Abstract
In this study, hydroxyl-modified graphitic carbon nitride materials were synthesized through thermal polymerization combined with the hydrothermal method, and the samples exhibiting the best catalytic activity for the ECH and CO2 cycloaddition reaction were selected. The structural evolution of the material before and after the catalytic reaction was analyzed using various characterization methods, such as Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS); the effects of reaction temperature, reaction time, CO2 pressure, and catalyst dosage on the catalytic performance of the materials were investigated systematically, and a possible reaction mechanism was proposed based on the above analysis.
1. Introduction
In the current wave of green and sustainable chemistry, developing efficient, stable, and recyclable multiphase catalysts to achieve high-value-added conversion of carbon dioxide (CO2) has received substantial research attention and constitutes a great challenge. Among different methods, the cycloaddition reaction of epoxy compounds with CO2 to form cyclic carbonates is highly favored due to its high atomic economy and wide range of product applications, such as polar non-proton solvents, polymer monomers, and lithium battery electrolytes.
The heterogeneous catalyst CN-HEIMBr, constructed with the bonding method, can effectively promote the conversion of various epoxy compounds to corresponding cyclic carbonates and exhibits good reusability. Its catalytic activity did not significantly decrease after multiple cycles. However, this type of catalyst uses complex organic synthesis methods to firmly anchor ionic liquids containing bromide anions (Br−), such as 1,3-diethylimidazolium bromide, on the carrier in the form of covalent bonds, forming stable catalytic active centers. The complexity of the synthesis process is reflected in the pretreatment of the carrier (such as acidification, amination to introduce reactive functional groups), followed by condensation or grafting reactions with ionic liquid precursors under strictly controlled anhydrous and anaerobic conditions, specific temperatures, and long periods of time (possibly up to tens of hours). These steps increase not only production time and economic costs but also the complexity of the process and the requirements for equipment, which, to some extent, limit the possibility of its large-scale industrial application.
Graphitic carbon nitride (g-C3N4), as a functional material with excellent thermal stability and low-cost advantages, has attracted much attention in the field of catalysis due to its simple preparation process and wide range of raw material sources. g-C3N4 is a graphene-like layered structure formed by the sp2 hybridization of carbon and nitrogen atoms. Its unique charm lies in the following: (1) Unparalleled preparation simplicity: it can usually be directly prepared by one-step high-temperature thermal shrinkage (usually at 500–600 °C) of nitrogen-rich precursors (such as urea, melamine, melamine, etc.). This process does not require tedious purification or complex post-processing, and the raw materials are cheap and easy to obtain, truly achieving “bottom-up” block synthesis. (2) Inherent nitrogen-rich structure: Its skeleton contains a large amount of tertiary nitrogen and edge amino groups (-NH2, -NH-), which are not only potential catalytic active centers, but also provide natural anchoring points for further chemical modifications (such as introducing hydroxyl groups). (3) Excellent physical and chemical stability: g-C3N4 can maintain structural stability at high temperatures (up to 600 °C) and in acidic and alkaline environments, laying a solid foundation for its long-term cyclic use as a multiphase catalyst. (4) Adjustable electronic and surface properties: Using molecular tailoring (such as thermal oxidation, acid treatment), element doping, or composite with other materials, the band structure, specific surface area, and surface functional groups can be effectively controlled, thereby “tailoring” materials with specific catalytic properties [,].
In summary, although CN-HEIMBr catalysts have excellent performance, their complex synthesis process constitutes a bottleneck for their practical application. g-C3N4 provides an ideal platform for constructing the next generation of heterogeneous catalysts for cycloaddition reactions with its unique advantages. By introducing hydroxyl functional groups to g-C3N4, a synergistic catalytic system similar to CN-HEIMBr but with simpler preparation can be constructed: the nitrogen-rich skeleton and stable structure of g-C3N4 provides a carrier basis and possible auxiliary active sites, while the introduced hydroxyl groups act as efficient epoxy activators, synergistically driving the entire catalytic cycle efficiently and stably with externally or internally fixed nucleophilic anions [,].
2. Results and Discussion
2.1. FT-IR Characterization
Figure 1 shows the infrared spectra of CN-480, CN-OH-T, and the used sample CN-OHepo (CN-OH-180). Analysis showed that, except for CN-OH-200, all other samples exhibited a characteristic peak at 808 cm−1, which was attributed to the stretching vibration of the triazine ring [,]. The absorption peaks observed in a wide wavelength range of 1200 to 1650 cm−1 are related to the vibrational modes of C-N heterocyclic compounds []. In addition, the sample exhibited a broad peak within the range of 3000 to 3650 cm−1, mainly due to the vibration of the terminal amino groups (-NH2 or =NH) and adsorbed water molecule hydroxyl groups (-OH) in the graphitic carbon nitride structure [,,,]. It is worth noting that when the hydrothermal treatment temperature reached 200 °C (CN-OH-200 sample), its spectrum underwent significant changes: the characteristic peak of the triazine ring at 808 cm−1 completely disappeared, accompanied by the appearance of a new absorption peak. This phenomenon clearly indicates that excessively high hydrothermal treatment temperatures damage the main skeleton structure of graphitic carbon nitride, resulting in the loss of characteristic vibration signals of triazine rings.

