Abstract
This study explores the potential of two marine-derived bacteria, Cytobacillus kochii and Marinobacter, through in silico analysis of their catechol 2,3-dioxygenase (C23O) enzymes. Molecular docking simulations were conducted using AutoDock Vina to assess the binding interactions between C23O enzymes and ten hydrocarbon pollutants, including monocyclic and polycyclic aromatic hydrocarbons (PAHs). Binding affinities ranged from −4 to −8.7 kcal/mol for Cytobacillus kochii, with the highest affinity observed for fluoranthene (−8.7 kcal/mol), followed by pyrene (−8.5 kcal/mol) and phenanthrene (−8.2 kcal/mol). In comparison, Marinobacter’s C23O showed binding affinities between −4.1 and −8 kcal/mol, with fluoranthene (−8 kcal/mol) and phenanthrene (−7.9 kcal/mol) being top performers. Despite slightly lower affinity, Marinobacter exhibits superior environmental resilience under high salinity and temperature, making it valuable for application in fluctuating marine conditions. Structural interaction analysis revealed consistent pi-pi stacking and hydrogen bonding within the active sites, further supporting enzyme–substrate compatibility. These computational findings underscore Cytobacillus kochii ’s superior catalytic potential and Marinobacter’s ecological robustness. The integration of both strains into a microbial consortium offers a promising synergistic approach, combining enzymatic efficiency and environmental adaptability for effective hydrocarbon degradation. While these computational assessments offer valuable predictive insights, further validation through in vitro and in vivo experiments would be beneficial to determine the actual hydrocarbon degradation efficiencies.
1. Introduction
Petroleum hydrocarbon contamination of marine and terrestrial habitats has become a growing worldwide problem, owing to increased industrialization, offshore drilling operations, and frequent oil spill accidents. Polycyclic aromatic hydrocarbons (PAHs), a major component of petroleum-derived pollutants, are persistent organic compounds that pose significant threats to human health and the environment due to their high toxicity, mutagenicity, and long-term stability in ecosystems [,]. These materials withstand natural degradation processes and accumulate in terrestrial and marine food chains, posing a threat to biodiversity and affecting food security in impacted areas.
Physical skimming, chemical dispersants, and in situ burning are common methods for cleaning up, although they do not always work well enough to totally eliminate the contaminant, particularly in isolated or sensitive habitats. Furthermore, these approaches sometimes result in secondary contamination or are cost-ineffective for large-scale applications []. On the other hand, bioremediation has emerged as a viable, environmentally friendly option, employing the metabolic capabilities of microorganisms to decompose complex hydrocarbons into non-harmful byproducts []. However, the success of bioremediation techniques depends on the identification and functional optimization of important catabolic enzymes able to break down stubborn hydrocarbon structures.
C23O is an essential meta-cleavage enzyme in the aerobic breakdown route of aromatic hydrocarbons. It promotes the ring-cleavage of catechols, a key intermediary in PAH degradation, thus transforming hazardous aromatic rings into intermediates amenable to further mineralization by microbial metabolic networks []. The efficacy, stability, and ecological adaptation of C23O enzymes are critical factors in achieving good bioremediation results.
Microorganisms isolated from hydrocarbon-rich or harsh environments frequently have strong enzymes with distinct structural and functional characteristics, allowing them to survive and digest complex contaminants. Marinobacter sp. have been thoroughly investigated and are acknowledged for their enzymatic versatility and ecological robustness in hydrocarbon degradation []. Cytobacillus kochii remains mostly unexplored. Modern studies reveal little on its ability to synthesise important catabolic enzymes, such as C23O, which is essential for the cleavage of aromatic rings in hydrocarbon degradation. Due to the increasing effects of hydrocarbon contamination and the constraints of existing bioremediation agents, it is necessary to find and assess new bacterial candidates such as Cytobacillus kochii.
