Reaction Mechanisms of Aqueous Methane Reforming by Continuous Flow Two-Phase Plasma Discharge
Abstract
1. Introduction
2. Results and Discussion
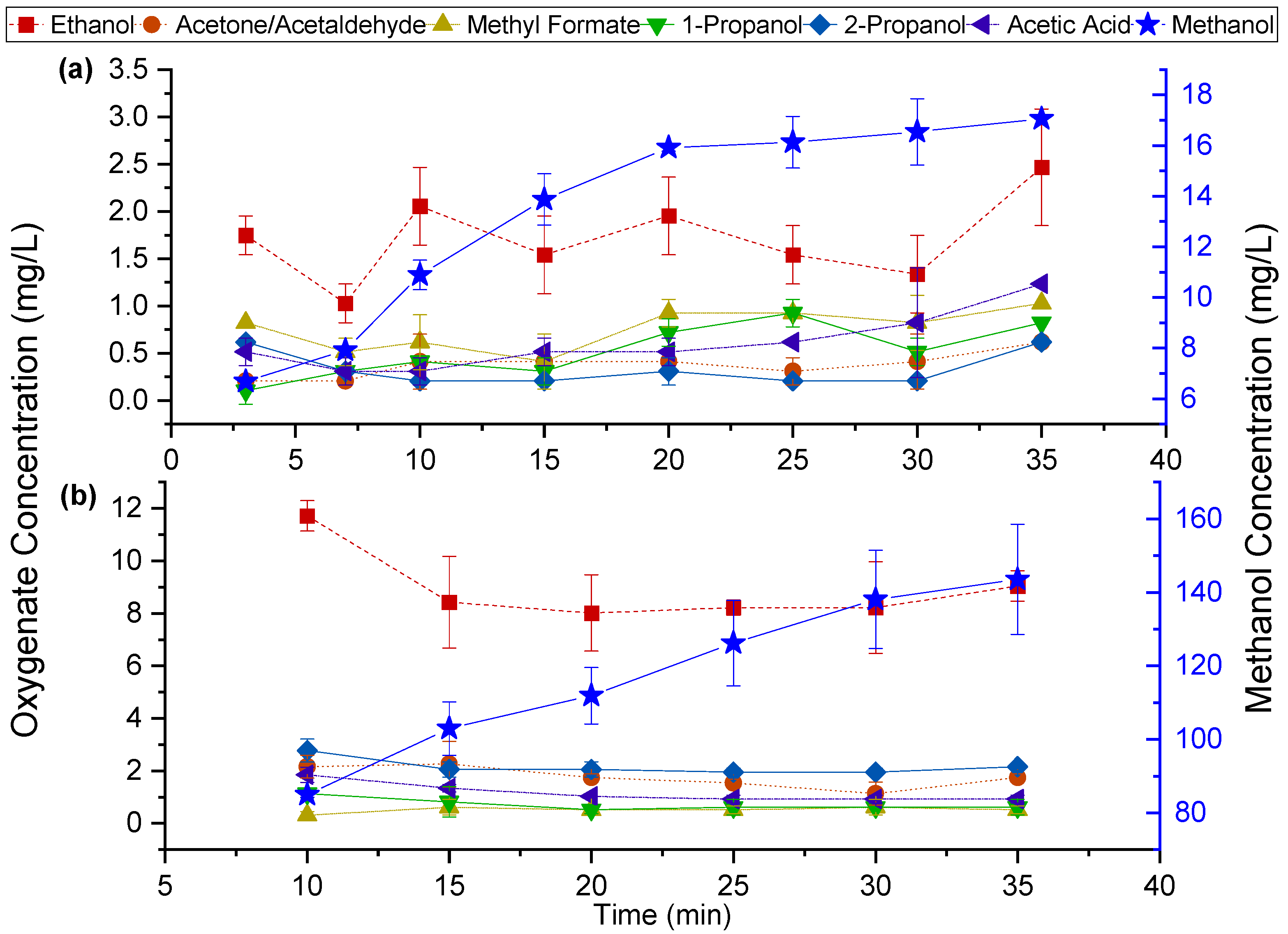
2.1. Characterization of Liquid Products
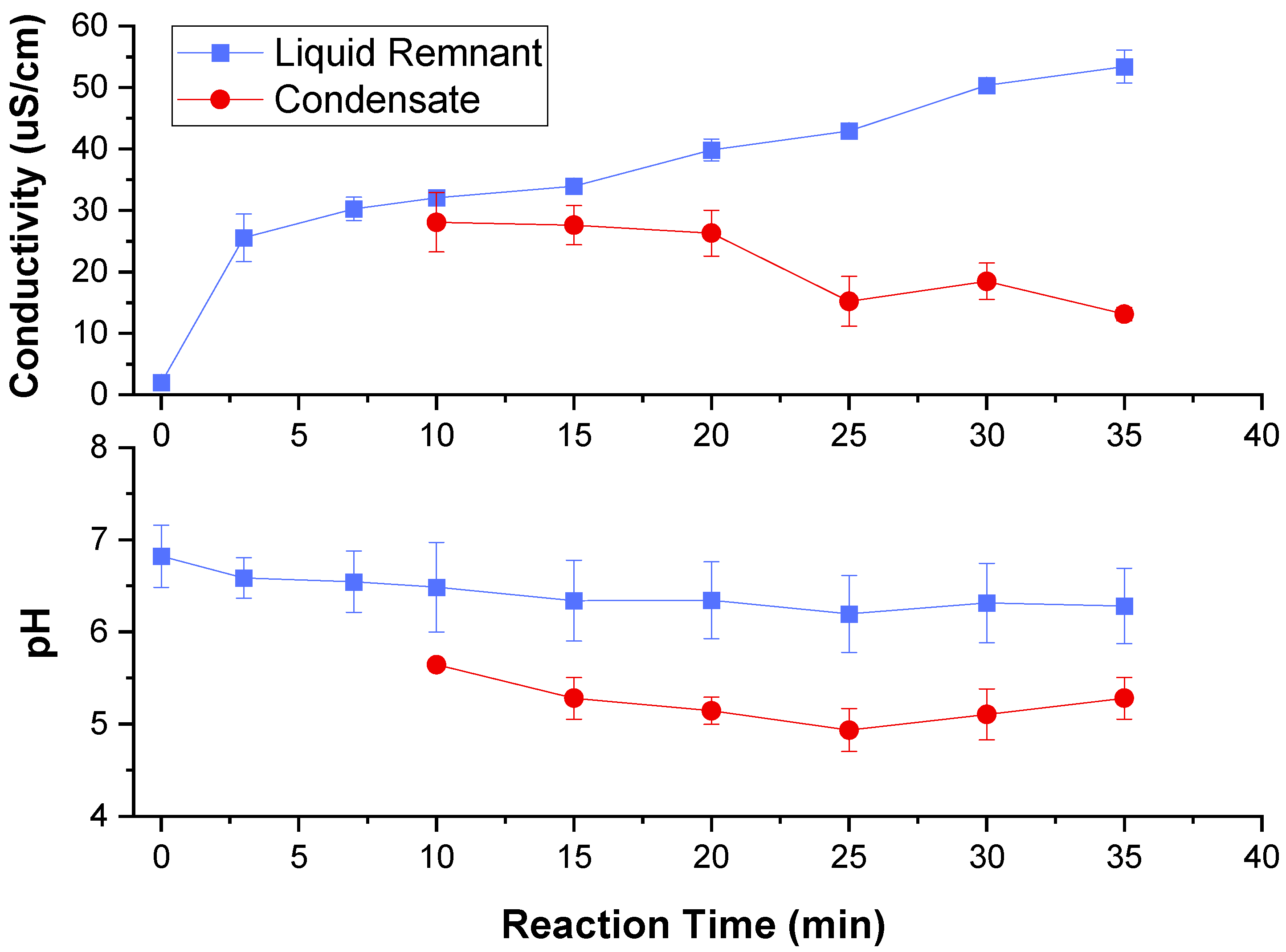
2.2. Conductivity and pH Trends
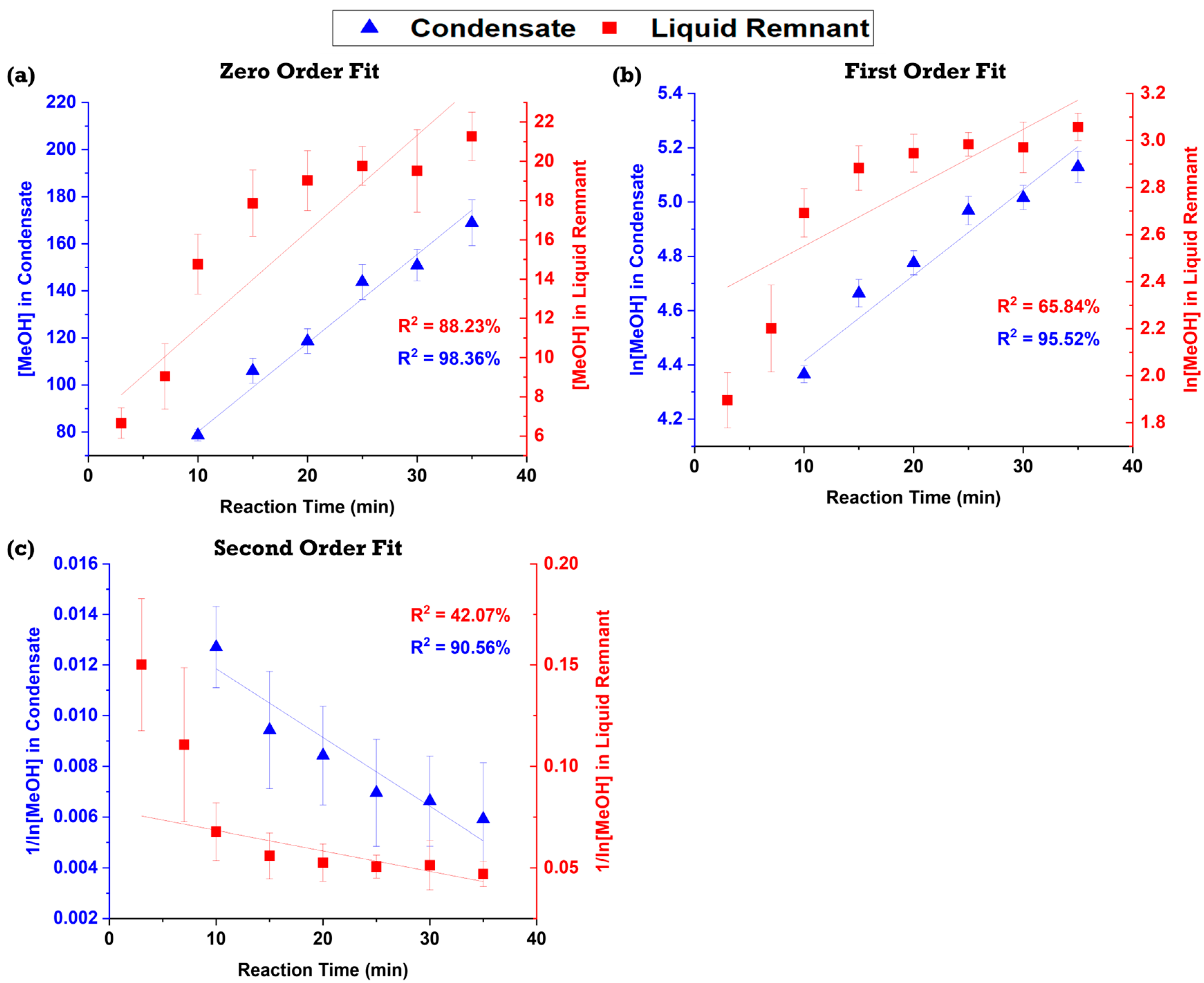
2.3. Methanol Reaction Kinetics
2.4. Characterization of Gas Products
2.5. Identification of Reactive Species in Plasma
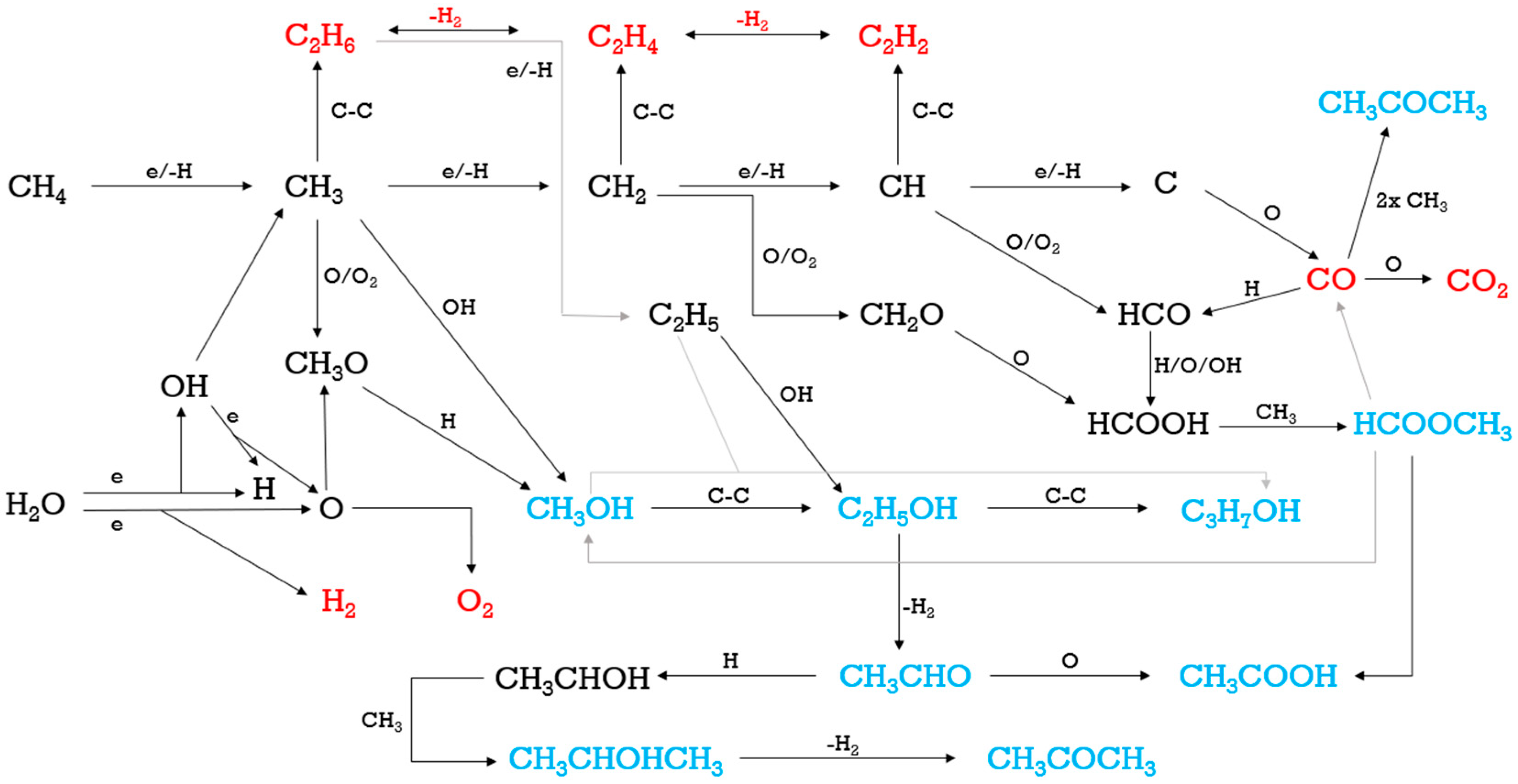
2.6. Reaction Pathways
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup and Operation
3.3. Sample Analysis
3.4. Plasma Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Worku, A.K.; Ayele, D.W.; Deepak, D.B.; Gebreyohannes, A.Y.; Agegnehu, S.D.; Kolhe, M.L. Recent advances and challenges of hydrogen production technologies via renewable energy sources. Adv. Energy Sustain. Res. 2024, 5, 2300273. [Google Scholar] [CrossRef]
- Hantoko, D.; Khan, W.U.; Osman, A.I.; Nasr, M.; Rashwan, A.K.; Gambo, Y.; Al Shoaibi, A.; Chandrasekar, S.; Hossain, M.M. Carbon–neutral hydrogen production by catalytic methane decomposition: A review. Environ. Chem. Lett. 2024, 22, 1623–1663. [Google Scholar] [CrossRef]
- Jia, T.; Wang, W.; Zhang, C.; Zhang, L.; Wang, W. Polydopamine-Mediated Contact-Electro-Catalysis for Efficient Partial Oxidation of Methane. Angew. Chem. Int. Ed. 2025, 64, e202413343. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Tao, F. Activation and catalytic transformation of methane under mild conditions. Chem. Soc. Rev. 2022, 51, 376–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Duan, R.M.; Wei, R.K.; Wang, X.H.; Zhang, G.R.; Duan, H.L.; Jin, W.Q. Hydrogen production in concentrated solar driven membrane reactors—A conception design and numerical analysis. Chem. Eng. J. 2025, 506, 159852. [Google Scholar] [CrossRef]
