
Performance metrics alone are insufficient for technology selection. Holistic life-cycle assessment (LCA) and techno-economic analysis (TEA) should be established to quantify specific desulfurization cost (e.g., USD bbl−1 ppm−1) and greenhouse-gas intensity, benchmarked against conventional HDS. Such data will guide policy formulation, investment decisions, and process optimization.
In addition, future investigations must push the frontiers of interfacial transport enhancement, devise stringent controls for residual catalyst removal to safeguard fuel quality, and explore multi-modal catalytic—for example, photo- or electro-assisted—routes to surmount the current limitations in ultra-deep desulfurization of recalcitrant species.
Author Contributions
X.Z.: Writing—Original Draft and Investigation; R.W.: Writing—Review and Editing and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province, China [ZR2018MEE048] and PetroChina Innovation Foundation [2010D-5006-0405].
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest in publishing the results.
References
- Dou, S.; Wang, R. Study on the Performance and Mechanism of Aerobic Oxidative Desulfurization Based on a Dual-Functional Material Possessing Catalytic and Adsorptive Properties. New J. Chem. 2019, 43, 3226–3235. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on Oxidative Desulfurization of Fuel by Supported Heteropolyacid Catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Haruna, A.; Merican, Z.M.A.; Musa, S.G. Recent Advances in Catalytic Oxidative Desulfurization of Fuel Oil—A Review. J. Ind. Eng. Chem. 2022, 112, 20–36. [Google Scholar] [CrossRef]
- Majid, M.F.; Mohd Zaid, H.F.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic Advance and Perspective of Deep Eutectic Solvent for Extractive Desulfurization of Fuel Oil: A Review. J. Mol. Liq. 2020, 306, 112870. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, Z.; Liu, J.; Qian, G.; Wang, D.; Gong, X. Review of Electrochemical Oxidation Desulfurization for Fuels and Minerals. Fuel 2021, 305, 121562. [Google Scholar] [CrossRef]
- Chehadeh, D.; Ma, X.; Al Bazzaz, H. Recent Progress in Hydrotreating Kinetics and Modeling of Heavy Oil and Residue: A Review. Fuel 2023, 334, 126404. [Google Scholar] [CrossRef]
- Kansha, Y.; Kato, S.; Tsuji, K. Analysis of Reactions during the Residue Desulfurization of Heavy Oil Based on a Data-Driven Method. Comput. Chem. Eng. 2022, 164, 107901. [Google Scholar] [CrossRef]
- Zaidi, Z.; Gupta, Y.; Gayatri, S.L.; Singh, A. A Comprehensive Discussion on Fuel Combustion and Desulfurization Technologies. Inorg. Chem. Commun. 2023, 154, 110964. [Google Scholar] [CrossRef]
- Guntida, A.; Jorqueira, D.S.S.; Nikitine, C.; Fongarland, P.; Thomas, K.; Maugé, F.; Aparicio, J. Catalytic Oxidative Desulfurization of Liquid Fuel: Impact of Oxidants, Extracting Agents, and Heterogeneous Catalysts with Prospects for Biodiesel Upgrading—A Mini Review. Biomass Bioenergy 2024, 188, 107341. [Google Scholar] [CrossRef]
- Adhami, M.; Movahedirad, S.; Sobati, M.A. Oxidative Desulfurization of Fuels Using Gaseous Oxidants: A Review. J. Sulfur. Chem. 2022, 43, 685–712. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Yu, H.; Han, S.; Wei, Y. Recent Advances of Anderson-Type Polyoxometalates as Catalysts Largely for Oxidative Transformations of Organic Molecules. Molecules 2022, 27, 5212. [Google Scholar] [CrossRef]
