Abstract
Transition metal borides (TMBs) are promising catalysts for hydrogen evolution reaction (HER). While the commercially available TMBs indicate poor HER performance due to powder electrode and low activity sites density, optimizing commercial TMBs for better HER performance is urgent. To break through the challenge, a new strategy is proposed to compose integral bulk electrodes with needle surfaces in TMBs. The integral bulk electrodes in TiB2, ZrB2, and HfB2 are formed under high pressure and high temperature (HPHT), and the nanoneedle morphology is constructed by chemical etching. In the three materials, the smallest overpotential is 346 mV at 10 mA cm−2 in the HCl etched bulk TiB2 electrode, which is about 61.9% higher than commercial TiB2 powder. Better performance arises from better conductivity of the integral bulk electrode, and the nano morphology exposes the edge sides of the structure which have high activity site density. This work is significant for developing new kinds of bulk TMBs catalysts.
1. Introduction
Hydrogen is a promising renewable energy candidate to replace conventional energy sources because of its high energy density and zero greenhouse gas emissions [1,2,3,4,5]. Water electrolysis is an ideal way to obtain highly pure hydrogen [6,7,8]. Suitable electrocatalysts can effectively improve hydrogen production and reduce overpotential [9]. Up to now, precious metal platinum-based materials are the most active electrocatalysts for hydrogen evolution reaction (HER), which have the lowest overpotential and Tafel slope [10]. However, the commercialization of water electrolysis is hampered due to precious metal electrocatalysts with high prices and scarce availability [11,12,13,14]. Thus, developing noble metal-free electrocatalysts is necessary.
In the past few decades, noble metal-free electrocatalysts including sulfides, carbides, phosphides, nitrides and alloys were extensively studied [15,16,17,18,19,20]. Among them, transition metal borides (TMBs) are favored by researchers because of their structural diversity, excellent electrical properties and stability. Mo-B, Ni-B, Co-B and Fe-B have shown excellent HER performance [21,22,23,24,25,26,27,28]. Meanwhile, commercially available TMBs are mostly sintered at high temperatures at present, which can exhibit the intrinsic catalytic performance of TMBs. However, many commercial TMBs exhibit poor HER performance because of their micron grain size, which is easy to fall off when preparing catalytic electrodes during HER and will hinder the exposure of activity sites [29,30]. Moreover, the Nafion adhesive can cause huge contact resistance between particles and electrodes, which impedes the development of TMBs catalysts [23,31]. Therefore, modifying TMBs and inventing a new kind of electrode is necessary.
Exploring integral bulk electrodes in TMBs for HER is an effective way to eliminate contact resistance. They are comparable with self-supported electrodes, which have excellent charge transport. However, it is difficult to obtain the dense bulk TMBs because of their high melting point and low diffusion coefficient. High pressure and high temperature (HPHT) is a key route to densify and modify TMBs. HPHT can effectively reduce the reaction temperature and reaction time under certain pressure and promote the densification of TMBs. More and more TMBs bulk are successfully synthesized by the HPHT method [32,33,34,35]. Therefore, HPHT is a suitable method to fabricate integral bulk electrodes. Besides, the AlB2-type TMBs are promising catalysts. Although α-MoB2 has the best HER performance, it cannot be purchased due to its high synthesis temperature of 1800 °C [23]. However, TiB2, ZrB2 and HfB2 are commercially available, and the transition metals of Ti, Zr, Hf are in the same group. Therefore, commercial TiB2, ZrB2 and HfB2 are suitable to design integral bulk electrodes.
Although the integral bulk electrode has excellent conductivity, the disadvantage is that the sample is bulky with a smaller surface area. As is known to all, the surface area of electrocatalysts can affect the number of active sites exposed, which directly dominates the catalyst performance. Typically, the bulk MoS2 has no HER performance, while nanosized MoS2 shows excellent catalytic activity [36]. Most of the high-performance electrocatalysts reported are nanosized at present [37]. Nano-morphology construction on the surface of TMBs is the key to further improving its electrocatalysts performance. Nevertheless, surface modification to form nano-morphology in bulk TMBs is a challenge because TMBs always have strong covalent bonds, which is difficult to process [38]. Moreover, the activity sites are located at some specific crystal planes thus surface modification should better expose the active crystal planes. Therefore, one strategy to achieve high HER performance in TMBs is composing integral bulk electrodes with nano morphology which exposes the active crystal planes.
To solve the above problems, in this work, commercially purchased AlB2-type TMBs (TiB2, ZrB2, HfB2) were densified by HPHT to obtain an integral bulk electrode. Then, chemical etching was used to construct the nanomorphology on the surface for the modified sample. The changes of the structure, morphology and valence state of the modified sample before and after nano-construction were studied, as well as the influence on HER performance. The theoretical calculation was used to analyze the internal mechanism of catalytic activity.
