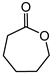
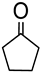
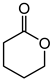
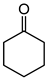
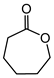
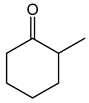
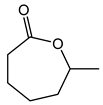
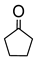
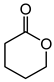
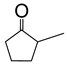
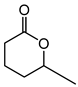
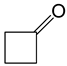
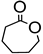
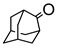
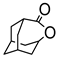
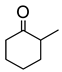
Abstract
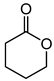
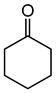
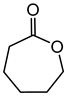
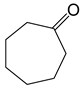
Baeyer–Villiger oxidation can synthesize a series of esters or lactones that have essential application value but are difficult to be synthesized by other methods. Cycloketones can be oxidized to lactones using molecular oxygen, peroxy acids, or hydrogen peroxide as an oxidant. Hydrogen peroxide is one of the environmental oxidants. Because of the weak oxidation ability of hydrogen peroxide, Bronsted acids and Lewis acids are used as catalysts to activate hydrogen peroxide or the carbonyl of ketones to increase the nucleophilic performance of hydrogen peroxide. The catalytic mechanisms of Bronsted acids and Lewis acids differ in the Baeyer–Villiger oxidation of cyclohexanone with an aqueous solution of hydrogen peroxide as an oxidant.
1. Introduction
The oxidation of ketones to lactones using KHSO5 as an oxidant was first reported in 1899 by Adolf Baeyer and Victor Villiger [1]. Lactones have significant commercial magnetism in the pharma, food, cosmetics, and perfumery industries [2,3]. The Baeyer–Villiger (BV) reaction can synthesize a series of esters or lactones that have essential application value but are difficult to be synthesized by other methods [4,5]. The BV reaction has been widely used in perfumes, pesticides, medicines, sterols, antibiotics, and pheromones, and for the synthesis of polymerized monomers, etc. [6,7,8,9].
Various oxidants and catalysts have been developed to improve BV oxidation. Peroxy benzoic acid, 3-chloroperbenzoic acid, pentafluoroperbenzoic acid, and trifluoroperacetic acid were the oxidants in traditional BV oxidation [10,11]. Peroxy acids produce one molecule of organic acid, the atom utilization rate is low, and the waste acid produced is difficult to handle and pollutes the environment. Current researches are focusing more attention on the replacement of the organic peroxy acids by more atom-efficient and environmental oxidants to overcome the problems. Molecular oxygen and hydrogen peroxide are more environmentally friendly oxidants, and they are used as substitutes for organic peroxy acids [12,13,14]. Although molecular oxygen and hydrogen peroxide have environmental advantages, their oxidation capacity is weak. To achieve a high conversion of ketones and high selectivity of esters or lactones, catalysts are required to catalyze the occurrence of the BV reaction of ketones [15,16,17,18]. The O-O bond in molecular oxygen is so stable that it is difficult to transfer oxygen atoms directly to ketones to form esters. Aldehydes were added as co-oxidants. Aldehydes were first oxidized by molecular oxygen to the corresponding peroxy acid, which oxidizes ketones to esters [19,20,21,22]. Although the O2/aldehydes system could avoid using peroxy acids for BV oxidation, large amounts of aldehydes were required as pro-oxidants for the oxidation of ketones, leading to the production of large amounts of organic acid [23,24]. The usage of alcohol and aldehydes is still the main drawback [25]. Developing highly efficient catalysts and green oxidants is an important issue. Hydrogen peroxide is one of the environmental oxidants. The product of hydrogen peroxide after the oxidation reaction is water. The hydrogen peroxide that does not participate in the oxidation reaction can be decomposed into water and oxygen. The oxidation ability of hydrogen peroxide is weak. It is difficult to directly oxidize ketones into esters with a high conversion rate. A high catalytic activity catalyst is required to activate hydrogen peroxide and the carbonyl of ketones to increase the nucleophilic performance of hydrogen peroxide.
The development of environmental chemical processes is required. For recyclable and high catalytic activity catalysts, Lewis acids and Bronsted acids with high catalytic activity were used as catalysts in the oxidation of cycloketones to lactones.
2. The Lewis Acid System
2.1. Homogeneous Systems
Markiton M. studied a range of metal triflates in the BV oxidation of 2-adamantanone. The results showed activity of Sn(OTf)2 was higher than other metal triflates. Both the conversion of 2-adamantanone and the yield of lactone were higher than 99% (0.67 mmol 2-adamantanone, 1.34 mmol 30 wt% H2O2, 10 mol% Sn(OTf)2, 6 mL toluene, 70 °C, 30 min). To increase the catalytic activity of Sn(OTf)2, it was immobilized on multi-walled carbon nanotubes (MWCNTs), and then hydrolyzed to triflic acid in the BV oxidation. The catalytic activity of the recycled catalyst decreased. The participation of both Lewis and Bronsted acid sites in the conversion of the ketone was postulated [26].
Ionic liquids were easily leached from porous solids during the reaction. The supported ionic liquid catalyst was prepared by a ship-in-a-bottle technique or organic-additive-instant seed technology. Modi [27] synthesized [BMIM]BF4@Co/ZSM-5 and [BMIM]BF4@Co/HY. They were tested in the BV oxidation of cyclohexanone using 30% H2O2 as an oxidant under solvent-free conditions. [BMIM]BF4@Co/HY showed high catalytic activity with the conversion of cyclohexanone being 54.88%, and the ε-caprolactone selectivity 86.36% (50 mg [BMIM]BF4@Co/HY, 0.03 mol cyclohexanone, 0.06 mol 30 wt% H2O2, 75 °C, 6 h). [BMIM]BF4@Co/HY interacts with carbonyl and hydrogen peroxide simultaneously. The nucleophile BF4− plays an essential role in the catalytic reaction. The supported ionic liquid catalysts prepared by the ship-in-a-bottle technique or organic additive-instant seed methods were unstable [28,29]. Although the selectivity of ε-caprolactone was maintained at about 80%, the activity loss is considerable after six recycling cycles.
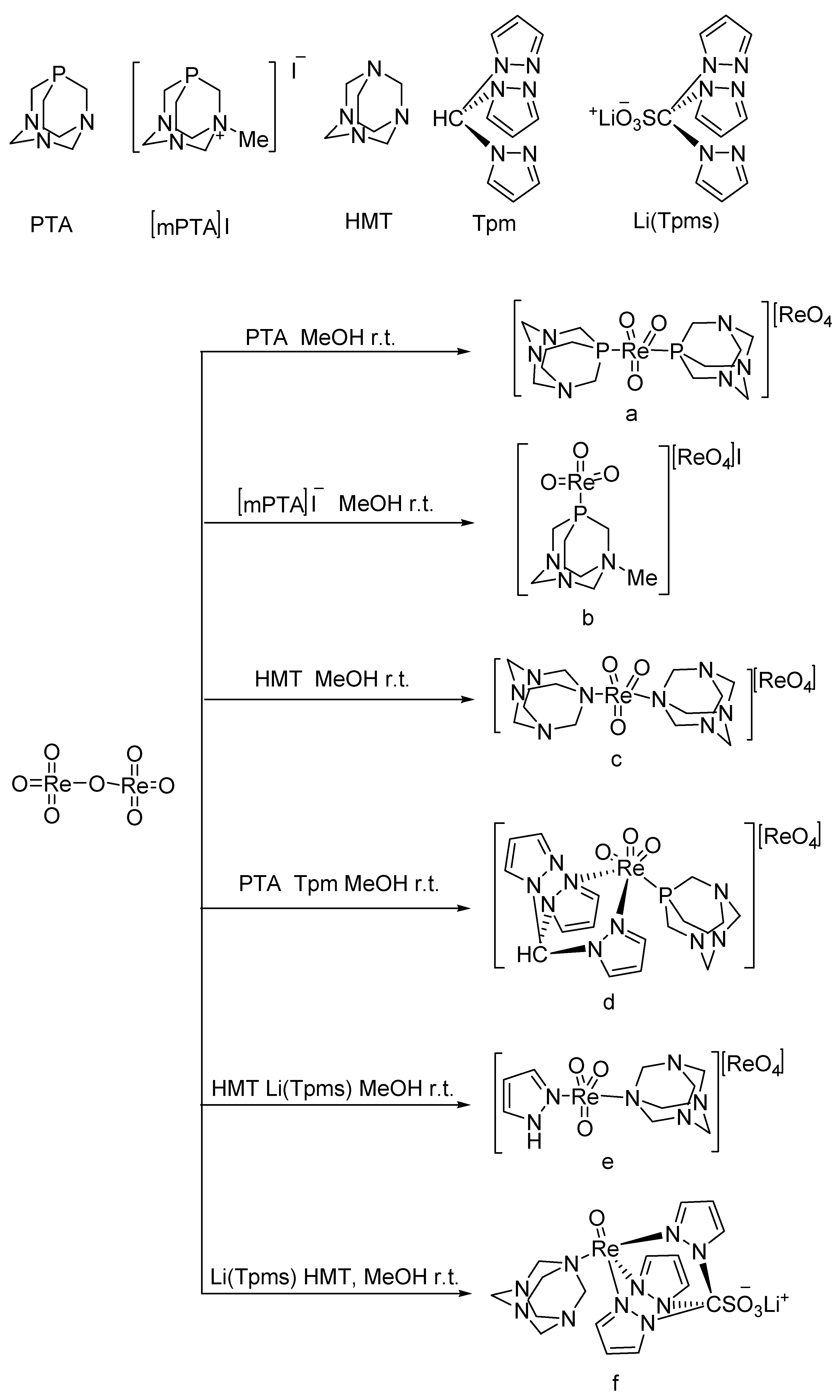
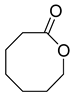
A series of oxo-rhenium complexes were prepared by reacting with Re2O7 using PTA, mPTA, HMT, Tpm, and Li(Tpms) as raw materials, respectively (Figure 1) [30]. These oxo-rhenium complexes’ catalytic activity was studied toward the BV oxidation of cyclic and linear ketones. These prepared oxo-rhenium complexes exhibit higher catalytic activity in the oxidation of ketones with H2O2 than the simple rhenium oxides K[ReO4] and Re2O7. The catalytic performances of oxo-rhenium complexes are shown Table 1. Although the oxo-rhenium complexes have good water solubility and are stable in an aqueous solution, the selectivity of the corresponding product ester is poor (<36%) when catalyzing the BV reaction of ketones.
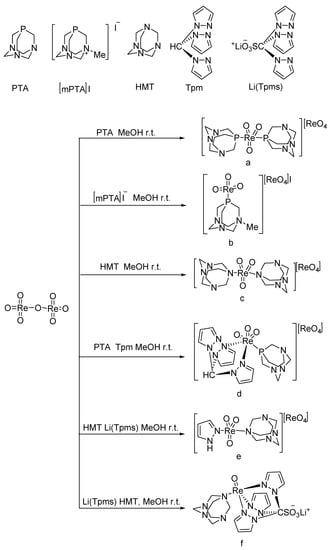
Figure 1.
