Abstract
In this study, one-pot microwave-assisted synthesis was used to fabricate the graphene (GR)-supported PtCoM catalysts where M = Mn, Ru, and Mo. The catalysts with the molar ratios of metals Pt:Co:Mn, Pt:Co:Ru, and Pt:Co:Mo equal to 1:3:1, 1:2:2, and 7:2:1, respectively, were prepared. Catalysts were characterized using Transmission Electron Microscopy (TEM). The electrocatalytic activity of the GR-supported PtCoMn, PtCoRu, and PtCoMo catalysts was evaluated toward methanol oxidation in an alkaline medium employing cyclic voltammetry and chrono-techniques. The most efficient electrochemical characteristics demonstrated the PtCoMn/GR catalyst with a current density value of 144.5 mA cm−2, which was up to 4.8 times higher than that at the PtCoRu(1:2:2)/GR, PtCoMo(7:2:1)/GR, and bare Pt/GR catalysts.
1. Introduction
Among the different fuel cells (FCs) developed so far, direct methanol fuel cells (DMFCs) are considered one of the most promising clean energy sources, especially for portable power supply [1,2]. They receive significant attention due to a whole range of benefits, including their superior specific energy, economical price, and physical state (liquid form at ambient conditions) that unambiguously determine the system’s operational safety. The main reactions occurring in the alkaline DMFCs are the oxidation of methanol (MOR) at the anode and the reduction of oxygen (ORR) at the cathode according to the following Equations (1) and (2), respectively [3]:
Anode reaction: CH3OH + 6OH− → CO2 + 5H2O + 6e−
Cathode reaction: 3/2O2 + 3H2O + 6e− → 6OH−
Overall reaction: CH3OH + 3/2O2 → CO2 + 2H2O.
However, the successful worldwide commercialization of DMFCs is related mainly to the Pt or Pt-based electrocatalysts that are expensive and generate considerable manufacturing costs. One more drawback is the relatively low resistance of such catalysts toward carbon monoxide adsorption (COads). Developing novel catalysts that substitute Pt in low-temperature fuel cell anodes and have low CO tolerance is vital. To reduce the use of precious metals, several metal catalysts have been investigated. The addition of a second or third metal and the formation of di- or trimetallic catalysts have been found to be an effective way to significantly increase the resistance of the catalyst to CO poisoning while increasing electrocatalytic activity and stability. Therefore, bimetallic noble systems such as Pt-Ru [4,5,6,7,8,9,10,11,12,13,14], Pt-Au [15,16], and Pt-Pd [10,14,17,18,19,20] have been developed for the oxidation of methanol. However, as was mentioned above, to reduce the amount of noble metal in the catalysts, Pt alloying with one or two non-noble (transition) metals, such as Co [14,21,22,23,24,25,26,27,28,29,30,31], Ni [14,21,24,27,32,33,34], Cu [24,35,36,37,38], Fe [39], Mn [40], Sn [10,40], and Zn [40] or W [41] was studied. Although cobalt was observed as one of the added metals to increase the activity of Pt catalysts, the addition of a third metal to the catalyst showed better catalytic properties of trimetallic catalysts due to the formation of metal alloys or a synergistic effect between the metals in the catalysts as well as for creating of cheaper catalysts.
There are some methods described as suitable for the preparation of binary or ternary PtM alloys. Shi et al. investigated flower-like structure PtRu catalysts for methanol oxidation in acidic media, where the catalysts were obtained from H2PtCl6·6H2O and RuCl3 aqueous solution after heating in a Teflon reactor at 140 °C for three h [12]. Tian et al. deposited PtRu alloys with different metal ratios from solutions using an electrochemical metal deposition. J. Lian et al. investigated PtMM (M = Cu, Ni) alloys. Researchers deposited metals from their salts aqueous solutions. A reaction occurred in the Teflon autoclave at a temperature of 190 °C for 12 h [42]. Li also investigated the activity of PtCu alloy for methanol oxidation reaction, where the catalyst was prepared from relevant salts aqueous solutions, and synthesis was executed in a Teflon autoclave at a temperature of 200 °C for 1 h [37]. However, heating in traditional autoclaves takes a lot of time, making catalyst formation not as easy as possible.
In recent years, microwave irradiation heating became more attractive for the formation of catalysts for fuel cells. In a microwave reactor, some liquids and solids transform electromagnetic radiation into heating. Many solvents such as water, alcohols, acetone, acetic acid, etc., are heated rapidly when microwave irradiation is used [43,44,45,46,47,48]. There are many advantages of using microwave heating instead of traditional heating. These include shorter reaction time, high efficiency, product purity, low cost, and the reduction of hazardous substances, which is very important nowadays [43,48]. Additionally, nanostructures with smaller sizes, narrower size distributions, and a higher degree of crystallization could be obtained under microwave heating than those in conventional oil-bath heating. By changing various experimental parameters, such as the concentration of metallic salt, the solvent, the reaction temperature, time, etc., the morphologies and sizes of nanostructures could be controlled. Çögenli et al. prepared PtM (M = Pd, Ru, Sn) bimetallic catalysts using microwave irradiation heating at 800 W for 120 s [10]. Kepenienė et al. investigated PtCo catalysts with different Pt:Co molar ratios deposited on a graphene substrate, using microwave irradiation heating method at a temperature of 170 °C for 30 min [23]. Kilmonis et al. synthesized the graphene-supported PtW efficient catalysts by the microwave irradiation method [41]. One-pot synthesis simplifies the catalysts’ preparation method compared to the synthesis, which requires multiple steps [12,13,23,34]. Moreover, all of the above-mentioned binary PtM alloys exhibited higher electrocatalytic activity toward MOR than bare Pt/C. However, various ternary or even quarternary PtM alloys have also emerged in the literature as effective MOR electro-catalysts [13,14,15]. Great attention has been attributed to the investigation of multi-component systems, such as PtNiCu [42], PtNiCo [49], PtCoRh [50], FePtCu [51], FePtSn [52], RuMPt (M = Fe, Co, Ni, and Cu) [53], PtRuFeCo [54], and PtRuCuW [55] as efficient catalysts for MOR. It should be noted that generally, the catalysts mentioned above allow reducing the amount of noble metal and lowering the catalysts’ overall cost. They demonstrated enhanced stability, higher resistance to catalyst poisoning, and markedly improved catalytic activity for MOR compared to those characteristics of monometallic catalysts. Zhao et al. investigated RuFePt, RuCoPt, RuNiPt, RuCuPt, and RuPt alloys, where RuCoPt showed three times greater mass activity toward MOR compared to that of Pt/C catalyst [53]. Rethinasabapathy et al. investigated the quaternary PtRuFeCo catalyst for MOR, which exhibited almost two times higher current density values than bare Pt catalyst [54]. Han et al. investigated PtCoRh catalyst for MOR in alkaline media, where this catalyst showed higher current density values for the bare Pt catalyst [50]. It was found that mass activity values for PtCoRh were two times higher than those for Pt/C catalyst.
