Abstract
The high dependence on and high cost of lithium has led to a search for alternative materials. Aluminum ion batteries (AIBs) have gained interest due to their abundance, low cost, and high capacity. However, the use of the expensive 1-ethyl-3-methylimidazolium chloride (EMIC) electrolyte in AIBs curtails its wide application. Recently, high-temperature batteries have also gained much attention owing to their high demand by industries. Herein, we introduce cost-effective 1T molybdenum sulfide grown on SP-1 graphite powder (1T-MoS2/SP-1) as a cathode material for high-temperature AIBs using the AlCl3-urea eutectic electrolyte (1T-MoS2/SP-1–urea system). The AIB using the 1T-MoS2/SP-1–urea system exhibited a capacity as high as 200 mAh/g with high efficiency of 99% over 100 cycles at 60 °C when cycled at the rate of 100 mA/g. However, the AIB displayed a capacity of 105 mAh/g when cycled at room temperature. The enhanced performance of the 1T-MoS2/SP-1–urea system is attributed to reduced viscosity of the AlCl3-urea eutectic electrolyte at higher temperatures with high compatibility of 1T-MoS2 with SP-1. Moreover, the electrocatalytic lithiation of 1T-MoS2 and its effect on the hydrogen evolution reaction were also investigated. We believe that our work can act as a beacon for finding alternative, cost-effective, and high-temperature batteries.
1. Introduction
Owing to the overshooting price of fossil fuels and climate change concerns, electric vehicles (EVs) have become popular, and people have started to recognize EVs as an eco-friendly and sustainable alternative. Currently, lithium-ion batteries (LIBs) are the main constituents of EVs owing to their high potential window, high capacity, and stability [1,2,3,4]. However, the low abundance of Li has sparked the search for a suitable alternative. Aluminum ion batteries (AIBs) are perceived to be promising next-generation batteries owing to their safety, high abundance, and large volumetric and gravimetric capacities [5,6,7]. In earlier days, carbon materials were used as cathodes for AIBs, and they achieved a capacity as high as 180 mAh/g using a eutectic electrolyte consisting of 1-ethyl-3-methylimidazolium chloride (EMIC) and aluminum chloride (AlCl3) [8,9,10,11]. The SP-1 natural graphite flake (SP-1) has exhibited a distinguishable charge–discharge profile, good capacity, and excellent stability [12]. Recently, metal oxides and sulfides have been employed as cathodes for AIBs and have achieved a capacity of more than 500 mAh/g [4,13,14,15,16].
Most studies are focused on the development of new rechargeable batteries that operate at room temperature. There are very few reports focusing on the development of a high-performance rechargeable battery that can operate at high temperatures [17,18]. This is because batteries are usually quite unstable due to the decomposition of the electrolyte and the structural instability of the active materials. However, rechargeable batteries that operate at high temperatures will be useful in industries if the capacity and stability of the battery can be retained or even improved at the high temperatures generated by the residual heat of the reactors used in many industries. Therefore, it is still important to explore new cathode materials and suitable electrolyte systems with good stability for AIBs operated at high temperatures.
Molybdenum disulfide (MoS2) has been established as an excellent energy-storage material owing to its layer structure and high active sites similar to those of graphene [19,20,21,22]. However, the performance of MoS2 is not adequate, thereby limiting its applications in AIBs [23,24]. For example, MoS2 (2H) microspheres displayed a capacity of 66.7 mAh/g after 100 cycles when cycled at a very low current density (40 mA/g) [24]. To improve the performance of MoS2 for AIBs, 2H-MoS2 nanosheets were grown directly on carbon nanofibers as cathode materials, achieving a capacity of 126.6 mAh/g after 200 cycles at the current density of 100 mA/g [25]. Similarly, 2H-MoS2 was also composited with MXenes, and a capacity of 166 mAh/g was achieved after 60 cycles [26]. The use of 1T-MoS2 with high metallic properties (high conductivity and high active sites) has been instrumental in the development of LIBs and sodium-ion batteries (NaIBs), exhibiting both high capacity and stability [27]. Therefore, it is worth developing the 1T-MoS2 nanostructure as a cathode for AIBs to achieve better performance with a higher discharging capacity and greater Faradaic efficiency (FE).
