Abstract
Biomass-derived carbons are emerging materials with a wide range of catalytic properties, such as large surface area and porosity, which make them ideal candidates to be used as heterogeneous catalysts and catalytic supports. Their unique physical and chemical properties, such as their tunable surface, chemical inertness, and hydrophobicity, along with being environmentally friendly and cost effective, give them an edge over other catalysts. The biomass-derived carbon materials are compatible with a wide range of reactions including organic transformations, electrocatalytic reactions, and photocatalytic reactions. This review discusses the uses of materials produced from biomass in the realm of heterogeneous catalysis, highlighting the different types of carbon materials derived from biomass that are potential catalysts, and the importance and unique properties of heterogeneous catalysts with different preparation methods are summarized. Furthermore, this review article presents the relevant work carried out in recent years where unique biomass-derived materials are used as heterogeneous catalysts and their contribution to the field of catalysis. The challenges and potential prospects of heterogeneous catalysis are also discussed.
1. Introduction
Over the years, catalysis scientists have long been interested in creating new, cutting-edge approaches to deal with problems related to pollution, sustainable energy, and climate change. In order to produce the desired product and achieve chemical specificity, catalysis makes it feasible to alter the speeds at which chemical bonds are created or broken [1]. Heterogeneous catalysis, which is one of the significant wings of catalysis, is the engine that powers numerous significant industrial processes due to its excellent capacity to accelerate the reaction at a low cost, high conversion rates, higher selectivity, easier separation, and recyclability. Since the heterogeneous catalysts possess these unique properties, a lot of importance is given to their tuning to make them better as they enable the green and sustainable manufacturing of more than 80% of the world’s chemicals [2,3,4]. The first few reported heterogeneous catalysts that were of industrial significance were those used in the Haber–Bosch process for the formation of ammonia, with iron as a catalyst; in the Ostwald’s process for the preparation of nitric acid, with platinum as a catalyst; and in the contact process for the formation of sulfuric acid, with vanadium pentoxide as a catalyst [5,6,7].
Beginning with using metal and metal oxides as heterogeneous catalysts, the use of nanomaterials since the 21st century has brought a real breakthrough in heterogeneous catalysis. In recent years, biowastes have been regarded as the most suitable material that can be used as carbon precursors for synthesizing a variety of highly valuable carbon structures, thereby turning waste into wealth. These materials are environmentally friendly and cost effective. Moreover, considering resource recycling and sustainability, natural biowaste serves as a promising material as compared to other carbon-rich precursors such as polymers, organic complexes [8], and carbohydrates [9]. The physical and mechanical characteristics of the bioprecursors will be reflected in the carbon-based materials that are produced: soft, compressible, and low-density biowastes, such as rice husks, ground nuts, peanut shells, soybean shells, etc., will produce low-density and flexible carbons; hard and dense biowastes, such as nut shells and fruit stones, will produce hard, high-density carbons [10]. Despite the fact that various carbon-based materials derived from lignocellulosic biomass, such as carbon nanospheres, activated carbon, porous carbons, carbon nanotubes, and so on are currently being investigated as heterogeneous catalysts in the fields of electrocatalysis, photocatalysis, and organic transformation (Figure 1), research in this area has a wide scope in terms of exploration specially in the domain of organic reactions.
Activated carbon is the most widely used carbon support derived from biomass to bring about various organic transformations on both the research and industrial scale. Other recently developed biomass-derived materials such as porous carbons and carbon nanotubes possess a better surface area, thermal stability, greater adsorption capacity, and electrical conductivity due to their small size and enhanced porosity, and they are still very new in the field of heterogeneous catalysis and need more optimization to replace their contemporaries, which are derived from synthetic carbon precursors [11]. However, since biomass is a renewable material, it is important to merge the gap between commonly used synthetic carbon precursors such as polymers with the biomass to synthesize materials such as porous carbon spheres and carbon nanotubes and utilize them in heterogeneous catalysis as the step towards sustainability. In this review, we will discuss the developments that took place in the area of biomass-derived materials in heterogeneous catalysis, starting with the various carbon materials designed and synthesized; the various techniques used in the catalyst preparation; their applications as heterogeneous catalysts in the organic transformation reaction, electrocatalysis, and photocatalysis; and also their future prospects.
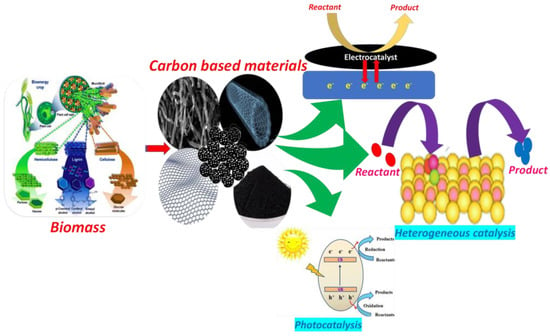
Figure 1.
Graphical illustration of various biomass-derived materials in heterogeneous catalysis [12,13].
2. Potential Biomass-Based Materials for Heterogeneous Catalysis
A wide range of functionalized heterogeneous catalysts, such as carbon materials, zeolites, metal-oxide-supported metals, magnetic iron oxides, metal–organic frameworks, solid phase ionic liquids, mesoporous silica, and organic polymers, have been created [14]. Carbon materials have been actively investigated over the last three decades in the disciplines of nanotechnology, materials science, and as support for active phases in catalysis due to its fascinating features relating to texture, conductivity, stability, and hydrophobicity.
Carbon materials have shown promise as both catalytic supports and metal-free active phase catalysts. A wide range of essential catalytic properties are present in them, including (i) a sizable high surface area, (ii) outstanding hydrothermal stability, (iii) effective crosslinking, (iv) adaptable surface composition and porous structures, and (v) chemical resistance in an acidic or alkaline medium [15]. Catalytic active phases have been stabilized using a variety of common carbon materials, including carbon black, carbon nanotubes, pyrolytic carbon, activated carbon, and carbon generated from polymers. The production of highly distributed metallic particles (Pd, Cu, Ni, Fe, Ag, Ru, etc. [16]) all across the catalytic matrices is made possible by the rich surface chemistry and large specific area of these carbon materials, which increase resistance to sintering even at high temperatures.
In view of sustainability, since biomass is a natural and renewable source of carbon, it can be extensively used to synthesize carbon structures that are potential candidates to bring about catalysis. Figure 2 illustrates the various carbon materials produced from biomass as precursors. Activated carbon, biochar, carbon nanotubes, carbon nanospheres, etc. are such carbon materials that are now being synthesized from biomass and finding their application in various reactions as catalysts and catalyst supports.
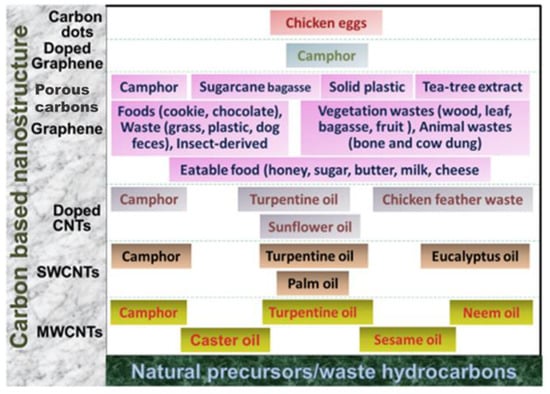
Figure 2.
Schematic representation of the natural precursors, eatable food, and waste hydrocarbons used for the synthesis of various carbon-based materials. Adapted with permission from ref. [17].
2.1. Activated Carbon
It is challenging to produce heterogeneous catalysts from activated carbon generated from biomass. Activated carbon made from biomass has a significant specific surface area, a very porous structure, and high stability. The high porosity, excellent electrical conductivity, surface oxygen functional groups, and heteroatoms of activated carbons derived from biomass feedstocks make them desirable as catalyst supports for use in sensors, energy storage, catalysis, and other applications in addition to being affordable and environmentally friendly [18,19]. By chemically activating carbonized materials with chemicals such as ZnCl2, H3PO4, KOH, or NaOH, they can be made.
Based on activated carbon from biomass, magnetic copper catalysts showed good catalytic activity in base-free Chan–Lam coupling and oxidations, Figure 3. Here, Shally Sharma et al. reported that abundant waste biomass (dead neem leaves) were carbonized to create biomass-derived activated carbon, which was then chemically activated with KOH. For the creation of affordable and ecologically friendly magnetic catalysts [Cu@KF-C/MFe2O4, M = Co, Cu, Ni, and Zn], such a porous carbon material was used as a low-cost and highly effective support material. Additionally, a KF modification was carried out to give the catalyst a basic character so that it could perform C-N coupling in base-free settings.
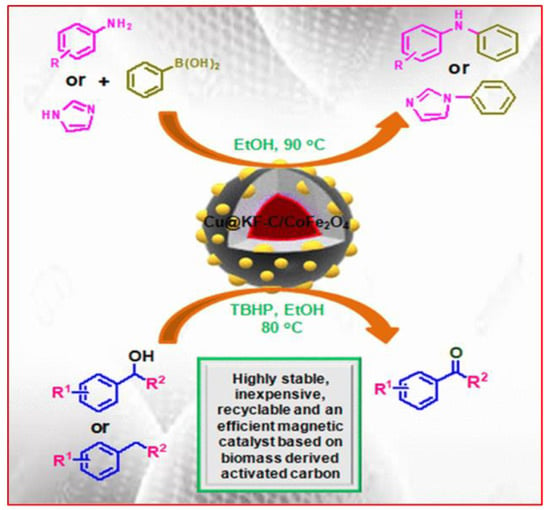
Figure 3.
Magnetic catalyst based on biomass-derived activated carbon. Adapted with permission from ref. [20].
Samira Bagheri et al. proposed the significance of biomass conversion, and catalytic pathways that include deoxygenation and -C-C coupling reactions have both been found to be efficient for converting biomass into chemicals and fuels, Figure 4. By using a catalytic approach, biomass is typically converted to carbon materials. Typically, this process begins with the hydrolysis of dehydrated cellulose chains [21] and proceeds to break down cellulose into monomer-soluble products [22]. As a result of linking and species transport from the solution to the nucleus, the soluble products undergo condensation or polymerization, aromatization of the resultant polymers, and growth.
Figure 4.
The route of AC derived from biomass. Adapted with permission from ref. [23].
2.2. Biochar
Biochar is a carbon-rich pyrogenic substance derived from carbon-neutral sources. It provides important CCS (carbon capture and storage) solutions and is a sustainable method of soil amendment. Preliminary studies into biochar’s catalytic potential and mechanistic procedures have been spurred by the recent identification of biochar as a flexible media for catalytic applications. Highly porous biochar with structurally bonded nitrogen groups are excellent supercapacitor electrode materials according to research [24]. However, the catalytic efficiency of biochar is significantly impacted by its inherent inorganics, matrix makeup, and surface functionality.
Biochar is an excellent option for many catalytic applications because it offers many advantages as a heterogeneous catalyst or support, including a wide surface area, cheaper cost, functional group tailoring, etc. The efficiency of biochar as a catalyst is influenced by a number of its inherent qualities. It comes from biomass and has an excellent thermal, stable structure as well as mechanical stability, and is structurally hierarchical. Catalysts made of biochar have the distinguishing qualities of (i) heterogeneity, which refers to the ability to separate the reaction mixture from the other reactants; (ii) bifunctionality, which refers to the involvement of transesterification and esterification; (iii) being recyclable; (iv) being porous; and (v) being nongraphitable, which refers to the fact that it does not crystallize at high temperatures [25]. When compared to other solid-based catalysts, biochar-based catalysts have the advantages of being affordable, environmentally beneficial, and simple to manufacture.
Xuefei Cao, et al. (Figure 5) reported the application of biochar-based catalysts in the upgrading of biomass, such as biochar solid acids for dehydration and biomass hydrolysis, biochars as catalyst supports for biomass pyrolysis, and biochar-based catalysts for the generation of biodiesel and bio-oil upgrading and gasification.
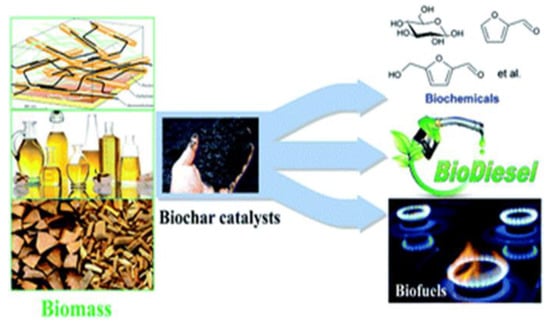
Figure 5.
Catalysts based on biochar are used in biomass upgrading. Adapted with permission from ref. [26].
The activated biochar can be further enhanced with additional functional groups or compounds to give it unique qualities such as adsorption on the surface and catalysis. They have been effectively employed in the production of biodiesel, biomass pyrolysis, gasification, and bio-oil upgrading. Biochars that have been activated and functionalized have enormous potential for usage as flexible catalysts and/or catalytic supports in biomass upgrading. However, the feedstock type, processing conditions, and activation or functionalization requirements all have a substantial impact on the physicochemical characteristics of the biochars. Future research should be conducted on the manufacturing of biochars with stable characteristics on an industrial scale.
2.3. Carbon Nanotubes
The Cinnamomum camphora tree’s crystalline latex was the first known renewable feedstock utilized to create CNTs [27]. Since then, a variety of items have been utilized, including solid biomasses such as grass and leaves, biodiesel, and plant oils. Recently, animal-sourced materials such as chicken fat and feathers have been used to create CNTs in addition to plant-based materials as an efficient carbon source.
Gao et al. successfully synthesized nitrogen-doped carbon nanotubes. The first step was to autoclave chicken feathers with NiAcTa (C4H14NiO8) and dry ice in a small pressure vessel for 2 h at 650 °C to produce N-doped carbon nanotubes. Ni3S2@CNF, or Ni3S2-carbon coaxial nanofibers, were created as a result. The second step involved treating the produced Ni3S2@CNF with hydrochloric acid at an ambient temperature for 12 h to eliminate the Ni3S2 particles that caused the N-doped carbon nanofibers to develop. Figure 6 shows a schematic illustration of the many steps involved in producing CNTs from chicken feathers.
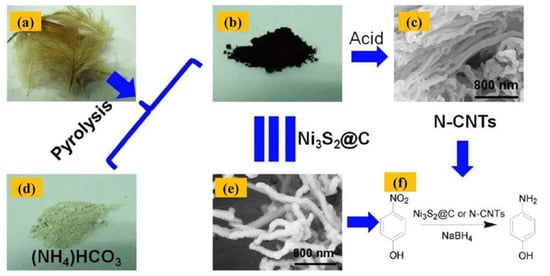
Figure 6.
