Abstract
The application of solid oxide electrolysis cell in CO2 electroreduction is a hot research topic at present, but the development of low−cost catalysts with high catalytic activity has always been a challenge for this work. Herein, we use NiCu alloy nanoparticles to modify the perovskite LSCM electrode to build a metal–oxide active interface to obtain high catalytic performance. At 850 °C, 4.66 mL min−1 cm−2 CO productivity and 97.7% Faraday current efficiency were obtained. In addition, the current remained stable during the 100 h long−term test, indicating that the active interface has the dual effect of improving catalytic performance and maintaining cell durability.
1. Introduction
Nowadays, the problems of environment and energy in the world have become increasingly prominent, and the use of fossil fuels has directly led to a large area of environmental pollution [1,2,3]. With the burning of fossil fuels, the concentration of CO2 in the atmosphere is increasing, resulting in serious environmental pollution problems, such as haze, acid rain and global warming [4,5,6]. Therefore, converting CO2 into economically valuable fuel can not only alleviate the global warming caused by greenhouse gas emissions, but also is the inevitable choice of the development of the times [7,8,9].
Electroreduction and conversion of CO2 is an effective way to realize CO2 recovery and reuse. The common methods for CO2 electroreduction are H−type cells, but they are susceptible to CO2 mass transport limitations and cannot achieve high product generation rates. As the limitation of CO2 gas transmission can be eliminated, flow cells are also widely used. Polymer electrolyte membrane (PEM) flow cells are the most promising device for CO2 reduction in practice, which are usually assembled from a cathode and anode current collector, cathode and anode flow plate and membrane electrode assembly (MEA). However, the lack of reference electrodes and the strong corrosion caused by high overpotentials can make potential measurements difficult. The microfluidic flow cells (MFC) can be used to evaluate the performance of catalysts for the electroreduction of CO2 under different operating conditions, but the structure is too sensitive to stresses caused by the transmembrane to be applied on a large scale [10,11,12,13]. Solid oxide electrolysis cells (SOEC) act as a kind of energy conversion device, which can convert electrical energy into chemical energy, so it greatly improves the energy efficiency. Compared with other electrolysis processes, CO2 electrolysis in a solid oxide electrolysis cell also has the advantages of carbon deposition resistance, all−solid−state design, and the use of non−precious metal catalysts [14,15,16].
At present, the conversion efficiency of CO2 is still limited by the low activity of the electrode catalyst. For this reason, researchers improve the catalytic activity by doping precious metals (such as Ru and Au) [17,18]. However, although the catalytic efficiency has been improved, it faces high costs. The non−noble metals are not only easy to obtain and cheap, but also easy to be exsolved to form active nanoparticles under the reducing atmosphere, which is the preferred choice of catalysts. La0.7Sr0.3Cr0.5Mn0.5(NiCu)xO3−δ(LSCrM(NC)x) is one of the commonly used cathode materials in SOEC because of its excellent electronic and ionic conductivity as well as high redox stability [19,20]. Zhang et al. proved that the electrolysis of LSCrM electrode in symmetrical cells has good current activation performance [21,22], with the electrolysis efficiency of CO2 reached 69%, 58% and 56% at 800 °C, at 1.0 V, 1.5 V and 2.0 V, respectively. Due to the poor catalytic activity of the traditional LSCrM cathode, its performance for electrocatalytic CO2 reduction is limited. In recent years, scientists have found that the doping of non−precious metal oxide significantly improves the electrochemical performance of LSCrM cathodes [23]. The exsolution of Ni nanoparticles from the catalyst has been proved to be an effective way to improve the catalytic activity of CO2 electrolysis [24]. However, the long−term instability of nanoparticles remains a major challenge because the agglomeration of nanoparticles at high temperatures leads to a decline in catalytic performance. According to recent studies, Cu nanoparticles have excellent CO2 adsorption properties and anti−coking properties [25]. Therefore, in order to improve the catalytic performance of LSCrM electrode, we designed an effective method to improve the performance of porous electrodes by immobilizing NiCu alloy catalytic active nanoparticles on the surface of the porous electrode. This alloy catalyst makes use of the interaction between nickel and copper to greatly improve the catalytic activity and anti−coking performance of LSCrM cathode materials in the electrolytic reaction of CO2.
