Abstract
Copper manganese oxide spinels and related (multiphase) materials with the formula CuxMn3−xO4 are the active catalysts in a wide variety of industrially important processes due to their great diversity in their phase relations, metal ion valence/site distribution, and chemical properties. In this review, we summarize the preparation methods and their effects on the composition, properties, and catalytic properties of various CuxMn3−xO4 catalysts with various Cu/Mn ratios. The main summarized catalytic reactions are the oxidation of carbon monoxide, nitrogen oxide, and hydrogen sulfide and the oxidative removal of organic solvents such as benzene, toluene, and xylene from the air. Some industrially important reactions (steam reforming of methanol or synthesis gas) and the manufacture of organic chemicals (methyl formate, propylene oxide, and benzyl alcohol) catalyzed by CuxMn3−xO4 spinels are also reviewed.
1. Introduction
From both fundamental and applicative points of view, copper manganese oxides, especially the copper manganese spinel compounds and related (multiphase) materials with CuxMn3−xO4 formula, are important catalysts because of their great diversity in their phase relations, metal ion valence/site distribution, and chemical properties. Copper and manganese oxide-based materials are widely used catalysts in many industrially important processes such as room-temperature CO conversions, exhaust gas purification, oxidation of harmful organic pollutants, and methanol conversions [1,2,3,4,5]. The most important representatives of the system are the Cu0.5Mn2.5O4, CuMn2O4, and Cu1.5Mn1.5O4 phases, but the solid solutions of CuxMn3−xO4 spinels (0 < x < 3), and composite materials with inhomogeneous phase distribution are also known, which have special properties due to stresses in the mixed crystals/solid solutions/phase boundaries [1,2,3,4,5].
Hopcalite was the first copper manganese oxide catalyst used in the long term for CO removal from air [6], and depending on the production conditions, may contain various amounts and kinds of CuxMn3−xO4 spinels together with copper, manganese, and copper manganese oxides. The presence of CuxMn3−xO4 components in various copper manganese oxide-based catalysts strongly depends on the synthesis conditions, and sometimes on the catalytic reaction condition due to the reductive/oxidative nature of the atmosphere, and reactants also have a strong influence on the composition/phase relations of the catalysts used. The crystallinity, composition, and chemical state of phases and surface layers in CuxMn3−xO4 catalysts, including the presence of amorphous materials, distorted crystal lattices, and the number of oxygen and metal vacancies are key parameters in the development of catalysts with high activity. The newest trends belong to the so-called “defect and synergic engineering” methods with the aim of enhancing the oxygen replenishment capacity of CuxMn3−xO4 phases. The amount and distribution of copper and oxygen vacancies are regulated with the preparation and annealing conditions. The valence of copper and manganese ions and the distribution of the CuI,II and MnII,III,IV ions between the crystalline and amorphous phases are key parameters, both in the bulk and surface layer, including the distribution of these ions between the tetrahedral and octahedral spinel sites. These features of CuxMn3−xO4 spinels can be controlled with a wide variety of reaction and annealing conditions, including the reaction routes, Cu/Mn ratios, metal valences, counter-ions, reagents, metal compound concentrations, temperature, time, and other factors.
In this review, we focused on the effect of synthesis routes (and consequently, the composition and structure) on the catalytic efficiency of CuxMn3−xO4 spinels. We summarized the influence of the preparation conditions on the composition, structure, and catalytic properties of CuxMn3−xO4 spinels and mixed-phase composites with various Cu/Mn ratios containing CuxMn3−xO4 spinel components (Tables 1–3). It is a widely accepted fact that the CuxMn3−xO4 spinel phase is one of the main active phases in the mixed Mn−Cu oxides in various catalytic oxidation reactions [7,8]. The adsorbed surface oxygen concentration and the Cu2+ + Mn3+ ↔ Cu+ + Mn4+ redox cycle play a key role in these processes, and oxygen vacancies that form ensure the regeneration ability of the copper manganese oxide catalysts. The presence and activity of the active spinel phases depend on the distribution of ions between the crystalline sites, indirectly on the synthesis conditions. Therefore, we summarized and compared the influence of the synthetic routes (ceramic, precipitation, redox, and combined methods) on the composition and catalytic properties of the copper manganese oxide spinel catalysts.
The reviewed catalytic reactions were the oxidation of carbon monoxide (Table 4), the removal of organic and inorganic volatiles such as benzene, toluene, trichloroethylene, NO, or H2S from air (Tables 5–9), the manufacturing of some organic chemicals (methyl formate, propylene oxide, and benzyl alcohol) (Tables 10–12) and the steam reforming of methanol (hydrogen production) (Table 13). These examples show the enormous industrial and environmental protection significance of these cheap and easily available noble metal-free spinel catalysts that contain copper and manganese oxide. Defect and synergism engineering can develop new kinds of copper manganese oxide catalysts with increased efficiency and selectivity in various reactions. Doping the known and newly prepared copper manganese oxide spinels is a prospective field of modifying and developing new noble metal-free catalyst systems.
2. Preparation Routes of CuxMn3−xO4 Spinels
The main spinel preparation methods consist of solid-phase reactions of copper and manganese oxides. These oxides can be prepared in situ from their precursors (hydroxide, carbonate, oxalate, or other compounds) precipitated from solutions with precipitation, gel-forming, and redox precipitation [9,10,11,12,13]. A special case of the stoichiometric CuMn2O4 synthesis is based on a solid-phase thermal decomposition of copper permanganate or its complexes with reducing ligands like ammonia [14].
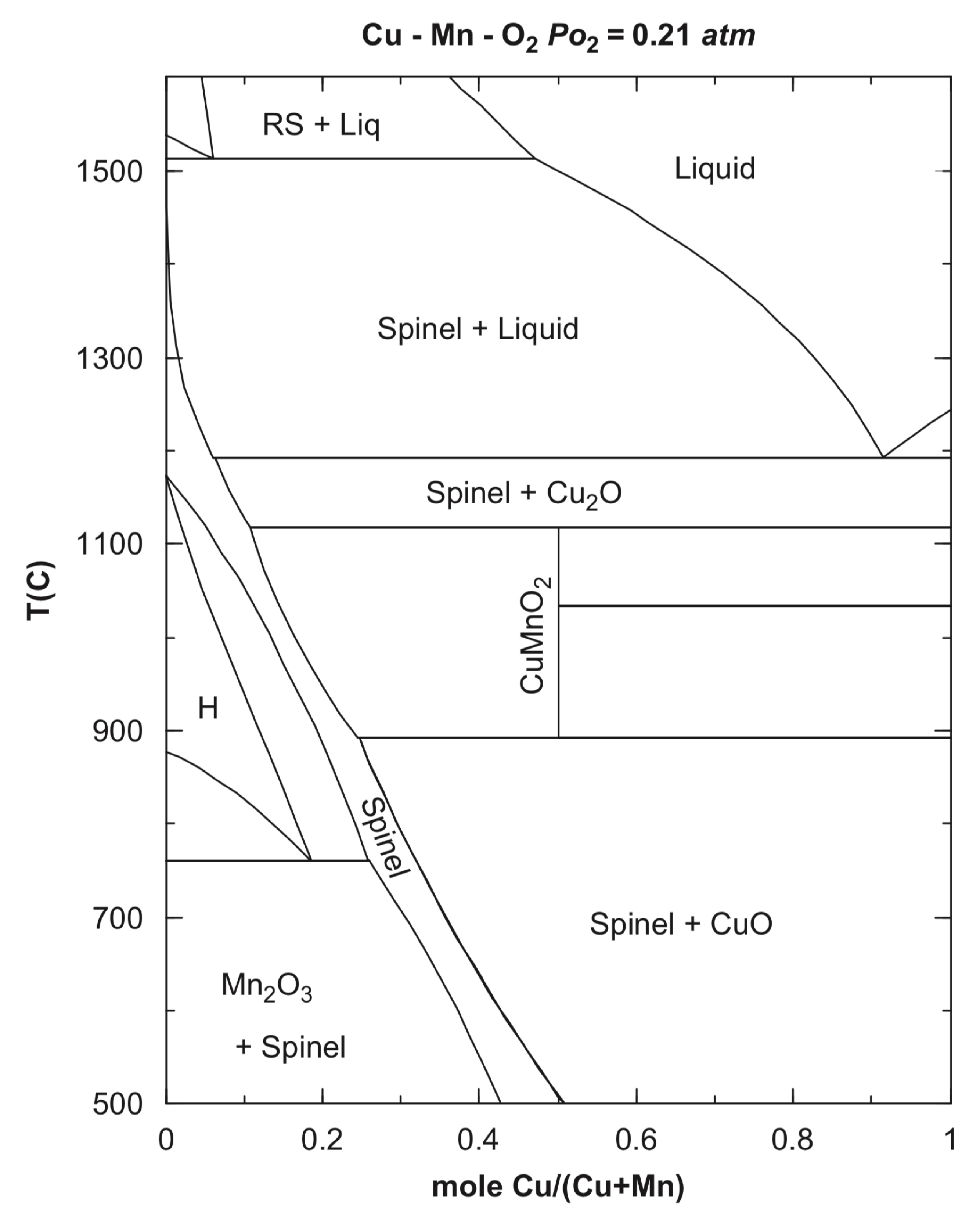
The calculated Cu–Mn–O2 phase diagram shows the temperature–composition relationships [10] (Figure 1).
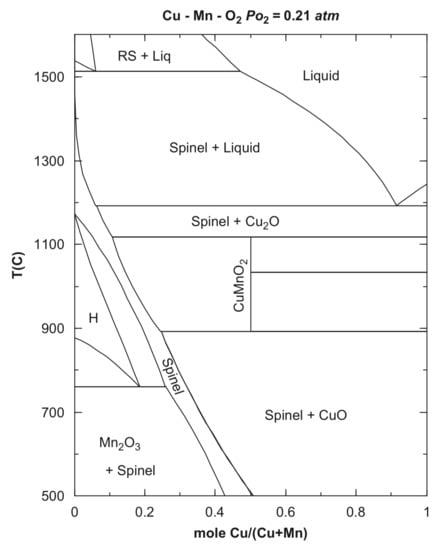
Figure 1.
The calculated phase diagram of the Cu–Mn–O2 system (reproduced from [10]).
In order to ensure homogeneous distribution of the reacting precursors, various methods have been developed over the simple grinding of oxide precursors, e.g.,
- -
- precipitation of copper and manganese hydroxides or carbonates or oxalates directly from aqueous solutions with bases or with special techniques such as spray drying, immersion, or alginate xerogel formation
- -
- hydrothermal and solvothermal methods with the transformation of the precursors containing metal into reactive materials; these methods include hydrolysis or co-precipitation with alkaline materials, such as ammonia that formed from the hydrolysis of urea or hexamethylenetetramine
- -
- solution and solid–phase redox reactions, such as oxidation of low-valence Cu or Mn compounds with permanganate or other oxidants, with subsequent heat treatment or the thermal decomposition of copper permanganate or its complexes that have reducing ligands such as ammonia.
2.1. Ceramic and Related Processes from Solid Precursors
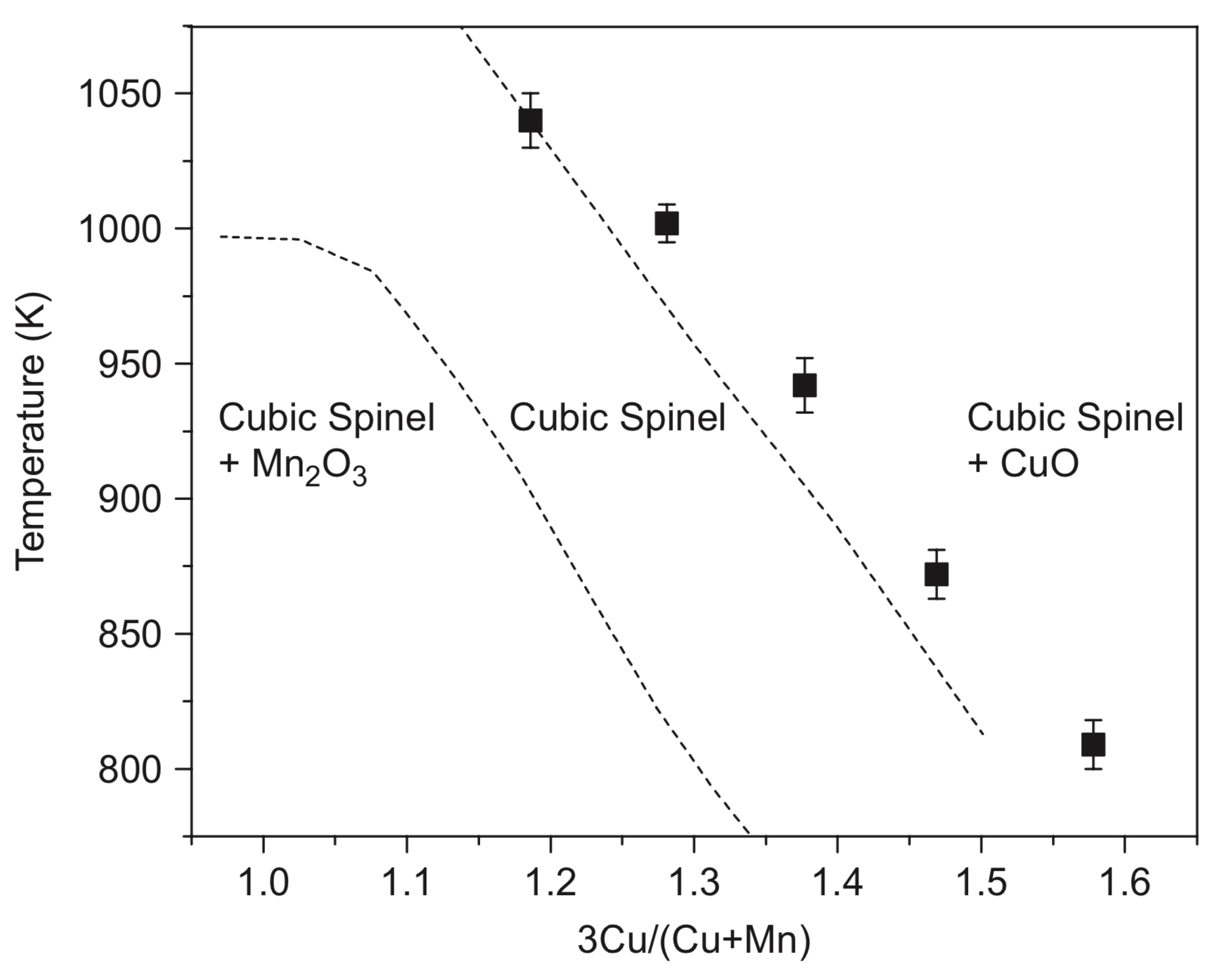
The stability area of the CuxMn3−xO4 spinels (Figure 2) shows that compounds with x > 1 at atmospheric oxygen pressure can be prepared from the corresponding copper and manganese oxides only above 900 °C. The copper-rich spinels such as Cu1.5Mn1.5O4 (x = 1.5) can be prepared below 900 °C only at elevated oxygen pressures, but due to the rather slow diffusion of copper and manganese ions in oxides below 900 °C, long enough sintering time and/or highly reactive precursors have to be used. In the preparation of manganese-rich spinels, such as CuMn2O4 (x = 1), the clustering of MnIII ions causes complications [9].