The several steps involved in creating CNTs from chicken feathers are represented schematically. Photograph of chicken feathers (a) in white and black products created in the autoclave in (b) and (c). Ni3S2@C and N-CNT images captured with a FESEM in (d) and (e), respectively. (f) The catalytic reaction of the synthesized Ni3S2@ C or N-CNTs. Adapted with permission from ref. [28].
The CNTs were produced by raising the reaction temperature to between 750 and 800 °C with the smallest diameters of 19.8–31.7 nm, 91% purity, and greater crystallinity; the 800 °C treatments generated the best yield. The reaction temperature was raised further (to 850–900 °C) and the diameters of the nanotubes increased while the quality of the CNTs decreased. The N content in the N-CNTs reached as high as 6.43%, which was responsible for the excellent catalytic performance of the material.
2.4. Carbon Nanospheres
The gold nanocatalysts intercalated into the nanospherical mesoporous carbons (Figure 7) displayed great activity and selectivity when hydrogenating nitroarenes into the respective amines by employing H2 as a reduction agent.
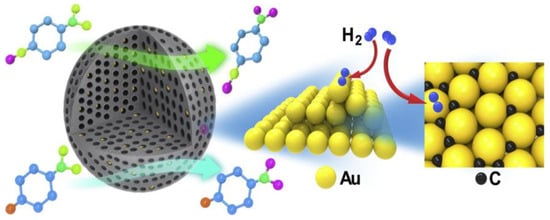
Figure 7.
As a heterogeneous catalyst for the selective reduction of aromatic nitro compounds, thermally reduced gold nanocatalysts were made by carbonizing ordered mesoporous carbon. Adapted with permission from ref. [29].
When using previously used Au/MCN catalysts, the initial reaction rate for 4-NP conversion and the product yields over a given period of time were equivalent to those obtained with a newly made catalyst, Figure 8. The initial reaction rate and product yield were sustained after five cycles, demonstrating the stability and reusability of the recycled catalyst. The amount of gold in the liquid phase that was collected after the solid catalyst separation was always below the instrument’s detection limit, suggesting that there was very little gold detachment during the recycling cycles for the catalyst. Here, a hydrothermal synthesis method was used for the first time to obtain ordered, mesoporous carbon nanospheres with dispersed gold nanoparticles (Au-NPs) of about 2.8 nm in size that had a diameter of about 90 nm.
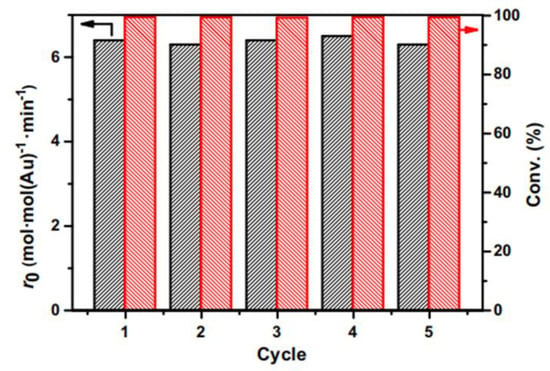
Figure 8.
Compilation of the starting reaction rate and product yield for the sequential hydrogenation of 4-NP using the recovered Au/MCN catalyst after 5.5 h. The reaction mixture for each run was 0.36 mmol of 4-NP or 0.32 mmol of p-CNB, 5.08 × 10−4 mmol of gold, 5.0 mL of ethanol as the solvent at 140 °C, and 4.0 MPa H2. Adapted with permission from ref. [29].
A similar application of carbon nanospheres obtained from biomass was reported, where S Supriya et al. proposed immobilizing Pd on carbon nanospheres made from areca nut kernels as a new heterogeneous catalyst was created (CNSs). Without any additional activation procedures, the CNSs could hold 3% of Pd on their surface. The Suzuki–Miyaura coupling of bromoarenes with aryl boronic acids and the reduction of nitroarenes both utilized the novel Pd/CNS material [30].
In Figure 9, under catalyst-free conditions, just 4% of nitrobenzene was converted to aniline, highlighting the role of the Pd/CNS catalyst in the process. Interestingly, when nitrobenzene was reduced using bioderived CNSs (in the absence of Pd), a 31% conversion was observed, most likely due to the porous nature of CNSs, which act as the reduction surface for the -NO2 group. Figure 9 also demonstrates the different catalytic loading of the Pd/CNS and the corresponding product conversion obtained. The Suzuki–Miyaura reactions produced conversions up to 98% at 150 °C with 10 mol% of the Pd/CNS catalyst under microwave irradiation. In addition, the Pd/CNS catalyst was found to be superior with respect to having a low catalytic loading (3% Pd on CNSs) in all the reactions compared to the commercially available 10%Pd on the activated carbon.
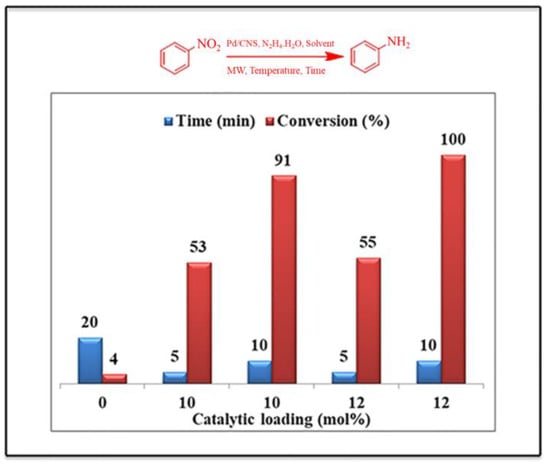
Figure 9.
The reaction conditions during the optimization of the reduction reaction of nitrobenzene in ethanol with different catalytic loadings and varying times. Adapted with permission from ref. [30].
3. Methods of Preparation of Carbon-Based Heterogeneous Catalysts and Its Importance
The method involved in catalyst preparation plays an important role in developing efficient catalysts. This is due to the fact that the catalyst preparation stage is very sophisticated and comprises numerous details that one must be aware of and understand. The preparation stage involves a lot of details, and these details surely have an impact on the catalyst’s final qualities, particularly its selectivity and catalytic efficiency. There are numerous preparation techniques that result in the same catalysts but with distinctive features linked to a specific preparation process, despite the fact that the precise details of the preparation process differ [31]. The catalyst preparation process as illustrated in Scheme 1 also includes certain crucial variables that fundamentally affect the different catalytic qualities, including precursors, composition, mixing outcomes, and so on. The economic value of catalysts is thus greatly influenced by these variables, which also have an impact on industrial and environmental applications.
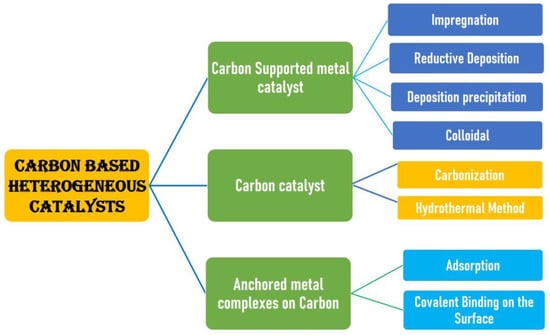
Scheme 1.
General scheme representing the various methods to prepare carbon-based catalysts.
Carbon-supported catalysts have been made from a variety of carbon materials, including carbon black, activated carbon (AC), glassy carbon, pyrolytic carbon, and carbon produced from polymers. Due to their large surface areas, the ACs and carbon black are the carbon-based materials that are most frequently utilized as the supports of the active phases [32]. Because of the great selectivity and activity of the catalyst, the main goal of the catalyst preparation process is to create a catalyst with precise specifications to carry out a particular catalytic reaction that would boost industrial or environmental productivity. The following are the general procedures for producing a heterogeneous catalyst: (a) choosing the necessary ingredients (support, precursors of the active components and promoters and water or solvent); (b) combining them (by coprecipitation, deposition, or impregnation); (c) drying; (d) blending with a forming agent, lubricant, or binder; (e) shape development; (f) canning; (g) the desired oxidation state is activated or reduced. Ceramic, precipitation or coprecipitation, ion exchange, adsorption, sol-gel, impregnation, and deposition–precipitation processes are the most frequently used (conventional) catalyst synthesis techniques. New techniques are also employed to manufacture catalysts, including the plasma processes, microemulsion, combustion, and electrospinning techniques [33].
3.1. Carbon-Supported Metal Catalyst
The most researched carbon-based material for supporting noble metals is activated carbon (AC). Large surface areas of ACs make it possible for the metallic phases to have excellent dispersion and stability. It has been common practice to use metal-supported ACs for C-C coupling processes, hydrogenations, oxidations, or carbonylations. In synthetic organic chemistry, the reactions that produce C-C bonds are crucial and useful transformations. Apart from this, Pd/C is also frequently used as a catalyst for many reactions. In order to make such carbon-supported catalysts economical and environmentally friendly, research is proceeding in the direction where carbon materials are developed from natural biomass precursors. The various techniques involved in the preparation of supported catalysts include impregnation, metal deposition, etc.
3.1.1. Impregnation
Impregnation is the easiest way to make supported metal catalysts out of all the preparation techniques [34]. In impregnation, a solution of a metal salt is applied to the support at a concentration that will provide the appropriate loading, as shown in Figure 10. The system is then aged, typically for a brief period of time, dried, and calcined. Wet impregnation (WI) refers to the process of producing a thin slurry by using a quantity of the precursor solution greater than the support’s pore volume. Dry or incipient wetness impregnation (DI) is the process of limiting the solution to merely fill the pore volume [35]. To achieve a uniform distribution of the catalyst precursor over the support, it is crucial to agitate the slurry continually throughout drying unless the catalyst precursor is heavily adsorbed on the support.
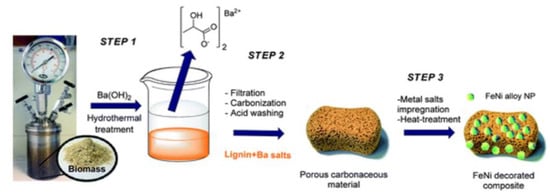
Figure 10.
Process involved in the synthesis of FeNi NPs-supported carbonaceous material composite derived from porous lignin. Adapted with permission from ref. [36].
3.1.2. Reductive Deposition
Nanoparticles can be produced directly from a number of precursor molecules that are soluble in a specific solvent; hence, the liquid phase reduction process is one of the simplest approaches for creating nanometer-sized particles [37]. In contrast to the deposition precipitation approach, reductive deposition occurs when precipitation is induced in the solution phase but near the surface rather than on the surface. Metal precursors are often reduced by utilizing a variety of reducing agents, including sodium borohydride, hydrazine, ethylene glycol, and ascorbic acid, through electrochemical processes, chemical pathways, and thermal degradation at high temperatures. The fact that the majority of powerful reducing agents are poisonous is one of this method’s drawbacks. Additionally, electrochemical approaches call for an additional electrochemical apparatus. As a result, easy and sustainable methods of reducing metal precursors are greatly wanted to increase productivity and ensure the sustainability of metal-based nanomaterial synthesis processes.
3.1.3. Deposition Precipitation
A highly soluble metal salt precursor is changed in the deposition precipitation approach into a less soluble compound that only precipitates on the support and not in the solution [38]. This procedure is typically accomplished by altering the pH of the solution, adding a precipitation agent, adding a reducing agent, or altering the concentration of a complexation agent. To guarantee that precipitation only happens on the support and not in the solution, two key requirements must be met: concentrations of the precursor in the solution must be regulated to prevent spontaneous precipitation and a robust interaction must occur between the soluble metal precursor and the surface of the support. The biggest flaw in this approach is the lack of adequate control over the metal distribution and surface composition, which makes it challenging to create real bimetallic catalysts with precise compositions. The precipitant must be added gradually and with vigorous mixing in order to prevent the creation of areas with a high local pH and to slow down rapid nucleation and growth in the majority of the solution. The optimum approach would seem to be to actively stir the suspension while slowly injecting the alkali solution through a hypodermic needle beneath the liquid’s surface. However, this situation offers an upscaling issue because highly effective mixing is challenging to implement on a wide scale [39].
3.1.4. Colloidal
Colloidal synthesis is a multistep “three-dimensional” process that includes the following: (a) the preparation of catalyst precursors in a solvent with a protective agent, such as a surfactant (e.g., cetyltrimethylammonium bromide-CTAB); (b) the deposition of the colloids into the support, and (c) the reduction of the mixture using chemicals. The colloidal approach can produce very fine particle sizes, but it requires the use of surfactants and protective agents, which means the catalyst must be repeatedly washed in the proper solvent or subjected to high temperatures in an inert environment to breakdown or eliminate these foreign chemicals. In order to avoid the accumulation of metal nanoparticles, the catalyst must first be adsorbed into the support. Therefore, using a different method to make small nanoparticles without the use of protective chemicals is preferable because it will make the process easier and prevent contamination [35].
3.2. Carbon Catalysts
Carbon-based compounds have long been recognized as valuable catalytic materials, either as catalysts themselves or as substrates for other catalysts. These carbon-based materials have several benefits for catalytic applications, including: (i) high thermal stability; (ii) easy recovery from the reaction mixture; (iii) high chemical stability in acid or basic media; (iv) hydrophobic nature; (v) low corrosion capability; and (vi) from an economic standpoint, their lower price.
Amorphous carbon, carbon fibers, activated carbon, ordered mesoporous carbon, graphene oxide, carbon black, carbon nanotubes, and carbon nanodots are just a few examples of the carbon-based materials that present new opportunities for the development of numerous catalytic supports and catalytic performances (Figure 11). Most often, graphitic or porous carbons are used in sorption, sensors, and catalysis. In addition, the regulated pore structure of carbons and the surface chemistry of carbons (surface oxygen, other heteroatoms, amphoteric character, and hydrophobicity) are crucial for catalysis applications [40].
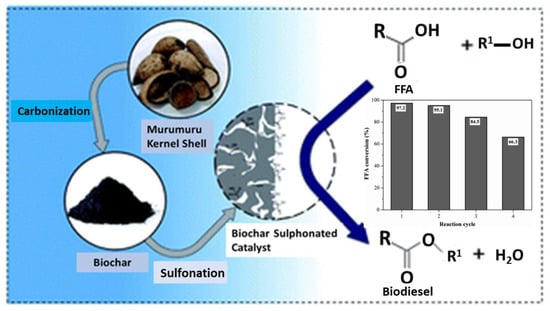
Figure 11.
