Electro-Absorbers: A Comparison on Their Performance with Jet-Absorbers and Absorption Columns
Abstract
- -
- Significant differences in the speciation related to the absorption step.
- -
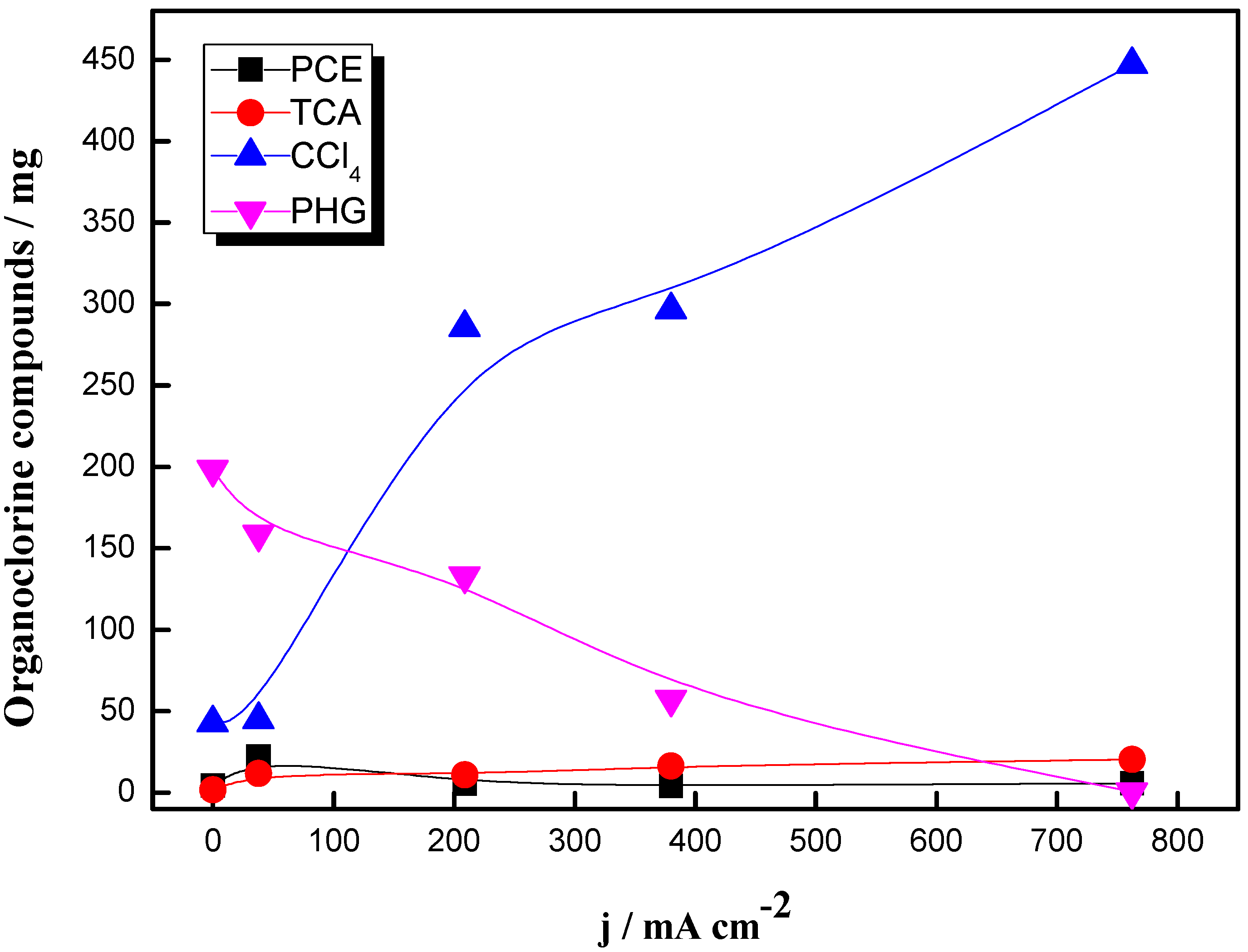
- PCE was not mineralized but transformed into other products.
