Abstract
Electrochemical nitrate reduction to ammonia offers a sustainable route for nitrogen fixation, yet achieving high efficiency and selectivity remains challenging. Here, a Sustainion-enabled Cu/Co tandem catalyst is developed to couple compositional synergy with ionomer-mediated interfacial regulation. The optimized Cu60Co40/Sus/C electrode delivers a Faradaic efficiency of 91.3% and an NH3 yield rate of 2.63 mmol gcat.−1 h−1 at −0.3 V vs. RHE, surpassing Cu-Co/Nafion/C and Cu-Co/C counterparts. Structural analyses confirm that Sustainion prevents nanoparticle aggregation and maintains robust Cu/Co interfaces. Electrochemical and in situ spectroscopic studies reveal that the cationic quaternary ammonium groups of Sustainion electrostatically enrich NO3−/NO2− intermediates, facilitating their adsorption and hydrogenation toward NH3 formation. The combined structural stabilization and intermediate modulation enable efficient tandem catalysis between Cu-driven nitrate activation and Co-mediated hydrogenation. This work provides molecular-level insight into ionomer–catalyst interactions and highlights interfacial engineering as a powerful strategy for sustainable ammonia synthesis.
1. Introduction
Ammonia (NH3) serves as a cornerstone of the global chemical industry, not only as the primary nitrogenous fertilizer supporting food production but also as an emerging carbon-neutral energy carrier for next-generation energy systems [1,2]. The industrial Haber–Bosch process, however, remains the dominant route for NH3 synthesis and operates under harsh thermodynamic conditions (300–500 °C, >200 atm), consuming over 2% of global energy and releasing nearly 1.8% of total anthropogenic CO2 emissions [3,4]. This process’s dependence on fossil-derived hydrogen and energy-intensive operation stands in stark contrast to the world’s commitment to carbon neutrality and sustainable chemical manufacturing. These challenges have motivated the search for alternative NH3 synthesis routes that can operate efficiently under mild conditions while minimizing the environmental impact [5,6].
Electrochemical NH3 synthesis has emerged as a promising approach that leverages renewable electricity to drive nitrogen conversion under ambient conditions. Early efforts focused primarily on the electrochemical nitrogen reduction reaction (NRR) [7], aiming to mimic the enzymatic nitrogen fixation pathway. However, the dissociation of the inert N≡N bond (bond energy ≈ 941 kJ mol−1) poses formidable kinetic barriers [8,9], leading to low conversion efficiencies and severe hydrogen evolution competition [10,11]. By contrast, the electrochemical nitrate reduction reaction (NO3−RR) has gained increasing attention as a thermodynamically and kinetically more favorable pathway toward NH3 synthesis. Nitrate, with a weaker N=O bond (≈204 kJ mol−1), can be reduced under milder conditions, offering faster reaction kinetics [12,13]. More importantly, NO3−RR not only enables sustainable NH3 production but simultaneously mitigates environmental pollution caused by excessive NO3− accumulation in soil and groundwater, thereby coupling clean energy conversion with water quality restoration and circular nitrogen management [14]. In comparison, other nitrate conversion technologies exhibit limitations to varying degrees. For instance, thermochemical nitrate conversion, which encompasses two core technologies—thermocatalytic reduction and high-temperature nitrate decomposition—requires operation under high-temperature (300–600 °C) and high-pressure conditions, and suffers from issues of high energy consumption and low ammonia (NH3) selectivity. While photocatalytic nitrate reduction can utilize solar energy at ambient temperature, its photogenerated charge carriers tend to recombine, resulting in low NH3 yield (<1 mmol·gcat.−1 h−1) and poor catalyst stability. By contrast, photoelectrochemical nitrate reduction can enhance NH3 selectivity to 85–90% via the application of a bias voltage, but it faces challenges of complex equipment and high costs [15,16].
Despite its promise, the electrochemical reduction of NO3− to NH3 is a multi-electron (8e−) and multi-proton (9H+) process that involves numerous intermediate species (NO2−, NO*, NHO*, NH2OH*, etc.) [17], posing stringent requirements on catalyst design. Efficient catalysts must activate the N-O bond, selectively guide intermediate transformations, and suppress side reactions such as hydrogen evolution. Among the various reported systems, Cu/Co tandem catalysts have recently demonstrated exceptional potential by integrating two complementary active centers. Cu efficiently catalyzes the initial NO3− activation and reduction to NO2− through N-O bond cleavage, while Co facilitates subsequent hydrogenation steps that convert NO2− to NH3 [18,19]. This tandem mechanism effectively divides the complex NO3−RR pathway into sequential steps, accelerating kinetics and enhancing selectivity. In current research, similar bimetallic catalysts are often combined with Metal–Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs). For instance, Cu-based MOFs can efficiently activate and convert nitrate species via their unique coordination environments [20]; similarly, constructing microenvironments with well-defined pore structures through the reticular structure engineering of COFs facilitates nitrate enrichment and confinement of reaction intermediates, ultimately achieving high-activity and high-selectivity ammonia synthesis [21]. However, current Cu/Co catalysts typically rely on complex nanostructuring or multi-step synthesis methods that limit their scalability [22]. In contrast, simple mechanical mixing of commercial Cu and Co powders represents a more practical and low-cost alternative. Yet, this approach often suffers from particle aggregation and poor interfacial contact, which decrease the active surface area and disrupt the delicate tandem synergy required for efficient NO3−RR [23]. Hence, maintaining a stable and well-dispersed Cu/Co interface is essential for sustaining catalytic performance and its future practical applications.
In addition to catalyst composition, the often-overlooked role of binders has recently emerged as a critical factor influencing the performance of electrochemical systems. The binder dictates catalyst dispersion, ion transport, and electrode–electrolyte interface properties. Nafion, the most widely used cation-exchange ionomer, consists of a hydrophobic perfluorinated backbone with terminal sulfonic acid groups [23,24]. While it provides excellent proton conductivity in acidic media, its hydrophobic backbone impedes electrolyte penetration and restricts the access of anionic reactants such as NO3− to active sites [25]. Moreover, in alkaline environments, the sulfonic acid groups of Nafion can accelerate surface oxidation of transition metals like Cu and Co, leading to deactivation of catalytic sites. The spatially localized sulfonic moieties also fail to regulate the adsorption and conversion of anionic intermediates such as NO2−, often resulting in the accumulation of undesired species and side reactions [26]. These limitations underscore the need for a new generation of ionomers capable of simultaneously enhancing mass transport, stabilizing metal surfaces, and modulating the microenvironment to guide intermediate transformations.