In this work, we synthesize a range of LSCrM(NC)x, doped excessive NiCu at the B position, and made NiCu exsolve from the lattice of LSCrM through reduction pretreatment to form active metal alloy nanoparticles, thus promoting the electrochemical conversion of CO2.
2. Results and Discussion
The general chemical formula of perovskite oxide is ABO3, in which a rare earth or alkaline earth metal element is at position A, and a transition metal element is at position B. Excellent ion electron mixed conductor properties can be obtained by doping. The transition at position B is easy to be exsolved under reducing atmosphere, and different transition metals are easy to form alloys [26,27]. A series of LSCrM(NC)x electrode materials were analyzed by XRD; the diffraction pattern is as shown in Figure 1a,b. Figure 1a is the XRD spectrum of LSCrM(NC)x synthesized in liquid phase, which displays a pure perovskite phase without other impurity peaks, indicating that excess metal Ni and Cu at the B site have successfully entered the lattice of LSCrM. The spectrogram of oxidized LSCrM(NC)x powder after reduction by 5% H2/Ar is shown in Figure 1b. Compared with Figure 1a, there is a new peak at 44.2° [14,28], which is confirmed to be NiCu alloy, illustrating that the NiCu at B site is exsolved from the lattice of LSCrM under the reducing atmosphere. In addition, except for the NiCu alloy peak, the phase of LSCrM remains unchanged after high−temperature reduction, exhibiting its excellent structural stability.
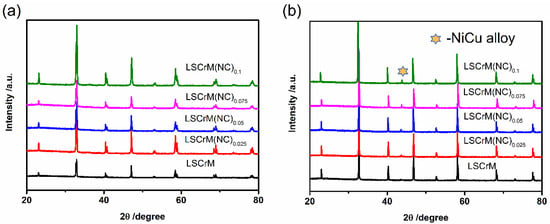
Figure 1.
XRD characterization of LSCrM(NC)x samples. (a) Unreduced and (b) reduced samples.
The XPS spectrum of NiCu for a proper amount of freshly fired LSCrM(NC)x liquid powder and a powder with 5% H2/Ar reduction is as shown in Figure 2. The doped Ni and Cu under oxidation state exist on LSCrM substrate in the form of 2+, as shown in Figure 2a,b, respectively. The two main peaks Ni2+ 2p3/2 and Ni2+ 2p1/2 are accompanied by two satellite peaks, as is Cu. After reduction, the binding energies of the peak positions of Ni and Cu are reduced, and coexistence of Ni0 and Cu0 is observed [29,30], indicating that NiCu alloy exists in the LSCrM(NC)x sample, which is in agreement with the results of previous XRD tests. These valence state changes caused by reduction treatment will theoretically lead to lattice distortion, thus promoting ion transport.
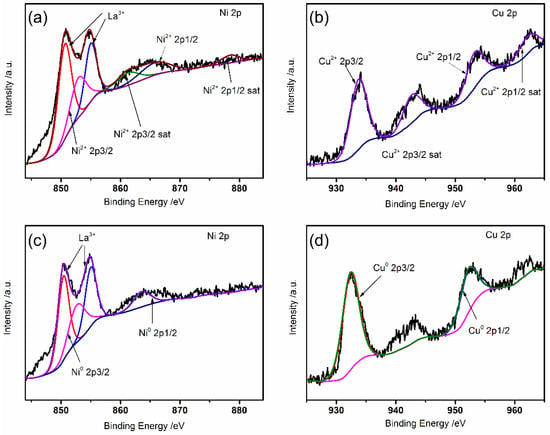
Figure 2.