Preparation of rhenium complexes [30].

Table 1.
Catalytic performance of Re complexes for the oxidation of cyclic ketones to lactones.
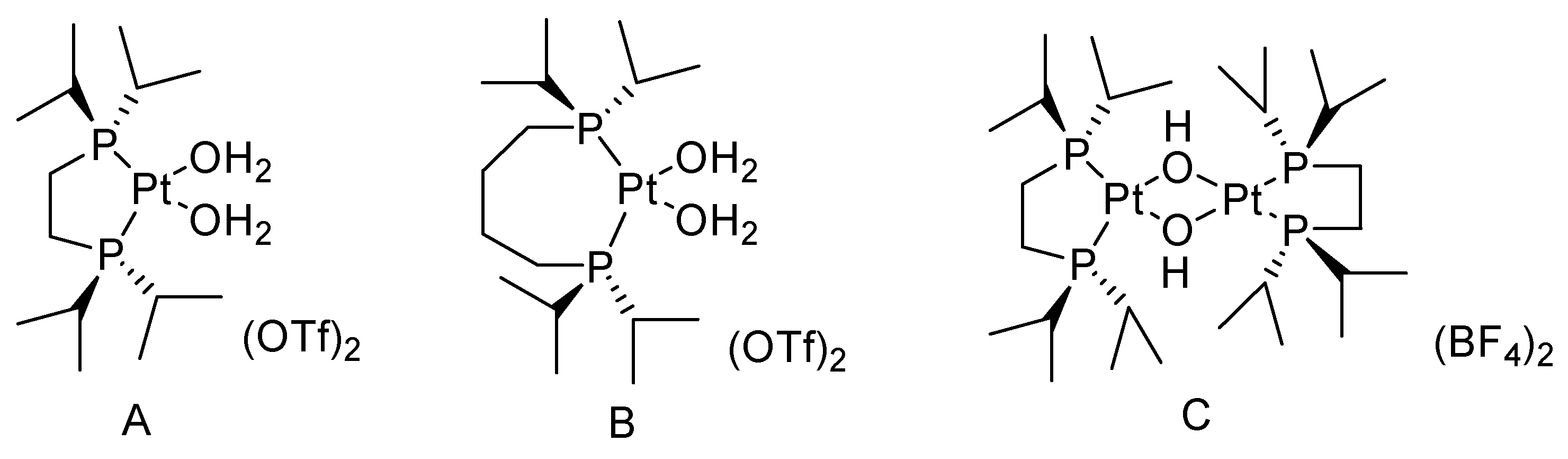
Frisone studied the BV oxidation of cyclic ketones with H2O2 catalyzed by platinum (II) complexes (Figure 2) [31]. (P-P)Pt(CF3)X (P-P = diphosphine; X = solvent, −OH) showed high activity for the BV oxidation of ketones using a commercial 32 wt% H2O2 solution. The conversion of cyclic ketones to the corresponding lactones is shown in Table 2. The solubility of the bis-cationic PtII catalyst in water led to hydrolysis with the release of one equivalent of H+. For more acid-sensitive lactones, the formation of the corresponding organic acid was increased because of H+-catalyzed hydrolysis of the intermediate lactone in water, higher temperatures, and the high catalyst loading result in lower yields of lactones.
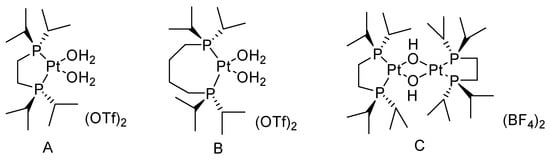
Figure 2.
Structure of the PtII water-soluble catalysts ((A): (P-P)Pt(H2O)(OTf)2 (P-P = 1,2-Bis(di-i-propylphosphino)ethane); (B): (P-P)Pt(H2O)(OTf)2 (P-P = 1,4-Bis(di-i-propylphosphino)butane); (C): (P-P)2Pt2(OH)2(BF4)2 (P-P = 1,2-Bis(di-i-propylphosphino)ethane)) [31].

Table 2.
Catalytic performance of (P-P)Pt(CF3)X for the oxidation of cyclic ketones to lactones.
2.2. Heterogeneous Systems
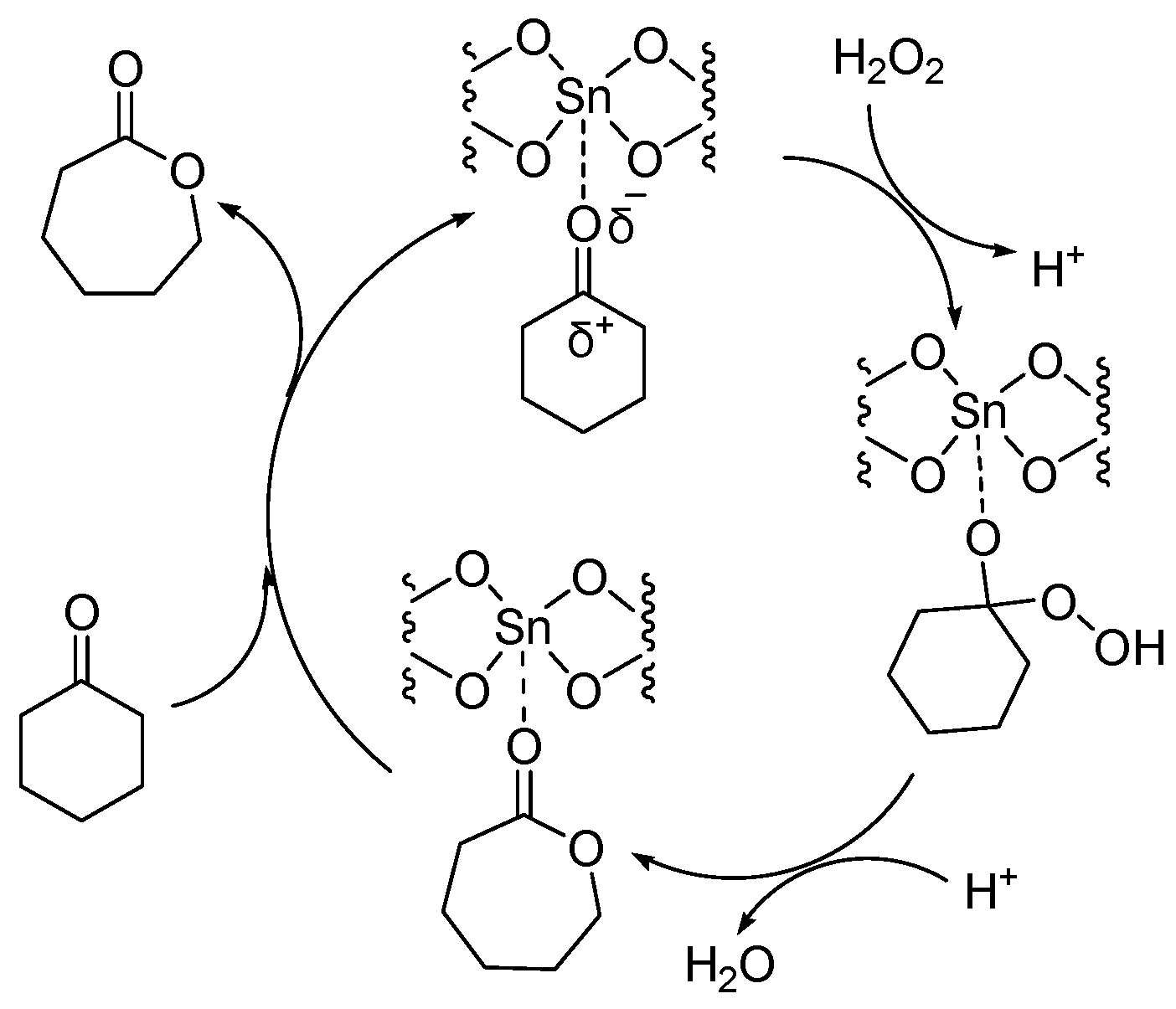
Sn-containing materials were extensively used as a highly active Lewis acid heterogeneous catalyst [32,33,34,35]. The catalytic performances of Sn-containing materials for the oxidation of cyclic ketones are shown Table 3 and Table 4. Because of the large specific surface area and various channel structures, mesoporous materials have attracted much attention in the field of catalysis. Corma A. synthesized a catalyst (Sn-zeolite beta) that had discrete and isolated Lewis sites [36]. Sn was incorporated into the zeolite framework as tetrahedrally coordinated Sn. The chemoselectivity of Sn-zeolite beta in the BV oxidation of ketones was high when using H2O2 as an oxidant. The selectivity for lactones was 100%. The mechanism of the BV reaction of cyclohexanone catalyzed by Sn-zeolite beta using aqueous hydrogen peroxide as an oxidant is shown in Figure 3. The oxygen atom in the ketone is coordinated with the Sn (Lewis acid center). Then it was easy for the oxygen atom of hydrogen peroxide to attack the carbon atom of carbonyl. The catalytic activity of Sn-zeolite beta in the BV oxidation of adamantanone and cyclohexanone was higher than the best homogeneous catalytic system. In addition, Sn-zeolite beta was filtered out and reused four times without special treatment, and its activity did not decrease in the BV oxidation of adamantanone and cyclohexanone. However, the preparation of Sn-zeolite beta was complex. The crystallization of Sn-zeolite beta takes 20 days at 140 °C in a stainless steel autoclave.
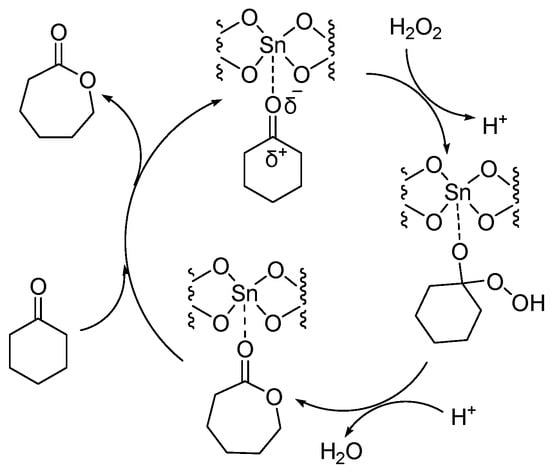
Figure 3.
Mechanism of BV reaction catalyzed by Sn-zeolite beta using aqueous hydrogen peroxide as oxidant [36].