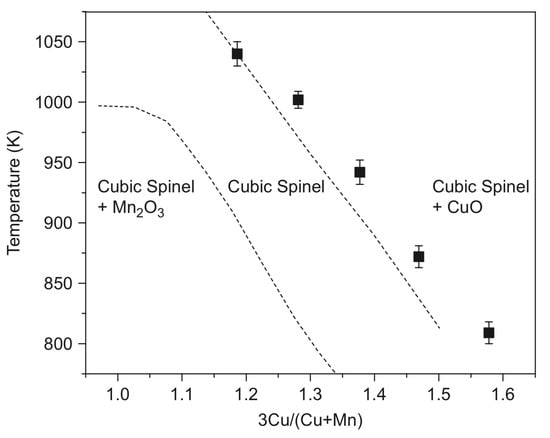
Figure 2.
Stability region of the cubic spinels in the Cu–Mn–O system in air (reproduced from [9]).
The high-temperature sintering of the finely divided copper and manganese oxides in the appropriate ratio is the most suitable method for the preparation of compounds CuxMn3−xO4 with x = ~1.0, because the reaction temperature can be selected as high as possible (Figure 2) [9]. For example, CuxMn3−xO4 compounds with 0.98 < x < 1.10 were prepared by sintering the mixtures of CuO and Mn3O4 in air at temperatures between 900 and 940 °C for 72 h. Quenching in water resulted in samples of slightly tetragonally distorted spinels with x < 1.06, whereas at x > 1.06 values (higher copper concentrations), cubic spinels are produced [9]. Spray granulation of CuO and Mn3O4 in a spouted bed system in a molar ratio of 1:2 with subsequent calcination at 1125 °C for 2 h resulted in Cu1.5Mn1.5O4 [15]. A solid-state reaction of CuO and MnO2 powders mixed in the desired proportions resulted in CuxMn3−xO4 spinels with x = 1.1–1.6 at 940 °C for 24 h in air with intermittent grindings [10]. Two cubic spinels, Cu1.15Mn1.85O4 and Cu1.47Mn1.53O4, were prepared similarly in the presence of 5 wt.% graphite as a pore-modifier additive at 950 °C for 6 h. These spinels contained macropores formed by the evolved CO2 gas [12]. Precursors of metal oxides, e.g., copper and manganese carbonates mixed at Mn:Cu 3:1, 1:1, and 1:3 ratios, were used to prepare Cu–Mn spinels by heating at 750 and 1000 °C [11,16]. The stoichiometric CuMn2O4 was prepared analogously at 700 °C for 240 h [17]. Spray-drying of water-soluble precursors, e.g., metal nitrates, ensured avoiding macroscopic separation. The homogeneous aq. solution of copper(II) and manganese(II) nitrate mixtures were nebulized and dried with a hydrogen burner at 300 °C. The obtained solid mixed metal nitrate particles were partially decomposed into oxides when kept at 350 °C for 24 h. A series of cubic CuxMn3−xO4 spinels with 1.1 < x < 1.5 [9] was prepared in this way.
A supported mixed-phase Cu1.4Mn1.6O4 catalyst with CuO and/or Mn2O3 content was synthesized by incipient wetness impregnation with the use of copper nitrate trihydrate and manganese(I) nitrate tetrahydrate and anatase. The aqueous solutions were mixed with anatase, the mixture was dried at 100 °C for 5 h, and calcined at 500 °C for 7 h in air [18].
A mechanochemical route to prepare solid spinel precursors was also developed [19]. Solid Mn(CH3COO)2·4H2O, copper acetate Cu(CH3COO)2·4H2O, and oxalic acid (H2C2O4·2H2O) were ball-milled in a molar ratio of MnII:CuII:oxalic acid of 1 − x:x:0.5 (x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5) at room temperature for 3 h. The powdered mixture was calcined at 550 °C for 2 h in air [19]. A similar process based on oxalate precursor formation is when solid Cu2CO3(OH)2 and MnCO3 were premixed in a 1:2 molar ratio with 20% stoichiometric excess of solid oxalic or citric acid, and the mixtures were ground under ambient conditions in a planetary mill at a speed of 600 rpm for 2 h. Calcination at 300 and 500 °C in air for 4 h resulted in CuMn2O4 [20].
A short summary of the ceramic (solid-state) preparation methods of catalysts containing CuxMn3−xO4 phases is given in Table 1. The solid-phase thermal decomposition of copper permanganates is discussed separately.

Table 1.
Synthesis conditions of CuxMn3−xO4 catalysts with solid-phase methods.
The solid-state methods started from oxides or carbonate, or nitrate oxide precursors require elevated temperatures (>700 °C); thus these methods are favorable for preparing crystalline spinels (Table 1). The spinels with x = 0.98–1.6 can be prepared with these solid-phase methods, and the solid precursor oxides can be homogenized with classical intermittent grindings [10] or spray drying [15]. Either cubic or tetragonally distorted spinels can be prepared by selecting the appropriate precursor composition and annealing temperature (Figure 1 and Figure 2) [20]. Pore-forming agents such as graphite resulted in porous materials [12]. The intensive milling of the solid oxalic or citric acid with solid carbonate or acetate salts resulted in mechanochemical reactions and the formation of oxalate/citrate precursors. These precursors were transformed into CuMn2O4 at lower temperatures (300–550 °C) [17,19] than the appropriate metal oxides or a mixture of oxides and carbonates (800–940 °C) [21], or carbonates (750–1000 °C) [16].
2.2. In Situ Precipitation of Solid Precursors from Solutions
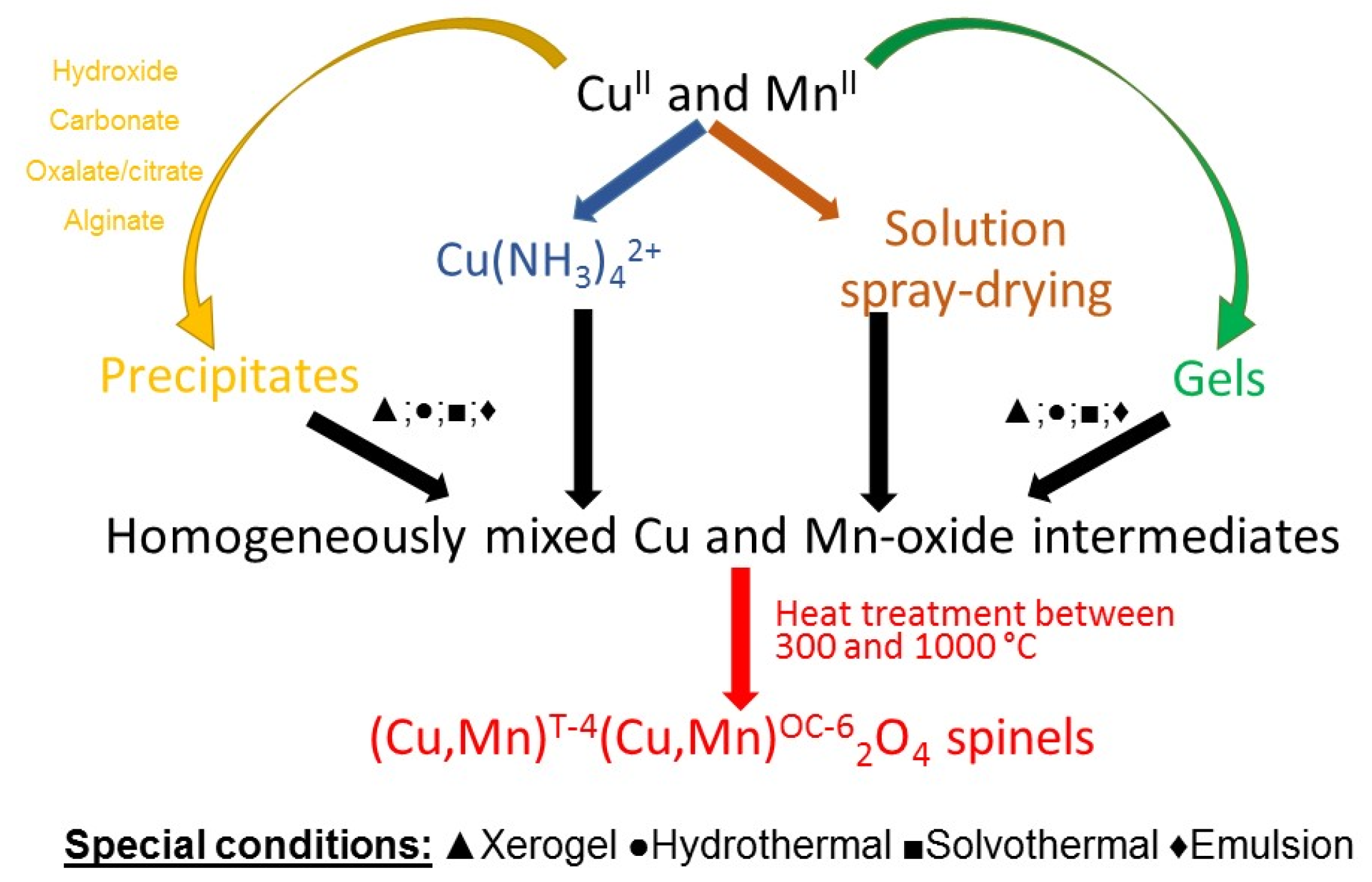
Mixing the dissolved water-soluble precursors in solutions and their transformation in situ into homogeneously mixed solid precursors—mainly hydroxides, carbonates, or oxalates—is one of the most frequently used methods (Scheme 1). Sodium hydroxide and ammonium hydroxide or sodium and ammonium carbonates are the most frequently used precipitation agents. Water-soluble copper and manganese salts (most frequently nitrates, sulfates, chlorides, and acetates) are used as precursors containing metal [21,22,23,24].
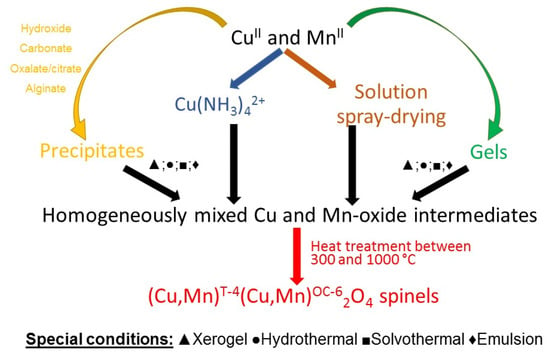
Scheme 1.
Summarizing the reaction routes to prepare CuxMn3−xO4 spinels with methods based on precipitation.
The single-phase cubic CuxMn3−xO4 spinels with 1.1 < x < 1.5 values were prepared by sintering the co-precipitated copper and manganese hydroxide mixtures in an oxygen atmosphere between 500 and 800 °C for 48 h [9]. Similarly, the aqueous solutions containing copper and manganese acetates or nitrates (1:2 molar ratio of copper to manganese) were mixed with 4 M aq. NaOH at 298 K until the final pH of 11 was reached. CuMn2O4 formed after 30 min aging at 298 K, drying in air at 50 °C for 24 h, and calcination at 300 °C for 2 h [25]. Instead of NaOH, organic bases can also be used, such as tetramethylammonium hydroxide. CuMn2O4 was prepared from the aqueous solutions of Cu(NO3)2·3H2O and 2 equiv. of Mn(NO3)2·4H2O [26,27], by adding aq. tetramethylammonium hydroxide at 25 °C for 20 min, followed by drying and calcining the precipitate at 100 °C for 24 h and 400 °C for 5 h, respectively [26].
If ammonium hydroxide acts as a hydroxide ion source in the synthesis of the spinel containing Cu, the ammonia has a double role: a precipitating and a complex-forming agent. The starting salts that contain copper immediately turn into [tetraamminecopper(II)] complexes, and the precipitation of copper(II) and manganese hydroxides arises from the self-protonation of the [tetraamminecopper(II)] ion [27,28]. A series of Cu–Mn mixed oxide catalysts with Cu/Mn molar ratios of 2:1, 1.5:1, 1:1, and 0.5:1 containing Cu1.5Mn1.5O4 as the active phase was prepared with this method. A 1 M Mn(NO3)2 aqueous solution with pH = 3–4 was dropped into an ammoniacal copper salt solution with pH = 9–10 at 30–40 °C. The precipitate was aged for 5 h and then calcined at 550 °C for 4 h [29,30]. The ammonia can also be generated in situ in hydrothermal/solvothermal reactions of urea or hexamethylenetetramine [13,31]. The advantages of the precipitation methods are combined with the positive effect of harsh reaction conditions (high temperature and pressure), and the hydrolysis with H2O. A hydrothermal reaction was used to prepare mixed oxide phases containing Cu1.5Mn1.5O4 with an overall Cu/Mn ratio < 0.2 from copper nitrate, manganese nitrate, citric acid, urea, and a KMnO4 solution (Mn(NO3)2/KMnO4 and urea/citric acid/MnII + KMnO4/Cu ratios were 1:2 and 1.5:1.5:1:0−0.2, respectively). Aqueous ammonia solution was added dropwise until a pH of 6–7 was reached. Then, the mixture was heated at 180 °C for 12 h in an autoclave. The precipitate was dried and calcined at 350 °C in air for 4 h [32].