Owing to the high cost of EMIC, Dai et al. proposed urea as the cost-effective alternative to EMIC in the ionic electrolyte system [28]. The discharging capacity of the AIB using the AlCl3-urea eutectic solution as its electrolyte was lower than that of the EMIC-AlCl3 electrolyte due to the higher viscosity of the urea-AlCl3 eutectic solution. The viscosity of the eutectic solution decreases with increasing temperature [29,30,31]. Considering some special environmental conditions, including high temperatures, batteries play a critical role in the industry, defense, nuclear reactors, and other fields where batteries need to operate under these circumstances. Therefore, it is worth studying the electrode stability, electrolyte dissociation, and safety concerns of AIB batteries using an AlCl3-urea eutectic solution at high temperatures.
On the other hand, the hydrogen evolution reaction (HER) has gained a tremendous response as H2 is considered the future source of energy. Recently, great efforts have been devoted to developing highly active, cost-effective, and stable catalysts for HER. Among various materials, MoS2 is recognized as a cost-effective and promising electrocatalyst for the hydrogen evolution reaction (HER). Many efforts have been implemented to improve the activity of MoS2 for HER by doping it with cations and anions and compositing it with carbon and other compounds. Recently, Sofer et al. studied the effect of cation intercalation on the electrocatalytic properties of metal sulfides [32]. Similarly, Hofmann et al. revealed the role of lithium (Li) in enhancing the HER activity of MoS2 [33]. Both of these reports involve the intercalation (doping) of cations by means of typical synthesis methods. Herein, we introduce the intercalation of Li+ into the MoS2 matrix using a facile and fast electrochemical method and investigate its influence on the HER activity of MoS2.
Here, the composite of 1T-MoS2 grown on SP-1 graphite powder (1T-MoS2/SP-1) using the facile single-step hydrothermal method was prepared in the same manner as the cathode materials in an AIB. The battery was assembled in a pouch cell using the low-cost electrolyte of the AlCl3-urea eutectic solution. The detailed characterizations of the cathode materials were confirmed using transmission electron microscopy, scanning electron microscopy, Raman spectroscopy, and powder X-ray diffraction. We found that the AIB created based on the 1T-MoS2/SP-1 cathode materials manifested a high capacity of 200 mAh/g and excellent stability of over 100 cycles with high FE of 99% at 60 °C on cycling at a current density of 100 mA/g. The AlCl3-urea electrolyte-based AIB provides promising opportunities to set up energy storage systems exhibiting low cost and high safety in high-temperature environments. Moreover, the lithiated 1T-MoS2-SP-1 composite displayed excellent HER activity by delivering 100 mA at 1.83 V and achieved a Tafel slope as low as 47 mV/dec. Overall, the 1T-MoS2/SP-1 composite demonstrated a dual function of energy storage at high temperatures and AIB and hydrogen evolution in the water-splitting system.
2. Results and Discussion
The 1T-MoS2/SP-1 composite was synthesized using the simple one-pot hydrothermal method. Briefly, 0.81 g of molybdic acid and 0.95 g of thiourea were dissolved in 40 mL of water. The resulting solution was mixed with 300 mg of SP-1 graphite powder. Then, the mixture was transferred to the Teflon container, and the hydrothermal reaction was carried out at 180 °C for 24 h. After cooling to room temperature, the final product was collected, washed with water and ethanol several times, and then dried at 70 °C for 12 h. As a comparison, only the 1T-MoS2 materials were synthesized without adding SP-1 graphite powder [19]. Figure 1a shows the X-ray diffraction (XRD) patterns of 1T-MoS2/SP-1. The peaks at 2θ~ 9.30°, 18.26°, and 32.80° were indexed to (002), (004), and (100) planes of 1T-MoS2 crystal structure, respectively [19,22]. The peak at 2θ~ 26.70° was indexed to the (002) plane of graphite. Figure 1b shows the Raman spectrum of 1T-MoS2/SP-1. The peaks at 150, 200, 290, and 350 cm−1 were ascribed to J1, J2, E1g, and J3 of 1T-MoS2, respectively [19,22]. The peak at 1580 cm−1 corresponded to the G band of SP-1 [9]. Thus, the XRD patterns and the Raman spectrum confirm the formation of the 1T-MoS2/SP-1 composite using the one-pot hydrothermal method. Figure S1 provides XRD, Raman and SEM images of bare 1T-MoS2.
