Non-Noble Metal Catalysts for Efficient Formaldehyde Removal at Room Temperature
Abstract
1. Introduction
2. Catalytic Materials
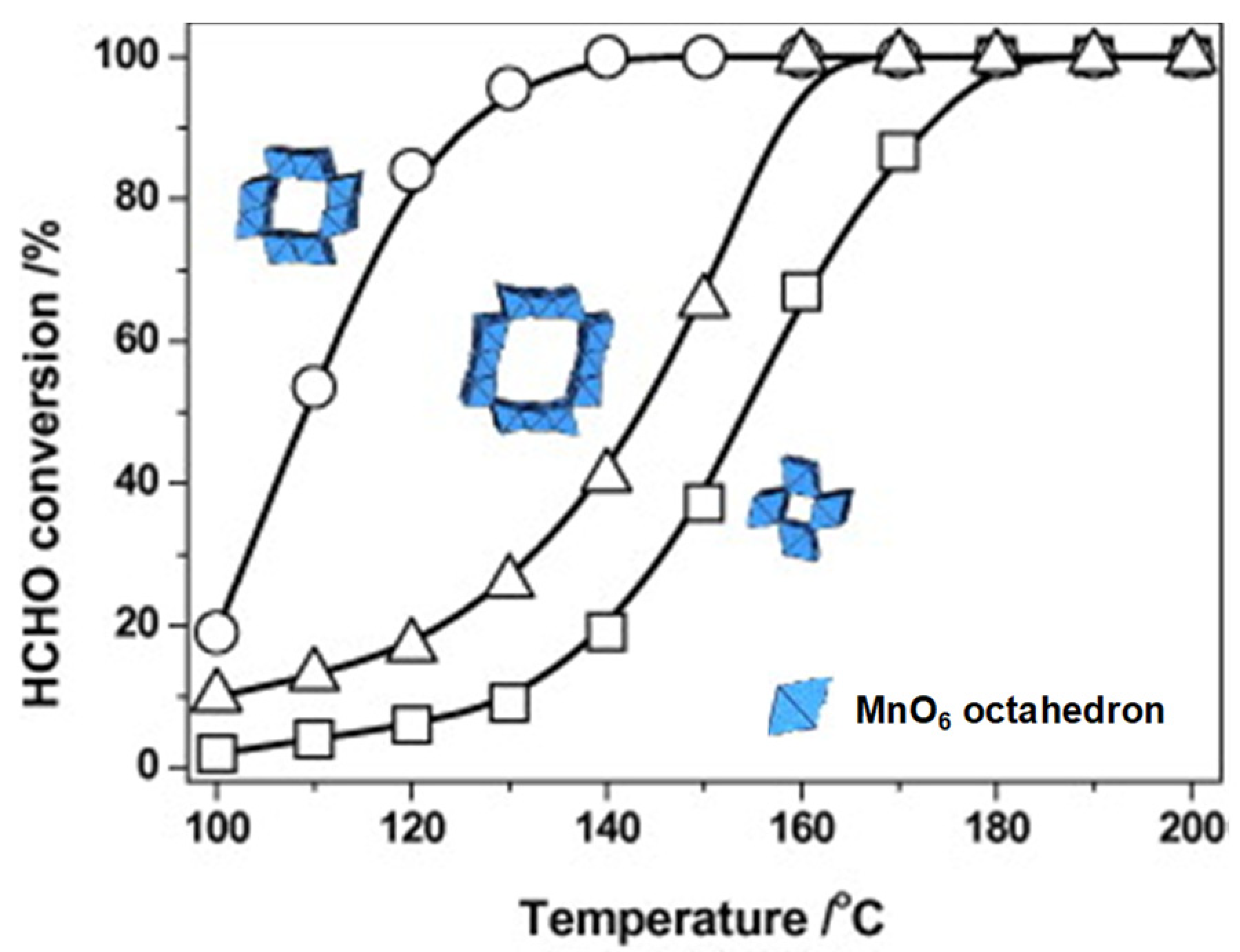
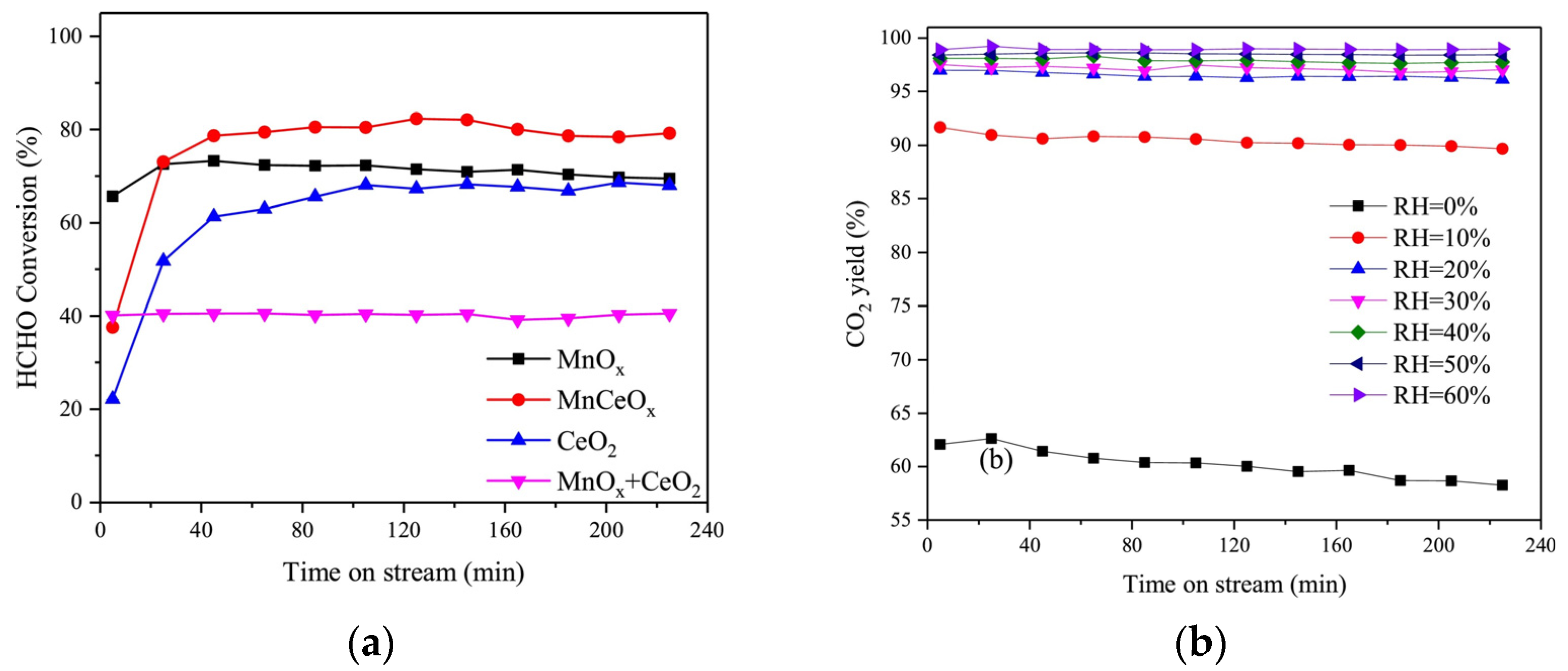
2.1. Manganese-Based Catalysts
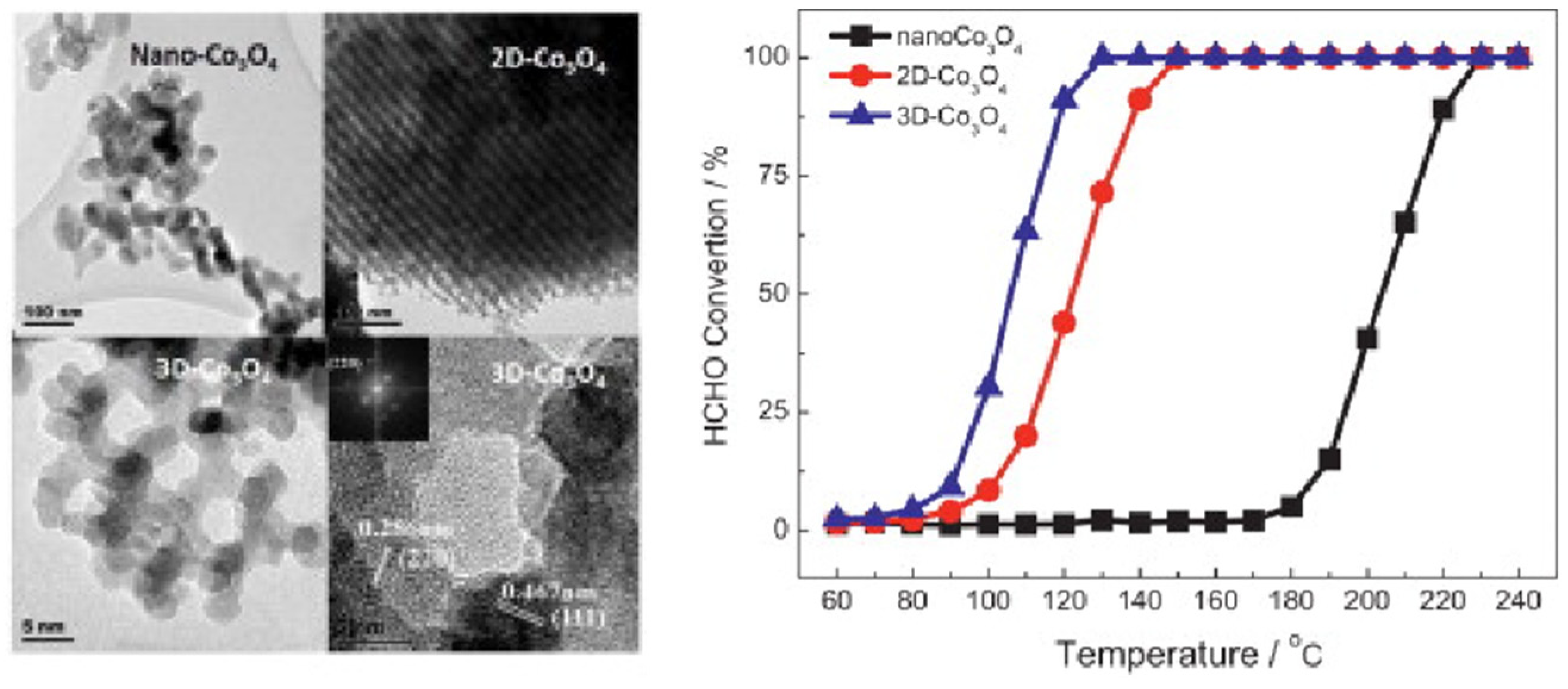
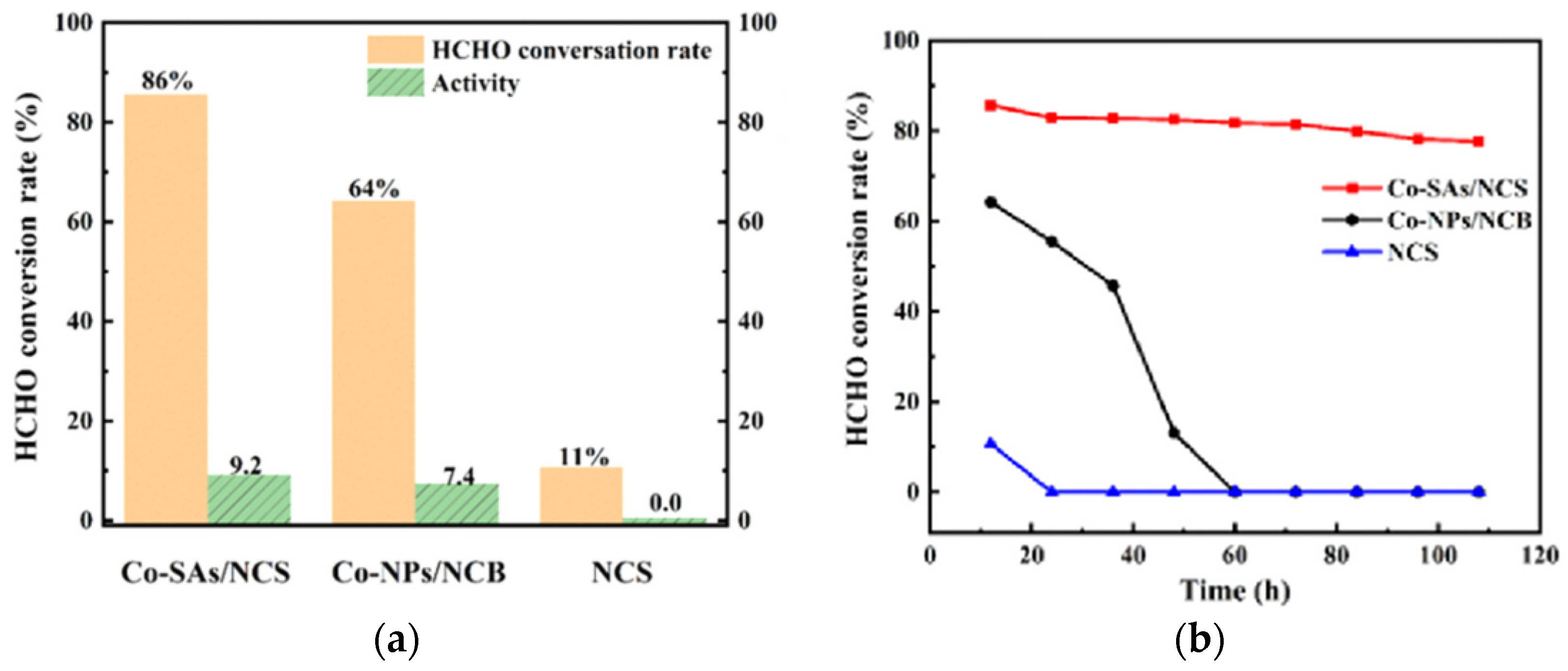
2.2. Cobalt-Based Catalysts
2.3. Other Transition Metal-Based Catalysts
3. Factors Affecting Catalytic Performance
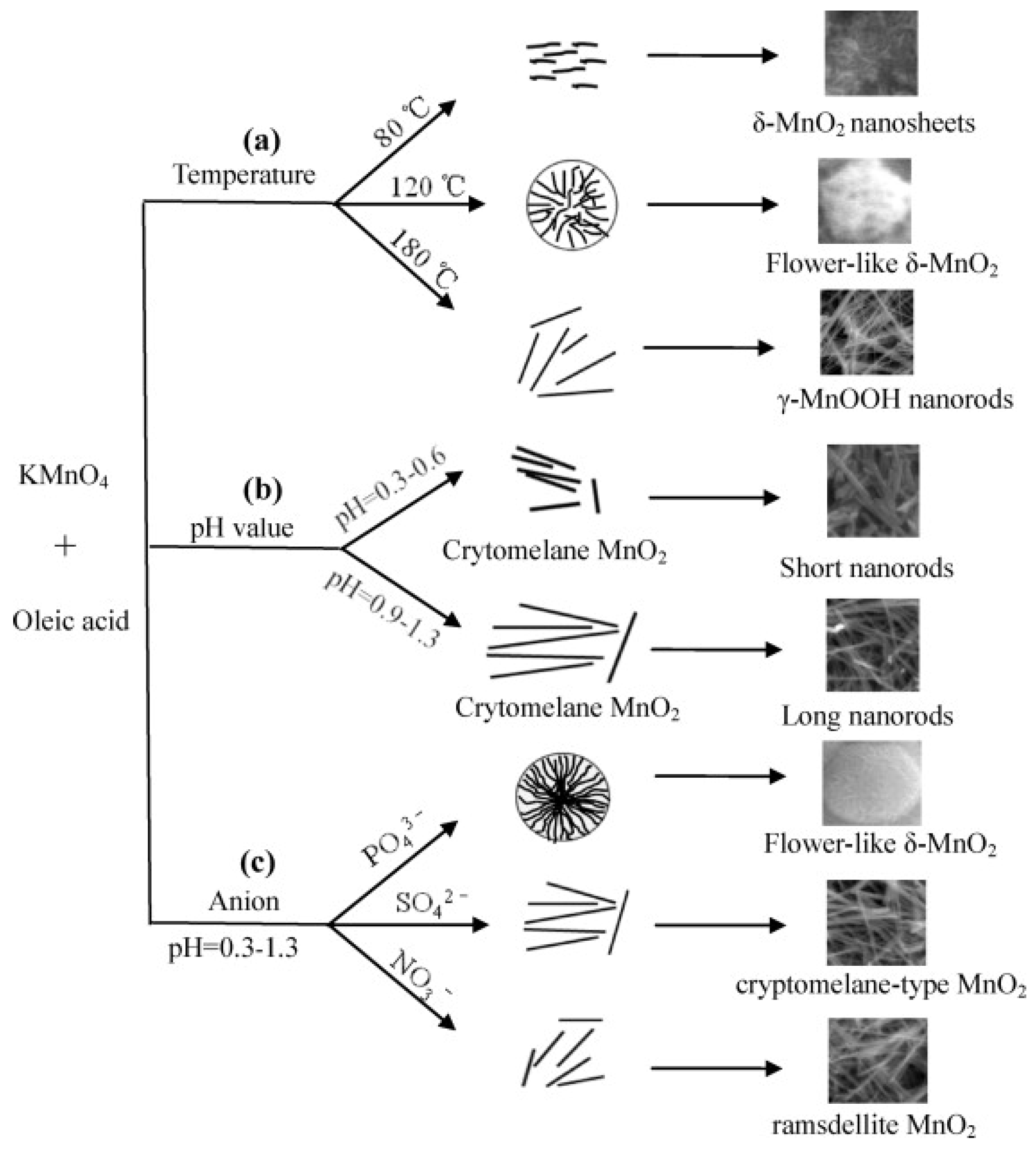
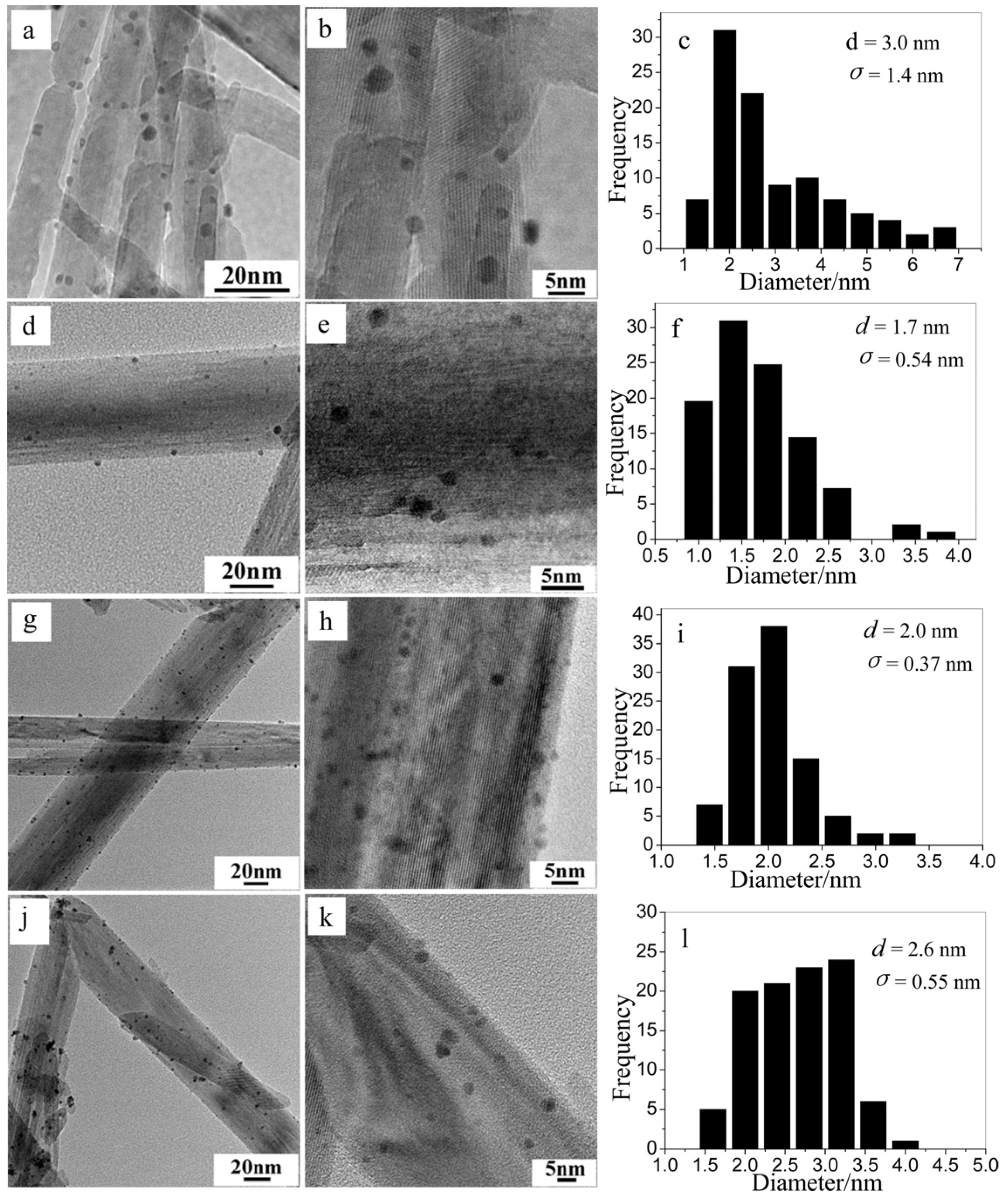
3.1. Preparation Methods
3.2. Catalyst Morphological Structure
3.3. Specific Surface Area
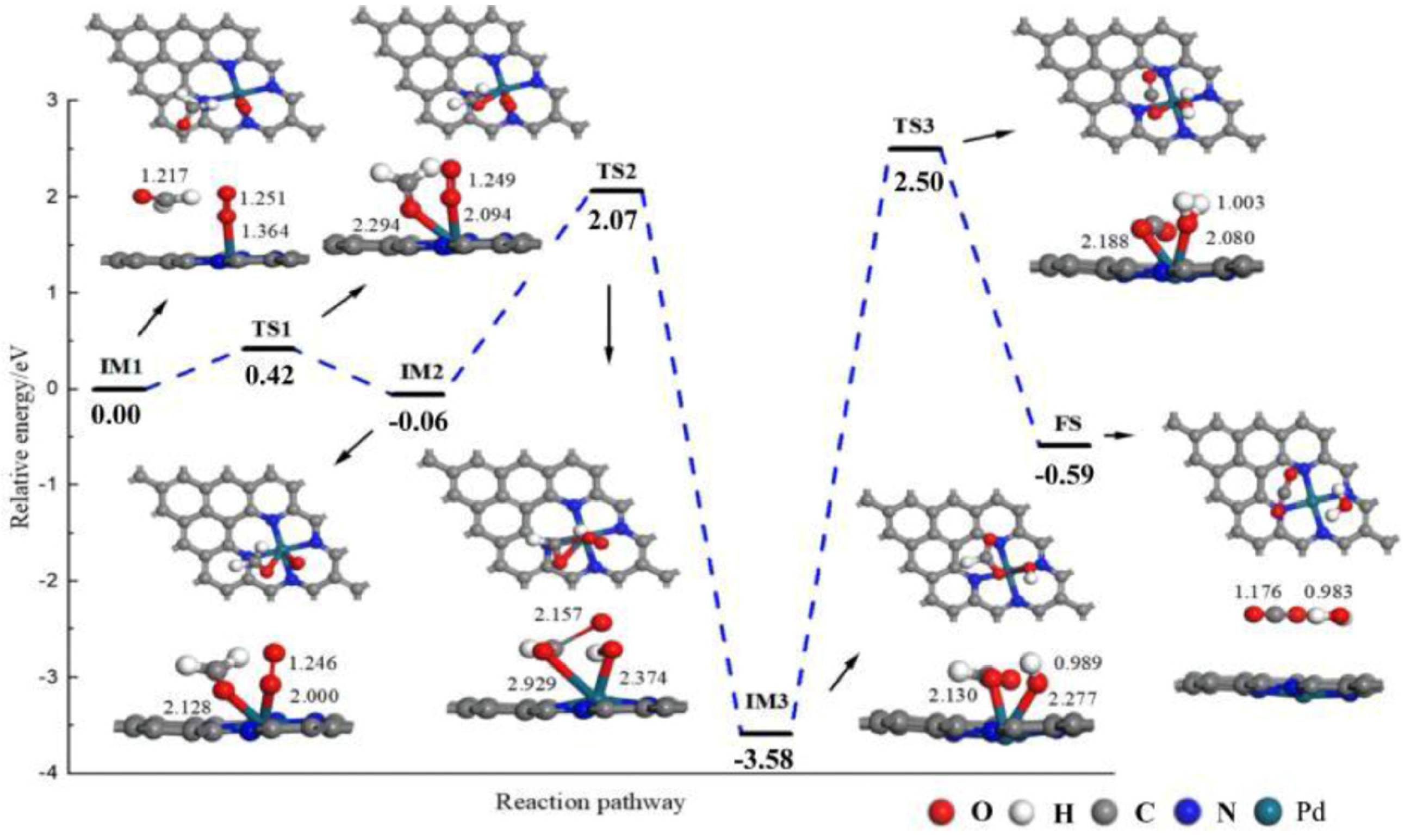
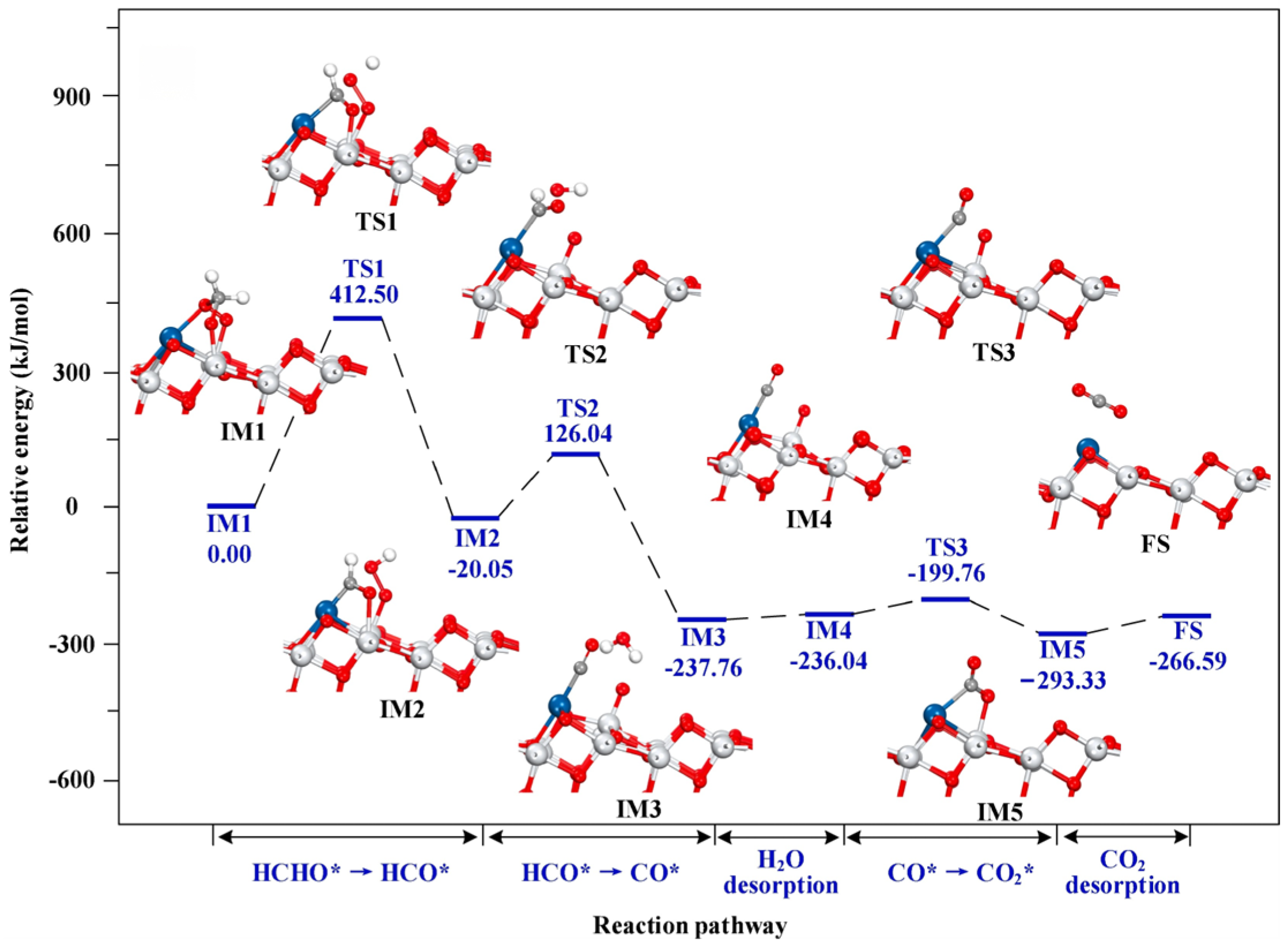
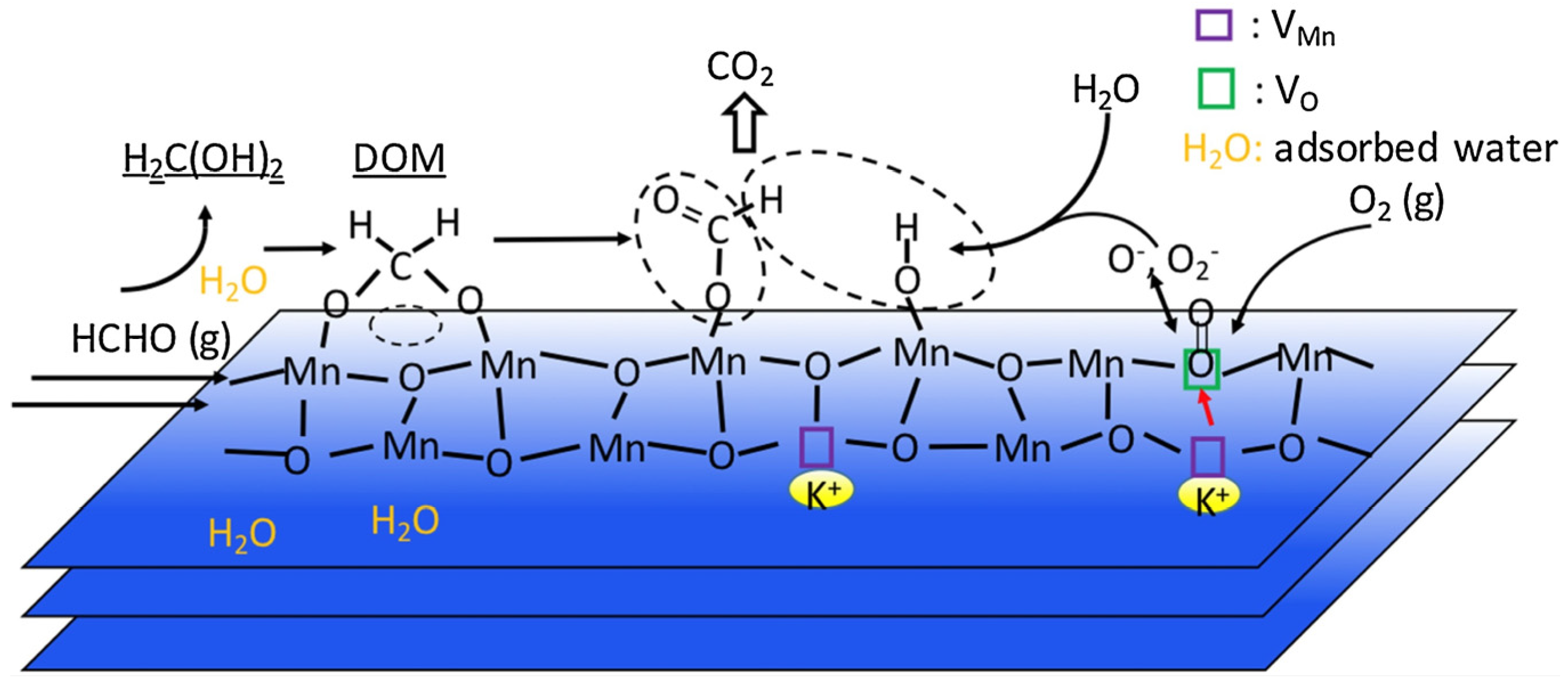
4. Catalytic Oxidation Mechanism of HCHO
4.1. Eley–Rideal Mechanism
4.2. Langmuir–Hinshelwood Mechanism
4.3. Mars–Van Krevelen Mechanism
5. Deactivation and Regeneration of HCHO Catalysts
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Li, K.; Xue, T.; Yang, Y.; Gong, Z.; Dong, F. Efficient and Durable Oxidation Removal of Formaldehyde over Layered Double Hydroxide Catalysts at Room Temperature. Environ. Sci. Technol. 2024, 58, 10378–10387. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.; Li, G.; Wang, P. Constructing α-MnO2/Mn2O3 Heterojunction for Formaldehyde Oxidation. Chemosphere 2024, 349, 140959. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Zhai, W.; Liu, X.; Xing, G.; Zhang, B.; Zang, J.; Yang, Y.; Ma, K.; Zhang, J. Room Temperature Catalysts for High Effective Degradation of Formaldehyde: Research Progresses and Challenges. ChemistrySelect 2024, 9, e202304418. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Recent Trend in Risk Assessment of Formaldehyde Exposures from Indoor Air. Arch. Toxicol. 2013, 87, 73–98. [Google Scholar] [CrossRef]
- La Torre, G.; Vitello, T.; Cocchiara, R.A.; Della Rocca, C. Relationship between Formaldehyde Exposure, Respiratory Irritant Effects and Cancers: A Review of Reviews. Public Health 2023, 218, 186–196. [Google Scholar] [CrossRef] [PubMed]
- de Falco, G.; Li, W.; Cimino, S.; Bandosz, T.J. Role of Sulfur and Nitrogen Surface Groups in Adsorption of Formaldehyde on Nanoporous Carbons. Carbon 2018, 138, 283–291. [Google Scholar] [CrossRef]
- Becker, A.; Israfilov, N.; Ehrstein, E.; Lara-Ibeas, I.; Planeix, J.-M.; Louis, B.; Le Calvé, S. Adsorption of Gaseous Formaldehyde on Y Zeolites and on Metal-Organic Frameworks. Microporous Mesoporous Mater. 2022, 343, 112136. [Google Scholar] [CrossRef]
- Su, G.; Xiong, J.; Li, Q.; Luo, S.; Zhang, Y.; Zhong, T.; Harper, D.P.; Tang, Z.; Xie, L.; Chai, X.; et al. Gaseous Formaldehyde Adsorption by Eco-Friendly, Porous Bamboo Carbon Microfibers Obtained by Steam Explosion, Carbonization, and Plasma Activation. Chem. Eng. J. 2023, 455, 140686. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Ho, W.; Han, S.; Wang, P.; Lee, S.; Zhang, Z. Modulation of Sulfur Vacancies at ZnIn2S4-δ/g-C3N4 Heterojunction Interface for Successive C-H Secession in Photocatalytic Gaseous Formaldehyde Complete Oxidation. Appl. Catal. B Environ. 