- -
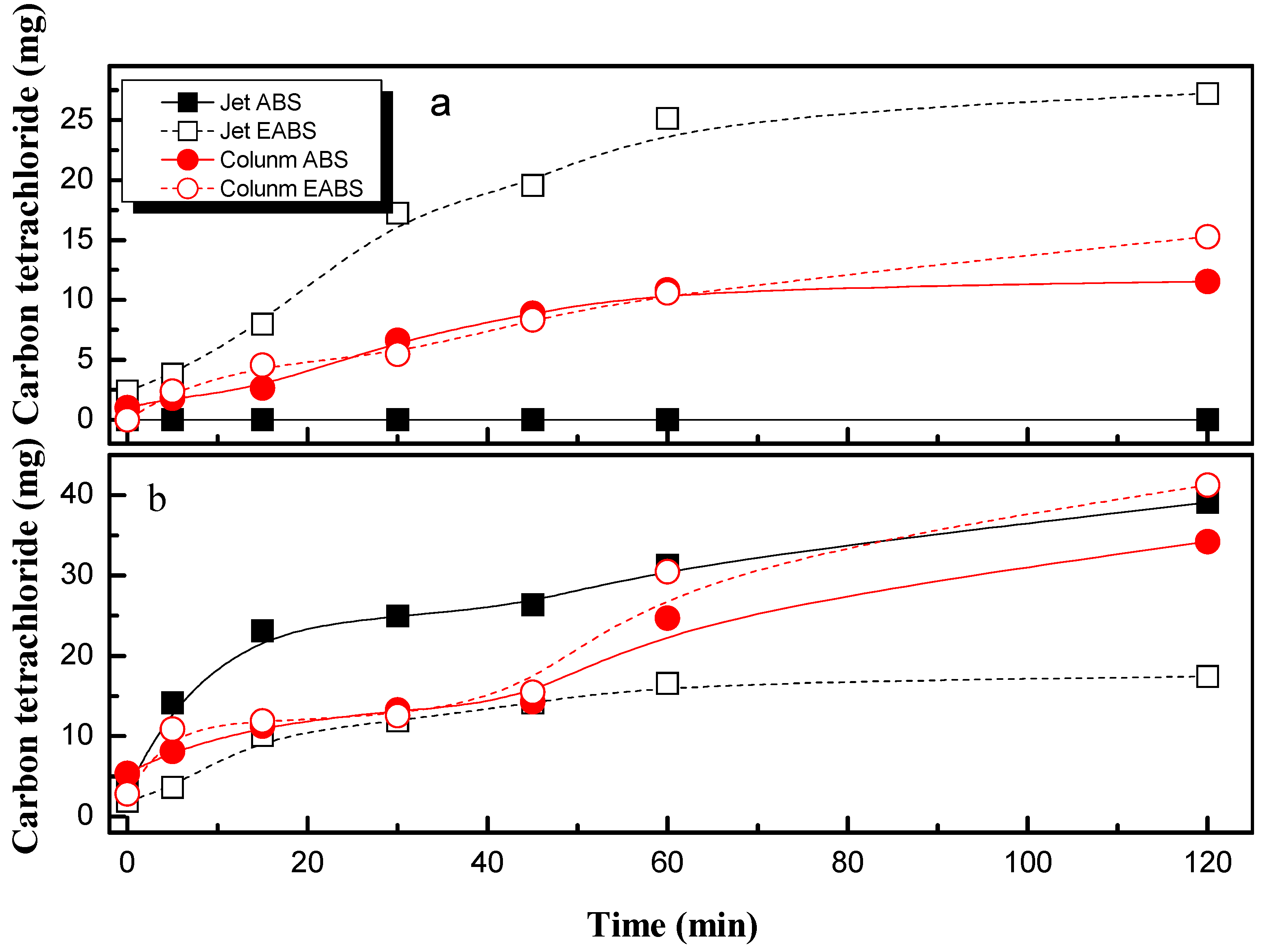
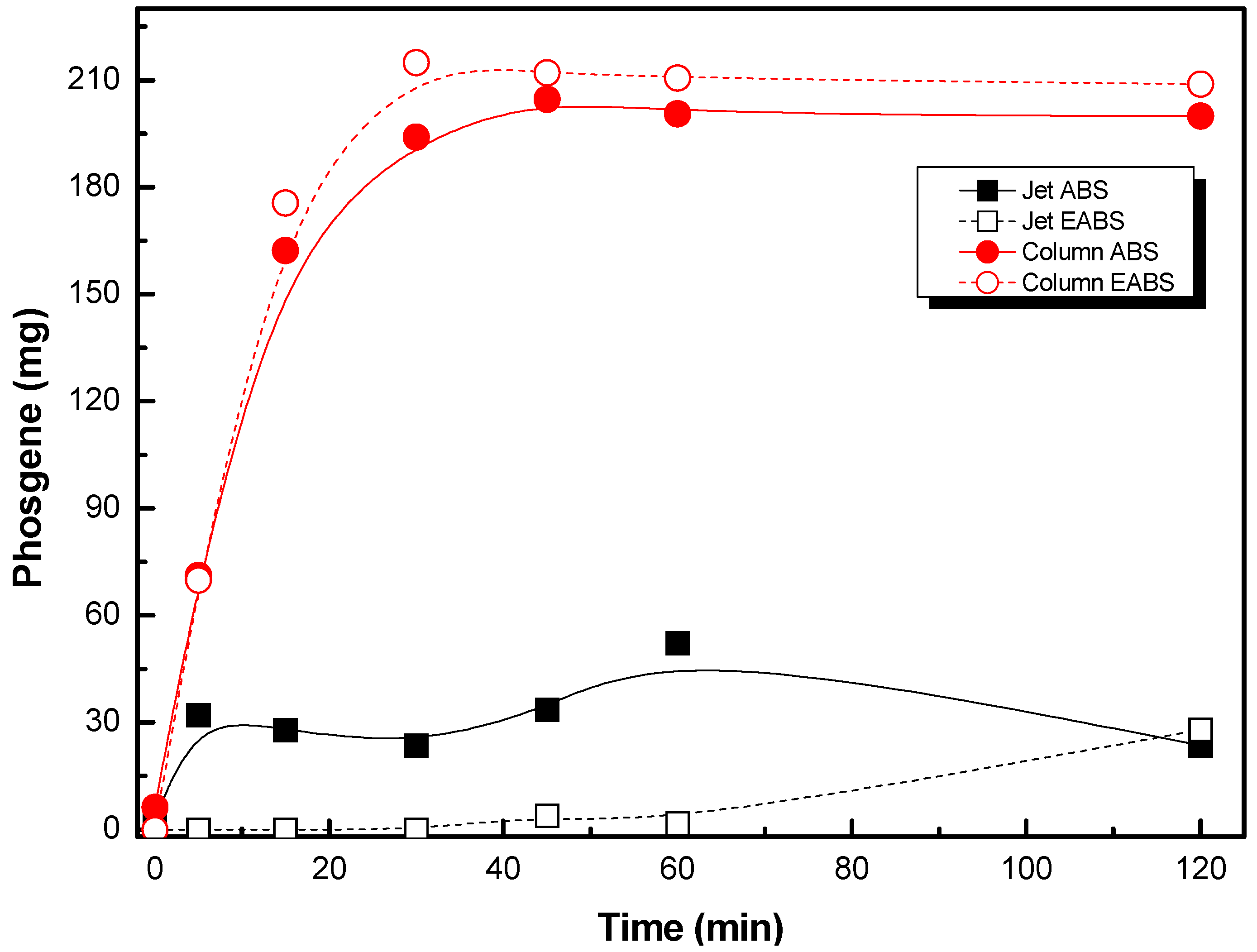
- Phosgene and carbon tetrachloride are the main products with the jet absorber.
