Abstract
This study compares the hydrotreating of the mixture of petroleum middle distillates and the same mixture containing 20 wt % of rapeseed oil. We also study the effect of the temperature and the weight hourly space velocity (WHSV) on the co-hydrotreating of gas oil and rapeseed oil mixture. The hydrotreating is performed over a commercial hydrotreating Ni-Mo/Al2O3 catalyst at temperatures of ca. 320, 330, 340, and 350 °C with a WHSV of 0.5, 1.0, 1.5, and 2.0 h−1 under a pressure of 4 MPa and at a constant hydrogen flow of 28 dm3·h−1. The total conversion of the rapeseed oil is achieved under all the tested reaction conditions. The content of the aromatic hydrocarbons in the products reached a minimum at the lowest reaction temperature and WHSV. The content of sulphur in the products did not exceed 10 mg∙kg−1 at the reaction temperature of 350 °C and a WHSV of 1.0 h−1 and WHSV of 0.5 h−1 regardless of the reaction temperature. Our results show that in the hydrotreating of the feedstock containing rapeseed oil, a large amount of hydrogen is consumed for the dearomatisation of the fossil part and the saturation of the double bonds in the rapeseed oil and its hydrodeoxygenation.
1. Introduction
High-quality biofuels, in particular, synthetic bio-jet fuel and synthetic biodiesel, can be produced by hydrotreating feedstocks containing triglycerides of higher fatty acids [1,2]. These raw materials include various vegetable oils, waste cooking oils and animal fats. Hydrotreating products are often referred to as hydrotreated vegetable oil (HVO) or green diesel in the case of diesel fuel or as hydroprocessed esters and fatty acids (HEFA) in the case of bio-jet fuel. These products mainly contain paraffinic hydrocarbons and almost no aromatics and sulphur and have excellent thermal oxidation stability and a high cetane number [1,3].
The most common renewable feedstock materials for the HVO production contain triglycerides formed by fatty acids with 14–22 carbon atoms, and maximally, three double bonds. The first step of the triglyceride hydrotreating is the hydrogenation of the double bonds present in the aliphatic chains of the acyl groups. The next step involves the hydrogenolysis of the ester bonds with the formation of diglycerides, monoglycerides, and finally, carboxylic acids and propane [4,5,6]. According to some authors, the intermediates then decompose into hydrocarbons via hydrodeoxygenation (HDO) and hydrodecarboxylation (HDCx) [7,8]. The CO2 generated by HDCx can be partially reduced by the hydrogen to CO and water (Equation (1)). In the opinion of other authors, in addition to HDO and HDCx, hydrodecarbonylation (HDCn) is also involved in the decomposition of the carboxylic acid into hydrocarbons [4,5,6].
Hydrodeoxygenation removes oxygen from the carboxylic acids in the form of water, and n-alkanes with the same number of carbon atoms as the starting fatty acids. Hydrodecarboxylation removes oxygen in the form of CO2, while hydrodecarbonylation removes oxygen in the form of CO and water. In both cases, the produced n-alkanes have one carbon atom less than the original carboxylic acids [4,5,6].
The molar ratio of the C17/C18 n-alkanes in the liquid products is usually used to determine the dominant pathway of the triglyceride conversion, i.e., whether HDO or HDCx/HDCn predominates. The reaction pathways of the triglyceride conversion are influenced by the reaction conditions. Under high hydrogen pressure, HDO occurs at a higher extent than HDCx/HDCn [9]. The CO2/CO ratio in the gaseous products is sometimes used to determine the selectivity of the HDCn and HDCx reactions, but it should be taken into account that methanation reaction, where the CO2 or CO reacts with hydrogen (Equations (2) and (3), respectively) to form methane, and the reaction between the carbon dioxide and hydrogen which forms carbon monoxide and water (Equation (1)) may affect the composition of gaseous products and hydrogen consumption [4,5,6].
Some oxygenates, such as carboxylic acids, alcohols, and esters, are usually formed under moderate reaction conditions when the conversion of the feedstock to hydrocarbons is not complete [8,10]. In some cases, hydrocarbons with a lower carbon number than that of the corresponding fatty acid are formed, due to the hydrocracking of the carbon chains of the intermediates originating from the triglyceride conversion, or due to the hydrocracking of the formed n-alkanes. The extent of hydrocracking depends on the acidity of the catalyst and the reaction conditions. Hydroisomerisation, cyclisation, and dehydrocyclisation with the formation of isoalkanes, alkyl cycloalkanes and alkyl aromatics, are also possible reactions. Their extent depends on the type of the catalyst and the reaction conditions [1,2]; however, n-alkanes are usually the predominant products of hydrotreating vegetable oil/animal fats. The hydroisomerisation of the n-alkanes takes place at the acidic centres of the catalysts and leads to an improvement in the low-temperature properties of the liquid product. However, the catalyst should not be too acidic because undesirable cracking reactions also take place at the acidic centres [1,2]. As hydroisomerisation requires lower reaction temperatures than the conversion of triglycerides to n-alkanes, it is, therefore, usually carried out in the second stage in the commercial production of HVO/HEFA [11,12].
A wide variety of catalysts for triacylglyceride hydrotreating have been investigated. Conventional catalysts consist of Mo and/or W with Co and Ni as promoters on various supports [2,13,14,15]. Various types of γ-alumina with different textural properties (surface area, pore volume and size) are the most common types of support. Recently, other supports, such as SiO2, TiO2, mixed oxides, zeolites, mesoporous supports based on SiO2, and carbon, have also been investigated [2,13,14,15]. Conventional catalysts typically provide high yields of hydrocarbon liquid fractions boiling in the diesel fuel range, but their low-temperature properties (cloud point and CFPP—cold filter plugging point) are often poor. These properties can be improved either by the additional processing on a hydroisomerisation catalyst or by using multifunctional catalysts directly for the hydrotreating [2,13,14,15].
Unsupported transition metal sulphide catalysts, mainly unsupported Ni/Co-Mo/W sulphide catalysts, were studied for the hydrotreating of petroleum fractions [16]. The performance studies identified several unsupported catalysts with higher activity and/or selectivity than traditionally supported Ni-Mo/γ-Al2O3 and Co-Mo/γ-Al2O3 catalysts. Even if the unsupported catalyst contains Group VIII and Group VI metals only, they are more expensive than the supported ones, because they contain a high concentration of the metals [16,17]. The commercial use of the unsupported catalysts is mainly limited to NEBULA catalysts [17].
The hydrotreating of a feedstock based on the triglycerides of fatty acids can be performed either in a separate unit or in a unit integrated into a conventional oil refinery (coprocessing or cohydrotreating) [18,19,20,21]. The commercial success of each process also depends on the development of an efficient catalyst, which must exhibit a stable activity in the presence of a relatively large amount of water that is generated as a by-product of the triglyceride conversion.
The coprocessing of vegetable oils/animal fats together with a conventional refinery feedstock provides flexibility to the refineries. However, it is not applied too often, mainly due to the inhibition of the hydrodesulphurisation (HDS) of the petroleum feedstock caused by the presence of large amounts of oxygen compounds or the inhibition of the hydrodeoxygenation (HDO) of triglycerides caused by the presence of sulphur compounds in the petroleum feedstock [21].
The hydrogen consumption during the hydrotreating of middle distillates depends on the composition of the feedstock, in particular, on the contents of sulphur, nitrogen and polyaromatics. The presence of triglycerides in the feedstock for cohydrotreating has a strong influence on hydrogen consumption. It mainly depends on the concentration of triglycerides in the feedstock and the preferred routes of the conversion of triglycerides to alkanes. The origin and structure of the triglycerides (mainly the amount of double bonds) also plays an important role. In the case of rapeseed oil with four double bonds in the molecule, 7 moles of hydrogen are consumed if 1 mole of rapeseed oil is converted by the HDCx route, in comparison with 10 and 16 moles of hydrogen consumed during the conversion of the same 1 mole of rapeseed oil via the HDCn and HDO routes, respectively.
The hydrogen consumption for the hydrotreating of the triglyceride-based feedstock into HVO is higher than the fossil middle distillates. The hydrogen consumption determined by Al-Darous and Ali [22] during the deep desulphurisation of gas oil (S: 1.0 wt %) over an Ni-Mo/Al2O3 catalyst was around 37 m3∙m−3. The hydrotreating of light gas oil (S: 1.27 wt %, N: 170 mg·kg−1, polyaromatics: 12 wt %) and its mixtures with rapeseed oil (15 and 25 wt %) over an Ni-Mo/Al2O3 catalyst was compared by Donnis et al. [23]. The hydrogen consumption was 47 m3·m−3 in the case of the pure gas oil hydrotreating and increased to 83 m3·m−3 and 103 m3·m−3 if the feedstocks contained 15 and 25 wt % of rapeseed oil, respectively. The hydrogen consumption for palm oil hydrotreating was estimated [24] to be 210 dm3·kg−1. Similarly, the hydrogen consumption for various triglycerides hydrotreating of 178–234 dm3·kg−1 was reported by Carmona et al. [25].
Although the investigation of the coprocessing of vegetable oils/animal fats with a conventional petroleum feedstock has already brought a number of new findings, there are still technical and operational problems, and further research is needed to solve these problems. It is necessary to investigate the compatibility of various vegetable oils and animal fats with different petroleum feedstocks, the long-term stability of the catalysts (over 1 year), the optimisation of the reaction conditions and the hydrogen consumption while maintaining the required product quality, the mutual influence of the heteroatoms contained in the petroleum feedstock (S, N), and the oxygenates derived from the biomass.
This work is focused on the investigation of the effect of the reaction conditions on the activity of a sulphided Ni-Mo/Al2O3 catalyst during the cohydrotreating of rapeseed oil and petroleum middle distillates in a 20:80 mass ratio to produce diesel fuel containing more than 10 % of biocomponent. Moreover, petroleum middle distillate that was processed contained 10 % of low-quality light cycle oil (LCO) and corresponded, thus, to a real refinery feedstock for the hydrotreating.
Not only the influence of the reaction temperature and the WHSV of the feedstock were studied, but also the influence of hydrogen to feedstock flow rate ratio, including coprocessing with lower hydrogen excess, was tested. The paper also brought a detailed overview of hydrogen consumption that is so rarely reported, especially in the field of vegetable oil coprocessing. Based on the mass flows of the individual components of the raw materials and the hydrotreating products formed from them, the approximate hydrogen consumptions for the individual reactions taking place during the hydrotreating were calculated.
2. Results
2.1. Influence of the Presence of Rapeseed oil in the Feedstock
The composition and properties of the gaseous products and stabilised liquid products of the hydrotreating of the F0 (0 wt % of rapeseed oil) and F20 (20 wt % of rapeseed oil) feedstocks at a WHSV of 1.0 h−1 were compared at first. The simulated distillation of the liquid products of the F20 feedstock confirmed the complete conversion of the rapeseed oil (RO) under all the tested reaction temperatures. The yields of the stabilised liquid products from the hydrotreating of the F0 and F20 feedstocks were around 96.5 and 92.5 wt %, respectively, and were not dependent on the reaction temperature. As was expected, the F20 feedstock provided less liquid product than the F0 one, since the RO hydrotreating produced more gaseous compounds as by-products, especially propane, CO2 and CO.
Group-type composition
The group type composition of the feedstocks and the stabilised liquid products are compared in Figure 1. A significant decrease in the content of the diaromatics and triaromatics and an increase in the content of the monoaromatics in all the products compared to the feedstock is evident at all the tested reaction temperatures regardless of the processed feedstock. The content of the diaromatics and triaromatics increased slightly with an increasing reaction temperature. At higher temperatures, the monoaromatics were hydrogenated to saturated hydrocarbons to a greater extent. As expected, the products obtained from the F20 feedstock containing 20 wt % of RO had a higher content of saturated hydrocarbons and a lower content of monoaromatics compared to the products obtained from the F0 feedstock at the comparable reaction temperature, because saturated hydrocarbons were formed from the RO. The content of the saturated hydrocarbons in the products obtained by the hydrotreating of the F20 feedstock varied only slightly with the reaction temperature. On the other hand, the content of the saturated hydrocarbons in the products obtained by the hydrotreating of the F0 feedstock increased with an increasing temperature, mainly at the expense of the monoaromatic content.
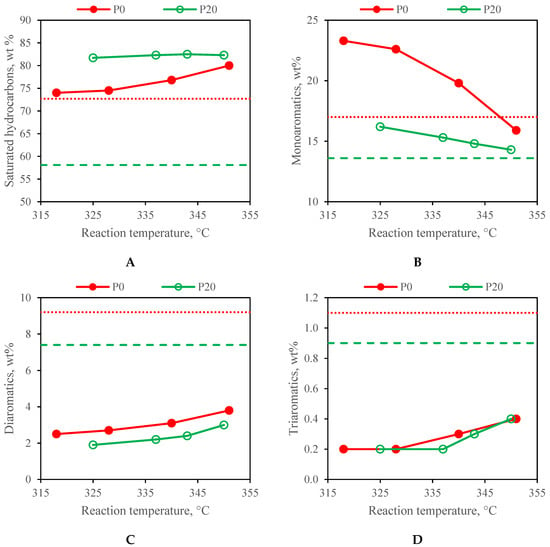
Figure 1.
Group-type composition of both feedstocks (dashed lines) and all the liquid products (solid lines) obtained at a WHSV of 1.0 h−1 (A) saturated hydrocarbons; (B) monoaromatics; (C) diaromatics; (D) triaromatics.
Content of n-alkanes
The contents of the n-alkanes present in the liquid products are listed in Table 1. As expected, the dominant n-alkanes present in the products obtained by hydrotreating the F20 feedstock were C17 and C18 n-alkanes, as acyls with 18 carbon atoms were predominant in the rapeseed oil. The slight increase in the n-C17/n-C18 ratio in the P20 products with the reaction temperature indicated that the HDCx/HDCn reactions proceeded at a higher rate at the expense of the HDO reaction. Linear alkanes almost do not undergo chemical reactions during hydrotreating, so their content in the products is independent of the reaction temperature.

