Abstract
The photocatalytic reduction of carbon dioxide (CO2) into CO and hydrocarbon fuels has been considered as an ideal green technology for solar-to-chemical energy conversion. The separation/transport of photoinduced charge carriers and adsorption/activation of CO2 molecules play crucial roles in photocatalytic activity. Herein, tetrakis (4-carboxyphenyl) porphyrin (H2TCPP) was incorporated with different metal atoms in the center of a conjugate macrocycle, forming the metalloporphyrins TCPP-M (M = Co, Ni, Cu). The as-obtained metalloporphyrin was loaded as a cocatalyst on commercial titania (P25) to form TCPP-M@P25 (M = Co, Ni, Cu) for enhanced CO2 photoreduction. Among all of the TCPP-M@P25 (M = Co, Ni, Cu), TCPP-Cu@P25 exhibited the highest evolution rates of CO (13.6 μmol⋅g−1⋅h−1) and CH4 (1.0 μmol⋅g−1⋅h−1), which were 35.8 times and 97.0 times those of bare P25, respectively. The enhanced photocatalytic activity could be attributed to the improved photogenerated electron-hole separation efficiency, as well as the increased adsorption/activation sites provided by the metal centers in TCPP-M (M = Co, Ni, Cu). Our study indicates that metalloporphyrin could be used as a high-efficiency cocatalyst to enhance CO2 photoreduction activity.
1. Introduction
In view of global warming and the energy crisis, the photocatalytic reduction of carbon dioxide (CO2) into CO and hydrocarbon fuels, which is known as artificial photosynthesis, has long been considered an ideal green technology for solar-to-chemical energy conversion [1,2,3]. Owing to its environmentally friendliness and sustainability, photocatalytic CO2 reduction has attracted tremendous attention in recent years [4,5,6]. So far, however, the efficiencies of the currently reported photocatalysis systems are still very low [7,8,9,10], which can mainly be attributed to inefficient photogenerated charge-carrier separation and migration, and the poor adsorption and activation of CO2 molecules [11,12,13]. Introducing cocatalysts provides a new perspective to overcome the weaknesses mentioned above [14,15]. Most of the efficient cocatalysts developed up to date consisted of noble metals, such as Au, Ag, Pt and their alloys, or bimetallic core-shell structures [16,17], which have large work functions that favor the separation of photogenerated electrons and holes [18]. However, their high cost and rarity restrict their further development and extensive application. In addition, such noble metals usually fail to exhibit an outstanding capacity for capturing and activating CO2 molecules [19]. In this context, exploring efficient noble-metal-free cocatalysts has aroused great interest [20,21,22].
In the natural photosynthesis process, chlorophyll serves as the photosynthetic center in green plants for fixing carbon dioxide in the atmosphere [23,24]. Among all the components of chlorophyll, porphyrin is believed to be the most effective one in harvesting sunlight, inspiring scientists to apply porphyrins and their derivatives to artificial photosynthesis [25]. It has been discovered that the electron fluidity within the conjugate macrocycle in porphyrin molecules renders them a class of excellent exciton transfer intermediate [26,27], and porphyrin rings are highly qualified for capturing CO2 molecules and consequently benefit the conversion of carbon dioxide [28,29]. In terms of fabrication, porphyrins are often designed to assemble metal-organic framework materials, or to coordinate with metals to form metalloporphyrins [30,31,32]. In fact, several previous researchers have demonstrated that earth-abundant-metal atoms (e.g., Co, Cu, Zn etc.) could be incorporated into porphyrin-based materials to serve as not only the catalytic sites but also as the adsorption center of CO2 molecules, thereby enhancing photocatalytic activity [33,34]. Thus, the above-mentioned facts suggest that metalloporphyrin can behave as a highly promising noble-metal-free cocatalyst to facilitate the photoinduced electron transfer process and to fix the CO2 molecules in photocatalytic CO2 reduction under sunlight irradiation.
In this work, we used 4,4′,4″,4‴-(porphyrin-5,10,15,20-tetrayl) tetra-benzoate (H2-TCPP) as the porphyrin matrix to incorporate various metal atoms M (M = Co, Ni, Cu), and the as-formed metalloporphyrin TCPP-M was loaded as a new noble-metal-free cocatalyst onto commercial titanium dioxide (P25) through a straightforward synthetic route. Owing to the remarkable photoconductivity of metalloporphyrin [35,36], the photocatalytic CO2 reduction activity of the assembled TCPP-M@TiO2 M (M = Co, Ni, Cu) nanocomposite was significantly improved. In the TCPP-Cu@TiO2, the CO and CH4 evolution rates were 13.6 μmol⋅g−1⋅h−1 and 1.0 μmol⋅g−1⋅h−1, which were 35.8-fold and 97.0-fold those of bare P25, respectively. The dramatically enhanced photocatalytic activity could be ascribed to the increased electron transfer from photoexcited TiO2 to the metal center of the porphyrin ring. In addition, the incorporated metal atoms also play an important role in the adsorption/activation of CO2 molecules, as evidenced by both theoretical calculation and experimental measurement. Our work has provided a new strategy for the enhancement of photocatalytic CO2 reduction by employing metalloporphyrin as an efficient noble-metal-free cocatalyst.
