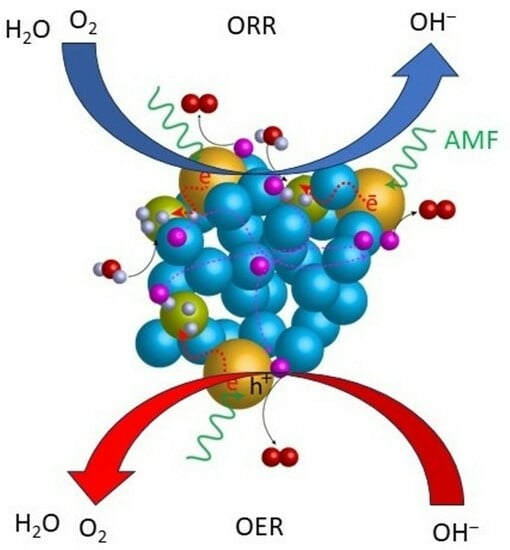
Molecular Catalysts for OER/ORR in Zn–Air Batteries
Abstract
:1. Introduction
| Top 5 Countries/Regions, Share of Commissioned Capacity, 2020 (747 GWh) | Leading Producers | Main Countries in Which Leading Producers Own Battery Manufacturing Plants |
|---|---|---|
| China 76% (568 GWh) U.S.A. 8% (60 GWh) Europe 7% (52 GWh) Republic of Korea 5% (37 GWh) Japan 4% (30 GWh) | CATL (Ningde, China) | China (Shenyang) JV “BBA” with BMW Group, Germany (Erfurt) |
| BYD (Shenzhen, China) | China (Pingshan in Shenzhen) France, Hungary | |
| Panasonic (Osaka, Japan) | Japan, China, USA (Nevada) | |
| LG (Seoul, Republic of Korea) | Republic of Korea (Ochang), China (Nanjing), USA (Holland, Michigan), Poland (Wroclaw) | |
| Samsung (Suwon, Republic of Korea) | Republic of Korea (Ulsan; Pohang), USA (Auburn Hill), China (Tianjin, Xi’an), Europe (Hungary, Austria), India, Malaysia, Vietnam | |
| SK Innovation (Seoul, Republic of Korea) Gotion High-Tech (Hefei, China), Envision (Shanghai, China), AESC (Yokohama, Japan) | Republic of Korea (Seosan), China (Changzhou, Jiangsu), USA (Commerce, Georgia; two locations in JV with Ford), Hungary (Komaron, Ivancsá) Japan (Kanagawa); USA (Tennessee), UK (Sunderland) and China (Jiangyin) China (Hefei), Germany (Salzgitter) |

2. Air Electrode Catalysts
2.1. Activated Carbon Materials
2.2. Monometallic Catalysts
