Abstract
Active granule (WC/Co3O4) doping Ti/Sb-SnO2/PbO2 electrodes were successfully synthesized by composite electrodeposition. The as-prepared electrodes were systematically characterized by scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), electrochemical performance, zeta potential, and accelerated lifetime. It was found that the doping of active granules (WC/Co3O4) can reduce the average grain size and increase the number of active sites on the electrode surface. Moreover, it can improve the proportion of surface oxygen vacancies and non-stoichiometric PbO2, resulting in an outstanding conductivity, which can improve the electron transfer and catalytic activity of the electrode. Electrochemical measurements imply that Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes have superior oxygen evolution reactions (OERs) relative to those of Ti/Sb-SnO2/PbO2 and Ti/Sb-SnO2/WC-PbO2 electrodes. A Ti/Sb-SnO2/Co3O4-PbO2 electrode is considered as the optimal modified electrode due to its long lifetime (684 h) and the remarkable stability of plating solutions. The treatment of copper wastewater suggests that composite electrodes exhibit low cell voltage and excellent extraction efficiency. Furthermore, pilot simulation tests verified that a composite electrode consumes less energy than other electrodes. Therefore, it is inferred that composite electrodes may be promising for the treatment of wastewater containing high concentrations of copper ions.
1. Introduction
The development of the nonferrous metal industry has improved social progress and economic development. However, the nonferrous metal industry also produces many pollutants, including wastewater containing copper, zinc, nickel, etc. [1,2]. The discharge of wastewater with high concentrations of metal ions can cause serious environmental problems if it is not properly treated. The technology of metal electrodeposition from solution is environmentally friendly and has attracted considerable attention with respect to resource recovery [3,4,5,6]. For example, copper can be extracted from copper-containing wastewater by the electrochemical method, and the product can be widely used in many fields, such as light industry, electrical, national defense, etc. [7,8,9,10]. In this procedure, low oxygen evolution overpotential is favorable for energy conservation.
Electrode material is a critical component in the electrochemical process [11,12,13] and can affect power consumption, production cost, current efficiency, and the quality of the product. Due to the strong oxidizing ability and the corrosivity of sulfuric acid in the electrodeposition process, electrode materials (lead-based alloys, Ti-based metal oxides, Al/PbO2, and SS/PbO2) with high conductivity, good stability, mechanical strength, and convenient processing have attracted considerable attention [14,15,16,17,18,19]. In particular, Ti-based insoluble electrode materials exhibit high corrosion resistance, long service life, excellent electrochemical performance, and electrocatalytic activity, which are highly valued with respect to environmental protection, metallurgy, and resource recovery [20,21,22]. Usually, Ti-based electrode material contains thinly coated noble (Ti/Ru, Ti/Ir, or Ti/Pt) electrodes and thickly coated Ti/PbO2 and Ti/MnO2 electrodes [23,24,25,26,27]. Ti/PbO2 electrodes possess well-established features such as low cost, ease of synthesis, good chemical stability, and long service life, and have great application potential to copper-containing wastewater. However, the high oxygen evolution overpotential of Ti/PbO2 electrodes causes energy waste during the copper electrodeposition process [28]. Reducing the oxygen evolution overpotential of the electrode is an important task for many researchers.
On the one hand, Co3O4 shows superior electrocatalytic performance towards oxygen evolution reactions (OERs) due to its spinal structure, with a Co2+ located in the tetrahedral site and two other Co3+ atoms in the octahedral site [29,30,31]. On the other hand, WC can improve the mechanical properties of coatings and is widely used in the fields of cemented carbide, electrocatalytics, and fuel cells [32,33]. Therefore, in this study, active granule Co3O4 and WC were introduced to Ti/Sb-SnO2/PbO2 electrodes by electrodeposition with the aim of decreasing the overpotential of oxygen evolution and enhancing the service life of the electrode. An amplified simulated experiment of electrolysis was conducted to investigate the change in voltage and temperature during this process, which is closely related to energy consumption. Furthermore, wastewater containing copper ions and sulfuric acid was employed as a model recyclable resource for the electrochemical extraction of copper. The concentration of copper ions was studied by inductively coupled plasma emission spectroscopy (ICPE), and the extraction efficiency was calculated by the mass of copper deposited on the cathode.
2. Results and Discussion
2.1. Surface Morphology Analysis of Electrodes
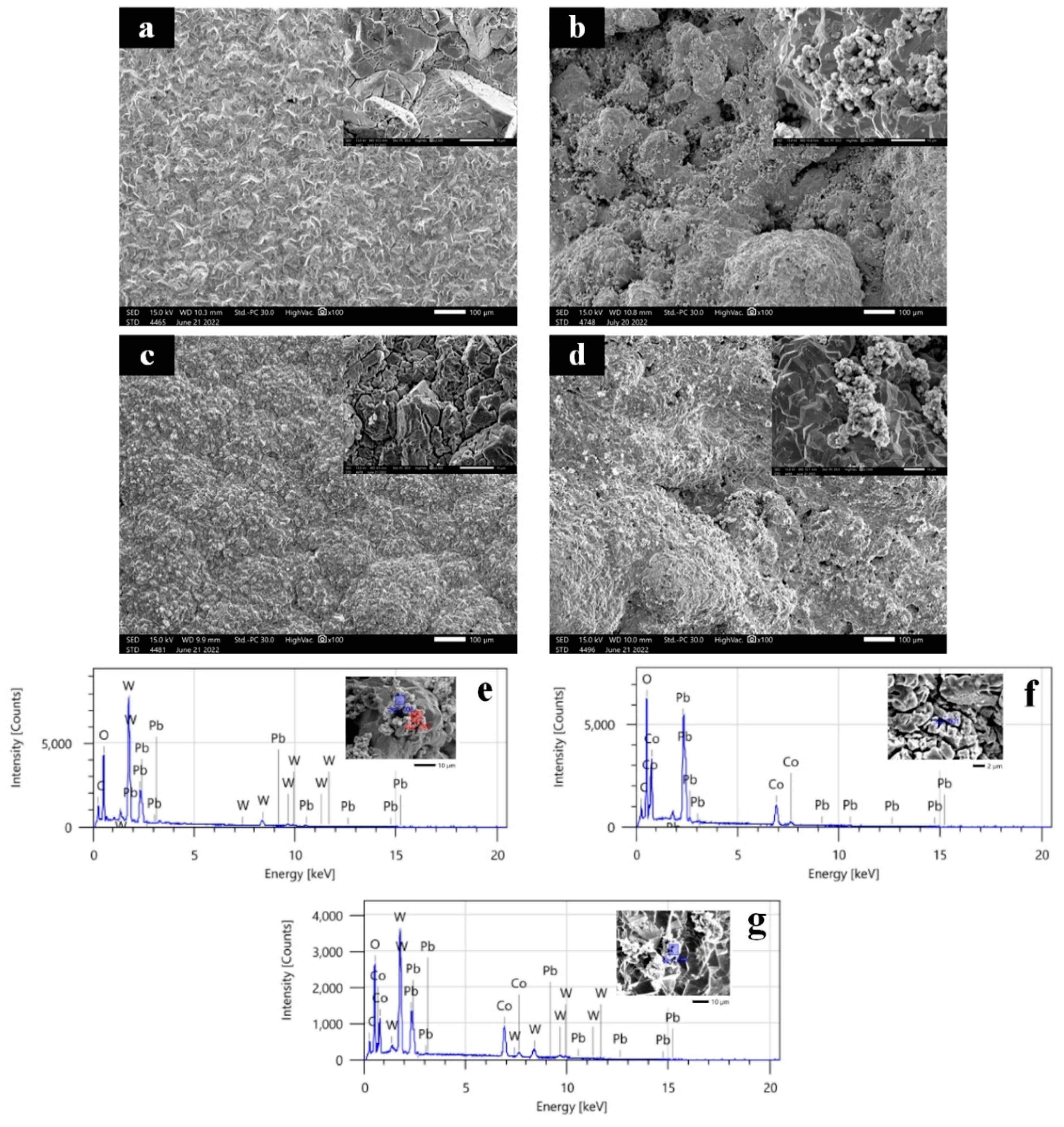
Figure 1 shows an SEM of an as-synthesized Ti/Sb-SnO2/PbO2 electrode, a Ti/Sb-SnO2/WC-PbO2 electrode, Ti/Sb-SnO2/Co3O4-PbO2 electrode, and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrode. It can be seen that the Ti/Sb-SnO2/PbO2 electrode shows a hill-like surface at the macroscopic level (Figure 1a). However, the hill-like surface becomes smooth after it is modified with active granules (WC/Co3O4), which indicates that the addition of active granules may affect the deposition process (Figure 1b–d). The microscopic morphology of the Ti/Sb-SnO2/PbO2 electrode displays a typical tetrahedron shape. Some small particles (WC or WC-Co3O4) are agglomerated on the surface of the Ti/Sb-SnO2/WC-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes, leading to an undulating crest of hillocks. Moreover, it was found that the surface uniformity of Ti/Sb-SnO2/Co3O4-PbO2 is better than that of Ti/Sb-SnO2/WC-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2, with a denser coating and better coverage. EDS measurements were employed to investigate the composition of particles deposited on the electrode surface (Figure 1e–g). W element was detected on the surface of the Ti/Sb-SnO2/WC-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes. Co element was discovered on the surface of the Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes. These results confirm that spherical Co3O4 granules and small WC particles were successfully doped into the composite electrode.
