Abstract
Ticks transmit diseases and harm animals worldwide, and their control primarily relies on pesticides. Resistance to these pesticides has developed consistently over centuries. Arginine Kinase (AK, EC 2.7.3.3) is a conserved, ancestral enzyme that provides reserve energy in emergency situations and a viable target for novel antiparasitic drugs. Our aim was to evaluate six carbamoyl carboxylic acid analogues (CCAs) as potential lead compounds by investigating their interaction with the active site of Rhipicephalus sanguineus AK (RsAK) using a structural modeling approach. The binding was characterized using fluorescence quenching (Stern–Volmer analysis) and molecular dynamics simulations. The simulations, performed with GROMACS using the CHARMM 26 force field over 100 ns, provided atomic-level insight into the ligand–protein interactions and stability. CCA4 exhibited the lowest dissociation constant (KD~13·10−6 M) among the analogues, which we attribute to its end moieties (carboxylate and a pyridine on the ends). Purely aromatic ends (CCA1) or those with dual carboxylates (CCA6) showed lower affinity, suggesting that electrostatic complementarity and steric fit are processes involved in the binding. Despite requiring optimization, the CCA scaffold represents a novel strategy for tick control. These compounds provide a foundation for developing synergistic agents to enhance the efficacy of sustainable acaricides.
1. Introduction
Hard ticks spread disease and damage livestock worldwide. While acaricides can help control infestations, resistance eventually emerges. Sustainable management requires new approaches. AK is considered an interesting target for tick control because it is an essential enzyme for energy metabolism in invertebrates, including chelicerates (and within this group, arachnids, acari, and ticks). Furthermore, it is a conserved enzyme, and it has been suggested by bioinformatic studies as a target in cotton pests (Helicoverpa, Aphis, Spodoptera) [1] and has been shown as essential for pupation in insects (e.g., red flour beetle, T. castaneum, and common house mosquito, C. pipiens) [2,3,4]. In the blacklegged tick (I. scapularis), the protein–protein interaction network of (AK) on the STRING database [5] shows its potential involvement in feeding efficiency, immune evasion, energy balance, and homeostasis (particularly maintaining ion gradients). Altogether, this evidence suggests that this enzyme is very important for arthropods in general. The emergence of hematophagy in both mosquitoes and ticks is more recent than AK’s [6,7]. Therefore, given its conserved nature and essential function, disrupting its activity should affect energy balance and other functions, making it a good control target. Furthermore, we can assume the viability of a structure-based computational strategy (including screening, docking, and dynamics) to find inhibitors for RsAK based in structural similarity to known inhibitors of other AKs [7]. It is known that flavonoids inhibit AK in beetles [2,8], locusts [9], and trypanosoma [10,11].
Recent ecological modeling [12] highlights that ixodid tick populations are primarily regulated by the availability of small hosts (e.g., rodents), which critically limits larval feeding success [13]. A seasonal matrix model, which incorporates complex life-history stages and density dependence, demonstrates that tick abundance is highly sensitive to host-driven bottlenecks, particularly during key seasonal windows [12]. Furthermore, the model reveals significant life-history plasticity, with populations in warmer southern climates exhibiting shorter generation times. This underscores that effective, sustainable control strategies should target these vulnerable points in the tick’s life cycle, such as the energy-intensive larval feeding phase, to disrupt population growth and reduce disease hazard. This modeling framework provides a valuable tool for assessing how factors like host availability, climate change, and land use influence tick population dynamics and, by extension, the transmission of tick-borne pathogens [12]. By elucidating the role of seasonal host interactions and life-history plasticity, recent literature [12,14] supports the need for integrated management strategies that target key regulatory factors (such as larval feeding success) to sustainably control tick populations and reduce associated disease hazards. This ecological perspective underscores that tick populations are critically dependent on successful energy acquisition and utilization, particularly in the larval stage. Disrupting a central enzyme in energy metabolism, such as arginine kinase, could exploit a key vulnerability in the tick’s life cycle at the larval stage.
Arginine Kinase (AK, E.C. 2.7.3.3) catalyzes the reversible transphosphorylation between N-phosphate-L-arginine and ADP [15,16]. It is a primary enzyme in cellular energy metabolism and ATP-using processes and plays an important role in maintaining constant ATP levels in invertebrate cells (Equation (1)). Arginine phosphate is the reserve molecule of the arthropod phosphagen system, from which energy is recovered. ATP can be utilized, for example, by the muscular tissue of a marine crustacean escaping from a predator [17,18] or an insect that has been exposed to a pesticide [2], providing energy for its detoxification system. Metabolic detoxification is also a well-described resistance mechanism in ticks [7,19,20,21], with the highlight that this system in ticks presents numerous gene duplications and redundancy [7,18,19]. Evidenced, for example, by an R. sanguineus genome presenting up to 37 esterase, 32 cytochrome P450 (CYP450), 15 glutathione S-transferase (GST), and 6 superoxide dismutase genes, among others [22]. These genes have been reported to be involved in detoxification [19,20,21,23,24,25,26,27,28,29,30]. AK is mentioned as a convenient target because it is essential for insects to emerge from pupation [31], it is a conserved enzyme [7,18], and is amenable to computational approaches to be successful [1].
Arginine + ATP −Mg+2 ⇌ ADP − Mg+2 + Arginine − PO4 + H+,
The Rhipicephalus sanguineus AK (RsAK) has two tryptophan residues, Trp203 and Trp221, the last is adjacent to the active site (Figure 1). Wang et al. described the interaction between Locusta AK’s Trp221 aromatic electrons and one hydroxyl group of luteolin and mentioned the interaction as the cause of it having better energy than the alternative [9]. Furthermore, mutagenesis studies on the sea cucumber Stichopus japonicus show that the substitution of tryptophan with alanine (Trp218Ala) leads to near-complete loss of enzymatic function and destabilizes the protein’s tertiary structure, indicating the essential role of this residue in both catalysis and conformational integrity [32]. In echinoderms, the same amino acid (Trp208) is responsible for dimerization [32]. Since AKs are monomers in arachnids, Trp203 does not have any obvious function. In RsAK is embedded inside the protein, in the vicinity of the active site.
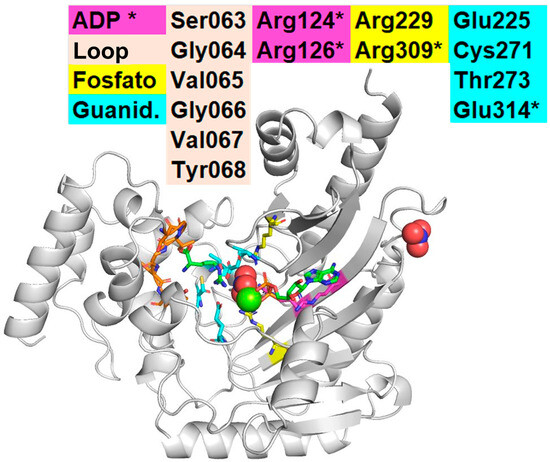
Figure 1.
Catalysis of AK in Chelicerates. Interaction observed in the crystal of AK from horseshoe crab Limulus polyphemus (LpAK, PDB: 1bg0) with Arg (substrate), NO3, and ADP [15]. The figure shows the four catalytic domains of Arginine Kinase. The ADP/ATP domain shows the two arginines that interact typically with ADP/ATP (Arg124, Arg126). The Phosphate (yellow) domain depicts the arginines that interact with the NO3 in this crystal, where the gamma phosphate of the ATP should be during catalysis (Arg229 and 309). * Indicates two amino acids that are in another region that also interact with ADP/ATP (Arg309, yellow; and Glu314, cyan). Notice the loop region on the left (Ser063 to Tyr068), and the Glu residues from the guanidine region (Glu225 and Glu314). Interaction between R229, R309, R124, and other amino acids on LpAK. The ribbon diagram shows RsAk with a fold with the catalytic amino acids, K (substrate) on the left and ADP on the right. The amino-terminal region is to the left, and the carboxy-terminal region is in the upper left corner. The two tryptophan residues (Trp203, Trp221) are depicted in light pink (center-bottom). The loop region (light-brown-S63 to Y68-, left): a hydrophobic region that “closes” the catalytic pocket. The guanidine region (cyan-E225, C271, T273, E314-, center) interacts with the gamma phosphate of ATP and the homonymous region on R (substrate). The ADP region (yellow-R229, R309-, pink-R124, R126-) has many R residues. These figures were represented using Chimera [1,15,33].