| Air Electrode Catalyst | Active Material | Max Power Density (mW cm−2) | Specific Capacity (mAh g−1) | Duration of Tests (h) | Ref |
|---|---|---|---|---|---|
| Fe/N–C | FeN4 embedded in N-doped carbon. | 225 | 636 | 260 | [72] |
| Fe–NCCs | Atomic Fe-Nx dispersed in carbon. | 66 | 705 | 67 | [73] |
| FeNx–PNC | FeNx on 2D N-doped carbon. | 278 | n/a | 40 | [74] |
| SA–Fe/NG | Fe-pyrrolic-N species on N-doped carbon. | 91 | n/a | 20 | [75] |
| Co/GO | Atomically dispersed Co on GO. | 225 | 795 | 50 | [76] |
| Zn/CoN–C | Zn and Co atoms coordinated via N on carbon. | 230 | n/a | 28 | [77] |
2.3. Mixed Metal Oxide Catalysts
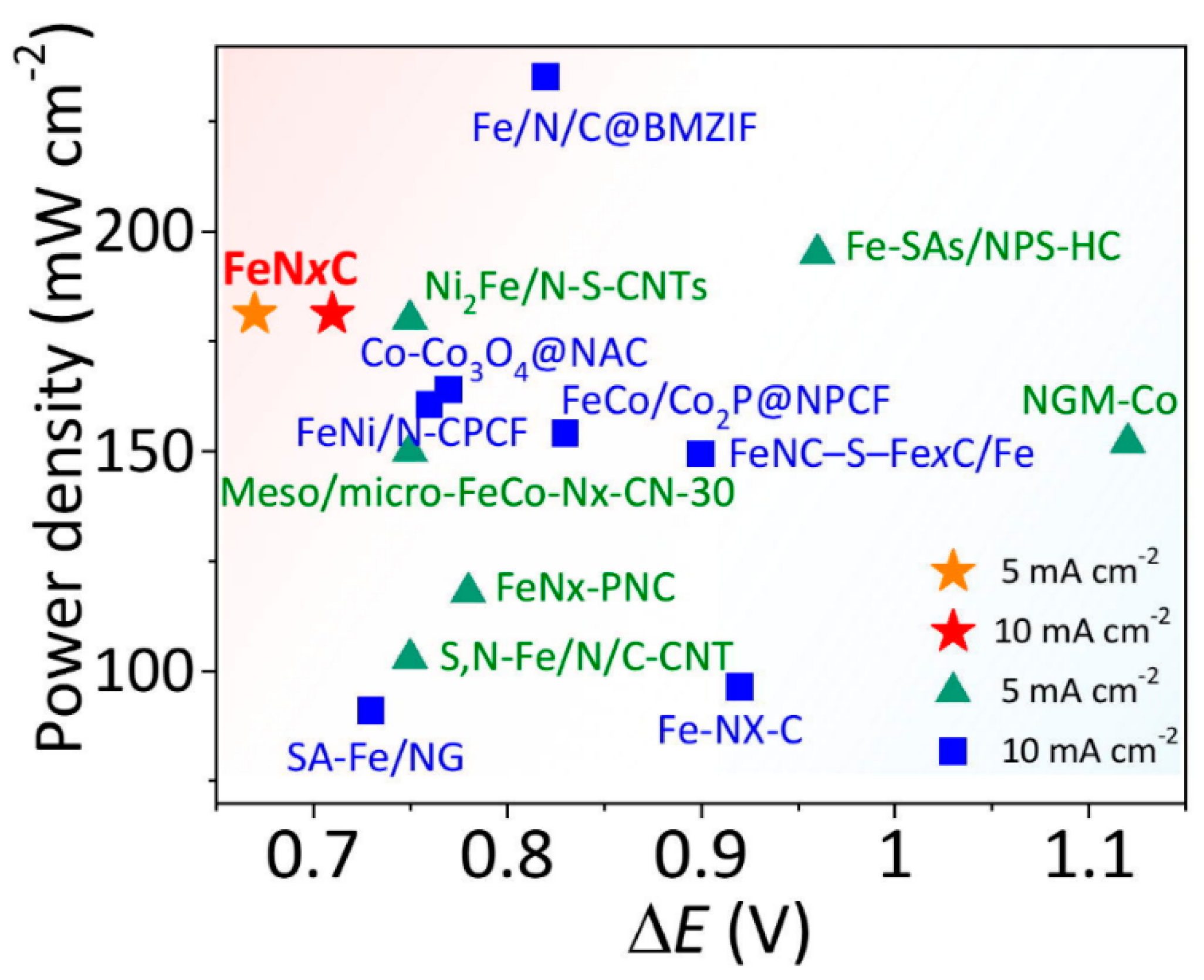
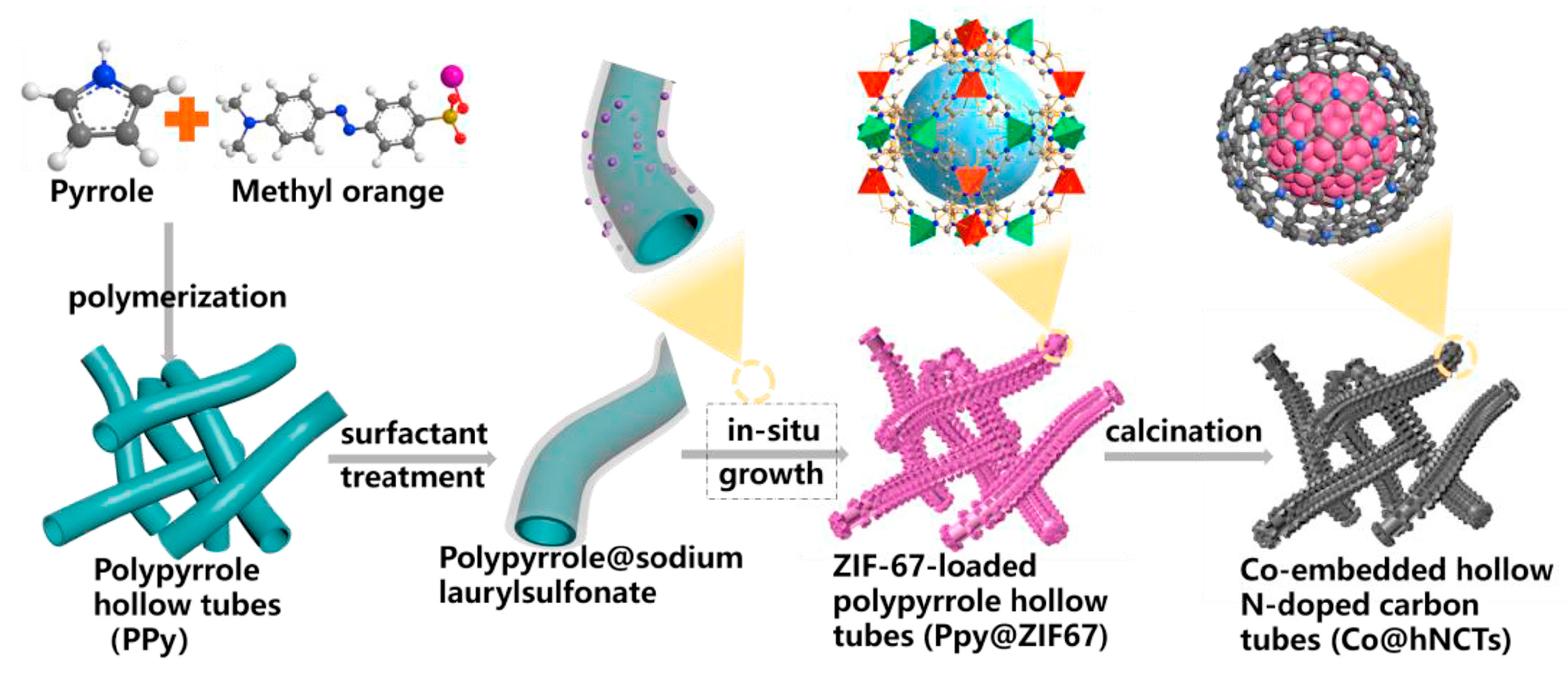
2.4. Bi- and Multi-Metallic Catalysts
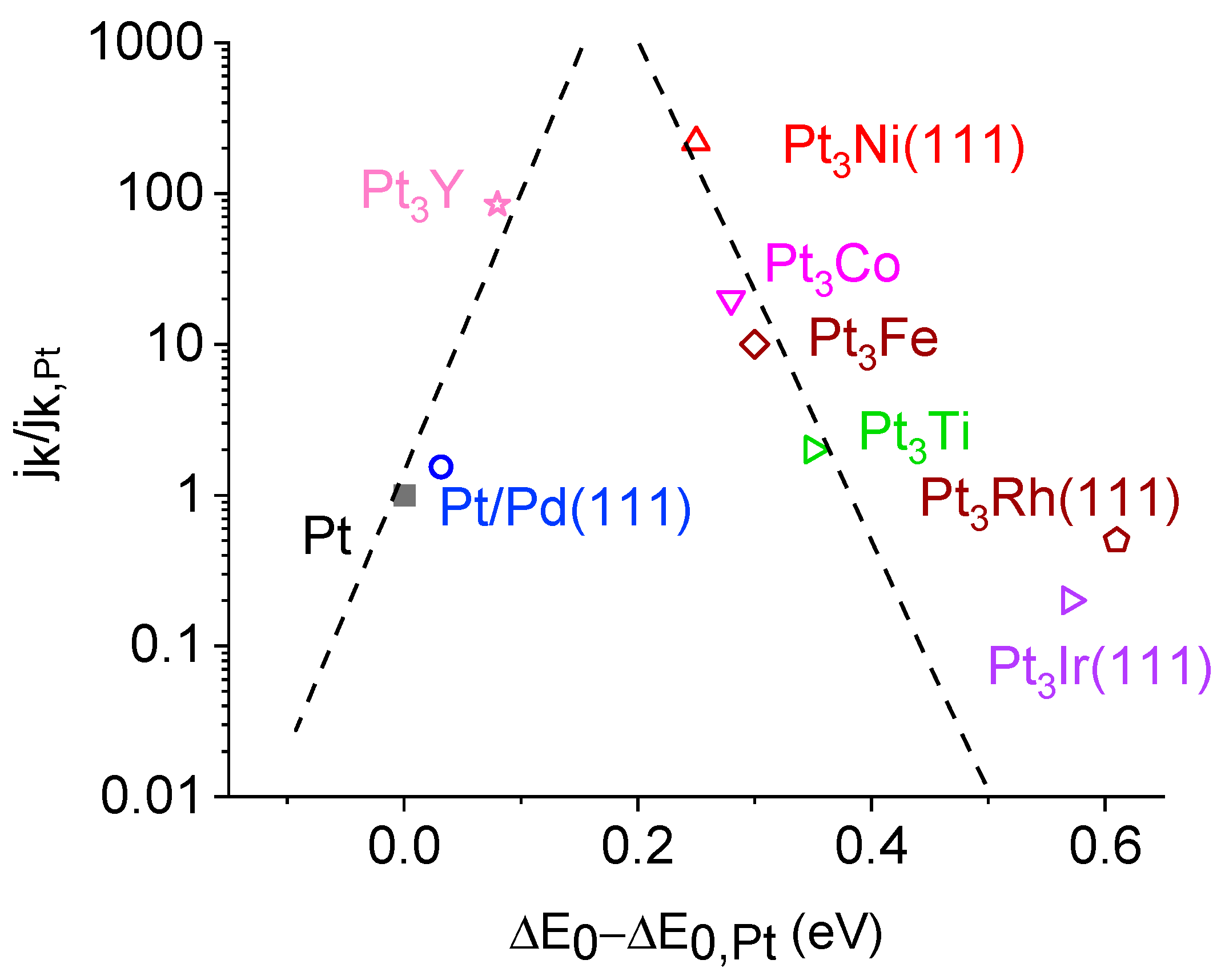
2.5. Layered Double Hydroxides
3. Effect of an External Magnetic Field
3.1. Spin Polarization Effect
3.2. Magnetothermal Effect
4. Conclusions and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Cole, W.; Frew, B.; Gagnon, P.; Reimers, A.; Zuboy, J.; Margolis, R. Envisioning a low-cost solar future: Exploring the potential impact of achieving the SunShot 2030 targets for photovoltaics. Energy 2018, 155, 690–704. [Google Scholar] [CrossRef]
- Bellini, E. Portuguese Government Confirms World Record Solar Price of $0.01316/kWh. PV Mag. Int. 2020. Available online: https://www.pv-magazine.com/2020/08/27/portuguese-government-confirms-world-record-solar-price-of-0-01316-kwh (accessed on 24 July 2023).