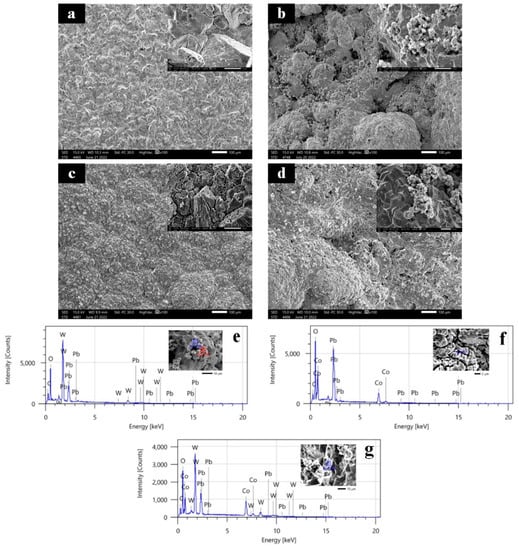
Figure 1.
SEM of the electrodes: (a) Ti/Sb-SnO2/PbO2; (b) Ti/Sb-SnO2/WC-PbO2; (c) Ti/Sb-SnO2/Co3O4-PbO2; (d) Ti/Sb-SnO2/WC-Co3O4-PbO2. EDS of the electrodes: (e) Ti/Sb-SnO2/WC-PbO2; (f) Ti/Sb-SnO2/Co3O4-PbO2; (g) Ti/Sb-SnO2/WC-Co3O4-PbO2.
2.2. XRD Structural Characterization
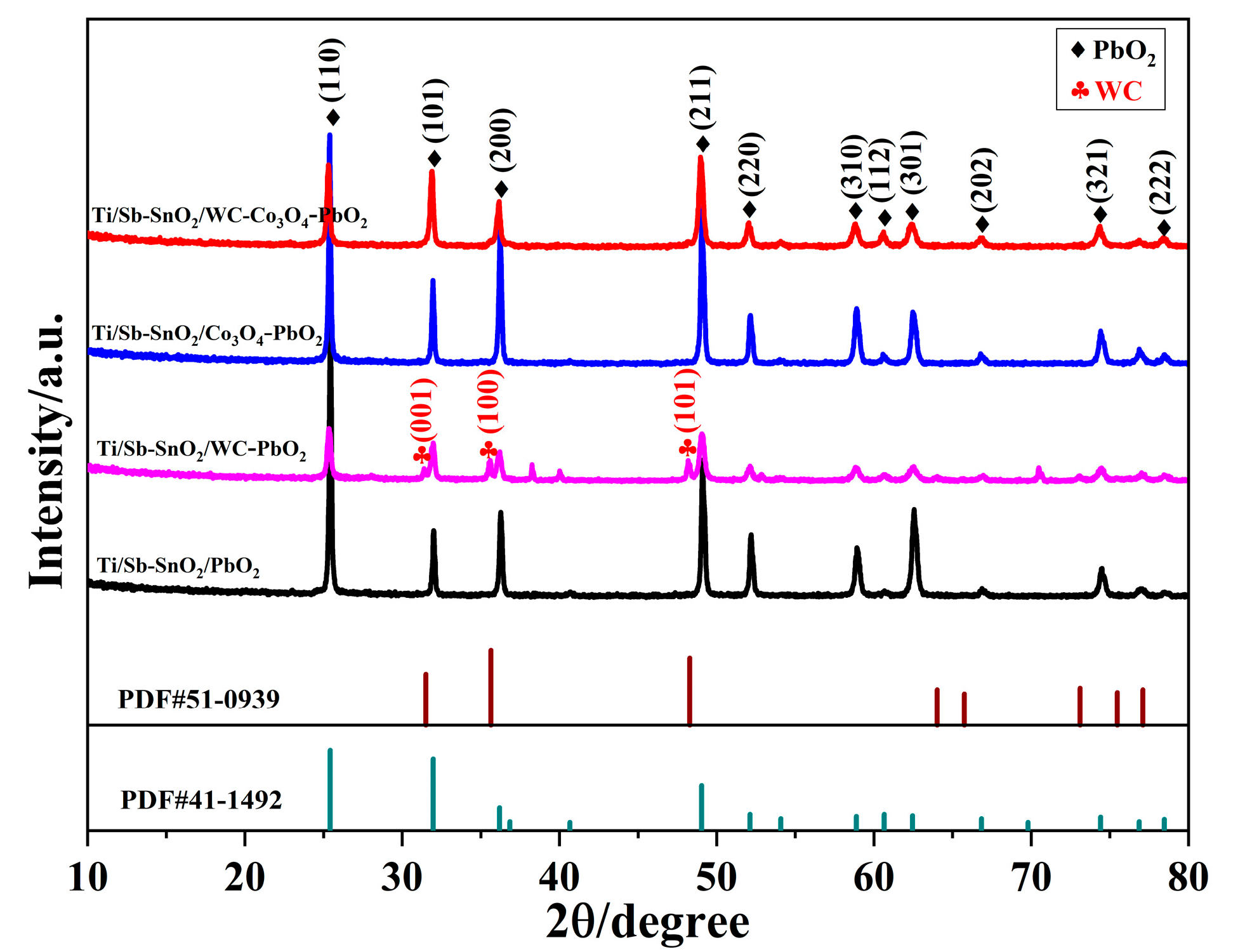
As shown in Figure 2, the XRD pattern of each electrode was obtained to compare the crystal structure and purity of electrodes. Figure 2 shows that bare Ti/Sb-SnO2/PbO2 exhibits the reflections of β-PbO2. The diffraction peaks at 25.4°, 32.0°, 36.2°, 49.0°, 52.1°, 58.9°, 60.7°, and 62.5° are assigned to the (110), (101), (200), (211), (220), (310), (112), and (301) planes of β-PbO2 (PDF#41-1492), respectively. No peaks corresponding to Sb or SnO2 were detected, which can be explained by two reasons. One is the low crystallinity of the Sb-SnO2 layer, and the other is the thick layer of β-PbO2 coating on the Sb-SnO2 layer. After doping with WC particles, new peaks appeared at 2θ = 31.5°, 35.6°, and 48.3° on the Ti/Sb-SnO2/WC-PbO2 electrode, which are assigned to the (001), (100), and (101) planes of WC (PDF#51-0939), respectively. However, no additional peaks were observed on Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/Co3O4-PbO2, which can be ascribed to the weak crystallinity, small particle size, and infinitesimal load of active particles. Moreover, the average grain sizes of Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 calculated by the Debye–Scherrer equation are 56.2 nm, 38.1 nm, 48.7 nm, and 37.5 nm, respectively. The doping of active granules can decrease the grain size, which is consistent with the SEM morphology. A smaller grain size indicates more active sites on the electrode surface, which is favorable for the enhancement of catalytic performance.
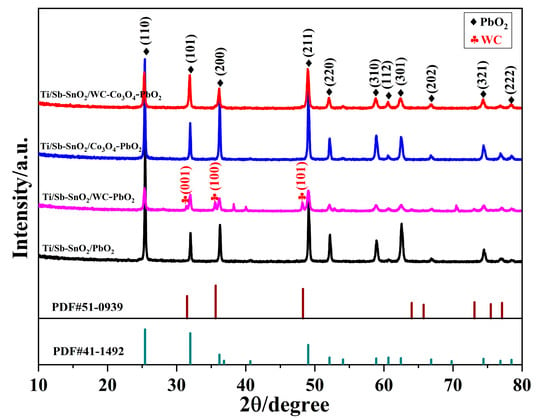
Figure 2.
XRD pattern of the different samples.