Different tin contents of Sn-Beta zeolites were synthesized by aerosol-seed-assisted hydrothermal methods. This method reduced the amount of template and mineralizer and the reaction time. The optimal condition was obtained through several synthesis parameters (1SiO2: 0.4TEAOH: 0.4HF: 5.6H2O with 3 wt% of seeds, 423 k, 6–9 days). The synthesized catalyst of Sn-Beta zeolite was tested in the BV oxidation of cyclohexanone using H2O2 as an oxidant. With the increase in tin content, the conversion of ketone increased, and the selectivity of ε-caprolactone decreased. The amount of the extra framework Sn sites increased with the increase in tin content, which may cause the oxidation of ε-caprolactone to acid [37].
Different Sn-zeolites (Sn-MCM-68, Sn-BEA, Sn-MWW, Sn-MFI) were synthesized and evaluated in the BV oxidation of 2-adamantanone. By comparison, Sn-MCM-68 possesses more catalytic activity because of its three-dimensional channels and relatively higher hydrophobicity [38]. After the reaction, Sn-MCM-68 and Sn-BEA were separated, washed with chlorobenzene, and calcined. The catalytic activity of the regenerated catalyst was comparable to the fresh catalyst.
Han Y. synthesized a series of Mg-Sn-W composite oxides with different W contents. These catalysts were tested in the BV oxidation of cyclohexanone. The tests of BV oxidation with Mg-Sn-W oxide catalysts showed high activity, and the selectivity of caprolactone approached 90%. The tungsten oxide was the main active component. Mg-Sn-W oxide was separated by filtration and used again immediately. The catalytic activity decreased significantly (conversion of cyclohexanone is 37%, selectivity of ε-caprolactone is 75%). Mg-Sn-W oxide was separated, washed with water and ethanol, and calcined at 400 °C. Although the structure of the recycled catalyst was not destroyed, the catalytic activity was still slightly worse than the fresh catalysts. (conversion: 59% (second), 55% (third), 50% (fourth); selectivity: 83% (second), 80% (third), 76% (fourth)) [39]. A series of magnesium–aluminum hydrotalcite-like compounds were prepared to catalyze the BV oxidation of cyclohexanone using H2O2 as oxidation. The results showed that the crystal size affected the catalytic activity. Enhanced hydrophilicity of poorly crystalline HT samples facilitates the approach and activation of H2O2 on basic surface centers. The catalytic activities of HT samples were different. The conversion of cyclohexanone increased from 30 to 37%, while the selectivity of ε-caprolactone decreased from 100 to 70% [40]. In addition, a series of tin-containing compounds of Sn-MCM-56 [41], Sn/HT [42], Sn-MCM-41 [43], Sn-palygorskite [44], P-PAMAM-HBA-Sn [45], Sn[N(SO2C8F17)2]4 [46], and Sn (salen)-NaY [47] were prepared. The catalytic activity tested in the BV reaction showed as being high. They are all heterogeneous catalysts and can be regenerated. Sn-MCM-56 was separated, washed with chlorobenzene, and calcined at 500 °C for 6 h. The catalytic activity of the regenerated catalyst was almost back to the initial level (conversion of 2-adamantanone: 40% (fresh), 38%(fifth)) (0.04 g Sn-MCM-56, n(adamantanone)/n(H2O2) = 2 mol/4 mol, 56 wt% H2O2, 4.5 g chlorobenzene, 90 °C, 2 h) [41]. Sn/HT was separated, and calcined at 400 °C for 2 h. The catalytic activity of the recycled Sn/HT was the same as that of the fresh one [42]. The catalytic activity of the regenerated P-PAMAM-HBA-Sn was lower than the fresh one (conversion of 2-adamantanone: 100% (fresh), 95 (second), 90 (third), 85 (fourth), 80% (fifth)) [45]. The catalytic activity of the regenerated Sn[N(SO2C8F17)2]4 was almost back to the initial level (conversion of 2-adamantanone: 93% (fresh), 92% (second), 90% (third), 90% (fourth)) [46]. Sn (salen)-NaY was separated by filtration and washed with 1,4-dioxane. The catalytic activity of the recycled Sn (salen)-NaY was the same as that of the fresh one (conversion of cyclohexanone: 75% (fresh), 74% (second), 75% (third)) [47]. The catalytic activity of Sn-bentonite formed by a simple procedure is low. It is necessary to add additives to improve the yield of lactones [48]. In addition, the toxicity of organotin compounds and tin chloride is high, and can induce DNA lesions. Although the toxicity of inorganic tin is low, its preparation uses tin chloride as a raw material [49]. Therefore new active, selective, and recyclable catalysts are still required, and a heterogeneous catalyst is an excellent option.

Table 3.
Catalytic performances of Sn-containing materials for the oxidation of cyclohexanone and adamantanone.
Table 3.
Catalytic performances of Sn-containing materials for the oxidation of cyclohexanone and adamantanone.
| Ketone | Product | Catalyst | Reaction Conditions | Conversion (%) | Yield (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
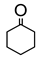 | 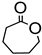 | Sn-zeolite beta | n(cyclohexanone)/n(H2O2) = 1.5 mol/1 mol, 35 wt% H2O2, 90 °C, 3 h | - | 52 | 98 | [36] |
| Sn-Beta-166 (Si/Sn = 166) | 50 mg catalyst; n(cyclohexanone)/n(H2O2) = 2 mol/3 mol, 30 wt% H2O2, 8 mL 1,4-dioxane, 80 °C, 3 h. | 29 | - | 68 | [37] | ||
| Sn-Beta-125 (Si/Sn = 125) | 50 mg catalyst; n(cyclohexanone)/n(H2O2) = 2 mol/3 mol, 30 wt% H2O2, 8 mL 1,4-dioxane, 80 °C, 3 h. | 36 | - | 66 | [37] | ||
| Sn-Beta-100 (Si/Sn = 100) | 50 mg catalyst; n(cyclohexanone)/n(H2O2) = 2 mol/3 mol, 30 wt% H2O2, 8 mL 1,4-dioxane, 80 °C, 3 h. | 39 | - | 62 | [37] | ||
| Mg-Sn-W | 96 g acetic acid, 75 g butyl acetate, 57 g cyclohexane, 54 g H2O2 (50 wt%), 60 °C—0.05 MPa, 5 h | 90 | - | 90 | [39] | ||
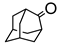 | 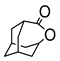 | Sn-zeolite beta | n(adamantanone)/n(H2O2) = 1 mol/1 mol, 35 wt% H2O2, 56 °C, 6 h | - | 94 | 98 | [36] |
| Sn-MCM-68 | 0.05 g catalyst; n(adamantanone)/n(H2O2) = 2 mol/4 mol, 30 wt% H2O2, 90 °C, 1 h | 78.1 | - | 99 | [38] | ||
| Sn-BEA | 0.05 g catalyst; n(adamantanone)/n(H2O2) = 2 mol/4 mol, 30 wt% H2O2, 90 °C, 1 h | 65.2 | - | 99 | [38] | ||
| Sn-MWW | 0.05 g catalyst; n(adamantanone)/n(H2O2) = 2 mol/4 mol, 30 wt% H2O2, 90 °C, 1 h | 32 | 99 | [38] | |||
| Sn-MFI | 0.05 g catalyst; n(adamantanone)/n(H2O2) = 2 mol/4 mol, 30 wt% H2O2, 90 °C, 1 h | 20.1 | - | 99 | [38] | ||
| Sn-MCM-56 | 0.04 g Sn-MCM-56, n(adamantanone)/n(H2O2) = 2 mol/4 mol, 56 wt% H2O2, 4.5 g chlorobenzene, 90 °C, 4 h | 55 | - | - | [41] |

Table 4.
Catalytic performances of Sn-containing materials for the oxidation of cycloketones.
Table 4.
Catalytic performances of Sn-containing materials for the oxidation of cycloketones.