In this study, we investigated ternary PtCoM (M = Mn, Ru, Mo) catalysts based on recent research on multi-component systems. Catalysts of this composition have not been extensively described. A one-pot microwave-assisted synthesis was used to prepare the GR-supported PtCoM (M = Mn, Ru, Mo) nanoparticle catalysts with different Pt:Co:M molar ratios as electrocatalysts for the oxidation of methanol in an alkaline medium. The prepared PtCoM/GR catalysts were characterized using Transmission Electron Microscopy (TEM), X-ray Energy Dispersive Analysis (EDX), and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The electrocatalytic activity of the graphene-supported PtCoM catalysts toward the oxidation of methanol in an alkaline medium was investigated using cyclic voltammetry and chrono-techniques.
2. Results
According to the synthesis conditions, using a one-pot microwave synthesis by microwave-assisted heating of Pt(IV), Co(II), Mn(II), Ru(III), and Mo(VI) salts in an ethylene glycol solution, the PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts were formed. The XRD patterns of the synthesized catalysts are shown in Figure 1. A sharp peak at 26.5° observed for investigated catalysts can be attributed to the graphite structure (002) plane of the supports. For PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts, the peaks corresponding to polycrystalline Pt and other metals cannot be clearly discerned, because the catalysts are amorphous, and PtCoMn, PtCoRu, and PtCoMo nanoparticles are too small, which results in the broadening of the small peaks.
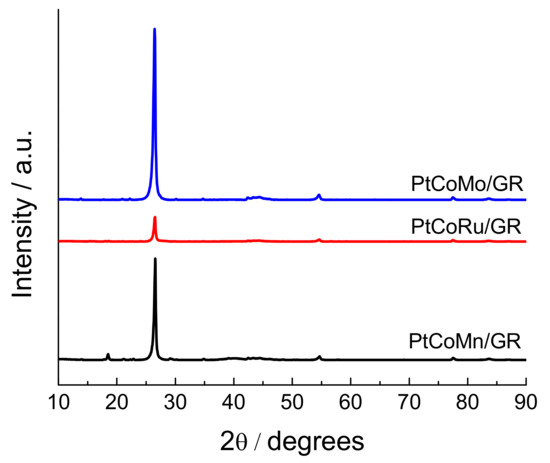
Figure 1.
XRD patterns of PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts.
The practical composition of synthesized catalysts was determined by ICP-OES and is given in Table 1. It was found that Pt:Co:Mn, Pt:Co:Ru, and Pt:Co:Mo molar ratios in the prepared PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts are 1:3:1, 1:2:2, and 7:2:1, respectively. It was determined that Pt loadings in the PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts were 103.8, 183.2, and 83.8 µg cm−2, respectively. The ESA values of Pt in the catalysts were determined from CVs recorded in the 0.5 M H2SO4 solution and calculating the charge associated with the hydrogen adsorption (220 µC cm−2) and are given in Table 1.

Table 1.
The composition of catalysts by ICP-OES, loading, and ESA values of Pt in the synthesized PtCoM/GR and Pt/GR.
The TEM examination was used to assess the size of reduced Pt nanoparticles in the investigated catalysts. Figure 2 shows TEM images and the corresponding particle size distribution of PtCoMn(1:3:1)/GR (a, a’), PtCoRu(1:2:2)/GR (b, b’), PtCoMo(7:2:1)/GR (c, c’), and Pt/GR (d, d’) catalysts. The particle size distribution was obtained by measuring the diameter of about 50–100 particles found in a randomly chosen area. Spherical and well-dispersed nanoparticles on graphene’s surface for all the investigated catalysts are observed (Figure 2). The average particle size is 1.4 nm for PtCoMn/GR (Figure 2a’) and 2.0 nm for PtCoRu/GR (Figure 2b’) and PtCoMo/GR (Figure 2c’) catalysts. The Pt/GR catalyst with an average Pt nanoparticles size of ca. 1.6 nm was synthesized, as evident from Figure 2d’. Additionally, crystalline PtCoM nanoparticles, i.e., lattice planes, are not observed in HRTEM of Figure 2a–d, which consists of the XRD analysis.
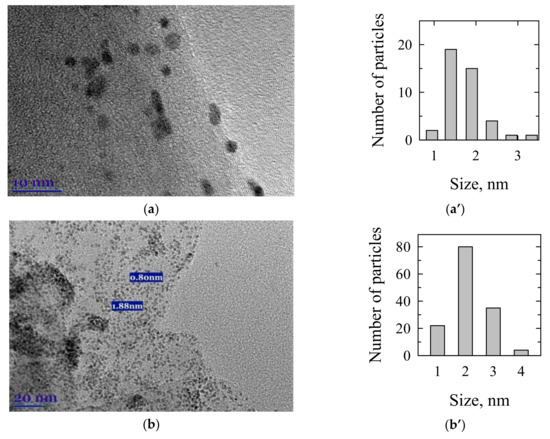
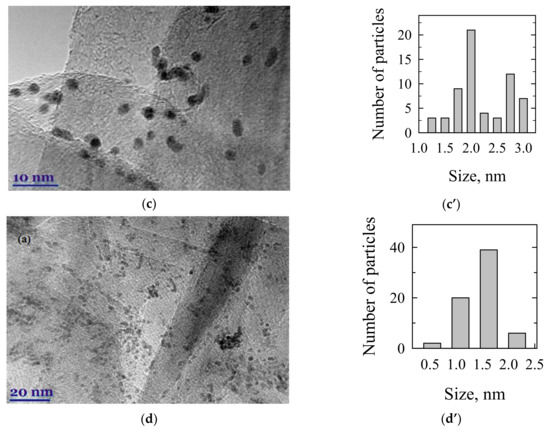
Figure 2.
TEM images and the corresponding particle size distribution of PtCoMn(1:3:1)/GR (a,a’), PtCoRu(1:2:2)/GR (b,b’), PtCoMo(7:2:1)/GR (c,c’), and Pt/GR (d,d’) catalysts.
The EDX analysis showed that all metals in the prepared catalysts are well-distributed on graphene’s surface. The images of metals distribution and amount of metal wt % from the mass spectrum of investigated catalysts are shown in Figure 3.
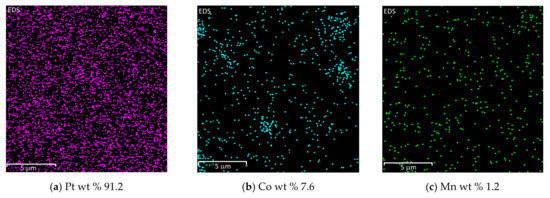
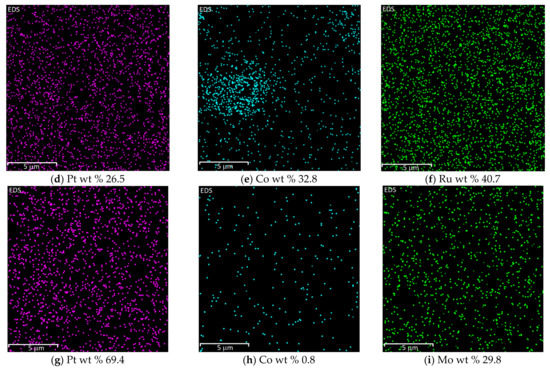
Figure 3.