- -
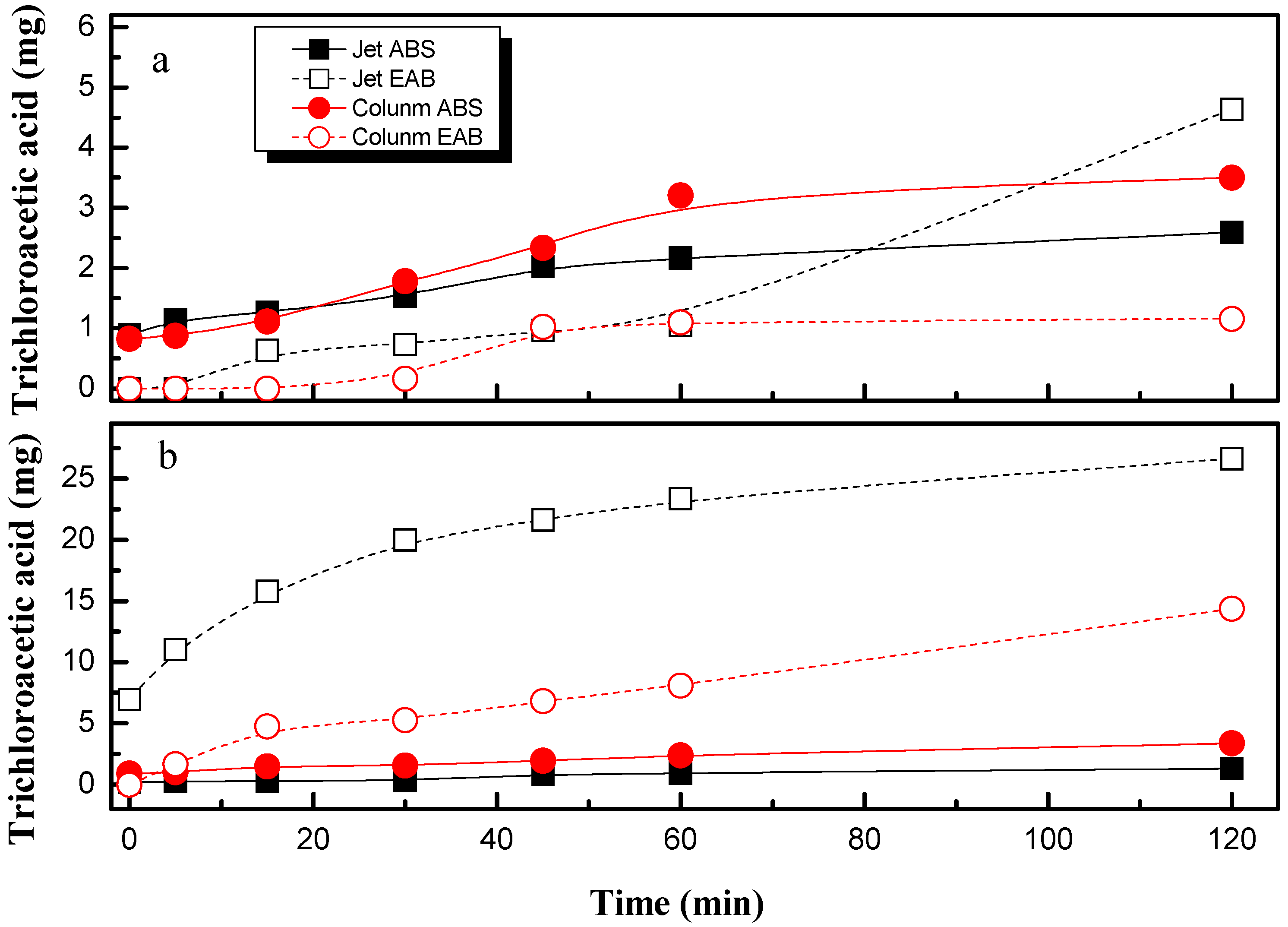
- Trichloroacetic acid is important when using the absorption column.
- -
- Scale up of electro-absorption with a stack cell was evaluated.
