2.1. Characterization Results
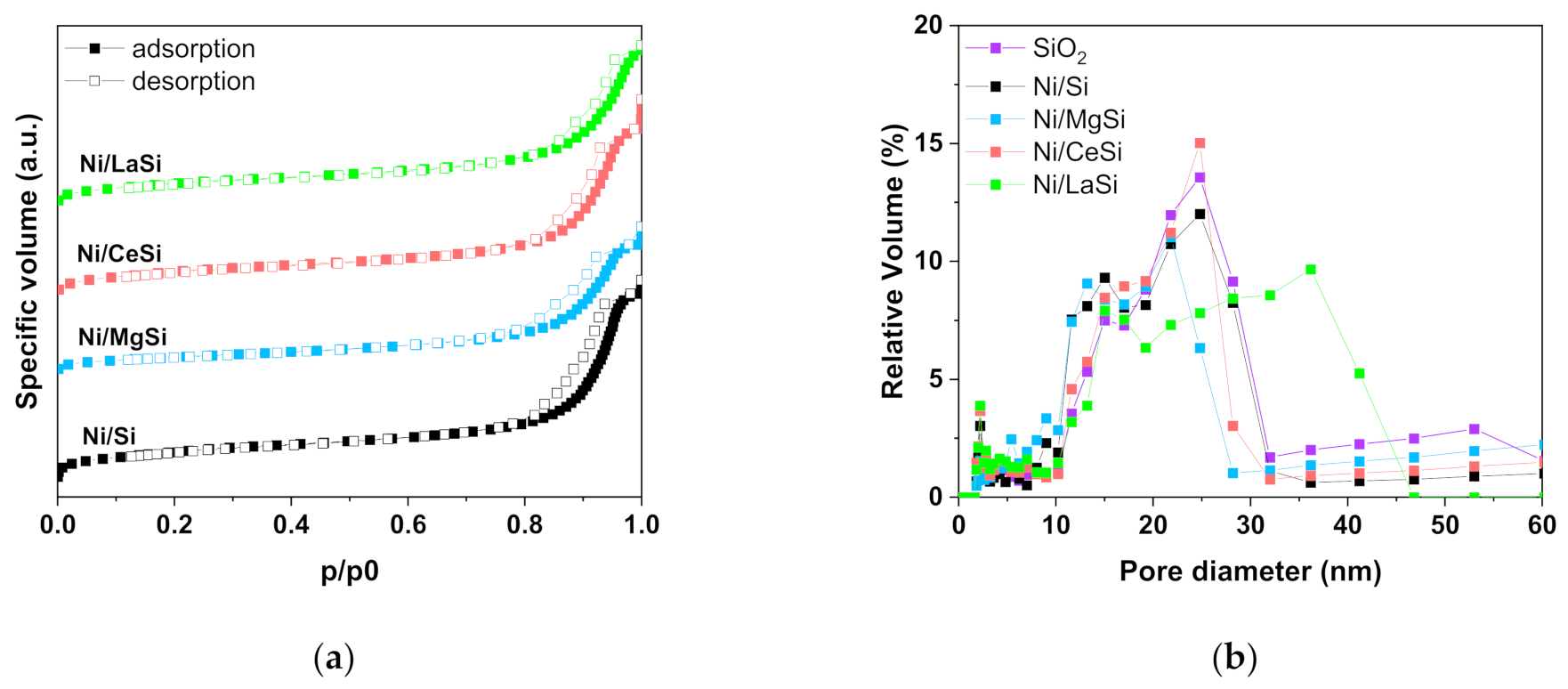
The porous structure of the prepared SiO
2 and corresponding Ni/Oxide-SiO
2 catalysts was investigated by means of N
2 adsorption-desorption isotherms (BET method). The type IV(a) isotherm shape with a H5 hysteresis loop is indicative for mesoporous materials with open and partially blocked pores [
36]. Upon addition of supplementary oxide, the shape of the isotherms is preserved as illustrated in
Figure 1a for the investigated catalysts. Surface area of the as prepared silica is 462.2 m
2/g, with a corresponding pore volume of 1.24 cm
3/g (
Table 1). For the mixed oxides supports, as well as for the corresponding Ni catalysts, the surface area and pore volume decrease in the order Ni/Si > Ni/CeSi > Ni/LaSi > Ni/MgSi (see
Table 1). In respect to the pore size distribution,
Figure 1b reveals a multimodal pore structure of the silica support: (i) small pores with average dimensions around 2.4 nm (around 10 % of the total number), and (ii) larger pores with sizes in the 10–30 nm range. In this latter region, two distinctive pore dimensions are also evidenced, that is 15 nm, and 25 nm average values, respectively. The pore size distribution of Ni/Oxide-SiO
2 materials is preserved to a large extent, with a more pronounced separation of the two pore sizes in the 10–30 nm window, as compared to SiO
2 alone. However, a certain influence of the MgO and La
2O
3 presence upon the pore size distribution can be distinguished: (i) some of the smallest pores collapsed, this being reflected by the broadening of the pores size distribution in this area (2.2–4.4 nm); (ii) the pores with intermediate size remained approximately unchanged (15 nm); and (iii) the dimension of larger pores decreased for Ni/MgSi, and significantly increased for Ni/LaSi.