Figure 1.
FT-IR spectra of CN-480, CN-OH-T, and CN-OHepo.
2.2. XRD Characterization
Figure 2 shows the X-ray diffraction patterns of CN-480, CN-OH-180, and CN-OHepo materials. All samples exhibit two distinct characteristic diffraction peaks at positions 13° and 27°. The peak near 13° belongs to the 100 crystal plane and originates from the periodic arrangement of triazine ring units in the material. The peak near 27° corresponds to the 002 crystal plane, reflecting the layered stacking structure of the triazine ring plane. These two peaks are direct evidence of the successful synthesis of graphitic carbon nitride. The CN-OH-200 sample subjected to hydrothermal treatment at 200 °C showed significant changes: in addition to the basic characteristic peaks, two new diffraction peaks were observed at 22° and 33.4°, indicating that the high temperature of 200 °C induced changes in the internal stacking mode and crystal structure reorganization of the original CN-480. It is worth noting that compared with the sample treated at 180 °C, a small new peak appeared at a lower angle of 6.1° in the 200 °C sample, which is likely related to the successful introduction of -OH groups during the hydrothermal process. The hydrothermal treatment temperature is not necessarily better when higher. The characteristic small peak at 6.1° disappeared in the CN-OHepo sample prepared at higher temperatures. This phenomenon clearly indicates that excessively high temperatures disrupt the specific ordered structure formed by the introduction of hydroxyl groups at 200 °C.

Figure 2.
XRD patterns of CN-480, CN-OH-T and CN-OHepo.
2.3. SEM Characterization
CN, CN-OH-180, and CN-OHepo materials were characterized with scanning electron microscopy (SEM). Figure 3 shows that both CN and CN-OH-180 samples exhibited significant hollow tubular stacking structures. In addition to the hollow tube structure, CN-OH-180 also exhibited a large amount of block-like stacking, which may be due to damage to the original morphology of graphite-phase nitride carbon caused by high temperature and high pressure during the hydrothermal synthesis process, resulting in partial rupture of the hollow tube wall and newly compact stacking. After the reaction, CN-OHepo exhibited a more complete morphological transformation. The degree of rupture of the air duct structure intensified, and the fragmented tubular segments piled up more densely. This significant morphological change may be closely related to the high-temperature and high-pressure environment and prolonged vigorous stirring process experienced by the fresh catalyst CN-OH-180 in the reaction vessel. The intense physical forces accelerated the fragmentation and reorganization of the original structure.

