Abstract
This contribution concerns the effect of the chemical composition of the brucite-type layer of bi-cationic LDH materials ZnxAl and MgxAl (x = 2–5) and tri-cationic LDH MgyZnzAl (y + z = 4, y = 1, 2, 3) on their catalytic activity for olefin epoxidation with H2O2 in the presence of acetonitrile. LDH materials were prepared by the standard method of co-precipitation at constant pH 10, using an aqueous solution of the corresponding metal nitrates and a basic solution containing NaOH and Na2CO3. The fresh LDHs were calcined to yield the corresponding mixed oxides and then the recovery of the LDH structure by hydration of the mixed oxides was performed. The resulting samples were characterized by AAS, XRD, DRIFT, DR-UV–Vis, BET and determination of basic sites. The results of the catalytic tests for olefin epoxidation were well correlated with the basicity of the samples, which was in turn related to the M2+/Al3+ ratio and the electronegativity of different bivalent metals in the brucite-type layer.
1. Introduction
In recent decades, the fine chemical industry started to show a real interest in using base-type heterogeneous catalysts due to the fact that this class of materials presents numerous advantages compared with homogeneous catalysts [1,2,3], namely: the separation of the catalyst from the reaction products is achieved by a simple filtration, the temperature range in which it can work is often wide, the thermal regeneration of the catalyst is often possible, etc. The layered double hydroxides (LDHs) (discovered in the 19th century [4]), can substitute with good results the corrosive and not environmentally friendly base homogeneous catalysts [4,5,6,7]. The LDH general formula is [M2+1−xM3+x(OH)2]x+[An−x/n]·mH2O where M2+ and M3+ represent divalent and trivalent cations in the brucite-type layers, A is the interlayer anion with charge n which compensates the excedentary positive charge brought by the isomorphic substitution of M2+ with M3+, x is the fraction of the trivalent cation (usually 0.20–0.33) and m is the number of crystallization water molecules [4]. In these structures, the cations adopt octahedral geometry. The mild calcination of LDHs converts them to mixed oxides having an important property named the “memory effect”, which consists in the spontaneous structural reconstruction of the original layered structure after hydration with water or aqueous solutions containing different anions [4,8,9]. Through this method, it is possible to include several anions in the interlayer space or to isomorphically exchange the existent cations with others having an adequate radius range. The mixed oxides obtained by calcinations at temperatures higher than 600 °C lose this property because the cations are placed in tetrahedral positions, which have a lower energy state compared to the octahedral ones [10]. Over time, these materials have found applications as catalysts in various fine chemical syntheses [4,11,12,13,14,15]. One class of important reactions are the oxidation ones, which can be classified into two types: one implying only a dehydrogenation and the second one involving both dehydrogenation and oxygen insertion into the hydrocarbon molecule [16]. The difficulties of controlling oxidation reactions in order to avoid total oxidation are due to the fact that usually any oxidation is accompanied by the release of heat, which can make undesirable side reactions thermodynamically possible. Until now, commonly used oxidation agents were NaOCl, alkylperoxides or peroxyacids [17]. However, due to environmental regulations, the actual trend is to replace these oxidants by others which are environmentally friendly, such as H2O2 or molecular oxygen, which are considered to be green oxidants [18]. Some oxidation reactions of alkenes give cyclic ethers in which both carbons of a double bond become bonded to the same oxygen atom, products called epoxides or oxiranes with a highly strained cycle which makes those epoxides more reactive than other ethers. Selective epoxidation of olefinic compounds is one of the important steps in organic synthesis of fine chemicals since, by ring opening reactions, epoxides are directly transformed into a wide variety of compounds with excellent yields [19,20,21]. Among epoxides, epoxycyclohexane is a valuable organic intermediate, used in the synthesis of pharmaceuticals, pesticides, epoxy paints, rubber promoters and stabilizers for chlorinated hydrocarbons [22]. Many publications present the oxidation of cyclohexene to cyclohexene oxide using different solids: magnetic core–shell type Fe3O4@chitosan-Schiff base Co(II), Cu(II) and Mn(II) complexes [23], vanadia-based catalysts [24], Ti(III)APO-5 materials [25], Fe nanocatalyst [26], nanostructured Au/SiO2 [27], Fe(Salen) intercalated-zirconium phosphate [28], niobium oxyhydroxide [29], etc. The green epoxidation of olefins with H2O2 was first studied by Payne who showed that a peroxycarboximidic acid serves as a terminal oxygen donor when a nitrile is present as a co-reactant in the presence of a homogeneous base catalyst [30]. The new trend, from the environmental point of view, is using H2O2 as an oxidizing agent and different types of LDH solid catalysts, such as: hydrotalcite (HT) [1,31], CuII(Sal-Ala)/MgAl-LDH and CuII(Sal-Phen)/MgAl-LDH [32], hydrotalcite-like compounds with low Mo-loading [33], Mg/Al; Mg,Zn/Al; Mg/Al,Ga hydrotalcite-like compounds [34,35], etc., or molecular oxygen and MIIMg/Al hydrotalcites and hydrotalcite-supported M(II) acetylacetonate (M(II) = Co, Cu or Ni) catalysts [36], cobalt-modified hydrotalcites [17], LDH hosted Fe and Mn sulfonato-salen complexes [37], etc.
The studies published until now concerning the Payne epoxidation with H2O2 using hydrotalcite catalysts were limited to the investigation of several factors affecting the selectivity to epoxide such as: the influence of the solvent and the activation agent [1], the influence of the Mg/Al ratio, the presence of pure hydrotalcite phase in the samples, the reconstruction rate of the HT-like phase during the reaction and the addition of water in the reaction mixture [33]. The Mg/Al ratio is a factor particularly influencing the basicity of the catalysts which increases with the Mg content. In a previous work, we investigated the effect of tuning the basicity of the HT structure by inclusion of very small amounts of Zn or Ga in the brucite-type layer on the epoxidation of cyclohexene in the presence of benzonitrile as a reductant agent, showing that Zn- and Ga-containing hydrotalcites presented higher activity than the Mg/Al hydrotalcite for the oxidation of cyclohexene to cyclohexene oxide in the initial stages of the process [34]. One of the issues occurring when Mg/Al LDHs are used as catalysts is that these structures are sensitive to the action of acids such as the peroxycarboximidic acid intermediate formed during Payne epoxidation with H2O2. An opportunity to overcome this issue would be the utilization of an LDH structure such as Zn/Al, which possesses the active basic sites but is also more resistant to acid action. To our knowledge, neither pure Zn/Al LDH nor ternary compositions of MgZn/Al with more than 5 mol% Zn in the brucite-type layer have been tested until now as catalysts for cyclohexene epoxidation. There are only two references concerning Zn/Al LDH used as a host for catalytic active guest species such as metallophtalocyanines [38], metatungstate and tungstoniobate [39] in the epoxidation of olefins with O2.