| Ketone | Product | Reaction Conditions | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
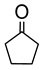 | 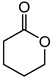 | 0.25 g catalyst; n(ketone)/n(H2O2) = 12.5 mol/50 mol, 30 wt% H2O2, 10 mL acetonitrile, 70 °C, 4 h [42]. | 100 [42] | 16 [42] |
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.15 mmol, 30% H2O2, 3 mL 1,4-dioxane, 70 °C, 24 h [44]. | 81 [44] | 100 [44] | ||
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.2 mmol, 30% H2O2, 3 mL 1.2-dichloroethane, 70 °C, 15 h [45]. | 99 [45] | 100 [45] | ||
| 0.03 mol catalyst, n(ketone)/n(H2O2) = 2 mmol/2 mmol, 35% H2O2, 3 mL 1.2-dichloroethane, 50 °C, 5 h [46]. | 70 [46] | 82 [46] | ||
| 50 mg catalyst, 1 g ketone, 2 mL tert-BuOOH; 5 mL 1,4-dioxane, 70 °C, 12 h [47]. | 100 [47] | 99 [47] | ||
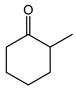 | 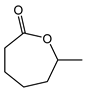 | 0.25 g catalyst; n(ketone)/n(H2O2) = 12.5 mol/50 mol, 30 wt% H2O2, 10 mL acetonitrile, 70 °C, 4 h [42]. | 100 [42] | 42 [42] |
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.15 mmol, 30% H2O2, 3 mL 1,4-dioxane, 90 °C, 24 h [44]. | 80 [44] | 100 [44] | ||
| 50 mg catalyst, 1 g ketone, 2 mL tert-BuOOH; 5 mL 1,4-dioxane, 90 °C, 24 h [47]. | 80 [47] | 99 [47] | ||
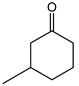 | 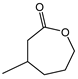 | 0.25 g catalyst; n(ketone)/n(H2O2) = 12.5 mol/50 mol, 30 wt% H2O2, 10 mL acetonitrile, 70 °C, 4 h [42]. | 100 [42] | 32 [42] |
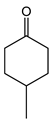 | 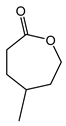 | 0.25 g catalyst; n(ketone)/n(H2O2) = 12.5 mol/50 mol, 30 wt% H2O2, 10 mL acetonitrile, 70 °C, 4 h [42]. | 100 [42] | 26 [42] |
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.15 mmol, 30% H2O2, 3 mL 1,4-dioxane, 90 °C, 24 h [44]. | 44 [44] | 100 [44] | ||
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.2 mmol, 30% H2O2, 3 mL ethanol, 70 °C, 15 h [45]. | 85 [45] | 100 [45] | ||
| 50 mg catalyst, 1 g ketone, 2 mL tert-BuOOH; 5 mL 1,4-dioxane, 90 °C, 24 h [47]. | 85 [47] | 99 [47] | ||
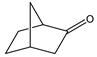 | 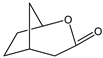 | 50 mg catalyst, n(ketone)/n(H2O2) = 1.0 mmol/1.5 mmol, 35 wt% H2O2, 3.00 g dioxane, 90 °C, 7 h [43]. | 71 [43] | 98 [43] |
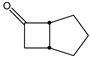 | 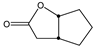 | 50 mg catalyst, 250 mg ketone, 150 mg H2O2, 50 wt% H2O2, 3.00 g dioxane, 40 °C, 7 h [43]. | 62 [43] | 98 [43] |
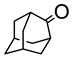 | 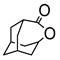 | 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.15 mmol, 30% H2O2, 3 mL nitrobenzene, 90 °C, 24 h [44]. | 100 [44] | 100 [44] |
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.2 mmol, 30% H2O2, 3 mL 1,4-dioxane, 90 °C, 15 h [45]. | 99 [45] | 100 [45] | ||
| 0.01 mol catalyst, n(ketone)/n(H2O2) = 2 mmol/2 mmol, 35% H2O2, 3 mL 1.2-dichloroethane, 25 °C, 2 h [46]. | 94 [46] | 99 [46] | ||
| 50 mg catalyst, 1 g ketone, 2 mL tert-BuOOH; 5 mL 1,4-dioxane, 90 °C, 20 h [47]. | 100 [47] | 99 [47] | ||
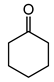 | 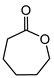 | 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.15 mmol, 30% H2O2, 3 mL 1,4-dioxane, 70 °C, 24 h [44]. | 16 [44] | 90 [44] |
| 3 mg catalyst, n(ketone)/n(H2O2) = 0.1 mmol/0.2 mmol, 30% H2O2, 3 mL 1.2-dichloroethane, 70 °C, 15 h [45]. | 70 [45] | 100 [45] | ||
| 0.01 mol catalyst, n(ketone)/n(H2O2) = 2 mmol/2 mmol, 35% H2O2, 3 mL 1.2-dichloroethane, 25 °C, 4 h [46]. | 73 [46] | 66 [46] | ||
| 50 mg catalyst, 1 g ketone, 2 mL tert-BuOOH; 5 mL 1,4-dioxane, 70 °C, 24 h [47]. | 75 [47] | 90 [47] |
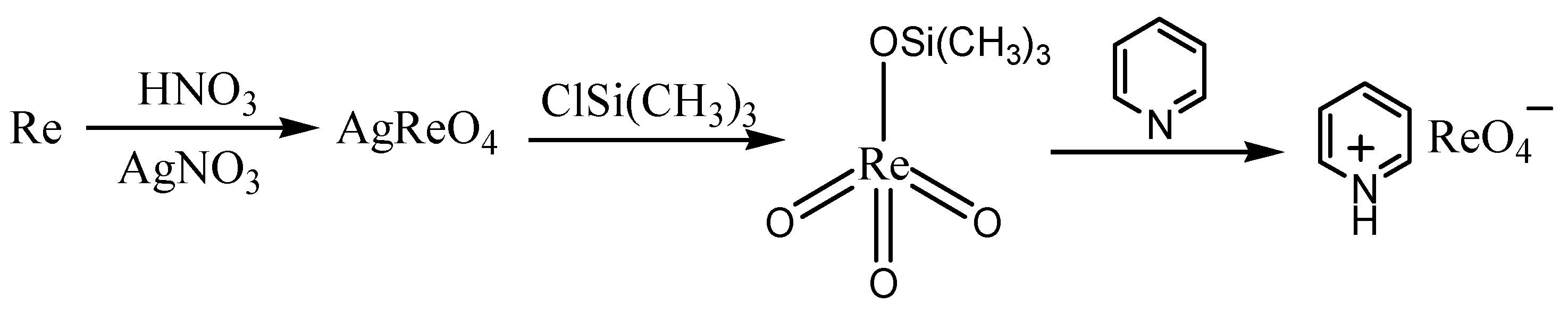
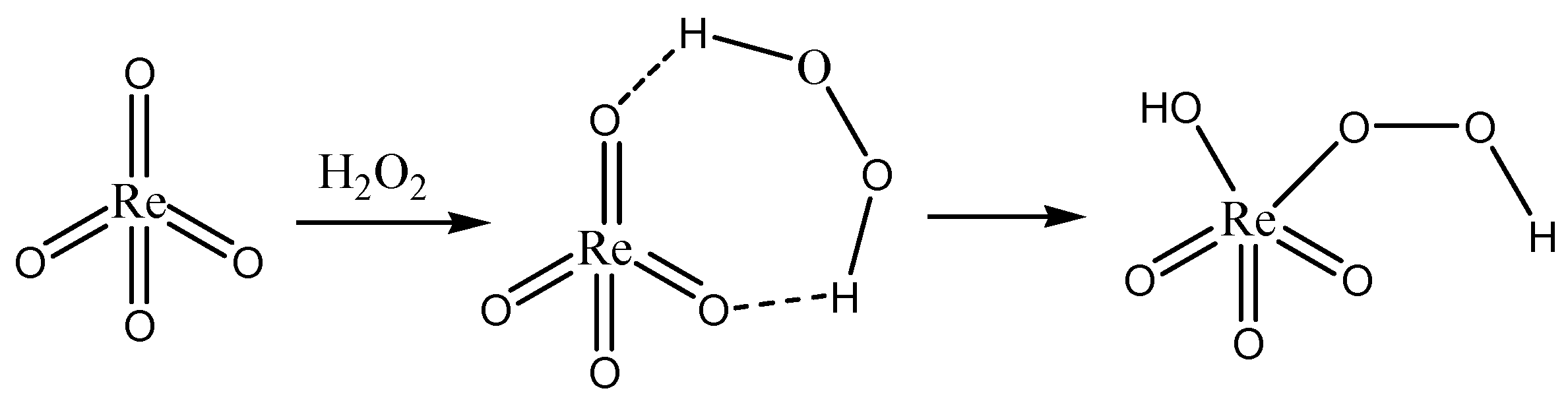
The synthesis of pyridinium perrhenate is shown in Figure 4 [50]. PyHReO4 was used to catalyze the BV oxidation of 2-adamantanone using 30% H2O2 as an oxidant, and the catalytic activity was high (yield: 90.02%; selectivity > 99%). PyHReO4 was separated by silica gel column chromatography. The catalytic activity of PyHReO4 in the oxidation of 2-adamantanone is attributed to the interaction between Re and hydrogen peroxide (Figure 5).
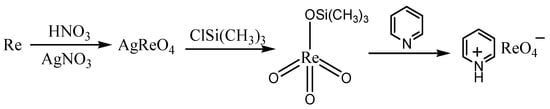
Figure 4.
Synthesis of pyridinium perrhenate [50].
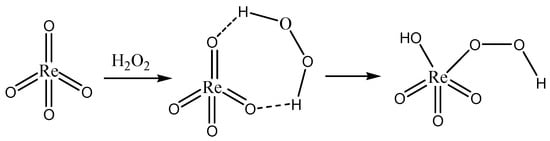
Figure 5.
Interaction between Re and hydrogen peroxide [50].
Silver supported on montmorillonite clay (Ag-NPs@mont) was synthesized and used as active catalysts in the BV oxidation of ketones with hydrogen peroxide as an oxidant under the solvent-free condition at room temperature [51]. The Ag-NPs@mont nanocatalyst was separated by filtration, washed with acetone, and dried. The recycled Ag-NPs@mont nanocatalyst was reused four times without significant loss of catalytic activity under the same conditions. The conversion of cyclohexanone was 99% under solvent-free conditions. The results in the oxidation of various ketones showed that the conversion of cyclic ketones was higher than aromatic ketones because aromatic ketones are more stable than cycloketones.
The magnetic composite Fe3O4@I-SR was prepared by loading Fe3O4 nanoparticles onto a porous illite silicon slag (I-SR) [52]. Fe3O4 nanoparticles were evenly distributed over the I-SR. Fe3O4@I-SR showed high catalytic activity in the oxidation of cyclohexanone. The conversion of cyclohexanone and the selectivity of ε-caprolactone were higher than 99%. Fe3O4@I-SR was separated, washed with acetone and ethanol, and dried. The catalytic activity of the recovered Fe3O4@I-SR was almost the same as that of the fresh one (conversion of cyclohexanone: 99% (fifth); selectivity of ε-caprolactone: 99% (fifth)). Fe3O4@I-SR can activate both the ketone group and H2O2, which can be used as catalysts to improve oxidation efficiency. Fe3O4@GO also showed high catalytic activity and selectivity for the BV oxidation of cyclohexanone without solvent [53].
Priya prepared mixed oxides of MoLaCeSm-Si [54]. MoLaCeSm-Si catalyst showed good activity and selectivity simultaneously in the oxidation of cyclohexanone to ε-caprolactone at room temperature. MoLaCeSm-Si was washed with acetone, dried, and activated at 400 °C for 1 h. The activity of the recovered MoLaCeSm-Si was not lost after six cycles. The conversion of cyclohexanone in the first cycle was 83.1%, and the selectivity of ε-caprolactone was 88.9%. The conversion of cyclohexanone was 79.1%, and the selectivity of ε-caprolactone was 81.2%, with the catalyst of MoLaCeSm-Si recovered from the sixth cycle. MoO3, La2O3, CeO2, and Sm2O3 in MoLaCeSm-Si each contribute their characteristic properties to increase the catalytic activity towards the oxidation of cyclohexanone at room temperature. Mo6+ in MoLaCeSm-Si provides the Lewis acidic center, [55] lanthanum induces primary sites on MoLaCeSm-Si [56], and ceriasamaria increases the oxygen vacancies [57] and performs the role of oxygen carrier between H2O2 and cyclohexanone.
