Abstract
The development of low-alcohol Baijiu is consistent with demand for the industry’s sustainable development. However, the ester aroma of low-alcohol Baijiu is insipid and unstable—mainly due to the hydrolysis of esters during shelf life—thus reducing the industry scale of low-alcohol Baijiu to a significantly small range. An electrochemical method for improving low-alcohol Baijiu’s ester concentration and stability was investigated from the aspects of thermodynamics and kinetics. The key finding is that the new Baijiu’s ester content obtained through distillation is relatively high, exceeding its content in the thermodynamic equilibrium state. Thus, the ester will be hydrolyzed during shelf life. The idea of applying electrochemical catalytic esterification technology to the production of low-alcohol Baijiu in this study is directly derived from the production practice of Baijiu factories; it provides a direction for the further optimization of low-alcohol Baijiu to facilitate the production of an alternative product that will contribute to public health.
1. Introduction
Distilled liquors have been consumed by humans for over one thousand years and are used for economic, social, and medicinal purposes; contributing to the development of ritual traditions and sophisticated culture throughout history [1,2]. However, the process of social development has prompted people to recognize the health damage caused by excessive drinking [3]. Recent studies have shown that excessive alcohol intake is closely related to a series of diseases, such as cardiovascular [4,5], cerebrovascular [6], and liver diseases [7,8]. Global alcohol consumption has increased significantly compared to 30 years ago, largely due to the growing phenomenon of large-scale drinking in China and India [9,10]. According to the current trend, global per capita alcohol consumption increased by 10% between 1990 and 2017, and will increase by 17% in the next 10 years [11]. Conversely, in many European countries—especially Eastern European ones—alcohol consumption has shown a significant downward trend [12]. The main reason for this is the implementation of alcohol control policies and the development of low-alcohol liquor [13]. In China, the global sale of Baijiu reached 7.16 billion L in 2021, according to data from the China National Bureau of Statistics [14]. The consumption market of Baijiu is dominated by high-alcohol Baijiu (ethanol content ranging from 40% to 65% in volume), while the market attraction of low-alcohol Baijiu (ethanol content controlled below 40% in volume) is extremely limited [15]. Therefore, developing low-alcohol Baijiu is beneficial for reducing alcohol consumption.
The unsatisfied market attraction of low-alcohol Baijiu is ascribed to its insipid ester flavor. Low-alcohol Baijiu usually uses high-alcohol Baijiu as a raw material, and the alcohol content is controlled below 40% by mixing Baijiu and water [1]. While alcohol was diluted during the mixing process, Baijiu’s ester flavor (ester content) inevitably decreased [16]. In our previous work, electrochemical esterification technology applied to improve low-alcohol Baijiu’s ester content was considered, and an enhanced flavor was obtained [17]. However, another important issue related to product quality is shelf life [18]. On April 2022, the Chinese national standard (GB/T 10781.1-2021) put forward the following new regulations on low-alcohol Baijiu: the content of ethyl hexanoate in low-alcohol Baijiu with a production date of less than or equal to (≤) one year shall not be less than 0.7 g/L, and the total acid content shall not be less than 0.3 g/L. However, for a production date longer than (>) one year from the production date, the total amount of ethyl hexanoate and hexanoic acid is not less than 0.8 g/L, and the content of ethyl hexanoate is no longer limited, as ethyl hexanoate will be hydrolyzed to acid during its shelf life. These standards have relaxed the restrictions on ester hydrolysis, reflecting the unstable characteristics of esters (spontaneous hydrolysis of esters) in low-alcohol Baijiu, which is a crucial problem that should be solved. Of course, high-alcohol Baijiu will also encounter the instability of esters; but relatively speaking, low-alcohol Baijiu has a high water content, increasing the speed of esters being hydrolyzed [19].
Therefore, this paper investigated the thermodynamics and kinetics [20] of the electrochemical esterification process in low-alcohol Baijiu. The esterification process of the main esters in low-alcohol Baijiu was investigated via thermodynamics. The equilibrium constant of the esterification process indicates the end point of the esterification reaction, providing a quantitative reference for the change trend of ester content. The results confirmed that the newly obtained Baijiu (obtained via distillation from fermented grains) contained relatively high amounts of ester. Its content exceeds the content in the thermodynamic equilibrium state. Therefore, esters will inevitably be hydrolyzed during Baijiu’s storage process, thus approaching the thermodynamic equilibrium state. The kinetic process revealed the essence of electrochemical esterification technology, provided a direction for further optimizing the electrochemically enhanced flavor of low-alcohol Baijiu, and attempted to obtain an alternative product, thus contributing to public health.
