Safety and Process Intensification of Catalytic Reduction of 4-Nitophenol Using Sodium Borohydride in Flow Microreactor System
Abstract
1. Introduction
2. Results and Discussion
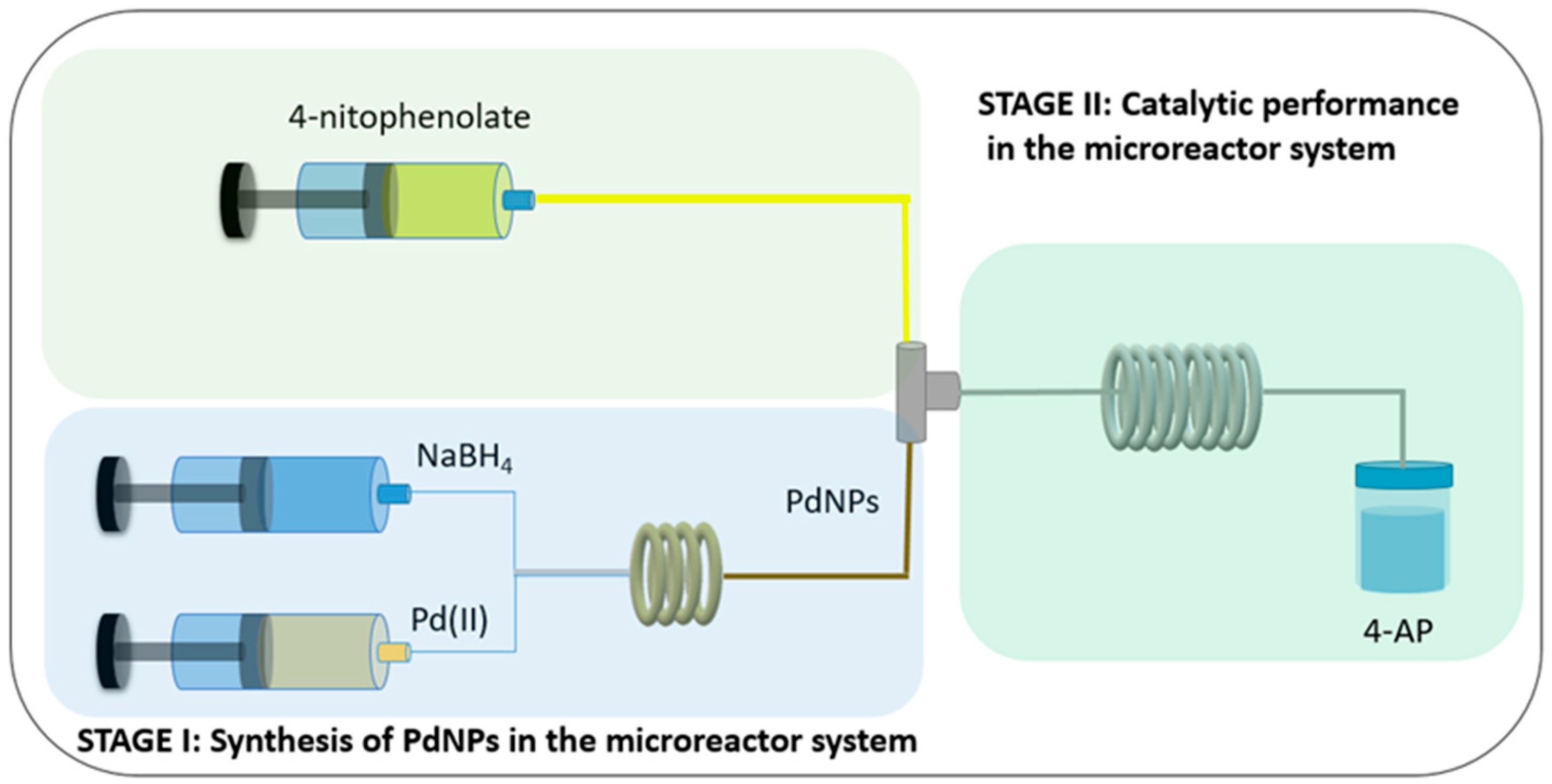
2.1. Set-Up of the Process Carried out in the Batch, Hybrid, and Microreactor Systems
2.2. Kinetic Background
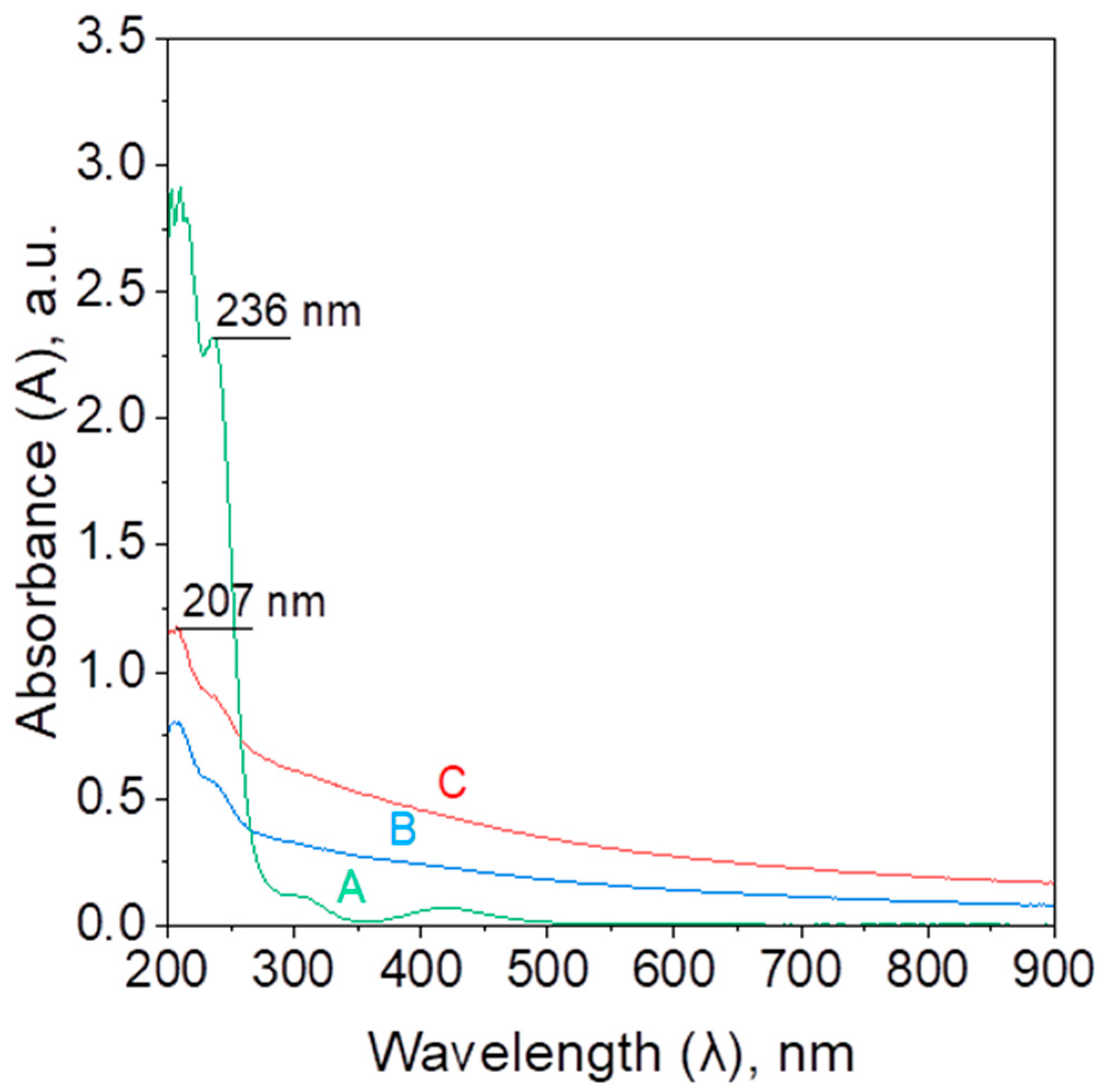
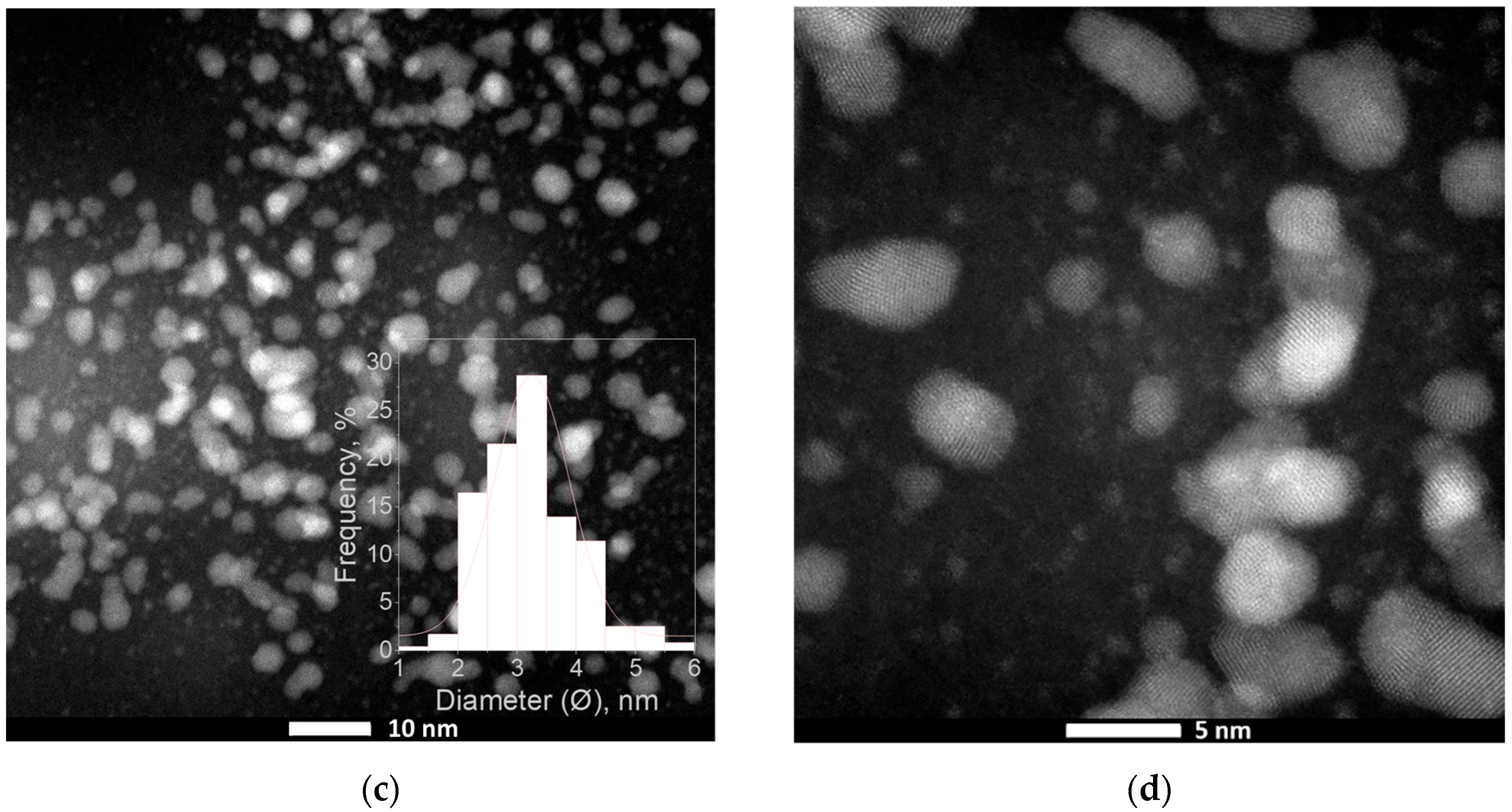
2.2.1. Synthesis of PdNPs—Kinetic, Mechanism, and Morphology Analysis
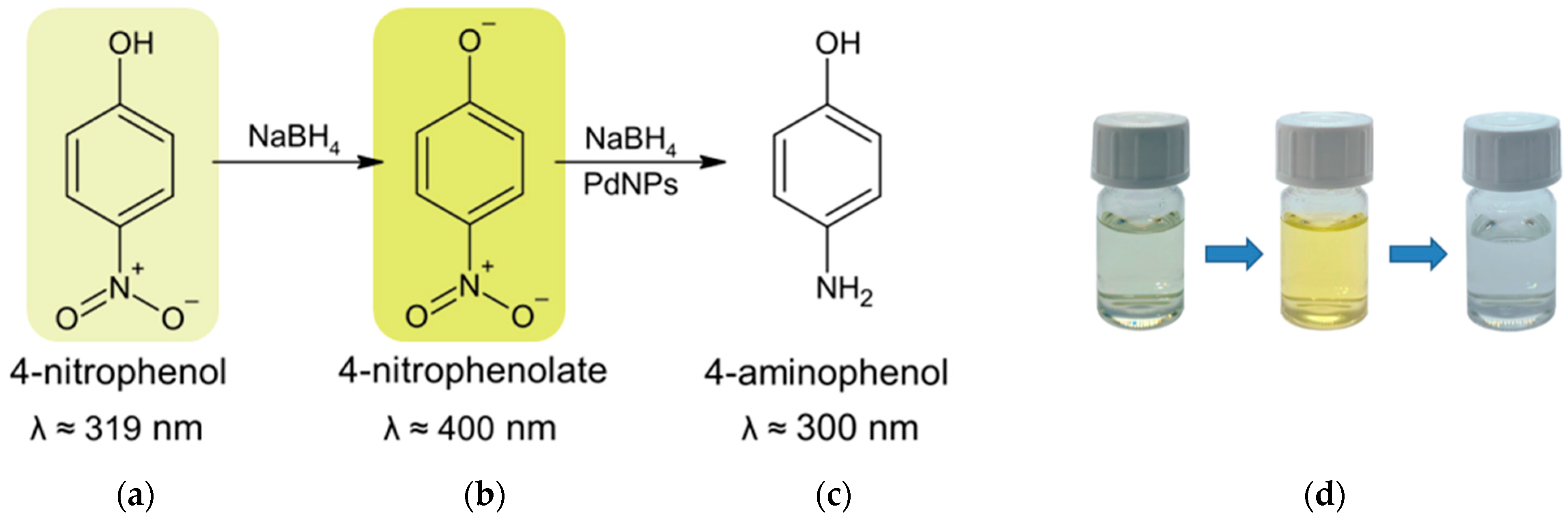
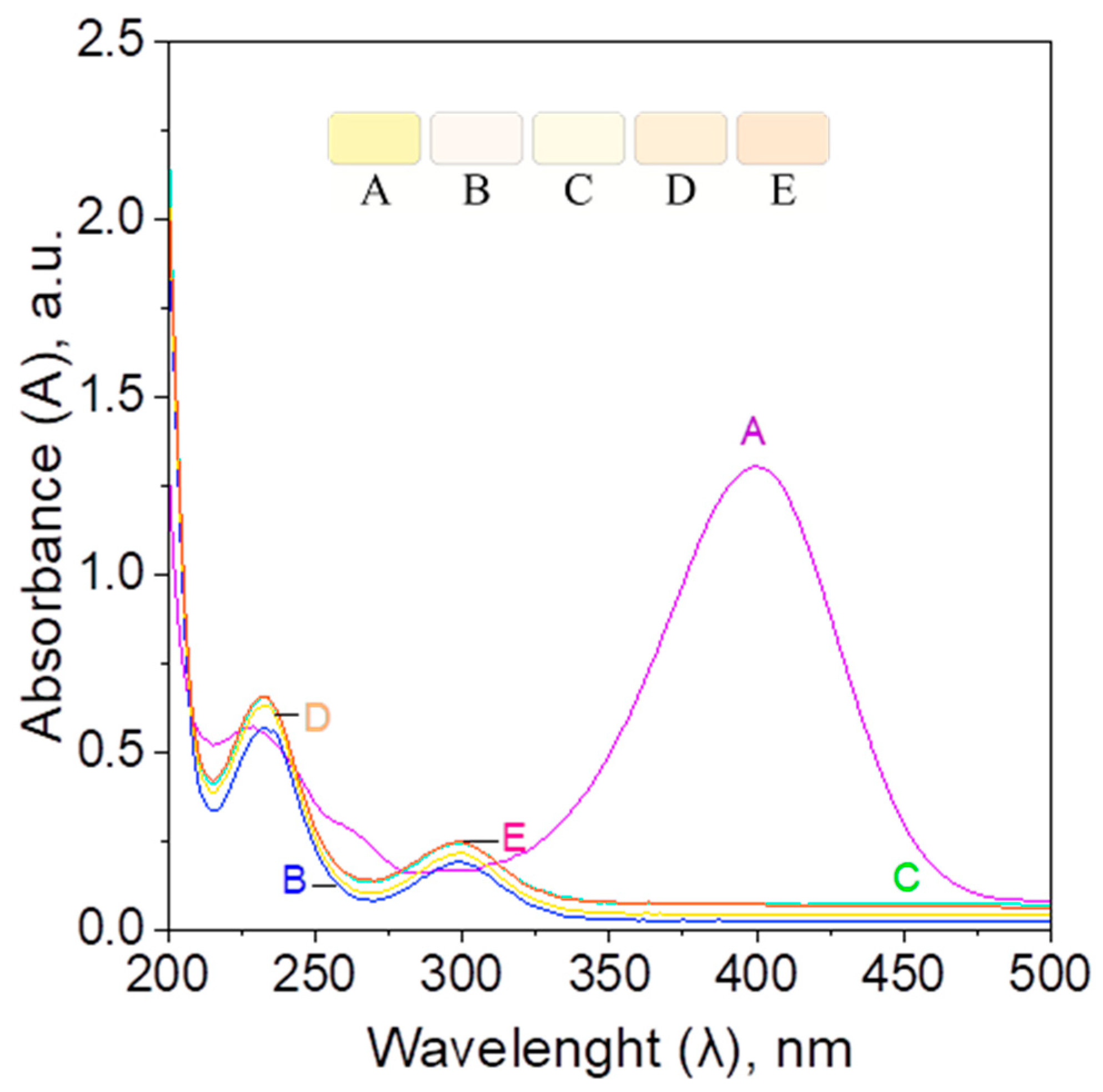
2.2.2. Reaction Between 4-Nitrophenol and Sodium Borohydride—Kinetics and Mechanism
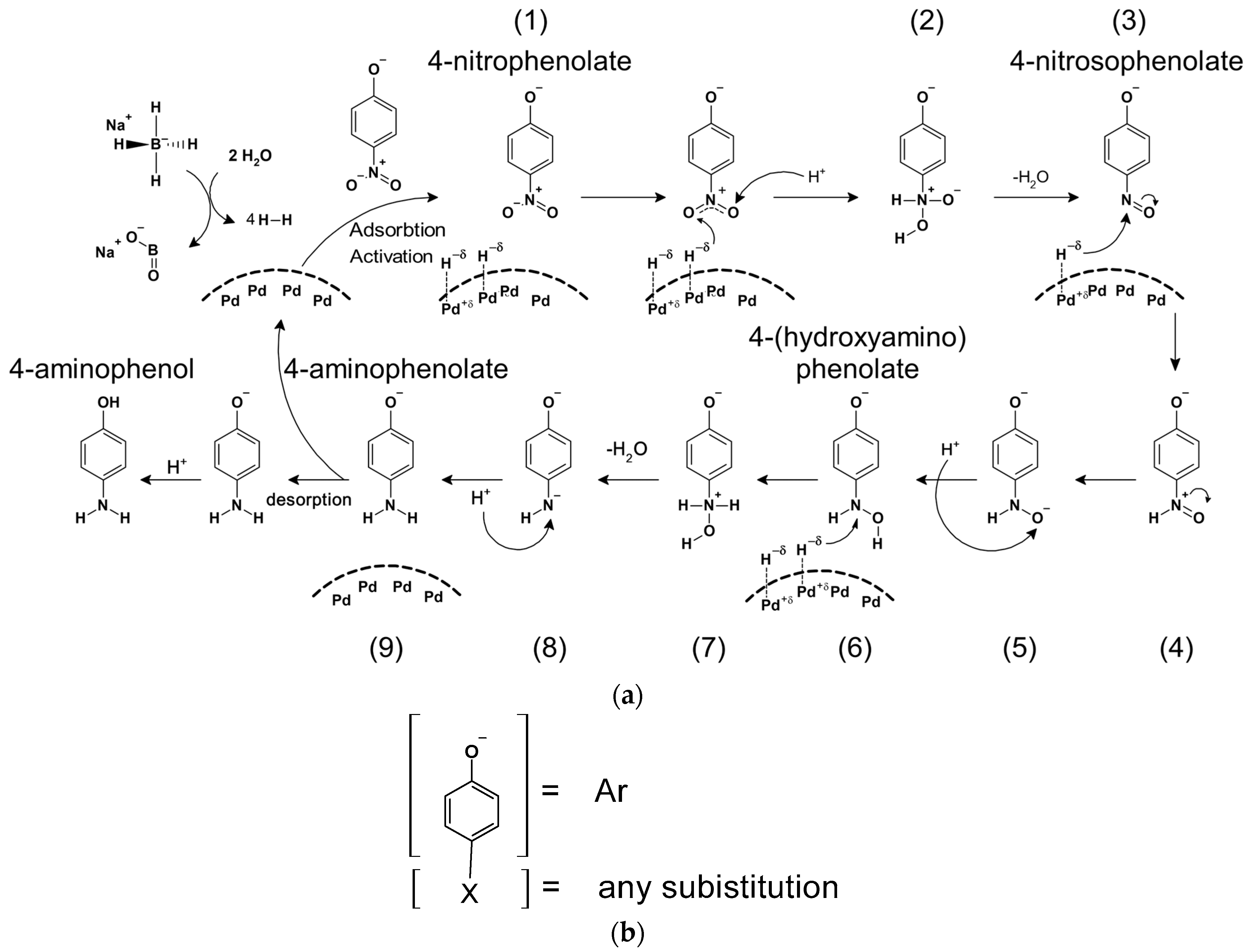
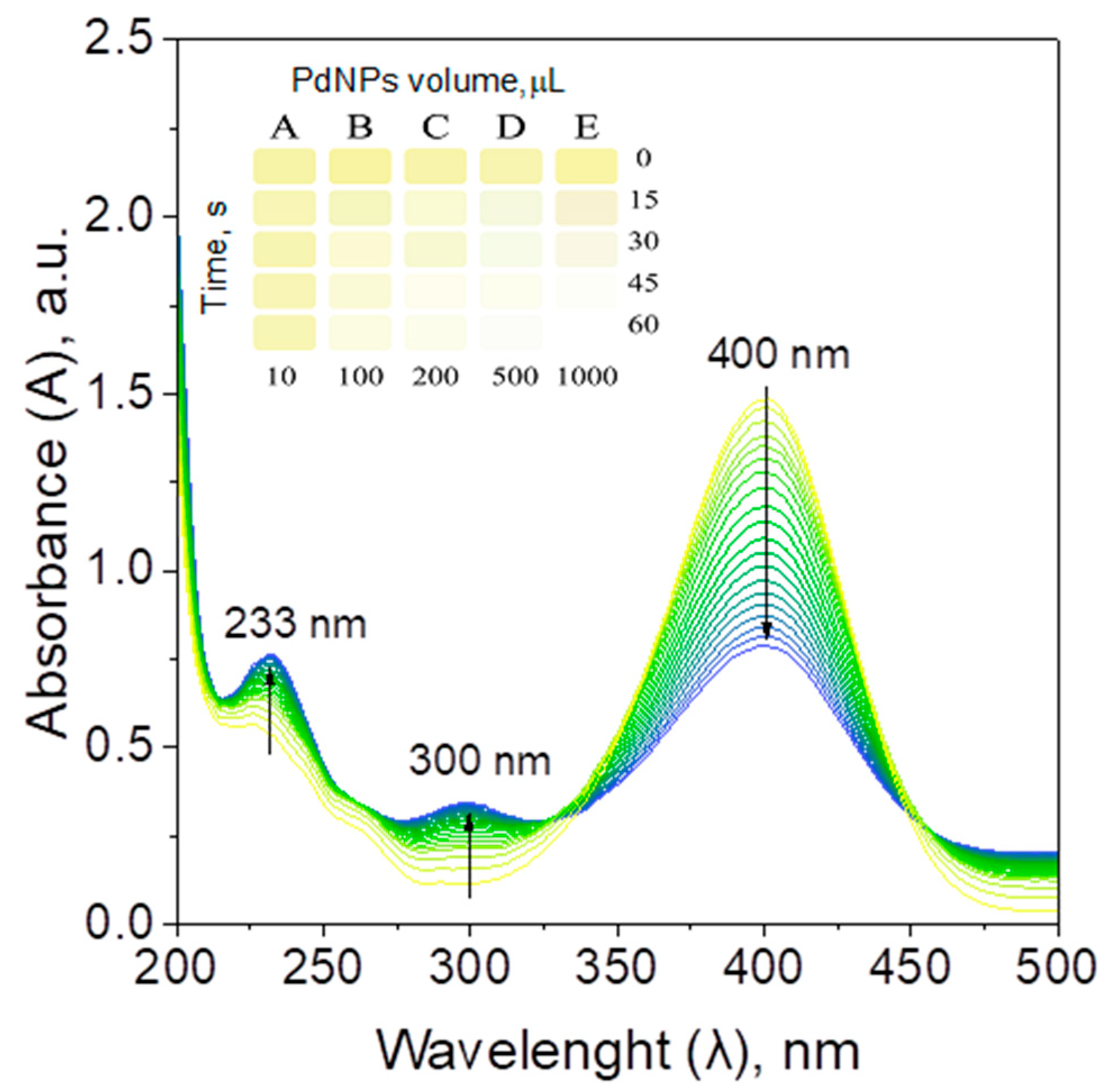
2.2.3. Reaction Between 4-Nitrophenolate and Sodium Borohydride in the Presence of Palladium Nanocatalyst—Kinetics and Mechanism
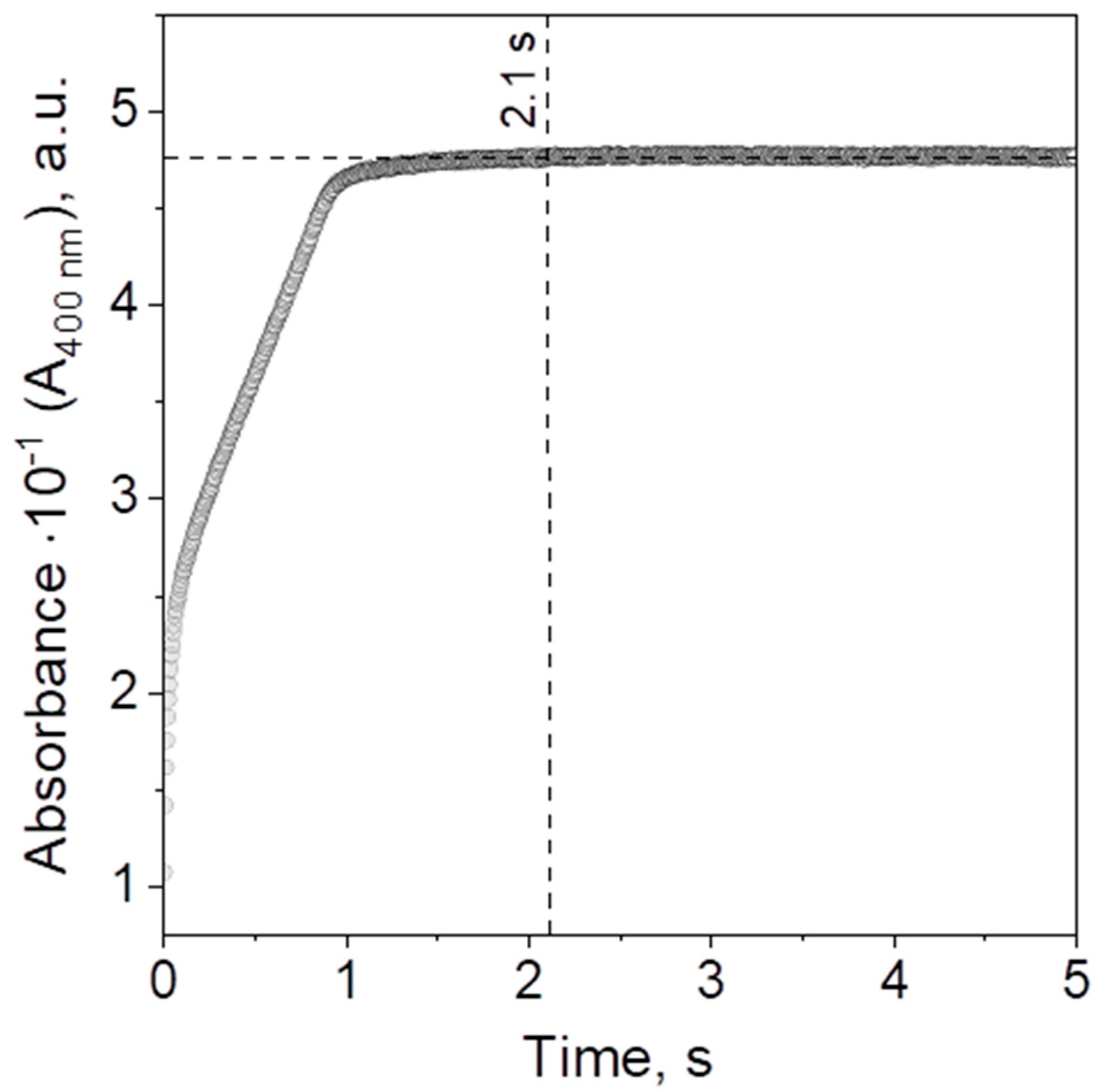
2.2.4. Catalyst Performance in the Batch, Hybrid, and Microreactor System—Kinetic Study
2.2.5. Comparison of the Kinetics of Heterogeneous Catalysis Carried out in the Batch, Hybrid, and Microreactor Systems
3. Materials and Methods
3.1. Chemicals
3.2. Method of Analysis
3.2.1. Spectra Registration and Kinetic Studies
3.2.2. Catalyst Analysis
3.2.3. Density Functional Theory Calculation
3.2.4. Color Coding and Data Processing
3.3. Batch, Hybrid, and Microreactor Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Li, M.; Chen, G. Revisiting Catalytic Model Reaction P-Nitrophenol/NaBH4 Using Metallic Nanoparticles Coated on Polymeric Spheres. Nanoscale 2013, 5, 11919–11927. [Google Scholar] [CrossRef]
- Yelboğa, M.; Akbayrak, M. Efficient Reduction of Highly Toxic 4-Nitrophenol with Ultra-Low Platinum Loading on Cobalt (II, III) Oxide Support: Facile Synthesis and High Turnover Frequency. Int. J. Hydrogen Energy 2025, 102, 800–815. [Google Scholar] [CrossRef]