2.3. XPS Analysis
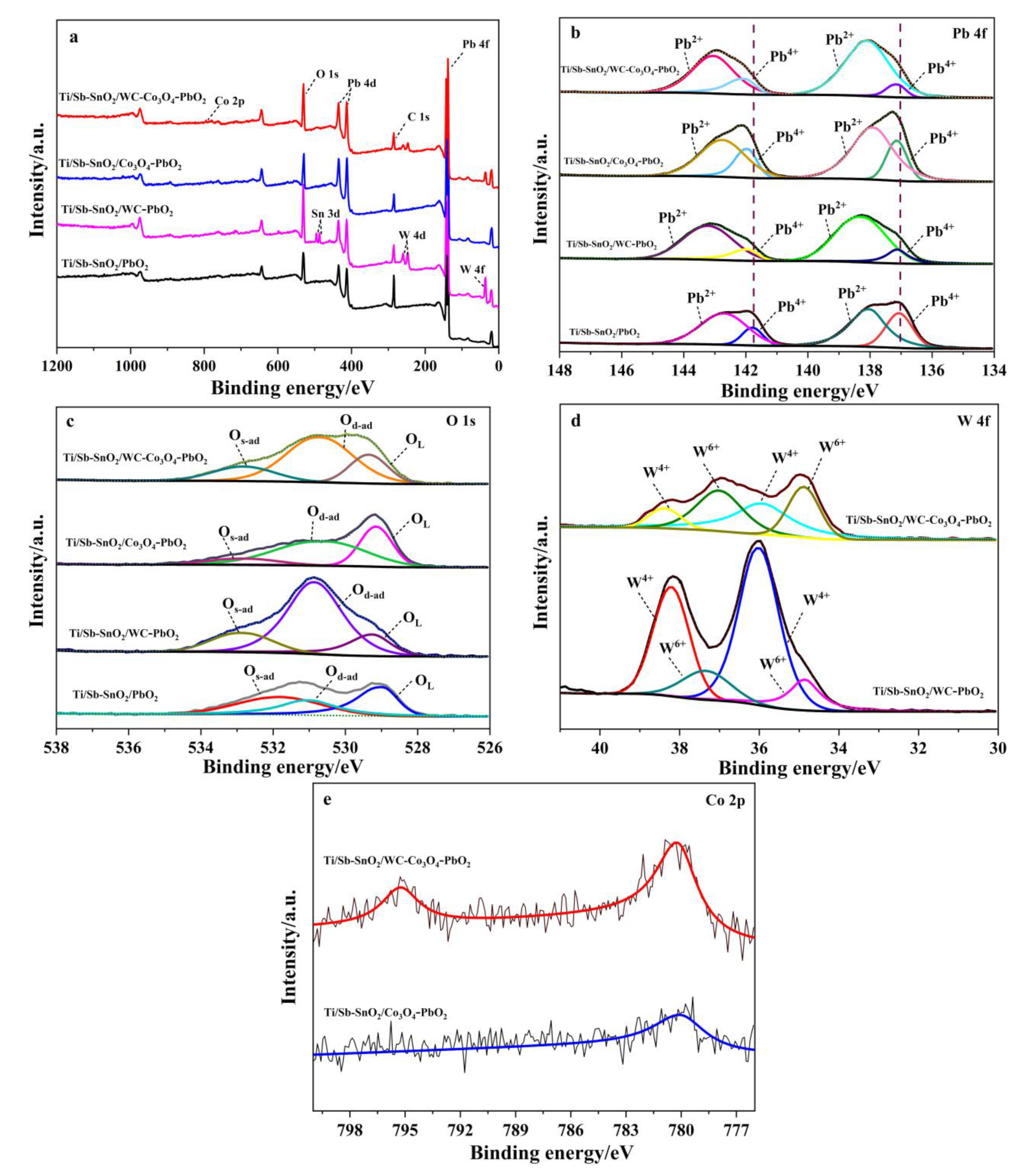
XPS measurements were performed to further analyze the chemical state of the elements on each electrode. In the survey, the XPS spectrum of each electrode and elemental peaks for Pb and O were observed on all electrodes (Figure 3a). A new elemental peak for W and Sn was found on the Ti/Sb-SnO2/WC-PbO2 electrode surface. The presence of Sn indicates that part of the coating is too thin; hence, and interlayer Sb-SnO2 film was detected by XPS. This suggests that the coating on Ti/Sb-SnO2/WC-PbO2 is ununiform. An additional peak for Co appeared on Ti/Sb-SnO2/Co3O4-PbO2, which confirms that Co3O4 was successfully doped in the modified PbO2 film. Moreover, peaks for W and Co were found on the Ti/Sb-SnO2/WC-Co3O4-PbO2 electrode, indicating the presence of WC and Co3O4. To identify the influence of active granule doping on PbO2 electrodeposition, XPS analysis of Pb, O, W, and Co was performed; the results are shown in Figure 3b–e.
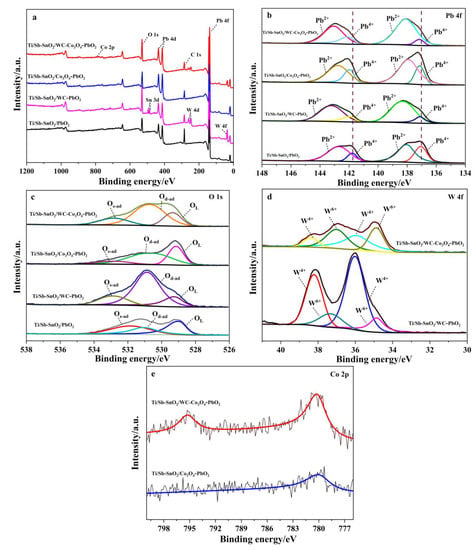
Figure 3.
XPS spectra of the as-prepared samples: (a) survey, (b) Pb 4f; (c) O 1s; (d) W 4f; (e) Co 2p.
In the undoped Ti/Sb-SnO2/PbO2 (Figure 3b), the binding energy peaks at 141.79 eV and 137.07 eV correspond to Pb4+, whereas the peaks at 142.70 eV and 138.02 eV are attributed to Pb2+ [34], as listed in Table 1. Moreover, the simultaneous presence of Pb4+ and Pb2+ in the PbO2 coating implies the formation of non-stoichiometric PbO2 during the electrodeposition process. After doping with active granules, the peaks of Pb4+ on modified electrodes shifted to the higher binding energy, which suggests that active granules have a strong interaction with Pb. The proportion of Pb4+ in Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 is 29.9%, 15.9%, 27.12%, and 15.61%, respectively. The decrease in Pb4+ after modification with active granules implies that part of the PbO2 converts to lower-valence compounds, enhancing the non-stoichiometric PbO2, which could improve the conductivity of the electrode.

Table 1.
The binding energy of Pb 4f on each electrode.
The O 1s core level shown in Figure 3c can be deconvoluted into four characteristic peaks of lattice oxygen species (528.9–530.4 eV for OL), surface oxygen vacancies, adsorbed oxygen (530.5–531.7 eV for Od-ad), and surface-adsorbed oxygen species (531.8–532.8 eV for Os-ad), as listed in Table 2. According to the literature [35], the formation of surface oxygen vacancies is closely related to highly oxidative oxygen species and is active for catalysis of OER. The proportions of Od-ad present on the electrode surface significantly increase after decoration with active granules, which is beneficial to the OER activity.

Table 2.
The binding energy of O 1s and its proportions on each electrode.
Figure 3d shows the W 4f spectrum; the binding energy peaks at 35.96 eV and 38.29 eV are assigned to W4+, whereas the peaks at 34.87 eV and 37.17 eV are ascribed to W6+. This consequence demonstrates the generation of WC. However, W6+ also appears on the electrode surface. The formation of W6+ can be attributed to the oxidation of WC during composite electrodeposition. Moreover, the XPS spectrum of Co 2p is very weak due to the extremely limited doping of active particles. It was found that two main peaks appeared on the Ti/Sb-SnO2/WC-Co3O4-PbO2 electrode. The peaks located at 795.26 eV and 780.23 eV are identical to Co 2p1/2 and Co 2p3/2, respectively (Figure 3e), which confirms the presence of Co3O4.