2. Results and Discussion
The initial transition metal borides (TMBs) were purchased commercially. The crystal structure and purity of the material were analyzed by X-ray diffraction (XRD) (Figure S1). The intensity and position of the diffraction peaks indicate that the purchased initial powders are pure phase TiB2, ZrB2 and HfB2 (PDF card 85-2083, 65-8704 and 65-8677). The polarization curves of HER for initial powders catalysts are recorded in Figure S2. The overpotential of initial TiB2, ZrB2 and HfB2 powder at a current density of 10 mA cm−2 (η10) exceeds 900 mV, indicating poor HER performance. The poor HER performance may come from the large connect resistance of the micron size powder electrode. HPHT was performed to generate bulk electrodes under 5 GPa and 1600 °C. The crystallinity and phase purity of the bulk materials were characterized by XRD. As shown in Figure 1a, TiB2, ZrB2 and HfB2 after HPHT have highly intense peaks, which mean they are highly crystalline, and no impurities exist. All three materials have a hexagonal crystal system (space group P6/mmm) and belong to the AlB2-type structure, which is alternately stacked by close-packed transition metal atoms and borophene-likes layers (Figure 1b).
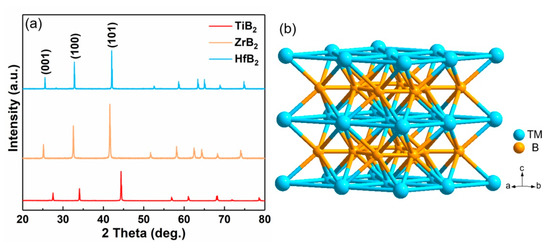
Figure 1.
(a) The XRD of TiB2, ZrB2 and HfB2 modified by HPHT, and (b) the crystal structure of AlB2 type materials.
The bulk samples are composed of high-density micron-size grains, which benefit conductivity (Figure 2a–c). High-resolution transmission electron microscopy (HRTEM) is used to observe finer structural information. All these structures have good crystallinity in Figure 2d–f. The spacing of 0.266 nm and 0.320 nm corresponds to (100) and (001) crystal planes in TiB2. The spacing of 0.219 nm, 0.278 nm, 0.175 nm and 0.215 nm matched the (101), (100) and (002), (101) crystal planes in ZrB2 and HfB2, respectively. All of the results confirmed that integral bulk electrodes of TiB2, ZrB2 and HfB2 are formed after HPHT treatment.
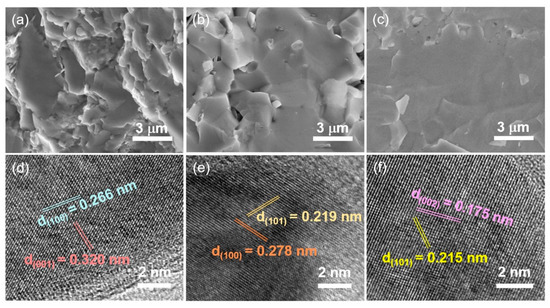
Figure 2.
The SEM images of (a) TiB2, (b) ZrB2 and (c) HfB2 after HPHT treatment, and the corresponding HRTEM images of (d) TiB2, (e) ZrB2 and (f) HfB2.
The electrochemical HER performance of TiB2, ZrB2, and HfB2 after HPHT employs a standard three-electrode system on CH Instruments 760E at the 0.5 M H2SO4 (pH 0) electrolyte. As shown in Figure 3a, the overpotential of TiB2, ZrB2 and HfB2 after HPHT treatment are 477, 627 and 563 mV, respectively. The results indicated that the HER performance of the materials after HPHT treatment is greatly improved, and TiB2 showed the best HER activity. The corresponding Tafel slope is shown in Figure 3b. TiB2, after HPHT treatment, also has the lowest Tafel slope (149.38 mV dec−1), which means a faster catalytic reaction rate for HER under acidic conditions. Moreover, the charge transport resistance of different catalysts was studied by electrochemical impedance spectroscopy (EIS), and the Nyquist curves are shown in Figure 3c. The charge transport resistance of TiB2 is much smaller than that of ZrB2 and HfB2, indicating that the charge transfer between TiB2 electrode and electrolyte is more effective at the interface during HER action. At the same time, the electrochemical surface area (ECSA) can be evaluated with electrochemical double-layer capacitance (Cdl). Figure 3d show the Cdl value of TiB2 is 1.97 mF cm−2, which is 3.4 and 5.3 times higher than ZrB2 and HfB2, respectively. It indicates that TiB2 has more catalytic active sites involved in the HER process. All of the results show that TiB2 has superior catalytic performance for HER.