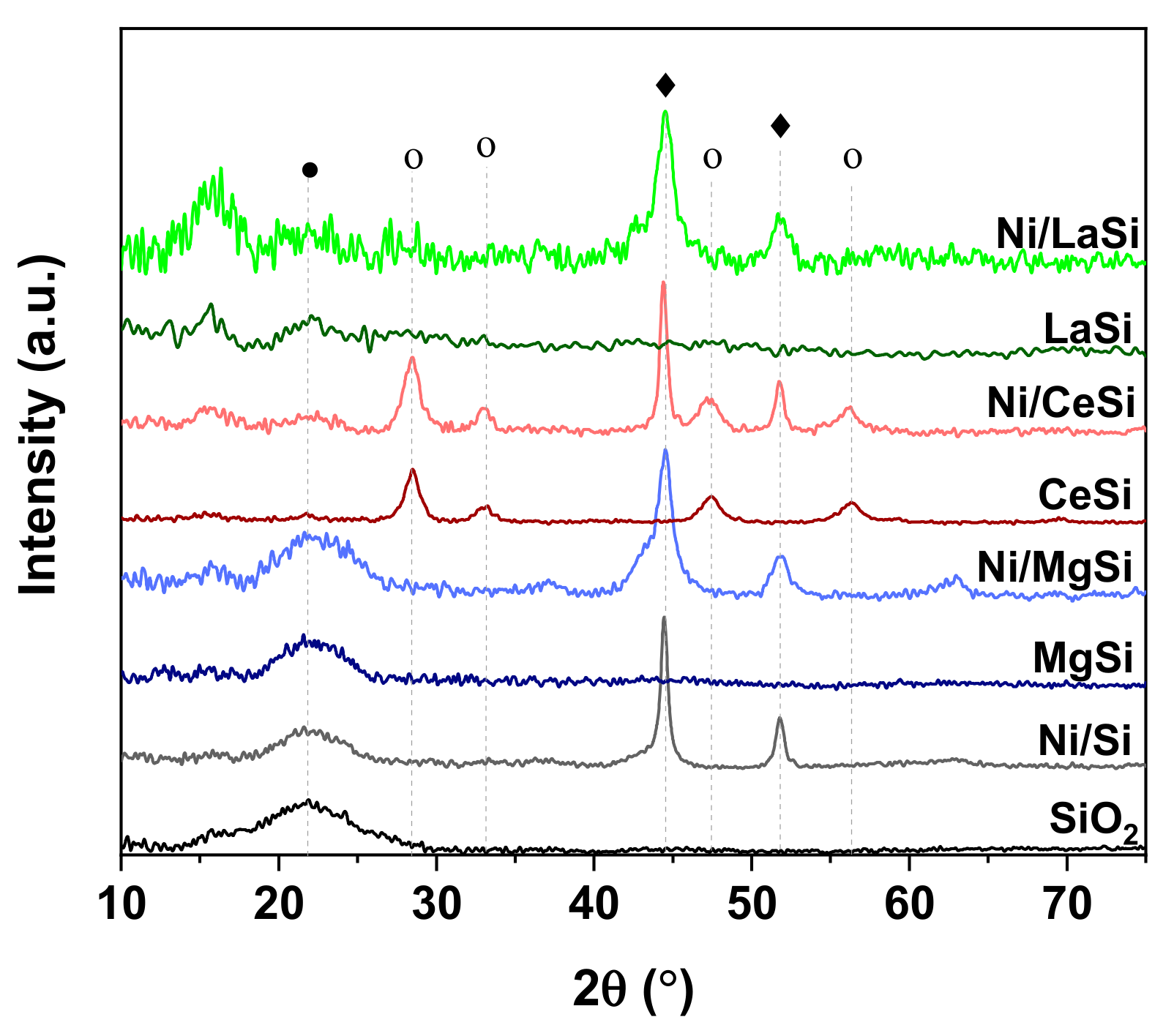
XRD analysis was used to evaluate the composition and crystallinity of the oxide-silica supports, as well as the size of Ni crystallites. The as-synthesized SiO
2 reveals an amorphous structure, with its typical large diffraction peak centered around 22° (
Figure 2) (JCPDS 89-3435). Upon addition of the second oxide, except for CeO
2-SiO
2, the XRD patterns of the Oxide-SiO
2 supports do not show distinctive diffraction peaks corresponding to the additional oxide. This might be an indication of either very good dispersion of the second oxide on silica, or its prevalence in a rather amorphous phase. In the case of the CeO
2-SiO
2 XRD pattern, the broad peak corresponding to SiO
2 is very low, in comparison to the sharp and high reflexions generated by the more crystalized ceria: CeO
2(111) at 28.5°, CeO
2(200) at 33.2°, CeO
2(220) at 47.4°, and CeO
2(311) at 56.7° [
37,
38] (JCPDS 43-1002). A partial coverage of SiO
2 by crystalline CeO
2 cannot be excluded. Upon reduction at 600 °C in H
2, all Ni based catalysts reveal diffraction lines corresponding to metallic Ni: Ni(111) at 44.5°, and Ni(200) at 51.8° (JCPDS 65-0380). For Ni/CeSi the XRD pattern reveals the presence of silica, CeO
2 and metallic Ni. For the lanthana containing catalyst, the “noisy” aspect of the XRD pattern at low 2θ angles suggests the presence of poorly crystalized La
2O
3 (JCPDS 065-3185), but also the possible presence of poorly crystalized La(OH)
3 (JCPDS 13-1481) and carbonated lanthana La
2O
2CO
3 (JCPDS 37-0804), which both present important reflexions in this 2θ range. In case of Ni/MgSi, except for the specific reflexions of SiO
2 and Ni, three additional peaks can be observed at 37.1°, 43.1° and 63.1°. These reflexions can be attributed to NiO (JCPDS 89-7390), but the presence of NiO-MgO mixed phase (JCPDS 24-0712) cannot be excluded. The XRD patterns of all other Ni based catalysts do not reveal the presence of any remanent NiO, proving thus the complete reduction. The incomplete reduction of NiO in Ni/MgSi is attributed to the higher interaction of NiO with the support constituents (MgO and SiO
2), leading thus to a lower reducibility of NiO. The very good dispersion of MgO and La
2O
3 on the silica surface was previously reported for silica [
29,
31], as well as for ordered mesoporous silica [
30,
33] supported catalysts.
Ni crystallite size was estimated by means of Scherrer’s equation, using the Ni(200) peak situated at 51.8° (see
Table 1). Ni crystallite sizes of 15.2 nm were obtained for Ni/Si, while catalysts with additional oxide reveal smaller Ni crystallites. It should be noted, however, that the decrease of crystallite size is quite small for the Ni/CeSi catalyst (14.2 nm), while the other two materials show crystallite dimensions around 6.2 nm. These data suggest a much better dispersion of Ni particles on the mixed oxides support for the magnesia and lanthana containing catalysts.
TEM images of Ni/Si revealed a non-ordered mesoporous structure, with irregularly shaped pores, which is maintained for the supplementary oxides containing catalysts, as well. By analyzing the TEM images of silica supported catalysts, a good dispersion of relatively uniform shaped and sized Ni nanoparticles (Ni NPs) is observed for each catalyst prepared in this work. EDX mapping revealed that MgO and La¬
2O
3 are very well dispersed on the catalysts’ surface, the Mg distribution for example, being very similar to that of Si and O. CeO
2 is evidenced as small islands of 20–30 nm, uniformly distributed on the catalyst surface. These results are in good agreement with the XRD patterns presented above, where the only additional oxide clearly present in the diffractograms is CeO
2. Ni NPs present a narrow size distribution (
Figure 3), the average size (
Table 1) being also in accordance with the Ni crystallite size determined from XRD. It is important to notice that the catalytic materials for which very high dispersion of the additional oxide is observed (Ni/MgSi and Ni/LaSi) also present an important decrease of Ni NPs size compared to the Ni/Si catalyst.