2.4. Electrochemical Properties
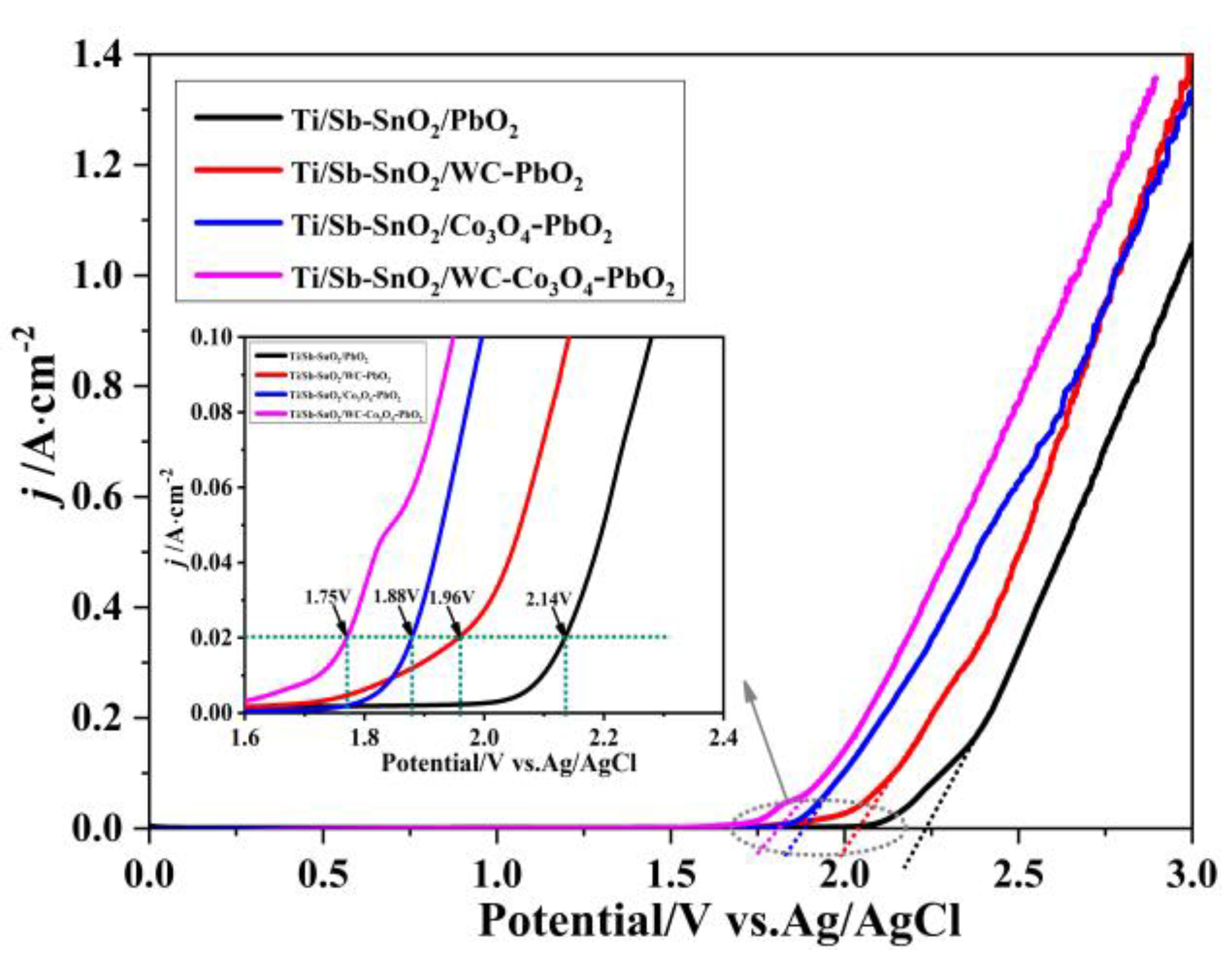
LSV measurements of the as-prepared samples were performed on an electrochemical workstation to investigate the OER performance of the electrodes. The onset potential for oxygen evolution potential (OEP) on Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 is 2.20 V, 2.12 V, 1.85 V, and 1.80 V (vs. Ag/AgCl), respectively. A low OER means that oxygen is more easily formed during the electrochemical process. Therefore, it is speculated that the modification of active granules (WC/Co3O4) can improve the OER properties of electrodes due to the presence of more active sites and increased electrocatalytic activity. Moreover, doping with Co3O4 is more efficient than doping with WC. This can be attributed to the special properties of Co3O4 and its better OER performance. Furthermore, the non-uniformity of the WC-PbO2 coating may enhance resistance, hindering the formation of oxygen. As shown in the illustration in Figure 4, when the current density is 200 A/m2, the potential of Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 is 2.14 V, 1.96 V, 1.88 V, and 1.75 V (vs. Ag/AgCl), respectively. This implies that Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 need lower potential than Ti/Sb-SnO2/PbO2 and Ti/Sb-SnO2/WC-PbO2 under the same current density. Low potential is more helpful for saving energy during electrolysis.
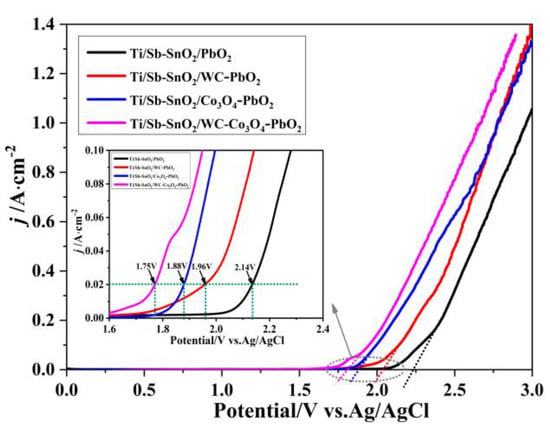
Figure 4.
LSV curves of the different electrodes.
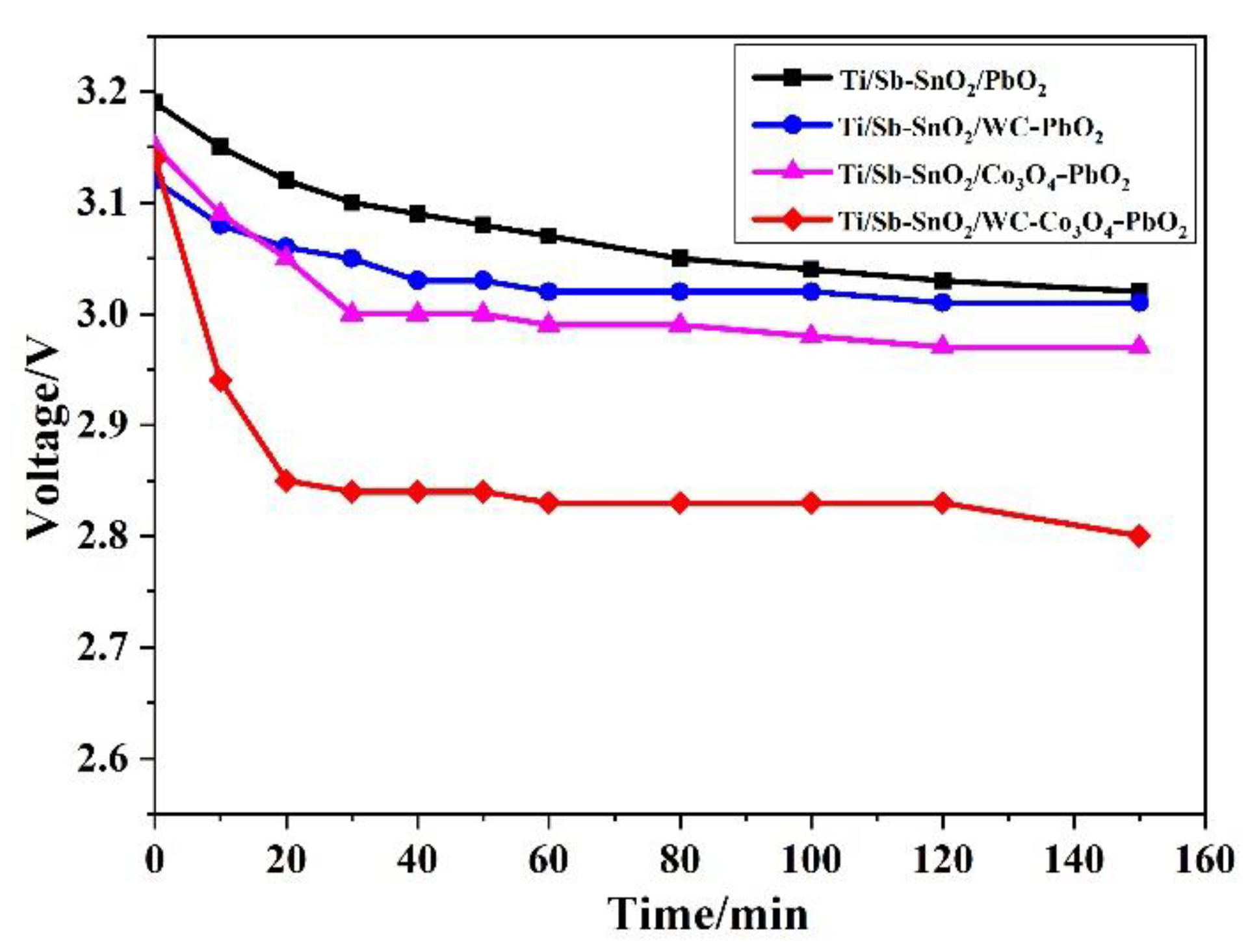
Energy conservation has become an important problem due to energy shortages. As is known to all, voltage is closely related to energy consumption. How to reduce the voltage of electrolysis is a crucial issue in the electrolytic industry. Therefore, a simulation experiment of electrolysis under a current density of 200 A/m2 was conducted to explore the variation in voltage, as displayed in Figure 5. It was found that the Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes have advantages over the Ti/Sb-SnO2/PbO2 and Ti/Sb-SnO2/WC-PbO2 electrodes. These results are consistent with the LSV measurements.
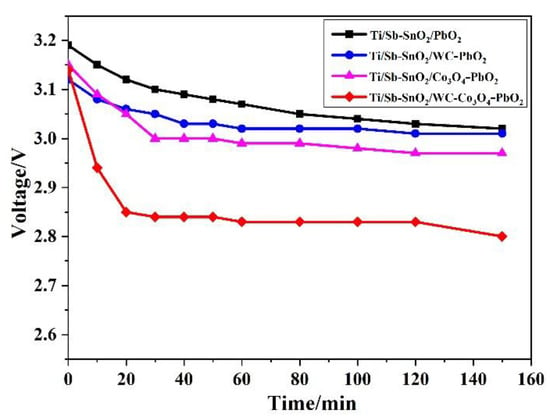
Figure 5.
The change in voltage during electrolysis (200 A/m2; 15% H2SO4 aqueous solutions).