Sustainion ionomer, a commercially available anion-exchange polymer, represents a promising alternative to Nafion [27,28]. Composed of a hydrophilic hydrocarbon backbone functionalized with cationic quaternary ammonium groups, Sustainion possesses unique physicochemical properties ideally suited for NO3−RR [29]. The positively charged ammonium sites can electrostatically attract anionic intermediates (NO3−, NO2−), enhancing their adsorption and promoting stepwise reduction at the catalyst surface [30]. This effect is consistent with electrostatic intermediate stabilization observed in other anion-involving electrocatalytic systems. Furthermore, the hydrophilic polymer backbone enables improved electrolyte wetting and ion mobility, thereby facilitating faster proton and electron transport [31]. Such features are particularly advantageous for the NO3−RR process, where mass transport and charge transfer efficiency directly govern catalytic activity and selectivity. Therefore, the incorporation of Sustainion as a binder is expected to not only improve catalyst utilization by preventing particle aggregation but also to regulate the local chemical environment through favorable electrostatic and solvation effects, thus enabling enhanced NH3 synthesis efficiency and catalyst durability [32,33]. Despite these advantages, the mechanistic understanding of how the Sustainion ionomer influences NO3−RR remains incomplete, and critical questions persist regarding how its molecular architecture affects the adsorption and hydrogenation of key intermediates, as well as how these interactions influence proton transfer dynamics. Furthermore, the extent to which Sustainion ionomer modulates the tandem catalytic pathways on Cu/Co interface remains poorly understood [34]. As a typical anionic ionomer, Sustainion has concentrated applications in electrocatalysis, primarily in CO2 reduction and alkaline electrolyzers. In current research on electrocatalytic nitrate reduction, Sustainion is rarely used as a binder during catalyst fabrication, with Nafion or carbon-based supports remaining the mainstream choices. Given its excellent ionic conductivity and interfacial stability, Sustainion, in our view, holds promising application prospects in this field [35]. Clarifying these relationships is essential for establishing molecular-level design principles for next-generation ionomer–catalyst interfaces in NO3−RR.
Herein, we systematically investigate the role of Sustainion ionomer as a functional binder in Cu/Co tandem catalysts for the electrochemical nitrate reduction reaction (NO3−RR) to ammonia (NH3). We confirm that Sustainion ionomer significantly modulates the physicochemical properties and catalytic behavior of the Cu/Co interface, and have characterized the Faradaic efficiency (FE) and NH3 yield of the modified Cu-Co/Sus/C catalyst. Comprehensive characterization demonstrates that Sustainion ionomer forms a dense polymeric coating, inhibiting catalyst aggregation and maintaining structural integrity during reaction. Electrochemical analyses reveal remarkable increases in electrochemically active surface area and reduced charge transfer resistance induced by the ionomer. In situ spectroscopic measurements further indicate that Sustainion ionomer accelerates the adsorption and hydrogenation of the key NO2− intermediate, facilitating efficient NH3 conversion. Collectively, this study uncovers the intrinsic mechanism by which ionomer–catalyst interactions regulate NO3−RR electroreduction at the molecular level, providing a generalizable strategy for designing high-performance, stable, and scalable tandem catalysts to advance sustainable NH3 synthesis and nitrate remediation technologies.
2. Results and Discussion
2.1. Catalyst Structure and Characterization
To elucidate the structural and compositional evolution of the Cu/Co tandem catalysts and the role of the Sustainion ionomer in regulating their morphology and surface states, a comprehensive suite of characterization techniques was employed, including scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). These analyses collectively provide multiscale insights into the morphology, crystallinity, and electronic structure of the active components, all of which are intimately related to the catalyst’s performance in electrochemical NO3−RR. ICP analysis was employed to characterize the formulated ink (Table S1), verifying that the content ratio of Cu to Co is essentially consistent with the expected value. Table S2 summarizes the nomenclature, core compositional characteristics, and implications of the names of the different catalysts employed in this study. SEM images reveal a pronounced contrast in surface morphology between the Cu-Co/C and Cu-Co/Sus/C catalysts (Figure 1a,b). The pristine Cu-Co nanoparticles (NPs) exhibited severe agglomeration, forming densely packed spherical aggregates with smooth surfaces and limited exposure of active sites. In sharp contrast, the Cu-Co/Sus/C electrode displayed a much finer and more porous structure, consisting of uniformly dispersed NPs with roughened surfaces. Such nanoscale texturing and homogeneous dispersion suggest that the cationic ionomer effectively prevents particle coalescence and facilitates better contact between the catalyst and the carbon substrate. The enhanced structural uniformity and interparticle spacing are expected to promote efficient electrolyte infiltration and ion diffusion, thereby increasing the availability of active sites for nitrate adsorption and reduction. The microscopic structure of the individual metallic components was further resolved by high-resolution TEM (Figure 1c,d). Distinct lattice fringes corresponding to Cu(200) and Co(002) planes were clearly observed, with interplanar spacings of 0.187 nm and 0.203 nm, respectively, confirming the presence of crystalline metallic Cu and Co phases.
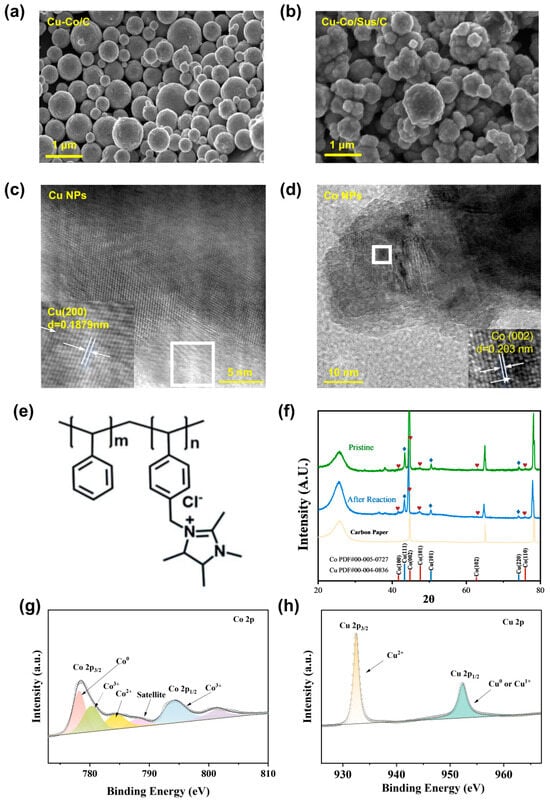
Figure 1.
