Abstract
Biodiesel is an alternative to conventional diesel. The use of heterogeneous catalysts in biodiesel production is promising, as it is easier to separate them from the product than homogeneous ones. It was determined that the calcined grape snail (Helix pomatia) shells show good catalytic efficiency in the rapeseed oil transesterification process with methanol. It was determined that the CaO concentration in calcined grape snail (Helix pomatia) shells was 97.74 ± 0.12%. Using the response surface methodology, the biodiesel production process was optimized. The influence of the interaction of independent variables and optimal conditions for the synthesis of rapeseed oil methyl ester was determined: an alcohol-to-oil molar ratio of 10.6:1, a catalyst concentration of 5.7 wt%, and a reaction duration of 7.8 h at a temperature of 64 °C. The physical and chemical properties of the produced biodiesel at optimal process conditions are presented, and their compliance with the requirements of the biodiesel standard is discussed. The produced biodiesel using snail shells, which are food processing waste, meets the requirements of the standard and can be used in diesel engines during the summer period.
1. Introduction
Population growth leads to a high demand for energy and, at the same time, dependence on limited fossil energy resources [1], the use of which pollutes the environment and increases global warming. It is predicted that in 2040, global energy consumption will increase by 56% compared with the 2010 level [2]. In the transport sector, a large share of vehicles are those that use mineral diesel.
Biodiesel is an alternative to conventional diesel. The advantages of biodiesel over diesel are better biodegradability, it has higher combustion efficiency, and less harmful substances are released during combustion, such as aromatic hydrocarbons, etc. [3,4] Another advantage is that biodiesel is miscible with mineral diesel in any ratio, so replacing at least part of mineral diesel with biodiesel reduces environmental pollution and increases energy independence on countries exporting oil or mineral fuels. It would also bring us closer to the Kyoto Protocol (1997) and the Paris Agreement (2015), which encourage the reduction of fossil fuel consumption and aim to implement one of the goals of the European Green Deal—reducing the negative impact on the environment of transport.
Biodiesel is obtained from renewable resources—oils or fats—by transesterifying them with alcohols and using catalysts to accelerate the process. Catalysts are divided into homogeneous and heterogeneous. Homogeneous catalysts such as NaOH or KOH are most often used in industrial biodiesel production thanks to their good catalytic activity and cheapness [4]. However, the disadvantage of homogeneous catalysts is the inevitable waste generated during the washing process, which cannot be reused, and most often during homogeneous reactions, several transesterification cycles are required to obtain high-quality biodiesel [5]. Alkaline catalysts are only suitable for use with low-acid oils, up to 2%, and are therefore not suitable for feedstocks with high free fatty acid contents (e.g., waste cooking oils) [5]. Acidic homogeneous (H2SO4, H2SO3, HCl) catalysts are most often used when the acidity of the oil is higher than 2%, but the acidic nature of these catalysts accelerates equipment corrosion, and they are also used once [6]. Some Fe(III)-based catalysts have been reported in the literature to be highly active at very low loadings (ppm range), enabling high biodiesel yields even with feedstocks rich in free fatty acids such as waste cooking oils. These catalysts also avoid problems of equipment corrosion typical of Brønsted acids and usually remain in residual concentrations below tolerated limits, eliminating the need for separation [7].
Multiple uses, better reaction rate and selectivity, easier catalyst separation from the final product and low cost are the advantages of heterogeneous catalysts. Due to these factors, more and more attention is paid to the search for solid-phase catalysts. Immobilized lipases are environmentally friendly materials that can be used in biodiesel synthesis, but their disadvantages are that lipases work most efficiently at temperatures of 40–45 °C, and at higher temperatures, denaturation processes begin. The transesterification process is most efficient when the temperature is close to the boiling point of alcohol [8,9]. What is more, transesterification is more efficient when excess alcohol is used, which can inactivate lipases [10]. As heterogeneous catalysts, alkali oxides, alkaline earth metal oxides are most often used. One of the oxides—CaO—is poorly soluble in methanol, easily available and cheap, and is also stable for a long time when used in biodiesel production [11]. It is promising to extract CaO from natural waste sources, which are large amounts of calcium carbonate [12]. There are many food production wastes, most of which consist of calcium carbonate. In biodiesel synthesis, heterogeneous catalysts can be oyster shells [13], crab shells [14], mussel shells [15], shrimp shells [16] or eggshells [17,18].
Snails belong to the classes Gastropoda and Phylum Mollusca. Every year, more and more snails are consumed worldwide. In 2014, the market was valued at approximately USD 12 billion, and the total consumption reached about 450,000 tons [19]. The value of the Lithuanian snail market in 2023 reached USD 3.7 million [20]. In Lithuania, grape snails grow in nature; they are collected, sold, and consumed. The development of the snail market is driven by the growing consumer interest in gourmet food, the recognition of the nutritional benefits of snails, and the increasing attention to sustainable food sources. After snails are consumed, the shells remain waste; therefore, research related to their use is relevant.
The aim of this study was to optimize the transesterification process of rapeseed oil with methanol using response surface methodology (RSM) and calcined grape snail (Helix pomatia) shells as a heterogeneous catalyst. In order to assess the influence of three independent variables on the biodiesel yield, multiple regression and correlation analysis were applied. The interaction effect of three independent variables—alcohol-to-oil molar ratio, concentration of the catalyst, and process duration—on the ester yield was evaluated. Furthermore, the optimal conditions were determined under which the maximum ester yield meeting the requirements of the standard was obtained.
