Abstract
In recent years, carbon dioxide hydrogenation leading to synthetic fuels and value-added molecules has been proposed as a promising technology for stabilizing anthropogenic greenhouse gas emissions. Methanation or Sabatier are possible reactions to valorize the CO2. In the present work, thermal CO2 methanation and non-thermal plasma (NTP)-assisted CO2 methanation was performed over 15Ni/CeO2 promoted with 1 and 5 wt% of cobalt. The promotion effect of cobalt is proven both for plasma and thermal reaction and can mostly be linked with the basic properties of the materials.
1. Introduction
Greenhouse gases such as CO2 are responsible for climate change and global warming. Thus, nowadays their control and utilization are an important subject from a scientific and economic point of view. CO2 emissions produced from an energy system based on fossil fuels are the main reason for climate change [1]. To overcome this problem and decrease these emissions in the atmosphere, several alternatives have been analyzed. Carbon capture and utilization (CCU) or storage (CCS) are among the solutions which took attention in the last decade for this aim. These processes will help to either turn CO2 emissions into valuable chemicals or make a closed cycle of carbon in industries (e.g., cement) where synthetic natural gas produced from their CO2 emissions through Sabatier reaction could be re-used for combustion processes [2]. Among the CO2 valorization strategies, CO2-to-methanol [3,4], Reverse Water Gas Shift (RWGS) reaction [5] and CO2 methanation [6] are the most studied. Indeed, methanolation and methanation are mature solutions already industrially implemented [7,8].
Regarding CO2 methanation, different types of metal-supported catalysts have been reported, with Ni, Ru, Co, Fe, Rh or Pd as the main active metals [9,10,11,12,13,14]. Among all, Ni-based catalysts are the most used due to their high activity, their availability, and their lower cost [15]. However, their catalytic activity depends on different factors, such as Ni loading, the type of support or the preparation method [16]. In terms of supports nature, alumina, silica, double layered mixed oxides, zeolites, ceria, or ceria-zirconia have been studied [6,11,14,17,18]. Among them, ceria (CeO2) can be considered as a promising support because of its properties such as oxygen mobility, which enhances CO2 activation and hinders carbon deposition [19,20].
Furthermore, the use of promoters such as Y, Mn, La, Cu or Co was reported as favorable for improving catalysts’ activity and stability [17,21,22,23,24]. Among them, cobalt incorporation has not been widely explored so far. Indeed, on bimetallic Co-Ni/Al2O3 catalysts, Liu et al. [25] showed that catalytic properties were enhanced for CO2 methanation by the formation of Co-Ni alloy, leading to a higher surface area and better Ni0 dispersion. Moreover, Alrafei et al. [16] also showed that for Co-Ni/Al2O3 catalytic systems, that the presence of Co improved Ni0 dispersion and Ni species reducibility. Furthermore, Summa et al. [17] concluded that the addition of cobalt in low amounts (0.5–1 wt%) resulted in an optimum improvement of the surface properties, such as basicity and hydrogen uptake, which increased the catalytic performance for CO2 methanation.
Besides the improvement of CO2 methanation catalysts formulation, plasma assisted catalysis has been developed for more than 10 years for improving this process [24,26,27]. Among the types of plasma reported, the dielectric-barrier discharge (DBD) is the most commonly used for CO2 methanation [24,25,26,27,28]. Moreover, as for thermal methanation, Ni based catalysts are the most used for methanation under plasma-assisted catalysis conditions [24,25,26,27,28]. In terms of used catalysts, different supports such as titania [29], ceria [19], zirconia [30], ceria-zirconia [31,32,33,34], alumina [35,36], zeolites [6,37,38], metal–organic frameworks [35] or mixed-oxides derived from hydrotalcites [18] have been reported. In terms of promoters, only cerium [6,7,8,9,10,11,12,13,14,15,16,17,18] and lanthanum [37] appeared to promote Ni-based catalysts performances for the DBD plasma methanation process over different supports. Accordingly, Chen et al. [37] demonstrated, compared to a non-promoted catalyst, that the addition of La resulted in an improvement of the turnover frequency and selectivity towards CH4. More recently, it was shown on DBD plasma using ceria-zirconia-based Ni catalysts that the promotion of elements such Cu, Co, Mn, La, Y, Gd and Sr can considerably alter both the physicochemical and the electrical features of the catalysts, resulting in different plasma-catalytic performance. Indeed, authors reported a real improvement with the studied elements except for Cu and Sr [39].
Furthermore, to our knowledge, so far, no studies have dealt with Co-promoted Ni catalysts supported on CeO2 for conventional thermal neither for plasma-assisted CO2 methanation. Thus, in this study, the activity and stability of Co-Ni/CeO2 using 2 Co loadings were investigated for CO2 methanation and a correlation between structure and activity was proposed.
2. Results and Discussion
2.1. Characterization Results
TPR-H2 profiles are presented in Figure 1. It is worth mentioning that 2 peaks were observed at 330–350 °C and 465–510 °C for Ni-containing catalysts, which could be attributed, based on literature, to the support reduction, more precisely to surface oxygen species in CeO2 and CeO2 bulk reduction, respectively [19].