Recent advancements in silico methodologies, especially molecular docking, have revolutionized the exploration of enzymatic potential. These techniques provide the accurate and rapid prediction of substrate-binding conformations, active site architecture, and structural characteristics, therefore diminishing dependence on laborious wet-lab experiments [,]. This paper presents a comparative computational analysis of C23O enzymes from Cytobacillus kochii and Marinobacter sp. by molecular docking techniques. Our objective is to analyze their interaction with hydrocarbon substrates, identify critical catalytic residues, and evaluate their structural characteristics. This work elucidates the enzymatic capability of the comparatively underexplored species Cytobacillus kochii in conjunction with the well-characterized genus Marinobacter, hence providing significant insights for broadening the array of biocatalysts suitable for hydrocarbon bioremediation.
2. Results and Discussions
2.1. Enzyme Selection and Physiochemical Properties of Enzymes
Two bacterial strains were chosen from different marine locations. One strain is derived from the Red Sea in Saudi Arabia (Marinobacter sp.), while the other originates from Indonesian coastal waters (Cytobacillus kochii). Both strains demonstrated hydrocarbon-degrading ability due to the presence of C23O, a crucial enzyme responsible for the breakage of aromatic rings in hydrocarbon biodegradation. Marinobacter is a reported genus known for its significant hydrocarbon-degrading capabilities in marine habitats. On the other hand, Cytobacillus kochii remained unexplored, with few studies reported in the literature.
A sequence alignment of the C23O enzymes from Cytobacillus kochii (214 amino acids) and Marinobacter (307 amino acids) revealed significant differences. Out of a total alignment length of 332 positions, the sequences shared 33.73% similarity (112/332) and contained a high gap percentage of 43.07% (143/332). Subsequently, the physicochemical parameters of both enzymes were examined with the ProtParam program from the ExPASy website. Table 1 presents a summary of the predicted parameters.

Table 1.
Physiochemical properties of C23O.
The comparison of physicochemical properties between C23O enzymes from Marinobacter and Cytobacillus kochii reveals interesting differences that may reflect how each bacterium has adapted to its specific environment. While both enzymes fulfill the identical fundamental role of degrading aromatic hydrocarbons, their structural characteristics indicate they may operate more efficiently under varying conditions. The Marinobacter identified for its role in marine oil biodegradation [], the enzyme comprises 307 amino acids and shows a theoretical isoelectric point (pI) of 5.04. The lower isoelectric point indicates that the enzyme is probably more negatively charged at neutral pH, perhaps supporting stability and interactions in saline marine conditions []. Conversely, the Cytobacillus kochii enzyme has 214 amino acids and possesses a pI of 5.90, resulting in a little more neutral charge profile that may indicate distinct environmental adaptations or intracellular circumstances.
The major distinction resides in the aliphatic index, which pertains to the thermal stability of a protein. The enzyme from Cytobacillus kochii exhibits a markedly elevated aliphatic index (90.70) relative to Marinobacter (75.86), indicating potentially greater stability at elevated temperatures []. This is particularly pertinent to bioremediation, where enzyme stability is crucial for reliable performance under challenging environmental circumstances. Both enzymes exhibit negative GRAVY (Grand Average of Hydropathicity) values, which suggests that they are generally hydrophilic and soluble in aqueous conditions []. The slightly lower negative result for Cytobacillus kochii (−0.347 compared to −0.410 for Marinobacter) indicates a modest decrease in hydrophilicity, potentially counterbalanced by its superior thermostability.
2.2. Three-Dimensional Modelling and the Validation
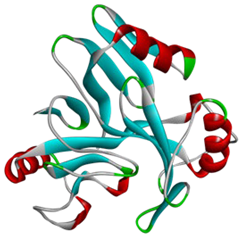
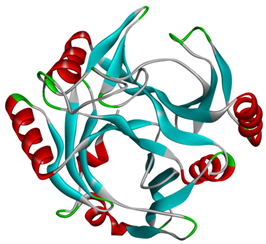
The AlphaFold2 was used to predict the three-dimensional structure of C23O enzymes from both bacterial sources. The predicted structure is presented in Table 2. The structures underwent further investigation through the Ramachandran plot, as illustrated in Figure 1.

Table 2.
Three-dimensional C23O enzymes.