Table 1.
The content of n-alkanes in the liquid products obtained at a WHSV of 1.0 h−1.
Sulphur and nitrogen content
The sulphur content of the stabilised liquid products decreased in all the cases as the reaction temperature increased (Figure 2). A satisfactory sulphur content (up to 10 mg∙kg−1 according to EN 590 standard requirement) in the products from the hydrotreating of both types of feedstock was achieved at a temperature of 350 °C only. The lower sulphur content in the P20 products in comparison with the P0 products gained at reaction temperatures of 320 and 330 °C were caused by the lower sulphur content in the F20 feedstock (2200 mg∙kg−1) compared to the F0 one (2800 mg∙kg−1) and by the higher real reaction temperatures at the F20 hydrotreating. The slight inhibition of the desulphurisation reactions at temperatures of 340 and 350 °C by the oxygen compounds resulting from the hydrotreating of the F20 feedstock was observed. A similar behaviour was described in the work of Tóth et al. [26]; however, the inhibition effect of oxygen compounds was observed at reaction temperatures of 360 and 380 °C. The desulphurisation of both types of feedstock was accompanied by deep denitrogenation (about 98.5 %) at all the tested reaction temperatures. The total nitrogen content in all products obtained at the WHSV of 1 h−1 did not exceed 3 mg∙kg−1. The nitrogen content is not monitored by EN 590, but a high content is undesirable from an environmental point of view.
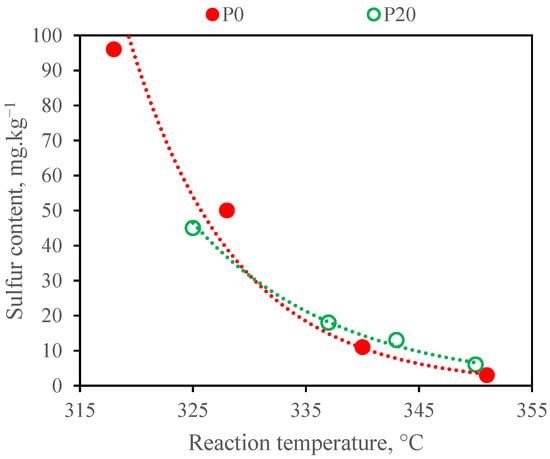
Figure 2.
The sulphur content in the liquid products from F0 and F20 feedstocks obtained at a WHSV of 1.0 h−1.
Physicochemical properties
The density, kinematic viscosity, cetane index and cold filter plugging point (CFPP) of the liquid products obtained at a WHSV of 1.0 h−1 are listed in Table 2.

