Synthesis of Graphene-Based Biopolymer TiO2 Electrodes Using Pyrolytic Direct Deposition Method and its Catalytic Performance
Abstract
1. Introduction
2. Results and Discussion
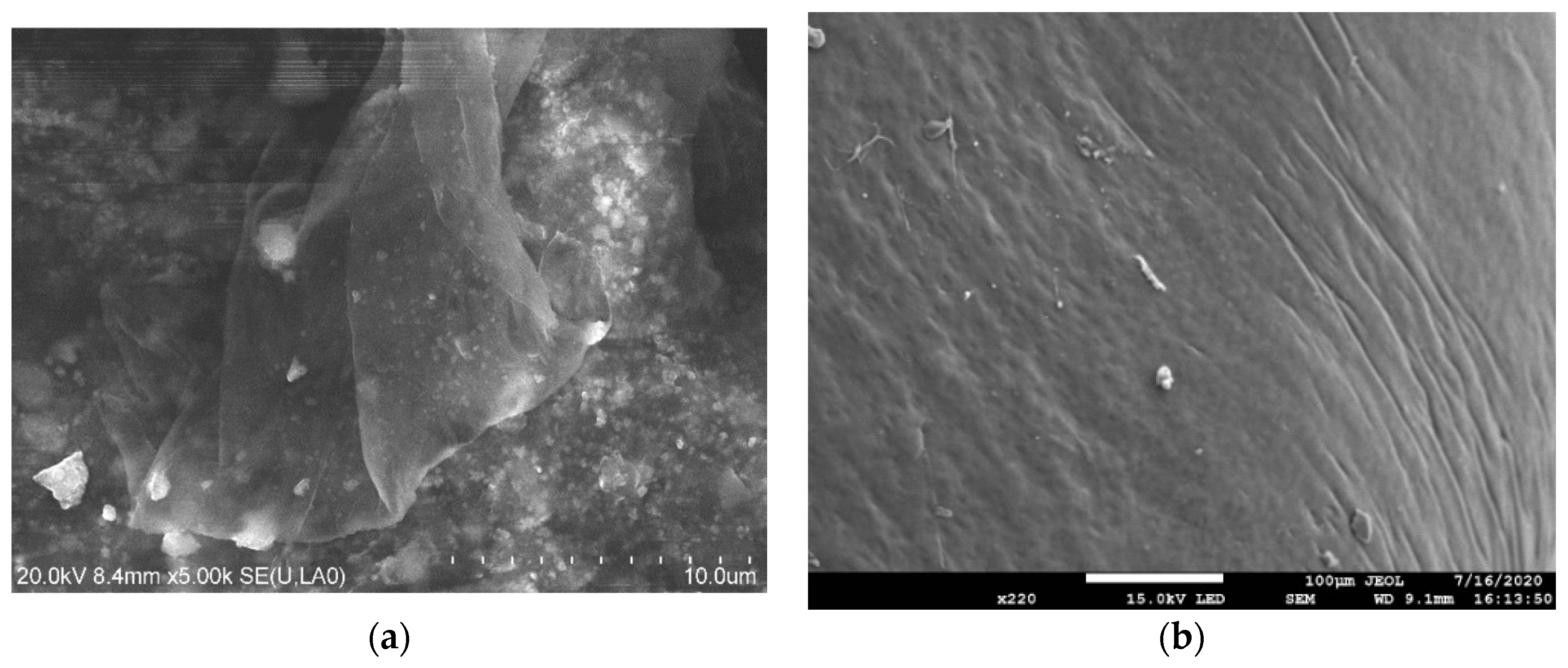
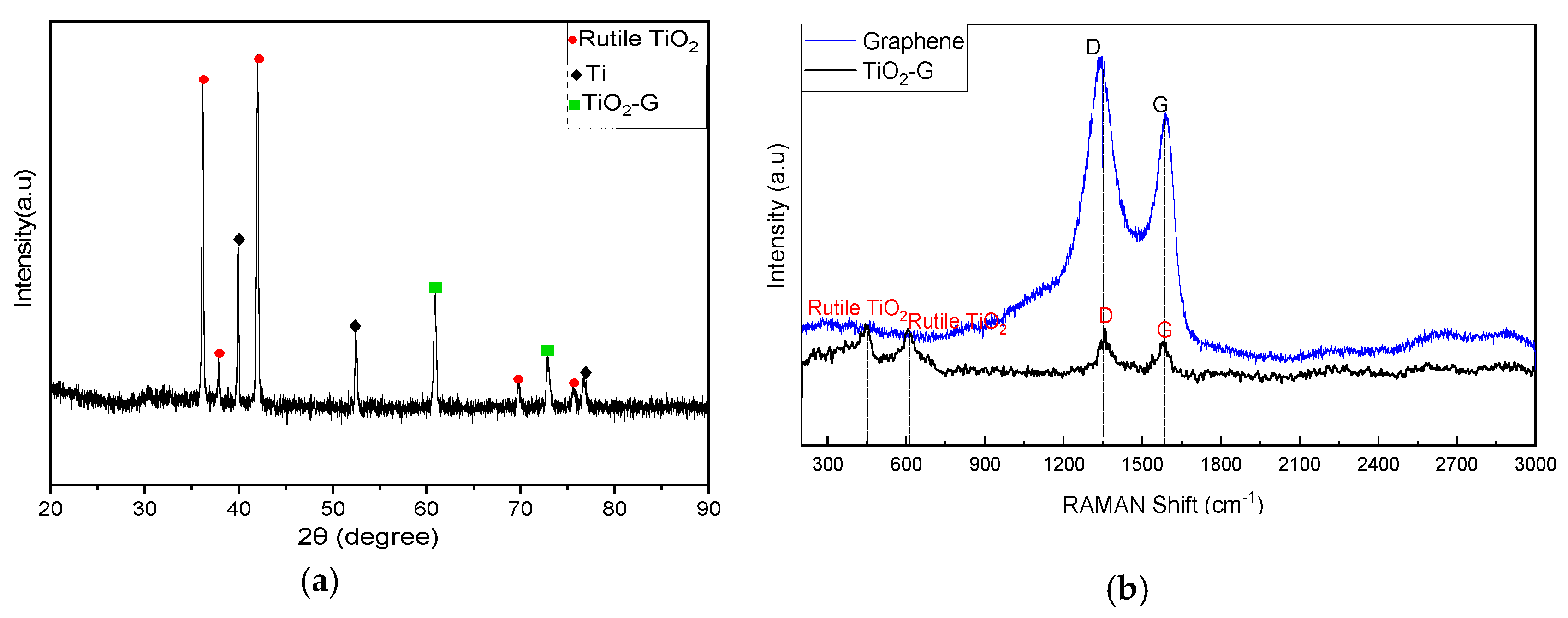
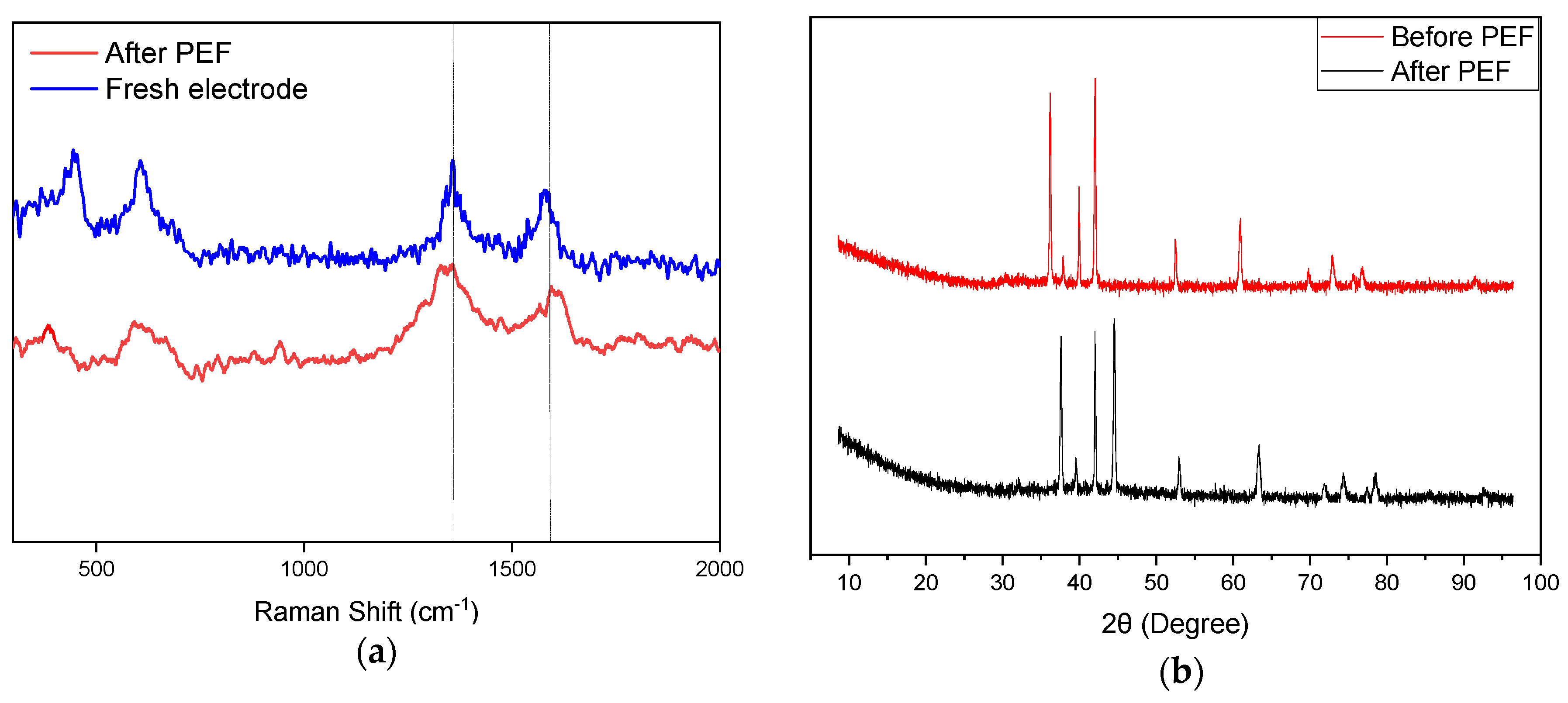
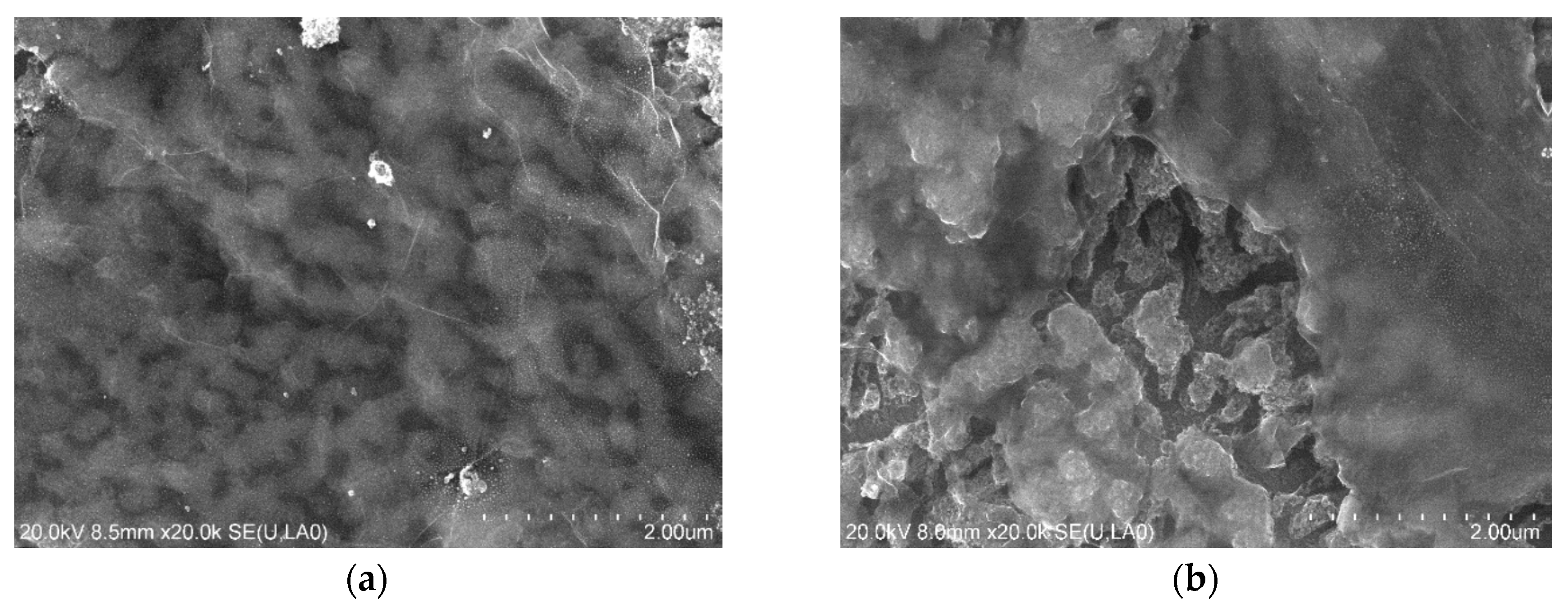
2.1. Characteristics of TiO2-G Electrode
2.2. Photo-Electrocatalytic Activity (PEA)
2.2.1. Model Fitting and Analysis of Variance
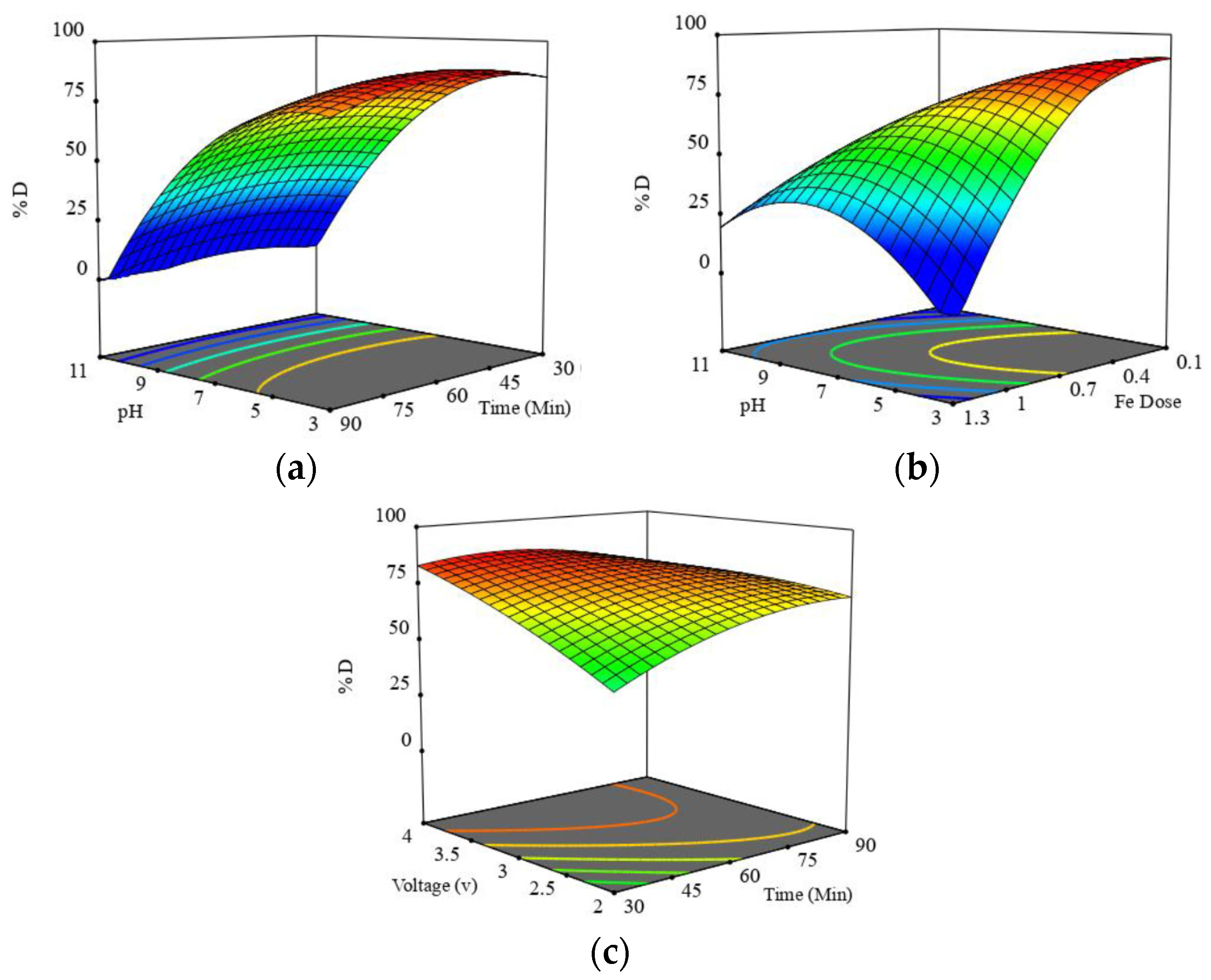
2.2.2. Effect of Operational Parameters
2.2.3. Optimization
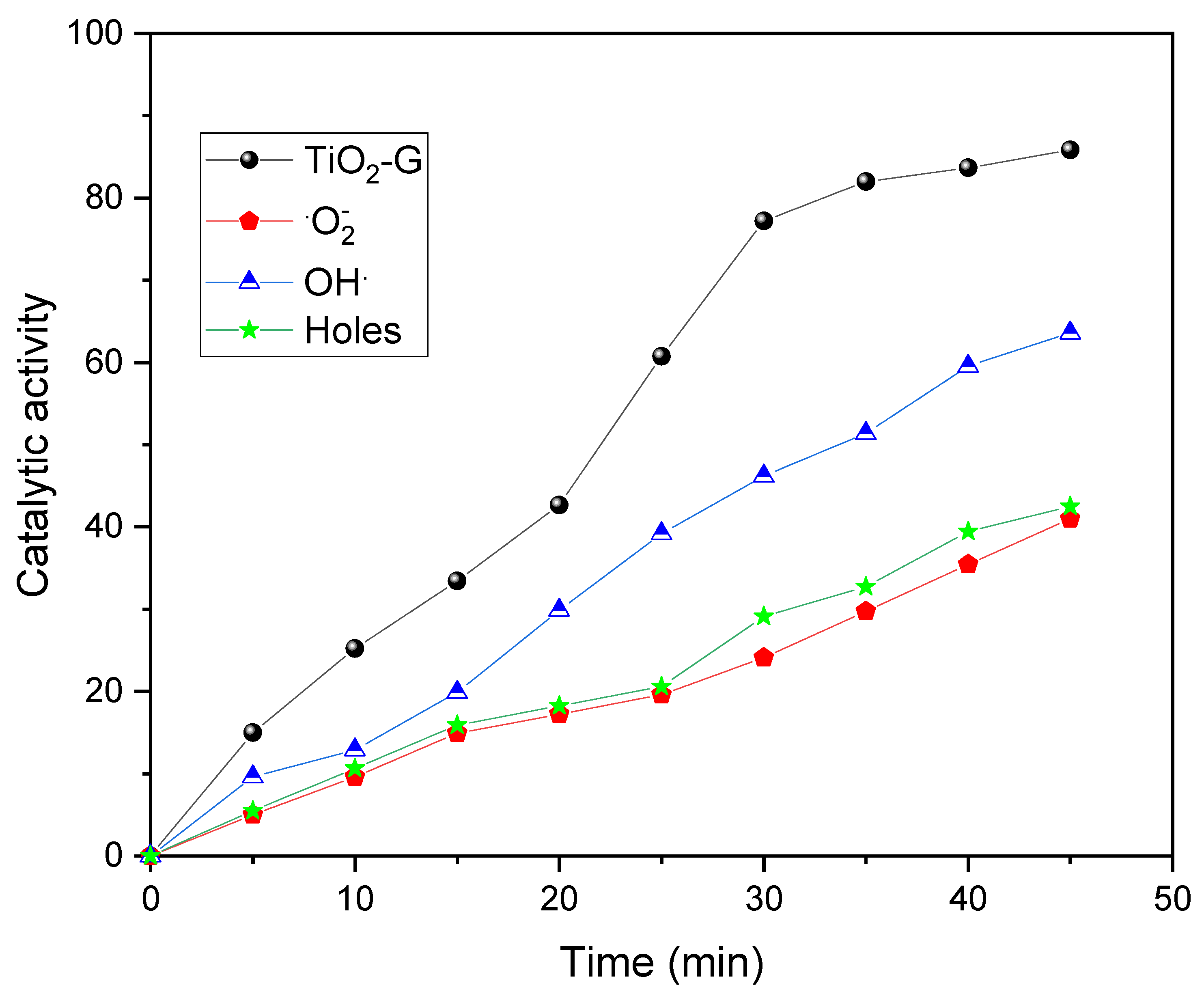
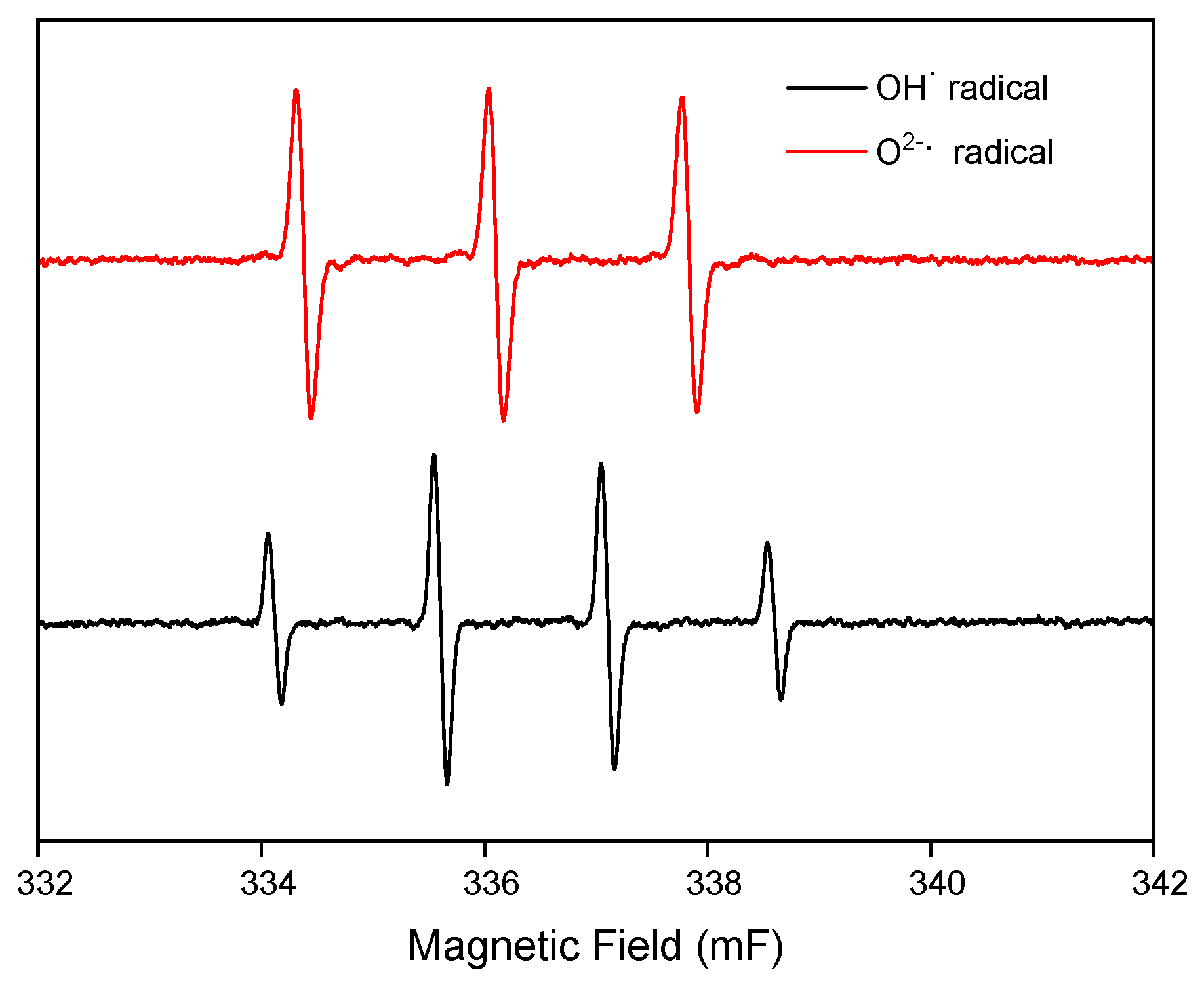
2.3. Degradation Mechanism
2.4. Removal of Rare Earth Metals and Arsenic from Simulated Wastewater
2.5. Degradation of Municipal Wastewater
2.6. Electrode Durability and Reusability
3. Experimental
3.1. Chemicals and Materials
3.2. Electrode Fabrication (TiO2-G Film)
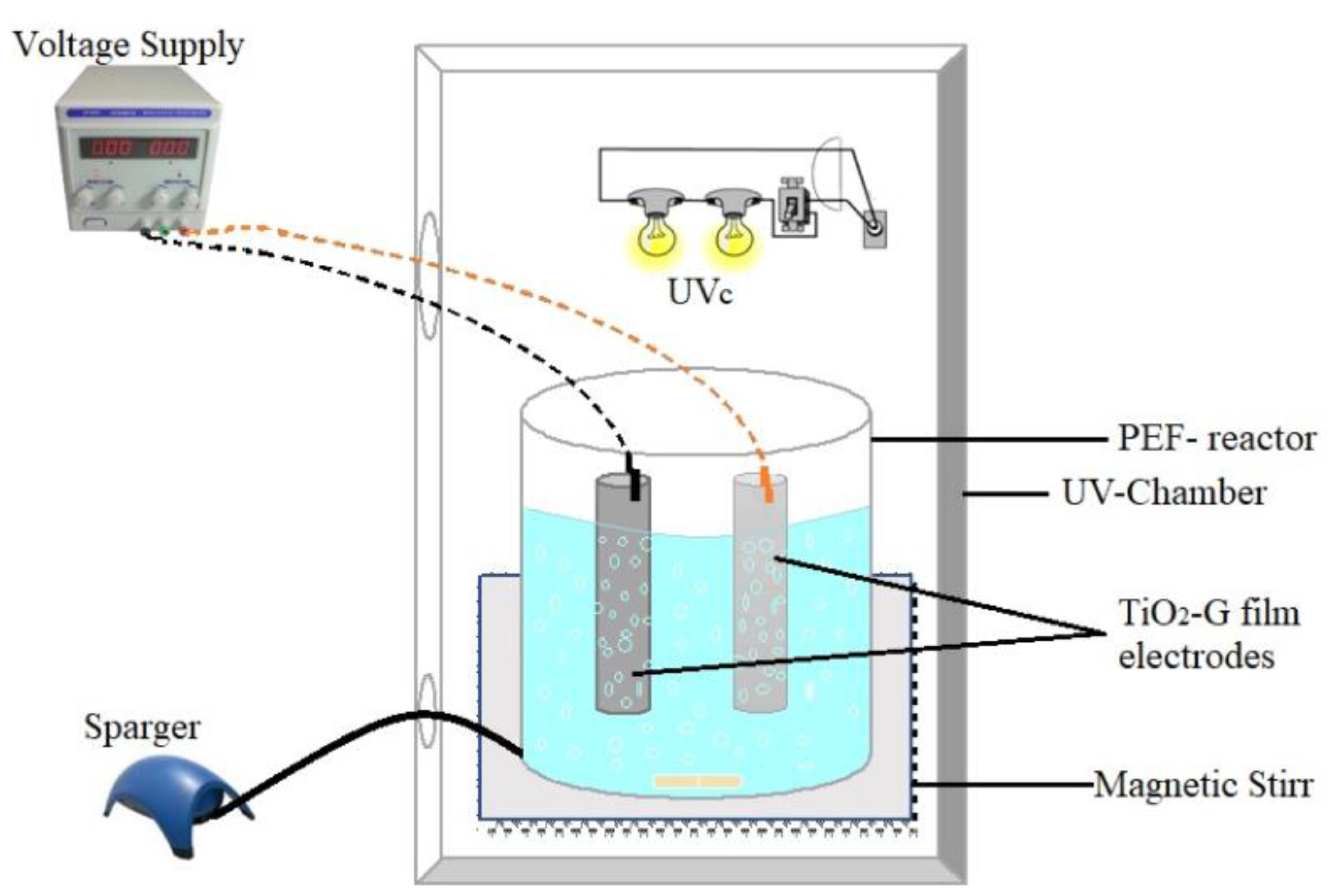
3.3. Design of Experiments, Experimental Setup and Procedure
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Meric, I.; Han, M.Y.; Young, A.F.; Ozyilmaz, B.; Kim, P.; Shepard, K.L. Current saturation in zero-bandgap, top-gated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Rana, F. Electron-hole generation and recombination rates for Coulomb scattering in graphene. Phys. Rev. B 2007, 76, 155431. [Google Scholar] [CrossRef]
- Saba, N.; Alothman, O.Y.; Almutairi, Z.; Jawaid, M.; Asad, M. Introduction of graphene-based nanotechnologies. In Graphene-Based Nanotechnologies Energy Environ; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–21. [Google Scholar]