The characterization of Cu/Co sample. SEM micrograph of (a) Cu-Co/C NPs and (b) Cu-Co/Sus/C NPs; TEM micrograph of (c) Cu NPs and (d) Co NPs; (e) The structural formula of Sustainion ionomer. (f) The XRD patterns of as-synthesized Cu-Co/Sus/C electrode before and after reaction (at −0.5 V vs. RHE for 1 h). (g) XPS spectrum of Cu. (h) XPS spectrum of Co.
The chemical structure of the binder Sustainion is shown in Figure 1e. The crystalline phase composition was examined via XRD (Figure 1f). The XRD pattern of the pristine electrode clearly exhibits characteristic diffraction peaks consistent with metallic Cu (PDF#04-0836), with the most intense (111) reflection appearing at approximately 43.3°. Concurrently, distinct peaks attributable to metallic Co (PDF#05-0727) are observed, notably the (002) plane diffraction at around 44.2°. Additional weaker reflections at approximately 50.4° and 74.1°, corresponding to Cu (200) and Cu (220) planes, respectively, further confirm the presence of the face-centered cubic Cu phase. Notably, the post-electrocatalysis pattern maintains all characteristic peaks of both metallic Cu and Co phases without the emergence of new diffraction features assignable to oxides or other side products. The absence of any new phases after the reaction further demonstrates the robustness of the Cu-Co/Sus/C interface and the protective role of the Sustainion ionomer during long-term operation. The minor peak broadening and attenuated intensity following reaction can be reasonably attributed to a slight degradation in crystallinity, a phenomenon commonly encountered during prolonged electrochemical testing. Meanwhile, it can be observed from the post-reaction scanning electron microscopy (SEM) images that the metal nanospheres in the catalysts incorporated with Sustainion binder still maintain a good surface morphology (Figure S1). XPS measurements were performed to probe the surface chemical states of the catalysts (Figure 1g,h). The Cu 2p spectrum displayed peaks at 932.6 and 952.4 eV, signifying the dominant presence of Cu0/Cu+ states. The lack of a distinct satellite peak near 942 eV effectively rules out substantial Cu2+ formation, highlighting a favorably reduced copper surface. Parallel examination of the Co 2p region showed signatures of metallic Co0 at 778.3 and 793.4 eV, with faint satellites hinting at minimal surface oxidation [36]. This co-existence of metallic Cu and Co centers establishes an optimal electronic structure for tandem catalysis. In addition, weak O 1s and N 1s signals originating from the Sustainion backbone confirmed the presence of quaternary ammonium and ether groups in close contact with the metal surface, implying strong interfacial interactions between the polymer and the NPs. These interactions not only anchor the metal particles firmly within the conductive matrix but also create a favorable electrostatic environment for the adsorption and transformation of anionic intermediates such as NO3− and NO2−.
2.2. Electrochemical Performance
The electrocatalytic nitrate reduction performance of the Cu-Co/Sus/C catalysts was systematically evaluated in 0.1 M KOH containing 0.1 M KNO3 using a conventional H-type electrochemical cell, with a Nafion membrane separating the cathodic and anodic compartments to prevent product crossover. Catalyst inks within 5% binder were prepared by dispersing the Cu and Co powders in isopropanol and drop-casting the suspension onto carbon paper electrodes (see Section 3). We evaluated the performance of binder-free Cu-Co/C, Cu-Co/Nafion/C and Cu-Co/Sus/C in electrochemical NO3−RR. Each catalyst was tested under a constant potential for one hour to assess their performance across a broad range of potentials. The main products on all three electrodes were NO2− and NH3, which were quantified by using the ultraviolet-visible (UV-Vis) spectroscopy (Figure S2). Linear sweep voltammetry (LSV) measurements clearly demonstrated the catalytic activity of the Cu/Co system toward NO3− reduction (Figure 2a). Upon the introduction of NO3− into the electrolyte, the cathodic current density increased sharply, indicating the activation of NO3−RR pathways rather than the hydrogen evolution reaction (HER). Notably, the Sustainion-containing electrode exhibited a higher current response than the binder-free electrodes, suggesting accelerated reaction kinetics and improved charge transfer at the electrode–electrolyte interface.
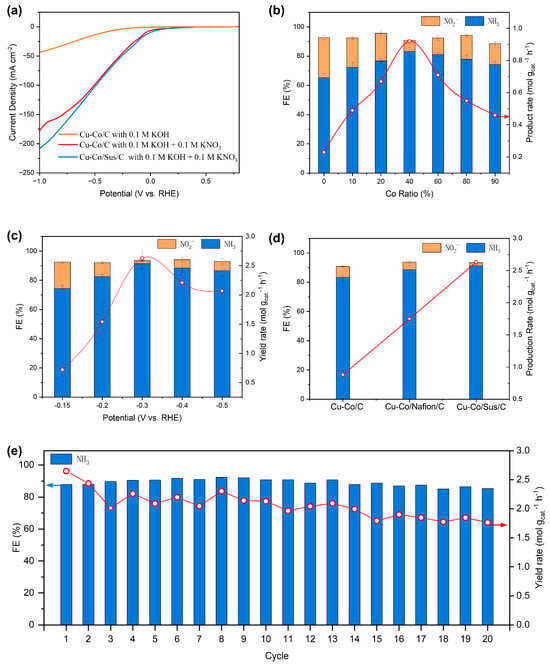
Figure 2.
Electrocatalytic performance of NO3−R. (a) The LSV analysis for Cu-Co/C and Cu-Co/Sus/C with different electrolytes. (b) The FE (column) of NO2−, NH3 and yield rate (red-line) of NH3 on pure Cu-Co/C electrodes as a function of Co mass fraction under −0.3 V vs. RHE in electrochemical NO3−RR. (c) The FE (column) of NO2−, NH3 and yield rate (red-line) of NH3 on Cu60-Co40/Sus/C electrode as a function of applied potential (vs. RHE). (d) The comparison of FE (column) of NO2−, NH3 and yield rate (red-line) of NH3 on binder-free Cu60-Co40/C, Cu60-Co40/Nafion/C and Cu60-Co40/Sus/C electrode at −0.3 V vs. RHE. (e) Electrocatalytic NO3−RR stability test (column: FE of NH3, red-line: yield rate) on Cu60-Co40/Sus/C at −0.3 V vs. RHE. The error bars represent the standard deviation of triplicate measurements.