Distribution of metals in PtCoMn(1:3:1)/GR (a–c), PtCoRu(1:2:2)/GR (d–f), and PtCoMo(7:2:1)/GR (g–i) catalysts obtained by EDX analysis.
Figure 4 presents the CVs of the PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, PtCoMo(7:2:1)/GR, and Pt/GR catalysts recorded in a 0.5 M H2SO4 solution. The highest ESA value was obtained for the PtCoMn/GR catalyst (Table 1). The ESA of Pt is 2.2, 2.9, and 3.1 times higher at PtCoMn/GR than those at Pt/GR, PtCoRu/GR, and PtCoMo/GR catalysts, respectively (Table 1).
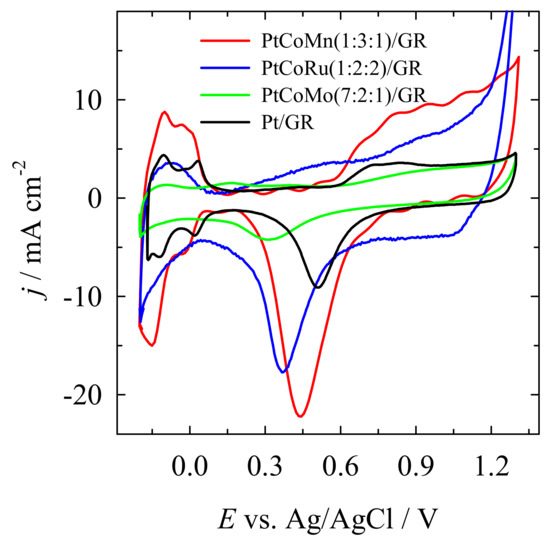
Figure 4.
CVs of the PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, PtCoMo(7:2:1)/GR, and Pt/GR catalysts recorded in a 0.5 M H2SO4 solution at a sweep rate of 50 mV s-1.
The electrocatalytic activity of the graphene-supported PtCoMn, PtCoRu, and PtCoMo catalysts toward the oxidation of methanol in an alkaline medium was investigated using cyclic voltammetry. Figure 5a–c shows long-term CVs for PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts recorded in a 1 M CH3OH + 1 M NaOH solution at a sweep rate of 50 mV s−1. As evident, in the forward sweep, anodic peaks A are observed at 0.2 V. They may be related to the direct oxidation of methanol in an alkaline medium. Anodic peaks B were detected at approximately −0.4 V for all the investigated catalysts in the reverse sweep. They may be attributed to the removal of the incompletely oxidized carbonaceous species formed in the forward sweep. During long-term cycling, the methanol oxidation current density values (anodic peak A) recorded at the investigated PtCoMn(1:3:1)/GR catalyst at first slightly are decreased and then stabilized (Figure 5a).
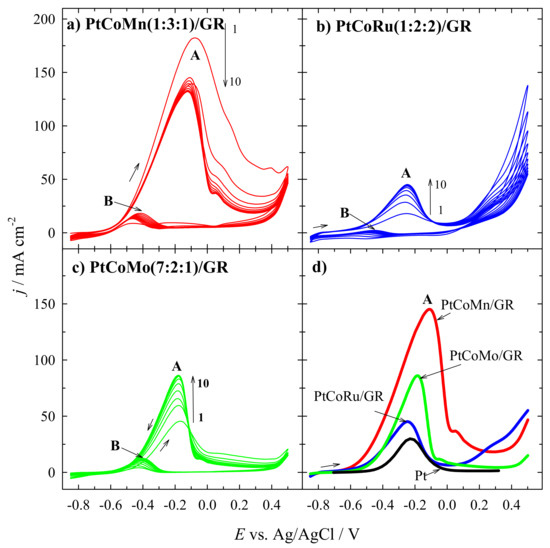
Figure 5.
CVs of PtCoMn(1:3:1)/GR (a), PtCoRu(1:2:2)/GR (b), and PtCoMo(7:2:1)/GR (c) catalysts recorded in 1 M CH3OH + 1 M NaOH at 50 mVs−1. Comparison of positive-potential going scans of the PtCoM/GR and Pt/GR catalysts (d).
The other way is for the PtCoRu(1:2:2)/GR and PtCoMo (7:2:1)/GR catalysts (Figure 5b,c). Current density values are rising and then stabilized. Figure 5d shows positive-potential going scans of the Pt/GR, PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts. The highest electrocatalytic activity for methanol oxidation reaction is noted for the PtCoMn(1:3:1)/GR catalyst (Figure 5d).
Furthermore, all ternary PtCoM/GR catalysts show more negative onset potential of MOR than the Pt/GR catalyst.
As well as methanol oxidation current densities are ca. 4.8, 1.5, and 2.8 times higher at ternary PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts than those at the bare Pt/GR catalyst. The comparison of current densities of our prepared catalysts with various similar catalysts described in the literature is given in Table 2.

Table 2.
The comparison of methanol oxidation current densities obtained for various catalysts presented in the literature and this study.
Figure 6 presents the specific (a) and mass (b) activities of the methanol oxidation reaction on Pt/GR and ternary PtCoM/GR catalysts. Methanol oxidation current densities were normalized by electrochemically active surface areas for each catalyst to represent the catalysts’ specific activity. As seen from the data obtained, the highest specific activity has PtCoMo(7:2:1)/GR. It is approximately two times higher than that at PtCoMn(1:3:1)/GR and PtCoRu(1:2:2)/GR catalysts and ca. four times higher than that at Pt/GR (Figure 6a). To obtain the mass activity data, methanol oxidation current densities at the potential values of peak A were normalized by the Pt loadings for the PtCoMn/GR, PtCoRu/GR, PtCoMo/GR, and Pt/GR catalysts (Figure 6b). The highest mass activity for methanol oxidation was calculated for PtCoMn(1:3:1)/GR and PtCoMo(7:2:1)/GR catalysts, and it was ca. 3 times higher than that at Pt/GR and PtCoRu(1:2:2)/GR catalysts (Figure 6b). Those catalysts also show significantly higher mass activity for methanol oxidation over reported similar materials.