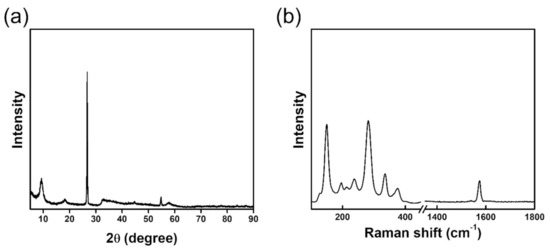
Figure 1.
(a) XRD patterns and (b) Raman spectrum of 1T-MoS2/SP-1.
Figure 2a shows the scanning electron microscopic (SEM) image of 1T-MoS2/SP-1. MoS2 has attained beautiful flower morphology with a layered structure. MoS2 is found to be grown homogeneously on SP-1 sheets yielding a high surface area and active sites. Figure 2b–d show the energy-dispersive X-ray (EDX) elemental mapping of 1T-MoS2/SP-1. Figure 2e shows the EDX spectrum of 1T-MoS2/SP-1. Both the EDX spectrum and the elemental mapping confirm the presence of Mo, S, and C in the as-synthesized sample. Figure 2f shows the high-resolution transmission electron microscopic (HR-TEM) image of 1T-MoS2/SP-1. The results showed that 1T-MoS2 exhibits good crystalline structures with well-defined lattice fringes. Overall results indicated that the layered structure of 1T-MoS2/SP-1 was fabricated during the facile hydrothermal reaction process.
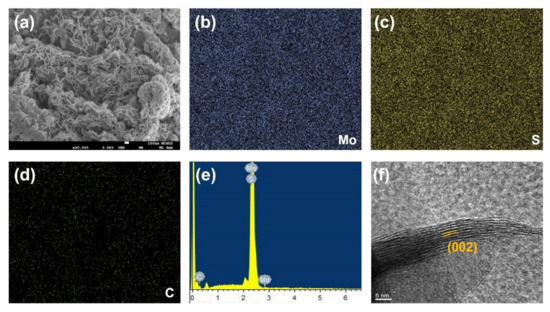
Figure 2.
(a) SEM image, (b–d) elemental mapping; (e) EDX spectrum. and (f) HR-TEM image of 1T-MoS2/SP-1.
The electrochemical properties of 1T-MoS2/SP-1 as a cathode for AIBs were investigated by assembling the materials in pouch cells. First, the slurry was prepared by dissolving 1T-MoS2/SP-1 and polyvinylidene fluoride (PVDF) in N-methyl-2-pyrrolidone (NMP) in a 9:1 ratio. It is important to know that the capacity was calculated based on the weight of 1T-MoS2 and SP-1 together. In the case of bare 1T-MoS2, an electrode was provided additionally in the mixture of MoS2, super P, and PVDF (the ratio is 7.5:1.5:1, respectively). The slurry was coated on carbon fiber paper (CFP) with a loading amount of 1 mg/cm2. The urea electrolyte was prepared by adding AlCl3 and urea in a 1.3:1 ratio and then stirring for 2 h at 60 °C. The pouch cells consisting of 1T-MoS2/SP-1 as a cathode and Al foil as an anode, along with the urea electrolyte, were assembled in an argon-filled glovebox similar to our earlier reported methods [34]. The cutoff potentials for galvanostatic investigations were set as 0.2 and 2.2 V (vs Al), and the investigations were carried out using a machine from Neware Technology Ltd. A hot plate was used for the high-temperature electrochemical investigations (60 ± 2 °C).
