Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts
Abstract
:1. Introduction
2. Results and Discussion
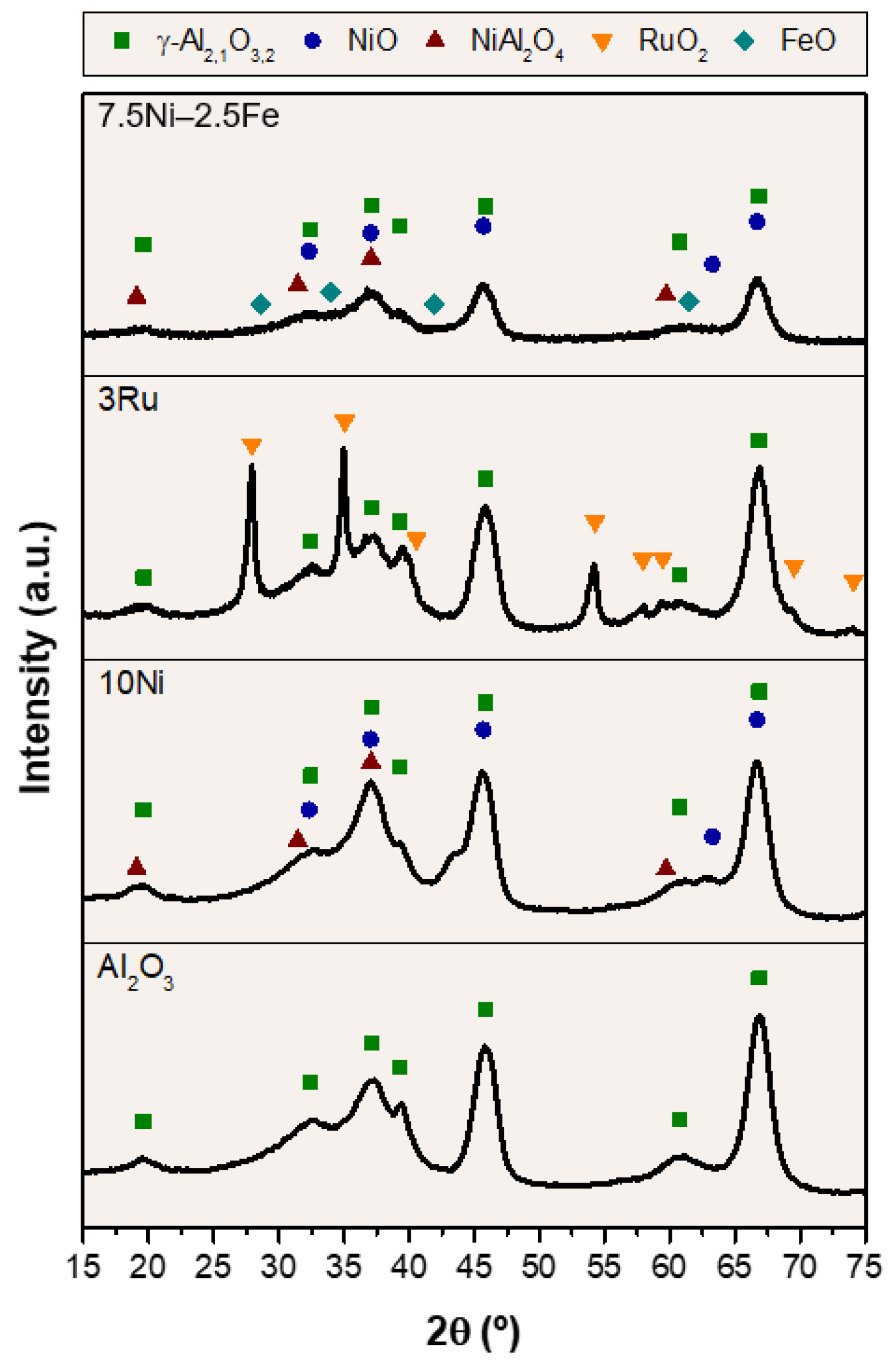