- Celik, A.; Müller, H.; Lott, P.; Deutschmann, O. Hydrogen Production and Carbon Capture By Pyrolysis of Methane and Biogas: From Reaction Engineering to Techno-Economic Studies. In Proceedings of the 2024 AIChE Annual Meeting, San Diego, CA, USA, 27–31 October 2024. [Google Scholar]
- Olivos-Suarez, A.I.; Szécsényi, À.; Hensen, E.J.; Ruiz-Martinez, J.; Pidko, E.A.; Gascon, J. Strategies for the direct catalytic valorization of methane using heterogeneous catalysis: Challenges and opportunities. Acs Catal. 2016, 6, 2965–2981. [Google Scholar] [CrossRef]
- Kishore, M.A.; Lee, S.; Yoo, J.S. Fundamental Limitation in Electrochemical Methane Oxidation to Alcohol: A Review and Theoretical Perspective on Overcoming It. Adv. Sci. 2023, 10, 2301912. [Google Scholar] [CrossRef]
- De Felice, G. Methane Valorisation Through Non-Thermal Plasma Technologies. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, September 2024. [Google Scholar]
- Das, A.; Peu, S.D. A comprehensive review on recent advancements in thermochemical processes for clean hydrogen production to decarbonize the energy sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Tomohiro, N.; Ken, O. Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Li, X.; Han, L.; Yan, M.; Zhong, Y.; Tu, X. Plasma activation of methane for hydrogen production in a N2 rotating gliding arc warm plasma: A chemical kinetics study. Chem. Eng. J. 2018, 345, 67–78. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Palomino, R.M.; Gutiérrez, R.n.A.; Grinter, D.C.; Vorokhta, M.; Liu, Z.; Ramírez, P.J.; Matolín, V.; Ganduglia-Pirovano, M.V.n.; Senanayake, S.D. Direct conversion of methane to methanol on Ni-Ceria surfaces: Metal–support interactions and water-enabled catalytic conversion by site blocking. J. Am. Chem. Soc. 2018, 140, 7681–7687. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; Van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tang, Y.; Li, X.; Dai, C.; Song, C.; Guo, X.; Ma, X. One-step direct conversion of methane to methanol with water in non-thermal plasma. Commun. Chem. 2022, 5, 124. [Google Scholar] [CrossRef]