2. Results and Discussion
From the FT–IR spectra in Figure 1a, the successful synthesis of TCPP@P25 and TCPP-M (M = Co, Ni, Cu) @P25 was confirmed by the characteristic bands of porphyrins from 1600 to 1400 cm−1, in which the bands at 1652, 1605 and 1556 cm−1 could be assigned to carboxylates (–COOM) [37]. Besides this, the broad and small bands of H2-TCPP ligand at 2700–2350 cm−1 are generally considered to be caused by the frequency multiplication of the O–H bond deformation vibration in the carboxylic group, which cannot be observed after bonding with P25 [38]. Compared to H2-TCPP, the hydroxy bands in TCPP@P25 and TCPP-M (M = Co, Ni, Cu) @P25 disappeared, indicating that the porphyrin molecules were jointed to the surface of the titanium dioxide by coordinating the carboxylic end group with Ti atoms.
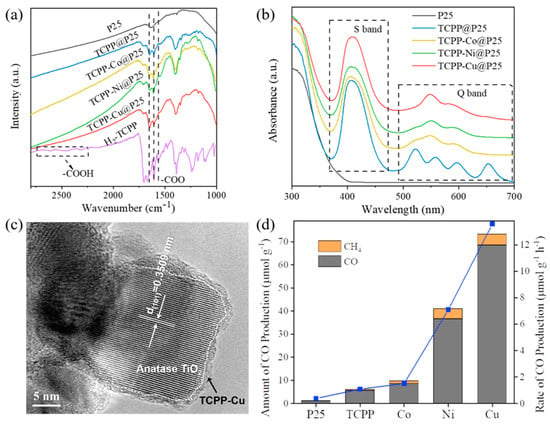
Figure 1.
(a) FT–IR spectra of P25, H2TCPP, TCPP@P25 and TCPP-M@P25 (M = Co, Ni, Cu); (b) UV–Vis spectra of P25, TCPP@P25 and TCPP-M@P25 (M = Co, Ni, Cu); (c) HRTEM image of TCPP-Cu@P25; (d) evolution rates of CO and CH4 over P25, TCPP@P25 and TCPP-M@P25 (M = Co, Ni, Cu) under Xe lamp irradiation.
In the UV–Vis diffuse reflectance spectra, the TCPP@P25 showed four weak Q absorption bands at 522, 558, 595 and 653 nm (see the blue curve in Figure 1b), respectively, which were characteristic for the porphyrin family. After metalation of the porphyrin ring with Co, Ni or Cu, only two Q bands were observed because of the higher symmetry of the porphyrin unit in TCPP-M@P25 (M = Co, Ni, Cu) [38]. The XPS results (Figure S1) further confirmed the existence of the central metal coordination. The original porphyrin ring in TCPP@P25 contains two chemically different types of nitrogen atoms, –NH (green peak in Figure S1a) and = N–(red peak in Figure S1a). In the XPS spectra of TCPP-Cu@P25 (Figure S1b), the peak for = N– shifted to a high value at 398.56 eV, which corresponded to the binding energy between copper and porphyrin [33], confirming the electron transfer from the porphyrin rings to coordinated metal atoms. In contrast, the green peak remained at ~ 400.10 eV, but the intensity decreased significantly, probably because a small percentage of N failed to coordinate with copper [32]. The above-mentioned FT–IR, UV–Vis and XPS results confirmed that the porphyrin coordinated with metal atoms in the center was indeed loaded onto P25 via carboxylic bonds.
From the XRD patterns (Figure S2), all the diffraction peaks of the as-prepared samples could be indexed as anatase (PDF#21-1272) and rutile titanium dioxide (PDF#21-1276), being consistent with the intrinsic two-phase P25. However, the TCPP in TCPP@P25 and TCPP-M (M = Co, Ni, Cu) @P25 hybrids could not be detected because of its small amount (<5 wt%). Almost no change in the morphology and particle size of P25 was observed from the SEM (Figure S3) and TEM (Figure S4) images after incorporating with TCPP-Cu. The lattice fringes in the TEM image corresponded to the (101) planar space of anatase TiO2 (Figure 1c).