1. Introduction
2. Results and Discussion
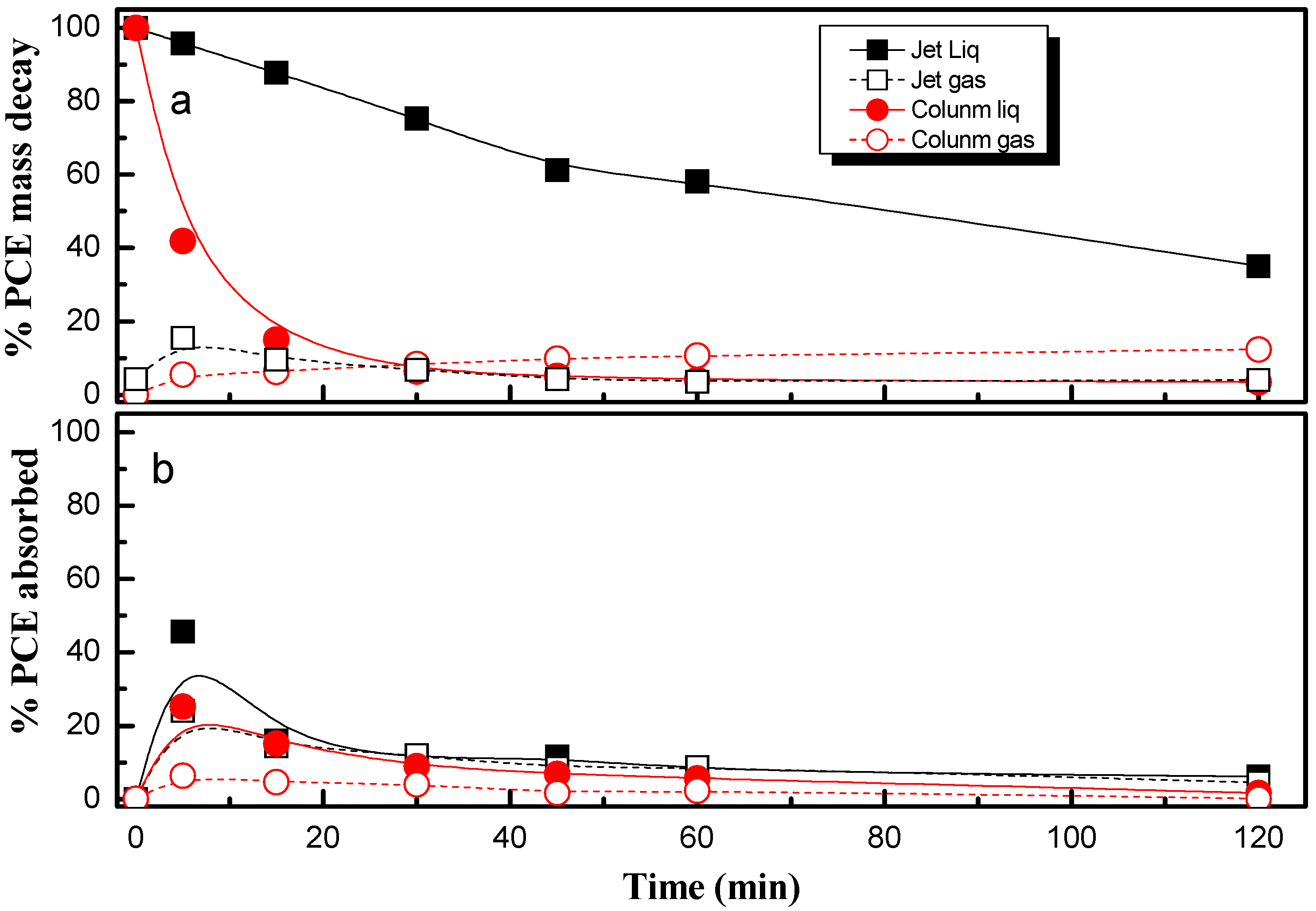
2.1. Absorption Using a Packed Column and Jet Absorber
2.2. Performance of Electro-Absorbers
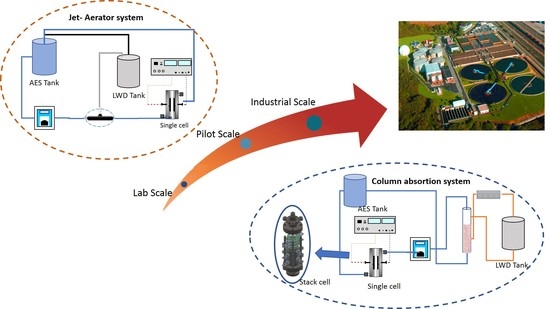
2.3. Scale up of Electro-Absorption Processes
3. Materials and Methods
