Abstract
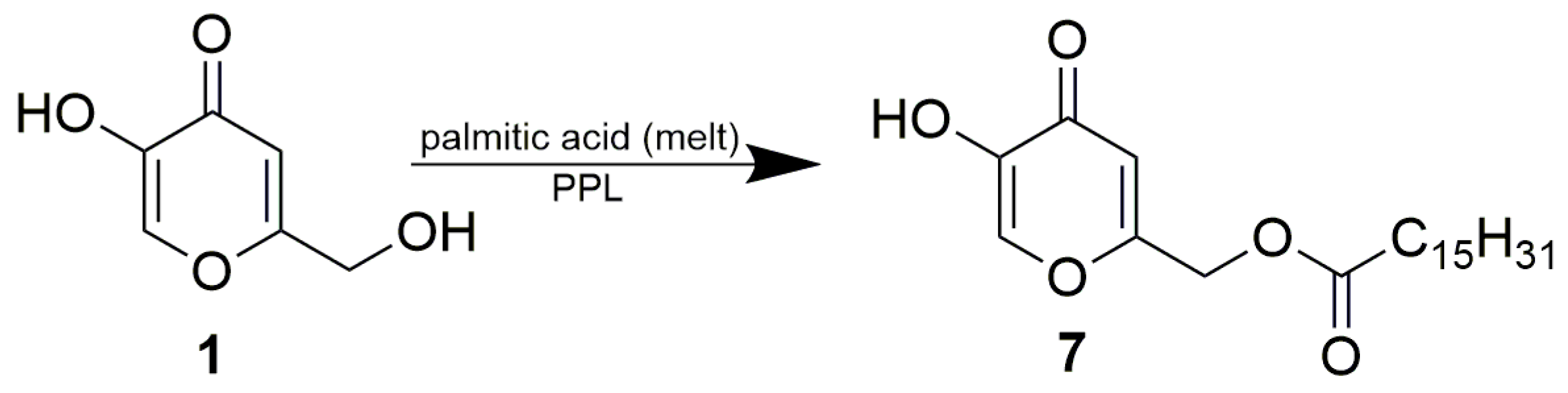
Kojic acid is a fungal metabolite and one of the strongest tyrosinase inhibitors. Its esters are used as lipid-compatible skin whitening components in cosmetic formulations. In this study, lipase PS, lipase AK, Lipolyve AN and pig pancreatic lipase catalyzed the acetylation of kojic acid under selective formation of the same product, kojic 7-acetate. However, the enzymes differed in their regioselectivity when catalyzing the alcoholysis of kojic acid diacetate. While lipase PS and lipase AK produced mixtures of both monoacetate regioisomers (7-acetate and 5-acetate of kojic acid), the pancreatic lipase almost exclusively produced 5-acetate. The enzyme displayed the same regioselectivity in the palmitoylation of kojic acid and in the alcoholysis of kojic acid dipalmitate. Simple reaction engineering with PPL as a catalyst thus provides the complementary monoesters of kojic acid. Kojic 7-acetate, 5-acetate, 7-palmitate and 5-palmitate were prepared with yields after purification of 57.3%, 38.2%, 31.7% and 31.4%, respectively.
1. Introduction
Kojic acid (5-hydroxy-2-hydroxymethyl-4H-pyran-4-one, 1) is a natural metabolite produced by a wide range of fungi and bacteria; it is frequently present in fermented foods, especially in Eastern Asia. Structurally, it is a multifunctional 4-pyranone bearing two hydroxyl groups differing in their chemical nature (Scheme 1). Together with the neighboring carbonyl, the phenolic hydroxyl is responsible for the chelating properties of kojic acid and related derivatives [1]. Kojic acid is probably the most studied inhibitor of tyrosinase and is oftentimes used as a standard in evaluations of new tyrosinase inhibitors [2]. Its inhibitory effect is due to chelation of the copper in the active site of the enzyme by the phenol-carbonyl couple [3]. Methylation of the phenolic hydroxyl therefore completely suppresses the inhibition; in contrast, derivatization of the primary hydroxyl in position 7-C with a hydrophobic substituent increases the inhibitory effect, probably due to hydrophobic interactions with a secondary binding domain of the enzyme [4]. According to the study of Lajis [5], kojic acid and its analogues also inhibit melanoma formation at the cell level. Due to the inhibitive effect of kojic acid against tyrosinase and melanoma formation, kojic dipalmitate is produced yearly at the multi-ton level for the cosmetic industry, since this substance is popular in Asian countries as a lipophilic skin whitening agent. The dipalmitate is hydrolyzed in situ to the active inhibitor by skin esterases.
Scheme 1.
Structure of kojic acid (1).
Free kojic acid is rather instable at ambient conditions. Several attempts have been made to improve its stability and water-solubility by enzymatic glycosylation [6,7,8,9,10,11], and also to widen its use in oil-based foods and cosmetic products by lipase catalyzed esterification [5,12,13,14,15,16,17,18,19,20,21,22,23,24]. Data on the enzymatic esterification (which is in fact an acylation) of kojic acid are, however, rather confusing with respect to the regioselectivity of the reaction. For example, Kobayashi et al. [18] published a paper on the semi-continual production of kojic 7-laurate by the direct esterification of kojic acid with free lauric acid using lipase from Candida antarctica. In contrast, Liu and Shaw [15] reported kojic 5-laurate and 5-oleate as the sole products of the direct esterification of the same reaction catalyzed by lipases from Pseudomonas (Burkholderia) cepacia and Penicillium cammemberti. However, the NMR data and positive reaction of the products with Fe3+ (characteristic of the free phenol moiety of kojic acid) suggest that the products were in fact esterified on the primary hydroxyl. A later work undertaken by authors from the latter team [22] describes the preparation of kojic acid mono laurate without specifying its positional structure.
Esterification of phenols by lipases occurs only rarely and has been reported, for example, for lipase from Chromobacterium viscosum [25] or Mucor miehei [26]. On the other hand, if the substrate comprises both phenolic and primary hydroxyls, the acylation would preferably proceed on the latter moiety, since primary alcohols are more reactive in enzymatic acylations. This is the expected situation also in case of kojic acid, and the products would therefore be acylated in position 7-O- of the kojic acid skeleton. Nevertheless, selective protection of the phenolic hydroxyl by acylation would also be of interest for organic chemists, since it gives rise to being able to chemically modify it on the unprotected primary alcohol, thereby potentially leading to new tyrosinase or melanoma inhibitors (after phenol deprotection).
2. Results and Discussion
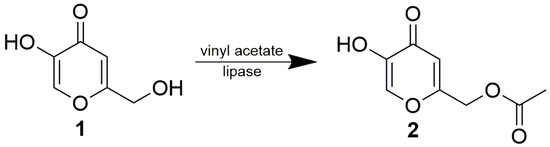
The objective of our work was to investigate the ability of lipases to directly and selectively acylate the phenolic moiety of kojic acid without reacting with the free primary hydroxyl occurring on the substrate. To simplify the experiment, acetylation with vinyl acetate was selected as the reaction model. The reaction is actually transesterification, which means that the process is fast, in contrast to direct acylation with free fatty acids. Moreover, it was easy to monitor the reaction, since all the theoretical reactants (kojic acid, two kojic monoacetates and kojic diacetate) could be estimated by HPLC in one analysis (Supplementary Materials, Figure S1).