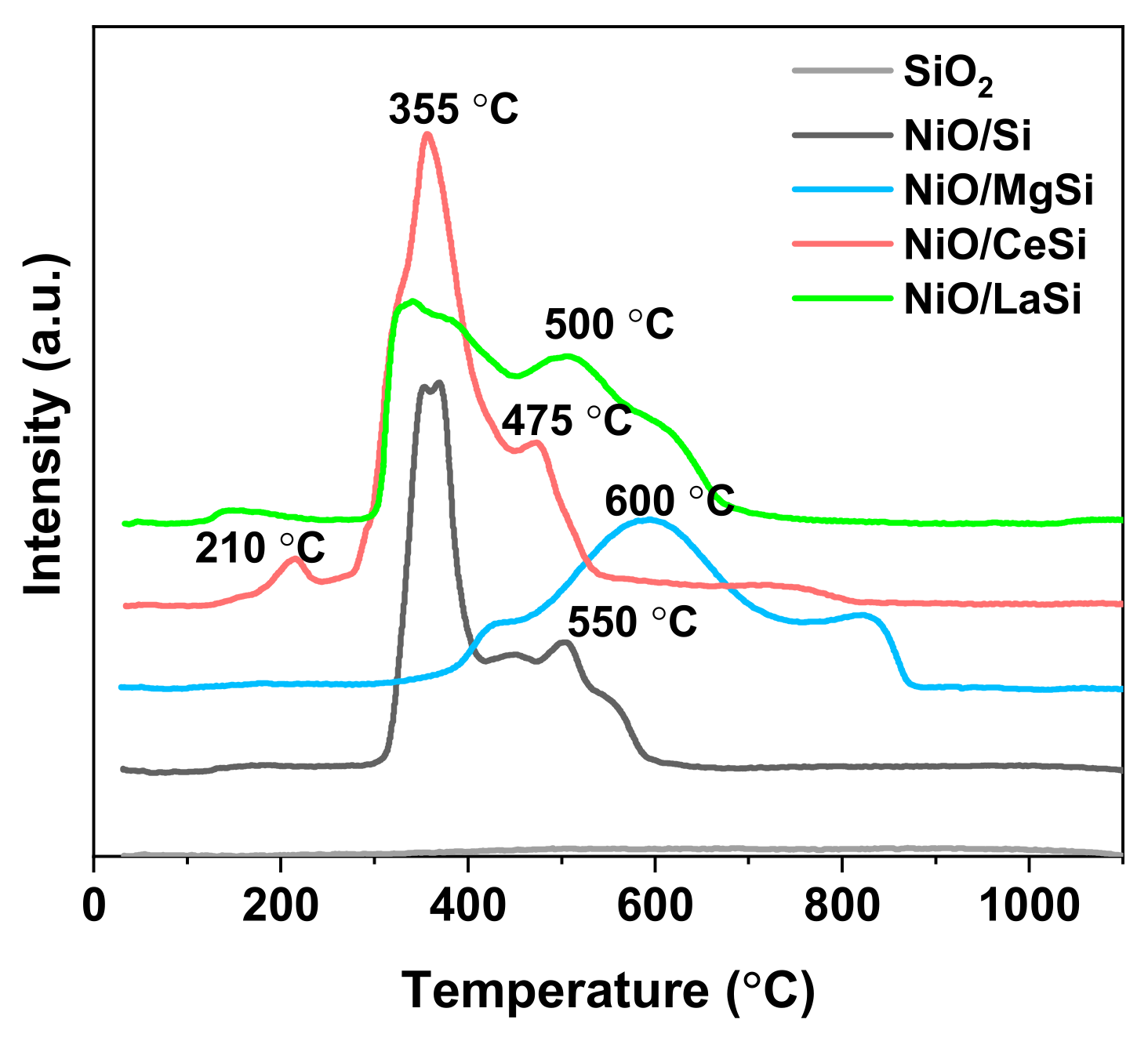
The reducibility of the supported NiO nanoparticles upon treatment in hydrogen is a valuable indicator of the strength of the NiO–support interaction and, at the same time, provides information about the metal–support interaction in the reduced catalysts. As NiO reduction is a one-step process, the multiple peaks observed in the TPR profiles (
Figure 4) are generated by the differences in the NiO interaction with the support. For silica supported nickel catalysts, previous publications identified a temperature threshold of 450 °C: the reduction peaks situated below this temperature indicate the presence of NiO NPs in low interaction with the support, while the peaks situated at higher temperatures correspond to the reduction of NiO strongly bonded to the silica support [
28]. For NiO/Si, the TPR profiles (
Figure 4) show the existence of NiO NPs with two different strengths of interaction with the support: low bonded NPs which correspond to the reduction peak situated at 360 °C, while the lower and broader peak situated at 500–550 °C corresponds to NiO NPs in strong interaction with the support. The addition of CeO
2 does not significantly change the reducibility of catalysts precursors, the difference being the lower proportion of strongly bonded NiO NPs, which are also reduced at a slightly lower temperature: 475 °C compared to 500 °C. Opposite to NiO/Si, NiO/LaSi and NiO/MgSi which contain only irreducible oxides alongside NiO, in case of NiO/CeSi the reduction of ceria can also occur during TPR measurements. Thus, the smaller peak situated at 210 °C was previously attributed either to the reduction of adsorbed oxygen species [
26], or to the surface reduction of ceria [
39]. A small reduction peak can be observed at high temperature (750 °C), and was attributed to the reduction of Ce
4+ to Ce
3+ [
26]. The reduction of ceria species alongside with NiO is reflected in the larger amount of consumed hydrogen in H
2-TPR experiments, as compared to the rest of the catalysts (
Table 2). Thus, for the ceria containing catalyst prepared in this work, the interaction of NiO with the CeO
2-SiO
2 support is similar to the interaction with SiO
2. This observation is in good correlation with the XRD and TEM results, which evidenced Ni crystallites/nanoparticles of similar size as the ones from Ni/Si catalysts, which are situated on materials with very similar surface area and pore size distribution. A similar effect of CeO
2 addition to electrospinning prepared silica [
28], and SBA-15 [
40] supported Ni catalyst was previously reported, while for Ni-CeO
2-MCM-41 [
25], and Ni-CeO
2-SBA-16 [
26] the reducibility is lowered by the presence of ceria. The addition of La
2O
3 and MgO, instead, drastically changes the TPR profile, proving the significant changes in the NiO interaction with MgO-SiO
2 and La
2O
3-SiO
2, compared to the bare SiO
2. For NiO/LaSi, the reduction peak situated at 550 °C for Ni/Si is slightly shifted to 600 °C, but the proportion of hard reducible NiO is significantly higher than in Ni/Si. This is an indication of the presence of higher proportion of strongly bonded Ni NPs in Ni/LaSi compared to Ni/Si. In other words, the La
2O
3-SiO
2 support better interacts with NiO NPs, this being reflected in smaller Ni NPs in the reduced catalyst, as revealed by the TEM images. A similar effect of Ni NPs dispersion and stabilization on the surface was observed on lanthana containing Ni-SBA-15 catalyst [
30]. In the case of Ni/MgSi, there are also two types of NiO, but their reducibility is much lower than for Ni/Si, the main reduction peaks being situated at 600 °C, and 850 °C. This very hard reducible NiO remained unreduced in Ni/MgSi as evidenced by XRD analysis (
Figure 2). The peak situated at 600 °C is given by the reduction of Ni
2+ from sub-surface layer of NiO-MgO solid solution, while the peak situated at 825 °C might be given by the reduction of Ni
2+ in the MgO matrix, indicating a more intimate interaction between MgO and NiO, or the existence of NiMgO
2 [
41,
42,
43]. The important decrease of catalyst reducibility upon MgO addition to Ni/oxide catalyst due to the difficulty of reduction of Ni
2+ species in intimate contact/chemical combinations with MgO is well documented [
24,
32,
43,
44].