2. Results and Discussion
2.1. Improve the Ester Content of Low-Alcohol Liquor by Electrochemical Esterification
An electrochemical esterification technique was designed to increase the ester content in liquor to improve the ester aroma of low-alcohol liquor. Figure 1a shows that the content of ethyl butyrate increased from 13.08 to 14.28, 13.92, 16.84, 16.62, and 16.02 mg/100 mL when the operating voltages of 0.2 V, 0.35 V, 0.5 V, 0.65 V, and 0.8 V, respectively, were applied to low-alcohol liquor. The content of ethyl hexanoate increased from 116.83 to 120.39, 126.71, 126.83, 130.21, and 132.01 mg/100 mL when the operating voltages of 0.2 V, 0.35 V, 0.5 V, 0.65 V, and 0.8 V were applied to low-alcohol liquor. Baijiu contains many esters, providing its main aroma and flavor substances [21,22,23]. These esters include ethyl formate, ethyl acetate, isoamyl acetate, ethyl propionate, ethyl butyrate, ethyl valerate, ethyl caproate, ethyl heptanoate, ethyl caproate, ethyl caproate, ethyl laurate, and ethyl lactate. The content of ethyl acetate, ethyl hexanoate, ethyl lactate, and ethyl butyrate accounts for more than 90% of the total ester content [24], the main esters in Baijiu known as the “four major esters.” Esters in Baijiu are basically ethyl esters, produced through the process of yeast fermentation using raw grain materials [25]. As seen, the contents of the major esters (ethyl acetate, ethyl hexanoate, ethyl lactate, and ethyl butyrate) have increased significantly after the electrochemical process. Typical strategies to enhance the ester aroma of low-alcohol liquor mainly include the mixing of different base liquors and the addition of foreign substances. However, the selection of base liquors is restricted by the limited diversity of base liquors. Therefore, electrochemical esterification technology (Figure 1b) applied to improve ester content provides a new strategy to improve the low-alcohol liquor’s quality.
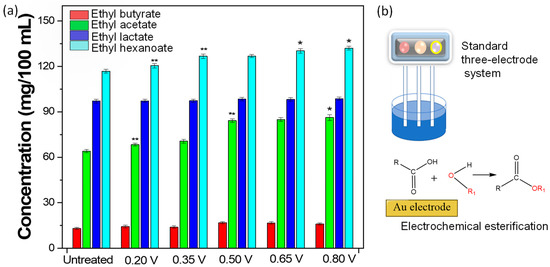
Figure 1.
(a) The content of ethyl butyrate, ethyl acetate, ethyl lactate, and ethyl hexanoate in the electrochemically treated low-alcohol Baijiu (operating voltages: 0.2 V, 0.35 V, 0.5 V, 0.65 V, and 0.8 V), ethanol concentration in the Baijiu: 40% in volume ratio. (* p < 0.05 and ** p < 0.01, compared with the control) (b) Schematic representation of electrochemical esterification technology.
The influence of alcohol and ester content on the taste and aroma of Baijiu was explored via sensory evaluation. Model liquors with different alcohol and ester contents were prepared with the blending method. Experienced tasters in the sensory evaluation team conducted a blind evaluation of the model liquors (Figure 2). The pungency intensity (taste score) increased as the alcohol content increased, and the change of aroma intensity (smell score) was not obvious for samples in which the alcohol content increased in turn and total ester content remained unchanged. In samples in which the total ester content increased in turn and alcohol content remained unchanged, the aroma intensity increased as the total ester content increased, and the change of pungency intensity was not obvious. Although Baijiu’s flavor characteristics depend on the coordination state of many substances mixed together, each substance has its own characteristics [26]. The amount of acid [27], for example, correlates positively with how long the taste lasts in the mouth. Overall, the results show that the alcohol content is positively correlated with the pungency intensity, while the ester content is positively correlated with the aroma intensity. It can be inferred that low-alcohol Baijiu’s insufficient aroma is due to the relatively low content of esters, and the elevation of the ester content can be the key strategy for the development of low-alcohol Baijiu.
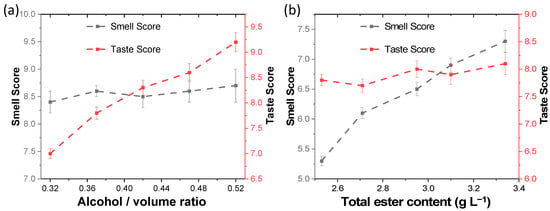
Figure 2.
Sensory evaluation of model liquors with different alcohol (a) and ester (b) contents.