Recent DFT studies reveal that arginine kinase catalyzes phosphoryl transfer by distorting the planar guanidinium group of arginine into a pyramidal, high-energy state via an extensive hydrogen-bond network [34]. This distortion disrupts resonance stabilization, polarizes the target nitrogen, and activates it for nucleophilic attack on ATP. The mechanism involves a pentavalent transition state, and the reverse reaction proceeds through a similar forced distortion of the phospho-arginine product. This process of nitrogen pyramidalization is a fundamental catalytic strategy, also observed in other arginine-modifying enzymes like methyltransferases [35]. Interestingly, it was demonstrated that the nitrate anion, often used in structural studies, is not a true transition state analog but a passive occupant of the active site [34], also suggested in earlier works [36,37]. Understanding this precise mechanism underscores the critical functional role of residues forming the active site hydrogen-bond network [34].
The Carbamoyl Carboxylic Acid Analogs (CCAs) used in this study were synthesized as previously described by Ochoa-Terán [38]. Specifically, we used 6 synthetic compounds that share structural similarities with the substrate and form a series that has either hydrogen, pyridine, amine, or carboxy on the outer rings. Thus, the outer rings have hydrogen in CCA1, pyridine (nitrogen) in CCA2, amino in CCA3, one pyridine and one carboxy in CCA4, one amino and one carboxy in CCA5, and two carboxy in CCA6 (Figure 2). These compounds share structural features with known arginine kinase inhibitors, featuring multi-anionic motifs, such as flavonoids [4,9,39], and have the potential to act as transition state analogs, likely by targeting amino acids involved in the intermediate state of the phosphate transfer reaction.
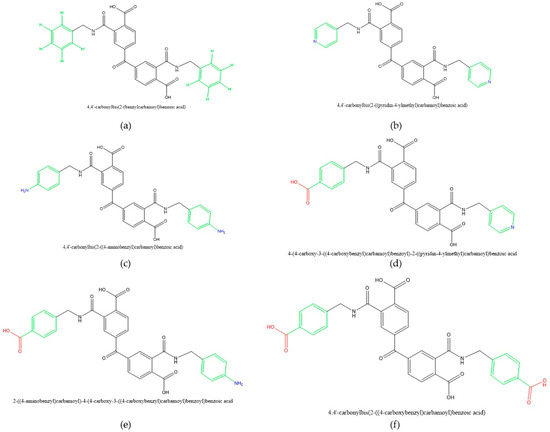
Figure 2.
Carbamoyl Carboxylic Acid Analogs: (a) 4,4′-Carbonyl Bis(2-(benzylcarbamoyl) benzoic acid), MW 534.52; (b) 4,4′-Carbonyl(2-((pyridin-4-ylmethyl)carbamoyl)benzoic acid), MW 536.50; (c) 4,4′-carbonyl(2-((4-aminobenzyl)carbamoyl)benzoic acid, MW 564.55; (d) 4-(4-carboxy-3-((4-carboxy-benzyl)carbamoyl)benzoyl)-2-((pyridin-4-ylmethyl)carbamoyl)benzoic acid, MW 575.49; (e) 2-((4-aminobenzyl)carbamoyl)-4-(4-carboxy-3-((4-carboxybenzyl)carbamoyl)benzoyl)benzoic acid, MW 592.54; (f) 4,4′-carbonylbis(2-((4-carboxybenzyl)carbamoyl)benzoic acid), MW 624.56. Molecular weights (MW) are presented in grams per mole (g/mol).
One of the ways to assess inhibitors of arginine kinase is fluorescence quenching, which uses the fluorescence of tryptophan to evaluate whether a protein interacts with a small molecule [9,39,40]. Furthermore, simulations were utilized to calculate the characteristics of the interaction of RsAK and CCAs. In the past, molecular dynamics (MD) and molecular docking [1,41] allowed the development of management strategies for agricultural pests. In this work, we describe the fluorescence properties of CCAs on binding to recombinant RsAK. These findings will contribute to the development of novel control strategies against R. sanguineus. In this work, we evaluated the interaction of six CCAs and RsAK, using fluorescence quenching and molecular dynamics simulations to evaluate their binding affinity, stability, and mechanism of action.
2. Results
Considering the analogous behavior observed across the six studied compounds, the following section presents a detailed mechanistic and structural analysis using CCA1 as a representative example. The key results for the remaining five compounds (CCA2 to 6) are presented in Table 1 (at the end of this section), which includes a summary of metrics. Experimental data and characterization are provided in Supplementary Materials.

Table 1.
Summary metrics of different compounds.
2.1. Interactions Between RsAK and CCAs by Fluorescence Quenching
Fluorescence emission spectra revealed that the CCAs exhibited an emission peak at 330 nm, which was progressively quenched upon the addition of fixed-volume aliquots of the corresponding CCA at increasing concentrations.
Fluorescence quenching was observed for RsAK (λmax = 330 nm) upon the addition of CCA1 (Figure 3). The interaction was quantified by fitting the data to a one-site specific binding model, which revealed a dissociation constant (KD) of 19.07 µM (95% CI: 15.24–24.19), indicating measurable binding affinity between the two molecules. A Stern–Volmer plot was constructed to further analyze the quenching mechanism. A diminishing fluorescence to higher quencher concentration is consistent with the quencher acting on the Trp203 and Trp221 to lower fluorescence at higher concentrations.
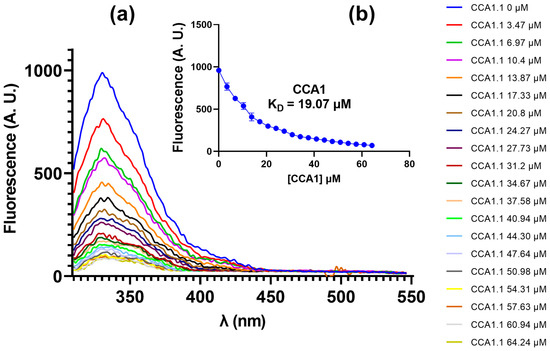
Figure 3.
Fluorescent emission spectra RsAK-CCA1: (a) fluorescence of RsAK upon titration with CCA1; (b) relationship between fluorescence emission and CCA1 concentration for KD calculation by fitting a non-linear regression.
All non-fluorescent CCAs behaved like CCA1 (Supplementary Figures S1, S5 and S9). The higher the quencher concentration, the lower the fluorescence, leading to the same hypothesis of fluorescence quenching.
The Stern–Volmer plot (SV) deviates slightly from the linear relationship predicted by the classic relationship for a single type of quenching (Figure 4a). A linear fit clearly shows an upward curvature that will be shown by most non-fluorescent CCAs (CCA1, 4, and 6). The double reciprocals plot (DR) shows a good fit to the linear model, with a slope of the fit of 14.39 (Figure 4b). The double logarithmic plot (DL) also presented a good linear fit (Figure 4c).