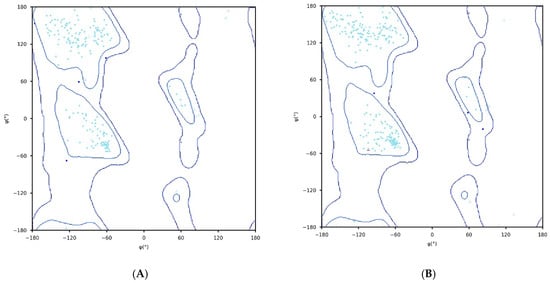
Figure 1.
Structure validation of C23O enzymes. (A) Cytobacillus kochii; (B) Marinobacter. The plots graphically represent the distribution of residues, where cyan, blue, and dots/triangles indicate torsion angles falling within favored, allowed, and disallowed regions, respectively. Dots represent residues other than glycine, while triangles correspond to glycine residues.
2.3. Virtual Screening of Hydrocarbon Pollutants Present in Environment
Ten hydrocarbon pollutants were selected for this investigation due to their recognized toxicity and environmental significance. These compounds are acknowledged for their detrimental impacts on ecosystems, alongside their adverse effects on human and animal health, attributed to their persistence, bioaccumulation, and potential to cause cancer. Table 3 displays the chosen hydrocarbons, including their molecular formulas, molecular weights, and three-dimensional structural representations.

Table 3.
Three-dimensional structures, molecular weight, and molecular formula of hydrocarbon pollutants.
The chosen hydrocarbon pollutants include various structural complexities and molecular weights, ranging from straightforward monoaromatic compounds such as benzene (78.11 g/mol) and toluene (92.14 g/mol) to more complex polycyclic aromatic hydrocarbons (PAHs) like pyrene and fluoranthene (both 202.25 g/mol), as well as long-chain alkanes including hexadecane (226.44 g/mol) and octadecane (254.49 g/mol). These substances are considered some of the most persistent and toxic petroleum pollutants, often identified in both marine and terrestrial oil spill locations [].
Aromatic hydrocarbons like naphthalene, anthracene, and phenanthrene raise significant concerns because of their carcinogenic properties, low solubility in water, and high potential for bioaccumulation []. They are difficult to break down naturally due to their structural stability, which highlights the significance of enzymatic approaches for environmental remediation. Similarly, saturated hydrocarbons such as hexadecane and octadecane, while exhibiting lower toxicity, are resistant to microbial degradation and play a role in the enduring ecological impact of oil spills [,].
2.4. The Active Sites
The active sites of C23O from Cytobacillus kochii and Marinobacter were identified using PrankWeb server, as shown in Figure 2 and Figure 3, respectively.
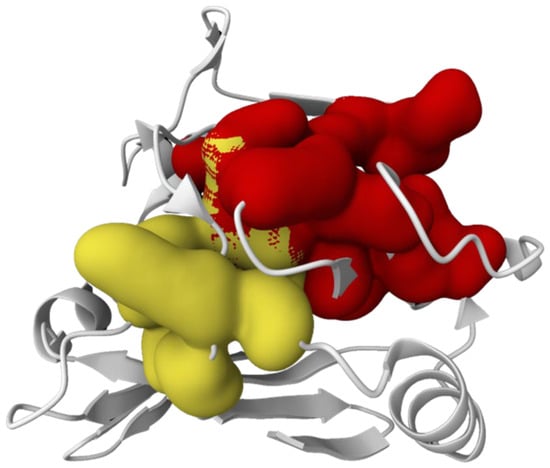
Figure 2.
The active sites (Red and Yellow) of catechol 2,3-dioxygenase from Cytobacillus kochii predicted using PrankWeb server.
