Abstract
Solid oxide fuel cells (SOFCs) represent a breed of eco-friendly, weather-independent, decentralized power generation technologies, distinguished for their broad fuel versatility and superior electricity generation efficiency. At present, SOFCs are impeded by a lack of highly efficient oxygen reduction catalysts, a factor that significantly constrains their performance. The double perovskites LnBaCo2O5+δ (Ln = Lanthanide), renowned for their accelerated oxygen exchange and conductivity features, are widely acclaimed as a promising category of cathode catalysts for SOFCs. This manuscript offers a novel perspective on the physicochemical attributes of LnBaCo2O5+δ accumulated over the past two decades and delineates the latest advancements in fine-tuning the composition and nanostructure for SOFC applications. It highlights surface chemistry under operational conditions and microstructure as emerging research focal points towards achieving high-performance LnBaCo2O5+δ catalysts. This review offers a comprehensive insight into the latest advancements in utilizing LnBaCo2O5+δ in the field of SOFCs, presenting a clear roadmap for future developmental trajectories. Furthermore, it provides valuable insights for the application of double perovskite materials in domains such as water electrolysis, CO2 electrolysis, chemical sensors, and metal–air batteries.
1. Introduction
The escalating issues of climate change and energy shortages, predominantly driven by the pervasive and inefficient use of fossil fuels, have intensified the search for novel energy conversion methodologies. Among various power generation technologies, solid oxide fuel cells (SOFCs) hold a unique position. They are particularly noted for their exceptional efficiency rates: 45% to 65% for independent applications and exceeding 85% for combined heat and power applications. Moreover, their fuel compatibility is versatile, ranging from hydrogen to hydrocarbons and even to carbon, making SOFCs a pivotal component in the design of innovative energy solutions [1,2,3]. An SOFC is a solid-state device comprising two porous electrodes and a dense electrolyte. This electrolyte conducts solid oxygen ions and is the key functional component of each individual cell. Oxygen introduced at the cathode side is reduced to form O2−. Driven by the concentration gradient, these O2− ions travel through the dense electrolyte layer to reach the anode. At the anode side, the fuel is directly oxidized to H2O and/or CO2 by O2−, releasing electrons to the external circuit [1,2]. Traditional SOFCs typically use the following materials: oxygen ion conductor yttria-stabilized zirconia (YSZ) for the electrolyte, the pure electronic conductors strontium-substituted manganites (LSM) for the cathode, and NiO–YSZ for the anode. Due to the inherent properties of these components, high operating temperatures, approximately 1000 °C, are required to achieve an economically viable power density [4]. However, such high operating temperatures result in significant fabrication and operational costs, severe material complications, and extended start–stop durations, all of which hinder the widespread commercialization of SOFCs [1,4,5].
In recent years, significant efforts have been made to lower the operational temperature of SOFCs to a range of 500–800 °C [1,4,6]. Identifying innovative oxygen catalysts with high catalytic activity at these reduced temperatures is a critical challenge for SOFCs, particularly due to the exceptionally high activation energy of LSM [1,2,5,7]. For instance, as the operational temperature decreases from 1000 °C to 500 °C, the polarization resistance of LSM increases dramatically from 1 Ω cm² to 2000 Ω cm². This decline in cathode catalytic performance is largely attributed to the limitation of the oxygen reduction reaction (ORR) to the narrow triple-phase boundary (TPB) at the interface between the cathode, electrolyte, and oxygen gas (air). Within this TPB, the transportation of electrons, oxygen vacancies or ions, and oxygen gas occurs, facilitating their movement to or from the reaction site [5,8].
Mixed ionic and electronic conductors (MIECs) that exhibit elevated oxygen ion conductivity within the temperature range of 500–800 °C have the potential to expand the oxygen reduction region from the TPB to several micrometers within the cathode. Consequently, these materials are anticipated to display exceptional catalytic activity for the ORR [9,10,11,12,13]. For instance, at a temperature of 700 °C, the area-specific resistance (ASR) of a La0.6Sr0.4Co0.2Fe0.8O3−δ cathode on a gadolinium-doped ceria (GDC) electrolyte has been reported to be approximately 0.1 Ω cm² [12]. Furthermore, Pang et al. reported a notably lower resistance of less than 0.035 Ω cm² for La0.5Ba0.5CoO3−δ on a GDC electrolyte under identical operating conditions [13]. In the search for advanced cathode materials for SOFCs, significant advancements have been realized over the past decade. However, polarization resistances at lower temperature ranges are often considered suboptimal. Notably, Hwang et al. observed a significant rise in the ASR of a La0.6Sr0.4Coe 0.2Fe0.8O3−δ cathode on a GDC electrolyte, increasing from ~0.1 Ω cm² at 700 °C to 12 Ω cm² at 500 °C [12]. The primary reason for this observation originates in the pronounced decrease in O2− conductivity as the temperature drops, due to the relatively high activation energies associated with oxygen transport and exchange processes [12,14]. As such, the ongoing quest for high-performance cathodes for SOFCs underscores the need for the development of materials that facilitate faster oxygen transport and surface exchange.
Over the past few years, owing to its unique crystal structure, considerable efforts have been directed towards investigating the MIEC double perovskite oxides LnBaCo2O5+δ (Ln = Lanthanide). These materials find potential applications across a multitude of domains, such as magnetism [15,16,17], SOFCs, proton-conductive ceramic fuel cells [18,19,20,21,22,23,24,25], water electrolysis [26,27,28], CO2 electrolysis [29], chemical sensors [30,31,32], ceramic semi-permeable membranes [33,34,35], metal–air batteries [36,37], soot combustion [38], supercapacitors [39], photocatalysis [40], and solar-driven thermal storage [41,42]. Given the diverse requirements in terms of physicochemical properties for each application, this article will exclusively focus on novel strategies employed in advancing double perovskites for use as cathode catalysts in SOFCs. It is worth noting that, due to the considerable interest in double perovskites, comprehensive reviews of these materials have previously been published [43,44]. However, the ongoing advancements in understanding the properties and applications of these materials underscore the need for updated reviews, such as the one presented in this article. Our discussions will span the exploration of physicochemical properties, optimization of composition, and enhancement of application methodologies. Additionally, we will deliberate on potential research breakthroughs concerning high-performance double perovskite-based cathode materials.
2. Physicochemical Properties of LnBaCo2O5+δ
As depicted in Figure 1, the LnBaCo2O5+δ compound exhibits a perovskite structure of the 112 type. Relative to their disordered analogs, these orderly structures have been widely reported to considerably enhance the rate of oxygen transport [45,46]. Notably, Taskin et al. [45] were pioneers in observing a notably high oxygen diffusion coefficient (Dchem) of approximately 3.0 × 10−⁹ cm² s−¹ at 350 °C and 10−⁵ cm² s−¹ at 600 °C for the GdBaCo2O5+δ double perovskite. The oxygen transport characteristics of the PrBaCo2O5+δ double perovskite were subsequently evaluated by Kim et al. [47,48]. Their results demonstrated appreciably higher rates of oxygen transport (Dchem) for PrBaCo2O5+δ in comparison to GdBaCo2O5+δ, suggesting an enhancement in oxygen transport properties corresponding to the increased size of the Ln cation. Tarancón et al. carried out a detailed comparative study between the double perovskite LnBaCo2O5+δ (Ln = Pr, Gd) and other classes of oxygen catalysts [44]. As illustrated in Figure 2, the double perovskite outperformed in terms of oxygen transport properties, emphasizing its considerable potential as a cutting-edge cathode material for SOFCs. It is important to recognize that significant variations exist in the LnBaCo2O5+δ oxygen tracer diffusion and the oxygen surface exchange coefficient as reported by different research groups [49]. Such disparities mainly stem from differences in the precise composition and/or microstructure of the samples used by distinct researchers. Thus, readers are encouraged to assess the data in Figure 2 judiciously and objectively.
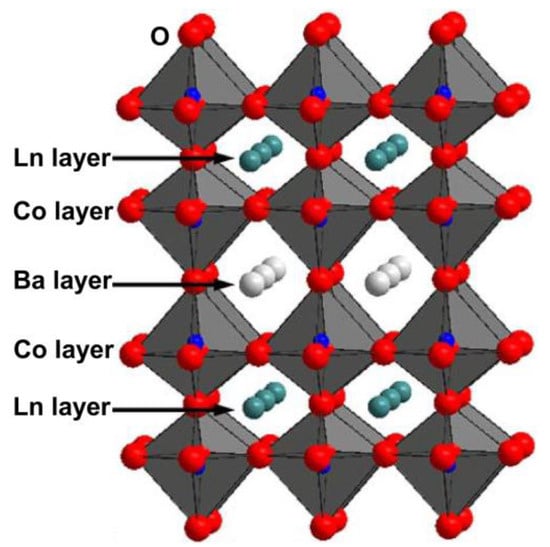
Figure 1.
Schematic diagram of crystal structure for double perovskite oxide LnBaCo2O5+δ.
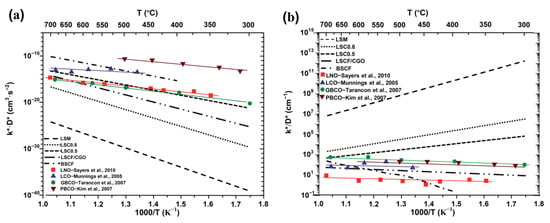
Figure 2.
Oxygen tracer diffusion and oxygen surface exchange for different layered oxide cathodes as a function of the temperature: (a) k*D*; (b) k*/D*. The materials used for comparison have been labeled as follows: La0.8Sr0.2MnO3−δ (LSM), La0.8Sr0.2CoO3−δ (LSC0.8), La0.5Sr0.5CoO3−δ (LSC0.5), La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Gd0.2O2−δ (LSCF/CGO), Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF), La2NiO4+δ (LNO) [50], La2CoO4+δ (LCO) [51], GdBaCo2O5+x (GBCO) [52], and PrBaCo2O5+x (PBCO) [47] . [Reprinted with permission from Ref. [44]. Copyright 2010, Royal Society of Chemistry].