2.2. Thermodynamic Study on Electrochemical Esterification
The ester content was increased by electrochemical esterification. However, after being treated by the electrochemical technology and stored for a period of time (less than one year), low-alcohol Baijiu’s ester content decreased significantly (more than 20%, data not shown) as the esters were hydrolyzed [28]. Esterification and hydrolysis are reversible reactions, and the reaction progress and speed are determined by thermodynamic and kinetic processes, respectively. Therefore, low-alcohol Baijiu’s esterification and hydrolysis processes were investigated from the thermodynamic and kinetic perspectives.
Under certain conditions, the heat released or absorbed in the process of chemical reactions is called the enthalpy change of chemical reaction (ΔH) [29]. The enthalpy change determines whether the reaction is exothermic or endothermic. The esterification process’s enthalpy change can be calculated according to the standard molar enthalpy of formation (ΔfH). As shown in Table 1, ΔH in the esterification process of ethyl butyrate and ethyl lactate is greater than 0 in the temperature range of 0 °C–100 °C, which is an exothermic reaction. According to the second law of thermodynamics [30], any chemical reaction in an isolated system always goes in the direction of increasing entropy (S). As shown in Table 1, compared with other esters, the S value of ethyl lactate increases the most. According to the change of H and S, the spontaneity of the reaction can be judged. Gibbs (G) energy is used to judge the spontaneity of the chemical reaction (Equation (1)) [31] to judge the reaction direction of the previously discussed esterification reactions under specific conditions.

Table 1.
Thermodynamic data of esterification reactions (ethyl acetate, ethyl hexanoate, ethyl lactate, and ethyl butyrate) in Baijiu, calculated by HSC Chemistry (version of 9.30) using the Reaction Equations module.
As shown in Table 1, the change of Gibbs energy (ΔG) in the esterification process of ethyl acetate and ethyl butyrate is greater than 0 in the temperature range of 0 °C–100 °C, which indicates these esterification processes occurs naturally under the conditions described above. Furthermore, when ΔG equals 0 (the reaction reaches equilibrium), the equilibrium constant (K) of the reaction can be calculated according to Equation (2) [32].
The relationship between the K of the esterification process of esters and temperature is shown in Figure 3. It can be seen that the K of the esterification process of the four esters fluctuates in the range of 0–17. The value of the K of the esterification process of ethyl butyrate is the smallest, increasing from 0.00092 to 0.147 in the temperature range of 0 °C–100 °C. The value of the K of the esterification process of ethyl lactate is the largest, increasing from 0.068 to 16.94 in the temperature range of 0 °C–100 °C. Under the common storage temperature of Baijiu (20 °C), the K of the esterification process of the four esters was 0.009, 0.13, 1.1, and 3.8. The esterification progress was 2.9%, 27.0%, 51.4%, and 66.1% under the condition of equal reactant (the ratio of alcohol to acid was 1:1). It can be seen from the thermodynamic data that only a small part of acid is converted to ester in the equilibrium state of the esterification reaction in Baijiu.
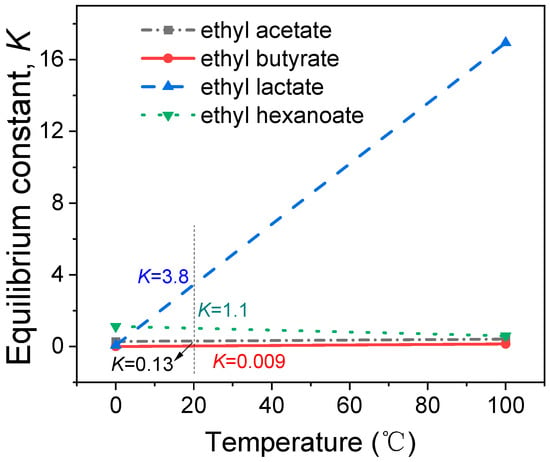
Figure 3.
Correlation between the K of the esterification process of Baijiu esters and temperature in the range of 0 °C–100 °C.
Baijiu was obtained following the distillation process. During distillation [33], the amount of alcohol, acid, ester, and water transferred into Baijiu is primarily determined by the boiling point and intermolecular interactions between the neighboring molecules. The boiling point of esters is relatively low; for example, ethyl acetate (its counterpart acid, acetic acid) has a boiling point of 77 °C and acetic acid has a boiling point of 118 °C. Thus, the ester content in new Baijiu obtained through distillation is relatively high. If the ester content exceeds its content in the thermodynamic equilibrium state, the ester will be hydrolyzed during Baijiu’s storage, thus approaching the thermodynamic equilibrium state. In a previous paper, a fluctuation phenomenon in ester content in aged Baijiu was observed and the correlation among chemical reactions, such as oxidation and esterification was interpreted [19]: If the content of a certain ester compound is high, the ester compound will undergo hydrolysis and its amount will be reduced so that the esterification can reach a balance. If the content of a certain ester compound is low, the oxidation makes the content of ester compound increase, so that the esterification can reach a balance. As seen, the previous paper suggested that ester content is related to esterification equilibrium. Thus, the thermodynamic equilibrium law in esterification is further revealed in this study.