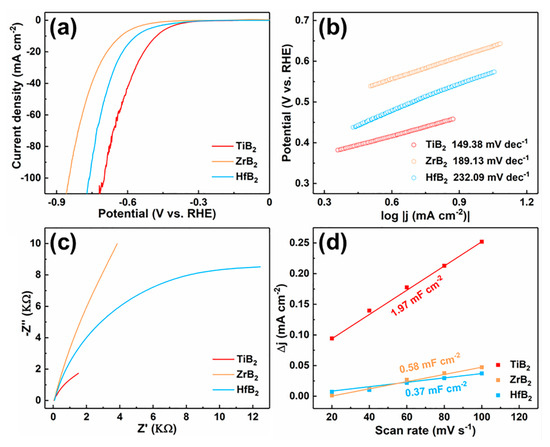
Figure 3.
(a) The linear sweep voltammetry (LSV) curves of TiB2, ZrB2 and HfB2 after HPHT treatment for HER in 0.5 M H2SO4 solution, (b) Tafel slopes of TiB2, ZrB2 and HfB2 after HPHT treatment, (c) EIS Nyquist plots of TiB2, ZrB2 and HfB2 after HPHT treatment, (d) ECSA fitting curves of TiB2, ZrB2 and HfB2 after HPHT treatment.
The better HER performance of TiB2, ZrB2 and HfB2 after HPHT treatment, the more dense integral bulk electrode, which brings better conductivity and lowers connected resistance, while these bulk electrodes sacrifice specific area. As we know, these TMB2 are layer structures with anisotropic HER performance in different crystal planes [39,40,41,42]. To further improve the HER performance, it is desirous to fabricate nano surface morphology and expose the crystal plane with active sites.
To uncover the activity crystal planes with the high HER performance, the Gibbs free energy (ΔG) of TiB2, ZrB2 and HfB2 are calculated (Figure 4). The results indicate the crystal planes of TiB2 (100), ZrB2 (100) and HfB2 (110) have the lowest ΔG. Additionally, all these structures show that the edge sides have a lower ΔG than the basal plane (001). This is consistent with MoB2, which has the best activity in the crystal plane (100) [23]. Therefore, exposing the edge sides on the surface of the integral bulk electrode is a good strategy to further optimize the HER performance of TiB2, ZrB2 and HfB2.
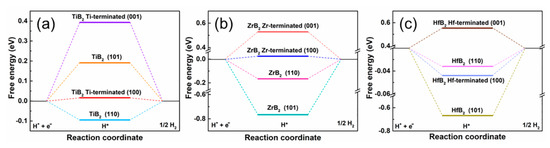
Figure 4.
The calculated free-energy diagram (at 25% hydrogen coverage) for HER over different surfaces of (a) TiB2, (b) ZrB2, (c) HfB2.
HCl etching is an effective way to modify the surface morphology of TMBs [34]. Thus, the surface of bulk samples (TiB2, ZrB2, and HfB2) are etched by HCl. The SEM results exhibit the surface change with different etching times (Figure 5). The nano needle-like morphology appears on the surface of TiB2 with a longer etching time (Figure 5a–d). Additionally, some humps are formed in ZrB2 with an etching time of 6 h (Figure 5e–h). HfB2 prefers to generate taper morphology after 4 h etched (Figure 5i–k). All of these morphologies can enlarge the specific area, which is of benefit to HER. Moreover, the XRD indicates all samples’ structures are still AlB2-type after etching (PDF card 85-2083, 65-8704 and 65-8677) and with no impurity (Figure 5l).
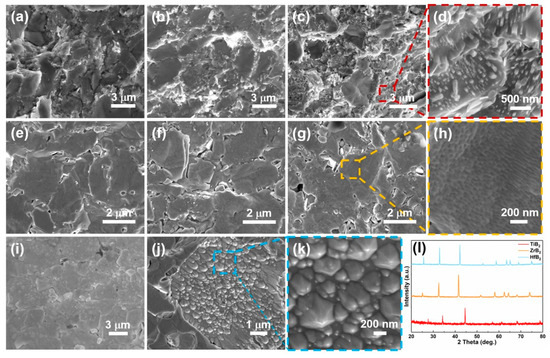
Figure 5.
The SEM images of TiB2 after HCl etched (a) 12 h, (b) 24 h, (c) 48 h, (d) the magnified images of the red dotted box in (c), the SEM images of ZrB2 after HCl etched (e) 2 h, (f) 4 h, (g) 6 h, (h) the magnified images of the yellow dotted box in (g), the SEM images of HfB2 after HCl etched (i) 2 h, (j) 4 h, (k) the magnified images of the light blue dotted box in (j), (l) the XRD of TiB2, ZrB2 and HfB2 after HCl etched.
According to HRTEM (Figure 6a,b), the crystal plane (001) can be found in the needle of TiB2, and the (001) is perpendicular to the direction of along the needle, which is consistent with before results that the direction along the needle is [001] [34]. These results also confirmed that the edge sides of the needle morphology are the edge sides of the structure. The preliminary research confirmed the corrosion mechanism of AlB2-type structures [34], thus the same exposed crystal planes in ZrB2 and HfB2. Therefore, the nano morphology with exposed edge sides was fabricated in some special crystal planes of TiB2, ZrB2 and HfB2 (Figure 6c).