Numerous experimental and theoretical studies have been undertaken to delve deeper into the oxygen diffusion behaviors in double perovskites [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. Seymour et al. utilized static atomistic simulations based on the Born model to methodically examine the intrinsic defect processes of the double perovskite LnBaCo2O5.5 (Ln = Y, La, Pr, Nd, Sm, Gd, Dy, Ho, Er, Yb) [53]. Their research indicated that the defect reaction with the lowest energy stemmed from the Ln/Ba antisite disorder energy, which diminishes with decreasing Ln size. This suggests that the ordered structure’s primary foundation is the size difference between the Ln and Ba cations [53]. Parfitt et al. combined molecular dynamics with Born model potentials to study the oxygen transport behavior of GdBaCo2O5+δ at 900 K [54,55]. They posited that A-site cation ordering, in contrast to its disordered equivalent, can amplify oxygen bulk diffusivity while decreasing transport in the c-axis direction [54,55]. Importantly, the distinctively anisotropic oxygen diffusion in the double perovskite GdBaCo2O5+δ takes place exclusively within the [GdOδ] and adjacent [CoO2] layers, as illustrated in Figure 3 [54,55,56,57]. Shiiba et al. probed the distribution of oxygen vacancies in GdBaCo2O5+δ under various oxygen vacancy concentrations (0 ≤ δ ≤ 1) and temperatures using a fusion of density functional theory and Monte Carlo simulation [57]. Their analysis showed that oxygen vacancies, which function as oxygen ion carriers, are restricted to the [GdOδ] and neighboring [CoO2] layers, reinforcing the anisotropic oxygen diffusion mechanism. Seymour et al. performed theoretical investigations on the oxygen transport properties of layered PrBaCo2O5+δ at 650 and 1000 °C, employing the MD method [59,60,61,62]. These proposed mechanisms for oxygen conducting were later confirmed experimentally via in situ high-temperature neutron powder diffraction and isotope exchange depth profile methods [59,60,61,62]. Additionally, it has been shown that PrBaCo2O5+δ has a lower energy barrier for oxygen diffusion perpendicular to the c-axis compared to Nd, suggesting enhanced oxygen ion diffusivity with larger Ln sizes [53,59]. Wang et al. detected rapid cobalt redox reactions in epitaxial LaBaCo2O5+δ within a temperature bracket of 260–700 °C, intimately tied to the processes of oxygen release and uptake processes [72]. This finding hints at the potential application of these films in SOFC cathodes. Notably, Wang et al. found the cobalt oxidation in the epitaxial thin films to be substantially swifter than the reduction process, denoting a more rapid oxygen uptake compared to the oxygen release (Figure 4) [72]. Bao et al.’s research further revealed a layer-by-layer oxygen transport mechanism in epitaxial double perovskites, specifically LnBaCo2O5+δ (Ln = Pr, Er), which likely originates in their intrinsic anisotropic oxygen diffusion properties [73].
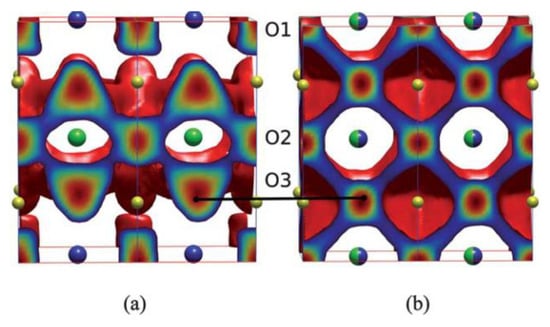
Figure 3.
(a) Calculated oxygen density profiles showing the oxygen migration pathways for (a) ordered and (b) disordered GdBaCo2O5.5 for δ = 0.5 at 900 K. [Reprinted with permission from Ref. [54]. Copyright 2011, Royal Society of Chemistry].
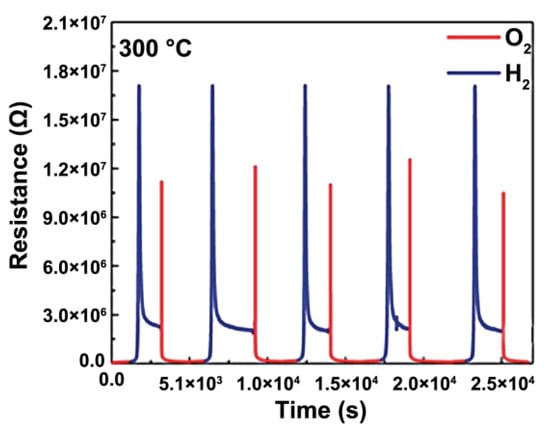
Figure 4.
R vs. t curves of redox reactions of the LBCO thin films under the switching flow of a reducing/oxidizing gas flow at 300 °C. [Reprinted with permission from Ref. [72]. Copyright 2014, Royal Society of Chemistry].
LnBaCo2O5+δ, owing to its exceptionally promising properties, has been extensively studied as a cathode material for SOFCs [34,74,75,76,77,78,79,80,81,82,83]. Researchers have undertaken thorough studies into the structural performance, thermal expansion behavior, electrical conductivity, and electrochemical performance of these double perovskites. Studies on ions such as La3+, Pr3+, Nd3+, Sm3+, and Gd3+ have shown that these oxides exhibit good chemical compatibility with commonly used electrolytes, including GDC, La0.8Sr0.2Ga0.8Mg0.2O2.8 (LSGM), and samarium oxide-doped ceria (SDC), at temperatures below 1000 °C [74,75,76,84]. After firing LnBaCo2O5+δ double perovskites at 850 °C in air for durations ranging from 60 to 100 h, no impurity phases or phase transitions were detected. This finding highlights the remarkable structural stability of these oxides under the standard operating conditions of SOFCs [34,77]. Additionally, the electrical conductivities of LnBaCo2O5+δ compounds tend to increase with growth in the size of the Ln ion, leading to a rise in the number of electronic holes created by interstitial oxygen [75,76]. The electrical conductivity values of these materials surpass 100 S cm−1 between 100 and 800 °C in air, meeting the electrical conductivity requirements for SOFC cathodes [34,75,76,77]. What is more, oxides with larger Ln ions exhibit superior electrochemical performance, stemming from enhanced oxygen transport and exchange rates [34,75]. For instance, as the Ln ion shifts from Gd3+ to La3+, the maximum power density (PPD) values of SOFCs utilizing these double perovskite cathodes increase from 443 to 516 mW cm2 [75].
Despite the numerous advantages of LnBaCo2O5+δ as a cathode catalyst for SOFCs, there are certain technical challenges that require further improvements. Firstly, enhancing the catalytic activity of these oxides for ORR is paramount. Chen et al. [74] observed that the ASR of PrBaCo2O5+δ on SDC electrolytes increases from 0.18 to 5.68 Ω cm2 as the temperature drops from 650 to 500 °C. Moreover, the PPD of SOFCs utilizing PrBaCo2O5+δ as the cathode material decreases from 866 mW cm2 (at 650 °C) to 115 mW cm2 (at 500 °C). Secondly, it is essential to minimize the thermal mismatch between these cobalt-based cathode materials and other SOFC components. Kim et al. [75] reported that the thermal expansion coefficients (TECs) of LnBaCo2O5+δ double perovskites increase from 16.6 × 10−6 K−1 (Ln = Gd3+) to 24.3 × 10−6 K−1 (Ln = Pr3+) with larger Ln sizes at 80–900 °C. Given that the TECs of standard electrolytes for SOFCs, such as GDC, SDC, and LSGM, are around 11 × 10−6 K−1, this notable thermal mismatch between LnBaCo2O5+δ and the electrolyte could adversely affect fuel cell stability. Thirdly, tuning the physicochemical properties of the surface is essential. The surface physicochemical properties serving as catalysts for the ORR significantly influence cathode performance. Findings by Téllez et al. [79] suggest that the surface composition and morphology of LnBaCo2O5+δ (Ln = Pr, Gd) double perovskites are profoundly influenced by exposure time, temperature, and ambient atmosphere. A quick covering of the electrocatalytic transition metal by inactive Ln3+ or Ba2+ cations, observed under certain conditions, can be detrimental to the ORR. Therefore, the subsequent sections will provide a comprehensive overview of advancements in studying the physicochemical property attributes of double perovskites and in adjusting the composition and nanostructure of LnBaCo2O5+δ.
3. Compositional Optimization of LnBaCo2O5+δ
To enhance the performance of double perovskite-based cathodes, extensive efforts have been made to optimize the composition of LnBaCo2O5+δ. These modifications involve A-site and B-site doping, or a combination of both, aiming to improve structural stability, enhance chemical compatibility with the electrolyte, increase electrocatalytic activity, and finely tune the TECs (Table 1) [73,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]. Marrero-Jerez et al. [85] found that substituting Sr for Ba in GdBaCo2O5+δ completely stabilizes the high-temperature tetragonal symmetry even at room temperature. Kim et al. [86] observed improved chemical stability when GDC and LSGM electrolytes are used. Numerous investigations have systematically examined the electrical and electrochemical properties of the LnBa1−xSrxCo2O5+δ system, with Ln representing La3+ [73], Pr3+ [87,92], Nd3+ [90,91], Sm3+ [87,88,89,112,114], and Gd3+ [85,86,87,111]. Kim et al. [86] demonstrated that the electrical conductivity of GdBa1−xSrxCo2O5+δ increases with rising Sr content, attributed to increased oxygen content, which is believed to be linked to the difference in A-site cation radii between (Ba1−xSrx)2+ and Gd3+. Additionally, Subardi et al. [88] found that the double perovskite SmBa0.6Sr0.4Co2O5+δ has a relatively high Dchem (1.63 × 10−6 cm2 s−1 at 500 °C and 1.41 × 10−5 cm2 s−1 at 700 °C) and a notably low activation energy (Ea = 68.03 kJ mol−1) for oxygen bulk diffusion at 500–700 °C. Jun et al. [89] demonstrated that Sr substitution in SmBa1−xSrxCo2O5+δ can boost the catalytic activity of double perovskites. For instance, on a GDC electrolyte, the ASR decreases from 0.192 Ω cm2 (x = 0.00) to 0.138 Ω cm2 (x = 0.75), and the maximum power density grows from 0.848 to 1.039 W cm−2 at 600 °C (Table 1).

Table 1.
Typical electrical conductivity, TEC, and ASR values of double perovskites.
The presence of A-site cation deficiency has been found to significantly influence the physical and chemical properties of perovskite oxides, as reported in previous studies [119]. Extensive investigations have been undertaken to understand the effects of Ba2+ [93,94,95,96,97,120,121] and Ln3+ [122,123,124,125] deficiencies on the crystal structure, oxygen content, electrical conductivity, and electrochemical performance of double perovskite LnBaCo2O5+δ. Pang et al. [94,95] observed that with an increase in Ba deficiency from x = 0.00 (0.181 Ω cm2) to x = 0.08 (0.093 Ω cm2) at 600 °C, the ASR value of PrBa1-xCo2O5+δ drops by approximately 50%. This indicates a substantial improvement in oxygen catalytic activity associated with A-site deficiency. Dong et al. [98] further revealed that a higher Ba deficiency in PrBa1−xCo2O5+δ oxides results in an increased concentration of oxygen vacancies, thus boosting oxygen transport and exchange kinetics. These findings strongly suggest that A-site cation deficiency can enhance the electrochemical performance of double perovskite LnBaCo2O5+δ.
