A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu)
Abstract
:1. Introduction
2. Results and Discussion
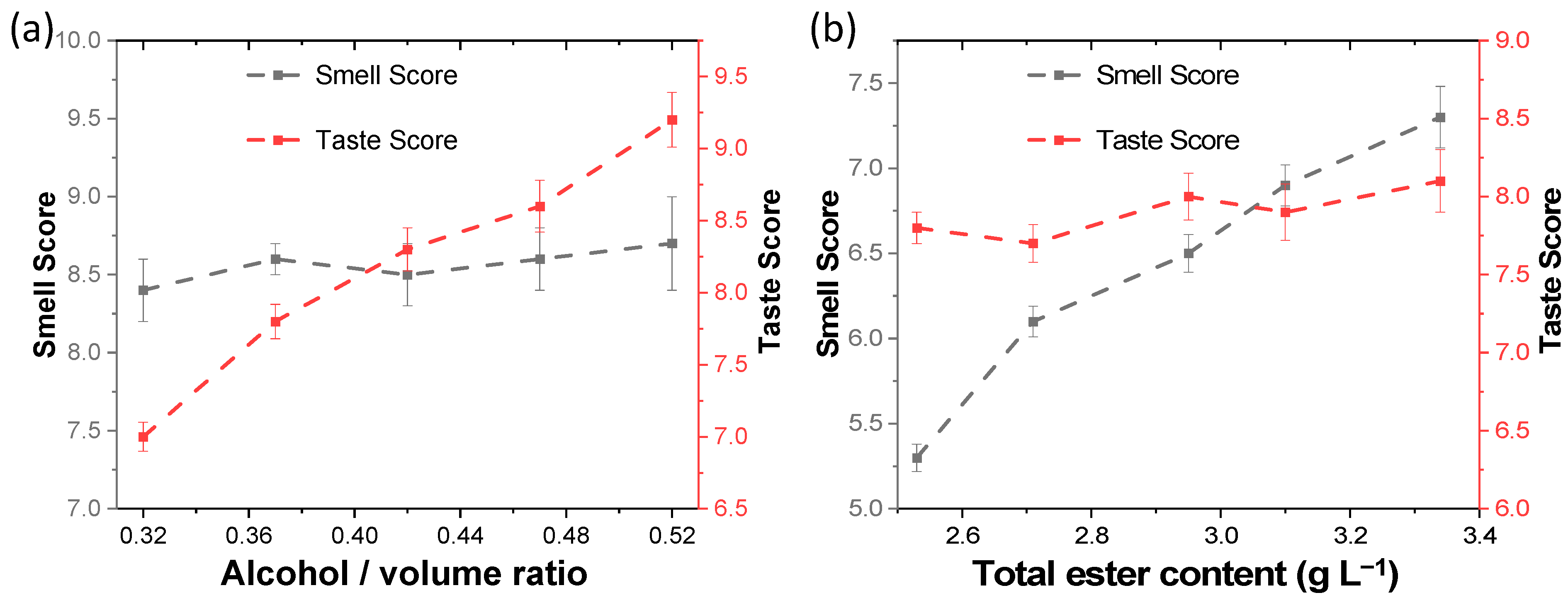
2.1. Improve the Ester Content of Low-Alcohol Liquor by Electrochemical Esterification
2.2. Thermodynamic Study on Electrochemical Esterification
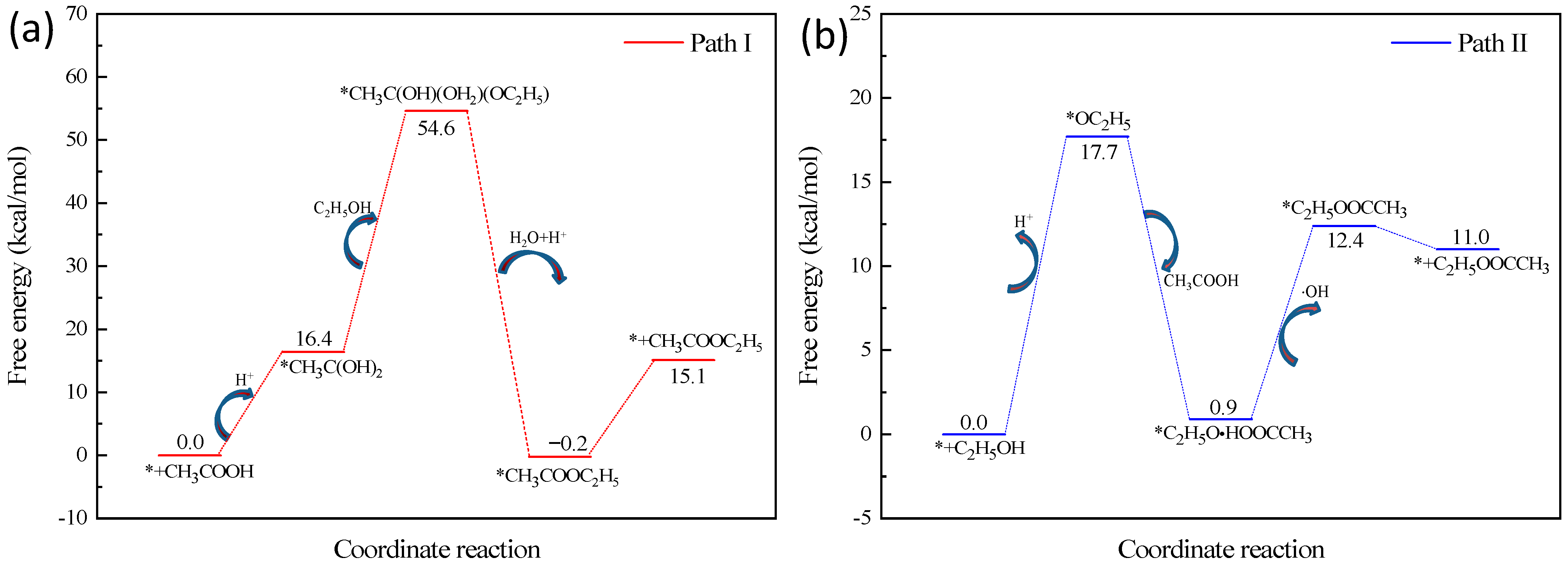
2.3. Kinetic Study on Electrochemical Esterification
3. Materials and Methods
3.1. Materials
3.2. Electrochemistry
3.3. Gas Chromatography (Arbitrating Method)
3.4. Sensory Evaluation
3.5. Equilibrium Thermodynamic Analysis
3.6. Kinetic Analysis
3.7. Statistical Analysis and Data Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tang, K.; Yu, X.; Chen, S.; Xu, Y. Identification of Compounds Contributing to Trigeminal Pungency of Baijiu by Sensory Evaluation, Quantitative Measurements, Correlation Analysis, and Sensory Verification Testing. J. Agric. Food Chem. 2022, 70, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Shan, L.; Wolfson, M.; Hall, M.; Chaloupka, F. Problem drinking as intentional risky behavior: Examining the association between state health insurance coverage and excessive alcohol consumption. Prev. Med. Rep. 2021, 24, 101556. [Google Scholar] [CrossRef]
- Krittanawong, C.; Isath, A.; Rosenson, R.; Khawaja, M.; Wang, Z.; Fogg, S.; Virani, S.; Qi, L.; Cao, Y.; Long, M.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230.e3. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, T.; Sørensen, H.; Jensen, M.; Furtado, J.; Dragsted, L.; Mukamal, K. Associations between Alcohol Consumption and HDL Subspecies Defined by ApoC3, ApoE and ApoJ: The Cardiovascular Health Study. Curr. Probl. Cardiol. 2023, 48, 101395. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Iida, H.; Fujiwara, H.; Dohi, S. Effects of alcohol infusion on smoking-induced cerebrovascular changes in rat in vivo. Alcohol 2003, 30, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Flamm, S. Alcohol-Related Liver Disease Including New Developments. Clin. Liver Dis. 2023, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.; Ghayour-Mobarhan, M.; Ferns, G.