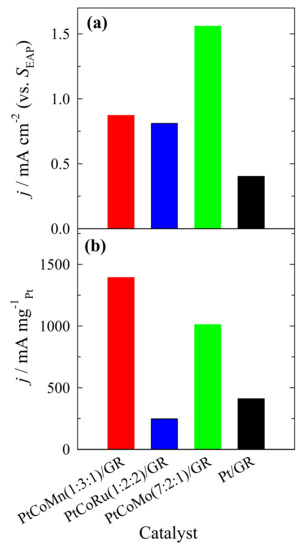
Figure 6.
Bar columns of PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, PtCoMo(7:2:1)/GR, and Pt/GR, catalysts recorded in a 1 M CH3OH + 1 M NaOH solution and normalized by the electrochemically surface area of Pt (a) and Pt loadings (b).
Figure 7 presents the Tafel plots for methanol oxidation derived from the data in Figure 5d. Tafel plots can be divided into two linear regions for Pt/GR, PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts. In the low potential range, a linear region of the Tafel plots on Pt/GR, PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts was found, giving the Tafel slopes of 159.9, 157.4, 239.6, and 152.1 mV dec−1, respectively (Figure 7).
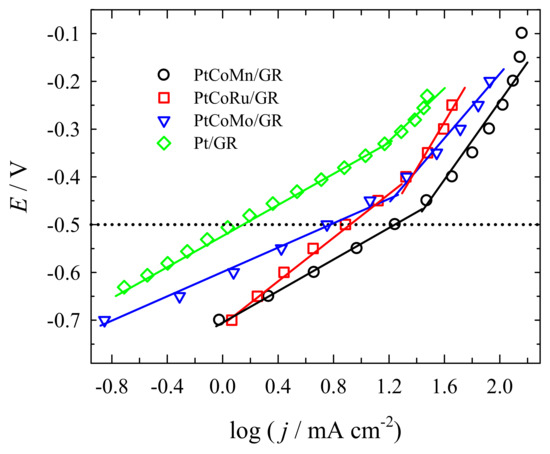
Figure 7.
Tafel plots for oxidation of methanol on various electrocatalysts in a 1 M CH3OH + 1 M NaOH solution.
The similar slopes indicate that the reaction mechanism is the same and corresponded to methanol adsorption and dehydrogenation [63]. Notably, PtCoMn/GR, PtCoRu/GR, and PtCoMo/GR catalysts are more active than Pt/GR. The PtCoMn/GR possess the highest output current density among the four electrocatalysts tested in this study under an identical polarization overpotential E = −0.5 V (Figure 7).
The linear region at high potential is related to oxidative removal of CO-like species [63]. At high potentials, the Tafel slopes of 360.3 mV dec−1 for PtCoMn/GR, 358.7 mV dec−1 for PtCoRu/GR, and 252.0 mV dec−1 for PtCoMo/GR were determined. A sharp change of the Tafel slope shows that removing poisonous species becomes the rate-determining process in the methanol oxidation reaction. The difference in the Tafel slopes at low and high potential ranges indicates a possible change of reaction mechanism or at least a change of rate-determining steps at different potential ranges [64].
Slope values of PtCoMn/GR and PtCoRu/GR are greater than that of PtCoMo/GR at high potential, indicating that the step of removing poisonous species on PtCoMn/GR and PtCoRu/GR is slower than on PtCoMo/GR.
Further, the electrochemical stability of the PtCoM/GR and Pt/GR catalysts for methanol oxidation was investigated using chrono-techniques. Figure 8 shows the CA data obtained at the PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts recorded at a constant potential of −0.2 V in a 1 M CH3OH + 1 M NaOH solution at a temperature of 25 ºC for 1800 s.
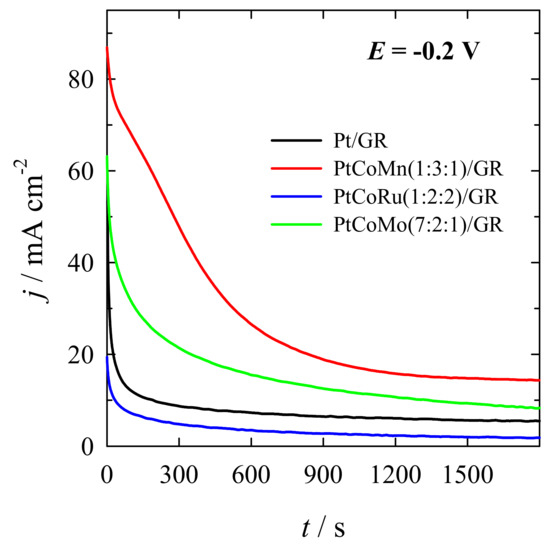
Figure 8.
Chronoamperometry data for PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, PtCoMo(7:2:1)/GR, and Pt/GR in a 1 M CH3OH + 1 M NaOH solution, at E = −0.2 V, t = 1800 s.
As seen in Figure 8, the investigated catalysts show current decay for the methanol oxidation reaction. However, after approximately 600 s, the current density stabilizes. At the end of the experimental period (t = 1800 s), the current densities recorded at the ternary catalysts are ca. 14, 2, and 8 mA cm−2 for PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR, respectively, and 5.5 mA cm−2 for Pt/GR (Figure 8).
At the end of the CA experimental period, calculated specific activity values are 1.8, 2.1, and 4.6 times higher for the PtCoMo(7:2:1)/GR catalyst than those of the PtCoMn(1:3:1)/GR, Pt/GR, and PtCoRu(1:2:2)/GR catalysts, respectively (Figure 9a). The calculated mass activity values are ca. 1.4, 1.8, and 13.7 times higher at PtCoMn(1:3:1)/GR than those at PtCoMo(7:2:1)/GR, Pt/GR, and PtCoRu(1:2:2)/GR catalysts (Figure 9b). As evident from Figure 9, the highest stability for methanol oxidation exhibits the PtCoMn(1:3:1)/GR and PtCoMo(7:2:1)/GR catalysts. These data confirm the results obtained by cyclic voltammetry.
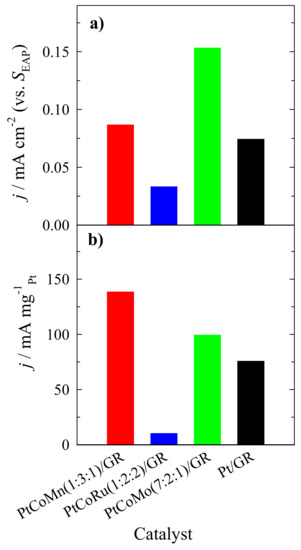
Figure 9.
Bar columns represent PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts specific (a) and mass (b) activity values in a 1 M CH3OH + 1 M NaOH solution, at E = −0.2 V, t = 1800 s.