- He, W.; King, M.; Luo, X.; Dooner, M.; Li, D.; Wang, J. Technologies and economics of electric energy storages in power systems: Review and perspective. Adv. Appl. Energy 2021, 4, 100060. [Google Scholar] [CrossRef]
- International Energy Agency Global EV Outlook 2020: Entering the Decade of Electric Drive? IEA Paris 2020. Available online: https://www.iea.org/reports/global-ev-outlook-2020 (accessed on 24 July 2023).
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–air batteries: From static to flow system. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Shaulova, E.; Biagi, L. Lithium Industry Worldwide. Statistics Report on the Global Lithium Industry. 2023. Available online: https://www.statista.com/study/40094/lithium-statista-dossier (accessed on 24 July 2023).
- Ellis, D. Lithium-Ion Batteries Demand to Grow 30% a Year—McKinsey. Mining 2022. Available online: https://miningdigital.com/supply-chain-and-operations/lithium (accessed on 24 July 2023).
- FCAB. Executive Summary. National Blueprint for Lithium Batteries 2021–2030. 2021. Available online: https://www.energy.gov (accessed on 24 July 2023).
- BMI. BYD Becomes Benchmark’s 7th Tier One EV Battery Manufacturer; 2nd China Cell Maker. 2021. Available online: https://www.benchmarkminerals.com/membership/byd-becomes-benchmarks-7th-tier-one-ev-battery-manufacturer-2nd-china-cell-maker-2/ (accessed on 19 May 2023).
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn–air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Khan, P.A.; Venkatesh, B. Economic Analysis of Chemical Energy Storage Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 277–291. [Google Scholar]
- O’Neill, A. World Bank Commodities Price Forecast; World Bank: Washington, DC, USA, 2022. [Google Scholar]
- Fu, J.; Liang, R.; Liu, G.; Yu, A.; Bai, Z.; Yang, L.; Chen, Z. Recent progress in electrically rechargeable zinc–air batteries. Adv. Mater. 2019, 31, 1805230. [Google Scholar] [CrossRef]
- Kiss, A.M.; Myles, T.D.; Grew, K.N.; Peracchio, A.A.; Nelson, G.J.; Chiu, W.K.S. Carbonate and bicarbonate ion transport in alkaline anion exchange membranes. J. Electrochem. Soc. 2013, 160, F994–F999. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, L.; Chen, J. Recent advances in isolated single-atom catalysts for zinc air batteries: A focus review. Nanomaterials 2019, 9, 1402. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Y.; Connell, J.W.; Cheng, H.; Gogotsi, Y.; Titirici, M.; Dai, L. Carbon-based metal-free catalysts for energy storage and Environmental Remediation. Adv. Mater. 2019, 31, 1806128. [Google Scholar] [CrossRef] [PubMed]
- Meffre, A.; Mehdaoui, B.; Kelsen, V.; Fazzini, P.F.; Carrey, J.; Lachaize, S.; Respaud, M.; Chaudret, B. A Simple Chemical Route toward Monodisperse Iron Carbide Nanoparticles Displaying Tunable Magnetic and Unprecedented Hyperthermia Properties. Nano Lett. 2012, 12, 4722–4728. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ge, X.; Goh, F.W.T.; Hor, T.S.A.; Geng, D.; Du, G.; Liu, Z.; Zhang, J.; Liu, X.; Zong, Y. Co3O4 nanoparticles decorated carbon nanofiber mat as binder-free air-cathode for high performance rechargeable zinc-air batteries. Nanoscale 2015, 7, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Yuxin, Z.; Ying, Y.; Chunzhen, Z.; Meipeng, Z.; Yue, W.; Fengliang, Z.; Lili, R. Air cathode catalyst layer ink suitable for aerosol printing and preparation method thereof. Patent CN113871761A, 31 December 2021. [Google Scholar]
- Pi, Y.-T.; Xing, X.-Y.; Lu, L.-M.; He, Z.-B.; Ren, T.-Z. Hierarchical porous activated carbon in OER with high efficiency. RSC Adv. 2016, 6, 102422–102427. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Li, C.; Li, Y.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of Nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angew. Chem. Int. Ed. 2014, 54, 588–593. [Google Scholar] [CrossRef]
- Fukuyama, H.; Terai, S. Preparing and characterizing the active carbon produced by steam and carbon dioxide as a heavy oil hydrocracking catalyst support. Catal. Today 2008, 130, 382–388. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, P.; Zhang, R.; Liu, K.; Liu, Y.; Liu, T.; Wang, X. A novel approach of binary doping sulfur and nitrogen into graphene layers for enhancing electrochemical performances of supercapacitors. J. Mater. Chem. A 2016, 4, 19053–19059. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, H.; Li, L.; Yao, Y.; Qu, H.; Zhang, C.; Liu, S.; Zhou, Y. Double soft-template synthesis of nitrogen/sulfur-codoped hierarchically porous carbon materials derived from protic ionic liquid for supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26088–26095. [Google Scholar] [CrossRef]
- Gupta, S.; Zhao, S.; Xu, H.; Wu, G. Highly stable nanocarbon catalysts for bifunctional oxygen reduction and evolution reactions in alkaline media. ECS Meet. Abstr. 2017, MA2017-01, 1423. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Higgins, D.; Nazar, L.; Chen, Z. Highly active graphene nanosheets prepared via extremely rapid heating as efficient zinc-air battery electrode material. J. Electrochem. Soc. 2013, 160, F910–F915. [Google Scholar] [CrossRef]
- Chengen, G.H.; Chao, G.X. Carbon-coated graphene/metal oxide composite material and preparation method thereof. Patent CN113690429A, 23 August 2021. [Google Scholar]
- Zhou, Q.; Zhang, Z.; Cai, J.; Liu, B.; Zhang, Y.; Gong, X.; Sui, X.; Yu, A.; Zhao, L.; Wang, Z.; et al. Template-guided synthesis of Co nanoparticles embedded in hollow nitrogen doped carbon tubes as a highly efficient catalyst for rechargeable Zn–air batteries. Nano Energy 2020, 71, 104592. [Google Scholar] [CrossRef]
- Long, J.; Chen, C. Fe-based bimetallic zinc-air battery cathode catalyst based on layered MOF. Patent CN113097513A, 2 April 2021. [Google Scholar]
- Jaksic, J.M.; Nan, F.; Papakonstantinou, G.D.; Botton, G.A.; Jaksic, M.M. Theory, substantiation, and properties of novel reversible electrocatalysts for oxygen electrode reactions. J. Phys. Chem. C 2015, 119, 11267–11285. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in Zn–Air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef]
- Sequeira, C.A.C. Carbon Anode in Carbon History. Molecules 2020, 25, 4996. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Meymian, M.-R.; Khanmohammadi Chenab, K.; Pourzolfaghar, H. Designing high-quality electrocatalysts based on CoO:MnO2@C supported on carbon cloth fibers as bifunctional air cathodes for application in rechargeable Zn–Air battery. ACS Appl. Mater. Interfaces 2022, 14, 55594–55607. [Google Scholar] [CrossRef]
- Song, Z.; Han, X.; Deng, Y.; Zhao, N.; Hu, W.; Zhong, C. Clarifying the Controversial Catalytic Performance of Co(OH)2 and Co3O4 for oxygen reduction/evolution reactions toward efficient Zn–air batteries. ACS Appl. Mater. Interfaces 2017, 9, 22694–22703. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, X.; Tu, T.; Zhang, P.; Li, J.; Zhou, Y.; Huang, L.; Sun, S. Controlled synthesis of FeNx-CoNx dual active sites interfaced with metallic Co nanoparticles as bifunctional oxygen electrocatalysts for rechargeable Zn–air batteries. Appl. Catal. B Environ. 2020, 278, 119259. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, P.; Chen, G.; Chen, J.; Yan, Y.; Qi, K.; Liu, H.; Xia, B.Y. 2D Nitrogen-doped carbon nanotubes/Graphene hybrid as bifunctional oxygen electrocatalyst for long-life rechargeable Zn–Air batteries. Adv. Funct. Mater. 2020, 30, 1906081. [Google Scholar] [CrossRef]