- Tanimu, A.; Tanimu, G.; Ganiyu, S.A.; Gambo, Y.; Alasiri, H.; Alhooshani, K. Metal-Free Catalytic Oxidative Desulfurization of Fuels─a Review. Energy Fuels 2022, 36, 3394–3419. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M. Oxidative Desulfurization of Liquid Fuels Using Polyoxometalate-Based Catalysts: A Review. Energy Fuels 2021, 35, 10347–10373. [Google Scholar] [CrossRef]
- Hossain, M.; Park, H.; Choi, H. A Comprehensive Review on Catalytic Oxidative Desulfurization of Liquid Fuel Oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef]
- Tugrul Albayrak, A.; Tavman, A. Sono-Oxidative Desulfurization of Fuels Using Heterogeneous and Homogeneous Catalysts: A Comprehensive Review. Ultrason. Sonochem. 2022, 83, 105845. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, A.; Sobati, M.A. Extraction of Sulfur Compounds from Middle Distillate Fuels Using Ionic Liquids and Deep Eutectic Solvents: A Critical Review. Fuel 2022, 310, 122279. [Google Scholar] [CrossRef]
- Abro, R.; Kiran, N.; Ahmed, S.; Muhammad, A.; Jatoi, A.S.; Mazari, S.A.; Salma, U.; Plechkova, N.V. Extractive Desulfurization of Fuel Oils Using Deep Eutectic Solvents—A Comprehensive Review. J. Environ. Chem. Eng. 2022, 10, 107369. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, W.; Liu, Z.H.; Yang, Z.Z.; Tan, J.J.; Xu, B.M. Recent Progress of Metal-Organic Frameworks for Liquid Fuels Desulfurization. Sep. Purif. Rev. 2024, 54, 128–148. [Google Scholar] [CrossRef]
- Saeed, M.; Firdous, A.; Zaman, M.S.; Izhar, F.; Riaz, M.; Haider, S.; Majeed, M.; Tariq, S. MOFs for Desulfurization of Fuel Oil: Recent Advances and Future Insights. J. Chin. Chem. Soc. 2023, 70, 789–824. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, R. Research Progress of Phase Transfer Catalysts Used in Oxidative Desulfurization of Fuel Oil. Mini-Rev. Org. Chem. 2021, 18, 626–648. [Google Scholar] [CrossRef]
- Khalid, H.; Umar, A.; Saeed, M.H.; Nazir, M.S.; Akhtar, T.; Ikhlaq, A.; Ali, Z.; Hassan, S.U. Advances in Fuel Oil Desulfurization: A Comprehensive Review of Polyoxometalate Catalysts. J. Ind. Eng. Chem. 2025, 141, 32–45. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, B.; Wang, R.; Kozhevnikov, I.V. New Adsorption Materials for Deep Desulfurization of Fuel Oil. Materials 2024, 17, 1803. [Google Scholar] [CrossRef]
- Ahmed, B.; Ahmad, Z.; Naz, S.; Ihsan, A.; Khan, B. Oxidative Desulfurization of Liquid Fuels Using Deep Eutectic Solvents as a Catalyst and Extractant: A Review. Chem. Eng. Res. Des. 2024, 211, 253–268. [Google Scholar] [CrossRef]
- Niu, A.; Xu, H.; Yuan, Q.; Wu, F.; Wei, X. Metal-Based Ionic Liquids and Solid-Loaded Catalysts in Fuel Oil Desulfurization:A Review. Mini-Rev. Org. Chem. 2024, 21, 704–716. [Google Scholar] [CrossRef]
- Jha, D.; Maheshwari, P.; Singh, Y.; Haider, M.B.; Kumar, R.; Balathanigaimani, M.S. A Comparative Review of Extractive Desulfurization Using Designer Solvents: Ionic Liquids & Deep Eutectic Solvents. J. Energy Inst. 2023, 110, 101313. [Google Scholar] [CrossRef]
- Azeez, M.O.; Ganiyu, S.A. Review of Biomass Derived-Activated Carbon for Production of Clean Fuels by Adsorptive Desulfurization: Insights into Processes, Modifications, Properties, and Performances. Arab. J. Chem. 2023, 16, 105182. [Google Scholar] [CrossRef]