- Pi, S.; Ma, F.; Cui, D.; Feng, L.; Zhou, L.; Li, A. Catalytic Reduction of 4-Nitrophenol by Green Silver Nanocomposites Assembled Using Microbial Extracellular Polymer Substances. Environ. Res. 2021, 197, 111006. [Google Scholar] [CrossRef]
- Kabiri, B.; Heidari, H. Synthesis and Catalytic Activity of Silver- Reduced Graphene Oxide and Silver- Magnetite- Reduced Graphene Oxide Nanocomposites in the Reduction of 4-Nitrophenol. Sci. Rep. 2025, 15, 14539. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ahmed, M.A.; Mohamed, A.A. Removal of 4-Nitrophenol and Indigo Carmine Dye from Wastewaters by Magnetic Copper Ferrite Nanoparticles: Kinetic, Thermodynamic and Mechanistic Insights. J. Saudi Chem. Soc. 2023, 27. [Google Scholar] [CrossRef]
- Lomonosov, V.; Asselin, J.; Ringe, E. Solvent Effects on the Kinetics of 4-Nitrophenol Reduction by NaBH4 in the Presence of Ag and Au Nanoparticles. React. Chem. Eng. 2022, 7, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Santos, B.M.; Faustino, R.F.C.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. C-Heterogenized Re Nanoparticles as Effective Catalysts for the Reduction of 4-Nitrophenol and Oxidation of 1-Phenylethanol. Catalysts 2022, 12, 285. [Google Scholar]
- Ehsani, A.; Nejatbakhsh, S.; Soodmand, A.M.; Farshchi, M.E.; Aghdasinia, H. High-Performance Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol Using M-BDC (M = Ag, Co, Cr, Mn, and Zr) Metal-Organic Frameworks. Environ. Res. 2023, 227, 115736. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Du, J. Reduction of 4-Nitrophenol Catalyzed by Silver Nanoparticles Supported on Polymer Micelles and Vesicles. RSC Adv. 2014, 4, 16425–16428. [Google Scholar] [CrossRef]
- Noël, S.; Bricout, H.; Addad, A.; Sonnendecker, C.; Zimmermann, W.; Monflier, E.; Léger, B. Catalytic Reduction of 4-Nitrophenol with Gold Nanoparticles Stabilized by Large-Ring Cyclodextrins. New J. Chem. 2020, 44, 21007–21011. [Google Scholar] [CrossRef]