- Samarathunga, D.N.; Gunathilake, C.A. Synthesis of Graphene Oxide from Graphite for Water Treatment. 2019. Available online: http://erepo.lib.uwu.ac.lk/bitstream/handle/123456789/239/202.pdf?sequence=1&isAllowed=y (accessed on 7 September 2020).
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Yi, X.; Yu, H. Edge-selectively amidated graphene for boosting H2-evolution activity of TiO2 photocatalyst. Appl. Catal. B Environ. 2020, 264, 118504. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhou, J.; Gao, N. Photocatalytic decomposition of bromate ion by the UV/P25-Graphene processes. Water Res. 2014, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew BY, Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, H.; Wang, L. Effect of morphology and defect density on electron transfer of electrochemically reduced graphene oxide. Appl. Surf. Sci. 2016, 390, 385–392. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, B.; Li, D.; Forsyth, M.; MacFarlane, D.R. Direct electro-deposition of graphene from aqueous suspensions. Phys. Chem. Chem. Phys. 2011, 13, 9187–9193. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Yan, C. Reduced graphene oxide with tunable C/O ratio and its activity towards vanadium redox pairs for an all vanadium redox flow battery. Carbon 2013, 55, 313–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Tseng, I.H.; Liu, Z.C.; Chang, P.Y. Bio-friendly titania-grafted chitosan film with biomimetic surface structure for photocatalytic application. Carbohydr. Polym. 2020, 230, 115584. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Liba, B.D.; Wang, Y.; Cheng, Y.; Rubloff, G.W.; Bentley, W.E.; David, L.; Montembault, A.; Royaud, I.; Payne, G.F. Electrodeposition of a biopolymeric hydrogel: Potential for one-step protein electroaddressing. Biomacromolecules 2012, 13, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, X.; Betz, J.; Buckhout-White, S.; Bekdash, O.