Figure 3.
SEM images of CN-480 (A,B), CN-OH-180 (C,D), and CN-OHepo (E,F).
It can be seen in Figure 4 that CN-OHepo exhibited a stub morphology both before and after the reaction. The degree of rupture in the air duct structure intensified, and the fragmented tubular segments piled up more densely. This significant morphological change may be closely related to the high-temperature and high-pressure environment and prolonged vigorous stirring process experienced by the fresh catalyst CN-OH-180 in the reaction vessel.
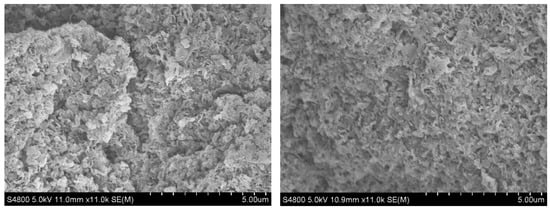
Figure 4.
SEM images of CN-OHepo before (left) and after (right) the reaction.
2.4. TG Characterization
Figure 5 shows the thermogravimetric analysis curves of the CN-OH-180 and CN-OHepo samples. CN-OH-180 decomposition proceeded in three consecutive stages: The mass loss in the first stage (0–134 °C) was due to the removal of adsorbed water (H2O) and carbon dioxide (CO2). The material quality remained highly stable in the second stage (134–276 °C). In the third stage (>276 °C), severe weight loss occurred, corresponding to the decomposition of the main structure of graphite-phase nitride carbon. Comprehensive analysis showed that CN-OH-180 had excellent thermal stability compared to CN-OHepo, and it did not undergo structural damage within the conventional catalytic reaction temperature range. In contrast, the first-stage weight loss of CN-OHepo was mainly caused by the removal of adsorbed water and surface-attached organic matter. The quality change in the second stage can be attributed to the shedding process of epoxy ionic liquid. The final high-temperature (>276 °C) decomposition stage is consistent with CN-OH-180, which also originates from the disintegration of the graphite-phase nitride carbon skeleton. The difference between the two materials is mainly reflected in the weight loss mechanism during the medium- and low-temperature stages.

Figure 5.
TG curves of CN-OH-180 and CN-OHepo.
2.5. XPS Characterization
Figure 6 shows the X-ray photoelectron spectroscopy (XPS) analysis results of CN-480, CN-OH-180, and CN-OHepo materials. In the C1s spectrum, the peaks of CN-480 at 284.7 eV and 288.1 eV are attributed to the C-C/C=C structure and the N-C=N bond in the triazine ring, respectively. After hydrothermal treatment, a new characteristic peak was observed at 288.7 eV in CN-OH-180, corresponding to the appearance of C-O bonds and confirming the successful introduction of hydroxyl groups. In contrast, the peak position of the C1s spectrum of CN-OHepo underwent significant changes, shifting to 286.1 eV and 286.7 eV. Analysis of the N1s spectrum provides further information. Both CN-480 and CN-OH-180 exhibited characteristic peaks at 398.5 eV (C-N=C structure) and 399.2 eV (N-(C)3 structure), while a peak attributed to the terminal amino group (-NH2) appeared near 400.5 eV. The N1s spectrum of CN-OHepo shows a significant difference, with a new peak appearing at 401 eV. These changes reveal key chemical reactions. The new peak at 401 eV and the bond energy shift in the C1s spectrum of CN-OHepo indicate the conversion of the terminal amino group to the quaternary ammonium salt group. Based on the overall variation characteristics of C1s and N1s spectra, it can be confirmed that during the reaction process between CN-OH-180 and epichlorohydrin (ECH), ECH was successfully grafted onto the material surface as an epoxy ionic liquid, ultimately resulting in the formation of a quaternary ammonium salt ionic liquid functionalized material CN-OHepo with chloride ions (Cl−) as anions.

Figure 6.
C1s (A) and N1s (B) XPS spectra of CN-480, CN-OH-180 and CN-OHepo.
3. Experimental Methods
3.1. Preparation of Graphite-Phase Carbon Nitride
Graphitic carbon nitride materials were prepared according to standard thermal polymerization procedures []. First, 25 g of urea was placed in a ceramic crucible, sealed with tin foil, and placed in a muffle furnace. The temperature was raised to 480 °C at a rate of 5 °C/min and maintained at a constant temperature for 4 h before naturally cooling to room temperature. The solid product obtained was labeled CN-480.
3.2. Preparation of Hydroxyl-Modified Graphite-Phase Carbon Nitride
First, 1.0 g of CN-480 and 80 mL of deionized water was added to a 100 mL PTFE liner, dispersed using ultrasound for 15 min, sealed in a stainless steel hydrothermal reactor, and then placed in a preset temperature oven for hydrothermal treatment for 4 h. After the hydrothermal treatment was complete, it was removed and left to cool naturally to room temperature. Then, the obtained solid was centrifuged, washed three times with deionized water, and finally dried in a 60 °C oven. The obtained product was labeled CN-OH-T (where “T” represents the hydrothermal treatment temperature). The complete synthesis path is shown in Figure 7.