Figure 3a shows the charge–discharge profile of 1T-MoS2/SP-1 when tested as a cathode for the AIBs. It displayed a broad plateau starting from 1.7 V and extending up to 2 V during the charging process in the first cycle and then displayed discharging plateaus of 1.6 V and 1 V. However, changes in the charge–discharge profile were observed after the fifth cycle with cycling yielding the typical MoS2 plateaus at 1.1 V and 0.7 V during the charging and discharging process, respectively [23,24,25]. Earlier reports have already proven that Al3+ is the major intercalating species when non-carbon materials are employed as cathodes [23,24,25], and due to the lack of intercalation behavior of graphite in the charge–discharge profile of 1T-MoS2/SP-1, Al3+ intercalating chemistry is supposed to be dominant in the 1T-MoS2/SP-1-urea battery. Based on Al3+ intercalating chemistry, the reaction mechanism can be represented as follows:
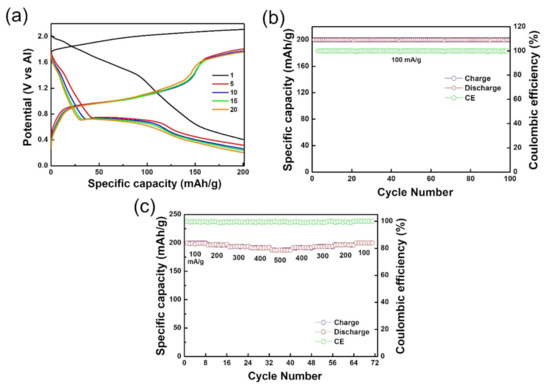
Figure 3.
(a) Charge–discharge profile, (b) cycling performance, and (c) rate capability of the 1T-MoS2/SP-1–urea battery at 60 °C.
During charging
Cathode:
Anode:
During discharge:
Cathode:
Anode:
Cathode: AlxMoS2 → MoS2 + 3xe− + xAl3+
Anode: 4Al2Cl7− + 3e− → Al + 7AlCl4−
During discharging
Cathode: MoS2 + 3xe− + xAl3+ → AlxMoS2
Anode: Al + 7AlCl4− → 4Al2Cl7− + 3e−
When a current density of 100 mA/g was applied in the potential window of 0.2 to 2.2 V (v Al3+/Al) at 60 °C, the 1T-MoS2/SP-1-urea battery exhibited a high capacity of 200 mAh/g in the first cycle, and the same capacity was maintained until nearly the end of 100 cycles, manifesting excellent stability (Figure 3b). The Coulombic efficiency of −100% was recorded throughout cycling, signifying the remarkable stability of the material. Furthermore, the rate capability of the 1T-MoS2/SP-1-urea battery investigated at 60 °C is shown in Figure 3c. The current density was increased stepwise from 100 to 500 mA/g and again decreased stepwise to 100 mA/g. The capacity was found to be undented irrespective of current density, demonstrating the high stability of the 1T-MoS2/SP-1 at 60 °C. Similarly, 1T-MoS2 was also tested at different current densities, as shown in Figure S2. It was found to drastically lose capacity under increasing current density and could not completely retain capacity upon the reduction in the current densities.
The electrochemical performance of 1T-MoS2/SP-1 was compared with the bare materials 1T-MoS2 and SP-1 at 60 °C, as shown in Figure 4a. The 1T-MoS2 exhibited a capacity of 200 mAh/g in the early cycles, but severe capacity decay yielded 90 mAh/g capacity at the end of 50 cycles when it was cycled at 100 mA/g. Similarly, SP-1 displayed a capacity of 183 mAh/g in the first cycle, but due to severe capacity decay, it displayed a capacity of 9 mAh/g at the end of 40 cycles. From the above results, it can be concluded that bare MoS2 and SP-1 urea batteries are not stable at high temperatures. Moreover, the electrochemical performance of 1T-MoS2/SP-1, 1T-MoS2, and SP-1 was also evaluated at room temperature (RT) to analyze the effect of temperature on the urea battery systems (Figure 4b). The results showed that 1T-MoS2/SP-1 delivered a capacity of 105 mAh/g in the early cycles and exhibited good stability over 100 cycles when cycled at 100 mA/g. Conversely, bare 1T-MoS2 and SP-1 delivered a capacity of 100 and 112 mAh/g in the first cycle, respectively. However, both MoS2 and SP-1 exhibited a gradual capacity decay, yielding 86 and 73 mAh/g at the end of 50 cycles on cycling at 100 mA/g. Thus, it can be concluded that the MoS2-SP-1 urea system demonstrates high stability at both high and room temperatures and delivers a very high capacity at high temperatures, whereas bare 1T-MoS2 and SP-1 urea systems were found to be unsteady in both reaction conditions.