Photocatalytic reactions over P25 with or without TCPP-M (M = Co, Ni, Cu) as cocatalyst were carried out using water as the electron donor. From Figure 1d and Figure S5, we can see that the pure P25 showed rather low activity (0.39 μmol⋅g−1⋅h−1 for CO; 0.01 μmol⋅g−1⋅h−1 for CH4). Loading TCPP with TiO2 increased the yields of both CO (1.08 μmol⋅g−1⋅h−1) and CH4 (0.06 μmol⋅g−1⋅h−1). More interestingly, when TCPP was incorporated with different metal atoms, the photoreduction activity of TCPP-M@P25 (M = Co, Ni, Cu) was further improved significantly. The dependence of photocatalytic performances on the loading amount of TCPP-Cu (Figure S6) showed that, when loading 1.0 wt% TCPP-Cu onto P25, the resultant sample exhibited a significant increase in photocatalytic activity for CO evolution (6.39 μmol⋅g−1⋅h−1), confirming the validity of the cocatalyst. With an optimal loading amount of TCPP-Cu (5 wt%), the TCPP-M@P25 showed the highest efficiency of CO2 reduction, with a CO evolution rate of 13.6 μmol⋅g−1⋅h−1, which was 35.8 times and 12.4 times those of bare P25 and TCPP@P25, respectively. The evolution rate of CH4 (1.0 μmol⋅g−1⋅h−1) was 97.0-fold and 16.5-fold that of bare P25 and TCPP@P25, respectively. As for TCPP-Co@P25, the optimal CO and CH4 generation rates were 1.51 μmol⋅g−1⋅h−1 and 0.27 μmol⋅g−1⋅h−1, respectively. For TCPP-Ni@P25, the optimal activities were 7.08 μmol⋅g−1⋅h−1 (CO) and 0.89 μmol⋅g−1⋅h−1 (CH4). The reason for the activities of TCPP-Co@P25 and TCPP-Ni@P25 being lower than TCPP-Cu@P25 will be discussed below, along with the adsorption results. We need to mention that further increasing the loading amount of TCPP-Cu decreased the activity, although TCPP itself could absorb a wide wavelength range of light [35]. However, no CO or CH4 could be detected when using an L42 filter to cut off the light with wavelengths shorter than 420 nm (Figure S7a). A photoluminescence (PL) investigation showed that no fluorescence peak of porphyrin (630–850nm) was observed in TCPP-Cu@P25 (see Figure S8), indicating that the porphyrin itself might not be excited upon light irradiation. Moreover, neither pure H2-TCPP ligand nor TCPP-Cu complex showed activity for CO2 photoreduction (Figure S7b). Thus, we could reasonably judge that loading excess TCPP would probably shield P25 from incident light, leading to reduced activity.
To exclude the possibility that the CO and CH4 were from the carbon contained in TCPP, TCPP-M or the adsorbed impurities on the TiO2, we performed a reaction by replacing the CO2 gas with Ar gas whilst keeping other conditions identical. Neither CO nor CH4 were detected (Figure S9), indicating that the obtained CO and CH4 originated from the photoreduction of CO2. Besides this, removing H2O from the reaction system also yielded neither CO nor CH4, verifying the role of H2O as the electron donor in the photoreduction of CO2. Although we did not detect O2 gas, the XPS result (see Table S1) indicated that the samples probably produced other forms of oxidized products during the process of the reaction, which is a common phenomenon in the photocatalytic reduction of CO2 over TiO2 [22,39]. The above results demonstrate clearly the significant role of TCPP-M as an effective cocatalyst in boosting the photocatalytic activity of TCPP-Cu@P25 for CO2 reduction.
It is well known that the charge carrier separation plays an important role in the photocatalytic reaction [18]. As shown in Figure 2a, the PL intensity decreased after loading with TCPP or TCPP-M, which could be attributed to improved charge separation efficiency [22]. Moreover, transient photocurrent (TPC) response and electrochemical impedance spectroscopy (EIS) were also studied. Figure 2b shows that TCPP-Cu@P25 exhibited the highest photocurrent density, followed by TCPP-Ni@P25, both of which were much higher than TCPP@P25 or pure P25. However, the photocurrent response of TCPP-Co@P25 was very close to that of TCPP@P25. The EIS graph (Figure 2c) shows the smaller semicircle diameters of TCPP-M@P25 (M = Co, Ni, Cu) in comparison with TCPP@P25 and pure P25, indicating enhanced charge transfer efficiency after the conjugation of metalloporphyrins [36]. The charge transfer process was also studied by Mott–Schottky (M–S) curves analysis (Figure 2d). All the samples showed a positive slope in the M–S plots, as expected for an n-type semiconductor. The TCPP-Cu@P25 showed the smallest slopes on the M–S plot, compared to TCPP-Ni@P25, TCPP-Co@P25 and TCPP@P25, confirming that the introduction of metalloporphyrin led to faster charge transfer, especially for the TCPP-Cu@P25. The above results verify that the metalloporphyrin-based cocatalyst greatly promoted the separation of photogenerated electron-hole pairs, thereby resulting in the dramatically enhanced activity of TCPP-Cu@P25.