Figure 7.
Preparation of hydroxyl-modified graphite-phase carbon nitride.
3.3. Cycloaddition of CO2 with Epichlorohydrin
Cycloaddition of CO2 with epichlorohydrin was conducted in a 100 mL magnetically stirred high-pressure autoclave (GY-100). A specified amount of catalyst and epichlorohydrin (30 mmol) were added to the autoclave, which was then sealed. The reactor was purged three times with high-purity CO2 (99.99%) to ensure complete removal of residual air. Following this, the system was pressurized with CO2 to the desired pressure, and the reaction was carried out at the set temperature and time, with a stirring speed of 400 rpm. After the reaction, the autoclave was cooled naturally to room temperature, and the excess CO2 was slowly vented. A precise quantity of n-butanol was introduced to the mixture as an internal standard. The mixture was homogenized, filtered, and the supernatant was analyzed using gas chromatography (Thermo, Waltham, MS, USA) equipped with an FID detector. The recovered catalyst was washed with ethanol, dried in an oven, and directly reused in subsequent cycles without further purification.
4. Catalytic Performance of ECH and CO2 Cycloaddition
4.1. Catalysts
According to the Table 1, as the hydrothermal treatment temperature gradually increased, the catalytic performance initially showed an upward trend. This is mainly attributed to the higher temperature promoting the introduction of hydroxyl groups on the catalyst surface. These added hydroxyl groups can effectively activate epoxide substrates, thereby enhancing catalytic activity. In the critical temperature range from 160 °C to 180 °C, the yield of the catalytic product CPC showed an especially sharp increase. Based on the analysis of material characterization results, this significant performance leap is attributed to the successful bonding between epoxy ionic liquid and hydroxyl-modified graphitic carbon nitride, which constructed efficient catalytic active sites. However, when the hydrothermal treatment temperature further increased to 200 °C, the catalytic performance underwent a turning point and significantly decreased. Excessive temperature causes irreversible damage to the main structure of graphitic carbon nitride, resulting in compromised key properties. In this case, the key precursor epichlorohydrin (ECH) cannot be effectively grafted onto the damaged carrier material as an epoxy ionic liquid, severely weakening the catalytic effect. The trend of comprehensive performance changes and the underlying mechanism analysis show that the catalyst CN-OH-180 prepared at a hydrothermal treatment temperature of 180 °C exhibited the best comprehensive catalytic performance and was confirmed as the best catalyst for this system.

Table 1.
Catalytic performance for cycloaddition of ECH with CO2 by different catalysts.
4.2. Reaction Temperature
Figure 8 shows the effects of the reaction temperature on the yield and selectivity of CPC. When the temperature rose from 100 °C to 130 °C, the CPC yield significantly increased, jumping from 26% to 97%. As the temperature continued to increase to 140 °C, the yield growth leveled off without significant changes. Based on the temperature response characteristics, 130 °C was determined as the optimal temperature condition for this reaction.

Figure 8.
Effects of reaction temperature on cycloaddition performance of ECH and CO2.
4.3. Reaction Time
In Figure 9, it can be seen that the CPC yield continued to increase as the reaction time was extended from 0.5 to 1.5 h. When the reaction reached 1.5 h, the peak yield reached 97%. When the reaction time was extended beyond this point, the yield stopped improving, and the magnitude of change weakened significantly. Taking into account both conversion efficiency and economy, the optimal reaction time was determined to be 1.5 h. This period ensured high conversion rates while avoiding ineffective energy consumption.

Figure 9.
Effects of reaction time on cycloaddition performance of ECH and CO2.
4.4. CO2 Pressure
Figure 10 shows the effect of carbon dioxide (CO2) pressure on the selectivity and yield of the product CPC. When the CO2 pressure was 0.5 MPa, the selectivity of CPC reached 92%. When the pressure rose to 1.5 MPa, the CPC yield reached its highest value of 97%. However, as the pressure continued to increase, the yield actually decreased. This may have been due to the excessive concentration of CO2 diluting the reactants, resulting in a decrease in the efficiency of CPC generation, which is consistent with the phenomenon reported in reference []. Therefore, considering both selectivity and yield, a CO2 pressure of 1.5 MPa is an ideal condition for this reaction.