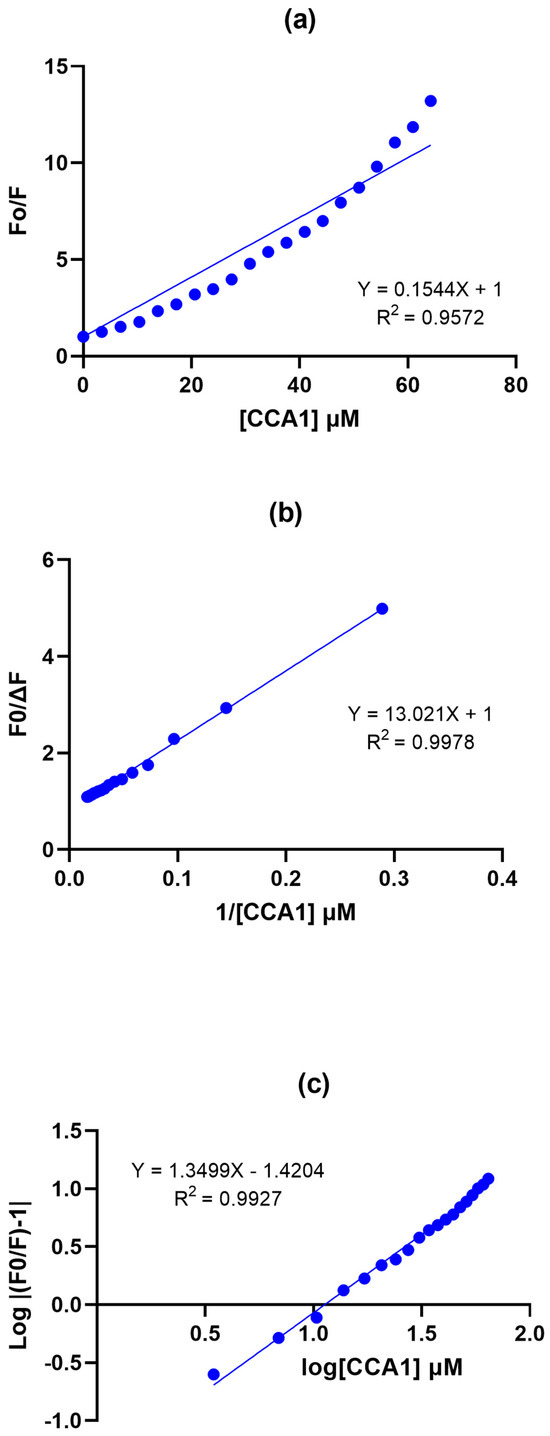
Figure 4.
Stern–Volmer Plots RsAK-CCA1: (a) Stern−Volmer plot. (b) Modified double-reciprocal Stern−Volmer plot. (c) Double logarithmic modified Stern−Volmer plot. Protein concentration 1 µM and CCA1 concentration ranged from 0 to 64.24 mM on a final volume reaction of 1.5 mL.
2.2. Interaction Stability Between RsAK and CCAs by Molecular Docking
Docking calculations were conducted within the predicted active site cleft, encompassing both substrate binding sites. To validate the method, we first docked the native substrates, arginine and ADP, into the RsAK active site to reproduce their known binding modes.
Figure 5a depicts the molecule of CCA1 docked into RsAK. 5b shows the interaction map: CCA1 not only binds in the catalytic pocket but also makes direct contact with key catalytic residues essential for ATP hydrolysis and phosphate transfer. The docking shows critical catalytic residues interacting with CCA1, like Arg126 (part of the ATP/ADP pocket); Glu225, Cys271, Thr273, and Arg309 (all the amino acids in the guanidino domain). Interestingly, some of the Loop amino acids (Val65 and Tyr68) are in the vicinity and show van der Waals interactions. The direct biological implication should be that if this small molecule remains in this configuration, the protein will not be able to catalyze its reaction. The scores of the 9 most stable poses of each CCA scored well below the arginine and phosphoarginine level, averaging from −9.0 to −8.0 kcal/mol (Supplementary Figure S12).
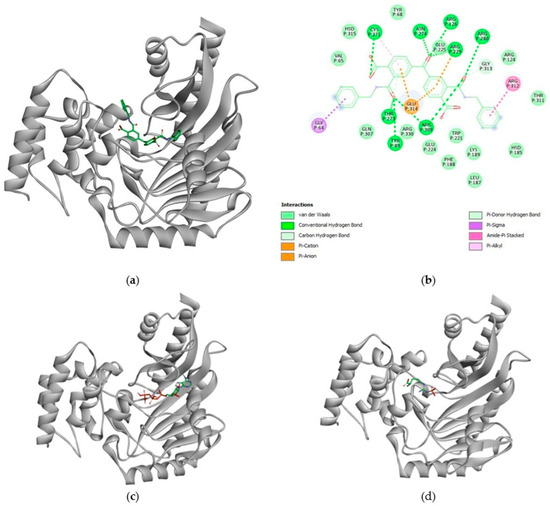
Figure 5.
Structural basis for CCA1 inhibition of RsAK: (a) surface representation of RsAK with CCA1 (stick representation) docked in the active site cleft; (b) two-dimensional diagram of specific interactions between CCA1 and the key residues of RsAK; (c) superposition of ATP (sticks), the natural substrate, bound in the RsAK active site, highlighting the overlapping binding site with CCA1. (d) Location of the product phospho-arginine, illustrating the catalytic site and the steric blockade imposed by CCA1 binding. This representation is to illustrate the general interaction scheme that we found in these molecules.
2.3. Interaction Stability Between RsAK and CCAs by Molecular Dynamics Simulations
Molecular dynamics (MD) simulations are used to assess the interaction stability of the protein–ligand complex. Since RsAK possesses two substrate-binding sites, the MD simulations were performed for each substrate and RsAK.
The interactions of RsAK with CCA1 have similar energy levels that compare to the level of energy of the interactions between RsAK and Arginine and RsAK and ATP (Figure 5a,b), with a slight increase at 25 ns that stabilizes later. The RMSDs of the RsAK-CCA1 interaction (~0.75 nm) are closer to Arg (~0.5 nm) than to ATP (~0.25 nm; Figure 5c,d). The decomposition analysis shows few residues are involved in the interaction between RsAK and CCA1. Namely Gly64, Val65, Arg280, and Arg309. ATP interacts mostly with Arg124, Arg126, Glu224, and other minor interactions.
These findings suggest that CCA1 binds to RsAK not as a standard substrate-like molecule, but as a somewhat stable ligand that occupies a distinct site, potentially acting as a regulatory molecule. Its binding mode and stability are more analogous to ATP than to Arginine, hinting at a functionally important role. Interestingly, the differential scores suggest a non-competitive mechanism for both catalytic sites (Figure 6, Supplementary Figure S18).
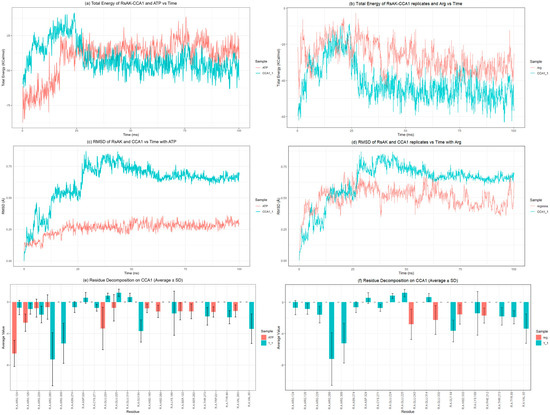
Figure 6.
Molecular dynamics and MMPBSA parameters in ATP- and Arg-binding sites: (a) binding free energy of CCA1-RsAK interaction vs. ATP; (b) binding free energy of CCA1−RsAK interaction vs. Arg; (c) CCA1-RsAK root mean square deviation (RMSD) vs. ATP; (d) CCA1 to RsAK root mean square deviation (RMSD) vs. Arg; (e) decomposition analysis by RsAK residue comparing binding of ATP and CCA1. (f) Decomposition analysis by RsAK residue comparing the binding of RsAK and Arg. All figures represent one simulation for ATPs and another for Arg. Four additional simulations for each CCA are shown in the Supplementary Material, confirming how each CCA behaves (Supplementary Figures S18–S23).
Binding CCA1 to RsAK appears to be an energetically favorable event. The slight increase and subsequent stabilization at 25 ns suggest the system undergoes a minor conformational adjustment before reaching a stabler bound state, which is a hallmark of a specific protein–ligand interaction. ATP has the lowest RMSD (~0.25 nm), indicating it is locked into a very rigid and precise pose, which is expected for a molecule that must be precisely positioned for phosphate transfer. CCA1’s RMSD (~0.75 nm) is closer to that of arginine (~0.5 nm). This suggests that while CCA1 is stable, it may have more conformational flexibility compared to the substrates (Supplementary Figure S18). This could be important if its binding needs to be modulated or if it induces specific conformational changes in the protein.