2023, 338, 123048. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, X.; Wang, F.; Qin, X.; Zhang, C.; He, H. High-Performance of Cu-TiO2 for Photocatalytic Oxidation of Formaldehyde under Visible Light and the Mechanism Study. Chem. Eng. J. 2020, 390, 124481. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Qin, R.; Zeng, Y.; Qu, R.; Zheng, C.; Tu, X. Plasma-Catalytic Removal of Formaldehyde over Cu–Ce Catalysts in a Dielectric Barrier Discharge Reactor. Appl. Catal. B Environ. 2015, 170–171, 293–300. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, J.; Xue, F.; Deng, J.; Dai, Y.; Li, L.; Cui, M.; Qiao, X.; Fei, Z. Plasma-Engraved Co3O4 Nanostructure toward Improved Formaldehyde Oxidation Performance: Insight into the Structure–Activity Relationship. Appl. Surf. Sci. 2022, 600, 154183. [Google Scholar] [CrossRef]
- Gao, E.; Jin, Q.; Zhang, T.; Han, L.; Li, N.; Xu, J.; Yao, S.; Wu, Z.; Li, J.; Zhu, J.; et al. Unraveling the Promotional Effects of K-Doping on the Mobility of Surface Oxygen Species of CoCr2O4 for Improved Formaldehyde Catalytic Oxidation: The Weakened Metal-Oxygen Bond Strength. Chem. Eng. J. 2023, 474, 145618. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, C.; Zhang, T.; Li, L.; Yang, Q.; Zhao, H. Sodium Doped SrTi1-xBxO3 (BMn, Co) for Formaldehyde Catalytic Oxidation: Flame Spray Pyrolysis Fabrication and Reaction Mechanism Elaboration. Fuel Process. Technol. 2023, 247, 107763. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, G.; Wang, M.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt/MnO2 Nanoflowers Anchored to Boron Nitride Aerogels for Highly Efficient Enrichment and Catalytic Oxidation of Formaldehyde at Room Temperature. Angew. Chem. Int. Ed. 2021, 60, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Zheng, L.; Zhang, C.; Wang, Y.; Shan, W.; Liu, F.; He, H. A Nonoxide Catalyst System Study: Alkali Metal-Promoted Pt/AC Catalyst for Formaldehyde Oxidation at Ambient Temperature. ACS Catal. 2021, 11, 456–465. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Cheng, B.; Jiang, C.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. Synergy between Platinum and Gold Nanoparticles in Oxygen Activation for Enhanced Room-Temperature Formaldehyde Oxidation. Adv. Funct. Mater. 2022, 32, 2110423. [Google Scholar] [CrossRef]
- Zhuo, Y.; Guo, X.; Cai, W.; Shao, T.; Xia, D.; Li, C.; Liu, S. Synergetic Modulation of Molecular Oxygen Activation and Surface Acidity/Basicity on Defective M/UiO-66m (M = Pt, Pd) for Advanced Oxidation of Gaseous Formaldehyde at Room Temperature. Appl. Catal. B Environ. Energy 2023, 333, 122789. [Google Scholar] [CrossRef]
- Vikrant, K.; Chung, M.W.; Boukhvalov, D.W.; Heynderickx, P.M.; Kim, K.-H.; Weon, S. A Platinum Ensemble Catalyst for Room-Temperature Removal of Formaldehyde in the Air. Chem. Eng. J. 2023, 475, 146007. [Google Scholar] [CrossRef]
- Gravvani, K.; Solakidou, M.; Louloudi, M. Highly-Efficient Reusable [Silica@Iminophosphine-FeII] Hybrids for Hydrogen Production via Formic Acid and Formaldehyde Dehydrogenation. Chem.—Eur. J. 2025, 31, e202404440. [Google Scholar] [CrossRef]