The degree of NiO–support interaction, reflected in the reducibility of nickel oxide, is not only influenced by the crystallites/nanoparticles size, but also by the NPs position on the mesoporous silica support. Analyzing the data provided by porosity measurements, XRD experiments, TEM images interpretation and TPR analysis, it was concluded that the hard-reducible NPs are most probably located inside the medium sized mesopores (14 nm), benefiting thus from the stabilizing effect of pore confinement. The NPs with lower interaction with the support are situated in the larger pores and/or on the grain surface. Ni/LaSi and Ni/MgSi are expected to have a significant proportion of Ni NPs inside the medium size mesopores. This observation is supported by: (i) the higher proportion of strongly bonded Ni NPs as revealed by H
2-TPR for both Ni/LaSi and Ni/MgSi; (ii) the Ni NPs size is significantly lower than the pore size (
Table 1), in contrast to Ni/Si for which the dimensions of the Ni NPs and of the support pores are more similar; and (iii) the lower surface area of these two catalysts compared to Ni/Si. This lower value of surface area is given by the presence of mesopores partially filled with Ni NPs, which are small enough to not produce the pore blockage. The observed H5 hysteresis loop also supports this affirmation [
36].
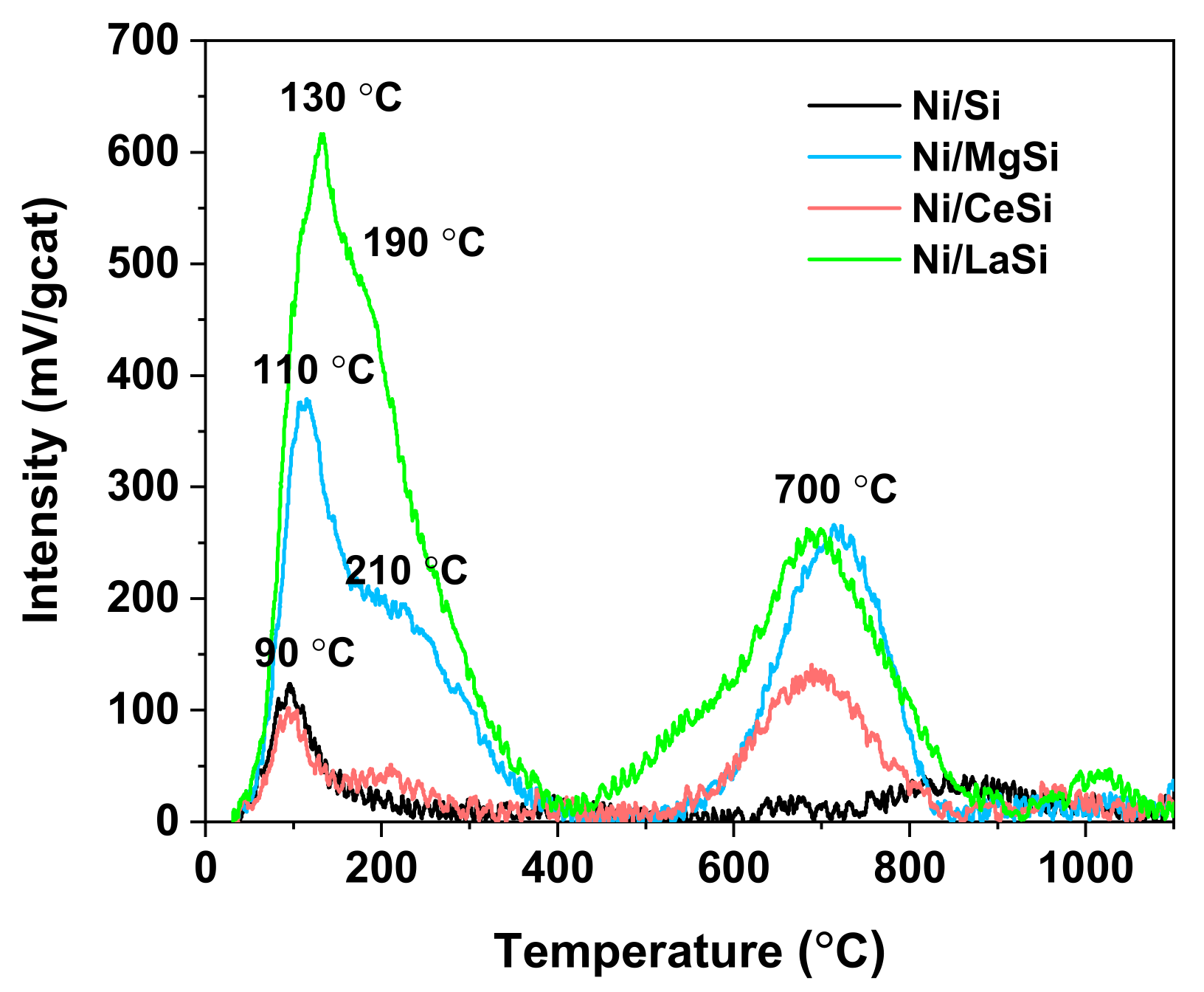
The strength of catalytic active sites for H
2 adsorption was studied by H
2-TPD. For all four catalysts studied in this work, a clear separation of two types of hydrogen desorption peaks was observed: those situated under 400 °C and denoted as type I, and those situated at temperatures higher than 400 °C and denoted as type II (
Figure 5). Type I peaks are generated by the desorption of hydrogen originated from metallic sites, while type II peaks correspond to the desorption of hydrogen from the support by reverse spillover [
45]. In the type I region of the spectra, one main desorption peak situated at 90 °C can be observed for Ni/Si, usually attributed to the removal of H
2 weakly chemisorbed to the surface. For the rest of the catalysts, two desorption peaks can be observed in this region, suggesting the presence of two types of hydrogen adsorption centers having different capacities to activate and bond hydrogen. For Ni/CeSi, the first peak is situated at 90 °C, similar to Ni/Si, and the second one at 210 °C. For Ni/MgSi, the first TPD peak is shifted to higher temperatures, that is 110 °C, while the position of the broad second peak is not significantly changed. For Ni/LaSi, the position of the first peak is further shifted to 130 °C, while the second peak appears at 190 °C. Based on the position of the desorption peaks it can be stated that the strength of catalytic active sites for hydrogen adsorption and activation decreases in the order: Ni/LaSi > Ni/MgSi > Ni/CeSi ≈ Ni/Si. The quantity of desorbed hydrogen from type I catalytic sites (
Table 2) is approximately the same for Ni/Si and Ni/CeSi, but increases dramatically for Ni/MgSi, and especially for Ni/LaSi. Ni dispersion, calculated from the quantity of desorbed hydrogen in the type I region, follows the same pattern, being approximately five times larger for Ni/LaSi compared to Ni/Si. The presence of both MgO and La
2O
3 on the catalyst support plays a significant role in enhancing the catalyst’s capacity to activate hydrogen by increasing the number of catalytic active sites. This information is in good correlation with XRD/TEM results, which both evidenced much lower crystallite/nanoparticles in Ni/LaSi, but also with H
2-TPR results which suggested a higher proportion of Ni NPs stabilized on the surface by strong interaction with the support. It is interesting to note that the presence of the second oxide significantly increases the intensity of the peaks in the type II region of the spectra, indicating the tendency of these materials to host strongly bonded hydrogen, either on support, or on sub surface layers. The reaction temperatures employed in this study are lower than those necessary to activate this type of hydrogen.