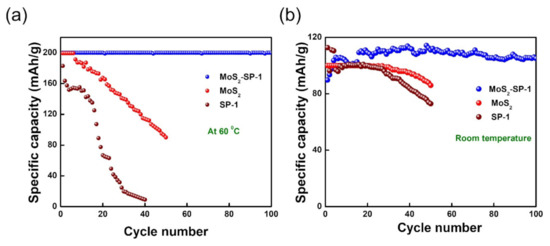
Figure 4.
(a,b) Cycling performance of 1T-MoS2/SP-1, 1T-MoS2, and SP-1 at 60 °C and room temperature, respectively.
At high temperatures, owing to the lower viscosity of the urea electrolyte and high ionic conductivity, the intercalation of Al3+ into the MoS2 matrix increased, yielding high capacity. However, the drastic capacity fall in the subsequent cycles can be ascribed to the structural distortion of the MoS2 matrix. To overcome this drawback, MoS2 was composited with SP-1 carbon to provide more stability to the structure and also enhance the area and conductivity. As a result, 1T-MoS2/SP-1 exhibited both stable and high capacity at high temperatures using the urea electrolyte. A comparison table (Table S1) shows different cathodes compared with respect to the cost of the electrolyte and its performance.
Furthermore, the instability of the EMIC electrolyte at a high temperature (60 °C) was investigated by adopting SP-1 as the cathode, as shown in Figure S3. It exhibited a capacity of 90 mAh/g in the first cycle, and a capacity of 40 mAh/g was attained at the end of 50 cycles, signifying the fragility of the EMIC electrolyte at high temperatures. Thus, it can be concluded that EMIC is not a suitable electrolyte for high-temperature AIBs. The excellent performance of the 1T-MoS2/SP-1-urea battery at a high temperature (60 °C) can be ascribed to the following: (1) the decreased viscosity of the urea electrolyte and enhanced ion transport. It is well known that the viscosity of the urea electrolyte decreases with increasing temperature, resulting in high-ion transport yielding enhanced capacity of the MoS2-urea battery. (2) The compatibility of 1T-MoS2 with SP-1. Bare 1T-MoS2 is found to be plagued with severe capacity decay; however, this deficiency was vanquished by commixing it with SP-1. Here, SP-1 acts as mechanical support for MoS2. Furthermore, the blending of 1T-MoS2 with SP-1 gives rise to high active sites and surface area, yielding enhanced interaction with the electrolyte. Moreover, the high capacity of 1T-MoS2/SP-1 is also attributed to the capacity storage properties and the conductivity of SP-1. This requires further study.
In addition, we introduced a facile and fast electrochemical method of lithiation into the 1T-MoS2 matrix. It involves conducting linear sweep voltammetry (LSV) from 0 to −0.3 V (RHE) in 0.5 M LiClO4 with 1T-MoS2 as a cathode and carbon and calomel as counter and reference electrodes, respectively. The intercalation of Li+ was confirmed by observing XRD patterns where the characteristic peak of 1T-MoS2 shifted from (2θ) 9.3° to 7.8°, as shown in Figure S4, and also from the emerging of the Li peak in XPS, as shown in our previous report [19]. Figure 5a shows the polarization curves of 1T-MoS2 and 1T-MoS2-SP-1 towards HER before and after lithiation. Here, HER was carried out using MoS2, carbon, and calomel as working, counter, and reference electrodes, respectively, in 0.5 M sulfuric acid (H2SO4). Before lithiation, 1T-MoS2 and 1T-MoS2-SP-1 displayed an onset potential of −0.134 and −0.129 V, respectively. The 1T-MoS2 and 1T-MoS2-SP-1 delivered 100 mA of current density at −0.254 and −0.250 V, respectively. After lithiation, the onset potential was shifted to −0.053 and −0.052 V for 1T-MoS2 and 1T-MoS2-SP-1, respectively. Similarly, both 1T-MoS2 and 1T-MoS2-SP-1 exhibited 100 mA of current density at −0.183 and −0.182 V, respectively. Tafel slopes were calculated for the LSV curves of 1T-MoS2 and 1T-MoS2-SP-1 before and after lithiation, as shown in Figure 5b. The Tafel slope of both 1T-MoS2 and 1T-MoS2-SP-1 was drastically improved from 64.41 and 57.66 mV/dec to 47.36 and 47.00 mV/dec, respectively. The enhancement in HER activity of 1T-MoS2 can be attributed to the edge sites of Li of 1T-MoS2 [33].