The chemical valence state of the elements. (a) Ni 2p and (b) Cu 2p XPS of unreduced LSCrM(NC)x; (c) Ni 2p and (d) Cu 2p XPS of reduced LSCrM(NC)x.
Figure 3a reveals the scanning electron microscope view of cross−section for a complete single cell. The dense LSGM electrolyte is conductive to the transmission of electrons and can isolate the reaction gas of the cathode and anode. The volatilization of organic matter at high temperature causes the porous structure of the electrode, which not only promotes the diffusion of reaction gas, but also ensures full contact between CO2 and catalyst [31]. Thermogravimetric analysis of LSCrM(NC)x electrode material in a reducing atmosphere (5% H2/Ar) is shown in Figure 3b. The sharp drop in weight within 100 °C is due to the evaporation of water in the material. The slow weight reduction in the second stage is owing to the continuous escape of lattice oxygen in the material, leaving oxygen vacancies that cause weight reduction. Among them, the weight loss of LSCM can be attributed to the reduction of Mn4+ to Mn3+, while the weight loss of LSCrM(NC)x is also affected by the alloy formed by the exsolution of doped NiCu from the lattice [32]. To further observe the metal nanoparticles, we take pictures of the microstructure of catalytic materials with different doping levels after reduction, as shown in Figure 3a,b. Metal alloy nanoparticles with average particle size less than 50 nm are evenly distributed, and the amount is positively correlated with the doping amount.
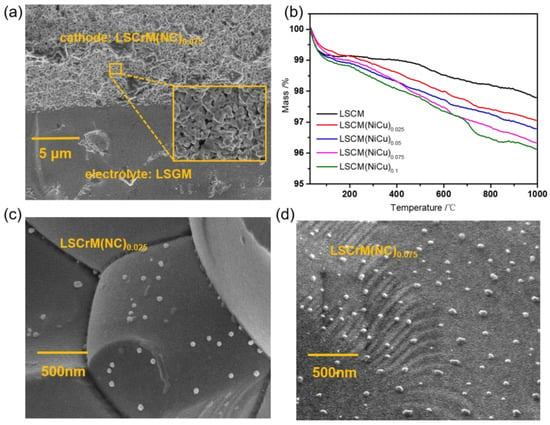
Figure 3.
Microscopic morphology and thermogravimetry. (a) SEM images of cell cross−sections, (b) the TGA of LSCrM(NC)x at 5% H2/Ar, SEM of reduced (c) LSCrM(NC)0.025 and (d) LSCrM(NC)0.075.
More evidence to prove the exsolution of NiCu alloy is shown in Figure 4a,b by HRTEM. Careful observation finds that the lattice stripes of the particles and the substrate are different, indicating that NiCu nanoparticles are exsolved from the substrate. NiCu alloy nanoparticles are anchored on the LSCrM skeleton, demonstrating that there is a strong interaction between metal particles and the interface. Figure 4b represents that the lattice stripes of NiCu alloy nanoparticles have a lattice spacing of 0.178 nm, corresponding to the (200) face of NiCu alloy. Furthermore, the atomic proportions of elements in the sample are analyzed by EDS, as shown in Figure 4c. In the table inserted in Figure 4c, except for the copper atom, the atomic proportion of other elements is basically consistent with the chemical formula and doping amount, while the large proportion of Cu atoms is attributed to the sample preparation on the copper mesh.
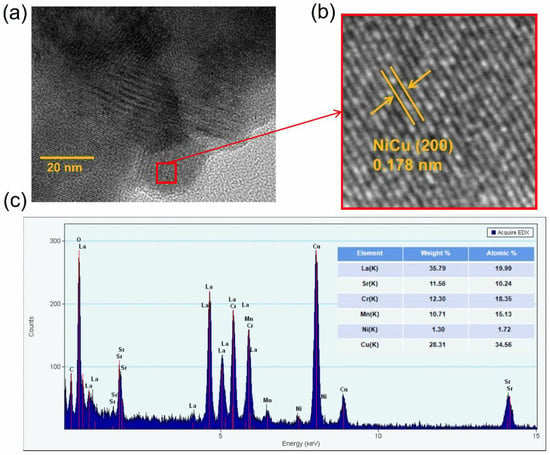
Figure 4.