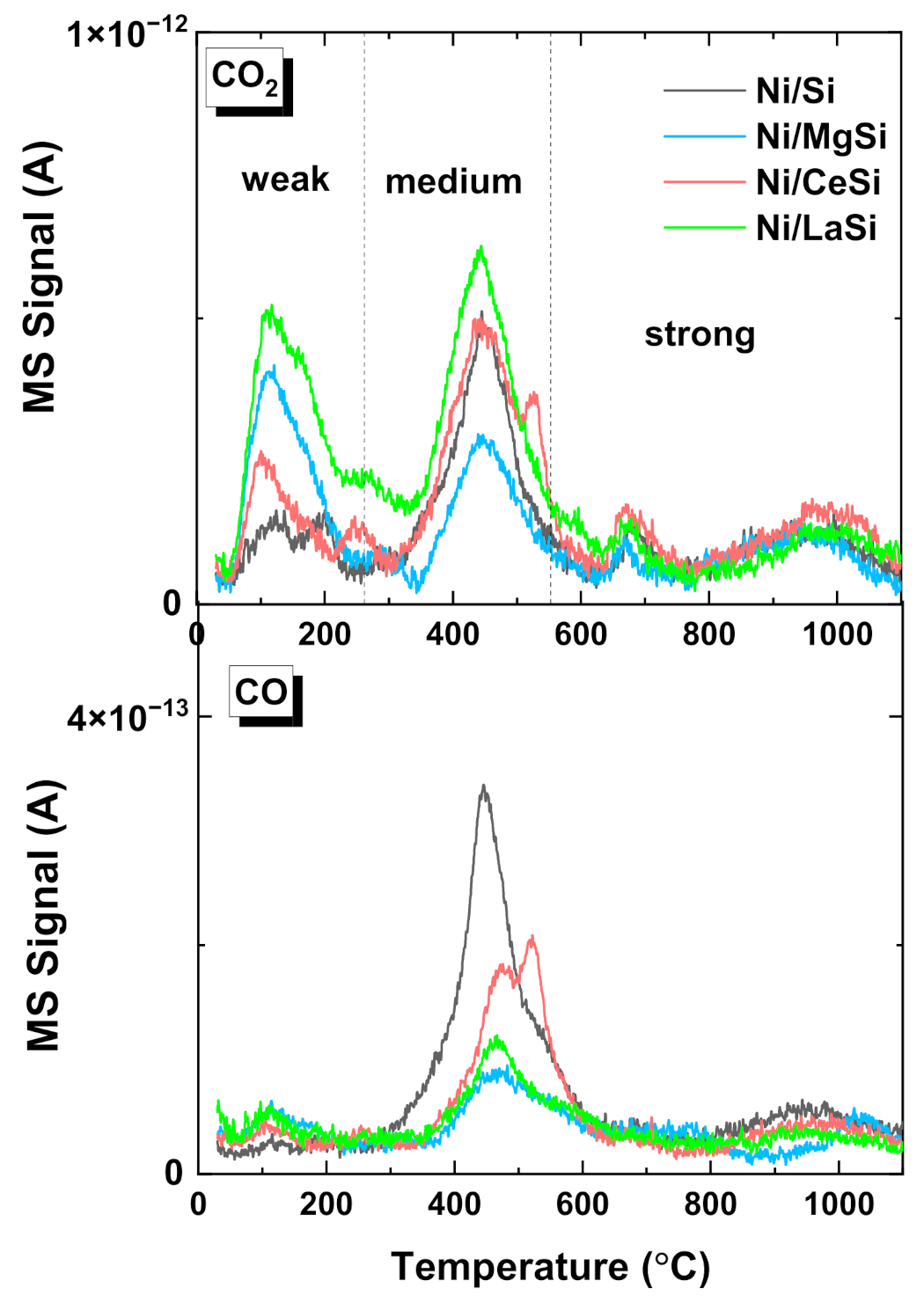
CO
2-TPD was used to assess the strength of active centers for CO
2 adsorption, and the influence of the additional oxide on the carbon dioxide activation in the reaction. Being a weak Lewis acid molecule, the adsorption of CO
2 is favored by the existence of basic sites, either on the support, or on the metal-support interface. For Ni deposited on acidic supports, the CO
2 adsorption on (O-terminated) Ni metallic sites, or unreduced NiO was also considered, motivated by the low capacity of these supports to adsorb CO
2 [
46]. Most authors agree to classify the strength of basic sites from the catalysts’ surface in correlation to the desorption temperatures, as follows: (i) weak sites corresponding to the desorption peaks situated below 200–250 °C; (ii) medium basic sites for the CO
2 desorbed between 250 °C and 550–600 °C; (iii) and strong basic sites for the peaks situated above 600 °C [
46,
47]. Low, moderate and strong basic sites correspond to surface OH
−, Lewis acid-base pairs, and low coordination surface O
2−, respectively [
48]. Although the mechanism of CO
2 transformation to methane essentially depends on the catalyst composition, and is still open to debate for some materials, it is generally accepted that CO
2 conversion depends on the presence and quantity of medium basic sites. The CO
2 weakly physisorbed could be insufficiently activated for the reaction, while the CO
2 chemically adsorbed to the strong sites do not participate in the reaction. For the silica supported materials prepared in this work there is a clear delimitation of the three types of basic sites, while the presence of supplementary oxides significantly influences their proportion (
Figure 6). For Ni/MgSi, a significant increase of weak adsorption centers is observed, suggesting that MgO creates additional weak basic centers on the catalyst, but the proportion of medium basic sites is significantly decreased compared to Ni/Si. For Ni/CeSi, an enhancement of weak and weak-medium adsorption is observed, but also the appearance of a supplementary desorption peak situated in the medium to strong region (527 °C) was noticed. An enhancement of weak and medium basic sites for the ceria containing material was reported for Ni-CeO
2(10%)-SBA-16, attributed to the CO
2 species adsorbed over Ce
3+ sites, and was considered to play an important role in the methanation reaction mechanism [
26]. For Ni/LaSi an important increase of both weak and moderate basic sites compared to all other materials prepared in this work was observed. This enhanced CO
2 adsorption was previously attributed to the formation of lanthanum oxycarbonate (La
2O
2CO
3), and formate species [
30]. Low concentrations of CO (one order of magnitude lower than for CO
2) were also detected, proving the existence of low proportion of dissociative CO
2 adsorption for all four investigated catalysts. CO desorption peaks are situated around 450–500 °C, their intensity in case of catalysts containing additional oxide being much lower than for Ni/Si.
2.2. Catalytic Activity Tests
All four silica supported materials prepared and characterized in this work were evaluated as catalysts for low temperature CO2 methanation using the temperature programmed reaction technique (TPRea), in the temperature window of 30–350 °C (2 °C/min), and at atmospheric pressure. Silica support was also tested, and no significant CO2 conversion was obtained in the studied temperature interval. The composition of the dried effluent gases was: CO2, H2, CH4, and CO. The first two are unreacted reagents, while the last ones are the products of the methanation reaction and of the reverse water gas shift reaction (RWGS), respectively.