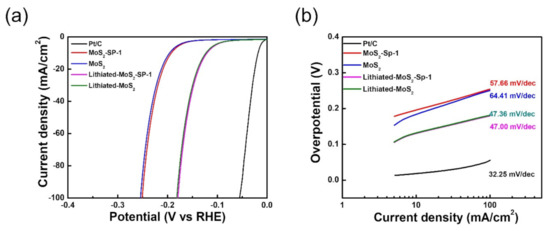
Figure 5.
(a,b) Polarization curves and Tafelplots of 1T-MoS2/SP-1 and 1T-MoS2 before and after lithiation, respectively.
Research shows that 1T-MoS2 is well established as an HER catalyst owing to its catalytic properties such as abundant active sites, defects, large area, and sheet-like structure. It is also known that the sulfides (S2−) of MoS2 are active centers for HER. Li+ intercalation yields change in the electronic properties, such as the conductivity of MoS2. Along with Li+ intercalation, adsorption of Li+ also plays a vital role in improving the HER activity of MoS2 by changing the ∆GH* in a favorable direction [33]. Overall, the lithiation of MoS2 resulted in enhanced HER activity by exhibiting a lower onset potential and Tafel slope.
3. Conclusions
Herein, we adopted a low-cost urea electrolyte for AIBs and investigated them at high temperatures. The 1T-MoS2/SP-1 was fabricated using simple one-pot hydrothermal synthesis and investigated as a cathode for the AIB using the urea electrolyte. The 1T-MoS2/SP-1 manifested a capacity as high as 200 mAh/g and remarkable stability (Coulombic efficiency ~100%) over 100 cycles when cycled at 100 mA/g at 60 °C. The excellent performance of 1T-MoS2/SP-1 was ascribed to the compatibility of 1T-MoS2 with SP-1 and the low viscosity of the urea electrolyte at high temperatures. The enhanced HER activity of 1T-MoS2 was also attributed to lithiation using a facile and fast method. Overall, the 1T-MoS2/SP-1 composite plays an important role in the high-temperature AIB and water splitting system.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11121547/s1. Figure S1: XRD, Raman and FESEM images of 1T-MoS2, Figure S2: Rate capability of 1T-MoS2-urea at 60 °C, Table S1: A comparison table comprising of different cathodes, Figure S3: Charge-discharge profile and cycling performance of SP-1-EMIC at 60 °C, Figure S4: XRD patterns of 1T-MoS2/SP-1 before and after lithiation.
Author Contributions
Conceptualization, S.B.P. and D.-Y.W.; methodology, S.B.P., J.-Y.A., Z.-J.L., Y.-C.W., S.M.G., H.-H.H. and Z.C.; validation, S.B.P., J.-Y.A., Z.-J.L., Y.-C.W., S.M.G., H.-H.H. and Z.C.; formal analysis, S.B.P., J.-Y.A., Z.-J.L., Y.-C.W., S.M.G., H.-H.H. and Z.C.; investigation, S.B.P., J.-Y.A., Z.-J.L., Y.-C.W., S.M.G., H.-H.H. and Z.C.; resources, S.B.P., J.-Y.A., Z.-J.L. and Y.-C.W.; data curation, S.B.P., J.-Y.A., Z.-J.L. and Y.-C.W.; writing—original draft preparation, S.B.P. and D.-Y.W.; writing—review and editing, S.B.P. and D.-Y.W.; visualization, S.B.P. and D.-Y.W.; supervision, D.-Y.W.; project administration, D.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financially supported by the Ministry of Science and Technology of Taiwan (MOST 106-2113-M-029-006-MY2, MOST 106-2632-M-029-001, and MOST 107-2622-M-029-001-CC2) and Tunghai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, D.; Chatterjee, A.; Man, L.H.; Or, S.W. In-Situ Arc Discharge-Derived FeSn2/Onion-Like Carbon Nanocapsules as Improved Stannide-Based Electrocatalytic Anode Materials for Lithium-Ion Batteries. Catalysts 2019, 9, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-H.; Wu, J.; Yu, Y.-X. Toward Large-Capacity and High-Stability Lithium Storages via Constructing Quinone–2D-MnO2-Pillared Structures. J. Phys. Chem. C 2021, 125, 3725–3732. [Google Scholar] [CrossRef]