Based on the above, the aim of this contribution was to bring new insights into the influence of the chemical composition of a brucite-type layer on the catalytic activity for selective cyclohexene epoxidation with H2O2 in the presence of acetonitrile by extending the studies from MgxAl to ZnxAl (x = 1–5) and MgyZnzAl (y + z = 4, y = 1, 2, 3)-type hydrotalcites. It was presumed that the utilization of LDH structures containing larger amounts of Zn in the brucite-type layer could bring the benefit of a better stability towards the action of the peroxycarboximidic acid intermediate. The catalytic performances were determined for all fresh, calcined and reconstructed LDH samples and were correlated with the physico-chemical characteristics of the solids in order to foresee a selection criterion of the optimal composition.
2. Results and Discussion
2.1. Characterization of Catalysts
The chemical compositions of the freshly synthesized samples are presented in Table 1. The results show that the amount of water in the interlayer ranges within 6–18% of the total weight, in concordance with the literature [4].

Table 1.
The chemical composition of the prepared solid samples.
HT MgxAl samples retain more water than HT ZnxAl ones. Looking at the columns where the molar ratios between each component and Al are presented, it is noticed that the preparation by co-precipitation at pH 10 leads to a lower incorporation of Zn than the one expected considering the molar ratios in the starting solutions, which is in agreement with literature data [40,41]. This effect is less pronounced for Mg, where the molar ratios of Mg/Al are closer to the theoretical ones. An explanation for this effect is that at pH 10, Zn species are more soluble than Mg ones [42,43]. The ratio of CO32−/Al always exceeds the theoretical value of 0.5, a fact that may be related to the excess carbonate used in the preparation as well as the high affinity of the cations to this anion. The presence of nitrate ions was evidenced in small concentrations only in the HT ZnxAl samples. This may be a consequence of the formation of a hydroxynitrate impurity during the co-precipitation and aging of Zn-containing samples [44].
The XRD patterns of the synthesized samples are presented in Figure 1, Figure 2 and Figure 3 and the calculated structural parameters are presented in the Supplementary Information (Tables S1–S3). The XRD patterns of HT MgxAl samples present the typical lines of layered materials, as shown in Figure 1 [4,8]. Their intensity decreases with the increase in the Mg/Al ratio because part of Mg forms an amorphous hydroxyde phase [45]. Meanwhile, in the diffraction patterns of HT ZnxAl, besides the typical diffraction lines of layered materials, some of the characteristic lines for a zinc hydroxy nitrate Zn(OH)4(NO3)2 impurity phase marked with * in Figure 1 are noticed. This additional phase increases its concentration along with x value from 2 to 5, but it is missing in the XRD patterns of HT MgyZnzAl samples. The partial substitution of Mg with Zn leads to the decrease in the crystallinity compared to Mg4Al and Zn4Al samples.
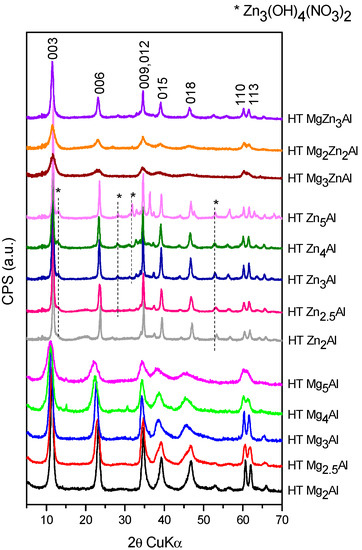
Figure 1.
The XRD patterns of the freshly prepared HT materials.
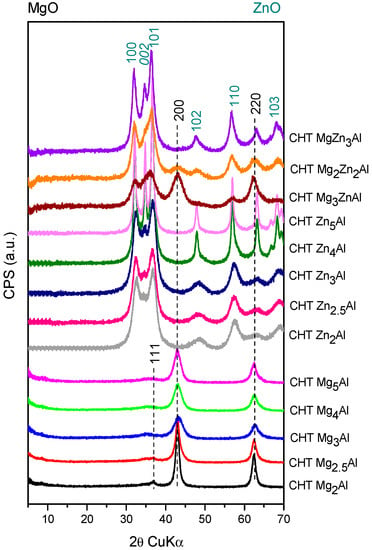
Figure 2.
The XRD patterns of the calcined materials.
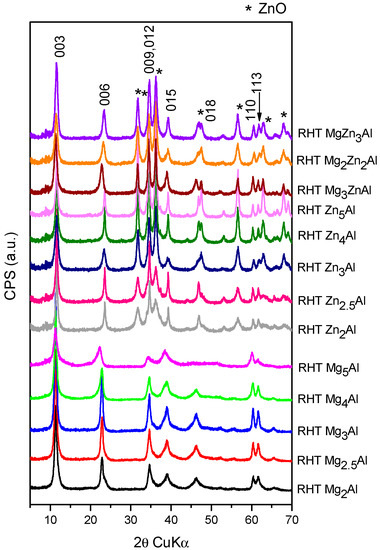
Figure 3.
The XRD patterns of the reconstructed samples.
The diffraction patterns of the calcined samples, shown in Figure 2, present the characteristic reflections of the mixed oxides Mg(Al)O (for CHT MgxAl) and zincite Zn(Al)O (for CHT ZnxAl) and a mixture of these oxides in the case of CHT MgyZnzAl samples. The intensity of the diffraction lines for MgO-periclase-type (JCPDS-45-0946) in the patterns of CHT MgxAl decreases with the increase in x. Meanwhile, in the diffraction patterns of CHT MgyZnzAl samples, the periclase-type phase intensity from the corresponding mixed oxides decreases with decreasing amounts of Mg cation to almost nothing for the CHT MgZn3Al sample.
The diffraction patterns in Figure 3 show that a good reconstruction by the memory effect is possible for a Mg/Al ratio lower than 5, the optimum ratio for a good reconstruction being 3 which is in agreement with literature [4]. For RHT ZnxAl samples, the reconstruction of the layered structure by the memory effect is less pronounced than that noticed for RHT MgxAl samples. The zincite phase is also always present as an independent phase in the XRD patterns of RHT ZnxAl and RHT MgyZnzAl samples, and the intensity of its corresponding maxima denoted with * in Figure 3 increases with Zn content. This aspect was also reported by other authors working with ZnAl LDHs [46].
The DR-UV–Vis spectra of the freshly prepared Zn-containing samples, HT ZnxAl and HT MgyZnzAl, shown in Figure 4A, show the presence of the band corresponding to Zn(Al)O at 208 nm, in agreement with the findings of XRD analyses. The intensity of this band increases with the Zn content. The spectra of the corresponding RHT samples, shown in Figure 4B, show multiple bands due to the preservation of the Zn(Al)O phase even after hydration. In the spectra of RHT MgyZnzAl, it is noticed that the increasing Mg content in the brucite-type layer leads to a shifting of λmax to higher wavelengths (274 < 292 < 300 < 304 nm), indicating a decrease in the M-O bond strength. As a partial conclusion, DR-UV–Vis spectra confirm the poor reconstruction of Zn-containing samples.