Heterogeneous acid catalysts synthesized from murumuru kernel shells for fatty acid esterification reactions. Adapted with permission from ref. [41].
3.3. Anchored Metal Complexes on Carbon (Heterogenized Homogeneous Catalysts)
Homogeneous catalysts, typically organometallic complexes, have demonstrated great activity and selectivity while remaining active under mild reaction conditions. They fall short, however, in terms of the simplicity with which the catalyst can be removed from products. The heterogenization of homogeneous catalysts is currently cheap economically, but it is also a toxicological and environmental concern [42]. Thus, there is a drive for metal complexes to be heterogenized, as this will combine the advantages of homogeneous and heterogeneous catalysts [43]. In contrast to homogenous systems, the activity and selectivity of the attached complexes may even be increased. This could be due to a site isolation effect in which the anchored complexes are prevented from reacting with one another as they might in a homogeneous medium, resulting in the formation of inactive dimeric species, or it could be due to a confinement effect, in which the interactions between the catalyst and reactant are enhanced when confined to the pore system of the support. Covalent bonding is the most popular method for heterogenizing metal complexes out of all the available techniques, especially when it comes to porous carbons. The metal complex may interact directly with the support’s surface functional groups [44] or indirectly through a spacer that has already been grafted. This approach results in the formation of a strong chemical bond to the support, which will prevent the leaching of the catalyst to the reaction medium [45].
4. Surface Modification of the Carbon Materials to Enhance Their Catalytic Property
Porous carbons have attracted a lot of attention because a large range of materials can be produced from relatively inexpensive and low-cost precursors. Due to their diverse porous structure, low cost, accessibility, good recyclability, surface hydrophobicity, resistance to acidic and basic environments as compared to silica and alumina supports, low density, and—most importantly—the ability to introduce various functionalities using a variety of activation, functionalization, and carbonization techniques, porous high-surface area carbons are typically used as good catalyst supports. Additionally, the textural and structural adaptability of carbon-based materials enables the development of distinctive adsorption sites [46].
In carbon-based materials, agglomeration results from high van der Waals forces between carbon particles. This tendency toward aggregation has hindered its application in numerous fields. Researchers have successfully developed a variety of surface modification/functionalization technologies in an effort to reduce this tendency toward agglomeration and to investigate potential application areas [47]. Surface modification refers to the process of changing a material’s surface by adding physical or chemical properties that are not currently present. The creation of functional groups on the surfaces of carbon-based materials is known as functionalization. Increased matrix–solvent interactions and a reduction in the long-range van der Waals force of interactions are two ways that functional groups contribute to a homogeneous dispersion. As a result, functionalization makes a material more reactive, tends to make carbon-based materials more soluble, and creates opportunities for additional chemical changes including the adsorption of ions, deposition of metal, grafting reactions, etc. Additionally, the functional groups have the capacity to act as anchoring groups, which allows for the bonding of two moieties followed by subsequent derivatization with other functional groups via chemical interactions [48]. In this regard, the chemical functionalization of porous carbons turns into a crucial technique for adding evenly distributed anchoring points, as shown in Figure 12. Meanwhile, as illustrated in Table 1, surface-modified carbon materials frequently show reduced aggregation compared to the pristine form, increasing dispersibility and improving catalytic activity [49].
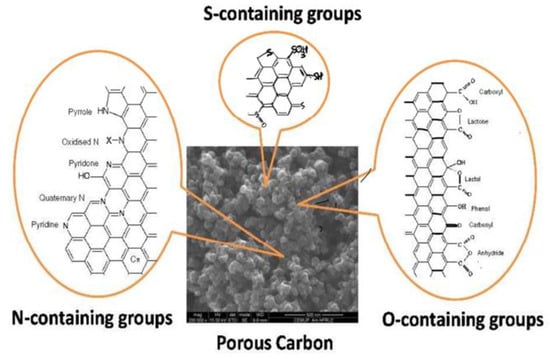
Figure 12.
Surface functional groups that act as anchoring sites for catalyst precursors or active sites for catalysis on porous carbons. Adapted with permission from ref. [45].

Table 1.
Property enhancement due to surface modification.
The surface properties influence the nature of the carbon materials in catalysis, which are in turn impacted by their structure. Since the porous carbons employed in catalysis have a graphitic structure, there are sp2 hybridized carbon atoms along the margins of the graphene layers and in basal plane defects. These atoms are highly susceptible to react with oxygen or nitrogen compounds and give rise to various surface functional groups, as illustrated in Figure 13 [58]. Additionally, these functionalities might operate as active sites for specific catalytic reactions.
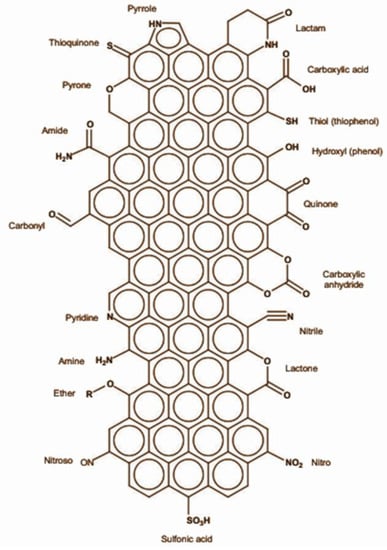
Figure 13.
Illustration of surface functionalities that can be introduced in porous carbon materials. Adapted with permission from ref. [59].
Owing to this, the surface modification techniques can be broadly classified into two types: covalent functionalization or chemical functionalization and noncovalent functionalization or physical modification. In the covalent functionalization technique, various functional groups are introduced on the surface of the carbon materials through covalent bonds, while in noncovalent functionalization, van der Waals interactions, π-π interactions, etc. are the driving force for the modification, and as the result the native structure and properties of the material is retained. The various techniques to noncovalently modify the surface include metal deposition, polymer wrapping, metal encapsulation, etc. [60].
4.1. Functionalization with Oxygen-Containing Groups
The oxygen-containing functionalities can be introduced on the carbon materials through a wet oxidation process or gas phase oxidation. In the wet oxidation process, the materials are chemically treated with strong oxidizing agents such as HNO3, HNO3 + H2SO4, H2SO4 + KMnO4, and H2SO4 + H2O2 [61]. The degree of oxidation is influenced by the oxidant as well as its concentration, temperature, and reaction time. Hydroxyl groups such as phenols often result from a lower degree of oxidation, whereas carboxyl groups are more likely to arise from higher degrees of oxidation. Wet oxidation is undesirable for industrial scale functionalization due to its risk to the environment and human health. The chemicals employed in this process are extremely toxic and challenging to work with; however, the oxidation of the carbon materials can be performed in the gas phase in the presence of oxygen, ozone, or plasma treatment [62], which is called dry oxidation or gas-phase oxidation. The most common oxidant in the gas phase is air, which is employed at temperatures between 473 K and 673 K. At lower temperatures, the oxidation effects are hardly noticeable; however, at higher temperatures, a degradation of the functional groups occurs.
4.2. Functionalization with Nitrogen-Containing Groups
Ammonia is frequently used in the gaseous phase to introduce amino functionalities to carbon materials. Amidation (materials containing oxidized carbon undergo amidation either through direct interactions with amine-containing molecules or through an intermediate reaction with thionyl chloride or a coupling agent) and ammoxidation, which is the reaction with ammonia/oxygen mixtures at temperatures between 200 °C and 450 °C, have also been utilized. In the liquid phase, porous carbon is more often treated with a nitrogen precursor such as urea [63], amines [64,65], melamine, etc. and is subsequently treated at elevated temperatures; a better outcome is typically obtained if the carbon has already undergone oxidation. After nitrogen functional groups such as amines, amides, pyrroles, and pyridines are produced, they often have a basic nature, which raises the pH of the carbon material at the point of zero charge [66]. Nitrogen can also be added to the surface of carbon-based materials via an HNO3 treatment. A similar result was seen upon oxidation in the gas phase with N2O.
4.3. Functionalization with Sulfur-Containing Groups
Carbon materials can be functionalized with sulfur-containing groups in the solid, gaseous, and liquid phase. In the gas phase, the carbon material is commonly heated in the presence of a sulfur-containing gas, primarily H2S; however, sulfonation can also be achieved using SO3 in the gas phase by heating fuming H2SO4 (a 50% SO3/H2SO4 solution) in an autoclave. This technique has been employed to sulfonate magnetic Fe3O4@carbon nanoparticles [67]. Another method, which eliminates the use of highly concentrated (or fuming) sulfuric acid, is the generation of SO3 via plasma by an electric discharge into a diluted sulfuric acid solution. This approach has been used for the sulfonation of carbon black [68]. While in the solid phase, carbon is usually heated in the presence of elemental sulfur. The carbon material can also be sulfonated by the reduction of a diazonium salt such as 4-benzene-diazoniumsulfonate with H3PO2 [69] or by using concentrated sulfuric acid [70]. Additionally, organic molecules with SO3H functions, such as p-toluenesulfonic acid (TsOH) and hydroxyethylsulfonic acid, can be used for sulfonation [71,72]. It is also reported that sulfur-functionalized carbon dots were produced utilizing a one-step hydrothermal process with an aqueous turmeric solution that contained ammonium persulfate as a sulfur source [73]. Similarly, carbon materials can also be functionalized with phosphorous [74,75], boron [76], or halogen functionalities [77].
Heteroatom functionalization on carbon materials can also be achieved by the method of doping. This type of doping can be conducted either in situ by mixing the carbon precursor and the dopant or by using a heteroatom-rich carbon precursor such as a pomelo peel [78] or fish waste.
5. Application of Biomass-Derived Materials in Various Heterogeneous Catalytic Systems
5.1. Biomass-Derived Carbon Materials in Electrocatalysis
Sustainable and clean energy generation depends heavily on the conversion and storage of electrochemical energy. Fuel cells and metal–air batteries, among other electrochemical systems, exhibit significant potential [79]. The most efficient catalysts for electrocatalytic reactions are those based on platinum, which has long been known. However, the expensive cost of Pt, the absence of long-term stability, and the susceptibility to surface poisoning by various compounds such as methanol and carbon monoxide enable the development of non-Pt group metal catalysts [80]. By doping carbon with different heteroatoms such as N, S, P, or B, or by codoping with N and Fe or N and Co, a range of non-Pt group catalysts have been developed to address these difficulties [81,82]. When doped with nitrogen, depending on the doping procedure, the nitrogen moieties may include graphitic-N along with pyrrolic and pyridinic nitrogen. Among these bonding configurations, graphitic-N induces an n-type doping effect, whereas pyrrolic-N and pyridinic-N may result in either weak n-type or p-type doping [83]; however, among all the possible nitrogen-doping configurations, the graphitic N doping could preserve the high carrier mobility due to minor distortion of the graphene lattice [84]. Moreover, it was also reported that N doping resulted in a shift of the conduction band to the fermi level, increasing the electrical conductivity of the material [85]. Hence, given the enormous capability of these catalysts, it is crucial to investigate the methods for producing them sustainably from biomass, which is an appealing renewable natural resource due to its characteristic abundance in carbon and nitrogen [86] and the presence of metal elements such as iron in some forms [87].
Soybeans and earth-abundant molybdenum were used to create a highly active electrocatalyst for hydrogen evolution by Wei-Fu Chen and colleagues. This catalyst drives the hydrogen evolution process (HER) with low overpotentials and is extremely durable in a corrosive acidic solution for more than 500 h. It is made up of a catalytic β-Mo2C phase and an acid-proof γ-Mo2N phase. The MoSoy catalyst, when supported on graphene sheets, displayed extremely fast charge transfer kinetics, and its performance was comparable to that of noble metal catalysts such as Pt for the generation of hydrogen. These findings challenge the dominance of platinum catalysts in the hydrogen economy by demonstrating that high-protein biomass, such as soybeans, may be used to make catalysts that incorporate an abundant transition metal [88]. It was observed in various studies of HER electrocatalysis that when carbon atoms of the carbon-based materials are replaced by heteroatoms, they modulate the charge distribution over the carbon network [89]. Additionally, changes in the crystal structure will cause defects and provide more adsorption sites and activity centers for HER intermediates. With the introduction of defect structures such as atom vacancies and heteroatom doping [90], the electron configuration of the catalyst site is efficiently modulated to promote the formation and transformation of intermediate states, thus facilitating specific electrochemical reactions [91]. In most cases, due to the modulation of the local regional electronic and surface configuration, defects are generally considered the active sites for electrocatalytic processes [92,93]. Additionally, these modifications may lead to the formation of a heterointerface, which will optimize the adsorption energy, regulate the electronic interactions, and expose more active sites, leading to a high electrochemical performance [94].
As illustrated in Figure 14, Jinghan Li and colleagues developed a low-cost and metal-free porous electrocatalyst for oxygen reduction reactions (ORR) using plum blossom, an environmentally friendly precursor, as a carbon source that is extremely effective and stable. The electrocatalysts demonstrated excellent stability with a current retention of 91.2% after 50,000 s, high catalytic activity (E1/2 = 0.82 V and JL = 5.96 mA cm−2), and methanol tolerance in alkaline media. When porous carbon materials are synthesized, they provide a larger surface area while inducing the defect sites. However, the size of the pore and the pore structure is also of importance, as smaller pores are responsible for providing a higher effective surface area that acts as active sites in enhancing the performance of the electrodes. Hence, increasing the surface area by optimizing the porosity in order to have more defect sites is key [95,96]. Furthermore, the extension of pore size could decrease the adsorption capability by decreasing the charge storage [97].
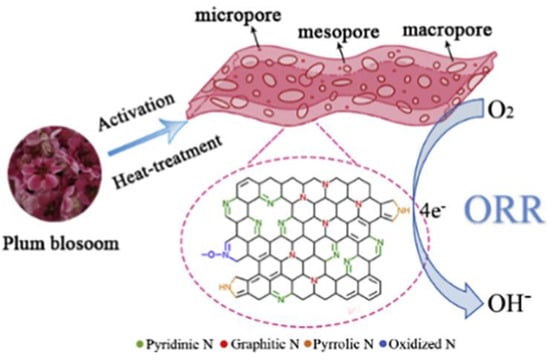
Figure 14.
Illustration of a nitrogen-doped porous carbon material prepared from fallen plum blossom via chemical activation and post-treatment and studied as the electrocatalyst for oxygen reduction reaction. Adapted with permission from ref. [98].