A chronopotentiometry study of methanol oxidation was also carried out on the investigated ternary catalysts in 1 M CH3OH + 1 M NaOH solution at a constant current density step of 2 mA cm−2 1800 s (Figure 10). It has been determined that the difference between the steady-state operating anode potential and that at open-circuit was the smallest one on the PtCoMn(1:3:1)/GR catalyst and was equal to ca. 0.0357 V, which was followed by the PtCoMo(7:2:1)/GR, PtCoRu(1:2:2)/GR, and Pt/GR, where it reached ca. 0.0406 V, 0.0911 V, and −0.138 V, respectively (Figure 10). It seems that the PtCoMn(1:3:1)/GR, PtCoMo(7:2:1)/GR, and PtCoRu(1:2:2)/GR catalysts note higher stability than Pt/GR.
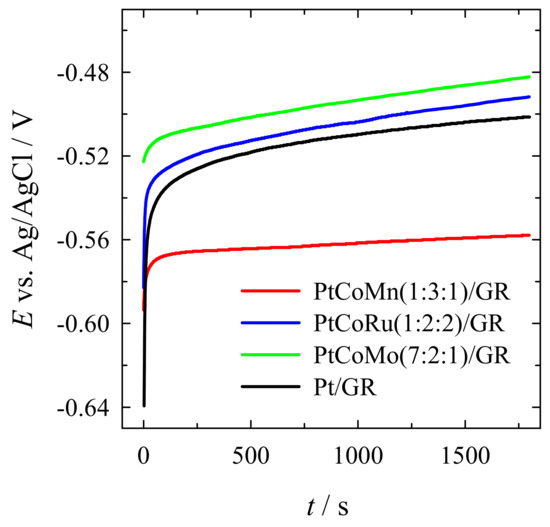
Figure 10.
Chronopotentiometric data obtained on PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts recorded in a 1 M CH3OH +1 M NaOH solution at 2 mA cm−2 for 1800 s.
Figure 11 presents CV data for PtCoMn(1:3:1)/GR (a), PtCoRu(1:2:2)/GR (b), and PtCoMo(7:2:1)/GR (c) catalysts recorded in a 1 M CH3OH +1 M NaOH solution at a sweep rate from 50 to 150 mV s−1.
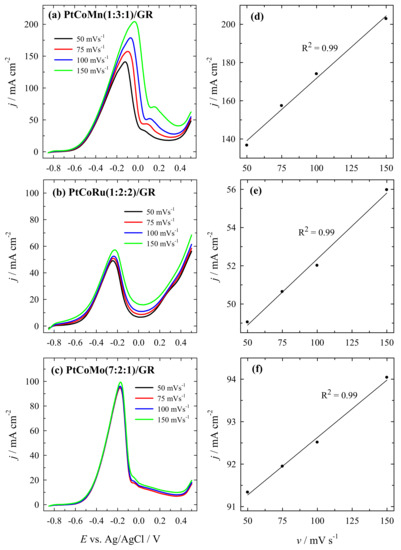
Figure 11.
CVs recorded on PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts in a 1 M CH3OH +1 M NaOH solution at different scan rates of 50–150 mV s−1 (a–c); The relationship between the peak current densities and the square root of the scan rates (d–f).
It is seen that the lowest current density is at 50 mV s−1 and increased at higher scan rates (Figure 11a–c). The plots of peak current density vs. square root of the sweep rate (Figure 11d–f) are linear over the entire range of scan rate studied, indicating a typical diffusion-controlled current in the methanol solution for all investigated catalysts.
3. Materials and Methods
3.1. Synthesis of Graphene-Supported PtCoM (M = Mn, Mo, Ru) Nanoparticle Catalysts
For the one-pot synthesis of PtCoM/GR (M = Ru, Co, Mn) nanoparticle catalysts, reaction mixtures containing constant amounts of H2PtCl6 and CoCl2, and required amounts of RuCl3, Na2MoO4, and MnCl2 were used. Graphene with a purity of 97% and specific surface area of 60 m2 g−1) was used as a substrate. The composition of reaction mixtures was as follows:
- PtCoRu/GR: 1.2 mM H2PtCl6, 12 mM CoCl2, 6 mM RuCl3, 0.1 g graphene powder, ethylene glycol;
- PtCoMo/GR: 1.2 mM H2PtCl6, 12 mM CoCl2, 6 mM Na2MoO4, 0.1 g graphene powder, ethylene glycol;
- PtCoMn/GR: 1.2 mM H2PtCl6, 12 mM CoCl2, 3.1 mM MnCl2, 0.1 g graphene powder, ethylene glycol.
The synthesis of the catalysts was carried out in a Monowave 300 (Anton Paar, Graz, Austria) reactor at a temperature of 170 °C for 30 min. Microwave output power was 750 W. After preparation, the synthesized catalysts were washed with acetone, filtered, and dried in a vacuum oven at 80 °C for two h. Pt/GR was prepared at the same conditions, but the synthesis duration was shortened to 30 s to prepare Pt particles with approximately similar ones in the created PtCoM/GR catalysts.
3.2. Characterization of Catalysts
The shape and size of catalyst particles were examined using a Transmission Electron Microscope Tecnai G2 F20 X-TWIN (FEI, Eindhoven, Netherlands) equipped with an EDAX spectrometer with an r-TEM detector. For microscopic examinations, 10 mg of sample was first sonicated in 1 mL of ethanol for 1 h and then deposited on the Cu grid covered with a continuous carbon film.
XRD patterns of studied powders were measured using an X-ray diffractometer D2 PHASER (Bruker, Karlsruhe, Germany). The measurements were conducted in the 2θ range 10–90°.
The Pt, Co, Mn, Mo, and Ru metal loadings were estimated using an ICP optical emission spectrometer Optima700DV (Perkin Elmer, Waltham, MA, USA).
The distribution of elements in the catalyst was analyzed using a scanning electron microscope TM4000Plus with an AZetecOne detector (Hitachi, Tokyo, Japan).
3.3. Electrochemical Measurements
All electrochemical measurements were performed with a Zennium electrochemical workstation (ZAHNER-Elektrik GmbH & Co.KG, Kronach, Germany). A three-electrode electrochemical cell was used for measurements. The working electrode was a glassy carbon electrode coated with a thin layer of Nafion-impregnated Pt/GR and PtCoM/GR catalysts. The Pt sheet was used as a counter electrode and Ag/AgCl as a reference electrode. The catalyst layer was obtained according to the following steps: at first, the required amounts of Pt/GR and PtCoM/GR catalysts were dispersed ultrasonically for 1 h in a solution containing 0.25 μL of 5 wt % Nafion and 0.75 μL deionized H2O to produce a homogeneous slurry. Then, 5 μL of the prepared suspension mixture was pipetted onto the polished surface of a glassy carbon electrode with a geometric area of a 0.07 cm2 and dried in the air for 12 h.
Cyclic voltammograms (CVs) were recorded at Pt/GR and PtCoM/GR in a deaerated 1 M CH3OH + 1 M NaOH solution at a potential sweep rate of 50 mV s−1. The presented current densities are normalized with respect to the geometric area of catalysts.