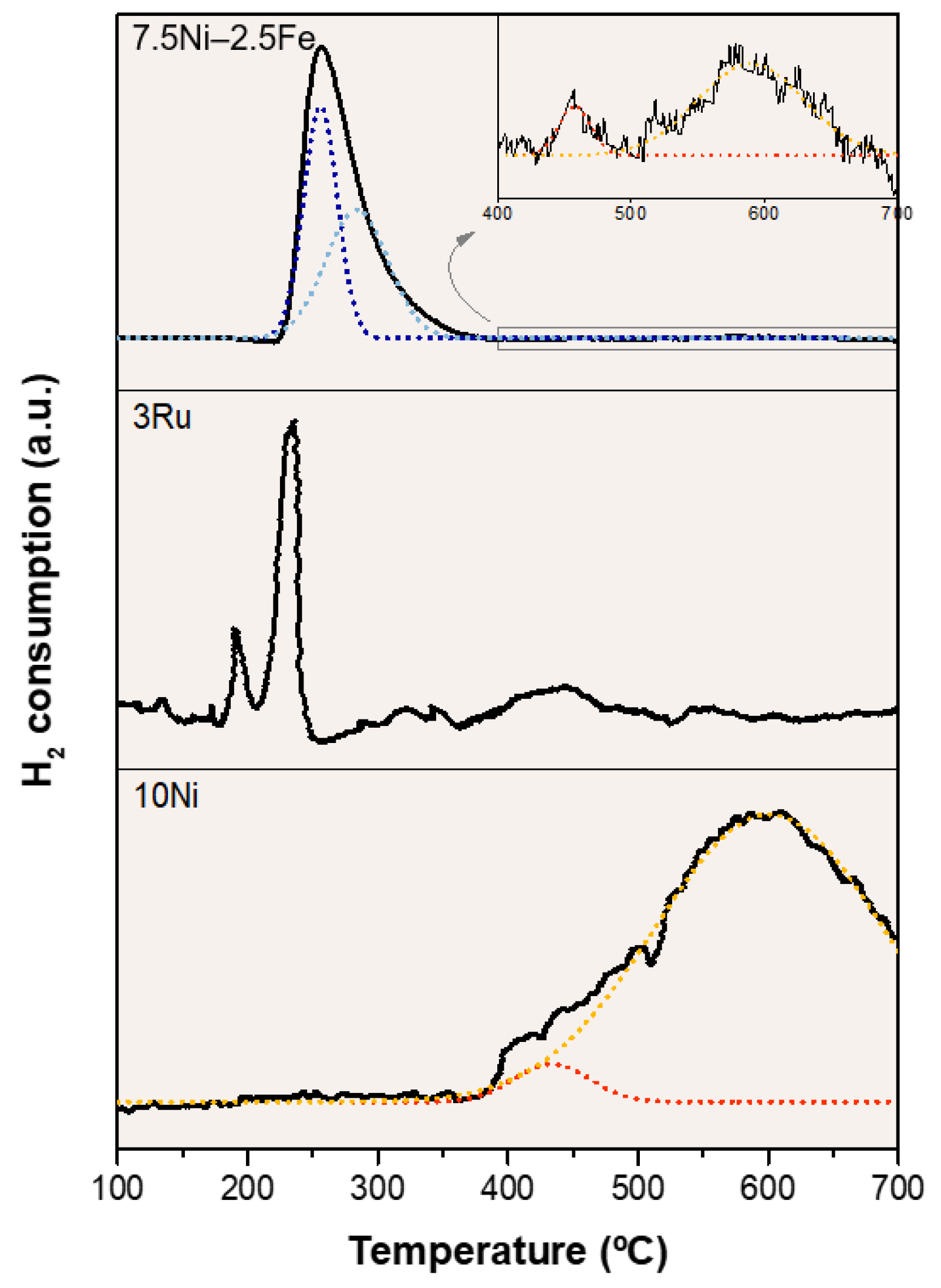
2.1. Catalyst Characterization
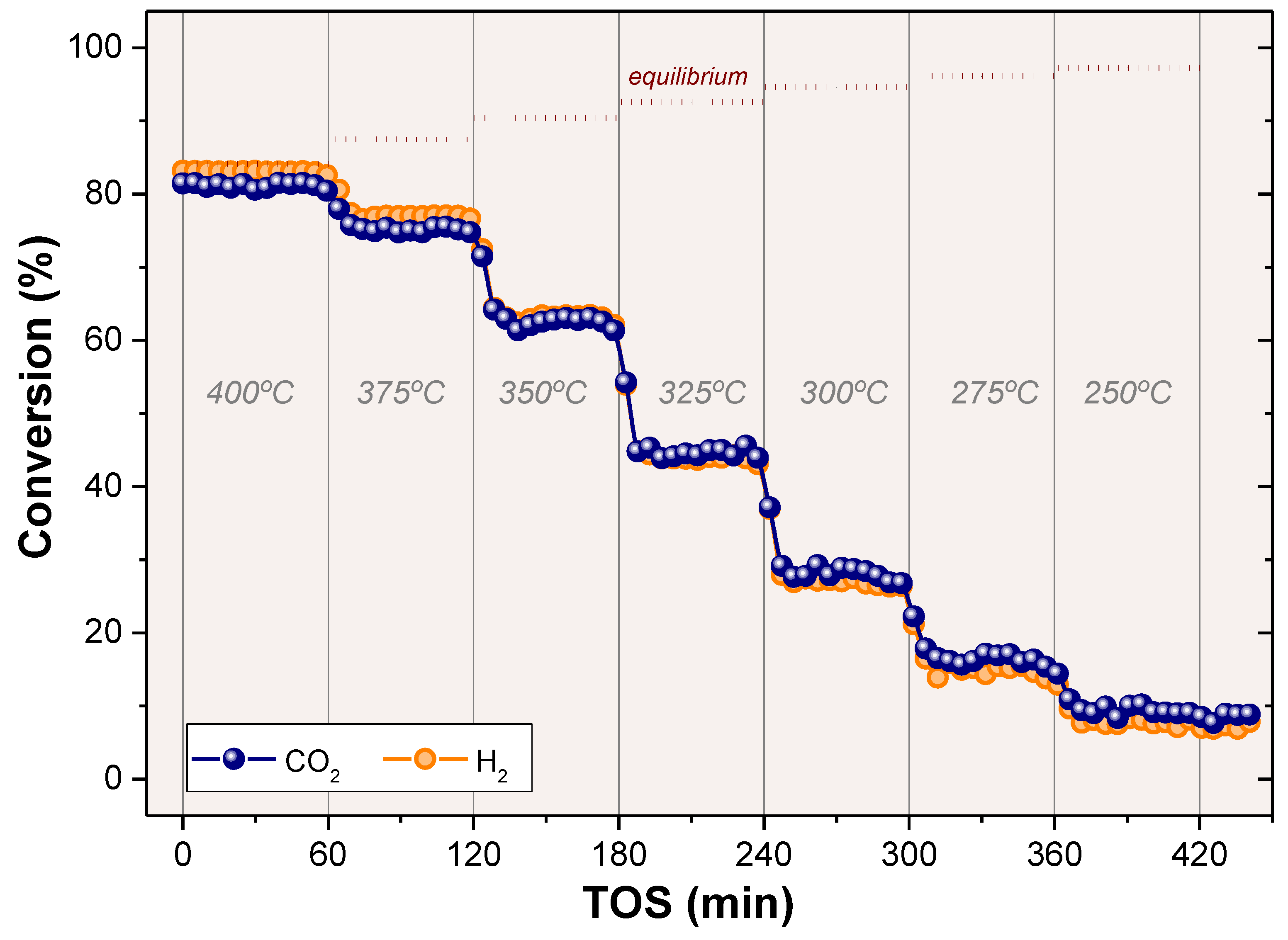
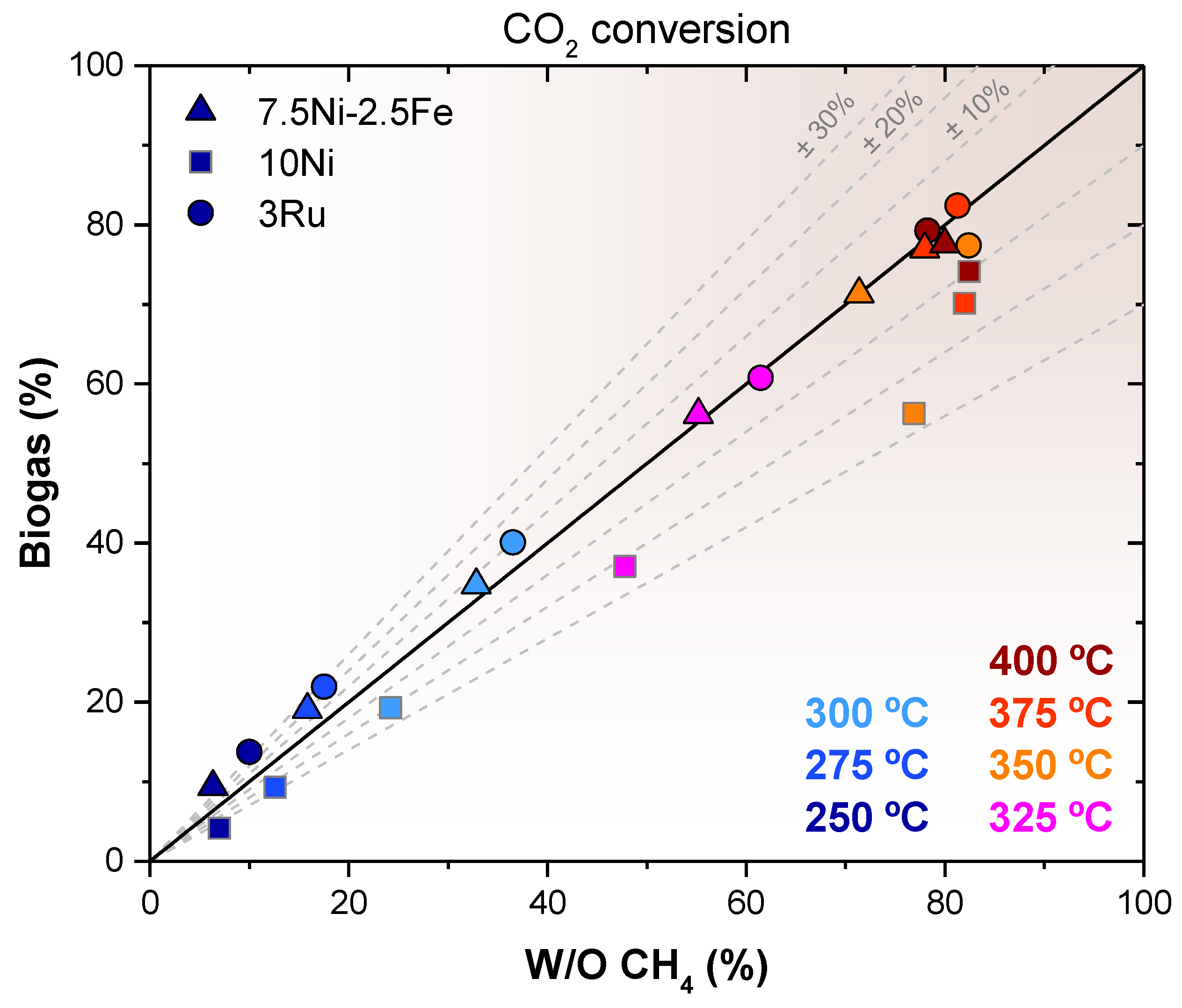