Ca doping and optimization of the Ln component have been identified as other effective strategies to boost the electrochemical performance of double perovskite cathodes [86,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153]. Yoo et al. proposed novel cathode materials, such as Ca-doped NdBaCo2O5+δ, that have ionic radii similar to Nd and demonstrated their impressive structural stability and outstanding electrochemical performance [99]. The ASR of NdBa1-xCaxCo2O5+δ double perovskites was observed to decrease from 0.091 Ω cm2 (x = 0.00) to 0.066 Ω cm2 (x = 0.25) at 600 °C. As illustrated in Figure 5, compared to the Ca-free sample, single cells using NdBa0.75Ca0.25Co2O5+δ as the cathode exhibited a significantly higher PPD of 2.114 W cm−2 at 600 °C. Moreover, while the power density of the single cell with an NdBaCo2O5+δ cathode experienced a decrease of approximately 50%, nearly no degradation in power density was seen for NdBa0.75Ca0.25Co2O5+δ, highlighting its remarkable stability. This result is believed to correlate with the increased electron affinity of mobile oxygen species in the presence of Ca.
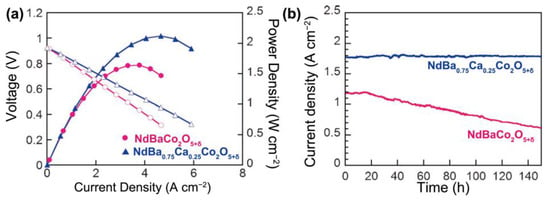
Figure 5.
Electrochemical performances and long-term stability data. (a) I–V curves and the corresponding power densities of test cells at 600 °C. The solid and hollow circles represent NdBaCo2O5+δ, while the solid and hollow triangles represent NdBa0.75Ca0.25Co2O5+δ. (b) Long term stability measurement at a constant cell voltage of 0.6 V at 550 °C. [Reprinted with permission from Ref. [99]. Copyright 2014, John Wiley and Sons].
Similar to other cobalt-based cathode materials, LnBaCo2O5+δ often displays relatively high TECs, typically ranging from 19 to 25 × 10−6 K−1 at 80–900 °C. These values are substantially higher than those of conventional electrolytes (10–13 × 10−6 K−1) [154] and sealing materials (11–14 × 10−6 K−1) [155]. Such differences can lead to significant compatibility issues between the double perovskites and other components of SOFCs during cell fabrication and thermal cycling, potentially causing performance degradation. Besides lattice anharmonic vibrations, the elevated TECs of cobalt-based oxides are mainly attributed to the conversion of smaller Co4+ ions to larger Co3+ ions at higher temperatures. This is due to the liberation of lattice oxygen upon heating and the spin-state changes of Co3+ ions [156,157]. To address these drawbacks, researchers have examined the substitution of cobalt with various elements, including Fe [100,101,102,158,159,160,161,162,163], Ni [103,104,164,165,166], Cu [105,106,107], Mn [167,168], Zn [169], Zr [170,171], W [172], Sc [173], Mo [174], Ga [175], and Bi [176]. Jo et al. [104] reported that partial substitution of Fe and Cu for Co in GdBaCo2O5+δ (GdBaCo2/3Fe2/3Cu2/3O5+δ) can reduce the TECs from 19.9 × 10−6 K−1 to 14.6 × 10−6 K−1 at 80–900 °C. Zhao et al. [100] conducted a comprehensive investigation into the impact of Fe content on the physicochemical properties of double perovskite PrBaCo2O5+δ, discovering a continuous decrease in TECs with higher Fe content. However, this substitution also led to reduced electrical conductivity, oxygen vacancy concentration, and electrochemical performance compared to the Fe-free compound. For an in-depth exploration of this subject, readers are encouraged to refer to relevant review articles [177].
The ability to incorporate dopants at both the A-site and B-site offers a broader spectrum for customizing double perovskite cathode materials [102,108,109,110,178,179,180]. As earlier elaborated, appropriate A-site doping in LnBaCo2O5+δ, like substituting Ba with Sr or inducing a Ba deficiency, has the potential to enhance electrical conductivity, oxygen bulk diffusivity, surface exchange kinetics, and the oxygen catalytic activity pertinent to ORR [86,89,94,181]. On the other hand, replacing Co with different metal ions, including Fe, Ni, and Cu, has proven to successfully enhance the structural resilience and thermal expansion coefficients, thus improving compatibility with the electrolyte [100,101,103,104,105]. For instance, Kim et al. [110] deftly adjusted the manganese content in NdBa0.5Sr0.5Co2-xMnxO5+δ to refine its physicochemical attributes as a cathode catalyst for SOFCs. They found that an increase in manganese content led to a decrease in TEC from 20.27 × 10−6 K−1 (x = 0.0) to 14.33 × 10−6 K−1 (x = 0.5), while maintaining acceptable electrochemical performance. Similarly, Choi et al. [108] documented a robust cathode material, PrBa0.5Sr0.5Co2-xFexO5+δ, which exhibited increased oxygen ion mobility and surface oxygen exchange reactions, superior electrochemical performance (~0.056 Ω cm−2 at 600 °C), and strong compatibility and stability with a GDC electrolyte. Persistent optimization of double perovskite composition is essential to uncover innovative cathode materials boasting excellent structural stability, advantageous chemical and thermal compatibility with the electrolyte, adequate electrical conductivities, swift oxygen transport and exchange kinetics, high catalytic ORR activity, and outstanding durability.
4. Nanostructure and Nanoscience of LnBaCo2O5+δ
Nanostructures offer significantly enhanced surface area-to-volume ratios and expanded interphase and interfacial areas. As such, they have the potential to augment electrochemical reaction sites. Perovskite oxides with nanostructured morphologies have been rigorously studied and employed in solid oxide fuel cells [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198] as well as other energy-related applications [199,200,201,202]. Reducing the operating temperature creates an opportunity to use nanostructured materials, which can sidestep the slow ORR and, in turn, boost the catalytic performance of the cathode [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198]. Infiltration is a common and straightforward method for developing nanostructured cathode materials tailored for SOFCs [182,183,184]. A nanostructured cathode material, represented by the formula SmBa0.5Sr0.5Co2O5+δ, was created by infusing its precursor solution into the porous LSGM framework, followed by calcining at 850 °C. This material showcased commendable electrochemical performance [185]. For instance, it showed an ASR as low as 0.12 Ω cm2 and a PPD of up to 0.70 W cm−2 at 500 °C. Electrospinning, praised for its scalability and precision, was utilized to fabricate a GdBaCo2O5+δ cathode material possessing a nanofiber configuration, achieving a comparatively low ASR, approximately 0.10 Ω cm2 at 700 °C [194].
Ding et al. [185] managed to produce unique needle-like nanospikes of the cathode material PrBaCo2O5+δ by applying a discharge voltage of 0.1 V to the anode-supported single cell, arranged as NiO-Sm0.2Ce0.8O1.9/Sm0.2Ce0.8O1.9/PrBaCo2O5+δ, and then firing the PrBaCo2O5+δ cathode slurry at 450 °C. As illustrated in Figure 6, these nanospikes, with an average diameter of 20 nm and lengths spanning from tens to hundreds of nanometers, are uniformly distributed along the pore boundaries of the porous cathode. For the single cell that used the nanospikes PrBaCo2O5+δ as the cathode, exceptionally high maximum power densities of 1.453 W cm−2 at 550 °C and 1.044 W cm−2 at 500 °C, coupled with excellent endurance, were recorded.
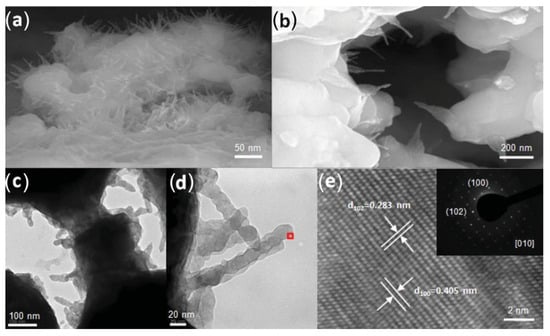
Figure 6.
(a,b) Cross-sectional view (field emission SEM image) of cathode–electrolyte interface at different locations. (c,d) TEM view of nanospikes growing from PrBaCo2O5+δ bulk. (e) HRTEM lattice fringe image of a nanospike tip (boxed area in (d)), and SAED pattern along [010] zone axis (insert). [Reprinted with permission from Ref. [185]. Copyright 2014, John Wiley and Sons].
The fabrication of double perovskites in a thin-film architecture not only facilitates fundamental studies to evaluate inherent properties of materials [73,183,203,204] but also illuminates a new avenue for the development of high-performing cathode materials [72,186,188]. The influence of orientations on the electrochemical performance of double perovskites was appraised by Gao et al. [186]. They produced PrBaCo2O5+δ thin films with different orientations, including (110), (001), and (111), using pulsed laser deposition. The thin film with the (111) orientation showed superior performance, achieving an ASR of 0.302 Ω cm² at 600 °C. Liu et al. [187,188] fabricated symmetric half-cells by coupling single-crystal, highly epitaxial LnBaCo2O5+δ (Ln = Pr, La) thin-film cathodes with Gd0.8Ce0.2O2:Y0.08Zr0.92O2 electrolytes and subsequently characterized their oxygen surface exchange and catalytic activity. For instance, the symmetric half-cell featuring the epitaxial LaBaCo2O5+δ thin film displayed remarkable properties, such as an impressively fast surface exchange rate of 0.017 cm s−1 at 600 °C and an exceptionally low activation energy value of 0.49 eV. These outcomes might be ascribed to the structural entropy arising from the nano-ordered oxygen vacancy framework.
5. Conclusions and Outlook
This manuscript addresses the development of novel strategies concerning the double perovskites LnBaCo2O5+δ, which possess rapid oxygen bulk diffusivity and a high surface exchange rate. These parameters serve as the cornerstone for achieving advanced catalytic activity for the ORR; hence, they are essential for cathode materials operating within intermediate-to-low temperature SOFCs. The discourse encompasses physicochemical characteristics, compositional fine-tuning, and the implementation of nanostructure and nanoscience within double perovskites. Potential research focuses for advancing high-performance double perovskite-based cathode materials include:
- (1)
- Surface Chemistry of LnBaCo2O5+δ Under Operating Conditions
The surface physicochemical properties of double perovskite LnBaCo2O5+δ, as catalysts for the ORR, are essential for their practical application. Téllez et al. [79] characterized the surface chemistry evolution in double perovskite LnBaCo2O5+δ (Ln = Pr, Gd) using low-energy ion scattering, spectrometry, and atomic force microscopy. They found that the surface composition and morphology of LnBaCo2O5+δ (Ln = Pr, Gd) are sensitive to their ambient environment. Inactive Ba-rich layers emerged on the double perovskite surface following annealing, adversely affecting oxygen surface exchange processes and, subsequently, the electrochemical performance. Druce et al. [205] observed a similar surface termination and subsurface restructuring for GdBaCo2O5+δ. According to Lee et al. [206], the segregation originates from the dopant’s elastic and electrostatic interactions with the host lattice. A slight size mismatch between the dopant and host cations could reduce this segregation, promoting a more stable cathode surface [205,206,207,208,209,210,211,212,213,214,215]. Nonetheless, the majority of research on the surface microstructure of double perovskite cathodes relies on basic sintering processes in the air, which differs significantly from actual battery operating conditions. Hence, guidance for the practical use of double perovskites remains limited. Investigating surface microstructure evolution under the SOFCs’ actual operational conditions and creating targeted enhancement strategies offer a promising path for practical deployment of these cathode materials.