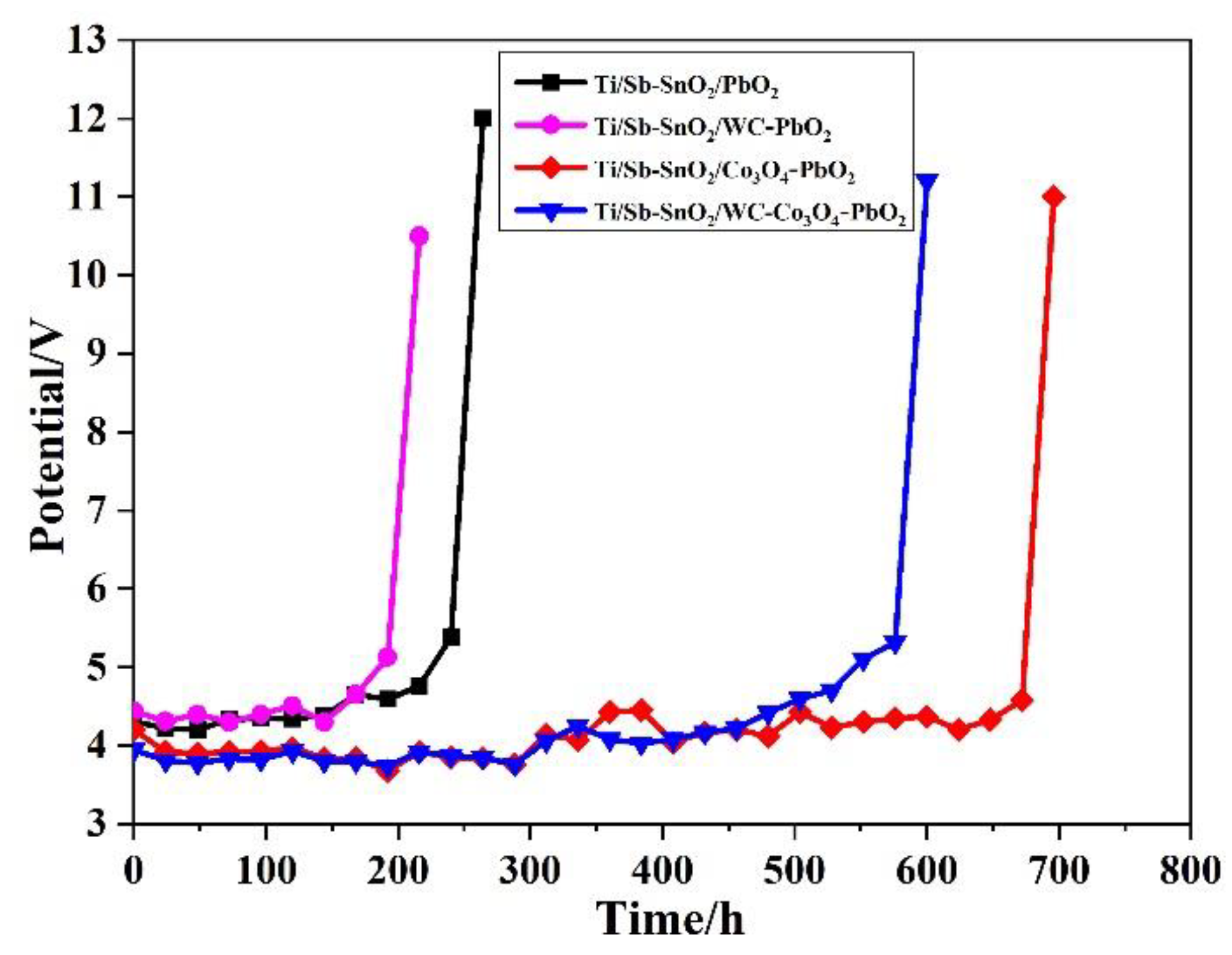
Moreover, an accelerated life test was carried out to evaluate the electrochemical stability of the electrodes. Figure 6 shows the time course of cell potential in the accelerated life test. It was observed that the Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 electrodes exhibited a lifetime of 252 h, 204 h, 684 h, and 592 h, respectively. The lifetime of Ti/Sb-SnO2/WC-PbO2 is shorter than that of Ti/Sb-SnO2/PbO2, which can be attributed to the non-uniformity of its coating. The lifetimes of Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/WC-Co3O4-PbO2 are increased by more than two-fold relative to that of Ti/Sb-SnO2/PbO2.
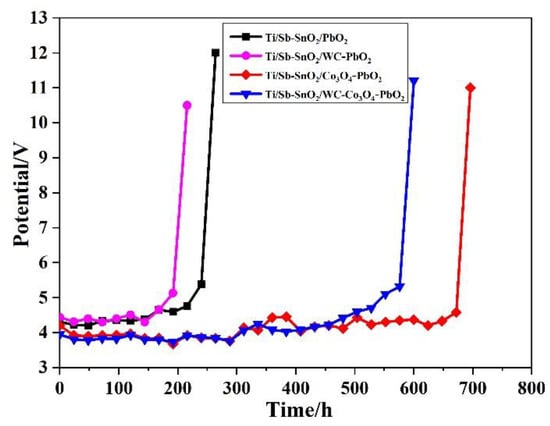
Figure 6.
Variation of the cell potential with testing time in an accelerated life test for the as-synthesized samples (10,000 A/m2; 15% H2SO4 aqueous solutions).
2.5. The Application of Modified Electrodes in Wastewater Containing Copper Ions
Deposition solutions containing active granules (WC/Co3O4) were tested with a zeta potential analyzer to study their stability [36]. The Zeta potential of deposition solutions with WC, Co3O4, and WC-Co3O4 are 3.9 mV, 5.5 mV, and 3.4 mV, respectively. It was found that a high absolute value of zeta potential indicates higher stability against coagulation, which implies excellent stability in such systems. Compared to other deposition systems, the plating solution with Co3O4 exhibits the best stability. Although Ti/Sb-SnO2/WC-Co3O4-PbO2 shows the lowest OER property, the stability of the plating solution with WC-Co3O4 is the worst. Therefore, considering the lifetime of the electrode and the stability of the solution, we selected the Ti/Sb-SnO2/Co3O4-PbO2 electrode as the optimal electrode for subsequent experiments.
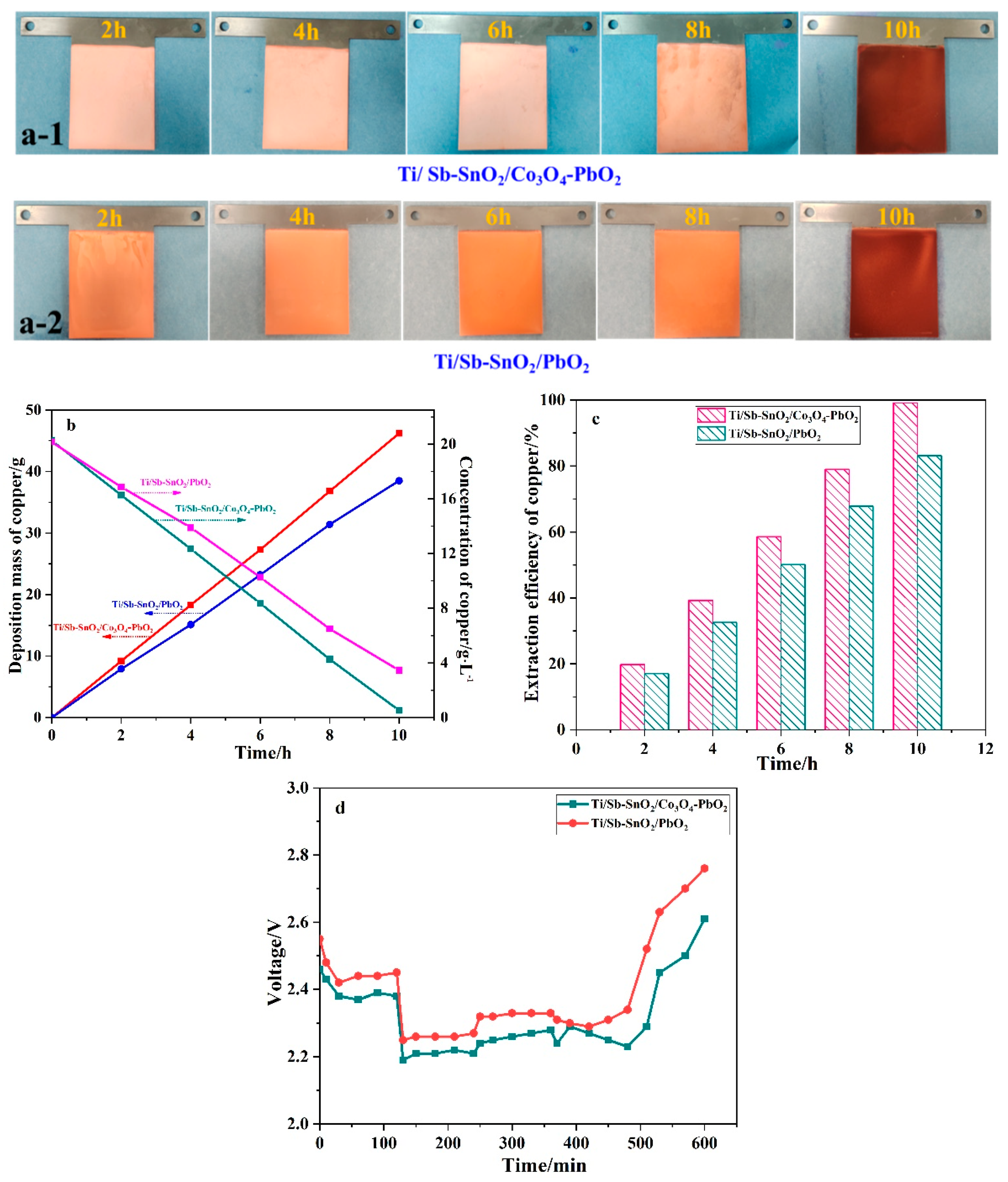
Electrochemical extraction experiments were carried out to investigate the application of electrodes to wastewater containing copper ions. This system contains two Ti/Sb-SnO2/Co3O4-PbO2 anodes and one SS cathode with magnetic stirring. As shown in Figure 7a, the color of copper deposited on the electrode becomes dark due to the ion impoverishment of copper ions. Usually, dark copper is generated when the copper ion concentration decreases to a certain value in actual production. Figure 7b displays the mass and concentration of copper over time; the mass of copper increases linearly with time, and the concentration of copper ions decreases with time. The Ti/Sb-SnO2/Co3O4-PbO2 electrode shows a higher mass and a lower concentration than the Ti/Sb-SnO2/PbO2 electrode. The extraction efficiency of Ti/Sb-SnO2/Co3O4-PbO2 and Ti/Sb-SnO2/PbO2 is 99.1% and 83.2%, respectively (Figure 7c). Moreover, the cell voltage of Ti/Sb-SnO2/Co3O4-PbO2 is lower than that of Ti/Sb-SnO2/PbO2, indicating less energy consumption (Figure 7d). These results reveal that the modified Ti/Sb-SnO2/Co3O4-PbO2 electrode has superior performance in the extraction of copper from wastewater by the electrochemical method.