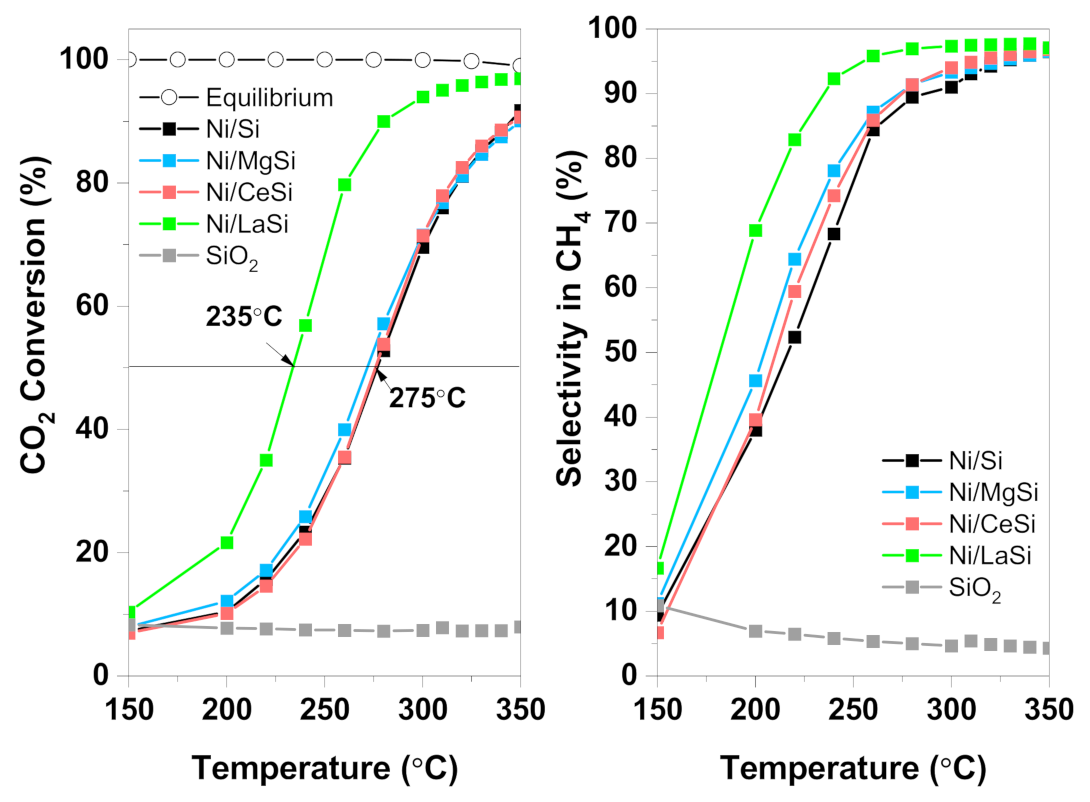
Up to 150 °C, very low CO
2 conversion was observed for all catalysts (
Figure 7). Above 200 °C the CO
2 conversion sharply increased, reaching values higher than 90% at 350 °C. At temperatures above 300 °C, no important differences between catalysts were observed, except for Ni/LaSi. Under these conditions, the reaction is controlled by temperature, and the catalysts’ properties which make the difference at lower temperature are no longer important. The addition of CeO
2 brought no improvement to the catalytic activity, while the addition of MgO only slightly improved the CO
2 conversion in the 200 °C–300 °C temperature range. Lanthana containing catalyst instead, performed remarkably well in the methanation of CO
2, both CO
2 conversion and CH
4 selectivity being significantly improved, especially at low temperatures. For this material, 50% conversion is reached at 235 °C, while for Ni/Si T
50% = 275 °C, and for Ni/MgSi it is 268 °C. CO
2 conversion of 95% is obtained for Ni/LaSi at temperatures as low as 300 °C. For comparison, Ni/Al
2O
3 tested in identical conditions reached 50% CO
2 conversion at 345 °C [
49]. In terms of CH
4 selectivity, the addition of MgO and CeO
2 to the Ni/Si catalyst brought some advantages at low temperatures. For example, at 240 °C, methane selectivity is 61.2% for Ni/Si, while 65.5% for Ni/CeSi, and 70% for Ni/MgSi. Above 300 °C instead, methane selectivity presents similar values for all three catalysts, being~91% at 300 °C, and 96% at 350 °C. For Ni/LaSi, CH
4 selectivity is significantly improved compared to Ni/Si, reaching 89% at 240 °C, and 97% at 350 °C. In order to evaluate the performance of silica supported catalysts investigated in this work in relation with other supported Ni catalysts, a comparative analysis was made with Ni/Al
2O
3 and Ni/MIL-101-Al
2O
3 which were tested in identical reaction conditions [
49]. In respect to both CO
2 conversion and methane selectivity, the results described in this work are better than those previously published for alumina supported catalysts. For example, in the case of Ni/Al
2O
3, 50% CO
2 conversion was reached at 345 °C, while for Ni/MIL-101-Al
2O
3 at 302 °C. Regarding methane selectivity at 350 °C, 72% was obtained for Ni/Al
2O
3, and 94% for Ni/MIL-101-Al
2O
3, compared to more than 95% for silica supported catalysts used in this work. However, comparison with other silica supported Ni catalysts tested in the methanation of CO
2 is difficult to be done due to the differences in testing conditions, as well as the different concentrations of Ni and/or additional oxide. For example, similar~60% CO
2 conversion at 250 °C was reported for Ni/La
2O
3-SBA-15 (10 wt.% Ni) [
30], but the reaction conditions used in this case are so different (nH
2/nCO
2 = 4:1, C
CO2 = 18%, 1 MPa, 6000 mL·h
−1·g
−1) that a comparison with our results is not relevant.
When comparing the behavior of silica and promoted silica supported Ni catalysts for CO
2 methanation, one must consider and analyze the two processes involved in the catalytic reaction: (i) the dissociative H
2 adsorption on the Ni active sites, and (b) the adsorption of CO
2 on the basic sites. In this study, the catalyst with best performances for CO
2 methanation was Ni/LaSi. This remarkable activity is due to the good structural characteristics of the catalyst (low Ni NPs size, very good dispersion of both La
2O
3 and Ni) which provide a high number of Ni active sites for hydrogen activation (
Table 2), but also to the enhanced proportion of weak-medium basic sites compared to all other catalysts used in this work (
Figure 6). Thus, for Ni/LaSi catalyst, both H
2 activation and CO
2 adsorption are considerably enhanced. Additionally, for Ni/LaSi employed in this study, the high improvement in catalytic activity is also due to the multimodal porous structure of this material. This helps: (i) to better stabilize the Ni NPs inside the medium size mesopores (as shown in the characterization section), and (ii) to guide the reagent molecules to the catalytic centers by the “tunnel effect” provided by the larger pores. These former types of pores are with one third larger for Ni/LaSi compared to the other materials in this work, facilitating thus the gas transport through the catalyst grain. Three studies related to CO
2 methanation over lanthana promoted silica supported nickel catalysts were recently reported [
29,
30,
31], two of them presenting also the lanthana involvement in the CO
2 methanation mechanism (Ni-La
2O
3-SiO
2 [
29], and Ni-La
2O
3-SBA-15 [
30]). A similar improvement of the catalytic performances for the lanthana containing catalysts compared to the unpromoted ones were observed in these studies, but a direct comparison of the results reported in these papers with the ones obtained in this work is difficult due to the differences in the composition and employed reaction conditions for both catalysts.