2. Results and Discussions
2.1. Concentration of Calcium Oxide in Snail Shells
It was found that the CaO concentration in uncalcined snail shells (Helix pomatia) was 47.14 ± 0.22%, while after calcination at 850 °C for 5 h, it reached 97.74 ± 0.12%. A slightly lower CaO content was obtained in snail shells of Helix Aspersa Maxima calcined under the same conditions (91.69 ± 0.43%) [11]. This proves that snail shells calcined under the same conditions produce different amounts of calcium oxide, which may be influenced not only by the type of snail but also by the duration of growth. In snail farms, snails grow within 9–12 months, while in nature, under natural conditions, on average, within 3 years, and in natural snail shells, a higher amount of calcium oxide is found. Similar results were obtained by Laskar et al., where snail shells (Pila spp.) were dried in an oven at 100 °C for 12 h before calcination (4 h at a temperature of 900 °C), and CaO content reached 98.017% [21]. Mohammed et al. obtained the optimal conditions for the preparation of snail shells at 900 °C and 3.5 h [22]. Trisupakitti et al. studied golden apple cherry snail shells (Pomacea canaliculata). They calcined crushed shells at 1050 °C for 2 h and determined 98.6% of CaO [23].
A lower CaO content of 70.113% was obtained by heating river snail shells at 800 °C for 4 h [24]. It is believed that the lower concentration of CaO was obtained because the calcination temperature was lower. However, Phewphong et al. investigated shells of golden apple snails (Pomacea canaliculata) and used a calcination temperature of 800 °C. It was found that the CaO concentration reaches 100% in treated snail shells with acid before calcination and 95.05% of CaO in untreated snail shells [25]. Trisupakitti et al. investigated golden apple cherry snail shells and obtained CaO of 99.5%. However, before calcination deproteination was done, ground shell was agitated with 4% w/v NaOH at 60 °C for 1 h and then allowed to precipitate, and the filtrate was washed with distilled water until the pH of the wash water was neutral, dried in an oven at 100 °C for 1 h, decolorized by boiling in acetone for 1 h, filtered and washed with methanol, and dried at 100 °C for 1 h [23]. Phewphong et al. [25] and Trisupakitti et al.’s [23] studies prove that treatment of snail shells before calcination leads to a 1–5% higher CaO concentration; nevertheless, additional reagents and energy costs are used during the treatment stage.
2.2. Optimal Reaction Conditions Modeling and Determination Using Response Surface Methodology
It is known that the main parameters determining the efficiency of biodiesel production are the molar ratio of alcohol to oil (A; mol/mol), catalyst concentration (B; wt%), and reaction duration (C; h). In order to evaluate the interaction effects (the total effect of these factors), experiments were carried out by varying the physical parameters according to the experimental design (Table 5). Multiple regression analysis applied to the data in Table 1 allowed the experimental results obtained according to the full factorial central composition design to be approximated by a second-order polynomial equation. The resulting regression model describing the synthesis of rapeseed methyl ester is presented in Equation (1), where the response variable (Y, ester yield; %) is expressed as the sum of the products of the independent variables and the regression coefficients.
where
Y = −182.02 + 13.90A + 19B + 27.40C − 0.32AB + 0.46AC + 0.26BC − 0.62A2 − 1.17B2 − 1.81C2
- Y—ester yield, %;
- A—alcohol-to-oil molar ratio, mol/mol;
- B—catalyst loading, wt%;
- C—reaction duration, h.

Table 1.
Result of experimental design matrix for ester yield.
Table 1.
Result of experimental design matrix for ester yield.
| Source of Variation | Sum of Squares | Degrees of Freedom (df) | Mean Squares | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 14,527.03 | 9 | 1614.11 | 273.49 | <0.0001 | Significant |
| A-molar ratio | 1899.94 | 1 | 1899.94 | 321.92 | <0.0001 | |
| B-catalyst | 143.28 | 1 | 143.28 | 24.28 | 0.0017 | |
| C-Duration | 7786.11 | 1 | 7786.11 | 1319.26 | <0.0001 | |
| AB | 184.22 | 1 | 184.22 | 31.21 | 0.0008 | |
| AC | 383.23 | 1 | 383.23 | 64.93 | <0.0001 | |
| BC | 43.11 | 1 | 43.11 | 7.30 | 0.0305 | |
| A2 | 1742.11 | 1 | 1742.11 | 295.18 | <0.0001 | |
| B2 | 814.71 | 1 | 814.71 | 138.04 | <0.0001 | |
| C2 | 1927.91 | 1 | 1927.91 | 326.66 | <0.0001 | |
| Residual | 41.31 | 7 | 5.90 | |||
| Lack of Fit | 40.43 | 5 | 8.09 | 18.25 | 0.0528 | Not significant |
| Pure Error | 0.8861 | 2 | 0.4430 | |||
| Cor Total | 14,568.34 | 16 | ||||
| C.V.% = 3.64 | R2 = 0.9972 | Adeq Precision = 44.764 | ||||
| R2Adj = 0.9935 | R2Pred = 0.9781 | |||||
Positive (+) coefficients indicate a positive correlation with the response variable, and negative (−) coefficients indicate a negative correlation.
To assess the variance (ANOVA) and to check the adequacy of the empirical model, a statistical analysis of the model was performed. Table 6 summarizes the ANOVA results obtained after applying the second-order response surface model using the mean squares method. The statistical significance of each coefficient was determined based on the p-values (probabilities, Prob > F), which also indicate the strength of the interaction of each parameter. Based on the data in Table 2, the p-value of the model is less than 0.0001, which indicates a high statistical significance of the model for predicting response values and the adequacy of the derived model. The probability that such a high F-value of the model would be random (due to noise or natural variability) is only 0.01%. The high F-value of the model (F = 273.49) and the correspondingly low p-value (p < 0.0001) confirm the high statistical significance of the constructed model. The “Lack of Fit” test assesses whether the selected model adequately describes the relationship between the independent variables and the response variable, or whether there is a systematic bias that the model misses. If the model imprecision is statistically significant (small p-value), this would indicate that the model is inappropriate and does not describe the data well enough, possibly because the model was too simple or important factors were omitted. An assessment of the discrepancy between the residual errors and the net error (F-value 18.25) showed that the model imprecision is not statistically significant (p = 0.0528 > 0.05). This result is desirable as it confirms that the selected second-order polynomial model is appropriate and adequately describes the experimental data, without significant systematic bias. The statistical significance of all model coefficients was determined by p-values and is presented in Table 1.