- Meng, W.; Song, X.; Bao, L.; Chen, B.; Ma, Z.; Zhou, J.; Jiang, Q.; Wang, F.; Liu, X.; Shi, C.; et al. Synergistic Doping and De-Doping of Co3O4 Catalyst for Effortless Formaldehyde Oxidation. Chem. Eng. J. 2024, 494, 153028. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Wu, Y.; Zhang, C.; Luo, J.; Chen, C.; Li, Y. Atomic Iron on Mesoporous N-Doped Carbon to Achieve Dehydrogenation Reaction at Room Temperature. Nano Res. 2020, 13, 3075–3081. [Google Scholar] [CrossRef]
- Lu, L.; Tian, H.; He, J.; Yang, Q. Graphene–MnO2 Hybrid Nanostructure as a New Catalyst for Formaldehyde Oxidation. J. Phys. Chem. C 2016, 120, 23660–23668. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, Y.; Ren, L.; Cheng, Z.; Niu, Y.; Li, Z.; Zhang, S. Pyrrolic Nitrogen Boosted H2 Generation from an Aqueous Solution of HCHO at Room Temperature by Metal-Free Carbon Catalysts. J. Phys. Chem. Lett. 2024, 15, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ying, J.; Mai, Y.; Zhang, J.; Chen, J.; Wen, M.; Yu, L. MOF-Derived Metal Oxide Composite Mn2Co1Ox/CN for Efficient Formaldehyde Oxidation at Low Temperature. Catal. Sci. Technol. 2019, 9, 5845–5854. [Google Scholar] [CrossRef]
- Sekine, Y.; Nishimura, A. Removal of Formaldehyde from Indoor Air by Passive Type Air-Cleaning Materials. Atmos. Environ. 2001, 35, 2001–2007. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Cheng, W.; Wu, F.; Lu, X.; Li, Z. Controlled Synthesis of Diverse Manganese Oxide-Based Catalysts for Complete Oxidation of Toluene and Carbon Monoxide. Chem. Eng. J. 2014, 244, 59–67. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, C.; Du, X.; Zhu, Y.; Zhang, Y.; Li, S. Catalytic Removal of Toluene over Manganese Oxide-Based Catalysts: A Review. Environ. Sci. Pollut. Res. 2020, 27, 2482–2501. [Google Scholar] [CrossRef]
- Sekine, Y. Oxidative Decomposition of Formaldehyde by Metal Oxides at Room Temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic Oxidation of Formaldehyde over Manganese Oxides with Different Crystal Structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Han, Z.; Wang, C.; Zou, X.; Chen, T.; Dong, S.; Zhao, Y.; Xie, J.; Liu, H. Diatomite-Supported Birnessite–Type MnO2 Catalytic Oxidation of Formaldehyde: Preparation, Performance and Mechanism. Appl. Surf. Sci. 2020, 502, 144201. [Google Scholar] [CrossRef]
- Kang Zhao, W.; Yu Zheng, J.; Bao Han, C.; Ruan, J.; Lu, Y.; Ling Zhou, K.; Rui Zhai, T.; Wang, H.; Yan, H. MnOx-PMMA Self-Powered Triboelectric Catalysts Based on Three-Dimensional Nanocomposite Structures for Formaldehyde Degradation at Room Temperature. Chem. Eng. J. 2022, 440, 135877. [Google Scholar] [CrossRef]
- Chen, T.; Dou, H.; Li, X.; Tang, X.; Li, J.; Hao, J. Tunnel Structure Effect of Manganese Oxides in Complete Oxidation of Formaldehyde. Microporous Mesoporous Mater. 2009, 122, 270–274. [Google Scholar] [CrossRef]
