Visible-Light-Driven CO2 Photoreduction Using Ruthenium (II) Complexes: Mechanisms, Hybrid Systems and Recent Advances
Abstract
1. Introduction
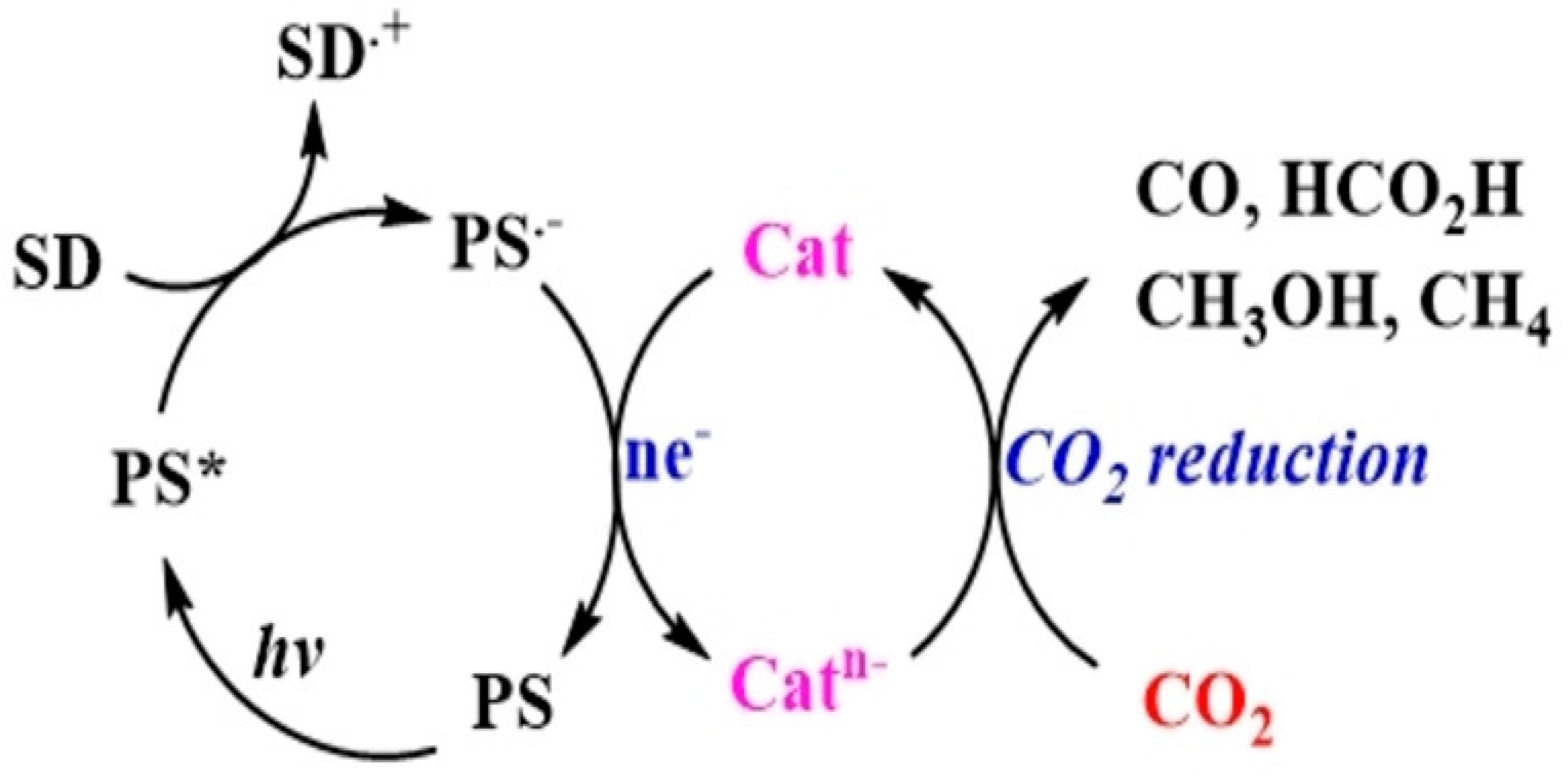
2. Mechanism of Photocatalytic CO2 Reduction
3. Photocatalytic CO2 Reduction Using Metal Molecular Catalysts
3.1. Electron Donors
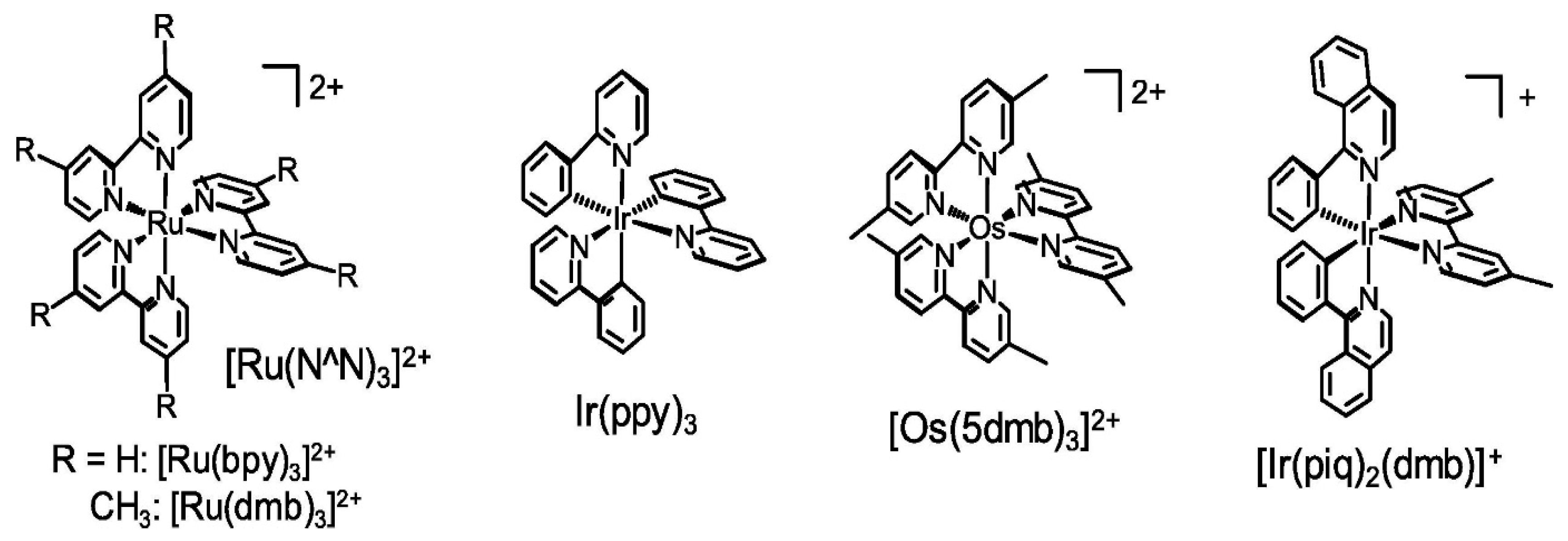
3.2. Photosensitizers
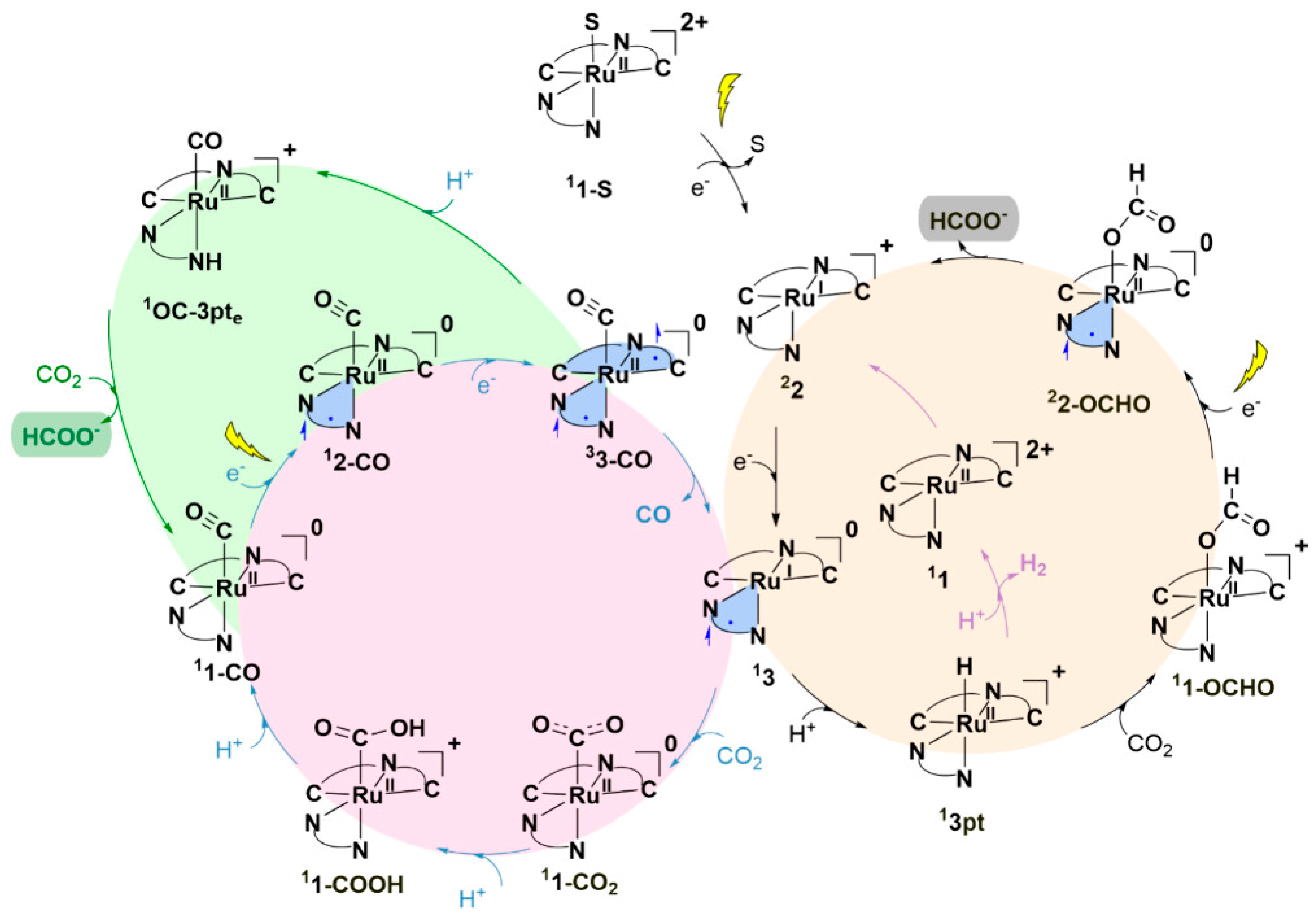
3.3. Catalytic Roles of [Ru(bpy)2(CO)2]2+ Complexes
4. Systematic Approaches to Ru-Driven CO2 Photoreduction
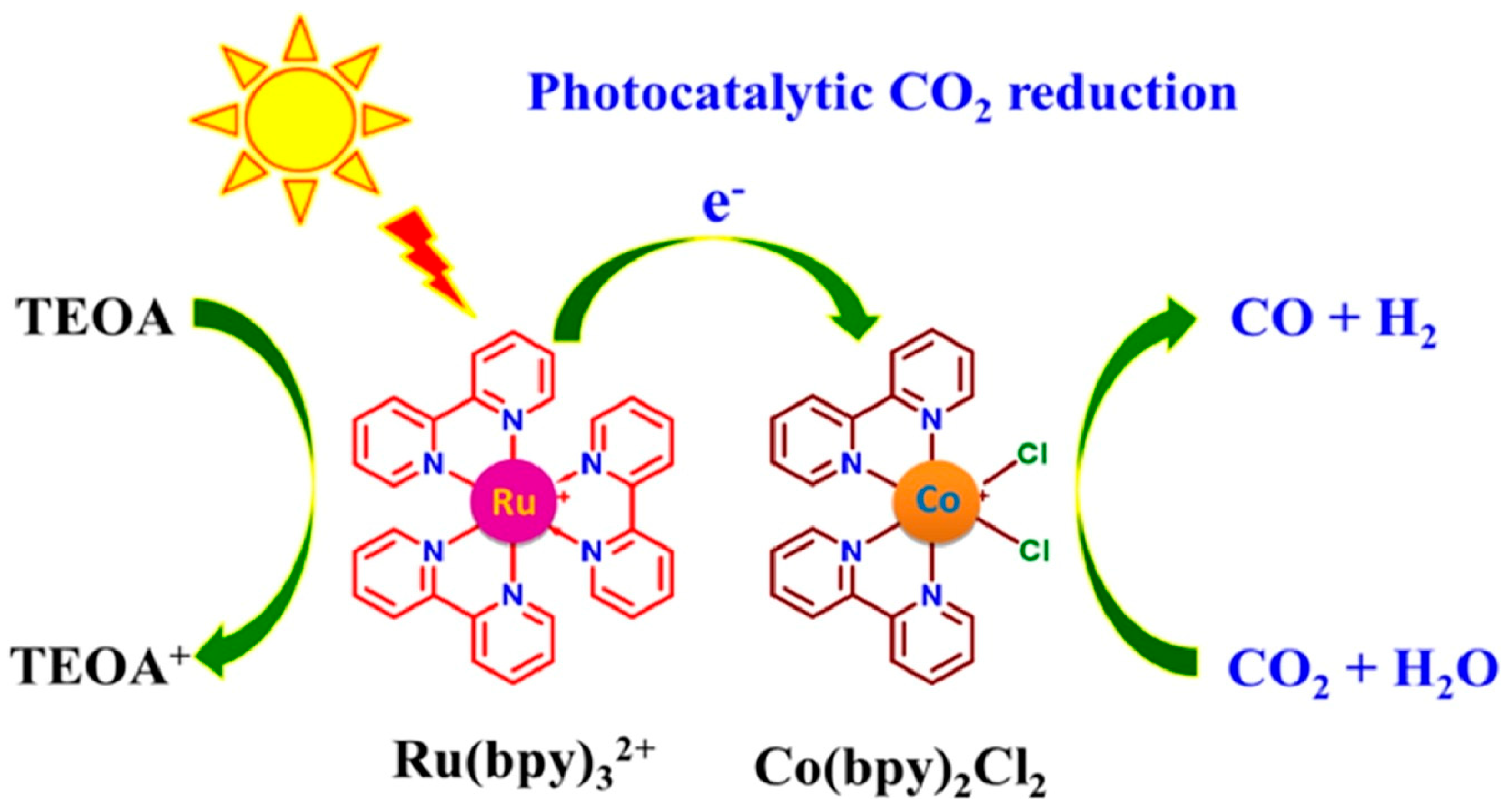
4.1. Photosensitizer/Catalyst Mixed Systems
4.2. Dyad CO2 Reduction Systems
4.3. Hybrid/Supramolecular Catalyst Systems
4.4. Artificial Photosynthesis Systems
5. Emerging Trends in Ru-Based CO2 Photocatalysis
6. Effect of Parameters
6.1. Effect of Donors
6.2. Effect of Solvent
6.3. Effect of Substituents Groups/Ligands
7. Limitations and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Kumar, P.; Aathira, M.S.; Singh, D.P.; Behera, B.; Jain, S.L. A Bridged Ruthenium Dimer (Ru–Ru) for Photoreduction of CO2 under Visible Light Irradiation. J. Ind. Eng. Chem. 2018, 61, 381–387. [Google Scholar] [CrossRef]
- Barrocas, B.; Oliveira, M.C.; Nogueira, H.I.S.; Fateixa, S.; Monteiro, O.C. A comparative study on emergent pollutants photo-assisted degradation using ruthenium modified titanate nanotubes and nanowires as catalysts. J. Environ. Sci. 2020, 92, 38–51. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Přech, J.; Filip Edelmannová, M.; Szaniawska, E.; Kočí, K.; Čejka, J. Titanosilicates enhance carbon dioxide photocatalytic reduction. Appl. Mater. Today 2022, 26, 101392. [Google Scholar] [CrossRef]
- Gu, J.; Chen, W.; Shan, G.G.; Li, G.; Sun, C.; Wang, X.L.; Su, Z. The Roles of Polyoxometalates in Photocatalytic Reduction of Carbon Dioxide. Mater. Today Energy 2021, 21, 100760. [Google Scholar] [CrossRef]
- Kuriki, R.; Maeda, K. Development of Hybrid Photocatalysts Constructed with a Metal Complex and Graphitic Carbon Nitride for Visible-Light-Driven CO2 Reduction. Phys. Chem. Chem. Phys. 2017, 19, 4938–4950. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-Efficiency g-C3N4 Based Photocatalysts for CO2 Reduction: Modification Methods. Adv. Fiber Mater. 2022, 4, 342–360. [Google Scholar] [CrossRef]
- Yoshino, S.; Takayama, T.; Yamaguchi, Y.; Iwase, A.; Kudo, A. CO2 Reduction Using Water as an Electron Donor over Heterogeneous Photocatalysts Aiming at Artificial Photosynthesis. Acc. Chem. Res. 2022, 55, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Srivastava, R. Catalytic Conversion of CO2 to Chemicals and Fuels: The Collective Thermocatalytic/Photocatalytic/Electrocatalytic Approach with Graphitic Carbon Nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Maeda, K. Metal-Complex/Semiconductor Hybrid Photocatalysts and Photoelectrodes for CO2 Reduction Driven by Visible Light. Adv. Mater. 2019, 31, 1808205. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Dar, A.H.; Shaikh, M.N.; Qurashi, A. Reticular-Chemistry-Inspired Supramolecule Design as a Tool to Achieve Efficient Photocatalysts for CO2 Reduction. ACS Omega 2021, 6, 29291–29324. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Chen, G.; Robert, M.; Lau, T.C. Electrocatalytic and Photocatalytic Reduction of Carbon Dioxide by Earth-Abundant Bimetallic Molecular Catalysts. ChemPhysChem 2021, 22, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Itabashi, J.; Toyama, M.; Ishida, H. Photochemical CO2 Reduction Catalyzed by Trans(Cl)-[Ru(2,2′-Bipyridine)(CO)2Cl2] Bearing Two Methyl Groups at 4,4′-, 5,5′- or 6,6′-Positions in the Ligand. ChemPhotoChem 2018, 2, 314–322. [Google Scholar] [CrossRef]
- Cancelliere, A.M.; Puntoriero, F.; Serroni, S.; Campagna, S.; Tamaki, Y.; Saito, D.; Ishitani, O. Efficient Trinuclear Ru(Ii)-Re(i) Supramolecular Photocatalysts for CO2 Reduction Based on a New Tris-Chelating Bridging Ligand Built around a Central Aromatic Ring. Chem. Sci. 2020, 11, 1556–1563. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Ishitani, O. Synthesis of Os(II)-Re(i)-Ru(II) Hetero-Trinuclear Complexes and Their Photophysical Properties and Photocatalytic Abilities. Chem. Sci. 2018, 9, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Grills, D.C.; Manbeck, G.F.; Polyansky, D.E. Understanding the Role of Inter- and Intramolecular Promoters in Electro- and Photochemical CO2 Reduction Using Mn, Re, and Ru Catalysts. Acc. Chem. Res. 2022, 55, 616–628. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Grills, D.C.; Polyansky, D.E.; Szalda, D.J.; Fujita, E. Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes. J. Am. Chem. Soc. 2020, 142, 2413–2428. [Google Scholar] [CrossRef]
- Kumagai, H.; Tamaki, Y.; Ishitani, O. Photocatalytic Systems for CO2 Reduction: Metal-Complex Photocatalysts and Their Hybrids with Photofunctional Solid Materials. Acc. Chem. Res. 2022, 55, 978–990. [Google Scholar] [CrossRef]
- Irikura, M.; Tamaki, Y.; Ishitani, O. Development of a Panchromatic Photosensitizer and Its Application to Photocatalytic CO2 reduction. Chem. Sci. 2021, 12, 13888–13896. [Google Scholar] [CrossRef]
- Deng, X.; Qin, Y.; Hao, M.; Li, Z. MOF-253-Supported Ru Complex for Photocatalytic CO2 Reduction by Coupling with Semidehydrogenation of 1,2,3,4-Tetrahydroisoquinoline (THIQ). Inorg. Chem. 2019, 58, 16574–16580. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction Mechanisms of Catalytic Photochemical CO2 Reduction Using Re(I) and Ru(II) Complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Livingstone, S.E. The Chemistry of Ruthenium, Rhodium, Palladium, Osmium, Iridium and Platinum, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–122. [Google Scholar]
- Hameed, Y.; Gabidullin, B.; Richeson, D. Photocatalytic CO2 Reduction with Manganese Complexes Bearing a Κ2-PN Lig and: Breaking the α-Diimine Hold on Group 7 Catalysts and Switching Selectivity. Inorg. Chem. 2018, 57, 13092–13096. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Takayama, T.; Sato, S.; Iwase, A.; Kudo, A.; Morikawa, T. Enhancement of CO2 Reduction Activity under Visible Light Irradiation over Zn-Based Metal Sulfides by Combination with Ru-Complex Catalysts. Appl. Catal. B 2018, 224, 572–578. [Google Scholar] [CrossRef]