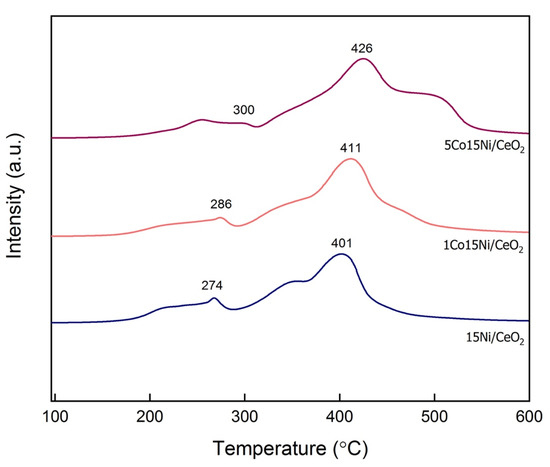
Figure 1.
H2-TPR profiles for calcined samples with different Co content.
One can also note that, with the introduction of both Ni and Co, CeO2, peaks shift towards lower temperatures. Moreover, for all the studied Ni catalysts, a well-defined peak is found at 200–300 °C, which shifts towards higher temperatures with increasing Co loadings. This peak is generally attributed to easily reducible NiO species [19]. Finally, a significant reduction peak is observed at ~500 °C on 5Co15Ni/CeO2 catalyst, which could be attributed to the reduction of CoOx or Co cationic species [40]. As reported in Table 1, the peaks of reduction identified in this study are comparable to those found in previous works. In addition, total H2 consumptions for the catalysts from this work are also reported in Table 2, with increasing Co loadings leading to higher values.

Table 1.
Comparison of reduction peaks for similar catalysts with different contents with present study.

Table 2.
Comparison of basicity of the samples in present study.
CO2-TPD profiles of the studied samples are shown in Figure 2. The profile of the reference sample (15Ni/CeO2) presents three main peaks at 138 °C, 206 °C and 400 °C, which can be ascribed to weak, medium, and strong basic sites, respectively. However, in the presence of Co only two peaks can be observed. The total basicity and the weak, medium, and strong basicity repartition are reported in Table 2.
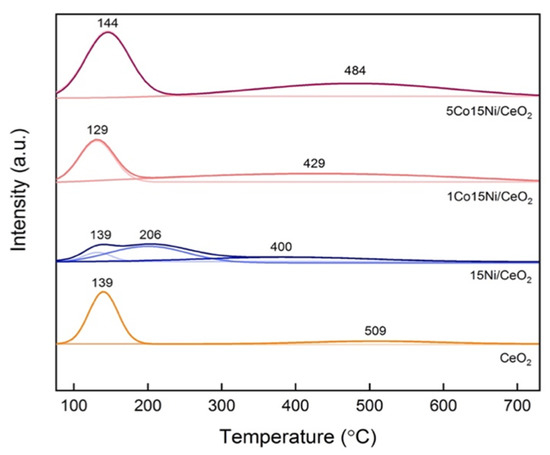
Figure 2.
CO2-TPD profiles for reduced catalysts with different Co content.
It is worth mentioning that Co addition led to a decrease in the total basicity, while the number of medium-strength basic sites increased in presence of 1 wt% Co and later decreased with 5 wt% Co. This might indicate that the addition of excess Co may cover some basic sites, resulting in an adverse effect on the CO2 chemisorption [25]. Additionally, it is observed that desorption peak temperatures shift towards higher values with the increase in Co content, suggesting a stronger interaction between CO2 and the active sites [17]. When compared with the literature, a Ni-Co catalyst supported over an hydrotalcite (HT1Co20Ni) [17] presented lower medium-strength basic sites compared to the 1Co15Ni/CeO2 catalyst of this study. As the medium basic sites are the most relevant for CO2 methanation [41], it can be concluded that, among the studied catalysts, 1Co15Ni/CeO2 presents the best basic properties.
In terms of textural properties (Table 3), assessed by N2 adsorption, the incorporation of metals over ceria support led to a reduction of the total pore volume (VP) and the BET surface area (SBET). When comparing the samples with 1 and 5 wt% Co, no significant differences in terms of textural properties were found, suggesting that the variation of Co loading did not induce a remarkable effect on these parameters.

Table 3.
Porous volume and external surface area for studied catalysts.
In addition, XRD diffractograms of calcined and reduced catalysts are presented in Figure 3. The presence of CeO2 diffraction lines (28.6, 33.1, 47.5, 56.4, 59.1, 68.1, 76.8 and 79.1°; JCPDS card 81-0792 [19,42]) is confirmed in all of the catalysts after calcination and reduction, indicating that Ce species nature was not affected by the reduction process. Regarding Ni species, NiO diffraction lines were clearly identified in calcined Ni and Co-Ni catalysts at 37, 43.2 and 62.9° (JCPDS card 78-0643) [19], while Ni0 phases were present after reduction (44.7 and 51.8°) [19]. We note that no Co3O4 diffraction lines (JCPDS card 78-1970) were identified in Co-containing catalysts. This could be due to the low metal loading used (1–5 wt%) or the presence of highly dispersed Co oxy-species on the catalysts. Based on CeO2 diffraction lines, an average crystallite size of 5 nm was determined (Table 4). Regarding NiO crystallite sizes, results are shown in Table 5 and suggest that Co incorporation leads to the formation of larger NiO crystallites after calcination (increase of 5 nm when comparing to 15Ni/CeO2). Furthermore, for reduced catalysts, the incorporation of 5 wt% Co led to the formation of smaller Ni0 crystallites (32 nm, lower than the 38–39 nm obtained for 15Ni/CeO2 and 1Co15Ni/CeO2 reduced catalysts; Table 4), indicating that Co could have an efficient effect in the prevention of agglomeration processes in Ni0 particles. Furthermore, when compared to other studies, it is worth mentioning that Benrabbah et al. [43] reported values of ~28 nm after reduction for 15Ni/CeZrO2. The higher values obtained in this work could be due to the preparation method, as these authors used a heating rate for calcination of 5 °C/min while in this study this step was carried out by 10 °C/min.