- (2)
- Microstructure of LnBaCo2O5+δ Cathode Materials
The structural design of double perovskite metal oxides offers advantages for their use in the cathodic parts of SOFCs. Nevertheless, in practical applications, these double perovskites often present as polycrystalline particles. It is believed that features such as grain orientation, microstructure, lattice strain, and chemical imperfections strongly influence their catalytic activity [72,73,183,186,188,189,193,203,216,217,218]. Consequently, a comprehensive study on the microstructure of these double perovskite particles is crucial for high-performance SOFC cathodes. For instance, Fu et al. found that a dual-phase cathode containing both double perovskite PrBa(Co1-xFex)2O5+δ and simple perovskite Pr0.5Ba0.5Co1-xFexO3-δ significantly enhanced the cathode’s oxygen catalysis [193]. Likewise, Pang et al. engineered a biomimetic ceramic catalyst resembling tree leaves, incorporating Ce0.9Gd0.1O1.95 “epidermis” and “veins” externally and inside the bulk of the PrBaCo2O5+δ [189]. This unique design substantially improved cell performance, inducing a 79% rise in the cell’s output power density, reversing the rapid decline trend, yielding a 23% power density gain in the initial 20 h, and stabilizing at 0.91 W cm−2 (at 750 °C and 0.7 V) [189].
Author Contributions
Conceptualization, S.P.; methodology, F.Z.; writing—original draft preparation, F.Z.; writing—review and editing, S.P.; visualization, F.Z.; supervision, S.P.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. U2032157) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20201425).
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, E.; Ishihara, T.; Kilner, J. Low-temperature of solid-oxide fuel cells. MRS Bulletin 2014, 39, 773–779. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Lang, Y.; Meng, G. Recent advances to the development of low-temperature solid oxide fuel cells. Fuel Cells 2004, 4, 41–47. [Google Scholar] [CrossRef]
- Xia, C.R.; Xia, M.L. Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 2002, 14, 521–523. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Intermediate-temperature solid-oxide fuel cells. Annu. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Zhou, W.; Sunarso, J.; Zhao, M.; Liang, F.; Klande, T.; Feldhoff, A. A highly active perovskites electrode for the oxygen reduction reaction below 600 °C. Angew. Chem. Int. Ed. 2013, 52, 14036–14040. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1-xSrxCoO3-δ electrodes. Solid State Ionics 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Hwang, H.J.; Moon, J.-W.; Lee, S.; Lee, E.A. Electrochemical performance of LSCF-based composite cathodes for intermediate temperature SOFCs. J. Power Sources 2005, 145, 243–248. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X. A comparative study of electrochemical performance of La0.5Ba0.5CoO3-δ and La0.5Ba0.5CoO3-δ-Gd0.1Ce0.9O1.95 cathodes. Int. J. Hydrogen Energy 2012, 37, 2157–2165. [Google Scholar] [CrossRef]
- Steele, B.C.H. Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ionics 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Bao, S.; Pang, S.; Wang, W.; Chen, J.; Chen, M.; Ma, J.; Nan, C.-W.; Chen, C. Ca doping effect on the magnetic and electronic transport properties in double perovskite PrBaCo2O5+δ films. Appl. Phys. Lett. 2017, 111, 232406. [Google Scholar] [CrossRef]
- Wu, J.; Guzman, R.; Bao, S.; Zhang, Y.; Chen, Y.; Shen, S.; Yu, P.; Nan, C.-W.; Zhou, W.; Chen, C.; et al. Mosaic growth induced magnetic anisotropy in double perovskite PrBaCo2O5+δ thin films. Acta Materialia 2022, 234, 118040. [Google Scholar] [CrossRef]
- Kudyakova, V.S.; Shalamova, A.M.; Politov, B.V.; Suntsov, A.Y. Specific interrelations of magnetic, thermodynamic and structural properties in highly non-stoichiometric PrBaMnFeO6−δ double perovskite. J. Alloys Compd. 2021, 886, 161133. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Du, L.; Zhu, R.; Xing, L.; Song, Z.; Yuan, B.; Zhao, L.; Aoki, Y.; Ye, S. Advanced cathode materials for protonic ceramic fuel cells: Recent progress and future perspectives. Adv. Energy Mater. 2022, 12, 2201882. [Google Scholar] [CrossRef]
- Teketel, B.S.; Beshiwork, B.A.; Luo, X.; Tian, D.; Zhu, S.; Desta, H.G.; Yang, Q.; Chen, Y.; Lin, B. A-site doping enabled higher-oxygen-vacancy cobalt-free layered perovskite cathode for higher-performing protonic ceramic fuel cells. Ceram. Int. 2022, 48, 37232–37241. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, S.; Nolan, A.M.; Mo, Y. First-principles computational design and discovery of novel double-perovskite proton conductors. Chem. Mater. 2021, 33, 8278–8288. [Google Scholar] [CrossRef]
- Cao, J.; Jia, Y.; Shao, Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Teketel, B.S.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Desta, H.G.; Kashif, K.; Chen, Y.; Lin, B. Promoted performance of layered perovskite PrBaFe2O5+δ cathode for protonic ceramic fuel cells by Zn doping. Catalysts 2022, 12, 488. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, K.; He, F.; Zhou, Y.; Sasaki, K.; Zhao, B.; Choi, Y.; Liu, M.; Chen, Y. Surface regulating of a double-perovskite electrode for protonic ceramic fuel cells to enhance oxygen reduction activity and contaminants poisoning tolerance. Adv. Energy Mater. 2022, 12, 2200761. [Google Scholar] [CrossRef]
- Malyshkin, D.; Novikov, A.; Ivanov, I.; Sereda, V.; Tsvetkov, D.; Zuev, A. The origin of triple conductivity and water uptake in layered double perovskites: A case study on lanthanum-substituted GdBaCo2O6-δ. J. Alloys Compd. 2020, 845, 156309. [Google Scholar] [CrossRef]
- Kim, B.-J.; Fabbri, E.; Castelli, I.E.; Borlaf, M.; Graule, T.; Nachtegaal, M.; Schmidt, T.J. Fe-doping in double perovskite PrBaCo2(1-x)Fe2xO6-δ: Insights into structural and electronic effects to enhance oxygen evolution catalyst stability. Catalysts 2019, 9, 263. [Google Scholar] [CrossRef]
- Xing, L.; Xia, T.; Li, Q.; Zhao, H.; Sun, L.; Huo, L.-H. High-Performance and CO2-durable composite cathodes toward electrocatalytic oxygen reduction: Ce0.8Sm0.2O1.9 Nanoparticle-decorated double perovskite EuBa0.5Sr0.5Co2O5+δ. ACS Sust. Chem. Eng. 2019, 7, 17907–17918. [Google Scholar] [CrossRef]
- Baral, A.K.; Sankar, K.V.; Matatyaho, A.; Kushnir, G.; Tsur, Y. Tri-functional double perovskite oxide catalysts for fuel cells and electrolyzers. ChemSusChem 2020, 13, 5671–5682. [Google Scholar] [CrossRef]
- Shin, T.H.; Myung, J.-H.; Verbraeken, M.; Kim, G.; Irvine, J.T.S. Oxygen deficient layered double perovskite as an active cathode for CO2 electrolysis using a solid oxide conductor. Faraday Discuss. 2015, 182, 227. [Google Scholar] [CrossRef]
- Wang, H.; Enriquez, E.; Collins, G.; Ma, C.; Liu, M.; Zhang, Y.; Dong, C.; Chen, C. Anomalous redox properties and ultrafast chemical sensing behavior of double perovskite CaBaCo2O5+δ thin films. J. Materiomics 2015, 1, 113–117. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Xia, Y.; Pang, S.; Xu, F.; Whangbo, M.-H.; Sun, L.; Chen, C. Anomaly negative resistance phenomena in highly epitaxial PrBa0.7Ca0.3Co2O5+δ thin films induced from superfast redox reactions. Catalysts 2021, 11, 1441. [Google Scholar] [CrossRef]
- Bao, S.; Ma, C.; Chen, G.; Xu, X.; Enriquez, E.; Chen, C.; Zhang, Y.; Bettis, J.L.; Whangbo, M.-H.; Dong, C.; et al. Ultrafast atomic layer-by-layer oxygen vacancy-exchange diffusion in double-perovskite LnBaCo2O5.5+δ thin films. Sci. Rep. 2014, 4, 4726. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.L.; Zakiryanov, P.O.; Sereda, V.V.; Mazurin, M.O.; Malyshkin, D.A.; Zuev, A.Y.; Tsvetkov, D.S. Nonstoichiometry, Defect chemistry and oxygen transport in Fe-doped layered double perovskite cobaltite PrBaCo2-xFexO6-δ (x = 0–0.6) membrane materials. Membranes 2022, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- Yagovitin, R.E.; Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Sereda, V.V.; Zuev, A.Y. Thermodynamics of Formation and Disordering of YBaCo2O6-δ Double Perovskite as a Base for Novel Dense Ceramic Membrane Materials. Membranes 2023, 13, 10. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhen, D.; Yoo, S.; Ding, Y.; Chen, D.; Chen, Y.; Zhang, Q.; Doyle, B.; Xiong, X.; et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 2017, 8, 14586. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, Y.-Q.; Yan, N.; Li, M.; Sun, Y.-F.; Chen, J.; Li, J.; Luo, J.-L. The excellence of both worlds: Developing effective double perovskite oxide catalyst of oxygen reduction reaction for room and elevated temperature applications. Adv. Funct. Mater. 2016, 26, 4106–4112. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In situ exsolution of Co/CoOx core-shell nanoparticles on double perovskite porous nanotubular webs: A synergistically active catalyst for soot efficient oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Chen, Y.; Yang, L.; Wang, Y.; Wei, M. A-site cation-ordered double perovskite PrBaCo2O5+δ oxide as an anion-inserted pseudocapacitor electrode with outstanding stability. J. Alloys Compd. 2019, 810, 151830. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, S.; Lu, C.; Xu, Z. Bandgap engineering of Gd0.8Ca0.2BaCo2O5+δ double perovskite for photocatalysis applications. Ceram. Int. 2018, 44, 15483–15489. [Google Scholar] [CrossRef]