Table 2.
Optimum parameters for rapeseed oil methyl ester production, predicted and experimental ester yield.
A higher F-value and a lower p-value indicate that the corresponding parameters are significant. p-Values “p > F” less than 0.05 mean that the model components are significant. In this model, A, B, C, AB, AC, BC, A2, B2, and C2 are statistically significant model components. A low coefficient of variation (CV) value (3.64%) indicates minimal data dispersion around the mean value, which indicates high experimental precision and model reliability. The coefficient of determination (R2) defines the proportion of the variance of the response variable that is explained by the regression model. The range of R2 values is from 0 to 1, where a value closer to 1 reflects a better fit of the model to the empirical data. Ideally, R2 = 1 would mean a complete explanation of the response variation by the model, while R2 = 0 would mean complete failure of the model to explain the variation. The resulting R2 value (0.9972) is extremely high, and indicates that the model explains 99.72% of the variation in the response variable, indicating an excellent fit of the model to the experimental data (Figure 1). The adjusted coefficient of determination (R2Adj) is a modification of R2 that adjusts the indicator for the number of independent variables in the model. Because R2 tends to increase artificially with an increasing number of variables, even if the newly added variables are not statistically significant, R2Adj introduces a correction factor that re-analyzes the addition of unnecessary variables. For this reason, R2Adj is considered a more reliable indicator of model fit, especially in models with a larger number of variables. For a good model, the value of R2Adj should be close to the value of R2. The obtained R2Adj value (0.9935) is extremely high and close to R2 (0.9972), which confirms the model fit and indicates that the model is not overfit with insignificant variables. A high R2Adj value reinforces the conclusion that the model is well-fitting, even considering the complexity of the model. The predicted coefficient of determination (R2Pred) assesses the model’s ability to predict response values for new, independent data. It is desirable that the R2Pred value be positive and close to the R2 and R2Adj values. The obtained R2Pred value (0.9781) is high and close to R2 and R2Adj, which indicates that the model has good predictive properties and can accurately predict response values for new data. The Adeq Precision value, reaching 44.764, shows a high signal-to-noise ratio, indicating that the model is statistically significant and reliable. This indicator allows us to conclude that the model is suitable for predicting response values and can be effectively used for optimizing reaction conditions. The high Adeq Precision value confirms the validity of the model and its value for further research.
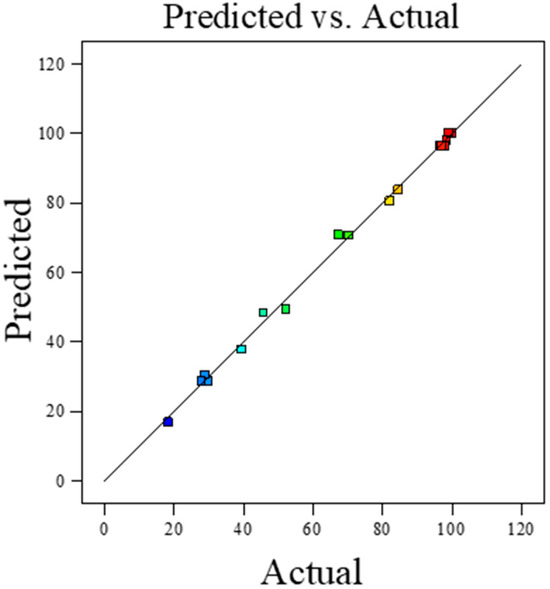
Figure 1.
Comparison of actual and predicted ester yield data.
Figure 1 shows the accuracy of the predictive model, assessed by comparing the experimentally determined and model-predicted values of ester yield. The visual graphical analysis presented shows the good fit and high predictive power of the regression model. The arrangement of the data points, close to the line of perfect fit, confirms the excellent fit of the model to the experimental data and the accuracy of the predicted values.
2.3. Effect of the Interaction of Independent Variables on the Effectiveness of Transesterification
Based on the results of the primary analysis, three-dimensional (3D) contour plots were constructed to visualize and identify the optimal conditions for the ester yield, as illustrated in Figure 2A–C. Each plot analyzes the dependencies between the process response (ester yield (%)) and the independent variables—catalyst concentration, alcohol-to-oil molar ratio, and reaction duration. Contour plots are constructed by fixing one independent variable at a stationary point and varying the remaining two independent variables on the X and Y axes to visually identify the conditions under which the response value is maximum. Figure 2 depicts the response surfaces for the ester yield, reflecting Figure 2A, the interaction between alcohol-to-oil molar ratio and catalyst concentration; Figure 2B shows the interaction between alcohol-to-oil molar ratio and the reaction duration; Figure 2C shows the interaction between the catalyst loading and the reaction duration.
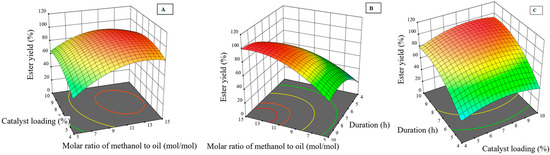
Figure 2.
Dependence of the ester yield on two independent variables: (A) alcohol-to-oil molar ratio and catalyst loading; (B) alcohol-to-oil molar ratio and reaction duration; (C) catalyst loading and reaction duration.