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter 2010, 6, 3177–3183. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemical deposition to construct a nature inspired multilayer chitosan/layered double hydroxides hybrid gel for stimuli responsive release of protein. J. Mater. Chem. B 2015, 3, 7577–7584. [Google Scholar] [CrossRef]
- Ghach, W.; Etienne, M.; Billard, P.; Jorand, F.P.; Walcarius, A. Electrochemically assisted bacteria encapsulation in thin hybrid sol–gel films. J. Mater. Chem. B 2013, 1, 1052–1059. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Wan, L.; Xie, Z.; Easton, C.D.; Bourgeois, L.; Wang, Z.; Bao, Q.; Zhu, Y.; Tao, S.; et al. Construction of porous N-doped graphene layer for efficient oxygen reduction reaction. Chem. Eng. Sci. 2019, 194, 36–44. [Google Scholar] [CrossRef]
- Primo, A.; Forneli, A.; Corma, A.; García, H. From biomass wastes to highly efficient CO2 adsorbents: Graphitisation of chitosan and alginate biopolymers. ChemSusChem 2012, 5, 2207–2214. [Google Scholar] [CrossRef]
- Wategaonkar, S.B.; Pawar, R.P.; Parale, V.G.; Nade, D.P.; Sargar, B.M.; Mane, R.K. Synthesis of rutile TiO2 nanostructures by single step hydrothermal route and its characterization. Mater. Today Proc. 2020, 23, 444–451. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Bouhfid, R.; Lahcini, M.; Garcia, H.; Bousmina, M.; El Kadib, A. Insightful understanding of the role of clay topology on the stability of biomimetic hybrid chitosan-clay thin films and CO2-dried porous aerogel microspheres. Carbohydr. Polym. 