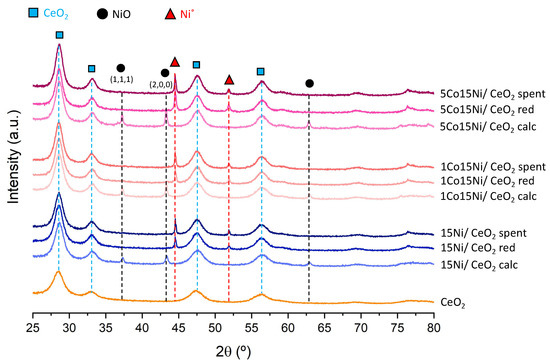
Figure 3.
X-ray diffractograms of the studied catalysts.

Table 4.
NiO and Ni0 crystallite sizes after calcination, reduction and reaction determined applying Scherrer equation.

Table 5.
Comparison between Ni-Co catalysts with different supports (conversion and selectivity are all considered at 300 °C).
TEM was then used to analyze the Ni0 dispersion on the support. Figure 4 shows TEM micrographs of the catalysts from this work with different resolutions. Due to the low contrast of the support, it was difficult to conclude on the distribution of Ni0 particles, so that EDS-TEM technique was also carried out (Figure 5). Based on the obtained results, it could be concluded that cobalt incorporation improves the dispersion of both Ni and Co species. However, the calculation the particle size distribution was not possible due to the low contrast between the metals and the support.
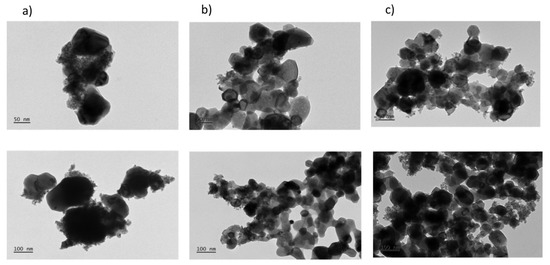
Figure 4.
TEM images of (a) 15Ni/CeO2; (b) 1Co15Ni/CeO2; (c) 5Co15Ni/CeO2 with different scales after reduction.
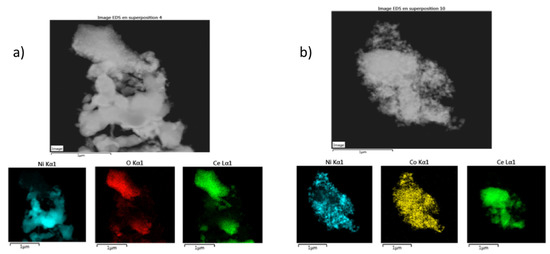
Figure 5.
EDS-TEM images of (a) Ni/CeO2; (b) Co-Ni/CeO2 after reduction.
2.2. Catalytic Results
Figure 6a reports the CO2 conversion as a function of temperature for the studied catalysts after a pre-reduction at 500 °C. One can note that, at low temperature (250 °C), the 15Ni/CeO2 catalyst shows the lower activity with a conversion of CO2 of 16%. However, 1Co15Ni/CeO2 and 5Co15Ni/CeO2 catalysts presents a significantly high conversion of around 74% and 62%, respectively, at the same temperature. By increasing the temperature, the conversion for 15Ni/CeO2 reaches 80%. On the other hand, the promoted catalysts show higher conversions, up to 85%.
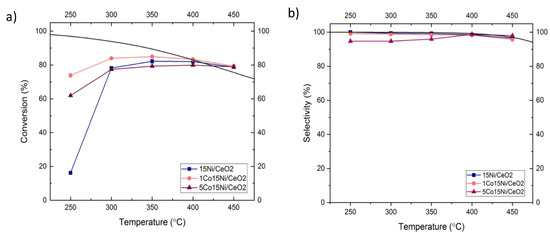
Figure 6.
(a) Conversion and (b) selectivity of Co-Ni/CeO2 catalyst in thermal CO2 methanation with different Co loadings.
Moreover, it is worth noting that the highest conversion was exhibited at 350 °C by 1Co15Ni/CeO2. Figure 6b reports CH4 selectivity of all of the samples. For 15Ni/CeO2 and 1Co15Ni/CeO2 catalysts, the selectivity is close to 100% while for 5Co15Ni/CeO2 the selectivity is around 94% at 250 °C and then with the increase in the temperature (350 °C) it reaches 100%. According to the literature, at a high temperature, reverse water gas shift starts to simultaneously occur with methanation. Then, methanation of CO begins, resulting in a decrease in CH4 selectivity [5]. Additionally, it could be expected that the catalyst with the higher H2 consumption has higher activity as H2 chemisorption plays an important role in CO2 methanation [25]. However, CO2 conversion is much higher for 1Co15Ni/CeO2 catalyst. Furthermore, there is another factor which has a remarkable impact. According to the result of number of basic sites, with the increase in Co loading the number of basic sites decreases, suggesting the covering of some sites by Co species, as previously discussed [25]. On the other hand, the promotion of 15Ni/CeO2 catalyst with 1% Co enhanced the number of medium strength sites, which present a more beneficial effect in the reaction [41], which could explain the more favorable performances exhibited by 1Co15Ni/CeO2 catalyst.