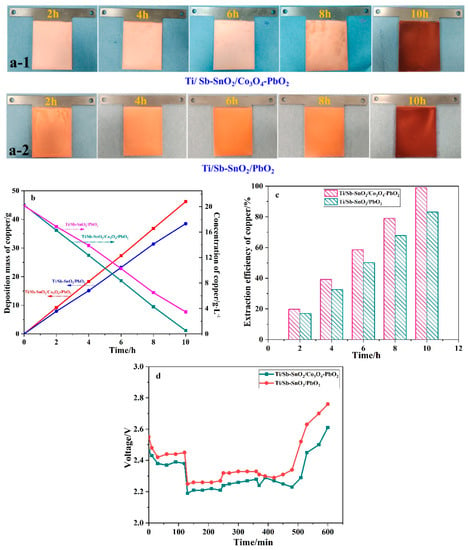
Figure 7.
(a) Digital images of copper deposited on Ti/Sb-SnO2/Co3O4-PbO2 (a-1) and Ti/Sb-SnO2/PbO2 (a-2) at different times. (b) Variation in the deposition mass and concentration of copper over time. (c) The extraction efficiency of copper during the electrochemical process. (d) The cell voltage of different electrodes during electrodeposition.
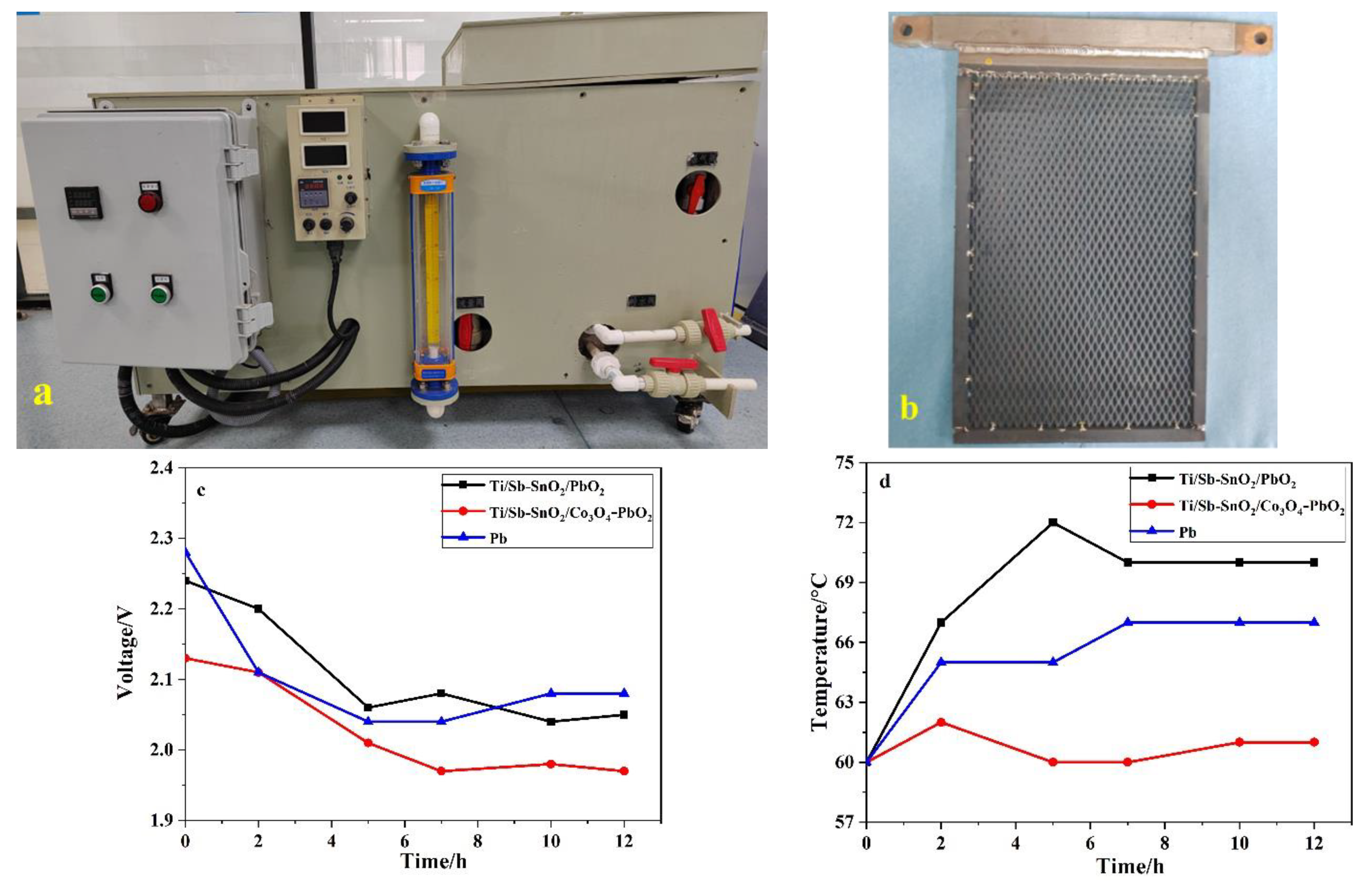
Laboratory experiments usually slightly deviate from actual working conditions. Therefore, we decided to build a magnifying system that is close to practical working conditions. To that end, an apparatus was manufactured and outsourcing factory consisting of a circular aeration, heat, and electric control (Figure 8a). Amplification tests were performed to research the energy consumption of this device. To simplify the experimental process, we employed 15% H2SO4 aqueous solutions to simulate acidic working conditions. The other parameters were similar to those used in the electrochemical extraction experiment. In the pilot simulation tests, the Ti/Sb-SnO2/Co3O4-PbO2, Ti/Sb-SnO2/PbO2, and Pb electrodes were employed as the anode, and SS was used as the cathode, all with dimensions of 200 mm × 290 mm × 4 mm (Figure 8b). It should be noted that Pb electrodes are traditionally used in the metallurgical industry. Ti/Sb-SnO2/Co3O4-PbO2 had a lower voltage and lower temperature (Figure 8c,d) than Ti/Sb-SnO2/PbO2 and Pb. These results suggest that Ti/Sb-SnO2/Co3O4-PbO2 consumes less energy than Ti/Sb-SnO2/PbO2 and Pb electrodes, which is in agreement with the results of the electrochemical extraction experiments. This indicates that Ti/Sb-SnO2/Co3O4-PbO2 electrodes may replace traditional Pb electrodes in the resource recovery of nonferrous metals.
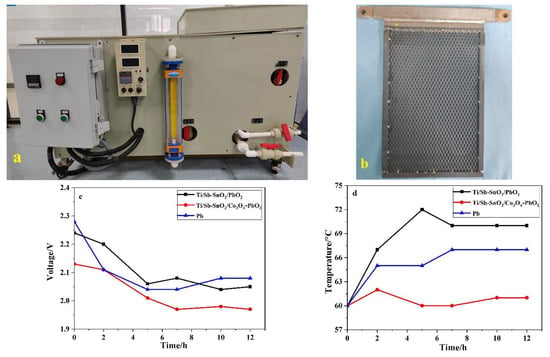
Figure 8.