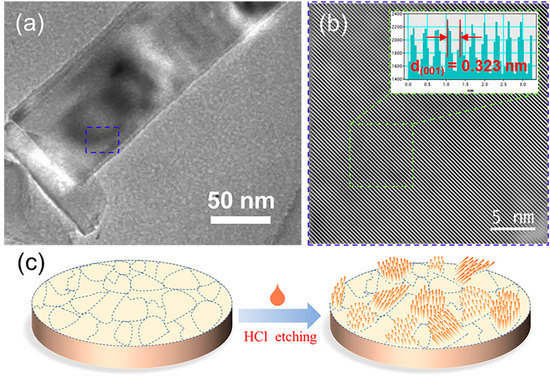
Figure 6.
(a) The TEM image of TiB2 nanorod, (b) the HRTEM image of TiB2 needle, (c) schematic diagram of sample surface morphology before and after HCl etched.
The XPS results before and after HCl etching are shown in Figure 7. Except for normal states of TMBs at lower binding energy, metal oxides states and boron oxides states with high binding energy on the surface of TiB2, ZrB2 and HfB2 are observed [43]. However, the oxides states are weaker after HCl etching. The HCl corrodes the surface of TMBs by destroying the boron atoms and transition metal atoms, but after corrosion, the remaining surface still maintains the atomic states of TMBs. Therefore, the improvement of HER performance is caused by specific areas and exposed edge sides.
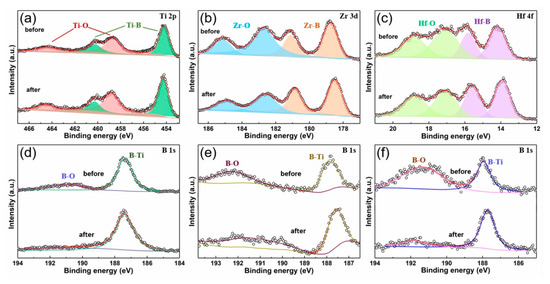
Figure 7.
The XPS spectra of TiB2 (a) Ti 2p, (d) B 1s, ZrB2 (b) Zr 3d, (e) B 1s and HfB2 (c) Hf 4f, (f) B 1s before and after HCl etched.
Uncovering the HER performance of integral bulk electrodes of TiB2, ZrB2 and HfB2 with nano morphology is urgent. Therefore, the electrochemical activity of TiB2, ZrB2 and HfB2 with different etching times were measured in 0.5 M H2SO4 solution. In 0.5 M H2SO4 solution, the LSV curves of TiB2 give overpotentials of 477, 434, 346 and 390 mV at current densities of 10 mA cm−2 with etching times of 0, 12, 24 and 48 h, respectively (Figure 8a). The optimal etching time is 24 h in TiB2. Meanwhile, ZrB2 needs 627, 598, 542 and 586 mV to get to 10 mA cm−2 with etching times of 0, 2, 4 and 6 h, respectively (Figure 8e). Additionally, HfB2 also needs higher overpotentials of 563, 515 and 518 mV to get to 10 mA cm−2 with etching times of 0, 2 and 4 h, respectively (Figure 8i). Those results are substantially better than the initial powder and the integral bulk electrodes before HCl etching. The HER performance of three structures is firstly improved and then becomes worse after increasing the etching time. Therefore, these structures have the optimal HER performance with different etching times. The structures can exhibit hydrophobicity with longer time HCl etching [34], which is the reason for worse HER performance with too much etching time. Figure 8b,f,j shows the Tafel plots of TiB2, ZrB2 and HfB2 with different etching times. The Tafel slope also indicates that TiB2, ZrB2 and HfB2 have the lowest slope when etching for 24, 4 and 2 h, respectively, which means the fastest catalytic reaction rate. The results of EIS and ECSA confirmed that the charge transport resistance of the catalysts decreases and the exposed surface area increases during different HCl etched times, which are beneficial for HER. The optimal HCl etched times of TiB2, ZrB2 and HfB2 also are 24, 4 and 2 h, respectively. All the results indicate that HCl etching is an effective route to enhance TMBs catalyst for HER performance. Compared with commercial powder, the HER performance of TiB2, ZrB2 and HfB2 are improved by about 61.9%, 40.3%, and 43.6%, respectively, after HCl etching. The better HER performance arises from better conductivity of the integral bulk electrode with HCl etching. The nano morphology enlarges the specific area, and the exposed edge sides of structures expose much more activity sites. The HER performance is superior to other TiB2, ZrB2 and HfB2, even better than some nanosized materials (Table S1). Therefore, this strategy for integral bulk electrodes with nano morphology is an effective way to optimize the HER performance.