Xiaojun Liu and colleagues developed a biomass-derived electrocatalyst where spinach was used as the precursor, melamine was used as a nitrogen promoter, and the combination of NaCl/KCl was used as the pore producer, and they successfully obtained a multielement-doped carbon with high ORR activity through pyrolysis at 900 °C under Ar. The resulting carbon composites exhibited a sheet-like shape, a specific area of 289.6 m2 g−1, and a high density of ORR (oxygen reduction reaction) active sites, as shown in Figure 15 [99]. The electrocatalytic oxidation of pyridyl carbinol at MnO2-Pi-PCN/CFP electrode with TEMPO as the mediator was studied by Mathew et al. in an aqueous–acidic medium. They synthesized porous carbon nanospheres from monkey pods as the carbon precursor and created a modified carbon fiber paper (CFP) electrode by electrodepositing manganese dioxide-phosphate (MnO2-Pi) on porous carbon nanospheres. The electrocatalytic efficiency of the MnO2-Pi-PCN/CFP electrode towards pyridyl carbinol oxidation significantly increased when evaluated utilizing CV. The study found that the MnO2-Pi-PCN/CFP electrode is superior to the bare CFP electrode in terms of effectiveness [100].
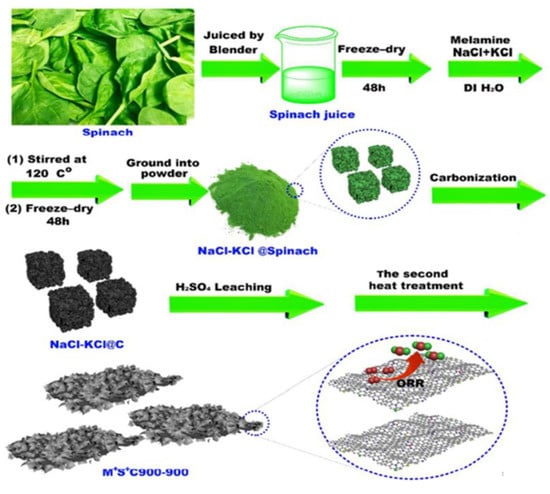
Figure 15.
Schematic procedure for preparation of carbon nanosheets derived from spinach as electrocatalysts for ORR. Adapted with permission from ref. [99].
Cobalt-catalyzed carbonization of biomass chitosan was described by Zhang et al. as a technique for producing cobalt and nitrogen codoped carbon nanotubes (Co-NCNT) exhibiting high activity for oxygen reduction reactions (ORR), as shown in Figure 16. In comparison to commercial Pt/C catalysts, the Co-NCNT catalyst with optimized synthetic conditions demonstrated an attractive ORR activity. This work also offers an effective synthetic method for the production of highly effective carbon-based ORR catalysts generated from biomass [101]. For a sustainable upgrading of oxygen reduction to hydrogen peroxide in UV-assisted electro-Fenton water treatment, Giorgia Daniel and colleagues developed a Chitosan-Derived Nitrogen-Doped Carbon Electrocatalyst. This research looked at the synthesis of N-doped mesoporous carbon produced from chitosan in both the absence (MC-C) and presence (N-MC-C) of 1,10-phenanthroline, a substance that acted as both a porogen and a second nitrogen source. N-MC-C had more significant surface N-functionalities than commercial carbon black, including the pyrrole group, as well as a higher level of graphitization and surface area (63 vs. 6 m2g−1). These qualities contributed to the high oxygen reduction reaction activity of N-MC-C [102].

Figure 16.
TEM images of (A) NCNS-T800, scale bar 50 nm, and (B) Co-NCNT-T800, scale bar 50 nm; (C) high-resolution TEM image of Co-NCNT-T800, scale bar 10 nm; (D) high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) image and element mapping of (E) carbon, (F) nitrogen, and (G) cobalt for Co-NCNT-T800, scale bar 50 nm. Adapted with permission from ref. [101].
In a study by Ma et al., heteroatom tridoped porous carbon was synthesized from fish waste using pyrolysis to create Pt-free electrocatalysts with a high catalytic activity and low cost for triiodide reduction. The carbon materials were naturally doped with nitrogen, phosphorus, and sulfur and had a remarkable BET surface area of 2933 m2 g−1 as the result of its porous structure. Additionally, the improved dye-sensitized solar cells (DSC) with a tridoped porous carbon electrode demonstrated a power conversion efficiency of 7.83%, which is comparable to the system with a Pt-based counter electrode with an efficiency of 8.34% [103].
Carbon-based electrocatalysts with intrinsic defects can be created for the effective and long-term electroreduction of CO2 using a straightforward two-step carbonization technique, according to the study by Mengjie Chen and colleagues. They used a nitrogen-rich silk cocoon as a precursor. The resulting electrocatalyst maintained good selectivity for around 10 days and catalyzed the CO2 reduction to CO with a Faradaic efficiency of 89% [104].
Consequently, there has been significant progress in recent years toward the development of carbon materials produced from biomass as electrocatalysts to fulfill the demands of high performance and as a potential replacement for costly noble metal-based electrocatalysts.
5.2. Biomass-Derived Materials in Photocatalysis
Heterogeneous photocatalysis is a viable technique in environmental remediation. Due to the abundance and environmental friendliness, carbon-based materials generated from biomass are thought to be a green and promising substitute for conventional photocatalysts, as shown in Figure 17 [105]. Photocatalysis is the term used to describe a chemical reaction that is photoactivated and occurs on a semiconductor surface. Titanium dioxide (TiO2), tungsten trioxide (WO3), zinc oxide (ZnO), iron oxide (Fe2O3), zirconia (ZrO2), and vanadium oxide (V2O5) are a few popular semiconductors that have been widely used. However, the majority of semiconductors have significant band gap energy (Eg), such as 3.37 eV and 3.2 eV for ZnO and TiO2, respectively, which indicates that they can function properly only when exposed to ultraviolet light. The rapid recombination of charge carriers may also limit these materials’ ability to act as photocatalysts. Hence, recent advancements in the doping and designing of hybrid material with carbon compounds sourced from biomass offer one practical and creative solution to these problems [106]. Moreover, the carbon-based nanostructured materials also feature good BET surface areas, tunable topologies, excellent charge carrier mobility, favorable recycling characteristics, excellent stability, and a high degree of porosity, which makes them suitable support frameworks for catalysts [107].
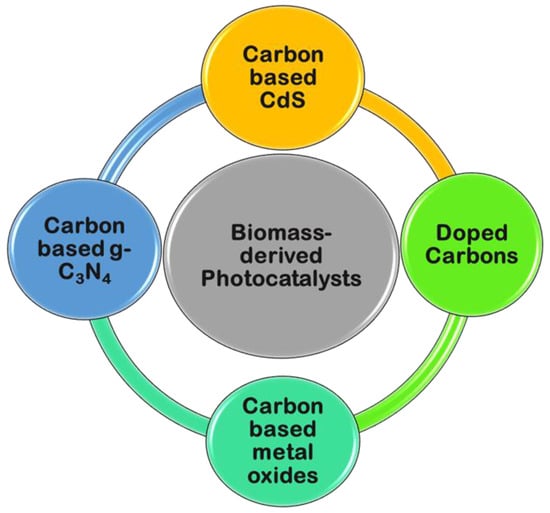
Figure 17.
Biomass-derived carbons as photocatalytic materials.
5.2.1. Doped Carbons
One of the most popular methods for enhancing the photocatalytic activity of the carbon compounds obtained from biomass is atom-doping modification. This implies that the surfaces of carbon materials are modified with chemical functional groups comprising oxygen, nitrogen, or sulfur atoms. It has been demonstrated that surface functionalization with atoms of oxygen, sulfur, and nitrogen has a significant effect on the surface properties of the carbon material, increasing the surface area and subsequently improving the ability to adsorb contaminants [108,109].
As illustrated in Figure 18, Zhou et al. developed a nitrogen and phosphorus self-doped porous carbon material using lotus pollen and a two-step calcination process. The N and P elements can significantly improve the photocatalytic H2 generation when exposed to visible light. It was also reported that the pollen carbon samples had special photoelectrochemical (PEC) characteristics after being annealed at 600 °C. Under a 0.8 V bias, LP-C-600 produced the most hydrogen (the hydrogen generation rate approached 3.5 µmol g−1h−1) and had the highest photocurrent (5.8 µA cm−2). The photo response range of the LP-C-600 was also very broad (300–600 nm), and its IPCE value achieved its greatest value of 0.11% at 350 nm under a 0.8V bias. The incident monochromatic photon current conversion efficiency (IPCE) of the sample increased sharply as the applied bias voltage increased, while its bandgap was noticeably reduced [110].
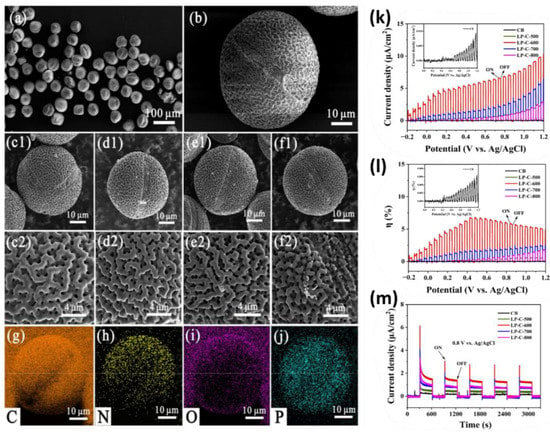
Figure 18.
SEM images of (a,b) LP-Et, (c1,c2) LP-C-500, (d1,d2) LP-C-600, (e1,e2) LP-C-700, and (f1,f2) LP-C-800. (g–j) Element mapping images of C, N, O, and P in LP-C-600. (k) Linear sweep voltammogram (LSV) curves, (l) photoelectric conversion efficiency, and (m) photocurrent density time plot at applied voltage of 0.8 V of samples under test conditions with 0.1 M Na2SO4. Adapted with permission from ref. [110].
Cristina Lavorato and colleagues demonstrated the synthesis of N-doped graphene derived from chitosan biomass using the technique of pyrolysis as a visible-light photocatalyst for hydrogen generation from water/methanol mixtures. The N-doped graphene exhibits a nearly neutral absorption spectrum, and the material demonstrates photocatalytic activity upon irradiation with UV- (355 nm) and visible light (532 nm), and can also generate hydrogen upon simulated sunlight illumination. In contrast to graphene, (N)G behaves like a semiconductor and demonstrates great efficiency for the photocatalytic synthesis of hydrogen from water/methanol mixtures without the use of Pt or any other metal and has a similar efficiency as utilizing UV or visible light. Additionally, this result is in complete contrast to the reported behavior of GO, which is exclusively active in UV light. One area in this study that deserves further attention is to increase the photostability of the material, which otherwise tends to deactivate upon extended irradiation [111].
5.2.2. Biomass-Derived Carbon-Based g-C3N4
A green technique that has recently demonstrated tremendous potential for enhancing the performance of g-C3N4 as a photocatalyst is the pairing of nontoxic and abundant biomass-derived carbon sources with g-C3N4 [112].
Li et al. reported that g-C3N4 and Ag nanoparticles combined with biomass-derived carbon from puffballs that have a hollow bird’s-nest-like shape and are produced using a one-step pyrolysis technique demonstrated notable photocatalytic activity towards the reduction of CO2 to CO under visible light. The Ag-g-C3N4/BN-C exhibited an impressively improved yield of 166.5 µmolg−1 for the total CO evolution, with an average evolution rate of 33.3 µmolg−1h−1 without the addition of any type of sacrificial reagents and photosensitizers, which was found to be superior to the pristine g-C3N4 and many reported g-C3N4-based counterparts [113].
Bin He and colleagues demonstrated the one-pot construction of chitin-derived carbon/g-C3N4 heterojunction for the enhancement of visible-light photocatalysis, as shown in Figure 19. According to the studies, the heterojunction performs a variety of functions, including increasing the photocatalytic performance by lowering the band gap and increasing the recombination rate. In addition, during the thermal polymerization of urea, chitin could act as a doping precursor and modify the terminal group and microstructure of g-C3N4, which results in the unbalanced electron density in the modified g-C3N4; additionally, it could lead to the tube structure of the g-C3N4 sheets and a subsequent increase in its surface area (49.1 m2/g) by almost ten times, which is attributed to the decomposition of chitin, which produced more pore structures during the process of urea thermal polymerization and thus facilitated the improved adsorption of the reactants. Subsequently, the visible-light-induced degradation of a common dye pollutant Rhodamine B was studied to examine the catalytic activity, where the modified g-C3N4 showed a considerable improvement of both the photocatalytic activity and reaction rate when compared to the pristine g-C3N4 [114].
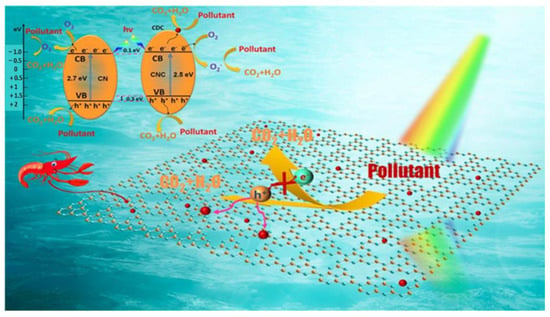
Figure 19.
A facile strategy to minimize the recombination of photon-generated carrier while simultaneously increasing the surface area by in situ constructing chitin-derived carbon/g-C3N4 metal-free heterojunction. Adapted with permission from ref. [114].
5.2.3. Biomass-Derived Carbon-Based Metal Oxides
Materials formed by the combination of biomass-derived carbon and metal oxides have the ability to quickly adsorb target pollutants molecules on the surface of the carbon material, followed by efficient photodegradation in the presence of metal oxides [115].
Wang et al. fabricated a high-performance photocatalyst using the combination of plane tree fluff (PTF) biomass-derived carbon and TiO2, which possessed trilevel hierarchical pores from the organic–inorganic network for organic dye degradation as well as water splitting for hydrogen production (Figure 20). In comparison to the benchmark photocatalyst P25, TF3 with a 1:3 proportion of titanium oxo acetate (TOA) and plane tree fluff (PTF) demonstrated 3.5 and 3.1 times the photocatalytic reactivity for the oxidation of organic dyes and the production of hydrogen for the splitting of water under UV light, respectively. With a photocurrent value of 11.3 mA cm2 under visible light irradiation, superior photoelectrochemical performances were also attained in TF3. TF3 loaded with 5 wt% Mo2C showed the highest hydrogen generation rates of 5.4 (UV) and 0.09 (vis) mmol g−1 h−1, which were significantly better than the same sample loaded with Pt [116].