Table 2.
The selected properties of the feedstocks and liquid products obtained at a WHSV of 1.0 h−1.
The products obtained by the hydrotreating of the F0 feedstock at temperatures of 320–340 °C had a higher density than the EN 590 standard requires for diesel fuel (820−845 kg∙m−3). Typically, 10–20 wt % of the hydrotreated kerosene with a lower density is, therefore, commonly added to the hydrotreated F0 feedstock during the production of diesel fuel. The density of the products obtained from the F0 feedstock decreased with an increasing reaction temperature and a decreasing content of the high-density aromatics (Figure 1). The products obtained from the F20 feedstock had a lower density than the products obtained from the F0 feedstock, because the hydrotreating of the RO mainly led to the formation of low-density C17 and C18 n-alkanes. The densities of all the products obtained from the F20 feedstock were in accordance with the EN 590 standard requirements. All the liquid products obtained from both feedstocks met the requirements of the EN 590 for kinematic viscosity (2.0–4.5 mm2·s−1).
The cetane index (CI) of all the liquid products was higher than the lower limit required by EN 590 specification (min. 46). While the F0 feedstock had a CI of 50, the products from its hydrotreating had a CI that were 3–5 units higher. As expected, the products obtained by the hydrotreating of the F20 feedstock had significantly higher CI values compared with those from the F0 feedstock. An increase in the CI of about 7–9 units was observed and was caused by the higher content of the linear alkanes (Table 1). A similar increase in the cetane number can be, therefore, expected too.
SRGO, as a major part of the F0 and F20 feedstocks, was taken from the refinery in the summer. Therefore, the CFPP of the liquid products obtained from both feedstocks met the requirements only for the summer diesel classes A and B (CFPP max. +5 and 0 °C, respectively). In spite of the fact, that the products obtained from the F20 feedstock contained higher amounts of the C17 and C18 n-alkanes, they had slightly lower CFPP values when compared to the products of the F0 feedstock. On the other hand, any clear dependence of the CFPP on the reaction conditions was not found. These results are at variance with the results of Tóth et al. [26]. In their work, an increasing CFPP with an increasing content of sunflower oil in the feedstock and an increasing CFPP with a decreasing temperature of hydrotreating was observed. On the other hand, Endisch et al. [27] found that the CFPP of the product obtained by the coprocessing of SRGO containing 10 wt % of Jatropha oil was lower by 3 °C than the CFPP of hydrotreated pure SRGO. Contrary, when the content of the Jatropha oil in the feedstock increased to 20 wt %, the CFPP was higher by 3 °C in comparison with the hydrotreated SRGO.
Composition of the gaseous products
The data given in Table 3 show that the gaseous products of the hydrotreating of the F20 feedstock differ from those of F0 ones mainly by the presence of CO, CO2 and propane, as expected. Furthermore, a higher content of methane was observed in the gaseous products of the F20 feedstock than the gaseous products of the F0 feedstock. The propane results from the conversion of the RO to carboxylic acids, CO2 and CO, are produced by their hydrodecarboxylation and hydrodecarbonylation, respectively. The methane content in the gaseous products of both feedstocks slightly increased with the reaction temperature because of the slightly higher extent of the cracking reactions. In addition to the cracking reactions, methanation reactions (Equations (2) and (3)) contributed to the methane formation during the hydrotreating of the F20 feedstock.