To elucidate the effect of composition on activity, a series of binder-free Cu-Co/C catalysts with different atomic ratios were evaluated under identical conditions (Figure 2b). Both the Faradaic efficiency (FE) and the NH3 production rate exhibited a volcano-shaped dependence on the Co content. As the Co fraction increased from 0% to 40%, the FE and NH3 yield steadily rose, reaching a maximum at Cu60Co40, which achieved an FE of 83.2% and an NH3 production rate of 0.92 mmol gcat.−1 h−1 at −0.3 V vs. RHE. Further increasing the Co content beyond 40% resulted in a pronounced decline in both metrics, reflecting the trade-off between Cu’s ability to activate NO3− and Co’s propensity to hydrogenate NO2− intermediates. In addition, as Figure 2b shows, Pure Cu (with a Co ratio of 0%) activates NO3− but poorly converts the formed NO2− to NH3, as evidenced by the high NO2− FE yet limited NH3 selectivity at low Co ratios. These results underscore the tandem mechanism between the two metals: Cu drives the NO3− → NO2− reaction to supply NO2− as an intermediate, while Co facilitates the subsequent NO2− → NH3 conversion; the enhanced NH3 FE and product rate at Cu/Co electrodes confirm this synergy, as Cu’s reaction product acts as Co’s reactant, allowing the two metals to sequentially leverage their complementary strengths for efficient NO3−-to-NH3 conversion. The optimal Cu/Co ratio thus ensures a balanced availability of both active sites, enabling efficient intermediate transfer and high overall selectivity. The potential-dependent activity profile of the Cu60Co40 catalyst (Figure 2c) further validated its superior tandem behavior. The FE for NH3 increased with increasing overpotential and reached its peak at −0.3 V vs. RHE, accompanied by a maximum FE of 91.3 and NH3 yield rate of 2.63 mmol gcat.−1 h−1. Beyond this potential, the FE gradually decreased, likely due to the enhanced competitive HER at more negative potentials. This observation highlights the importance of achieving a potential window where NO3− activation and intermediate hydrogenation are both kinetically favorable, yet HER is suppressed. To assess the role of binders in modulating catalytic performance, three types of electrodes—binder-free Cu-Co/C, Cu-Co/Nafion/C, and Cu-Co/Sus/C—were compared under identical conditions (Figure 2d). The incorporation of Sustainion ionomer resulted in a remarkable improvement in both activity and selectivity.
At −0.3 V vs. RHE, the Cu-Co/Sus/C electrode exhibited an FE of 91.3%, which was 8% and 4% higher than those of the Cu-Co/C and Cu-Co/Nafion/C electrodes, respectively. Concomitantly, the NH3 production rate of the Cu-Co/Sus/C electrode reached 2.63 mmol gcat.−1 h−1, which corresponded to approximately 2.9- and 1.6-fold increases compared to the Cu-Co/Nafion/C and Cu-Co/C counterparts, respectively. Moreover, the sustained superiority of Cu-Co/Sus/C across the entire potential range suggests that its beneficial effect is not limited to physical binding but is rooted in its chemical functionality (Figure 2c). The cationic quaternary ammonium groups of Sustainion can attract anionic species such as NO3− and NO2− via electrostatic interactions, enhancing their adsorption and subsequent conversion. In addition, its hydrophilic hydrocarbon backbone facilitates electrolyte infiltration and ion transport, improving overall charge transfer efficiency. By contrast, Nafion’s perfluorinated backbone and sulfonic acid groups hinder anionic diffusion and can induce partial oxidation of metallic Cu and Co under alkaline conditions, thereby compromising activity and stability. Consequently, Sustainion establishes a more favorable microenvironment that promotes tandem catalysis by coupling enhanced mass transport with intermediate stabilization.
The long-term stability of the optimized Cu60-Co40/Sus/C electrode was evaluated through continuous electrolysis for 20 successive cycles (1 h per cycle) at −0.3 V vs. RHE, with periodic electrolyte replacement (Figure 2e). The FE for NH3 consistently remained above 85% throughout the test, while the NH3 production rate exhibited only a slight decline from 2.63 to 1.76 mmol gcat.−1 h−1 after 20 h of operation. This minimal degradation demonstrates the excellent structural robustness and interfacial stability of the Cu-Co/Sus/C electrode. Overall, the electrochemical results reveal that the Cu-Co/C tandem architecture integrated with the Sustainion ionomer achieves a synergistic enhancement in activity, selectivity, and durability. These findings demonstrate that rational binder design, when coupled with tandem catalysis, offers a powerful strategy for advancing the efficiency and stability of electrochemical nitrate-to-ammonia conversion.
2.3. Mechanism Analysis
To further elucidate the origin of the superior catalytic performance, the electrochemically active surface area (ECSA) of the different electrodes was quantified through double-layer capacitance measurements (Figure S3). As shown in (Figure 3a), the double-layer capacitance (Cdl) values derived from these plots are 46.2 μF cm−2 for bind-free Cu-Co/C, 36.5 μF cm−2 for Cu-Co/Nafion/C, and 135.1 μF cm−2 for Cu-Co/Sus/C, respectively. The increase in ECSA on Cu-Co/Sus/C configuration enables more uniform exposure of active sites and ensures efficient charge exchange across the electrode–electrolyte interface, which is crucial for sustaining high reaction rates in multi-electron transfer processes. Electrochemical impedance spectroscopy (EIS) further revealed the beneficial impact of Sustainion ionomer on charge-transfer dynamics. The Nyquist plots (Figure 3b) display a substantially smaller semicircular diameter for the Cu-Co/Sus/C electrode compared with its binder-free and Nafion-modified counterparts, indicating a pronounced decrease in charge-transfer resistance (Rct). This reduction suggests that the ionomer enhances electron mobility and facilitates rapid interfacial charge transfer, consistent with its cationic nature and hydrophilic polymer backbone. The contact angle measurements were performed to assess the hydrophobicity of different Cu/Co-based electrodes, as illustrated in Figure S4. The specific results are as follows: The average water contact angle (indicating hydrophobicity) of the binder-free Cu-Co electrode is 140.7°, that of the Cu-Co/Nafion/C electrode is 133.6°, and that of the Cu-Co/Sus/C electrode is 128.8°. Comparison of the three sets of data reveals that the water contact angle (hydrophobicity index) of the Cu-Co/Sustainion/C electrode is significantly smaller than those of the other two electrodes. This result confirms that Sustainion modification can effectively reduce the hydrophobicity of the Cu-Co electrode, which facilitates an increased contact probability between the electrolyte and the active sites on the catalyst surface, and is of great significance for enhancing the catalytic performance.