HRTEM with element dispersive X−ray spectroscopy analysis. (a) HRTEM image of reduced LSCrM(NC)x and lattice spacing micro−image of the exsolved (b) NiCu alloy, and (c) EDS analysis of the corresponding regions.
The single cell after reduction pretreatment starts to test its electrochemical performance and catalytic performance. Figure 5a manifests the instantaneous current density of the cell at 0.6–1.6 V, the increases of voltage play a positive role in the enhancement of current density. On the one hand, the increase of voltage strengthens the electric field of the conductor, and on the other hand, the active interface formed by the exsolved alloy particles and the oxide substrate contributes to the directional migration of electrons [33]. Figure 5b is the current density of the cell running for 10 min under different voltages. The current density of the electrode LSCrM(NC)0.075 doped with NiCu is stable at 0.68 A cm−2 at 1.6 V, which is much higher than that of the pure LSCM 0.31 A cm−2. In general, the electrochemical performance of the electrode loaded with metal nanoparticles is better than that of the electrode without metal nanoparticles, as well as the CO2 conversion performance, see Figure 5c,d for details. LSCrM(NC)0.075−SDC electrode used for CO2 electrolysis has the best catalytic effect in this series of materials, with the production of CO reaching 4.66 mL min−1 cm−2, and the current efficiency up to 97.7%, which is much higher than that of pure phase LSCrM−SDC electrode. Under the same conditions, the trends of current density, CO productivity and current efficiency are consistent. These results prove that NiCu alloy nanoparticles, as the active site of the reaction, can promote the electroreduction of CO2. However, too much doping will be counterproductive, for example, the performance of LSCrM(NC)0.1−SDC electrode will be degraded due to the inevitable agglomeration of alloy nanoparticles. Table 1 shows the electrochemical performance of relevant literatures with different metal doping, mainly including the single metal element and its multiple element co−doping.
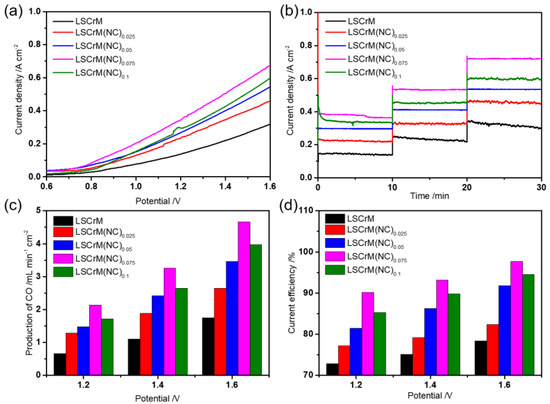
Figure 5.
Performance diagram of LSCrM(NC)x−SDC. (a) Transient current, (b) short−term current, (c) CO yield from electrolytic CO2 at different voltages and (d) corresponding Faraday current efficiency.

Table 1.
The summary of electrochemical performance of the perovskite cathode doped with different metal elements.
Figure 6a–e reveal the in situ AC impedance spectra of each electrode material at a specific voltage, which are often used to analyze the reasons for the improvement of cell performance [38]. With the increase of voltage, the impedance shows a negative correlation, because the increase of voltage can accelerate the transfer of electrons. The total polarization resistance of each electrode is shown in Figure 6e, the LSCrM(NC)0.075−SDC electrode again emerges with excellent performance, with the lowest impedance value of 0.51 Ω cm2. This result attests that the reduction of electrode polarization resistance is closely related to the effect of alloy nanoparticles.