The interaction on this specific iteration (1) seemed to favor some catalytic amino acids in the loop region (Gly64, Val65) and close to the guanidinium region (Arg309), as well as an arginine located in the vicinity, likely involved in the pyramidalization of the substrate. If this interaction is stable over time, it could affect the enzyme’s catalytic rate.
The information about the other iterations can be found in Supplementary Figure S18. It showed that the energy behavior was consistent with the first iteration, stabilizing between −25 and −50 kcal/mol, comparable to the interaction with ATP energy-wise.
The RMSD shows CCA1 several types of behavior, which ranged from very similar to arginine, in the 4th iteration, with RMSD~0.5 nm, to a rather large RMSD over 1 nm. ATP had a much lower RMSD, always keeping below 0.4 nm.
Amino acids that were most relevant to the interaction included Arg280 and 309, as well as Lys189. The interaction with loop amino acids happened only in the 1st iteration, and not in the others.
From the summary metrics (Table 1), the fluorescence (Supplementary Figures S1–S10) and MD data (Supplementary Figures S18–S23), we can say that the best performing small molecule was CCA4, with the lowest KD, and the highest binding affinity of the group. From fluorescence data, it appears that CCA1, CCA2, CCA4, and CCA6 interact with RsAK; in all cases, an increasing concentration caused RsAK fluorescence to diminish (Figure 3, Supplementary Figures S1, S3, S5, S7 and S9). From the affinity data (Table 1), it seems that the combination of COOH and pyridine moieties was the best fit for the enzyme. The COOH group is good for binding at the solvent pH; it can act as both a strong hydrogen bond donor and acceptor and can form ionic bonds with basic amino acids (e.g., Lys, Arg, His) on the protein. The pyridine nitrogen can also act as a weak H-bond acceptor. This combination provides multiple specific interaction points. It is possible that the better performance of CCA4 is due to electrostatic interactions from the end groups, pyridine and COOH. The benzene rings present in CCA1 are aromatic. They drive binding through hydrophobic interactions and van der Waals forces, but the rest of the molecule also has nitrogen and carboxy groups that could justify its interaction with RsAK. The comparatively high KD in CCA4 and CCA1 could be attributed to a better steric fit rather than ionic interactions by themselves, considering the modest KD found in CCA6, which had 2 COOH groups.
While the carboxyl (COOH) group is a strong binding group, having one on each end may be counterproductive due to electrostatic repulsion. Additionally, a desolvation energetic penalty may occur, along with an incorrect orientation of the side chains.
3. Discussion
The growing problem of acaricide resistance necessitates the exploration of novel molecular targets and chemical alternatives for tick control. There are several works showing that the detoxification system of ticks has gene redundancies, a rich pool of strategies to control oxidative stress [18,19,20,30,42].
This study provides evidence supporting AK as a viable target and introduces CCAs as a promising new class of inhibitor scaffolds. Our approach, combining fluorescence quenching with MD simulations, demonstrates measurable binding to RsAK, with CCA4 (KD = 13.30 µM) having the highest affinity. The simulations further suggest that binding occurs near the active site, involving residues critical for catalysis and substrate stabilization (Supplementary Figures S18–S23).
The analyzed compounds with the highest theoretical binding affinity (CCA1, CCA4) interact with a cluster of arginine residues (Arg280, Arg309, Arg330), yet they do not achieve a highly stable protein–ligand complex. Recent work demonstrates that AK catalysis relies on an extensive hydrogen-bonding network and precise conformational changes, such as the movement of a flexible loop, polarizing the arginine substrate and achieving transition state geometry, including Arg280 and Arg330 [34]. We propose that even transient interactions of CCAs with this region could perturb this delicate dynamic network. This model of dynamic interference aligns with an emerging paradigm in enzymology, where effective inhibition targets essential conformational landscapes [43,44] rather than static binding pockets [40,45]. An illustrative example is found in the bacteriophage T7 replication machinery. The T7 DNA polymerase forms a rigid, stable complex with its DNA substrate through extensive complementary interactions [46], while the T7 RNA polymerase undergoes major rearrangements, such as promoter release, that are critical for its function [43,44]. Similarly, RsAK likely requires precise conformational changes, like the movement of a flexible loop, to achieve a catalytically competent state. We propose that the CCAs, by binding transiently near the catalytic arginine cluster, act not as perfect substrate mimics but as dynamic impediments to these essential rearrangements, potentially forcing the enzyme to an inadequate conformation.
This hypothesis is supported by the consistent involvement of catalytic and other residues across all simulation iterations (Table 2). For instance, Arg309, a critical catalytic residue, appears in interactions with CCA1, CCA4, CCA5, and CCA6. Furthermore, Arg280 has also been reported in simulations of interactions of an ATP with spider AK [40]. This recurring pattern of interaction (Table 2) suggests that CCAs preferentially engage with the enzyme’s functional core. Apparently, the increased RMSD occurs because the molecule moves significantly relative to its initial position in the external part, resulting in high positional changes. There are other reported mechanisms in AK where there is aggregation by positive cations like lead [47], silver and gold nanoparticles [48].

Table 2.
Residue decomposition involvement in all the iterations.
Tick AK inhibition through this dynamic mechanism could impair critical physiological processes reliant on rapid ATP regeneration, such as energy buffering for detoxification [7,18], possibly feeding, since it is potentially involved in muscle function, and osmoregulation, due to a likely involvement of electron transporters (Figure 7). By disrupting the energy metabolism, CCAs could compromise the tick’s ability to rapidly upregulate ATP-dependent detoxification pathways (e.g., Cytochrome P450, GST, etc.) during pesticide exposure. This would lead to the accumulation of toxic compounds, effectively sensitizing the tick to acaricides.
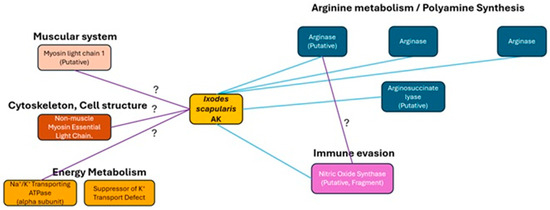
Figure 7.
Protein Interaction Network of Ixodes scapularis AK. Systems and proteins likely to interact with IsAK by examining the STRING database [5]. The blue line indicates connections made via databases and the purple is from data analyzed from experimental papers. The interrogation symbol shows relations that have a likelihood score under 0.9.
AK is responsible for rapid ATP regeneration during periods of high energy demand, being homologous to Creatin Kinase [49,50]. Inhibiting tick AK could impair critical physiological processes like energy balance, feeding efficiency, immune evasion, and homeostasis (particularly maintaining ion gradients). The implications are effects on energy buffering, which can provide ATP for energy-intensive processes that require immediate energy. This is particularly important for metabolic detoxification [7,18], as the degradation via detoxification enzymes (Cytochrome P450, GST, esterases, etc.) is highly dependent on ATP. Impaired energy buffering would compromise the tick’s ability to rapidly upregulate these detoxification pathways during pesticide exposures, leading to the accumulation of toxic compounds. If the arginase pathway is affected, other processes like growth and repair for post-feeding tissue development [51,52], and digestion [52] would be affected. This pathway also modulates tick-specific processes like vasoconstriction [53] and the Toll pathway, which manages defensins, a protein that modulates Gram-negative bacteria behavior when ingested by ticks [54]. The nitric oxide signaling pathway is also involved in host manipulation, in processes such as vasodilation and immunomodulation [55]. Even though direct references linking arginase to cuticle growth in chelicerates specifically are rare, the core biochemistry (Arg to Ornithine to Pro conversion via arginase) is conserved in arthropods, implying a similar role in chelicerate cuticle biology [56].