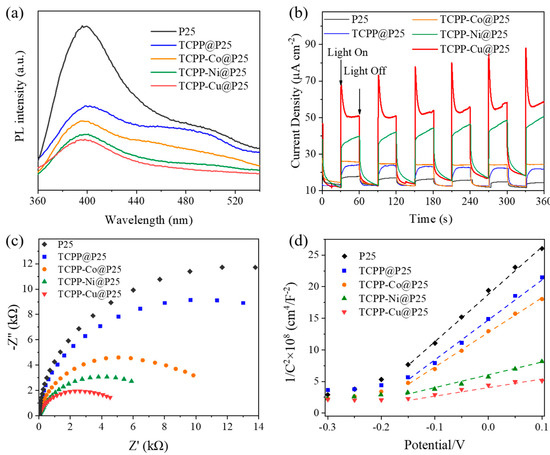
Figure 2.
(a) PL spectra, (b) transient photocurrent responses, (c) EIS Nyquist plots, and (d) Mott–Schottky curves of P25, TCPP@P25 and TCPP-M@P25 (M = Co, Ni, Cu).
Owing to the large dissociation energy (ca. 750 kJ mol−1) of the C=O bond, CO2 is an extremely stable molecule in terms of thermodynamics [40]. Therefore, the adsorption/activation of CO2 molecules into the reactive intermediates (e.g., HCO3−, HCO2− or CO32−) is of vital importance to the subsequent photocatalytic reduction process. While loading metalloporphyrins on P25 decreased the specific surface area (Figure S10), the incorporated metal atoms could provide active sites for increasing the adsorption of CO2 molecules (see Figure 3a) [32]. The first principle calculation also proved that the metal centers could bring down the adsorption energy from −0.02 eV (for TCPP@P25) to −0.11 eV (for TCPP-Cu@P25). To clarify whether the CO2 molecules captured by the porphyrin rings were activated or not, a CO2-TPD (Temperature Programmed Desorption) measurement was conducted. As shown in Figure 3b, the small peaks which appeared below 180 °C could be ascribed to the physically adsorbed CO2 molecules, which could not be activated for subsequent photocatalytic reduction reactions. The two peaks at 380–550 °C and 550–600 °C correspond to the desorption of bidentate carbonates (b-CO32−) and monodentate carbonates (m-CO32−), respectively [41]. The b-CO32− and m-CO32− species were generated from CO2 molecules combined with oxygen atoms, or both the oxygen and metal atoms of the cocatalysts. While the surface area of bare P25 was as high as 84.69 m2 g−1, only a small amount of CO2 was adsorbed, indicating a weak interaction between CO2 and TiO2. As for TCPP@P25, the appearance of the peaks at higher temperatures indicated that the introduction of porphyrin strengthened the interaction between CO2 and TiO2. Further coordination of metal atoms remarkably enhanced the intensity of the desorption peaks at 380–550 °C and 550–600 °C, suggesting that the anchored metal atoms greatly promoted the chemisorption of CO2. We noticed that the b-CO32− peak for TCPP-Cu@P25 was higher than that for either TCPP-Co@P25 or TCPP-Ni@P25. In addition, the peak for TCPP-Cu@P25 shifted to a higher temperature, suggesting that the binding strength between Cu sites and the adsorbed CO2 molecules was higher than in the cases of TCPP-Co@P25 and TCPP-Ni@P25. As a result, TCPP-Cu@P25 exhibited the highest photocatalytic activity for CO2 reduction. As mentioned above, the incorporation of a metal center in the porphyrin ring plays a crucial role in the adsorption and activation of CO2 molecules for significantly enhanced photocatalytic CO2 reduction over TCPP-M@P25 (M = Co, Ni, Cu). In particular, the strongest bond between Cu-porphyrin and CO32− endowed the highest activity of TCPP-Cu@P25.
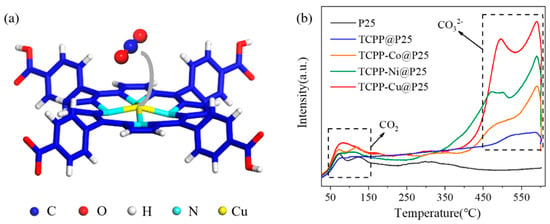
Figure 3.