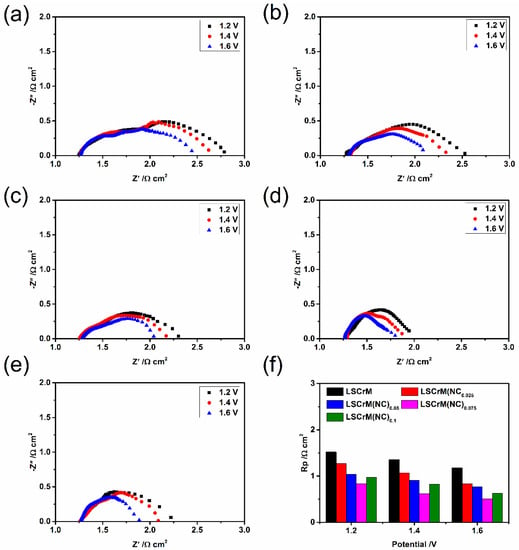
Figure 6.
Impedance spectra of LSCrM(NC)x at different voltages. (a) LSCrM, (b) LSCrM(NC)0.025, (c) LSCrM(NC)0.05, (d) LSCrM(NC)0.075, (e) LSCrM(NC)0.01, and (f) the corresponding polarised resistance of LSCrM(NC)x.
Long−term tests with CO2 electrolysed by reduction pretreated LSCrM(NC)0.075−SDC is shown in Figure 7a. The current density is stable at 0.33 A cm−2 at 850 °C and 1.2 V even at 100 h without decay, indicating this exsolved cathode material has excellent long−term stability. Figure 7b shows a micrograph of the cell after 100 h of CO2 electrolysis. We can clearly see that the NiCu alloy nanoparticles are still attached to the almost unchanged porous electrode surface, suggesting that the metal oxide interface formed by the combination of the exsolved NiCu alloy nanoparticles and the structurally stable LSCrM substrate is the key to maintaining superior performance.
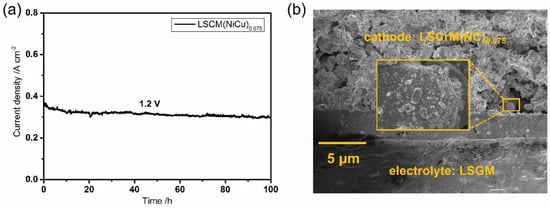
Figure 7.
Long−term tests and morphology. (a) Long−term performance of electrolysis of CO2 100 h and (b) micrographs after electrolysis.
3. Experimental
The perovskite LSCrM(NC)x powders are synthesized by the glycine–nitrate method [39,40]. Appropriate amounts of lanthanum nitrate, strontium nitrate, chromium nitrate, manganese nitrate, nickel nitrate, copper nitrate and glycine were weighed and dissolved in ultrapure water according to the stoichiometric ratio. Then they were heated at 200 °C until evaporation and combustion. The resulting powder was placed in a muffle furnace, heated at a rate of 3 °C per minute and held at 1300 °C for 6 h. Ce0.8Sm0.2O3−δ(SDC) powders are synthesized though a glycine−nitrate combustion method with appropriate amounts of Ce(NO3)3·6H2O and Sm2O3 followed by 800 °C for 3 h in air. La0.9Sr0.1Ga0.8Mg0.2O3 (LSGM) powder is prepared by a solid−state reaction, by mixing appropriate amounts of the corresponding oxides and heating to 1300 °C in a muffle furnace and holding for 6 h. The resulting sample was weighed 0.65 g and pressed into a 2 cm diameter disc, then heated to 1450 °C in the furnace and held for 10 h to obtain a dense electrolyte carrier.
The appropriate amount of LSCrM and SDC powder is added to the paste consisting of α−pinoresinol, tapioca starch and ethyl cellulose in the ratio of 65:35 by mass. Then they are mixed and ground for 2 h. The obtained samples are coated on both sides of the LSGM surface and heated at 1000 °C for 3 h to form cell pellets. Finally, the silver wire is fixed to both sides of the cell pellet by silver paste and then maintained in the furnace at 550 °C for half an hour, and the resulting sample is the LSCrM−SDC cell. The cell consists of about 1 mm electrolyte and about 40 μm porous anode and cathode, among them, the active area of the electrode is about 0.5 cm−2, which is an important data for the later calculation of impedance density and current density. Before testing, the LSCrM−SDC cell is pretreated by reduction with 5% H2/Ar in a tube furnace and then electrochemically measured by CO2 electrolysis at 850 °C. The reaction gas CO2 of cathode is cracked into CO and O2− under the action of high temperature and applied voltage, while the O2− is transmitted to the anode through a dense electrolyte to form oxygen. The specific reaction procedure is as follows: CO2 + 2e−→CO + O2− (cathode), O2−−2e−→1/2O2 (anode).