After the tests, catalysts were analyzed by XRD (Figure 3), being only Ni0 diffraction lines and no NiO phases observed. This indicates that no reoxidation of Ni0 species occurred during the experiments. Regarding Ni0 crystallite sizes before and after the reaction (Table 4), the differences (1–2 nm) are negligible for 1 and 5 wt% Co-containing catalysts, which means that no remarkable sintering processes occurred. On the contrary, for 15Ni/CeO2 catalyst, an increase in the Ni0 crystallite size from 38 nm to 45 nm before and after reaction, respectively, was observed. This indicates that Co presents a positive effect in the prevention of Ni0 sintering processes. When comparing to literature for monometallic Ni catalysts, Mikhail et al. [45] studied 15Ni/CeZrO2 catalysts and found out that Ni0 size increased from ~24 to ~46 nm after reaction, which was attributed to the occurrence of sintering. In other studies, regarding Ni-based zeolite catalysts from Guo et al. [46], an increase in Ni0 particle size from ~14 to ~29 nm was observed for 10Ni/ZSM-5 before and after reaction, being this again attributed to sintering effects. Consequently, the increase in the Ni0 crystallite size verified for 15Ni/CeO2 catalyst is in accordance with the results in the literature.
Spent samples were also analyzed by TGA (Figure 7). As observed, a mass variation process was observed below 400 °C in the catalysts, corresponding to the loss of adsorbed water in the samples. On the other hand, at higher temperatures (>600 °C), where coke should be decomposed, the mass loss is negligible, indicating that no carbonaceous species were deposited in the catalysts during the tests [47].
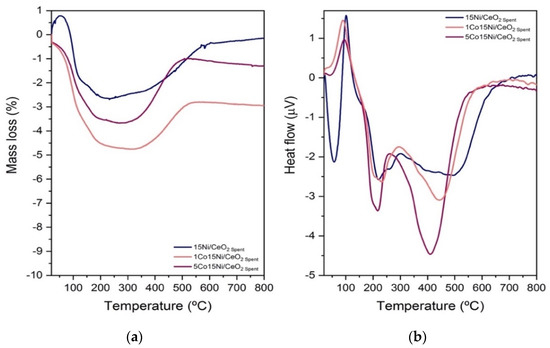
Figure 7.
TGA analysis with O2 for (a) Mass loss and (b) Heat loss of Co-Ni/CeO2 catalyst with different Co content.
Furthermore, stability tests were performed for the studied samples, being the results presented in Figure 8. Tests were carried out at 250 °C for 5 h with the same conditions used in the tests performed at variable temperatures. It can be observed that in the first 20 min all samples lose some activity (<9%). However, afterwards, all of the catalysts became stable. Moreover, the stability tests showed that 1Co15Ni/CeO2 catalyst presented the higher activity.
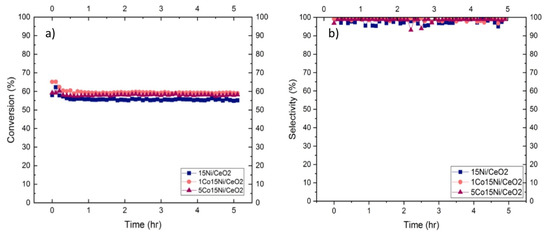
Figure 8.
Stability tests: (a) CO2 Conversion and (b) CH4 Selectivity of Co-Ni/CeO2 catalysts for 5 h run at 250 °C.
Table 5 presents the results obtained for Co-Ni samples with different supports under CO2 methanation conditions at 300 °C for different catalysts. When comparing 5Co15Ni/CeO2 catalyst to 5Co15Ni/Al2O3 [16], which has the same loadings for the active metal and promoter, it can be seen that the conversion is higher by 5%, while GHSV for the catalyst of this study is much higher than that used for the alumina support. This indicates that 5Co15Ni/CeO2 catalyst presents a higher efficiency.
Moreover, for the 1Co15Ni/CeO2 catalyst, when comparing to HT1Co20Ni [17], higher conversion with much higher GHSV can be observed. It is worth mentioning that when comparing the basicity of these two catalysts, the number of basic sites (especially medium ones) for the 1 wt% Co catalyst of this study is higher, which is in a good agreement with the reported effect of the catalyst’s basicity on the performances [41].
2.3. Plasma Assisted Catalytic Methanation
Figure 9a–c report the CO2 conversion as a function of voltage applied in keeping the frequency constant and equal to 12.3 kHz. We note that for all the studied catalysts that the conversion of CO2 and the methane selectivity increased with the increase in the voltage; similarly to the thermal catalysis, as reported in the Figure 9a. the 15Ni/CeO2 catalyst showed the lower activity with a conversion of CO2 of 16% at low voltage. However, for higher voltage the conversion of CO2 for 15Ni/CeO2 catalyst is higher than the one obtained for 5Co15Ni/CeO2 one.
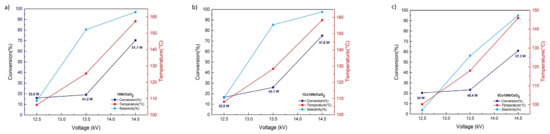
Figure 9.
CO2 conversion, Temperature measured and CH4 selectivity of (a) 15Ni/CeO2, (b) 1Co15Ni/CeO2, (c) 5Co15Ni/CeO2, catalysts as function of voltage.