- Sun, X.; Gong, Q.; Liang, Y.; Wu, M.; Xu, N.; Gong, P.; Sun, S.; Qiao, J. Exploiting a high-performance “double-carbon” structure Co9S8/GN bifunctional catalysts for rechargeable Zn–Air batteries. ACS Appl. Mater. Interfaces 2020, 12, 38202–38210. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Li, J.; Huang, H.; Qiao, J. In situ growth of CoP nanoparticles anchored on (N,P) co-doped porous carbon engineered by MOFs as advanced bifunctional oxygen catalyst for rechargeable Zn–air battery. J. Mater. Chem. A 2020, 8, 19043–19049. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, H.; Niu, T.; Wang, S.; Fu, G.; Jin, W.; Ma, T. Sulfurated Metal–Organic framework-derived nanocomposites for efficient bifunctional oxygen electrocatalysis and rechargeable Zn–air battery. ACS Sustain. Chem. Eng. 2020, 8, 9226–9234. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, S.; Luo, Y.; Pang, H. Synthesis of confining cobalt nanoparticles within SiOx /nitrogen-doped carbon framework derived from sustainable bamboo leaves as oxygen electrocatalysts for rechargeable Zn–air batteries. Chem. Eng. J. 2020, 401, 126005. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Hu, C.; Ji, M.; Wang, M.; Yu, J.; Liu, H.; Zhu, C.; Xu, J. Co/Co9S8@carbon nanotubes on a carbon sheet: Facile controlled synthesis, and application to electrocatalysis in oxygen reduction/oxygen evolution reactions, and to a rechargeable Zn–air battery. Inorg. Chem. Front. 2021, 8, 368–375. [Google Scholar] [CrossRef]
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Xu, X.; Tadé, M.O.; Shao, Z. A Porous nano-micro-composite as a high-performance bi-functional air electrode with remarkable stability for rechargeable Zinc–Air batteries. Nano-Micro Lett. 2020, 12, 130. [Google Scholar] [CrossRef]
- Wang, X.; Ge, L.; Lu, Q.; Dai, J.; Guan, D.; Ran, R.; Weng, S.-C.; Hu, Z.; Zhou, W.; Shao, Z. High-performance metal-organic framework-perovskite hybrid as an important component of the air-electrode for rechargeable Zn-Air battery. J. Power Sources 2020, 468, 228377. [Google Scholar] [CrossRef]
- Tan, J.; Thomas, T.; Liu, J.; Yang, L.; Pan, L.; Cao, R.; Shen, H.; Wang, J.; Liu, J.; Yang, M. Rapid microwave-assisted preparation of high-performance bifunctional Ni3Fe/Co-N-C for rechargeable Zn–air battery. Chem. Eng. J. 2020, 395, 125151. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Cui, Z.; Zhang, Q.; Chen, X.; Sui, J.; Dong, H.; Yu, L.; Dong, L. Hierarchical architecture derived from two-dimensional zeolitic imidazolate frameworks as an efficient metal-based bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. Electrochim. Acta 2020, 331, 135394. [Google Scholar] [CrossRef]
- Yu, N.-F.; Wu, C.; Huang, W.; Chen, Y.-H.; Ruan, D.-Q.; Bao, K.-L.; Chen, H.; Zhang, Y.; Zhu, Y.; Huang, Q.-H.; et al. Highly efficient Co3O4/Co@NCs bifunctional oxygen electrocatalysts for long life rechargeable Zn–air batteries. Nano Energy 2020, 77, 105200. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Yang, H.; Zhang, L.; Shao, J.; Huang, W.; Liu, B.; Dong, X. Anchoring Mn3O4 Nanoparticles on oxygen functionalized carbon nanotubes as bifunctional catalyst for rechargeable zinc-air battery. ACS Appl. Energy Mater. 2018, 1, 963–969. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, X.; Gu, X.; Li, G.; Mu, Y.; Wang, N.; Ithisuphalap, K.; Wang, H.; Guo, Z.; Shi, Z.; et al. Mn3O4 quantum dots supported on nitrogen-doped partially exfoliated multiwall carbon nanotubes as oxygen reduction electrocatalysts for high-performance Zn–Air batteries. ACS Appl. Mater. Interfaces 2018, 10, 23900–23909. [Google Scholar] [CrossRef]
- Sidhureddy, B.; Prins, S.; Wen, J.; Thiruppathi, A.R.; Govindhan, M.; Chen, A. Synthesis and electrochemical study of mesoporous nickel-cobalt oxides for efficient oxygen reduction. ACS Appl. Mater. Interfaces 2019, 11, 18295–18304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Peng, J.; Peng, K. Bifunctional NiCo2O4 porous nanotubes electrocatalyst for overall water-splitting. Electrochim. Acta 2019, 318, 762–769. [Google Scholar] [CrossRef]
- Dilshad, K.A.J.; Rabinal, M.K. Rationally designed Zn-anode and Co3O4-cathode nanoelectrocatalysts for an efficient Zn–air battery. Energy Fuels 2021, 35, 12588–12598. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Teshager, M.A.; Workineh, Z.G. Recent progress in MnO2-based oxygen electrocatalysts for rechargeable zinc-air batteries. Mater. Today Sustain. 2021, 13, 100072. [Google Scholar] [CrossRef]
- Rittiruam, M.; Buapin, P.; Saelee, T.; Khajondetchairit, P.; Kheawhom, S.; Alling, B.; Praserthdam, S.; Ektarawong, A.; Praserthdam, P. First-principles calculation on effects of oxygen vacancy on α-MnO2 and β-MnO2 during oxygen reduction reaction for rechargeable metal-air batteries. J. Alloys Compd. 2022, 926, 166929. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Nie, Y.; Chen, S.; Shi, F.; Xiong, K.; Ding, W.; Qi, X.; Hu, J.; Wei, Z.; et al. Insight into the effect of oxygen vacancy concentration on the catalytic performance of MnO2. ACS Catal. 2015, 5, 4825–4832. [Google Scholar] [CrossRef]
- Gupta, P.K.; Bhandari, A.; Saha, S.; Bhattacharya, J.; Pala, R.G.S. Modulating oxygen evolution reactivity in MnO2 through polymorphic engineering. J. Phys. Chem. C 2019, 123, 22345–22357. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Hu, L.; Tian, R.; Wang, Y.; Arandiyan, H.; Chen, F.; Li, M.; Wan, T.; Han, Z.; et al. A facile approach to tailor electrocatalytic properties of MnO2 through tuning phase transition, surface morphology and band structure. Chem. Eng. J. 2022, 438, 135561. [Google Scholar] [CrossRef]
- Bera, K.; Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Kundu, S. Enhancement of the OER kinetics of the less-explored α-MnO2 via nickel doping approaches in alkaline medium. Inorg. Chem. 2021, 60, 19429–19439. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Zhao, Y.; Li, X.; Sun, Y.; Qian, J.; Xu, Q.; Gao, P.; Wu, D.; Kato, K.; et al. Single atomic Ag enhances the bifunctional activity and cycling stability of MnO2. Chem. Eng. J. 2019, 366, 631–638. [Google Scholar] [CrossRef]
- Zhang, J.-N. (Ed.) Carbon-Based Nanomaterials for Energy Conversion and Storage. In Springer Series in Materials Science; Springer Nature Singapore: Singapore, 2022; Volume 325, ISBN 978-981-19-4624-0. [Google Scholar]
- Singh, B.; Gawande, M.B.; Kute, A.D.; Varma, R.S.; Fornasiero, P.; McNeice, P.; Jagadeesh, R.V.; Beller, M.; Zbořil, R. Single-atom (Iron-based) catalysts: Synthesis and applications. Chem. Rev. 2021, 121, 13620–13697. [Google Scholar] [CrossRef]
- Global Industrial Catalyst Market Outlook to 2028. Available online: https://www.researchandmarkets.com/reports/5778274/global-industrial-catalyst-market-outlook (accessed on 11 July 2023).