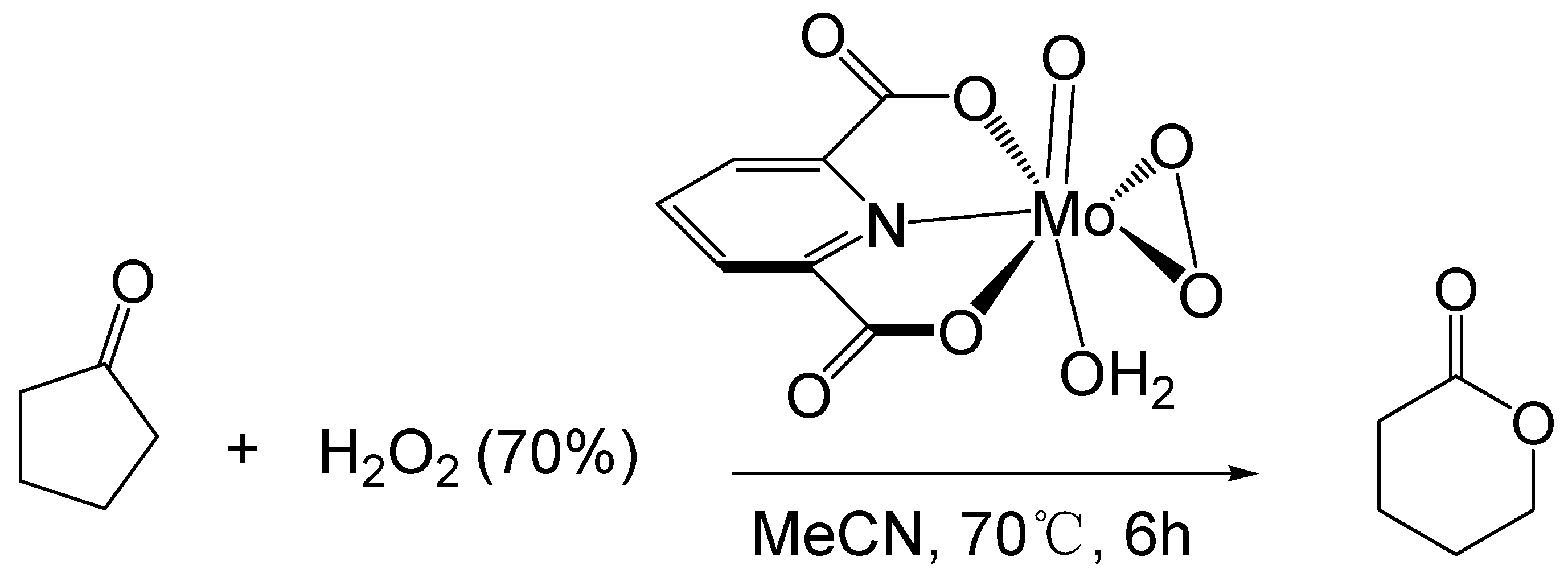
Furia [58] studied the BV reaction of ketones catalyzed by transition metals. They used a 70% hydrogen peroxide solution as an oxidant and a molybdenum peroxy complex as a catalyst to oxidize cyclopentanone to δ-valerolactone (Figure 6). The maximum conversion of cyclopentanone is 65%, and the maximum yield of δ-valerolactone is 54%. (0.09 M catalyst, 1.4 M cyclopentanone, 2.2 M H2O2, 70 °C, 6 h)
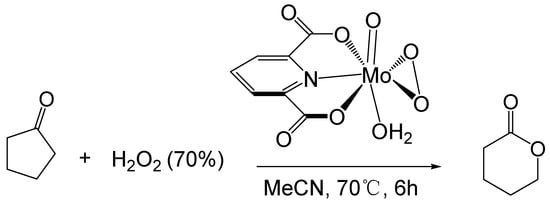
Figure 6.
Oxidation of cyclic ketones catalyzed by molybdenum peroxy complex [58].
MoO3, WO3, and [{γ-SiW10O32(H2O)2}2(μ-O)2]4− have high catalytic activity and selectivity in the BV oxidation of cyclic ketones [59,60]. The catalytic activity of the recycled [{γ-SiW10O32(H2O)2}2(μ-O)2]4− was the same as that of the fresh one (yields of lactone: 99% (third run)). The conversion of cyclic ketones and the yields of corresponding lactones are shown in Table 5. Cyclic ketones with tertiary carbon atoms adjacent to the ketone carbonyl group are more likely to undergo the BV reaction than cyclic ketones with secondary carbon atoms adjacent to the ketone carbonyl group, and the selectivity for the formation of corresponding lactones is also higher.

Table 5.
Catalytic performances of different catalysts for the oxidation of cyclic ketones to lactones.
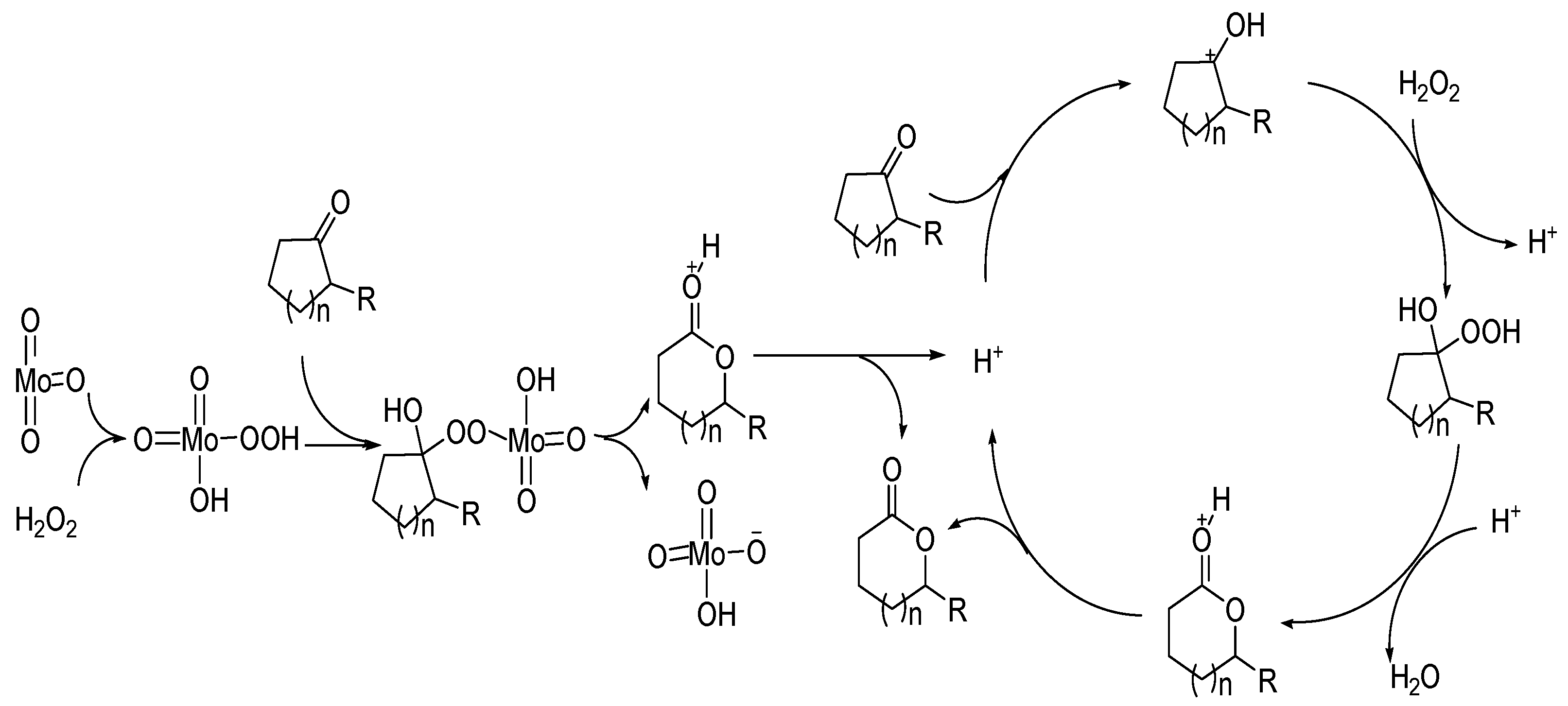
The high catalytic activity of MoO3 is due to its reaction with hydrogen peroxide to form peroxy acids. The possible reaction process is shown in Figure 7.
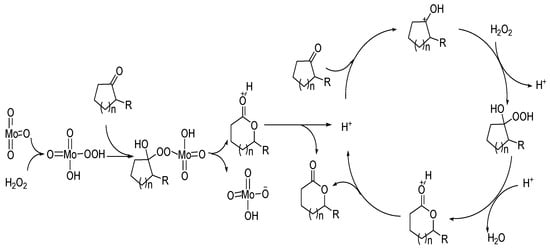
Figure 7.
A possible mechanism for Baeyer–Villiger oxidation over transition metal oxides [59].
The catalytic mechanisms of Bronsted acids and Lewis acids are different. The good catalytic performance of transition metal oxides can be attributed to the coordination of the Lewis acid center of metal with carbonyl oxygen, and the reaction with hydrogen peroxide to form peroxy acids.
3. The Bronsted Acid System
3.1. Homogeneous Systems
Bronsted acid showed high catalytic activity in the BV oxidation. The conversion of 2-heptylcyclopentanone to δ-dodecalactone was catalyzed by different Bronsted acids (CH3COOH, CF3COOH, CH3SO3H, TsOH, H3PW12O40, and H3PMo12O40) using aqueous hydrogen peroxide (30 wt%) as oxidant [61]. The catalytic activity of H3PW12O40 was higher than the other acids. Although the catalytic activity was affected by acid strength, it was not the only factor. Although the conversion of cyclopentanone, cyclohexanone, and cycloheptanone was high, the selectivity for the corresponding lactones was very poor. The low selectivity of H3PW12O40 was ascribed to its acid sites, which catalyzed the hydrolysis and oxidation of lactones, leading to the formation of acids. It is difficult to separate H3PW12O40 from the reaction system.
The study by Mouheb L. found the Keggin-type polyoxometalates H3PMo12O40, H2.98Sn0.1PMo12O40, Cs1.5Sn0.75PMo12O40, Cs2.14Sn0.43PMo12O40, and Cs3PMo12O40 involve acidic sites of the Bronsted and Lewis type [62]. The catalytic activity of POMs was tested in the homogenous oxidation of cyclohexanone using hydrogen peroxide as an oxidant. The products of the reaction are adipic (AA), glutaric (GA), succinic (SA) acids, butane-1,3-diol (Diol), and ε-caprolactone (ε-CL). The results showed that the whole Keggin-type polyoxometalates have a high catalytic activity (conversion of cyclohexanone is 98–100%) (Table 6) [62]. The substrate was activated by the Bronsted acidity [63]. H3PMo12 was oxidized to {PO4[MoO(O2)2]4}3−, {PMo3Om}n− and {PO4[MoO(O2)2]2}2−, peroxo species in the presence of H2O2 [64]. POM oxygen atoms were replaced by the “peroxo” (O22−) from the hydrogen peroxide, Peroxo ions are distributed in the Keggin anion without disturbing its structure. The good catalytic performance of POMs was attributed to the formation of Peroxo-POM.

Table 6.
Catalytic performances of POMs for cyclohexanone oxidation.
3.2. Heterogeneous Systems
KHPW was prepared with a potassium content of 1.3 wt%. Although the chemical structure of KHPW was similar to that of HPW, it has better thermal stability and can be separated from the reaction solution by filtration. After repeated use three times in the oxidation of 2-heptylcyclopentanone, the catalytic activity of KHPW did not decrease significantly. In the BV oxidation of 2-adamantanone and cyclododecanone, the corresponding lactones are obtained in good yields [65]. The oxidation of cyclic ketones to corresponding lactones with 30% H2O2 in the presence of HPW and KHPW is shown in Table 7.

Table 7.
Catalytic performances of HPW and KHPW for the oxidation of cyclic ketones to lactones.