- Thawarkar, S.R.; Thombare, B.; Munde, B.S.; Khupse, N.D. Kinetic Investigation for the Catalytic Reduction of Nitrophenol Using Ionic Liquid Stabilized Gold Nanoparticles. RSC Adv. 2018, 8, 38384–38390. [Google Scholar] [CrossRef]
- Neal, R.D.; Inoue, Y.; Hughes, R.A.; Neretina, S. Catalytic Reduction of 4-Nitrophenol by Gold Catalysts: The Influence of Borohydride Concentration on the Induction Time. J. Phys. Chem. C 2019, 123, 12894–12901. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mandal, M.; Kundu, S.; Nath, S.; Pal, T. Bimetallic Pt–Ni Nanoparticles Can Catalyze Reduction of Aromatic Nitro Compounds by Sodium Borohydride in Aqueous Solution. Appl. Catal. A Gen. 2004, 268, 61–66. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Zang, W.; Wang, L.; Zhang, X. Catalytic Activity of Shaped Platinum Nanoparticles for Hydrogenation: A Kinetic Study. Catal. Sci. Technol. 2014, 4, 3290–3297. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, S.K. Size-Dependent Catalytic Activity of PVA-Stabilized Palladium Nanoparticles in p-Nitrophenol Reduction: Using a Thermoresponsive Nanoreactor. ACS Omega 2021, 6, 20746–20757. [Google Scholar] [CrossRef]
- Berahim, N.; Basirun, W.J.; Leo, B.F.; Johan, M.R. Synthesis of Bimetallic Gold-Silver (Au-Ag) Nanoparticles for the Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol. Catalysts 2018, 8, 412. [Google Scholar] [CrossRef]
- König, R.Y.G.; Schwarze, M.; Schomäcker, R.; Stubenrauch, C. Catalytic Activity of Mono- and Bi-Metallic Nanoparticles Synthesized via Microemulsions. Catalysts 2014, 4, 256–275. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, B.; Zhang, J.; Wang, R.; Sun, H.; Wang, L.; Gao, D. Polyethyleneimine-Stabilized Palladium Nanoparticles for Reduction of 4-Nitrophenol. Transit. Met. Chem. 2019, 44, 655–662. [Google Scholar] [CrossRef]