- Qiuying, W.; Jiaqi, W.; Tonghui, Z.; Xiaomei, Z.; Bing, S. Characteristics of methane wet reforming driven by microwave plasma in liquid phase for hydrogen production. Int. J. Hydrog. Energy 2021, 46, 34105–34115. [Google Scholar]
- Qi, C.; Bi, Y.; Wang, Y.; Yu, H.; Tian, Y.; Zong, P.; Zhang, Q.; Zhang, H.; Wang, M.; Xing, T. Unveiling the mechanism of plasma-catalyzed oxidation of methane to C2+ oxygenates over Cu/UiO-66-NH2. ACS Catal. 2024, 14, 7707–7716. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Sajjadi, B. Non-thermal plasma enhanced catalytic conversion of methane into value added chemicals and fuels. J. Energy Chem. 2024, 97, 265–301. [Google Scholar] [CrossRef]
- Indarto, A. Partial oxidation of methane to methanol with nitrogen dioxide in dielectric barrier discharge plasma: Experimental and molecular modeling. Plasma Sources Sci. Technol. 2016, 25, 025002. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, C.; Tu, X. Direct conversion of methanol to n-C4H10 and H2 in a dielectric barrier discharge reactor. Green Chem. 2016, 18, 5658–5666. [Google Scholar] [CrossRef]
- Schweer, S.M.; Gawrilow, M.; Nejad, A.; Suhm, M.A. Formic acid–methanol complexation vs. esterification: Elusive pre-reactive species identified by vibrational spectroscopy. Phys. Chem. Chem. Phys. 2023, 25, 29982–29992. [Google Scholar] [CrossRef]
- Indu, B.; Ernst, W.R.; Gelbaum, L.T. Methanol-formic acid esterification equilibrium in sulfuric acid solutions: Influence of sodium salts. Ind. Eng. Chem. Res. 1993, 32, 981–985. [Google Scholar] [CrossRef]
- Laas, J.C.; Garrod, R.T.; Herbst, E.; Weaver, S.L.W. Contributions from grain surface and gas phase chemistry to the formation of methyl formate and its structural isomers. Astrophys. J. 2011, 728, 71. [Google Scholar] [CrossRef]