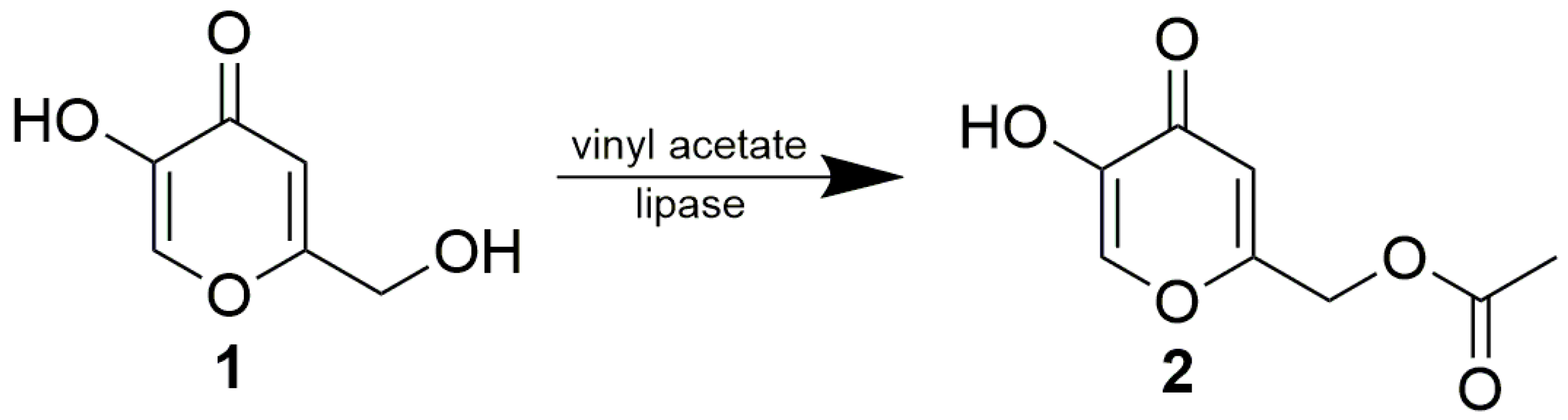
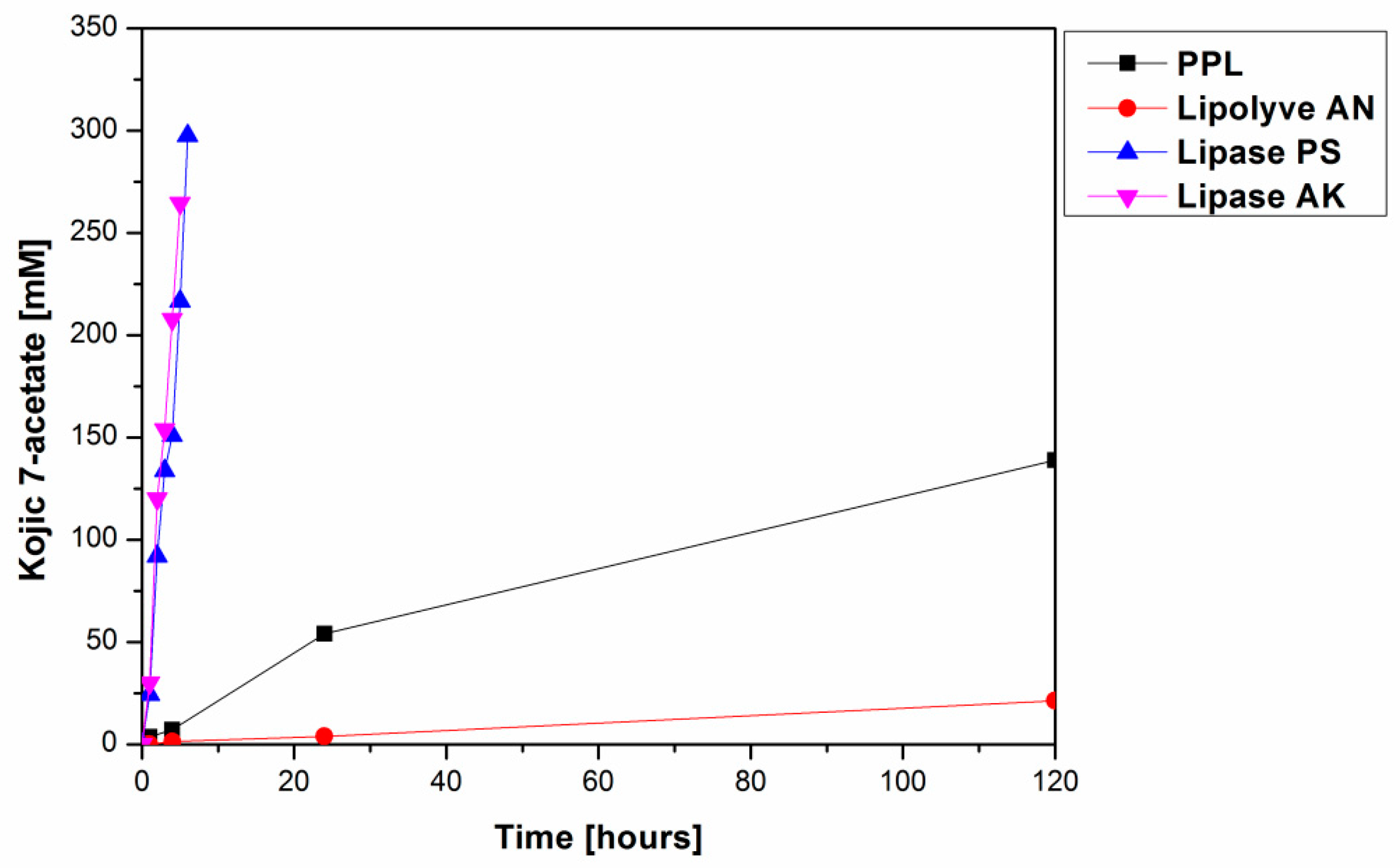
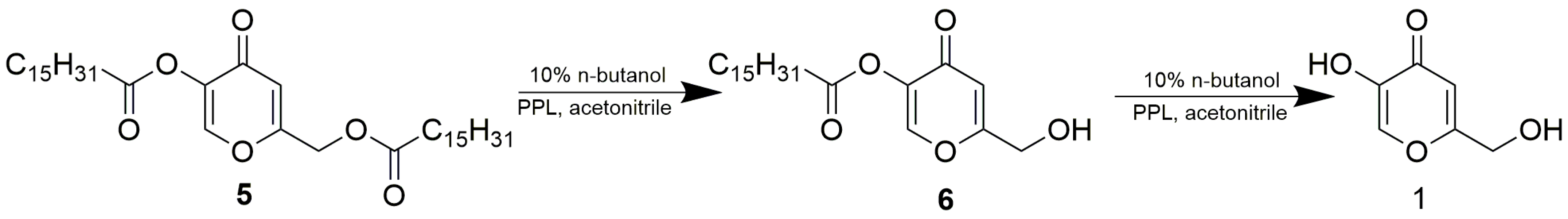
From among the eight tested lipases possessing acetyl esterase activity (Supplementary Materials, Table S1), only lipase AK, lipase PS, Lipolyve AN and pig pancreatic lipase (PPL) acetylated kojic acid to a reasonable extent. All four lipases, representing bacterial, fungal and animal enzymes, catalyzed the acetylation of the primary hydroxyl of kojic acid, thereby solely forming 7-acetate 2 (Scheme 2, Figure 1). Formation of the complementary monoacetate 3 was not observed. Preparative acetylation catalyzed by PPL produced monoacetate 2 with a yield of 57.3%; its structure was confirmed by NMR (see Section 3.4 and Supplementary Materials Figures S2 and S3). This observation supports our presumption that some of the published lipase-catalyzed acylations of phenolic hydroxyl of kojic acid possibly present incorrect product structures.