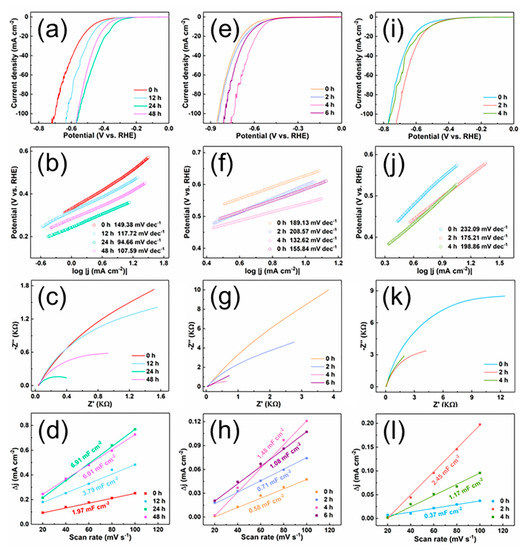
Figure 8.
The LSV curves of (a) TiB2, (e) ZrB2, (i) HfB2 with different etched time for HER in 0.5 M H2SO4 solution, the tafel slopes of (b) TiB2, (f) ZrB2, (j) HfB2 with different etched time, EIS Nyquist plots of (c) TiB2, (g) ZrB2, (k) HfB2 with different etched time, ECSA fitting curves of (d) TiB2, (h) ZrB2, (l) HfB2 with different etched time.
3. Materials and Methods
3.1. Materials
The powder of TiB2 (99%), ZrB2 (99.5%) and HfB2 (99%) were purchased from Aladdin (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Sulfuric acid (98%), hydrochloric acid (37%) and silver paste were purchased from Beijing Chemical Factory (Beijing, China). All reagents were not purified before being used. Highly purified water (>18 MΩ cm resistivity) was provided by a PALL PURELAB Plus system (Sichuan Delishi Technology Co., Ltd., Chengdu, China). The experimental flow chart is shown in Figure 9.
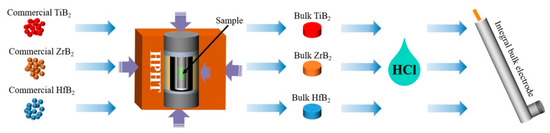
Figure 9.
The experimental flow chart. (Preparation process and HER diagram of TiB2, ZrB2 and HfB2.).
3.2. Preparation of TiB2, ZrB2 and HfB2 Bulk Materials
The preparation of bulk materials was consistent with our previous work [34]. Briefly, the sample powders were cold-pressed into a tablet shape with a diameter of 4 mm and thickness of 2.5 mm. Subsequently, the tablets samples were placed in a cubic anvil apparatus (6 × 14,400 KN) under the pressure of 5 GPa; the temperature was 1600 °C for 30 min, 3 h and 5 h for ZrB2, HfB2, and TiB2, respectively.
3.3. Constructed the Surface Morphology of TiB2, ZrB2 and HfB2 Bulk Materials
The bulk samples were etched in concentrated hydrochloric acid (12 M) for different times. TiB2 was etched for 12, 24, 48 h, ZrB2 was etched for 2, 4, 6 h, and HfB2 was etched for 2, 4 h. After etching, the surface of the sample was thoroughly rinsed with deionized water to remove the residual acid and finally dried in a vacuum oven at 60 °C overnight.
3.4. Characterization
The powder X-ray diffraction (XRD) was performed by a Rigaku D/Max 2550 X-ray diffractometer operating with Cu Kα X-ray radiation (λ = 1.5418 Å). The scanning electron microscope (SEM) images were obtained with an FEI Magellan 400L microscope operating at 18 KV. A high-resolution transmission electron microscope (HRTEM) was obtained with a JEM-2200FS TEM operating at 200 KV. The X-ray Photoelectron Spectroscopy (XPS) were recorded on an ESCALAB 250 analyzer with Al Kα radiation. All the peaks were corrected by C 1 s line at 284.8 eV as standard, and curve fitting and background subtraction were accomplished.
3.5. Preparation of the Working Electrode
Powder material. The preparation of the electrode was similar to that previously reported in the literature [44]: 2 mg powder material was dispersed in isopropanol (200 μL) and then ultrasonicated for 20 min to obtain a homogeneous ink. Drop-casting 2 μL of homogeneous ink onto a glassy carbon electrode with a diameter of 3 mm, and finally coating the dried samples with 2 μL of 0.3% Nafion solution and letting to dry. The mass loading of the materials was 0.28 mg cm−2.
Bulk material. The preparation of the electrode was similar to that previously reported in the literature [23]: the bulk material was fixed on an L-type copper electrode with conductive silver paste. After the paste was dried, the copper electrode and exposed silver paste were sealed with a modified acrylate adhesive. The effective area of the working electrode was measured with the Leica M125 C system.