- Beltramo, G.L.; Shubina, T.E.; Koper, M.T. Oxidation of formic acid and carbon monoxide on gold electrodes studied by surface-enhanced Raman spectroscopy and DFT. ChemPhysChem 2005, 6, 2597–2606. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Ren, Y.; Gu, R.; Chen, Z.-X.; Zeng, G. Mechanistic Insight into Acceptorless Dehydrogenation of Methanol to Syngas Catalyzed by MACHO-Type Ruthenium and Manganese Complexes: A DFT Study. Inorg. Chem. 2023, 62, 19516–19526. [Google Scholar] [CrossRef]
- Webley, P.A.; Tester, J.W. Fundamental kinetics of methane oxidation in supercritical water. Energy Fuels 1991, 5, 411–419. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Roy, K.; van Bokhoven, J.A.; Artiglia, L. Role of water on the structure of palladium for complete oxidation of methane. ACS Catal. 2020, 10, 5783–5792. [Google Scholar] [CrossRef]
- Ollis, D.; Silva, C.G.; Faria, J. Simultaneous photochemical and photocatalyzed liquid phase reactions: Dye decolorization kinetics. Catal. Today 2015, 240, 80–85. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of photocatalyzed reactions: Five lessons learned. Front. Chem. 2018, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.R. Methane. In Encyclopedia of Toxicology, 3rd ed.; Philip, W., Ed.; Academic Press: Oxford, UK, 2014; pp. 235–237. [Google Scholar]
- Yawut, N.; Mekwilai, T.; Vichiansan, N.; Braspaiboon, S.; Leksakul, K.; Boonyawan, D. Cold plasma technology: Transforming food processing for safety and sustainability. J. Agric. Food Res. 2024, 18, 101383. [Google Scholar] [CrossRef]
- Huo, J.; Zhu, B.; Ma, C.; You, L.; Cheung, P.C.-K.; Pedisić, S.; Hileuskaya, K. Effects of chemically reactive species generated in plasma treatment on the physico-chemical properties and biological activities of polysaccharides: An overview. Carbohydr. Polym. 2024, 342, 122361. [Google Scholar] [CrossRef]