- Liao, W.M.; Zhang, J.H.; Hou, Y.J.; Wang, H.P.; Pan, M. Visible-Light-Driven CO2 Photo-Catalytic Reduction of Ru(II) and Ir(III) Coordination Complexes. Inorg. Chem. Commun. 2016, 73, 80–89. [Google Scholar] [CrossRef]
- Huang, A.; Zhou, T.; Zhang, J.; Zhang, Y.; Wu, Y.; Wang, Y.; Luo, W. Competing CO and HCOOH Pathways in CO2 Electroreduction. ChemCatChem 2024, 16, e202400504. [Google Scholar] [CrossRef]
- Ishida, H. Electrochemical/Photochemical CO2 Reduction Catalyzed by Transition Metal Complexes. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Karamé, I., Shaya, J., Srour, H., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Zhang, Y.-Q.; Wu, T.; Zhang, Y.; Liao, R.-Z. Theoretical Study on Photocatalytic CO2 Reduction to Formate by a Ruthenium CNC Pincer Complex. J. Phys. Chem. A 2025, 129, 8357–8369. [Google Scholar] [CrossRef]
- Bharti, J.; Chen, L.; Guo, Z.; Cheng, L.; Wellauer, J.; Wenger, O.S.; von Wolff, N.; Lau, K.C.; Lau, T.C.; Chen, G.; et al. Visible-Light-Driven CO2 Reduction with Homobimetallic Complexes. Cooperativity between Metals and Activation of Different Pathways. J. Am. Chem. Soc. 2023, 145, 25195–25202. [Google Scholar] [CrossRef]
- Ma, K.; Li, J.; Liu, J.; Li, C.; Chen, X.B.; Li, Z.; Wang, L.; Shi, Z.; Feng, S. Covalent Triazine Framework Featuring Single Electron Co2+ Centered in Intact Porphyrin Units for Efficient CO2 Photoreduction. Appl. Surf. Sci. 2023, 629, 157453. [Google Scholar] [CrossRef]
- Xiong, Z.; Huang, L.; Peng, J.; Hou, Y.; Ding, Z.; Wang, S. Spinel-Type Mixed Metal Sulfide NiCo2S4 for Efficient Photocatalytic Reduction of CO2 with Visible Light. ChemCatChem 2019, 11, 5513–5518. [Google Scholar] [CrossRef]
- Ishizuka, T.; Hosokawa, A.; Kawanishi, T.; Kotani, H.; Zhi, Y.; Kojima, T. Self-Photosensitizing Dinuclear Ruthenium Catalyst for CO2 Reduction to CO. J. Am. Chem. Soc. 2023, 145, 23196–23204. [Google Scholar] [CrossRef]
- Li, N.X.; Chen, Y.M.; Xu, Q.Q.; Mu, W.H. Photocatalytic Reduction of CO2 to CO Using Nickel(II)-Bipyridine Complexes with Different Substituent Groups as Catalysts. J. CO2 Util. 2023, 68, 102385. [Google Scholar] [CrossRef]
- Hong, D.; Kawanishi, T.; Tsukakoshi, Y.; Kotani, H.; Ishizuka, T.; Kojima, T. Efficient Photocatalytic CO2 Reduction by a Ni(II) Complex Having Pyridine Pendants through Capturing a Mg2+ Ion as a Lewis-Acid Cocatalyst. J. Am. Chem. Soc. 2019, 141, 20309–20317. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, Y.; Ye, L.; Chen, H.; Sun, L. Highly Efficient Photocatalytic Reduction of CO2 and H2O to CO and H2 with a Cobalt Bipyridyl Complex. J. Energy Chem. 2018, 27, 502–506. [Google Scholar] [CrossRef]
- Sun, M.; Wang, C.; Sun, C.Y.; Zhang, M.; Wang, X.L.; Su, Z.M. Ultra Stable Multinuclear Metal Complexes as Homogeneous Catalysts for Visible-Light Driven Syngas Production from Pure and Diluted CO2. J. Catal. 2020, 385, 70–75. [Google Scholar] [CrossRef]