- Tao, Y.; Li, R.; Huang, A.-B.; Ma, Y.-N.; Ji, S.-D.; Jin, P.; Luo, H.-J. High Catalytic Activity for Formaldehyde Oxidation of an Interconnected Network Structure Composed of δ-MnO2 Nanosheets and γ-MnOOH Nanowires. Adv. Manuf. 2020, 8, 429–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhang, Z.; Jiang, Z.; Shangguan, W.; Einaga, H. Simultaneously Catalytic Decomposition of Formaldehyde and Ozone over Manganese Cerium Oxides at Room Temperature: Promotional Effect of Relative Humidity on the MnCeOx Solid Solution. Catal. Today 2019, 327, 323–333. [Google Scholar] [CrossRef]
- Duan, C.; Zhou, Y.; Meng, M.; Huang, H.; Ding, H.; Zhang, Q.; Huang, R.; Yan, M. Research on the Elimination of Low-Concentration Formaldehyde by Ag Loaded onto Mn/CeO2 Catalyst at Room Temperature. Phys. Chem. Chem. Phys. 2023, 25, 24495–24507. [Google Scholar] [CrossRef]
- Xing, G.; Liu, X.; Jia, Y.; Wu, J.; Chai, L.; Zhai, W.; Wu, Z.; Kong, J.; Zhang, J. Oxygen Vacancy–Rich K-Mn3O4@CeO2 Catalyst for Efficient Oxidation Degradation of Formaldehyde at near Room Temperature. J. Colloid Interface Sci. 2025, 677, 417–428. [Google Scholar] [CrossRef]
- Liu, R.Y.; Man Trinh, M.; Chuang, H.T.; Chang, M.B. Ozone Catalytic Oxidation of Low-Concentration Formaldehyde over Ternary Mn-Ce-Ni Oxide Catalysts Modified with FeOx. Environ. Sci. Pollut. Res. 2023, 30, 32696–32709. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhou, K.L.; Zhao, W.K.; Wang, Y.; He, J.; Wang, X.; Wang, H.; Yan, H.; Han, C.B. Enhanced the Synergistic Degradation Effect between Active Hydroxyl and Reactive Oxygen Species for Indoor Formaldehyde Based on Platinum Atoms Modified MnOOH/MnO2 Catalyst. J. Colloid Interface Sci. 2022, 628, 359–370. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, W.; Li, W.; Cong, P.; Gao, D.; Jiang, X.; Hu, R.; Wang, R.; Chen, G. Insight into Formaldehyde Decomposition over MOFs-Derived CeO2-MnOx Bimetallic Oxides. Sep. Purif. Technol. 2025, 358, 130287. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Li, J.; Zhang, M.; Li, K.; Zheng, Y.; Ji, H. Mn/HZSM-5 Catalyst with High Content of Mn4+ and Surface Hydroxyls for Formaldehyde Oxidation at Room Temperature. Appl. Surf. Sci. 2023, 637, 157917. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Zhao, C.; Zhang, Y.; Mahmud, S.; Zhang, J. Ceramic Fiber Paper-Based Manganese Oxides Catalyst for Room Temperature Formaldehyde Oxidation. Catal. Lett. 2025, 155, 65. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef]
- Yang, J.; Sasaki, T. Morphological Control of Single Crystalline Co3O4 Polyhedrons: Selective and Nonselective Growth of Crystal Planes Directed by Differently Charged Surfactants and Solvents. Cryst. Growth Des. 2010, 10, 1233–1236. [Google Scholar] [CrossRef]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 Catalysts for Low-Temperature Oxidation of Trace Ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the Performance for Oxidation of Formaldehyde on Nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 Catalysts. Appl. Catal. B Environ. 