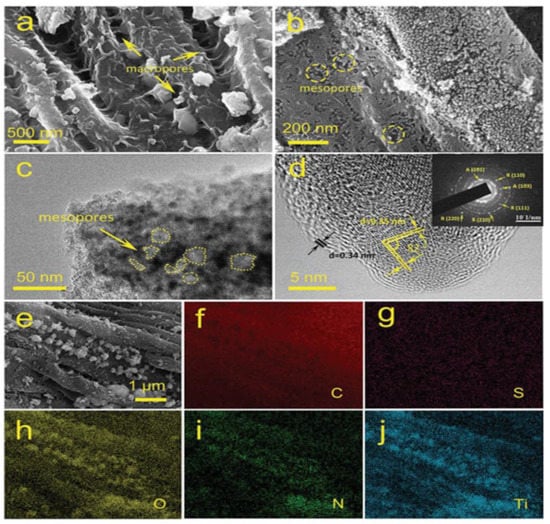
Figure 20.
(a,b) SEM images of TF3 with 1:3 proportion of titanium oxo acetate (TOA) and plane tree fluff (PTF), with low and high magnifications; (c,d) bright-field and high-resolution TEM images of TF3 with the inset as the selected area electron diffraction (SAED). Anatase is noted as A and rutile as R; (e–j) EDX mapping of different component elements. Adapted with permission from ref. [116].
Faisal et al. designed a unique ternary material via the combination of semiconducting metal oxide (ZnO), activated carbon (AC) derived from date seeds, and noble metallic AuNPs to further explore the possibilities to improve the efficacy of ZnO in the visible light region. In this work, for the first time, AuNPs and ZnO were developed as smart photocatalysts and used to help destroy the drugs gemifloxacin mesylate and methylene blue (MB) dye with the use of visible light illumination. With 98.0% of the target gemifloxacin mesylate drug molecule destroyed after 35 min of visible light irradiation and nearly all of the target MB molecule removed in just 30 min, the Novel AC@Au/ZnO photocatalyst was shown to be extremely effective [117].
5.2.4. CdS/Biomass-Derived Carbon Nanocomposites
Cadmium sulfide (CdS) has recently been used to design nanomaterials that are extensively utilized in photocatalysis. CdS possesses an optimum band gap energy of 2.4 eV, indicating its excellent activation capacity in the visible range [118,119]. However, since CdS is unstable, it gets converted to toxic Cd2+ ions through photocorrosion, thereby minimizing its practical usage. Recent research has shown that producing highly stable CdS composite nanomaterials by depositing CdS on natural-biomass-based carbon materials is an efficient method for overcoming this limitation.
Chen and colleagues prepared a CdS supported on carbon as a photocatalyst with a high performance of dye photodegradation using cadmium-enriched Perilla frutescens biomass. It was observed that the CdS@CP included cubic and hexagonal mixed-phase CdS nanoparticles. The estimated band gap energy of CdS@CP (2.21 eV) was significantly lower than that of pure CdS (2.57 eV), indicating that the proposed synthesis of CdS@CP could be stimulated by visible light more readily and requires less energy. Additionally, Rhodamine B (RhB) was photodegraded by CdS@CP at a rate of nearly 98% in 90 min, and its photocatalytic effectiveness was almost 22 times higher than that of pure CdS. The fact that the photocatalytic activation could continue to be over 75% even after four cycles of testing and that the structure of CdS remained essentially constant supported the photostability and reusability of CdS@LAC-800 [120].
Huang et al. developed the activated carbon materials (denoted as LAC–T), which were derived from the lotus leaf at different temperatures (T = 600, 700 and 800 °C). This resulted in carbonaceous materials with various microstructures and porosity. Through the deposition of nano-CdS precursors on LAC-T supports (CdS@LAC-T), these microporous carbonaceous materials were subsequently developed as excellent platforms for cadmium sulfide (CdS) composite photocatalysts. It was identified that the CdS@LAC-T nanocomposites showed an improved photocatalytic efficacy toward the degradation of several organic dyes under visible light as compared to the nano-CdS. The best photocatalytic efficiency was provided more particularly by CdS@LAC-800, which was made from a carbonaceous support with the highest BET. Compared to nano-CdS (2.22 eV), the estimated band gap energy for CdS@LAC-800 was much lower (2.01 eV). Additionally, a significant portion of the outstanding photocatalytic ability of CdS@LAC-800 was due to the generation and transportation of photogenerated carriers (h+, e−), as well as catalytically free radicals (OH• and O2•−) [121].
As illustrated in Figure 21, Li et al. recycled cadmium from wastewaters using Pistia stratiotes, first through phytoaccumulation and then through carbonization and a hydrothermal reaction that produced a carbon-supported cadmium sulfide photocatalyst (CdS@C). A blend of cubic and hexagonal CdS with a reduced band gap energy (2.14 eV) and a good electron hole separation efficiency made up the CdS@C photocatalyst, which suggests that it has an outstanding photoresponse and catalytic efficiency. The effective photodegradation of organic contaminants showcased that CdS@C has remarkable stability and photocatalytic efficiency. The main active species for the breakdown of organic contaminants during CdS@C photocatalysis were confirmed to be •OH and O2•-. After exposure to visible light for 90 min during the experiment, the extent of degradation of RhB in the presence of CdS was 32%, while in the presence of the CdS@CP photocatalyst, the rate of degradation was close to 99%, which was much higher than the single-CdS degradation efficiency. Recycling studies that tested the efficiency of the CdS@CP catalyst revealed that it could maintain a photocatalytic efficiency of more than 75% of the initial value even after four consecutive treatments. These results indicated that the photocatalyst had a considerably stable photocatalytic performance and reusability [122].
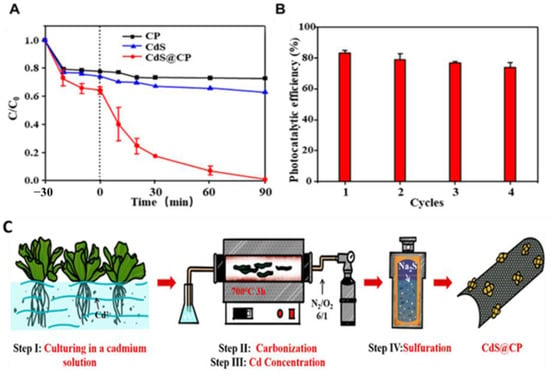
Figure 21.
(A) RhB degradation efficiency of CP (Pistia stratiotes-derived biochar), CdS, and CdS@CP; (B) recycling reactions when using CdS@CP; (C) schematic illustration of the procedures for CdS@CP photocatalyst composites. Adapted with permission from ref. [122].
5.3. Biomass-Derived Carbon Materials in Organic Reactions
Homogeneous catalytic reactions are considered commercially nonviable compared with heterogeneous catalysis owing to the tedious processes involved in the catalyst recovery. Noble metals have thus been extensively immobilized on solid supports in order to act as heterogeneous catalysts for a variety of organic processes. Since biomass-derived carbon has several advantages over commercially available, highly expensive carbon materials (graphene, carbon nanotubes, etc.), including low cost, ease of availability, high quality, and environmental suitability, biomass-derived carbons have been widely investigated recently. The biomass-derived carbons have been successfully used as heterogeneous catalysts in various organic transformation reactions such as oxidation reactions, reduction reactions, and cross-coupling reactions to name a few.
5.3.1. Oxidation and Reduction Reaction
Biomass-derived heteroatom-doped carbon materials and metal-deposited carbons have been reported to bring about the oxidation of alcohols, reduction of olefins, nitro-amine reduction, etc. The most advanced research in catalytic science prefers these forms of catalysts because they can address energy and environmental crises by substituting metal-based catalysts and have a catalytic performance that is on par with or even better than that of these metal catalysts.
In this regard, Liu et al. developed a N-doped porous carbon (NKC) made from readily available, inexpensive radish, which can be used as adaptable, effective bifunctional catalysts for both the oxidation of styrene (SOR) and the catalytic reduction of 4-nitrophenol (NRR), as illustrated in Figure 22. Using radish as the carbon source and varied urea dosages (mass ratio of urea to carbon precursor 1:1:x (x = 1, 2, 3)) and carbonization temperatures (y = 700, 800, 900), multiple NKC catalysts were developed in this study. The obtained catalysts were used as attractive bifunctional catalysts for the NRR and SOR. To find the ideal reaction conditions and better comprehend the catalytic process, a number of reaction parameters, including reaction temperature, reaction time, catalyst dose, and amount of reactants, were thoroughly examined. It was observed that the carbon framework with graphitic N content was crucial for both reactions. The NKC-3-800 is the catalyst that is best suited for these two reactions out of the other NKC catalysts, providing appealing catalytic results that are on par with many metal or even precious metal catalysts. In this study, it was encouraging to observe that the TOF value in the NRR could reach up to 1.67 × 10−4 mmol mg−1 min−1, while the SOR achieved a high SO (styrene oxide) yield of 64.0% with a styrene conversion of 83.5% and an SO selectivity of 76.7% [123].
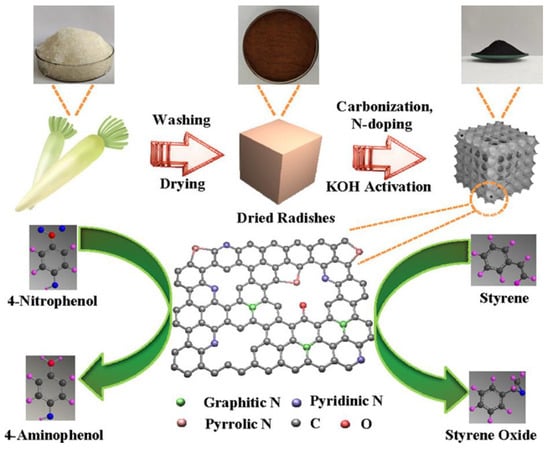
Figure 22.
Schematic representation of the synthesis of N-doped porous carbon derived from cheap and abundant radish which was employed as an efficient bifunctional catalyst for the catalytic reduction of 4-Nitrophenol and oxidation of styrene. Adapted with permission from ref. [123].
A carbon material doped with phosphorus possessing a highly porous structure and an appreciable surface area (>1600 m2 g−1) was reported by Xiwei Hu and colleagues. It was made by utilizing a practical and scalable method employing readily available soluble starch and phosphoric acid. First, a number of catalysts were prepared and tested for their ability to catalyze the oxidation of a variety of alcohols, including those that were only nitrogen doped (NC-700) with urea, those that were only phosphorus doped at various pyrolysis temperatures (PC-500, PC-600, PC-700, and PC-800), and those that were codoped with nitrogen and phosphorous (NPC-700). During the oxidation of benzyl alcohol under aerobic conditions, the as-fabricated PC-700 demonstrated excellent efficiency as a metal-free catalyst with full conversion (>99%) and impressive selectivity (>99%). The phosphorous-doped sites were shown to be the active sites for the oxidation reaction in this work based on the associated characterizations and experimental results. Additionally, the PC-700 exhibits exceptional performance in terms of its recyclability and stability, and it continues to maintain a high level of reactivity even after eight cycles according to the recycling test and characterizations of the used catalyst [124].
Qingjie Tang and colleagues created a carbon-supported Ni catalyst generated from biomass that is an efficient heterogeneous catalyst containing non-noble metal for nitro compound hydrogenation. In this study, a one-pot pyrolysis method was used to easily prepare a carbon-material-supported Ni catalyst (Ni/C) using medical absorbent cotton as the carbon precursor. The as-prepared Ni/C catalyst showed a noticeable catalytic activity for the hydrogenation of nitro compounds into primary amines even at room temperature. Additionally, the Ni/C catalyst proved to be extremely stable throughout the recycling trials without losing its catalytic activity [125].
As demonstrated in Figure 23, Zhu Yin and colleagues developed a P-doped porous carbon with inherent N functionality from waste peanut shells using phosphoric acid (NPC-T/NPC-800) and tested their application as heterogeneous catalysts in the metal-free aerobic oxidation of alcohols. The study also revealed a synergistic effect of N and P species on the catalytic efficiency of carbon materials. The amount of graphitic N and C3PO species on the surface of NPC-T were shown to be correlated with catalytic activity. NPC-800 achieved 100% conversion and 99.7% selectivity along with a high TOF value (2.49 × 10–3 mol·g–1·h–1) for the oxidation of benzyl alcohol (BA) to benzaldehyde (BAD). Even when applied to various alcohols, the NPC-800 showed good catalytic activity [126].
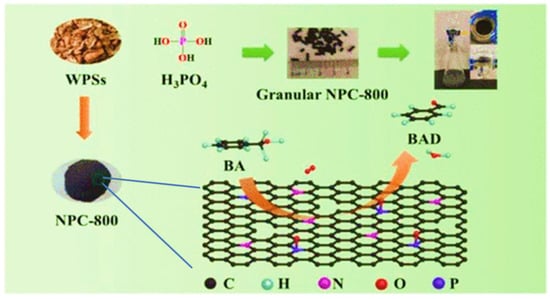
Figure 23.
P-doped porous carbon catalysts from waste peanut shells with inherent N functionality and their application in metal-free aerobic alcohol oxidation. Adapted with permission from ref. [126].
A biowaste chitosan-derived heterogeneous catalyst with cobalt for the hydrogenation of terminal and internal olefins was reported by Florian Korbinian Scharnagl and his team. When this non-noble metal composite was used, the hydrogenation of terminal C=C double bonds was achieved under incredibly moderate and safe conditions (methanol or water, 40 to 60 °C). The efficacy of Co@Chitosan-700 was demonstrated through its effective hydrogenation of the commercially important compounds of diisobutene, fatty acids, and their triglycerides. Additionally, the substance exhibited magnetic properties, and because water served as the solvent, the process of product separation and catalyst recycling was also made simpler [127].
5.3.2. Coupling Reactions
In recent years, a number of heterogeneous catalytic systems have been developed and put forth for the coupling reactions with the goal of minimizing the loss of extremely valuable palladium while simultaneously making the separation of the catalyst from the reaction mixtures simpler.
Patel et al. produced chemically activated graphitic mesoporous carbon (RAGC) from rice husk and then immobilized it with palladium nanoparticles (Pd NPs). The material was ascertained to have a hollow mesoporous structure, high surface areas (1019 m2g−1), and pores with an average size of 3.5 nm. As shown in Figure 24, the hybrid nano-Pd@RAGC material’s catalytic applications in significant carbon–carbon bond formation processes, such as Suzuki/Mizoroki–Heck/Sonogashira cross-coupling reactions in the absence of a ligand under microwave conditions, were investigated. In cross-coupling reactions, the RAGC gives exceptional stability to Pd NPs. Additionally, as green solvents, ethanol or water were used in all reactions. The nano-Pd@RAGC catalyst was also easily separated from the reaction mixture by filtration and was utilized for at least ten consecutive reactions [128].