- Yu, Y.-X. Can all nitrogen-doped defects improve the performance of graphene anode materials for lithium-ion batteries? Phys. Chem. Chem. Phys. 2013, 15, 16819–16827. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.-L.; Lei, H.; Yu, Z.; Chen, L.-L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, M.; Lin, M.C.; Yuan, C.; Angell, M.; Huang, L.; Wang, D.Y.; Zhang, X.; Yang, J.; Hwang, B.J. 3D graphitic foams derived from chloroaluminate anion intercalation for ultrafast aluminum-ion battery. Adv. Mater. 2016, 28, 9218–9222. [Google Scholar] [CrossRef]
- Ellingsen, L.A.-W.; Holland, A.; Drillet, J.-F.; Peters, W.; Eckert, M.; Concepcion, C.; Ruiz, O.; Colin, J.-F.; Knipping, E.; Pan, Q. Environmental screening of electrode materials for a rechargeable aluminum battery with an AlCl3/EMIMCl electrolyte. Materials 2018, 11, 936–959. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.B.; Liao, H.-J.; Wang, D.-Y. Challenges and prospects of polyatomic ions’ intercalation in the graphite layer for energy storage applications. Phys. Chem. Chem. Phys. 2020, 22, 24842–24855. [Google Scholar] [CrossRef]
- Lee, T.-S.; Patil, S.B.; Kao, Y.-T.; An, J.-Y.; Lee, Y.-C.; Lai, Y.-H.; Chang, C.-K.; Cheng, Y.-S.; Chuang, Y.-C.; Sheu, H.-S. Real-time observation of anion reaction in high performance Al ion batteries. ACS Appl. Mater. Interfaces 2019, 12, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-T.; Patil, S.B.; An, C.-Y.; Huang, S.-K.; Lin, J.-C.; Lee, T.-S.; Lee, Y.-C.; Chou, H.-L.; Chen, C.-W.; Chang, Y.J. A quinone-based electrode for high-performance rechargeable aluminum-ion batteries with a low-cost AlCl3/urea ionic liquid electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 25853–25860. [Google Scholar] [CrossRef]
- Stadie, N.P.; Wang, S.; Kravchyk, K.V.; Kovalenko, M.V. Zeolite-templated carbon as an ordered microporous electrode for aluminum batteries. ACS Nano 2017, 11, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Wei, C.-Y.; Lin, M.-C.; Pan, C.-J.; Chou, H.-L.; Chen, H.-A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Lv, C.; Wang, L.; Cui, W.; Zhang, L.; Dinh, K.N.; Tan, H.; Wu, C.; Wu, T.; Ren, Y. Architecting a stable high-energy aqueous al-ion battery. J. Am. Chem. Soc. 2020, 142, 15295–15304. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, S.; Tu, J.; Song, W.-L.; Xiao, X.; Li, S.; Wang, M.; Lei, H.; Tian, D.; Chen, H. Rechargeable ultrahigh-capacity tellurium–aluminum batteries. Energy Environ. Sci. 2019, 12, 1918–1927. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Wang, H.; Zhang, F.; Li, Q.; Li, H. Nonaqueous aluminum ion batteries: Recent progress and prospects. ACS Mater. Lett. 2020, 2, 887–904. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Varma, R.S.; Farha, O.K.; Shokouhimehr, M. Recent advances in rechargeable aluminum-ion batteries and considerations for their future progress. ACS Appl. Energy Mater. 2020, 3, 6019–6035. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Solid Electrolytes for High-Temperature Stable Batteries and Supercapacitors. Adv. Energy Mater. 2021, 11, 2002869. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent development in separators for high-temperature lithium-ion batteries. Small 2019, 15, 1901689. [Google Scholar] [CrossRef]