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Jiao, Y.; Jing, C.; Wang, Y.; Yao, F.; Ye, G.; Wang, X.; Zhao, G.; Peng, W.; Huang, H.; Ye, D. Electrospinning Synthesis of Co3O4 Porous Nanofiber Monolithic Catalysts for the Room-Temperature Indoor Catalytic Oxidation of Formaldehyde at Low Concentrations. Appl. Surf. Sci. 2023, 639, 158215. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Du, B.; Wen, F.; Zhao, Y.; Yang, H.; Zhang, C. Efficient and Long-Lasting Removal of High-Level Formaldehyde at Room Temperature over a Cobalt Single-Atom Catalyst Anchored on Graphene-like Carbon Nanosheets. ACS EST Eng. 2025, 5, 1868–1878. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, G.; Wang, Z.; He, Q.; He, X.; Ji, H. Superior Performance of Formaldehyde Complete Oxidation at Ambient Temperature over Co Single-Atom Catalysts. Appl. Catal. B Environ. Energy 2023, 333, 122774. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Jia, L.; Wang, F.; Liu, T.; Xia, Y.; Xue, H. Regulation of Oxygen Vacancies in Ceria-Zirconia Nanocatalysts by Pluronic P123-Templated for Room Temperature Formaldehyde Total Oxidation. Catal. Lett. 2024, 154, 503–512. [Google Scholar] [CrossRef]
- Wang, X.; Yang, F.; Yi, J.; Kong, J.; Gong, J.; Yuan, A.; Rui, Z.; Ji, H. Constructing Synergy of Sufficient Hydroxyl and Oxygen in PtNi/AlO Enables Room-Temperature Catalytic HCHO Oxidation. AIChE J. 2023, 69, e17895. [Google Scholar] [CrossRef]
- Zhou, Z.; Ng, Y.H.; Xu, S.; Yang, S.; Gao, Q.; Cai, X.; Liao, J.; Fang, Y.; Zhang, S. A CuNi Alloy–Carbon Layer Core–Shell Catalyst for Highly Efficient Conversion of Aqueous Formaldehyde to Hydrogen at Room Temperature. ACS Appl. Mater. Interfaces 2021, 13, 37299–37307. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; He, X.; Ma, Z.; Yue, J.; Wang, W.; Li, C. The Composite of Zr-Doped TiO2 and MOF-Derived Metal Oxide for Oxidative Removal of Formaldehyde at the Room Temperature. Microporous Mesoporous Mater. 2022, 336, 111892. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.; Li, C.; Liu, S. Synergetic Molecular Oxygen Activation and Catalytic Oxidation of Formaldehyde over Defective MIL-88B(Fe) Nanorods at Room Temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Liu, L.; Wang, D.; Hao, Z.; Ma, C. Highly Active Manganese Oxide Catalysts for Low-Temperature Oxidation of Formaldehyde. Microporous Mesoporous Mater. 2012, 151, 397–402. [Google Scholar] [CrossRef]
- Bathla, A.; Kukkar, D.; Heynderickx, P.M.; Younis, S.A.; Kim, K.-H. Removal of Gaseous Formaldehyde by Portable Photocatalytic Air Purifier Equipped with Bimetallic Pt@Cu-TiO2 Filter. Chem. Eng. J. 2023, 469, 143718. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Rochard, G.; Giraudon, J.-M.; Liu, J.; Lamonier, J.-F. Mesoporous MnO2 Hollow Spheres for Enhanced Catalytic Oxidation of Formaldehyde. Sustain. Mater. Technol. 2019, 20, e00091. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Q.; Liu, H.; Wei, S.; Wang, L. Effect of Fe Doping on the Surface Properties of δ-MnO2 Nanomaterials and Its Decomposition of Formaldehyde at Room Temperature. J. Environ. Chem. Eng. 2022, 10, 108277. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Synthesis of Three-Dimensional Ordered Mesoporous MnO2 and Its Catalytic Performance in Formaldehyde Oxidation. Chin. J. Catal. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; He, J.; Hu, Y.; Tian, H. Control over the Morphology and Structure of Manganese Oxide by Tuning Reaction Conditions and Catalytic Performance for Formaldehyde Oxidation. Mater. Res. Bull. 2011, 46, 1714–1722. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Wang, W.; Jiang, Y.; Cao, K. PdZn Alloys Decorated 3D Hierarchical Porous Carbon Networks for Highly Efficient and Stable Hydrogen Production from Aldehyde solution. Int. J. Hydrogen Energy 2021, 46, 33429–33437. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, T.; Chen, J.; Zhang, J.; Mai, Y. Hydroxy-Modified Hierarchical Porous Na-CoOx/CN Material for Low-Concentration High-Throughput Formaldehyde Oxidation at Room Temperature. ChemPlusChem 2022, 87, e202200218. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Hou, B.; He, Y.; Weng, W.; Zhu, Y.; Wang, Z. Catalytic Ozonation of Formaldehyde with an Oxygen-Vacancy-Rich MnOx/γ-Al2O3 Catalyst at Room Temperature. Catalysts 2024, 14, 885. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, S.; Li, Q.; Wu, Z.; Zhang, J. Evaluation of an ε-Manganese (IV) Oxide/Manganese Vanadium Oxide Composite Catalyst Enriched with Oxygen Vacancies for Enhanced Formaldehyde Removal. Appl. Catal. B Environ. 2023, 320, 121994. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Li, J.; Jiang, C.; Yunus, R.; Kim, J. Room-Temperature Oxidation of Formaldehyde by Layered Manganese Oxide: Effect of Water. Environ. Sci. Technol. 2015, 49, 12372–12379. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese Oxides with Rod-, Wire-, Tube-, and Flower-Like Morphologies: Highly Effective Catalysts for the Removal of Toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Hou, Y.; Fu, X.; Yang, J.; Li, Q. The Catalytic and Adsorptive Synergistic Effect of Oxygen-Vacancy-Rich MnOx/C Catalysts for HCHO Oxidation at Room Temperature. Ind. Eng. Chem. Res. 2024, 63, 5125–5134. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, C.; Liu, Y.; Huang, Q. Enhancement of Photocatalytic Property of ZnO for Gaseous Formaldehyde Degradation by Modifying Morphology and Crystal Defect. J. Alloys Compd. 2013, 550, 190–197. [Google Scholar] [CrossRef]
- Yu, X.; He, J.; Wang, D.; Hu, Y.; Tian, H.; He, Z. Facile Controlled Synthesis of Pt/MnO2 Nanostructured Catalysts and Their Catalytic Performance for Oxidative Decomposition of Formaldehyde. J. Phys. Chem. C 2012, 116, 851–860. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Li, D.; Zhou, S.; Du, Y.; Zhang, H.; Hang, C.; Tian, Y.; Suga, T. Fabrication of Ag@Ag2O-MnOx Composite Nanowires for High-Efficient Room-Temperature Removal of Formaldehyde. J. Mater. Sci. Technol. 2021, 91, 5–16. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, M.; Zhang, B.; Gao, Y.; Lu, W.; Guo, Y. Controlled Synthesis of Porous Co3O4 Nanofibers by Spiral Electrospinning and Their Application for Formaldehyde Oxidation. RSC Adv. 2016, 6, 102127–102133. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Liu, L.; Wang, D. Effects of Textural Parameters and Noble Metal Loading on the Catalytic Activity of Cryptomelane-Type Manganese Oxides for Formaldehyde Oxidation. Ceram. Int. 2013, 39, 315–321. [Google Scholar] [CrossRef]