- Ashraf, J.; Khan, G.A.; Ain, M.U.; Ghanem, M.A.; Mohammed, K.M.H.; Ahmed, W. Synthesis and Catalytic Activity of Core-Shell Gold-Palladium Nanoworms towards the Catalytic Reduction of 4-Nitrophenol and Methyl Orange. Inorg. Chem. Commun. 2024, 169, 113039. [Google Scholar] [CrossRef]
- Zhao, L.; Du, X.; Gao, F.; Hua, Y.; Li, H.; Zhang, X.; Di, L.-B. Oxygen Plasma Synthesis of Pd/GO with Enhanced Co-Adsorption Ability of 4-Nitrophenol and NaBH4 for Boosting 4-Nitrophenol Reduction Activity. Appl. Catal. A Gen. 2025, 696, 120203. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Hou, T.; Wang, D.; Tu, W. Efficient AuPd Catalysts with Layered Material Supporters for the Reduction of 4-Nitrophenol. J. Nanoparticle Res. 2022, 24, 108. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of Hydrolysis of Sodium Borohydride for Hydrogen Production in Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]
- Shin, S.; Kim, Y.; Jin, J.-H.; Jung, J. Heat-Induced Dry Hydrolysis of Sodium Borohydride/Oxalic Acid Dihydrate Composite for Hydrogen Production. ACS Omega 2022, 7, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Luty-Błocho, M.; Varon Hoyos, M.; Hessel, V. Implications of the Use of Functional Nanomaterials for the Environment and Health. In Functional Nanomaterials for Sensors; CRC Press: Boca Raton, FL, USA, 2023; pp. 289–319. ISBN 9781003263852. [Google Scholar]
- Mejía, Y.R.; Reddy Bogireddy, N.K. Reduction of 4-Nitrophenol Using Green-Fabricated Metal Nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Haswell, S.J. The Application of Micro Reactors for Organic Synthesis. Chem. Soc. Rev. 2005, 34, 235–246. [Google Scholar] [CrossRef]