- Vasilev, M.; Suiter, J.; Bohl, D.; Thagard, S.M. Caffeine degradation in a plasma-liquid reactor with the lateral liquid flow: Elucidating the effects of mass transport on contaminant removal. Chem. Eng. J. 2023, 473, 144833. [Google Scholar] [CrossRef]
- Vandevelde, T.; Wu, T.-D.; Quaeyhaegens, C.; Vlekken, J.; D’Olieslaeger, M.; Stals, L. Correlation between the OES plasma composition and the diamond film properties during microwave PA-CVD with nitrogen addition. Thin Solid Film. 1999, 340, 159–163. [Google Scholar] [CrossRef]
- Fendrych, F.; Taylor, A.; Peksa, L.; Kratochvilova, I.; Vlcek, J.; Rezacova, V.; Petrak, V.; Kluiber, Z.; Fekete, L.; Liehr, M. Growth and characterization of nanodiamond layers prepared using the plasma-enhanced linear antennas microwave CVD system. J. Phys. D Appl. Phys. 2010, 43, 374018. [Google Scholar] [CrossRef]
- Chingsungnoen, A.; Wilson, J.; Amornkitbamrung, V.; Thomas, C.; Burinprakhon, T. Spatially resolved atomic excitation temperatures in CH4/H2 and C3H8/H2 RF discharges by optical emission spectroscopy. Plasma Sources Sci. Technol. 2007, 16, 434. [Google Scholar] [CrossRef]
- Jun-Feng, Z.; Xin-Chao, B.; Qiang, C.; Fu-Ping, L.; Zhong-Wei, L. Diagnosis of methane plasma generated in an atmospheric pressure DBD micro-jet by optical emission spectroscopy. Chin. Phys. Lett. 2009, 26, 035203. [Google Scholar] [CrossRef]
- Yoo, S.; Seok, D.; Jung, Y.; Lee, K. Hydrophilic surface treatment of carbon powder using CO2 plasma activated gas. Coatings 2021, 11, 925. [Google Scholar] [CrossRef]
- Scurtu, A.; Ticoş, D.; Mitu, M.L.; Diplașu, C.; Udrea, N.; Ticoș, C.M. Splitting CO2 in Intense Pulsed Plasma Jets. Int. J. Mol. Sci. 2023, 24, 6899. [Google Scholar] [CrossRef]