The analysis of Figure 2A, where the reaction duration is fixed at 6.94 h, shows the interaction effect of the alcohol-to-oil molar ratio and catalyst loading on the ester yield. The graph shows that the ester yield increases with increasing both the alcohol-to-oil molar ratio and the catalyst loading. The contour plots of the surface response methodology provide a detailed visual analysis of the efficiency of the snail shells CaO catalyst. The snail shells CaO catalyst acts as a solid material, and the catalytic reaction occurs on its surface. A higher catalyst loading in the reaction mixture means that a larger amount of solid catalyst particles is introduced into the system. These particles obtained after calcination are characterized by a porous structure and a large specific surface area [11]. The active catalytic sites responsible for the transesterification reaction (most often basic CaO surface sites, such as O2− ions) are located precisely on this surface. Therefore, increasing the catalyst loading proportionally increases the total surface area of the catalyst in the reaction mixture, and with it the total number of available active sites on which the reacting oil and alcohol molecules can adsorb and the transesterification reaction takes place. As a result, the reaction rate accelerates, and a larger portion of the oil is converted to ester during the same reaction time. A higher alcohol-to-oil molar ratio pushes the reaction equilibrium in the direction of ester formation according to Le Chatelier’s principle, also increasing the probability of collision of the reactants with the catalyst [12]. The highest yield, exceeding 98%, is achieved at the highest tested alcohol amount and catalyst loading, confirming the synergistic effect of these parameters, and the shape of the contour lines in the graph highlights this interaction, consistent with trends reported in the scientific literature for heterogeneous catalysis. For example, Trisupakitti et al. [23] obtained a biodiesel yield of 95.2% with a golden apple snail shell catalyst using a 0.8 wt% catalyst loading and a 12:1 methanol-to-oil molar ratio, while Laskar et al. [21] reached a 98% ester yield with a Pila spp. snail shell catalyst using a 3 wt% catalyst loading and a 6:1 methanol-to-oil molar ratio, highlighting the potential of snail shells as a promising catalyst source. These quantitative values show a direct correlation between catalyst loading, alcohol-to-oil molar ratio, and ester yield, emphasizing the critical role of catalyst loading for an efficient transesterification process.
The effect of the alcohol-to-oil molar ratio and the reaction duration on the ester yield, where the catalyst loading is fixed at 4.24%, is given in Figure 2B. It can be seen that the ester yield increases in both cases, with increasing the alcohol-to-oil molar ratio and the reaction duration. The effect of reaction duration is particularly important in heterogeneous catalysis, where the reaction takes place on the catalyst surface. A longer reaction duration provides more time for molecules of the oil and alcohol to diffuse into the catalyst pores, adsorb on the active sites, and react, especially when the catalyst concentration is relatively low [14]. A higher alcohol-to-oil molar ratio, as in Figure 2A, further shifts the reaction equilibrium towards the formation of esters. The highest ester yield, exceeding 99%, is again achieved at the highest tested reaction duration values, emphasizing that the longer reaction duration compensates for the lower catalyst concentration, allowing for obtaining high ester yield. The shape of the contour lines and the localization of the highest ester yield in the graph emphasize the synergistic effect of the parameters and are consistent with the trends described in the scientific literature for heterogeneous catalysis in biodiesel production. Kaewdaeng et al. [24] achieved 92.5% oil conversion to ester after a 1 h reaction with a river snail shell catalyst, while Birla et al. [26] obtained the optimal reaction duration of 7 h, achieving 99.58% conversion with a snail shell catalyst. It can be noticed that the reaction duration is one of the essential parameters to achieve a high ester yield, especially when using heterogeneous catalysts.
The effect of catalyst loading and reaction duration, when the alcohol-to-oil molar ratio is fixed at 8.4 mol/mol, on the ester yield is presented in Figure 2C. It can be seen that the ester yield increases with increasing both catalyst loading and reaction duration, and optimal conditions are achieved by combining these parameters. When the alcohol content is fixed, catalyst loading and reaction duration become the main factors determining the ester yield. Higher catalyst loading increases the number of active sites, while longer reaction duration provides sufficient time for the reaction to proceed to completion, especially when the alcohol content is limited [11]. The highest ester yield, exceeding 98%, is again achieved at the highest tested catalyst loading, close to 9–10 wt%, and reaction duration reaching 9–10 h, confirming the synergistic effect of these parameters. For example, Kaewdaeng et al. investigated the use of a river snail shell catalyst for the synthesis of fatty acid methyl ester [24]. The optimal catalyst loading of 3 wt% and a reaction time of 1 h allowed the achievement of 92.5% of fatty acid methyl ester yield, and subsequent optimization tests showed that the ester yield can reach up to 98.19%. Karkal et al. (2023) optimized the process with a crab shell as a heterogeneous catalyst, achieving a biodiesel yield of 88.56 wt% with a catalyst loading of 3 wt% and a reaction duration of 60 min, demonstrating efficiency even in short reaction duration [14]. Alsabi et al. (2024) achieved an extremely high, 99.36%, fatty acid methyl ester yield with a mussel shell as a catalyst, optimizing the methanol-to-oil molar ratio to 18:1, a catalyst loading of 6 wt%, and a reaction duration of 6 h [15]. Laskar et al. (2018) obtained a biodiesel yield of 98% while using snail shells as a catalyst (3 wt%); the reaction took 7 h, and the methanol-to-oil molar ratio was 6:1 [21].
2.4. Optimization of Rapeseed Oil Methyl Ester Synthesis Process
The influence of three independent variables on the rapeseed oil methyl ester yield was evaluated, and the process was optimized. The optimal conditions were determined (process temperature is 64 °C): the methanol-to-oil molar ratio is 10.6:1, snail shells loading is 5.7 wt%, and reaction duration is 7.8 h. The predicted ester yield was 99.81 wt%, and the experimentally determined ester content was slightly lower at 98.80 ± 0.30 wt%, while the obtained experimental ester content was 97.15 ± 0.25 wt%; however, it meets the requirements of the standard EN 14214 [27]. The data are presented in Table 2.