(a) The optimized structure for CO2 adsorption on a TCPP-Cu unit; (b) CO2-TPD spectra of P25, TCPP@P25, TCPP-Co@P25, TCPP-Ni@P25 and TCPP-Cu@P25.
To unravel the electron transfer process in TCPP-Cu@P25, some blank experiments were performed over Cu@P25 and Cu@TCPP/P25 under conditions identical to TCPP-Cu@P25, as described in Figure 1d. The sample denoted as Cu@P25 was obtained by the photodeposition of Cu onto P25 (Figure S11). Low photoactivity was observed on Cu@P25, primarily due to the inefficient separation/migration of photoexcited electron-hole pairs. For Cu@TCPP/P25, Cu was photodeposited onto the surface of TCPP/P25, rather than coordinating in the center of porphyrin ring. The sample Cu@TCPP/P25 showed a much lower photoactivity than TCPP-Cu@P25 (Figure S11). Thus, we could infer that only when a Cu atom was coordinated in the center of porphyrin ring could it serve as the destination of the electron transfer process. In fact, the ESR measurement demonstrated that Cu2+ was partially reduced to Cu+ after light irradiation, which was evidenced by a decline in the Cu2+ peak at g = 2.06, as shown in Figure 4a [42,43]. Furthermore, the long-term course of CO2 reduction over TCPP-Cu@P25 running for three cycles showed stable CO evolution (see Figure 4b), suggesting that the conversion from Cu2+ to Cu+ was invertible. Based on the above-mentioned results and discussion, we propose in Figure 5 the process of photocatalytic CO2 reduction over TCPP-Cu@P25. Upon light irradiation, photoelectrons generated in P25 quickly transfer to Cu2+ in the center of porphyrin ring, where the CO2 molecules are preferentially adsorbed and activated, and finally reduced into CO and CH4 photocatalytically.
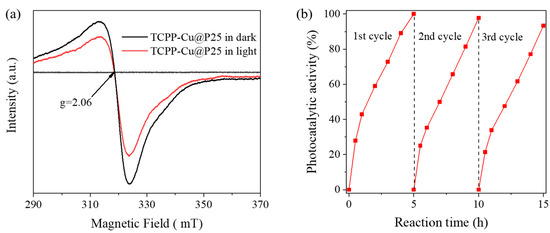
Figure 4.
(a) ESR spectra of TCPP-Cu@P25 with (red curve) and without (black curve) light irradiation; (b) long-term course of CO evolution from CO2 reduction over TCPP-Cu@P25 (50 mg) under Xe lamp irradiation.
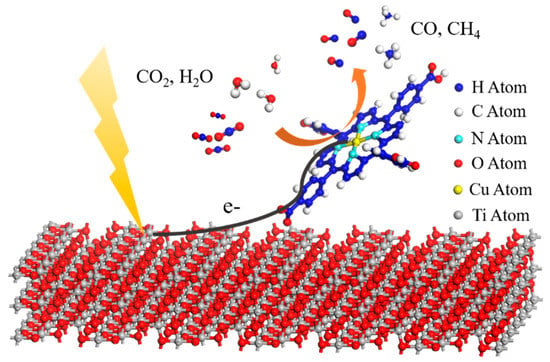
Figure 5.
Schematic illustration for photocatalytic CO2 reduction over TCPP-Cu@P25. An anatase TiO2 structure is used to represent P25.
3. Materials and Methods
3.1. Materials and Synthesis
Titanium (IV) dioxide (TiO2, P25 99%) was purchased from Sinopharm Chemical Reagent Co., Ltd. Tetrakis (4-carboxyphenyl) porphyrin (H2TCPP, 97%) and N,N-dimethylformamide (DMF, 97%) were purchased from TCI (Tokyo Chemical Industry). Other reagents of analytical grade were used without further purification.
TCPP@P25 was synthesized via a ‘one-pot’ solvothermal method. Titanium dioxide (P25, 500 mg) was first added to N,N-dimethylformamide (DMF, 30 mL) and sonicated for 30 min, then tetrakis (4-carboxyphenyl) porphyrin (25 mg) was added to the solution. The mixture was transferred into a Teflon-lined autoclave and heated at 150 °C for 12 h. The as-obtained product was washed and centrifugated three times with DMF and six times with ethanol. Finally, the TCPP@P25 was obtained after drying in a vacuum oven at 60 °C for 48 h. TCPP-M (M = Co, Ni, Cu) @P25 was sythesized following the same procedures as those of TCPP@P25. The raw materials were titanium dioxide (P25, 500 mg), tetrakis (4-carboxyphenyl) porphyrin (25 mg) and cobalt acetate tetrahydrate (C4H6CoO4 4H2O, 7.9 mg, for TCPP-Co@P25), nickel acetate tetrahydrate (C4H6NiO4 4H2O, 7.9 mg, for TCPP-Ni@P25), copper acetate hydrate (C4H6CuO4 H2O, 6.3 mg, for TCPP-Cu@P25) and DMF (30 mL) as the solvent for each sample.