- Bišćan, M.; Kregar, Z.; Krstulović, N.; Milošević, S. Time resolved spectroscopic characterization of aC: H deposition by methane and removal by oxygen inductively coupled RF plasma. Plasma Chem. Plasma Process. 2010, 30, 401–412. [Google Scholar] [CrossRef]
- Rezaei, F.; Abbasi-Firouzjah, M.; Shokri, B. Investigation of antibacterial and wettability behaviours of plasma-modified PMMA films for application in ophthalmology. J. Phys. D Appl. Phys. 2014, 47, 085401. [Google Scholar] [CrossRef]
- Shi, Y.C.; Zhang, Q.J.; Li, J.J.; Chen, G.C. Optical Emission Spectroscopy Measurement of Ar\H2\CH4 RF Plasma for Nano-Crystal Diamond Film Deposition. Adv. Mater. Res. 2014, 1035, 373–378. [Google Scholar] [CrossRef]
- Yanguas-Gil, A.; Focke, K.; Benedikt, J.; Von Keudell, A. Optical and electrical characterization of an atmospheric pressure microplasma jet for Ar/CH4 and Ar/C2H2 mixtures. J. Appl. Phys. 2007, 101, 103307. [Google Scholar] [CrossRef]
- Catherine, Y.; Pastol, A.; Athouel, L.; Fourrier, C. Diagnostics of CH/sub 4/plasmas used for diamond-like carbon deposition. IEEE Trans. Plasma Sci. 1990, 18, 923–929. [Google Scholar] [CrossRef]
- Feng, X.; WANG, Y.-m.; Fan, L.; NIE, X.-y.; ZHU, L.-h. Hydrogen production by the steam reforming and partial oxidation of methane under the dielectric barrierdischarge. J. Fuel Chem. Technol. 2021, 49, 367–373. [Google Scholar] [CrossRef]
- Nozaki, T.; Hattori, A.; Okazaki, K. Partial oxidation of methane using a microscale non-equilibrium plasma reactor. Catal. Today 2004, 98, 607–616. [Google Scholar] [CrossRef]
- Yu, T.; Yi, C.; Gaosheng, R.; Ke, M.; Xiaoxun, M.; Chengyi, D.; Chunshan, S. One-step synthesis of methanol and hydrogen from methane and water using non-thermal plasma and Cu-Mordenite catalyst. Fuel Process. Technol. 2023, 244, 107722. [Google Scholar]