Table 3 presents comparative results of studies by scientists who analyzed the process of transesterification of oil with methanol using snail shells as a catalyst. Different oils were used. Rapeseed oil was used in this research and Gaide et al.’s [11] study; soybean oil was used by Laskar et al. [21], Das et al. [28], and Ouafi et al. [29]. Transesterification of palm oil was studied by Viriya-empikul et al. [30], Phewphong et al. [25], and Birla et al. [26]. Kaewdaeng et al. [24] and Mohammed et al. [22] produced fatty acid methyl ester from waste frying/cooking oil. The obtained ester yield ranged from 85.5 to 98.15 wt%. The lowest ester yield was 85.5 wt%, 87.28 wt%, and 87.5 wt% for the transesterification of waste cooking oil [25], waste frying oil [26], and palm oil [21], respectively. The lower ester yield is probably due to the lower quality of the oil used for frying.

Table 3.
Comparison of optimum conditions for biodiesel production when different snail shells are used as heterogeneous catalysts.
Many researchers have performed the synthesis of methyl esters at 60–65 °C; in this research, 64 °C was used. It is believed that temperatures close to the boiling point of methanol (64.7 °C) were chosen, as it is known that transesterification reactions are most efficient under temperatures close to alcohols boiling. Only Laskar et al. synthesized biodiesel at a low temperature of 28 °C and obtained an ester yield of 98 wt% [21].
For the transesterification reaction to proceed, a minimum methanol-to-oil molar ratio of 3:1 is required; all researchers used an excess of methanol-to-oil molar ratio from 21:5 (4.2:1) [22] to 12:1 [23,25,29,30]. In this study, the optimal methanol-to-oil molar ratio was found to be 10.6:1.
Very different data were obtained by other researchers when determining the optimal amount of catalyst and process duration. Trisupakitti et al. (2018) obtained 92.5 wt% of ester yield in 6 h, while using 0.8 wt% of golden apple snail shells [23]. While the same snails were used by Viriya-empikul et al. [30], however, the optimal catalyst amount was 10 wt%, although the process duration was 2 h, and a similar ester yield of 93.2 wt% was obtained. In this study, the optimum reaction duration was 7.8 h and 5.7 wt% of Helix pomatia snail shells.
Although in this study optimized process conditions were similar to the optimal conditions described in the literature, significantly higher ester yield (98.80 wt%) was achieved, and ester content (97.15 wt%), exceeding the requirements of the EN 14214 standard.
2.5. Physical and Chemical Properties of Obtained Rapeseed Oil Methyl Ester
Biofuels can be used in the transport sector if they meet the requirements of the standard EN 14214 [27]. The compliance of physical and chemical properties of the produced methyl ester and their comparison with the requirements of the standard are presented in Table 4.

Table 4.
The physical and chemical properties of rapeseed oil methyl ester.
The ester content shows how many fatty acid methyl esters were formed from triglycerides. The ester content of the obtained biodiesel 97.15 ± 0.25 wt% meets the requirements of the standard (not less than 96.5%). The density of biodiesel is higher (885 ± 2.00 kgm−3) than that of mineral diesel and meets the requirements of the standard (860–900 kgm−3) (at 15 °C). Viscosity affects the fuel supply and combustion process. It should be 3.5–5.0 mm2s−1 (at 40 °C). The obtained value is 4.70 ± 0.10 mm2s−1. The maximum acid value in the European standard 0.5 mg KOHg−1. If biodiesel contains a higher number of acids, engine corrosion and sediment formation may occur; the resulting RME acid value is 0.22 ± 0.05 mg KOHg−1. Water in biodiesel can cause microbiological processes, during which the sludge formed can clog filters. Moisture content must be a maximum of 500 mgkg−1. In this study, the RME moisture content obtained is 305 ± 2.10 mgkg−1.
Iodine value and linolenic acid methyl ester content depend on the fatty acid composition of the oils or fats used. It is determined that if biodiesel consists of a large amount of mono- and poly-unsaturated acids, it polymerizes when heated and sediment is formed. The iodine value should be a maximum of 120 g J2100−1g−1, and the obtained value is 15 ± 0.20 g J2100−1g−1. The linolenic acid methyl ester content should be a maximum of 12%, and the obtained value is 8.86 ± 0.10%. Monoglyceride, diglyceride, triglyceride, free and total glycerol content should be no less than 0.8%, 0.2%, 0.2%, 0.02%, and 0.25%, respectively, and the obtained values are 0.27 ± 0.03%, 0.05 ± 0.02%, 0.06 ± 0.01%, 0.007%, and 0.19 ± 0.10%, respectively. If these indicators are exceeded, sediment may form, and they also directly affect the increase in viscosity. Excess methanol is used for transesterification, so its removal is a very important stage of the biodiesel purification process. Methanol content should not exceed 0.2%, and in the resulting biodiesel, it is 0.10 ± 0.05%. Another indicator that depends on the oil used is the phosphorus content. Phosphorus in esters may remain due to phospholipids contained in the raw material. Phosphorus content is limited to 10 ppm; the determined value is 7.5 ± 0.10 ppm. The content of alkali metals must not exceed 5 mgkg−1, and the obtained is 3.5 ± 0.25 mgkg−1. Because snail shells calcined to CaO were used as catalysts in this study, it is very important that the biodiesel is purified well, and no calcium remains. It is important that the fuel maintains the required properties during storage and transportation, as oxidation processes occur when it comes into contact with oxygen, and the properties of the fuel change. Oxidation stability must be a minimum of 8 h at 110 °C, and was obtained at 8.5 ± 0.15 h. Cetane number determines combustion quality. According to the standard requirements, it is a minimum of 51, and it was obtained at 52 ± 0.20. Low-temperature properties are very important to use the fuel not only in the summer, but also in the cold period. In different countries, depending on climatic conditions, different requirements apply for the cold filter plugging point. In this study, the RME cold filter plugging point obtained is −9.8 ± 0.04 °C. The cold filter plugging point of fuels used in the summer period is a minimum of −5 °C in Lithuania.