The electrochemically active surface areas (ESAs) of Pt in the prepared catalysts were determined from the CVs of the PtCoM/GR and Pt/GR catalysts recorded in a deaerated 0.5 M H2SO4 solution at a potential sweep rate of 50 mV s−1 by calculating the charge associated with hydrogen adsorption (220 µC cm−2) [65]. The ESA values of Pt in the prepared catalysts were calculated by the following Equation (4):
SESA (cm2) = Q (µC)/QH (220 µC cm−2).
The stability of the PtCoM/GR (M = Mn, Mo, Ru) and Pt/GR catalysts was examined using chrono-techniques. Chronoamperometric curves (CA) were recorded in 1 M CH3OH + 1 M NaOH solution at a constant potential value of 0.2 V for 30 min. Chronopotentiometric curves (CP) were recorded at a constant current density of 2 mA cm−2 for 30 min in the same solution.
The methanol oxidation current density values were normalized by the electrochemically active surface areas of Pt and Pt loadings for each catalyst, respectively, to evaluate the specific activity (mA cm−2) and mass activity (mA mgPt−1) of the PtCoM/GR and Pt/GR catalysts.
4. Conclusions
The graphene-supported PtCoMn, PtCoRu, and PtCoMo catalysts were prepared using one-pot microwave-assisted synthesis with the molar ratios of metals Pt:Co:Mn, Pt:Co:Ru, and Pt:Co:Mo equal to 1:3:1, 1:2:2, and 7:2:1, respectively. It has been found that the Pt nanoparticles with an average size of approximately 2.5 nm were deposited in the PtCoMn(1:3:1)/GR, PtCoRu(1:2:2)/GR, and PtCoMo(7:2:1)/GR catalysts. In addition, all metals in the prepared catalysts were uniformly distributed on the surface of graphene. All investigated ternary catalysts showed enhanced electrocatalytic activity toward methanol oxidation than the pure Pt/GR catalyst. However, the highest current density, activity, and stability toward methanol oxidation demonstrated the PtCoMn(1:3:1)/GR catalyst. The results suggest that the enhanced electrocatalytic activity of investigated ternary PtCoM/GR (M = Mn, Ru, Mo) catalysts might be related to the synergistic effect between metals in the catalyst composition.
Author Contributions
This study was conducted through the contributions of all authors. Conceptualization, L.T.-T.; V.K. and E.N.; methodology, T.K.; M.S. and J.V.; formal analysis, T.K.; M.S. and J.V.; investigation, A.B.; J.J.; A.N. and R.S.; data curation, A.B.; D.U. and R.S.; writing—original draft preparation, L.T.-T., A.N. and V.K.; writing—review and editing, E.N.; visualization, J.J. and D.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union Structural Funds project “Development of Doctoral Studies” under grant No. 09.3.3.-ESFA-V-711-01-0001.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goor, M.; Menkin, S.; Peled, E. High power direct methanol fuel cell for mobility and portable applications. Int. J. Hydrog. Energy 2019, 44, 3138–3143. [Google Scholar] [CrossRef]
- Yang, L.; Ge, J.; Liu, C.; Wang, G.; Xing, W. Approaches to improve the performance of anode methanol oxidation reaction—A short review. Curr. Opin. Electrochem. 2017, 4, 83–88. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Du, Q.; Yin, Y.; Jiao, K. Transient investigation of passive alkaline membrane direct methanol fuel cell. Appl. Therm. Eng. 2016, 100, 1245–1258. [Google Scholar] [CrossRef]
- Kakati, N.; Lee, S.H.; Maiti, J.; Yoon, Y.S. Ru decorated Pt nanoparticles by a modified polyol process for enhanced catalytic activity for methanol oxidation. Surf. Sci. 2012, 606, 1633–1637. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; Yin, G.; Tang, Z.; Liu, S. Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 3719–3724. [Google Scholar] [CrossRef]
- Chen, W.; Wei, X.; Zhang, Y. A comparative study of tungsten-modified PtRu electrocatalysts for methanol oxidation. Int. J. Hydrog. Energy 2014, 39, 6995–7003. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, L.; Zhu, J.; Xing, W. Rapid synthesis of a PtRu nano-sponge with different surface compositions and performance evaluation for methanol electrooxidation. Nanoscale 2015, 7, 9467–9471. [Google Scholar] [CrossRef]
- Li, M.; Zheng, H.; Han, G.; Xiao, Y.; Li, Y. Facile synthesis of binary PtRu nanoflowers for advanced electrocatalysts toward methanol oxidation. Catal. Commun. 2017, 92, 95–97. [Google Scholar] [CrossRef]
- Lu, S.; Eid, K.; Ge, D.; Guo, J.; Wang, L.; Wang, H.; Gu, H. One-pot synthesis of PtRu nanodendrites as efficient catalysts for methanol oxidation reaction. Nanoscale 2017, 9, 1033–1039. [Google Scholar] [CrossRef]
- Çögenli, M.S.; Yurtcan, A.B. Catalytic activity, stability and impedance behavior of PtRu/C, PtPd/C and PtSn/C bimetallic catalysts toward methanol and formic acid oxidation. Int. J. Hydrog. Energy 2018, 43, 10698–10709. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Q.; Qu, K.; Guo, L.; Hu, H.; Zang, Y. Decorated PtRu Electro-catalyst for Concentrated Direct Methanol Fuel Cells. Chem. Cat. Chem. 2019, 11, 1238–1243. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, W.; Shi, H.; Liao, F.; Fan, Z.; Shao, M. Mesocrystal PtRu supported on reduced graphene oxide as catalysts for methanol oxidation reaction. J. Colloid Interface Sci. 2019, 557, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Shi, S.; Shen, Y.; Yin, H. PtRu alloy nanoparticles supported on nanoporous gold as an efficient anode catalyst for direct methanol fuel cell. Electrochim. Acta 2019, 293, 390–398. [Google Scholar] [CrossRef]
- Burkan, H.; Cellat, K.; Yilmaz, G.; Şen, F. Direct methanol fuel cells (DMFCs). In Direct Liquid Fuel Cells; Akay, R.G., Yurtcan, A.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 71–94. [Google Scholar] [CrossRef]
- Chang, G.; Cai, Z.; Jia, H.; Zhang, Z.; Liu, X.; Liu, Z.; Zhu, R.; He, Y. High electrocatalytic performance of a graphene-supported PtAu nanoalloy for methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 12803–12810. [Google Scholar] [CrossRef]
- Prabu, N.; Jeyakumar, D. Superior Electrocatalytic Performance of Au-Pt Graded Nano-Alloys towards Alcohol Oxidation Reaction. Chem. Sel. 2018, 3, 13207–13216. [Google Scholar] [CrossRef]
