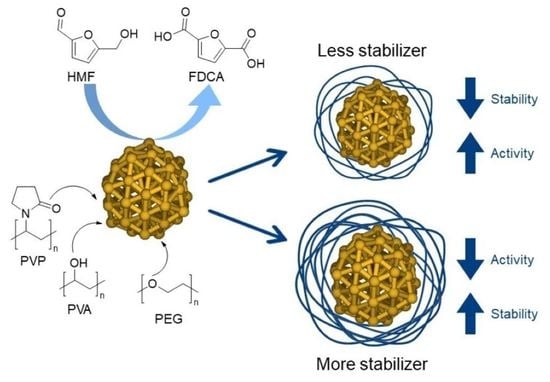
Effect of Capping Ligands for the Synthesis of Gold Nanoparticles and on the Catalytic Performance for the Oxidation of 5-Hydroxymethyl-2-furfural
Abstract
1. Introduction
2. Results and Discussion
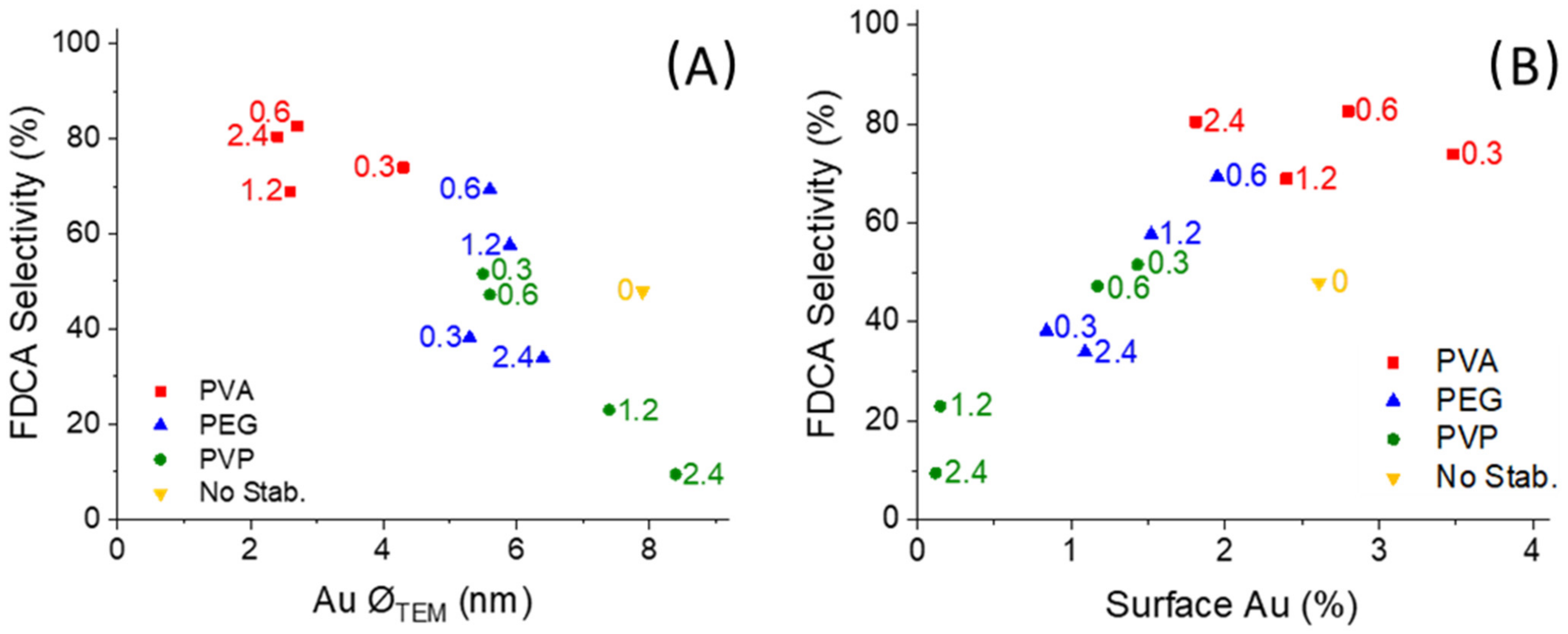
2.1. Catalysts Characterization
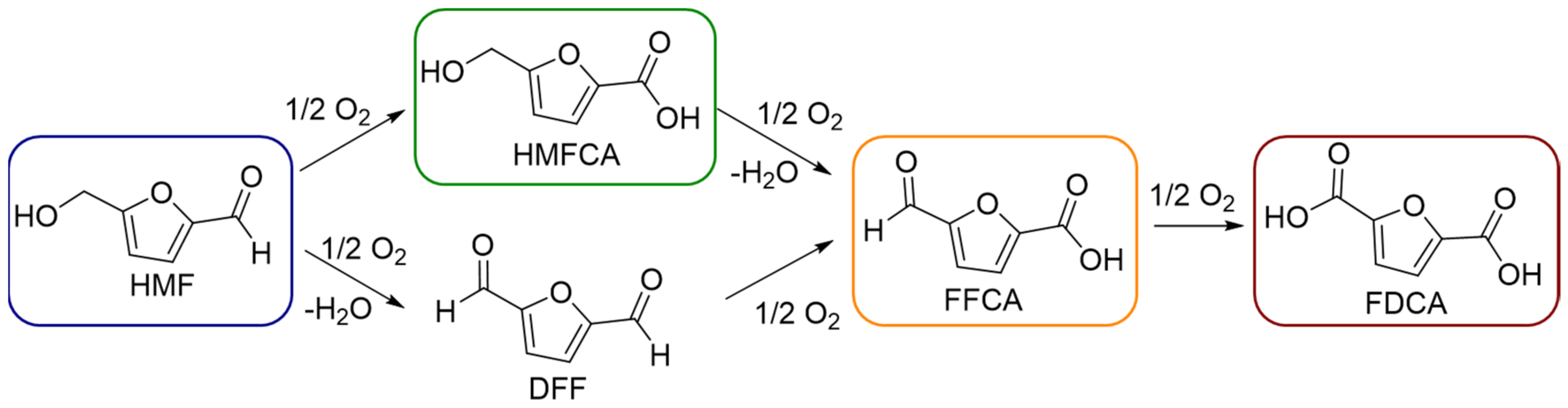
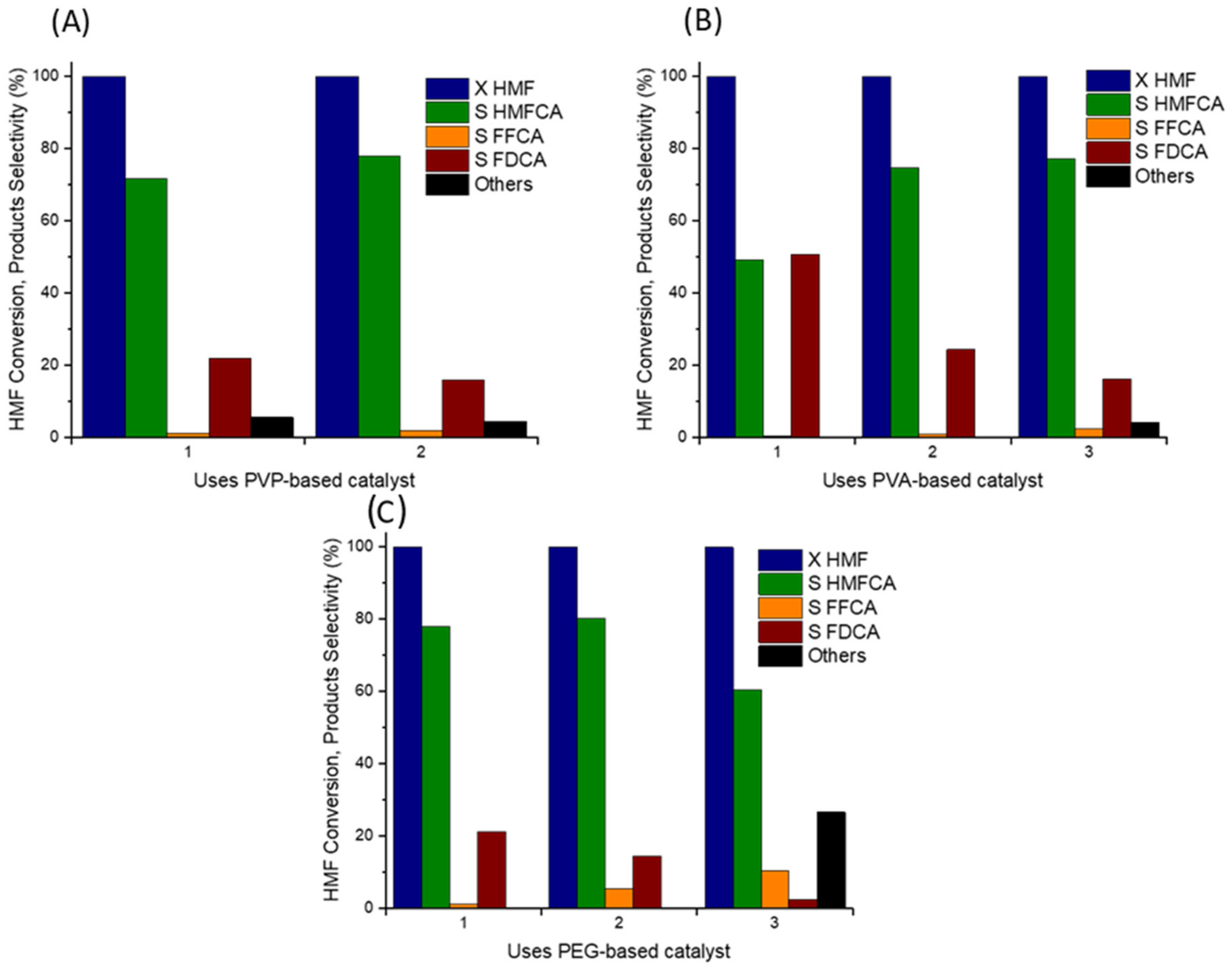
2.2. Catalytic Tests
2.3. Characterization of Catalysts for Evaluating the Difference in the Catalytic Activity
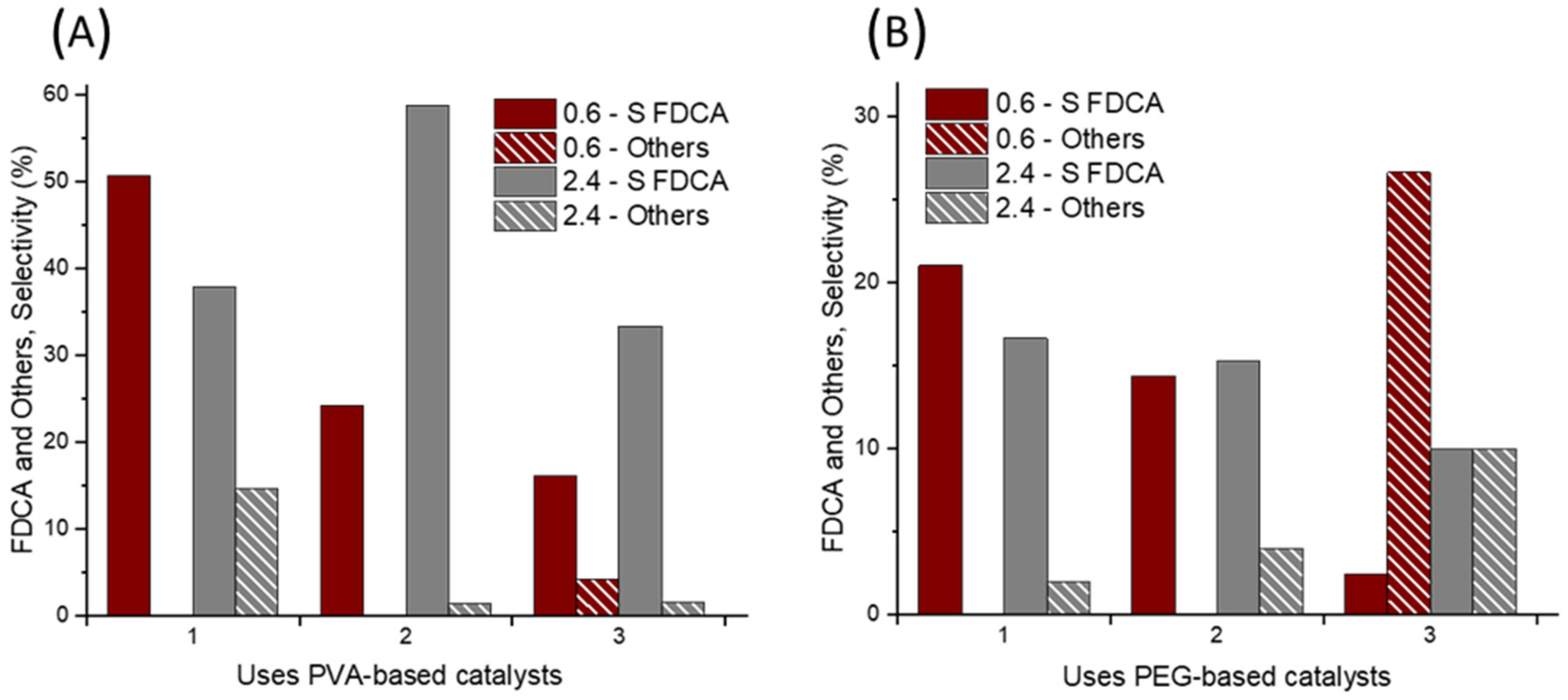
2.4. Catalyst Reusability
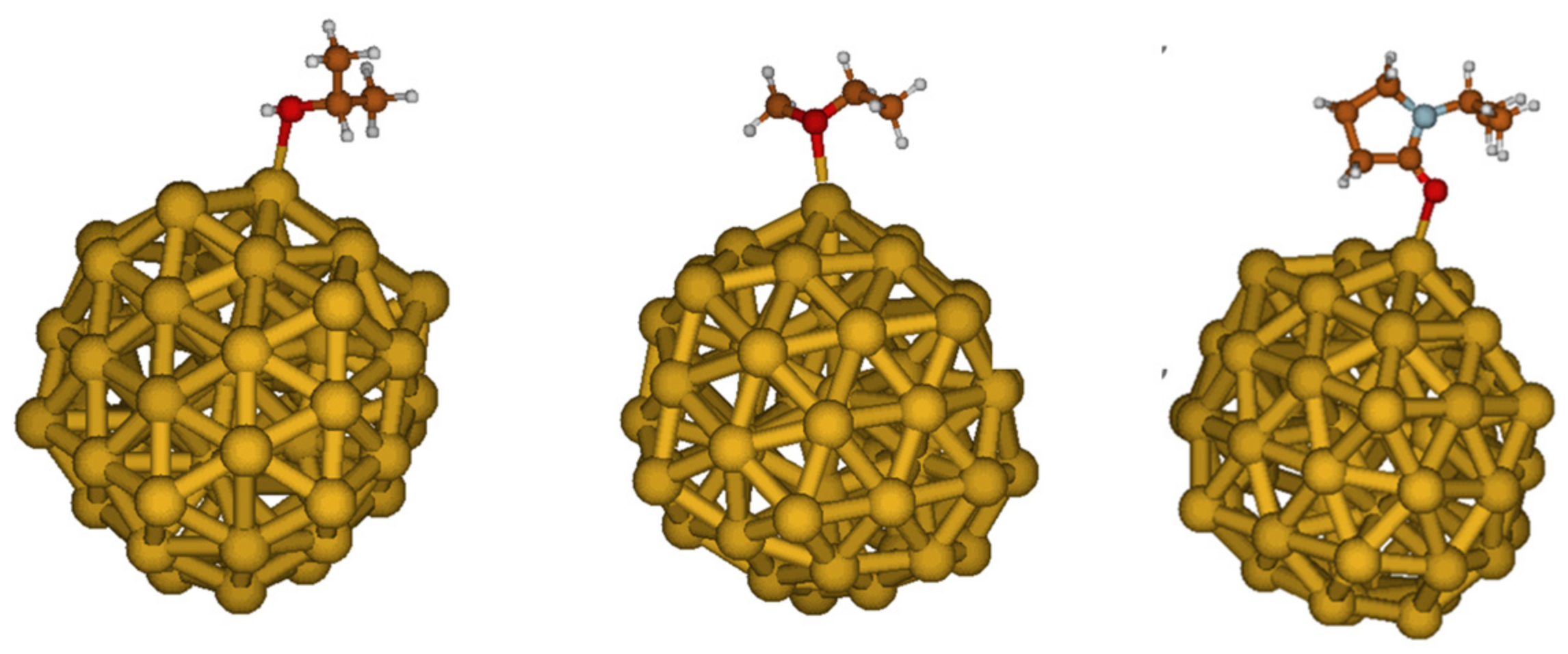
2.5. Interaction between Stabilizing Agents and Au NPs: A Computational Study
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Characterization
3.4. 5-Hydroxymethylfurfural Oxidation Tests
3.5. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1896. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, R. Heterogeneous Catalysis by Gold and Gold-Based Bimetal Nanoclusters. Nano Today 2018, 18, 86–102. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions. Chem. Soc. Rev. 2012, 41, 5577. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Dimitratos, N.; Villa, A.; Prati, L.; Hammond, C.; Chan-Thaw, C.E.; Cookson, J.; Bishop, P.T. Effect of the Preparation Method of Supported Au Nanoparticles in the Liquid Phase Oxidation of Glycerol. Appl. Catal. Gen. 2016, 514, 267–275. [Google Scholar] [CrossRef]
- Hutchings, G.J. Selective Oxidation Using Supported Gold Bimetallic and Trimetallic Nanoparticles. Catal. Today 2014, 238, 69–73. [Google Scholar] [CrossRef]