Furthermore, for all the range of studied voltage from 25 to 29 kV peak to peak which corresponds to 12.5 kV to 14.5 kV peak voltage in Figure 9, the 1Co15Ni/CeO2 catalyst exhibited higher conversion (Figure 9b). This conversion is of course increase with the voltage. Finally, the higher conversion was found to be 75% at 29 kV. The conversion is also linked with the temperature measured for the different operating conditions. Indeed, the higher temperature was observed for the higher obtained conversion. It was also already reported on promoted Ni/CeZrOx catalysts [39] that depending on the type of the promoter, a modification in the basicity distribution could be observed. Thus, the investigation of basic properties showed that the addition of the promoters (Gd, Y and Co) not only led to an increase in the total number of basic sites of the catalyst, but also the number of moderate basic sites which are reported to be the predominant ones for methanation reaction. In our study, among the Co promoted materials the 1Co15Ni/CeO2 catalyst exhibited both the higher number of basic sites but also the higher number of medium basic ones. Thus, in agreement with thermal methanation, the basic sites presented on our materials play an important role both in thermal and plasma assisted methanation reaction on Ni-Co/CeO2 catalysts. On the other hand, the synergistic effects are also an important factor regarding different behavior of catalyst. Furthermore, the plasma can lower the activation barrier which leads to a faster reaction occurrence. Finally, the plasma can also change the surface reaction pathway with the presence of excited species which results in a change in the transition state, as proposed elsewhere [24].
3. Materials and Methods
3.1. Catalysts Synthesis
Ni/CeO2 and Co-Ni/CeO2 catalysts with different cobalt loadings were prepared by wet impregnation method. For this purpose, a certain amount of Ni nitrate hexahydrate (Ni(NO3)2·6H2O) was dissolved in distilled water. Following this, a certain amount of commercial cerium oxide (CeO2) and Co nitrate hexahydrate (Co(NO3)2·6H2O) added to this solution. Then, the mentioned solution was stirred at 50 °C for 4 h and then dried at 100 °C overnight. Subsequently, the samples were calcined at 500 °C for 4 h in static air. Table 6 reports the studied catalysts and summarize the nominal metal loadings.

Table 6.
Studied catalysts.
3.2. Characterization Methods
Catalysts were characterized by temperature-programmed reduction with hydrogen (TPR-H2), temperature-programmed desorption of CO2 (CO2-TPD), N2 adsorption, X-ray diffraction (XRD), transmission electron microscopy (TEM) and thermogravimetric analysis (TGA).
TPR-H2 experiments were carried out on a BELCAT-M equipped with a thermal conductivity detector (TCD) to characterize catalysts’ reducibility (BEL Japan, Inc., Osaka, Japan). For this purpose, 60 mg of calcined sample were first degassed in helium atmosphere at 100 °C for 130 min and then reduced in 5% H2/Ar mixture with a heating rate of 10 °C/min starting from 100 °C to 700 °C. TPD-CO2 was performed after TPR-H2 run, using the same device. CO2 was adsorbed at 80 °C for 1 h from a mixture of 10% CO2/He. Then, helium flow was applied for 15 min in order to desorb weakly adsorbed CO2. Finally, the materials were heated from 80 °C to 800 °C in helium to analyze their basic properties based on the desorption temperature. TPD profiles were deconvoluted into three Gaussian peaks corresponding to weak, medium, and strong basic sites in agreement with literature [25].
N2 adsorption was carried out for reduced catalysts on an Autosorb iQ equipment (Quantachrome, Odelzhausen, Germany) at −196 °C. Catalysts (80–100 mg) were degassed under vacuum prior to the experiments at 90 °C (1 h) and 350 °C (4 h). Total pore volumes (VP) were measured at a relative pressure (p/p0) of 0.97 while surface areas (SBET) were obtained by BET method.
XRD patterns were obtained from a Bruker AXS Advance D8 diffractometer combined with a 1D detector (SSD 160), using a Ni filter (Bruxer, Oeiras, Portugal). The scanning range was from 5 to 80° (2θ). The step time and step size were 0.5 s and 0.03°, respectively. CeO2, NiO and Ni0 average crystallite sizes were calculated in applying the Scherrer equation [47]. For this purpose, the considered diffraction lines were: 28.6, 33.1, 47.5 and 56.4° for CeO2; 37.3° and 43.3° for NiO; and 44.5 and 52.1° for Ni0.
High-Resolution TEM was performed using an JEOL JEM-2010 equipment with EDS to characterize the deposited carbon and to obtain a precise analysis of the planes registered at the nanometer scale (JEOL, Gmbh, Freising, Germany). The specimens were prepared by dropwise addition of a colloidal solution in ethanol onto a copper grid covered with amorphous carbon film [47].
Finally, TGA analysis were carried out using a Setsys Evolution TGA (Setaram instruments, SPECANALITICA LDA, Cracavelos, Portugal). Experiments were carried out for spent catalysts using a mass of 20 mg and heating the samples from 20 to 800 °C (heating rate = 10 °C/min) under air flow [47].