Table 3.
The composition of the gaseous products obtained at a WHSV of 1.0 h−1.
The shares of the HDO, HDCx and HDCn reactions in the RO conversion (AHDO, AHDCx, and AHDCn, respectively) are shown in Table 4. The hydrogen consumption for individual reactions together with the total hydrogen consumption and hydrogen excess are summarised in Table 5. It must be emphasised that the calculation of the hydrogen consumption comes from many data and from several assumptions, so it could be determined with a relatively larger error compared to the other data. All calculations concerning hydrogen consumption are described in detail in Supplementary Material.

Table 4.
The shares of the HDO, HDCx and HDCn reactions during the RO conversion in the hydrotreating experiments at a WHSV of 1.0 h−1.

Table 5.
The hydrogen consumption (m3·m−3) for the individual reactions, total hydrogen consumption and hydrogen excess (ExH) in the hydrotreating at a WHSV of 1.0 h−1.
The share of the HDCn reaction in the RO conversion increased at the expense of the HDO reaction with an increasing reaction temperature. The addition of 20 wt % of RO to the feedstock significantly increased the total hydrogen consumption. Due to the higher hydrogen consumption for the hydrotreating of the F20 feedstock compared to the hydrotreating of the F0 feedstock, the excess of hydrogen decreased from 6.4–9.0 to about 2.9.
In the hydrotreating of the F0 feedstock, hydrogen was consumed mainly for the dearomatisation of the feedstock (Table 5). In the hydrotreating of the F20 feedstock, a large amount of hydrogen was consumed for the dearomatisation as well, but also for the saturation of the double bonds (DB) in RO and its HDO. The hydrogen consumption related to the total conversion of pure RO into hydrocarbons was 288–298 dm3·kg−1 (312–327 dm3·kg−1, including methanation), which is more than was reported by Templis et al. [24] for palm oil hydrotreating (210 dm3·kg−1). This difference can be easily explained by different level of unsaturation of rapeseed oil and palm oil. Palm oil had an average of 1.8 double bonds per molecule [28], while RO had 3.9 double bonds per molecule.
For the F0 feedstock hydrotreating, the total hydrogen consumption increased with an increasing reaction temperature as the total aromatics, and sulphur content in the liquid products decreased (Figure 1 and Figure 2). For the F20 feedstock hydrotreating, the total hydrogen consumption did not change much with an increasing reaction temperature. In total, around 15 and 35 wt % of hydrogen entering the reactor were consumed in case of the highest reaction temperature with the F0 and F20 feedstock, respectively. The decreasing share of the HDO in the RO conversion and the lower dearomatisation slightly reduced the hydrogen consumption for these reactions in the F20 feedstock hydrotreating at higher reaction temperatures (Table 4 and Table 5). On the other hand, the increasing methanation reaction and increasing share of the HDCn in the RO conversion increased the hydrogen consumption needed for these partial reactions. These opposing trends resulted in a nearly constant total hydrogen consumption that was independent of the reaction temperature (Table 5).
2.2. The Influence of WHSV and Hydrogen on the Feedstock Ratio on the Composition and Properties of the Hydrotreating Products
The activity of the catalyst was additionally tested under a variable WHSV (0.5, 1.5 and 2.0 h−1) and a constant hydrogen flow rate of 28 dm3∙h−1 (variable hydrogen/feedstock flow rate ratio in the range of 120−480 m3∙m−3). The aim of using a relatively low hydrogen to feedstock ratio of 120 m3∙m−3 was to find out how the hydrotreating of the feedstock containing 20 wt % of RO takes place with a small excess of hydrogen. The obtained products were compared with the products gained at a WHSV of 1.0 h−1 that were described in the previous section.
The content of the diaromatics and triaromatics in the liquid products only increased slightly with an increasing WHSV (Figure 3). At a WHSV of 0.5 h−1, the hydrogenation of the diaromatics into monoaromatics and the hydrogenation of the monoaromatics into saturated hydrocarbons reached their maximum. The monoaromatics content decreased, and the diaromatics content increased with an increasing reaction temperature at all WHSV used. The total aromatic content was the lowest at a WHSV of 0.5 h−1. The lowest aromatic content in the liquid products at the lowest WHSV was also observed by Palanisamy et al. [29].
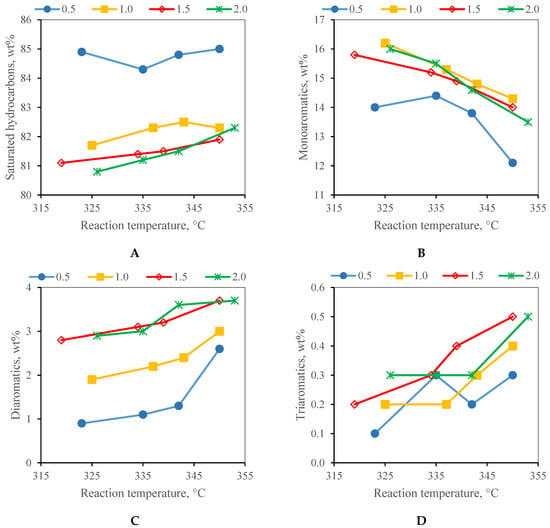
Figure 3.
Group-type composition of all the liquid products obtained by the hydrotreating of F20 feedstock at the WHSV of 0.5–2.0 h−1 (A) saturated hydrocarbons; (B) monoaromatics; (C) diaromatics; (D) triaromatics.
Due to the full deoxygenation of the RO at all the flow rates and reaction temperatures, the dominant products formed from RO were C17 and C18 n-alkanes (Table 6). The n-heptadecane content in the liquid products slightly increased at the expense of n-octadecane with an increasing reaction temperature. The content of the other n-alkanes was not strongly influenced by the reaction temperature, WHSV and hydrogen/feedstock ratio.