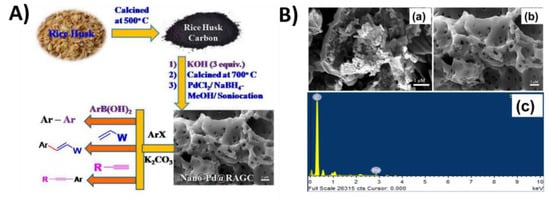
Figure 24.
(A) Fabrication of nano-Pd@RAGC (from rice husk biomass-derived chemically activated mesoporous graphitic carbon) and its applications in cross-coupling reactions; (B) FESEM images of biomass-derived carbon (a) before KOH activation and (b) after KOH activation, and (c) FESEM-EDAX image of nano-Pd@RAGC. Adapted with permission from ref. [128].
The preparation of palladium deposited on the waste-derived pine needle biochar (PiNe) and the use of cyclopentyl methyl ether (CPME) as a solvent produced from petrochemical waste that forms a heterogeneous aqueous azeotrope and boils at 83 °C as a heterocatalytic system was reported by Francesco Ferlin and colleagues (Figure 25). A Pd/PiNe catalyst was used to study the continuous-flow copper-free Sonogashira reaction, enabling a multigram-scale production that not only optimized the recovery and reuse of the catalytic system and reaction media but also reduced the amount of waste created. With an average throughput of 0.95 mmol per hour, the flow reactor demonstrated steady efficiency and negligible metal leaching during its 15 days of continuous operation [129].
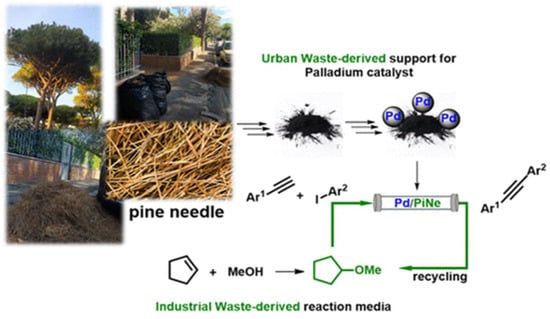
Figure 25.
Features of the Pd/PiNe-catalyzed continuous-flow Sonogashira reaction [129].
Aminosilane-functionalized low-cost mesoporous biomass carbon (BC) from waste radish leaves was synthesized for the first time with a specific surface area of 703.99 m2 g−1 by Kempasiddaiah and colleagues. The heterogeneous catalyst was prepared by first chemically oxidizing the biomass carbon (BC) with hydrogen peroxide (H2O2) followed by treatment with N-[3-(trimethoxysilyl)propyl]ethylenediamine (TPEA); then, it was employed as support in the preparation of the palladium-based (BC-TPEA@Pd) heterogeneous catalyst. Here, a greener reaction technique was used for Suzuki–Miyaura cross-coupling reactions employing the basic extract of radish leaves (BERL) under base, ligand, promoter, and additive-free reaction conditions. The recyclability studies demonstrated that recycling and reusing the BCTPEA@Pd catalyst is very efficient up to the sixth recycle under optimal circumstances, but somewhat reduced yields were achieved after the seventh recycle. According to recyclability studies, a BCTPEA@Pd catalyst may be recycled and reused up to six times under ideal conditions. Unfortunately, yields started to decline after the seventh recycle. In addition, the BC-TPEA@Pd catalyst was recovered after the reaction was finished, and an ICP-OES analysis of the reaction mass revealed a less than 0.1 ppm Pd concentration. This demonstrated that the Pd NPs are securely attached to the catalyst support, making them less vulnerable to the leaching of Pd NPs from the support surface during the reaction [130].
5.3.3. Specific Organic Reactions
In a solvent-free Henry reaction carried out at room temperature, Musa acuminata (banana) peel ash (MAPA) was used as a heterogeneous catalyst for the production of C-C bonds. Rajkumari et al. studied the Henry reactions of several aromatic and aliphatic aldehydes with nitroalkanes in this work and achieved a great yield of β-nitroalcohols with no dehydrated product within 15–30 min. Through a series of reactions, the recyclability of the MAPA catalyst was investigated, and even after the tenth cycle, 95% of the product was generated. A hot filtering method was also used to evaluate the catalyst’s heterogeneity, and the results showed that none of the active metallic sites of the catalyst leached into the reaction media [131].
Ojer and colleagues reported, for the first time, acid biomass-derived carbons from vegetal biomass Hedychium gardnerianum through chemical activation with H3PO4 with impregnation ratios (g biomass: g H3PO4) of 1:1 and 3:1; i.e., RCB2 and RCB1 possessed a highly developed porosity obtained through the combination of pyrolysis and surface phosphonation. With a BET area of 2197 m2 g−1 and a pore volume of 1.39 cm3g−1, the obtained carbon material (RCB2) demonstrated highly promising textural properties comprising both micro- and mesopores. Under aerobic conditions, the material produced was able to efficiently catalyze the synthesis of quinoxalines from 1,2-diamines and ∝-hydroxy ketones, as shown in Figure 26. The results indicated that the combination of the acid function strength and textural properties were the main factors that influenced the conversion and selectivity towards the reaction. In the synthesis of quinoxaline from o-phenylendiamine and benzoin, these biomass-derived carbons demonstrated exceptional activity and selectivity, with a 94% conversion and 83% selectivity. Additionally, recycling experiments were carried out using an RCB2 sample, and the authors observed a diminished conversion values of around 10% for the second and third cycles with maintained selectivity (first run: 82%; second run: 72%; third run: 74%) [132].
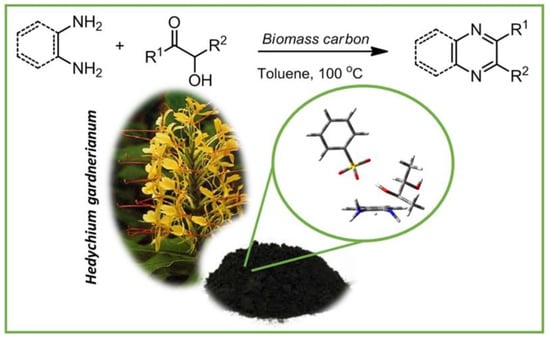
Figure 26.
Acid biomass-derived carbons from Hedychium gardnerianum biomass efficiently catalyze the synthesis of quinoxalines. Adapted with permission from ref. [132].
Bio oil, which is a condensable liquid obtained during the pyrolysis of the biomass, not only serves as a feedstock for the biofuel production, but it can also be used for the synthesis of functional carbon materials [133]. One such work was conducted by Ballotin and colleagues. They synthesized an amphiphilic carbon catalyst using the reaction of bio oil with sulfuric acid and used it for the room temperature, solvent free ketalization reaction of glycerol with 2-propanone to produce solketal (2,2-dimethyl-1,3-dioxolane-4-methanol) with a high catalytic activity of 93 ± 5%. The reuse reactions suggested that the material possessed a heterogeneous nature, as the material was stable under the reaction medium even after four uses [134].
Supriya and coworkers reported that the porous-nanocarbon spheres (PNCSs) can break down metal and oxidant-free azo compounds. These PNCSs were made from kitchen waste. The PNCSs produced by the pyrolysis of an onion peel at 1000 °C were discovered to be efficient catalysts for the reductive degradation of azo dyes in the presence of hydrazine hydrate, as shown in Figure 27. Under the influence of microwave radiation, azo linkages (-N=N-) were reductively cleaved. UV–visible spectroscopy was used to monitor the degradation process, which required 10 to 40 min to complete. It is interesting to note that the reductive degradation of the azo dyes led to the formation of corresponding amines, which were successfully employed to manufacture new azo compounds [135].
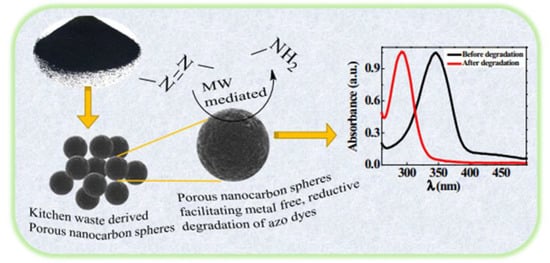
Figure 27.
Porous nanocarbon spheres derived from kitchen waste for metal-free azo dyes degradation. Adapted with permission from ref. [135].
In a one-pot microwave-induced pyrolysis process using a corn stover as a carbon precursor, Zhang and colleagues reported the synthesis of activated carbons (ACs) with P-containing functional groups. These ACs were then tested as the heterogeneous catalyst for selective monophenol production from cellulose pyrolysis, as shown in Figure 28. It was found that the porosity and peak intensities of the P-containing functional groups in the resulting ACs increased along with the phosphoric acid to corn stover ratios. Based on various mass ratios of phosphoric acid to corn stover, four distinct activated carbons were produced; these were designated as AC1, AC2, AC3, and AC4. The obtained ACs had an outstanding catalytic performance in the production of phenol, but when catalytic performance and phosphoric acid consumption were considered, the acid-to-biomass ratio of 0.85:1, i.e., AC3, was regarded as the optimal ratio [136].
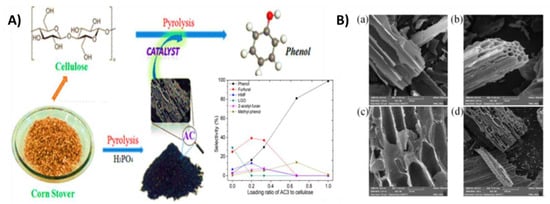
Figure 28.
(A) Schematic representation of fabrication of the activated carbons (ACs) enriched in P-containing functional groups from corn stover. (B) SEM images of corn-stover-derived activated carbons, AC1 (a), AC2 (b), AC3 (c), and AC4 (d). Adapted with permission from ref. [136].
6. Conclusions and Future Prospects
Carbon-based catalysts have recently become a research study focus due to their low cost, simple synthesis method, and ecologically friendly nature. Given that biomass is a renewable, abundant, and affordable precursor, it is reasonable to presume that making carbonaceous compounds directly from biomass would have a potential advantage. In addition to choosing the right biomass precursor, the synthesis method plays a significant role in producing carbon materials with superior physicochemical qualities. To develop biomass-derived carbon compounds with the characteristics required for future catalysis-related applications, it is necessary to put more effort into the development of greener synthetic methods. Hydrothermal carbonization is a new emerging method of the synthesis of biomass-derived carbons along with the considerably older technique of the controlled pyrolysis process. These synthetic techniques will assist in preserving the inherent, ordered porosity structure of the natural raw biomass, which can contribute distinguished textural qualities to the finished carbon materials and meet the high demands of numerous potential technological applications which otherwise are difficult to achieve in synthetic carbon precursors. There is a need to promote the synthesis of various other structural carbon materials such as carbon nanotubes, carbon nanospheres, etc. from biomass feedstock, which is now commonly made from synthetic sources that are comparatively expensive. The preparation of heterogeneous catalysts from biomass-derived carbons plays a significant role in the efficiency of the catalysts as it influences the uniform distribution of deposited metal or the number of the active sites that will be made available during the reaction. Surface-modified or -functionalized carbonaceous materials have already been demonstrated in a wide range of advanced catalytic applications. Hence, newer and simpler methods need to be developed that can consistently modify the surface of biomass-derived carbon and make them more effective as catalysts. Various examples of catalytic applications of carbonaceous materials derived from biomass illustrate their potential in the area of heterogeneous catalysis. The future of a more sustainable and benign-by-design processing of carbon materials as a potential replacement for current industrial catalytic systems will benefit from the development of nanomaterials and nanocomposites from biomass for a variety of applications in catalysis.