Scheme 2.
Enzymatic acetylation of free kojic acid.
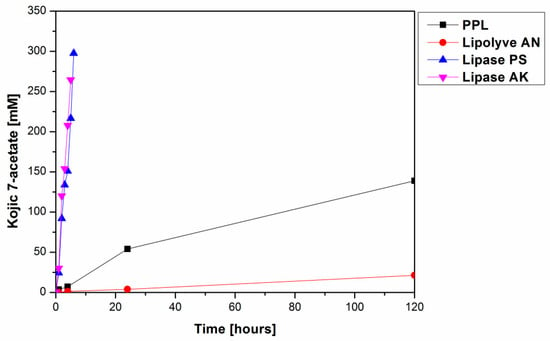
Figure 1.
Time course of lipase catalyzed formation of kojic acid 7-acetate (2) via acetylation of 1 at 37 °C. The reaction mixtures comprised 50 mg lipase, 75 mg kojic acid, 2 mL chloroform and 0.2 mL vinyl acetate.
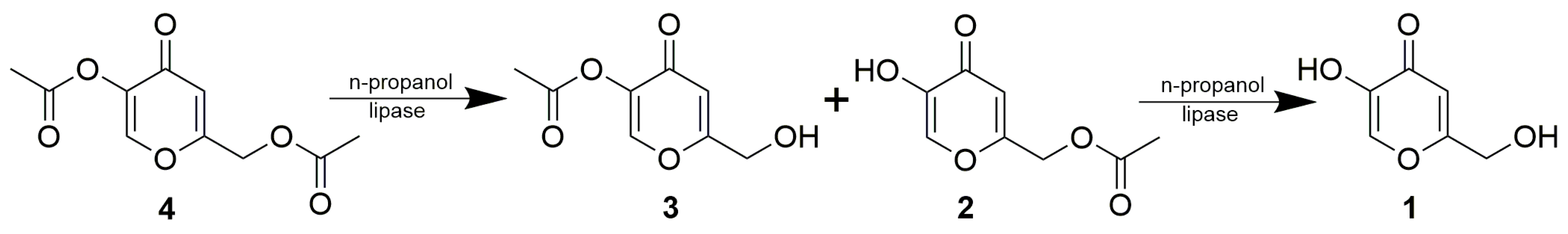
The regioselectivity of the tested lipases is probably the result of their chemoselectivity favoring the acetylation of primary alcohol over phenolic hydroxyl. If identical chemoselectivity occured in the hydrolysis or the alcoholysis of kojic acid diesters, it would be possible to obtain the complementary 5-monoacetate 3. In order to test this idea, alcoholysis in organic solvents instead of hydrolysis in water was carried out as a more controllable reaction format, enabling variation of alcohol content and overall polarity of the reaction medium through selection of the solvent. Three lipases (PPL, PS and AK) were used for the alcoholysis of kojic diacetate 4 by 10% n-propanol in methyl tert-butyl ether. The latter two enzymes simultaneously produced both monoesters 2 and 3 (with a prevalence of 2), which, after reaching a maximum concentration, degraded to free 1 (Scheme 3, Figure 2). In contrast, PPL continually produced 3 with only limited formation of free kojic acid and a negligible amount of 2. This means that only PPL retains its chemoselectivity with respect to the primary alcohol/ester, both in the acetylation of kojic acid and the alcoholysis of its diacetate 4.
Scheme 3.
Lipase-catalyzed alcoholysis of kojic acid diacetate (4).
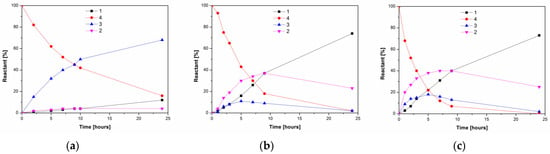
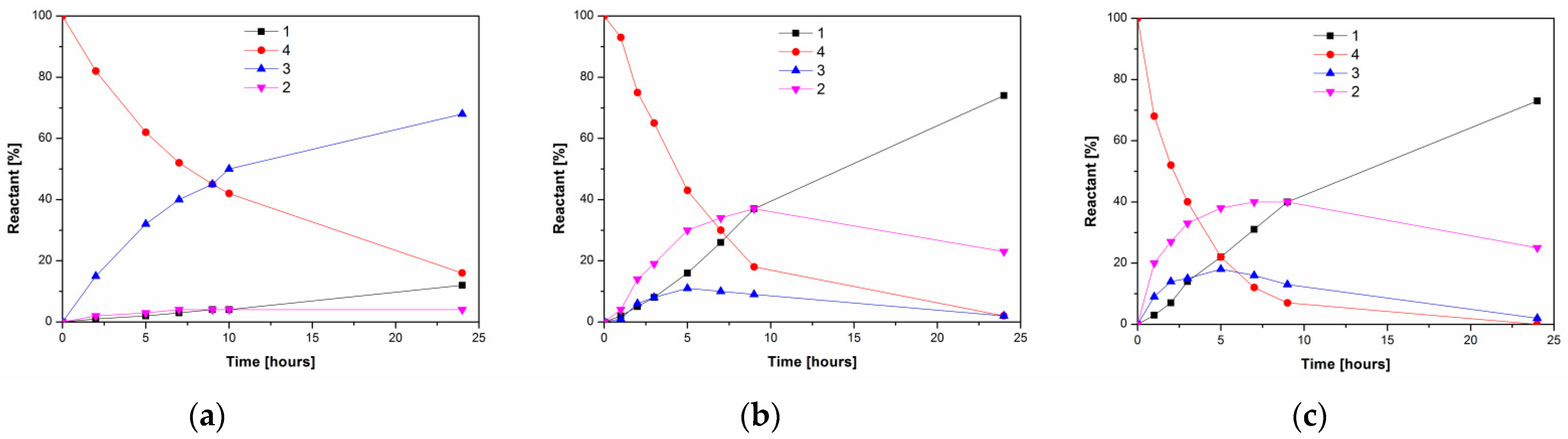
Figure 2.
Time course of lipase-catalyzed alcoholysis of kojic acid diacetate 4 and product formation at 37 °C. (a) PPL; (b) lipase PS; (c) lipase AK. The reaction mixtures comprised lipase (2.5 mg/mL), 4 (6.25 mg/mL) and 10% n-propanol in MTBE.