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.; Jiang, H. From Metal–organic frameworks to single-atom Fe implanted N-doped porous carbons: Efficient oxygen reduction in both alkaline and acidic media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.-A.; Xue, X.; Yin, H.; Cheng, W.; Cheng, J.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Kim, K.; Kim, S.; Lee, S.; Han, J.W.; Lee, J. Versatile strategy for tuning ORR activity of a single Fe-N4 site by controlling electron-withdrawing/donating properties of a carbon plane. J. Am. Chem. Soc. 2019, 141, 6254–6262. [Google Scholar] [CrossRef]
- Liu, K.; Fu, J.; Lin, Y.; Luo, T.; Ni, G.; Li, H.; Lin, Z.; Liu, M. Insights into the activity of single-atom Fe-N-C catalysts for oxygen reduction reaction. Nat. Commun. 2022, 13, 2075. [Google Scholar] [CrossRef]
- Shen, H.; Thomas, T.; Rasaki, S.A.; Saad, A.; Hu, C.; Wang, J.; Yang, M. Oxygen Reduction Reactions of Fe-N-C Catalysts: Current Status and the Way Forward. Electrochem. Energy Rev. 2019, 2, 252–276. [Google Scholar] [CrossRef]
- Li, Y.; Xu, K.; Zhang, Q.; Zheng, Z.; Li, S.; Zhao, Q.; Li, C.; Dong, C.; Mei, Z.; Pan, F.; et al. One-pot synthesis of FeNxC as efficient catalyst for high-performance zinc-air battery. J. Energy Chem. 2022, 66, 100–106. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Jia, N.; Xu, Q.; Zhao, F.; Gao, H.-X.; Song, J.; Chen, P.; An, Z.; Chen, X.; Chen, Y. Fe/N Codoped Carbon nanocages with single-atom feature as efficient oxygen reduction reaction electrocatalyst. ACS Appl. Energy Mater. 2018, 1, 4982–4990. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Huang, Y.; Liang, G.; Mo, F.; Yang, Q.; Su, J.; Gao, Y.; Zapien, J.A.; et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc–Air Battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, D.; Xu, H.; Zeng, X.; Wan, X.; Shui, J.; Xiang, Z.; Cao, D. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 6626–6631. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Chen, N.; Jia, Y.; Cai, R.; Theis, W.; Yang, X.; Xia, Y.; Yang, D.; Yao, X. Scalable and controllable synthesis of atomic metal electrocatalysts assisted by an egg-box in alginate. J. Mater. Chem. A 2018, 6, 18417–18425. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, B.; Hu, Y.; Liu, W.; Zhao, Y.; Yang, R.; Li, Z.; Luo, J.; Chi, B.; Jiang, Z.; et al. An isolated zinc–cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. 2019, 131, 2648–2652. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Fang, G.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.-J. Multicomponent spinel metal oxide nanocomposites as high-performance bifunctional catalysts in Zn–Air batteries. ACS Appl. Energy Mater. 2020, 3, 7710–7718. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Song, W.; Ren, Z.; Chen, S.-Y.; Meng, Y.; Biswas, S.; Nandi, P.; Elsen, H.A.; Gao, P.-X.; Suib, S.L. Ni- and Mn-promoted mesoporous Co3O4: A stable bifunctional catalyst with surface-structure-dependent activity for oxygen reduction reaction and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 20802–20813. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese–cobalt oxide and Graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Qiao, J.; Zhang, X.; Ma, C.; Jian, S.; Liu, Y.; Pei, P. Morphology controlled La2O3/Co3O4/MnO2–CNTs hybrid nanocomposites with durable bi-functional air electrode in high-performance zinc–air energy storage. Appl. Energy 2016, 175, 495–504. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, X.; Liang, Y.; Zhang, G.; Wang, X.; Yan, Y.; Li, X.; Yan, G.; Wang, J. Anchoring NiCo2O4 nanowhiskers in biomass-derived porous carbon as superior oxygen electrocatalyst for rechargeable Zn–air battery. J. Power Sources 2020, 476, 228684. [Google Scholar] [CrossRef]
- Go, Y.; Min, K.; An, H.; Kim, K.; Eun Shim, S.; Baeck, S.-H. Oxygen-vacancy-rich CoFe/CoFe2O4 embedded in N-doped hollow carbon spheres as a highly efficient bifunctional electrocatalyst for Zn–air batteries. Chem. Eng. J. 2022, 448, 137665. [Google Scholar] [CrossRef]