- Huang, D.B.; Yuan, Q.; Wang, H.H.; Zhou, Z.Y. Facile synthesis of PdPt nanoalloys with sub-2.0 nm islands as robust electrocatalysts for methanol oxidation. Chem. Commun. 2014, 50, 13551–13554. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Zhao, X.; An, K.; Ou, Z.; Fang, Y. One-step synthesis of Pt-Pd catalyst nanoparticles supported on few-layer graphene for methanol oxidation. Curr. Appl. Phys. 2018, 18, 898–904. [Google Scholar] [CrossRef]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: An efficient anode catalyst for direct methanol fuel cells. J. Alloys Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Aziz, F.; Rahman, N.A. One-pot synthesis of efficient reduced graphene oxide supported binary Pt-Pd alloy nanoparticles as superior electrocatalyst and its electrocatalytic performance toward methanol electro-oxidation reaction in direct methanol fuel cell. J. Alloys Compd. 2019, 793, 232–246. [Google Scholar] [CrossRef]
- Xu, C.; Hou, J.; Pang, X.; Li, X.; Zhu, M.; Tang, B. Nanoporous PtCo and PtNi alloy ribbons for methanol electrooxidation. Int. J. Hydrog. Energy 2012, 37, 10489–10498. [Google Scholar] [CrossRef]
- Zheng, J.N.; He, L.L.; Chen, C.; Wang, A.-J.; Ma, K.-F.; Feng, J.-J. One-pot synthesis of platinum3cobalt nanoflowers with enhanced oxygen reduction and methanol oxidation. J. Power Sources 2014, 268, 744–751. [Google Scholar] [CrossRef]
- Kepeniene, V.; Tamasauskaite-Tamasiunaite, L.; Jablonskiene, J.; Semasko, M.; Vaiciuniene, J.; Vaitkus, R.; Norkus, E. One-pot synthesis of graphene supported platinum-cobalt nanoparticles as electrocatalysts for methanol oxidation. Mat. Chem. Phys. 2016, 171, 145–152. [Google Scholar] [CrossRef]
- Lei, F.; Li, Z.; Zhang, L.; Wang, Y.; Xu, S.; Lin, S. Facile synthesis of Pt-Cu (Ni, Co)/GNs-CD and their enhanced electrocatalytic activity for methanol oxidation. J. Electrochem. Soc. 2016, 163, F913–F918. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrog. Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Ding, J.; Ji, S.; Wang, H.; Pollet, B.G.; Wang, R. Tailoring nanopores within nanoparticles of PtCo networks as catalysts for methanol oxidation reaction. Electrochim. Acta 2017, 255, 55–62. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, Y.; Qin, L.; Zhao, M. Synthesis of Pt-Co micro/nanoporous array with high activity for methanol electrooxidation. Mater. Lett. 2018, 216, 166–169. [Google Scholar] [CrossRef]
- Ren, W.; Zang, W.; Zhang, H.; Bian, J.; Chen, Z.; Guan, C.; Cheng, C. PtCo bimetallic nanoparticles encapsulated in N-doped carbon nanorod arrays for efficient electrocatalysis. Carbon 2019, 142, 206–216. [Google Scholar] [CrossRef]
- Liu, S.; Qin, L.; Liu, G.; Li, J.; Zhang, Q.; Zhao, J. Controlled growth of PtCo nanoparticles on Ni bowl-like pore arrays as an electrocatalyst for methanol oxidation. Solid State Sci. 2020, 107, 106358. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Wang, X.; Hu, J.; Liu, Y.; Fu, G.; Tang, Y. Concave PtCo nanocrosses for methanol oxidation reaction. Appl. Catal. B Environ. 2020, 277, 119135. [Google Scholar] [CrossRef]
- Macias-Ferrer, D.; Melo-Banda, J.A.; Silva-Rodrigo, R.; Lam-Maldonado, M.; Páramo-García, U.; Verde-Gómez, J.Y.; Del-Ángel-Vicente, P. Highly dispersed PtCo nanoparticles on micro/nano-structured pyrolytic carbon from refined sugar for methanol electro-oxidation in acid media. Catal. Today 2020, 349, 159–167. [Google Scholar] [CrossRef]
- Tamašauskaitė-Tamašiūnaitė, L.; Balčiūnaitė, A.; Vaiciukevičienė, A.; Selskis, A.; Norkus, E. Investigation of electrocatalytic activity of titania nanotube supported nanostructured Pt-Ni catalyst towards methanol oxidation. J. Power Sources 2013, 225, 20–26. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, X.; Hu, H.; Liu, Y.; Zheng, H.; Yang, A.; Chen, L.; Cao, M.; Xu, Y.; Min, Y.; et al. Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation. Adv. Funct. Mater. 2018, 28, 1704774. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Liang, S.; Wang, Y.-H.; Zang, H.-Y. One step synthesis of PtNi electrocatalyst for methanol oxidation. Inorg. Chem. Commun. 2019, 106, 104–110. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Y.; Chen, D.; Fan, Y.; Wang, X.; Wang, W. One-pot synthesis of reduced graphene oxide supported PtCuy catalysts with enhanced electrocatalytic activity for the methanol oxidation reaction. Electrochim. Acta 2014, 136, 292–300. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Chen, B.; Wei, C.; Zhao, J.; Chen, J.; Zhang, X.; Lai, Z.; Fan, Z.; Tan, C.; et al. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef]
- Li, R.; Li, S.; Liu, Y.; Ochiai, T.; Latthe, S.S.; Nakata, K.; Xinga, R.; Liu, S. Polyelectrolyte-assisted soft reduced process for Pt-Cu nanoclusters with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Phys. Chem. Solids 2019, 124, 361–366. [Google Scholar] [CrossRef]
- Long, X.; Yin, P.; Lei, T.; Wang, K.; Zhan, Z. Methanol electro-oxidation on Cu@Pt/C core-shell catalyst derived from Cu-MOF. Appl. Catal. B Environ. 2020, 260, 118187. [Google Scholar] [CrossRef]
- Lv, Q.; Xiao, Y.; Yin, M.; Ge, J.; Xing, W.; Liu, C. Reconstructed PtFe alloy nanoparticles with bulk-surface differential structure for methanol oxidation. Electrochim. Acta 2014, 139, 61–68. [Google Scholar] [CrossRef]
- El-Khatib, K.M.; Abdel Hameed, R.M.; Amin, R.S.; Fetohi, A.E. Core-shell structured Pt-transition metals nanoparticles supported on activated carbon for direct methanol fuel cells. Microchem. J. 2019, 145, 566–577. [Google Scholar] [CrossRef]
- Kilmonis, T.; Nacys, A.; Šimkūnaitė, D.; Tamašauskaitė-Tamašiūnaitė, L.; Balčiūnaitė, A.; Norkus, E. Investigation of methanol electro-oxidation on graphene supported platinum–tungsten catalyst. Chemija 2019, 30, 146–153. [Google Scholar] [CrossRef]
- Lian, J.; Li, C.; Liu, T.; Yuan, Q. One-step synthesis of porous PtNiCu trimetallic nanoalloy with enhanced electrocatalytic performance toward methanol oxidation. J. Saudi Chem. Soc. 2019, 23, 43–51. [Google Scholar] [CrossRef]
- Ravichandran, S.; Karthikeyan, E. Microwave synthesis—A potential tool for green chemistry. Int. J. Chem. Tech. Res. 2011, 3, 466–470. Available online: http://sphinxsai.com/Vol.3No.1/chem_jan-mar11/pdf/CT=72(466-470)%20JMCT11.pdf (accessed on 7 October 2021).