3.3. Catalytic Runs
3.3.1. Thermal Experiments
CO2 methanation reaction was conducted in a tubular fixed-bed glass U-type reactor (8 mm inner diameter) at atmospheric pressure with a K-type thermocouple inserted in the catalyst bed. Before reaction, catalysts (15mg) were reduced in situ at 500 °C for 1 h with a flow of 5%H2/Ar. Afterwards, a reaction was performed within the temperature range of 250–450 °C, with each temperature being kept for 30 min to reach steady-state operating conditions. The heating ramp between each step was 10 °C/min. An inlet flow of 100 mL/min with the composition of CO2/H2/Ar = 1.5/6/2.5 was used, corresponding to a GHSV of 52,000 h−1. An online micro-chromatograph (Varian GC4900) equipped with a TCD was used to analyze the reaction products. Additionally, stability tests were performed at 250 °C for 5 h, using the same operating conditions of the reaction.
3.3.2. Plasma-Assisted Experiments
Plasma catalytic tests were also carried out. Prior to the experiments, catalysts were reduced ex-situ in the presence of 100 mL/min 5%H2/Ar at 500 °C for 1 h. Subsequently, 15 mg of each catalyst was loaded in the DBD plasma reactor, being a cleaning step performed by flowing hydrogen on the surface for 10 min at 12.3 kHz and 21 kV. The same GHSV was kept for the tests, to allow a proper comparison with the results from the thermal experiments. Quartz wool was placed on both sides of the catalyst to fix it, by preventing its mobility. In this study, the feed gas entering the reactor was a H2/CO2 = 4/1 mixture.
In the plasma-catalytic hybrid process, the energy was supplied by a high voltage 30 kHz (MiniPuls 6) and voltages applied ranged from 21 kV to 29 kV (peak to peak). The applied voltage was measured by a digital picoscope (series 3000, PicoTechnology) with a probe (ELDITEST GE 3830). A capacitor (3.32 nF) was inserted between the reactor and the grounded electrode to measure the power provided to the plasma reactor by Q-V Lissajous method [48]. The temperature was measured by a temperature sensor (Pt 100) placed on the outer surface of the quartz tube, in the middle of the ground electrode. The outlet flow was analyzed by a gas chromatograph (Agilent MicroGC 490) equipped with a thermal conductivity detector (TCD). The flowrate was measured with a bubble flowmeter.
For both thermal and plasma conditions, CO2 conversion () and CH4 selectivity () were calculated by following Equations (1) and (2), respectively.
which Fi is the molar flow of each component calculated from the total flowrate and the concentration of gases.
4. Conclusions
Ni-based catalysts are proper materials for CO2 methanation. Adding Co as a promoter significantly increased the catalytic performances, such as conversion and methane selectivity both in thermal catalytic system and DBD plasma-catalytic one. The increase in such activity could be explained by the increase in the number of medium basic sites, which are well known to be a key factor for CO2 methanation. The 1Co15Ni/CeO2 was the catalyst which presented the higher number of medium basic sites and showed the best performances both in plasma and non-plasma methanation reaction. A further investigation on electric parameters of the materials will be carried out in order to determine their importance in the plasma assisted methanation reaction. Finally, a deep investigation on Ni dispersion will be carried out by the means of CO chemisorption as reported elsewhere [49] in order to avoid the H2 adsorption on Ceria support.
Author Contributions
Conceptualization, C.H. and P.D.C.; methodology, G.H.; M.C.B., C.H. and P.D.C.; validation, M.C.B., C.H. and P.D.C.; formal analysis, G.H.; M.C.B., C.H. and P.D.C.; investigation, G.H.; M.C.B., C.H. and P.D.C.; resources, P.D.C. and C.H.; writing—original draft preparation, G.H.; M.C.B., C.H. and P.D.C.; writing—review and editing, G.H.; M.C.B., C.H. and P.D.C.; visualization, M.C.B.; supervision, M.C.B.; C.H.; P.D.C.; project administration, C.H. and P.D.C.; funding acquisition, P.D.C. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union's Horizon 2020 research and innovation program, grant number PIONEER-ITN MSCA 813393.
Acknowledgments
This work was carried out in the framework of Plasma Catalysis CO2 Recycling, a PIONEER project which has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 813393. Authors thank also to Fundação para a Ciência e Tecnologia (FCT) for CQE funding (UIDB/00100/2020 and UIDP/00100/2020) and M. Carmen Bacariza’ contract (2020.00030.CEECIND).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon Capture and Utilization Technologies: A Literature Review and Recent Advances. Energy Sources Part Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Strah Štefančič, N.; Lašič Jurković, D.; Dasireddy, V.D.B.C.; Likozar, B. Unravelling the Mechanisms of CO2 Hydrogenation to Methanol on Cu-Based Catalysts Using First-Principles Multiscale Modelling and Experiments. Catal. Sci. Technol. 2017, 7, 5900–5913. [Google Scholar] [CrossRef] [Green Version]