- Wilson-Kovacs, R.; Acevedo, O. Computational Advances in Ionic-Liquid-Mediated Extractive Desulfurization of Fuel: A Minireview. Energy Fuels 2024, 38, 20224–20241. [Google Scholar] [CrossRef]
- Omar, R.A.; Verma, N. Review of Adsorptive Desulfurization of Liquid Fuels and Regeneration Attempts. Ind. Eng. Chem. Res. 2022, 61, 8595–8606. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Ishaq, M.; Yang, W.; Hussain, M. Recent Breakthroughs in Deep Aerobic Oxidative Desulfurization of Petroleum Refinery Products. J. Clean. Prod. 2021, 294, 125731. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, W.; Wu, G.; Xin, F.; Qin, K.; Lu, Y.; Zhang, L.; Li, M. Kinetics toward Mechanism and Real Operation for Ultra-deep Hydrodesulfurization and Hydrodenitrogenation of Diesel. AlChE J. 2021, 67, e17188. [Google Scholar] [CrossRef]
- Hosseini, A.; Khoshsima, A.; Sabzi, M.; Rostam, A. Toward Application of Ionic Liquids to Desulfurization of Fuels: A Review. Energy Fuels 2022, 36, 4119–4152. [Google Scholar] [CrossRef]
- Bhasarkar, J.B.; Singh, M.; Moholkar, V.S. Mechanistic Insight into Phase Transfer Agent Assisted Ultrasonic Desulfurization. RSC Adv. 2015, 5, 102953–102964. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, H.; Wang, J.; Yang, Y.; Zhao, Y. Kinetics and Mechanism of Quaternary Ammonium Salts as Phase-Transfer Catalysts in the Liquid−liquid Phase for Oxidation of Thiophene. Energy Fuels 2007, 21, 2543–2547. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C.L.; Halpern, M.E. Phase-Transfer Catalysis; Springer: Dordrecht, The Netherlands, 1994; ISBN 978-94-010-4297-0. [Google Scholar]
- Mąkosza, M.; Fedoryński, M. Interfacial Processes—The Key Steps of Phase Transfer Catalyzed Reactions. Catalysts 2020, 10, 1436. [Google Scholar] [CrossRef]
- Sato, K.; Hyodo, M.; Aoki, M.; Zheng, X.-Q.; Noyori, R. Oxidation of Sulfides to Sulfoxides and Sulfones with 30% Hydrogen Peroxide under Organic Solvent- and Halogen-Free Conditions. Tetrahedron 2001, 57, 2469–2476. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Gaggero, N.; Prenga, K. Synthesis of 3,4-Dihydropyridin-2-Ones via Domino Reaction under Phase Transfer Catalysis Conditions. Catalysts 2023, 13, 170. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Sun, G.; You, Q.; Jiang, X.; Cui, Y. Epoxidation of Allyl Chloride with H2O2 Catalyzed by Three Structurally Related Quaternary Ammonium Modified Polyoxophosphotungstates. Appl. Catal. A 2020, 608, 117846. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Chen, S.; Dong, F.; Hu, J.; Ge, S. A Novel Dendrobine-derived Phase-transfer Catalyst. ChemistrySelect 2025, 10, e202405664. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, R.; Zheng, Y.; Liu, Y.; Zhang, Q.; Zhang, J.; Chen, C.; Duan, E. Fluorescence Visualization of CO2-Responsive Phase Transfer Materials Targeting at the Heterogeneous Interfacial Reactions in Advanced Oxidation of Naphthenic Acid in Wastewater. Sci. Total Environ. 2024, 933, 173235. [Google Scholar] [CrossRef]
- Zuwei, X.; Ning, Z.; Yu, S.; Kunlan, L. Reaction-Controlled Phase-Transfer Catalysis for Propylene Epoxidation to Propylene Oxide. Science 2001, 292, 1139–1141. [Google Scholar] [CrossRef]