- Wang, J.W.; Huang, H.H.; Sun, J.K.; Ouyang, T.; Zhong, D.C.; Lu, T.B. Electrocatalytic and Photocatalytic Reduction of CO2 to CO by Cobalt(II) Tripodal Complexes: Low Overpotentials, High Efficiency and Selectivity. ChemSusChem 2018, 11, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, L.; Chen, G.; Guo, Z.; Wang, L.; Fan, H.; Robert, M.; Lau, T.C. A Highly Active and Robust Iron Quinquepyridine Complex for Photocatalytic CO2 reduction in Aqueous Acetonitrile Solution. Chem. Commun. 2020, 56, 6249–6252. [Google Scholar] [CrossRef]
- Dong, Y.L.; Liu, H.R.; Wang, S.M.; Guan, G.W.; Yang, Q.Y. Immobilizing Isatin-Schiff Base Complexes in NH2-UiO-66 for Highly Photocatalytic CO2 Reduction. ACS Catal. 2023, 13, 2547–2554. [Google Scholar] [CrossRef]
- Zhong, W.; Sa, R.; Li, L.; He, Y.; Li, L.; Bi, J.; Zhuang, Z.; Yu, Y.; Zou, Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2019, 141, 7615–7621. [Google Scholar] [CrossRef]
- Li, M.; Fu, Y.; You, S.; Yang, Y.; Qin, C.; Zhao, L.; Su, Z. Hexanuclear Nickel-Based [P4Mo11O50] with Photocatalytic Reduction of CO2 Activity. Inorg. Chem. Commun. 2021, 134, 109009. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Chen, L.; Chao, D. Water-Assisted Highly Efficient Photocatalytic Reduction of CO2 to CO with Noble Metal-Free Bis(Terpyridine)Iron(II) Complexes and an Organic Photosensitizer. Inorg. Chem. 2021, 60, 5590–5597. [Google Scholar] [CrossRef]
- Ohkubo, K.; Yamazaki, Y.; Nakashima, T.; Tamaki, Y.; Koike, K.; Ishitani, O. Photocatalyses of Ru(II)–Re(I) Binuclear Complexes Connected through Two Ethylene Chains for CO2 Reduction. J Catal. 2016, 343, 278–289. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fukaya, K.; Yoshida, M.; Ishida, H. Trans-(Cl)-[Ru(5,5′-Diamide-2,2′-Bipyridine)(CO)2Cl2]: Synthesis, Structure, and Photocatalytic CO2 Reduction Activity. Chem. Eur. J. 2015, 21, 10049–10060. [Google Scholar] [CrossRef]
- Hameed, Y.; Rao, G.K.; Ovens, J.S.; Gabidullin, B.; Richeson, D. Visible-Light Photocatalytic Reduction of CO2 to Formic Acid with a Ru Catalyst Supported by N,N″-Bis(Diphenylphosphino)-2,6-Diaminopyridine Ligands. ChemSusChem 2019, 12, 3453–3457. [Google Scholar] [CrossRef]
- Das, S.; Rodrigues, R.R.; Lamb, R.W.; Qu, F.; Reinheimer, E.; Boudreaux, C.M.; Webster, C.E.; Delcamp, J.H.; Papish, E.T. Highly Active Ruthenium CNC Pincer Photocatalysts for Visible-Light-Driven Carbon Dioxide Reduction. Inorg. Chem. 2019, 58, 8012–8020. [Google Scholar] [CrossRef]
- Chen, S.; Kong, P.; Niu, H.; Liu, H.; Wang, X.; Zhang, J.; Li, R.; Guo, Y.; Peng, T. Co-Porphyrin/Ru-Pincer Complex Coupled Polymer with Z-Scheme Molecular Junctions and Dual Single-Atom Sites for Visible Light-Responsive CO2 Reduction. Chem. Eng. J. 2022, 431, 133357. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Sekine, M.; Kitamura, K.; Maegawa, Y.; Goto, Y.; Shirai, S.; Inagaki, S.; Ishida, H. Photocatalytic CO2 Reduction by Periodic Mesoporous Organosilica (PMO) Containing Two Different Ruthenium Complexes as Photosensitizing and Catalytic Sites. Chem. Eur. J. 2017, 23, 10301–10309. [Google Scholar] [CrossRef] [PubMed]
- Benseghir, Y.; Solé-Daura, A.; Mialane, P.; Marrot, J.; Dalecky, L.; Béchu, S.; Frégnaux, M.; Gomez-Mingot, M.; Fontecave, M.; Mellot-Draznieks, C.; et al. Understanding the Photocatalytic Reduction of CO2 with Heterometallic Molybdenum(V) Phosphate Polyoxometalates in Aqueous Media. ACS Catal. 2022, 12, 453–464. [Google Scholar] [CrossRef]