- Mason, B.P.; Price, K.E.; Steinbacher, J.L.; Bogdan, A.R.; McQuade, D.T. Greener Approaches to Organic Synthesis Using Microreactor Technology. Chem. Rev. 2007, 107, 2300–2318. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Sandre, O.; Cabuil, V. Microfluidics in Inorganic Chemistry. Angew. Chemie Int. Ed. 2010, 49, 6268–6286. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Grzonka, J.; Kurzydłowski, K.J. The Synthesis of Stable Platinum Nanoparticles in the Microreactor. Arch. Metall. Mater. 2014, 59, 509–512. [Google Scholar] [CrossRef]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P.J. Nanoparticle Synthesis in Microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Pach, A.; Szot, A.; Fitzner, K.; Luty-Błocho, M. Opportunities and Challenges in the Synthesis of Noble Metal Nanoparticles via the Chemical Route in Microreactor Systems. Micromachines 2024, 15, 1119. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Pacławski, K.; Fitzner, K. The Synthesis of Platinum Nanoparticles and Their Deposition on the Active Carbon Fibers in One Microreactor Cycle. Chem. Eng. J. 2013, 226, 46–51. [Google Scholar] [CrossRef]
- Pach, A.; Zaryczny, A.; Michałek, T.; Kamiński, H.; Kutyła, D.; Tokarski, T.; Chat-Wilk, K.; Hessel, V.; Luty-Błocho, M. One-Step Synthesis of Pt–Pd@ACF Catalyst in the Microreactor System for the Hydrogen Evolution Reaction. Ind. Eng. Chem. Res. 2024, 63, 7018–7030. [Google Scholar] [CrossRef]
- Ašperger, S. Chemical Kinetics and Inorganic Reaction Mechanisms; Springer Science & Business Media: Cham, Switzerland, 2003. [Google Scholar] [CrossRef]
- Stevens, W.F. Chemical Engineering Kinetics. Use in the Scale-Up of Chemical Processes. Ind. Eng. Chem. 1958, 50, 591–593. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Włoch, G.; Fitzner, K. Green Method for Efficient PdNPs Deposition on Carbon Carrier in the Microreactor System. J. Nanoparticle Res. 2018, 20, 239. [Google Scholar] [CrossRef]