Additionally, the high-alcohol Baijiu was diluted with water to obtain low-alcohol Baijiu. Adding water promoted the hydrolysis reaction, so the ester content in low-alcohol Baijiu would further reduce. Therefore, electrochemical esterification technology only solved the problem of insufficient ester aroma in low-alcohol Baijiu in one respect (ester content). The electrochemical esterification technology can only maintain the enhanced ester aroma of low-alcohol Baijiu for a short period of time, which is unfavorable for shelf life. To solve the problem of insufficient ester aroma and short shelf life for low-alcohol Baijiu, it is necessary to combine electrochemical esterification technology and aroma blending technology. According to the calculated equilibrium constant using the aroma blending method, the esterification reaction in low-alcohol Baijiu can reach the equilibrium state and contribute to low-alcohol Baijiu with a sufficient ester aroma and long shelf life, providing the framework for further research.
2.3. Kinetic Study on Electrochemical Esterification
Next, the energy of the transition states in the esterification process was calculated using the transition state theory [34]. For different esters, the esterification mechanism is similar, thus ethyl acetate serves as an example for the esterification process. Ethanol and acetic acid undergo an electrocatalytic process on the surface of a gold electrode with applied voltage to produce ethyl acetate. There are two possible mechanisms of electrocatalytic esterification [35]. The first possible mechanism is that a positively charged carbon ion is formed during the transition state, which is the rate-controlled process of esterification. Under the catalysis of the voltage pulsed electrode, the carboxylic acid gives electrons to the electrode surface, forming a positively charged carbon ion in the presence of hydrogen ions. The carbonium ion forms a covalent bond with the lone pair of electrons of the oxygen atom in the alcohol. Finally, a water molecule and a hydrogen ion are removed to form an ester. The calculation result of the transition state energy of the reaction is shown in the Figure 4a, and the energy of the intermediate transition state is 54.6 kcal/mol. The higher the transition state energy is, the lower the probability of completing the effective collision [36], indicating that the esterification mechanism of carbocation is not suitable for explaining the described electrocatalytic esterification process.
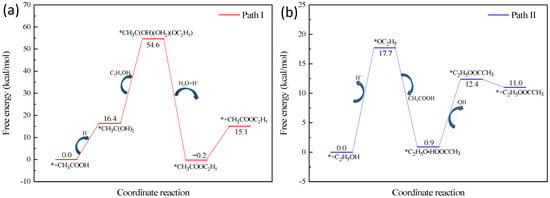
Figure 4.
Calculation result of the transition state energy of the carbonium ion formed process (a) and free radical formed process (b). The asterisk (*) represents the active sites on the electrode’s surface.
The second possible mechanism is that free radicals are formed as the transition state, which is the rate-controlled process of esterification. The lone pair electrons of oxygen in alcohol combine with hydrogen atoms to form a free radical and a hydrogen ion on the gold surface, as shown in Figure 5. Free radicals attack the carbon of acid molecules to form free radical transition states. The transition state loses one molecule of water and one hydrogen ion to obtain ester. The calculation result of the transition state energy of this process is shown in the Figure 4b, and the energy of the intermediate transition state is 17.7 kcal/mol. It can be seen that the energy of this transition state is relatively low, which is ~1/3 of the transition state energy in the first mechanism. At the same time, it should be noted that the anode not only occurs in the ester formation, but also occurs as a side reaction of oxygen formation. This is also in line with the relationship between ester yield and voltage value, as shown in Figure 1a. The ethyl butyrate content decreased from 16.84 mg/100 mL to 16.02 mg/100 mL when the operating voltage of 0.5 V increased to 0.8 V. Therefore, it is not the case that the higher the voltage, the higher the extent of esterification. The activity of oxygen generation reaction on a gold surface is high and presents an onset potential of ~1.3 V [37]. Thus, the higher the voltage, the faster the oxygen generation rate may be, reducing the esterification rate. The results of this study are consistent with the assumption that the electrocatalytic esterification undergoes a free radical formed process to complete the esterification reaction, which is also supported by the esterification reaction that undergoes a free radical process in the water medium [38].The conventional strategy for enhancing the ester aroma of low-alcohol Baijiu mainly includes mixing and blending different base Baijiu and adding foreign substances. However, it is not possible to solve the problem of reduced ester aroma of low-alcohol Baijiu only by the mixing of different base Baijiu, as the amount of Baijiu compounds in the base Baijiu depends on microbial fermentation and distillation processes, which is difficult to regulate through artificial factors.