- Xiang, K.; Liu, H.; Yang, B.; Zhang, C.; Yang, S.; Liu, Z.; Liu, C.; Xie, X.; Chai, L.; Min, X. Selenium Catalyzed Fe(III)-EDTA Reduction by Na2SO3: A Reaction-Controlled Phase Transfer Catalysis. Environ. Sci. Pollut. Res. 2016, 23, 8113–8119. [Google Scholar] [CrossRef]
- Weng, Z.H.; Wang, J.Y.; Jian, X.G. Efficient Oxidation of Benzyl Alcohol with Heteropolytungstate as Reaction-Controlled Phase-Transfer Catalyst. Chin. Chem. Lett. 2007, 18, 936–938. [Google Scholar] [CrossRef]
- Yang, X.; Gao, S.; Xi, Z. Reaction-Controlled Phase Transfer Catalysis for Styrene Epoxidation to Styrene Oxide with Aqueous Hydrogen Peroxide. Org. Process Res. Dev. 2005, 9, 294–296. [Google Scholar] [CrossRef]
- Malakooti, R.; Feghhi, A. MoOx—Pyridine Organic–Inorganic Hybrid Wires as a Reusable and Highly Selective Catalyst for the Oxidation of Alcohols: A Comparison Study between Reaction-Controlled Phase-Transfer Catalysis and Heterogeneous Catalysis. New J. Chem. 2017, 41, 3405–3413. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, H.; Wang, C.; Wang, X.; Wang, X.; Huo, M. Amphiphilic Peroxo Polyoxometalate as Reaction Control Phase Transfer Catalyst for Efficient Epoxidation of Olefins. Micro Nano Lett. 2021, 16, 615–620. [Google Scholar] [CrossRef]
- Xu, L.; Li, B.-G.; Jie, S.; Li, Z.; Bu, Z. 110th Anniversary: The Epoxidation of Polybutadiene via Reaction-Controlled Phase-Transfer Catalysis. Ind. Eng. Chem. Res. 2019, 58, 13085–13092. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, W. The Oxidation of Pyridine and Alcohol Using the Keggin-Type Lacunary Polytungstophosphate as a Temperature-Controlled Phase Transfer Catalyst. J. Mol. Catal. A Chem. 2011, 337, 45–51. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Wang, Y.; Xu, J. Kinetic Studies and Reaction Network in the Epoxidation of Styrene Catalyzed by the Temperature-Controlled Phase-Transfer Catalyst [(C18 H37)2 (CH3)2 N]7 [PW11 O39]. Ind. Eng. Chem. Res. 2022, 61, 10747–10755. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, W.; Ma, B.; Ding, Y. Efficient Oxidation of Sulfides Catalyzed by a Temperature-Responsive Phase Transfer Catalyst [(C18H37)2(CH3)2N]7 PW11O39 with Hydrogen Peroxide. Catal. Commun. 2012, 29, 73–76. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Wang, Y.; Yue, L.; Li, W.; Wu, L. A Photo-Driven Polyoxometalate Complex Shuttle and Its Homogeneous Catalysis and Heterogeneous Separation. J. Am. Chem. Soc. 2013, 135, 14500–14503. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, M.; Wu, X.; Chen, C.; Ma, W.; Dong, Q.; Yuan, M.; Hou, Z. A pH-responsive Soluble-polymer-based Homogeneous Ruthenium Catalyst for Highly Efficient Asymmetric Transfer Hydrogenation (ATH). ChemPlusChem 2016, 81, 541–549. [Google Scholar] [CrossRef]
- Starks, C.M. Phase-Transfer Catalysis. I. Heterogeneous Reactions Involving Anion Transfer by Quaternary Ammonium and Phosphonium Salts. J. Am. Chem. Soc. 1971, 93, 195–199. [Google Scholar] [CrossRef]
- Shabestary, N.; Khazaeli, S.; Hickman, R. Phase Transfer Catalytic Reactions: A Physical Chemistry Laboratory Experiment. J. Chem. Educ. 1998, 75, 1470. [Google Scholar] [CrossRef]
- Saha, B.; Brahma, S.; Basu, J.K.; Sengupta, S. Optimization of Ultrasound-Assisted Oxidation of Thiophene Using Phase Transfer Catalyst. Indian Chem. Eng. 2017, 59, 200–214. [Google Scholar] [CrossRef]