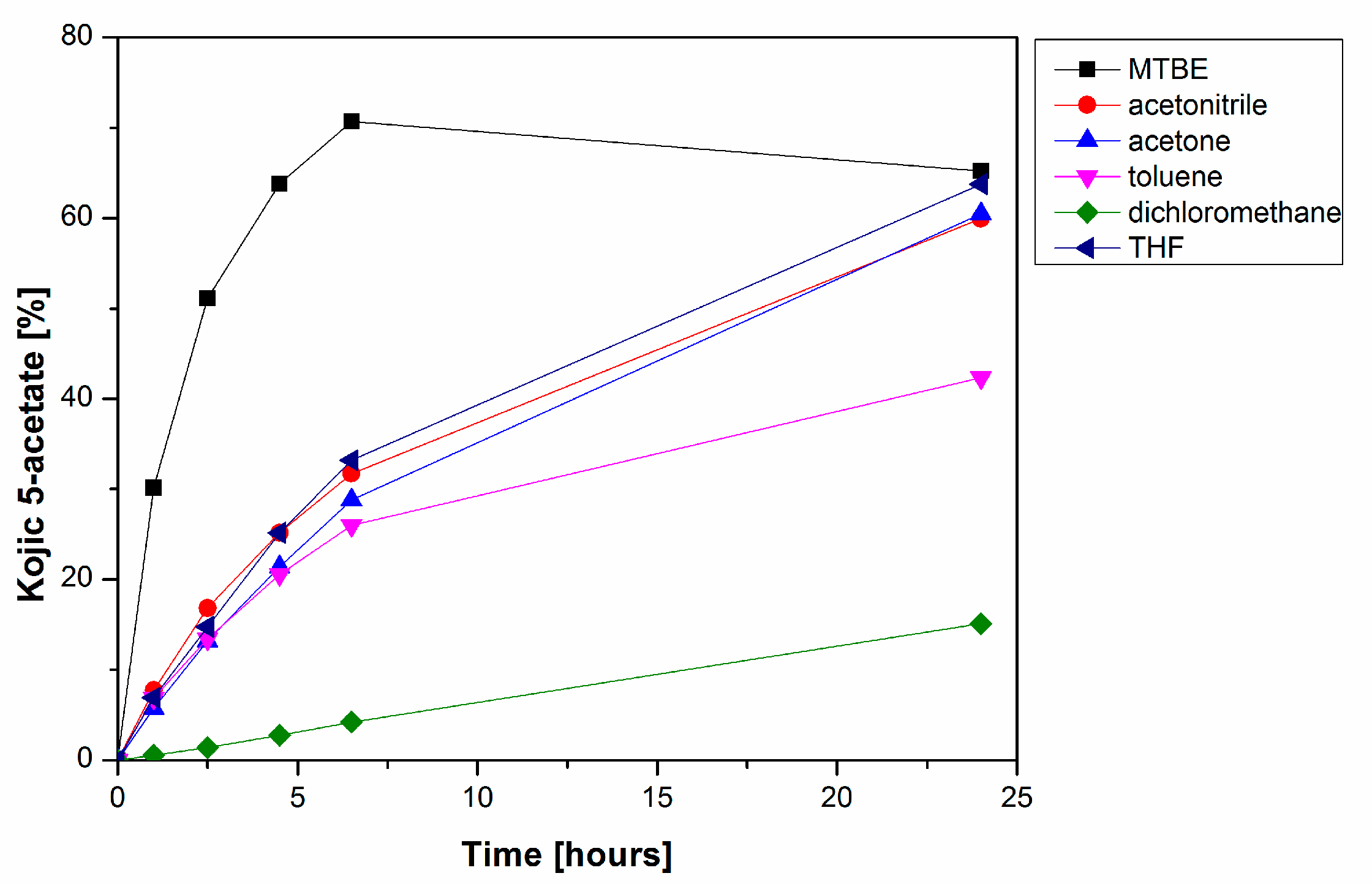
The PPL-catalyzed deacetylation of 4 was further studied in order to achieve higher speed, selectivity and yield of the reaction. Using an organic solvent as a reaction environment can significantly affect the performance of lipases due to the effect of its polarity, potential inhibition of the enzyme or its ability to solubilize substrates and products of the enzymatic reaction. Six solvents (toluene, tetrahydrofuran, acetonitrile, acetone, dichloromethane and MTBE) were tested to find the best reaction media for the deacetylation of kojic diacetate 4. We did not observe any direct relation between the polarity of the solvents used and the productivity of 3, although ethers such as THF and especially MTBE were excellent media for alcoholysis (Figure 3). All reactions in this set of experiments produced only negligible amounts of the undesired 2.
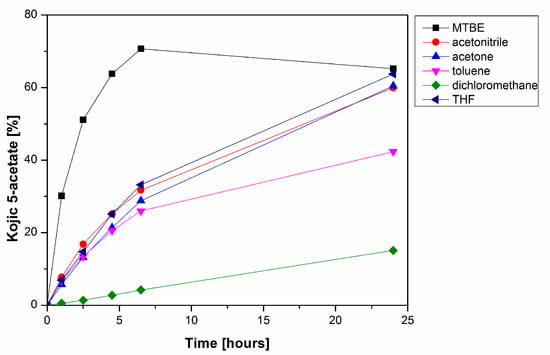
Figure 3.
Time course of PPL-catalyzed formation of kojic acid 5-acetate 3 in organic solvents. The reaction mixtures comprised PPL (2.5 mg/mL), kojic diacetate 4 (6.25 mg/mL) and 10% n-propanol in organic solvent.
Similarly to the organic solvent, the presence of alcohol or water can influence polarity of the reaction system and modulate the activity of the biocatalyst. To compare the performance of PPL in the alcoholysis and hydrolysis of 4, the deacetylation was carried out in neat water and in 10% n-propanol or 10% water in acetonitrile as a water-miscible solvent (Figure 4).
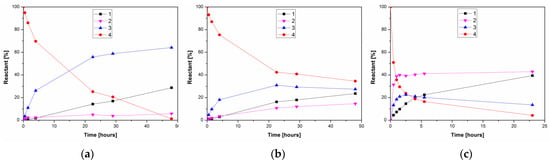
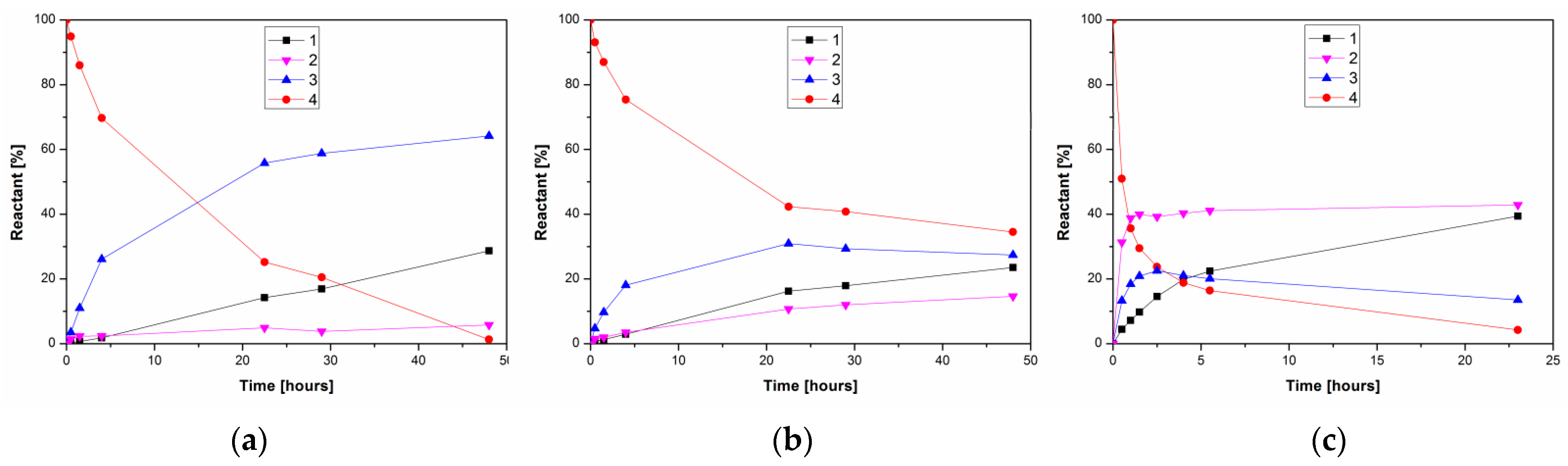
Figure 4.
Comparison of time course of PPL-catalyzed alcoholysis/hydrolysis of kojic acid diacetate 4 in: (a) 10% n-propanol in acetonitrile, (b) 10% water in acetonitrile and (c) neat water at 37 °C. The reaction mixtures comprised 1.5 mg/mL PPL and 6.25 mg/mL 4.