Given their current potency, the most immediate application for CCAs lies in synergism. They could be used alongside commercial pesticides to overcome metabolic resistance by “disarming” the tick’s ATP-dependent detoxification machinery, analogous to the use of piperonyl butoxide (PBO) [57,58]. More strategically, CCAs could be integrated with “green” acaricides into a multi-target approach in two ways:
- -
- Combination with Botanical AK Inhibitors: Formulating CCAs with natural AK inhibitors such as flavonoids [39,57], catechins [10,40], or resveratrol [11] could create a synergistic blend. Attacking the same essential target (AK) with distinct chemical scaffolds could enhance efficacy and potentially delay the emergence of resistance.
- -
- Enhanced Efficacy of Essential Oils: Many green acaricides based on essential oils act rapidly but are quickly metabolized [59]. By impairing the tick’s energy metabolism, CCAs could slow the tick’s recovery from the sub-lethal effects of these botanicals, effectively increasing their kill rate and residual activity.
The conservation of key catalytic residues and structural motifs in AK across invertebrate species suggests that insights gained from studying RsAK could have broad implications for developing novel acaricides. By targeting this dynamic functional network, we may circumvent resistance mechanisms that often arise from mutations in static active-site residues.
Our work suggests that CCAs represent a novel class of arginine kinase ligands that may operate through a dynamic, allosteric mechanism. This model, involving the disruption of conformational landscapes rather than statically interacting with the active site [43,44], offers a strategy that could circumvent resistance mechanisms arising from mutations in static residues. Therefore, the objective for future work is reframed from finding a tight-binding substrate analog to designing compounds that specifically and persistently disrupt the enzyme’s dynamic workflow. Future directions should include biochemical validation through enzymatic activity assays to determine IC50. Rational design of bivalent inhibitors that bridge the arginine and ATP subsites to enhance affinity. Advanced simulations and structural biology (e.g., X-ray crystallography) to visualize the precise binding modes and conformational states stabilized by these ligands.
4. Materials and Methods
4.1. Fluorescence Quenching
The intrinsic tryptophan fluorescence quenching method was used to explore interactions between RsAK and ACC1 through ACC6, following the protocol published by our group [40]. Briefly, Briefly, the experiments consisted of shining a specific wavelength of light on the protein, causing it to fluoresce. They then gradually added increasing amounts of the ACC molecule that was being tested and monitored how the protein’s fluorescence changed. The decrease in fluorescence intensity as more ACC was added indicated that the two molecules were binding together. By analyzing this change in fluorescence, the strength of the interaction was calculated, determining both the binding affinity (dissociation constant, KD) and the efficiency of the fluorescence quenching (Ksv).
Fluorometric titrations were conducted in a Tris-HCl buffer solution (50 mM, pH 8.4) at 25 °C with 1 μM RsAK, gradually increasing ACC concentrations by adding 2 µL of 2.6 mM ACC, dissolved in ultrapure water, and then adding it to the buffer. For ACC3 and ACC5 we used 1 and 2.6 mM, dissolved in ultrapure water, and then added them to the buffer. RsAK’s steady-state fluorescence was measured in the absence and presence of ACC. The excitation wavelength (λex) was set at 295 nm, and the emission spectra (λem) were recorded from 300 to 500 nm using a QM-2003 spectrofluorometer (Photon Technology International, Edison, NJ, USA), with slit widths for excitation and emission monochromators set at 5 nm and 10 nm, respectively. For ACC3 and ACC5 we had to use 5 nm excitation and 5 nm emission monochromators. Emission spectra were smoothed with the Savitzky–Golay filter (2nd order, nine neighboring data points), and analyses were conducted in GraphPad Prism v9.5.1 (GraphPad Software, San Diego, CA, USA). The dissociation constant (Kd) of ACC was determined by fitting the fluorescence intensity change in RsAK at a peak emission wavelength (λem) of 325 nm against ACC concentration using a non-linear one-site binding model. Corrections for inner filter effects were applied (Equation (2)), and the quenching constant (Ksv) was calculated using the linear Stern–Volmer equation [60,61] (Equation (3)).
Fcorr denotes the fluorescence adjusted for inner filter effects, Fobs is the observed fluorescence emission, and ODex and ODem are the absorbance values at the excitation and emission wavelengths, respectively.
Here, F0 and F refer to the fluorescence intensities of RsAK without and with ACC, respectively. [Q] denotes the concentration of ACC, and KSV represents the Stern–Volmer constant. The modified Stern–Volmer equation [62] (Equation (4)) was used on ACC, which contains two W residues, one accessible and one inaccessible to the quencher.
where ΔF is the difference between F0 and F, fa (intercept) represents the fraction of fluorophore accessible to ACC is represented, and Kq (the slope of the F0/ΔF) versus 1/[Q] plot) becomes the modified Stern–Volmer quenching constant, similar to the binding association constant. Lastly, Fluorescence measurements are valuable for obtaining quantifiable insights into protein–ligand interactions. A double logarithmic equation can be used in proteins with multiple potential binding sites (Equation (5)).
where Ka represents the binding constant for the interaction between the macromolecule and the quencher, along with n, the number of binding sites per macromolecule, which can be determined from the intercept and slope of the double logarithmic regression curve.
4.2. Molecular Dockings
Rigid docking was performed with auto dock vina 1.2 [63,64] with the optimized models of ACCs (referenced on [38]), and the library of naphthalene diimide-type compounds (link) functionalized with polar groups, some of which have been previously described by Ochoa-Terán [65], and other commercially available water-soluble natural compounds known to inhibit AK, such as rutin, quercetin, resveratrol, tagetone, pyrethrin I and other flavonoids and terpenes. The region used for the interaction corresponds to the arginine pocket and its locality, closer to the N-terminus, shown in blue in Figure 1. It was set using (5.486, 2.612, 3.167) and 2.1 nm on each side, executed on Ubuntu 22.04 terminal using a Perl script. The binding energies to the ternary state analog complex (TSAC) of RsAK determine the most favorable ones. The compounds not available in PubChem [66] were created in ChemDraw 12.0 according to specifications and converted with open babel [67] to mol2.
4.3. Molecular Dynamics
Molecular dynamics (MD) simulations were performed to assess the stability of the substrate controls (ATP and arginine) and the RsAK-CCA complexes. Briefly, to understand how stable the interactions are between the protein (RsAK) and the candidate inhibitor molecules (CCA1-6), computer simulations that mimic the natural motion of atoms over time were used. A virtual model of the protein was created, with a molecule bound to it, and it was placed in a simulated water and salt environment. Then calculations were made to observe how the complex behaved. To ensure their findings were reliable, they repeated each simulation multiple times (4). By analyzing these simulations, they measured the overall stability of the complex and identified the specific chemical forces, like hydrogen bonds, that hold the molecule to the protein, which helps evaluate the strength of the interaction between the molecule and the protein.
The stability of these complexes, inferred from metrics like root mean square deviation (RMSD), was used to evaluate their potential for inhibition.
The topologies for the docked RsAK-CCA complexes (CCA1 to CCA6) were generated using Chimera 1.17 and prepared via the CHARMM-GUI web server [68]. All systems were parameterized using the CHARMM36m force field. Each system was solvated in a TIP3P water box and neutralized with ions to achieve physiological pH (7.0) and ionic strength.
The simulation protocol consisted of an energy minimization step (5000 steps of the steepest descent algorithm), followed by equilibration in the NVT and NPT ensembles for 200 ps. During equilibration, temperature was maintained at 303.15 K using the Nose-Hoover thermostat, and pressure was held at 1 bar using the Parrinello-Rahman barostat. Finally, production MD simulations were run for 100 ns per replica.
To ensure the robustness and statistical significance of our results, we performed four independent replicate simulations for each compound, starting from different initial velocities.
Long-range electrostatic interactions were handled using the Particle Mesh Ewald (PME) method, and short-range van der Waals interactions were calculated with a Verlet cutoff scheme at 1.0 nm. The LINCS algorithm was used to constrain all bonds involving hydrogen atoms. All molecular dynamics simulations were performed using the open source GROMACS software (versions 2023.4 and 2024.1).