- Lu, Y.; Dai, T.; Lu, C.; Cao, C.; Zhang, W.; Xu, W.; Min, H.; Yang, X. Fabrication of doped SmBaCo2O5+δ double perovskites for enhanced solar driven interfacial evaporation. Ceram. Int. 2019, 45, 24903–24908. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Wei, L.; Lu, C.; Ni, Y.; Xu, Z. Specific features of spectral and electrical properties of double-perovskite LnBaCo2O5+δ (Ln=lanthanides) under solar irradiation. Ceram. Int. 2017, 43, 1186–1192. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Tarancόn, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett. 2005, 86, 091910. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Transport and magnetic properties of GdBaCo2O5+δ single crystals: A cobalt oxide with square-lattice CoO2 planes over a wide range of electron and hole doping. Phys. Rev. B 2005, 71, 134414. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Yang, Z.; Donner, W.; Chen, C.L.; Reimus, L.; Brodersen, P.; Mims, C.A. Oxygen exchange kinetics of epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2006, 88, 024103. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ananjev, M.V.; Eremin, V.A.; Zuev, A.Y.; Kurumchin, E.K. Oxygen nonstoichiometry, defect structure and oxygen diffusion in the double perovskite GdBaCo2O6−δ. Dalton Trans. 2014, 43, 15937–15943. [Google Scholar] [CrossRef]
- Sayers, R.; De Souza, R.A.; Kilner, J.A.; Skinner, S.J. Low temperature diffusion and oxygen stoichiometry in lanthanum nickelate. Solid State Ionics 2010, 181, 386–391. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G. Whitfield, P.S;. Davidson, I.J. Oxygen transport in the La2Ni1−xCoxO4+δ system. Solid State Ionics 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Tarancón, A.; Morata, A.; Dezanneau, G.; Skinner, S.J.; Kilner, J.A.; Estradé, S.; Hernández-Ramírez, F.; Peiró, F.; Morante, J.R. GdBaCo2O5+x layered perovskite as an intermediate temperature solid oxide fuel cell cathode. J. Power Sources 2007, 174, 255–263. [Google Scholar] [CrossRef]
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, D.; Chroneos, A.; Tarancόn, A.; Kilner, J.A. Oxygen ion diffusion in cation ordered/disordered GdBaCo2O5+δ. J. Mater. Chem. 2011, 21, 2183–2186. [Google Scholar] [CrossRef]
- Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J. Oxygen diffusion in ordered/disordered double perovskites. Ecs Trans. 2011, 35, 1151–1154. [Google Scholar] [CrossRef]
- Hermet, J.; Geneste, G.; Dezanneau, G. Molecular dynamics simulations of oxygen diffusion in GdBaCo2O5.5. Appl. Phys. Lett. 2010, 97, 174102. [Google Scholar] [CrossRef]
- Shiiba, H.; Nakayama, M.; Kasuga, T.; Grimes, R.W.; Kilner, J.A. Calculation of arrangement of oxygen ions and vacancies in double perovskite GdBaCo2O5+δ by first-principles DFT with monte carlo simulations. Phys. Chem. Chem. Phys. 2013, 15, 10494–10499. [Google Scholar] [CrossRef]
- Zapata, J.; Burriel, M.; Carcía, P.; Kilner, J.A.; Santiso, J. Anisotropic O18 tracer diffusion in epitaxial films of GdBaCo2O5+δ cathode material with different orientations. J. Mater. Chem. A 2013, 1, 7408–7414. [Google Scholar] [CrossRef]
- Seymour, I.D.; Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ionics 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Burriel, M.; Peña-Martínez, J.; Chater, R.J.; Fearn, S.; Berenov, A.V.; Skinner, S.J.; Kilner, J.A. Anisotropic oxygen ion diffusion in layered PrBaCo2O5+δ. Chem. Mater. 2012, 24, 613–621. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yashima, M.; Peña-Martínez, J.; Kilner, J.A. Experimental visualization of the diffusional pathway of oxide ions in a layered perovskite-type cobaltite PrBaCo2O5+δ. Chem. Mater. 2013, 25, 2638–2641. [Google Scholar] [CrossRef]
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Yu, C.; Wang, X.; Gong, W.; Jacobson, A.J.; McIntosh, S. An in-situ neutron diffraction study of the crystal structure of PrBaCo2O5+δ at high temperature and controlled oxygen partial pressure. Solid State Ionics 2013, 249–250, 34–40. [Google Scholar] [CrossRef]
- Hu, Y.; Hernandez, O.; Broux, T.; Bahout, M.; Hermet, J.; Ottochian, A.; Ritter, C.; Geneste, G.; Dezanneau, G. Oxygen diffusion mechanism in the mixed ion-electron conductor NdBaCo2O5+x. J. Mater. Chem. 2012, 22, 18744–18747. [Google Scholar] [CrossRef]
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Kim, J.-H.; Yu, C.; Wang, X.; Jacobson, A.J.; Mcintosh, S. Visualizing oxygen anion transport pathways in NdBaCo2O5+δ by in situ neutron diffraction. J. Mater. Chem. 2013, 1, 3091–3100. [Google Scholar] [CrossRef]
- Hermet, J.; Dupé, B.; Dezanneau, G. Simulations of REBaCo2O5.5 (RE = Gd, La, Y) cathode materials through energy minimization and molecular dynamics. Solid State Ionics 2012, 216, 50–53. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Defect structure and charge transfer in the double perovskite GdBaCo2O6−δ. Solid State Ionics 2011, 192, 215–219. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-site cation ordering on chemical stability, oxygen stoichiometry and electrical conductivity in layered LaBaCo2O5 double perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef]
- Anjum, U.; Khan, T.S.; Agarwal, M.; Haider, M.A. Identifying the origin of the limiting process in a double perovskite PrBa0.5Sr0.5Co1.5Fe0.5O5+δ Thin-film electrode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 25243–25253. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ionics 2010, 180, 1620–1625. [Google Scholar] [CrossRef]
- Pang, S.; Wang, W.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Chen, C. Synergistic effect of A-site cation ordered-disordered perovskite as a cathode material for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2017, 164, F775–F780. [Google Scholar] [CrossRef]
- Zhou, Y.; Lü, Z.; Xu, S.; Wei, B.; Xu, D.; Yang, Z. The electronic structure and the oxygen adsorption at BaO terminated surface of GdBaCo2O5.5: A first principles study. Solid State Commun. 2020, 311, 113871. [Google Scholar] [CrossRef]
- Wang, H.B.; Bao, S.Y.; Liu, J.; Collins, G.; Ma, C.R.; Liu, M.; Chen, C.L.; Dong, C.; Whangbo, M.-H.; Guo, H.M.; et al. Ultrafast chemical dynamic behavior in highly epitaxial LaBaCo2O5+δ thin films. J. Mater. Chem. C 2014, 2, 5660–5666. [Google Scholar] [CrossRef]
- Subardi, A.; Liao, K.Y.; Fu, Y.P. Oxygen transport, thermal and electrochemical properties of NdBa0.5Sr0.5Co2O5+δ cathode for SOFCs. J. Eur. Ceram. Soc. 2019, 39, 30–40. [Google Scholar] [CrossRef]
- Chen, D.J.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z.P. Intermediate-temperature electrochemical performance of a polycrystalline PrBaCo2O5+δ cathode on samarium-doped ceria electrolyte. J. Power Sources 2009, 188, 96–105. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Su, Z.X.; Xu, H.X.; Xu, Q.L.; Chen, C.L. Characterization of cation-ordered perovskite oxide LaBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 6836–6843. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Wang, Q.X.N.; Zhang, Q.Y. Structural stability and high-temperature electrical properties of cation-oredered/disordered perovskite LaBaCoO. Mater. Chem. Phys. 2012, 131, 642–646. [Google Scholar] [CrossRef]
- Chen, D.J.; Ran, R.; Shao, Z.P. Effect of firing temperature on the microstructure and performance of PrBaCo2O5+δ cathodes on Sm0.2Ce0.8O1.9 electrolytes fabricated by spray deposition-firing processes. J. Power Sources 2010, 195, 4667–4675. [Google Scholar] [CrossRef]
- Téllez, H.; Druce, J.; Ju, Y.-W.; Kilner, J.; Ishihara, T. Surface chemistry evolution in LnBaCo2O5+δ double perovskites for oxygen electrodes. Int. J. Hydrogen Energy 2014, 39, 20856–20863. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Pérez-Coll, D.; Peña-Martínez, J.; Garcia-Martín, S. New insights into the GdBaCo2O5+δ material: Crystal structure, electrical and electrochemical properties. J. Power Sources 2014, 263, 90–97. [Google Scholar] [CrossRef]
- Ishizawa, N.; Asaka, T.; Kudo, T.; Fukuda, K.; Yasuhara, A.; Abe, N.; Arima, T. Structural evolution of GdBaCo2O5+δ (δ = 7/18) at elevated temperatures. Chem. Mater. 2014, 26, 6503–6517. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Gavrilova, L.Y.; Yaremchenko, A.A.; Cherepanov, V.A.; Kharton, V.V. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+δ and SmBaCo2O5+δ. Mater. Res. Bull. 2010, 45, 1288–1292. [Google Scholar] [CrossRef]
- Shi, Z.; Xia, T.; Meng, F.; Wang, J.; Lian, J.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C.; Meng, J. A layered perovskite EuBaCo2O5+δ for intermediate-temperature solid oxide fuel cell cathode. Fuel Cells 2013, 14, 979–990. [Google Scholar] [CrossRef]
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6-δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells: Stability and cation interdiffusion. Energies 2019, 12, 417. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Peña-Martínez, J.; Nñez, P. Study of the oxygen desorption from GdBa1-xSrxCo2O5+δ (x = 0, 0.25, 0.5 and 1): Effect of the Sr-content on the oxidation state of cobalt ions. J. Alloys Compd. 2014, 606, 269–272. [Google Scholar] [CrossRef]
- Kim, J.-H.; Prado, F.; Manthiram, A. Characterization of GdBa1-xSrxCo2O5+δ (0 ≤ x ≤ 1.0) double perovskites as cathodes for solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B1023–B1028. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cassidy, M.; Irvine, J.T.S.; Bae, J. Advanced electrochemical properties of LnBa0.5Sr0.5Co2O5+δ (Ln = Pr, Sm, and Gd) as cathode materials for IT-SOFC. J. Electrochem. Soc. 2009, 156, B682–B689. [Google Scholar] [CrossRef]
- Subardi, A.; Cheng, M.-H.; Fu, Y.-P. Chemical bulk diffusion and electrochemical properties of SmBa0.6Sr0.4Co2O5+δ cathode for intermediate solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 20783–20790. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Optimization of Sr content in layered SmBa1-xSrxCo2O5+δ perovskite cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 18381–18388. [Google Scholar] [CrossRef]