- Jose, V.; Hu, H.; Edison, E.; Manalastas, W.; Ren, H.; Kidkhunthod, P.; Sreejith, S.; Jayakumar, A.; Nsanzimana, J.M.V.; Srinivasan, M.; et al. Modulation of Single Atomic Co and Fe Sites on hollow carbon nanospheres as oxygen electrodes for rechargeable Zn–Air batteries. Small Methods 2021, 5, 2000751. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Liu, R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-N-C nanofibers with embedding FeCo alloy nanoparticles. Appl. Catal. B Environ. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Ni3FeN Nanoparticles Derived from Ultrathin NiFe-Layered Double Hydroxide Nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Jensen, K.D.; Tymoczko, J.; Rossmeisl, J.; Bandarenka, A.S.; Chorkendorff, I.; Escudero-Escribano, M.; Stephens, I.E.L. Elucidation of the oxygen reduction volcano in alkaline media using a copper-platinum (111) alloy. Angew. Chem. Int. Ed. 2018, 57, 2800–2805. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shan, H.; Zhou, Z.; Yan, Y.; Chen, W.; Yang, Y.; Liu, Y.; Tian, H.; Wu, J.; Zhang, H.; et al. Tuning surface structure and strain in Pd-Pt core-shell nanocrystals for enhanced electrocatalytic oxygen reduction. Small 2017, 13, 1603423. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Fu, G.; Li, Y.; Goodenough, J.B. Ni3FeN-Supported Fe3Pt intermetallic nanoalloy as a high-performance bifunctional catalyst for Metal–Air Batteries. Angew. Chem. Int. Ed. 2017, 56, 9901–9905. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, F.; Huang, M.; Guan, L. Ternary PtZnCu Intermetallic Nanoparticles as an efficient oxygen reduction electrocatalyst for fuel cells with ultralow Pt loading. ACS Appl. Energy Mater. 2022, 5, 12219–12226. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, W.; Liu, S.; Wang, X.; Li, G.; Shi, B.; Wang, K.; Wang, X.; Wang, Q.; Jia, Y. A magnetic field strategy to porous Pt-Ni nanoparticles with predominant (111) facets for enhanced electrocatalytic oxygen reduction. J. Energy Chem. 2021, 53, 192–196. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Karlberg, G.S.; Jaramillo, T.; Nørskov, J.K. Steady state oxygen reduction and cyclic voltammetry. Faraday Discuss. 2009, 140, 337–346. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Klinger, E.A.; Berenguer-Murcia, A.; Sulman, E.M.; Schouten, J.C. Selective hydrogenation of 2-Methyl-3-butyne-2-ol in a wall-coated capillary microreactor with a Pd25Zn75/TiO2 Catalyst. Org. Process Res. Dev. 2009, 13, 991–998. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Rebrov, E.V. Novel synthesis of thick wall coatings of titania supported Bi poisoned Pd catalysts and application in selective hydrogenation of acetylene alcohols in capillary microreactors. Lab Chip 2015, 15, 1952–1960. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, H.; Zhao, M.; DiSalvo, F.J. High-Performance Pd3Pb Intermetallic Catalyst for Electrochemical Oxygen Reduction. Nano Lett. 2016, 16, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Slanac, D.A.; Hardin, W.G.; Johnston, K.P.; Stevenson, K.J. Atomic ensemble and electronic effects in Ag-rich AgPd nanoalloy catalysts for oxygen reduction in alkaline media. J. Am. Chem. Soc. 2012, 134, 9812–9819. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Merisalu, M.; Kasikov, A.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Oxygen electroreduction in alkaline solution on Pd coatings prepared by galvanic exchange of copper. Electrocatalysis 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Betancourt, L.E.; Rojas-Pérez, A.; Orozco, I.; Frenkel, A.I.; Li, Y.; Sasaki, K.; Senanayake, S.D.; Cabrera, C.R. Enhancing ORR performance of bimetallic PdAg electrocatalysts by designing interactions between Pd and Ag. ACS Appl. Energy Mater. 2020, 3, 2342–2349. [Google Scholar] [CrossRef]
- Biao, K.; Dongwei, L.; Yanjun, H.; Zeng, W.M. Au/Cu2O composite material, super-assembly preparation method and application. Patent No. CN113707890A, 17 August 2021. [Google Scholar]
- Wang, Q.; O’Hare, D. Recent Advances in the synthesis and application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Huang, Y. Electron-rich NiFe layered double hydroxides via interface engineering for boosting electrocatalytic oxygen evolution. Appl. Catal. B Environ. 2021, 297, 120453. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Recent Development of Ni/Fe-based micro/nanostructures toward Photo/Electrochemical water oxidation. Adv. Energy Mater. 2020, 10, 1900954. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Akram, B.; Wang, X. Fabrication of NiFe layered double hydroxide with well-defined laminar superstructure as highly efficient oxygen evolution electrocatalysts. Nano Res. 2019, 12, 1327–1331. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially amorphous nickel–iron layered double hydroxide nanosheet arrays for robust bifunctional electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, K.; Pi, Y.; Huang, X. Superior Electrochemical Oxygen Evolution Enabled by Three-Dimensional Layered Double hydroxide nanosheet superstructures. ChemCatChem 2017, 9, 84–88. [Google Scholar] [CrossRef]