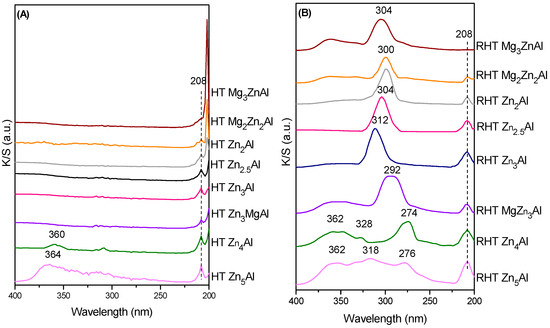
Figure 4.
DR-UV–Vis spectra of the Zn-modified layered materials; (A) freshly prepared HT samples and (B) reconstructed samples.
For HT MgxAl samples, shown in Figure 5A, it was found that DRIFTS spectra present, in the region 3500–3700 cm−1, a broad band due to the stretching vibration of the OH groups present in the lamellar structures. The position of the maximum is shifted to higher wavenumbers as the Mg/Al ratio increases. A shoulder of this broad band at approx. 3167 cm−1 is due to the hydrogen bonds formed with carbonate anions in the interlayer space. The characteristic deformation vibration of water at approx. 1600 cm−1 is slightly shifted to higher wavenumbers due to the bridged-type interaction of CO32−-H2O between water molecules and carbonate anions from the interlayer. The effects due to the interaction of carbonate counter ions with superficial hydroxyl groups observed in the spectral region beyond 3000 cm−1 are confirmed by specific bands characteristic of the asymmetric stretching vibration (ν3) from free carbonate groups in D3h symmetry in the region from 1420 to 1360 cm−1. The increasing intensity of this band characteristic for carbonate groups certifies the increasing concentration of this compensation anion in the system as the Mg/Al ratio decreases. This conclusion is also supported by the fact that the characteristic peak for carbonate ν4 vibration (700 cm−1) is observed only for samples with Mg/Al ratio < 3 and the characteristic maximum derived from the overlapping of carbonate ν2 and ν1 bands shifts its position from 935 to 1007 cm−1 when the Mg/Al ratio decreases (i.e., as the interlayer carbonate amount increases). An additional band at about 1500 cm−1 appears in the spectra of the samples that contain an excedentary brucite-like phase [47,48].
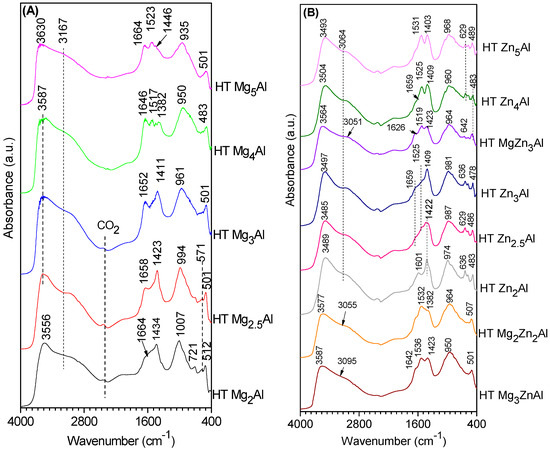
Figure 5.
DRIFT spectra of freshly prepared HT samples: (A) MgxAl, (B) ZnxAl and MgyZnzAl.
The DRIFT spectra profiles of HT ZnxAl, shown in Figure 5B, are similar to those obtained for MgxAl compounds. In the region 3700–3000 cm−1, the band corresponding to hydroxyl groups is shifted towards lower wavenumbers with decreasing Zn/Al ratio (3493; 3507; 3497; 3485 and 3489 cm−1 for Zn/Al ratios = 5; 4; 3; 2.5; 2) as in the case of MgxAl compounds. Compared to MgxAl solids, the position of the maximum of bands is located at smaller wavenumbers, suggesting a lower basicity of Zn-containing solids. The shift of the shoulder from 3167 cm−1 at 3064 cm−1 (which is more pronounced for Zn3Al) indicates that the hydrogen bridges of OH ions with the counter anions and water molecules in the interlayer are less intense than in HT MgxAl series. The band at 1601 cm−1 (δH2O) is clearly visible only for the sample richest in Al, Zn2Al, indicating a lower confinement of water in the interlayer of HT ZnxAl compared to HT MgxAl. In the spectra of the HT ZnxAl samples, the band characteristic of carbonate around 1423 cm−1 overlaps those characteristic of nitrate. A shift of the maximum to lower wavenumbers is clearly noticed for HT Zn3Al, HT Zn4Al and HT Zn5Al samples which, according to the results of the chemical analyses, contain higher concentrations of nitrate (see Table 1). By comparing the DRIFT of HT MgxAl and HT ZnxAl, shown in Figure 5A,B, it can be concluded that the first class of materials possess OH groups with a more pronounced basic character.
The DRIFT spectra of the corresponding mixed oxides, shown in Figure 6A,B, show that the relative intensity of the bands in the region 4000–3000 cm−1 and in the mid-infrared region decreases significantly compared to those in the 1000–400 cm−1 region, indicating that OH groups and even small amounts of carbonate are still present in both series of samples. This fact confirms the literature data claiming that the calcination of the solids at 460 °C does not allow total dehydroxylation and decarbonation [49].

Figure 6.
DRIFT spectra of mixed oxide CHT samples, (A) MgxAl, (B) ZnxAl and MgyZnzAl.
The spectra of RHT MgxAl, shown in Figure 7A, present the maximum of the band corresponding to νOH shifted by 100 cm−1 to higher wavelengths due to the larger number of OH− introduced by the memory effect compared to the spectra of the RHT ZnxAl samples, shown in Figure 7B, a fact that suggests an increased basicity of the Mg-containing samples. After reconstruction, in the spectra of Zn-containing samples, the band for νOH is less shifted to higher wavenumbers compared to its position in the spectra of corresponding HT samples.
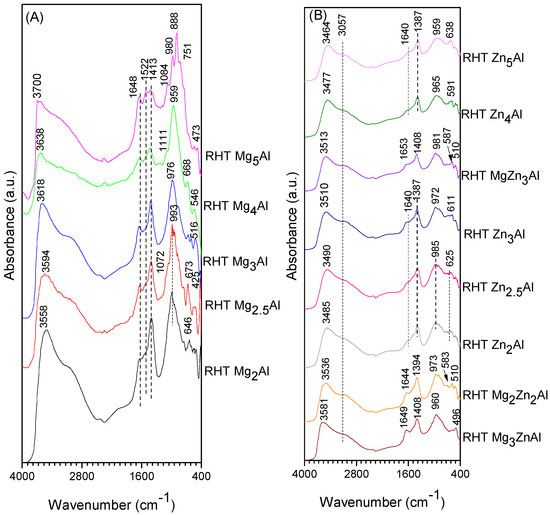
Figure 7.