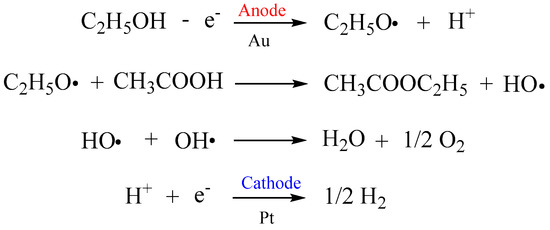
Figure 5.
Schematic representation of the free radicals formed during the esterification process.
To sum up, from the perspective of methodology, this study explored the thermodynamic and kinetic laws of esterification in Baijiu and proposes that a new method be applied to regulate its aroma intensity without adding foreign substances. From the perspective of engineering, the idea of applying electrochemical catalytic esterification technology to the production of low-alcohol Baijiu in this study is directly derived from the production practice of Baijiu factories, and the scientific problems raised directly focus on the bottleneck, due to insufficient and unstable ester aroma. This provided a direction for further optimization of low-alcohol Baijiu in order to obtain an alternative product and thus contribute to public health.
3. Materials and Methods
3.1. Materials
Baijiu samples were collected from a local Baijiu manufacturer (Xiangjiao Group Ltd., Shaoyang, China). Mention of the Baijiu brand does not indicate research contact with the manufacturer, nor is for advertising purposes. The ethanol content of the raw Baijiu sample was ~63%, and the low alcohol Baijiu (ethanol content: 32%, 37%, 42%, 47%, and 52%) was obtained through mixing the raw sample with a certain amount of ultra-pure water (with a resistivity of 18.2 MΩ·cm−1), in accordance with the dilution technology that commonly used in Baijiu manufacturers [1]. The same batch of baijiu was used for dilutions. The model Baijiu used for sensory evaluation was obtained through a blending process, according to the concentration of the main ester component (ethyl hexanoate, ethyl acetate, and ethyl lactate) recorded in Table 2. The ester standards (ethyl butyrate, ethyl acetate, ethyl lactate, and ethyl hexanoate) used for gas chromatography were purchased from Merck (Darmstadt, Germany). HPLC-grade solvents were purchased from Aladdin (Shanghai, China) and used as received.

Table 2.
Sensory evaluation of model Baijiu with different alcohol and ester contents.
3.2. Electrochemistry
A typical three-electrode electrochemical system was used in this work, performed with the CHI660E electrochemical workstation (CH Instruments, Inc., Shanghai, China). Chronoamperometry with different potentials (0.20 V, 0.35 V, 0.50 V, 0.65 V, and 0.80 V) was used for the electrochemical esterification of Baijiu. A polycrystalline gold electrode (1.0 mm in radius, CHI101) was employed as the working electrode, an Ag/AgCl electrode with saturated KCl solution (CHI111) as the reference electrode, and a platinum wire (CHI115) as the counter electrode. Before the recording of the chronoamperometry curve, the working electrode was polished with alumina powder in different sizes (1, 0.3 and 0.05 mm) to achieve a smooth surface, followed by ultrasonic cleaning in ethanol and water. To avoid the oxygen-induced oxidation, oxygen dissolved in the electrolyte was removed from the sample by passing of nitrogen gas no less than 15 min before the recording of chronoamperometry. The treatment time for electrochemical esterification process was 20 min. More details about electrochemical configuration can be found in the previous work [17].
3.3. Gas Chromatography (Arbitrating Method)
According to the arbitrating method recorded in Chinese national standard (GB/T 10345-2007), gas chromatography with authentic internal standards was selected to determine the contents of Baijiu compounds. Gas chromatography was performed with an Agilent 7890B gas chromatography (Agilent Technologies Co., Ltd., Shanghai, China). The quantitative analysis was performed using internal standards, 2-ethylbutyric acid and n-pentyl acetate (which was chemically similar to the analytes of interest). An Agilent CP-Wax 57 CB capillary column with the size 0.25 mm × 50 m × 0.2 μm was used for gas chromatography separation, with a flow rate of 1.0 mL min−1. Gas chromatography temperature parameters were well-optimized as follows: initially maintained at 40 °C and held for 5 min, 50 °C held for 6.5 min, 90 °C held for 5 min, 130 °C held for 2 min, 190 °C held for 1.4 min, and finally 195 °C held for 20 min.