- Lü, S.Q.; Meng, X.W.; Ji, Y.; Fu, C.W.; Sun, C.C.; Zhao, H.Y. Electrochemical performances of NdBa0.5Sr0.5Co2O5+x as potential cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 8094–8096. [Google Scholar] [CrossRef]
- Yoo, S.; Choi, S.; Kim, J.; Shin, J.; Kim, G. Investigation of layered perovskite type NdBa1-xSrxCo2O5+δ (x = 0, 0.25, 0.5, 0.75, and 1.0) cathodes for intermediate-temperature solid oxide fuel cells. Electrochimica Acta 2013, 100, 44–50. [Google Scholar] [CrossRef]
- Ding, H.P.; Xue, X.J. PrBa0.5Sr0.5Co2O5+δ layered perovskite cathode for intermediate temperature solid oxide fuel cells. Electrochimica Acta 2010, 55, 3812–3816. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Xu, H.X.; Jiang, L.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Structure and properties of layered-perovskite LaBa1-xCo2O5+δ (x = 0-0.15) as intermediate-temperature cathode material. J. Power Sources 2013, 240, 54–59. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X. Characterization of Ba-deficient PrBa1-xCo2O5+δ as cathode material for intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 204, 53–59. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X.; Zhang, Q.Y. Highly enhanced electrochemical performance of PrBa0.92Co2O5+δ cathode by introducing Ba cationic-deficiency. Int. J. Hydrogen Energy 2012, 37, 3998–4001. [Google Scholar] [CrossRef]
- Jiang, L.; Li, F.; Wei, T.; Zeng, R.; Huang, Y.H. Evaluation of Pr1+xBa1-xCo2O5+δ (x = 0 − 0.3) as cathode materials for solid-oxide fuel cells. Electrochimica Acta 2014, 133, 364–372. [Google Scholar] [CrossRef]
- Jiang, X.N.; Shi, Y.C.; Zhou, W.L.; Li, X.N.; Su, Z.X.; Pang, S.L.; Jiang, L. Effects of Pr3+-deficiency on structure and properties of PrBaCo2O5+δ cathode material-A comparison with Ba2+-deficiency case. J. Power Sources 2014, 272, 371–377. [Google Scholar] [CrossRef]
- Wang, J.P.; Meng, F.C.; Xia, T.; Shi, Z.; Lian, J.; Xu, C.B.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C. Superior electrochemical performance and oxygen reduction kinetics of layered perovskite. Int. J. Hydrogen Energy 2014, 39, 18392–18404. [Google Scholar] [CrossRef]
- Yoo, S.; Jun, A.; Ju, Y.-W.; Odkhuu, D.; Hyodo, J.; Jeong, H.Y.; Park, N.; Shin, J.; Ishihara, T.; Kim, G. Development of Double-perovskite compounds as cathode materials for low-temperature solid oxide fuel cells. Angew. Chem. Int. Ed. 2014, 53, 13064–13067. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.C.; He, B.B.; Chen, F.L.; Xia, C.R. Synthesis, characterization and evaluation of PrBaCo2-xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.-H.; Manthiram, A. Effects of Fe substitution on the structure and properties of LnBaCo2-xFexO5+δ (Ln = Nd and Gd) cathodes. J. Power Sources 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Jun, A.; Lim, T.-H.; Shin, J.; Kim, G. Electrochemical properties of B-site Ni doped layered perovskite cathodes for IT-SOFCs. Int. J. Hydrogen Energy 2014, 39, 20791–20798. [Google Scholar] [CrossRef]
- Hu, Y.; Bogicevic, C.; Bouffanais, Y.; Giot, M.; Hernandez, O.; Dezanneau, G. Synthesis, physical-chemical characterization and electrochemical performance of GdBaCo2-xNixO5 (x = 0-0.8) as cathode materials for IT-SOFC application. J. Power Sources 2013, 242, 50–56. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Jia, D.C.; Huang, X.Q.; Zhang, Y.H.; Su, W.H. Thermal expansion and electrochemical properties of Ni-doped GdBaCo2O5+δ double-perovskite type oxides. Int. J. Hydrogen Energy 2010, 35, 3775–3782. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2-xNixO5+δ (0 ≤ x ≤1.0) perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2011, 158, B276–B282. [Google Scholar] [CrossRef]
- Lü, S.Q.; Long, G.H.; Ji, Y.; Meng, X.W.; Zhao, H.Y.; Sun, C.C. SmBaCoCuO5+x as cathode material based on GDC electrolyte for intermediate-temperature solid oxide fuel cells. J. Alloy Compd. 2011, 509, 2824–2828. [Google Scholar] [CrossRef]
- Jo, S.H.; Muralidharan, P.; Kim, D.K. Enhancement of electrochemical performance and thermal compatibility of GdBaCo2/3Fe2/3Cu2/3O5+δ cathode on Ce1.9Gd0.1O1.95 electrolyte for IT-SOFCs. Electrochem. Commun. 2009, 11, 2085–2088. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, S.; Kim, J.; Park, S.; Jun, A.; Sengodan, S.; Kim, J.; Shin, J.; Jeong, H.Y.; Choi, Y.; et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2-xFexO5+δ. Sci. Rep. 2013, 3, 2426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jun, A.; Shin, J.; Kim, G. Effect of Fe Doping on Layered GdBa0.5Sr0.5Co2O5+δ Perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Am. Ceram. Soc. 2014, 97, 651–656. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Park, S.; Kim, C.; Shin, J.; Kim, G. Effect of Mn on the electrochemical properties of a layered perovskite NdBa0.5Sr0.5Co2-xMnxO5+δ (x = 0, 0.25, and 0.5) for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2013, 112, 712–718. [Google Scholar] [CrossRef]
- Pramana, S.S.; Cavallaro, A.; Li, C.; Handoko, A.D.; Chan, K.W.; Walker, R.J.; Regoutz, A.; Herrin, J.S.; Yeo, B.S.; Payne, D.J.; et al. Crystal structure and surface characteristics of Sr-doped GdBaCo2O6-δ double perovskites: Oxygen evolution reaction and conductivity. J. Mater. Chem. A 2018, 6, 5335–5345. [Google Scholar] [CrossRef]
- Subardi, A.; Chen, C.C.; Cheng, M.H.; Chang, W.K.; Fu, Y.P. Electrical, thermal and electrochemical properties of SmBa1-xSrxCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2016, 204, 118–127. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Performance of double-perovskite YBa0.5Sr0.5Co2O5+δ as cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 6894–6898. [Google Scholar] [CrossRef]
- Subardi, A.; Indra, A.; Setiawan, J.; Fu, Y.-P. Structural and Electrochemical Analysis of SmBa0.8Sr0.2Co2O5+δ Cathode Oxide for IT-SOFCs. Int. J. Integr. Eng. 2023, 15, 173–179. [Google Scholar] [CrossRef]
- Zan, J.; Wang, S.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Characterization and functional application of PrBa0.5Sr0.5Co1.5Fe0.5O5+δ cathode material for IT-SOFC. Mater. Res. Bull. 2021, 137, 111173. [Google Scholar] [CrossRef]
- Costilla-Aguilar, S.U.; Escudero, M.J.; Cienfuegos-Pelaes, R.F.; Aguilar-Martinez, J.A. Double perovskite La1.8Sr0.2CoFeO5+δ as a cathode material for intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2021, 862, 158025. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Sun, S.; Lu, C.; Wang, B.; Liu, G.; Gao, S.; Niu, B. Novel CO2-tolerant Co-based double perovskite cathode for intermediate temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2023, 43, 1028–1038. [Google Scholar] [CrossRef]
- Li, M.; Chen, K.; Hua, B.; Luo, J.-l.; Rickard, W.D.A.; Li, J.; Irvine, J.T.S.; Jiang, S.P. Smart utilization of cobaltite-based double perovskite cathodes on barrier-layer-free zirconia electrolyte of solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 19019–19025. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, L.Z.; Han, M.-F. A-site deficient Ba1−xCo0.7Fe0.2Ni0.1O3-δ cathode for intermediate temperature SOFC. J. Power Sources 2011, 196, 868–871. [Google Scholar] [CrossRef]
- Chen, T.; Pang, S.; Shen, X.; Jiang, X.; Wang, W. Evaluation of Ba-deficient PrBa1-xFe2O5+δ oxides as cathode materials for intermediate-temperature solid oxide fuel cells. RSC Adv. 2016, 6, 13829–13836. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Chen, S.; Lang, X.; Meng, J.; Cai, K. Evaluation of A-site Ba-deficient PrBa0.5-xSr0.5Co2O5+δ (x = 0, 0.04 and 0.08) as cathode materials for solid oxide fuel cells. J. Alloys Compd. 2021, 883, 160759. [Google Scholar] [CrossRef]
- Idrees, A.; Jiang, X.; Jiang, L.; Zhang, Q. Properties of composite cathodes composed of Pr3+-deficient perovskite oxide and ionic conductor Ce0.8Sm0.2O1.9. Ceram. Int. 2020, 46, 17532–17539. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Sun, N.; Li, J.; Shen, Y.; Wang, F.; Li, J. Nd3+-deficiency double perovskite Nd1-xBaCo2O5+δ and performance optimization as cathode materials for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2021, 47, 33886–33896. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.-M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6-δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- Lü, S.; Zhu, Y.; Fu, X.; Huang, R.; Guo, Y.; Zhang, W.; Li, H.; Hou, L.; Meng, X. A-site deficient Fe-based double perovskite oxides PrxBaFe2O5+δ as cathodes for solid oxide fuel cells. J. Alloys Compd. 2022, 911, 165002. [Google Scholar] [CrossRef]
- Nie, Z.; Wang, J.; Xia, T.; Wang, G. A-site Ca-doped layered double perovskite Pr1-xCaxBa0.94Co2O5+δ as high-performance and stable cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2022, 905, 164191. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Lang, X.; Cai, K. Ca-doped PrBa1-xCaxCoCuO5+δ (x = 0–0.2) as cathode materials for solid oxide fuel cells. Ceram. Int. 2022, 48, 7652–7662. [Google Scholar] [CrossRef]
- Asensio, A.M.; Clematis, D.; Cademartori, D.; Carpanese, M.P.; Viviani, M.; Carbone, C.; Barbucci, A. Calcium doping in double perovskite SmBa1−xCaxCo2O5+δ to enhance the electrochemical activity of solid oxide cell reversible oxygen electrode. J. Alloys Compd. 2023, 933, 167731. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Liu, X.; Sun, N.; Li, J.; Shen, Y.; Wang, F.; Yang, L.; Chu, X.; Xu, M.; et al. Effect of calcium doping on Sm1–xCaxBaCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2021, 390, 138830. [Google Scholar] [CrossRef]
- Jin, F.; Liu, X.; Chu, X.; Shen, Y.; Li, J. Effect of nonequivalent substitution of Pr3+/4+ with Ca2+ in PrBaCoFeO5+δ as cathodes for IT-SOFC. J. Mater. Sci. 2021, 56, 1147–1161. [Google Scholar] [CrossRef]