DRIFT spectra of reconstructed RHT samples, (A) MgxAl, (B) ZnxAl and MgyZnzAl.
The results in Table 2 point out that the samples in the ZnxAl series have a smaller specific surface, smaller specific pore volume and larger mesopores compared to those in the MgxAl series. The same trend is noticed for the total number of base sites and the weak and medium-strength base sites. As for the number of strong base sites, HT Zn4Al and HT Zn5Al have more base sites than the related MgxAl samples due to the presence of the zincite phase that has O2− strong base sites.

Table 2.
Textural properties and basicities of HT samples.
The samples with ternary composition, HT Mg3ZnAl and HT Mg2Zn2Al, have a larger specific surface area and greater porosity than the reference binary compositions. The total basicity of ternary samples is higher than that of HT Zn4Al due to the presence of Mg that has a lower Pauling electronegativity than Zn (e.g., 1.31 < 1.65).
The data in Table 3 show a trend of the textural properties and the basicity variation for the calcined samples in the ZnxAl series compared to those in the MgxAl series, similar to that suggested by the data in Table 2 concerning HT samples.

Table 3.
Textural properties and basicities of CHT samples.
The textural data displayed in Table 4 show that in the case of the reconstructed samples, the specific surface areas are significantly smaller than those of the HT samples, as was also noticed by other authors who investigated the memory effect [50,51]. However, the trends of the textural properties and the basicity variation for the ZnxAl series compared to those in the MgxAl series are different than those noticed for HT samples (Table 2) and CHT samples (Table 3).

Table 4.
Textural properties and basicities of RHT samples.
The fact that Zn-containing samples have larger specific surface areas than the corresponding ones containing Mg may be the consequence of the presence of the zincite secondary phase (indicated in Figure 3) which remains between the reconstructed HT-phase crystallites.
2.2. Catalytic Activity
According to Scheme 1, besides the epoxide compound which is the main product, other by-products appear after some successive and parallel reactions.
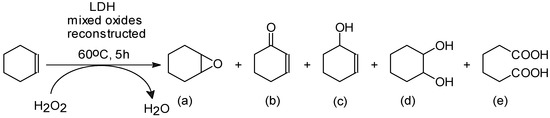
Scheme 1.
Possible products for the oxidation of cyclohexene (epoxycyclohexane (a); 2-cyclohexene-1-one (b); 2-cyclohexene-1-ol (c); cyclohexane-1,2-diol (d); adipic acid (e)).
The blank test performed without catalyst at 60 °C for 5 h leads to a conversion of cyclohexene of 9.2%, and yield to cyclohexene oxide of 2.5%. The decomposition of hydrogen peroxide in the presence of the catalysts under similar conditions in the absence of cyclohexene and acetonitrile was found to be in the 4–5% range and therefore it was considered that the influence of the solid base catalysts on the decomposition of hydrogen peroxide is low and does not significantly influence the catalytic oxidation of cyclohexene.
The results of the catalytic tests are presented in Figure 8A–C. Since all catalysts were extremely selective for epoxidation, and the total concentration of by-products in the reaction mixture was in the range of 0.5–1 mol%, we have presented the yields to cyclohexeneoxide after 5 h.
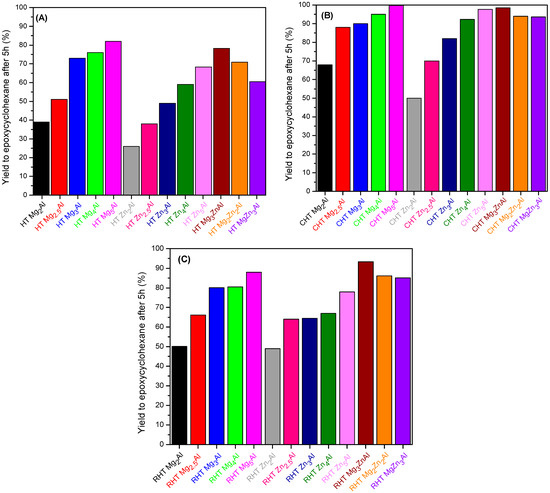
Figure 8.
The variation of yield to epoxycyclohexane after 5 h on the investigated catalysts, (A) freshly prepared HT samples; (B) calcined CHT samples; (C) reconstructed RHT samples.
The increasing of the Mg/Al ratio from 2 to 5, shown in Figure 8A, led to the increase in the yield to epoxide from 38%, for HT Mg2Al, up to 82% for HT Mg5Al. The same trend, but with values lower by around 10%, is noticed for the increasing of the Zn/Al ratio in the same range (e.g., 26% for HT Zn2Al and 69% for HT Zn5Al). The lower yield values obtained are a consequence of both the smaller surface areas and the inferior basicity of HT ZnxAl samples compared to HT MgxAl considering the slightly higher electronegativity of Zn (1.65) compared to Mg (1.31). It could also be due to the presence in these samples of the zinc hydroxynitrate impurity. The yield to cyclohexene oxide from the samples with tricationic compositions of MgyZnzAl varies similarly to Mg content, and it is higher than the one obtained from HT Zn4Al. Only the tricationic HT Mg3ZnAl sample leads to higher yields than both HT Zn4Al and HT Mg4Al. This fact may be a consequence of its larger specific surface area compared to the reference bi-cationic samples. The mixed oxides show the highest activity of cyclohexeneoxide formation compared to the homologue materials HT and RHT, mostly due to their larger specific surface area and more emphasized basic character, as shown in Figure 8B. The variation of the yield as a function of the chemical composition follows the same trend as the one noticed for the freshly prepared HT materials.
This trend is not noticed for Zn-containing reconstructed samples, shown in Figure 8C. In this case, the sample with the highest activity is RHT Mg3ZnAl instead of RHT Mg5Al. All the RHT samples have higher activity than the corresponding freshly prepared HT samples. As shown in Table 4, the overall basicity of these samples is higher than that of HT (Table 2), even though the specific surface area is smaller. The type of base site is also very important. Di Cosimo et al. [52] showed that the strongest base sites are O2− anions, followed by OH−, bridge cation-O and hydrogen carbonate or carbonate anions, which are considered as being medium-strength and weak base sites. In several of our early works, we found that the epoxidation is favored mostly according to the number of weak and medium-strength base sites [33,34]. A correlation of the yield to cyclohexeneoxide and the number of weak and medium-strength base sites of the investigated catalysts is presented in Figure 9.

Figure 9.
Variation of the yield to epoxide vs. the number of low- and medium-strength base sites (the colors of the symbols represent the sample compositions: Mg2Al, Mg2.5Al, Mg3Al, Mg4Al, Mg5Al, Zn2Al, Zn2.5Al, Zn3Al, Zn4Al, Zn5Al, Mg3ZnAl, Mg2Zn2Al, MgZn3Al).