- Bentea, L.; Watzky, M.; Finke, R. Sigmoidal Nucleation and Growth Curves Across Nature Fit by the Finke–Watzky Model of Slow Continuous Nucleation and Autocatalytic Growth: Explicit Formulas for the Lag and Growth Times Plus Other Key Insights. J. Phys. Chem. C 2017, 121. [Google Scholar] [CrossRef]
- Finke, R.G.; Watzky, M.A.; Whitehead, C.B. Response to “Particle Size Is a Primary Determinant for Sigmoidal Kinetics of Nanoparticle Formation: A ‘Disproof’ of the Finke–Watzky (F-W) Nanoparticle Nucleation and Growth Mechanism. Chem. Mater. 2020, 32, 3657–3672. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.Z.; Liu, J.W.; Yao, X.D.; Wang, H.; Liu, Z.W.; Zhu, M. Hydrolysis and Regeneration of Sodium Borohydride (NaBH4)—A Combination of Hydrogen Production and Storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Fu, M.; Li, M.; Zhao, Y.; Bai, Y.; Fang, X.; Kang, X.; Yang, M.; Wei, Y.; Xu, X. A Study on the High Efficiency Reduction of P-Nitrophenol (4-NP) by a Fe(OH)3/Fe2O3@Au Composite Catalyst. RSC Adv. 2021, 11, 26502–26508. [Google Scholar] [CrossRef] [PubMed]
- Reddy Bogireddy, N.K.; Mejia, Y.R.; Aminabhavi, T.M.; Barba, V.; Becerra, R.H.; Ariza Flores, A.D.; Agarwal, V. The Identification of Byproducts from the Catalytic Reduction Reaction of 4-Nitrophenol to 4-Aminophenol: A Systematic Spectroscopic Study. J. Environ. Manag. 2022, 316, 115292. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.I.; Luart, D.; Dris, A.O.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Alshehri, A.A.; Abomuti, M.A.; Danish, E.Y.; Patel, R. Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol. Toxics 2021, 9, 103. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Song, Y.; Xu, X.; Cai, M.; Li, P.; Yuan, W.; Xiahou, Y. Functionalized Agarose Hydrogel with in Situ Ag Nanoparticles as Highly Recyclable Heterogeneous Catalyst for Aromatic Organic Pollutants. Environ. Sci. Pollut. Res. Int. 2023, 30, 43950–43961. [Google Scholar] [CrossRef]