Keggin-type heteropolyacids (HPAs) always show higher catalytic activity than common acids. HPAs have strong acidity and redox capacity, and their crystal structures are adjustable [66]. HPAs have been used in many reactions such as hydrations [67], isomerization [68,69], esterification [70], Fries rearrangement [71], Friedel–Crafts acylation [72], and etherification [73], etc. Three mesoporous materials were prepared by loading silicotungstic acids on SBA-15, MCM-41, and MCM-48 [66]. These prepared materials can be recycled and showed excellent catalytic activity in the BV oxidation of cyclic ketones using 30% H2O2 aqueous solution as oxidant. The conversion of cyclic ketones to the corresponding lactones was up to 90% under the optimum reaction conditions. Cyclic ketones were easily introduced into the hole of MCM-41 and SBA-15, so the catalytic activity of HSiW/MCM-41 and HSiW/SBA-15 was higher than HSiW/MCM-48 in the BV oxidation.
Ionic liquids are often used as green solvents or catalysts because of their low volatility, good thermal stability, good solubility, and convenient separation at room temperature. Bronsted acidic heteropolyanion-based ionic liquid ([MIMPS]3PW12O40) exhibited higher catalytic activity in the BV reaction of cyclohexanone to produce ε-caprolactone. The phosphotungstic anion (PW12O403−) was successfully modified by the organic precursor MIMPS, and SO3H was introduced into the catalyst ([MIMPS]3PW12O40). MIMPS]3PW12O40 could be dissolved in the reaction system as a liquid, then precipitated after the reaction [74]. The conversion of cyclohexanone was 93.51%, the yield of ε-caprolactone was 93.26%, and the selectivity of ε-caprolactone was 99.73% under optimum reaction conditions (0.8 g [MIMPS]3PW12O40, n (H2O2): n (cyclohexanone) = 6: 1, 25 g cyclohexane, 70 °C, 1 h). Tungsten and SO3H play a very crucial role in promoting the yield of ε-caprolactone because tungsten and SO3H are oxidized to peroxyacid in the existence of H2O2. The W element in [MIMPS]3PW12O40 showed a noticeable loss after several cycle tests. This leads to catalyst deactivation. Stabilizing the active components of the catalyst is a critical problem to be solved in catalyst preparation.
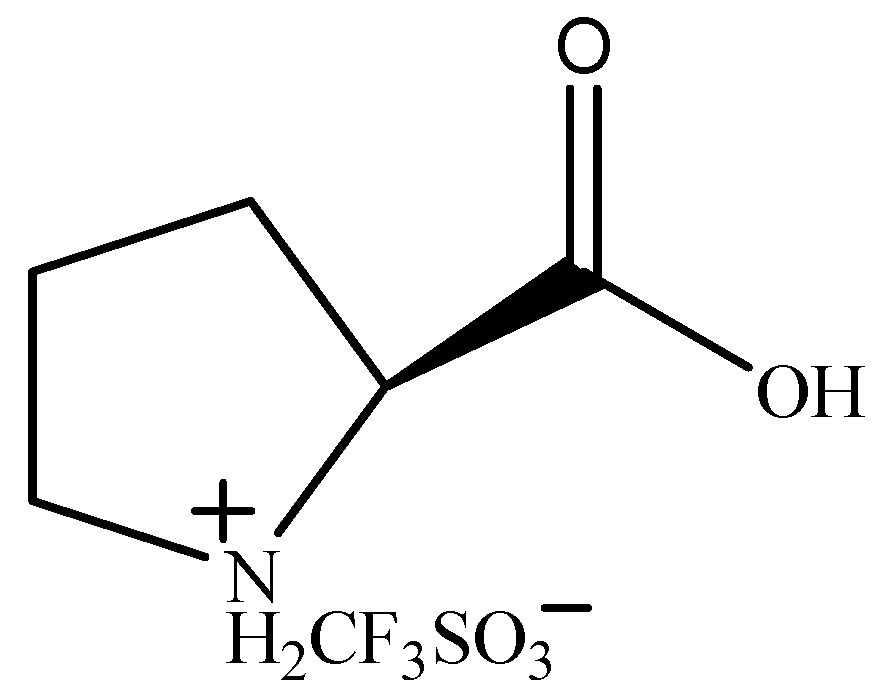
Yu F.L. synthesized a series of amino-acid-based ionic liquids. (Figure 8) [75] They were used as catalysts for the BV oxidation of cyclopentanone. The proline-based ionic liquid [ProH]CF3SO3 showed the best catalytic activity. Under the optimum conditions (n(cyclopentanone):n(catalyst):n(H2O2) = 1:0.06:4, 60 °C, 6 h), the conversion of cyclopentanone was 96.57%, and the selectivity for δ-valerolactone was 73.01%. The catalytic activity of [ProH]CF3SO3 in the BV oxidation of cyclopentanone increases with increasing acidity, but the increase in acidity can lead to the hydrolysis of δ-valerolactone. The carbonyl was activated by H+ to facilitate the oxidation of hydrogen peroxide. The possible reaction process is shown in Figure 9.
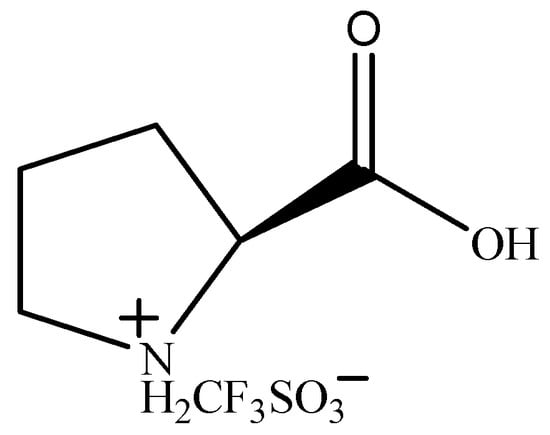
Figure 8.
Structure of [ProH]CF3SO3 [75].
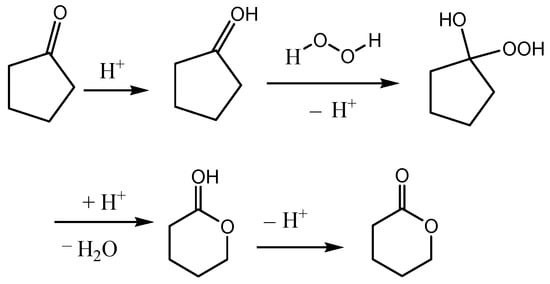
Figure 9.
A possible mechanism for Baeyer–Villiger oxidation over Bronsted acid [75].
Bronsted acid catalysts of silica/A153–SO3H [76], silica-VTMO–OSO3H [77], and Fe–Co/SPS [78] were prepared. The acidity of silica/A153–SO3H was 2.34 mmol g−1. The acidity of silica-VTMO–OSO3H was 1.39 mmol g−1. The catalytic activity of [ProH]CF3SO3, silica/A153–SO3H, silica-VTMO–OSO3H, and Fe–Co/SPS in the BV oxidation of various cyclic ketones with 30% H2O2 is shown in Table 8.

Table 8.
Oxidation of cyclic ketones to lactones.
4. Conclusions
The BV oxidation of cycloketones could be catalyzed by Bronsted acids and Lewis acids. The obtained catalysts have different catalytic activities in the BV oxidation of cycloketones using hydrogen peroxide as the oxidant. The catalytic mechanisms of Bronsted acids and Lewis acids are different. The excellent catalytic performance of Lewis acids can be attributed to the coordination of the Lewis acid center of metal with carbonyl oxygen. The high catalytic activity of Bronsted acids is due to their reaction with hydrogen peroxide to form peroxy acids. The carbonyl was also activated by H+ to facilitate oxidation.
In the oxidation of cyclohexanone, the catalytic activity and selectivity of Lewis acids are higher than those of Bronsted acids. The low selectivity of Bronsted acids is ascribed to their acid sites, which catalyzed the hydrolysis and oxidation of ε-caprolactone. Among the Lewis acid catalysts mentioned in this paper, the catalytic activity and selectivity of the heterogeneous catalysts are higher than those of the homogeneous catalysts. In particular, the heterogeneous catalysts can be easily recycled and reused, and the catalytic activity and the selectivity of the recycled catalysts are almost the same as those of the fresh catalysts. The catalytic activity and the selectivity of Ag-NPs@montnano, Fe3O4@I-SR, and Mg-Sn-W are higher than those of other catalysts, but the preparation of Mg-Sn-W uses the toxic tin chloride, the preparation is complex, and it is not economical to use Ag-NPs@montnano as a catalyst. Fe3O4@I-SR is a better choice in the BV oxidation of cyclohexanone.