The produced biodiesel meets the requirements of the EN 14214 standard and can be used in diesel engines during the summer period. The results obtained substantiate the relevance and novelty of the research in obtaining biodiesel that meets the requirements of the standard, while the works of other scientists who studied the use of different snail shells in the production of biodiesel do not contain detailed studies of the properties of the resulting biodiesel; therefore, it is unclear whether it can be used in the transport sector.
3. Materials and Methods
3.1. Preparation of the Catalyst
Grape snails (Helix pomatia) were collected in Lithuania, Panevezys distr. Snail shells are a natural material consisting mainly of calcium carbonate. When calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide. Snail shells were prepared according to the studies conducted by Gaide and colleagues. Snails were calcined in a muffle furnace (AB UMEGA SNOL 8.2/1100, Utena, Lithuania) for 5 h at 850 °C [31]. Before calcination, snail shells were crushed, sieved through a sieve, and a 0.315–0.1 mm fraction was used for the experiment.
3.2. Determination of Calcium Oxide in Snail Shells
Crushed snail shells were dissolved in aqua regia (a mixture of nitric and hydrochloric acid in a 1:3 volume ratio). A total of 25 mL of the test solution was poured into a conical flask, and 60–70 mL of H2O and 10 mL of KOH (2 mol/L) were added. After mixing well, the indicator of calcium carboxylic acid was added to the flask, mixed and titrated with trilon B. A color change from raspberry to blue was observed. Equation (2) was used for the calculation of the CaO content:
where
- V—volume of trilon B used for calcium titration, mL;
- m—mass of the snail shells sample, g;
- K—trilon B correction factor.
3.3. Oil Transesterification Process
Rapeseed oil purchased from a local market was used for transesterification. It met the requirements for edible oil. The transesterification process was carried out in a laboratory reactor equipped with mixing and heating elements and a reflux condenser.
Before adding methanol (Analytical pure Lach-Ner) and the prepared catalyst, the oil was heated to the temperature required for the reaction, then the required amount of catalyst and alcohol was added. The experiments were performed at 64 °C, i.e., close to the boiling point of methanol, which is most often chosen for research, because increasing the temperature is associated with an increase in the reaction rate, but higher than the boiling point is undesirable due to possible alcohol losses and increased energy consumption.
After the reaction, the resulting mixture was filtered through cellulose paper, washed once with H3PO4 (5%) (10% of the volume of the mixture) and twice with distilled water (10% of the volume of the mixture), after which the aqueous part was separated. The acidic water neutralizes any residual base catalyst. It also helps convert soap (formed from free fatty acids + base) back into free fatty acids, which can then separate out instead of emulsifying with biodiesel. A rotary evaporator was used to separate the excess alcohol and water residues.
3.4. Determination of Ester Content
The content of rapeseed oil methyl ester in the product obtained during the synthesis was analyzed by gas chromatography methods using a Clarus 500 chromatograph (Perkin Elmer) with a flame ionization detector. The content of methyl ester up to 80% was determined according to the methodology given in the standard EN 14105 [32]. The analysis conditions for determining the content of partial (mono-, di-, tri-glyceride) glycerides were as follows: Restek MXT Biodiesel TG capillary column (14 m-0.53 mm-0, and 16 μm); initial thermostat temperature 50 °C, held for 1 min. Then, the temperature was raised by 15 °C/min to 180 °C, then 7 °C/min to 230 °C and 30 °C/min to 370 °C, and held for 5 min; detector temperature—380 °C; carrier gas—hydrogen, the flow rate of which was 4 mL/min. The ester yield (%) was calculated based on the determined amount of partial glycerides [33].
In the case of ester content of more than 80% in a final product, determination was done based on the requirements of the EN 14103 standard [34]. The following conditions were used for the analysis: Alltech AT-FAME capillary column (30 m-0.25 mm-0.25 µm), the initial oven temperature was 210 °C, held for 5 min, then at a rate of 20 °C/min it was raised to 230 °C and held for 12 min; the carrier gas (H2) flow rate was 3 mL/min; the injector and detector temperature was 250 °C.
3.5. Response Surface Analysis: Optimization and Statistical Analysis of the Transesterification Process
In order to optimize the transesterification process and determine the influence of the molar ratio of methanol to rapeseed oil (A), catalyst loading (B), and reaction duration (C) (Table 5) on the ester yield, a 3-factor experiment was performed using the Central composite design (CCD). The CCD experiment consisted of 17 trials (Table 6). The ester yield (%) was used as a response indicator in the model. The obtained data were analyzed by variance (ANOVA) and graphical analysis using Design-Expert 13 (Stat-Ease, Minneapolis, MN, USA) software. The experimental data (Table 6) were analyzed using the response surface regression (RSREG) method of the Statistical Analysis System (SAS). This method is based on a second-order polynomial model (Equation (3)). The RSREG methodology includes canonical analysis to determine the stationary values of each factor. Based on the fitted model, response surface contour plots were constructed for each pair of factors under study, fixing the third factor at its calculated stationary value. To validate the model, an optimization procedure of the reaction conditions was performed using combinations of independent variables that were not included in the original experimental design.

Table 5.
Independent variables used in the Central composite design for the synthesis of rapeseed oil methyl ester.

Table 6.
Central composite design matrix with three independent variables and experimental and predicted results.
The quadratic polynomial regression equation was used to estimate the model parameters and predict the response:
where
- Y—the response (dependent variable);
- Xi and Xj—the independent variables;
- β0, βi, βii bei βj, and βij—constant coefficients.