- Cui, J.; Li, W.; Song, X.; Zhang, Z.; Yu, H.; Shan, W.; Xiong, Y. Microwave-assisted one-pot rapid synthesis of mesoporous silica-chitosan composites for efficient recovery of rhenium(Ⅶ). Sep. Purif. Technol. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Siakavelas, I.; Charisiou, N.D.; AlKhoori, S.; AlKhoori, A.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable nickel catalysts for the CO2 methanation reaction based over supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation. Appl. Catal. B-Environ. 2021, 282, 119562. [Google Scholar] [CrossRef]
- AlKhoori, A.; Polychronopoulou, K.; Belabbes, A.; Jaoude, M.A.; Vega, L.F.; Sebastian, V.; Hinder, S.; Baker, M.A.; Zedan, A.F. Cu, Sm co-doping effect on the CO oxidation activity of CeO2. A combined experimental and density functional study. Appl. Surf. Sci. 2020, 521, 146305. [Google Scholar] [CrossRef]
- Siakavelas, I.; Charisiou, N.D.; AlKhoori, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable Ni/La-M (M=Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation. J. CO2 Util. 2021, 51, 101618. [Google Scholar] [CrossRef]
- Menendez, J.A.; Arenillas, A.; Fidalgo, B.; Fernandez, Y.; Zubizarreta, L.; Calvo, E.G.; Bermudez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Elrouby, M.; AbdEl-Lateef, H.M.; Sadef, M. Electrodeposited Pt nanorods on a novel flowered-like nanostructured Ni-Co alloy as an electrocatalyst for methanol oxidation. Int. J. Hydrog. Energy 2019, 44, 13820–13834. [Google Scholar] [CrossRef]
- Han, Z.; Wang, A.-J.; Zhang, L.; Wang, Z.-G.; Fang, K.-M.; Yin, Z.-Z.; Feng, J.-J. 3D highly branched PtCoRh nanoassemblies: Glycine-assisted solvothermal synthesis and superior catalytic activity for alcohol oxidation. J. Colloid Interface Sci. 2019, 554, 512–519. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Liu, C.-F.; Yang, M.-L.; Zhao, X.-H.; Xue, Z.-X.; Xia, Y.-Z. Concave Pt-Cu-Fe ternary nanocubes: One-pot synthesis and their electrocatalytic activity of methanol and formic acid oxidation. Chin. Chem. Lett. 2017, 28, 60–64. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, H.; Liang, H.; Ma, J.; Li, S.; Song, Y.; Wang, R. Microfluidic synthesis and characterization of FePtSn/C catalysts with enhanced electrocatalytic performance for direct methanol fuel cells. Electrochim. Acta 2017, 230, 245–254. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, G.; Xu, G.; Li, Y.; Liu, B.; Gong, X.; Zheng, D.; Zhang, J.; Wang, Q. Synthesis of highly active and dual-functional electrocatalysts for methanol oxidation and oxygen reduction reactions. Appl. Surf. Sci. 2016, 389, 181–189. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Kang, S.-M.; Haldorai, Y.; Jonna, N.; Jankiramab, M.; Lee, G.-W.; Jang, S.-C.; Natesan, B.; Roh, C.; Huh, Y.S. Quaternary PtRuFeCo nanoparticles supported N-doped graphene as an efficient bifunctional electrocatalyst for low-temperature fuel cells. J. Ind. Eng. Chem. 2019, 69, 285–294. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Wang, Y.; Bai, Q.; Gao, Y.; Zhang, Z. Synthesis and electrocatalytic performance of multi-component nanoporous PtRuCuW alloy for direct methanol fuel cells. Catalysts 2015, 5, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Ahmed, R.; Sohail, M.; Khan, S.A.; Ansari, M.S. Co@Pt core-shell nanoparticles supported on carbon nanotubes as promising catalyst for methanol electro-oxidation. J. Ind. Eng. Chem. 2015, 28, 344–350. [Google Scholar] [CrossRef]
- Li, B.; Higgins, D.C.; Zhu, S.; Li, H.; Wang, H.; Ma, J.; Chen, Z. Highly acive Pt-Ru nanowire network catalysts for the methanol oxidation reaction. Catal. Commun. 2012, 18, 51–54. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, C.M. Electrocatalysis of Pd-Co supported on carbon black or ball-milled carbon nanotubes towards methanol oxidation in alkaline media. Appl. Catal. B. 2010, 99, 229–234. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, C.; Chao, L.; Xiong, X.; Liu, H.; Cheng, Y.; Xie, O. Preparation of a thin-film Pt electrocatalyst by MnO2 electrodeposition and galvanic replacement reaction for oxidation of methanol. J. Electroanal. Chem. 2019, 853, 113553. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, H.; Liu, J.; Yao, Y.; Huang, Z.; Fu, C.; Kuang, Y. Preparation of Pd/MnO2-reduced graphene oxide nanocomposite for methanol electro-oxidation in alkaline media. Electrochem. Commun. 2013, 26, 63–66. [Google Scholar] [CrossRef]
- Xu, C.; Su, Y.; Tan, L.; Liu, Z.; Zhang, J.; Chen, S.; Jiang, S.P. Electrodeposited PtCo and PtMn electrocatalysts for methanol and ethanol electrooxidation of direct alcohol fuel cells. Electrochim. Acta 2009, 54, 6322–6326. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Zhang, W.; Huo, M.; Yin, J.; Dang, G.; Ren, Z.; Zhang, Q.; Xie, J.; Mao, S.S. Morphology-dependent electrocatalytic performance of Fe2(MoO4)3 for electro-oxidation of methanol in alkaline medium. J. Materiomics 2017, 3, 135–143. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Liu, M.; Guo, Y. Fabrication of a novel PtPbBi/C catalyst for ethanol electro-oxidation in alkaline medium. Electrochim. Acta 2012, 83, 1–6. [Google Scholar]
- Wang, W.; Li, Y.; Wang, H. Tin oxide nanoparticle-modified commercial PtRu catalyst for methanol oxidation. Micro. Nano Lett. 2013, 8, 23–26. [Google Scholar] [CrossRef]
- Angerstein-Kozlowska, H.; Conway, B.E.; Sharp, W.B.A. The real condition of electrochemically oxidized platinum surfaces: Part I. Resolution of component processes. J. Electroanal. Chem. 1973, 43, 9–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).