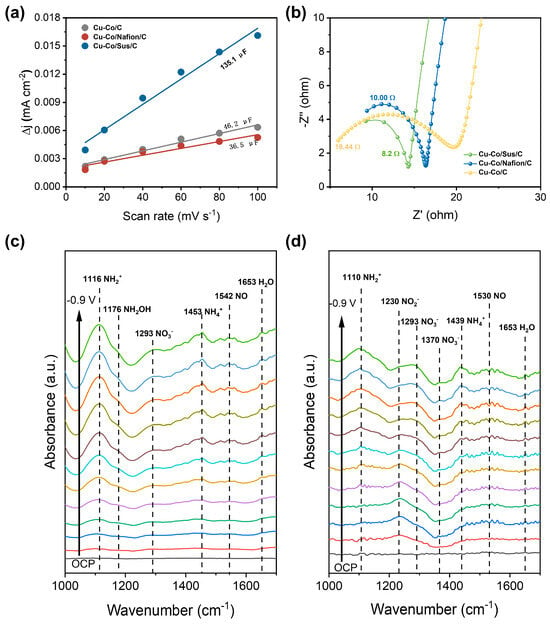
Figure 3.
The mechanism study. (a) Electrochemical Active Surface Area (ECSA) Measurement of Binder-Free and Binder-Modified Cu-Co Electrodes. (b) Electrochemical Impedance Spectroscopy (EIS) Nyquist Plots of Binder-Free and Binder-Modified Cu-Co Electrodes. (c) In Situ FTIR Spectra of the Cu-Co/C Catalyst. (d) In Situ FTIR Spectra of the Cu-Co/Sus/C Catalyst.
To gain molecular-level insight into the reaction mechanism and the specific role of Sustainion in modulating intermediate transformations, in situ spectroscopic analyses were conducted under operating conditions (Figure S5). The NO3−RR typically proceeds via a sequence of proton-coupled electron transfer steps, with NO2− serving as the key rate-determining intermediate. The general reaction pathway involves the initial two-electron reduction of NO3− to NO2−, followed by successive hydrogenation through surface-adsorbed intermediates such as NO*, NHO*, and NH2OH*, culminating in the formation of NH3. Hence, efficient adsorption and subsequent hydrogenation of NO2− are crucial for achieving high overall activity and selectivity. The in situ infrared spectra provide compelling evidence for the mechanistic differences induced by Sustainion ionomer. For the binder-free Cu-Co/C catalyst (Figure 3c), distinct vibrational bands were observed at 1116 cm−1 (NH2+, C-N stretching), 1176 cm−1 (NH2OH, N-O stretching), 1293 cm−1 (weakly adsorbed NO3−, ν3 symmetric stretching), 1453 cm−1 (NH4+, δ4 bending), 1542 cm−1 (adsorbed NO, stretching vibration), and 1653 cm−1 (H2O, bending of the solvent background). The relatively strong NO2−-related signals and weak NH4+ peaks indicate accumulation of intermediates and sluggish conversion to NH3, consistent with limited surface adsorption strength and suboptimal hydrogenation kinetics. In contrast, the Cu-Co/Sus/C catalyst (Figure 3d) exhibited a distinctly different spectral profile. Peaks at 1110 cm−1 (NH2+, C–N stretching), 1230 cm−1 (NO2−, ν3 symmetric stretching), 1293 cm−1 (residual NO3−), 1370 cm−1 (adsorbed NO3−, ν3 asymmetric stretching), 1439 cm−1 (NH4+, δ4 bending), 1530 cm−1 (adsorbed NO), and 1653 cm−1 (H2O) were observed. Notably, the attenuated intensity of the NO2− band at 1230 cm−1, coupled with the enhanced NH4+ signal at 1439 cm−1, demonstrates that the presence of Sustainion ionomer accelerates the hydrogenation of NO2−—the rate-determining step—thereby facilitating its conversion to NH3. Simultaneously, the stronger NO3− peak at 1370 cm−1 suggests electrostatic enrichment of NO3− at the catalyst surface, promoted by the cationic quaternary ammonium groups within the ionomer matrix. These findings clearly indicate that Sustainion ionomer serves not only as a passive binder but as an active participant in modulating the catalytic microenvironment. Its positively charged functional groups interact electrostatically with anionic intermediates such as NO3− and NO2−, effectively increasing their surface residence time and promoting activation through favorable charge polarization. Such interactions lower the activation barrier for hydrogenation steps and stabilize transition states along the NO3− → NO2− → NH3 pathway (Figure S6). Furthermore, the hydrophilic polymer backbone enhances local proton availability and facilitates water access, both of which are essential for sustaining rapid proton-coupled electron transfer reactions under alkaline conditions. In addition, the complementary catalytic functions of Cu and Co are also reinforced by these mechanistic observations. Cu sites primarily catalyze the reduction of NO3− to NO2− via N–O bond cleavage, whereas Co centers are highly active for subsequent hydrogenation of NO2− and related intermediates to NH3. The presence of Sustainion ensures intimate interfacial contact between Cu and Co domains, maintaining nanoscale proximity that enables efficient intermediate transfer between the two metals. This structural coherence prevents particle aggregation and spatial segregation, thereby preserving the tandem catalytic synergy necessary for efficient multi-step reduction.
3. Experimental Procedures
3.1. Chemicals
Copper powder (Cu, 99.9%, 60–100 nm) was sourced from Macklin Biochemical Technology Co., Ltd. (Shanghai, China), while cobalt powder (Co, 99.9%, 30 nm) was obtained from Adamas-beta. Potassium hydroxide (KOH, ≥95%), potassium nitrate (KNO3, AR), and isopropanol ((CH3)2CHOH, AR) were all purchased from Aladdin Ltd. (Shanghai, China). The Sustainion solution (5 wt%) was supplied by The Chemours Company (formerly DuPont, Wilmington, NC, USA). Carbon paper (P75T) was procured from Avcarb Ltd. (Lowell, MA, USA). All aqueous solutions were prepared using Milli-Q water (resistivity 18.25 MΩ·cm).