3.1. Chemicals
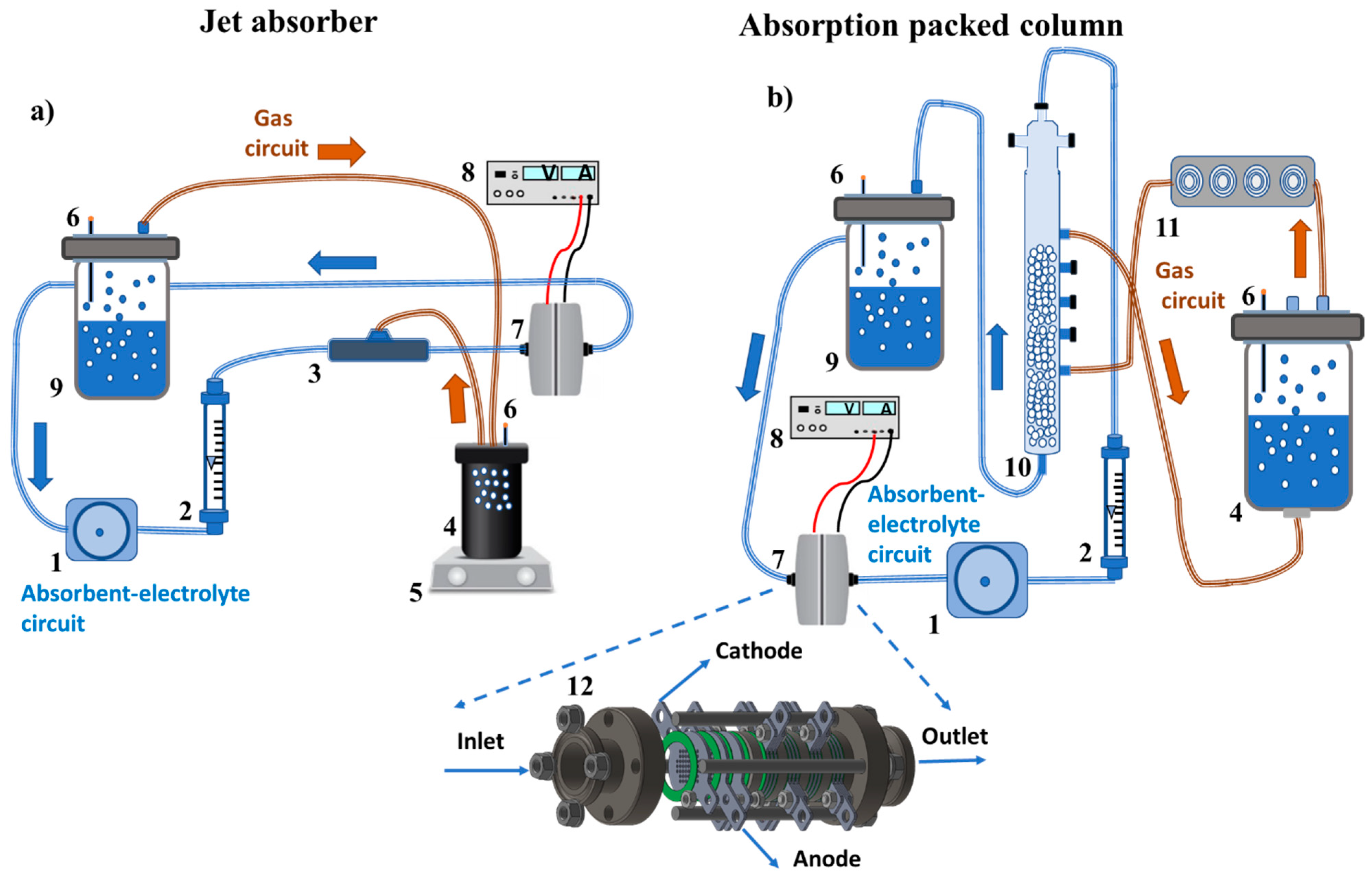
3.2. Experimental Setup
3.3. Analysis Procedures
4. Conclusions
- Both jet absorbers and packed column absorbers can be used for the absorption stage of the removal of PCE with electro-absorbers. Packed columns reached more successful results.