The most frequently used method to prepare Cu and Mn-containing intermediates for spinel synthesis is the precipitation of a mixture of Cu and Mn carbonates/basic carbonates with the use of water-soluble carbonate compounds, mainly with sodium and ammonium carbonate. For example, CuMn2O4 was prepared from an aqueous solution containing copper nitrate trihydrate and manganese nitrate hexahydrate in a 1:2 molar ratio by treatment with 2 M Na2CO3 until pH = 8.3 was reached, with subsequent calcination of the precipitate formed at 400–500 °C for 2–18 h [33]. The Cu1.5Mn1.5O4 spinel was prepared from copper and manganese acetates dissolved in a ratio of 1:1 in water by co-precipitation with an aqueous solution of sodium carbonate at room temperature. The solid obtained was dried and calcined at 500 °C for 6 h. In a similar route but varying the molar ratio of Cu to Mn from 5:1 to 1:5 and the calcination temperature between 400 and 800 °C, mixed-phase catalysts with Cu1.5Mn1.5O4 content were prepared [34]. The effect of aging of the precipitates formed from aqueous Cu(NO3)2·3H2O and Mn(NO3)·6H2O solutions (0.25 M each, mixed in a 1:2 ratio) at 80 °C with aqueous Na2CO3 (0.25 M) at pH = 8.9 was studied in detail. Cu1.4Mn1.6O4 and CuMn2O4 spinels were formed (80 °C, pH = 8.9) by aging the precursor precipitate between 0 to 1440 min, after drying (120 °C, 16 h) and calcination at 500 °C for 17 h. [35]. Different variations in the Na2CO3 and metal salts concentrations, heat treatment times, and temperatures were used to prepare Cu–Mn spinels with various properties from nitrate salts of manganese and copper (80 °C, pH = 8.3, calcination at 700 °C for 7 h [36] and pH = 8.5, drying in air at 110 °C for 12 h, calcination at 300 or 500 °C in air for 4 h [20]) with an aqueous solution of Na2CO3 as precipitation agent. Instead of sodium carbonate, ammonium carbonate can also be used, especially in preparing metal-doped spinels to avoid odium contamination. For example, La-doped Cu1.5Mn1.5O4 was also prepared by co-precipitation from Mn(CH3COO)2 4H2O and Cu(NO3)2·3H2O solutions spiked with La(NO3)3·6H2O in a water bath at 60 °C with 1.5 M (NH4)2CO3 solution at a pH of 8. Calcination was done at 550 °C in air for 2 h [37].
Polycarboxylic acids such as citric acid or oxalic acid (or its precursor, e.g., ethylene glycol, under oxidative conditions) are used as precipitating and complex or gel-forming reagents in Cu–Mn spinel synthesis. The mixed copper–manganese oxalates, as thermally easily decomposable and reactive compounds, are important precursors of direct spinel synthesis. According to this, copper and manganese nitrates were dissolved in water and mixed with an aq. solution of oxalic acid in 20% excess at room temperature. The precipitate was dried at 110 °C overnight and calcined at 300–500 °C for 4 h [20]. Gel/xerogel/polymer forming is also a convenient way to prepare homogeneously mixed quasi–solid precursors with a controlled Cu and Mn salt content. Cu1.5Mn1.5O4 and its composites with CuO (50 and 77% CuO) were prepared with the use of 2% ionotropic sodium alginate and 0.1 M (total metal salt) CuCl2 and MnCl2 solutions in various (including 1:1) Cu:Mn stoichiometric ratios by stirring at room temperature for 12 h and by subsequent alcoholic dehydration (with increasing alcohol concentration from 10 to 100%). The xerogel formed was calcined at 450 °C for 8 h [38]. A gel-like intermediate was prepared from citric acid monohydrate, copper nitrate trihydrate, and a 50% aq. manganese(II) nitrate solution (the nitrate:citric acid molar ratio was 1:1.2) and ethylene glycol (the nitrate:ethylene glycol molar ratio was 1:3). The mixture was stirred at 70 °C, and the gel that formed was calcined at 500 °C for 3 h [19]. Copper and manganese nitrates were dissolved in a mixture of ethylene glycol, water, and concentrated nitric acid. The solution was stirred under reflux (105 °C) for 2 h and dried at the same temperature for 16 h [39]. The method resulted in the formation of polymeric precursors consisting of ethylene glycol and its oxidation product (oxalic acid). The drying procedure was adjusted to the different hydrolysis rates of the precursor complexes resulting in variations in phase compositions and crystallinity. Calcination was done at a temperature of 350 and 500 °C for 5 h [39].
A simple way to prepare CuxMn3−xO4 spinels is based on the autoignition of a mixed solution of urea with manganese nitrate and copper nitrate (75% excess of urea) in an open muffle furnace at 550 °C for 1 h [36,40]. Manganese(II) acetate and a calculated amount of copper nitrate (and dopant metal precursors) were dissolved in water, then a KMnO4 solution was added at room temperature, then the precipitate that formed was calcined to CuxMn3−xO4 spinels in air at 300 °C for 2 h [41]. The precipitation agent (ammonia) can be prepared in situ from the hydrolysis of hexamethylenetetramine [13].
The results of the preparation of CuxMn3−xO4 spinels from precursors made in solution-phase reactions are summarized in Table 2.

Table 2.
Synthesis conditions of CuxMn3−xO4 catalyst with solution-phase methods.
The solution-phase spinel precursor syntheses ensure the complete distribution of copper and manganese ions and the monophase spinel synthesis. The reactive species can be transformed into spinels at lower temperatures (>250 °C) than the solid oxides or oxide precursors. These methods are typical in the preparation of copper-rich spinels [9,13,21,22,23,38,50]. The copper manganese oxide spinels with x ≥ 1 were prepared from the appropriate sulfate, chloride, and acetate salts with such precipitating agents as NaOH, NaOH, Na2CO3, sodium oxalate, or sodium alginate. The most frequently used precursor salts are nitrate salts. For example, Cu1.5Mn1.5O4 can be prepared from the Mn(II) acetate and Cu(II) nitrate via precipitating the solid precursors with ammonium carbonate or tetramethylammonium hydroxide, or with redox precipitation by KMnO4 [20,37,41]. The solid precursors for the synthesis of spinels with x = 1.5 and 1.8 were deposited from manganese(II) nitrate solution with the use of [tetraamminecopper(II)] hydroxide, and ammonium hydroxide (in reverse microemulsions of water–surfactant–n-octanol–cyclohexane) as precipitating agents, respectively [29,30,47].
The CuxMn3−xO4 spinels with x = 0.3–1.5 can be prepared from copper(II) and manganese(II) nitrate solutions with various methods. The precursors for heat treatment were precipitated with Na2CO3 [20,33,35] Me4NOH [26,42], NaOH [9,21,22,23,25,31], or oxalic acid [20]. Direct impregnation methods including dip-coating, incipient wetness impregnation and spray drying were also used with subsequent thermal decomposition of the solid metal nitrates into active oxide precursors [9,18,44]. Gel-forming materials such as urea, an ethylene-glycol–nitric acid mixture, citric acid [32,39,46], or autoignition methods with organic fuels were also used to prepare the CuxMn3−xO4 spinels with x ≥ 1 [36,40,43].
2.3. Thermal Decomposition of the Hydrated and Ammonia-Complexed Copper Permanganate
Anhydrous copper(II) permanganate has not been isolated yet, because the hydrated and the ammonia-complexed copper(II) permanganates decompose at lower temperatures than their ligand loss temperatures [14]. The hydrates of copper permanganate (Cu(MnO4)2·nH2O (n = 2, 3, 4, 6, 8), however, were prepared easily via various synthesis methods [14,51]. Its composition ensures an optimal Cu:Mn = 1:2 ratio to prepare the regular CuMn2O4 spinel. Its thermal decomposition starts at 80 °C with oxygen evolution in an autocatalytic process [52]. The decomposition of the permanganate ion and water release are parallel processes during heating. Thus, the water content of copper permanganate (n = 1.7–4.1 with evaporation at 290–330 K in vacuum) does not influence the decomposition temperature or the composition of the thermal decomposition products. The product of the first decomposition step is a mixed-phase copper manganese oxide with a composition of CuMn2O5.45 [53]. Kinetic studies performed in the range of 335 and 370 K showed that in the median range of thermal decomposition, the dominant decomposition feature is the constant reaction rate without any acceleratory behavior, and the induction period was also short. The mixed-phase decomposition intermediate decomposes further when the temperature is increased to 695 K, and the amorphous product formed corresponds to the CuMn2O5 formula. Magnetic susceptibility measurements showed that the valence state of the copper above 725 K is exclusively CuI. Thus “CuMn2O5” cannot be a mixture of CuO + 2MnO2. When the temperature was increased to 1175 K, the expected CuMn2O4 and its partial decomposition product, Mn2O3, were formed [54].
The basic copper(II) permanganate, Cu2(OH)3MnO4, was prepared by an anion exchange of the layered Cu2(OH)3OAc with 1 M KMnO4 in a day [55]. The thermal decomposition of Cu2(OH)3MnO4 can be described as:
Cu2(OH)3MnO4 = 0.5CuMn2O4 + 1.5CuO + 1.5H2O + O2
The amorphous mixed copper manganese oxide that formed catalyzes the oxidation of CO at 30 °C. The amorphous mixed copper manganese oxide crystallizes at 440 °C, and loses its catalytic activity above 500 °C but can be reactivated in oxygen at 400 °C. It decomposes at 940 °C into CuMnO2 [55].
We developed a solid-phase reaction to obtain CuMn2O4 spinel materials with x = 1 at a low temperature (between 100 and 500 °C), via a quasi-intramolecular redox reaction of the ammonia ligand and permanganate anion in [Cu(NH3)4](MnO4)2 [56]. The decomposition reaction of [tetraamminecopper(II)] permanganate strongly depends on the reaction conditions. It explodes on fast heating at 65 °C and ignites at >8 atm O2 in a violent reaction (dark red flame, 1500 K combustion temperature) [57], according to the equation.
[Cu(NH3)4](MnO4)2 = Cu(l) + 2/3 Mn3O4(l) + 5.34H2O(g) + 0.44NH3 + 1.78N2
Thermogravimetric studies on the decomposition of [tetraamminecopper(II)] permanganate showed a stepwise ligand and oxygen-releasing process [58], and the solid decomposition end-product was identified on the basis of weight loss as CuMn2O4 [58]. Although the decomposition mechanism declared was proven to be incorrect, the end-product of the decomposition was an amorphous material with CuMn2O4 composition [56]. The decomposition residue did not dissolve in HNO3 at all, and the decomposition process was formally written as [56].
[Cu(NH3)4](MnO4)2 = CuMn2O4 + 2NH3 + NH4NO3 + H2O
NH4NO3 = N2O + 2H2O
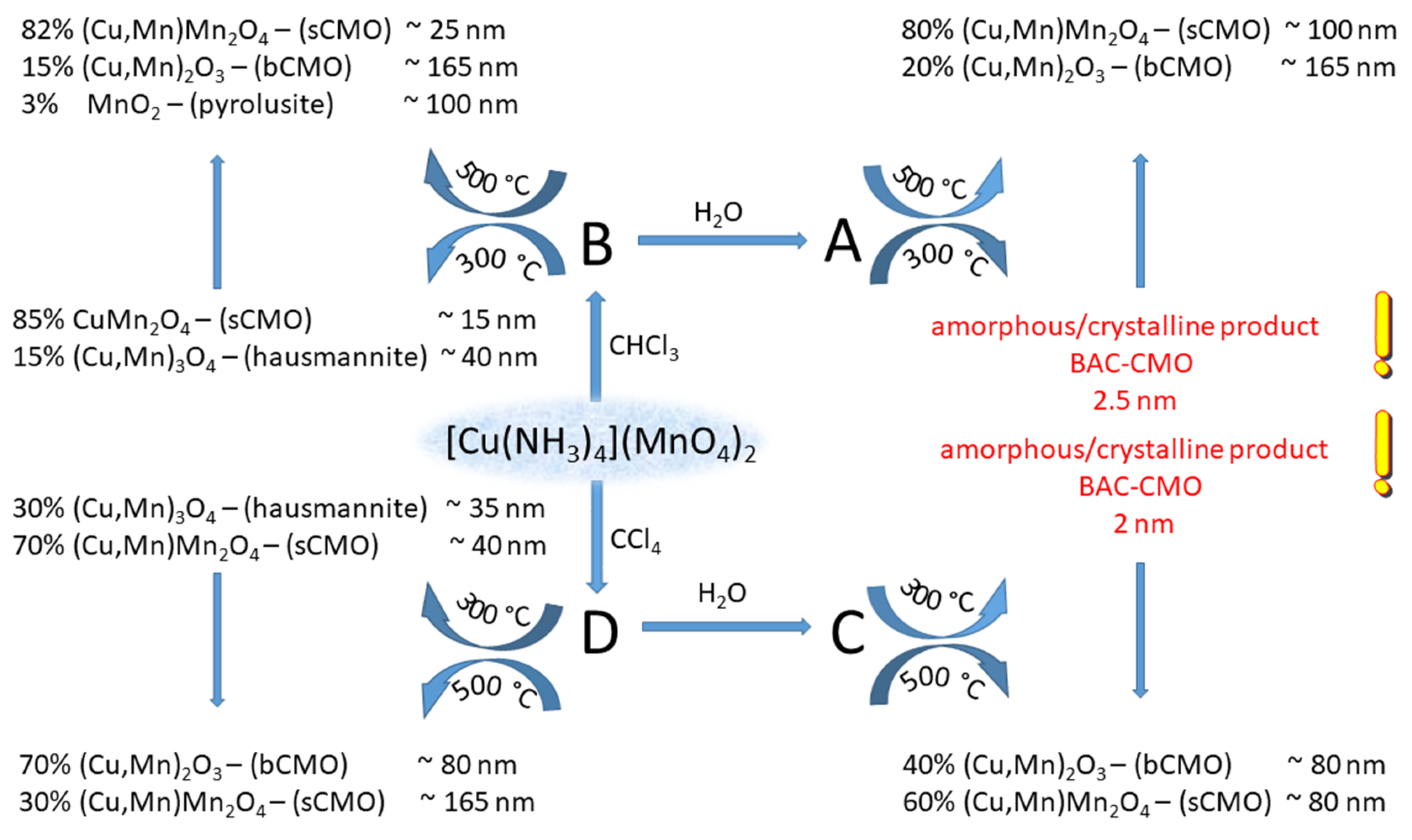
Solid-phase decomposition is frequently explosion-like; therefore, to control the decomposition process, the decomposition reaction was performed under inert solvents like CHCl3 and CCl4. In this way, the decomposition temperature cannot exceed the boiling point of the solvent, because the evaporation heat of the solvents absorbs the exothermic reaction heat. The solid products are the expected spinel and ammonium nitrate; every other reaction product is gas. Since ammonium nitrate can be dissolved out with water and decomposed thermally as well, we studied the effect of the synthesis temperature (CHCl3–61 °C, CCl4–77 °C) and the isolation methods (aqueous extraction or thermal decomposition of ammonium nitrate) on the composition and properties of the resulting spinel compounds [59,60].
This temperature-limited process prevents nucleation and crystallization of the formed copper manganese oxides driven by the exothermicity-derived reaction heat (289.8 kJ/mol) [57]. The amorphous copper manganese oxides were characterized with the formula of CuMn2O4+x from x = 0 (CuMn2O4,spinel structure) to x = 0.5 (Cu0.89Mn1.78□0.32O4 or Cu0.67Mn1.33MnO3) with defect structure (□ is a defect in the spinel structure). Depending on the removal method of ammonium nitrate (washing with water or thermal decomposition) and the temperature and time of the subsequent heat treatments, the size and phase composition of the products can be controlled (Scheme 2). This method ensures a safe and easy route to prepare copper manganese oxide nanoparticles in various compositions [60]. The samples containing copper manganese oxide and ammonium nitrate prepared in CCl4 at 77 °C were extracted with water to remove ammonium nitrate. Subsequent heat treatment of the extraction residue up to 400 °C for 1–8 h resulted in copper manganese oxides, which d(001) peak shows the initial stage of the formation of the spinel structure with 2 nm crystallite sizes. The crystallization of the spinels starts at 500 °C when crystallites with the size of 80 nm were obtained [60].