- Elcheikh Mahmoud, M.; Audi, H.; Assoud, A.; Ghaddar, T.H.; Hmadeh, M. Metal-Organic Framework Photocatalyst Incorporating Bis(4′-(4-Carboxyphenyl)-Terpyridine)Ruthenium(II) for Visible-Light-Driven Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 7115–7121. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Chang, C.H.; Zhang, T.; Guan, X.; Liu, Q.; Zhang, L.; Wen, P.; Tang, I.; Zhang, Y.; et al. Engineering Single Ni Sites on 3D Cage-like Cucurbit[n]Uril Ligands for Efficient and Selective CO2 Photocatalytic Reduction. Angew. Chem. Int. Ed. 2025, 64, 202417384. [Google Scholar] [CrossRef]
- Verma, P.; Rahimi, F.A.; Samanta, D.; Kundu, A.; Dasgupta, J.; Maji, T.K. Visible-Light-Driven Photocatalytic CO2 Reduction to CO/CH4 Using a Metal–Organic “Soft” Coordination Polymer Gel. Angew. Chem. Int. Ed. 2022, 61, e202116094. [Google Scholar] [CrossRef]
- Du, J.; Ma, Y.; Xin, X.; Na, H.; Zhao, Y.; Tan, H.; Han, Z.; Li, Y.; Kang, Z. Reduced Polyoxometalates and Bipyridine Ruthenium Complex Forming a Tunable Photocatalytic System for High Efficient CO2 Reduction. Chem. Eng. J. 2020, 398, 125518. [Google Scholar] [CrossRef]
- Zhang, P.; Sui, X.; Wang, Y.; Wang, Z.; Zhao, J.; Wen, N.; Chen, H.; Huang, H.; Zhang, Z.; Yuan, R.; et al. Surface Ru-H Bipyridine Complexes-Grafted TiO2 Nanohybrids for Efficient Photocatalytic CO2 Methanation. J. Am. Chem. Soc. 2023, 145, 5769–5777. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zhu, X.; Peng, L.; Fu, Y.; Ma, R.; Lu, X.; Zhang, F.; Zhu, W.; Fan, M. Boosting Photocatalytic CO2 Reduction over a Covalent Organic Framework Decorated with Ruthenium Nanoparticles. Chem. Eng. J. 2021, 405, 127011. [Google Scholar] [CrossRef]
- Kumar, A.; Ananthakrishnan, R. Visible Light-Assisted Reduction of CO2 into Formaldehyde by Heteroleptic Ruthenium Metal Complex-TiO2 Hybrids in an Aqueous Medium. Green Chem. 2020, 22, 1650–1661. [Google Scholar] [CrossRef]
- Maeda, K.; An, D.; Kuriki, R.; Lu, D.; Ishitani, O. Graphitic Carbon Nitride Prepared from Urea as a Photocatalyst for Visible-Light Carbon Dioxide Reduction with the Aid of a Mononuclear Ruthenium(II) Complex. Beilstein J. Org. Chem. 2018, 14, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Du, L.; Gao, H. Coordination Synergy between Metal Nanoclusters and Ruthenium Photosensitizers for Photocatalytic Cycloaddition of CO2 under Mild Conditions. Mol. Catal. 2025, 577, 114977. [Google Scholar] [CrossRef]
- Saito, D.; Tamaki, Y.; Ishitani, O. Photocatalysis of CO2 Reduction by a Ru(II)-Ru(II) Supramolecular Catalyst Adsorbed on Al2O3. ACS Catal. 2023, 13, 4376–4383. [Google Scholar] [CrossRef]
- Muraoka, K.; Uchiyama, T.; Lu, D.; Uchimoto, Y.; Ishitani, O.; Maeda, K. A Visible-Light-Driven Z-Scheme CO2 Reduction System Using Ta3N5 and a Ru(II) Binuclear Complex. Bull. Chem. Soc. Jpn. 2019, 92, 124–126. [Google Scholar] [CrossRef]
- Morikawa, T.; Sato, S.; Sekizawa, K.; Suzuki, T.M.; Arai, T. Solar-Driven CO2 Reduction Using a Semiconductor/Molecule Hybrid Photosystem: From Photocatalysts to a Monolithic Artificial Leaf. Acc. Chem. Res. 2022, 55, 933–943. [Google Scholar] [CrossRef]