- Bal, D.K.; Bhasarkar, J.B. Mechanistic Investigation of Sono–Phosphotungstic Acid/Phase Transfer Agent Assisted Oxidative Desulfurization of Liquid Fuel. Asia-Pac. J. Chem. Eng. 2019, 14, e2271. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Wan, M.-W.; Golosinda, L.R.; Futalan, C.M.; Lu, M.-C. Kinetics of Mixing-Assisted Oxidative Desulfurization of Dibenzothiophene in Toluene Using a Phosphotungstic Acid/Hydrogen Peroxide System: Effects of Operating Conditions. Energy Fuels 2017, 31, 9923–9929. [Google Scholar] [CrossRef]
- Wan, M.-W.; De Luna, M.D.G.; Golosinda, L.R.; Futalan, C.M.; Phatai, P.; Lu, M.-C. Oxidative Desulfurization of Dibenzothiophene via High-Shear Mixing with Phosphotungstic Acid: The Influence of Calcination Temperature on Kinetics and Catalytic Activity. Clean. Technol. Environ. Policy 2019, 21, 1459–1469. [Google Scholar] [CrossRef]
- Haboc, M.M.; Dugos, N.P.; Choi, A.E.S.; Wan, M.-W. Enhancing Oxidative Desulfurization Using Sludge-Derived Ferrate (VI) for Dibenzothiophene: An Optimization Study. J. Clean. Prod. 2024, 470, 143307. [Google Scholar] [CrossRef]
- Haboc, M.M.; Dugos, N.P.; Choi, A.E.S.; Wan, M.-W. An Innovative Approach for Oxidative Desulfurization Advancement through High Shear Mixing: An Optimization Study on the Application of Benzothiophene. ACS Omega 2024, 9, 41279–41288. [Google Scholar] [CrossRef]
- Desai, K.; Bhatt, S.; Dharaskar, S.; Unnarkat, A.; Sasikumar Jampa, S.; Khalid, M. Butyl Triphenyl Phosphonium Bromide as an Effective Catalyst for Ultrasound Assisted Oxidative Desulfurization Process. Mater. Today Proc. 2020, 28, 836–841. [Google Scholar] [CrossRef]
- Roustaei, S.; Taheri, A. Deep Extractive and Oxidative Removal of 2- and 3-Methylthiophene Using a Hierarchical Architecture of α-Keggin-Type Polyoxotungstate. J. Clean. Prod. 2023, 384, 135405. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Ardeshiri, H.H.; Aghasadeghi, Z. Extractive–Oxidative Desulfurization of Real and Model Gasoline Using (Gly)3 H[SiW12 O40]⊂CoFe2 O4 as a Recoverable and Efficient Nanocatalyst. Energy Fuels 2023, 37, 2245–2254. [Google Scholar] [CrossRef]
- Balaraman, H.; Rathnasamy, S. Synergetic Ultrasound Assisted Catalytic Oxidative Extractive Desulfurization of Tire Pyrolysis Oil Employing Sustainable Protic Deep Eutectic Solvents. Fuel 2023, 351, 129031. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Khandan, S.; Sabahi, N. Oxidative Desulfurization of Gas Oil Catalyzed by (TBA)4 PW11 Fe@PbO as an Efficient and Recoverable Heterogeneous Phase-Transfer Nanocatalyst. Energy Fuels 2017, 31, 5472–5481. [Google Scholar] [CrossRef]
- Ali Rezvani, M.; Noori Oghoulbeyk, Z.; Khandan, S.; Gabriel Mazzei, H. Synthesis and Characterization of New Nano Organic-Inorganic Hybrid (TBA)4PW11Fe@TiO2@PVA as a Promising Phase-Transfer Catalyst for Oxidative Desulfurization of Real Fuel. Polyhedron 2020, 177, 114291. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Afshari, P.; Aghmasheh, M. Deep Catalytic Oxidative Desulfurization Process Catalyzed by TBA-PWFe@NiO@BNT Composite Material as an Efficient and Recyclable Phase-Transfer Nanocatalyst. Mater. Chem. Phys. 2021, 267, 124662. [Google Scholar] [CrossRef]