Figure 10.
Effects of CO2 pressure on cycloaddition performance of ECH and CO2.
4.5. Catalyst Dosage
Figure 11 shows that as the catalyst dosage increased, the selectivity of the target product CPC decreased, while its yield first increased and then decreased. Specifically, when the catalyst/ECH mass ratio was 0.018, the CPC yield was 94%. When the ratio increased to 0.036, the yield reached a peak of 96%. The decrease in yield at lower ratios may be related to insufficient catalyst dosage and an inability to provide sufficient active sites. Therefore, a catalyst-to-ECH mass ratio of 0.036 was determined as the optimal condition.
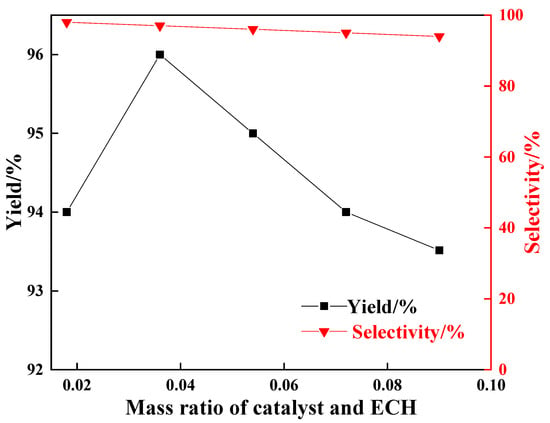
Figure 11.
Effects of catalyst and ECH mass ratio on cycloaddition performance of ECH and CO2.
5. Catalytic Performance of CN-OH-180 for ECH and CO2 Cycloaddition Recycling
The regenerated CN-OH-180 catalyst (washed and dried) was used to catalyze the cycloaddition reaction of CO2 and epichlorohydrin (ECH). As shown in Figure 12, the catalyst maintained stable catalytic performance over five consecutive cycles, with no significant decrease in the ECH conversion rate or selectivity for propylene carbonate (CPC). This proves that the active components of the catalyst were not lost during the reaction process, exhibiting excellent stability and good potential for recycling.
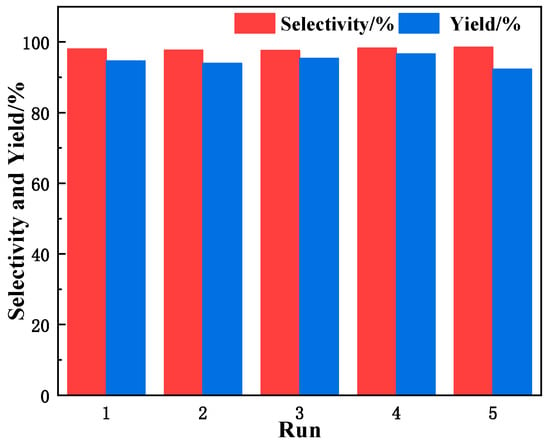
Figure 12.
Reusability of CN-OH-180 in the cycloaddition of ECH with CO2.
Reaction conditions: 30 mmol of ECH, temperature of 130 °C, reaction time of 1.5 h, 1.5 MPa of CO2 pressure, 0.1 g of catalyst.
6. Possible Reaction Mechanism of CN-OH-180-Catalyzed ECH and CO2 Cycloaddition
To investigate the mechanism of CO2 and epichlorohydrin (ECH) cycloaddition, styrene oxide (SO) was selected as the probe substrate to evaluate the reaction activity (Table 2). Research has found that when using the CN-OH-180 catalyst, its catalytic efficiency for SO is relatively low. However, when using CN-OHepo catalyst to catalyze the reaction between SO and CO2, the yield of styrene carbonate (SC) increased to 25%. Based on relevant characterization analysis, it is speculated that ECH may react with the fresh catalyst CN-OHepo to generate an active new species that can efficiently catalyze the cycloaddition of CO2 and ECH.

Table 2.
Cycloaddition of SO with CO2 under different conditions using CN-OH-180.
Based on experimental data and the literature [,], Figure 13 shows the detailed reaction pathway of CN-OH-180 catalyzing the synthesis of cyclic carbonates (CPCs) from CO2 and epoxides (ECHs). The reaction begins with the interaction between CN-OH-180 and ECH, forming a quaternary ammonium salt catalyst CN-OHepo, with chloride ions (Cl−) as counterions. Subsequently, CN-OHepo exhibits dual-functional catalytic activity: the hydroxyl groups on the surface of the material effectively activate the C-O bond in the epoxide, reducing its ring-opening energy barrier. Meanwhile, the amino group (-NH2) activates the CO2 molecule, generating the key intermediate—aminoformate ester (intermediate product 1). In the core step of the reaction, Cl−, released by CN-OHepo, acts as a strong nucleophile, selectively attacking carbon atoms with lower steric hindrance in epoxides, achieving ring opening and forming intermediate product 2. Subsequently, activated CO2 reacts with intermediate product 2 to produce intermediate chlorocarbonate (intermediate product 3). In the end, intermediate product 3 undergoes intramolecular cyclization to efficiently generate the target product cyclic carbonate (CPC), accompanied by the regeneration of catalyst CN-OHepo, which completes the entire catalytic cycle. This mechanism clearly explains the complete catalyst formation process, synergistic activation, nucleophilic ring opening, CO2 addition, and closed-loop regeneration.