- Yan, X.; Bao, J.; Yuan, C.; Wheeler, J.; Lin, W.-Y.; Li, R.; Jang, B.W.-L. Gold on Carbon and Titanium Oxides Composites: Highly Efficient and Stable Acetylene Hydrogenation in Large Excess of Ethylene. J. Catal. 2016, 344, 194–201. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37, 2096. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Liu, B.; Duay, S.; He, J. Engineering Surface Ligands of Noble Metal Nanocatalysts in Tuning the Product Selectivity. Catalysts 2017, 7, 44. [Google Scholar] [CrossRef]
- Bell, A.T. The Impact of Nanoscience on Heterogeneous Catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Laoufi, I.; Saint-Lager, M.-C.; Lazzari, R.; Jupille, J.; Robach, O.; Garaudée, S.; Cabailh, G.; Dolle, P.; Cruguel, H.; Bailly, A. Size and Catalytic Activity of Supported Gold Nanoparticles: An in Operando Study during CO Oxidation. J. Phys. Chem. C 2011, 115, 4673–4679. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Kamiński, M.; Glajcar, W.; Woźnica, N.; Julienne, F.; Bartczak, P.; Polański, J.; Lelątko, J.; Zubko, M.; Burian, A. Paracrystalline Structure of Gold, Silver, Palladium and Platinum Nanoparticles. J. Appl. Crystallogr. 2018, 51, 411–419. [Google Scholar] [CrossRef]
- Bujak, P.; Bartczak, P.; Polanski, J. Highly Efficient Room-Temperature Oxidation of Cyclohexene and d-Glucose over Nanogold Au/SiO2 in Water. J. Catal. 2012, 295, 15–21. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Veith, G.M.; Vindigni, F.; Prati, L. Sol Immobilization Technique: A Delicate Balance between Activity, Selectivity and Stability of Gold Catalysts. Catal. Sci. Technol. 2013, 3, 3036–3041. [Google Scholar] [CrossRef]
- Quintanilla, A.; Butselaar-Orthlieb, V.C.L.; Kwakernaak, C.; Sloof, W.G.; Kreutzer, M.T.; Kapteijn, F. Weakly Bound Capping Agents on Gold Nanoparticles in Catalysis: Surface Poison? J. Catal. 2010, 271, 104–114. [Google Scholar] [CrossRef]