- Shokri Aghbolagh, Z.; Khanmohammadi Khorrami, M.R.; Rahmatyan, M.S. Oxidative Desulfurization and Denitrogenation of Simulated Fuels Catalyzed by TBAPMo11 Cu@CuO as a High-Performance and Recoverable Heterogeneous Phase-Transfer Catalyst. Energy Fuels 2020, 34, 16366–16380. [Google Scholar] [CrossRef]
- Aghbolagh, Z.S.; Khorrami, M.R.K.; Rahmatyan, M.S. Sum of Ranking Differences in Studies on High Performance of Catalytic Oxidative Denitrogenation and Desulfurization of Model Fuel Using Efficient Organic–Inorganic Hybrid Nanocatalyst. J. Iran. Chem. Soc. 2022, 19, 219–230. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Wang, R. Homogeneous Oxidative Desulfurization Catalyzed by a Recoverable Reaction-Controlled Phase Transfer Catalyst Based on Trilacunary Keggin Polyoxometalate. New J. Chem. 2019, 43, 7363–7370. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Aghmasheh, M. Catalytic Oxidative Desulphurization of Gasoline Using Amphiphilic Polyoxometalate@polymer Nanocomposite as an Efficient, Reusable, and Green Organic–Inorganic Hybrid Catalyst. Environ. Technol. 2020, 41, 1219–1231. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Gao, C.-Y.; Yang, K.-G.; Wang, Z.-J.; Duan, Y.-H.; Feng, S.-X.; Xu, H. Mild and Deep Oxidative Extraction Desulfurization Using Dual-Function Imidazolium Peroxydisulfate Ionic Liquid. Energy Fuels 2019, 33, 10728–10733. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Shokri Aghbolagh, Z.; Babaei, R. Oxidative Desulfurization of Gasoline Catalyzed by IMID@PMA@CS Nanocomposite as a High-performance Amphiphilic Nanocatalyst. Environ. Prog. Sustain. Energy 2018, 37, 1891–1900. [Google Scholar] [CrossRef]
- Ali Rezvani, M.; Aghmasheh, M. Synthesis of a Nanocomposite Based on Chitosan and Modified Heteropolyanion as a Nanocatalyst for Oxidative Desulfurization of Real and Thiophenic Model Fuels. J. Coord. Chem. 2020, 73, 1407–1424. [Google Scholar] [CrossRef]
- Yue, S.; Song, W.; Pan, S.; Liu, D.; Li, C.; Zang, J.; Nan, J.; Gui, J. Efficient Oxidative Desulfurization over [Mo8 O26 ]4– -Based Hybrids: Phase Transfer Catalysis. Energy Fuels 2023, 37, 8988–8998. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yin, J.; Zhu, T.; Dai, C.; Jiang, W.; Li, H.; Li, H. Unraveling the Mechanism of Extractive Desulfurization to Rationally Design Functional Ionic Liquids and Deep Eutectic Solvents: A Comprehensive Review. Energy Fuels 2025, 39, 4095–4118. [Google Scholar] [CrossRef]
- Desai, K.; Dharaskar, S.; Khalid, M.; Gupta, T.C.S.M. Triphenyl Methyl Phosphonium Tosylate as an Efficient Phase Transfer Catalyst for Ultrasound-Assisted Oxidative Desulfurization of Liquid Fuel. Environ. Sci. Pollut. Res. 2021, 28, 26747–26761. [Google Scholar] [CrossRef]
- Vafaee, F.; Jahangiri, M.; Salavati-Niasari, M. A New Phase Transfer Nanocatalyst NiFe2 O4 –PEG for Removal of Dibenzothiophene by an Ultrasound Assisted Oxidative Process: Kinetics, Thermodynamic Study and Experimental Design. RSC Adv. 2021, 11, 31448–31459. [Google Scholar] [CrossRef]
- Ghahremani, H.; Nasri, Z.; Eikani, M.H. Ultrasound-Assisted Oxidative Desulfurization (UAOD) of Iranian Heavy Crude Oil: Investigation of Process Variables. J. Pet. Sci. Eng. 2021, 204, 108709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).