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Jiang, H.; Dowling, R.; Guo, L.; Zhao, X.; Hao, W.; Xiang, X. The transition of alcohol control in China 1990–2019: Impacts and recommendations. Int. J. Drug Policy 2022, 105, 103698. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.; Rylett, M.; Hasan, O.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Malisauskaite, G.; Klein, A. Drinking under communism: Why do alcohol consumption habits in Eastern Europe differ from the west in the long-run? J. Comp. Econ. 2018, 46, 821–837. [Google Scholar] [CrossRef]
- Neufeld, M.; Rovira, P.; Ferreira-Borges, C.; Kilian, C.; Sassi, F.; Veryga, A.; Rehm, J. Impact of introducing a minimum alcohol tax share in retail prices on alcohol-attributable mortality in the WHO European Region: A modelling study. Lancet Reg. Health Eur. 2022, 15, 100325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Huang, C.; Ge, X.; Xi, B.; Mao, J. Chinese Baijiu distiller’s grains resourcing: Current progress and future prospects, Resources. Conserv. Recycl. 2022, 176, 105900. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Qian, M. Current Practice and Future Trends of Aroma and Flavor Research in Chinese Baijiu. In Sex, Smoke, and Spirits: The Role of Chemistry; American Chemical Society: New York, NY, USA, 2019; pp. 145–175. [Google Scholar]
- Li, Z.; Haigh, A.; Wang, P.; Soutis, C.; Gibson, A. Characterisation and analysis of alcohol in baijiu with a microwave cavity resonator. LWT 2021, 141, 110849. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, A.; Yu, Y.; Zheng, Q. Electrochemical esterification in distilled liquor via gold catalysis and its application for enhancing ester aroma of low-alcohol liquor. Curr. Res. Food Sci. 2022, 5, 1769–1776. [Google Scholar] [CrossRef]