- Chawdhury, P.; Wang, Y.; Ray, D.; Mathieu, S.; Wang, N.; Harding, J.; Bin, F.; Tu, X.; Subrahmanyam, C. A promising plasma-catalytic approach towards single-step methane conversion to oxygenates at room temperature. Appl. Catal. B Environ. 2021, 284, 119735. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.; Zhou, X.; Gong, J.; Yin, Y.; Zheng, H.; Guo, H. Direct oxidation of methane to hydrogen peroxide and organic oxygenates in a double dielectric plasma reactor. ChemSusChem 2011, 4, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Zhang, H.; Zhou, X. Cubic platinum nanoparticles capped with Cs2 [closo-B12H12] as an effective oxidation catalyst for converting methane to ethanol. J. Colloid Interface Sci. 2020, 566, 135–142. [Google Scholar] [CrossRef]
- DeWilde, J.F.; Czopinski, C.J.; Bhan, A. Ethanol dehydration and dehydrogenation on γ-Al2O3: Mechanism of acetaldehyde formation. ACS Catal. 2014, 4, 4425–4433. [Google Scholar] [CrossRef]
- Pargoletti, E.; Rimoldi, L.; Meroni, D.; Cappelletti, G. Photocatalytic removal of gaseous ethanol, acetaldehyde and acetic acid: From a fundamental approach to real cases. Int. Mater. Rev. 2022, 67, 864–897. [Google Scholar] [CrossRef]
- Muggli, D.S.; McCue, J.T.; Falconer, J.L. Mechanism of the photocatalytic oxidation of ethanol on TiO2. J. Catal. 1998, 173, 470–483. [Google Scholar] [CrossRef]
- Jürling-Will, P.; Botz, T.; Franciò, G.; Leitner, W. A “Power-to-X” route to acetic acid via palladium-catalyzed isomerization of methyl formate. ChemSusChem 2022, 15, e202201006. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Kim, Y. Methyl formate as a new building block in C1 chemistry. Appl. Catal. 1990, 57, 1–30. [Google Scholar] [CrossRef]
- Ai, M. The production of methyl formate by the vapor-phase oxidation of methanol. J. Catal. 1982, 77, 279–288. [Google Scholar] [CrossRef]
- Taylor, C.; Noceti, R. New developments in the photocatalytic conversion of methane to methanol. Catal. Today 2000, 55, 259–267. [Google Scholar] [CrossRef]