The possibility of recycling was investigated only for the catalysts that gave yields to cyclohexeneoxide higher than 95% (e.g., CHT Mg4Al, CHT Zn5Al, CHTMg3ZnAl, CHT Mg5Al). The results obtained in five consecutive cycles are presented in Table 5. For the CHT Mg5Al sample, the yield decreases after the first cycle with 10%, and falls as low as 75% after the fifth cycle. For CHT Mg4Al, the yield decreases from 95% to 82% after the first cycle and finally to 64% after the fifth reaction cycle. This behavior is due, on one hand, to the structural modification by reconstruction under the influence of the water and, on the other hand, to the partial dissolution of Mg species by the peroxymidic acid intermediate. The analysis by AAS of the cations from the liquid reaction mixtures obtained after five reaction cycles revealed that the amount of Mg leached from CHT Mg5Al was around 60 ppm, which corresponded to 2.5% w/w Mg loss from the catalyst, while for CHT Mg4Al, the leached Mg was 40 ppm, which corresponded to 1.8% w/w Mg loss from the catalyst. The level of Al leached in the reaction mixture was under the detection limit. On the contrary, for CHT Zn5Al, the yield decreases only by 4.1% after the first reaction cycle (93.6%) and drops to 85.2% after the fifth cycle. This fact is due to the better stability towards the action of the acid intermediate and poor reconstruction ability of the solid. A similar trend is noticed for CHT Mg3ZnAl which loses only 10% of the yield to cyclohexeneoxide after five reaction cycles, suggesting a synergetic effect between Mg and Zn in this ternary composition. In the case of Zn-containing samples, the levels of cation concentration in the reaction mixtures recovered after five reaction cycles were all under the detection limit. Hence, it seems that the presence of Zn stabilizes the Mg in the framework. This fact was also indicated by the XRD patterns of the materials used in the catalytic tests before and after their regeneration by re-calcination (Figure 10).

Table 5.
Results obtained in repeated reaction cycles.
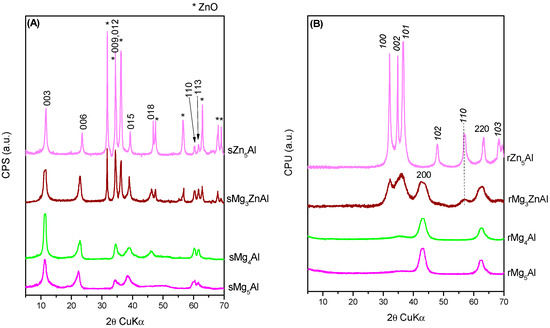
Figure 10.
XRD patterns of the spent (A) (the prefix “s” added to the names of the samples) and recycled samples (B) (the prefix “r” added to the names of the samples, diffraction lines corresponding to Mg(Al)O—normal text, diffraction lines of Zn(Al)O—italics).
The XRD patterns of the solid samples recovered after the five reaction cycles presented in Figure 10A show that the LDH structure is recovered to some extent during the reaction. Meanwhile, the XRD patterns of the re-calcined samples regenerated after the reaction (Figure 10B) exhibit similar features to those of the parent CHT samples (see Figure 2), showing that the structure is maintained after regeneration.
3. Materials and Methods
3.1. Catalyst Preparation
The catalysts were synthesized using chemicals of analytical purity grade, Mg(NO3)2∙6H2O, Zn(NO3)2∙6H2O, Al(NO3)3∙9H2O, NaOH and Na2CO3 were purchased from Merck, Darmstadt, Germany. Three sets of LDH materials were prepared by co-precipitation at pH 10 under low supersaturation conditions [53,54]: (i) MgxAl; (ii) ZnxAl (with x = 2; 2.5; 3; 4; 5) and (iii) MgyZnzAl (y + z = 4, y = 1, 2, 3). Two aqueous solutions were prepared using the metal nitrate precursors (solution MN) and a mixture of NaOH and Na2CO3 (solution B). Solution MN contained the required amounts of dissolved metal nitrates for the desired values of x, y and z, at a total concentration of cations of 1.5 M. Solution B contained a molar amount of Na2CO3 1.5 times higher than the molar amount of Al in the MN solution while the amount of NaOH was adjusted in order to reach a total Na concentration of 3 M. Both solutions were simultaneously fed into the precipitation reactor with a feeding flow of 60 mL∙h−1 at room temperature under vigorous stirring at 600 rpm. The obtained gel was aged for 18 h at 75 °C in mother liquor, and cooled afterwards to room temperature. Then, the solid was separated by vacuum filtration and washed with bi-distilled water until the conductivity of the washing water dropped below 100 µS∙cm−1. The washed solids were dried at 90 °C for 24 h under air flow. The freshly prepared solid samples were called HT MgxAl, HT ZnxAl and HT MgyZnzAl. These materials were calcined at 460 °C for 18 h in air flow to obtain the mixed oxides (CHT MgxAl, CHT ZnxAl, CHT MgyZnzAl). The reconstruction of the hydrotalcite-like structure was performed by immersion of these calcined solids in bi-distilled water for 24 h at 25 °C, followed by drying at 90 °C for 24 h in air flow. The reconstructed materials were named RHT MgxAl, RHT ZnxAl and RHT MgyZnzAl.
3.2. Catalyst Characterization
The chemical composition of the samples was determined by atomic absorption spectrometry (AAS) for the determination of Mg, Zn and Al using a Thermoelemental Solar AAS spectrophotometer (ThermoFischer SCIENTIFIC, Waltham, MA, USA). The inorganic carbon analysis (TIC) was performed with the UV-persulfate oxidation method using a HiPerTOC carbon analyzer (ThermoFischer Scientific, Waltham, MA, USA), which measures the IR absorbance of carbon dioxide produced, and determining weight loss between 105 and 200 °C to determine the amount of hydration water. The total nitrogen content was also determined with the HiPerTOC analyzer using a method based on the catalytic combustion of the samples when the nitrogen is converted into nitric oxide (NO). The NO in the gas outlet from the combustion reactor was further oxidized with ozone in a reaction chamber, generating excited nitrogen dioxide (NO2*). The emitted radiation by the nitrogen dioxide when returning to the ground state was then quantified by a photomultiplier tube detector.
Powder X-ray diffraction (XRD) patterns were collected on a PANalytical X’Pert MPD system (Almelo, Netherlands) equipped with monchromatic CuKα radiation (λ = 1.5418 Å) in a continuous scan mode (counting 2 s/ 0.02° 2θ) over a 5–70° 2θ range. The PANalytical HighScore software package was used for the analysis of the XRD patterns.
DR-UV–Vis spectra were recorded with a Shimadzu 3600 UV–Vis NIR spectrometer (Shimadzu, Kyoto, Japan) equipped with an integration sphere using BaSO4 as white reference.