- Du, Z.; Yan, C.; Zhao, H.; Zhang, Y.; Yang, C.; Yi, S.; Lu, Y.; Świerczek, K. Effective Ca-doping in Y1-xCaxBaCo2O5+δ cathode materials for intermediate temperature solid oxide fuel cells. J. Mater. Chem. A 2017, 5, 25641–25651. [Google Scholar] [CrossRef]
- Pang, S.; Su, Y.; Yang, G.; Shen, X.; Zhu, M.; Wu, X.; Li, S.; Yang, X.; Xi, X. Enhanced electrochemical performance of Ca-doped NdBa1-xCaxCoCuO5+δ as cathode material for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2018, 44, 21902–21907. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y.; Li, J. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode material for IT-SOFCs. Electrochim. Acta 2020, 364, 137274. [Google Scholar] [CrossRef]
- Li, J.; Sun, N.; Liu, X.; Shen, Y.; Wang, F.; Li, J.; Shi, K.; Jin, F. Investigation on Nd1–xCaxBaCo2O5+δ double perovskite as new oxygen electrode materials for reversible solid oxide cells. J. Alloys Compd. 2022, 913, 165245. [Google Scholar] [CrossRef]
- Wang, L.; Xie, P.; Bian, L.; Liu, X.; Chou, K. Performance of Ca-doped GdBa1-xCaxFe2O5+δ (x=0, 0.1) as cathode materials for IT-SOFC application. Catal. Today 2018, 318, 132–136. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, J.; Li, S.; Xia, T.; Wang, G. Positive effects of calcium-doping on the cathode performance of layered perovskite Eu1-xCaxBaCo2O5+δ for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2019, 801, 220–228. [Google Scholar] [CrossRef]
- Wang, W.; Pang, S.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Xi, X.; Xiang, J. The effect of calcium on the properties of SmBa1−xCaxCoCuO5+δ as a cathode material for intermediate-temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, C.; Chen, X.; Chen, F.; Xiong, X.; Liu, Y.; Yan, J.; Fujita, T. A-site double-lanthanide-doped La1-xPrxBaCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. J. Mater. Sci. 2022, 57, 14398–14412. [Google Scholar] [CrossRef]
- Bangwal, A.S.; Jha, P.K.; Chauhan, M.; Singh, S.; Sinha, A.S.K.; Jha, P.A.; Singh, P. Compositional effect on oxygen reduction reaction in Pr excess double perovskite Pr1-xBa1-xCo2O6-δ cathode materials. Int. J. Hydrogen Energy 2020, 45, 23378–23390. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Xiong, X.; Pan, J.; Cai, D.; Wei, Z.; Chen, X.; Liu, Y.; Luo, N.; Yan, J.; et al. Doping strategy on improving the overall cathodic performance of double perovskite LnBaCo2O5+δ (Ln=Pr, Gd) as potential SOFC cathode materials. J. Mater. 2023, 9, 825–837. [Google Scholar] [CrossRef]
- Zhu, F.; He, F.; Xu, K.; Chen, Y. Enhancing the oxygen reduction reaction activity and durability of a double-perovskite via an A-site tuning. Sci. China Mater. 2022, 65, 3043–3052. [Google Scholar] [CrossRef]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5+δ (Ln = La, Pr, Nd and Sm) as cathode materials for IT-SOFC. J. Electroanal. Chem. 2021, 886, 115144. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, D.; Liu, R.; Wu, H.; Chen, Y.; Ding, Y.; Lu, X.; Lin, B. Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=La and Nd) for SOFCs. Int. J. Hydrogen Energy 2021, 46, 5630–5641. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, B.; Hao, X.; Wang, Y.; Liu, J.; Jiang, P.; He, T. Layered oxygen-deficient double perovskite GdBaFe2O5+δ as electrode material for symmetrical solid-oxide fuel cells. Electrochim. Acta 2021, 370, 137807. [Google Scholar] [CrossRef]
- Saccoccio, M.; Jiang, C.; Gao, Y.; Chen, D.; Ciucci, F. Nb-substituted PrBaCo2O5+δ as a cathode for solid oxide fuel cells: A systematic study of structural, electrical, and electrochemical properties. Int. J. Hydrogen Energy 2017, 42, 19204–19215. [Google Scholar] [CrossRef]
- Akande, S.O.; Chroneos, A.; Schwingenschlögl, U. O vacancy formation in (Pr/Gd)BaCo2O5.5 and the role of antisite defects. Phys. Chem. Chem. Phys. 2017, 19, 11455–11459. [Google Scholar] [CrossRef] [PubMed]
- Anjum, U.; Vashishtha, S.; Agarwal, M.; Tiwari, P.; Sinha, N.; Agrawal, A.; Basu, S.; Haider, M.A. Oxygen anion diffusion in double perovskite GdBaCo2O5+δ and LnBa0.5Sr0.5Co2-xFexO5+δ (Ln = Gd, Pr, Nd) electrodes. Int. J. Hydrogen Energy 2016, 41, 7631–7640. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Eremin, V.A.; Tsvetkov, D.S.; Porotnikova, N.M.; Farlenkov, A.S.; Zuev, A.Y.; Fetisov, A.V.; Kurumchin, E.K. Oxygen isotope exchange and diffusion in LnBaCo2O6−δ (Ln = Pr, Sm, Gd) with double perovskite structure. Solid State Ionics 2017, 304, 96–106. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, F.; Shen, Y.; He, T. Performances of LnBaCo2O5+x–Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 2174–2181. [Google Scholar] [CrossRef]
- Malyshkin, D.; Novikov, A.; Tsvetkov, D.; Zuev, A. Preparation, oxygen nonstoichiometry and defect structure of double perovskite LaBaCo2O6–δ. Mater. Lett. 2018, 229, 324–326. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Li, Y.; Yu, B. REBaCo2O5+δ (RE = Pr, Nd, and Gd) as promising oxygen electrodes for intermediate-temperature solid oxide electrolysis cells. RSC Adv. 2017, 7, 16332–16340. [Google Scholar] [CrossRef]
- Politov, B.V.; Suntsov, A.Y.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. Thermodynamic analysis of defect equilibration in double perovskites based on PrBaCo2O6–δ cobaltite. J. Solid State Chem. 2017, 249, 108–113. [Google Scholar] [CrossRef]
- Jin, F.; Liu, J.; Shen, Y.; He, T. Improved electrochemical performance and thermal expansion compatibility of LnBaCoFeO5+δ-Sm0.2Ce0.8O1.9 (Ln=Pr and Nd) composite cathodes for IT-SOFCs. J. Alloys Compd. 2016, 685, 483–491. [Google Scholar] [CrossRef]
- Zając, W.; Świerczek, K.; Molenda, J. Thermochemical compatibility between selected (La,Sr)(Co,Fe,Ni)O3 cathodes and rare earth doped ceria electrolytes. J. Power Sources 2007, 173, 675–680. [Google Scholar] [CrossRef]
- Zhu, J.H.; Geng, S.J.; Ballard, D.A. Evaluation of several low thermal expansion Fe–Co–Ni alloys as interconnect for reduced-temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2007, 32, 3682–3688. [Google Scholar] [CrossRef]
- Señarís-Rodríguez, M.A.; Goodenough, J.B. Magnetic and Transport Properties of the System La1-xSrxCoO3-δ (0 < x ≤ 0.50). J. Solid State Chem. 1995, 118, 323–336. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.Y.; Goodenough, J.B. Sr- and Ni- Doped LaCoO3 and LaFeO3 perovskites: New cathode materials for solid-oxide fuel cells. J. Electrochem. Chem. 1998, 145, 3220–3227. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Zuev, A.Y. Crystal structure and oxygen content of the double perovskites GdBaCo2-xFexO6-δ. J. Solid State Chem. 2013, 199, 154–159. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Double-perovskites YBaCo2−xFexO5+δ cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2011, 196, 3729–3735. [Google Scholar] [CrossRef]
- Zou, J.; Park, J.; Kwak, B.; Yoon, H.; Chung, J. Effect of Fe doping on PrBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Solid State Ionics 2012, 206, 112–119. [Google Scholar] [CrossRef]
- Joo, S.; Kim, J.; Shin, J.; Lim, T.-H.; Kim, G. Investigation of a layered perovskite for IT-SOFC cathodes: B-site Fe-doped YBa0.5Sr0.5Co2-xFexO5+δ. J. Electrochem. Soc. 2016, 163, F1489–F1495. [Google Scholar] [CrossRef]
- Son, S.J.; Kim, D.; Park, H.J.; Joo, J.H. Investigation of oxygen ion transport and surface exchange properties of PrBaFe2O5+δ. J. Eur. Ceram. Soc. 2021, 41, 2691–2698. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Yang, J.-X.; Wang, P.-F.; Yao, C.-G.; Yu, X.-D.; Shi, F.-N. Novel cobalt-free perovskite PrBaFe1.9Mo0.1O5+δ as a cathode material for solid oxide fuel cells. Solid State Ionics 2023, 391, 116144. [Google Scholar] [CrossRef]
- Politov, B.V.; Suntsov, A.Y.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. High-temperature defect thermodynamics of nickel substituted double-perovskite cobaltite PrBaCo2-xNixO6-δ (x = 0.2). J. Alloys Compd. 2017, 727, 778–784. [Google Scholar] [CrossRef]
- Xia, L.-N.; You, J.; He, Z.-P.; Huang, X.-W.; Yu, Y. Performances of nickel-doped SmBaCo2O5+δ-Sm0.2Ce0.8O1.9 composite cathodes for IT-SOFC. Int. J. Hydrogen Energy 2016, 41, 1176–1186. [Google Scholar] [CrossRef]
- Xia, L.-N.; He, Z.-P.; Huang, X.W.; Yu, Y. Synthesis and properties of SmBaCo2-xNixO5+δ perovskite oxide for IT-SOFC cathodes. Ceram. Int. 2016, 42, 1272–1280. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Sayagués, M.J.; Gotor, F.J. A Novel, Simple and highly efficient route to obtain PrBaMn2O5+δ double perovskite: Mechanochemical synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef]
- Huang, X.; Feng, J.; Abdellatif, H.R.S.; Zou, J.; Zhang, G.; Ni, C. Electrochemical evaluation of double perovskite PrBaCo2-xMnxO5+δ (x = 0, 0.5, 1) as promising cathodes for IT-SOFCs. Int. J. Hydrogen Energy 2018, 43, 8962–8971. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the electrochemical performance of Fe-based layered double perovskite cathodes by Zn2+ doping for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef]
- Sun, C.; Kong, Y.; Niu, Y.; Yin, X.; Zhang, N. Probing Zr substituting effects on the oxygen reduction reaction of Fe-based double perovskite cathodes for solid oxide fuel cells. ACS Appl. Energy Mater. 2022, 5, 4486–4495. [Google Scholar] [CrossRef]
- Sun, C.; Kong, Y.; Shao, L.; Zhang, Q.; Wu, X.; Zhang, N.; Sun, K. Significant zirconium substitution effect on the oxygen reduction activity of the cathode material NdBaCo2O5+δ for solid oxide fuel cells. ACS Sust. Chem. Eng. 2019, 7, 11603–11611. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, Y.; Hua, Z.; Tang, K.; Xia, C. Tungsten-doped PrBaFe2O5+δ double perovskite as a high-performance electrode material for symmetrical solid oxide fuel cells. ACS Appl. Energy Mater. 2021, 4, 8401–8409. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Kwon, O.; Lim, C.; Sengodan, S.; Shin, J.; Kim, G. Scandium doping effect on a layered perovskite cathode for low-temperature solid oxide fuel cells (LT-SOFCs). Appl. Sci. 2018, 8, 2217. [Google Scholar] [CrossRef]