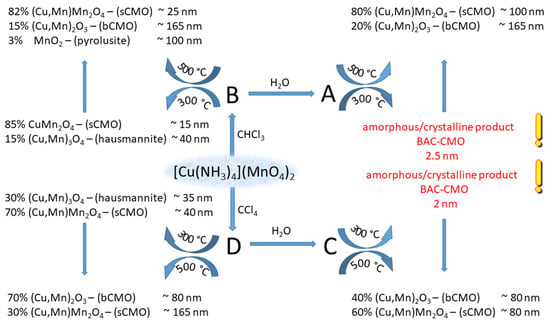
Scheme 2.
Decomposition of [tetraamminecopper(II)] permanganate under CHCl3 (reaction products A and B) and CCl4 (reaction products C and D) with (reaction products A and C) and without (reaction products B and D) the removal of NH4NO3 by aqueous leaching (reproduced from [60]).
3. Composition and Crystal Structure of CuxMn3−xO4 Spinels
Hausmannite (MnIIMnIII2O4) occurs in both tetragonal (room temperature) and cubic (high temperature, >>1000 K) forms. The two phases co-exist in a wide temperature range [61]. The cubic spinel phase can be stabilized at lower temperatures by doping with copper [21] despite the fact that both MnIII([Ar]d4) and CuII([Ar]d9) ions show Jahn–Teller activity in both octahedral and tetrahedral spinel sites. These CuxMn3−xO4 mixed oxides are solid solutions, where copper substitutes manganese in the tetragonal lattice of hausmannite (Mn3O4, MnIIMnIII2O4). Depending on the amount, site, and valence of copper (CuI or CuII), and manganese species (MnII, MnIII, and MnIV), distorted cubic or tetragonal spinel lattices are built up. The CuxMn3−xO4 spinels with x ≈ 1 have a tetragonally deformed structure with a c/a ratio slightly varying between 1.05 and 1.03. Above x = 1.06, a cubic spinel structure was found with a diminishing lattice parameter upon increasing copper content [9]. Formally, the stoichiometric spinel structures can be formulated as [62,63,64]:
CuII[MnIII2O4] and CuI[MnIIIMnIVO4]
In octahedral field symmetry, MnIII and CuII d levels are split into t2g3eg1 and (t2g6)(eg2)eg1 levels. In tetrahedral symmetry, they split into e2t22 and (e4)(t24)(t21), respectively [10]. The mixing of copper and manganese valences in the spinel lattice leads to subcritical levels of Jahn–Teller active ions, thereby causing no distortion [21,65]. As a result of that, the ionic configurations in CuxMn3−xO4 spinels can be written as:
CuIaMnIIbMnIII1−a−b[CuIIx−aMnIII3−2x−bMnIVx+a+b−1]O4
The tetrahedral sites of the spinel lattice can mainly be occupied by CuI, MnII, and MnIII, whereas the octahedral sites are mainly occupied by CuII, MnIII, and MnIV ions. Thus, only the MnIII ions can disperse between the A (tetrahedral) and B (octahedral) spinel sites, in any proportion; CuI and MnII strongly favor the tetrahedral, whereas CuII and MnIV the octahedral sites. The electrical conductivity of cubic Mn3O4 was explained by small polaron hopping between MnIII and MnIII on octahedral sites [66] and the disproportionation reaction between two MnIII ions, resulting in MnII and MnIV [67] at the octahedral sites. The MnII in the tetrahedral sites is the consequence of the equation,
which is shifted to the MnII + MnIV side [24] in the manganese-rich compounds.
MnIIIA + MnIIIB = MnIIA + MnIVB
CuII appeared in the octahedral site above 300 °C due to the reaction of tetrahedral CuI components and octahedral MnIV components [24], according to the equation.
CuIA +MnIVB = CuIIB + MnIIIA
Furthermore, a thermally activated site exchange reaction was also detected by neutron diffraction measurement on the CuMn2O4 samples quenched from 1213 K due to the strong octahedral site preference of the Jahn–Teller CuII–ion compared to that of MnII [68]. As a result of these reactions, various cation distributions were found experimentally in the samples formally from the normal (MIIMIII2O4) to inverse spinel (MIII[MIIMIII]O4 structures. Therefore, the cation valence and site distributions observed at room temperature strongly depends not only on the synthesis method but also on the annealing conditions and cooling rates as well [66,67,68,69,70,71,72]. For example, the CuMn2O4 crystals obtained from a 1:1 mixture of CuO and Mn2O3 in vacuum at 1323 K and with cooling at 6 K/h to room temperature resulted in CuI0.2MnII0.8[CuII0.8MnIII0.2MnIV1.0]O4 [65], whereas the room temperature magnetic susceptibility measurements on quenched CuMn2O4 showed that all Cu is monovalent, and all manganese is tetravalent [69].
Neutron diffraction experiments showed that CuMn2O4 prepared in a solid-state reaction of oxides at 700 °C for 240 h has a partially inverted cation distribution (12–13% of copper ions located on octahedral sites [17]). Since the copper ions are mainly expected to be monovalent CuI at the tetrahedral sites, it means that all Mn4+ and Cu2+ ions should be located at the octahedral sites [13]. The magnetic susceptibility and Curie constant parameters of CuxMn3−xO4 spinels confirm the CuI(T–4)–MnIV(OC–6) configuration, and according to this, at x = 1.5 (Cu1.5Mn1.5O4), the favored configuration is CuI[CuII0.5MnIV1.0MnIII0.5]O4. The cubic structure (x > 1.06) is an indicative parameter of the low concentration of distorting MnIII and CuII cations.
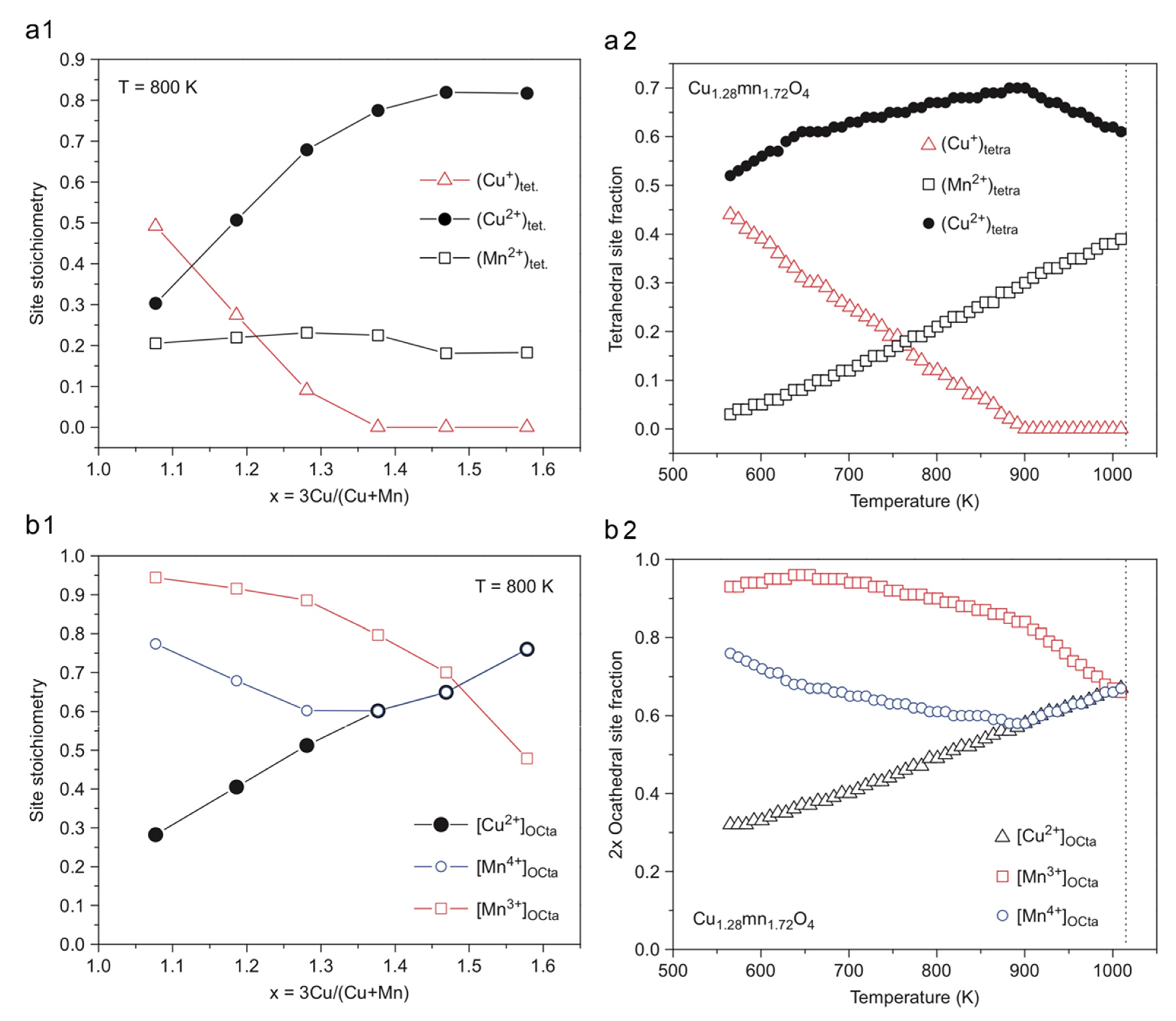
The surface composition of the copper manganese oxides is not identical to their bulk composition, e.g., typically, CuII is enriched at the surface layer of CuMn2O4 and Cu1.5Mn1.5O4 [44]. Thin films of copper–manganese oxides were prepared on aluminum foil by dip coating from nitrate salts dissolved in ethanol at room temperature. The coatings were decomposed and annealed at 300 °C and between 400 and 500 °C, respectively, for periods between 15 and 90 min. The results summarized in Table 3 show that the preparation method of copper manganese oxide spinels has a strong influence on the amount and distribution of the copper and manganese species with various valence at the different spinel crystal lattice sites. Furthermore, the precipitation and ceramic methods resulted in copper manganese oxide spinels with completely different metal ion valences and distributions, depending on the synthesis methods used. Not only the preparation method, but within one preparation route, the post-treatment conditions such as annealing time and temperature also have an enormous influence on the composition and properties of the CuxMn3−xO4 films (Figure 3).

Table 3.
Distribution of copper ions between the T–4 and OC–6 sites of the spinel lattice in CuxMn3−xO4 samples.
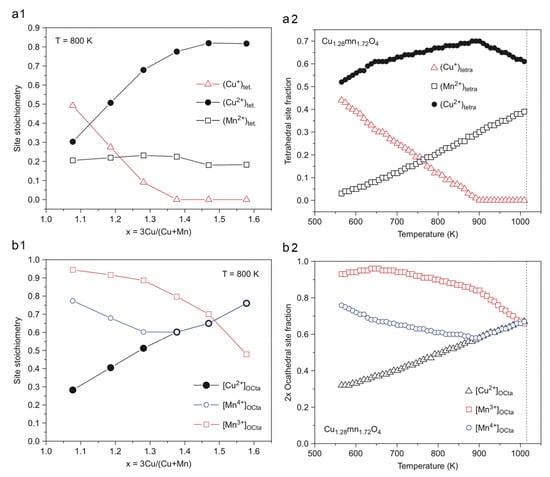
Figure 3.
Variation of the cation valence and distribution on tetrahedral and octahedral sites with temperature and composition for Cu1.28Mn1.72O4. The vertical dashed line corresponds to the onset of CuO precipitation (reproduced from [10]).
For example, for the preparation of Cu1.5Mn1.5O4, with the dip-coating of nitrates, the reaction has to be carried out above 450 °C and the full conversion of Cu and Mn oxides into Cu1.5Mn1.5O4 requires at least 60 min. The high conductivity of the films prepared in this way may be attributed to the presence of Mn3+ and Mn4+ in octahedral and Cu+ and Cu2+ in tetrahedral sites, respectively. This is detected by XPS and may be attributed to the electron hopping between CuI and MnIV ions [44]. This unexpected distribution of copper valences in the film is similar to the distribution of copper valences in the surface layer of the bulk compound with the same composition.
The relative amount of copper, manganese, and the lattice defects, and also the distribution of valence states of copper and manganese ions and the locations of each type of ions at various spinel sites are one of the key factors in the catalytic activity of CuxMn3−xO4 spinels.
4. Overview of the Catalytic Activity of CuxMn3−xO4 Spinels
One of the oldest and most important application areas of copper manganese oxide catalysts, including CuxMn3−xO4 spinels and mixtures containing a CuxMn3−xO4 spinel is the removal of carbon monoxide from air. The CuxMn3−xO4 spinels, however, proved to be very active catalysts in such important processes, such as the recovery of aromatic and chlorinated hydrocarbons from air, methanol reformation to produce hydrogen, and in a series of industrially important reactions, which are summarized in Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13.

Table 4.
Removal of carbon monoxide from the air in the presence of CuxMn3−xO4 catalysts.

Table 5.
Copper manganese oxide spinel catalysts and their activity in NO oxidation reactions.

Table 6.
Desulfurization with CuxMn3−xO4 catalysts.

Table 7.
Removal of chlorinated hydrocarbons from air in the presence of CuxMn3−xO4 catalysts.

Table 8.
Removal of benzene from the air in the presence of CuxMn3−xO4 catalysts.
4.1. Removal of Carbon Monoxide from the Air
The Hopcalite catalyst used for the removal of CO from air is a mixed copper manganese oxide, the composition of which strongly depends on the synthesis conditions. Hopcalite was developed by the USA Chemical Warfare Service for gas masks against carbon monoxide during World War I. The heat treatment history of the Hopcalite catalyst is a key factor to ensure its catalytic activity in CO removal at room temperature [33,45]. The samples prepared between 400 and 500 °C for a calcination time of 2–18 h resulted in catalysts containing CuMn2O4, but their activity dropped to zero if the sample was prepared at 500 °C in 18 h. The sample containing crystalline CuMn2O4 was active for the room temperature oxidation of CO, but the amorphous precursors prepared by calcination at 300 °C showed higher activity than CuMn2O4 [33].
The lowest Cu content (x = 0.5) among the studied CuxMn3−xO4 catalysts proved to be active in CO oxidation was found in the samples prepared by the heat treatment of basic copper(II) permanganate, Cu2(OH)3MnO4. This catalyst was active for CO removal at 30 °C [55]. The catalyst with the same composition prepared by precipitation from copper and manganese nitrates solution with NaOH and heated at 300 °C reached quantitative CO conversion in air at 120 °C [25].