Propanolysis of kojic diacetate in acetonitrile once again almost exclusively produced 3 (Figure 4a), while hydrolysis with 10% water also produced increased amounts of 2 (Figure 4b). On the other hand, hydrolysis in neat water simultaneously produced both monoacetates, with 2 as the major product (Figure 4c). Our results suggest that the organic environment fixes the chemoselectivity of PPL towards the primary acyl of kojic diacetate, while the replacement of water by alcohol is important to suppress formation of the minor byproduct 2. In contrast, the only published report thus far describing the enzymatic deacetylation of kojic diacetate, from Peña-Montes et al. [27], describes the hydrolysis of 4 by two esterases from Aspergillus nidulans. One of these enzymes was selective against the 5-O-acetyl moiety of 4, thereby producing the 7-monoacetate 2.
To assess if alcoholysis of 4 in MTBE can be affected by the nature of the alcohol, we compared the effectiveness of dry methanol, 99% ethanol, 99.8% n-propanol and 99.8% n-butanol in the reaction (Table 1). Within 6 h, the highest production of 3 was reached by use of n-butanol and dry methanol. Since we had previously experienced a lower reproducibility of results with different batches of dried methanol, n-butanol was selected as a reliable alcoholysis reagent.

Table 1.
Effect of type of alcohol on alcoholysis of kojic diacetate 4: percentage content of monoacetate 3 and unreacted 4.
A concentration of butanol in the reaction mixture did not substantially influence the maximum formation of 3 within the first 6 h, with the exception of a slower reaction in neat butanol. The alcoholysis of diacetate 4 in this case was slower, so the content of 3 was increasing during the whole reaction time interval (25 h). Alcoholysis in 10% butanol was selected for further experiments to simplify the workup of reaction mixtures. This choice obviously requires monitoring of the reaction to stop it before the point at which alcoholysis of 3 prevails.
The preparative alcoholysis of 4 in a 200 mL scale was stopped after 6.5 h (with the conversion of 4 to 3 being 72%). The product was purified by flash chromatography to give 0.39 g (38%) of pure 3. The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S4 and S5).
In this work, preparing kojic monoacetates served as an experimental model for exploring lipase regioselectivity. In contrast to long-chain fatty esters of kojic acid, kojic acetates do not find practical application in the cosmetic industry. Therefore, we have been interested in whether PPL maintains the same regioselectivity in the synthesis of kojic acid monopalmitates. Again, the esterification of free kojic acid and the alcoholysis of kojic diester (namely kojic dipalmitate 5) were studied. Since we did not find any HPLC method compatible with a whole mixture of free kojic and palmitic acids, kojic monopalmitates and kojic dipalmitate, the course of the reaction was monitored only qualitatively by TLC, and chemical yields of products were estimated by weight after separation and purification. Kojic dipalmitate is not soluble in MTBE, so alcoholysis with 10% butanol was tested in acetonitrile. The reaction was finished after 4 days and the product after isolation and purification was obtained with a yield of 31.4%. Structural analysis by NMR confirmed that the product was kojic 5-palmitate 6 and, therefore, the alcoholysis proceeded on the primary hydroxyl esterified with palmitic acid (Scheme 4). Thus, PPL maintains the same regioselectivity in the alcoholysis of both kojic diesters 4 and 5.
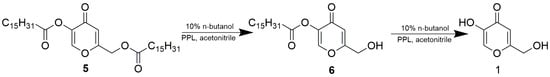
Scheme 4.
PPL-catalyzed alcoholysis of kojic acid dipalmitate 5. The reaction mixture comprised 12.5 mg/mL PPL and 17.2 mg/mL 5.
We were obviously also interested in the regioselectivity of PPL in the palmitoylation of free kojic acid. Due to the inhibitive effect of acetic acid, lipase-catalyzed acetylations are usually performed as transesterifications, where acetic esters serve as the acetate donor. However, acylation with long fatty acids such as palmitic acid can be executed as a direct esterification with free acid, which is an inexpensive and non-toxic way of ester formation. Zero or minimal inhibition occurs; however, the esterification requires a long reaction time. All reactants should be dissolved (at least partly) in the reaction medium, since the enzyme is suspended in the reaction mixture as a solid material. Our initial attempts to palmitoylate kojic acid in acetonitrile upon catalysis of either lipase PS or PPL were complicated by the formation of a thick mixture during the course of the reaction. To avoid this problem and to accelerate the reaction by using an excess of acyl donor in combination with an elevated temperature, the palmitoylation of kojic acid was carried out as a reaction in a solvent-free melt of palmitic acid catalyzed by PPL (Scheme 5). Isolation and purification by flash chromatography provided a 31.7% yield of the product, 7. NMR analysis confirmed esterification of the primary hydroxyl of kojic acid, and the same regioselectivity of PPL was thus proven in the acetylation and palmitoylation of free kojic acid. This regioselectivity may be linked to the natural catalytic sn-1,3 specificity of pig pancreatic lipase in the hydrolysis of triglycerides, which is used routinely in the regiospecific analysis of fatty acids in triglyceride structures [28]. Thus, PPL is a universal catalyst which enables the preparation of complementary monoesters of kojic acid by simple reaction engineering, switching between two reaction formats: the esterification of kojic acid and the alcoholysis of its diesters.
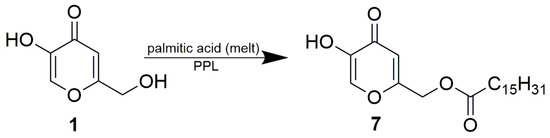
Scheme 5.
PPL-catalyzed palmitoylation of kojic acid. The reaction mixture consisted of 1 g PPL and 1 g kojic acid in a melt of palmitic acid (3 g).
NMR techniques were chosen as an instrument to distinguish the position of esterification on kojic acid, and the chemical shift of methylene protons at C-7 were used as the marker since the binding of carboxyl has a downfield effect on the chemical shift of methylene protons H-7. As can be seen from Table 2, chemical shifts between 4.4 and 4.5 ppm are typical for H-7 of the free hydroxymethyl moiety of 1, 3 and 6, while signals between 4.9 and 5.02 ppm are characteristic for the acylation of this group in 2, 4, 5 and 7. Acetylation of the phenolic hydroxyl also resulted in a change of chemical shift of H-6 (neighboring the phenol) from 7.95–7.98 (free phenol at 1 and 2) to 8.26–8.29 ppm (acetylated phenol at 3 and 4). However, this downfield effect on H-6 was not observed in the spectra of kojic palmitates. Our results were supported by a selective INEPT technique applied to all three kojic acetates.

Table 2.
Key proton signals for distinguishing acylation of primary and phenolic hydroxyl of kojic acid in 1H NMR.
Proton NMR spectra can thus help to quickly distinguish regioisomers of kojic monoesters in the enzymatic acylation of 1. Assignment of the correct structure is especially important in cases where kojic monoesters are used in the study of their inhibition mechanism in tyrosinase reactions or melanoma formation.
3. Materials and Methods
3.1. General
Pig pancreatic lipase (PPL) was purchased from Sigma-Aldrich (St. Louis, MO, USA); lipase PS (Burkholderia cepacia), lipase AYS (Candida rugosa), lipase A (Aspergillus niger), lipase F (Rhizopus oryzae) and lipase AK (Pseudomonas fluorescens) were kindly donated by Amano Enzymes (Elgin, IL, USA); and Lipolyve CC (Candida cylindracea) and Lipolyve AN (Aspergillus niger) were a kind gift from Lyven (Colombelles, France). Their acetyl esterase activity was estimated according to Biely et al. [29].