3.6. Electrochemical Measurement
All electrochemical measurements were performed with a typical three-electrode system in 0.5 M H2SO4 using a CH Instrument (Model 760E). The electrolyte was purged with high-purity N2 gas. A carbon rod and a saturated calomel electrode (SCE) were employed as the counter electrode and reference electrode, respectively. The overpotential is expressed as the value versus the reversible hydrogen electrode (RHE) in this work, according to Evs.RHE = Evs.SCE + 0.241. For the LSV measurements, the scan rate was set to 1 mV s−1. All of the data were without iR correction in this work. In order to probe the electrochemical double-layer capacitance of the material, cyclic voltammetry (CV) was used at non-Faradaic overpotentials as the means for estimating the effective electrochemical surface areas. With this procedure, a series of CV measurements were performed at various scan rates (20, 40, 60, 80, 100 mV s−1, etc.) from −0.2 to −0.3 V vs. RHE region, in which the sweep segments were set to 40 to ensure consistency. By plotting the difference of current density (J) between the anodic and cathodic sweeps (Janodic − Jcathodic) at −0.25 V vs. RHE against the scan rate, a linear trend was observed. The slope of the fitting line is equal to twice the geometric double-layer capacitance (Cdl), which is proportional to the cyclic voltammetry (CV) was used at non-faradaic overpotentials as the means for estimating the effective electrochemical surface areas. The slope of the fitting line was equal to twice the geometric double-layer capacitance (Cdl), which was proportional to the electrochemically active surface area (ECSA) of the material.
Electrochemical Impedance Spectroscopy (EIS) on the different materials were performed at a potential of −0.25 V versus RHE. A sinusoidal voltage with an amplitude of 5 mV and scanning frequency ranging from 105 Hz to 1 Hz was applied to carry out the measurements.
3.7. DFT Calculations
The density functional theory (DFT) computations were performed by employing the generalized gradient approximation (GGA) in the form of the Perdew–Burke–Ernzerhof for the exchange-correlation functional within the Cambridge Serial Total Energy Package (CASTEP) code [45,46]. The cutoff energy for the plane wave calculations and the energy convergence threshold was set to 400 eV and 10−4 eV, respectively. The residual force was set to less than 0.02 eV/Å. The Brillouin zones were sampled using a Monkhorst-Pack 9 × 9 × 9 and 5 × 5 × 1 k-point grid for all geometric optimization of bulk and slab models, and the 15 Å vacuum layer was used to decouple the periodic images [39].
The value of Gibbs free energy (ΔGH*) for hydrogen adsorption on the different crystal planes of TMBs can be estimated by
ΔGH* = ΔEH* + ΔEZPE − TΔSH*
Here, ΔEZPE and TΔSH* represent the changes of zero-point energy and entropy of hydrogen adsorption, respectively. In this work, ΔEZPE is usually very small, about 0.05 eV, as previously reported [47]. While TΔSH* is obtained by TΔSH* ≈ −1/2 , where = 130.7 J/mol·K is the entropy of H2 at 298 K and 1 atm [40]. Therefore, the equation can be rewritten as ΔGH* = ΔEH* + 0.25 eV. ΔEH* is the adsorption energy was calculated by
Here, E[surface+nH*] and E[surface+(n−1)H*] represent the total energy of n and (n − 1)H* atoms adsorbed on the surface, respectively. is the total energy of a gas phase H2 molecule.
4. Conclusions
In conclusion, we reported a new strategy to design high HER performance in TMBs. High-density integral bulk electrodes are composed of TMBs (TiB2, ZrB2, and HfB2), which indicate better HER performance than commercial powder electrodes. Moreover, nano morphology which exposes high active crystal planes, was fabricated in integral bulk electrodes of TiB2, ZrB2 and HfB2 by HCl etching. The best HER of TiB2, ZrB2 and HfB2 are 346 mV, 542 mV, 515 mV at 10 mA cm−2, respectively, 61.9%, 40.3%, and 43.6% higher than the commercial powder electrodes. The reasons for better HER performance are the better conductivity of the integral bulk electrode, larger specific area, and much more exposed activity sites. This work is significant to fabricate new high HER performance TMBs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12020222/s1, Figure S1: The XRD of commercial TiB2, ZrB2, and HfB2; Figure S2: The linear sweep voltammetry (LSV) curves of commercial TiB2, ZrB2, and HfB2 for HER in 0.5 M H2SO4 solution; Table S1: Comparison of the catalytic activity of TiB2, ZrB2, HfB2 obtained herein with those reported previously. (The Current density is 10 mA cm−2.) [48,49,50,51,52].
Author Contributions
Conceptualization, W.Z. and Q.T.; data curation, D.X., Y.C. and X.W.; formal analysis, W.Z., J.C., Q.T. and S.D.; funding acquisition, Q.T. and P.Z.; investigation, W.Z., D.X., Q.T. and P.Z.; methodology, Y.C., C.Y. and Q.T.; project administration, P.Z.; resources, P.Z. and Q.T.; supervision, P.Z.; validation, X.W.; visualization, P.Z.; writing—original draft, W.Z.; writing—review and editing, Y.C., Q.T. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (under grant Nos. 11904119, 11974131), China postdoctoral Science Foundation (No. 2016M601374), the Open Project of State Key Laboratory of Superhard Materials (Jilin University) (201911), the National Key R&D Program of China (2018YFA0703400).