- Baker, L.R.; Kennedy, G.; Krier, J.M.; Van Spronsen, M.; Onorato, R.M.; Somorjai, G.A. The Role of an Organic Cap in Nanoparticle Catalysis: Reversible Restructuring of Carbonaceous Material Controls Catalytic Activity of Platinum Nanoparticles for Ethylene Hydrogenation and Methanol Oxidation. Catal. Lett. 2012, 142, 1286–1294. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The Role and Fate of Capping Ligands in Colloidally Prepared Metal Nanoparticle Catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Yang, N.; Pattisson, S.; Douthwaite, M.; Zeng, G.; Zhang, H.; Ma, J.; Hutchings, G.J. Influence of Stabilizers on the Performance of Au/TiO2 Catalysts for CO Oxidation. ACS Catal. 2021, 11, 11607–11615. [Google Scholar] [CrossRef]
- Garcia, M.A.S.; Ibrahim, M.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; dos Santos, E.N.; Philippot, K.; Rossi, L.M. Study of the Influence of PPh3 Used as Capping Ligand or as Reaction Modifier for Hydroformylation Reaction Involving Rh NPs as Precatalyst. Appl. Catal. Gen. 2017, 548, 136–142. [Google Scholar] [CrossRef]
- García-Aguilar, J.; Navlani-García, M.; Berenguer-Murcia, Á.; Mori, K.; Kuwahara, Y.; Yamashita, H.; Cazorla-Amorós, D. Evolution of the PVP–Pd Surface Interaction in Nanoparticles through the Case Study of Formic Acid Decomposition. Langmuir 2016, 32, 12110–12118. [Google Scholar] [CrossRef]
- Sang, B.; Li, J.; Tian, X.; Yuan, F.; Zhu, Y. Selective Aerobic Oxidation of the 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Gold Nanoparticles Supported on Graphitized Carbon: Study on Reaction Pathways. Mol. Catal. 2019, 470, 67–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Tacacima, J.; Derenzo, S.; Poco, J.G.R. Synthesis of HMF from Fructose Using Purolite® Strong Acid Catalyst: Comparison between BTR and PBR Reactor Type for Kinetics Data Acquisition. Mol. Catal. 2018, 458, 180–188. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Optimization of the Process of Chemical Hydrolysis of Cellulose to Glucose. Cellulose 2014, 21, 2397–2407. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Zang, H.; Wang, K.; Zhang, M.; Xie, R.; Wang, L.; Chen, E.Y.-X. Catalytic Coupling of Biomass-Derived Aldehydes into Intermediates for Biofuels and Materials. Catal. Sci. Technol. 2018, 8, 1777–1798. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In ACS Symposium Series; Smith, P.B., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. ISBN 978-0-8412-2767-5. [Google Scholar]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing Fossil Based PET with Biobased PEF: Process Analysis, Energy and GHG Balance. Energy Environ. Sci. 2012, 5, 6407. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A Facile Method to Synthesize High-Molecular-Weight Biobased Polyesters from 2,5-Furandicarboxylic Acid and Long-Chain Diols. J. Polym. Sci. Part Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of Bio-Based 2,5-Furan Dicarboxylate Polyesters: Recent Progress and Critical Aspects in Their Synthesis and Thermal Properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2− and ZrO2− Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Nocito, F.; de Giglio, E.; Cometa, S.; Altomare, A.; Dibenedetto, A. Tunable Mixed Oxides Based on CeO2 for the Selective Aerobic Oxidation of 5-(Hydroxymethyl)Furfural to FDCA in Water. Green Chem. 2018, 20, 3921–3926. [Google Scholar] [CrossRef]
- German, D.; Pakrieva, E.; Kolobova, E.; Carabineiro, S.A.C.; Stucchi, M.; Villa, A.; Prati, L.; Bogdanchikova, N.; Cortés Corberán, V.; Pestryakov, A. Oxidation of 5-Hydroxymethylfurfural on Supported Ag, Au, Pd and Bimetallic Pd-Au Catalysts: Effect of the Support. Catalysts 2021, 11, 115. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Cavani, F. Insights into the Reaction Mechanism for 5-Hydroxymethylfurfural Oxidation to FDCA on Bimetallic Pd–Au Nanoparticles. Appl. Catal. Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Albonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J.; et al. Selective Oxidation of 5-Hydroxymethyl-2-Furfural over TiO2-Supported Gold–Copper Catalysts Prepared from Preformed Nanoparticles: Effect of Au/Cu Ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-Furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef]
- Monti, E.; Ventimiglia, A.; Forster, L.; Rodríguez-Aguado, E.; Cecilia, J.A.; Ospitali, F.; Tabanelli, T.; Albonetti, S.; Cavani, F.; Rivalta, I.; et al. Influence of Stabilisers on the Catalytic Activity of Supported Au Colloidal Nanoparticles for the Liquid Phase Oxidation of Glucose to Glucaric Acid: Understanding the Catalyst Performance from NMR Relaxation and Computational Studies. Green Chem. 2023, 25, 2640–2652. [Google Scholar] [CrossRef]
- Scurti, S.; Allegri, A.; Liuzzi, F.; Rodríguez-Aguado, E.; Cecilia, J.A.; Albonetti, S.; Caretti, D.; Dimitratos, N. Temperature-Dependent Activity of Gold Nanocatalysts Supported on Activated Carbon in Redox Catalytic Reactions: 5-Hydroxymethylfurfural Oxidation and 4-Nitrophenol Reduction Comparison. Catalysts 2022, 12, 323. [Google Scholar] [CrossRef]
- Scurti, S.; Monti, E.; Rodríguez-Aguado, E.; Caretti, D.; Cecilia, J.A.; Dimitratos, N. Effect of Polyvinyl Alcohol Ligands on Supported Gold Nano-Catalysts: Morphological and Kinetics Studies. Nanomaterials 2021, 11, 879. [Google Scholar] [CrossRef]
- Monti, E.; Ventimiglia, A.; Garcia Soto, C.A.; Martelli, F.; Rodríguez-Aguado, E.; Cecilia, J.A.; Sadier, A.; Ospitali, F.; Tabanelli, T.; Albonetti, S.; et al. Effect of the Colloidal Preparation Method for Supported Preformed Colloidal Au Nanoparticles for the Liquid Phase Oxidation of 1,6-Hexanediol to Adipic Acid. Catalysts 2022, 12, 196. [Google Scholar] [CrossRef]