The kojic acid (1) was from LikoSpol (Bratislava, Slovakia), the palmitic acid was from Merck (Bratislava, Slovakia), molecular sieves (4Å, 1–2 mm) were from ThermoFisher (Dreieich, Germany), and Silica gel 60 for flash chromatography and 4-nitrophenyl acetate were from Fluka (Buchs, Switzerland). All other solvents and reagents were from local suppliers.
High-performance liquid chromatography was performed on Agilent 1200 Series apparatus. Flash chromatography was performed on Isolera One with UV detection from Biotage (Uppsala, Sweden). Spectrophotometric assays of acetyl esterase activity were performed on Spectrophotometer UV-1800 (Shimadzu Co., Ltd., Kyoto, Japan). 1H NMR (400 MHz) and 13C NMR (101 MHz) spectra were recorded with 400 MHz Bruker AVANCE III HD 400 MHz (Bruker GmbH, Karlruhe, Germany).
The production and degradation of kojic acetates were monitored at 254 nm by HPLC on MZ–Aqua Perfect C18 5µm reverse-phase column (250 × 4.0 mm2) from MZ-Analysentechnik (Mainz, Germany), equilibrated and eluted with a mixture of acetic acid (0.15%) and 1-butanol (1%) in water with a flow rate of 1 mL/min.
3.2. Synthesis of Kojic Acid Diesters
3.2.1. Kojic Acid Diacetate 4
Kojic acid (12 g) and potassium carbonate (11 g) were mixed with 80 mL of acetic anhydride. The mixture was stirred under calcium chloride stopper at 65 °C for 100 min. The reaction was quenched by pouring onto ice and left for 2 h to precipitate the product, which was then separated by filtration and recrystallized from ethanol to give 7.47 g (39%) of white crystalline material. The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S6 and S7)
3.2.2. Kojic Dipalmitate 5
Kojic acid (14.21 g) was suspended in 800 mL of dry dichloromethane, followed by 24.29 g (33.3 mL) triethylamine and 2.4 g 4-dimethylaminopyridine, and then the mixture was cooled to 10 °C. Palmitoyl chloride (60.47 g, 66.5 mL) in 200 mL dichloromethane was added dropwise. The reaction mixture became homogenous, and after 5 min, the product started to precipitate. The whole mixture was stirred for an additional 4 h at laboratory temperature. Next, 1 L of 95% ethanol was added, and the crystalline product was separated by filtration and recrystallized from ethanol to give 58.26 g (94%) of white crystalline material. The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S8 and S9)
3.3. Enzymatic Reactions
3.3.1. Enzymatic Acetylations of 1
Reaction mixtures in 4 mL flasks stoppered by septum comprised 2 mL chloroform, 0.2 mL vinyl acetate, 75 mg kojic acid and 50 mg lipase. The blank reaction did not contain any enzyme. The acetylations (ran in triplicates) were stirred at 37 °C. In preselected time intervals, 50 µL aliquots of the reaction mixture were mixed with 950 µL of methanol to stop the reaction, vortexed, passed through a 0.22 µm nylon syringe filter, diluted 20 times with the eluent and injected onto HPLC. The concentrations of the reactants were calculated from calibration curves of standards prepared by non-optimized enzymatic reactions (5- and 7-monoacetates of kojic acid, 3 and 2, respectively) or chemical acetylation (kojic acid diacetate, 4).
3.3.2. Preparative Enzymatic Acetylation of 1
A mixture of 0.5 g 1, 0.5 g pig pancreatic lipase, 2 mL vinyl acetate and 1 g molecular sieves in 20 mL chloroform were stirred for 4 days at laboratory temperature. The reaction mixture was then filtered and concentrated on a rotary evaporator. The product, 7-acetate of kojic acid (2), was purified by flash chromatography on silica gel in a gradient of methanol in chloroform. The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S2 and S3)
3.3.3. Enzymatic Deacetylation of Kojic Acid Diacetate 4—General Procedure
The deacetylations of 4 were run in 10 mL Duran flasks comprising pre-weighted amounts of enzyme, the substrate (4, typically 6.25 mg/mL), 9 mL of solvent and 1 mL alcohol or water. The reactions were optimized according to the type of enzyme and concentration, type of solvent, type and concentration of alcohol, and the concentration of 4. The reaction mixtures were analyzed exactly the same way as in the acetylation of kojic acid.
3.3.4. Preparative Enzymatic Alcoholysis of 4
A mixture of 1.25 g 4, 2.5 g pig pancreatic lipase and 200 mL of 10% n-butanol in MTBE was stirred for 6.5 h at 37 °C. The reaction mixture was then filtered and the solvents were removed by evaporation under reduced pressure. The product was purified by flash chromatography on silica gel by a gradient of methanol in chloroform to give 0.39 g (38.2%) of pure kojic 5-acetate (3), as confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S4 and S5).
3.3.5. Preparative Enzymatic Alcoholysis of Kojic Dipalmitate (5)
A mixture of 5 (1.72 g), PPL (1.25 g) and 10% n-butanol in acetonitrile (100 mL) was stirred at 37 °C for 4 days, filtered and concentrated by vacuum evaporation. The product was isolated by flash chromatography on silica gel by a gradient of methanol in chloroform. Fractions comprising solely the monoester (as verified by TLC) were combined and concentrated in vacuo to give 0.33 g (31.4%) of kojic 5-palmitate (6). The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S10 and S11).
3.3.6. Enzymatic Preparation of Kojic Acid 7-Palmitate (7)
A mixture of 1 g of kojic acid, 3 g of palmitic acid and 1 g of PPL was stirred in a round flask on an alumina nest at 74 °C. After 5 days, an additional 1 g of PPL was added to the melt and the reaction was run for another 5 days, while being monitored by TLC (ethyl acetate/toluene/acetic acid, 1:1:0.1, visualization under UV lamp). The melt was then suspended in chloroform and the enzyme was removed by filtration and washed with chloroform. The filtrate and washings were pooled, concentrated in vacuo and fractionated by flash chromatography on silica gel by a gradient of methanol in chloroform. Fractions comprising solely the monoester (as verified by TLC) were combined and concentrated in vacuo to give 0.79 g (31.7%) of 7. The structure of the product was confirmed by NMR (see Section 3.4. and Supplementary Materials Figures S12 and S13).
3.4. Interpretation of NMR Spectra of Kojic Acid and Its Monoesters and Diesters
- Kojic acid (1, NMR spectra available in Supplementary Materials, Figures S14 and S15)(2-hydroxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 7.95 (s, 1H, H-6), 6.50 (t, J = 0.9 Hz, 1H, H-3), 4.41 (d, J = 0.9 Hz, 1H, CH2-7).13C NMR (101 MHz, CD3OD): δ = 176.8 (C-4), 170.4 (C-2), 147.4 (C-6), 141.0 (C-5), 110.7 (C-3), 61.2 (CH2-7).
- Kojic acid 7-acetate (2)(2-acetoxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 7.98 (s, 1H, H-6), 6.48 (s, 1H, H-3), 4.96 (s, 2H, CH2-7), 2.12 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 176.4 (C-4), 171.6 (COCH3), 164.5 (C-2), 147.7 (C-6), 141.4 (C-5), 113.0 (C-3), 62.5 (CH2-7), 20.4 (COCH3).