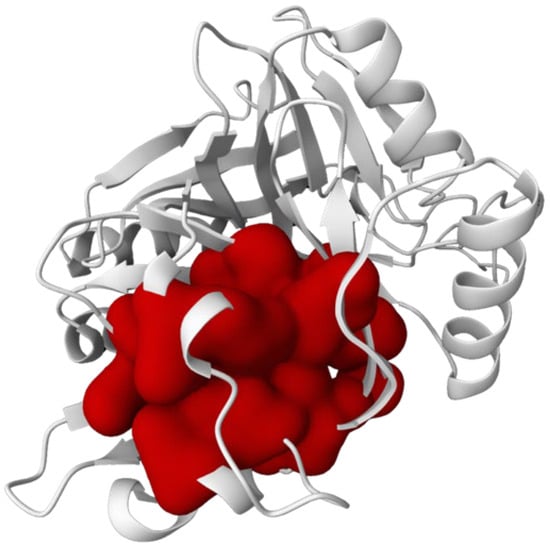
Figure 3.
The active sites Yellow of C23O from Marinobacter predicted using PrankWeb server.
Finding active sites in C23O enzymes from Cytobacillus kochii and Marinobacter offers significant insights into their catalytic capabilities in aromatic hydrocarbon degradation. The predicted active sites were visualized and localized using the PrankWeb server, which employs the P2Rank algorithm to anticipate ligand-binding pockets based on surface geometry and evolutionary conservation (Figure 2 and Figure 3). The residues constitute the catalytic core that accommodates catechol intermediates and facilitates the meta-cleavage of aromatic rings, a crucial process in the aerobic degradation of polycyclic aromatic hydrocarbons (PAHs) [].
Based on Figure 2 and Figure 3, both enzymes exhibited comparable pocket localization; the distribution and density of predicted binding residues appeared slightly distinct. This variation may indicate variances in tertiary structure and amino acid content, potentially influencing substrate orientation and enzymatic effectiveness. For example, Marinobacter C23O exhibited a more pronounced and accessible binding cavity, potentially correlating with its established catalytic effectiveness in hydrocarbon breakdown in marine environments []. Meanwhile, the anticipated active site of Cytobacillus kochii was marginally more compact, potentially suggesting a restricted substrate selectivity or differing catalytic kinetics. The structural details are significant as the shape, depth, and chemical environment of the binding pocket directly affect substrate affinity and reaction kinetics []. The existence of hydrophobic residues, hydrogen bond donors, and acceptors in the active sites indicates that both enzymes are adept at interacting with aromatic substrates.
2.5. Comparative Structural and Interaction Studies
The comparative studies began with a structural analysis to establish model reliability and provide a basis for functional comparison. First, the AlphaFold models were validated against known PDB structures, confirming their high fidelity. The Cytobacillus kochii model aligned to its closest homolog, Toxoflavin Lyase (PDB ID: 3PKV), with a low C-alpha RMSD of 0.880 Å, and the Marinobacter model showed exceptional fidelity, aligning to its homolog, catechol 2,3 dioxygenase from Pseudomonas putida (PDB ID: 1MPY) with an RMSD of 0.658 Å. A subsequent comparison between these validated models confirmed they are structurally non-homologous, yielding a high C-alpha RMSD of 16.84 Å. These structural comparisons are visualized in Figure 4. This finding of significant structural divergence served as the foundation for the subsequent molecular docking analysis to compare their binding capabilities.
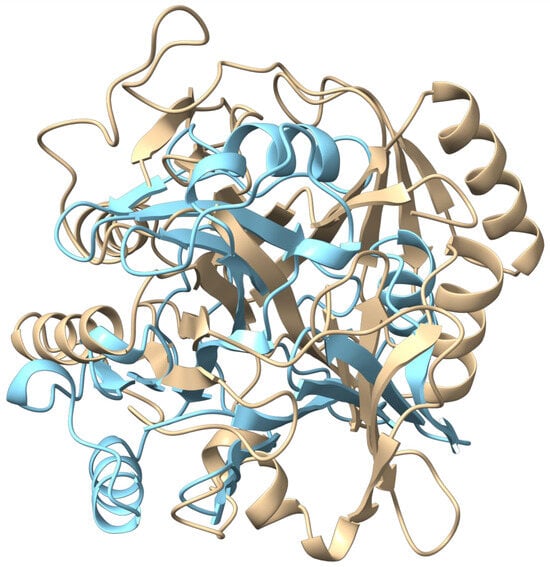
Figure 4.
Structural superposition of the Cytobacillus kochii (light blue) and Marinobacter (light beige) C23O enzymes.