2016, 146, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Huston, L.Q.; Tang, C.; Wei, L.L.; Sheppard, L.R.; Chen, H.; Frankcombe, T.J.; Bradby, J.E.; Liu, Y. Chemical Synthesis and High Pressure Reaction of Nb5+ Mono-Doped Rutile TiO2 Nanocrystals. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Kaur, P.; Kushwaha, J.P.; Sangal, V.K. Electrocatalytic oxidative treatment of real textile wastewater in continuous reactor: Degradation pathway and disposability study. J. Hazard. Mater. 2018, 346, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon: Optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015, 5, 18438–18450. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Z.; Zhang, Y.; Wang, J.; Shi, H.; Shen, Q.; Wei, G.; Zhao, G. Simultaneous photoelectrocatalytic aromatic organic pollutants oxidation for hydrogen production promotion with a self-biasing photoelectrochemical cell. Electrochim. Acta 2017, 254, 140–147. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Behfar, R.; Davarnejad, R. Pharmaceutical wastewater treatment using UV-enhanced electro-Fenton process: Comparative study. Water Environ. Res. 2019, 91, 1526–1536. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Cardoso, J.C.; Da Silva, B.F.; Zanoni MV, B. Combination of photoelectrocatalysis and ozonation: A novel and powerful approach applied in Acid Yellow 1 mineralization. Appl. Catal. B Environ. 2016, 180, 161–168. [Google Scholar] [CrossRef]
- Berger, T.; Lana-Villarreal, T.; Monllor-Satoca, D.; Gómez, R. Charge transfer reductive doping of nanostructured TiO2 thin films as a way to improve their photoelectrocatalytic performance. Electrochem. Commun. 2006, 8, 1713–1718. [Google Scholar] [CrossRef]