DRIFTS spectra obtained from accumulation of 400 scans in the domain 400–4000 cm−1 were recorded with a NICOLET 4700 spectrometer (ThermoFisher Scientific, Waltham, MA, USA). A KBr spectrum was used as background.
N2 adsorption–desorption isotherms were determined using a Micromeritics ASAP 2010 instrument (Micromeritics, Eindhoven, Netherlands). Prior to nitrogen adsorption, samples were degassed under vacuum for 24 h.
The basic character of the catalysts was determined using a method based on the irreversible adsorption of organic acids of different pKa values corresponding to the total number of base sites, e.g., acrylic acid, pKa = 4.2, and strong base sites, e.g., phenol pKa = 9.9 [55,56,57,58]. The number of weak and medium base sites is given by the difference between the amounts of adsorbed acrylic acid and phenol. The acrylic acid and phenol amounts that remained in solution were determined by UV–Vis spectrometry using the Jasco V650 UV–Vis spectrometer (Jasco, Tokyo, Japan). Three sets of determinations were performed for each solid and the error domaine was +/− 1%.
3.3. Catalytic Tests
The catalytic activity of the solids was tested in the oxidation of cyclohexene with hydrogen peroxide using acetonitrile as a reductant, as shown in Scheme 2 [30,31]. The reactions were performed in a stirred flask (50 mL) at 60 °C, for a 5 h reaction time. In a typical experiment, cyclohexene (4 mmol) and acetonitrile (32 mmol) were dissolved in 20 mL of solvent (mixture of equal volumes of water and acetone). The amount of H2O2 was calculated in order to reach a molar ratio of cyclohexene/H2O2 of 1/32 and was added dropwise during the reaction. All reagents were purchased from Merck (Darmstadt, Germany). In all reactions, the catalyst concentration was 1% (w/w) in the reaction mixture. The reaction was monitored in time using a GC K072320 Thermo Quest Chromatograph (ThermoFisher Scientific, Waltham, MA, USA) equipped with an FID detector and a capillary column of 30 m in length with DB5 stationary phase. The oxidation products were identified by comparison with a standard sample (retention time in GC). The reaction products were also identified by mass spectrometer coupled chromatography, using a GC/MS/MS VARIAN SATURN 2100 T equipped with a CP-SIL 8 CB Low Bleed/MS column of 30 m in length and 0.25 mm in diameter (Varian, Palo Alto, CA, USA). H2O2 consumption was determined by an iodometric titration at the end of the reaction. The oxidation process can lead to several products, Scheme 1, such as: epoxycyclohexane (a); 2-cyclohexene-1-one (b); 2-cyclohexene-1-ol (c); cyclohexane-1,2-diol (d); adipic acid (e).
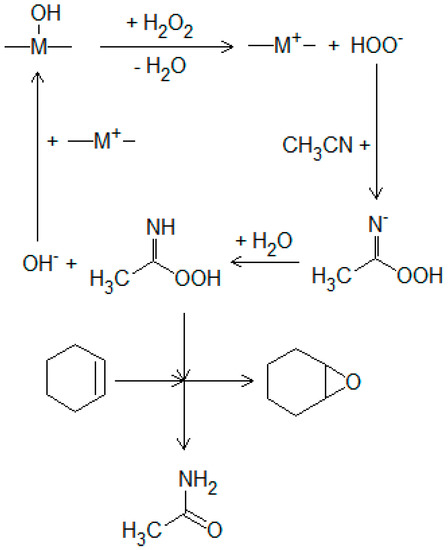
Scheme 2.
Reaction mechanism on Brönsted base sites of HT solids.
3.4. Catalyst Recycling
The most active calcined catalysts were selected for recycling tests. The solid catalysts were separated from the reaction mixture by centrifugation and were dried for 5 h at 90 °C and re-calcined at 460 °C for 5 h before being used in the consecutive cycle. XRD patterns of the dried and re-calcined samples after the fifth reaction cycle were recorded. After being analyzed by GC at the end of each cycle, the liquid reaction mixtures recovered for each catalyst were mixed in order to determine the content of cations leached in the liquid using AAS. In order to perform the analysis by AAS, the cations from the liquid samples were extracted in distilled water acidified with HNO3 (pH 5), and the volume of the aqueous extract was brought to 100 mL in volumetric flasks.
4. Conclusions
The freshly prepared HT ZnxAl samples contain zinc hydroxynitrate Zn3(OH)4(NO3)2 as an impurity. This impurity is not present in the samples that contain both Mg and Zn. The calcination of dried samples leads to partial removal of OH groups under H2O and CO32− under the CO2 form. The reconstruction of the initial structure is not total for Zn-containing LDHs since there are still Zn(Al)O species. The reconstruction is also poor for hydrotalcite samples at a Mg/Al ratio higher than 3. All LDH-derived solids investigated were very selective for cyclohexene epoxidation with H2O2 under mild reaction conditions. The catalytic activity and the basicity of the samples varies in the order HT (OH−/CO32−) < RHT (majority OH−) < CHT (O2−). The yield to epoxycyclohexane increases almost linearly when the number of weak and medium-strength base sites in the brucite-type layer rises in the range 4.5–8.5 mmol·g−1. The basicity of Zn-containing samples decreases proportionally with the amount of Zn incorporated, but Zn improves the stability of the solids towards the action of the acid reaction intermediate and hence the maintenance of the catalytic activity. The strength of the base sites is influenced by the electronegativity of the elements in the brucite-type layer (Mg-containing LDHs have stronger base sites than Zn-containing LDHs) (e.g., Mg 1.31 < Zn 1.65). Based on the obtained results, it could be concluded that the optimal composition of the catalyst enabling a high activity and stability would be that with the ratio Mg/Zn = 3/1 and (Mg + Zn)/Al = 4/1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12020145/s1, Table S1. Structural parameters of freshly prepared samples; Table S2. Structural parameters of calcined samples; Table S3. Structural parameters of reconstructed samples.