X−ray powder diffraction (XRD, Cu−Kα, Miniflex600, Rigaku, Tokyo, Japan) is used to characterized the phase of the generated material (LSCrM(NiCu)x). X−ray photoelectron spectroscopy (XPS, AlKa, ESCALAB 250Xi, Waltham, MA, USA) is used to characterize the changes in valence and bonding of samples. High−resolution transmission electron microscopy (HRTEM, Tecnai F20, Hillsboro, OR, USA) and a scanning electron microscope (SEM, SU−8010, Tokyo, Japan) are used to characterize the microstructure of the electrodes samples and the composition of the electrode material is analyzed by energy dispersive X−ray spectrometer (EDS) of SEM. The relevant electrical performance tests are recorded by an electrochemical workstation (IM6, Zahner, Kansas, MO, USA). The yield of carbon monoxide produced by electrolysis of CO2 is analyzed by gas chromatography (GC−2014, Shimadzu, Kyoto, Japan).
4. Conclusions
In conclusion, by doping at the B position, alloy metal nanoparticles are exsolved from the oxide substrate to serve as the active site of the reaction, and form an active metal−oxide interface with the substrate to promote the adsorption and decomposition of CO2, thus accelerating the CO2 electrolysis reaction. The electrode with the best doping amount, LSCrM(NC)0.075, has outstanding performance, including 4.66 mL min−1 cm−2 CO yield and 100 h of long−term stable operation. The results suggest that the application of interface engineering in SOEC has a good prospect.
Author Contributions
Conceptualization, G.M. and Y.X.; methodology, K.X.; software, G.M.; validation, G.M.; formal analysis, Y.X.; investigation, K.X.; resources, K.X.; data curation, G.M.; writing—original draft preparation, G.M.; writing—review and editing, G.M.; visualization, Y.X.; supervision, Y.X.; project administration, K.X.; funding acquisition, K.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0700102), the Natural Science Foundation of China (91845202), and Strategic Priority Research Program of Chinese Academy of Sciences (XDB2000000).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shindell, D.; Smith, C.J. Climate and Air-Quality Benefits of a Realistic Phase-out of Fossil Fuels. Nature 2019, 573, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The Role of New Energy in Carbon Neutral. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-Gas Emission Targets for Limiting Global Warming to 2 °C. Nature 2009, 458, 1158–1162. [Google Scholar] [CrossRef]
- Manzone, M.; Calvo, A. Woodchip Transportation: Climatic and Congestion Influence on Productivity, Energy and CO2 Emission of Agricultural and Industrial Convoys. Renew. Energy 2017, 108, 250–259. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Sanyal, S. Air Quality in a Cleaner Energy World. Curr. Pollut. Rep. 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Najjar, Y.S.H. Gaseous Pollutants Formation and Their Harmful Effects on Health and Environment. Innov. Energy Policies 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic Carbon Dioxide Hydrogenation to Methane: A Review of Recent Studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Electrocatalytic Conversion of CO2 to Liquid Fuels Using Nanocarbon-Based Electrodes. J. Energy Chem. 2013, 22, 202–213. [Google Scholar] [CrossRef]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic Cell Design for Electrochemical CO2 Reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Wu, D.; Jiao, F.; Lu, Q. Progress and Understanding of CO2/CO Electroreduction in Flow Electrolyzers. ACS Catal. 