(a) Pilot apparatus of the simulation test. (b) Part of the sample used in the pilot test. (c) The variation in cell voltage during the electrochemical process. (d) The change in temperature during the electrochemical process.
3. Experimental Details
3.1. Materials
All chemicals were of analytical grade and were used without any further purification. SnCl2∙2H2O and SbCl3 were provided by Xilong Scientific Co., Ltd. (Chaoshan, China). Ethanol and tert-butyl alcohol were offered by Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China). Pb(NO3)2, Cu(NO3)2, NaF, and some additives were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). HNO3, H2SO4, and HCl were produced from Xi’an Sanpu Chemical Reagants Co., Ltd. (Xi’an, China). WC and Co3O4 reagents were purchased from MACKLIN (Shanghai, China). All solutions were prepared with deionized water (DI). Titanium foil (Baoji Baite Metal Co., Ltd., Baoji, China, 1.5 mm thickness) was cut into pieces with effective dimensions of 80 mm × 100 mm before experiments.
3.2. Fabrication of Ti/Sb-SnO2/PbO2 Electrode by Active Granules
A Ti sheet was subjected to pretreatment before the experiment, including polishing, degreasing, and etching in boiling oxalic acid for 2 h, to obtain a gray surface with uniform roughness. Sb-SnO2 was introduced as a conductive transition layer between the PbO2 film layer and the Ti substrate by the thermal decomposition approach. Sb-SnO2 precursor solutions were prepared by dissolving a SnCl4 and SbCl3 mixture in a mixed solvent at a molar ratio of 10:1. The coating liquids were evenly brushed on the Ti sheet. Then, the Ti sheet was dried at 100 °C and annealed at 450 °C. The brushing, drying, and calcining steps were repeated several times.
PbO2 coatings with active granules (Co3O4 and WC) were synthesized by composite electrodeposition with a current density of 200 A/m2 for 2 h at 60 °C. The deposition solutions containing Pb(NO)3, Cu(NO)3, HNO3, active granules (WC/Co3O4), and other additives were ultrasonicated for 30 min. The as-prepared electrodes were rinsed thoroughly with DI and are denoted as Ti/Sb-SnO2/PbO2, Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2.
3.3. Characterization
A scanning electron microscope (SEM, JSM-IT200, JEOL, Tokyo, Japan) equipped with an energy-dispersive X-ray spectroscopy (EDS, JEOL) detector was employed to study the surface morphology and composition of the as-prepared samples. X-ray diffraction (XRD, D8 Advance, Bruker, Karlsruhe, Germany) measurement was conducted on an X’pert PRO MRD diffractometer using a Cu-Ka source (λ = 0.15416 nm) with a scanning angle (2θ) range of 10–80°. Chemical states of Pb, O, W, and Co in the composite electrodes were identified by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, MA, USA) on an Ultra DLD Electron Spectrometer (Al Ka radiation; hν = 1486.71 eV). XPS data were calibrated using the binding energy of C1s (284.8 eV) as the standard and were fitted using commercial software (Thermo Avantage). The zeta potential of the deposition solutions containing active granules (WC/Co3O4) was tested on a zeta potential analyzer (Stabino zeta, Microtrac MRB, Dusseldorf, Germany) to study the stability of the solutions.
Electrochemical performance was evaluated on an electrochemical workstation (Corrtest CS2350, Corrtest Instruments Corp., Ltd., Wuhan, China) using the traditional three-electrode system. A Pt sheet is used as counter electrode, a saturated Ag/AgCl electrode is employed as reference electrode, and the as-prepared electrode served as working electrode. Linear sweep voltammetric (LSV) characterization was measured in 15% H2SO4 aqueous solutions at a scan rate of 10 mV/s. simulated electrochemical experiment is also tested in 15% H2SO4 aqueous solutions to study the change in voltage during electrolysis. Accelerated life tests were carried out to research the stability and lifetime of the as-prepared electrodes in 15% H2SO4 aqueous solutions with a current density of 10,000 A/m2. In this procedure, the experiment was considered finished when the cell voltage exceeded 10 V.
3.4. Electrochemical Treatment of Copper-Containing Wastewater
Wastewater containing copper ions was treated by electrochemical approaches in a large beaker equipped with a water bath and magnetic stirrer, as shown in Figure 9. Two pieces of Ti/Sb-SnO2/Co3O4-PbO2 electrodes were used as the anode, and stainless steel (SS) was employed as the cathode. The temperature was set to 60 °C, and the current density was set to 200 A/m2. Simulated wastewater with a high concentration of copper ions (20 g/L Cu2+) was prepared by dissolving CuSO4∙5H2O in 15% H2SO4 aqueous solutions. During experiments, samples were taken out of the beaker every 2 h for ICPE measurement, and the SS cathode was weighed every 2 h to calculate the mass of copper deposited on the cathode. The variation in cell voltage during this chemical process was also recorded to compare the energy consumption. The extraction efficiency of copper was calculated as follows:
where m0 and mt are the mass of copper before and after an electrolysis time of t, respectively; C0 is the initial concentration of copper ions, which can be obtained by the ICPE technique; and V is the volume of the solutions.
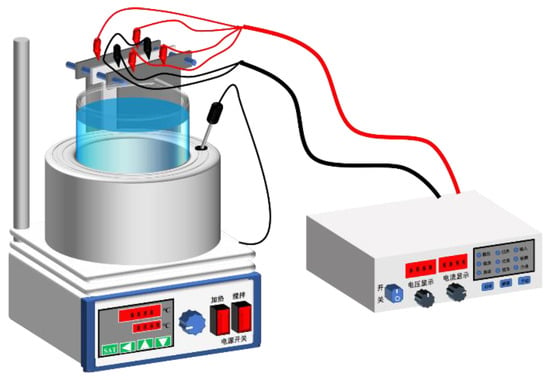
Figure 9.
Schematic diagram of treatment of wastewater containing copper ions.
4. Conclusions
In summary, active granule (WC/Co3O4) modified Ti/Sb-SnO2/PbO2 electrodes were fabricated by composite electrodeposition. EDS and XPS characterization of the as-prepared electrodes suggests that active granules (WC/Co3O4) were successfully deposited on the surface of Ti/Sb-SnO2/WC-PbO2, Ti/Sb-SnO2/Co3O4-PbO2, and Ti/Sb-SnO2/WC-Co3O4-PbO2 composite electrodes. The introduction of active granules (WC/Co3O4) can decrease the average grain size and enhance the proportions of Od-ad present on the electrode surface, leading to more active sites on the electrode surface. Moreover, the excellent conductivity of the electrode caused by the presence of non-stoichiometric PbO2 was also observed, which is favorable for improving the electron transfer and catalytic activity of the electrode. LSV measurements show that Ti/Sb-SnO2/Co3O4-PbO2 (1.85 V) and Ti/Sb-SnO2/WC-Co3O4-PbO2 (1.80 V) have lower OER values than Ti/Sb-SnO2/PbO2 (2.20 V) and Ti/Sb-SnO2/WC-PbO2 (2.12 V). Ti/Sb-SnO2/Co3O4-PbO2 is regarded as the optimal modified electrode due to its long service lifetime (684 h) and the good stability of its plating solutions. Wastewater containing copper ions was employed as a model pollutant, and Ti/Sb-SnO2/Co3O4-PbO2 was used as the anode to study the electrocatalytic activity of the modified electrodes. The experimental results demonstrate that the Ti/Sb-SnO2/Co3O4-PbO2 composite electrode exhibits remarkable extraction efficiency and low cell voltage. The color of copper deposited on the cathode became dark due to the impoverishment of copper ions. Furthermore, pilot simulation tests were carried out to research the electrodes in practical applications. A low cell voltage further verified that the composite electrodes consume less energy than other electrodes. Therefore, it is deduced that composite electrodes may be promising for the treatment of wastewater containing high concentrations of copper ions. Furthermore, it is expected that Ti/Sb-SnO2/Co3O4-PbO2 electrodes may substitute traditional Pb electrodes in resource recovery of nonferrous metals. Further studies exploring modified electrodes in specific pilot tests will be conducted in the future.
Author Contributions
Conceptualization, X.K. and S.X.; Methodology, X.K. and B.J.; Software, Q.F.; Validation, X.K., J.W., Z.W. and S.X.; Formal analysis, X.K., J.W. and Z.W.; Investigation, X.K. and Z.W.; Resources, X.K. and Q.F.; Data curation, J.W.; Writing—original draft, J.W.; Writing—review and editing, Y.W.; Supervision, X.K. and Q.F.; Project administration, B.J.; Funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21878242).
Data Availability Statement
All data supporting this study are available from authors upon request.