3.4. Sensory Evaluation
Sensory evaluation of model Baijiu with different alcohol and ester contents was performed by trained sensory tasters, according to the procedures stipulated in Chinese national standard GB/T 33404-2016. Sensory tasters were trained in descriptive sensory analysis before the formal evaluation. The evaluation team included ten trained tasters (five males and five females) aged from 20 to 50 years old, in a specific air-conditioned room at 20 °C, equipped with separated booths and standardized glasses; and the smell score and taste score of model Baijiu were independently voted on. At each evaluation session, Baijiu samples were presented to tasters at random. The sensory evaluation was repeated three times and the reported data was the mean value.
3.5. Equilibrium Thermodynamic Analysis
The equilibrium thermodynamic in the esterification process of Baijiu esters (ethyl acetate, ethyl hexanoate, ethyl lactate, and ethyl butyrate) in the temperature range of 0 to 100 °C was investigated using the HSC Chemistry® 9.30 thermodynamic program [39]. In this regard, the simulations were carried out using the Reaction Equation module which is based on a Gibbs Energy Minimization method. HSC Chemistry software was used only for the equilibrium simulation and is not used to predict reaction rates because it does not take into account the reaction kinetics and the non-ideality of solutions.
3.6. Kinetic Analysis
Kinetic analysis was carried out using the density functional theory (DFT) simulations, performed with a Dmol3 module of Material Studio 2020 [40]. The interactions between core and electrons were described with the generalized gradient approximation (GGA) method using the Perdew-Burke-Ernzerhof (PBE) function. The force and energy convergence criterion were set to 0.002 Ha Å−1 and 10−5 Ha, respectively. The frequency calculations were performed when the optimization was completed. The well-known linear synchronous transit (LST) and quadratic synchronous transit (QST) methods were utilized to locate the transition states. The frequency calculations were performed after the LST/QST calculations and a true transition state from LST/QST calculations was confirmed by a single negative frequency. Further, the free energy corrections were accomplished with Dmol3 at a temperature of 298.15 K, hence the free energy of reaction path was obtained.
3.7. Statistical Analysis and Data Treatment
Samples were analyzed in triplicate and the data were presented as the mean value of the measurements (n = 3, error bars indicate standard deviations). One simple t test was carried out with Origin 2022b (OriginLab Co., Northampton, MA, USA) to evaluate significant differences of the assay (* p < 0.05 and ** p < 0.01, compared with the control).
4. Conclusions
Thermodynamic study confirmed that only a small part of acid is converted to ester in the equilibrium state of esterification reaction in Baijiu. However, the content of esters in the new Baijiu obtained through distillation is relatively high as the boiling point of esters is relatively low. That is to say, the ester content exceeds its content in the thermodynamic equilibrium state. As a result, the ester will be hydrolyzed during the storage of Baijiu, thus approaching the thermodynamic equilibrium state. Low-alcohol Baijiu was obtained through mixing high alcohol Baijiu and water, which promoted the hydrolysis reaction, so the content of esters in low-alcohol Baijiu would be further reduced. Kinetic study confirmed that the electrocatalytic esterification undergoes a free radical formed process to complete the esterification reaction. Electrochemical esterification technology can be used to improve the ester content of low-alcohol Baijiu; however, the issue of the unsatisfactory shelf life of aroma-enhanced Baijiu should be addressed by an additional aroma blending process, which is a suggestion for future research.
Author Contributions
H.X.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper; Q.L.: Performed the experiments; Analyzed and interpreted the data; Y.Y.: Review & editing, Supervision; Q.Z.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hunan Provincial Technician Foundation [2021GK5056], Hunan Provincial Natural Science Foundation of China [2022JJ50230], and Innovation Foundation For Postgraduate of Shaoyang University [CX2022SY081].