3.6. Studies of the Physical and Chemical Properties of Biodiesel
The physical and chemical properties of the obtained biodiesel were evaluated based on the requirements of the EN 14214 standard. Linolenic acid methyl ester and ester content were determined by gas chromatography according to the standard test method, EN 14103 [34]. ISO 3675, the standard method, was used for the determination of the density at 15 °C [35]. The standard EN ISO 3104 was used to determine the kinematic viscosity of a biodiesel at 40 °C [36]. The acid value was determined according to ISO 660 [37], and the moisture content according to ISO 665 [38]. The international standard ISO 3961 was used to determine the iodine value [39]. Glyceride contents (glycerol, monoglycerides, diglycerides, and triglycerides) were determined by gas chromatography using a Perkin Elmer Clarus 500 (Boston, MA, USA)(detector—FID) gas chromatograph according to the requirements of the EN 14105 standard. EN 14110 was used for the determination of methanol in rapeseed oil methyl ester [40]. Calcium, magnesium, and phosphorus were determined by ICP-OES (standards EN 14109 [41] and EN 14107 [42]). The oxidation stability at 110 °C of a biodiesel was determined by the Rancimat method (EN 14112) [43]. The cetane number was determined by EN 590 [44]. The cold filter plugging point (CFPP) of biodiesel was determined based on the requirements of EN 116, which vary according to the season to ensure fuel efficiency at low temperatures [45]. CFPP is the lowest temperature at which fuel will clog the filter due to crystallization.
4. Conclusions
Calcium oxide is a suitable catalyst for biodiesel synthesis. CaO concentration was 97.74 ± 0.12% in calcined for 4 h at 850 °C grape snail shells (Helix pomatia). Transesterification studies were performed by varying three independent variables (methanol-to-oil molar ratio, loading of catalyst, and reaction duration) in order to determine their influence on the efficiency of the transesterification process and to select optimal conditions. The studies were performed at a temperature of 64 °C. The optimal conditions for the synthesis of rapeseed oil methyl ester were determined by using the response surface methodology: alcohol-to-oil molar ratio 10.6:1, loading of catalyst 5.7 wt%, and process duration 7.8 h. Under the determined optimal conditions, the ester yield reached 97.15 wt%. The produced biodiesel meets the requirements of the EN 14214 standard and can be used in diesel engines during the summer period.
Author Contributions
Conceptualization, E.S. and V.M.; methodology, E.S. and M.G.; software, G.G. and M.G.; validation, E.S. and V.M.; formal analysis, E.S., M.G. and V.M.; investigation, G.G., I.G., M.G. and K.K.; resources, E.S.; data curation, E.S. and M.G.; writing—original draft preparation, E.S., I.G., M.G. and V.M.; writing—review and editing, E.S. and V.M.; visualization, E.S., G.G. and M.G.; supervision, E.S. and V.M.; project administration, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aghbashlo, M.; Peng, W.; Tabatabaei, M.; Kalogirou, S.A.; Soltanian, S.; Hosseinzadeh-Bandbafha, H.; Mahian, O.; Lam, S.S. Machine learning technology in biodiesel research: A review. Prog. Energy Combust. Sci. 2021, 85, 100904. [Google Scholar] [CrossRef]
- CEEC the Future. Available online: https://www.ceecthefuture.org/resources/world-energy-use-to-rise-by-56-percent-driven-by-growth-in-the-developing-world (accessed on 3 April 2025).
- Gaide, I.; Grigas, A.; Makareviciene, V.; Sendzikiene, E. Life cycle assessment and biodegradability of biodiesel produced using different alcohols and heterogeneous catalysts. Green Chem. Lett. Rev. 2024, 17, 2394503. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. BioEnergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Karčauskiene, D.; Sendžikiene, E.; Makarevičiene, V.; Zaleckas, E.; Repšiene, R.; Ambrazaitiene, D. False flax (Camelina sativa L.) as an alternative source for biodiesel production. Agriculture 2014, 101, 161–168. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Langellotti, V.; Melchiorre, M.; Cucciolito, M.E.; Esposito, R.; Grieco, D.; Pinto, G.; Ruffo, F. Biodiesel from Waste Cooking Oil: Highly Efficient Homogeneous Iron(III) Molecular Catalysts. Catalysts 2023, 13, 1496. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Xia, S.; Lin, J.; Sayanjali, S.; Shen, C.; Cheong, L.Z. Lipase-catalyzed production of biodiesel: A critical review on feedstock, enzyme carrier and process factors. Biofuels Bioprod. Biorefining 2024, 18, 291–309. [Google Scholar] [CrossRef]
- Gumbytė, M.; Makareviciene, V.; Sendzikiene, E. Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol. Materials 2023, 16, 185. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Snail Shells as a Heterogeneous Catalyst for Biodiesel Fuel Production. Processes 2023, 11, 260. [Google Scholar] [CrossRef]
- Mazaheri, H.H.C.; Ong, H.C.; Amini, Z.; Masjuki, H.H.; Mofijur, M.; Su, C.H.; Anjum Badruddin, I.; Khan, T.M.Y. An Overview of Biodiesel Production via Calcium Oxide Based Catalysts: Current State and Perspective. Energies 2021, 14, 3950. [Google Scholar] [CrossRef]
- De la Cruz-De, M.; De la cruz-Burelo, P.; Hernández-Núñez, E.; Santiago-González, R.E.; Valerio-Cárdenas, C.; Villegas-Cornelio, V.M. Using discarded oyster shells to obtain biodiesel. Agro Product. 2022. [Google Scholar] [CrossRef]
- Karkal, S.S.; Rathod, D.R.; Jamadar, A.S.; Shivaramu, M.S.; Kudre, T.G. Production optimization, scale-up, and characterization of biodiesel from marine fishmeal plant oil using Portunus sanguinolentus crab shell derived heterogeneous catalyst. Biocatal. Agric. Biotechnol. 2023, 47, 102571. [Google Scholar] [CrossRef]
- Alsabi, H.A.; Shafi, M.E.; Almasoudi, S.H.; Mufti, F.A.M.; Alowaidi, S.A.; Sharawi, S.E.; Alaswad, A.A. From Waste to Catalyst: Transforming Mussel Shells into a Green Solution for Biodiesel Production from Jatropha curcas Oil. Catalysts 2024, 14, 59. [Google Scholar] [CrossRef]
- Karkal, S.S.; Rathod, D.R.; Jamadar, A.S.; Shivaramu, M.S.; Kudre, T.G. Exploitation of freshwater fish waste as feedstock and Fenneropeneus indicus shrimp shell as catalyst source for biodiesel production. Biofuels 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Gumbytė, M. Rapeseed Oil Transesterification Using 1-Butanol and Eggshell as a Catalyst. Catalysts 2023, 13, 302. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E. Effectiveness of Eggshells as Natural Heterogeneous Catalysts for Transesterification of Rapeseed Oil with Methanol. Catalysts 2022, 12, 246. [Google Scholar] [CrossRef]
- Available online: https://touchstonesnailfranchise.com/snail-market/?utm_source (accessed on 25 August 2025).