- Torchio, F.; Segade, S.; Gerbi, V.; Cagnasso, E.; Giordano, M.; Giacosa, S.; Rolle, L. Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines. Food Res. Int. 2012, 48, 491–498. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, A.; Zhao, K.; Hu, Y.; Kuang, B.; Xiong, X.; Yang, Z.; Yu, Y.; Zheng, Q. Mechanisms of the regulation of ester balance between oxidation and esterification in aged Baijiu. Sci. Rep. 2020, 10, 17169. [Google Scholar] [CrossRef]
- Agu, C.; Agulanna, A.; Kadurumba, C.; Nnaji, P.; Udokporo, E.; Menkiti, M. Characterization, kinetics and thermodynamics of epoxidation-esterification of Irvingia gabonensis kernel oil methyl ester. Heliyon 2022, 8, e09520. [Google Scholar] [CrossRef]
- Jia, W.; Di, C.; Zhang, R.; Shi, L. Ethyl carbamate regulate esters degradation by activating hydrolysis during Baijiu ripening. Food Res. Int. 2022, 156, 111157. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Wang, J.; Hong, J.; Tian, W.; Zhao, D.; Sun, J.; Huang, M.; Li, H.; Zheng, F.; et al. Uncover the Flavor Code of Roasted Sesame for Sesame Flavor Baijiu: Advance on the Revelation of Aroma Compounds in Sesame Flavor Baijiu by Means of Modern Separation Technology and Molecular Sensory Evaluation. Foods 2022, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhao, D.; Sun, B. Research Progress on the Profile of Trace Components in Baijiu. Food Rev. Int. 2021, 1–27. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Huang, H.; Guo, X.; Li, X.; Zou, W.; Li, W.; Zhang, C.; Huang, M. Biodegradation of phthalate esters by Pantoea dispersa BJQ0007 isolated from Baijiu. J. Food Compos. Anal. 2022, 105, 104201. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wu, Y.; Zhao, D. Uncover the flavor code of strong-aroma baijiu: Research progress on the revelation of aroma compounds in strong-aroma baijiu by means of modern separation technology and molecular sensory evaluation. J. Food Compos. Anal. 2022, 104499, 109. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Y.; Chen, H.; Wang, J.; Zhang, C.; Zhao, Z.; Ao, R.; Huang, H.; Hong, J.; Zhao, D.; et al. “Key Factor” for Baijiu Quality: Research Progress on Acid Substances in Baijiu. Foods 2022, 11, 2959. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Meng, L.-J.; Lu, Z.-M.; Chai, L.-J.; Wang, S.-T.; Shi, J.-S.; Shen, C.-H.; Xu, Z.-H. Identification of age-markers based on profiling of Baijiu volatiles over a two-year maturation period: Case study of Lu-flavor Baijiu. LWT 2021, 141, 110913. [Google Scholar] [CrossRef]
- Hoang, N.H.; Rodrigues, D.; Bonvin, D. Revisiting the concept of extents for chemical reaction systems using an enthalpy balance. Comput. Chem. Eng. 2020, 136, 106652. [Google Scholar] [CrossRef]
- Demetrius, L.; Wolf, C. Directionality theory and the second law of thermodynamics. Phys. A Stat. Mech. Its Appl. 2022, 598, 127325. [Google Scholar] [CrossRef]
- Wu, J.; Wu, W.; Liu, L.; Tong, J. Measurement of evaporation entropy, evaporation enthalpy, and Gibbs free energy for the [C4Dmim]Gly and [C4Dmim]Ala. J. Mol. Liq. 2022, 346, 117142. [Google Scholar] [CrossRef]
- Awasthi, N.; Ritschel, T.; Lipowsky, R.; Knecht, V. Standard Gibbs energies of formation and equilibrium constants from ab-initio calculations: Covalent dimerization of NO2 and synthesis of NH3. J. Chem. Thermodyn. 2013, 62, 211–221. [Google Scholar] [CrossRef]
- He, F.; Yang, S.; Zhang, G.; Xu, L.; Li, H.; Sun, J.; Huang, M.; Zheng, F.; Sun, B. Exploration of key aroma active compounds in strong flavor Baijiu during the distillation by modern instrument detection technology combined with multivariate statistical analysis methods. J. Food Compos. Anal. 2022, 110, 104577. [Google Scholar] [CrossRef]