Ni/MgSi presents equally good structural characteristics as Ni/LaSi: Ni NPs of similar small size as Ni/LaSi, most probably placed inside the medium size mesopores, and very good dispersion of MgO, but its catalytic activity still is much lower than that of Ni/LaSi, being actually only slightly better than Ni/Si. This can be explained by the hard-reducible nature of this material, which leads to the existence of Ni in unreduced form in the final catalyst. This fact is reflected in the number of active sites for hydrogen activation, which, although significantly larger than for Ni/Si, is still low compared to Ni/LaSi (
Table 2). Regarding the activation of CO
2 for the reaction, CO
2-TPD profile for Ni/MgSi showed the lowest proportion of moderate basic sites in the catalyst series employed in this study. The positive effect of higher number of active sites for hydrogen activation is negatively balanced by the low proportion of medium sites for CO
2 activation. The performances of this material emphasize the need for an optimum catalyst composition, which can provide the best equilibrium between the two processes of the catalytic reaction.
Surprising results were obtained for Ni/CeSi compared to Ni/Si, for which only a slight improvement in CH
4 selectivity was noticed, and no influence on the conversion of CO
2 was observed. This can be explained by taking into account the catalyst’s structure. In contrast to magnesia and lanthana containing catalysts prepared in this work following the same procedure, Ni/CeSi catalyst presents very few structural enhancements compared to Ni/Si. No improvement in Ni NPs size or dispersion were obtained, and the number of Ni catalytic active sites is very similar to Ni/Si. This fact is reflected in a very similar behavior of these two catalysts for hydrogen adsorption. CeO
2 is not as well dispersed on the silica surface as MgO or La
2O
3, and the involvement of ceria in the catalytic process by creating oxygen vacancies on the surface to effectively activate CO
2 for the reaction is dependent on the ceria dispersion. The size distribution of Ni NPs suggests that a significant proportion of NPs are probably placed in larger mesopores, or on the grain surface, being thus less stabilized on the surface. The slight improvement in methane selectivity can be due to the ceria activity for the water gas shift (WGS) reaction, and subsequent inhibition of the reverse water gas shift (RWGS) reaction (CO
2+H
2→CO+H
2O) [
50].
This behavior of Ni/CeSi is opposite to the results recently reported for CO
2 methanation using Ni-CeO
2-SiO
2 [
28], Ni-CeO
2-MCM-41 [
25], and Ni-CeO
2-SBA-16 [
26], for which the ceria containing catalysts presented significantly better results than the Ni/silica counterparts, especially at low temperatures. Analyzing the mentioned reported results, it was obvious that in all cases the presence of ceria brought significant better structural characteristics accompanied by enhanced capacity to adsorb hydrogen, and by more medium basic sites for CO
2 activation. It was concluded that the method used in this work is not suitable for the preparation of Ni-CeO
2-SiO
2 materials with improved structural and functional properties for CO
2 methanation. The results obtained in this study for Ni/CeSi emphasize the importance of optimum correlation between catalyst composition, selected preparation method, and catalysts’ structural and functional properties.
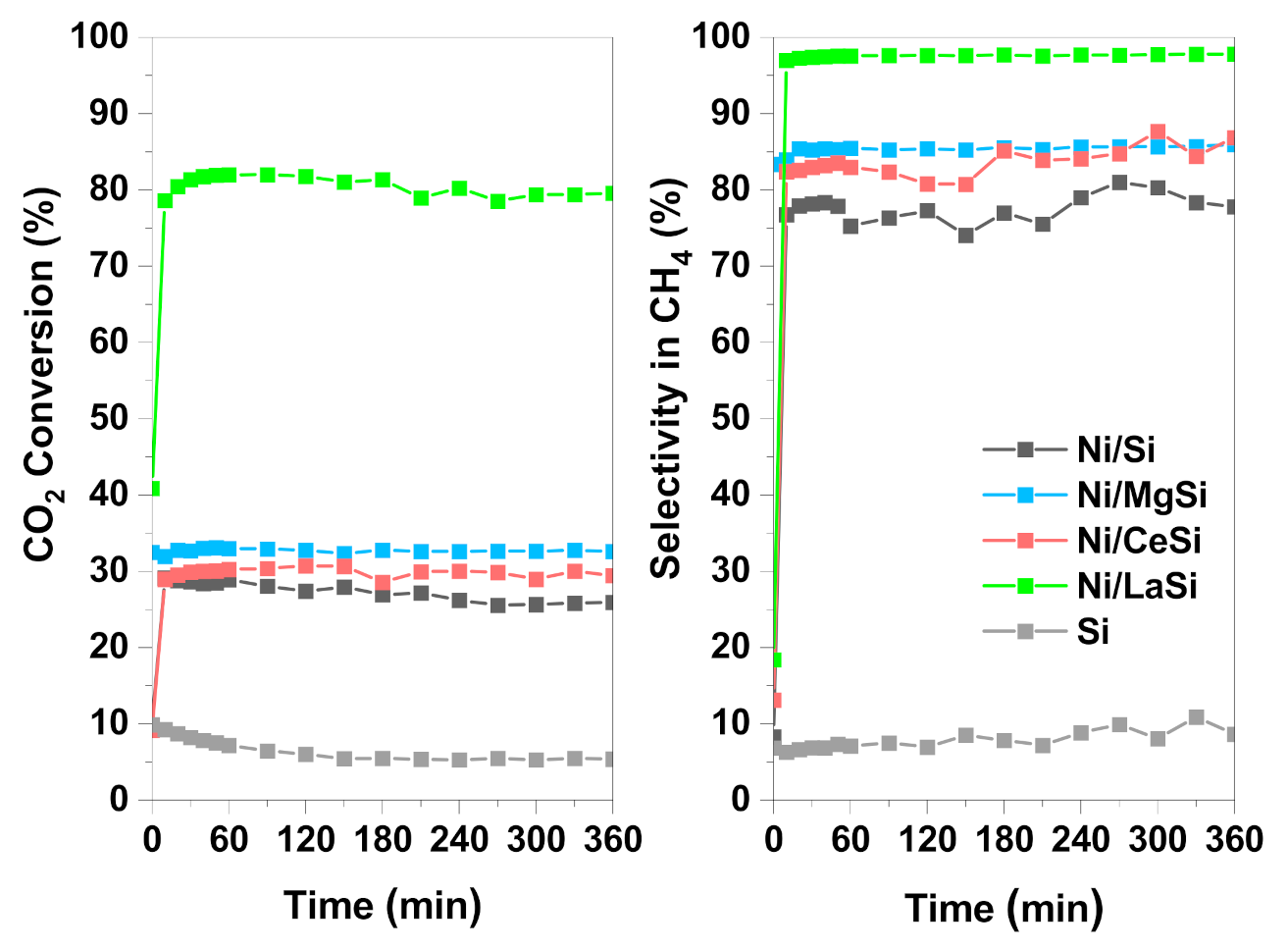
All four materials studied in this work were tested under steady state conditions, for 6 h time on stream (TOS), at 250 °C, and at atmospheric pressure (
Figure 8). The low reaction temperature was selected in order to have the proper conditions to study the catalyst influence, but also to demonstrate the very good performances of Ni/LaSi for low temperature CO
2 methanation. As expected, the catalytic activity of the studied materials followed the same trend as in TPRea runs: Ni/LaSi > Ni/MgSi > Ni/CeSi > Ni/Si. In stability tests, the differences in catalytic performances (both CO
2 conversion and CH
4 selectivity) between Ni/Si, Ni/CeSi, and Ni/MgSi are more pronounced than those corresponding to the TPRea runs, most probably due to the fact that under steady state conditions, catalysts show their complete working potential. No obvious deactivation is observed for the catalysts which contain additional basic oxide. In the case of the Ni/Si catalyst, a slight decrease of conversion from 29% to 25% was registered after 6 h TOS.