- Meng, X.; Li, R.; Yang, J.; Xu, S.; Zhang, C.; You, K.; Ma, B.; Guan, H.; Ding, Y. Hexanuclear Ring Cobalt Complex for Photochemical CO2 to CO Conversion. Chin. J. Catal. 2022, 43, 2414–2424. [Google Scholar] [CrossRef]
- Qin, J.; Lin, L.; Wang, X. A Perovskite Oxide LaCoO3 Cocatalyst for Efficient Photocatalytic Reduction of CO2 with Visible Light. Chem. Commun. 2018, 54, 2272–2275. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, S.; Tomanec, O.; Petr, M.; Zhu Chen, J.; Miller, J.T.; Varma, R.S.; Gawande, M.B.; Zbořil, R. Carbon Nitride-Based Ruthenium Single Atom Photocatalyst for CO2 Reduction to Methanol. Small 2021, 17, 2006478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kim, S.; Eom, Y.K.; Valandro, S.R.; Schanze, K.S. Polymer Chromophore-Catalyst Assembly for Photocatalytic CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 7030–7039. [Google Scholar] [CrossRef]
- Sakakibara, N.; McQueen, E.; Sprick, R.S.; Ishitani, O. Photocatalytic CO2 Reduction in Aqueous Media Using a Silver-Loaded Conjugated Polymer and a Ru(II)-Ru(II) Supramolecular Photocatalyst. Bull. Chem. Soc. Jpn. 2025, 98, uoaf017. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Wang, J.; Shen, Q.; Zhang, Y.; Ding, C.; Bai, Y.; Jiang, G.; Li, Z.; Gaponik, N. Boosting Photocatalytic CO2 Reduction on CsPbBr3 Perovskite Nanocrystals by Immobilizing Metal Complexes. Chem. Mater. 2020, 32, 1517–1525. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Chen, H.; Weng, Y.X.; Tang, H.; Chen, Z.; Zhu, W.; She, Y.; Xia, J.; Li, H. Unique Z-Scheme Carbonized Polymer Dots/Bi4O5Br2 Hybrids for Efficiently Boosting Photocatalytic CO2 Reduction. Appl. Catal. B 2021, 293, 120182. [Google Scholar] [CrossRef]
- Lin, J.; Qin, B.; Zhao, G. Effect of Solvents on Photocatalytic Reduction of CO2 Mediated by Cobalt Complex. J. Photochem. Photobiol. A Chem. 2018, 354, 181–186. [Google Scholar] [CrossRef]
- Kuriki, R.; Ishitani, O.; Maeda, K. Unique Solvent Effects on Visible-Light CO2 Reduction over Ruthenium(II)-Complex/Carbon Nitride Hybrid Photocatalysts. ACS Appl. Mater. Interfaces 2016, 8, 6011–6018. [Google Scholar] [CrossRef]
- Thompson, W.A.; Sanchez Fernandez, E.; Maroto-Valer, M.M. Review and Analysis of CO2 Photoreduction Kinetics. ACS Sustain. Chem. Eng. 2020, 8, 4677–4692. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, S.; Peter, S.C. Systematic Assessment of Solvent Selection in Photocatalytic CO2 Reduction. ACS Energy Lett. 2021, 6, 3270–3274. [Google Scholar] [CrossRef]




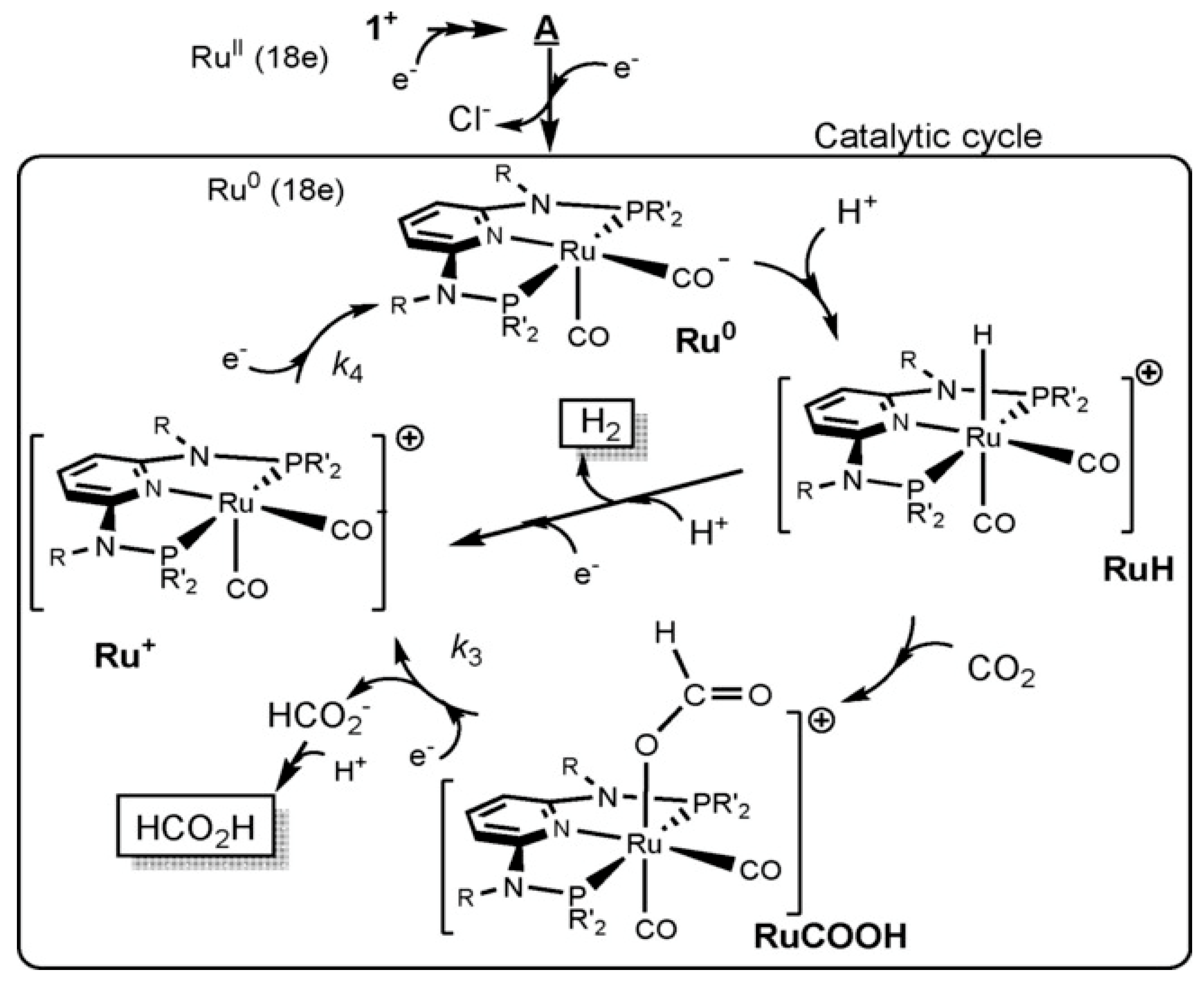
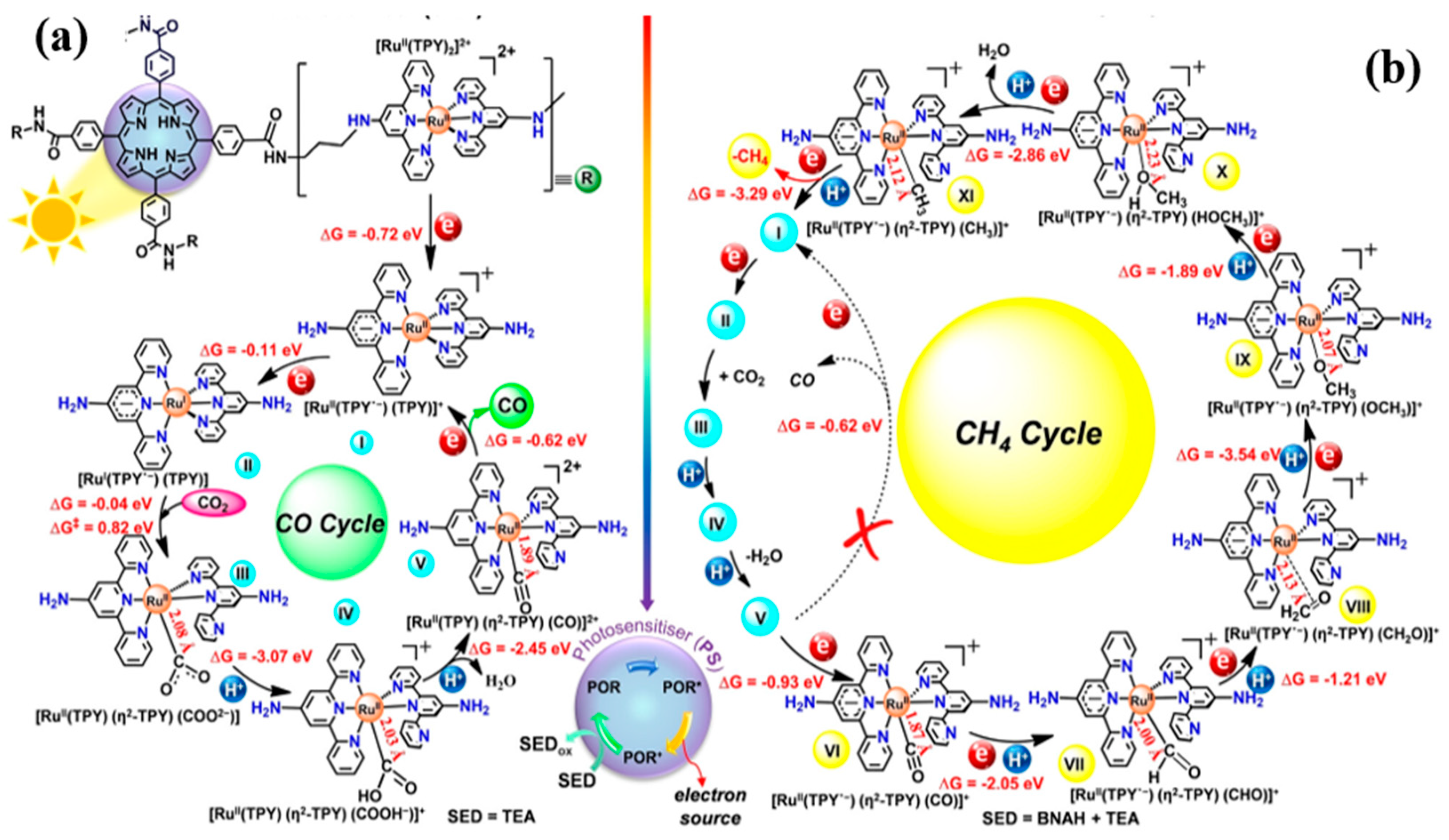
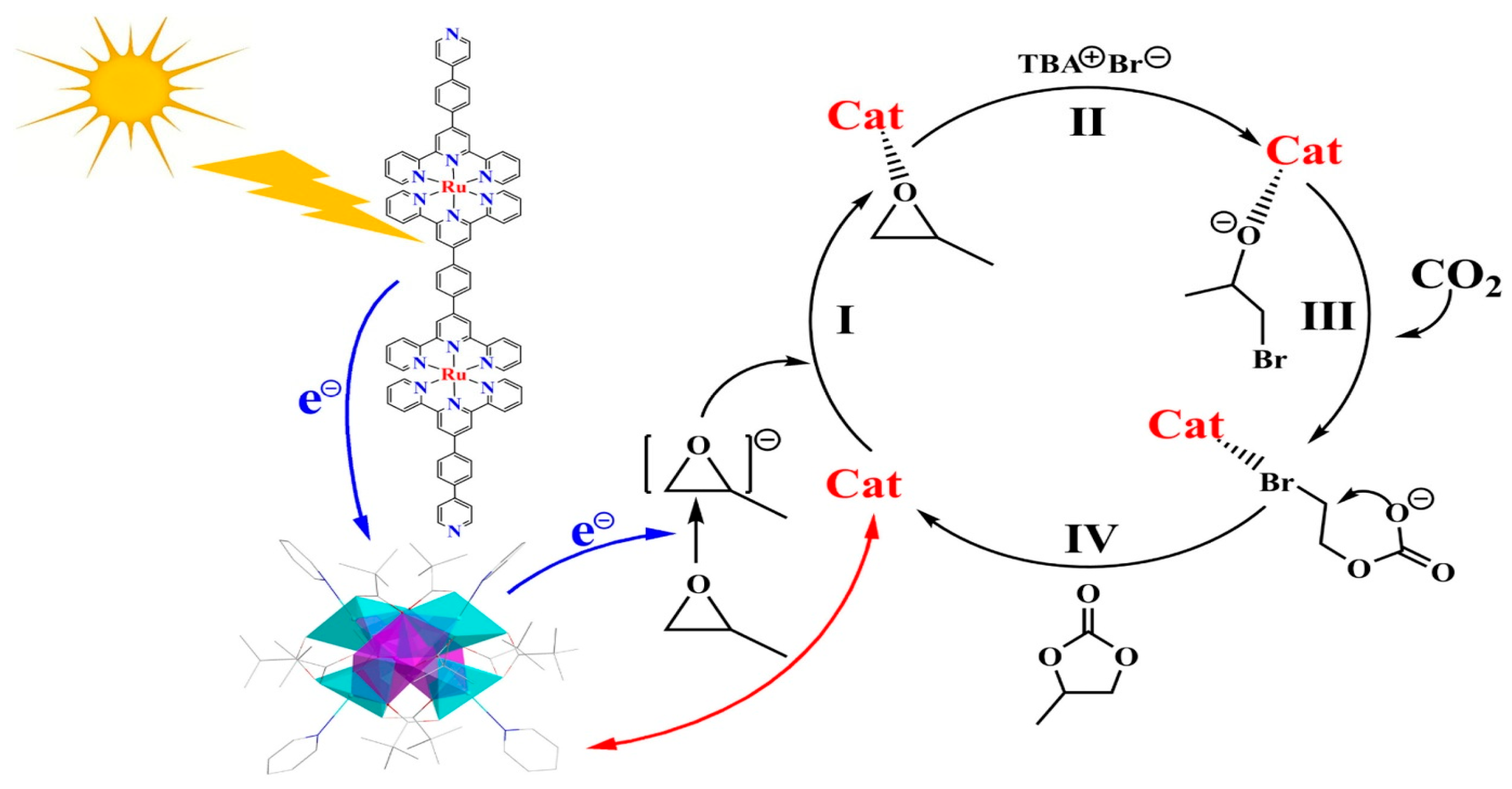

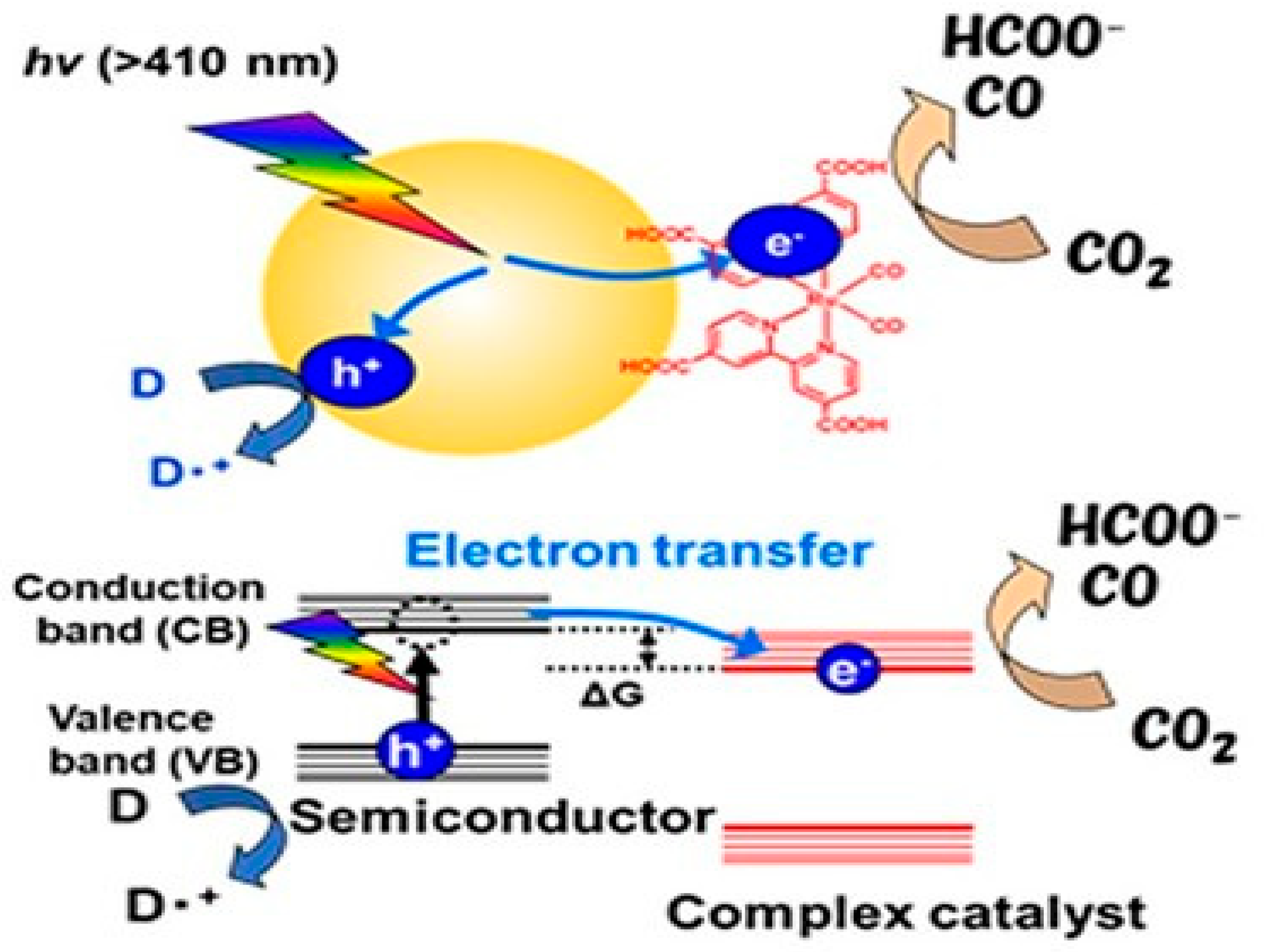
| Ru Complex | Chemical Structure | Anchor | Charge | CO2 Reduction Potential [V vs. NHE] a |
|---|---|---|---|---|
| [Ru(dcbpy)2] | [Ru(dcbpy)2(CO)2]2+ b | COOH | cationic | −0.8 |
| [Ru(dpbpy)(bpy)] | [Ru(dpbpy)(bpy)(CO)2]2+ c,d | PO3H2 | cationic | −0.8 |
| [Ru(dpbpy)] | Ru(dpbpy)(CO)2Cl2 c | PO3H2 | neutral | −1.0 |