- Indexbox Lithuania—Snails (Except Sea Snails)—Market Analysis, Forecast, Size, Trends and Insights. Available online: https://www.indexbox.io/store/lithuania-snails-except-sea-snails-market-analysis-forecast-size-trends-and-insights/?utm_source (accessed on 19 March 2025).
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, S.L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.K.; Alkhafaje, Z.A.; Rashid, I.M. Heterogeneously catalyzed transesterification reaction using waste snail shell for biodiesel production. Heliyon 2023, 9, e17094. [Google Scholar] [CrossRef] [PubMed]
- Trisupakitti, S.; Ketwong, C.; Senajuk, W.; Phukapak, C.; Wiriyaumpaiwong, S. Golden apple cherry snail shell as catalyst for heterogeneous transesterification of biodiesel. Braz. J. Chem. Eng. 2018, 35, 1283–1291. [Google Scholar] [CrossRef]
- Kaewdaeng, S.; Sintuya, P.; Nironsin, R. Biodiesel production using calcium oxide from river snail shell ash as catalyst. Energy Procedia. 2017, 138, 937–942. [Google Scholar]
- Phewphong, S.; Roschat, W.; Moonsin, P.; Promarak, V.; Yoosuk, B. Biodiesel production process catalyzed by acid-treated golden apple snail shells (Pomacea canaliculata)-derived CaO as a high-performance and green catalyst. Eng. Technol. Appl. Sci. Res. 2021, 49, 36–46. [Google Scholar]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef]
- EN 14214; Automotive fuels—Fatty acid methyl esters (FAME) for diesel engines—Requirements and test methods. European Committee for Standardization: Brussels, Belgium, 2019.
- Das, S.; Anal, J.M.H.; Kalita, P.; Saikia, L.; Rokhum, S.L. Process Optimization of Biodiesel Production Using Waste Snail Shell as a Highly Active Nanocatalyst. Int. J. Energy Res. 2023, 2023, 6676844. [Google Scholar] [CrossRef]
- Ouafi, R.; Haldhar, R.; Mehdaoui, I.; Asri, M.; AlObaid, A.A.; Warad, I.; Taleb, M.; Rais, Z.; Kim, S.C. Waste snail shells-derived mixed oxide catalyst for efficient transesterification of vegetable oil: Towards sustainable biodiesel production. Mater. Today Commun. 2024, 39, 109128. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis. Catalysts 2021, 11, 384. [Google Scholar] [CrossRef]
- EN 14105; Fat and oil derivatives—Fatty Acid Methyl Esters (FAME)—Determination of free and total glycerol and mono-, di-, triglyceride contents. European Committee for Standardization: Brussels, Belgium, 2020.
- Bailer, J.; Hödl, P.; de Hueber, K.; Mittelbach, M.; Plank, C.; Schindlbauer, H. Handbook of Analytical Methods for Fatty Acid Methyl Esters Used as Diesel Fuel Substitutes; Fichte-Research Institute for Chemistry and Technology of Petroleum Products, University of Technology: Vienna, Austria, 1994. [Google Scholar]
- EN 14103; Fat and oil derivatives—Fatty Acid Methyl Esters (FAME)—Determination of ester and linolenic acid methyl ester contents. European Committee for Standardization: Brussels, Belgium, 2020.
- ISO 3675; Crude petroleum and liquid petroleum products—Laboratory determination of density—Hydrometer method. The International Organization for Standardization: Genève, Switzerland, 1998.
- ISO 3104; Petroleum products—Transparent and opaque liquids—Determination of kinematic viscosity and calculation of dynamic viscosity. The International Organization for Standardization: Genève, Switzerland, 2023.
- ISO 660; Animal and vegetable fats and oils—Determination of acid value and acidity. The International Organization for Standardization: Genève, Switzerland, 2020.
- ISO 665; Oilseeds—Determination of moisture and volatile matter content. The International Organization for Standardization: Genève, Switzerland, 2020.
- ISO 3961; Animal and vegetable fats and oils—Determination of iodine value. The International Organization for Standardization: Genève, Switzerland, 2024.
- EN 14110; Fat and oil derivatives—Fatty Acid Methyl Esters—Determination of methanol content. European Committee for Standardization: Brussels, Belgium, 2019.
- EN 14109; Fat and oil derivatives—Fatty Acid Methyl Esters (FAME)—Determination of potassium content by atomic absorption spectrometry. European Committee for Standardization: Brussels, Belgium, 2003.
- EN 14107; Fat and oil derivatives. Fatty acid methyl esters (FAME)—Determination of phosphorous content by inductively coupled plasma (ICP) emission spectrometry. European Committee for Standardization: Brussels, Belgium, 2003.
- EN 14112; Fat and oil derivatives—Fatty Acid Methyl Esters (FAME)—Determination of oxidation stability. European Committee for Standardization: Brussels, Belgium, 2020.
- EN 590; Automotive fuels—Diesel -Requirements and test methods. European Committee for Standardization: London, UK, 2025.
- EN 116; Diesel and domestic heating fuels—Determination of cold filter plugging point—Stepwise cooling bath method. European Committee for Standardization: Brussels, Belgium, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).