- Zeng, L.; Song, W.; Li, M.; Zeng, D.; Xie, C. Catalytic Oxidation of Formaldehyde on Surface of HTiO2/HCTiO2 without Light Illumination at Room Temperature. Appl. Catal. B Environ. 2014, 147, 490–498. [Google Scholar] [CrossRef]
- Zhao, W.K.; Zheng, J.Y.; Wang, X.; Ma, T.; Zhou, K.L.; Wang, H.; Yan, H.; Han, C.B. Removal of Formaldehyde by Triboelectric Charges Enhanced MnOx-PI at Room Temperature. Appl. Surf. Sci. 2021, 541, 148430. [Google Scholar] [CrossRef]
- Ahmad, W.; Park, E.; Lee, H.; Kim, J.Y.; Kim, B.C.; Jurng, J.; Oh, Y. Defective Domain Control of TiO2 Support in Pt/TiO2 for Room Temperature Formaldehyde (HCHO) Remediation. Appl. Surf. Sci. 2021, 538, 147504. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Wang, W.; Chen, M.; Li, H.; Lee, S.; Ho, W.; Huang, T.; Cao, J. Oxygen Vacancy–Engineered δ-MnOx/Activated Carbon for Room-Temperature Catalytic Oxidation of Formaldehyde. Appl. Catal. B Environ. 2020, 278, 119294. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Wang, H.; Chen, P.; Dong, X.; Sun, Y.; Zhou, Y.; Dong, F. Photocatalytic Reaction Mechanisms at a Gas–Solid Interface for Typical Air Pollutant Decomposition. J. Mater. Chem. A 2021, 9, 20184–20210. [Google Scholar] [CrossRef]
- Liu, S.; Niu, S.; Liu, J.; Wang, D.; Wang, Y.; Han, K. Mechanism of Formaldehyde Oxidation Catalyzed by Doped Graphene Single Atom Catalysts: Density Functional Theory Study. Mol. Catal. 2022, 528, 112516. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Jiang, Q.; Zhao, W.; Ao, Z.; An, T. Density Functional Theory Calculations on Single Atomic Catalysis: Ti-Decorated Ti3C2O2 Monolayer (MXene) for HCHO Oxidation. Chin. J. Catal. 2020, 41, 1633–1644. [Google Scholar] [CrossRef]
- Mang, C.; Li, G.; Rao, M.; Zhang, X.; Luo, J.; Jiang, T. An Ab Initio Investigation on the Mechanism of Formaldehyde Oxidation: A Case of Heterogeneous Catalytic Reaction over Graphene-like MnO2 Monolayer Anchored Different Single Atoms (Fe, Co, and Ni). Appl. Surf. Sci. 2023, 608, 154964. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Liu, J.; Wang, Z. Catalytic Reaction Mechanism of Formaldehyde Oxidation by Oxygen Species over Pt/TiO2 Catalyst. Chemosphere 2020, 248, 125980. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Mo, J.; Zhou, Y.; Fan, M.; Chen, Y.; Liu, J.; Xu, Y.; Shen, S. Study of Manganese–Cerium Composite Oxide Catalysed Oxidation for Low Concentration Formaldehyde at Room Temperature. Mater. Chem. Phys. 2022, 285, 126151. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Liu, Y.; Chu, X. Alkali Metal Doped MnOx Catalysts for Formaldehyde Oxidation. Catal. Lett. 2024, 155, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Lee, S.C.; Cao, J. The Mechanism of Room Temperature Catalytic C–H Dissociation and Oxygenation of Formaldehyde over Nano-Zirconia Phase-Junction. Chem. Eng. J. 2020, 380, 122498. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The Effect of Manganese Vacancy in Birnessite-Type MnO2 on Room-Temperature Oxidation of Formaldehyde in Air. Appl. Catal. B Environ. 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; Romero-Sáez, M.; González-Marcos, J.A.; González-Velasco, J.R. Catalytic Activity of Regenerated Catalyst after the Oxidation of 1,2-Dichloroethane and Trichloroethylene. Chem. Eng. J. 2014, 241, 200–206. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst Deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Neyestanaki, A.K.; Klingstedt, F.; Salmi, T.; Murzin, D.Y. Deactivation of Postcombustion Catalysts, a Review. Fuel 2004, 83, 395–408. [Google Scholar] [CrossRef]
- Abbas, N.; Hussain, M.; Russo, N.; Saracco, G. Studies on the Activity and Deactivation of Novel Optimized TiO2 Nanoparticles for the Abatement of VOCs. Chem. Eng. J. 2011, 175, 330–340. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-Temperature Catalytic Combustion of Trichloroethylene over Cerium Oxide and Catalyst Deactivation. Appl. Catal. B Environ. 2008, 81, 192–202. [Google Scholar] [CrossRef]
- Kułażyński, M.; van Ommen, J.G.; Trawczyński, J.; Walendziewski, J. Catalytic Combustion of Trichloroethylene over TiO2-SiO2 Supported Catalysts. Appl. Catal. B Environ. 2002, 36, 239–247. [Google Scholar] [CrossRef]
- Kießling, D.; Schneider, R.; Kraak, P.; Haftendorn, M.; Wendt, G. Perovskite-Type Oxides—Catalysts for the Total Oxidation of Chlorinated Hydrocarbons. Appl. Catal. B Environ. 1998, 19, 143–151. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- HafezKhiabani, N.; Fathi, S.; Shokri, B.; Hosseini, S.I. A Novel Method for Decoking of Pt–Sn/Al2O3 in the Naphtha Reforming Process Using RF and Pin-to-Plate DBD Plasma Systems. Appl. Catal. A Gen. 2015, 493, 8–16. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Liu, J.-L.; Liu, J.-B.; Zhu, X.; Zhu, A.-M. In-Situ Regeneration of Au Nanocatalysts by Atmospheric-Pressure Air Plasma: Significant Contribution of Water Vapor. Appl. Catal. B Environ. 2015, 179, 69–77. [Google Scholar] [CrossRef]
- Kim, H.H.; Tsubota, S.; Daté, M.; Ogata, A.; Futamura, S. Catalyst Regeneration and Activity Enhancement of Au/TiO2 by Atmospheric Pressure Nonthermal Plasma. Appl. Catal. A Gen. 2007, 329, 93–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wang, R. Non-Noble Metal Catalysts for Efficient Formaldehyde Removal at Room Temperature. Catalysts 2025, 15, 723. https://doi.org/10.3390/catal15080723
Feng Y, Wang R. Non-Noble Metal Catalysts for Efficient Formaldehyde Removal at Room Temperature. Catalysts. 2025; 15(8):723. https://doi.org/10.3390/catal15080723
Chicago/Turabian StyleFeng, Yiqing, and Rui Wang. 2025. "Non-Noble Metal Catalysts for Efficient Formaldehyde Removal at Room Temperature" Catalysts 15, no. 8: 723. https://doi.org/10.3390/catal15080723
APA StyleFeng, Y., & Wang, R. (2025). Non-Noble Metal Catalysts for Efficient Formaldehyde Removal at Room Temperature. Catalysts, 15(8), 723. https://doi.org/10.3390/catal15080723