| [Ru(dcbpy)] | Ru(dcbpy)(CO)2Cl2 b | COOH | neutral | N.D. f |
| [Ru(pypcbpy)] | [Ru(pypcbpy)(CO)(MeCN)Cl2 e | - | neutral | −0.6 |
| Entry | Ru-Complex Catalyst | Photosensitizer | Solvent | Donor | Main Product | TON | S (%) | AQY (%) | Time/h | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Homogeneous | ||||||||||
| 1 | ([NiCl2(4,4′-dichloro-2,2′-bipyridine)2]) | [Ru(bpy)3]2+ | MeCN/TEOA | BIH | CO | 2409 | 84 | 0.24 | n.d | [33] |
| 2 | [Ni(bpetpy2)(H2O)2]-(Mg2+(ClO4)2) | [Ru(bpy)3]2+ | DMA/H2O | BIH | CO | 120 | 99.7 | 11.1 | 1 | [34] |
| 3 | Co(bpy)2Cl2 | [Ru(bpy)3]2+ | MeCN/TEOA | TEOA | CO/H2 | 6230/6990 | n.d | 2.1 | 4 | [35] |
| 4 | [Co5(btz)6(NO3)4(H2O)4] | [Ru(bpy)3]2+ | MeCN/TEOA | TEOA | CO/H2 | 1000/300 | n.d | 0.07/0.18 | 10 | [36] |
| 5 | [Co(BQPA)CI]CIO4 | [Ru(phen)3]2+ | MeCN/H2O | TEOA | CO | 10,650 | 98 | 0.27 | 12 | [37] |
| 6 | [Fe(qnpy)(H2O)2]2+ | [Ru(phen)3]2+ | MeCN/H2O | BIH | CO | 14,095 | 98 | 2.3 | 130 | [38] |
| 7 | [L3CuCu](ClO4)2 | [Ru(phen)3]2+ | MeCN/TEOA | BIH/TEOA | HCOO−/CO | 766 | 60/28 | n.d | 28 | [29] |
| 8 | Ru(bpy)(CO)2Cl2 | [(mbip)Os(mtpy)]2+ | DMA | BI(OH)H | HCOO−/CO | 81/3 | 96 | 0.06 | 40 | [19] |
| Heterogeneous | ||||||||||
| 9 | NiCo2S4 | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | CO | n.d | 70.2 | 1.45 | 1 | [31] |
| 10 | 66-IS-Ni (Ni/Co/Cu) | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | CO | n.d | 87 | n.d | 6 | [39] |
| 11 | Ni-TpBpy | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | CO | 13.62 | 96 | 0.3 | 5 | [40] |
| 12 | [Ni6(trz)2(Htrz)13][H4P4Mo11O50]·7H2O | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | CO | n.d | n.d | n.d | 1 | [41] |
| 13 | FeCoS2–CoS2 | [Ru(bpy)3]2+ | MeCN/H2O | TOEA | CO | n.d | n.d | n.d | 3 | [42] |
| 14 | CTFTfOH-Co | [Ru(bpy)3]2+ | MeCN/TEOA | TEOA | CO | (2.6) | 61 | n.d | 6 | [30] |
| Entry | Ru-Complex Catalyst | Solvent | Donor | Main Product | TON/Rate a | S(%) | AQY (%) | Time/h | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | trans-(Cl)-[Ru(L)(CO)2Cl2] | (DMA)/H2O | BNAH | CO/HCOO− | 3000 | n.d | n.d | 4 | [44] |
| 2 | [Ru(k3-{2,6-(Ph2PNMe)2NC5H3})(CO)2Cl]+ (1+) | DMF | TEOA | HCOO− | 380 | 100 | 12.9 | 24 | [45] |
| 3 | [(CNC)Ru(bpy)(CH3CN)]-(OTF)2 | TEA/MeCN | TEA/BIH | CO | 33,000 | n.d | 0.00005 | 1 | [46] |
| 4 | (CoPor-RuN3) | DMF | BIH | CO/CH4 | 37.1 a/1.57 a | n.d | n.d | n.d | [47] |
| 5 | [Ru(bpy)2(bpm)Ru(CO)2Cl2] (Ru–Ru) | DMF/H2O | TEOA | COHCOO− | 54/28 | n.d | n.d | 12 | [26] |
| 6 | trans(Cl)-[Ru(bpy)(CO)2Cl2](Ru(PS)x-Ru(Cat)y-BPy-PMO) | DMA)/H2O | BNAH | CO/HCOO− | 95/67 | n.d | n.d | n.d | [48] |
| 7 | Fe–Mn and Ru(bpy)–Mn | TEOA/H2O | TEOA | CH4/CO | n.d | 92.6/85.2 | n.d | n.d | [49] |
| Entry | Ru-Complex Catalyst | Solvent | Donor | Main Product | TON/Tof b Rate a | S (%) | AQY (%) | Time/h | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Supramolecular | |||||||||
| 1 | MOF-253-Ru(dcbpy)2 | MeCN/THIQ | HCOO− | 61.8 | n.d | n.d | 5 days | [20] | |
| 2 | AUBM-4 (Zr-MOF-(Ru(cptpy)2)) | MeCN/TEOA | TEOA | HCOO− | 366 b | n.d | n.d | 6 h | [50] |
| 3 | Cucurbit[n]urils (CB[n] (CB[7]-Ni) | MeCN/H2O) | TEOA | CO | n.d | 97.9 | 1.34 | 0.5 | [51] |