- Tomishige, K.; Honda, M.; Sugimoto, H.; Liu, L.; Yabushita, M.; Nakagawa, Y. Recent Progress on Catalyst Development for Ring-Opening C-O Hydrogenolysis of Cyclic Ethers in the Production of Biomass-Derived Chemicals. Carbon Neutrality 2024, 3, 17. [Google Scholar] [CrossRef]




| Synthesis of Catalyst Nanoparticles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst Composition: | PdNPs | Pdn-PEI NPs | Au-Pd Core–Shell (Pd@Au) NWs | Pd/GO- O2P | Graphene- Supported AuPd (1:3) | |||||
| Precursor concentration | 0.2 mm H2PdCl4 | 2 mm Na2PdCl4aq | H2PdCl4 (2.0 mm) | 0.0554 g/mL H2PdCl4 | 0.8 mm HAuCl4 | |||||
| HAuCl4 (0.1 M) | 2.5 mm H2PdCl6 | |||||||||
| Volume of metal salt solutions | 10 mL | 110 µL | Molar ratio Na2PdCl4:PEI = 25, 100, 150 | 80 μL | 0.185 mL | 3 mL | ||||
| 50 μL | ||||||||||
| Reducing agent of the precursor | 0.01 mg/mL NaBH4 | Fivefold molar excess of NaBH4 | Ascorbic acid (0.1 M) | Plasma treatment | NaBH4 (8 × 10−5 mol) | |||||
| Reducing agent volume (mL) | 10 | - | 0.28 | - | 3 | |||||
| Particle size (nm) | 4.0 ± 0.5 | 3.0 ± 0.5 | 4.0 ± 0.5 | 3.0 ± 0.5 | 3.0 ± 0.5 | 3.64~6.20 | (66 ± 22) × (13 ± 1) | 2.6 | 3.03 | |
| Catalyst information | ||||||||||
| Catalyst volume | 50 µL | 50 µL | 1 mL | 1 mL | 90 µL | 25 μL | 100 µL | - | - | |
| Catalyst mass (mg) | 1.1 × 10−3 | 1.1 × 10−3 | 2.1 × 10−2 | 2.1 × 10−2 | 1.9 × 10−3 | * 6 × 10−3 | * 2.7 × 10−8 | - | - | |
| Catalytic test conditions | ||||||||||
| System | Batch- reactor | Hybrid | Batch reactor | Hybrid | Micro reactor | Quartz cuvette | - | Batch | - | |
| Temperature | 20 °C | RT | - | - | 29.9 °C | |||||
| Time | 34 min | 22 min | 140 s | 45 s | 5 s | 10 min | 60 s | - | ||
| Reagent concentrations and volumes | ||||||||||
| [4-NP] (mm) | 0.5 | 0.6 | 0.1 | 0.03 | 2 | |||||
| V4-NP (mL) | 1.5 | 1.5 | 0.25 | 0.25 | 0.0225 | 0.25 | 0.2 | 3 | ||
| [NaBH4] (M) | 0.04 | 0.5 | 0.1 | 0.25 | 0.25 | |||||
| VNaBH4 (mL) | 0.75 | 0.75 | 0.125 | 0.125 | 0.011 | 1 | 1.0 | - | 20 | |