Author Contributions
Conceptualization, A.S. (Apoorva Shetty) and G.H.; methodology, V.M. and A.S. (Apoorva Shetty); validation, A.S. (Apoorva Shetty), V.M. and A.S. (Aman Sharma); formal analysis, A.S. (Apoorva Shetty); investigation, G.H.; writing—original draft preparation, A.S. (Apoorva Shetty); writing—review and editing, A.S. (Apoorva Shetty), V.M., A.S. (Aman Sharma), and G.H.; supervision, G.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, A.X.; Yip, C.K. Heterogeneous Catalysis: Enabling a Sustainable Future. Front. Catal. 2021, 1, 667675. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Padil, V.V.T.; Černík, M. Major Advances and Challenges in Heterogeneous Catalysis for Environmental Applications: A Review. Ecol. Chem. Eng. S 2018, 25, 9–34. [Google Scholar] [CrossRef]
- Li, J.; Stephanopoulos, M.F.; Xia, Y. Introduction: Heterogeneous Single-Atom Catalysis. Chem. Rev. 2020, 120, 11699–11702. [Google Scholar] [CrossRef]
- Van Houten, J. A Century of Chemical Dynamics Traced through the Nobel Prizes. 1998: Walter Kohn and John Pople. J. Chem. Educ. 2002, 79, 1297. [Google Scholar] [CrossRef]
- Fechete, I. Paul Sabatier—The father of the chemical theory of catalysis. Comptes Rendus Chim. 2016, 19, 1374–1381. [Google Scholar] [CrossRef]
- Friedrich, B. Fritz Haber: Chemist, Nobel laureate, German, Jew. By Dietrich stoltzenberg. Angew. Chem. Int. Ed. Engl. 2005, 44, 3957–3961. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Song, P.; Fu, J.; Jin, Z.; Ma, L.; Ge, J.; Liu, C.; Chen, Z.; Xing, W. Highly polarized carbon nano-architecture as robust metal-free catalyst for oxygen reduction in polymer electrolyte membrane fuel cells. Nano Energy 2018, 49, 23–30. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Wen, X.; Chen, X.; Wen, Y.; Shi, X.; Mijowska, E. High yield conversion of biowaste coffee grounds into hierarchical porous carbon for superior capacitive energy storage. Sci. Rep. 2020, 10, 3518. [Google Scholar] [CrossRef]
- Paraskeva, P.; Kalderis, D.; Diamadopoulos, E. Production of activated carbon from agricultural by-products. J. Chem. Technol. Biotechnol. 2008, 83, 581–592. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Ding, W.; Wu, M.; Liang, M.; Ni, H.; Li, Y. Sensitive Hydrazine Electrochemical Biosensor Based on a Porous Chitosan–Carbon Nanofiber Nanocomposite Modified Electrode. Anal. Lett. 2015, 48, 1551–1569. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-based heterogeneous catalysts for biodiesel production: A comprehensive review. Int. J. Energy Res. 2022, 46, 3782–3809. [Google Scholar] [CrossRef]
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Critical design of heterogeneous catalysts for biomass valorization: Current thrust and emerging prospects. Catal. Sci. Technol. 2016, 6, 7364–7385. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Veerakumar, P.; Veeramani, V.; Chen, S.M.; Madhu, R.; Liu, S.-B. Palladium Nanoparticle Incorporated Porous Activated Carbon: Electrochemical Detection of Toxic Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 1319–1326. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Z.; Na, C. Hierarchical carbon nanotube membrane-supported gold nanoparticles for rapid catalytic reduction of p-nitrophenol. ACS Sustain. Chem. Eng. 2013, 1, 746–752. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, M.; Sharma, C.; Choudhary, A.; Paul, S. Biomass-Derived Activated Carbon-Supported Copper Catalyst: An Efficient Heterogeneous Magnetic Catalyst for Base-Free Chan–Lam Coupling and Oxidations. ACS Omega 2021, 6, 19529–19545. [Google Scholar] [CrossRef]
- Samus, T.; Lang, B.; Rohn, H. Assessing the natural resource use and the resource efficiency potential of the Desertec concept. Sol. Energy 2013, 87, 176–183. [Google Scholar] [CrossRef]
- Rosales, L.; González, J.W. Transport properties of two finite armchair graphene nanoribbons. Nanoscale Res. Lett. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Julkapli, N.M.; Hamid, S.B.A. Functionalized Activated Carbon Derived from Biomass for Photocatalysis Applications Perspective. Int. J. Photoenergy. 2015, 2015, 218743. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Wang, Z.; Horton, R.; Mendivelso-Perez, D.; Smith, E.A.; Webster, T.E.; Laird, D.A.; van Leeuwen, J.H. Macroporous Carbon Supported Zerovalent Iron for Remediation of Trichloroethylene. ACS Sustainable Chem. Eng. 2017, 5, 1586–1593. [Google Scholar] [CrossRef]
- Guo, F.; Peng, K.; Liang, S.; Jia, X.; Jiang, X.; Qian, L. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis. Fuel 2019, 258, 116204. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology. J. Phys. Conf. Ser. 2007, 61, 643. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Sui, X.; Li, R.; Chen, C.; Chen, Q. Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ. Sci. Technol. 2014, 48, 10191–10197. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; Wang, Y.; Chen, S.; Wan, Y. Thermally reduced gold nanocatalysts prepared by the carbonization of ordered mesoporous carbon as a heterogeneous catalyst for the selective reduction of aromatic nitro compounds. J. Catal. 2016, 344, 313–324. [Google Scholar] [CrossRef]
- Supriya, S.; Ananthnag, G.S.; Shetti, V.S.; Nagaraja, B.M.; Hegde, G. Cost-effective bio-derived mesoporous carbon nanoparticles-supported palladium catalyst for nitroarene reduction and Suzuki–Miyaura coupling by microwave approach. Appl. Organomet. Chem. 2020, 34, e5384. [Google Scholar] [CrossRef]
- Deraz, N. The Importance of catalyst preparation. J. Ind. Environ. Chem. 2018, 2, 16–18. [Google Scholar]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials: Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Ertl, G.; Knozinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag: Weinheim, Germany, 1997. [Google Scholar]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for preparation of catalytic materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A review of preparation methods for supported metal catalysts. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2017; Volume 61, pp. 1–35. [Google Scholar]
- Chieffi, G.; Fechler, N.; Esposito, D. Valorization of lignin waste from hydrothermal treatment of biomass: Towards porous carbonaceous composites for continuous hydrogenation. RSC Adv. 2015, 5, 63691–63696. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Yamamoto, K.; Takahashi, H.; Muramatsu, A. Liquid-phase reductive deposition as a novel nanoparticle synthesis method and its application to supported noble metal catalyst preparation. Catal. Today 2008, 132, 81–87. [Google Scholar] [CrossRef]
- Regalbuto, J. Catalyst Preparation: Science and Engineering, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–488. [Google Scholar]
- Van, S.R.A.; van Leeuwen, P.W.N.M.; Moulijn, J.A.; Averill, B.A. Chapter 10. Preparation of supported catalysts. In Catalysis: An Integrated Approach; Elsevier: Amsterdam, The Netherlands, 1999; Volume 123, pp. 459–485. [Google Scholar]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.C. A metal-free carbon-based catalyst: An overview and directions for future research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; de Lecea, C.S.M. Heterogenization of homogeneous catalysts on carbon materials. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 55–78. [Google Scholar]
- Becerra, J.A.; González, L.M.; Villa, A.-L. A bio-inspired heterogeneous catalyst for the transformation of limonene from orange peel waste biomass into value-added products. Catal. Today 2018, 302, 250–260. [Google Scholar] [CrossRef]
- Mavrogiorgou, A.; Papastergiou, M.; Deligiannakis, Y.; Louloudia, M. Activated carbon functionalized with Mn(II) Schiff base complexes as efficient alkene oxidation catalysts: Solid support matters. J. Mol. Catal. A Chem. 2014, 393, 8–17. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Rahaman, M.; Giri, R. Surface Modification/Functionalization of Carbon Materials by Different Techniques: An Overview. In Carbon-Containing Polymer Composites; Rahaman, M., Khastgir, D., Aldalbahi, A.K., Eds.; Springer: Singapore, 2019; pp. 65–98. [Google Scholar]
- Noordadi, M.; Mehrnejad, F.; Sajedi, R.H.; Jafari, M.; Ranjbar, B. The potential impact of carboxylic-functionalized multi-walled carbon nanotubes on trypsin: A Comprehensive spectroscopic and molecular dynamics simulation study. PLoS ONE 2018, 13, e0198519. [Google Scholar] [CrossRef] [PubMed]
- Beltrama, A.; Melchionnaa, M.; Montini, T.; Nasi, L.; Gortec, R.J.; Pratoa, M.; Fornasiero, P. Improved activity and stability of Pd@CeO2 core–shell catalysts hybridized with multi-walled carbon nanotubes in the water gas shift reaction. Catal. Today 2015, 253, 142–148. [Google Scholar] [CrossRef]
- Tian, W.; Sun, H.; Duan, X.; Zhang, H.; Ren, Y.; Wang, S. Biomass-derived functional porous carbons for adsorption and catalytic degradation of binary micropollutants in water. J. Hazard. Mater. 2020, 389, 12188. [Google Scholar] [CrossRef] [PubMed]
- Peymanfar, R.; Moradi, F. Functionalized carbon microfibers (biomass-derived) ornamented by Bi2S3 nanoparticles: An investigation on their microwave, magnetic, and optical characteristics. Nanotechnology 2021, 32, 065201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, W.B.; Wang, J.T.; Liu, G.F.; Cao, H.L.; Lü, J. Amino-functionalized biomass-derived porous carbons with enhanced aqueous adsorption affinity and sensitivity of sulfonamide antibiotics. Bioresour. Technol. 2019, 277, 128–135. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, N.; Yin, F.; Yang, T.; Wu, P.; Dang, Z.; Liu, M.; Wei, X. Biomass-derived heteroatoms-doped mesoporous carbon for efficient oxygen reduction in microbial fuel cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.; Pan, Z.; Li, S.; Li, Q.; Lin, W. Preparation of carbonyl, hydroxyl, and amino-functionalized microporous carbonaceous nanospheres from syrup-based waste to remove sulfamethazine. Environ. Sci. Pollut. Res. Int. 2022, 29, 27688–27702. [Google Scholar] [CrossRef]
- Yang, H.; Nie, R.; Xia, W.; Yu, X.; Jin, D.; Lu, X.; Zhou, D.; Xia, Q. Co embedded within biomass-derived mesoporous N-doped carbon as an acid-resistant and chemoselective catalyst for transfer hydrodeoxygenation of biomass with formic acid. Green Chem. 2017, 19, 5714–5722. [Google Scholar] [CrossRef]
- Hao, X.; An, X.; Wang, P.; Ma, X.; Du, X.; Hao, X.; Abudula, A.; Guan, G. Biomass-Derived N-Doped Carbon for Efficient Electrocatalytic CO2 Reduction to CO and Zn-CO2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 3738–3747. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Gera, P.; Jha, M.K. Process optimization with acid functionalised activated carbon derived from corncob for production of 4-hydroxymethyl-2,2-dimethyl-1,3-dioxolane and 5-hydroxy-2,2-dimethyl-1,3-dioxane. Sci. Rep. 2021, 11, 8567. [Google Scholar] [CrossRef] [PubMed]
- José Luís Figueiredo, M.F.R.P. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Pérez Mayoral, E.; Matos, I.; Bernardo, M.; Durán-Valle, C.; Fonseca, I. Functional porous carbons: Synthetic strategies and catalytic application in fine chemical synthesis. In Emerging Carbon Materials for Catalysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 299–352. [Google Scholar]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Chen, Y.; Wang, F.; Hong, R.Y. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar]
- Zhang, B.B.; Chen, Y.; Wang, F.; Hong, R.Y. Surface modification of carbon black for the reinforcement of polycarbonate/acrylonitrile–butadiene–styrene blends. Appl. Surf. Sci. 2015, 351, 280–288. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Gontarek-Castro, E.; Ollik, K.; Lieder, M. Biomass-Derived Nitrogen Functionalized Carbon Nanodots and Their Anti-Biofouling Properties. Processes 2020, 9, 61. [Google Scholar] [CrossRef]
- Faisal, M.; Pamungkas, A.Z.; Krisnandi, Y.K. Study of Amine Functionalized Mesoporous Carbon as CO2 Storage Materials. Processes 2021, 9, 456. [Google Scholar] [CrossRef]
- Atta-Obeng, E.; Dawson-Andoh, B.; Felton, E.; Dahle, G. Carbon Dioxide Capture Using Amine Functionalized Hydrothermal Carbons from Technical Lignin. Waste Biomass Valorization 2019, 10, 2725–2731. [Google Scholar] [CrossRef]
- Straten, J.W.; Schleker, P.; Krasowska, M.; Veroutis, E.L.; Granwehr, J.; Auer, A.A.; Hetaba, W.; Becker, S.; Schlögl, R.; Heumann, S. Nitrogen-Functionalized Hydrothermal Carbon Materials by Using Urotropine as the Nitrogen Precursor. Chem. Eur. J. 2018, 24, 12298–12317. [Google Scholar] [CrossRef]
- Zheng, F.C.; Chen, Q.W.; Hu, L.; Yan, N.; Kong, X.K. Synthesis of sulfonic acid-functionalized Fe3O4@C nanoparticles as magnetically recyclable solid acid catalysts for acetalization reaction. Dalton Trans. 2014, 43, 1220–1227. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Pires, E.; Fraile, J.M. Carbon materials functionalized with sulfonic groups as acid catalysts. In Emerging Carbon Materials for Catalysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–298. [Google Scholar]
- Wang, X.; Liu, R.; Waje, M.M.; Chen, Z.; Bozhilov, K.N.; Feng, P. Sulfonated Ordered Mesoporous Carbon as a Stable and Highly Active Protonic Acid Catalyst. Chem. Mater. 2007, 19, 2395–2397. [Google Scholar] [CrossRef]
- Anderson, J.M.; Johnson, R.L.; Schmidt-Rohr, K.; Shanks, B.H. Solid state NMR study of chemical structure and hydrothermal deactivation of moderate-temperature carbon materials with acidic SO3H sites. Carbon N. Y. 2014, 74, 333–345. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ren, J.; Liu, X.; Lu, G.; Wang, Y. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, M.; Qi, C. One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon N. Y. 2010, 48, 1844–1848. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.W.; Molaei, R. Preparation of turmeric-derived sulfur-functionalized carbon dots: Antibacterial and antioxidant activity. J. Mater. Sci. 2022, 57, 2941–2952. [Google Scholar] [CrossRef]
- Fan, X.; Yu, C.; Ling, Z.; Yang, J.; Qiu, J. Hydrothermal synthesis of phosphate-functionalized carbon nanotube-containing carbon composites for supercapacitors with highly stable performance. ACS Appl. Mater. Interfaces 2013, 5, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Grishchenko, L.; Novychenko, N.S.; Matushko, I.; Zaderko, A.N.; Tsapyuk, G.G.; Mischanchuk, O.V.; Diyuk, V.E.; Lisnyak, V.V. Catalytic efficiency of activated carbon functionalized with phosphorus-containing groups in 2-propanol dehydration. Fr.-Ukr. J. Chem. 2021, 7, 46–56. [Google Scholar] [CrossRef]
- Park, S.E.; Yang, S.Y.; Kim, K.J. Boron-functionalized carbon felt electrode for enhancing the electrochemical performance of vanadium redox flow batteries. Appl. Surf. Sci. 2021, 546, 14894. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Zhou, J.; Lin, P.; Chen, C.; Chen, J.; Feng, H. Facile synthesis of halogenated multi-walled carbon nanotubes and their unusual photoluminescence. J. Mater. Chem. 2012, 22, 22113–22119. [Google Scholar] [CrossRef]