- Monti, E.; Ventimiglia, A.; Soto, C.A.G.; Martelli, F.; Rodríguez-Aguado, E.; Cecilia, J.A.; Maireles-Torres, P.; Ospitali, F.; Tabanelli, T.; Albonetti, S.; et al. Oxidative Condensation/Esterification of Furfural with Ethanol Using Preformed Au Colloidal Nanoparticles. Impact of Stabilizer and Heat Treatment Protocols on Catalytic Activity and Stability. Mol. Catal. 2022, 528, 112438. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Au-Based Catalysts: Optimization of Active Phase and Metal–Support Interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the Mechanism of Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Supported Pt and Au Catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Odriozola, J.A. Au/Al2O3—Efficient Catalyst for 5-Hydroxymethylfurfural Oxidation to 2,5-Furandicarboxylic Acid. Catal. Today 2019, 333, 169–175. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Lolli, A.; Bonincontro, D.; Penkova, A.; Albonetti, S.; Cavani, F.; Odriozola, J.A.; Ivanova, S. Effect of Gold Particles Size over Au/C Catalyst Selectivity in HMF Oxidation Reaction. ChemCatChem 2020, 12, 1177–1183. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, L.; Medrano, J.A.; Ross, J.R.H.; Lefferts, L. Supported Pd Catalysts Prepared via Colloidal Method: The Effect of Acids. ACS Catal. 2013, 3, 2341–2352. [Google Scholar] [CrossRef]
- Tierney, G.F.; Alijani, S.; Panchal, M.; Decarolis, D.; de Gutierrez, M.B.; Mohammed, K.M.H.; Callison, J.; Gibson, E.K.; Thompson, P.B.J.; Collier, P.; et al. Controlling the Production of Acid Catalyzed Products of Furfural Hydrogenation by Pd/TiO2. ChemCatChem 2021, 13, 5121–5133. [Google Scholar] [CrossRef]