It is important to notice the very good performances of Ni/LaSi at low temperature: CO2 conversion is more than 80%, and methane is the only reaction product (methane selectivity more than 98%).
The catalysts used in stability tests for CO2 methanation were characterized to assess the modifications of catalysts’ structure in the tested conditions, and to check the possible carbon deposition on the catalyst surface.
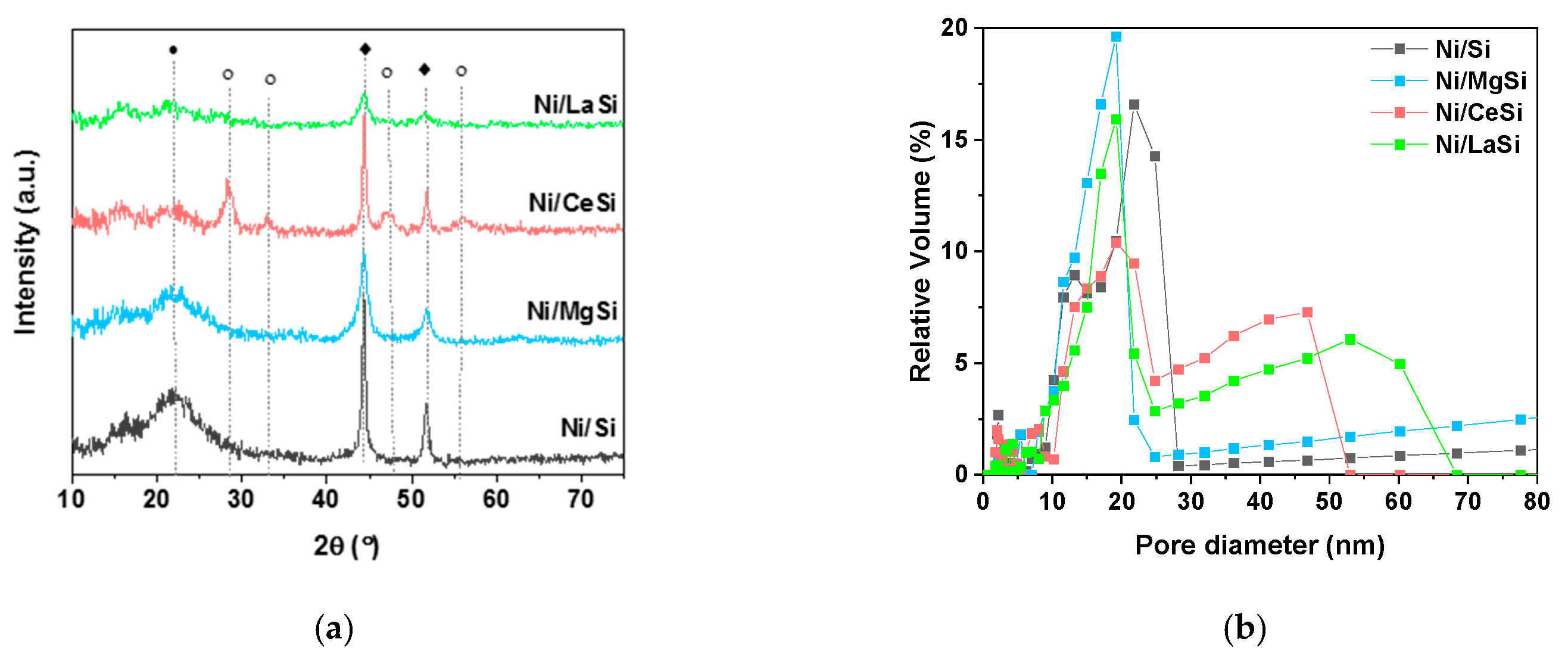
The XRD patterns of used catalysts (
Figure 9a) present the same characteristic reflexions as for the fresh materials (as described in Characterization
Section 2.1). Ni XRD peaks were used to estimate the Ni crystallites size (
Table 3). Only a slight increase was observed for Ni/Si and Ni/MgSi catalysts, while an important increase of Ni crystallites was obtained for Ni/CeSi (approximately 33% larger crystallites for used catalysts compared to fresh ones). A very good stability of the Ni crystallite size was observed for Ni/LaSi, for which the estimated values of Ni crystallites in the fresh and used catalyst are the same. These observations confirm the assumptions made for the confinement of Ni NPs inside the porous structure: for Ni/LaSi, the embedment of NPs inside the medium mesopores leads to their stability against agglomeration and sintering during the catalytic process, while for Ni/CeSi the placement of NPs predominantly on the catalyst grain cannot avoid their agglomeration. For Ni/Si and Ni/MgSi, for which the Ni NPs are situated both inside and outside the medium mesopores, a partial agglomeration and sintering was observed. No carbon deposition could be observed in the XRD patterns of the used catalysts, considering that characteristic diffraction lines of Si and C are superposed, corroborated with possible low intensity C diffraction peaks.
TGA measurements were carried out for the used catalysts in order to assess the mass loss associated with the oxidation of deposited carbon on the catalysts’ surface. For all catalysts, except for the desorption of water and other adsorbed gases which appear at temperatures lower than 100 °C, no other mass loss was observed. Due to the fact that the oxidation of Ni produces a low mass gain in the same temperature region in which the carbon oxidation would occur, a possible mass loss produced by the oxidation of small amounts of deposited carbon cannot be observed in TGA. To further investigate the possible carbon deposition on used catalysts, the corresponding TEM images were analyzed. No carbon deposits, either as fibers (wires), or as amorphous carbon were observed. In conclusion, for the catalysts tested in this work no observable carbon deposition occurs.
Total surface area and pore size distribution of silica supported catalysts after stability tests at 250 °C in CO
2 methanation were calculated from N
2 adsorption-desorption isotherms. The shape of the isotherms corresponding to the used catalysts is very similar to the fresh ones, suggesting the preservation of the catalysts’ porous structure. For all catalysts, the multimodal porous structure is maintained (
Table 3 and
Figure 9b). In the case of used Ni/Si and Ni/MgSi, the pore size remained relatively unchanged, but the proportion of larger pores increased. Instead, for the used Ni/CeSi and Ni/LaSi, the pore size distribution is changed. While the medium mesopores sizes are in the same region as in the fresh catalysts, the larger pores present a much broader distribution and a shift towards higher values. This can be due to the collapsing of the walls of some larger pores during the stability tests. However, it is important to notice that the general multimodal porosity of the samples is maintained even after catalysts testing.