Table 6.
The content of n-alkanes in P20 liquid products.
From the sulphur and nitrogen contents in the liquid products summarised in Table 7, it is evident that a WHSV higher than 1.0 h−1 led to insufficient desulphurisation. The sulphur content in the products obtained at the highest reaction temperature of 350 °C and at a WHSV of 1.5 and 2.0 h−1 was 35 and 118 mg∙kg−1, respectively. A higher temperature and possibly a higher ratio of hydrogen to feedstock would have to be used at a WHSV 1.5 and 2.0 h−1 to obtain a product with a sulphur content below 10 mg∙kg−1. If a lower temperature is required, hydrotreating at a lower WHSV is, thus, necessary. The degree of desulphurisation was satisfactory for all the products obtained at a WHSV of 0.5 h−1 regardless of the reaction temperature.

Table 7.
The sulphur and nitrogen contents in the F20 feedstock and the P20 liquid products.
The selected physicochemical properties of the P20 products obtained at a variable WHSV are summarised in Table 8. The lower density of the products obtained at a WHSV of 0.5 h−1 was observed, due to the greater extent of the dearomatisation reactions caused by the prolonged reaction time on the catalyst surface and the highest hydrogen to feedstock volume ratio. The density and viscosity of all the products were in accordance with the EN 590 requirements (density at 15 °C 820–845 kg∙m−3, kinematic viscosity at 40 °C 2.0–4.5 mm2·s−1). The very high cetane index (60–63) was partly caused by the hydrogenation of the aromatics in the fossil part of the feedstock, but predominantly by the formation of the high-cetane n-alkanes from the RO. The cetane index decreased slightly with an increasing WHSV, i.e., with decreasing hydrogen to feedstock ratio, depending on a slight increase in the aromatics content (Figure 3). The CFPP of all the liquid products obtained from the F20 feedstock met the requirements for the diesel classes A and B (CFPP max. +5 and 0 °C, respectively).

Table 8.
The selected properties of the F20 feedstock and the P20 liquid products.
The changes in the contents of key compounds of the gaseous products with the reaction temperature and WHSV are summarised in Figure 4. The maximum contents of ethane and C4+ hydrocarbons were 0.3 vol% and are not, therefore, discussed. The influence of an increasing WHSV at a constant hydrogen flow rate was evident in the case of the increasing propane and CO2 concentrations at the expense of the hydrogen concentration in the gaseous products. The changes in the content of the other gaseous compounds with an increasing WHSV were smaller. The methane to the CO + CO2 content ratio presented in Figure 5 was in the range of 0.06–0.67 and increased with the reaction temperature and decreased with an increasing WHSV. The lowest value of 0.06 was observed at a WHSV of 1.5 and 2.0 h−1 and at the reaction temperature of 320 °C. The values obtained at a WHSV of 1.0 h−1 are comparable with the results of the work by Gong et al. [30] where methane to CO + CO2 ratio of 0.03 was reported in hydrotreating over a similar type of catalyst and reaction temperature. The low value of this ratio indicates the small extent of the methanation reactions. As expected, the extent of the methanation reactions increased with an increasing temperature and declined with an increasing WHSV. An increase in the methanation with an increasing reaction temperature and a decrease with an increasing WHSV was also observed by Palanisamy et al. [29]. It was explained by the dissociation of CO that is a rate-determining step of methanation; hence, the rate of methanation increases at low space velocity. The CO disproportionation reaction produces C-radicals which then react with hydrogen to form methane [29].
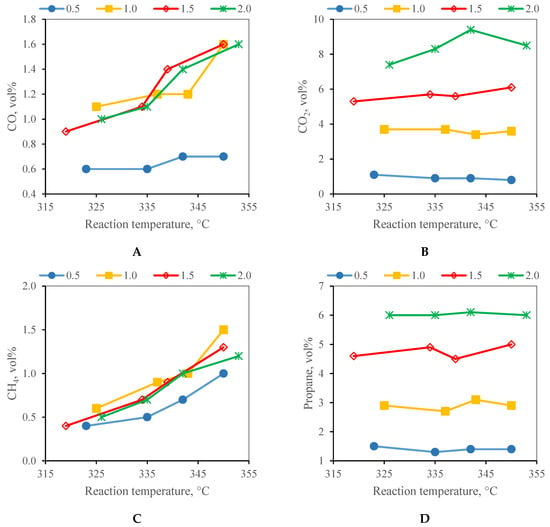
Figure 4.
The composition of the P20 gaseous products at WHSV of 0.5–2.0 h−1 (A) carbon monoxide; (B) carbon dioxide; (C) methane; (D) propane.
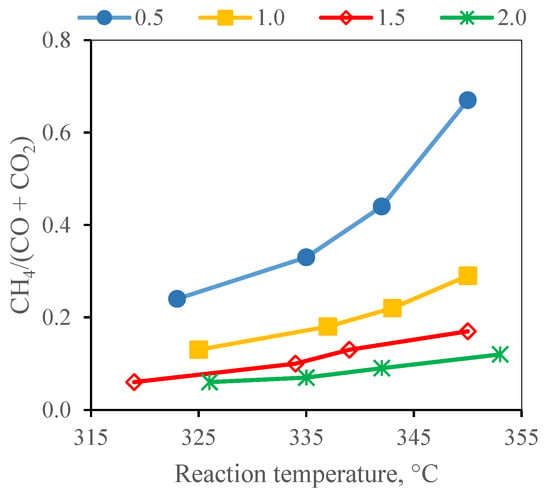
Figure 5.
Methane to (CO + CO2) ratio at WHSV of 0.5–2.0 h−1.
The shares of the HDO, HDCx and HDCn reactions in the RO conversion (AHDO, AHDCx and AHDCn, respectively) are shown in Table 9. The hydrogen consumption for individual reactions together with the total hydrogen consumption and hydrogen excess are summarised in Table 10. The share of the HDCn reaction in the RO conversion increased at the expense of the HDO reaction with an increasing reaction temperature at all the WHSV used. The share of the HDCx reaction in the RO conversion increased with an increasing WHSV.

Table 9.
The shares of the HDO, HDCx and HDCn reactions in the RO conversion during the hydrotreating of the F20 feedstock.