Trajectory analysis was conducted to evaluate binding stability. This included calculating the root mean square deviation (RMSD) of the RsAK backbone C-α atoms (using the GROMACS gmx rms) and monitoring the number of hydrogen bonds between RsAK and each CCA (using the GROMACS gmx hbond). The binding free energy and its per-residue decomposition (for residues within 4 nm of the ligand) were calculated for the entire 100 ns trajectory using the GROMACS gmx_MMPBSA tool [17,26,27,28,29,69,70].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15100982/s1, Figure S1: Fluorescent emission spectra of RsAK-CCA2; Figure S2: Stern-Volmer Plots RsAK-CCA2; Figure S3: Fluorescent emission spectra RsAK-CCA3; Figure S4: Stern-Volmer plots of the interaction of CCA3 with RsAK; Figure S5: Fluorescent emission spectra; Figure S6: Stern-Volmer plots of the interaction of CCA4 with RsAK; Figure S7: Fluorescent emission spectra; Figure S8: Stern-Volmer plots of the interaction of CCA5 with RsAK; Figure S9: Fluorescent emission spectra of CCA6; Figure S10: Stern-Volmer plots of the interaction of CCA6 with RsAK; Figure S11: Interaction region of arginine kinase and small molecules; Figure S12: Average energy of docked RsAK with standard deviation; Figure S13: Structural basis for CCA2 inhibition of RsAK; Figure S14: Structural basis for CCA3 inhibition of RsAK; Figure S15: Structural basis for CCA4 inhibition of RsAK; Figure S16: Structural basis for CCA5 inhibition of RsAK; Figure S17: Structural basis for CCA6 inhibition of RsAK; Figure S18: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA1 in RsAK; Figure S19: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA2 in RsAK; Figure S20: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA3 in RsAK; Figure S21: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA4 in RsAK; Figure S22: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA5 in RsAK; Figure S23: Molecular dynamic and MMPBSA parameters in ATP and Arg binding site for CCA6 in RsAK.
Author Contributions
Conceptualization, A.O.-T. and R.R.S.-M.; Data curation, J.F.R.-C.; Formal analysis, J.F.R.-C.; Funding acquisition, R.R.S.-M.; Investigation, J.F.R.-C., E.N.M.-C. and A.Á.-A.; Methodology, E.N.M.-C. and R.R.S.-M.; Project administration, R.R.S.-M.; Resources, C.L.C.-R., H.S.-O., A.O.-T. and R.R.S.-M.; Software, J.F.R.-C. and A.Á.-A.; Supervision, A.M.-A., A.A.L.-Z., A.O.-T. and R.R.S.-M.; Validation, E.N.M.-C.; Visualization, J.F.R.-C.; Writing—original draft, J.F.R.-C., A.O.-T. and R.R.S.-M.; Writing—review & editing, J.F.R.-C., E.N.M.-C., A.Á.-A., C.L.C.-R., A.M.-A., A.A.L.-Z., H.S.-O., A.O.-T. and R.R.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SECIHTI, grant number CF-2019-610264 and the APC was funded by CIAD Coordinación de Investigación.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at https://doi.org/10.5281/zenodo.17317099.
Acknowledgments
The authors thank the former Mexico’s National Council of Science, Humanities, and Technology (CONAHCYT) for the Ciencia de Frontera grant CF-2019-610264 to R.R.S.-M. The scholarship for doctoral students and postdoctoral fellows was provided by Mexico’s Secretary of Science, Humanities, Technology and Innovation (SECHTI). The publication APC was funded by the Office of Research at the Research Center in Food and Development (Centro de Investigación en Alimentación y Desarrollo, A.C., Coordinación de Investigación).
Conflicts of Interest
The authors declare no conflicts of interest. The funding agency had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| Enzymes, Proteins, and Molecules | |
| AK | Arginine Kinase |
| RsAK | Rhipicephalus sanguineus Arginine Kinase |
| LpAK | Limulus polyphemus (horseshoe crab) Arginine Kinase |
| ATP | Adenosine Triphosphate |
| ADP | Adenosine Diphosphate |
| CCAs | Carbamoyl Carboxylic Acid Analogues (the six studied compounds: CCA1 to CCA6) |
| CYP450 | Cytochrome P450 |
| Biological and Chemical Terms | |
| E.C. GST | Enzyme Commission number (a numerical classification scheme for enzymes) Glutathione S-transferase |
| MD | Molecular Dynamics |
| RMSD | Root Mean Square Deviation |
| KD | Dissociation Constant |
| Ka | Association Constant |
| KSV | Stern–Volmer Constant |
| Kq | Quenching Constant |
| IC50 | Half Maximal Inhibitory Concentration |
| Ki | Inhibition Constant |
| ODex | Optical Density at excitation wavelength |
| ODem | Optical Density at emission wavelength |
| PME | Particle Mesh Ewald (method to calculate long-range electrostatic interactions) |
| LINCS | Linear Constraint Solver (an algorithm for constraining bond lengths) |
| NVT | Canonical Ensemble (constant Number of particles, Volume, and Temperature) |
| NPT | Isothermal-Isobaric Ensemble (constant Number of particles, Pressure, and Temperature) |
| Tick Species | |
| R. sanguineus | Rhipicephalus sanguineus (the brown dog tick) |
| Computational and Analytical Methods | |
| PDB | Protein Data Bank |
| MMPBSA | Molecular Mechanics Poisson–Boltzmann Surface Area (a method to calculate binding free energies) |
| Other General Abbreviations | |
| e.g. | For example (exempli gratia) |
| i.e. | That is (id est) |
| et al. | And others (et alii) |
| R2 | Coefficient of Determination (a statistical measure) |
| CI | Confidence Interval |
| SV | Stern–Volmer (plot) |
| DR | Double Reciprocal (plot) |
| DL | Double Logarithmic (plot) |
| TSAC | Transition State Analog Complex |
References
- Sakthivel, S.; Habeeb, S.K.M.; Raman, C. Screening of Broad Spectrum Natural Pesticides against Conserved Target Arginine Kinase in Cotton Pests by Molecular Modeling. J. Biomol. Struct. Dyn. 2019, 37, 1022–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, J.; Jiang, H.; Ge, H.; Zheng, Y.; Meng, X.; Qian, K.; Wang, J. Knockdown or Inhibition of Arginine Kinases Enhances Susceptibility of Tribolium Castaneum to Deltamethrin. Pestic. Biochem. Physiol. 2022, 183, 105080. [Google Scholar] [CrossRef]
- Brown, A.E.; Grossman, S.H. The Mechanism and Modes of Inhibition of Arginine Kinase from the Cockroach (Periplaneta americana). Arch. Insect Biochem. Physiol. 2004, 57, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Guan, Q.; Zhang, H.; Zhang, N.; Meng, X.; Liu, H.; Wang, J. RNAi-Mediated Knockdown of Arginine Kinase Genes Leads to High Mortality and Negatively Affect Reproduction and Blood-Feeding Behavior of Culex pipiens pallens. PLoS Negl. Trop. Dis. 2022, 16, e0010954. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Ellington, W.R. Evolution and Physiological Roles of Phosphagen Systems. Annu. Rev. Physiol. 2001, 63, 289–325. [Google Scholar] [CrossRef]