- Viegas, L. Gas-phase OH-oxidation of 2-butanethiol: Multiconformer transition state theory rate constant with constrained transition state randomization. Chem. Phys. Lett. 2022, 803, 139829. [Google Scholar] [CrossRef]
- Zhang, S.; Lian, F.; Xue, M.; Qin, T.; Li, L.; Zhang, X.; Xu, K. Electrocatalytic Dehydrogenative Esterification of Aliphatic Carboxylic Acids: Access to Bioactive Lactones. Org. Lett. 2017, 19, 6622–6625. [Google Scholar] [CrossRef] [PubMed]
- Williams, I. Gibbs energies of activation for reacting systems with multiple reactant-state and transition-state conformations. J. Phys. Org. Chem. 2022, 35, e4312. [Google Scholar] [CrossRef]
- Perini, N.; Ticianelli, E. Oxygen evolution on gold: The effects of alkali-metal cations and iron impurities from alkaline electrolytes. J. Catal. 2019, 378, 277–282. [Google Scholar] [CrossRef]
- Osman, A.; Khodair, A.; El-Cheikh, F.; Swelim, A.; Aly, I. Studies on electrosynthesis of organic compounds: I. Electrolytic esterification in aqueous solutions. J. Appl. Chem. Biotechnol. 1975, 25, 859–861. [Google Scholar] [CrossRef]
- Yang, J.; Yuling, L.; Penghe, Z.; Hao, S.; Chuanchuan, D.; Ruihao, S.; Feifei, L. Chemical thermodynamic and catalytic mechanism analysis of Cu-BTC-derived CuOx/C catalyst for selective catalytic reduction (SCR). Mol. Catal. 2022, 531, 112710. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, T.; Su, Y.; Zheng, L.; Zeng, H.; Ren, J.; Yu, H.; Meng, P. Effective response of multiple kinetic models and DFT calculations to the ultrafast adsorption behaviors of cellulose-titanium dioxide composites. Ind. Crops Prod. 2022, 178, 114673. [Google Scholar] [CrossRef]

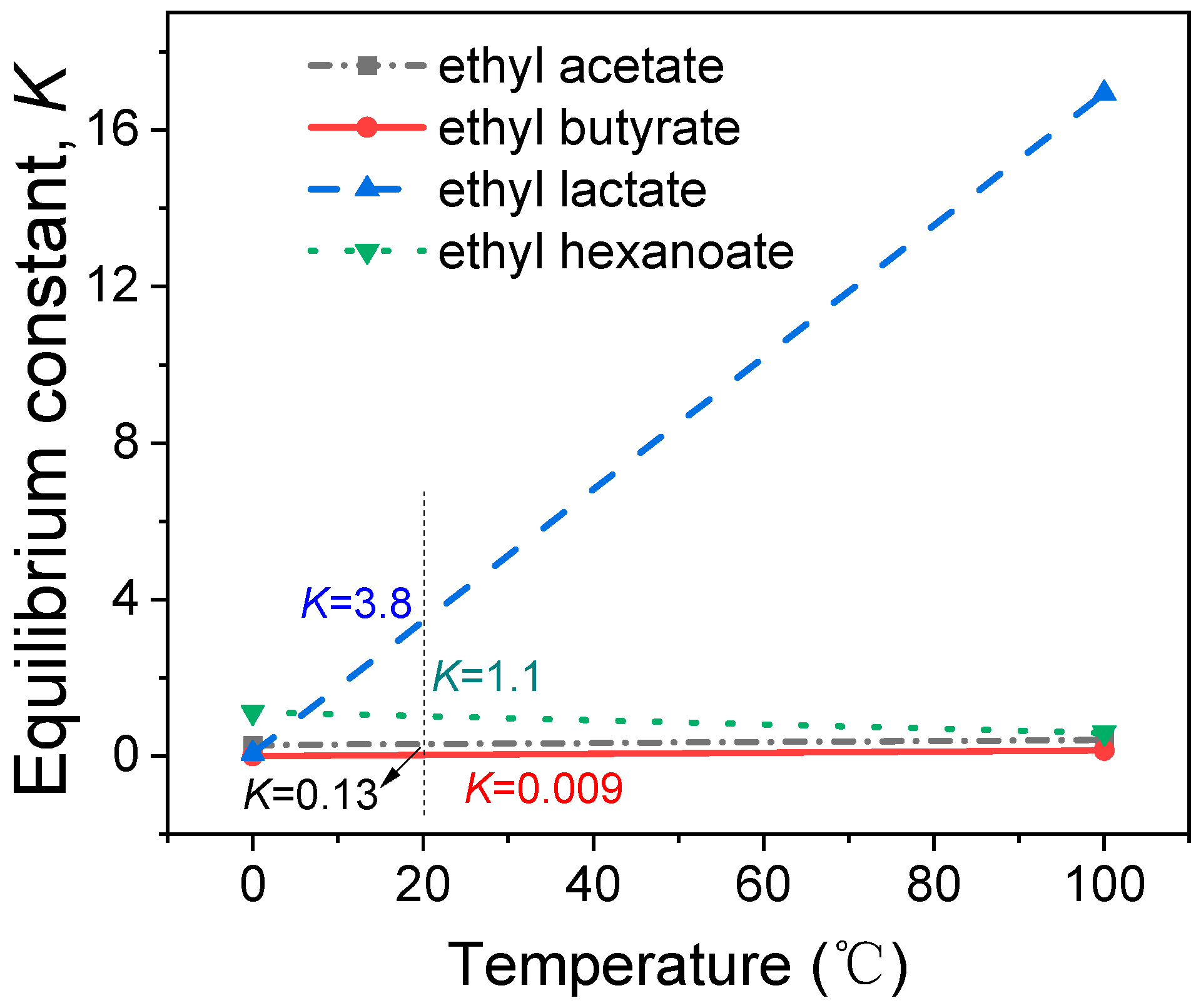

| Reaction | T | ΔH | ΔS | ΔG | K | Log K |
|---|---|---|---|---|---|---|
| °C | kcal | cal/K | kcal | |||
| C2H5OH + CH3COOH = C4H8O2 + H2O | 0.000 | −1.503 | −8.013 | 0.686 | 0.28 | −0.549 |