The CuMn2O4 catalysts with x = 1, which are active in CO oxidation reactions, were prepared in various reaction routes (Table 4), including precipitation from metal nitrates with Na2CO3 [33,35], via the formation of complexes with citric acid and ethylene glycol (oxalate precursor) [39], and by the thermal decomposition of [Cu(NH3)4](MnO4)2 in CHCl3 and CCl4 at 61 and 77 °C [60]. The thermal decomposition of [Cu(NH3)4](MnO4)2 under inert solvents resulted in nanosized CuMn2O4 with a defect structure. The best catalytic performance in CO oxidation was found for the catalysts prepared in CHCl3 and CCl4 with the aqueous extraction of the NH4NO3 intermediate with subsequent annealing at 300 °C for 1 h (80 and ~95%, respectively). If the ammonium nitrate was removed by thermal decomposition, the catalytic activity of the Cu–Mn spinels in the course of CO oxidation [60] decreased. A catalyst (CCl4, aq. leaching, 300 °C) was tested in 5 cycles (each cycle was 22–25 h). Its catalytic activity decreased with increasing reaction time from 5 × 10−3 to 2.5 × 10−3 mol CO2 m−2 h−1 at 125 °C, mainly in the first three hours. This kind of activity profile was found for commercial Hopcalite catalysts as well [75].
Mixed-phase CuxMn3−xO4 spinel catalysts containing Mn2O3 were developed for fuel cells PROX (preferential CO oxidation in CO + H2 mixtures). Copper and manganese nitrates were refluxed in a mixture of ethylene glycol, water, and concentrated nitric acid for 2 h and dried at 105 °C for an additional 16 h followed by calcination at a temperature of 350 and 500 °C for 5 h. The obtained samples converted 20 and 17% CO in 1 h at room temperature, respectively [39].
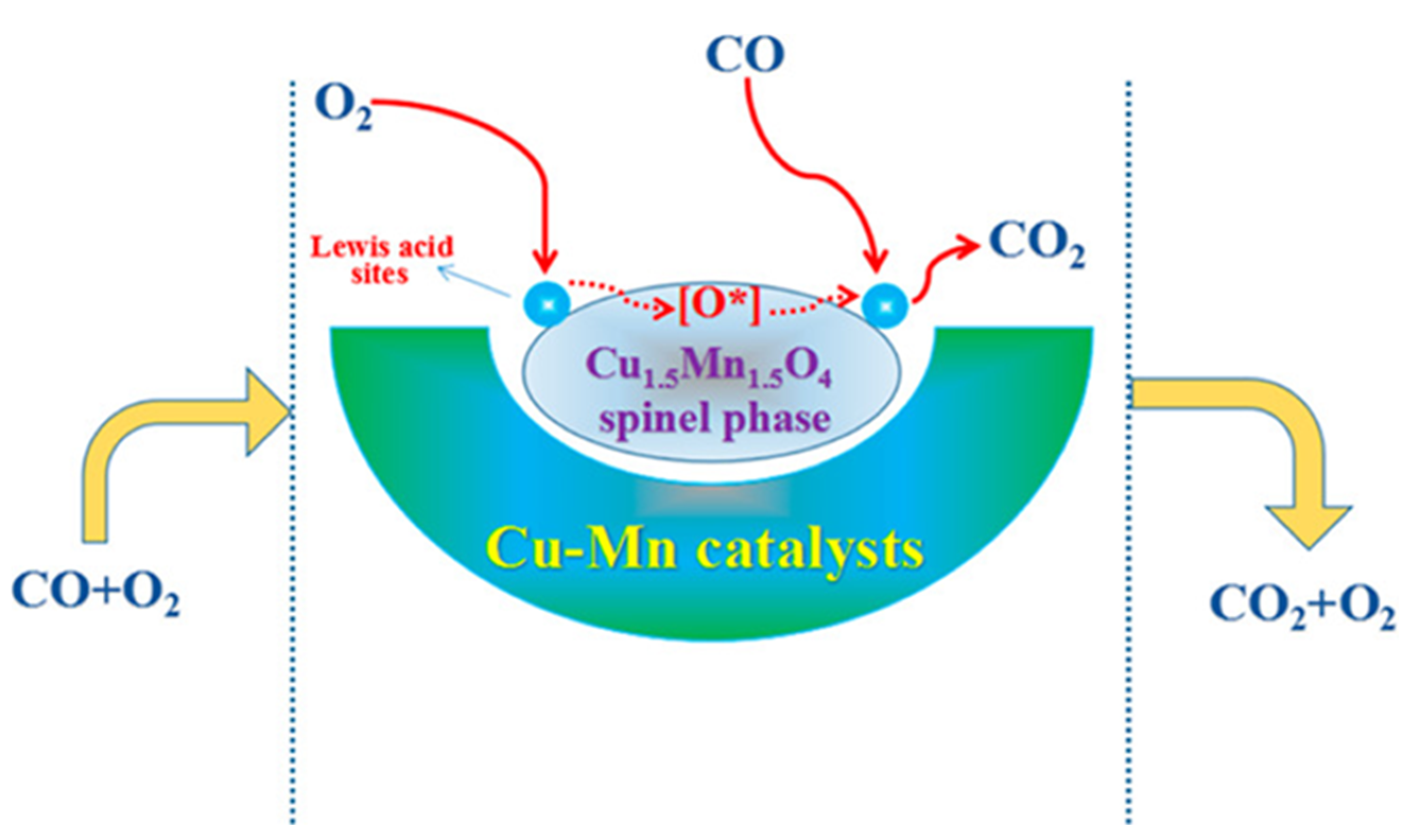
The precipitation method using Na2CO3 and metal nitrates resulted in Cu–Mn spinels with x = 1.0–1.4, depending on the aging time (from 0 to 1440 min). The conversion of CO was found to be ca. 50% for the unaged catalyst at room temperature and decreased with increasing aging time, but catalytic performance is restored after 720 min and reaches its maximum (the highest CO conversion). For this aging time, the composition of the catalyst is characterized by x = 1 (CuMn2O4) [35]. The copper-rich catalysts with x = 1.4–1.5 were prepared by precipitation methods, starting from the appropriate metal nitrates [35,41], acetates [25,41] and chlorides [13], by redox methods using KMnO4 [41] and by combined methods using KMnO4 and hydrothermal treatments with complex forming reagents, such as citric acid or oxalate precursors (ethylene glycol) [32]. Their detailed catalytic activities in CO oxidation are given in Table 4. The Cu1.5Mn1.5O4 catalysts synthesized by the co-precipitation method from acetate or nitrate salt solutions of copper and manganese with NaOH and Na2CO3) were studied in detail [25] to maximize the efficiency of CO oxidation at room temperature. The activity of the oxidation of CO strongly depended on the combination of precipitant and precursor anions, ranking in the order (acetate + carbonate) > (nitrate + carbonate) > (acetate + hydroxide) > (nitrate + hydroxide) [25]. The redox reaction of Mn-acetate, Cu-nitrate, and KMnO4 solution with calcination of the reaction product in air at 300 °C for 2 h resulted in a catalyst with the same composition (Cu1.5Mn1.5O4), which was used in the low-temperature oxidation of CO. The presence of other transition metals added to the catalysts improved the catalytic properties. For example, the T50 temperatures of CO conversion were 34 and 50 °C with Co- and Fe-doping, respectively [41]. The catalyst was prepared with a hybrid method, with the use of KMnO4 as oxidant and citric acid as a complex-forming agent, together with ethylene glycol as oxalate precursor under hydrothermal conditions (180 °C for 12 h) and by calcination at 350 °C in air for 4 h contained nanosized (5–10 nm) particles [32]. The mechanism of the oxidation of CO with this catalyst is shown in Figure 4.
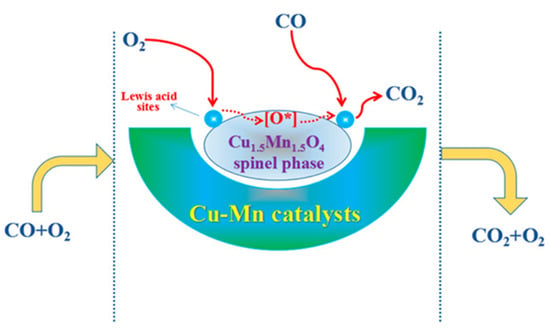
Figure 4.
Mechanism of CO oxidation catalyzed by Cu1.5Mn1.5O4 prepared with a hybrid (redox, complex forming, and hydrothermal) method (reproduced from ref. [32]).
This catalyst shows activity even at room temperature, but 100% CO oxidation was achieved only at a temperature of 65 °C [32]. A plausible tentative mechanism for the carbon monoxide oxidation reaction over the copper manganese oxide spinel catalysts includes two main processes:
- (1)
- Molecular oxygen is preferentially adsorbed on the spinel surface and subsequently forms surface-active species containing adsorbed oxygen (O*) such as O2−(ads) and O−(ads).
- (2)
- CO molecules are oxidized by O* species with the formation of gaseous carbon dioxide. The two-step reaction can be formulated as e.g., CO + O−(ads) → CO2 + e− and CO + 2O−(ads) → CO32−(ads).
The cooperative effect of the redox cycle between the metal centers in the spinel catalyst (Cu+ + Mn4+ ↔ Cu2+ + Mn3+) involving the Cu2+ and Mn3+ ions of the distorted spinel structure promotes the generation of Lewis acid sites on the surface, which play a beneficial role in the activation of reactants to form active species. These lead to the enhancement of catalytic activity (performance) in the CO oxidation reactions. The spinel catalyst transports the surface O* species. The Cu2+–O–Mn3+ units lose some oxygen to form CO2 from CO and form oxygen defects. The spinel phase captures some oxygen from the gas phase to form surface-active O* species again. The detailed mechanism is given in [32].
4.2. Removal of NO from Air by Carbon Monoxide
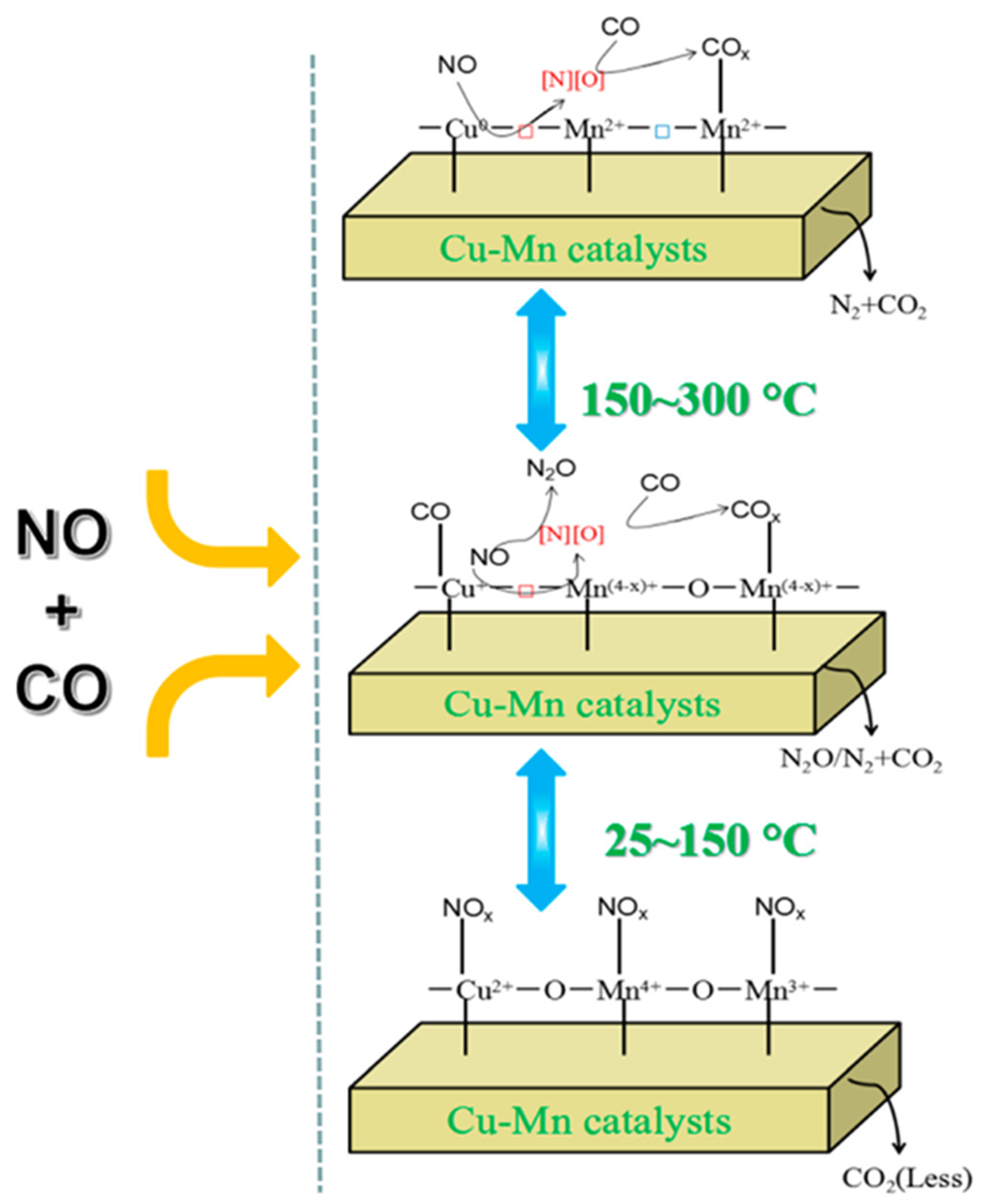
The reaction of NO with carbon monoxide in the presence of copper manganese oxide catalysts, especially Cu1.5Mn1.5 spinels, results in CO2 and N2. The aging time of samples prepared from metal nitrates with sodium carbonate has crucial importance in the preparation of a catalyst for the NO SCR with carbon monoxide at ambient temperature [35]. The Cu1.5Mn1.5O4 nanocatalyst given in Table 5 was prepared with a hybrid method. It can adsorb NOx gases due to the presence of Cu+–CO active species. The surface-dispersed Cux+–O2−–Mny+ active species can be reduced to Cu+–□–Mn(4−x)+ active species at increasing adsorption temperature, and the synergetic effects between Cu and Mn (Cux+–O2−–Mny+ species) also play a role in the NO + CO SCR reaction [16]. The mechanism of the reaction is shown in Figure 5.