2.2. CO2 Methanation
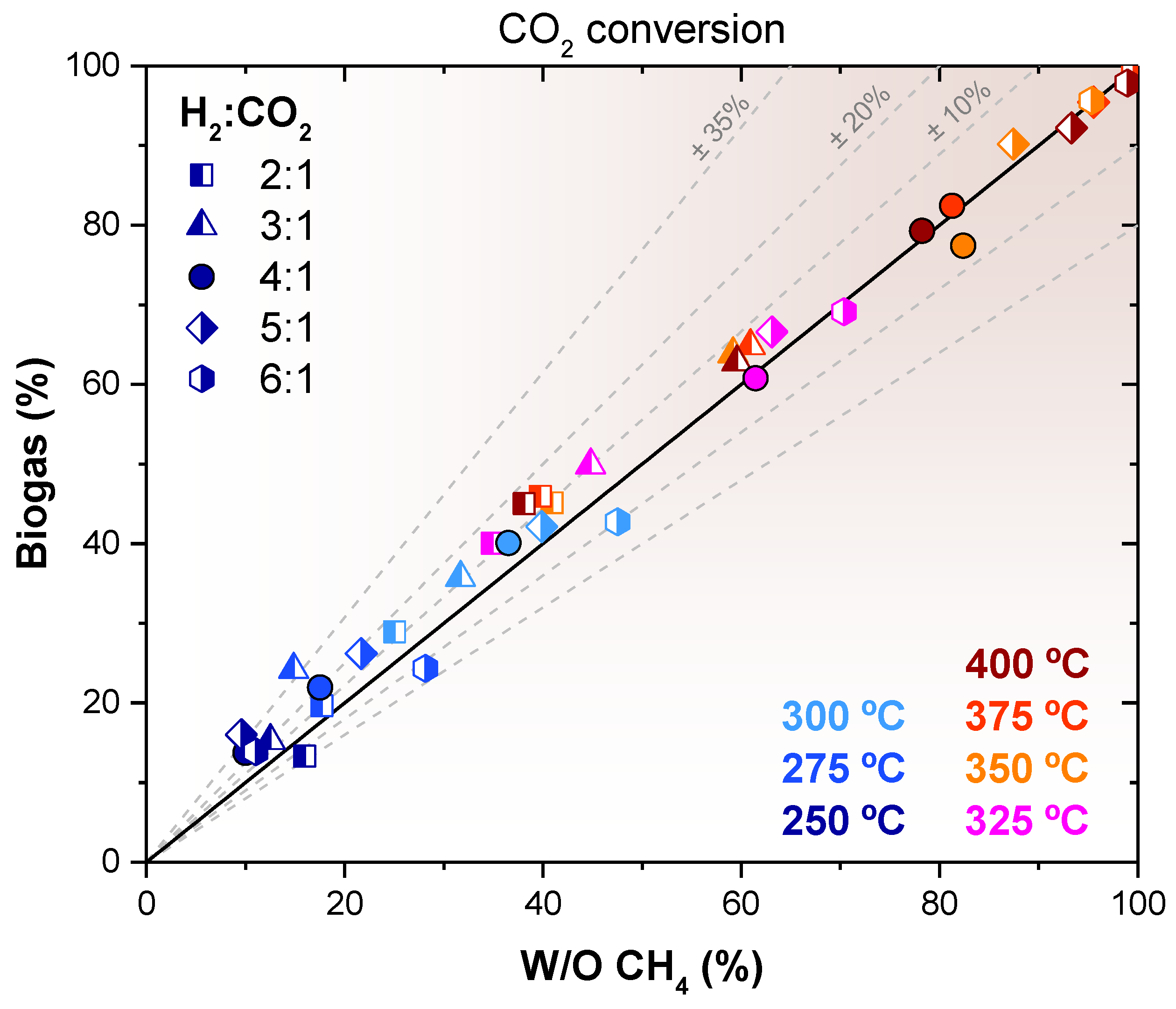
2.3. Biogas Methanation
3. Materials and Methods
3.1. Preparation and Characterization of Catalysts
3.2. Catalytic Activity Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The Paris Agreement. 2015. Available online: https://unfccc.int/process/conferences/pastconferences/paris-climate-change-conference-november-2015/paris-agreement (accessed on 10 March 2022).