Data Availability Statement
The data that support the findings are available from corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge the financial support from the Hunan Provincial Technician Foundation (2021GK5056), the Hunan Provincial Natural Science Foundation of China (2022JJ50230), and the Innovation Foundation for Postgraduates of Shaoyang University (CX2022SY081).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tang, K.; Yu, X.; Chen, S.; Xu, Y. Identification of Compounds Contributing to Trigeminal Pungency of Baijiu by Sensory Evaluation, Quantitative Measurements, Correlation Analysis, and Sensory Verification Testing. J. Agric. Food Chem. 2022, 70, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Shan, L.; Wolfson, M.; Hall, M.; Chaloupka, F. Problem drinking as intentional risky behavior: Examining the association between state health insurance coverage and excessive alcohol consumption. Prev. Med. Rep. 2021, 24, 101556. [Google Scholar] [CrossRef]
- Krittanawong, C.; Isath, A.; Rosenson, R.; Khawaja, M.; Wang, Z.; Fogg, S.; Virani, S.; Qi, L.; Cao, Y.; Long, M.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230.e3. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, T.; Sørensen, H.; Jensen, M.; Furtado, J.; Dragsted, L.; Mukamal, K. Associations between Alcohol Consumption and HDL Subspecies Defined by ApoC3, ApoE and ApoJ: The Cardiovascular Health Study. Curr. Probl. Cardiol. 2023, 48, 101395. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Iida, H.; Fujiwara, H.; Dohi, S. Effects of alcohol infusion on smoking-induced cerebrovascular changes in rat in vivo. Alcohol 2003, 30, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Flamm, S. Alcohol-Related Liver Disease Including New Developments. Clin. Liver Dis. 2023, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.; Ghayour-Mobarhan, M.; Ferns, G.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Jiang, H.; Dowling, R.; Guo, L.; Zhao, X.; Hao, W.; Xiang, X. The transition of alcohol control in China 1990–2019: Impacts and recommendations. Int. J. Drug Policy 2022, 105, 103698. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.; Rylett, M.; Hasan, O.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Malisauskaite, G.; Klein, A. Drinking under communism: Why do alcohol consumption habits in Eastern Europe differ from the west in the long-run? J. Comp. Econ. 2018, 46, 821–837. [Google Scholar] [CrossRef]
- Neufeld, M.; Rovira, P.; Ferreira-Borges, C.; Kilian, C.; Sassi, F.; Veryga, A.; Rehm, J. Impact of introducing a minimum alcohol tax share in retail prices on alcohol-attributable mortality in the WHO European Region: A modelling study. Lancet Reg. Health Eur. 2022, 15, 100325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Huang, C.; Ge, X.; Xi, B.; Mao, J. Chinese Baijiu distiller’s grains resourcing: Current progress and future prospects, Resources. Conserv. Recycl. 2022, 176, 105900. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Qian, M. Current Practice and Future Trends of Aroma and Flavor Research in Chinese Baijiu. In Sex, Smoke, and Spirits: The Role of Chemistry; American Chemical Society: New York, NY, USA, 2019; pp. 145–175. [Google Scholar]
- Li, Z.; Haigh, A.; Wang, P.; Soutis, C.; Gibson, A. Characterisation and analysis of alcohol in baijiu with a microwave cavity resonator. LWT 2021, 141, 110849. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, A.; Yu, Y.; Zheng, Q. Electrochemical esterification in distilled liquor via gold catalysis and its application for enhancing ester aroma of low-alcohol liquor. Curr. Res. Food Sci. 2022, 5, 1769–1776. [Google Scholar] [CrossRef]
- Torchio, F.; Segade, S.; Gerbi, V.; Cagnasso, E.; Giordano, M.; Giacosa, S.; Rolle, L. Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines. Food Res. Int. 2012, 48, 491–498. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, A.; Zhao, K.; Hu, Y.; Kuang, B.; Xiong, X.; Yang, Z.; Yu, Y.; Zheng, Q. Mechanisms of the regulation of ester balance between oxidation and esterification in aged Baijiu. Sci. Rep. 2020, 10, 17169. [Google Scholar] [CrossRef]
- Agu, C.; Agulanna, A.; Kadurumba, C.; Nnaji, P.; Udokporo, E.; Menkiti, M. Characterization, kinetics and thermodynamics of epoxidation-esterification of Irvingia gabonensis kernel oil methyl ester. Heliyon 2022, 8, e09520. [Google Scholar] [CrossRef]