Data Availability Statement
All relevant data are contained in the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.-J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Landman, A.; Dotan, H.; Shter, G.E.; Wullenkord, M.; Houaijia, A.; Maljusch, A.; Grader, G.S.; Rothschild, A. Photoelectrochemical water splitting in separate oxygen and hydrogen cells. Nat. Mater. 2017, 16, 646–651. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef]
- Arif, M.; Yasin, G.; Shakeel, M.; Mushtaq, M.A.; Ye, W.; Fang, X.; Ji, S.; Yan, D. Highly active sites of NiVB nanoparticles dispersed onto graphene nanosheets towards efficient and pH-universal overall water splitting. J. Energy Chem. 2021, 58, 237–246. [Google Scholar] [CrossRef]
- Liu, C.; Gong, T.; Zhang, J.; Zheng, X.; Mao, J.; Liu, H.; Li, Y.; Hao, Q. Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution. Appl. Catal. B Environ. 2020, 262, 118245. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 4174–4192. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nano-Micro Lett. 2022, 14, 43. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, B.; Wang, Z.; Tao, W.; Shen, S.; Huang, L.; Zhang, J.; Meng, F.; Zhang, Q.; Gu, L.; et al. Planar-Coordination PdSe2 Nanosheets as Highly Active Electrocatalyst for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2102321. [Google Scholar] [CrossRef]
- Wei, Y.; Soomro, R.A.; Xie, X.; Xu, B. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes. J. Energy Chem. 2021, 55, 244–255. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Murthy, A.P.; Lee, S.J.; Karuppasamy, K.; Arumugam, S.R.; Yu, Y.; Hanafiah, M.M.; Kim, H.-S.; Mittal, V.; Choi, M.Y. Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion. Ceram. Int. 2021, 47, 4404–4425. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Lee, E.; Fokwa, B.P.T. Nonprecious Metal Borides: Emerging Electrocatalysts for Hydrogen Production. Acc. Chem. Res. 2022, 55, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jung, H.; Jun, H.; Woo, D.Y.; Han, J.W.; Lee, J. Design of grain boundary enriched bimetallic borides for enhanced hydrogen evolution reaction. Chem. Eng. J. 2021, 405, 126977. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, G.; Chen, W.; Liu, Y.; Li, G.D.; Zhu, P.; Tao, Q.; Li, Q.; Liu, J.; Shen, X.; et al. Highly Active, Nonprecious Electrocatalyst Comprising Borophene Subunits for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 12370–12373. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Li, Y.; Li, Z.; Lv, F.; Lang, Z.; Zhao, K.; Zhou, L.; Moskaleva, L.; Guo, S.; Mai, L. MoB/g-C3N4 Interface Materials as a Schottky Catalyst to Boost Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 496–500. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, Y.; Zhang, G.; Zhu, X.; Wang, S.; Wang, A.-L. A crystalline/amorphous CoP@CoB hierarchical core-shell nanorod array for enhanced hydrogen evolution. J. Mater. Chem. A 2021, 9, 19719–19724. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Feng, T.; Wang, L.; Yuan, W.; Huang, Q.; Guo, Y. Electronically modulated nickel boron by CeOx doping as a highly efficient electrocatalyst towards overall water splitting. Dalton Trans. 2022, 51, 675–684. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Cheng, S.; Zhao, X.; Zhang, J.; Ao, Z.; Zhao, C.; Li, B.; Wang, S.; Wang, S.; et al. Nitrogen defects/boron dopants engineered tubular carbon nitride for efficient tetracycline hydrochloride photodegradation and hydrogen evolution. Appl. Catal. B Environ. 2022, 303, 120932. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, R.; Graś, M.; Wei, W.; Lota, G.; Chen, H.; Ni, B.-J. Tuning electronic property and surface reconstruction of amorphous iron borides via W-P co-doping for highly efficient oxygen evolution. Appl. Catal. B Environ. 2021, 288, 120037. [Google Scholar] [CrossRef]
- Mazanek, V.; Nahdi, H.; Luxa, J.; Sofer, Z.; Pumera, M. Electrochemistry of layered metal diborides. Nanoscale 2018, 10, 11544–11552. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Y.; Tao, Q.; Yang, L.; Cheng, J.; Liu, X.; Cao, J.; Fan, H.; Wei, M.; Zhu, P.; et al. Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions. Catalysts 2021, 11, 1265. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Yu, T.; Liu, J.; Luo, L.; Yin, S. Three-Phase Heterojunction NiMo-Based Nano-Needle for Water Splitting at Industrial Alkaline Condition. Nano-Micro Lett. 2022, 14, 20. [Google Scholar] [CrossRef]