Author Contributions
Conceptualization, X.P.; writing—original draft preparation, Q.M.; writing—review and editing, Y.X. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi, grant number 2019L0919. This work was also supported by the Fund for Shanxi “1331 Project” and the National Natural Science Fund of China (grant number 22072105).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hannes, L.; Krista, M.; Peter, C.; Lau, K. Baeyer-Villiger monooxygenases: More than just green chemistry. Chem. Rev. 2011, 111, 4165–4222. [Google Scholar]
- Syed, N.; Singh, S.; Chaturvedi, S.; Nannaware, A.D.; Khare, S.K.; Rout, P.K. Production of lactones for flavoring and pharmacological purposes from unsaturated lipids: An industrial perspective. Crit. Rev. Food Sci. 2022, 62, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.F.; Wang, C.C.; Han, F.; Zhang, Z.W. Synthesis of the ester side chains of homoharringtonine and harringtonine using lactones as building blocks. Synth. Commun. 2021, 51, 317–323. [Google Scholar] [CrossRef]
- Diot-Neant, F.; Mouterde, L.M.M.; Couvreur, J.; Brunois, F.; Miller, S.A.; Allais, F. Green synthesis of 2-deoxy-D-ribonolactone from cellulose-derived levoglucosenone (LGO): A promising monomer for novel bio-based polyesters. Eur. Polym. J. 2021, 159, 110745. [Google Scholar] [CrossRef]
- Xue, A.; Gao, P.P.; Zhao, S.S.; Zhu, L.; You, X.T.; Li, C.Y.; Zhang, Q.R.; Shan, L.H. Biotransformation of androst-4-ene-3,17-dione by three fungal species Fusarium solani BH1031, Aspergillus awamori MH18 and Mucor circinelloides W12. Nat. Prod. Res. 2021, 35, 428–435. [Google Scholar]
- Xu, H.; Jiang, J.; Yang, B.; Wu, H.; Wu, P. Effective Baeyer-Villiger oxidation of ketones over germanosilicates. Catal. Commun. 2014, 55, 83–86. [Google Scholar] [CrossRef]
- Mallin, H.; Wulf, H.; Bornscheuer, U.T. A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ε-caprolactone from cyclohexanol. Enzym. Microb. Technol. 2013, 53, 283–287. [Google Scholar] [CrossRef]
- Heinrich, M.R.; Steglich, W. Effective syntheses of quinoline-7,8-diol, 5-amino-L-DO-PA, and 3-(7,8-dihydroxyquinolin-5-yl)-L-alanine. Tetrahedron 2003, 59, 9231–9237. [Google Scholar] [CrossRef]
- Wright, S.E.; Clarkson, A.; Korns, J.M.; Haljun, E.; Lofving, L.; Ourgessa, M.; Getzler, Y.D.Y.L. An efficient synthesis of the inimer gamma-(2-bromo-2-methylpropionate)-epsilon-caprolactone (BMPCL). Green Chem. Lett. Rev. 2022, 15, 683–688. [Google Scholar] [CrossRef]
- Jenner, G. High pressure mechanistic diagnosis in Baeyer-Villiger oxidation of aliphatic ketone. Tetrahedron Lett. 2001, 42, 8969–8970. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Egorova, T.M.; Aminov, R.I.; Dzhemilev, U.M. Pentafluoroperbenzoic acid as the efficient reagent for Baeyer-Villiger oxidation of cyclic ketones. Mendeleev Commun. 2018, 28, 644–645. [Google Scholar] [CrossRef]
- Yakabi, K.; Mathieux, T.; Milne, K.; Lopez-Vidal, E.M.; Buchard, A.; Hammond, C. Continuous Production of Biorenewable, Polymer-Grade Lactone Monomers through Sn-b-Catalyzed Baeyer-Villiger Oxidation with H2O2. ChemSusChem 2017, 10, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Olszowka, J.; Karcz, R.; Napruszewska, B.D.; Duraczynska, D.; Gawel, A.; Bahranowski, K.; Serwicka, E.M. Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile over hydrotalcite-like catalysts: Effect of Mg/Al ratio on the ε-caprolactone yield. Catal. Commun. 2017, 100, 196–201. [Google Scholar] [CrossRef]
- Guan, F.F.; Ma, T.T.; Yuan, X.; Zeng, H.Y.; Wu, J. Sn-Modified NaY Zeolite Catalysts Prepared by Post-Synthesis Methods for Baeyer-Villiger Oxidation. Catal. Lett. 2018, 148, 443–453. [Google Scholar] [CrossRef]
- Ogibin, Y.N.; Alexandre, O.; Terent, A.O.; Kutkin, A.V.; Nikishin, G.I. A rearrangement of 1-hydroperoxy-2-oxabicycloalkanes into lactones of -acyloxy-(-3)-hydroxyalkanoic acids related to the Criegee reaction. Tetrahedron Lett. 2002, 43, 1321–1324. [Google Scholar] [CrossRef]
- Iyer, P.; Ghosh, S.K. An expeditious synthesis of a silylethanol anchoring group by a silicon directed Baeyer-Villiger oxidation on solid-phase. Tetrahedron Lett. 2002, 43, 9437–9440. [Google Scholar] [CrossRef]
- Borah, J.M.; Chowdhury, P. Expedited Baeyer-Villiger oxidation of steroidal ketones by microwave irradiation. Steroids 2011, 76, 1341–1345. [Google Scholar] [CrossRef]
- Demir, A.S.; Aybey, A. Synthesis of chiral acetoxy lactones via the Baeyer-Villiger oxidation of cyclic aromatic acetoxy ketones. Tetrahedron 2008, 64, 11256–11261. [Google Scholar] [CrossRef]
- Staudt, S.; Bornscheuer, U.T.; Menyes, U.; Hummel, W.; Gröger, H. Direct biocatalytic one-pot-transformation of cyclohexanol with molecular oxygen into ε-caprolactone. Enzym. Microb. Technol. 2013, 53, 288–292. [Google Scholar] [CrossRef]
- Zarrabi, S.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Baeyer-Villiger oxidation of cyclic ketones utilizing potassium peroxydisulfate (K2S2O8) or sodium perborate (NaBO3) in acidic media. Chin. Chem. Lett. 2009, 20, 1400–1404. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Sinha, A.; Pahari, S.K.; Sutradhar, N.; Bajaj, H.C.; Panda, A.B. Room temperature Baeyer-Villiger oxidation using molecular oxygen over esoporous zirconium phosphate. Catal. Sci. Technol. 2012, 2, 2375–2382. [Google Scholar] [CrossRef]
- Liu, G.; Sun, L.; Luo, W.; Yang, Y.; Liu, J.H.; Wang, F.; Guild, C.J. Aerobic Baeyer-Villiger oxidation of ketones over mesoporous Mn-Ce and Mn-Co composite oxides in the presence of benzaldehyde: The effect of valence state. Mol. Catal. 2018, 458, 9–18. [Google Scholar] [CrossRef]
- Zheng, C.M.; Chang, S.B.; Yang, C.W.; Lian, D.Y.; Ma, C.; Zhang, C.R.; Fan, X.R.; Xu, S.X.; Sun, X.H. Enhanced shape selective catalysis of mixed cyclic ketones in aerobic Baeyer-Villiger oxidation with magnetic Cu-Fe3O4 supported mesoporous silica microspheres. Tetrahedron 2018, 74, 2608–2616. [Google Scholar] [CrossRef]
- Hazra, S.; Martins, N.M.R.; Mahmudov, K.; Zubkov, F.I.; Silva, M.F.C.G.; Pombeiro, A.J.L. A tetranuclear diphenyltin(IV) complex and its catalytic activity in the aerobic Baeyer-Villiger oxidation of cyclohexanone. J. Organomet. Chem. 2018, 867, 193–200. [Google Scholar] [CrossRef]
- Du, R.F.; Yuan, H.R.; Zhao, C.X.; Wang, Y.T.; Yao, J.; Li, H.R. ε-Caprolactone manufacture via efficient coupling Baeyer-Villiger oxidation with aerobic oxidation of alcohols. Mol. Catal. 2020, 490, 110947. [Google Scholar] [CrossRef]
- Markitona, M.; Szelwicka, A.; Boncelb, S.; Jurczykc, S.; Chrobok, A. Superactive tin(II) triflate/carbon nanotube catalyst for the Baeyer-Villiger oxidation. Appl. Catal. Gen. 2018, 556, 81–91. [Google Scholar] [CrossRef]
- Modi, C.K.; Panwala, S.; Vithalani, R.; Patel, D. Ionic liquid infiltrated within metal loaded zeolites for Baeyer-Villiger oxidation reaction under solvent-free condition. J. Porous Mater. 2018, 25, 871–883. [Google Scholar] [CrossRef]
- Ntais, S.; Moschovi, A.M.; Paloukis, F.; Neophytides, S.; Burganos, V.N.; Dracopoulos, V.; Nikolakis, V.J. Preparation and ion transport properties of NaY zeolite–ionic liquid composites. J. Power Sources 2011, 196, 2202. [Google Scholar] [CrossRef]
- Yu, Y.; Mai, J.; Huang, L.; Wang, L.; Li, X. Ship in a bottle synthesis of ionic liquids in NaY supercages for CO2 capture. RSC Adv. 2014, 4, 12756. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Piotr, S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Oxorhenium complexes bearing the water-soluble tris(pyrazol-1-yl)methanesulfonate, 1,3,5-triaza-7-phosphaadamantane, or related ligands, as Catalysts for Baeyer-Villiger oxidation of ketones. Inorg. Chem. 2013, 52, 4534–4546. [Google Scholar] [CrossRef]
- Frisone, M.D.T.; Pinna, F.; Strukul, G. Lactonization of cyclic ketones with hydrogen peroxide catalyzed by platinum(II) complexes. Stud. Surf. Sci. Catal. 1991, 66, 405–410. [Google Scholar]
- Zhu, Z.G.; Ma, H.K.; Li, M.T.; Su, T.; Lv, H.Y. Fluoride-free hydrothermal synthesis of nanosized Sn-Beta zeolite via interzeolite transformation for Baeyer-Villiger oxidation. Appl. Catal. Gen. 2020, 590, 117370. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A.; Renz, M.; Sastre, G.; Pedro, M.; ViruelaHazra, P.M. A Multisite Molecular Mechanism for Baeyer-Villiger Oxidations on Solid Catalysts Using Environmentally Friendly H2O2 as Oxidant. Chem. Eur. J. 2005, 11, 6905–6915. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Yuan, H.R.; Liu, H.; Li, J.B.; Wang, Y.; Wang, Y.; Yao, J.; Li, H.R. One-pot Baeyer-Villiger oxidation of cyclohexanone with in situ generated hydrogen peroxide over Sn-Beta zeolites. Green Chem. Eng. 2021, 2, 294–300. [Google Scholar] [CrossRef]
- Alavijeh, M.K.; Amini, M.M. Tin and copper species dispersed on a metal-organic framework as a new catalyst in aerobic Baeyer-Villiger oxidation: An insight into the mechanism. Catal. Commun. 2020, 139, 105985. [Google Scholar] [CrossRef]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef]
- Meng, Q.R.; Liu, J.X.; Xiong, G.; Liu, X.Y.; Liu, L.P.; Guo, H.C. Aerosol-seed-assisted hydrothermal synthesis of Sn-Beta zeolite and its catalytic performance in Baeyer-Villiger oxidation. Microporous Mesoporous Mater. 2018, 266, 242–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Y.L.; Tang, K.; Xu, W.; Lu, X.Q.; Ma, R.; Fu, Y.H.; Zhu, W.D. Role of the pore-opening structure and hydrophobicity of stannosilicate zeolites in Baeyer-Villiger oxidation. J. Catal. 2021, 394, 8–17. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.N.; Ding, R.; Xu, W.J.; Zhang, G.X. Baeyer-Villiger oxidation of cyclohexanone catalyzed by cordierite honeycomb washcoated with Mg-Sn-W composite oxides. Chin. J. Chem. Eng. 2019, 27, 564–574. [Google Scholar] [CrossRef]