- Van Den Bossche, M. DFTB-Assisted Global Structure Optimization of 13- and 55-Atom Late Transition Metal Clusters. J. Phys. Chem. A 2019, 123, 3038–3045. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bonincontro, D.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Odriozola, J.A. 5-Hydroxymethyl-2-Furfural Oxidation Over Au/CexZr1-XO2 Catalysts. Front. Chem. 2020, 8, 461. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-71227-6. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z =11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Jansen, H.B.; Ros, P. Calculations Monoxide. Chem. Phys. Lett. 1969, 3, 140–143. [Google Scholar] [CrossRef]
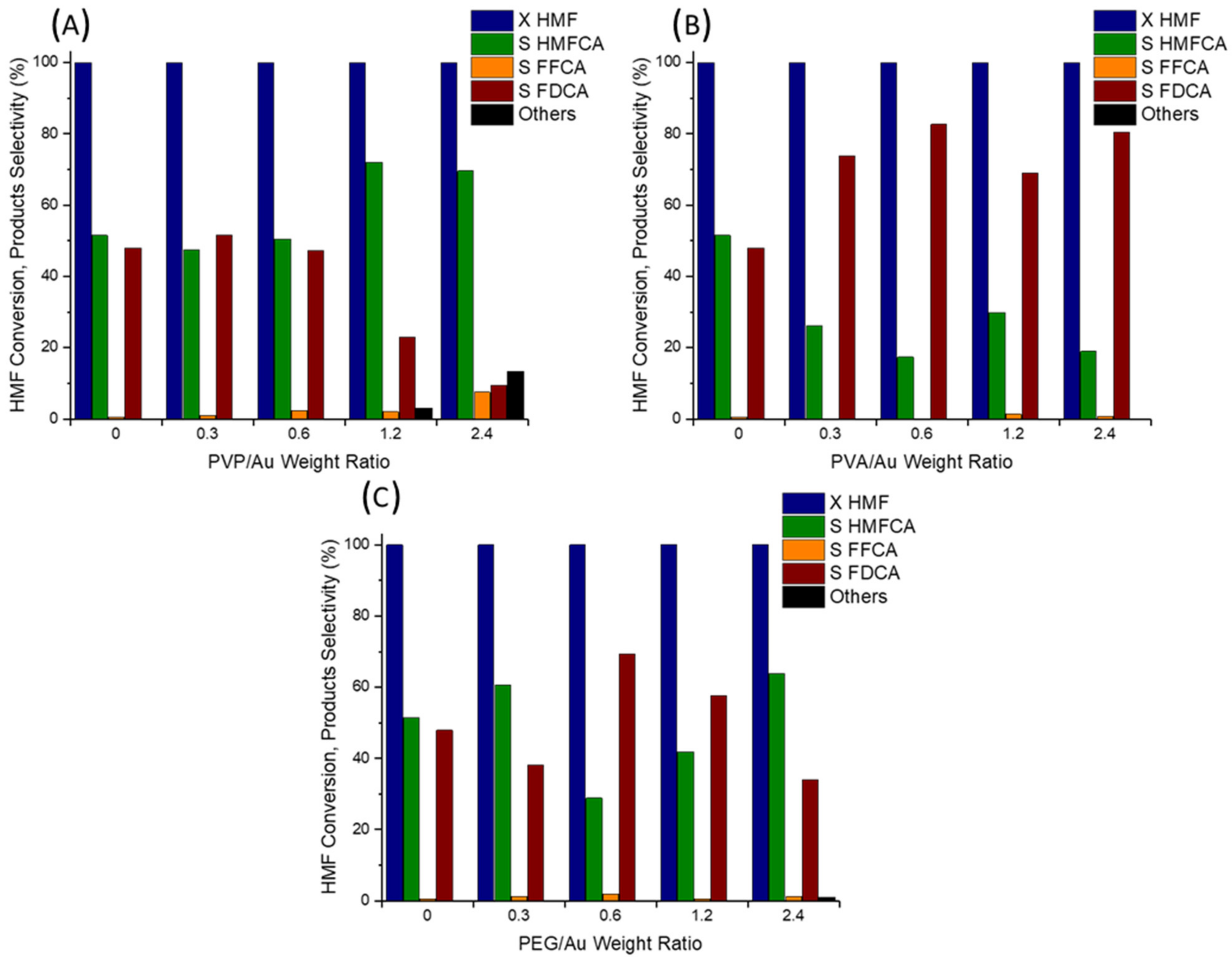
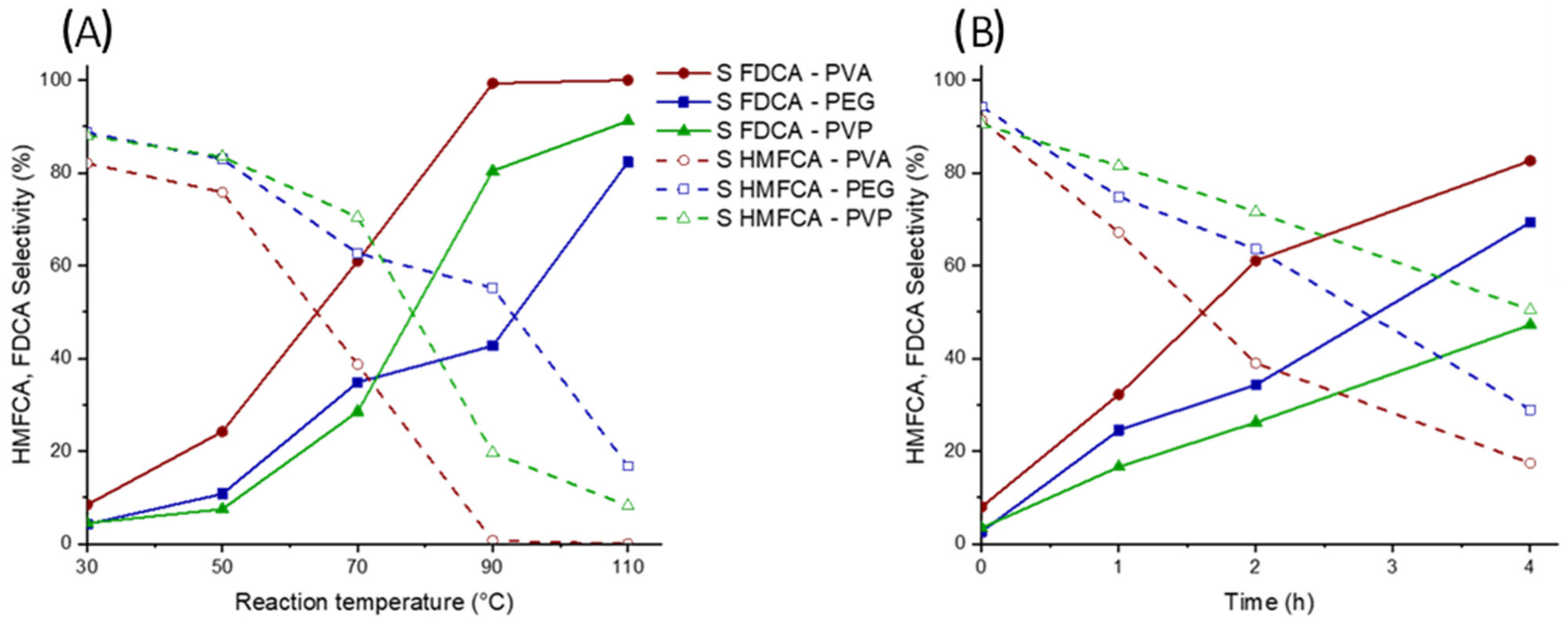

| Sample | Employed Polymer | Polymer:Au Weight Ratio | Au on Surface [at%] | Au NPs TEM Diameter (nm) |
|---|---|---|---|---|
| Au/AC _0 | None | 0 | 2.61 | 7.9 ± 6.3 |
| Au/AC_PVA_0.3 | PVA | 0.3 | 3.48 | 4.3 ± 3.6 |
| Au/AC_PVA_0.6 | PVA | 0.6 | 2.80 | 2.7 ± 1.6 |
| Au/AC_PVA_1.2 | PVA | 1.2 | 2.40 | 2.6 ± 2.1 |
| Au/AC_PVA_2.4 | PVA | 2.4 | 1.81 | 2.4 ± 1.2 |
| Au/AC_PEG_0.3 | PEG | 0.3 | 0.84 | 5.3 ± 2.0 |
| Au/AC_PEG_0.6 | PEG | 0.6 | 1.95 | 5.6 ± 2.2 |
| Au/AC_PEG_1.2 | PEG | 1.2 | 1.52 | 5.9 ± 2.3 |
| Au/AC_PEG_2.4 | PEG | 2.4 | 1.09 | 6.4 ± 2.2 |
| Au/AC_PVP_0.3 | PVP | 0.3 | 1.43 | 5.5 ± 3.6 |
| Au/AC_PVP_0.6 | PVP | 0.6 | 1.17 | 5.6 ± 3.9 |
| Au/AC_PVP_1.2 | PVP | 1.2 | 0.15 | 7.4 ± 4.7 |
| Au/AC_PVP_2.4 | PVP | 2.4 | 0.12 | 8.4 ± 4.9 |
| Sample | Au on Surface [at%] | Au NPs TEM Diameter (nm) |
|---|---|---|
| Au/AC_PVA_0.6 | 2.80 | 2.7 ± 1.6 |
| Au/AC_PVA_0.6 3 uses | 0.18 | 20 ± 10 |
| Au/AC_PVA_2.4 | 1.81 | 2.4 ± 1.2 |
| Au/AC_PVA_2.4 3 uses | 0.24 | 9.4 ± 5.7 |
| Au/AC_PEG_0.6 | 1.95 | 5.6 ± 2.2 |