The photodeposition of Cu nanoparticles onto P25 was conducted by dispersing P25 (100 mg) and CuSO4 (0.8 mg) with a magnetic stirrer in an aqueous methanol solution (220 mL of H2O and 70 mL of methanol) under 300 W Xe lamp irradiation for 1 h. After washing, centrifugation and desiccation, Cu@P25 was obtained. The synthesis procedure of Cu@TCPP/P25 was same as that of Cu@P25, replacing P25 (100 mg) with the pre-synthesized TCPP@P25 (100 mg).
3.2. Characterization
The Fourier transform infrared (FT–IR) spectrum was measured on a spectrometer (Nicolet-6700, Thermo Scientific) in the range of 3000–400 cm−1. The UV–Vis diffuse reflectance spectrum was recorded on a spectrophotometer (UV-2700, Shimadzu) using BaSO4 as the standard reference, and then converted into absorption spectra via the Kubelka–Munk function. Morphology and microstructure were observed on a field emission scanning electron microscope (FESEM; S4800, Hitachi, Japan) and a transmission electron microscope (TEM, FEI Tecnai-G2-F20). X-ray diffraction (XRD) patterns were acquired on a diffractometer (Rigaku D/MAX-2500) equipped with Cu-Kα radiation (λ = 0.1538 nm). X-ray photoelectron spectra (XPS) were detected on a Thermo Scientific Escalab 250Xi, and all binding energies were calibrated by the C 1s peaks (284.6 eV) of the surface adventitious carbon. The photoluminescence (PL) spectrum was measured on a spectrofluorometer (Fluorolog-2, Horiba Jobin Yvon); the excitation wavelength was 335 nm.
Electron spin resonance (ESR) was conducted on an ESR spectrometer (JES-FA 200, JEOL) under the following conditions: central field, 330 mT; scanning frequency, 9.45 GHz; scanning width, 20 mT; scanning power, 0.998 mW; scanning temperature, 25 °C. The Brunauer–Emmett–Teller (BET) surface area was analyzed on a Quantachrome Autosorb iQ2 using N2 adsorption isotherms at 77 K. CO2 adsorption isotherm was obtained on a BEL SORP-mini II. CO2-TPD (Temperature Programmed Desorption) was measured on a multifunction chemisorption analyzer (Pengxiang Technology, PX 200A) with a quartz U-tube reactor, and was monitored by a thermal conductivity detector. The sample (100 mg) was heated to 250 °C at a rate of 10 °C/min and then cooled to room temperature. After the pretreatment, CO2 flow (20 mL/min) passed through the catalyst bed for 30 min, then the TPD analysis was performed by heating the sample to 620 °C at a rate of 10 °C/min. The whole process of TPD measurement was performed under Ar gas flow (28 mL/min).
3.3. Photocatalytic Performance Evaluation and Photoelectrochemical Measurement
The photocatalytic experiment was carried out in a vacuum reaction system with a total volume of about 330 mL. In the center of the reaction cell, a 50 mg sample was uniformly dispersed on a porous quartzose slice fixed on the stage of the reactor. In total, 3 mL of deionized water was injected into the cell as sacrificial agent. After evacuating the system, 70 kPa CO2 was introduced and a 300 W Xe lamp was employed as the light source. with a water filter to cut off infrared light. The incident light intensity in the reaction was measured to be 83.5 mW cm−2.
Photoelectrochemical performance was tested on an electrochemical workstation (CHI 660D, Chenhua, Shanghai) connected to a three-electrode quartz glass cell, using a piece of Pt plate as the counter electrode and an Ag/AgCl electrode as the reference electrode. In total, 2 mg of the sample was spin-coated on an area of ~ 1 cm2 of thin FTO film, forming the photoelectrode. Photoresponse was then measured in 0.1 M K2SO4 aqueous solution under 300 W Xe lamp irradiation.
3.4. Computational Details
The theoretical calculations were carried out with the Vienna Ab initio Simulation Package (VASP), based on density functional theory (DFT) [44]. The projector augmented wave (PAW) pseudopotentials and the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional were adopted in the calculations [45,46]. The Grimme method (DFT-D2) was used for the van der Waals (VdW) interaction corrections [47]. The wave-functions were expanded by plane-wave with a cutoff energy of 450 eV, and the energy and force convergence threshold were set to 10−5 eV and 10−2 eV Å−1, respectively. The 1 × 1 × 1 Monkhorst–Pack k-points mesh was employed for the molecular model simulation, with a vacuum layer of 16 Å in three directions.