- Lin, P.; Shen, J.; Tang, H.; Lin, Z.; Jiang, Y. Enhanced photocatalytic H2 evolution of ultrathin g-C3N4 nanosheets via surface shuttle redox. J. Alloys Compd. 2019, 810, 151918. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Jiang, H.; Jiang, W.; Su, R.; Luo, S.; Luo, Y. Photoelectrocatalytic oxidation of bisphenol A over mesh of TiO2/graphene/Cu2O. Appl. Catal. B Environ. 2016, 183, 75–85. [Google Scholar] [CrossRef]
- Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J. Electro-oxidation of As (III) with dimensionally-stable and conductive-diamond anodes. J. Hazard. Mater. 2012, 203, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.; Kim, G.Y.; Yoon, D.; Lee, S. Uranium recovery via electrochemical deposition with a liquid zinc cathode followed by electrochemical oxidation of rare earth metals. J. Nucl. Mater. 2019, 520, 245–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Schulz, S.; Lindoy, L.F.; Du, H.; Zheng, S.; Wenzel, M.; Weigand, J.J. Separation and recovery of rare earths by in situ selective electrochemical oxidation and extraction from spent fluid catalytic cracking (FCC) catalysts. Hydrometallurgy 2020, 105300. [Google Scholar] [CrossRef]
- Kaur, P.; Kushwaha, J.P.; Sangal, V.K. Transformation products and degradation pathway of textile industry wastewater pollutants in Electro-Fenton process. Chemosphere 2018, 207, 690–698. [Google Scholar] [CrossRef]
- Fouad, D.E.; Zhang, C.; El-Didamony, H.; Yingnan, L.; Mekuria, T.D.; Shah, A.H. Improved size, morphology and crystallinity of hematite (α-Fe2O3) nanoparticles synthesized via the precipitation route using ferric sulfate precursor. Results Phys. 2019, 12, 1253–1261. [Google Scholar] [CrossRef]