- Leonzio, G. State of Art and Perspectives about the Production of Methanol, Dimethyl Ether and Syngas by Carbon Dioxide Hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Jurković, D.L.; Pohar, A.; Dasireddy, V.D.B.C.; Likozar, B. Effect of Copper-Based Catalyst Support on Reverse Water-Gas Shift Reaction (RWGS) Activity for CO2 Reduction. Chem. Eng. Technol. 2017, 40, 973–980. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Biset-Peiró, M.; Graça, I.; Guilera, J.; Morante, J.; Lopes, J.M.; Andreu, T.; Henriques, C. DBD Plasma-Assisted CO2 Methanation Using Zeolite-Based Catalysts: Structure Composition-Reactivity Approach and Effect of Ce as Promoter. J. CO2 Util. 2018, 26, 202–211. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Vasilev, K. High Active and Selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 Catalysts for Oxy-Steam Reforming of Methanol. Catalysts 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.; Chu, W.; Zhou, Y.; Köhler, K. CO2 Methanation over Supported Ru/Al2O3 Catalysts: Mechanistic Studies by In Situ Infrared Spectroscopy. ChemistrySelect 2016, 1, 3197–3203. [Google Scholar] [CrossRef]
- Schubert, M.; Pokhrel, S.; Thomé, A.; Zielasek, V.; Gesing, T.M.; Roessner, F.; Mädler, L.; Bäumer, M. Highly Active Co–Al2O3-Based Catalysts for CO2 Methanation with Very Low Platinum Promotion Prepared by Double Flame Spray Pyrolysis. Catal. Sci. Technol. 2016, 6, 7449–7460. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on Iron Based Catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 Hydrogenation at Low Temperature over Rh/γ-Al2O3 Catalysts: Effect of the Metal Particle Size on Catalytic Performances and Reaction Mechanism. Appl. Catal. B Environ. 2012, 113–114, 237–249. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A Highly Dispersed Pd–Mg/SiO2 Catalyst Active for Methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Yatagai, K.; Shishido, Y.; Gemma, R.; Boll, T.; Uchida, H.-H.; Oguri, K. Mechanochemical CO2 Methanation over LaNi-Based Alloys. Int. J. Hydrogen Energy 2020, 45, 5264–5275. [Google Scholar] [CrossRef]
- Alrafei, B.; Polaert, I.; Ledoux, A.; Azzolina-Jury, F. Remarkably Stable and Efficient Ni and Ni-Co Catalysts for CO2 Methanation. Catal. Today 2020, 346, 23–33. [Google Scholar] [CrossRef]
- Summa, P.; Świrk, K.; Wang, Y.; Samojeden, B.; Rønning, M.; Hu, C.; Motak, M.; Da Costa, P. Effect of Cobalt Promotion on Hydrotalcite-Derived Nickel Catalyst for CO2 Methanation. Appl. Mater. Today 2021, 25, 101211. [Google Scholar] [CrossRef]
- Nizio, M.; Benrabbah, R.; Krzak, M.; Debek, R.; Motak, M.; Cavadias, S.; Gálvez, M.E.; Da Costa, P. Low Temperature Hybrid Plasma-Catalytic Methanation over Ni-Ce-Zr Hydrotalcite-Derived Catalysts. Catal. Commun. 2016, 83, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni Supported on CeO2 as Selective Bimetallic Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of Surface Ni and Ce Species of Ni/CeO2 Catalyst in CO2 Methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Sun, C.; Świrk, K.; Wierzbicki, D.; Motak, M.; Grzybek, T.; Da Costa, P. On the Effect of Yttrium Promotion on Ni-Layered Double Hydroxides-Derived Catalysts for Hydrogenation of CO2 to Methane. Int. J. Hydrogen Energy 2021, 46, 12169–12179. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Garbarino, G.; Chen, W.; Parastaev, A.; Longo, A.; Pidko, E.A.; Hensen, E.J.M. Ni-Mn Catalysts on Silica-Modified Alumina for CO2 Methanation. J. Catal. 2020, 382, 358–371. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. Novel Ni-La-Hydrotalcite Derived Catalysts for CO2 Methanation. Catal. Commun. 2016, 83, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Dębek, R.; Azzolina-Jury, F.; Travert, A.; Maugé, F. A Review on Plasma-Catalytic Methanation of Carbon Dioxide—Looking for an Efficient Catalyst. Renew. Sustain. Energy Rev. 2019, 116, 109427. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, B.; Fan, J.; Yang, J. Cobalt Doped Ni Based Ordered Mesoporous Catalysts for CO2 Methanation with Enhanced Catalytic Performance. Int. J. Hydrogen Energy 2018, 43, 4893–4901. [Google Scholar] [CrossRef]
- Ashford, B.; Wang, Y.; Wang, L.; Tu, X. Plasma-Catalytic Conversion of Carbon Dioxide. In Plasma Catalysis: Fundamentals and Applications; Tu, X., Whitehead, J.C., Nozaki, T., Eds.; Springer Series on Atomic, Optical, and Plasma Physics; Springer International Publishing: Cham, Switzerland, 2019; pp. 271–307. ISBN 978-3-030-05189-1. [Google Scholar]
- Snoeckx, R.; Bogaerts, A. Plasma Technology—A Novel Solution for CO2 Conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, P.; Hasrack, G.; Bonnety, J.; Henriques, C. Ni-Based Catalysts for Plasma-Assisted CO2 Methanation. Curr. Opin. Green Sustain. Chem. 2021, 32, 100540. [Google Scholar] [CrossRef]