4. Conclusions
In summary, porphyrin-based noble-metal-free cocatalysts were developed via a facile solvothermal synthesis route. With metal atoms anchored in the center of porphyrin rings, the metalloporphyrin TCPP-M (M = Co, Ni, Cu) was loaded onto TiO2 through bonding between the end group carboxyl and Ti atoms, forming TCPP-M@TiO2 (M = Co, Ni, Cu) nanohybrids. TCPP-Cu@TiO2 was found to show the best activity for photocatalytic CO2 reduction into CO (13.6 μmol⋅g−1⋅h−1) and CH4 (1.0 μmol⋅g−1⋅h−1), which were 35.8 times and 97.0 times those of bare P25, respectively. The remarkably enhanced activity of TCPP-Cu@TiO2 could be attributed to the excellent photogenerated charge carrier separation/transport and the CO2 adsorption/activation ability endowed by the Cu atoms coordinated in the center of porphyrin rings in TCPP-Cu. The present study suggests that metalloporphyrin could be employed as an efficient noble-metal-free cocatalyst for photocatalytic CO2 reduction.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/6/654/s1.
Figure S1: XPS N 1s spectra (a) before the implantation of Cu (TCPP@P25); (b) after the implantation of Cu (TCPP-Cu@P25); Figure S2: XRD pattern for P25 and prepared samples: TCPP@P25 and TCPP-M (M = Co, Ni, Cu) @P25; Figure S3: SEM images of (a) P25 and (b) TCPP-Cu@P25; Figure S4: TEM images of (a) P25 and (b) TCPP-Cu@P25 at low magnification; Figure S5: Time dependent of CO and CH4 evolution over P25, TCPP@P25, TCPP-Co@P25, TCPP-Ni@P25 and TCPP-Cu@P25; Figure S6: Photocatalytic CO production performance over TCPP-Cu@P25 with different cocatalyst mass fraction; Figure S7: Time course of CO evolution (a) with (black line) and without (red line) L42 filter; (b) over TCPP-Cu@P25, H2-TCPP ligand and TCPP-Cu complex (the other experimental conditions were identical to those in Figure 1d); Figure S8: PL spectra over TCPP-Cu@P25 and H2TCPP ligand in the range of porphyrin fluorescence peak. After coupling with P25, the porphyrin fluorescence peak could not be observed; Figure S9. Time dependent of CO yield TCPP-Cu@P25 under different conditions: with CO2 gas and deionized water (black); with Ar gas and deionized water (red); with CO2 gas but without water (blue), other conditions remain the same with Figure 1d; Table S1: Relative quantitative analysis of O 1s in the XPS spectra of TCPP@P25 and TCPP-Cu@P25 before (B.R.) and after (A.R.) the photoreduction reaction; Figure S10: N2 adsorption and desorption curves and BET surface areas of P25, TCPP@P25 and TCPP-M (M = Co, Ni, Cu) @P25; Figure S11: Time course of CO yield over TCPP-Cu@P25, Cu@P25 (photodeposited Cu on P25) and Cu@TCPP/P25 (photodeposited Cu on TCPP@P25).
Author Contributions
Conceptualization, X.W., Z.W. and D.W.; computation and analysis, W.Z.; methodology and experimental investigation, Z.W., X.W., X.Z., H.C., H.H.; discussion, L.L., X.W., X.Z.; writing, Z.W., D.W.; project supervision, D.W.; funding acquisition, D.W., J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (51572191, 21633004).