Table 10.
The hydrogen consumption (m3·m−3) for the individual reactions, total hydrogen consumption and hydrogen excess (ExH) in the hydrotreating of the F20 feedstock.
The hydrogen consumption related to 1 m3 of the feedstock decreased slightly with an increasing WHSV and decreasing hydrogen to feedstock ratio mainly due to the decreased extent of the hydrodearomatisation and methanation reactions. It can be conducted from the data in Table 10, from the increase in the total aromatic content in the liquid products (Figure 3) and from the decrease in the methane to CO + CO2 ratio in the gaseous products with an increasing WHSV (Figure 5). Depending on the increasing hydrogen consumption, the excess of hydrogen decreased with an increasing WHSV. At a WHSV of 2.0 h−1, the excess of hydrogen was 1.6.
3. Materials and Methods
3.1. Materials
Straight run gas-oil (SRGO) from crude oil distillation, light cycle oil (LCO) from fluid catalytic cracking and commercial rapeseed oil (RO) were used as components of the two types of feedstock in this work. The first type of feedstock (F0) was a mixture of SRGO and LCO in a mass ratio of 90:10. The second type of feedstock (F20) was composed of SRGO, LCO and RO in a mass ratio of 72:8:20. For the given SRGO and LCO quality, the usual amount of LCO in the feedstock for the production of summer diesel fuel is 10–12 wt %. The properties of both feedstocks are summarised in Table 11.

Table 11.
The properties of the feedstocks for hydrotreating.
The commercial RO contained 4 mg∙kg−1 of sulphur and 8 mg∙kg−1 of nitrogen. The C18 acyl groups represented more than 91 wt % of the total acyl groups in the RO (Table 12).

Table 12.
The distribution of the acyl groups in the RO used as a component of the F20 feedstock (wt %).
3.2. Hydrotreating
The fossil feedstock (F0) was hydrotreated at a pressure of 4.0 MPa, with a commonly used hydrogen/feedstock flow rate ratio of 240 m3∙m−3 (hydrogen flow rate of 28 dm3∙h−1), a weight hourly space velocity (WHSV) of about 1.0 h−1, and temperatures around 320, 330, 340 and 350 °C (Table 13).

Table 13.
The reaction conditions and labelling of the products obtained by hydrotreating the F0 feedstock.
The hydrotreating of the F20 feedstock was carried out at the same reaction pressure and similar temperatures as the F0 feedstock. The hydrogen flow rate of 28 dm3∙h−1 was kept constant for all the experiments. The WHSV of 0.5, 1.0, 1.5 and 2.0 h−1 were tested, and thus, the hydrogen/feedstock flow rate ratio varied from 480 to 120 m3∙m−3. Sixteen liquid products from the hydrotreating of the F20 feedstock were obtained in total and are listed in Table 14.

Table 14.
The reaction conditions and labelling of the products obtained by hydrotreating the F20 feedstock.
The hydrotreating was carried out in a tubular fixed-bed reactor with the cocurrent flow of the feedstock and hydrogen. A simplified schematic diagram of the preheater and electrically heated reactor system was described in a previous article [31].
The total length of the reactor tube was 658 mm, and its internal diameter was 30 mm. The reactor was divided into three zones: An upper preheat zone, a catalytic bed zone, and a bottom zone below the catalytic bed. The catalytic bed zone was filled with a commercial hydrotreating Ni-Mo/Al2O3 catalyst with a particle size reduced to the range of 0.25–0.42 mm. The total catalyst volume in the bed was 95 cm3, corresponding to a catalyst mass of 99 g. The catalyst was mixed with silicon carbide (particle size of 0.25–0.30 mm) in the volume ratio of 1:1. The activation, composition and properties of the catalyst were described in detail by Vozka et al. [31].
The whole experiment lasted about 250 h. The catalyst activation lasted for about 24 h; it was followed by the stabilisation of the catalyst activity, in which the SRGO hydrotreating was performed, and the sulphur content in the liquid products was measured every 4 h. When the sulphur content of the products did not change for at least 20 h, the individual experiments were performed, each lasting about 10 h. The setting and control of the reaction conditions took about 1 h, rinsing the reactor and separator with the reaction products took 6–8 h, the collection of the liquid product took 1.5–4.0 h (ca 200 ml of the liquid product was collected). The gaseous products were also simultaneously collected into Tedlar sampling bags with a volume of 5 dm3 during the collection of the liquid product. After the last hydrotreating conditions, the conditions from the first experiment were adjusted to verify the same catalyst activity at the beginning and the end of the experiment. Both samples showed the same level of desulphurisation.
3.3. Processing of the Liquid Products
The liquid products were processed considering their use for diesel fuel production. At first, they were purged with hydrogen with a flow rate of 0.5 dm3∙min−1 for 2 h to remove the hydrogen sulphide and ammonia (the products of refining the hetero compounds). If the hydrogen sulphide remained in the product, it would be oxidised into elemental sulphur during further processing, which is undesirable because of the evaluation of the desulphurisation efficiency.
The fraction boiling up to 150 °C was afterwards removed from the liquid products by the distillation in a Fischer HMS 500 distillation apparatus. The liquid product was first distilled at atmospheric pressure and then under a reduced pressure of 5 kPa until the temperature at the head of the column reached 64 °C, which is the boiling point corresponding to 150 °C under atmospheric pressure. The obtained stabilised liquid products were consequently analysed.
3.4. Analysis of the Liquid and Gaseous Products
The conversion of the rapeseed oil to hydrocarbons was verified by the simulated distillation of the liquid products using a TRACE GC ULTRA gas chromatograph (Thermo Fisher, Milano, Italy) according to the extended ASTM D2887 standard. The parameters of the simulated distillation are listed in Table 15. The cetane index was calculated according to EN ISO 4264 using the density and the simulated distillation data converted to the ASTM D86 equivalent results.