- Zhou, R.; Rui, N.; Fan, Z.; Liu, C. Effect of the Structure of Ni/TiO2 Catalyst on CO2 Methanation. Int. J. Hydrogen Energy 2016, 41, 22017–22025. [Google Scholar] [CrossRef]
- Mikhail, M.; Da Costa, P.; Cavadias, S.; Tatoulian, M.; Ognier, S.; Galvez, M.E. Nickel Supported Modified Zirconia Catalysts for CO2 Methanation in DBD Plasma Catalytic Hybrid Process. Mater. Sci. Forum 2021, 1016, 894–899. [Google Scholar] [CrossRef]
- Nizio, M.; Albarazi, A.; Cavadias, S.; Amouroux, J.; Galvez, M.E.; Da Costa, P. Hybrid Plasma-Catalytic Methanation of CO2 at Low Temperature over Ceria Zirconia Supported Ni Catalysts. Int. J. Hydrogen Energy 2016, 41, 11584–11592. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, M.; Wang, B.; Jalain, R.; Cavadias, S.; Tatoulian, M.; Ognier, S.; Gálvez, M.E.; Da Costa, P. Plasma-Catalytic Hybrid Process for CO2 Methanation: Optimization of Operation Parameters. React. Kinet. Mech. Catal. 2019, 126, 629–643. [Google Scholar] [CrossRef]
- Biset-Peiró, M.; Guilera, J.; Zhang, T.; Arbiol, J.; Andreu, T. On the Role of Ceria in Ni-Al2O3 Catalyst for CO2 Plasma Methanation. Appl. Catal. Gen. 2019, 575, 223–229. [Google Scholar] [CrossRef]
- Wang, B.; Mikhail, M.; Cavadias, S.; Tatoulian, M.; Da Costa, P.; Ognier, S. Improvement of the Activity of CO2 Methanation in a Hybrid Plasma-Catalytic Process in Varying Catalyst Particle Size or under Pressure. J. CO2 Util. 2021, 46, 101471. [Google Scholar] [CrossRef]
- Chen, H.; Mu, Y.; Shao, Y.; Chansai, S.; Xiang, H.; Jiao, Y.; Hardacre, C.; Fan, X. Nonthermal Plasma (NTP) Activated Metal–Organic Frameworks (MOFs) Catalyst for Catalytic CO2 Hydrogenation. AIChE J. 2020, 66, e16853. [Google Scholar] [CrossRef]
- Zeng, Y.; Tu, X. Plasma-Catalytic Hydrogenation of CO2 for the Cogeneration of CO and CH4 in a Dielectric Barrier Discharge Reactor: Effect of Argon Addition. J. Phys. Appl. Phys. 2017, 50, 184004. [Google Scholar] [CrossRef]
- Chen, H.; Mu, Y.; Shao, Y.; Chansai, S.; Xu, S.; Stere, C.E.; Xiang, H.; Zhang, R.; Jiao, Y.; Hardacre, C.; et al. Coupling Non-Thermal Plasma with Ni Catalysts Supported on BETA Zeolite for Catalytic CO2 Methanation. Catal. Sci. Technol. 2019, 9, 4135–4145. [Google Scholar] [CrossRef]
- Jwa, E.; Lee, S.B.; Lee, H.W.; Mok, Y.S. Plasma-Assisted Catalytic Methanation of CO and CO2 over Ni–Zeolite Catalysts. Fuel Process. Technol. 2013, 108, 89–93. [Google Scholar] [CrossRef]
- Mikhail, M.; Da Costa, P.; Amouroux, J.; Cavadias, S.; Tatoulian, M.; Gálvez, M.E.; Ognier, S. Tailoring Physicochemical and Electrical Properties of Ni/CeZrOx Doped Catalysts for High Efficiency of Plasma Catalytic CO2 Methanation. Appl. Catal. B Environ. 2021, 294, 120233. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S. Temperature Programme Reduction (TPR) Studies of Cobalt Phases in -Alumina Supported Cobalt Catalysts. J. Pet. Technol. Altern. Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; He, T.; Han, D.; Li, G.; Zhao, R.; Wu, J. Plasma-Assisted CO2 Methanation: Effects on the Low-Temperature Activity of an Ni–Ce Catalyst and Reaction Performance. R. Soc. Open Sci. 2019, 6, 190750. [Google Scholar] [CrossRef] [Green Version]
- Sayyed, S.A.A.R.; Beedri, N.I.; Kadam, V.S.; Pathan, H.M. Rose Bengal-Sensitized Nanocrystalline Ceria Photoanode for Dye-Sensitized Solar Cell Application. Bull. Mater. Sci. 2016, 39, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Benrabbah, R.; Cavaniol, C.; Liu, H.; Ognier, S.; Cavadias, S.; Gálvez, M.E.; Da Costa, P. Plasma DBD Activated Ceria-Zirconia-Promoted Ni-Catalysts for Plasma Catalytic CO2 Hydrogenation at Low Temperature. Catal. Commun. 2017, 89, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 Methanation over CoNi Bimetal-Doped Ordered Mesoporous Al2O3 Catalysts with Enhanced Low-Temperature Activities. Int. J. Hydrogen Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Mikhail, M.; Da Costa, P.; Amouroux, J.; Cavadias, S.; Tatoulian, M.; Ognier, S.; Gálvez, M.E. Electrocatalytic Behaviour of CeZrOx-Supported Ni Catalysts in Plasma Assisted CO2 Methanation. Catal. Sci. Technol. 2020, 10, 4532–4543. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Amjad, S.; Teixeira, P.; Lopes, J.M.; Henriques, C. Boosting Ni Dispersion on Zeolite-Supported Catalysts for CO2 Methanation: The Influence of the Impregnation Solvent. Energy Fuels 2020, 34, 14656–14666. [Google Scholar] [CrossRef]
- Hołub, M. On the Measurement of Plasma Power in Atmospheric Pressure DBD Plasma Reactors. Int. J. Appl. Electromagn. Mech. 2012, 39, 81–87. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, L.; Hu, H.; Zuo, S.; Yang, L. Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst. Nanomaterials 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).