Figure 13.
Possible mechanism for cycloaddition of CO2 and ECH by CN-OH-180.
It is important to note that the catalyst is specifically designed for ECH, with a unique activation step: during the first reaction with ECH, the catalyst transforms to generate its active sites (Figure 13). This initial activation is crucial, as it prepares the catalyst for effective performance in later reuse cycles. Meanwhile, a concise comparison of the performance of different catalysts reported previously with those in the current work is presented. As shown in Table 3, under similar reaction conditions, the catalyst activity reported in this work is significantly comparable to that in the existing literature. At the same time, while maintaining the same activity, the reaction conditions (reaction pressure, reaction temperature, etc.) in this work are milder than those reported previously [,,,,].

Table 3.
Comparison of the performance of different catalysts.
Furthermore, related calculations were carried out to verify the roles of hydroxyl and amino groups in CO2 activation (Figure 14). The calculation results indicate that CO2 is activated by different catalysts via van der Waals forces, with CN-480 activating the CO2 bond angle to 177.01° and CN-OH-T activating the CO2 bond angle to 176.61°.

Figure 14.
IRI analysis of the interaction between (a) CN-480, (b) CN-OH-T and CO2.
Thermal filtration experiments were conducted to demonstrate the stability of amino and hydroxyl groups in the catalyst structure. As shown in Figure 15, after a given reaction period, we filtered and separated the catalyst before continuing the reaction, and we found no significant change in reaction activity. This indicates that there was no dissolution phenomenon of the active sites, such as Cl− or quaternary ammonium groups.