Acknowledgments
The Northwest Institute for Non-ferrous Metal Research is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, B.; Zhang, G. Estimates of electricity saving potential in chinese nonferrous metals industry. Energy Policy 2013, 60, 558–568. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Wang, M.; Yang, Q.; Liu, X.; Shen, J.; Liu, G.; Zheng, M. Concentrations and profiles of persistent organic pollutants unintentionally produced by secondary nonferrous metal smelters: Updated emission factors and diagnostic ratios for identifying sources. Chemosphere 2020, 255, 126958. [Google Scholar] [CrossRef]
- Sorour, N.; Zhang, W.; Gabra, G.; Ghali, E.; Houlachi, G. Electrochemical studies of ionic liquid additives during the zinc electrowinning process. Hydrometallurgy 2015, 157, 261–269. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, N.; Jiang, L.; Xu, F. The impact mechanism of Mn2+ ions on oxygen evolution reaction in zinc sulfate electrolyte. J. Electroanal. Chem. 2018, 811, 53–61. [Google Scholar] [CrossRef]
- Karbasi, M.; Eskandar, K.A.; Elaheh, A.D. Electrochemical performance of Pb−Co composite anode during zinc electrowinning. Hydrometallurgy 2018, 183, 51–59. [Google Scholar] [CrossRef]
- Chen, B.; Wang, S.; Liu, J.; Huang, H.; Dong, C.; He, Y.; Yan, W.; Guo, Z.; Xu, R.; Yang, H. Corrosion resistance mechanism of a novel porous Ti/Sn-Sb-RuOx/β-PbO2 anode for zinc electrowinning. Corros. Sci. 2018, 144, 136–144. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ni, S.; Bie, C.; Zhi, H.; Sun, X. A clean process for selective recovery of copper from industrial wastewater by extraction-precipitation with p-tert-octyl phenoxy acetic acid. J. Environ. Manag. 2022, 304, 114164. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Chen, P. An environmentally friendly gradient treatment system of copper-containing wastewater by coupling thermally regenerative battery and electrodeposition cell. Sep. Purif. Technol. 2022, 295, 121243. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S. Enhanced copper extraction in the chalcopyrite bioleaching system assisted by microbial fuel cells and catalyzed by silver-bearing ores. J. Environ. Chem. Eng. 2022, 10, 108827. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Lyu, X.; Gao, W.; Su, H.; Li, C. Extraction and separation of copper and iron from copper smelting slag: A review. J. Clean. Prod. 2022, 368, 133095. [Google Scholar] [CrossRef]
- Kim, H.K.; Jang, H.; Jin, X.; Kim, M.G.; Hwang, S.J. A crucial role of enhanced Volmer-Tafel mechanism in improving the electrocatalytic activity via synergetic optimization of host, interlayer, and surface features of 2D nanosheets. Appl. Catal. B Environ. 2022, 312, 121391. [Google Scholar] [CrossRef]
- Abedini, A.; Valmoozi, A.A.E.; Afghahi, S.S.S. Anodized graphite as an advanced substrate for electrodeposition of PbO2. Mater. Today Commun. 2022, 31, 103464. [Google Scholar] [CrossRef]
- Kandasamy, K. Electrochemical degradation of sago wastewater using Ti/PbO2 electrode: Optimisation using response surface methodology. Int. J. Electrochem. Sci. 2014, 10, 1506–1516. [Google Scholar]
- Torres, J.E.; Sierra, A.; Pena, D.Y.; Uribe, I.; Estupinan, H. Corrosion rate in a lead based alloy in a sulfuric acid solution at different temperatures. Matéria 2014, 19, 183–196. [Google Scholar]
- Li, H.; Yuan, T.; Li, R.; Wang, W.; Zheng, D.; Yuan, J. Electrochemical properties of powder-pressed Pb-Ag-PbO2 anodes. Trans. Nonferrous Met. Soc. China 2019, 29, 2422–2429. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Zhang, F.; Zhang, G.; Chen, L. A novel functional lead-based anode for efficient lead dissolution inhibition and slime generation reduction in zinc electrowinning. J. Clean. Prod. 2021, 284, 124767. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Jiang, W.; Chen, C.; Yu, B.; Xu, R. Facile synthesis MnCo2O4 modifying PbO2 composite electrode with enhanced OER electrocatalytic activity for zinc electrowinning. Sep. Purif. Technol. 2021, 272, 118916. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Wang, S.; Yan, W.; He, Y.; Guo, Z.; Xu, R. Ag doping to boost the electrochemical performance and corrosion resistance of Ti/Sn-Sb-RuOx/α-PbO2/β-PbO2 electrode in zinc electrowinning. J. Alloys Compd. 2020, 815, 152551. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, J.; Jin, W.; Xu, F.; Jiang, L.; Xi, D.; Wen, Y.; Li, J.; Yu, Z.; Li, Z.; et al. Size effect of γ-MnO2 precoated anode on lead-containing pollutant reduction and its controllable fabrication in industrial-scale for zinc electrowinning. Chemosphere 2022, 287, 132457. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, X.; Wang, P.; Ren, Y.; Meng, X.; Ding, Y.; Zhang, T.; Jiang, W. Electrochemical degradation of doxycycline hydrochloride on Bi/Ce co-doped Ti/PbO2 anodes: Efficiency and mechanism. J. Environ. Chem. Eng. 2022, 10, 108430. [Google Scholar] [CrossRef]
- Duan, P.; Qian, C.; Wang, X.; Jia, X.; Jiao, L.; Chen, Y. Fabrication and characterization of Ti/polyaniline-Co/PbO2-Co for efficient electrochemical degradation of cephalexin in secondary effluents. Environ. Res. 2022, 214, 113842. [Google Scholar] [CrossRef]
- Shao, D.; Li, W.; Wang, Z.; Yang, C.; Xu, H.; Yan, W.; Yang, L.; Wang, G.; Yang, J.; Feng, L.; et al. Variable activity and selectivity for electrochemical oxidation wastewater treatment using a magnetically assembled electrode based on Ti/PbO2 and carbon nanotubes. Sep. Purif. Technol. 2022, 301, 122008. [Google Scholar] [CrossRef]
- Macounová, K.M.; Pittkowski, R.K.; Nebel, R.; Zitolo, A.; Krtil, P. Selectivity of Ru-rich Ru-Ti-O oxide surfaces in parallel oxygen and chlorine evolution reactions. Electrochimi. Acta. 2022, 427, 140878. [Google Scholar] [CrossRef]
- Preez, S.P.; Jones, D.R.; Warwick, M.E.A.; Falch, A.; Sekoai, P.T.; das Neves Quaresma, A.M.; Bessarabov, D.G.; Dunnill, C.W. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrog. Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Ma, D.; Ngo, V.; Raghavan, S.; Sandoval, S. Degradation of Ir-Ta oxide coated Ti anodes in sulfuric acid solutions containing fluoride. Corros. Sci. 2020, 164, 108358. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wang, C.; Meng, X.; Zhang, W.; Chen, Z.; Crittenden, J. Electrochemical degradation of methylisothiazolinone by using Ti/SnO2-Sb2O3/α, β-PbO2 electrode: Kinetics, energy efficiency, oxidation mechanism and degradation pathway. Chem. Eng. J. 2019, 374, 626–636. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Luo, S.; Wang, Z.; Li, X. Effect of SnO2 intermediate layer on performance of Ti/SnO2/MnO2 electrode during electrolytic-manganese process. Trans. Nonferrous Met. Soc. China 2017, 27, 1417–1422. [Google Scholar] [CrossRef]
- Hakimi, F.; Rashchi, F.; Ghalekhani, A.; Dolati, A.; Astaraei, F.R. Effect of a synthesized pulsed electrodeposited Ti/PbO2-RuO2 nanocomposite on zinc electrowinning. Ind. Eng. Chem. Res. 2021, 60, 11737–11748. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.M.; Schmidt, W.; Spliethoff, B.; Schüth, E.B.F. Weakly ferromagnetic ordered mesoporous Co3O4 synthesized by nanocasting from vinyl-functionalized cubic Ia3d mesoporous silica. Adv. Mater. 2004, 17, 53–56. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-Khathami, N.D.; Mohamed, R.M. Co3O4 nanoparticles accommodated mesoporous TiO2 framework as an excellent photocatalyst with enhanced photocatalytic properties. Opt. Mater. 2022, 134, 112643. [Google Scholar] [CrossRef]
- Dan, Y.; Lu, H.; Liu, X.; Lin, H.; Zhao, J. Ti/PbO2 + nano-Co3O4 composite electrode material for electrocatalysis of O2 evolution in alkaline solution. Int. J. Hydrogen Energy 2011, 36, 1949–1954. [Google Scholar] [CrossRef]
- He, S.; Xu, R.; Hu, G.; Chen, B. Electrosynthesis and performance of WC and Co3O4 co-doped α-PbO2 electrodes. RSC Adv. 2016, 6, 3362–3371. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.; Wang, F.; Niu, H.; Yu, L.; Xue, J. Influence of a MnO2-WC interlayer on the stability and electrocatalytic activity of titanium-based PbO2 anodes. Electrochim. Acta 2020, 331, 135381. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, L.; Wu, C.; Lin, L.; Liu, X. Bi5+ doping improves the electrochemical properties of Ti/SnO2-Sb/PbO2 electrode and its electrocatalytic performance for phenol. J. Clean. Prod. 2022, 380, 135005. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Kosmulski, M.; Mączka, E. Zeta potential and particle size in dispersions of alumina in 50-50 w/w ethylene glycol-water mixture. Colloid. Surfaces A 2022, 654, 130168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).