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Conti, C.; Mancusi, M.L.; Sanna-Randaccio, F.; Sestini, R.; Verdolini, E. Transition towards a green economy in Europe: Innovation and knowledge integration in the renewable energy sector. Res. Policy 2018, 47, 1996–2009. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission: The European Green Deal; COM: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52019DC0640 (accessed on 10 March 2022).
- Korberg, A.D.; Skov, I.R.; Mathiesen, B.V. The role of biogas and biogas-derived fuels in a 100% renewable energy system in Denmark. Energy 2020, 199, 117426. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calbry-Muzyka, A.S.; Gantenbein, A.; Schneebeli, J.; Frei, A.; Knorpp, A.J.; Schildhauer, T.J.; Biollaz, S.M.A. Deep removal of sulfur and trace organic compounds from biogas to protect a catalytic methanation reactor. Chem. Eng. J. 2019, 360, 577–590. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent progress towards in-situ biogas upgrading technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, P.; Senderens, J.B. New methane synthesis. Compt. Rend. Acad. Sci. 1902, 134, 514–516. [Google Scholar]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Fazlollahi, F.; Nikoo, M.K.; Sefidkon, F.; Klemes, J.J.; Baxter, L.L. Mechanisms and kinetics of CO2 hydrogenation to value-added products: A detailed review on current status and future trends. Renew. Sustain. Energy Rev. 2017, 80, 1292–1311. [Google Scholar] [CrossRef]
- Zhuang, Y.; Simakov, D.S.A. Single-pass conversion of CO2/CH4 mixtures over the low-loading Ru/γ-Al2O3 for direct biogas upgrading into renewable natural gas. Energy Fuels 2021, 35, 10062–10074. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ 2012, 113–114, 237–249. [Google Scholar] [CrossRef]
- Martins, J.; Batail, N.; Silva, S.; Rafik-Clemnent, S.; Karelovic, A.; Debecker, D.P.; Chaumonnot, A.; Uzio, D. CO2 hydrogenation with shape-controlled Pd nanoparticles embedded in mesoporous silica: Elucidating stability and selectivity issues. Catal. Commun. 2015, 58, 11–15. [Google Scholar] [CrossRef]
- Yu, K.P.; Yu, W.Y.; Kuo, M.C.; Liou, Y.C.; Chien, S.H. Pt/titania-nanotube: A potential catalyst for CO2 adsorption and hydrogenation. Appl. Catal. B Environ. 2008, 84, 112–118. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalyst. Appl.Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Guo, X.; Traitagwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon dioxide methanation over nickel-based catalysts supported on various mesoporous material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Zhang, L.; Gao, J.; Shao, Y.; Dong, D.; Zhang, S.; Liu, Q.; Xu, L.; Hu, X. Impacts of alkali or alkaline earth metals addition on reaction intermediates formed in methanation of CO2 over cobalt catalysts. J. Energy Inst. 2020, 93, 1581–1596. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Parametric study of CO2 methanation for Synthetic Natural Gas production. Energy Technol. 2019, 7, 1900795. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.H.; Nomanbhay, S.; Shamsuddin, A.; Park, Y.-K.; Hernándes-Cocoletzi, H.; Show, P.L. Current developments in catalytic methnation of carbon dioxide-A review. Front. Energy Res. 2022, 9, 795423. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Gao, J.; Li, H.; Xu, G.; Su, F. Coking-resistant Ni-ZrO2/Al2O3 catalyst for CO methanation. J. Energy Chem. 2014, 23, 761–770. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.D. Potential of an alumina-supported Ni3Fe catalyst in the methanation of CO2: Impact of alloy formation on activity and stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Tsitsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-based catalysts for CO2 methanation: A review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Brooks, K.P.; Holladay, J.D.; Howe, D.T.; Simon, T.M. Catalyst development for microchannel reactors for martian in situ propellant production. Catal. Today 2007, 125, 103–110. [Google Scholar] [CrossRef]
- Burger, T.; Koschany, F.; Thomys, O.; Köhler, K.; Hinrichsen, O. CO2 methanation over Fe- and Mn-promoted co-precipitated Ni-Al catalysts: Synthesis, characterization and catalysis study. Appl. Catal. A Gen. 2018, 558, 44–54. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Carbon dioxide methanation over Ni-M/Al2O3 (M: Fe, Co, Zr, La and Cu) catalysts synthesized using the one-pot sol-gel synthesis method. Int. J. Hydrogen Energy 2018, 43, 16522–16533. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Abate, S.; Chen, S.; Sierra, A.F.; Perathoner, S.; Krebs, F.; Palkovits, R.; Centi, G. Enhanced catalytic activity of iron-promoted nickel on γ-Al2O3 nanosheets for carbon dioxide methanation. Energy Technol. 2018, 6, 1196–1207. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Wang, X.; Jin, R.; Yan, W.; Li, H.; Wang, Z.-j. An Al2O3-supported NiFe bimetallic catalyst derived from hydrotalcite precursors for efficient CO2 methanation. Catal. Today. 2022, 402, 38–44. [Google Scholar] [CrossRef]
- US EPA. An Overview of Renewable Natural Gas from Biogas; EPA 456-R-20-001: Washington, DC, USA, 2020. Available online: https://www.epa.gov/sites/default/files/2020-07/documents/lmop_rng_document.pdf (accessed on 10 March 2022).
- ISO 13686; Natural Gas–Quality Designation. International Organization for Standardization: Geneva, Switzerland, 2013.
- Gutiérrez-Martín, F.; Rodríguez-Antón, L.M.; Legrand, M. Renewable power-to-gas by direct catalytic methanation of biogas. Renew. Energy 2020, 162, 948–959. [Google Scholar] [CrossRef]
- Canu, P.; Pagin, M. Biogas upgrading by 2-steps methanation of its CO2-thermodynamics analysis. J. CO2 Util. 2022, 63, 102123. [Google Scholar] [CrossRef]
- Boggula, R.R.; Fischer, D.; Casaretto, R.; Born, J. Methanation potential: Suitable catalyst and optimized process conditions for upgrading biogas to reach gas grid requirements. Biomass Bioenergy 2020, 133, 105447–105454. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 methanation of biogas over 20 wt% Ni-Mg-Al catalyst: On the effect of N2, CH4, and O2 on CO2 conversion rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Patel, V.; Le Saché, E.; Reina, T.R. CO2 methanation in the presence of methane: Catalysts design and effect of methane concentration in the reaction mixture. J. Energy Inst. 2020, 93, 415–424. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Slowik, G.; Pennemann, H.; Neuberg, S.; Zapf, R.; Kolb, G. Direct conversion of carbon dioxide to methane over ceria- and alumina-supported nickel catalysts for biogas valorization. Chempluschem 2021, 86, 889–903. [Google Scholar] [CrossRef]