- Jia, W.; Di, C.; Zhang, R.; Shi, L. Ethyl carbamate regulate esters degradation by activating hydrolysis during Baijiu ripening. Food Res. Int. 2022, 156, 111157. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Wang, J.; Hong, J.; Tian, W.; Zhao, D.; Sun, J.; Huang, M.; Li, H.; Zheng, F.; et al. Uncover the Flavor Code of Roasted Sesame for Sesame Flavor Baijiu: Advance on the Revelation of Aroma Compounds in Sesame Flavor Baijiu by Means of Modern Separation Technology and Molecular Sensory Evaluation. Foods 2022, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhao, D.; Sun, B. Research Progress on the Profile of Trace Components in Baijiu. Food Rev. Int. 2021, 1–27. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Huang, H.; Guo, X.; Li, X.; Zou, W.; Li, W.; Zhang, C.; Huang, M. Biodegradation of phthalate esters by Pantoea dispersa BJQ0007 isolated from Baijiu. J. Food Compos. Anal. 2022, 105, 104201. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wu, Y.; Zhao, D. Uncover the flavor code of strong-aroma baijiu: Research progress on the revelation of aroma compounds in strong-aroma baijiu by means of modern separation technology and molecular sensory evaluation. J. Food Compos. Anal. 2022, 104499, 109. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Y.; Chen, H.; Wang, J.; Zhang, C.; Zhao, Z.; Ao, R.; Huang, H.; Hong, J.; Zhao, D.; et al. “Key Factor” for Baijiu Quality: Research Progress on Acid Substances in Baijiu. Foods 2022, 11, 2959. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Meng, L.-J.; Lu, Z.-M.; Chai, L.-J.; Wang, S.-T.; Shi, J.-S.; Shen, C.-H.; Xu, Z.-H. Identification of age-markers based on profiling of Baijiu volatiles over a two-year maturation period: Case study of Lu-flavor Baijiu. LWT 2021, 141, 110913. [Google Scholar] [CrossRef]
- Hoang, N.H.; Rodrigues, D.; Bonvin, D. Revisiting the concept of extents for chemical reaction systems using an enthalpy balance. Comput. Chem. Eng. 2020, 136, 106652. [Google Scholar] [CrossRef]
- Demetrius, L.; Wolf, C. Directionality theory and the second law of thermodynamics. Phys. A Stat. Mech. Its Appl. 2022, 598, 127325. [Google Scholar] [CrossRef]
- Wu, J.; Wu, W.; Liu, L.; Tong, J. Measurement of evaporation entropy, evaporation enthalpy, and Gibbs free energy for the [C4Dmim]Gly and [C4Dmim]Ala. J. Mol. Liq. 2022, 346, 117142. [Google Scholar] [CrossRef]
- Awasthi, N.; Ritschel, T.; Lipowsky, R.; Knecht, V. Standard Gibbs energies of formation and equilibrium constants from ab-initio calculations: Covalent dimerization of NO2 and synthesis of NH3. J. Chem. Thermodyn. 2013, 62, 211–221. [Google Scholar] [CrossRef]
- He, F.; Yang, S.; Zhang, G.; Xu, L.; Li, H.; Sun, J.; Huang, M.; Zheng, F.; Sun, B. Exploration of key aroma active compounds in strong flavor Baijiu during the distillation by modern instrument detection technology combined with multivariate statistical analysis methods. J. Food Compos. Anal. 2022, 110, 104577. [Google Scholar] [CrossRef]
- Viegas, L. Gas-phase OH-oxidation of 2-butanethiol: Multiconformer transition state theory rate constant with constrained transition state randomization. Chem. Phys. Lett. 2022, 803, 139829. [Google Scholar] [CrossRef]
- Zhang, S.; Lian, F.; Xue, M.; Qin, T.; Li, L.; Zhang, X.; Xu, K. Electrocatalytic Dehydrogenative Esterification of Aliphatic Carboxylic Acids: Access to Bioactive Lactones. Org. Lett. 2017, 19, 6622–6625. [Google Scholar] [CrossRef] [PubMed]
- Williams, I. Gibbs energies of activation for reacting systems with multiple reactant-state and transition-state conformations. J. Phys. Org. Chem. 2022, 35, e4312. [Google Scholar] [CrossRef]
- Perini, N.; Ticianelli, E. Oxygen evolution on gold: The effects of alkali-metal cations and iron impurities from alkaline electrolytes. J. Catal. 2019, 378, 277–282. [Google Scholar] [CrossRef]
- Osman, A.; Khodair, A.; El-Cheikh, F.; Swelim, A.; Aly, I. Studies on electrosynthesis of organic compounds: I. Electrolytic esterification in aqueous solutions. J. Appl. Chem. Biotechnol. 1975, 25, 859–861. [Google Scholar] [CrossRef]
- Yang, J.; Yuling, L.; Penghe, Z.; Hao, S.; Chuanchuan, D.; Ruihao, S.; Feifei, L. Chemical thermodynamic and catalytic mechanism analysis of Cu-BTC-derived CuOx/C catalyst for selective catalytic reduction (SCR). Mol. Catal. 2022, 531, 112710. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, T.; Su, Y.; Zheng, L.; Zeng, H.; Ren, J.; Yu, H.; Meng, P. Effective response of multiple kinetic models and DFT calculations to the ultrafast adsorption behaviors of cellulose-titanium dioxide composites. Ind. Crops Prod. 2022, 178, 114673. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).