- Rojas-Cabeza, J.F.; Moreno-Cordova, E.N.; Ayala-Zavala, J.F.; Ochoa-Teran, A.; Sonenshine, D.E.; Valenzuela, J.G.; Sotelo-Mundo, R.R. A Review of Acaricides and Their Resistance Mechanisms in Hard Ticks and Control Alternatives with Synergistic Agents. Acta Trop. 2025, 261, 107519. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, H.; Meng, X.; Qian, K.; Liu, Y.; Song, Q.; Stanley, D.; Wu, J.; Park, Y.; Wang, J. Broad-Complex Transcription Factor Mediates Opposing Hormonal Regulation of Two Phylogenetically Distant Arginine Kinase Genes in Tribolium castaneum. Commun. Biol. 2020, 3, 631. [Google Scholar] [CrossRef]
- Wang, H.-R.; Zhu, W.-J.; Wang, X. Mechanism of Inhibition of Arginine Kinase by Flavonoids Consistent with Thermodynamics of Docking Simulation. Int. J. Biol. Macromol. 2011, 49, 985–991. [Google Scholar] [CrossRef]
- Paveto, C.; Güida, M.C.; Esteva, M.I.; Martino, V.; Coussio, J.; Flawiá, M.M.; Torres, H.N. Anti-Trypanosoma cruzi Activity of Green Tea (Camellia sinensis) Catechins. Antimicrob. Agents Chemother. 2004, 48, 69–74. [Google Scholar] [CrossRef]
- Valera Vera, E.A.; Sayé, M.; Reigada, C.; Damasceno, F.S.; Silber, A.M.; Miranda, M.R.; Pereira, C.A. Resveratrol Inhibits Trypanosoma Cruzi Arginine Kinase and Exerts a Trypanocidal Activity. Int. J. Biol. Macromol. 2016, 87, 498–503. [Google Scholar] [CrossRef]
- Vindenes, Y.; Mysterud, A. A Seasonal Matrix Population Model for Ixodid Ticks with Complex Life Histories and Limited Host Availability. Ecology 2025, 106, e4511. [Google Scholar] [CrossRef]
- van Duijvendijk, G.; Sprong, H.; Takken, W. Multi-Trophic Interactions Driving the Transmission Cycle of Borrelia afzelii between Ixodes ricinus and Rodents: A Review. Parasit. Vectors 2015, 8, 643. [Google Scholar] [CrossRef]
- Makwarela, T.G.; Seoraj-Pillai, N.; Nangammbi, T.C. Tick Control Strategies: Critical Insights into Chemical, Biological, Physical, and Integrated Approaches for Effective Hard Tick Management. Vet. Sci. 2025, 12, 114. [Google Scholar] [CrossRef]
- Zhou, G.; Somasundaram, T.; Blanc, E.; Parthasarathy, G.; Ellington, W.R.; Chapman, M.S. Transition State Structure of Arginine Kinase: Implications for Catalysis of Bimolecular Reactions. Proc. Natl. Acad. Sci. USA 1998, 95, 8449–8454. [Google Scholar] [CrossRef]
- Gomez-Yanes, A.C.; Moreno-Cordova, E.N.; Garcia-Orozco, K.D.; Laino, A.; Islas-Osuna, M.A.; Lopez-Zavala, A.A.; Valenzuela, J.G.; Sotelo-Mundo, R.R. The Arginine Kinase from the Tick Rhipicephalus sanguineus Is an Efficient Biocatalyst. Catalysts 2022, 12, 1178. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Beis, I.; Leech, A.R.; Zammit, V.A. The Role of Creatine Kinase and Arginine Kinase in Muscle. Biochem. J. 1978, 172, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, B.M.; Guimarães Ribeiro, V.; Campos, N.d.N.; da Silva Romão Mota, L.G.; Moreira, M.F. A Comprehensive Review of Arginine Kinase Proteins: What We Need to Know? Biochem. Biophys. Rep. 2024, 40, 101837. [Google Scholar] [CrossRef]
- Sabadin, G.A.; Salomon, T.B.; Leite, M.S.; Benfato, M.S.; Oliveira, P.L.; da Silva Vaz, I. An Insight into the Functional Role of Antioxidant and Detoxification Enzymes in Adult Rhipicephalus microplus Female Ticks. Parasitol. Int. 2021, 81, 102274. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, V.L.; Klafke, G.M.; Torres, T.T. Detoxification Mechanisms Involved in Ivermectin Resistance in the Cattle Tick, Rhipicephalus (Boophilus) Microplus. Sci. Rep. 2018, 8, 12401. [Google Scholar] [CrossRef] [PubMed]
- Duscher, G.G.; Galindo, R.C.; Tichy, A.; Hummel, K.; Kocan, K.M.; de la Fuente, J. Glutathione S-Transferase Affects Permethrin Detoxification in the Brown Dog Tick, Rhipicephalus sanguineus. Ticks Tick. Borne Dis. 2014, 5, 225–233. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.-F.; Wang, Q.; Zhang, B.; Ji, P.; et al. Large-Scale Comparative Analyses of Tick Genomes Elucidate Their Genetic Diversity and Vector Capacities. Cell 2020, 182, 1328–1340.e13. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Cunninghamella spp. Produce Mammalian-Equivalent Metabolites from Fluorinated Pyrethroid Pesticides. AMB Express 2021, 11, 101. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial Cytochrome P450 Involvement in the Biodegradation of Fluorinated Pyrethroids. J. Xenobiot. 2025, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamaguchi, M. Characterization of a Novel Superoxide Dismutase in Nilaparvata lugens. Arch. Insect Biochem. Physiol. 2022, 109, e21862. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the Role of Esterases in the Biodegradation of Organophosphate, Carbamate, and Pyrethroid Pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef] [PubMed]
- Villarino, M.A.; Waghela, S.D.; Wagner, G.G. Biochemical Detection of Esterases in the Adult Female Integument of Organophosphate-Resistant Boophilus microplus (Acari: Ixodidae). J. Med. Entomol. 2003, 40, 52–57. [Google Scholar] [CrossRef][Green Version]
- Gaudêncio, F.N.; Klafke, G.M.; Tunholi-Alves, V.M.; Ferreira, T.P.; Coelho, C.N.; da Fonseca, A.H.; da Costa Angelo, I.; Pinheiro, J. Activity of Carboxylesterases, Glutathione-S-Transferase and Monooxygenase on Rhipicephalus microplus Exposed to Fluazuron. Parasitol. Int. 2017, 66, 584–587. [Google Scholar] [CrossRef]
- Hernandez, E.P.; Kusakisako, K.; Talactac, M.R.; Galay, R.L.; Hatta, T.; Fujisaki, K.; Tsuji, N.; Tanaka, T. Glutathione S-Transferases Play a Role in the Detoxification of Flumethrin and Chlorpyrifos in Haemaphysalis longicornis. Parasit. Vectors 2018, 11, 460. [Google Scholar] [CrossRef]
- Ruiz-May, E.; Álvarez-Sánchez, M.E.; Aguilar-Tipacamú, G.; Elizalde-Contreras, J.M.; Bojórquez-Velázquez, E.; Zamora-Briseño, J.A.; Vázquez-Carrillo, L.I.; López-Esparza, A. Comparative Proteome Analysis of the Midgut of Rhipicephalus microplus (Acari: Ixodidae) Strains with Contrasting Resistance to Ivermectin Reveals the Activation of Proteins Involved in the Detoxification Metabolism. J. Proteom. 2022, 263, 104618. [Google Scholar] [CrossRef]
- Qi, X.-L.; Su, X.-F.; Lu, G.-Q.; Liu, C.-X.; Liang, G.-M.; Cheng, H.-M. The Effect of Silencing Arginine Kinase by RNAi on the Larval Development of Helicoverpa armigera. Bull. Entomol. Res. 2015, 105, 555–565. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, F.; Guo, S.-Y.; Wang, X. The Tryptophane Residues of Dimeric Arginine Kinase: Roles of Trp-208 and Trp-218 in Active Site and Conformation Stability. Biochimie 2004, 86, 379–386. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera–A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Falcioni, F.; Molt, R.W.; Jin, Y.; Waltho, J.P.; Hay, S.; Richards, N.G.J.; Blackburn, G.M. Arginine Kinase Activates Arginine for Phosphorylation by Pyramidalization and Polarization. ACS Catal. 2024, 14, 6650–6658. [Google Scholar] [CrossRef]
- Aranda, J.; Zinovjev, K.; Roca, M.; Tuñón, I. Dynamics and Reactivity in Thermus aquaticus N6-Adenine Methyltransferase. J. Am. Chem. Soc. 2014, 136, 16227–16239. [Google Scholar] [CrossRef]