| Au/AC_PEG_0.6 3 uses | 0.68 | 8.5 ± 4.9 |
| Au/AC_PEG_2.4 | 1.09 | 6.4 ± 2.2 |
| Au/AC_PEG_2.4 3 uses | 0.71 | 9.9 ± 4.6 |
| Au/AC_PVP_0.6 | 1.17 | 5.6 ± 3.9 |
| Au/AC_PVP_0.6 3 uses | 0.25 | 9.1 ± 5.4 |
| Stabilizing Agent | Eads (kCal/mol) | Distance Au-O (Å) |
|---|---|---|
| PVA | −28.7 | 2.50 |
| PEG | −31.6 | 2.47 |
| PVP | −36.8 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liuzzi, F.; Ventimiglia, A.; Allegri, A.; Rodríguez-Aguado, E.; Cecilia, J.A.; Rivalta, I.; Dimitratos, N.; Albonetti, S. Effect of Capping Ligands for the Synthesis of Gold Nanoparticles and on the Catalytic Performance for the Oxidation of 5-Hydroxymethyl-2-furfural. Catalysts 2023, 13, 990. https://doi.org/10.3390/catal13060990
Liuzzi F, Ventimiglia A, Allegri A, Rodríguez-Aguado E, Cecilia JA, Rivalta I, Dimitratos N, Albonetti S. Effect of Capping Ligands for the Synthesis of Gold Nanoparticles and on the Catalytic Performance for the Oxidation of 5-Hydroxymethyl-2-furfural. Catalysts. 2023; 13(6):990. https://doi.org/10.3390/catal13060990
Chicago/Turabian StyleLiuzzi, Francesca, Alessia Ventimiglia, Alessandro Allegri, Elena Rodríguez-Aguado, Juan Antonio Cecilia, Ivan Rivalta, Nikolaos Dimitratos, and Stefania Albonetti. 2023. "Effect of Capping Ligands for the Synthesis of Gold Nanoparticles and on the Catalytic Performance for the Oxidation of 5-Hydroxymethyl-2-furfural" Catalysts 13, no. 6: 990. https://doi.org/10.3390/catal13060990
APA StyleLiuzzi, F., Ventimiglia, A., Allegri, A., Rodríguez-Aguado, E., Cecilia, J. A., Rivalta, I., Dimitratos, N., & Albonetti, S. (2023). Effect of Capping Ligands for the Synthesis of Gold Nanoparticles and on the Catalytic Performance for the Oxidation of 5-Hydroxymethyl-2-furfural. Catalysts, 13(6), 990. https://doi.org/10.3390/catal13060990