The binding affinities and interaction profiles of ten hydrocarbon pollutants with C23O enzymes from Cytobacillus kochii and Marinobacter were evaluated through molecular docking simulations using AutoDock Vina 1.1.2, followed by analysis with BIOVIA Discovery Studio Visualizer 25.1, as detailed in Table 4. The molecular docking results highlighted that among the tested compounds, aromatic and polycyclic aromatic hydrocarbons (PAHs), particularly anthracene, phenanthrene, pyrene, and fluoranthene, exhibited the highest binding affinities for both enzymes, with Cytobacillus kochii consistently exceeding Marinobacter in nearly all cases.

Table 4.
Comparison results of the C23O enzyme ability from both bacteria against hydrocarbon pollutants.
The docking scores ranged from −4.0 to −8.7 kcal/mol for Cytobacillus kochii, and from −4.1 to −8.0 kcal/mol for Marinobacter. Among these, fluoranthene exhibited the highest binding affinity to Cytobacillus kochii (−8.7 kcal/mol), while the same ligand showed a slightly lower affinity to Marinobacter (−8.0 kcal/mol). Similarly, pyrene and phenanthrene displayed high binding affinities in Cytobacillus kochii (−8.5 and −8.2 kcal/mol, respectively), compared to Marinobacter (−7.9 and −7.2 kcal/mol). These results align with studies showing that aromatic compounds form stable complexes with dioxygenases through pi–pi interactions and are more readily attacked by enzymes with aromatic-rich binding sites [,].
Interaction mapping revealed that the dominant interaction modes across both enzymes were pi–pi stacking, pi-alkyl, and van der Waals interactions, which are characteristic of hydrophobic ligands. In Cytobacillus kochii, ligands frequently interacted with PHE 92, PHE 172, TRP 191, ILE 192, and TYR 10. These residues formed a hydrophobic environment ideal for pi–pi and pi-alkyl interactions, consistent with prior findings that aromatic-rich residues enhance PAH docking []. In contrast, Marinobacter’s interactions relied more on HIS 214, ARG 141, TRP 139, and GLU 220, which contribute more polar or electrostatic contacts. While these residues may assist in catalysis through metal ion coordination (e.g., Fe2+), they offer less hydrophobic surface for stable PAH binding. These differences support previous insights that enzyme-substrate recognition is strongly influenced by pocket hydrophobicity and shape complementarity [].
This clear structural divergence is further illuminated by the docking results for the non-substrate alkanes. The seemingly weak binding affinities for these compounds are an expected result as C23O is a specialized enzyme in the meta-cleavage pathway that acts on aromatic intermediates (catechols), not directly on aliphatic alkanes. The resulting weak scores for hexadecane and octadecane in both Cytobacillus kochii (−4.8 and −4.9 kcal/mol, respectively) and Marinobacter (−4.3 and −4.2 kcal/mol) confirm this specificity. This pattern is highly consistent with previous literature showing low dioxygenase affinity for non-aromatic hydrocarbons [].
However, when assessing the target aromatic substrates, a clear functional divergence was revealed. Despite Marinobacter’s well-characterized hydrocarbon metabolism, Cytobacillus kochii outperformed it in ligand affinity. This suggests that Cytobacillus kochii’s enzyme may have evolved higher structural specificity for PAHs. A similar trend was observed by Meckenstock et al. (2015), who emphasized the role of environmental pressures in shaping substrate specificity in dioxygenases isolated from distinct niches [].
3. Materials and Methods
3.1. Materials
The base strains used in this study were Marinobacter (NCBI accession no. MT820493), isolated from Jeddah coast, Saudi Arabia [], and Cytobacillus kochii (NCBI accession no. PQ395181), isolated from Tanjung Emas Port, Semarang, Java, Indonesia []. The protein sequences of C23O enzymes from both strains were retrieved from the UniProt https://www.uniprot.org (accessed on 6 May 2025) database.