- Stanjek, H. XRD peak migration and apparent shift of cell-edge lengths of nano-sized hematite, goethite and lepidocrocite. Clay Miner. 2002, 37, 629–638. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Moaddel, H. Structural modification of chitosan biopolymer as a novel polyelectrolyte membrane for green power generation. Polym. Adv. Technol. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Chang, R.; Ma, S.; Guo, X.; Xu, J.; Zhong, C.; Huang, R.; Ma, J. Hierarchically Assembled Graphene Oxide Composite Membrane with Self-Healing and High-Efficiency Water Purification Performance. ACS Appl. Mater. Interfaces 2019, 11, 46251–46260. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef]
| Factor | Units | Coded Low (−2) | Coded High (+2) |
|---|---|---|---|
| pH | 3.00 | 11.00 | |
| Time | Min | 30.00 | 90.00 |
| Voltage | V | 1.0000 | 5.00 |
| Fe Conc | mM | 0.1000 | 1.30 |
| Standard | Run | pH | Time (min) | Voltage (V) | Fe Dose (mM) | %D | %D chloramphenicol | %D nadolol,218 | %D nadolol, 270 | %D nadolol, 278 |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 1 | 7 | 60 | 5 | 0.7 | 70.14 | 69.12 | 69.99 | 69.98 | 71.47 |
| 17 | 2 | 3 | 60 | 3 | 0.7 | 62.31 | 61.23 | 61.67 | 62.11 | 64.23 |
| 26 | 3 | 7 | 60 | 3 | 0.7 | 70.63 | 69.45 | 70.13 | 69.77 | 73.17 |
| 9 | 4 | 5 | 45 | 2 | 1 | 25.01 | 25.16 | 25.14 | 25.22 | 24.52 |
| 15 | 5 | 5 | 75 | 4 | 1 | 50.66 | 50.12 | 50.22 | 50.34 | 51.96 |
| 11 | 6 | 5 | 75 | 2 | 1 | 44.61 | 43.67 | 44.17 | 44.43 | 46.17 |
| 16 | 7 | 9 | 75 | 4 | 1 | 45.94 | 44.21 | 44.92 | 44.82 | 49.81 |
| 3 | 8 | 5 | 75 | 2 | 0.4 | 65.1 | 64.32 | 64.87 | 64.54 | 66.67 |
| 23 | 9 | 7 | 60 | 3 | 0.1 | 47.76 | 46.63 | 47.66 | 46.89 | 49.86 |
| 20 | 10 | 7 | 90 | 3 | 0.7 | 70.51 | 69.98 | 70.77 | 70.01 | 71.28 |
| 19 | 11 | 7 | 30 | 3 | 0.7 | 50.1 | 50.1 | 50.23 | 49.99 | 50.08 |