- Li, K.; Guo, D.; Kang, J.; Wei, B.; Zhang, X.; Chen, Y. Hierarchical hollow spheres assembled with ultrathin CoMn double hydroxide nanosheets as trifunctional electrocatalyst for overall water splitting and Zn air battery. ACS Sustain. Chem. Eng. 2018, 6, 14641–14651. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, T.; Ji, G.; Hu, N.; Xu, C.; Zhang, Y. Core/shell design of efficient electrocatalysts based on NiCo2O4 nanowires and NiMn LDH nanosheets for rechargeable zinc–air batteries. J. Mater. Chem. A 2018, 6, 10243–10252. [Google Scholar] [CrossRef]
- Loizou, K.; Mourdikoudis, S.; Sergides, A.; Besenhard, M.O.; Sarafidis, C.; Higashimine, K.; Kalogirou, O.; Maenosono, S.; Thanh, N.T.K.; Gavriilidis, A. rapid millifluidic synthesis of stable high magnetic moment FexCy nanoparticles for hyperthermia. ACS Appl. Mater. Interfaces 2020, 12, 28520–28531. [Google Scholar] [CrossRef] [PubMed]
- Rebrov, E.V.; Gao, P.; Verhoeven, T.M.W.G.M.; Schouten, J.C.; Kleismit, R.; Turgut, Z.; Kozlowski, G. Structural and magnetic properties of sol–gel Co2xNi0.5−xZn0.5−xFe2O4 thin films. J. Magn. Magn. Mater. 2011, 323, 723–729. [Google Scholar] [CrossRef]
- Houlding, T.K.; Gao, P.; Degirmenci, V.; Tchabanenko, K.; Rebrov, E.V. Mechanochemical synthesis of TiO2/NiFe2O4 magnetic catalysts for operation under RF field. Mater. Sci. Eng. B 2015, 193, 175–180. [Google Scholar] [CrossRef]
- Yan, B.; Gao, P.; Lu, Z.; Ma, R.; Rebrov, E.V.; Zheng, H.; Gao, Y. Effect of Pr3+ substitution on the microstructure, specific surface area, magnetic properties and specific heating rate of Ni0.5Zn0.5PrxFe2-xO4 nanoparticles synthesized via sol–gel method. J. Alloys Compd. 2015, 639, 626–634. [Google Scholar] [CrossRef]
- Houlding, T.K.; Rebrov, E.V. Application of alternative energy forms in catalytic reactor engineering. Green Process. Synth. 2012, 1, 19–31. [Google Scholar] [CrossRef]
- Liu, H.; Ren, Y.; Wang, K.; Mu, X.; Song, S.; Guo, J.; Yang, X.; Lu, Z. Magnetic-field-induced strain enhances electrocatalysis of FeCo alloys on anode catalysts for water splitting. Metals 2022, 12, 800. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, T.; Liu, Y.; Zhang, W.; Yin, Z.; Ji, Z.; Wei, J. Magnetic field-enhanced 4-electron pathway for well-aligned Co3O4 /electrospun carbon nanofibers in the oxygen reduction reaction. ChemSusChem 2018, 11, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Peng, J.; Zhang, W.; Peng, K. Magnetic field enhancing electrocatalysis of Co3O4/NF for oxygen evolution reaction. J. Power Sources 2019, 433, 226704. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Z.; Wang, X. Understanding the mechanism of the oxygen evolution reaction with consideration of spin. Electrochem. Energy Rev. 2021, 4, 136–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Li, S.; Sun, J.; Wang, W.; Song, B.; Yang, X.; Wang, X.; Jiang, Z.; Wu, G.; et al. Magnetic field assisted electrocatalytic oxygen evolution reaction of nickel-based materials. J. Mater. Chem. A 2022, 10, 1760–1767. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Xie, J.; Gao, P.; Li, D.; Rebrov, E.V.; Qin, H.; Liu, X.; Xiao, H. Enhanced alkaline oxygen evolution using spin polarization and magnetic heating effects under an AC magnetic field. ACS Appl. Mater. Interfaces 2022, 14, 34627–34636. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, H.; Yang, T.; Yue, S.; Gao, P.; Liu, X.; Xiao, H. AC magnetic field enhancement oxygen evolution reaction of bimetallic metal-organic framework. Int. J. Hydrogen Energy 2022, 47, 18675–18687. [Google Scholar] [CrossRef]
- Wu, T.; Xu, Z.J. Oxygen evolution in spin-sensitive pathways. Curr. Opin. Electrochem. 2021, 30, 100804. [Google Scholar] [CrossRef]