The three-dimensional structure of hydrocarbon pollutants was downloaded from Protein Data Bank (https://www.rcsb.org) (accessed on 6 May 2025) and pubchem (https://pubchem.ncbi.nlm.nih.gov) (accessed on 6 May 2025) database. The software and tools used for preparation until molecular docking analysis included AlphaFold2 (https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb) (accessed on 6 May 2025), Ramplot (https://www.ramplot.in) (accessed on 6 May 2025), ExPASy ProtParam (https://web.expasy.org/protparam/) (accessed on 6 May 2025), PrankWeb (https://prankweb.cz/) (accessed on 6 May 2025), VectorBuilder (https://en.vectorbuilder.com/tool/sequence-alignment.html) (accessed on 6 November 2025), Autodock Tools 1.5.7, PyMol 3.1.4.1, BIOVIA Discovery Studio Visualizer 25.1, Open Babel 3.1.1, ChimeraX 1.10.1, and Autodock Vina 1.1.2.
3.2. Methods
3.2.1. Structure Modelling and Physiochemical Properties
To ensure the reliability of the selected C23O enzymes for downstream in silico degradation analyses, both structural and physicochemical evaluations were performed. The three-dimensional structures of the enzymes were predicted using AlphaFold2 (https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb) (accessed on 6 May 2025), a state-of-the-art deep learning algorithm known for its exceptional accuracy in protein structure prediction []. Structural validity was assessed using per-residue confidence scores, specifically the predicted Local Distance Difference Test (pLDDT) values, to evaluate the confidence of each region within the protein model. In addition, physicochemical characteristics such as molecular weight, theoretical isoelectric point (pi), aliphatic index, and the grand average of hydropathicity (GRAVY) were calculated using the ExPASy ProtParam tool []. Additionally, the protein sequences of the Cytobacillus kochii and Marinobacter enzymes were aligned online using the VectorBuilder platform (https://en.vectorbuilder.com/tool/sequence-alignment.html) (accessed on 6 November 2025) to assess their similarity.
3.2.2. Structure Validation
The predicted three-dimensional structures were validated to ensure their structural accuracy and reliability. Stereochemical quality was evaluated using Ramachandran plot analysis via the Ramplot server (https://www.ramplot.in) (accessed on 6 May 2025), which assesses the distribution of backbone dihedral angles phi (ϕ) and psi (ψ) to identify residues in favored, allowed, and disallowed regions. This analysis provides a crucial check for structural plausibility, as a high proportion of residues in favored regions indicates a well-modeled protein structure [].
3.2.3. Virtual Screening of Hydrocarbon Pollutants Present in Environment
Ten hydrocarbons commonly detected in oil-contaminated environments were selected, including alkanes, monoaromatic hydrocarbons, and polycyclic aromatic hydrocarbons (PAHs). These substances are recognized for their environmental persistence, bioaccumulative potential, and ecological toxicity, especially in marine and terrestrial ecosystems [,].
3.2.4. Ligand Preparation
The three-dimensional structures of selected hydrocarbon pollutants were retrieved in Structure Data File (SDF) format from publicly available databases, including the RCSB Protein Data Bank (RCSB PDB) (https://www.rcsb.org) (accessed on 6 May 2025) and PubChem (https://pubchem.ncbi.nlm.nih.gov) (accessed on 6 May 2025). These compounds were then geometrically optimized (energy-minimized) using the AutoDock Tools (ADT) suite to ensure proper conformation for docking analysis. Following minimization, the ligands were converted into the AutoDock-compatible pdbqt format.
3.2.5. Comparative Structural and Interaction Studies
To compare the two enzymes, both structural and interaction analyses were performed. The 3D structures of the Cytobacillus kochii and Marinobacter models were structurally aligned using the MatchMaker tool in UCSF ChimeraX 1.10.1 to quantify their structural divergence. Following this, the results from molecular docking were analyzed. The top-ranked docked complex for each ligand, selected based on the lowest binding energy score, was subjected to a detailed interaction analysis using BIOVIA Discovery Studio Visualizer 25.1. This platform was used to generate high-resolution 3D visualizations and identify key non-covalent interactions, including hydrogen bonding patterns, hydrophobic contacts, and pi-pi stacking.