Table 15.
The chromatographic conditions of the simulated distillation.
The nitrogen and sulphur contents in the liquid products were determined on a Xplorer-NS (Trace Elemental Instruments, Delft, The Netherlands) according to the ASTM D4629 and the ASTM D5453 procedures, respectively. The density was measured according to EN ISO 12185 via an DMA 4000 (Anton Paar, Graz, Austria), the kinematic viscosity was measured according to ASTM D7042 via an SVM 3000 (Anton Paar, Graz, Austria) and the cold filter plugging point (CFPP) was measured according to EN 116 on a Callisto 100 (Anton Paar, Blankenfelde-Mahlow, Austria) coupled to a FL 601 cryostat (Julabo, Seelbach, Germany).
The content of the n-alkanes in the liquid products was determined using an Agilent 6890N gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionisation detector (GC-FID). The n-alkanes were identified by comparing their retention times with those of a standard mixture of the C6-C30 n-alkanes analysed under the same conditions as the samples. The content of each n-alkane was calculated as the relative ratio of the individual n-alkane area to the total product area. The GC-FID parameters were provided in a previous article [32].
The group-type composition of the liquid products was determined according to the EN 12916 procedure via high-performance liquid chromatography (HPLC) with a refractometric detection and a normal phase arrangement (Shimadzu Corporation, Kyoto, Japan).
The analysis of the gaseous products was performed using an Agilent 6890N gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with two detectors: A flame ionisation detector (FID) for the detection of the hydrocarbons (C1–C5) and a thermal conductivity detector (TCD) for the detection of the permanent gases. The effluent flowing out of the column was split into the detectors using a Y-piece Siltek MXT Connector (Restek, Bellefonte, PA, USA). The experimental parameters of the analysis are listed in Table 16.