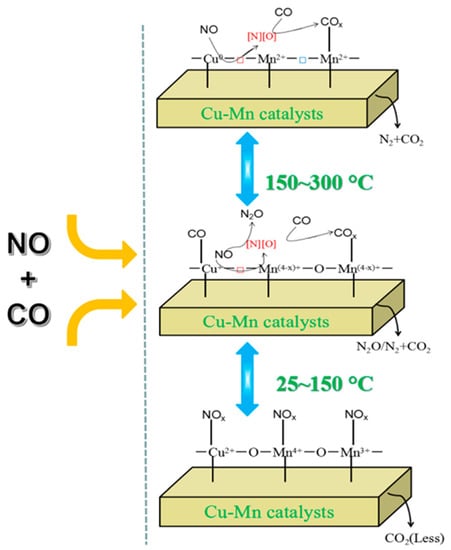
Figure 5.
The mechanism of NO reduction with adsorbed CO on Cu–Mn–spinel catalysts (reproduced from [32]).
A possible tentative reaction mechanism for the NO + CO reaction over copper manganese oxide spinel catalysts (illustrated in Figure 5) is given in [32]. The formation of surface oxygen vacancies in the spinel catalysts contributes to the activation of NO and CO gases via the formation of highly reactive species. NO molecules are preferentially adsorbed on the spinel surface with the formation of surface NOx species, thus only a small part of carbon monoxide molecules is oxidized by surface active oxygen (O*) derived from Cu–O–Mn linkages. The surface NOx species are gradually dissociated into N and O atoms, with the formation of N2O as an intermediate product [32], and surface O vacancies are also produced due to the synergistic effect between Cu and Mn.
The high or intermediate oxidation-state metal ions such as Mn4+, Mn3+, and Cu2+ are partially reduced by carbon monoxide with the formation of low or intermediate valence metal species, such as Mn3+, Mn2+, Cu+ or Cu0. The interaction between divalent and monovalent metal ions and surface COx species forms Mn2+–COx and Cu+–CO species. The higher the temperature, the more oxygen vacancies are generated, resulting in an enhancement of catalytic activity/performance. The catalytic cycle is when the CO molecules and the intermediate N2O turn into gaseous CO2 and N2.
4.3. Removal of Other Pollutants from the Air and Other Gases
Copper manganese spinel catalysts are widely used materials in the oxidative recovery of various other inorganic and organic pollutants such as H2S (Table 6), chlorinated hydrocarbons (Table 7), and aromatic hydrocarbons (Table 8 and Table 9). The recovery of hydrogen sulfide with porous copper-rich CuxMn3−xO4 spinel catalysts prepared from finely divided CuO, MnO2, and graphite (x = 1.47 and x = 1.15) were tested from hot coal gas in the presence and absence of water. The pores in the catalysts are produced due to the release of the CO2 gas that formed from the graphite and oxide phases. The regeneration of catalysts was performed with a regeneration gas containing 3 vol% O2 (balance N2) at 720 °C [12].
The oxidative removal of trichloroethylene from air was studied in the presence of Cu1.5Mn1.5O4 catalyst carried out on SiC support. The decomposition of trichloroethylene in moist air (relative humidity was 15%) was done by post-plasma catalysis above 150 °C. Trichloroethylene removal is 19% and 50% at 250 °C and 300 °C, respectively. Up to 250 °C, the selectivity of the catalyst into CO2 is 100%, whereas, at 300 °C, C2Cl4, CO, and CO2 formed (Table 7) [58].
4.4. Removal of Aromatic Hydrocarbons from Air
The strong carcinogen effect of benzene generated a demand to remove it from the air. This may be done in the presence of CuxMn3−xO4 spinels with either synthetic air [46] or ozone [42]. The high reactivity of ozone towards benzene ensures a much lower reaction temperature (70 °C) than that of synthetic air (Table 8) [42,46]. The ozonation reaction was catalyzed with a mixed oxide phase containing CuMn2O4 prepared from Mn and Cu–nitrate solutions with NaHCO3, by calcination between 400 and 600 °C. Oxidation with synthetic air was tested with a series of CuxMn3−xO4 spinels with x = 0.5, 1.0, 1.5, and 2. The CuII/CuI or MnIII/MnIV ratios for the spinels with x = 0.5, 1.0, 1.5, and 2.0 were found to be 12.0, 20.6, 4.5, and 7.8 or 2.0, 2.5, 1.4, and 0.6, respectively. The catalytic oxidation of benzene was complete at a temperature lower than 200 °C. The MnIII-occupied octahedral positions of the spinel lattice greatly improve catalytic activity, and the surface lattice O-species play a key role in the process [46].
The removal of toluene from air in the presence of various CuxMn3−xO4 spinels (x = 0.5–1.5) was studied in detail (Table 9). These catalysts were synthesized from the appropriate nitrate, acetate, and chloride salts by precipitation with hydroxides (NaOH [49], NH4OH [47], Me4NOH [26]) or carbonates (Na2CO3 [13,26] or (NH4)2CO3) [37], including reverse emulsion formation [47] or hydrothermal reactions [49]. Incipient wetness impregnation and direct pyrolysis on γ–Al2O3 [76] and anatase [18] were used to prepare supported catalysts. A series of TiO2-supported mixed-phase catalysts containing Cu1.4Mn1.6O4 showed high catalytic activity with the total conversion (100%) of toluene into carbon dioxide above 240 °C, without any deactivation of the catalyst. The mobility of the network oxygen in the structure of the catalyst is a key factor in the catalytic process. The gradual loss of catalytic activity below 240 °C can be attributed to the adsorption of carbonaceous compounds on the surface, and according to this, catalytic activity can easily be regenerated by heating the sued catalysts in airflow at 300 °C [18].
Alcoholic dehydration of the xerogel made with sodium alginate from Cu(II) and Mn(II) salt solutions resulted in cubic nanosized Cu1.5Mn1.5O4 [38]. This nanosized cubic Cu1.5Mn1.5O4 proved to be a high-performance catalyst in the oxidation of toluene at atmospheric pressure. The CuMn2O4 prepared from copper acetate and manganese nitrate in reverse microemulsion (Triton–X–100 surfactant, n–octanol, and cyclohexane) by adding NH4OH and calcining at 450–1000 °C in oxygen also catalyzed toluene combustion (0.35% toluene and 9% O2 in inert gas). The ignition temperature of 210 °C and a combustion temperature of 220 °C resulted in the complete oxidation of toluene [47]. The oxidation of toluene in the presence of this catalyst was complete at ∼240 °C [38].
The catalyst containing stoichiometric CuMn2O4, which was prepared from nitrate salts with tetramethylammonium hydroxide, was active in toluene oxidation with the formation of CO2 as the main product with 22 (10.45) and 1.9 (0.04) μmol g−1 h−1 (μmol m−2 h−1) decomposition rate at 170 and 150 °C, respectively. Only a minor amount of benzene and CO formed [26]. Oxygen is adsorbed by the surface (Oads), but the subsurface oxygen content also plays a key role in the activity of the catalyst [26]. The rod-like nanosized (10–20 nm) copper manganese mixed oxides containing CuMn2O4 were prepared from copper and manganese acetate with Na2CO3 under hydrothermal conditions. These catalysts have significantly higher catalytic activity for toluene combustion at a temperature of 210 °C than that found for CuMn2O4 prepared without hydrothermal post-treatment even at a combustion temperature of 250 °C [49]. It was attributed to the higher surface concentration of high valence ionic species such as Cu2+, Mn3+, and Mn4+, and surface oxygen concentration in the nanorods, than those in the CuMn2O4 made without hydrothermal treatment [23]. The hydrothermal process increases the surface Mn4+ concentration up to 40% in the hydrothermally prepared nanorods due to the Cu2+ + Mn3+ ↔ 2 Cu+ + Mn4+ [77]. The excess surface oxygen combines more easily with toluene molecules than the adsorbed oxygen on the catalyst surface [78]. The π bond of the aromatic ring interacts with Cu2+ with the formation of a transition complex, and the d electrons of Cu2+ can be fed back to the π bond system of the toluene molecule [34].
The catalytic activity of the copper-rich Cu1.5Mn1.5O4 spinels in toluene oxidation can be increased by La-loading [37]. Lanthanum acts as a structure promoter and increases the surface area and diminishes the crystallinity of the catalyst. As an electron promoter, lanthanum changes the valence state and redox properties of copper and manganese located at various spinel sites. The reducibility of the La-doped catalysts is significantly improved, and there was also a significant increase in the surface oxygen concentration [37].

Table 9.
Removal of toluene from air in the presence of CuxMn3−xO4 catalysts.
Table 9.
Removal of toluene from air in the presence of CuxMn3−xO4 catalysts.
| CuxMn3−xO4 | Precursors, Preparation Method, and Conditions | Catalyst Efficiency and Reaction Conditions | Refs. | Remarks |
|---|---|---|---|---|
| x = 1.0 | Cu–nitrate, Mn–acetate, Cu:Mn = 1:2, γ–Al2O3, 500 °C for 5 h | 1000 ppm toluene in 7:3 N2:O2, 120,000 h−1 space velocity, T50/T90 = 369/474 °C, resp. | [76] | Supported on Al2O3, SBET = ~101.3 m2 g−1 |
| x = 1.0 | Cu and Mn(II) acetate, co-precipitation at 80 °C with aq. Na2CO3, 500 °C | 0.35% toluene and 9% O2 in Ar, 250 °C | [49] | Mixed phase, SBET = 73 m2 g−1 |
| x = 1.0 | Cu and Mn(II) acetate, hydrothermal treatment in 10 M NaOH at 110 °C for 20 h, 250 °C | 0.35% toluene and 9% O2 in Ar, GHSV 36,000, 210 °C; T10/T50/T95 are 150, 170, 200 °C, resp. | [49] | Nanorods, mixed phase, SBET = 221 m2 g−1 |
| x = 1.0 | Mn and Cu nitrates, co-precipitation with Me4NOH, 400 °C for 5 h | 800 ppm toluene in air, 150 °C for 24 h, GHSV = 27,800 mL g−1 h−1, T10/T50/T90 = 185/195/200 °C, resp. | [26] | SBET = 48 m2 g−1, Vp = 0.30 cm3 g−1 |
| x = 1.2 | Cu–nitrate, Mn–acetate, Cu:Mn = 1:0.5, γ–Al2O3, 500 °C for 5 h | 1000 ppm toluene in 7:3 N2:O2, 120,000 h−1 space velocity, T50/T90 = 330/383 °C, resp. | [76] | Supported on Al2O3, SBET = ~109.2 m2 g−1 |
| x = 1.3 | Cu–nitrate, Mn–acetate, Cu:Mn = 1:1.5, γ–Al2O3, 500 °C for 5 h | 1000 ppm toluene in 7:3 N2:O2, 120,000 h−1 space velocity, T50/T90 = 271–303/293–329 °C, resp., depending on loaded amount on Al2O3 | [76] | Supported on Al2O3, SBET = ~99.0 m2 g−1, TOF = 0.05–0.28 |
| x = 1.4 | Mn and Cu–nitrates, anatase, incipient wetness impregnation, 500 °C for 7 h in air | Atmospheric pressure, 500 ppm toluene in air, 150–300 °C, 5 h, GHSV 5000 h−1 | [18] | Mixed phase, SBET = 34–48 m2 g−1, supported on TiO2 |
| x = 1.4 | Cu:Mn = 1:1, MnCl2, CuCl2, Na2CO3, 450 °C for 5 h | 500 ppm toluene in humid air, GHSV = 50,000 mL g−1 h−1, T50/T90 =265/280 °C, resp., reaction rate = 0.001 mmol g−1 h−1 and 0.005 mmol m−2 h−1 at 200 °C | [13] | SBET = 18 m2 g−1, Pv = 0.02 cm3 g−1, APD = 13 |
| x = 1.5 | Cu–nitrate, Mn–acetate, Cu:Mn = 1:2, γ–Al2O3, 500 °C for 5 h | 1000 ppm toluene in 7:3 N2:O2, 120,000 h−1 space velocity, T50/T90 = 300/343 °C, resp. | [76] | Supported on Al2O3 SBET = ~107.4 m2 g−1 |
| x = 1.5 | ionotropic sodium alginate, metal chlorides, alcoholic dehydration, and calcining the xerogel at 450 °C | Toluene in dry air, 1000 ppm, GHSV = 46,000 mL g−1 h−1, reaction rate = 1.03 mmol g−1 h−1 and 24.52 mmol m−2 h−1 at 200 °C | [38] | cubic, ∼10 nm size, SBET = 42 m2 g−1 |
| x = 1.5 | Mn–acetate, Cu–nitrate, 1.5 M ammonium carbonate, pH = 8, 2 h, calcining at 550 C for 2 h in air | 1000 ppm toluene in air, GHSV 30,000 h−1, T50/T90 = 245/274 °C, resp. | [37] | Mixed phase, undoped, SBET = 784.1 m2 g−1, Vp (meso) = 0.246 and Vp (all) 0.25 cm3 g−1 |
| x = 1.5 | Mn–acetate, Cu–nitrate, La–nitrate, 1.5 M ammonium carbonate, pH = 8, 2 h, calcining at 550 C for 2 h in air | 1000 ppm toluene in air, GHSV 30,000 h−1. T50/T90 for 2, 4, and 6% La content were 218/268, 217/255, and –/280 °C, respectively. | [37] | Mixed phase, 4% La-doping, SBET = 164.2 m2 g−1, Vp (meso) 0.426 and Vp (all) 0.45 cm3 g−1 |
SBET—specific surface area; Pv—the total adsorption pore volume at P/P0 = 0.995; APD—average pore diameter; TOF—turnover frequency; GHSV—gas hourly space velocity.
The Cu1.4Mn1.6O4 spinel catalyst showed activity in the combustion of benzene, toluene, and xylene. The acidity of the catalyst used in the oxidation of toluene decreased from ca. 89% to 79% after 2500 min, and the surface concentrations of CuII and MnIV were 56.8 and 56.9%. Catalytic activity decreased when H2O, CO2, and SO2 were added, due to the formation of surface species. However, the deactivation is reversible. The aromatic hydrocarbons were oxidized into carbon dioxide, and only a minor amount of CO was detected as a consequence of the incomplete conversion of CO into CO2. This means the catalyst is less efficient in the oxidation of CO than in the combustion of aromatic hydrocarbons [13].
5. Copper Manganese Oxides as Catalysts in Industrial Production Processes
Hydrogen gas is an essential raw material for numerous industrial processes, including ammonia and fertilizer synthesis and fine chemical industry, therefore converting the carbon monoxide content of water gas into carbon dioxide and hydrogen, and the methanol steam reforming processes have a huge importance. These processes can be catalyzed with copper manganese oxide spinels [20,36,40]. The hydrogen-rich CO:H2 mixtures (e.g., synthesis gas), however, resulted in the formation of important organic intermediates such as methyl formate in the presence of these copper–manganese oxide catalysts and the alkanol–DMF organic solvents [29,30].