4. Conclusions
This study provides insights into the bioremediation potential of C23O enzymes derived from Cytobacillus kochii and Marinobacter through molecular docking analyses. Ten hydrocarbon pollutants, including several polycyclic aromatic hydrocarbons (PAHs), were docked to both enzymes to assess binding affinity and substrate specificity. The results revealed that Cytobacillus kochii C23O consistently demonstrated higher binding affinity toward complex aromatic hydrocarbons such as anthracene, fluoranthene, and phenanthrene, with binding energies ranging from −8 to −8.7 kcal/mol, suggesting a more efficient enzymatic degradation potential than Marinobacter. Structural analysis further indicated that Cytobacillus kochii possesses a well-defined active site architecture conducive to stable substrate interaction, making it a promising candidate for hydrocarbon breakdown in the marine environment. The potential synergistic action of both bacterial enzymes could significantly enhance the scope and efficiency of hydrocarbon degradation. Cytobacillus kochii may target complex aromatic structures with high specificity, and Marinobacter may support broader degradation through its stability and auxiliary metabolic capabilities. Their combined application as a microbial consortium could create a more resilient and adaptive bioremediation system capable of tackling diverse hydrocarbon pollutants in contaminated marine environments. A valuable next step would be to explore co-cultivation strategies and metabolic pathway integration to optimize this synergy. These findings would subsequently require validation through dedicated wet-lab (i.e., in vitro and in vivo) experiments. This work not only deepens the understanding of marine-derived dioxygenases in pollutant degradation but also lays a foundation for developing next-generation microbial consortia for sustainable and efficient oil spill mitigation.
Author Contributions
M.B.A.: writing—original draft, software, data curation, investigation. M.O.: writing—review and editing, validation, supervision, investigation. M.T.J.: writing—review and editing, supervision, project administration, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Dean of Scientific Research (DSR), King Abdulaziz University, under the grant no. IPP:1201-188-2025.
Data Availability Statement
All data are present within the manuscript.
Acknowledgments
This research article was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under the Grant no. (IPP:1201-188-2025). The authors, therefore, thank DSR for providing technical and financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.; Semor, N.; Babele, P.K.; Sagar, S.; Bhatia, R.K.; Walia, A. Recent advancements in hydrocarbon bioremediation and future challenges: A review. Biotech 2022, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.M.; Gestler, R.; Dworatzek, S.M. Bioremediation of Petroleum Hydrocarbons in the Subsurface. In Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R., Eds.; Springer: Cham, Switzerland, 2024; pp. 479–502. [Google Scholar]
- Harayama, S.; Kok, M.; Neidle, E.L. Functional and evolutionary relationships among diverse oxygenases. Annu. Rev. Microbiol. 1992, 46, 565–601. [Google Scholar] [CrossRef] [PubMed]
- Duran, R. Marinobacter. In Handbook of Hydrocarbon and Lipid Microbiology; Eckey, C., Fabiani, S., Eds.; SPI-Publishing: Pondicherry, India, 2010; pp. 1725–1735. [Google Scholar]
- Chen, G.; Seukep, A.J.; Guo, M. Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs. Mar. Drugs 2020, 18, 545. [Google Scholar] [CrossRef]
- Temelkovski, D.; Kiss, T.; Terstyanszky, G.; Greenwell, P. Building Science Gateways for Analysing Molecular Docking Results Using a Generic Framework and Methodology. J. Grid. Comput. 2020, 18, 529–546. [Google Scholar] [CrossRef]
- Raddadi, N.; Giacomucci, L.; Totaro, G.; Fava, F. Marinobacter sp. from marine sediments produce highly stable surface-active agents for combatting marine oil spills. Microb. Cell Fact. 2017, 16, 186. [Google Scholar] [CrossRef]