- Azzi, A.; Clark, S.A.; Ellington, W.R.; Chapman, M.S. The Role of Phosphagen Specificity Loops in Arginine Kinase. Protein Sci. 2004, 13, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.-P.; Pfeiffer, I.P.-M.; Mordhorst, S. Methyltransferases from RiPP Pathways: Shaping the Landscape of Natural Product Chemistry. Beilstein J. Org. Chem. 2024, 20, 1652–1670. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Terán, A.; Estrada-Manjarrez, J.; Martínez-Quiroz, M.; Landey-Álvarez, M.A.; Alcántar Zavala, E.; Pina-Luis, G.; Santacruz Ortega, H.; Gómez-Pineda, L.E.; Ramírez, J.-Z.; Chávez, D.; et al. A Novel and Highly Regioselective Synthesis of New Carbamoylcarboxylic Acids from Dianhydrides. Sci. World J. 2014, 2014, e725981. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Q.; Zhu, W.-J.; Lü, Z.-R.; Xia, Y.; Yang, J.-M.; Zou, F.; Wang, X.-Y. The Effect of Rutin on Arginine Kinase: Inhibition Kinetics and Thermodynamics Merging with Docking Simulation. Int. J. Biol. Macromol. 2009, 44, 149–155. [Google Scholar] [CrossRef]
- Moreno-Cordova, E.N.; Alvarez-Armenta, A.; Garcia-Orozco, K.D.; Arvizu-Flores, A.A.; Islas-Osuna, M.A.; Robles-Zepeda, R.E.; Lopez-Zavala, A.A.; Laino, A.; Sotelo-Mundo, R.R. Binding of Green Tea Epigallocatechin Gallate to the Arginine Kinase Active Site from the Brown Recluse Spider (Loxosceles laeta): A Potential Synergist to Chemical Pesticides. Heliyon 2024, 10, e34036. [Google Scholar] [CrossRef]
- Li, Y.-J.; Gu, F.-M.; Chen, H.-C.; Liu, Z.-X.; Song, W.-M.; Wu, F.-A.; Sheng, S.; Wang, J. Binding Characteristics of Pheromone-Binding Protein 1 in Glyphodes Pyloalis to Organophosphorus Insecticides: Insights from Computational and Experimental Approaches. Int. J. Biol. Macromol. 2024, 260, 129339. [Google Scholar] [CrossRef]
- De Rouck, S.; İnak, E.; Dermauw, W.; Van Leeuwen, T. A Review of the Molecular Mechanisms of Acaricide Resistance in Mites and Ticks. Insect Biochem. Mol. Biol. 2023, 159, 103981. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Nayak, D.; Brieba, L.G.; Sousa, R. Major Conformational Changes During T7RNAP Transcription Initiation Coincide with, and Are Required for, Promoter Release. J. Mol. Biol. 2005, 353, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Corbella, M.; Pinto, G.P.; Kamerlin, S.C.L. Loop Dynamics and the Evolution of Enzyme Activity. Nat. Rev. Chem. 2023, 7, 536–547. [Google Scholar] [CrossRef]
- Peng, Y.; Hansen, A.L.; Bruschweiler-Li, L.; Davulcu, O.; Skalicky, J.J.; Chapman, M.S.; Brüschweiler, R. The Michaelis Complex of Arginine Kinase Samples the Transition State at a Frequency That Matches the Catalytic Rate. J. Am. Chem. Soc. 2017, 139, 4846–4853. [Google Scholar] [CrossRef]
- Doublié, S.; Tabor, S.; Long, A.M.; Richardson, C.C.; Ellenberger, T. Crystal Structure of a Bacteriophage T7 DNA Replication Complex at 2.2 Å Resolution. Nature 1998, 391, 251–258. [Google Scholar] [CrossRef]
- Si, Y.-X.; Lee, J.; Cai, Y.; Yin, S.-J.; Yang, J.-M.; Park, Y.-D.; Qian, G.-Y. Molecular Dynamics Simulations Integrating Kinetics for Pb2+-Induced Arginine Kinase Inactivation and Aggregation. Process Biochem. 2015, 50, 729–737. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Whiteley, C.G. Interaction of Metal Nanoparticles with Recombinant Arginine Kinase from Trypanosoma Brucei: Thermodynamic and Spectrofluorimetric Evaluation. Biochim. Et. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 701–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blethen, S.L.; Kaplan, N.O. Characteristics of Arthropod Arginine Kinases. Biochemistry 1968, 7, 2123–2135. [Google Scholar] [CrossRef]
- Ellington, W.R.; Hines, A.C. Mitochondrial Activities of Phosphagen Kinases Are Not Widely Distributed in the Invertebrates. Biol. Bull. 1991, 180, 505–507. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Miranda, C.; Flint, C.; Harris, E.; Wu, G. Nutrients Limit Production of Insects for Food and Feed: An Emphasis on Nutritionally Essential Amino Acids. Anim. Front. 2023, 13, 64–71. [Google Scholar] [CrossRef]
- Martins, J.C.S.; Assunção Romão, H.A.; Canettieri, C.K.; Cercilian, A.C.; Oliveira, P.R.O.; Ferreira, C.; Terra, W.R.; de Oliveira Dias, R. The Loss of the Urea Cycle and Ornithine Metabolism in Different Insect Orders: An Omics Approach. Insect Mol. Biol. 2025, 34, 632–644. [Google Scholar] [CrossRef]
- Pekáriková, D.; Rajská, P.; Kazimírová, M.; Pecháňová, O.; Takáč, P.; Nuttall, P.A. Vasoconstriction Induced by Salivary Gland Extracts from Ixodid Ticks. Int. J. Parasitol. 2015, 45, 879–883. [Google Scholar] [CrossRef]
- Jalovecka, M.; Malandrin, L.; Urbanova, V.; Mahmood, S.; Snebergerova, P.; Peklanska, M.; Pavlasova, V.; Sima, R.; Kopacek, P.; Perner, J.; et al. Activation of the Tick Toll Pathway to Control Infection of Ixodes Ricinus by the Apicomplexan Parasite Babesia Microti. PLoS Pathog. 2024, 20, e1012743. [Google Scholar] [CrossRef]
- Kitsou, C.; Fikrig, E.; Pal, U. Tick Host Immunity: Vector Immunomodulation and Acquired Tick Resistance. Trends Immunol. 2021, 42, 554–574. [Google Scholar] [CrossRef] [PubMed]
- Dzik, J.M. Evolutionary Roots of Arginase Expression and Regulation. Front. Immunol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, P.; Chu, X.; Hao, L.; Guo, X.; Xu, B. Isolation of Arginine Kinase from Apis Cerana Cerana and Its Possible Involvement in Response to Adverse Stress. Cell Stress. Chaperones 2015, 20, 169–183. [Google Scholar] [CrossRef]
- Higa, L.D.O.S.; Garcia, M.V.; Rodrigues, V.D.S.; Bonatte-Junior, P.; Barradas-Piña, F.T.; Barros, J.C.; Andreotti, R. Effects of Cypermethrin, Chlorpyrifos and Piperonyl Butoxide-Based Pour-on and Spray Acaricides on Controlling the Tick Rhipicephalus Microplus. Syst. Appl. Acarol. 2019, 24, 278. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; González, M.G.; González, J.; Sánchez, M.; González-Coloma, A.; Sanchis, J.; Elhachimi, L.; Olmeda, A.S.; Tercero, J.M.; et al. Acaricidal and Repellent Effects of Essential Oils against Ticks: A Review. Pathogens 2021, 10, 1379. [Google Scholar] [CrossRef]
- Stern, O.; Volmer, M. Über Die Abklingungszeit Der Fluoreszenz. Phys. Z. 1919, 20, 183–188. [Google Scholar] [CrossRef]
- Gehlen, M.H. The Centenary of the Stern-Volmer Equation of Fluorescence Quenching: From the Single Line Plot to the SV Quenching Map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Lehrer, S. Solute Perturbation of Protein Fluorescence. Quenching of the Tryptophyl Fluorescence of Model Compounds and of Lysozyme by Iodide Ion. Biochemistry 1971, 10, 3254–3263. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Alatorre-Barajas, J.A.; Ramírez-Zatarain, S.D.; Ochoa-Terán, A.; Cordova, J.; Reynoso-Soto, E.A.; Chávez, D.; Miranda-Soto, V.; Labastida-Galván, V.; Ordoñez, M. An Efficient Method for the Synthesis of New Non-Symmetrical Naphthalenediimides. ChemistrySelect 2018, 3, 11943–11949. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 Update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).