3.2. Sustainion
Sustainion is an imidazolium-based anionic ionomer, whose name is derived from its functional and chemical properties (Figure 1e). The component ‘Sustain’ denotes its ability to maintain or enhance ion transport performance, while ‘ion’ refers to its core role as an ionic carrier—this role is associated with the electrostatic attraction of quaternary ammonium or imidazolium cationic groups in its molecular structure toward species such as NO3− and NO2−.
3.3. Preparation of Cu-Co Catalyst Ink
The catalyst ink was prepared as follows: First, Cobalt powder and copper powder were accurately weighed using an analytical balance and homogenously mixed to a total mass of 100 mg, where the mass fractions of cobalt powder were precisely controlled at 0%, 10%, 20%, 40%, 60%, 80%, and 90%, respectively. Then, a mixed solvent consisting of 10 mL of deionized water and 10 mL of isopropanol was added. To systematically investigate the influence of binders on the microstructure and performance of the catalytic layer, three different ink formulations were prepared in parallel: the first was added with 250 μL of Nafion solution, the second with 250 μL of Sustainion solution, and the third without any binder as a control. Finally, each mixture was ultrasonicated for 30 min to obtain a homogeneously dispersed and stable catalyst ink.
3.4. Fabrication of the Cu-Co Tandem Catalyst
Carbon paper was cut into 4 cm × 4 cm pieces, weighed, and fixed on a heated plate. The catalyst ink was then uniformly sprayed onto the carbon paper using an airbrush, with the catalyst loading controlled at approximately 0.5 mg/cm2. After spraying, the coated carbon paper was cut into 1 cm × 2 cm strips to obtain the final Cu-Co tandem catalyst samples. This procedure was repeated for each of the three ink variants, yielding three distinct types of catalysts for comparative evaluation.
3.5. Physical Characterizations
The morphology of the catalyst coated on carbon paper was examined using a scanning electron microscope (SEM, Phenom Pharos G2, Phenom-World B.V., Eindhoven, The Netherlands) at an accelerating voltage of 15 kV. The sample morphology was studied by Transmission Electron Microscope (TEM, a Tecnai G 2 F20 S-Twin, FEI Company, Hillsboro, OR, USA) at 200 kV accelerating voltage. Samples were fixed on conductive tape without additional conductive coating. The crystal structure was analyzed by X-ray diffraction (XRD, Rigaku MiniFlex600-C, Rigaku Corporation, Tokyo, Japan) using Cu Kα radiation (λ = 1.5406 Å). Data were collected in the 2θ range from 20° to 80° at a scanning rate of 5°/min. Surface chemical analysis was performed by X-ray photoelectron spectroscopy (XPS, Thermo Scientific Nexsa, Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with a monochromatic Al Kα X-ray source (1486.6 eV). All spectra were acquired under ultra-high vacuum conditions (<5 × 10−8 Torr), with high-resolution scans recorded at a pass energy of 50 eV.
3.6. Electrochemical Measurements
The electrochemical measurements were performed using a three-electrode system connected to the DH7001B electrochemical workstation (manufactured by Donghua Analytical Instrument Co., Ltd., Taizhou, China) in a homemade H-type cell (separated by Nafion 117 membrane (Wilmington, DE, USA); with magnetic stirring of 500 rpm). The Nafion 117 was pre-processed according to the reported procedures. The Cu-Co electrodes were used as working electrodes, and a Pt plate and Hg/HgO electrode (filled with 1 M KOH solution) were used as counter and reference electrode, respectively. A 0.1 M KOH aqueous solution containing 0.1 M KNO3 was used as an electrolyte, which was bubbled with Ar to remove O2 and N2 for 20 min before the experiment. LSV was performed from 0 V to −2.0 V (vs. RHE) at a scan rate of 10 mV s−1 to validate the catalytic activity of the Cu-Co system for nitrate reduction. CA was employed to assess catalytic stability and Faradaic efficiency by applying a constant potential of −0.5 V (vs. RHE) for 20 consecutive cycles (1 h per cycle), with periodic electrolyte replenishment. The charge (Q) passed during the reaction was calculated from the i-t data for subsequent product analysis. The cyclic voltammetry curves in electrochemical double-layer capacitance (Cdl) determination were measured in a potential window where no Faradaic process occurred in an electrolyte of 0.1 M KOH at different scanning rates of 10, 20, 40, 60, 80, and 100 mV s−1. EIS measurements were conducted at a frequency range from 1000 kHz to 0.1 Hz. The obtained Nyquist plots were fitted using an appropriate equivalent circuit to extract the charge transfer resistance. All the potentials were converted to the RHE reference scale by E (V vs. RHE) = E (V vs. Hg/HgO) + 0.0591 × pH + 0.098. Note that NH3 volatilization is negligible during the 1-h electrolysis.
3.7. Product Measurements Analysis
An ultraviolet-visible (UV-vis, UV-2600i, Shimadzu Corporation, Kyoto, Japan) spectrophotometer was employed for determining ion concentrations in electrolytes, with small aliquots of the electrolyte collected as samples after 1, 2, 3, and 4 h of testing. For nitrite-nitrogen (NO2−-N) quantification, absorbance at 540 nm was recorded, and this measurement relied on a chromogenic reagent mixture. The mixture was composed of 4 g p-aminobenzenesulfonamide, 0.2 g N-(1-naphthyl)ethylenediamine dihydrochloride, 50 mL ultrapure water, and 10 mL phosphoric acid. Ammonia-nitrogen (NH3-N) was analyzed via the indophenol blue method, which involved extracting 1 mL of the test electrolyte at the selected time intervals. Specifically, 1 M sodium hydroxide (NaOH) solution—containing 5 wt% salicylic acid and 5 wt% sodium citrate—was first added to the sample aliquots. After this, 1 mL of 0.05 M sodium hypochlorite (NaClO) and 0.1 mL of a 1 wt% aqueous solution of C5FeN6Na2O were sequentially added to 1 mL of the samples. The formation of indophenol blue was detected by measuring absorbance at a wavelength of 655 nm. All calibration curves for NO2−-N, and NH3-N exhibited excellent linearity in absorbance-concentration plots. To ensure sample concentrations fell within the linear range of each corresponding calibration curve, all samples were subjected to appropriate dilution (Figure S2).