- Petra, P.H.; Bradshaw, R.A.; Walsh, K.A.; Neurath, H. Identification of the Amino Acid Replacements Characterizing the Allotypic Forms of Bovine Carboxypeptidase A. Biochemistry 1969, 8, 2762–2768. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, H.; Gao, C.; Zang, J.; Yang, D. The Distinct Properties of the Consecutive Disordered Regions Inside or Outside Protein Domains and Their Functional Significance. Int. J. Mol. Sci. 2021, 22, 10677. [Google Scholar] [CrossRef]
- Payá, G.; Bautista, V.; Camacho, M.; Esclapez, J.; Bonete, M.J. Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain. Microorganisms 2023, 11, 1196. [Google Scholar] [CrossRef]
- ATASDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1995; pp. 11–64. [Google Scholar]
- Chakrabarti, C.; Khimani, M.; Patel, V.; Parekh, P.; Pillai, S.; Mata, J.; Vekariya, R.L.; Bhadja, P.; Muddassir, M. Solubilization of polycyclic aromatic hydrocarbons (PAHs) in PEO-PPO-PEO type linear and star block copolymers. J. Mol. Liq. 2021, 325, 115177. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Zhang, J.; Li, S.; Liu, J.; Ghalib, H.B.; Zhang, X.; Ma, F. Biodegradation of n-hexadecane by Aspergillus sp. RFC-1 and its mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.; Tachibana, S. Crude oil and n-octadecane degradation under saline conditions by Fusarium sp., F092. J. Environ. Sci. Technol. 2013, 6, 29–40. [Google Scholar] [CrossRef]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554481/ (accessed on 6 May 2025).
- Zhang, Z.; Cai, Y.; Zheng, N.; Deng, Y.; Gao, L.; Wang, Q.; Xia, X. Diverse models of cavity engineering in enzyme modification: Creation, filling, and reshaping. Biotechnol. Adv. 2024, 72, 108346. [Google Scholar] [CrossRef]
- Weiß, R.G.; Chudoba, R.; Setny, P.; Dzubiella, J. Affinity, Kinetics, and Pathways of Anisotropic Ligands Binding to Hydrophobic Model Pockets. J. Chem. Phys. 2018, 149, 094902. [Google Scholar] [CrossRef]
- Vaillancourt, F.H.; Bolin, J.T.; Eltis, L.D. The ins and outs of ring-cleaving dioxygenases. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 241–267. [Google Scholar] [CrossRef]
- Saragi, R.T.; Calabrese, C.; Juanes, M.; Pinacho, R.; Rubio, J.E.; Pérez, C.; Lesarri, A. π-Stacking Isomerism in Polycyclic Aromatic Hydrocarbons: The 2-Naphthalenethiol Dimer. J. Phys. Chem. Lett. 2023, 14, 207–213. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding—A review. J. Adhes. Sci. Technol. 2017, 31, 910–931. [Google Scholar] [CrossRef]
- Stank, A.; Kokh, D.B.; Fuller, J.C.; Wade, R.C. Protein Binding Pocket Dynamics. Acc. Chem. Res. 2016, 49, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Semana, P.; Powlowski, J. Four Aromatic Intradiol Ring Cleavage Dioxygenases from Aspergillus niger. Appl. Environ. Microbiol. 2019, 85, e01786-19. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Elsner, M.; Griebler, C.; Lueders, T.; Stumpp, C.; Aamand, J.; Agathos, S.N.; Albrechtsen, H.J.; Bastiaens, L.; Bjerg, P.L.; et al. Biodegradation: Updating the Concepts of Control for Microbial Cleanup in Contaminated Aquifers. Environ. Sci. Technol. 2015, 49, 7073–7081. [Google Scholar] [CrossRef]
- Jamal, M.T.; Pugazhendi, A. Isolation and characterization of halophilic bacterial consortium from seagrass, Jeddah coast, for the degradation of petroleum hydrocarbons and treatment of hydrocarbons—Contaminated boat fuel station wastewater. Clean Technol. Environ. Policy 2021, 23, 77–88. [Google Scholar] [CrossRef]
- Alim, M.B.; Oves, M.; Jamal, M.T.; Kunarso, K. Comparative Hydrocarbon Biodegradation by Bacillus haikouensis and Cytobacillus kochii: Pure Culture versus Consortium Performance. J. Pure Appl. Microbiol. 2025, 19, 2069–2086. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., III; de Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: φ, ψ and Cβ deviation. Proteins Struct. Funct. Genet. 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).