- Jing, H.; Shi, Y.; Wu, D.; Liang, S.; Song, X.; An, Y.; Hao, C. Well-defined heteroatom-rich porous carbon electrocatalyst derived from biowaste for high-performance counter electrode in dye-sensitized solar cells. Electrochim. Acta 2018, 281, 646–653. [Google Scholar] [CrossRef]
- Hao, M.; Dun, R.; Su, Y.; He, L.; Ning, F.; Zhou, X.; Li, W. In situ self-doped biomass-derived porous carbon as an excellent oxygen reduction electrocatalyst for fuel cells and metal–air batteries. J. Mater. Chem. A 2021, 9, 14331–14343. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Ji, S.; Feng, H.; Linkov, V.; Wang, R. Biomass-derived activated carbon as high-performance non-precious electrocatalyst for oxygen reduction. RSC Adv. 2013, 3, 12039–12042. [Google Scholar] [CrossRef]
- Wang, W.; Jia, Q.; Mukerjee, S.; Chen, S. Recent Insights into the Oxygen-Reduction Electrocatalysis of Fe/N/C Materials. ACS Catal. 2019, 9, 10126–10141. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Ma, J.; Dong, X.; Ku, W.; Wang, M.; Sun, H.; Liang, S.; Lu, G. Nitrogen and cobalt-doped porous biocarbon materials derived from corn stover as efficient electrocatalysts for aluminum-air batteries. Energy 2018, 162, 453–459. [Google Scholar] [CrossRef]
- Bianco, G.V.; Sacchetti, A.; Grande, M.; D’Orazio, A.; Milella, A.; Bruno, G. Effective hole conductivity in nitrogen-doped CVD-graphene by singlet oxygen treatment under photoactivation conditions. Sci. Rep. 2022, 12, 8703. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Yuan, Q.; Li, Q.; Zhang, J.; Sun, L.; Rui, D.; Chen, Z.; Jia, K.; Wang, M.; et al. Nitrogen cluster doping for high-mobility/conductivity graphene films with millimeter-sized domains. Sci Adv. 2019, 5, eaaw8337. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, e2004689. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Zhou, W.; Li, L.; Huang, S.; Chen, S. Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale 2015, 7, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ru, X.; Yang, X.; Bai, Z.; Yang, L. Red Blood Cells-Derived Iron Self–Doped 3D Porous Carbon Networks for Efficient Oxygen Reduction. Catalysts 2022, 12, 273. [Google Scholar] [CrossRef]
- Chen, W.F.; Iyer, S.; Iyer, S.; Sasaki, K.; Wang, C.H.; Zhu, Y.; Muckerman, J.T.; Fujita, E. Biomass-derived electrocatalytic composites for hydrogen evolution. Energy Environ. Sci. 2013, 6, 1818–1826. [Google Scholar] [CrossRef]
- Zhang, R.; Du, X.; Li, S.; Guan, J.; Fang, Y.; Li, X.; Dai, Y.; Zhang, M. Application of heteroatom doping strategy in electrolyzed water catalytic materials. J. Electroanal. Chem. 2022, 921, 116679. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Cao, J.; Guo, S.; Zheng, X.; Feng, J.; Qi, J. Activating and optimizing the activity of NiCoP nanosheets for electrocatalytic alkaline water splitting through the V doping effect enhanced by P vacancies. J. Mater. Chem. A 2019, 7, 24486–24492. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Xu, T.; Liu, B.; Huang, K.; Qiao, L.; Liu, S.; Cao, J.; Jun, S.C.; Yamauchi, Y.; et al. Atomic-level platinum filling into Ni-vacancies of dual-deficient NiO for boosting electrocatalytic hydrogen evolution. Adv. Energy Mater. 2022, 12, 2200434. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, C.; Wang, Y.; Chen, R.; Wang, S. Recent advances in defect electrocatalysts: Preparation and characterization. J. Energy Chem. 2021, 53, 208–225. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, e1804672. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, L.; Koh, S.W.; Ge, J.; Fei, J.; Yao, M.; Hong, W.; Liu, S.; Yamauchi, Y.; Li, H. Photovoltaic-powered supercapacitors for driving overall water splitting: A dual-modulated 3D architecture. Carbon Energy 2022, 4, 1262–1273. [Google Scholar] [CrossRef]
- Raut, A.S.; Parker, C.B.; Stoner, B.R.; Glass, J.T. Effect of porosity variation on the electrochemical behavior of vertically aligned multi-walled carbon nanotubes. Electrochem. Commun. 2012, 19, 138–141. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Wang, Y.; Xun, X.; Liao, T.; Du, Y.; Bai, Z.; Dou, S. Defect Sites-Rich Porous Carbon with Pseudocapacitive Behaviors as an Ultrafast and Long-Term Cycling Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 9353–9361. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.G.; Arulraj, A.; Jagadish, P.; Khalid, M.; Nasrollahzadeh, M.; Fen, R.; Yang, C.C.; Hegde, G. Pore size matters!—A critical review on the supercapacitive charge storage enhancement of biocarbonaceous materials. Crit. Rev. Solid State Mater. Sci. 2022, 1–56. [Google Scholar] [CrossRef]
- Li, J.; Gao, S.; Li, B.; Li, Y.; Cheng, C.; Maouche, C.; Wu, Y.; Rahman, N.; Zhou, Y.; Yang, J. Biomass-derived nitrogen-doped porous carbons with ultra-high surface area for electrocatalytic oxygen reduction reaction. J. Electroanal. Chem. 2020, 878, 114542. [Google Scholar] [CrossRef]
- Liu, X.; Culhane, C.; Li, W.; Zou, S. Spinach-Derived Porous Carbon Nanosheets as High-Performance Catalysts for Oxygen Reduction Reaction. ACS Omega 2020, 5, 24367–24378. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.T.; Hegde, S.; Akshaya, K.B.; Kannan, P.; Varghese, A.; Hegde, G. MnO2-Pi on Biomass Derived Porous Carbon for Electro-Catalytic Oxidation of Pyridyl Carbinol. J. Electrochem. Soc. 2020, 167, 155513. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Zhang, S.; Lv, Z.; Yang, D.; Liu, J.; Chen, Y.; Tian, X.; Jin, H.; Song, W. Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 5740–5745. [Google Scholar] [CrossRef]
- Daniel, G.; Zhang, Y.; Lanzalaco, S.; Brombin, F.; Kosmala, T.; Granozzi, G.; Wang, A.; Brillas, E.; Sirés, I.; Durante, C. Chitosan-Derived Nitrogen-Doped Carbon Electrocatalyst for a Sustainable Upgrade of Oxygen Reduction to Hydrogen Peroxide in UV-Assisted Electro-Fenton Water Treatment. ACS Sustain. Chem. Eng. 2020, 8, 14425–14440. [Google Scholar] [CrossRef]
- Ma, P.; Lu, W.; Yan, X.; Li, W.; Li, L.; Fang, Y.; Yin, X.; Liu, Z.; Lin, Y. Heteroatom tri-doped porous carbon derived from waste biomass as Pt-free counter electrode in dye-sensitized solar cells. RSC Adv. 2018, 8, 18427–18433. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Zhang, H.; Zhang, P.; Tian, Z.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Intrinsic defects in biomass-derived carbons facilitate electroreduction of CO2. Nano Res. 2020, 13, 729–735. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, C.W.; Shak, K.P.Y.; Lim, S.; Cheam, W.C.; Koo, C.H.; Abdullah, A.Z. Biomass-Based Photocatalysts for Environmental Applications. In Nanophotocatalysis and Environmental Applications: Detoxification and Disinfection; Inamuddin Asiri, A.M., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 30, pp. 55–86. [Google Scholar]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Łomot, D.; Chernyayeva, O.; Lisovytskiy, D. Novel biomass-derived hybrid TiO2/carbon material using tar-derived secondary char to improve TiO2 bonding to carbon matrix. J. Anal. Appl. Pyrolysis 2018, 131, 35–41. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, H.; Wang, C.; Jin, C.; Sun, Q. One Step Construction of Nitrogen–Carbon Derived from Bradyrhizobium japonicum for Supercapacitor Applications with a Soybean Leaf as a Separator. ACS Sustain. Chem. Eng. 2018, 6, 4695–4704. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Chen, J. Revealing the role of surface elementary doping in photocatalysis. Catal. Sci. Technol. 2022, 12, 3634–3638. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef]
- Zhou, J.W.; Jiang, X.; Chen, Y.X.; Lin, S.W.; Lu, C.Z. N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol-Water Mixtures Photocatalytic Hydrogen Production. Nanomaterials 2022, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, C.; Primo, A.; Molinari, R.; Garcia, H. N-doped graphene derived from biomass as a visible-light photocatalyst for hydrogen generation from water/methanol mixtures. Chem. Eur. J. 2014, 20, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Y.; Li, Y.; Meng, Q.; Duan, T. Functionally integrated g-C3N4@wood-derived carbon with an orderly interconnected porous structure. Appl. Surf. Sci. 2021, 540, 148440. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, Z.; Wang, Q. Renewable biomass-derived carbon-supported g-C3N4 doped with Ag for enhanced photocatalytic reduction of CO2. J. Colloid Interface Sci. 2022, 606, 1311–1321. [Google Scholar] [CrossRef]
- He, B.; Feng, M.; Chen, X.; Zhao, D.; Sun, J. One-pot construction of chitin-derived carbon/g-C3N4 heterojunction for the improvement of visible-light photocatalysis. Appl. Surf. Sci. 2020, 527, 146737. [Google Scholar] [CrossRef]
- Son, B.T.; Long, N.V.; Nhat Hang, N.T. The development of biomass-derived carbon-based photocatalysts for the visible-light-driven photodegradation of pollutants: A comprehensive review. RSC Adv. 2021, 11, 30574–30596. [Google Scholar] [CrossRef]
- Wang, H.; Qu, M.; Wang, S.; Liu, X.; Li, L. Fabrication of high-performance biomass derived carbon/metal oxide photocatalysts with trilevel hierarchical pores from organic–inorganic network. Adv. Sustain. Syst. 2019, 3, 1800169. [Google Scholar] [CrossRef]
- Faisal, M.; Alsaiari, M.; Rashed, M.A.; Harraz, F.A. Highly efficient biomass-derived carbon@Au/ZnO novel ternary photocatalyst for ultra-fast degradation of gemifloxacin drug. J. Mater. Res. Technol. 2021, 14, 954–967. [Google Scholar] [CrossRef]
- Ma, R.M.; Dai, L.; Qin, G.G. High-performance nano-Schottky diodes and nano-MESFETs made on single CdS nanobelts. Nano Lett. 2007, 7, 868–873. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Pan, X.; Fu, X.; Liu, S.; Xu, Y.J. Assembly of CdS Nanoparticles on the Two-Dimensional Graphene Scaffold as Visible-Light-Driven Photocatalyst for Selective Organic Transformation under Ambient Conditions. J. Phys. Chem. C 2011, 115, 23501–23511. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Xing, R.; Wang, Y.; Wang, S.; Wei, D.; Li, J.; Chen, Z.; Lü, J. Preparation of carbon-supported CdS photocatalysts with high performance of dye photodegradation using cadmium-enriched Perilla frutescens biomass. Inorg. Chem. Commun. 2019, 109, 107559. [Google Scholar] [CrossRef]
- Huang, H.B.; Wang, Y.; Jiao, W.B.; Cai, F.Y.; Shen, M.; Zhou, S.G.; Cao, H.L.; Lü, J.; Cao, R. Lotus-Leaf-Derived Activated-Carbon-Supported Nano-CdS as Energy-Efficient Photocatalysts under Visible Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 7871–7879. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, R.L.; Pan, Z.J.; Liao, Y.; Xiong, C.B.; Chen, M.L.; Huang, R.; Pan, X.H.; Chen, Z. Preparation of CdS@C Photocatalyst Using Phytoaccumulation Cd Recycled From Contaminated Wastewater. Front Chem. 2021, 9, 717210. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ye, R.; Yan, X.; Wang, L.; Jian, P. Versatile bifunctional nitrogen-doped porous carbon derived from biomass in catalytic reduction of 4-nitrophenol and oxidation of styrene. Chin. J. Catal. 2020, 41, 1217–1229. [Google Scholar] [CrossRef]
- Hu, X.; Fan, M.; Zhu, Y.; Zhu, Q.; Song, Q.; Dong, Z. Biomass-derived phosphorus-doped carbon materials as efficient metal-free catalysts for selective aerobic oxidation of alcohols. Green Chem. 2019, 21, 5274–5283. [Google Scholar] [CrossRef]
- Tang, Q.; Yuan, Z.; Jin, S.; Yao, K.; Yang, H.; Chi, Q.; Liu, B. Biomass-derived carbon-supported Ni catalyst: An effective heterogeneous non-noble metal catalyst for the hydrogenation of nitro compounds. React. Chem. Eng. 2019, 5, 58–65. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, X.; Wu, F.; Lu, B.; Huang, B.; Chen, Y.; Lin, G. Fabrication of P-Doped Porous Carbon Catalysts, with Inherent N Functionality, from Waste Peanut Shells and Their Application in the Metal-Free Aerobic Oxidation of Alcohols. ACS Sustainable Chem. Eng. 2022, 10, 911–922. [Google Scholar] [CrossRef]
- Scharnagl, F.K.; Hertrich, M.F.; Ferretti, F.; Kreyenschulte, C.; Lund, H.; Jackstell, R.; Beller, M. Hydrogenation of terminal and internal olefins using a biowaste-derived heterogeneous cobalt catalyst. Sci. Adv. 2018, 4, eaau1248. [Google Scholar] [CrossRef]
- Patel, A.R.; Asatkar, A.; Patel, G.; Banerjee, S. Synthesis of rice husk derived activated mesoporous carbon immobilized palladium hybrid nano-catalyst for ligand-free mizoroki-heck/Suzuki/sonogashira cross-coupling reactions. Chem. Select. 2019, 4, 5577–5584. [Google Scholar] [CrossRef]
- Ferlin, F.; Valentini, F.; Sciosci, D.; Calamante, M.; Petricci, E.; Vaccaro, L. Biomass Waste-Derived Pd–PiNe Catalyst for the Continuous-Flow Copper-Free Sonogashira Reaction in a CPME–Water Azeotropic Mixture. ACS Sustain. Chem. Eng. 2021, 9, 12196–12204. [Google Scholar] [CrossRef]
- Kempasiddaiah, M.; Sree Raj, K.A.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Yelamaggad, C.V.; Sekhar, R.C.; Patil, S.A. Waste biomass-derived carbon-supported palladium-based catalyst for cross-coupling reactions and energy storage applications. Appl. Surf. Sci. 2021, 570, 151156. [Google Scholar] [CrossRef]
- Rajkumari, K.; Das, D.; Pathak, G.; Rokhum, S.L. Waste-to-useful: A biowaste-derived heterogeneous catalyst for a green and sustainable Henry reaction. N. J. Chem. 2019, 43, 2134–2140. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; Blazquez-García, R.; Matos, I.; Bernardo, M.; Fonseca, I.M.; Pérez Mayoral, E. Porous carbons-derived from vegetal biomass in the synthesis of quinoxalines. Mechanistic insights. Catal. Today 2020, 354, 90–99. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, G.; Tian, H.; Gao, Z.; Zhang, S.; Xu, L.; Hu, X. Carbon materials derived from polymerization of bio-oil as a catalyst for the reduction of nitrobenzene. Sustain. Energy Fuels 2021, 5, 2952–2959. [Google Scholar] [CrossRef]
- Carvalho Ballotin, F.; da Silva, M.J.; Paula de Carvalho Teixeira, A.; Montero Lago, R. Amphiphilic acid carbon catalysts produced by bio-oil sulfonation for solvent-free glycerol ketalization. Fuel 2020, 274, 117799. [Google Scholar] [CrossRef]
- Supriya, S.; Ananthnag, G.S.; Maiyalagan, T.; Hegde, G. Kitchen Waste Derived Porous Nanocarbon Spheres for Metal Free Degradation of Azo Dyes: An Environmental Friendly, Cost Effective Method. J. Cluster Sci. 2022, 251, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, H.; Yang, Z.; Qian, K.; Villota, E. Renewable High-Purity Mono-Phenol Production from Catalytic Microwave-Induced Pyrolysis of Cellulose over Biomass-Derived Activated Carbon Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 5349–5357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).