| VWater (mL) | 3.75 | 3.75 | 0.625 | 0.625 | 0.056 | 1 | - | - | - | |
| Catalytic activity | ||||||||||
| k (min−) | 0.085 | 0.172 | 3.48 | 5.7 | 64.56 | 0.28 | 0.5 | 3.47 | 10.5 | - |
| Conversion (%) | 98.62 | 99.35 | - | - | 100 | 90 | - | - | - | - |
| Ref. | ||||||||||
| References | Herein | [20] | [21] | [22] | [23] | |||||
| Year | 2025 | 2019 | 2024 | 2025 | 2022 | |||||
| Process | Flow Rate of the Stream Containing Pd(II) Ions | Flow Rate of the Stream Containing NaBH4 | Diameter mm | Length cm | Flow Rate of the Stream Containing 4-Nitrophenolate * + PdNPs | Contact Time s | System |
|---|---|---|---|---|---|---|---|
| Synthesis of PdNPs | 1.795 mL/min | 1.795 mL/min | 0.8 | 25 | 7.18 mL/min | 2.1 | Hybrid and Microreactor |
| Catalytic tests performance | 1.795 mL/min | 1.795 mL/min | 1.25 | 49 | 5 | Microreactor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhadad, A.I.; Luty-Błocho, M. Safety and Process Intensification of Catalytic Reduction of 4-Nitophenol Using Sodium Borohydride in Flow Microreactor System. Catalysts 2025, 15, 1038. https://doi.org/10.3390/catal15111038
Elhadad AI, Luty-Błocho M. Safety and Process Intensification of Catalytic Reduction of 4-Nitophenol Using Sodium Borohydride in Flow Microreactor System. Catalysts. 2025; 15(11):1038. https://doi.org/10.3390/catal15111038
Chicago/Turabian StyleElhadad, Ahmed Ibrahim, and Magdalena Luty-Błocho. 2025. "Safety and Process Intensification of Catalytic Reduction of 4-Nitophenol Using Sodium Borohydride in Flow Microreactor System" Catalysts 15, no. 11: 1038. https://doi.org/10.3390/catal15111038
APA StyleElhadad, A. I., & Luty-Błocho, M. (2025). Safety and Process Intensification of Catalytic Reduction of 4-Nitophenol Using Sodium Borohydride in Flow Microreactor System. Catalysts, 15(11), 1038. https://doi.org/10.3390/catal15111038