Figure 15.
Hot filtration experiment.
7. Conclusions
In this study, a hydroxyl-modified graphitic carbon nitride catalyst was successfully prepared using thermal polymerization combined with the hydrothermal method. The effect of hydrothermal treatment temperature on its catalytic performance for the cycloaddition reaction of epichlorohydrin (ECH) and carbon dioxide (CO2) was systematically investigated, and CN-OH-180, the catalyst with the best performance, was selected. By optimizing the reaction temperature, reaction time, CO2 pressure, and catalyst dosage, the reaction mechanism was thoroughly explored. The findings are as follows:
(1) Material characterization indicates that CN-OH-180 undergoes significant structural changes after catalytic reaction. Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) analysis confirmed that the epoxy ionic liquid was successfully anchored on the hydroxyl-modified graphite-phase nitride carbon skeleton through chemical bonding, forming a catalytically active quaternary ammonium salt ionic liquid structure.
(2) Process optimization achieved the optimal reaction conditions: reaction temperature of 130 °C, reaction time of 1.5 h, 1.5 MPa of CO2 pressure, and a catalyst-to-ECH mass ratio of 0.036. Under these conditions, the conversion rate of ECH reached 99%, and the yield of the target product chloropropene carbonate (CPC) reached 96%. The catalyst exhibited excellent recyclability, and after simple washing, drying, and five cycles of repeated use, its catalytic activity did not exhibit a significant decrease.
(3) Based on the experimental results, a possible synergistic catalytic mechanism is proposed: In the reaction, the hydroxyl (-OH) and amino (-NH2) functional groups in the structure of melamine oligomers work together with the chloride ions (Cl−) in the introduced epoxy ionic liquid to effectively activate CO2 and ECH molecules, synergistically promoting the efficient progress of the cycloaddition reaction. This catalyst is simple to prepare, its performance is stable and efficient, and it shows good application prospects in CO2 resource utilization.
Author Contributions
Y.S.: formal analysis, investigation, writing—original draft; L.C.: investigation, writing—original draft; A.M.: writing—review and editing, data curation; H.D.: writing—review and editing; H.W.: funding acquisition, conceptualization, methodology, resources; G.G.: funding acquisition, conceptualization, methodology, resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number 51904157, 21927815, 51834007.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Jiang, P.; Wang, Z.; Wang, X.; Liu, H.; Ma, Y. Synthesis of graphite-like carbon citride in different atmospheres and its thermal stability. Mater. Rep. 2021, 35, 6048–6053. [Google Scholar]
- Zhang, J.; Wang, B.; Wang, X. Chemical synthesis and applications of graphitic carbon nitride. Acta Phys.-Chim. Sin. 2013, 29, 1865–1876. [Google Scholar]
- Sun, J.; Zhang, S.J.; Cheng, W.G.; Ren, J. Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008, 49, 3588–3591. [Google Scholar] [CrossRef]
- Denizalti, S. Imidazolium based ionic liquids bearing a hydroxyl group as highly efficient catalysts for the addition of CO2 to epoxides. RSC Adv. 2015, 5, 45454–45458. [Google Scholar] [CrossRef]
- Kharlamov, A.; Bondarenko, M.; Kharlamova, G.; Fomenko, V. Synthesis of reduced carbon nitride at the reduction by hydroquinone of water-soluble carbon nitride oxide (g-C3N4)O. J. Solid State Chem. 2016, 241, 115–120. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Fan, J.; Xiang, Q. Highly crystalline carbon nitride hollow spheres with enhanced photocatalytic performance. Chin. J. Catal. 2021, 42, 627–636. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Lu, Y.; Li, H.; Chen, X.; Wei, G.; Wu, T.; Maguire, D.-J.; Ye, G.; Chen, J. Sunlight-induced uranium extraction with triazine-based carbon nitride as both photocatalyst and adsorbent. Appl. Catal. B Environ. 2020, 282, 119523. [Google Scholar] [CrossRef]
- Su, Q.; Sun, J.; Wang, J.; Yang, Z.; Cheng, W.; Zhang, S. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Technol. 2014, 4, 1556–1562. [Google Scholar] [CrossRef]
- Yue, S.; Wang, P.; Hao, X.; Zang, S. Dual amino-functionalized ionic liquids as efficient catalysts for carbonate synthesis from carbon dioxide and epoxide under solvent and cocatalyst-free conditions. J. CO2 Ultilization 2017, 21, 238–246. [Google Scholar] [CrossRef]
- Yue, C.T.; Su, D.; Zhang, X.; Wu, W.; Xiao, L. Amino-functional imidazolium ionic liquids for CO2 activation and conversion to form cyclic carbonate. Catal. Lett. 2014, 144, 1313–1321. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S.; Yang, H.-J.; Ban, B.; Wei, Z.; Wang, L.; Lei, B. Highly efficient fixation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis-(quaternary ammonium) ionic liquids as metal-free catalysts under mild conditions. Fuel 2018, 224, 481–488. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Lu, C.; Wang, F.; Ma, C.; Chen, Z.; Yang, G. Imidazolium-Based Polymeric Ionic Liquids for Heterogeneous Catalytic Conversion of CO2 into Cyclic Carbonates. Microporous Mesoporous Mater. 2020, 292, 109751. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Lyu, Y.; Chen, X.; Li, C.; Zhang, Y.; Song, X.; Ding, Y. Highly Recyclable Polymer Supported Ionic Liquids as Efficient Heterogeneous Catalysts for Batch and Flow Conversion of CO2 to Cyclic Carbonates. RSC Adv. 2017, 7, 2836–2841. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Xiao, M.; Liu, L.; Liu, Y.; Liu, X.; Gai, H. Design of Novel Poly(Ionic Liquids) for the Conversion of CO2 to Cyclic Carbonates under Mild Conditions without Solvent. ACS Sustain. Chem. Eng. 2019, 7, 9489–9497. [Google Scholar] [CrossRef]
- Du, H.; Ye, Y.; Xu, P.; Sun, J. Experimental and Theoretical Study on Dicationic Imidazolium-Derived Poly(Ionic Liquid)s for Catalytic Cycloaddition of CO2-Epoxide. J. CO2 Ultilization 2023, 67 (Suppl. C), 102325. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Y.; Cheng, L.; Fang, C.; Li, H.; Ding, J.; Wan, H.; Guan, G. Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate. Catalysts 2025, 15, 406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).