- Kojic acid 5-acetate (3)(2-hydroxymethyl-5-acetoxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 8.26 (s, 1H, H-6), 6.57 (t, J = 1.0 Hz, 1H, H-3), 4.46 (d, J = 1.0 Hz, 2H, CH2-7), 2.28 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 175.5 (C-4), 171.7 (COCH3), 169.4 (C-2), 150.9 (C-6), 142.0 (C-5), 113.2 (C-3), 60.9 (CH2-7), 20.1(COCH3).
- Kojic acid 5,7-diacetate (4)(2-acetoxymethyl-5-acetoxy-4H-pyran-4-one)1H NMR (400 MHz, CD3OD): δ = 8.29 (s, 1H, H-6), 6.57 (t, J = 0.7 Hz, 1H, H-3), 5.02 (d, J = 0.7 Hz, 2H, CH2-7), 2.28 (s, 3H, CH3), 2.14 (s, 3H, CH3).13C NMR (101 MHz, CD3OD): δ = 175.0 (C-4), 171.5 (COCH3), 169.3 (COCH3), 165.7 (C-2), 151.2 (C-6), 142.2 (C-5), 115.5 (C-3), 62.2 (CH2-7), 20.3 (COCH3), 20.1 (COCH3).
- Kojic Acid 5,7-Dipalmitate (5)(2-palmitoyloxymethyl-5-palmitoyloxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.86 (s, 1H, H-6), 6.47 (s, 1H, H-3), 4.91 (s, 2H, CH2-7), 2.59 (t, J = 7.5 Hz, 2H, CH2-1’), 2.39 (t, J = 7.6 Hz, 2H, CH2-1”), 1.73 (p, J = 7.6 Hz, 2H, CH2-2’), 1.65 (p, J = 7.2 Hz, 2H, CH2-2”), 1.43–1.35 (m, 2H, CH2-3’), 1.33–1.28 (m, 2H, CH2-3”), 1.32 (s, 44H, 22 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3).13C NMR (101 MHz, CDCl3): δ = 172.6 (C-4), 172.3 (COCH2-), 170.7 (COCH2-), 162.5 (C-2), 147.7 (C-6), 141.3 (C-5), 115.1 (C-3), 60.7 (CH2-7), 33.9 (CH2-1’), 33.7 (CH2-1”), 31.9 (CH2-2’, CH2-2”), 29.7 (4 × CH2), 29.7 (2 × CH2), 29.6 (2 × CH2), 29.6 (2 × CH2), 29.6 (CH2), 29.6 (CH2), 29.4 (2 × CH2), 29.4 (2 × CH2), 29.2 (CH2), 29.2 (CH2), 29.1 (CH2), 29.0 (CH2), 24.8 (CH2), 24.7 (CH2), 22.7 (2 × CH2), 14.1 (2 × CH3).
- Kojic Acid 5-Palmitate (6)(2-hydroxymethyl-5-palmitoyloxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.86 (s, 1H, H-6), 6.55 (s, 1H, H-3), 4.49 (s, 2H, CH2-7), 2.59 (t, J = 7.5 Hz, 2H, CH2-1’), 1.73 (p, J = 7.5 Hz, 2H, CH2-2’), 1.35–1.43 (m, 2H, CH2-3’), 1.28–1.33 (m, 2H, CH2-4’), 1.26 (bs, 20H, 10 × CH2), 0.88 (t, J = 6.7 Hz, 3H, CH3).13C NMR (101 MHz, CDCl3): δ = 173.2 (C-4), 170.9 (COCH2-), 168.2 (C-2), 147.8 (C-6), 141.0 (C-5), 113.1 (C-3), 60.7 (CH2-7), 33.6 (CH2-1’), 31.9 (CH2-2’), 29.7 (3 × CH2), 29.6 (3 × CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 29.0 (CH2), 24.7 (CH2), 22.7 (CH2),14.1 (CH3).
- Kojic Acid 7-Palmitate (7)(2-palmitoyloxymethyl-5-hydroxy-4H-pyran-4-one)1H NMR (400 MHz, CDCl3): δ = 7.85 (s, 1H, H-6), 6.50 (s, 1H, H-3), 4.93 (s, 2H, CH2-7), 2.40 (t, J = 7.6 Hz, 2H, CH2-1’), 1.65 (p, 2H, J = 7.3 Hz, CH2-2’), 1.34–1.28 (m, 8H, 4 × CH2), 1.26 (bs, 16H, 8 × CH2), 0.88 (t, J = 6.8 Hz, CH3).13C NMR (101 MHz, CDCl3): δ = 174.0 (C-4), 172.7 (COCH2-), 163.0 (C-2), 145.9 (C-6), 138.2 (C-5), 111.2 (C-3), 61.1 (CH2-7), 33.9 (CH2-1’), 31.9 (CH2-2’), 29.7 (3 × CH2), 29.6 (3 × CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 29.1 (CH2), 24.8 (CH2), 22.7 (CH2), 14.1 (CH3).
4. Conclusions
Demand for lipid-compatible derivatives of kojic acid as skin whitening agents inhibiting tyrosinase activity and meloma formation provoked efforts to prepare them via enzymatic esterification as a green alternative to chemical processes. Monoesters of kojic acid were prepared under catalysis by lipases and proteases. However, the published data are rather inconsistent regarding the correct structure of prepared monoesters, frequently claiming the less probable acylation of the phenolic hydroxyl of kojic acid. In our model of acetylations of kojic acid by four lipases of various origin, the acetylation proceeded strictly on the primary hydroxyl in position 7-O- of the kojic acid structure, and palmitoylation with pig pancreatic lipase (PPL) proceeded with the same regioselectivity. Interestingly, this enzyme differs in its selectivity in the alcoholysis of kojic acid diesters, almost exclusively providing kojic 5-monoesters instead of mixtures of 5-O- and 7-O-acylated kojic acid, as produced by two other studied lipases. This affinity of PPL towards acylation and deacylation of the primary hydroxyl/ester enables us to prepare the complementary regioisomers of monoacylated kojic acid as pure substances at a preparative scale. A chemical shift of methylene protons at C-7 in 1H NMR spectra was found to be a reliable indicator for distinguishing the regioisomers of kojic acid monoesters.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11121430/s1. Figure S1: HPLC chromatogram of reaction mixture from alcoholysis of 4. Retention times: 1: 1.984 min, 3: 2.737 min, 2: 5.198 min, 4: 7.780 min. Figure S2: 1H NMR spectra of kojic acid 7-acetate (2). Figure S3: 13C NMR spectra of kojic acid 7-acetate (2). Figure S4: 1H NMR spectra of kojic acid 5-acetate (3). Figure S5: 13C NMR spectra of kojic acid 5-acetate (3). Figure S6: 1H NMR spectra of kojic acid diacetate (4). Figure S7: 13C NMR spectra of kojic acid diacetate (4). Figure S8: 1H NMR spectra of kojic acid dipalmitate (5). Figure S9: 13C NMR spectra of kojic acid dipalmitate (5). Figure S10: 1H NMR spectra of kojic acid 5-palmitate (6). Figure S11: 13C NMR spectra of kojic acid 5-palmitate (6). Figure S12: 1H NMR spectra of kojic acid 7-palmitate (7). Figure S13: 13C NMR spectra of kojic acid 7-palmitate (7). Figure S14: 1H NMR spectra of kojic acid (1). Figure S15: 13C NMR spectra of kojic acid (1). Table S1: Acetyl esterase activity of lipases used in acetylation of 1.