3.8. Analysis of Ammonia and Nitrate
Chronoamperometry (CA) was employed to systematically evaluate the performance of Cu-Co catalysts with different atomic ratios in the nitrate reduction reaction. A 0.5 mL aliquot of the post-electrolysis catholyte was diluted by a factor of 20 to a final volume of 10 mL, and the pH was adjusted to 2–7. The ammonium content was analyzed by cation chromatography. The Faradaic efficiency and yield for ammonia were calculated based on the charge passed and the quantity of ammonia produced. To investigate the effect of potential, the optimal Cu60Co40 catalyst was tested at five different potentials (−0.15, −0.20, −0.30, −0.40, and −0.50 V vs. RHE). Furthermore, to evaluate the influence of binders, three catalyst configurations- Cu-Co/C, Cu-Co/Nafion/C, and Cu-Co/Sus/C—were compared at a potential of −0.3 V vs. RHE.
4. Conclusions
This study demonstrates that integrating Sustainion ionomer with Cu-Co tandem catalysts enables highly efficient and durable electrochemical nitrate reduction to ammonia. The Cu60Co40/Sus/C electrode achieves a Faradaic efficiency of 91% and a production rate of 2.63 mmol gcat.−1 h−1, outperforming both Cu-Co/Nafion/C and Cu-Co/C systems. Structural analyses reveal that Sustainion forms a conformal polymer matrix that prevents nanoparticle aggregation, preserves metallic Cu0/Co0 species, and maintains structural stability during operation. Electrochemical and in situ spectroscopic studies confirm that Sustainion not only enhances active site accessibility and charge-transfer kinetics but also accelerates the key NO2− hydrogenation step via electrostatic intermediate stabilization. By bridging compositional synergy with interfacial regulation, this work provides a mechanistic foundation for the rational design of ionomer–catalyst interfaces. The insights gained here establish a generalizable strategy for constructing efficient, scalable, and stable electrocatalysts for nitrate reduction and other multi-electron nitrogen conversion reactions, advancing the broader goal of sustainable ammonia synthesis within a circular nitrogen economy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15121156/s1, Figure S1: SEM images of binder-free Cu-Co/C and Cu-Co/Sus/C after one hour of reaction; Figure S2: Quantification of NO2− (a,b) and NH3 (c,d) in a 0.1 M KOH background solution using the indophenol blue method. The UV-vis absorption spectra (left) and corresponding calibration curve (right); Figure S3: Cyclic voltammograms of Ni electrodes in Ar-saturated 0.1 M NaOH solution; Figure S4: The contact angle measurement images for the bind-free Cu-Co/C (top row), Cu-Co/Nafion/C and Cu-Co/Sus/C (bottom row) electrodes; Figure S5: The picture of electrochemical in situ infrared enhanced cell; Figure S6: Schematic diagram of the reaction mechanism; Table S1: The Co and Cu contents determined by ICP-OES; Table S2: Names and Characteristics.
Author Contributions
Conceptualization, Q.Z. and C.Z.; methodology, C.Z.; validation, Q.Z., L.Z. and C.Z.; formal analysis, Q.Z., L.Z. and C.Z.; data curation, Q.Z., Q.W. and C.Z.; writing—original draft preparation, Q.Z., L.Z., Z.W. and Q.W.; writing—review and editing, Q.Z. and C.Z.; supervision, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Dataset is available on request from the authors.
Acknowledgments
The technical and scientific staff of Université Claude Bernard Lyon 1, CNRS IRCELYON and Università degli Studi di Milano (SmartMatLab Project 2013-1776, Cariplo Foundation, and Laboratorio di Analisi of Dipartimento di Chimica) are acknowledged for the valuable assistance in physico-chemical characterizations. Nadia Santo of Unitech NOLIMITS at Università degli Studi di Milano is gratefully acknowledged for TEM analyses on unmodified HAP.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lim, J.; Fernández, C.A.; Lee, S.W.; Hatzell, M.C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 2021, 6, 3676–3685. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Lin, B.-L.; Tsunemi, K. Assessment of ammonia as an energy carrier from the perspective of carbon and nitrogen footprints. ACS Sustain. Chem. Eng. 2019, 7, 15359–15368. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Jiang, H.; Li, L. Green ammonia: Revolutionizing sustainable energy for a carbon-free future. J. Mater. Chem. A 2024, 12, 33334–33361. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Li, W.; Zhou, C.; Huo, X.; Pan, Z.; Chen, R.; An, L. Techno-economic analysis of using ammonia as an energy carrier for renewable energy conversion and storage. Int. J. Hydrog. Energy 2025, 162, 150784. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Endo, Y.; Nishibayashi, Y. Catalytic ammonia formation from dinitrogen, water, and visible light energy. Nat. Commun. 2025, 16, 4540. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wark, M. A recent review on photochemical and electrochemical nitrogen reduction to ammonia: Strategies to improve NRR selectivity and faradaic efficiency. Appl. Mater. Today 2024, 39, 102253. [Google Scholar] [CrossRef]
- Ali, T.; Muhammad, N.; Qian, Y.; Liu, S.; Wang, S.; Wang, M.; Qian, T.; Yan, C. Recent advances in material design and reactor engineering for electrocatalytic ambient nitrogen fixation. Mater. Chem. Front. 2022, 6, 843–879. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, H.; Zheng, M.; Huang, Z.; Kang, F.; Li, J.; Lv, R. Oxidation state modulation of bismuth for efficient electrocatalytic nitrogen reduction to ammonia. Adv. Funct. Mater. 2021, 31, 2102572. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, P.; Gao, H. Observation of continuum state dissociation enables the determination of N2 bond dissociation energy to spectroscopic accuracy. J. Phys. Chem. Lett. 2023, 14, 10974–10979. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, X.; Zhi, X.; Jiao, Y. New mechanistic insights into electrokinetic competition between nitrogen reduction and hydrogen evolution reactions. Adv. Energy Mater. 2024, 14, 2470118. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Ding, L.-X.; Wang, H. Competing hydrogen evolution reaction: A challenge in electrocatalytic nitrogen fixation. Mater. Chem. Front. 2021, 5, 5954–5969. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, S.; Ahmad, M.S.; Sharif, H.M.A.; Liu, Q.; Kida, T.; Shafique, A.; Rehman, M.U.; Wang, G.; Qiu, J. Investigating the role of oxygen vacancies in metal oxide for enhanced electrochemical reduction of NO3− to NH3: Mechanistic insights. Inorg. Chem. Front. 2023, 10, 6440–6488. [Google Scholar] [CrossRef]