- Patil, S.B.; Chou, H.-L.; Chen, Y.-M.; Hsieh, S.-H.; Chen, C.-H.; Chang, C.-C.; Li, S.-R.; Lee, Y.-C.; Lin, Y.-S.; Li, H. Enhanced N 2 affinity of 1T-MoS 2 with a unique pseudo-six-membered ring consisting of N–Li–S–Mo–S–Mo for high ambient ammonia electrosynthesis performance. J. Mater. Chem. A 2021, 9, 1230–1239. [Google Scholar] [CrossRef]
- Patil, S.B.; Raghu, M.; Kishore, B.; Nagaraju, G. Enhanced electrochemical performance of few-layered MoS 2–rGO nanocomposite for lithium storage application. J. Mater. Sci. Mater. Electron. 2019, 30, 316–322. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Lee, P.-C.; Tsao, C.-W.; Lee, L.-H.; Wang, D.-Y.; Wen, C.-Y. In situ Scanning Electron Microscopy Observation of MoS2 Nanosheets during Lithiation in Lithium Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 7066–7072. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.-C.; Wang, D.-Y.; Kuo, T.-R. Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Tu, J.; Xiao, X.; Wang, M.; Jiao, S. Hierarchical flower-like MoS2 microspheres and their efficient al storage properties. J. Phys. Chem. C 2019, 123, 26794–26802. [Google Scholar] [CrossRef]
- Li, Z.; Niu, B.; Liu, J.; Li, J.; Kang, F. Rechargeable aluminum-ion battery based on MoS2 microsphere cathode. ACS Appl. Mater. Interfaces 2018, 10, 9451–9459. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lu, H.; Cao, Y.; Xu, B.; Deng, Y.; Cai, W. Flexible free-standing MoS2/carbon nanofibers composite cathode for rechargeable aluminum-ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 4861–4867. [Google Scholar] [CrossRef]
- Tan, B.; Lu, T.; Luo, W.; Chao, Z.; Dong, R.; Fan, J. A Novel MoS2-MXene Composite Cathode for Aluminum-Ion Batteries. Energy Fuels 2021, 35, 12666–12670. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Angell, M.; Pan, C.-J.; Rong, Y.; Yuan, C.; Lin, M.-C.; Hwang, B.-J.; Dai, H. High Coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl. Acad. Sci. USA 2017, 114, 834–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Greaves, T.L.; Warr, G.G.; Atkin, R. Mixing cations with different alkyl chain lengths markedly depresses the melting point in deep eutectic solvents formed from alkylammonium bromide salts and urea. Chem. Commun. 2017, 53, 2375–2377. [Google Scholar] [CrossRef] [Green Version]
- Simeonov, S.; Afonso, C.A. Basicity and stability of urea deep eutectic mixtures. RSC Adv. 2016, 6, 5485–5490. [Google Scholar] [CrossRef]
- Smith, P.J.; Arroyo, C.B.; Lopez Hernandez, F.; Goeltz, J.C. Ternary deep eutectic solvent behavior of water and urea–choline chloride mixtures. J. Phys. Chem. B 2019, 123, 5302–5306. [Google Scholar] [CrossRef] [PubMed]
- Luxa, J.; Voseckyɓ, P.; Mazaȥnek, V.; Sedmidubskyɓ, D.; Pumera, M.; Sofer, Z.K. Cation-controlled electrocatalytical activity of transition-metal disulfides. ACS Catal. 2018, 8, 2774–2781. [Google Scholar] [CrossRef]
- Wu, L.; Dzade, N.Y.; Yu, M.; Mezari, B.; van Hoof, A.J.; Friedrich, H.; de Leeuw, N.H.; Hensen, E.J.; Hofmann, J.P. Unraveling the role of lithium in enhancing the hydrogen evolution activity of MoS2: Intercalation versus adsorption. ACS Energy Lett. 2019, 4, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-Y.; Huang, S.-K.; Liao, H.-J.; Chen, Y.-M.; Wang, S.-W.; Kao, Y.-T.; An, J.-Y.; Lee, Y.-C.; Chuang, C.-H.; Huang, Y.-C. Insights into dynamic molecular intercalation mechanism for AlC battery by operando synchrotron X-ray techniques. Carbon 2019, 146, 528–534. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).