- Li, S.; Ahmed, R.; Yi, Y.; Bogaerts, A. Methane to Methanol through Heterogeneous Catalysis and Plasma Catalysis. Catalysts 2021, 11, 590. [Google Scholar] [CrossRef]
- Pruett, R.L.; Kacmarcik, R.T. Reactions of formic acid. 1. The iridium-catalyzed synthesis of acetic acid from methyl formate. Organometallics 1982, 1, 1693–1699. [Google Scholar] [CrossRef]
- Ekow, A.-O.; Ahmad, M.; Sarah, W. Plasma-enabled methane upgrading in a gas-liquid reactor: Optimizing a one-step methanol production process. J. Ind. Eng. Chem. 2025, 151, 682–691. [Google Scholar]
- Du, C.; Mills, J.P.; Yohannes, A.G.; Wei, W.; Wang, L.; Lu, S.; Lian, J.-X.; Wang, M.; Guo, T.; Wang, X. Cascade electrocatalysis via AgCu single-atom alloy and Ag nanoparticles in CO2 electroreduction toward multicarbon products. Nat. Commun. 2023, 14, 6142. [Google Scholar] [CrossRef]
| Reaction | Products |
|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agyekum-Oduro, E.; Hossain, M.M.; Mukhtar, A.; Wu, S. Reaction Mechanisms of Aqueous Methane Reforming by Continuous Flow Two-Phase Plasma Discharge. Catalysts 2025, 15, 980. https://doi.org/10.3390/catal15100980
Agyekum-Oduro E, Hossain MM, Mukhtar A, Wu S. Reaction Mechanisms of Aqueous Methane Reforming by Continuous Flow Two-Phase Plasma Discharge. Catalysts. 2025; 15(10):980. https://doi.org/10.3390/catal15100980
Chicago/Turabian StyleAgyekum-Oduro, Ekow, Md. Mokter Hossain, Ahmad Mukhtar, and Sarah Wu. 2025. "Reaction Mechanisms of Aqueous Methane Reforming by Continuous Flow Two-Phase Plasma Discharge" Catalysts 15, no. 10: 980. https://doi.org/10.3390/catal15100980
APA StyleAgyekum-Oduro, E., Hossain, M. M., Mukhtar, A., & Wu, S. (2025). Reaction Mechanisms of Aqueous Methane Reforming by Continuous Flow Two-Phase Plasma Discharge. Catalysts, 15(10), 980. https://doi.org/10.3390/catal15100980