| 1 | 12 | 5 | 45 | 2 | 0.4 | 65.32 | 64.87 | 65.81 | 64.09 | 66.51 |
| 10 | 13 | 9 | 45 | 2 | 1 | 30.66 | 29.72 | 31.32 | 30.42 | 31.18 |
| 13 | 14 | 5 | 45 | 4 | 1 | 58.01 | 57.3 | 57.99 | 57.8 | 58.95 |
| 7 | 15 | 5 | 75 | 4 | 0.4 | 86.39 | 86.21 | 85.44 | 86.56 | 87.35 |
| 5 | 16 | 5 | 45 | 4 | 0.4 | 80.8 | 80.9 | 80.65 | 80.78 | 80.87 |
| 8 | 17 | 9 | 75 | 4 | 0.4 | 39.55 | 39.09 | 39.51 | 38.89 | 40.71 |
| 27 | 18 | 7 | 60 | 3 | 0.7 | 69.22 | 69.38 | 68.64 | 69.33 | 69.53 |
| 18 | 19 | 11 | 60 | 3 | 0.7 | 20.33 | 20.65 | 20.19 | 20.11 | 20.37 |
| 4 | 20 | 9 | 75 | 2 | 0.4 | 38.23 | 37.42 | 38.17 | 38.5 | 38.83 |
| 6 | 21 | 9 | 45 | 4 | 0.4 | 47.71 | 47.55 | 47.62 | 47.68 | 47.99 |
| 29 | 22 | 7 | 60 | 3 | 0.7 | 60.28 | 60.81 | 60.22 | 59.56 | 60.53 |
| 14 | 23 | 9 | 45 | 4 | 1 | 50.55 | 50.21 | 50.44 | 51.02 | 50.53 |
| 12 | 24 | 9 | 75 | 2 | 1 | 50.89 | 50.76 | 50.53 | 50.78 | 51.49 |
| 28 | 25 | 7 | 60 | 3 | 0.7 | 70.99 | 70.09 | 71.54 | 70.98 | 71.35 |
| 24 | 26 | 7 | 60 | 3 | 1.3 | 30.19 | 30.33 | 30.21 | 29.49 | 30.73 |
| 25 | 27 | 7 | 60 | 3 | 0.7 | 69.5 | 69.85 | 69.99 | 68.19 | 69.97 |
| 21 | 28 | 7 | 60 | 1 | 0.7 | 40.15 | 40.19 | 40.32 | 39.23 | 40.86 |
| 2 | 29 | 9 | 45 | 2 | 0.4 | 24.8 | 24.9 | 25.25 | 24.41 | 24.64 |
| 30 | 30 | 7 | 60 | 3 | 0.7 | 69.46 | 69.49 | 69.94 | 68.43 | 69.98 |
| %D | |||||
|---|---|---|---|---|---|
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
| Model | 14 | 8481.14 | 605.80 | 27.38 | <0.0001 |
| pH | 1 | 2233.59 | 2233.59 | 100.94 | <0.0001 |
| Time | 1 | 262.22 | 262.22 | 11.85 | 0.0036 |
| Voltage | 1 | 1275.60 | 1275.60 | 57.65 | <0.0001 |
| Fe dose | 1 | 668.98 | 668.98 | 30.23 | <0.0001 |
| pH × Time | 1 | 0.6683 | 0.6683 | 0.0302 | 0.8644 |