- Nieß, S.; Armbruster, U.; Dietrich, S.; Klemm, M. Recent advances in catalysis for methanation of CO2 from biogas. Catalysts. 2022, 12, 374. [Google Scholar] [CrossRef]
- Gaikwad, R.; Villadsen, S.N.B.; Rasmussen, J.P.; Grumsen, F.B.; Nielsen, L.P.; Gildert, G.; Møller, P.; Fosbøl, F.L. Container-sized CO2 to methane: Design, construction and catalytic tests using raw biogas to biomethane. Catalysts 2020, 10, 1428. [Google Scholar] [CrossRef]
- Aragüés-Aldea, P.; Sanz-Martínez, A.; Durán, P.; Francés, E.; Peña, J.A.; Herguido, J. Improving CO2 methanation performance by distributed feeding in a Ni-Mn catalyst fixed bed reactor. Fuel 2022, 321, 124075. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 formation on Ni/Al2O3 stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Y.; Zhang, X.; Zhang, Q.; Wang, T.; Ma, L. Effect of Ru particle size on hydrogenation/decarbonylation of propanoic acid over supported Ru catalysts in aqueous phase. Catal. Lett. 2017, 147, 29–38. [Google Scholar] [CrossRef]
- Burger, T.; Augenstein, H.M.S.; Hnyk, F.; Döblinger, M.; Köhler, K.; Hinrichsen, O. Targeted Fe-doping of Ni-Al catalysts via the surface redox reaction technique for unravelling its promoter effect in the CO2 methanation reaction. ChemCatChem 2020, 12, 649–662. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2020, 265, 118538. [Google Scholar] [CrossRef]
- Durán, P.; Esteban, I.; Francés, E.; Herguido, J.; Peña, J.A. CO2 methanation using Ru based catalysts for storage and distribution of energy: Activation of catalyst strategies. In Proceedings of the European Hydrogen Energy Conference (EHEC), Malaga, Spain, 14–16 March 2018; pp. 243–244. [Google Scholar]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/ Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]


| Solid | BET Area (m2·g−1) | XRF (wt%) | ||
|---|---|---|---|---|
| Ni | Ru | Fe | ||
| Al2O3 | 200.6 ± 0.4 | - | - | - |
| 10Ni | 174.5 ± 0.3 | 10.3 ± 0.1 | - | - |
| 3Ru | 190.2 ± 0.6 | - | 3.7 ± 0.1 | - |
| 7.5Ni–2.5Fe | 167.4 ± 0.4 | 7.4 ± 0.1 | - | 2.1 ± 0.1 |
| Variable (Units) | Base Value | Studied Range |
|---|---|---|
| Catalyst (-) | 3Ru | 10Ni, 3Ru, 7.5Ni–2.5Fe |
| T (°C) | - | 400–250 * |
| Wcat (g)/Winert (g) | 0.5/2.0 | - |
| q0 (STPmL·min−1) | 250 | - |
| WHSV (STPmL·gcat−1·h−1) | 30,000 | - |
| H2:CO2 molar ratio (-) | 4:1 | 2:1, 3:1, 4:1, 5:1, 6:1 |
| CH4:CO2 molar ratio ** (-) | 7:3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Martínez, A.; Durán, P.; Mercader, V.D.; Francés, E.; Peña, J.Á.; Herguido, J. Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts. Catalysts 2022, 12, 1609. https://doi.org/10.3390/catal12121609
Sanz-Martínez A, Durán P, Mercader VD, Francés E, Peña JÁ, Herguido J. Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts. Catalysts. 2022; 12(12):1609. https://doi.org/10.3390/catal12121609
Chicago/Turabian StyleSanz-Martínez, Andrés, Paul Durán, Víctor D. Mercader, Eva Francés, José Ángel Peña, and Javier Herguido. 2022. "Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts" Catalysts 12, no. 12: 1609. https://doi.org/10.3390/catal12121609
APA StyleSanz-Martínez, A., Durán, P., Mercader, V. D., Francés, E., Peña, J. Á., & Herguido, J. (2022). Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts. Catalysts, 12(12), 1609. https://doi.org/10.3390/catal12121609