- Xu, J.; Cai, H.; Hao, G.; Zhang, L.; Song, Z.; Long, W.; Zhang, L.; Wang, L. Characterization of high-valence Mo-doped PrBaCo2O5+δ cathodes for IT-SOFCs. J. Alloys Compd. 2020, 842, 155600. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Han, H.; Tang, K.; Xia, C. Cobalt-free double perovskite oxide as a promising cathode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2023, 15, 8253–8262. [Google Scholar] [CrossRef]
- Bao, X.; Su, X.; Wang, S.; Pan, B.; Wang, L.; Zhang, L.; Song, Z.; Long, W.; Li, C. Effects of Bi-doping on structure and properties of YBaCo2O5+δ layered perovskite cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2023, 965, 171391. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature solid oxide fuel cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Yu, F.; Pan, Z.; Yang, H.; Guo, L. Ca and Fe co-doped SmBaCo2O5+δ layered perovskite as an efficient cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2017, 696, 964–970. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, W.; Jia, L.; Pu, J.; Chi, B.; Li, J. High performance and stability of double perovskite-type oxide NdBa0.5Ca0.5Co1.5Fe0.5O5+δ as an oxygen electrode for reversible solid oxide electrochemical cell. J. Energy Chem. 2020, 43, 108–115. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Novel PrBa0.9Ca0.1Co2-xZnxO5+δ double-perovskite as an active cathode material for high-performance proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 31009–31016. [Google Scholar] [CrossRef]
- Dong, F.F.; Ni, M.; Chen, Y.B.; Chen, D.J.; Tadé, M.O.; Shao, Z.P. Structural and oxygen-transport studies of double perovskites PrBa1-xCo2O5+δ (x = 0.00, 0.05, and 0.10) toward their application as superior oxygen reduction electrodes. J. Mater. Chem. A 2014, 2, 20520–20529. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.X.; Lai, S.Y.; Gerdes, K.; Liu, M.L. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, S.; Jeong, H.Y.; Liu, M.L.; Kim, B.-S.; Kim, G. Highly efficient layer-by-layer-assisted infiltration for high-performance and cost-effective fabrication of nanoelectrodes. ACS Appl. Mater. Interfaces 2014, 6, 17352–17357. [Google Scholar] [CrossRef]
- Han, D.; Wu, H.; Li, J.L.; Wang, S.R.; Zhan, Z.L. Nanostructuring of SmBa0.5Sr0.5Co2O5+δ cathodes for reduced-temperature solid oxide fuel cells. J. Power Sources 2014, 246, 409–416. [Google Scholar] [CrossRef]
- Ding, H.P.; Xue, X.J. An Interfacial nanospike-structured cathode for low temperature solid oxide fuel cells. Adv. Mater. Interfaces 2014, 1, 1400008. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.J.; Chen, C.; Shao, Z.P.; Ciucci, F. Oriented PrBaCo2O5+δ thin films for solid oxide fuel cells. J. Power Sources 2015, 278, 623–629. [Google Scholar] [CrossRef]
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L. Superfast oxygen exchange kinetics on highly epitaxial LaBaCo2O5+δ thin films for intermediate temperature solid oxide fuel cells. APL Mater. 2013, 1, 031101. [Google Scholar] [CrossRef]
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L.; He, J.; Jiang, J.C.; Meletis, E.I. Ultrafast oxygen exchange kinetics on highly epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2012, 100, 193903. [Google Scholar] [CrossRef]
- Pang, S.; Long, C.; Tang, X.; Fang, T.; Ke, L.; Yang, G.; Song, Y.; Chen, C. Highly active and robust biomimetic ceramic catalyst for oxygen reduction reaction: Inspired by plant leaves. Ceram. Int. 2023, 49, 20273–20280. [Google Scholar] [CrossRef]
- Kim, S.; Jun, A.; Kwon, O.; Kim, J.; Yoo, S.; Jeong, H.Y.; Shin, J.; Kim, G. Nanostructured double perovskite cathode with low sintering temperature for intermediate temperature solid oxide fuel cells. ChemSusChem 2015, 8, 3153–3158. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Oxygen content, cobalt oxide exsolution and defect structure of the double perovskite PrBaCo2O6-δ. J. Mater. Chem. A 2016, 4, 1962–1969. [Google Scholar] [CrossRef]
- Pang, S.; Song, Y.; Cui, M.; Tang, X.; Long, C.; Ke, L.; Yang, G.; Fang, T.; Guan, Y.; Chen, C. Rapid and durable oxygen reduction reaction enabled by a perovskite oxide with self-cleaning surface. J. Energy Chem. 2023, 83, 333–340. [Google Scholar] [CrossRef]
- Fu, M.; Lin, X.; Tan, L.; Zhang, P.; Xie, H.; Tao, Z. Self-assembled Fe-doped PrBaCo2O5+δ composite cathodes with disorder transition region for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 15229–15237. [Google Scholar] [CrossRef]
- Jiang, X.N.; Xu, H.X.; Wang, Q.; Jiang, L.; Li, X.N.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Fabrication of GdBaCo2O5+δ cathode using electrospun composite nanofibers and its improved electrochemical performance. J. Alloys Compd. 2013, 557, 184–189. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on fabrication techniques for porous electrodes of solid oxide fuel cells by sacrificial template methods. Renew. Sust. Energ. Rev. 2017, 77, 1221–1239. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, Y.; Zhao, B.; Zhang, Y.; Ding, D.; Hu, R.; Wei, T.; Rainwater, B.; Ding, Y.; Chen, F.; et al. A durable, high-performance hollow-nanofiber cathode for intermediate-temperature fuel cells. Nano Energy 2016, 26, 90–99. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 2017, 29, 1700132. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Zhen, D.; Zhao, B.; Shin, H.-C.; Bu, Y.; Ding, Y.; He, G.; Liu, M. Electrospun porous perovskite La0.6Sr0.4Co1–xFexO3–δ nanofibers for efficient oxygen evolution reaction. Adv. Mater. Interfaces 2017, 4, 1700146. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, M.; Wang, G.Q.; Lu, H.L.; Yang, T.Z.; Guo, H.M.; Ma, C.R.; Xu, X.; Zhang, M.H.; Jiang, J.C.; et al. Step terrace tuned anisotropic transport properties of highly epitaxial LaBaCo2O5.5+δ thin films on vicinal SrTiO3 substrates. ACS Appl. Mater. Interfaces 2014, 6, 6704–6708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Collins, G.; Chen, C.L.; Jiang, X.N.; Gong, W.Q.; Jacobson, A.J.; He, J.; Jiang, J.C.; Meletis, E.I. Epitaxial nature and transport properties in (LaBa)Co2O5+δ thin films. Chem. Mater. 2010, 22, 799–802. [Google Scholar] [CrossRef]
- Druce, J.; Téllez, H.; Burriel, M.; Sharp, M.D.; Fawcett, L.J.; Cook, S.N.; Mcphail, D.S.; Ishihara, T.; Brongersma, H.H.; Kilner, J.A. Surface termination and subsurface restructuring of perovskite-based solid oxide electrode materials. Energy Environ. Sci. 2014, 7, 3593–3599. [Google Scholar] [CrossRef]
- Lee, W.; Yildiz, B. Factors that influence cation segregation at the surfaces of perovskite oxides. ECS Trans. 2013, 57, 2115–2123. [Google Scholar] [CrossRef]
- Lee, W.; Han, J.W.; Chen, Y.; Cai, Z.H.; Yildiz, B. Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 2013, 135, 7909–7925. [Google Scholar] [CrossRef]
- Mebane, D.S. A variational approach to surface cation segregation in mixed conducting perovskites. Comput. Mater. Sci. 2014, 103, 231–236. [Google Scholar] [CrossRef]
- Pang, S.; Xu, J.; Su, Y.; Yang, G.; Zhu, M.; Cui, M.; Shen, X.; Chen, C. The role of A-site cation size mismatch in tune the catalytic activity and durability of double perovskite oxides. Appl. Catal. B Environ. 2020, 270, 118868. [Google Scholar] [CrossRef]
- Anjum, U.; Agarwal, M.; Khan, T.S.; Haider, M.A. Mechanistic elucidation of surface cation segregation in double perovskite PrBaCo2O5+δ material using MD and DFT simulations for solid oxide fuel cells. Ionics 2020, 26, 1307–1314. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Lü, Z.; Feng, J.; Xu, L.; Gao, H.; Zhang, Y.; Huang, X. Performance degradation of double-perovskite PrBaCo2O5+δ oxygen electrode in CO2 containing atmospheres. Appl. Surf. Sci. 2017, 416, 649–655. [Google Scholar] [CrossRef]
- Wei, B.; Chen, K.; Wang, C.C.; Lü, Z.; Jiang, S.P. Performance degradation of SmBaCo2O5+δ cathode induced by chromium deposition for solid oxide fuel cells. Electrochim. Acta 2015, 174, 327–331. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, S.; Murphy, R.; Chen, Y.; Ding, Y.; Pei, K.; Zhao, B.; Kim, G.; Choi, Y.M.; Liu, M. Promotion of oxygen reduction reaction on a double perovskite electrode by a water-induced surface modification. Energy Environ. Sci. 2021, 14, 1506–1516. [Google Scholar] [CrossRef]
- Wei, B.; Schroeder, M.; Martin, M. Surface cation segregation and chromium deposition on the double perovskite oxide PrBaCo2O5+δ. ACS Appl. Mater. Interfaces 2018, 10, 8621–8629. [Google Scholar] [CrossRef] [PubMed]
- Druce, J.; Téllez, H.; Hyodo, J. Surface segregation and poisoning in materials for low-temperature SOFCs. MRS Bull. 2014, 39, 810–815. [Google Scholar] [CrossRef]
- Lu, F.; Xia, T.; Li, Q.; Wang, J.; Huo, L.; Zhao, H. Heterostructured simple perovskite nanorod-decorated double perovskite cathode for solid oxide fuel cells: Highly catalytic activity, stability and CO2-durability for oxygen reduction reaction. Appl. Catal. B Environ. 2019, 249, 19–31. [Google Scholar] [CrossRef]
- Ke, L.; Pang, S.; Long, C.; Fang, T.; Yang, G.; Song, Y.; He, X.; Ma, S.; Qian, Y.; Shen, X.; et al. Quenching-induced surface reconstruction of perovskite oxide for rapid and durable oxygen catalysis. Chem. Eng. J. 2023, 463, 142509. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Pang, S.; Cui, M.; Shen, X. Tuning interfacial chemistry and electrochemical properties of solid oxide cells via cation interdiffusion. Ceram. Int. 2020, 46, 12044–12049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).