Table 16.
The conditions of the chromatographic analysis of the gaseous products.
The results of the determination of the CO, CO2, and hydrocarbons were corrected to zero content of nitrogen and oxygen, which entered the sampling bags in small quantities during the collection of the gaseous hydrotreating products. As hydrogen provides a small response on the TCD detector if helium is used as a carrier gas, its direct determination can have a relatively large error. Therefore, the hydrogen content was calculated as the rest to 100 vol%.
3.5. Calculation of Hydrogen Consumption
The hydrogen and other compounds contained in the gaseous products can be dissolved in varying amounts in the liquid hydrotreating products. It can affect the correct determination of these compounds. Therefore, the consumption of hydrogen could not be precisely calculated from the amount and composition of the gaseous products. The consumption of the hydrogen was, thus, calculated from the mass balance and from the composition of the feedstocks and the liquid and gaseous products (excluding the hydrogen content). Moreover, the calculation made it possible to determine the approximate hydrogen consumption for the individual reactions taking place during the hydrotreating and is described in the Supplementary Material.
4. Conclusions
This study compared the hydrotreating of the mixture of petroleum middle distillates and the same mixture containing 20 wt % of rapeseed oil (RO). Experiments were carried out in a fixed-bed reactor over a commercial Ni-Mo/γAl2O3 catalyst at a temperature range of 320–350 °C, a pressure of 4 MPa, a WHSV of 1.0 h−1 and at a ratio of hydrogen to feedstock of 240 m3·m−3.
The total conversion of the RO to hydrocarbons, CO, CO2, CH4, propane and water was achieved at all the tested reaction temperatures. The content of the saturated hydrocarbons was, therefore, higher in the hydrotreating products of the RO containing feedstock compared to the hydrotreating products of the neat petroleum feedstock. A higher reaction temperature favoured the hydrodecarbonylation and/or hydrodecarboxylation of the RO prior to its hydrodeoxygenation. As a result, the content of n-heptadecane in the liquid products increased with an increasing reaction temperature at the expense of the n-octadecane. The slight inhibition of desulphurisation, caused by the oxygen-containing compounds arising from the hydrotreating of the RO was observed at reaction temperatures of 340 and 350 °C. In the hydrotreating of the pure petroleum feedstock, hydrogen was mainly consumed for the dearomatisation of the feedstock. In the hydrotreating of the feedstock containing 20 wt % of RO, a large amount of hydrogen was consumed for the dearomatisation as well, but also for the saturation of the double bonds in the RO and its hydrodeoxygenation.
The hydrotreating of the feedstock containing 20 wt % of the RO under a variable WHSV (0.5, 1.0, 1.5 and 2.0 h−1) and a constant hydrogen flow rate of 28 dm3∙h−1 (a variable hydrogen/feedstock flow rate ratio in the range of 120–480 m3∙m−3) was further compared. At a WHSV of 0.5 h−1, the hydrogenation of the diaromatics into monoaromatics and the monoaromatics into saturated hydrocarbons reached its maximum rate, due to the considerable time for the reactions and the largest hydrogen/feedstock ratio. It was proved that for the deep desulphurisation of the feedstock to the sulphur level below 10 mg∙kg−1, a reaction temperature of at least 350 °C and a WHSV not higher than 1 h−1 are necessary. On the other hand, the highest tested WHSV of 2.0 h−1 combined with the lowest tested hydrogen/feedstock ratio of 120 m3∙m−3 were still sufficient for the complete RO conversion and to get diesel fuel with the acceptable other parameters (density, kinematic viscosity, cetane index and CFPP) required by the EN 590 specification.
The hydrogen consumption related to 1 m3 of the feedstock decreased slightly with an increasing WHSV and decreasing hydrogen to feedstock ratio, mainly due to the decreased extent of the hydrodearomatisation and methanation reactions. Depending on the increasing hydrogen consumption, the hydrogen excess decreased with an increasing WHSV.
Supplementary Materials
The supplementary equations and reaction details are available online at https://www.mdpi.com/article/10.3390/catal11040442/s1.
Author Contributions
Conceptualization, J.B.; methodology, J.B.; validation, D.T. and T.I.; formal analysis, P.S., P.Š.; investigation, D.T., T.I.; resources, J.B.; data curation, P.S., J.B.; writing—original draft preparation, D.T. and J.B.; writing—review and editing, P.S., P.Š., J.B.; visualization, P.S.; supervision, J.B.; project administration, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
The work was supported by the Ministry of Education, Youth and Sports of the Czech Republic from the National Sustainability Programme (NPU I LO1613, MSMT-43760/2015) and institutional support of the research organisation (CZ60461373).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neste Corporation. Neste Renewable Diesel Handbook; Neste Oil Proprietary Publication: Espoo, Finland, 2016. [Google Scholar]
- Triantafyllidis, K.; Lappas, A.; Stocker, M. The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Triantafzllidis, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0444563309. [Google Scholar]
- Šimáček, P.; Souček, I.; Pospíšil, M.; Vrtiška, D.; Kittel, H. Impact of hydrotreated vegetable oil and biodiesel on properties in blends with mineral diesel fuel. Therm. Sci. 2019, 23, 1769–1777. [Google Scholar] [CrossRef]
- Váchová, V.; Vozka, P. Processing of vegetable oils to diesel fuel. Paliva 2015, 7, 66–73. (In Czech) [Google Scholar] [CrossRef]
- Pattanaik, B.-P.; Misra, R.-D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review. Renew. Sustain. Energy Rev. 2017, 73, 545–557. [Google Scholar] [CrossRef]
- Hermida, L.; Abdullah, A.-Z.; Mohamed, A.-R. Deoxygenation of fatty acid to produce diesel-like hydrocarbons: A review of process conditions, reaction kinetics and mechanism. Renew. Sustain. Energy Rev. 2015, 42, 1223–1233. [Google Scholar] [CrossRef]
- Melero, J.-A.; Garcia, A.; Iglesias, J. Biomass catalysis in conventional refineries. In Advances in Clean Hydrocarbon Fuel Processing: Science and Technology; Khan, M.-R., Ed.; Woodhead Publishing Limited: Oxford, UK, 2011; pp. 220–221. ISBN 978-1-84569-727-3. [Google Scholar]
- Horáček, J.; Tišler, Z.; Rubáš, V.; Kubička, D. HDO catalysts for triglycerides conversion into pyrolysis and isomerization feedstock. Fuel 2014, 121, 57–64. [Google Scholar] [CrossRef]
- Kubička, D.; Tukač, V. Hydrotreating of triglyceride-based feedstocks in refineries. In Advances in Chemical Engineering; Murzin, D.-Y., Ed.; Academic Press: London, UK, 2013; Volume 42, pp. 141–194. [Google Scholar]
- Kubička, D.; Kaluža, L. Deoxygenation of vegetable oils over sulfided Ni, Mo and NiMo catalysts. Appl. Catal. A Gen. 2010, 372, 199–208. [Google Scholar] [CrossRef]
- Neste: Hydrotreated Vegetable Oil (HVO)—Premium Renewable Biofuel for Diesel Engines; Neste Oil Proprietary Publication: Espoo, Finland, 2014.
- Topsoe: Renewables. Available online: https://renewables.topsoe.com/haldor-tops%C3%B8e-renewables-about (accessed on 28 January 2020).
- Vozka, P.; Váchová, V.; Blažek, J. Catalysts for hydrotreating of liquid products from processing of biomass. Paliva 2015, 7, 59–65. (In Czech) [Google Scholar] [CrossRef]
- Boyás, R.-S.; Zárraga, F.-T.; Hernández Loyo, F.-J. Hydroconversion of triglycerides into green liquid fuels. In Hydrogenation; Karamé, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 187–216. [Google Scholar]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Eijsbouts, S.; Mayo, S.; Fujita, K. Unsupported transition metal sulfide catalysts: From fundamentals to industrial application. Appl. Catal. A Gen. 2007, 322, 58–66. [Google Scholar] [CrossRef]
- Théodet, M. New Generation of” Bulk” Catalyst Precursors for Hydrodesulfurization Synthesized in Supercritical Fluids. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, 2010. Available online: https://tel.archives-ouvertes.fr/tel-00559113/ (accessed on 4 June 2020).
- UOP: Honeywell Green Diesel. Available online: https://www.uop.com/processing-solutions/renewables/green-diesel/ (accessed on 28 January 2020).
- Eni: What Is Ecofining? Available online: https://www.eni.com/en_IT/results.page?question=what+is+ecofining%3F (accessed on 3 February 2020).
- Axens: Vegan®—Technology for Premium Quality, Drop-In Biofuels from Renewable Oils & Fats. Available online: https://www.axens.net/product/process-licensing/11008/vegan.html (accessed on 3 February 2020).
- Douvartzides, S.-L.; Charisiou, N.-D.; Kyriakos, N.; Papageridis, K.-N.; Maria, A.; Goula, M.-A. Green diesel: Biomass feedstocks, production technologies, catalytic research, fuel properties and performance in compression ignition internal combustion engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef]
- Al-Daous, M.-A.; Ali, S.-A. Deep desulfurization of gas oil over NiMo catalysts supported on alumina-zirconia composites. Fuel 2012, 97, 662–669. [Google Scholar] [CrossRef]
- Donnis, B.; Egeberg, R.-G.; Blom, P.; Knudsen, K.-G. Hydroprocessing of bio-oils and oxygenates to hydrocarbons. Understanding the reaction routes. Top. Catal. 2009, 52, 229–240. [Google Scholar] [CrossRef]
- Templis, C.; Vonortas, A.; Sebos, I.; Papayannakos, N. Vegetable oil effect on gasoil HDS in their catalytic co-hydroprocessing. Appl. Catal. B Environ. 2011, 104, 324–329. [Google Scholar] [CrossRef]
- De Paz Carmona, H.; de la Torre Alfaro, O.; Brito Alayón, A.; Romero Vázquez, M.-A.; Macías Hernández, J.-J. Co-processing of straight run gas oil with used cooking oil and animal fats. Fuel 2019, 254, 115583. [Google Scholar] [CrossRef]
- Tóth, C.; Baladincz, P.; Kovács, S.; Hancsók, J. Producing clean diesel fuel by co-hydrogenation of vegetable oil with gas oil. Clean Technol. Environ. Policy. 2011, 13, 581–585. [Google Scholar] [CrossRef]
- Endisch, M.; Kuchling, T.; Roscher, J. Process Balances of Vegetable Oil Hydrogenation and Coprocessing Investigations with Middle-Distillates. Energy Fuels 2013, 27, 2628–2636. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072. [Google Scholar] [CrossRef]
- Palanisamy, S.; Gevert, B.-S. Hydrodeoxygenation of fatty acid methyl ester in gas oil blend-NiMoS/alumina catalyst. Green Process. Synth. 2018, 7, 260–267. [Google Scholar] [CrossRef]
- Gong, S.; Shinozaki, A.; Qian, E.-Q. Role of support in hydrotreating of jatropha oil over sulfided NiMo catalysts. Ind. Eng. Chem. Res. 2012, 51, 13953–13960. [Google Scholar] [CrossRef]
- Vozka, P.; Orazgaliyeva, D.; Šimáček, P.; Blažek, J.; Kilaz, G. Activity comparison of Ni-Mo/Al2O3 and Ni Mo/TiO2 catalysts in hydroprocessing of middle petroleum distillates and their blend with rapeseed oil. Fuel Process. Technol. 2017, 167, 684–694. [Google Scholar] [CrossRef]
- Kochetkova, D.; Blažek, J.; Šimáček, P.; Staš, M.; Beňo, Z. Influence of rapeseed oil hydrotreating on hydrogenation activity of CoMo catalyst. Fuel Process. Technol. 2016, 142, 319–325. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).