2022, 12, 12993–13020. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Tufa, R.A.; Chanda, D.; Ma, M.; Aili, D.; Demissie, T.B.; Vaes, J.; Li, Q.; Liu, S.; Pant, D. Towards Highly Efficient Electrochemical CO2 Reduction: Cell Designs, Membranes and Electrocatalysts. Appl. Energy 2020, 277, 115557. [Google Scholar] [CrossRef]
- Fu, X.Z.; Melnik, J.; Low, Q.X.; Luo, J.L.; Chuang, K.T.; Sanger, A.R.; Yang, Q.M. Surface Modified Ni Foam as Current Collector for Syngas Solid Oxide Fuel Cells with Perovskite Anode Catalyst. Int. J. Hydrog. Energy 2010, 35, 11180–11187. [Google Scholar] [CrossRef]
- Dong, C.; Jiang, F.; Yang, L.; Wang, C.; Xie, K. Enhancing Electrocatalytic Reforming of CO2/CH4 with in Situ Exsolved Metal-Oxide Interfaces in a Solid Oxide Electrolysis Cell. Sep. Purif. Technol. 2022, 299, 121714. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, W.; Wang, Z.; Ren, C.; Wang, Y.; Ding, M.; Liu, T. Efficient Electrochemical CO2 Reduction Reaction on a Robust Perovskite Type Cathode with In-Situ Exsolved Fe-Ru Alloy Nanocatalysts. Sep. Purif. Technol. 2023, 304, 122287. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, S.; Dong, Q.; Li, Y.; Zhang, X.; Ta, N.; Liu, Z.; Zhao, J.; Yang, F.; Wang, G.; et al. Oxygen Evolution Reaction over the Au/YSZ Interface at High Temperature. Angew. Chem.-Int. Ed. 2019, 58, 4617–4621. [Google Scholar] [CrossRef]
- Li, W.; Luo, J.L. High-Temperature Electrochemical Devices Based on Dense Ceramic Membranes for CO2 Conversion and Utilization. Electrochem. Energy Rev. 2021, 4, 518–544. [Google Scholar] [CrossRef]
- Ye, L.; Xie, K. High-Temperature Electrocatalysis and Key Materials in Solid Oxide Electrolysis Cells. J. Energy Chem. 2021, 54, 736–745. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Yao, W.; Dong, D.; Xie, K. Direct Electrolysis of CO2 Using an Oxygen-Ion Conducting Solid Oxide Electrolyzer Based on La0.75Sr0.25Cr 0.5Mn0.5O3−δ Electrode. J. Power Sources 2013, 230, 115–121. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, L.; Hu, J.; Li, J.; Jiang, W.; Tseng, C.J.; Xie, K. Perovskite LSCM Impregnated with Vanadium Pentoxide for High Temperature Carbon Dioxide Electrolysis. Electrochim. Acta 2016, 212, 32–40. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Guan, F.; Zhou, Y.; Lv, H.; Wang, G.; Bao, X. Enhancing Electrocatalytic CO2 Reduction in Solid Oxide Electrolysis Cell with Ce0.9Mn0.1O2−δ Nanoparticles-Modified LSCM-GDC Cathode. J. Catal. 2018, 359, 8–16. [Google Scholar] [CrossRef]
- Gunduz, S.; Deka, D.J.; Ozkan, U.S. Advances in High-Temperature Electrocatalytic Reduction of CO2 and H2O, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 62, ISBN 9780128150887. [Google Scholar]
- Yang, X.; Sun, W.; Ma, M.; Xu, C.; Ren, R.; Qiao, J.; Wang, Z.; Li, Z.; Zhen, S.; Sun, K. Achieving Highly Efficient Carbon Dioxide Electrolysis by in Situ Construction of the Heterostructure. ACS Appl. Mater. Interfaces 2021, 13, 20060–20069. [Google Scholar] [CrossRef]