- Wu, T.; Ren, X.; Sun, Y.; Sun, S.; Xian, G.; Scherer, G.G.; Fisher, A.C.; Mandler, D.; Ager, J.W.; Grimaud, A.; et al. Spin pinning effect to reconstructed oxyhydroxide layer on ferromagnetic oxides for enhanced water oxidation. Nat. Commun. 2021, 12, 3634. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, H.; Wang, Y.; Gao, P.; Liu, X.; Rebrov, E.V. Fabrication of magnetic superstructure NiFe2O4 @MOF-74 and its derivative for electrocatalytic hydrogen evolution with AC magnetic field. ACS Appl. Mater. Interfaces 2020, 12, 45987–45996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Yue, S.; Zheng, H.; Liu, X.; Gao, P.; Qin, H.; Xiao, H. Effects of alternating magnetic fields on the OER of heterogeneous core–shell structured NiFe2O4@(Ni,Fe)S/P. ACS Appl. Mater. Interfaces 2023, 15, 11631–11641. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Q.; Zhang, H.; Zhang, M.; Jiang, H.; Zheng, C.; Li, J. Recent advances in catalyst design and activity enhancement induced by a magnetic field for electrocatalysis. J. Mater. Chem. A 2023, 11, 7802–7832. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, L.; He, J.; Ding, D.; Wang, T.; Li, J.; Li, M.; Liu, Y.; Li, Y.; Yuan, M.; et al. Regulating the Spin State of Fe III Enhances the Magnetic Effect of the Molecular Catalysis Mechanism. J. Am. Chem. Soc. 2022, 144, 8204–8213. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Chen, R.R.; Ren, X.; Liu, J.; Ong, S.J.H.; Xu, Z.J. Ferromagnetic–antiferromagnetic coupling core–shell nanoparticles with spin conservation for water oxidation. Adv. Mater. 2021, 33, 2101091. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Jiang, Z.; Zeng, W.; Hu, C.; Luo, X.; Lei, W.; Yuan, C. Alternating magnetic field induced magnetic heating in ferromagnetic cobalt Single-Atom Catalysts for efficient oxygen evolution reaction. Nano Lett. 2022, 22, 9411–9417. [Google Scholar] [CrossRef]
- Niether, C.; Faure, S.; Bordet, A.; Deseure, J.; Chatenet, M.; Carrey, J.; Chaudret, B.; Rouet, A. Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles. Nat. Energy 2018, 3, 476–483. [Google Scholar] [CrossRef]
- Peng, D.; Hu, C.; Luo, X.; Huang, J.; Ding, Y.; Zhou, W.; Zhou, H.; Yang, Y.; Yu, T.; Lei, W.; et al. Electrochemical reconstruction of NiFe/NiFeOOH superparamagnetic core/catalytic shell heterostructure for magnetic heating enhancement of oxygen evolution reaction. Small 2023, 19, 2205665. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Qiao, H.; Li, C.; Huang, Z.; Luo, S.; Qi, X. Magnetic field enhanced surface activity of ferromagnetic Cr2Ge2Te6 nanosheets for electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2023, 637, 157899. [Google Scholar] [CrossRef]
- Su, M.; Zhou, W.; Liu, L.; Chen, M.; Jiang, Z.; Luo, X.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C. Micro eddy current facilitated by screwed MoS2 structure for enhanced hydrogen evolution reaction. Adv. Funct. Mater. 2022, 32, 2111067. [Google Scholar] [CrossRef]
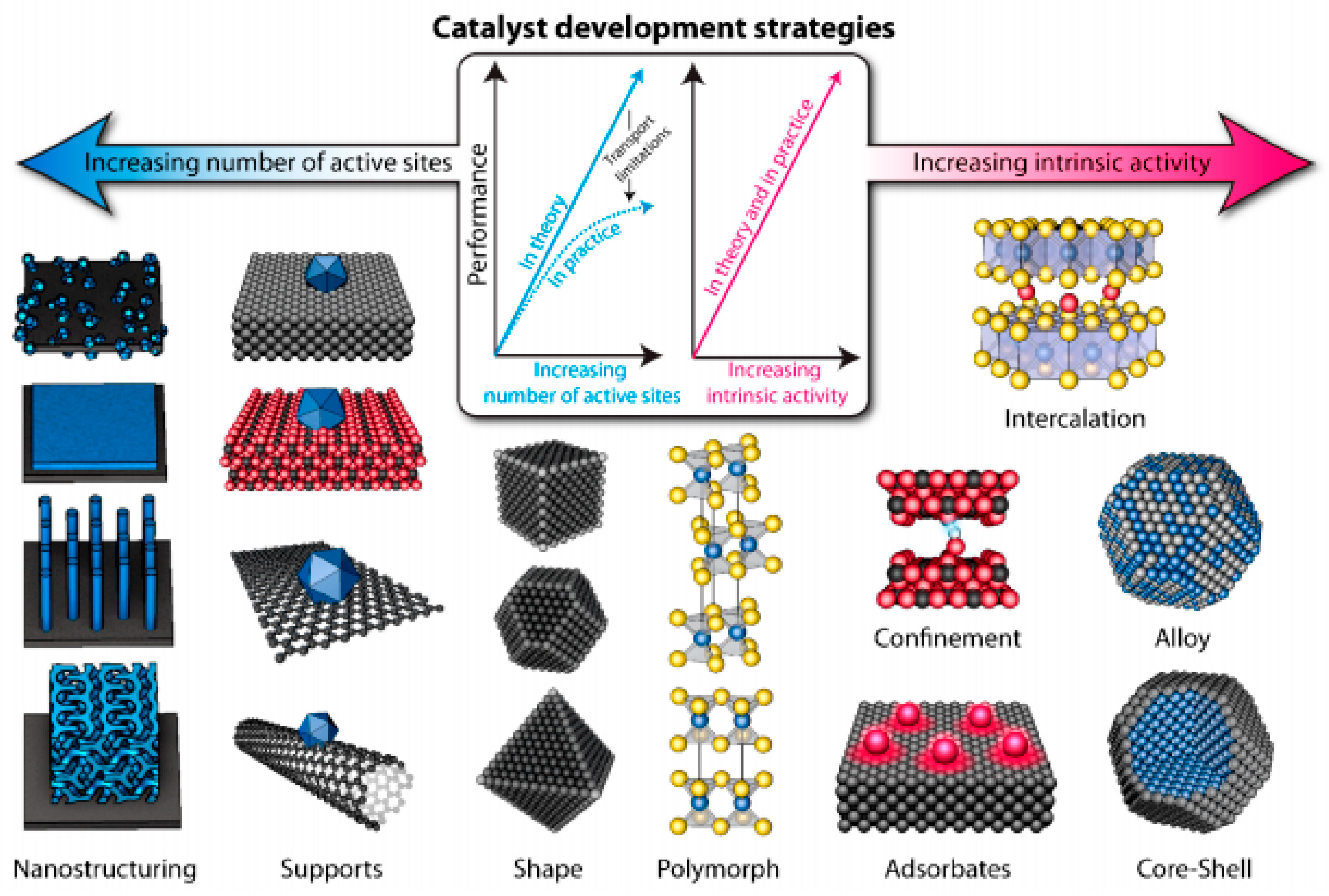
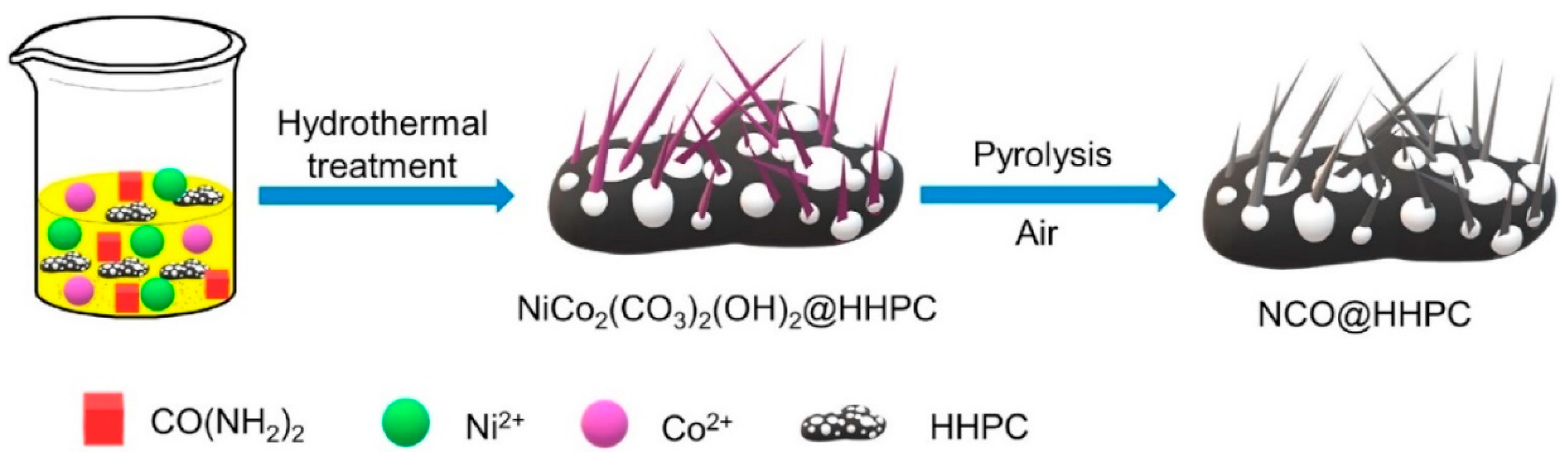
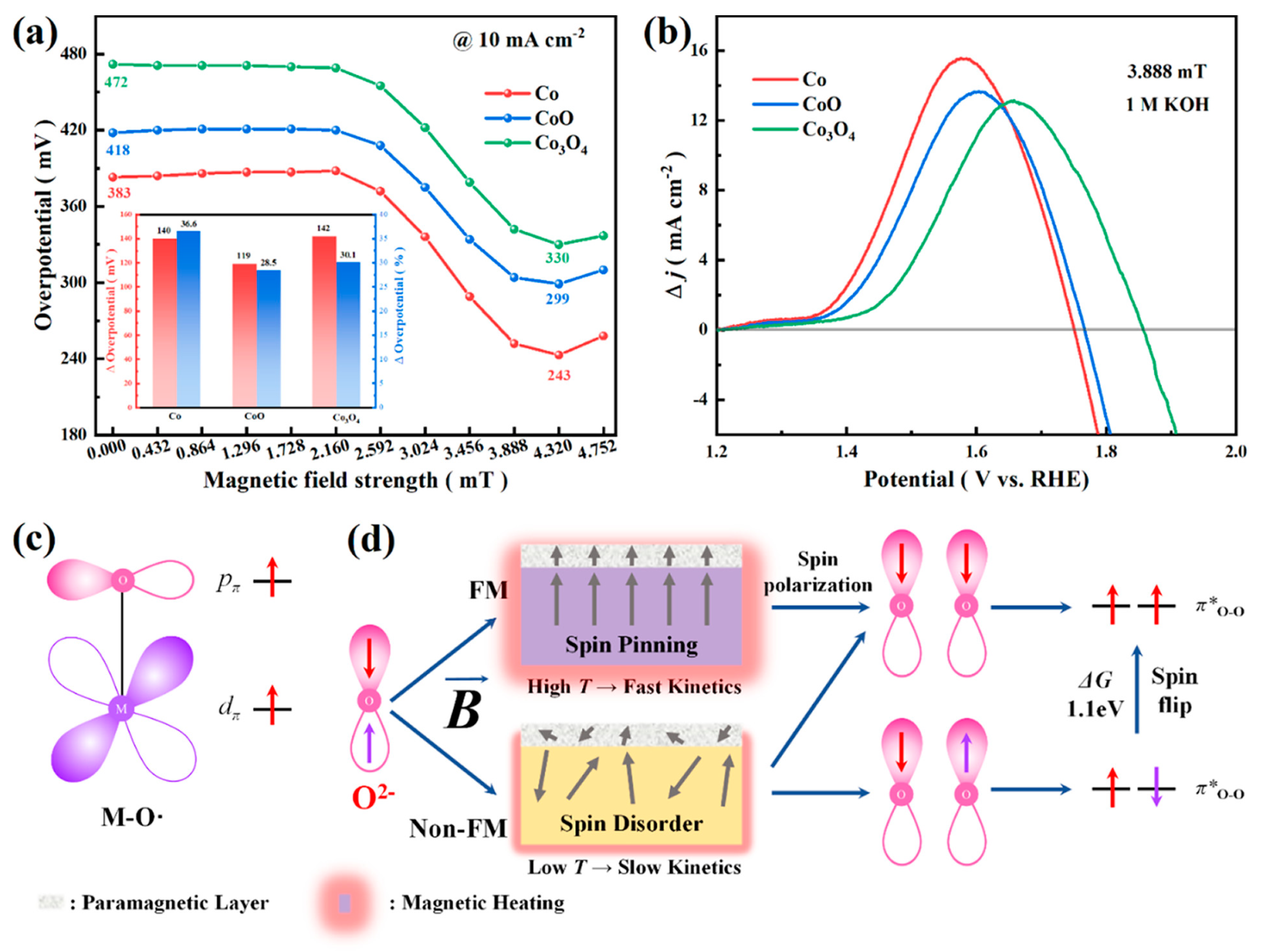
| Catalyst | OCV (V) | PPD (mW cm−2) | Discharge-Charge Voltage Gap (V) at Current Density | Cycling Stability | Ref |
|---|---|---|---|---|---|
| HCo@FeCo/N/C | 1.45 | 125 | 0.84 (10 mA cm−2) | 200 h (10 mA cm−2) | [39] |
| Co@N-CNT hollow nitrogen-doped carbon nanotubes | 1.45 | 149 | 0.85 (5 mA cm−2) | 500 h (5 mA cm−2) | [30] |
| Co/N-doped CNT/graphene hybrid | 1.48 | 253 | 0.76 (5 mA cm−2) | 9000 cycles, 3000 h (5 mA cm−2) | [40] |
| ZIF-derived Co9S8/GN graphene nanosheet | 186 | 0.52 (2 mA cm−2) | 2000 cycles, 147 h (2 mA cm−2) | [41] | |
| CoP/(N,P) codoped porous carbon | 1.4 | 186 | 1.00 (2 mA cm−2) | 80 h (2 mA cm−2) | [42] |
| Ni–Co–S/(N,S) codoped porous carbon | 1.43 | 137 | 0.73 (10 mA cm−2) | 180 cycles (10 mA cm−2) | [43] |
| Co@SiOx/N-doped carbon | 138 | 0.82 (5 mA cm−2) | 400 h (5 mA cm−2) | [44] | |
| Co/Co9S8@CNT | 1.44 | 185 | 0.75 (5 mA cm−2) | 50 cycles, 2000 h (5 mA cm−2) | [45] |
| Ba0.5Sr0.5Co0.8Fe0.2O3 | 0.83 (5 mA cm−2) | 1800 cycles, 300 h (5 mA cm−2) | [46] | ||
| Co-MOF/LaCoO3-δ hybrid | 1.44 | 126 | 0.67 (5 mA cm−2) | 120 h (5 mA cm−2) | [47] |
| Ni3Fe/Co–N–C | 1.39 | 68 | 0.75 (10 mA cm−2) | 65 h (10 mA cm−2) | [48] |
| Co-NCS@nCNT | 1.42 | 90 | 0.89 (5 mA cm−2) | 480 cycles, 80 h (5 mA cm−2) | [49] |
| Co3O4/Co@NC | 1.5 | 124 | 0.94 (10 mA cm−2) | 3600 cycles, 600 h (10 mA cm−2) | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebrov, E.V.; Gao, P.-Z. Molecular Catalysts for OER/ORR in Zn–Air Batteries. Catalysts 2023, 13, 1289. https://doi.org/10.3390/catal13091289
Rebrov EV, Gao P-Z. Molecular Catalysts for OER/ORR in Zn–Air Batteries. Catalysts. 2023; 13(9):1289. https://doi.org/10.3390/catal13091289
Chicago/Turabian StyleRebrov, Evgeny V., and Peng-Zhao Gao. 2023. "Molecular Catalysts for OER/ORR in Zn–Air Batteries" Catalysts 13, no. 9: 1289. https://doi.org/10.3390/catal13091289
APA StyleRebrov, E. V., & Gao, P.-Z. (2023). Molecular Catalysts for OER/ORR in Zn–Air Batteries. Catalysts, 13(9), 1289. https://doi.org/10.3390/catal13091289