5.1. Copper Manganese Oxide Spinel-Catalyzed Reactions of CO:H2 Mixtures
The water–gas shift reaction is an enormously important industrial process for hydrogen generation through the conversion of CO gas produced by the steam reforming of hydrocarbons:
CO + H2O ↔ CO2 + H2, ΔH298 = −41 kJ mol−1.
The catalytic performance of the catalysts containing Cu1.5Mn1.5O4, which were prepared by the autoignition of copper and manganese nitrates with an excess of urea and by precipitation with sodium carbonate [36], was studied in the oxidative conversion of carbon monoxide (Table 10). The measured CO conversion strongly depended on the preparation method. The sample prepared by autoignition showed 90% conversion at 220 °C. The catalyst prepared by co-precipitation was less active in the 160–240 °C interval, and ca. 80% CO conversion was reached at 240 °C. The effect of space velocity on CO conversion over the catalyst prepared by the autoignition method was studied at 180 °C when a significant decrease of CO conversion by ∼17 and 32% was observed at GHSV = 6000 and 8000 h−1, respectively. The conversion CO at 180 °C increases when the partial pressure of water is increased from 5 to 10 kPa [26].

Table 10.
The water–gas shift reaction in the presence of CuxMn3−xO4 catalysts.
5.2. Synthesis of Methyl Formate
The copper manganese spinels with x = 0.5 prepared from manganese nitrate and [tetraamminecopper(II)] hydroxide solutions with subsequent calcination at 450, 550, and 650 °C were tested in the synthesis of methyl formate from synthesis gas at 160 °C in 30 min with the MeOH:DMF solvent [29,30] (Table 11).
2CO + 2H2 = HC(=O)OCH3

Table 11.
Methyl formate synthesis from synthesis gas catalyzed by copper manganese oxide spinel containing catalysts.
When the molar ratio of CO:H2 was adjusted to 1:2, the selectivity of methyl formate formation (byproducts were CO2, EtOH, and formaldehyde) was >70% [30] whereas using a CaO–ZrO2 co-catalyst resulted in an increase in selectivity up to >80% [30]. The best methyl formate space–time yield and selectivity, 4.19 g L−1 h−1 and 88.1%, respectively, were obtained on the spinel formed at 450 °C due to its high specific surface area. CO conversion in ethanol/DMF was higher than in methanol/DMF as solvent. The hydrogenation of methyl formate into methanol according to the equation
was catalyzed with the spinel prepared at 550 °C. Methyl formate showed high conversion (86.5%) with high methanol space-time yield and selectivity (Table 11) [30].
HCOOCH3 + 2H2 = 2CH3OH
5.3. Methanol Steam Reforming Process
The steam reforming of methanol is a reaction that produces high H2 yield/per mole methanol:
CH3OH + H2O ↔ CO2 + 3H2
A series of CuxMn3−xO4 catalysts with x = 0.3–1.05 prepared by the urea–nitrate combustion method at 550 °C were tested in the methanol steam reforming reaction under atmospheric pressure. Dilute (5% MeOH and 7.5% H2O) gas stream was used, and quantitative methanol conversion was achieved at 280 °C with the copper manganese oxide spinel catalysts with x = 0.6–1.05. The superior catalysts resulted in H2 and CO2 between 240 and 280 °C, and only a small amount of CO was formed (CO and H2 selectivity were between 1.7–6.4 and 91.9–97.5%, respectively, depending on the value of x and the reaction temperature [40] (Table 12).

Table 12.
Steam reforming of methanol in the presence of catalysts containing copper manganese oxide spinel.
The catalytic activities of nanostructured Cu–Mn spinel oxides prepared in various reactions including the mechanochemical reaction of solid oxalic acid and citric acid with basic copper carbonate and manganese carbonate with subsequent calcination at 300 and 500 °C) were compared in the steam reforming reaction of methanol (Table 12). The catalytic activity of the copper–manganese oxide spinels prepared by mechanochemical reaction routes strongly depended on the milling intensity and the chemical nature of the precursors. The Cu1.5Mn1.5O4 spinel prepared with solid oxalic acid was extremely active and converted more than 92% of methanol at temperatures of 240 and 260 °C. The spinel prepared from citric acid precursors with the same composition converted only 68% of methanol. The samples prepared at 300 °C had much higher catalytic activity than those calcined at 500 °C. The superior activity of the spinel prepared by grinding oxalic acid and calcining it at 300 °C was attributed to the generation of the active Cu1.5Mn1.5O4 spinel phase and the high surface area, which provides high component dispersion. The catalytic performance of the spinels obtained with the same stoichiometry by the conventional co-precipitation with aq. Na2CO3 is much inferior to the samples prepared by the mechanochemical methods, due to the low surface areas and the presence of less of the active phase of Cu1.5Mn1.5O4 spinel [20].
5.4. Other Industrial Processes Catalyzed by CuxMn3−xO4 Spinels
The copper-rich catalysts containing Cu1.5Mn1.5O4 spinel prepared with the conventional precipitation technique with sodium carbonate from the appropriate metal acetates were tested in the oxidation of toluene into benzyl alcohol. The catalysts (heat-treated between 400 and 800 °C) proved to be effective in the oxidation of toluene with molecular oxygen without using any solvents and additives, combining the advantages of minimized separation difficulties and equipment corrosion [34]. The main products were benzyl alcohol, benzaldehyde, and benzoic acid. The conversion of toluene (~17%) was higher than that found in the presence of other copper or manganese-based bimetallic Cu–(Zn, Si, Zr, Cr) or Mn–(Co, Ce, Fe) catalysts under 1.0 MPa oxygen pressure at 190 °C for 2 h. The distributions of the oxidation products strongly depend on the phase composition (heat treatment temperature and molar ratio of Cu and Mn acetate). The highest conversion of toluene (21.6%) was found with the spinel prepared at a 1:1 Cu:Mn ratio and the sintering temperature of 700 °C [34]. The benzyl alcohol was formed in 30 min in a high amount but transformed gradually into benzoic acid in 180 min. The highest selectivity to benzoic acid was in the sample made at 600 °C [34,43] (Table 13).

Table 13.
Other industrial-scale reactions catalyzed by CuxMn3−xO4 spinels.
A series of mixed-phase catalysts containing CuxMn3−xO4 (1 < x < 2) doped with NaCl were tested in the propylene epoxidation reaction. The catalysts were prepared by combustion synthesis (self–propagating reaction) with the use of a variety of fuels such as glycerol, maleic acid, citric acid, or ethyl acetoacetate. The optimal propylene oxide selectivity of 30−37% was achieved at 1.3−1.5% propylene conversion using a catalyst prepared with glycerol at 550 °C [43].
The total combustion of solid coals with inherent CO2 separation was performed with the Chemical Looping with Oxygen Uncoupling (CLOU) process performed in the presence of mixed-phase catalysts containing Cu1.5Mn1.5O4 as oxygen carriers. Full combustion was reached, but CO2 capture efficiencies depended on coal rank [15].
6. Characterization and Operando Techniques Used in the Studying of CuxMn3−xO4 Spinel Catalysts
The phase characterization of CuxMn3−xO4 spinels was performed with powder and single-crystal XRD studies. Vibrational spectroscopy including IR, far-IR, and Raman methods was also used.
The surface of the catalysts was characterized by X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and N2 BET. Porosity was also characterized with BET isotherms measurements. Temperature-programmed desorption of O2, H2, and other gases used in the catalytic reactions (CO, NO, etc.) was also used. The decomposition reaction routes were monitored with DSC, TG, and TG–MS methods with simultaneous characterization of the solid phase by XRD, XPS, IR, SEM, EDX, and transmission electron microscopy (TEM).
Operando methods used to monitor the changes in the catalysts and conversion of reactants were mainly IR and GC methods. In situ diffuse-reflectance FT-IR spectra (DRIFTS) of fine powdery catalysts were recorded in in situ chambers. The reaction/desorption studies were performed by heating the adsorbed species and obtaining DRIFTS spectra at various targets.
The catalytic tests with gases were performed in various kinds of flow reactors, at atmospheric and high-pressure conditions and various temperatures. The liquid oxidation reactions were performed in an autoclave by adjusting the temperature/pressure conditions. The conversions were monitored by LC–MS or GC–MS.
7. Conclusions
The preparation and catalytic activity of CuxMn3−xO4 spinels and multiphase materials containing CuxMn3−xO4 spinels in a wide variety of industrial and environmental protection processes are reviewed. The great diversity of metal ion valences and distribution in the spinel lattice, and the relation of the preparation and annealing methods on the crystallinity, composition, and chemical state of phases and surface layers play an important role in the catalytic activity of the CuxMn3−xO4 spinels. The presence of amorphous materials, lattice distortions, and the number of oxygen and metal vacancies are key factors in their catalytic activity. These features of CuxMn3−xO4 spinels can be controlled by the synthesis conditions, including the reaction routes, Cu/Mn ratios, starting metal compound valences, counter-ions, reagents, metal compound concentrations, temperature, time, and a wide variety of other reaction and annealing conditions.
The properties and catalytic activities and performances of the catalyst prepared by traditional solid-phase and solution-phase precipitating reactions were compared with the properties of the catalyst prepared new and promising synthesis routes were developed. The low-temperature methods (<100 °C) based on a solvent-mediated thermal decomposition of [tetraamminecopper(II)] permanganate results in amorphous phases, which can be transformed into regular spinel or bixbyite-like copper manganese oxides with a defect structure. The size and crystallinity of the copper manganese oxides prepared in this reaction route can be controlled with annealing time and temperature, and nanosized catalysts can be prepared even at 500 °C. These kinds of catalysts have been tested only in CO oxidation reactions, yet their studying in other industrially important reactions promises exciting possibilities to increase the performance of copper manganese oxide spinel catalysts in these processes. This reaction to prepare spinel oxides can be made with co-crystals or solid solutions made with [tetraamminecopper(II)] permanganate and other analog salts with various divalent cations or anions containing metal (e.g., perrhenate). In this way, a wide variety of metal-doped copper manganese oxide spinels can be prepared, which gives an almost infinite possibility to change the properties of amorphous or partially crystalline nanosized catalysts.
These perspectives pose new challenges to defect and synergic engineering to enhance the oxygen replenishment capacity of the doped CuMn2O4 phases and control the amount and distribution of copper and oxygen vacancies in the spinel lattice sites. Developing (multi)-doped copper–manganese oxide catalysts to increase their efficiency and selectivity in various reactions helps substitute expensive and hardly available noble metal catalysts.