| 4 | Ru-TPY-POR CPG | MeCN/H2O | TEA | CO | 92.7 | >99 | 0.037 | 12 | [52] |
| TEA/BNAH | CH4 | 208.3 | >95 | 0.0767 | 14 | ||||
| 5 | 1/Ru(bpy)/2 Rubpy {P4Mo6}/Ru(bpy) | TEOA/H2O | TEOA | CO/CH4 | 3.28/2.81 b | 96.3/96.4 | 10 | [53] | |
| Hybrid | |||||||||
| 6 | TiO2-4,4′-bpy−RuH | H2, CO2 | H2 | CH4 | 0.605 a | 93.4 | 1.15 | 4 | [54] |
| 7 | Ru/TpPa-1 | MeCN/TEOA | TEOA | HCOO− | 108.8 b | n.d | n.d | n.d | [55] |
| 8 | TiO2/RuL1OH) | TEOA/H2O | TEOA | n.d | n.d | 720 | 68 | 8 | [56] |
| 9 | RuP/Ag/g-C3N4 | DMA/TEOA | TEOA | HCOO− | 5775 | 95 | 4.2 | n.d | [57] |
| 10 | RuPyL2/Mn2Ni4 | MeCN | TBAB | cyclic CO32− | 1723 | n.d | n.d | 12 | [58] |
| 11 | [Ru(dpbpy)]/ZnS:Ni | MeCN/TEOA | TEOA | HCOO− | 126 | n.d | 0.92 | 16 | [24] |
| 12 | RuRu+Ru)/Al2O3 (RuRu) | DMA/TEOA | BNAH | HCOO− | 520 | n.d | n.d | 60 | [59] |
| 13 | RuRu/Ag/Ta3N5 | MeCN/TEOA | TEOA | HCOO− | n.d | >99 | n.d | 15 | [60] |
| Entry | Ru-Complex Catalyst | Photosensitizer | Solvent | Donor | Main Product | TON/Rate a | S(%) | AQY (%) | Time/h | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | {K2[CoO3PCH2N(CH2CO2)2]}6 | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | 300 | 78 | 0.81 | 4 | [62] | |
| 2 | LaCoO3 | [Ru(bpy)3]2+ | MeCN/H2O | TEOA | CO/H2 | 28.5/9.1 | 76 | 0.01 | 12 | [63] |
| 3 | RuSA–mC3N4 | DMF/H2O | H2O | HCOO− | 1500 a | n.d | n.d | 6 | [64] | |
| 4 | N-Ta2O5/[Ru(dcbpy)2(CO)2]2+ | MeCN/TEOA | TEOA | HCOO− | 89 | >75 | n.d | n.d | [8] | |
| 5 | 2·(PF6)4 ([Ru2 (1)2]4+ (2)) 1 = (bpy3Bz) | DMA/H2O | BIH | CO | 2400 | 99.1 | 19.7 | 26 | [32] | |
| 6 | PS-RuC/RuCat | DMF/TEOA | BNAH | CO/H2 | 23/6.1 | n.d | 6.7 | 1 | [65] | |
| 7 | RuRu′/Ag/P10 | MeCN/TEOA/H2O | TEOA | HCOO− | 38,000 | 71 | 4.2 | 48 | [66] |
| Entry | Solvent | V(cp) | Sol (mol/L) | CO (umol) | H2 (mol) | S (%) |
|---|---|---|---|---|---|---|
| 1 | Acetonitrile (MeCN) | 0.4 | 0.3 | 42.6 | 7.4 | 85.2 |
| 2 | Dimethylformamide (DMF) | 0.9 | 0.20 | 29.4 | 4.8 | 86.0 |
| 3 | Tetrahydrofuran (THF) | 0.6 | 0.20 | 24.7 | 2.6 | 90.5 |
| 4 | dimethylsulfoxide (DMSO) | 2.2 | 0.10 | 8.9 | 8.0 | 52.7 |
| 5 | Water (H2O) | 1.0 | 0.03 | 0.2 | 1.9 | 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncube, P.; Mochane, M.J. Visible-Light-Driven CO2 Photoreduction Using Ruthenium (II) Complexes: Mechanisms, Hybrid Systems and Recent Advances. Catalysts 2025, 15, 1036. https://doi.org/10.3390/catal15111036
Ncube P, Mochane MJ. Visible-Light-Driven CO2 Photoreduction Using Ruthenium (II) Complexes: Mechanisms, Hybrid Systems and Recent Advances. Catalysts. 2025; 15(11):1036. https://doi.org/10.3390/catal15111036
Chicago/Turabian StyleNcube, Pauline, and Mokgaotsa Jonas Mochane. 2025. "Visible-Light-Driven CO2 Photoreduction Using Ruthenium (II) Complexes: Mechanisms, Hybrid Systems and Recent Advances" Catalysts 15, no. 11: 1036. https://doi.org/10.3390/catal15111036
APA StyleNcube, P., & Mochane, M. J. (2025). Visible-Light-Driven CO2 Photoreduction Using Ruthenium (II) Complexes: Mechanisms, Hybrid Systems and Recent Advances. Catalysts, 15(11), 1036. https://doi.org/10.3390/catal15111036