Author Contributions
Conceptualization, V.M.; methodology, V.M.; investigation, K.K. and M.M.; writing—original draft preparation, V.M.; writing—review and editing, M.M.; supervision, M.M.; project administration, V.M. and M.M.; funding acquisition, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovak Research and Development Agency, grant number APVV-18-0188. It was also funded by the Slovak Grant Agency for Science VEGA, grant number 2/0126/19.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the contribution of COST Action Greenering (CA18224), supported by COST (European Cooperation in Science and Technology), in promoting scientific interaction and exchange of knowledge in the field of green technologies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Chen, Y.H.; Lu, P.J.; Hulme, C.; Shaw, A.Y. Synthesis of kojic acid-derived copper-chelating apoptosis inducing agents. Med. Chem. Res. 2013, 22, 995–1003. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Goh, M.; Lee, J.; Ahn, S.M.; Yeon, J.; Yoo, D.S.; Kim, D.H.; Kim, H.G.; Cho, J.Y. Ester derivatives of kojic acid and polyphenols containing adamantane moiety with tyrosinase inhibitory and anti-inflammatory properties. Bull. Korean Chem. Soc. 2011, 32, 1411–1414. [Google Scholar] [CrossRef][Green Version]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef]
- Hassan, M.A.; Ismail, F.; Yamamoto, S.; Yamada, H.; Nakanishi, K. Enzymatic synthesis of galactosylkojic acid with immobilized β -galactosidase from Bacillus circulans. Biosci. Biotechnol. Biochem. 1995, 59, 543–545. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Giridhar, R.; Wu, W.T. Regioselective formation of kojic acid-7-O-alpha-D-glucopyranoside by whole cells of mutated Xanthomonas campestris. Enzyme Microb. Technol. 2007, 40, 324–328. [Google Scholar] [CrossRef]
- Kitao, S.; Sekine, H. Syntheses of two kojic acid glucosides with sucrose phosphorylase from Leuconostoc mesenteroides. Biosci. Biotechnol. Biochem. 1994, 58, 419–420. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Hamada, H. Functional glucosylation of kojic acid and daidzein with the eucalyptus membrane-associated UDP-glucosyltransferase reaction system. J. Biosci. Bioeng. 2001, 92, 469–471. [Google Scholar] [CrossRef]
- Nishimura, T.; Kometani, T.; Takii, H.; Terada, Y.; Okada, S. Acceptor specificity in the glucosylation reaction of Bacillus subtilis X-23 α-amylase towards various phenolic compounds and the structure of kojic acid glucoside. J. Ferment. Bioeng. 1994, 78, 37–41. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nakanishi, K.; Hassan, M.A. Chromatographic separation of galactosylkojic acid. J. Ferment. Bioeng. 1997, 84, 82–85. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, W.; Fu, J.; Li, Z.; Wang, Z.; Lü, P. Double-lipase catalyzed synthesis of kojic dipalmitate in organic solvents. Chem. Res. Chinese Univ. 2017, 33, 903–907. [Google Scholar] [CrossRef]
- Raku, T.; Tokiwa, Y. Regioselective synthesis of kojic acid esters by Bacillus subtilis protease. Biotechnol. Lett. 2003, 25, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Norddin, F.A.A.; Azhar, S.N.A.S.; Ashari, S.E. Evaluation of direct esterification of fatty acid derivative of kojic acid in co-solvent system: A statistical approach. J. Chem. Eng. Process Technol. 2017, 8, 1000331. [Google Scholar] [CrossRef]
- Liu, K.; Shaw, J. Lipase-catalyzed synthesis of kojic acid esters in organic solvents. J. Am. Oil Chem. Soc. 1998, 75, 1507–1511. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Lipase-catalyzed synthesis of kojic acid derivative in bioreactors and the analysis of its depigmenting and antioxidant activities. Cosmetics 2017, 4, 22. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Comparative study of stirred and fluidized tank reactor for hydroxyl-kojic acid derivatives synthesis and their biological activities. Turk. J. Biochem. 2018, 43, 205–219. [Google Scholar] [CrossRef]
- Kobayashi, T.; Adachi, S.; Nakanishi, K.; Matsuno, R. Semi-continuous production of lauroyl kojic acid through lipase-catalyzed condensation in acetonitrile. Biochem. Eng. J. 2001, 9, 85–89. [Google Scholar] [CrossRef]
- Khamaruddin, N.H.; Basril, M.; Lian, G.E.C.; Salleh, A.B.; Rahman, R.N.Z.R.; Ariff, A.; Mohamad, R.; Awang, R. Enzymatic synthesis and characterization of palm-based kojic acid ester. J. Oil Palm Res. 2008, 20, 461–469. [Google Scholar]
- Jumbri, K.; Al-Haniff Rozy, M.F.; Ashari, S.E.; Mohamad, R.; Basri, M.; Fard Masoumi, H.R. Optimisation and characterisation of lipase catalysed synthesis of a kojic monooleate ester in a solvent-free system by response surface methodology. PLoS ONE 2015, 10, e0144664. [Google Scholar] [CrossRef]
- Ishak, N.; Lajis, A.F.B.; Mohamad, R.; Ariff, A.B.; Mohamed, M.S.; Halim, M.; Wasoh, H. Kinetics and optimization of lipophilic kojic acid derivative synthesis in polar aprotic solvent using lipozyme RMIM and its rheological study. Molecules 2018, 23, 501. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Liu, K.-J.; Lou, Y.-H.; Shieh, C.-J. Optimisation of kojic acid monolaurate synthesis with lipase PS from Pseudomonas cepacia. J. Sci. Food Agric. 2002, 82, 601–605. [Google Scholar] [CrossRef]
- El-Boulifi, N.; Ashari, S.E.; Serrano, M.; Aracil, J.; Martínez, M. Solvent-free lipase-catalyzed synthesis of a novel hydroxyl-fatty acid derivative of kojic acid. Enzyme Microb. Technol. 2014, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ashari, S.E.; Mohamad, R.; Ariff, A.; Basri, M.; Salleh, A.B. Optimization of enzymatic synthesis of palm-based kojic acid ester using response surface methodology. J. Oleo Sci. 2009, 58, 503–510. [Google Scholar] [CrossRef]
- Nicolosi, G.; Piattelli, M.; Sanfilippo, C. Acetylation of phenols in organic solvent catalyzed by a lipase from Chromobacterium viscosum. Tetrahedron 1992, 48, 2477–2482. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Ma, C.Y.; Lou, Z.X.; Chen, S.W.; Dai, J.; Wang, H.X. Optimization of lipase-catalyzed synthesis of acetylated EGCG by response surface methodology. J. Mol. Catal. B Enzym. 2013, 97, 87–94. [Google Scholar] [CrossRef]
- Peña-Montes, C.; Lange, S.; Castro-Ochoa, D.; Ruiz-Noria, K.; Cruz-García, F.; Schmid, R.; Navarro-Ocaña, A.; Farrés, A. Differences in biocatalytic behavior between two variants of StcI esterase from Aspergillus nidulans and its potential use in biocatalysis. J. Mol. Catal. B Enzym. 2009, 61, 225–234. [Google Scholar] [CrossRef]
- Luddy, F.E.; Barford, R.A.; Herb, S.F.; Magidman, P.; Riemenschneider, R.W. Pancreatic lipase hydrolysis of triglycerides by a semimicro technique. J. Am. Oil Chem. Soc. 1964, 41, 693–696. [Google Scholar] [CrossRef]
- Biely, P.; Côté, G.L.; Kremnický, L.; Weisleder, D.; Greene, R.V. Substrate specificity of acetylxylan esterase from Schizophyllum commune: Mode of action on acetylated carbohydrates. Biochim. Biophys. Acta 1996, 1298, 209–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).