Author Contributions
Conceptualization, L.K.; writing—original draft preparation, L.K.; writing—review and editing, K.A.B., V.M.P. and L.B.; visualization, L.B.; supervision, L.K. and V.M.P.; project administration, L.B.; funding acquisition, L.K. and K.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund (VEKOP-2.3.2-16-2017-00013) (L.K.) and the ÚNKP-21-3 and 22-3 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund (K.A.B.).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, K.; Ding, H.; Pan, W.; Mu, X.; Qiu, K.; Ma, J.; Zhao, Y.; Song, J.; Zhang, Z. Research Progress of a Composite Metal Oxide Catalyst for VOC Degradation. Environ. Sci. Technol. 2022, 56, 9220–9236. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, L.; Ling, H.; Ge, Z.; Lin, X.; Dai, X.; Chen, H. Critical review of thermochemical energy storage systems based on cobalt, manganese, and copper oxides. Renew. Sustain. Energy Rev. 2022, 158, 112076. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Chen, J.; Shen, Y.; Xiong, K.; Zhou, Y.; Li, X.; Hu, Y. Different Active Phases of CuMnOx for Total Oxidation and Partial Oxidation. Cailiao Daobao/Mater. Rep. 2020, 34, 9028–9033. [Google Scholar]
- Yuvaraj, S.; Selvan, R.K.; Lee, Y.S. An overview of AB2O4– and A2BO4–structured negative electrodes for advanced Li–ion batteries. RSC Adv. 2016, 6, 21448–21474. [Google Scholar] [CrossRef]
- Gao, P.-X.; Shimpi, P.; Gao, H.; Liu, C.; Guo, Y.; Cai, W.; Liao, K.-T.; Wrobel, G.; Zhang, Z.; Ren, Z.; et al. Hierarchical assembly of multifunctional oxide–based composite nanostructures for energy and environmental applications. Int. J. Mol. Sci. 2012, 13, 7393–7423. [Google Scholar] [CrossRef]
- Lamb, A.B.; Bray, W.C.; Frazer, J.C.W. The Removal of Carbon Monoxide from Air. J. Ind. Eng. Chem. 1920, 12, 213–221. [Google Scholar] [CrossRef]
- Lu, H.F.; Kong, X.X.; Huang, H.F.; Zhou, Y.; Chen, Y.F. Cu–Mn–Ce Ternary Mixed–Oxide Catalysts for Catalytic Combustion of Toluene. J. Environ. Sci. 2015, 32, 102–107. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, P.; Moreno, S. Cu–Mn and Co–Mn Catalysts Synthesized from Hydrotalcites and Their Use in the Oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Broemme, A.D.D.; Brabers, V.A.M. Preparation and properties of copper– and manganese-containing mixed. Solid State Ion. 1985, 16, 171–178. [Google Scholar] [CrossRef]
- Martin, B.E.; Petric, A. Electrical properties of copper–manganese spinel solutions and their cation valence and cation distribution. J. Phys. Chem. Solids 2007, 68, 2262–2270. [Google Scholar] [CrossRef]
- Múčka, V.; Silber, R. Decomposition of hydrogen peroxide on nickel–silver two–component catalyst. Collect. Czech. Chem. Commun. 1984, 49, 2222–2230. [Google Scholar] [CrossRef]
- Garcıa, E.; Palacios, J.M.; Alonso, L.; Moliner, R. Performance of Mn and Cu Mixed Oxides as Regenerable Sorbents for Hot Coal Gas Desulfurization. Energy Fuels 2000, 14, 1296–1303. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Kotai, L.; Gacs, I.; Sajo, I.E.; Sharma, P.K.; Banerji, K.K. Beliefs and Facts in Permanganate Chemistry–An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates. Trends Inorg. Chem. 2009, 11, 25–104. [Google Scholar] [CrossRef]
- Adánez–Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.F.; Adánez, J. CLOU process performance with a Cu–Mn oxygen carrier in the combustion of different types of coal with CO2 capture. Fuel 2018, 212, 605–612. [Google Scholar] [CrossRef]
- Shaheen, W.M.; Selim, M.M. Effect of thermal treatment on physicochemical properties of pure and mixed manganese carbonate and basic copper carbonate. Thermochim. Acta 1998, 322, 117–128. [Google Scholar] [CrossRef]
- Aoki, I. Cation Distribution in CuMn2O4. J. Phys. Soc. Japan 1965, 20, 871. [Google Scholar] [CrossRef]
- Vu, V.H.; Belkouch, J.; Ould-Dris, A.; Taouk, B. Catalytic oxidation of volatile organic compounds on manganese and copper oxides supported on titania. AIChE. J. 2008, 54, 1585–1591. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Sun, P.; Zhang, P.; Zeng, Z.; Liang, S.; Zhu, X. Nanostructured Mn–Cu binary oxides for supercapacitor. J. Alloys Compd. 2014, 598, 166–170. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.-C.; Chen, M.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Waste–free Soft Reactive Grinding Synthesis of High–Surface–Area Copper–Manganese Spinel Oxide Catalysts Highly Effective for Methanol Steam Reforming. Catal. Lett. 2008, 121, 144–150. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Robbrecht, G.G.; Brabers, V.A.M. On the stability of the cubic spinel structure in the system Cu–Mn–O. Mater. Res. Bull. 1973, 8, 571–580. [Google Scholar] [CrossRef]
- Loginova, M.V.; Stogova, V.A.; Sheftel, I.T. Structural transformations and electrical conductivity of copper–manganese spinel. Izv. Ak. Nauk SSSR Ser. Neorg. Mater. 1971, 1, 120. [Google Scholar]
- Beley, M.; Padel, L.; Bernier, J.C. Etudes et Proprietes des Composes CuxMn3−xO4. Ann. Chim. Fr. 1978, 3, 429–452. [Google Scholar]
- Jarrige, J.; Mexmain, J. Préparation et stabilité des manganites de cuivre. Bull. Soc. Chim. Fr. 1976, 405–409. [Google Scholar]
- Cai, L.N.; Guo, Y.; Lu, A.H.; Branton, P.; Li, W.C. The choice of precipitant and precursor in the co–precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J. Mol. Catal. A Chem. 2012, 360, 35–41. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.-M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.-F. Influence of the preparation method on the activity of copper–manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Kótai, L.; Horváth, T.; Szentmihályi, K.; Keszler, Á. Evidenceforquasi–intramolecular acid–base reactions in solutions of transition metal ammine complexes. Transit. Met. Chem. 2000, 25, 293–294. [Google Scholar] [CrossRef]
- Kótai, L.; Gács, I.; Kazinczy, B.; Sajó, I.E. Quasi–intramolecular acid–base reactions in aqueous solutions of metal–complexes of basic ligands I. Generalized theoretical considerations on the deammoniation of [MLm]Xn type ammonia complexes. Transit. Met. Chem. 2003, 28, 292–295. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, K.; Zhou, J.; Lin, M.; Sun, Y. Direct synthesis of methyl formate from syngas on Cu–Mn mixed oxide catalyst. Int. J. Hydrog. Energy 2016, 41, 8819–8828. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, K.; Dong, F.; Lin, M.; Sun, Y.; Tang, Z. Textual properties of Cu–Mn mixed oxides and application for methyl formate synthesis from syngas. J. Ind. Eng. Chem. 2017, 54, 117–125. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Lakshmi, S.M.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Electrochemical properties of rice–like copper manganese oxide (CuMn2O4) nanoparticles for pseudocapacitor applications. J. Alloys Compd. 2017, 723, 115–122. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Wei, L.; Shi, Z.; Han, L.; Yuan, H.; Li, B.; Dong, L.; Wang, F.; Sun, C. Preparation and Evaluation of Copper–Manganese Oxide as a High–Efficiency Catalyst for CO Oxidation and NO Reduction by CO. J. Phys. Chem. C. 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Jones, C.; Cole, K.J.; Taylor, S.H.; Crudace, M.J.; Hutchings, G.J. Copper manganese oxide catalysts for ambient temperature carbon monoxide oxidation: Effect of calcination on activity. J. Mol. Catal. A Chem. 2009, 305, 121–124. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Zhou, L.; Wang, F.; Gao, J.; Chen, C.; Ning, J.; Ma, H. Liquid–phase oxidation of toluene by molecular oxygen over copper manganese oxides. Catal. Lett. 2006, 110, 149–154. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Shaterian, H.R.; Habibi, M.; Hutchings, G.J.; Taylor, S.H. Characterisation of copper–manganese oxide catalysts: Effect of precipitate aging upon the structure and morphology of precursors and catalysts. Appl. Catal. A Gen. 2003, 253, 499–508. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Ioannides, T. Highly active copper catalyst for low–temperature water–gas shift reaction prepared via a Cu–Mn spinel oxide precursor. Appl. Catal. A Gen. 2013, 451, 184–191. [Google Scholar] [CrossRef]
- Pan, J.; Du, W.; Liu, Y.; Cheng, Y.; Yuan, S. Lanthanum–doped CuMn composite oxide catalysts for catalytic oxidation of toluene. J. Rare Earths 2019, 37, 602–608. [Google Scholar] [CrossRef]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu–Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar] [CrossRef]
- Krämer, M.; Schmidt, T.; Stöwe, K.; Müller, F.; Natter, H.; Maier, W.F. Structural and catalytic aspects of sol–gel derived copper manganese oxides as low–temperature CO oxidation catalyst. Appl. Catal. A Gen. 2006, 302, 257–263. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steam reforming of methanol over copper–manganese spinel oxide catalysts. Catal. Commun. 2005, 6, 497–501. [Google Scholar] [CrossRef]
- Choi, K.-H.; Lee, D.-H.; Kim, H.-S.; Yoon, Y.-C.; Park, C.-S.; Kim, Y.H. Reaction Characteristics of Precious–Metal–Free Ternary Mn–Cu–M (M = Ce, Co, Cr, and Fe) Oxide Catalysts for Low–Temperature CO Oxidation. Ind. Eng. Chem. Res. 2016, 55, 4443–4450. [Google Scholar] [CrossRef]
- Einaga, H.; Kiya, A.; Yoshioka, S.; Teraoka, Y. Catalytic properties of copper manganese mixed oxides prepared by co–precipitation using tetramethylammonium hydroxide. Catal. Sci. Technol. 2014, 4, 3713–3722. [Google Scholar] [CrossRef]
- Seubsai, A.; Kahn, M.; Zohour, B.; Noon, D.; Charoenpanich, M.; Senka, S. Copper–Manganese Mixed Metal Oxide Catalysts for the Direct Epoxidation of Propylene by Molecular Oxygen. Ind. Eng. Chem. Res. 2015, 54, 2638–2645. [Google Scholar] [CrossRef]
- Bayón, R.; Vicente, G.S.; Maffiotte, C.; Morales, Á. Characterization of copper–manganese–oxide thin films deposited by dip–coating. Sol. Energy Mater. Sol. Cells 2008, 92, 1211–1216. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Mirzaei, A.A.; Joyner, R.W.; Siddiqui, M.R.H.; Taylor, S.H. Effect of preparation conditions on the catalytic performance of copper manganese oxide catalysts for CO oxidation. Appl. Catal. A Gen. 1998, 166, 143–152. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.; Wu, X.; Zhao, F.; Wang, D.; Zha, X.; Li, S.; Liu, H.; Chen, Y. Low–temperature catalytic oxidation of benzene over nanocrystalline Cu–Mn composite oxides by facile sol–gel synthesis. New J. Chem. 2020, 44, 2442–2451. [Google Scholar] [CrossRef]
- Li, W.B.; Chu, W.B.; Zhuang, M.; Hua, J. Catalytic oxidation of toluene on Mn–containing mixed oxides prepared in reverse microemulsions. Catal. Today 2004, 93–95, 205–209. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; Ye, Z.; Giraudon, J.-M.; De Geyter, N.; Morent, R.; Lamonier, J.-F. Plasma-assisted Cu–Mn mixed oxide catalysts for trichloroethylene abatement in moist air. J. Hazard. Mater. 2019, 379, 120781. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, Z.X.; Liu, R.F.; Chen, J.L.; Xu, B.Q. Rod–like CuMnOx transformed from mixed oxide particles by alkaline hydrothermal treatment as a novel catalyst for catalytic combustion of toluene. Phys. Chem. Chem. Phys. 2016, 18, 22794–22798. [Google Scholar] [CrossRef]
- Jarrige, J.; Mexmain, J. Propriétés du Manganite de Cuivre Cu1.5Mn1.5O4. Bull. Soc. Chim. Fr. 1980, 9–10, I-363–I-369. [Google Scholar]
- Kótai, L.; Sajó, I.E.; Gács, I.; Sharma, P.K.; Banerji, K.K. Convenient Routes for the Preparation of Barium Permanganate and other Permanganate Salts. Z. Anorg. Allg. Chem. 2007, 633, 1257–1260. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Roginskii, S.Z.; Shmuk, E.I. Kinetics of the Thermal Decomposition of Solid Permanganates. Izv. AN SSSR Otdel. Khim. Nauk. 1950, 5, 469–474. [Google Scholar]
- Galwey, A.K.; Fakiha, S.A.A.; Abd El-Salaam, K.M. A kinetic and mechanistic study of the thermal decomposition of copper(II) permanganate. Thermochim. Acta 1994, 239, 225–232. [Google Scholar] [CrossRef]
- Ball, M.C.; Dowen, P.; Galwey, A.K. Chemical and physical properties of the solid products of thermal decomposition of copper(II) and nickel(II) permanganates. J. Mater. Chem. 1997, 7, 315–318. [Google Scholar] [CrossRef]
- Yamanaka, S. Anion exchange reactions in layer structured crystals. Stud. Surf. Sci. Catal. 1994, 83, 147–153. [Google Scholar]
- Kótai, L.; Banerji, K.K.; Sajó, I.; Kristóf, J.; Sreedharm, B.; Holly, S.; Keresztury, G.; Rockenbauer, A. An Unprecedented-Type Intramolecular Redox Reaction of Solid [Tetraamminecopper(2+)] Bis (permanganate)([Cu(NH3)4](MnO4)2)–A Low-Temperature Synthesis of Copper Dimanganese Tetraoxide-Type (CuMn2O4) Nanocrystalline Catalyst Precursors. Helv. Chim. Acta 2002, 85, 2316–2327. [Google Scholar] [CrossRef]
- Gorbunov, V.V.; Shmagin, L.F. On the combustion of salts of tetramine copper (II). Fiz. Goreniya Vzriva 1972, 8, 523–526. [Google Scholar]
- Seferiadis, N.; Dubler, E.; Oswald, H.R. Structure of [tetraamminecopper(II)] dipermanganate. Acta Cryst. Sect. C. 1986, 42, 942–945. [Google Scholar] [CrossRef]
- Kótai, L.; Németh, P.; Kocsis, T.; Sajó, I.E.; Pasinszki, T. A new route to synthesize controlled–size MMn2O4–type transition metal (M = Cd, Zn, Cu) nanomanganites. Nano Stud.—Sci. J. 2016, 13, 7–13. [Google Scholar]
- Solt, H.E.; Németh, P.; Mohai, M.; Sajó, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature–Limited Synthesis of Copper Manganites along the Borderline of the Amorphous/Crystalline State and Their Catalytic Activity in CO Oxidation. ACS Omega 2021, 6, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Pollert, E. Tetragonal–to–cubic transformation of hausmannite. J. Solid State Chem. 1980, 33, 305–308. [Google Scholar] [CrossRef]
- Miyahara, S. Jahn–Teller distortion in magnetic spinels. J. Phys. Soc. Japan 1962, 17 (Suppl. BI), 181–184. [Google Scholar]
- Radhakrishnan, N.K.; Biswas, A.B. A Neutron Diffraction Study of Cation Distribution in Some Manganites. J. Ind. Chem Soc. 1974, 37, 274–281. [Google Scholar]
- Sinha, A.P.B.; Sanjana, N.R.; Biswas, A.P.B. The crystal structure of copper manganite. J. Phys. Chem. 1958, 62, 191–194. [Google Scholar] [CrossRef]
- Waskowska, A.; Gerward, L.; Olsen, J.S.; Steenstrup, S.; Talik, E. CuMn2O4: Properties and the high–pressure induced Jahn–Teller phase transition. J. Phys. Condens. Matter 2001, 13, 2549–2562. [Google Scholar] [CrossRef]
- Dorris, S.E.; Mason, T.O. Electrical Properties and Cation Valencies in Mn3O4. J. Am. Ceram. Soc. 1988, 71, 379–385. [Google Scholar] [CrossRef]
- Vetter, K.M.; Manecke, G. Der Einstellungsmechanismus des Mn+++/Mn++++–Redoxpotentials an Platin. Z. Phys. Chem. 1950, 195, 270–337. [Google Scholar] [CrossRef]
- Buhl, R. Manganites spinelles purs d’elements de transition preparations et structures cristallographiques. J. Phys. Chem. Solids 1969, 30, 805–812. [Google Scholar] [CrossRef]
- Blasse, G. Ferromagnetism and ferrimagnetism of oxygen spinels containing tetravalent manganese. J. Phys. Chem. Solids 1966, 27, 383–389. [Google Scholar] [CrossRef]
- Miller, A. Determination of the valence state of copper in cubic CuMn2O4 spinel by X–ray absorption edge measurements. J. Phys. Chem. Solids 1967, 29, 633–639. [Google Scholar] [CrossRef]
- Lenglet, M.; D’Huysser, A.; Kasperek, J.; Bonnelle, J.P.; Durr, J. Characterization of the oxidation states of copper and manganese in some manganites by the analysis of the XPS spectrum, X emission, and X absorption thresholds. Mater. Res. Bull. 1985, 20, 745–757. [Google Scholar] [CrossRef]
- Di Castro, V.; Furlani, C.; Gargano, M.; Rossi, M. XPS characterization of the CuO/MnO2 catalyst. Appl. Surf. Sci. 1987, 28, 270–278. [Google Scholar] [CrossRef]
- Radhakrishnan, N.K.; Biwas, A.B. A neutron diffraction study of the spinel oxide CuMn2O4. Phys. Status Solidi (A) Appl. Res. 1977, 44, 45–49. [Google Scholar] [CrossRef]
- Gillot, B.; Buguet, S.; Kester, E.J. Oxidation mechanism and valence states of copper and manganese in tetragonal CuMn2O4. Mater. Chem. 1997, 7, 2513–2517. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Hahn, T. Synergy effect between copper and manganese oxides in hopcalite catalysts. Stud. Surf. Sci. Catal. 2001, 138, 315–322. [Google Scholar]
- Wang, H.; Lu, Y.; Han, Y.X.; Lu, C.; Wan, H.; Xu, Z.; Zheng, S. Enhanced catalytic toluene oxidation by the interaction between copper oxide and manganese oxide in Cu–O–Mn/γ–Al2O3 catalysts. Appl. Surf. Sci. 2017, 420, 260–266. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx–CeOx on carbon nanotubes for the low–temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takeguchi, T.; Kikuchi, R.; Eguchi, K. Influence of preparation method and additive for Cu–Mn spinel oxide catalyst on water gas shift reaction of reformed fuels. Appl. Catal. A Gen. 2005, 279, 59–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).