Author Contributions
Conceptualization, R.Z. and O.D.P.; methodology, R.Z.; investigation, C.B., R.B., O.D.P., R.Z., M.F. and A.C.; resources, R.Z. and M.F.; data curation, R.B.; writing—original draft preparation, R.Z. and O.D.P.; writing—review and editing, R.Z. and O.D.P.; visualization, A.C. and M.F.; supervision, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
APC was sponsored by MDPI.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romero, M.D.; Calles, J.A.; Ocaña, M.A.; Gómez, J.M. Epoxidation of cyclohexene over basic mixed oxides derived from hydrotalcite materials: Activating agent, solvent and catalyst reutilization. Microporous Mesoporous Mater. 2008, 111, 243–253. [Google Scholar] [CrossRef]
- Schmidt, F. New catalyst preparation technologies- observed from an industrial viewpoint. Appl. Catal. A Gen. 2001, 221, 15–21. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Studer, M. The role of catalysis for the clean production of fine chemicals. Appl. Catal. A Gen. 1999, 189, 191–204. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Costantino, U.; Curini, M.; Montanari, F.; Nocchetti, M.; Rosati, O. Hydrotalcite-like compounds as catalysts in liquid phase organic synthesis: I. Knoevenagel condensation promoted by [Ni0.73Al0.27(OH)2](CO3)0.135. J. Mol. Catal. A Chem. 2003, 195, 245–252. [Google Scholar] [CrossRef]
- Mantilla, A.; Tzompantzi, F.; Manríquez, M.; Mendoza, G.; Fernández, J.L.; Gómez, R. ZnAlFe mixed oxides obtained from LDH type materials as basic catalyst for the gas phase acetone condensation. Adv. Mat. Res. 2010, 132, 55–60. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G., Jr.; Mo, X. Transesterification of poultry fat with methanol using Mg–Al hydrotalcite derived catalysts. Appl. Catal. A Gen. 2007, 331, 138–148. [Google Scholar] [CrossRef]
- Pavel, O.D.; Bîrjega, R.; Che, M.; Costentin, G.; Angelescu, E.; Şerban, S. The activity of Mg/Al reconstructed hydrotalcites by “memory effect” in the cyanoethylation reaction. Catal. Commun. 2008, 9, 1974–1978. [Google Scholar] [CrossRef]
- Pavel, O.D.; Cojocaru, B.; Angelescu, E.; Pârvulescu, V.I. The activity of yttrium-modified Mg, Al hydrotalcites in the epoxidation of styrene with hydrogen peroxide. Appl. Catal. A Gen. 2011, 403, 83–90. [Google Scholar] [CrossRef]
- Lavikainen, L.P.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Interaction of octahedral Mg(II) and tetrahedral Al(III) substitutions in aluminium-rich dioctahedral smectites. Theor. Chem. Acc. 2016, 135, 85. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Miquel, S.; Primo, J. Catalysts for the production of fine chemicals: Production of food emulsifiers, monoglycerides, by glycerolysis of fats with solid base catalysts. J. Catal. 1998, 173, 315–321. [Google Scholar] [CrossRef]
- Ono, Y. Solid base catalysts for the synthesis of fine chemicals. J. Catal. 2003, 216, 406–415. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Li, F.; Duan, X. Layered double hydroxides as catalytic materials: Recent development. Catal. Surv. Asia 2008, 12, 253–265. [Google Scholar] [CrossRef]
- Tichit, D.; Lutic, D.; Coq, B.; Durand, R.; Teissier, R. The aldol condensation of acetaldehyde and heptanal on hydrotalcite-type catalysts. J. Catal. 2003, 219, 167–175. [Google Scholar] [CrossRef]
- Jiao, N.; Stahl, S.S. (Eds.) Green Oxidation in Organic Synthesis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Angelescu, E.; Ionescu, R.; Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Luculescu, C.R.; Florea, M.; Olar, R. Epoxidation of cyclohexene with O2 and isobutyraldehyde catalysed by cobalt modified hydrotalcites. J. Mol. Catal. A Chem. 2010, 315, 178–186. [Google Scholar] [CrossRef]
- Litter, M.I.; Candal, R.J.; Meichtry, J.M. (Eds.) Advanced Oxidation Technologies Sustainable Solutions for Environmental Treatments; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781138072886. [Google Scholar]
- Philip, R.M.; Radhika, S.; Abdulla, C.M.A.; Anilkumar, G. Recent trends and prospects in homogeneous manganese-catalysed epoxidation. Adv. Synth. Catal. 2021, 363, 1272–1289. [Google Scholar] [CrossRef]
- Dusi, M.; Mallat, T.; Baiker, A. Epoxidation of functionalized olefins over solid catalysts. Catal. Rev. Sci. Eng. 2007, 42, 213–278. [Google Scholar] [CrossRef]
- Hauser, S.A.; Cokoja, M.; Kühn, F.E. Epoxidation of olefins with homogeneous catalysts–Quo vadis? Catal. Sci. Technol. 2013, 3, 552–561. [Google Scholar] [CrossRef]
- Ouidri, S.; Guillard, C.; Caps, V.; Khalaf, H. Epoxidation of olefins on photoirradiated TiO2-pillared clays. Appl. Clay Sci. 2010, 48, 431–437. [Google Scholar] [CrossRef]
- Cai, X.; Wang, H.; Zhang, Q.; Tong, J.; Lei, Z. Magnetically recyclable core–shell Fe3O4@chitosan-Schiff base complexes as efficient catalysts for aerobic oxidation of cyclohexene under mild conditions. J. Mol. Catal. A Chem. 2014, 383–384, 217–224. [Google Scholar] [CrossRef]
- El-Korso, S.; Khaldi, I.; Bedrane, S.; Choukchou-Braham, A.; Thibault-Starzyk, F.; Bachir, R. Liquid phase cyclohexene oxidation over vanadia based catalysts with tert-butyl hydroperoxide: Epoxidation versus allylic oxidation. J. Mol. Catal. A Chem. 2014, 394, 89–96. [Google Scholar] [CrossRef]
- Alfayate, A.; Márquez-Álvarez, C.; Grande-Casas, M.; Sánchez-Sánchez, M.; Pérez-Pariente, J. Ti(III)APO-5 materials as selective catalysts for the allylic oxidation of cyclohexene: Effect of Ti source and Ti content. Catal. Today 2014, 227, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Habibia, D.; Faraji, A.R.; Arshadi, M.; Fierro, J.L.G. Characterization and catalytic activity of a novel Fe nano-catalyst as efficient heterogeneous catalyst for selective oxidation of ethylbenzene, cyclohexene, and benzylalcohol. J. Mol. Catal. A Chem. 2013, 372, 90–99. [Google Scholar] [CrossRef]
- Bujak, P.; Bartczak, P.; Polanski, J. Highly efficient room-temperature oxidation of cyclohexene and d-glucose over nanogold Au/SiO2 in water. J. Catal. 2012, 295, 15–21. [Google Scholar] [CrossRef]
- Khare, S.; Chokhare, R. Synthesis, characterization and catalytic activity of Fe(Salen) intercalated α-zirconium phosphate for the oxidation of cyclohexene. J. Mol. Catal. A Chem. 2011, 344, 83–92. [Google Scholar] [CrossRef]