- Olszowka, J.E.; Karcz, R.; Napruszewska, B.D.; Michalik-Zym, A.; Duraczynska, D.; Ciak-Czerwenka, J.K.; Niecikowska, A.; Bahranowski, K.; Serwicka, E.M. Effect of Mg-Al hydrotalcite crystallinity on catalytic Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile. Catal. Commun. 2018, 107, 48–52. [Google Scholar] [CrossRef]
- Liu, G.Q.; Jiang, J.G.; Yang, B.; Fang, X.Q.; Xu, H.; Peng, H.G.; Xu, L.; Liu, Y.M.; Wu, P. Hydrothermal synthesis of MWW-type stannosilicate and its post-structural transformation to MCM-56 analogue. Microporous Mesoporous Mater. 2013, 165, 210–218. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Sn-exchanged hydrotalcites as catalysts for clean and selective Baeyer-Villiger oxidation of ketones using hydrogen peroxide. J. Mol. Catal. Chem. 2003, 191, 93–100. [Google Scholar] [CrossRef]
- Corma, A.; Navarro, M.T.; Renz, M. Lewis acidic Sn(IV) centers-grafted onto MCM-41—As catalytic sites for the Baeyer-Villiger oxidation with hydrogen peroxide. J. Catal. 2003, 219, 242–246. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, Q.; Luo, J.; He, X. Baeyer-Villiger oxidation of ketones with hydrogen peroxide catalyzed by Sn-palygorskite. Tetrahedron Lett. 2005, 46, 3505–3508. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Yang, Z.; Hu, Z.; Lei, Z. Baeyer-Villiger oxidation of ketones with hydrogen peroxide catalyzed by cellulose-supported dendritic Sn complexes. Catal. Commun. 2007, 8, 1202–1208. [Google Scholar] [CrossRef]
- Hao, X.; Yamazaki, O.; Yoshida, A.; Nishikido, J. Tin(IV) bis(perfluoroalkanesulfonyl)-amide complex as a highly selective Lewis acid catalyst for Baeyer-Villiger oxidation using hydrogen peroxide in a fluorous recyclable phase. Tetrahedron Lett. 2003, 44, 4977–4980. [Google Scholar] [CrossRef]
- Dutta, B.; Jana, S.; Bhunia, S.; Honda, H.; Koner, S. Heterogeneous Baeyer-Villiger oxidation of cyclic ketones using tert-BuOOH as oxidant. Appl. Catal. Gen. 2010, 382, 90–98. [Google Scholar] [CrossRef]
- Ma, Q.G.; Li, L.; Cao, Y.X.; Peng, X.H. Sn-bentonite-induced Baeyer-Villiger oxidation of 2-heptylcyclopentanone to d-dodecalactone with aqueous hydrogen peroxide. Res. Chem. Intermed. 2015, 41, 2249–2256. [Google Scholar] [CrossRef]
- Ferancova, A.; Adamovski, M.; Gründler, P.; Zima, J.; Barek, J.; Mattusch, J.; Wennrich, R.; Labuda, J. Interaction of tin(II) and arsenic(III) with DNA at the nanostructure film modified electrodes. Bioelectrochemistry 2007, 71, 33–37. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Zhang, D.; Li, Y.C.; Dong, Y.N.; Wang, S.J.; Song, S.W.; Wang, J.; Wang, G. Rhenium-containing compound(PyHReO4): Synthesis, characterization and catalytic application in olefin epoxidation and baeyer-villiger oxidation. J. Chem. Sci. 2022, 134, 3. [Google Scholar] [CrossRef]
- Borah, S.J.; Das, D.K. Efficient Baeyer-Villiger oxidation catalysed by silver nanoparticles stabilized on modified montmorillonite. Catal. Lett. 2018, 148, 3669–3677. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, D.D.; Liu, Y.; Chen, S.; Meng, W.; Jiang, N.Z. Study on synthesis of acid-washed illite supported Fe3O4 nanometer catalyst and Baeyer-Villiger oxidation reaction of cyclohexanone. Catal. Lett. 2019, 149, 1111–1117. [Google Scholar] [CrossRef]
- Xiao, G.S.; Gao, X.; Yan, W.T.; Wu, T.; Peng, X.H. Baeyer-Villiger Oxidation of cyclohexanone by hydrogen peroxide with Fe3O4@GO as catalyst under solvent free conditions. Catal. Lett. 2019, 149, 1765–1771. [Google Scholar] [CrossRef]
- Selva Priya, A.; Sunaja Devi, K.R.; Mohan, M.K.; Sugunan, S. Role of mesoporous silica supported mixed oxides of ceria and samaria for the synthesis of ε-caprolactone at room temperature. J. Porous Mater. 2020, 27, 1027–1038. [Google Scholar] [CrossRef]
- Gonzalez, J.; Wang, J.A.; Chen, L.; Manríquez, M.; Salmones, J.; Limas, R.; Arellano, U. Quantitative determination of oxygen defects, surface lewis acidity, and catalytic properties of mesoporous MoO3/SBA-15 catalysts. J. Solid State Chem. 2018, 263, 100–114. [Google Scholar] [CrossRef]
- Sugunan, S.; Sherly, K.B. Basicity and electron donor properties of lanthanum oxide and its mixed oxided with alumina. Indian J. Chem. 1993, 32, 689–692. [Google Scholar]
- Li, J.G.; Ikegami, T.; Mori, T. Solution-based processing of Sc2O3 nanopowders yielding transparent ceramics. J. Mater. Res. 2004, 19, 733–736. [Google Scholar] [CrossRef]
- Campestrini, S.; Furia, F.D. Baeyer-Villiger oxidation of ketones by peroxomolybd-enum complexes: A revisit. J. Mol. Catal. 1993, 79, 13–19. [Google Scholar] [CrossRef]
- Ma, Q.G.; Xing, W.Z.; Xu, J.H.; Peng, X.H. Baeyer-Villiger oxidation of cyclic ketones with aqueous hydrogen peroxide catalyzed by transition metal oxides. Catal. Commun. 2014, 53, 5–8. [Google Scholar] [CrossRef]
- Yoshida, A.; Yoshimura, M.; Uehara, K.; Hikichi, S.; Mizuno, N. Formation of S-shaped disilicoicosatungstate and efficient Baeyer-Villiger oxidation with hydrogen peroxide. Angew. Chem. Int. Ed. 2006, 45, 1956–1960. [Google Scholar] [CrossRef]
- Ma, Q.G.; Zhao, J.R.; Xing, W.Z.; Peng, X.H. Baeyer-Viiliger oxidation of cyclic ketones using aqueous hydrogen peroxide catalyzed by heteropolyacids in solvent-free system. J. Adv. Oxid. Technol. 2014, 17, 212–216. [Google Scholar] [CrossRef]
- Mouheb, L.; Dermeche, L.; Essayem, N.; Rabia, C. Keggin-Type mixed polyoxomolybdates catalyzed cyclohexanone oxidation by hydrogen peroxide: In situ IR pyridine adsorption. Catal. Lett. 2020, 150, 3327–3334. [Google Scholar] [CrossRef]
- Amitouche, D.; Haouas, M.; Mazari, T.; Mouanni, S.; Canioni, R.; Rabia, C.; Marchal-Roch, C. The primary stages of polyoxomolybdate catalyzed cyclohexanone oxidation by hydrogen peroxide as investigated by in situ NMR. Substrate activation and evolution of the working catalyst. Appl. Catal. 2018, 561, 104–116. [Google Scholar] [CrossRef]
- Moudjahed, M.; Dermeche, L.; Benadji, S.; Mazari, T.; Rabia, C. Dawson-type polyoxometalates as green catalysts for adipic acid synthesis. J. Mol. Catal. Chem. 2016, 414, 72–77. [Google Scholar] [CrossRef]
- Ma, Q.G.; Xing, W.Z.; Xu, J.H.; Peng, X.H. Baeyer-Villiger oxidation of cyclic ketones using aqueous hydrogen peroxide catalyzed by potassium salts of tungstophosphoric acid. Chem. Lett. 2014, 43, 941–943. [Google Scholar] [CrossRef]
- Hu, S.P.; Niu, L.T.; Wei, Y.L.; Chen, L.N.; Yang, Z.W. Catalytic properties of mesoporous materials supported heteropoly acids for Baeyer-Villiger oxidation of cyclic ketones. Mol. Phys. 2020, 118, e1759832. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Prasad, P.S.S.; Lingaiah, N. Solvent-free hydration of alkynes over a heterogeneous silver exchanged silicotungstic acid catalyst. Green Chem. 2012, 14, 1507–1514. [Google Scholar] [CrossRef]
- Pinto, T.; Arquilliere, P.; Dufaud, V. Isomerization of n-hexane over Pt-H3PW12O40/SBA-15 bifunctional catalysts: Effect of the preparation method on catalytic performance. Appl. Catal. Gen. 2016, 528, 44–51. [Google Scholar] [CrossRef]
- Alazman, A.; Belic, D.; Kozhevnikova, E.F. Isomerisation of n-hexane over bifunctional Pt-heteropoly acid catalyst: Enhancing effect of gold. J. Catal. 2018, 357, 80–89. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, H.Y.; Shi, W.; Cheng, M.X.; Wang, X.H.; Li, S.W. A Bronsted-Lewis-surfactant-combined heteropolyacid as an environmental benign catalyst for esterification reaction. Catal. Commun. 2012, 20, 103–106. [Google Scholar] [CrossRef]
- Yadav, G.D.; George, G. Single step synthesis of 4-hydroxybenzophenone via esterification and Fries rearrangement: Novelty of cesium substituted heteropoly acid supported on clay. J. Mol. Catal. Chem. 2008, 292, 54–61. [Google Scholar] [CrossRef]
- Ammar, M.; Jiang, S.; Ji, S. Heteropoly acid encapsulated into zeolite imidazolate framework (ZIF-67) cage as an efficient heterogeneous catalyst for Friedel-Crafts acylation. J. Solid State Chem. 2016, 233, 303–310. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G. Cesium exchanged tungstophosphoric acid supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]
- Li, X.S.; Liu, S.H.; Chen, Y.; Zhang, G.X. Brønsted acidic Heteropolyanion-Based ionic liquid: A highly efficient reaction-induced self-separation catalyst for Baeyer-Villiger reaction. Tetrahedron Lett. 2022, 105, 154042. [Google Scholar] [CrossRef]
- Yu, F.L.; Chi, Y.J.; Gao, C.; Chen, R.R.; Xie, C.X.; Yu, S.T. Baeyer-Villiger Oxidation of cyclic ketones catalyzed by amino acid ionic liquids. Chem. Res. Chin. 2020, 36, 865–869. [Google Scholar] [CrossRef]
- Xing, W.Z.; Ma, Q.G.; Peng, X.H. Silica/A153-SO3H: An efficient catalyst for oxidation of cyclic ketones with hydrogen peroxide. Cr–Chim. 2015, 18, 581–585. [Google Scholar] [CrossRef]
- Xing, W.Z.; Ma, Q.G.; Xu, J.H.; Peng, X.H. Baeyer-Villiger oxidation of cyclic ketones with hydrogen peroxide catalyzed by silica–VTMO–OSO3H. J. Porous Mater. 2015, 22, 487–492. [Google Scholar] [CrossRef]
- Wang, Y.T.; Huang, J.F.; Xia, X.M.; Peng, X.H. Fe-Co/sulfonated polystyrene as an efficient and selective catalyst in heterogeneous Baeyer-Villiger oxidation reaction of cyclic ketones. J. Saudi Chem. Soc. 2018, 22, 129–135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).