Acknowledgments
The authors are grateful to Qianjin Guo and Qingyuan Luo for their assistance with the TEM and PL measurements, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2011, 24, 229–251. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amala, R.; Qiao, S.; Wu, J.-B.; Yin, Z. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent advances in Hheterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Vu, N.; Kaliaguine, S.; Do, T.-O. Critical aspects and recent advances in structural engineering of photocatalysts for sunlight-driven photocatalytic reduction of CO2 into fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Z.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 2019, 7, 865–887. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Liu, F.; Feng, N.; Wang, Q.; Xu, J.; Qi, G.; Wang, C.; Deng, F. Transfer channel of photoinduced holes on a TiO2 surface as revealed by solid-state nuclear magnetic resonance and electron spin resonance spectroscopy. J. Am. Chem. Soc. 2017, 139, 10020–10028. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Li, H.; Li, P.; Zou, Z. Au@TiO2yolk-shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field. Nanoscale 2015, 7, 14232–14236. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient photocatalysts of TiO2 nanocrystals-supported PtRu alloy nanoparticles for CO2 reduction with H2O: Synergistic effect of Pt-Ru. Appl. Catal. B Environ. 2018, 236, 445–457. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B Environ. 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Raskó, J. FTIR study of the photoinduced dissociation of CO2 on titania-supported noble metals. Catal. Lett. 1998, 56, 11–15. [Google Scholar] [CrossRef]
- Meng, X.; Ouyang, S.; Kako, T.; Li, P.; Yu, Q.; Wang, T.; Ye, J. Photocatalytic CO2 conversion over alkali modified TiO2 without loading noble metal cocatalyst. Chem. Commun. 2014, 50, 11517–11519. [Google Scholar] [CrossRef]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, photons, protons and Earth-abundant metal complexes for molecular catalysis of CO2 reduction. ACS Catal. 2016, 7, 70–88. [Google Scholar] [CrossRef]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the photocatalytic activity of P25 for carbon dioxide reduction by using a surface-alkalinized titanium carbide MXene as cocatalyst. ChemSusChem 2018, 11, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Frischmann, P.D.; Mahata, K.; Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 2013, 42, 1847–1870. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.; Kang, F. Porphyrin-based nanostructures for photocatalytic applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Zhao, G.; Pang, H.; Liu, G.; Li, P.; Liu, H.; Zhang, H.; Shi, L.; Ye, J. Co-porphyrin/carbon nitride hybrids for improved photocatalytic CO2 reduction under visible light. Appl. Catal. B Environ. 2017, 200, 141–149. [Google Scholar] [CrossRef]
- Lian, S.; Kodaimati, M.S.; Weiss, E.A. Photocatalytically active superstructures of quantum dots and iron porphyrins for reduction of CO2 to CO in water. ACS Nano 2018, 12, 568–575. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Kumar, S.; Wani, M.Y.; Arranja, C.T.; Silva, J.D.A.E.; Avula, B.; Sobral, A.J.F.N. Porphyrins as nanoreactors in the carbon dioxide capture and conversion: A review. J. Mater. Chem. A 2015, 3, 19615–19637. [Google Scholar] [CrossRef]
- Chen, E.-X.; Qiu, M.; Zhang, Y.-F.; Zhu, Y.S.; Liu, L.-Y.; Sun, Y.-Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and base resistant zirconium polyphenolate-metalloporphyrin scaffolds for efficient CO2 photoreduction. Adv. Mater. 2017, 30, 1704388. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal-organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Feng, W.; Lei, H.; Li, J. Preparation of Cu (ii) porphyrin–TiO2 composite in one-pot method and research on photocatalytic property. RSC Adv. 2017, 7, 52738–52746. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wang, A.; Wu, L.; Wang, Q. Facile synthesis and photocatalytic activity of a novel titanium dioxide nanocomposite coupled with zinc porphyrin. Nanomater. Nanotechnol. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, D.; Shen, S.; Wan, J.; Zheng, X.; Yu, L.; Phillips, D.L. The photocatalytic activity and degradation mechanism of methylene blue over copper (ii) tetra (4-carboxyphenyl) porphyrin sensitized TiO2 under visible light irradiation. RSC Adv. 2014, 4, 28978–28986. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhou, W.; Liu, L.; Ye, J.; Wang, D. An ultrathin porphyrin-based metal-organic framework for efficient photocatalytic hydrogen evolution under visible light. Nano Energy 2019, 62, 250–258. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.-Y.; Wu, J.; Wang, S.; Yu, L.; Zhu, Q.-Y.; Dai, J. Perylene carboxylate-modified titanium–oxide gel, a functional material with photoswitchable fluorescence properties. J. Mater. Chem. C 2013, 1, 7973–7978. [Google Scholar] [CrossRef]
- Kung, C.-W.; Chang, T.-H.; Chou, L.-Y.; Hupp, J.T.; Farha, O.K.; Ho, K.-C. Post metalation of solvothermally grown electroactive porphyrin metal–organic framework thin films. Chem. Commun. 2015, 51, 2414–2417. [Google Scholar] [CrossRef]
- Yan, S.; Ouyang, S.; Xu, H.; Zhao, M.; Zhang, X.; Ye, J. Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. J. Mater. Chem. A 2016, 4, 15126–15133. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B Environ. 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Verani, C.N.; Bill, E.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. Electronic structure of bis (o-iminobenzosemiquinonato) metal complexes (Cu, Ni, Pd). The art of establishing physical oxidation states in transition-metal complexes containing radical ligands. J. Am. Chem. Soc. 2001, 123, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).