- Tsekouras, G.; Neagu, D.; Irvine, J.T.S. Step-Change in High Temperature Steam Electrolysis Performance of Perovskite Oxide Cathodes with Exsolution of B-Site Dopants. Energy Environ. Sci. 2013, 6, 256–266. [Google Scholar] [CrossRef]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on Exsolution and Its Driving Forces in Perovskites. J. Phys. Energy 2020, 2, 032001. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J. Catalytic Performance of Cu-Ni/La0.75Sr0.25Cr0.5Mn0.5O3−δ for Dry Methane Reforming. Int. J. Energy Res. 2022, 46, 10522–10534. [Google Scholar] [CrossRef]
- Wei, H.; Xie, K.; Zhang, J.; Zhang, Y.; Wang, Y.; Qin, Y.; Cui, J.; Yan, J.; Wu, Y. In Situ Growth of NixCu1−x Alloy Nanocatalysts on Redox-Reversible Rutile (Nb,Ti)O4 towards High-Temperature Carbon Dioxide Electrolysis. Sci. Rep. 2014, 4, 5156. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu-Ni Interactions on Reduced Copper-Nickel-Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Ma, L.; Li, W.; Liu, X. Degradation of Solid Oxide Electrolysis Cells: Phenomena, Mechanisms, and Emerging Mitigation Strategies—A Review. J. Mater. Sci. Technol. 2020, 55, 35–55. [Google Scholar] [CrossRef]
- Wan, J.; Zhu, J.H.; Goodenough, J.B. La0.75Sr0.25Cr0.5Mn0.5O3−δ + Cu Composite Anode Running on H2 and CH4 Fuels. Solid State Ionics 2006, 177, 1211–1217. [Google Scholar] [CrossRef]
- Cao, T.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells. Nanomaterials 2020, 10, 2445. [Google Scholar] [CrossRef]
- Qian, B.; Liu, C.; Wang, S.; Yin, B.; Zheng, Y.; Ge, L.; Chen, H.; Zhang, C. Ca-Doped La0.75Sr0.25Cr0.5Mn0.5O3 Cathode with Enhanced CO2 Electrocatalytic Performance for High-Temperature Solid Oxide Electrolysis Cells. Int. J. Hydrog. Energy 2021, 46, 33349–33359. [Google Scholar] [CrossRef]
- Xing, R.; Wang, Y.; Zhu, Y.; Liu, S.; Jin, C. Co-Electrolysis of Steam and CO2 in a Solid Oxide Electrolysis Cell with La0.75Sr0.25Cr0.5Mn0.5O3−δ -Cu Ceramic Composite Electrode. J. Power Sources 2015, 274, 260–264. [Google Scholar] [CrossRef]
- Ruan, C.; Xie, K. A Redox-Stable Chromate Cathode Decorated with in Situ Grown Nickel Nanocatalyst for Efficient Carbon Dioxide Electrolysis. Catal. Sci. Technol. 2015, 5, 1929–1940. [Google Scholar] [CrossRef]
- Qian, B.; Wang, S.; Zheng, Y.; Ni, Q.; Chen, H.; Ge, L.; Yang, J. Ca-Fe Co-Doped La0.75Sr0.25Cr0.5Mn0.5O3 Cathodes with High Electrocatalytic Activity for Direct CO2 Electrolysis in Solid Oxide Electrolysis Cells. J. CO2 Util. 2023, 67, 102305. [Google Scholar] [CrossRef]
- Nechache, A.; Cassir, M.; Ringuedé, A. Solid Oxide Electrolysis Cell Analysis by Means of Electrochemical Impedance Spectroscopy: A Review. J. Power Sources 2014, 258, 164–181. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, C.; Xie, K.; Gan, L. High Performance, Coking-Resistant and Sulfur-Tolerant Anode for Solid Oxide Fuel Cell. J. Power Sources 2018, 406, 1–6. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Chen, T.; Yuan, C.; Zhou, Y.; Wang, S.; Huang, J. Performance of the Nano-Structured Cu-Ni (Alloy)-CeO2 Anode for Solid Oxide Fuel Cells. J. Power Sources 2015, 274, 730–735. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).