| 100.000 | 4.267 | 9.688 | 0.652 | 0.41 | −0.382 | |
| C2H5OH + C3H7COOH = C6H12O2 + H2O | 0.000 | 14.604 | 39.577 | 3.794 | 0.00092 | −3.036 |
| 100.000 | 4.696 | 8.784 | 1.419 | 0.147 | −0.831 | |
| C2H5OH + C3H6O3 = C5H10O3 + H2O | 0.000 | 14.611 | 48.164 | 1.454 | 0.068 | −1.164 |
| 100.000 | 7.250 | 25.051 | −2.098 | 16.94 | 1.229 | |
| C2H5OH + C5H11COOH = C8H16O2 + H2O | 0.000 | 3.840 | 14.292 | −0.064 | 1.12 | 0.051 |
| 100.000 | −8.197 | −22.997 | 0.385 | 0.59 | −0.225 |
| No. | Alcohol /Volume Ratio | Total Ester g L −1 | Ethyl Hexanoate g L −1 | Ethyl Acetate g L −1 | Ethyl Lactate g L −1 | Smell Score | Taste Score |
|---|---|---|---|---|---|---|---|
| 1 | 32% | 3.90 | 2.46 | 0.63 | 0.38 | 8.4 | 7.0 |
| 2 | 37% | 3.92 | 2.30 | 0.72 | 0.44 | 8.6 | 7.8 |
| 3 | 42% | 3.89 | 2.20 | 0.81 | 0.49 | 8.5 | 8.3 |
| 4 | 47% | 3.93 | 1.92 | 0.90 | 0.58 | 8.6 | 8.6 |
| 5 | 52% | 3.92 | 1.82 | 1.02 | 0.63 | 8.7 | 9.2 |
| 6 | 42% | 2.53 | 1.35 | 0.60 | 0.32 | 5.3 | 7.8 |
| 7 | 42% | 2.71 | 1.42 | 0.63 | 0.34 | 6.1 | 7.7 |
| 8 | 42% | 2.95 | 1.56 | 0.67 | 0.35 | 6.5 | 8.0 |
| 9 | 42% | 3.10 | 1.68 | 0.71 | 0.39 | 6.9 | 7.9 |
| 10 | 42% | 3.34 | 1.78 | 0.79 | 0.42 | 7.3 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, Q.; Yu, Y.; Zheng, Q. A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu). Catalysts 2023, 13, 478. https://doi.org/10.3390/catal13030478
Xu H, Li Q, Yu Y, Zheng Q. A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu). Catalysts. 2023; 13(3):478. https://doi.org/10.3390/catal13030478
Chicago/Turabian StyleXu, Haiyue, Qu Li, Yougui Yu, and Qing Zheng. 2023. "A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu)" Catalysts 13, no. 3: 478. https://doi.org/10.3390/catal13030478
APA StyleXu, H., Li, Q., Yu, Y., & Zheng, Q. (2023). A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu). Catalysts, 13(3), 478. https://doi.org/10.3390/catal13030478