- Wang, P.; Kumar, R.; Sankaran, E.M.; Qi, X.; Zhang, X.; Popov, D.; Cornelius, A.L.; Li, B.; Zhao, Y.; Wang, L. Vanadium Diboride (VB2) Synthesized at High Pressure: Elastic, Mechanical, Electronic, and Magnetic Properties and Thermal Stability. Inorg. Chem. 2018, 57, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Chen, Y.; Lian, M.; Xu, C.; Li, L.; Feng, X.; Wang, X.; Cui, T.; Zheng, W.; Zhu, P. Modulating Hardness in Molybdenum Monoborides by Adjusting an Array of Boron Zigzag Chains. Chem. Mater. 2019, 31, 200–206. [Google Scholar] [CrossRef]
- Gan, Q.; Liu, H.; Zhang, S.; Wang, F.; Cheng, J.; Wang, X.; Dong, S.; Tao, Q.; Chen, Y.; Zhu, P. Robust Hydrophobic Materials by Surface Modification in Transition-Metal Diborides. ACS Appl. Mater. Interfaces 2021, 13, 58162–58169. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, F.; Liang, H.; Guan, S.; Liang, W.; Zhang, L.; Huang, M.; Tang, Y.; He, D. Synthesis and sintering of tungsten tetraboride and tantalum-bearing tungsten tetraboride under ultra high temperature and high pressure. Int. J. Refract. Met. Hard Mater. 2022, 102, 105701. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, X.; Han, J.; Li, J.; Han, W. Electronic structure, elasticity and hardness of diborides of zirconium and hafnium: First principles calculations. Comput. Mater. Sci. 2008, 44, 411–421. [Google Scholar] [CrossRef]
- Chen, Y.; Rong, J.; Wang, Z.; Tao, Q.; Gan, Q.; Wang, F.; Ye, Y.; Liu, X.; Cao, J.; Fan, H.; et al. Tailoring the d-band center by borophene subunits in chromic diboride toward the hydrogen evolution reaction. Inorg. Chem. Front. 2021, 8, 5130–5138. [Google Scholar] [CrossRef]
- Jothi, P.R.; Zhang, Y.; Scheifers, J.P.; Park, H.; Fokwa, B.P.T. Molybdenum diboride nanoparticles as a highly efficient electrocatalyst for the hydrogen evolution reaction. Sustain. Energy Fuels 2017, 1, 1928–1934. [Google Scholar] [CrossRef]
- Park, H.; Zhang, Y.; Lee, E.; Shankhari, P.; Fokwa, B.P.T. High-Current-Density HER Electrocatalysts: Graphene-like Boron Layer and Tungsten as Key Ingredients in Metal Diborides. ChemSusChem 2019, 12, 3726–3731. [Google Scholar] [CrossRef]
- Jothi, P.R.; Zhang, Y.; Yubuta, K.; Culver, D.; Conley, M.P.; Fokwa, B.P.T. Abundant vanadium diboride with graphene-like boron layers for hydrogen evolution. ACS Appl. Energy Mater. 2019, 2, 176–181. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Feng, L.-L.; Yu, G.; Wu, Y.; Li, G.-D.; Li, H.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. High-index Faceted Ni3S2 Nanosheet Arrays as Highly Active and Ultrastable Electrocatalysts for Water Splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef] [PubMed]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Xiao, B.; Min, T.; Yang, Y.; Ma, S.; Yi, D. The electronic, mechanical properties and theoretical hardness of chromium carbides by first-principles calculations. J. Alloys Compd. 2011, 509, 5242–5249. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-D. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Zheng, S.; Fu, Y.; Zheng, L.; Zhu, Z.; Yu, G.; Yang, D. Polypyrrole encapsulating TiB2 as newly-emerged electrocatalyst for highly boosted hydrogen evolution reaction. Ceram. Int. 2019, 45, 23298–23303. [Google Scholar] [CrossRef]
- Li, Q.; Zou, X.; Ai, X.; Chen, H.; Sun, L.; Zou, X. Revealing Activity Trends of Metal Diborides Toward pH-Universal Hydrogen Evolution Electrocatalysts with Pt-Like Activity. Adv. Energy Mater. 2019, 9, 1803369. [Google Scholar] [CrossRef]
- Lim, C.S.; Sofer, Z.; Mazánek, V.; Pumera, M. Layered titanium diboride: Towards exfoliation and electrochemical applications. Nanoscale 2015, 7, 12527–12534. [Google Scholar] [CrossRef]
- Sitler, S.J.; Raja, K.S.; Charit, I. ZrB2-HfB2 Solid Solutions as Electrode Materials for Hydrogen Reaction in Acidic and Basic Solutions. Mater. Lett. 2017, 188, 239–243. [Google Scholar] [CrossRef][Green Version]
- Mete, B.; Peighambardoust, N.S.; Aydin, S.; Sadeghi, E.; Aydemir, U. Metal-substituted zirconium diboride (Zr1–xTMxB2; TM = Ni, Co, and Fe) as low-cost and high-performance bifunctional electrocatalyst for water splitting. Electrochim. Acta 2021, 389, 138789. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).