| pH × voltage | 1 | 83.95 | 83.95 | 3.79 | 0.0704 |
| pH × Fe dose | 1 | 1351.85 | 1351.85 | 61.09 | <0.0001 |
| Time × Voltage | 1 | 285.36 | 285.36 | 12.90 | 0.0027 |
| Time × Fe dose | 1 | 18.55 | 18.55 | 0.8385 | 0.3743 |
| Voltage × Fe dose | 1 | 3.07 | 3.07 | 0.1388 | 0.7147 |
| pH2 | 1 | 1171.48 | 1171.48 | 52.94 | <0.0001 |
| Time2 | 1 | 87.79 | 87.79 | 3.97 | 0.0649 |
| Voltage2 | 1 | 260.04 | 260.04 | 11.75 | 0.0037 |
| Fe dose2 | 1 | 1391.09 | 1391.09 | 62.87 | <0.0001 |
| Residual | 15 | 331.92 | 22.13 | ||
| Lack of Fit | 10 | 251.31 | 25.13 | 1.56 | 0.3259 |
| Pure Error | 5 | 80.60 | 16.12 | ||
| Cor Total | 29 | 8813.06 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Frindy, S.; Park, Y.; Sillanpää, M.; Imteaz, M.A. Synthesis of Graphene-Based Biopolymer TiO2 Electrodes Using Pyrolytic Direct Deposition Method and its Catalytic Performance. Catalysts 2020, 10, 1050. https://doi.org/10.3390/catal10091050
Kaur P, Frindy S, Park Y, Sillanpää M, Imteaz MA. Synthesis of Graphene-Based Biopolymer TiO2 Electrodes Using Pyrolytic Direct Deposition Method and its Catalytic Performance. Catalysts. 2020; 10(9):1050. https://doi.org/10.3390/catal10091050
Chicago/Turabian StyleKaur, Parminder, Sana Frindy, Yuri Park, Mika Sillanpää, and Monzur A. Imteaz. 2020. "Synthesis of Graphene-Based Biopolymer TiO2 Electrodes Using Pyrolytic Direct Deposition Method and its Catalytic Performance" Catalysts 10, no. 9: 1050. https://doi.org/10.3390/catal10091050
APA StyleKaur, P., Frindy, S., Park, Y., Sillanpää, M., & Imteaz, M. A. (2020). Synthesis of Graphene-Based Biopolymer TiO2 Electrodes Using Pyrolytic Direct Deposition Method and its Catalytic Performance. Catalysts, 10(9), 1050. https://doi.org/10.3390/catal10091050