- Chagas, P.; Oliveira, H.S.; Mambrini, R.; Le Hyaric, M.; de Almeida, M.V.; Oliveira, L.C.A. A novel hydrofobic niobium oxyhydroxide as catalyst: Selective cyclohexene oxidation to epoxide. Appl. Catal. A Gen. 2013, 454, 88–92. [Google Scholar] [CrossRef]
- Payne, G.B. Reactions of Hydrogen Peroxide. VII. Alkali-catalyzed epoxidation and oxidation using a nitrile as co-reactant. J. Org. Chem. 1961, 26, 659–663. [Google Scholar] [CrossRef]
- Kirm, I.; Medina, F.; Rodriguez, X.; Cesteros, Y.; Salagre, P.; Sueiras, J. Epoxidation of styrene with hydrogen peroxide using hydrotalcites as heterogeneous catalysts. Appl. Catal. A Gen. 2004, 272, 175–185. [Google Scholar] [CrossRef]
- Mureşeanu, M.; Georgescu, I.; Bibire, L.E.; Cârjă, G. CUII(Sal-Ala)/MgAlLDH and CUII(Sal-Phen)/MgAlLDH as novel catalytic systems for cyclohexene oxidation by H2O2. Catal. Commun. 2014, 54, 39–44. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Bȋrjega, R.; Pavel, O.D.; Cruceanu, A.; Alifanti, M. Hydrotalcite like compounds with low Mo-loading active catalysts for selective oxidation of cyclohexene with hydrogen peroxide. Appl. Catal. A Gen. 2005, 286, 211–220. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.D.; Bȋrjega, R.; Florea, M.; Zăvoianu, R. The impact of the “memory effect” on the catalytic activity of Mg/Al; Mg,Zn/Al; Mg/Al,Ga hydrotalcite-like compounds used as catalysts for cycloxene epoxidation. Appl. Catal. A Gen. 2008, 341, 50–57. [Google Scholar] [CrossRef]
- Palomeque, J.; Figueras, F.; Gelbard, G. Epoxidation with hydrotalcite-intercalated organotungstic complexes. Appl. Catal. A Gen. 2006, 300, 100–108. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Ionescu, R.; Pavel, O.D.; Bîrjega, R.; Angelescu, E. Comparison between MeIIMg/Al hydrotalcites and hydrotalcite-supported Me(II) acetylacetonates (Me(II) = Co, Cu or Ni) catalysts for the epoxidation of cyclohexene with molecular oxygen. Appl. Clay Sci. 2011, 52, 1–10. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Anderson, J.A. Comparison of the epoxidation of cyclohexene, dicyclopentadiene and 1,5-cyclooctadiene over LDH hosted Fe and Mn sulfonato-salen complexes. J. Mol. Catal. A Chem. 2006, 249, 103–110. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Chen, Y.; Cui, A.; Sun, F.; He, M.; Xu, Z.; Chen, Q. Metallophthalocyanine intercalated layered double hydroxides as an efficient catalyst for the selective epoxidation of olefin with oxygen. Appl. Catal. A Gen. 2017, 542, 191–200. [Google Scholar] [CrossRef]
- Carriazo, D.; Lima, S.; Martín, C.; Pillinger, M.; Valente, A.A.; Rives, V. Metatungstate and tungstoniobate-containing LDHs: Preparation, characterisation and activity in epoxidation of cyclooctene. J. Phys. Chem. Solids 2007, 68, 1872–1880. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; De Witte, K.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Mantilla, A.; Banũelos, F.; Fernández, J.L.; Gómez, R. Improved photocatalytic degradation of phenolic compounds with znal mixed oxides obtained from LDH Materials. Top. Catal. 2011, 54, 257–263. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Scholz, F.; Kahlert, H. The calculation of the solubility of metal hydroxides, oxide-hydroxides, and oxides, and their visualisation in logarithmic diagrams. ChemTexts 2015, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Moezzi, A.; Lee, P.-S.; McDonagh, A.M.; Cortie, M.B. On the thermal decomposition of zinc hydroxide nitrate, Zn5(OH)8(NO3)2⋅2H2O. J. Solid State Chem. 2020, 286, 121311. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.D.; Zavoianu, R.; Birjega, R. Cyanoethylation of ethanol over mixed oxides obtained from hydrotalcite, precursors. Rev. Roum. Chim. 2004, 49, 367–375. [Google Scholar]
- Starukh, G.; Rozovik, O.; Oranska, O. Organo/Zn-Al LDH nanocomposites for cationic dye removal from aqueous media. Nanoscale Res. Lett. 2016, 11, 228. [Google Scholar] [CrossRef] [Green Version]
- Navajas, A.; Arzamendi, G.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A.; Gandía, L.M. DRIFTS study of methanol adsorption on Mg–Al hydrotalcite catalysts for the transesterification of vegetable oils. Catal. Commun. 2012, 17, 189–193. [Google Scholar] [CrossRef]
- Kocík, J.; Hájek, M.; Tišler, Z.; Strejcová, K.; Velvarská, R.; Bábelová, M. The influence of long-term exposure of Mg–Al mixed oxide at ambient conditions on its transition to hydrotalcite. J. Solid State Chem. 2021, 304, 122556. [Google Scholar] [CrossRef]
- Yi, H.; Zhao, S.; Tang, X.; Ning, P.; Wang, H.; He, D. Influence of calcination temperature on the hydrolysis of carbonyl sulfide over hydrotalcite-derived Zn–Ni–Al catalyst. Catal. Commun. 2011, 12, 1492–1495. [Google Scholar] [CrossRef]
- Takehira, K.; Kawabata, T.; Shishido, T.; Murakami, K.; Ohi, T.; Shoro, D.; Honda, M.; Takaki, K. Mechanism of reconstitution of hydrotalcite leading to eggshell-type Ni loading on Mg single bondAl mixed oxide. J. Catal. 2005, 231, 92–104. [Google Scholar] [CrossRef]
- Palomeque, J.; Lopez, J.; Figueras, F. Epoxydation of activated olefins by solid bases. J. Catal. 2002, 211, 150–156. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Fornes, V.; Rey, F. Hydrotalcites as Base Catalysts: Influence of the Chemical Composition and Synthesis Conditions on the Dehydrogenation of Isopropanol. J. Catal. 1994, 148, 205–212. [Google Scholar] [CrossRef]
- Jyothi, T.M.; Raja, T.; Sreekumar, K.; Talawar, M.B.; Rao, B.S. Influence of acid–Base properties of mixed oxides derived from hydrotalcite-like precursors in the transfer hydrogenation of propiophenone. J. Mol. Catal. A Chem. 2000, 157, 193–198. [Google Scholar] [CrossRef]
- Debecker, D.; Gaigneaux, E.M.; Busca, G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Ionescu, R.; Pavel, O.D.; Bîrjega, R.; Zăvoianu, R.; Angelescu, E. Epoxidation of cyclohexene with H2O2 and acetonitrile catalyzed by Mg–Al hydrotalcite and cobalt modified hydrotalcites. Catal. Lett. 2010, 134, 309–317. [Google Scholar] [CrossRef]
- Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E. The effect of ageing step elimination on the memory effect presented by Mg0.75Al0.25 hydrotalcites (HT) and their catalytic activity for cyanoethylation reaction. Catal. Commun. 2011, 12, 845–850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).