- Shen, Z.; Yan, J.; Wang, M.; Xing, L.; Huang, B.; Zhou, H.; Li, W.; Chen, L.; Shi, J. Cu/Cu+ synergetic effect in Cu2O/Cu/CF electrocatalysts for efficient nitrate reduction to ammonia. ACS Sustain. Chem. Eng. 2023, 11, 9433–9441. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, R.; Yu, L.; Zhao, Z.; Liu, L.; Xi, J. Enhancing compatibility of two-step tandem catalytic nitrate reduction to ammonia over P-Cu/Co(OH)2. Adv. Mater. 2024, 36, 2403702. [Google Scholar] [CrossRef]
- Li, Y.C.; Ke, Z.J.; Xiao, X.H. Photoelectrochemical nitrate denitrification towards acidic ammonia synthesis on copper-decorated black silicon. Energy Environ. Sci. 2024, 17, 1892–1905. [Google Scholar] [CrossRef]
- Xue, B.J.; Tian, L.; Mao, Y.P. Enhanced nitrate reduction in hypotrophic waters with integrated photocatalysis and biodegradation. Environ. Sci. Ecotechnol. 2024, 12, 100345. [Google Scholar] [CrossRef]
- Meng, X.; Tan, X.; Ma, Y.; Obisanya, A.A.; Wang, J.; Xiao, Z.; Wang, D. Recent progress in cobalt-based electrocatalysts for efficient electrochemical nitrate reduction reaction. Adv. Funct. Mater. 2024, 35, 2400031. [Google Scholar] [CrossRef]
- Sun, L.; Yao, H.; Wang, Y.; Zheng, C.; Liu, B. Mesostructures engineering to promote selective nitrate-to-ammonia electroreduction. Adv. Energy Mater. 2023, 13, 2302154. [Google Scholar] [CrossRef]
- Yang, W.; Chang, Z.; Yu, X.; Wu, P.; Shen, R.; Wang, L.; Cui, X.; Shi, J. Cu-Co dual sites tandem synergistic effect boosting neutral low concentration nitrate electroreduction to ammonia. Adv. Sci. 2025, 12, e2416386. [Google Scholar] [CrossRef]
- Chen, Y.; Ying, T.; Qian, Y.; Bo, W. Copper-nickel-MOF/nickel foam catalysts grown in situ for efficient electrochemical nitrate reduction to ammonia. J. Hazard. Mater. 2024, 480, 136036. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Wang, M.; Xing, H.-R. Efficient ammonia electrosynthesis from pure nitrate reduction via tuning bimetallic sites in redox-active covalent organic frameworks. Angew. Chem. Int. Ed. 2025, 64, e202505580. [Google Scholar] [CrossRef]
- Wen, W.; Yan, P.; Sun, W.; Zhou, Y.; Yu, X. Metastable phase Cu with optimized local electronic state for efficient electrocatalytic production of ammonia from nitrate. Adv. Funct. Mater. 2022, 33, 2206811. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, M.; Wang, S.; Wang, Y.; Zhou, J.; Hao, F.; Liu, F.; Yan, Y.; Meng, X.; Guo, L.; et al. Atomic scale cooperativity of alloy nanostructures for efficient nitrate electroreduction to ammonia in neutral media. Adv. Funct. Mater. 2024, 35, 2400134. [Google Scholar] [CrossRef]
- Cui, H.; Chen, Y.-X. Regulating sulfonic acid group adsorption for enhanced oxygen reduction reaction activity at Pt(111)-Nafion. J. Power Sources 2025, 643, 237083. [Google Scholar] [CrossRef]
- Avvari, V.D.; Sreekanth, P.S.R.; Shanmugam, R.; Salunkhe, S.; Cep, R.; Nasr, E.A.; Kimmer, D. Exploring electrospun Nafion nanofibers: Bibliographic insights, emerging possibilities, and diverse applications. AIP Adv. 2024, 14, 075110. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Deng, N.; Wang, X.; Xiang, H.; Cheng, B.; Kang, W. Electrospun multi-scale nanofiber network: Hierarchical proton-conducting channels in Nafion composite proton exchange membranes. Cellulose 2021, 28, 6567–6585. [Google Scholar] [CrossRef]
- Yu, C.; Lei, T.; Xu, L.; Jin, C.; Yi, J.; Liu, S.; Lin, S.; Yang, Y.; Song, H.; Wang, K.; et al. Regulating the selectivity through ionomer-catalyst interactions for high-efficiency electrocatalytic CO2 reduction. J. Mater. Chem. A 2024, 12, 17181–17192. [Google Scholar] [CrossRef]
- Masel, R.I.; Liu, Z.; Sajjad, S.D. Sustainion imidazolium-functionalized polymers for carbon dioxide electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Wang, B.; Lei, F.; Li, L.; Chan, A.W.M.; et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef]
- Varcoe, J.R. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Bird, A.; Guo, F.; Ilavsky, J.; Liu, Y.; Cullen, D.A.; Kusoglu, A.; Weber, A.Z.; Ferreira, P.J.; et al. Unraveling the core of fuel cell performance: Engineering the ionomer/catalyst interface. Energy Environ. Sci. 2023, 16, 2348–2360. [Google Scholar] [CrossRef]
- Cao, H.; Pan, J.; Yan, F. Interaction regulation between ionomer binder and catalyst: Active triple-phase boundary and high performance catalyst layer for anion exchange membrane fuel cells. Adv. Sci. 2021, 8, e2101744. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Lou, Y.-Y.; Wu, Z.; Huang, X.J.; Sun, S.-G. Spatially separated Cu/Ru on ordered mesoporous carbon for superior ammonia electrosynthesis from nitrate over a wide potential window. J. Am. Chem. Soc. 2024, 146, 24966–24977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, K.; Liu, C.; Zhang, Z.; He, W.-Q.; Huang, H.; Liu, J. Electron-rich Au nanocrystals/Co3O4 interface for enhanced electrochemical nitrate reduction into ammonia. J. Colloid Interface Sci. 2023, 650, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, X.; He, W.; Zheng, Z.; Dang, X.; Zhang, Y. Electrodeposition of FeCoNiCuMn high-entropy alloy nanoparticles as efficient bifunctional electrolytic water catalyst. Surf. Interfaces 2024, 46, 104084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).