- The column absorber favors the formation of trichloroacetic acid.
- Electrooxidation process with diamond electrodes increases the reduction of PCE but enhances the generation of more dangerous and toxic intermediates. Trichloroacetic acid and carbon tetrachloride are the main compounds detected. A removal pathway for PCE degradation related to the absorption efficiency in both set-ups can be proposed.
- The use of a cell stack with five electrodes does not show remarkable differences in the removal efficiency of PCE as compared to the singles cell. However, TCA formation is not promoted with the scaled-up system. In addition, current density affects importantly to results. Low current densities lead to the formation of higher amounts of phosgene and higher current densities to the less efficient removal of PCE.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, H.; Tan, H.; Zhao, Y.; Wang, Y.; Wang, X.; Li, Y.; Lu, W.; Wang, H. Statistical correlations on the emissions of volatile odorous compounds from the transfer stage of municipal solid waste. Waste Manag. 2019, 87, 701–708. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, M.; Streese, J.; Biedermann, W.; Herold, T.; Stegmann, R. Odour control at biowaste composting facilities. Waste Manag. 2005, 25, 917–927. [Google Scholar] [CrossRef]
- Dalton, P. How people sense, perceive and react to odors. BioCycle 2003, 44, 26–29. [Google Scholar]
- Muthuraman, G.; Chung, S.J.; Moon, I.S. The combined removal of methyl mercaptan and hydrogen sulfide via an electro-reactor process using a low concentration of continuously regenerable Ag(II) active catalyst. J. Hazard. Mater. 2011, 193, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Govindan, M.; Chung, S.-J.; Jang, J.-W.; Moon, I.-S. Removal of hydrogen sulfide through an electrochemically assisted scrubbing process using an active Co(III) catalyst at low temperatures. Chem. Eng. J. 2012, 209, 601–606. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, J.H.; Lin, Y.C. A novel two-stage scrubbing technology for odor control of kitchen waste composting. Aerosol Air Qual. Res. 2012, 12, 1386–1397. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Ruokojärvi, A.; Ruuskanen, J.; Martikainen, P.J.; Olkkonen, M. Oxidation of Gas Mixtures Containing Dimethyl Sulfide, Hydrogen Sulfide, and Methanethiol Using a Two-Stage Biotrickling Filter. J. Air Waste Manag. Assoc. 2001, 51, 11–16. [Google Scholar] [CrossRef]
- Muthuraman, G.; Thirumavalavan, M.; Il Shik, M. In situ electrochemically generated peroxymonophosphoric acid as an oxidant for the effective removal of gaseous acetaldehyde. Chem. Eng. J. 2017, 325, 449–456. [Google Scholar] [CrossRef]
- Govindan, M.; Adam Gopal, R.; Moon, I.S. Efficient removal of gaseous trichloroethylene by continuous feed electro-scrubbing using a new homogeneous heterobimetallic electro-catalyst. Chem. Eng. J. 2017, 308, 1145–1153. [Google Scholar] [CrossRef]
- Fang, P.; Cen, C.; Tang, Z.; Zhong, P.; Chen, D.; Chen, Z. Simultaneous removal of SO2 and NOX by wet scrubbing using urea solution. Chem. Eng. J. 2011, 168, 52–59. [Google Scholar] [CrossRef]
- Muthuraman, G.; Moon, I.-S. A review on an electrochemically assisted-scrubbing process for environmental harmful pollutant’s destruction. J. Ind. Eng. Chem. 2012, 18, 1540–1550. [Google Scholar] [CrossRef]
- Yang, J.; Liu, K.; Jia, J.; Cao, L. Electro-scrubbing volatile organic carbons in the air stream with a gas diffusion electrode. J. Hazard. Mater. 2011, 188, 125–131. [Google Scholar] [CrossRef]
- González-Pérez, O.; Muñoz-Morales, M.; Souza, F.L.; Saez, C.; Cañizares, P.; Rodrigo, M.A. Jet electro-absorbers for the treatment of gaseous perchloroethylene wastes. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Pérez, J.F.; Llanos, J.; Sáez, C.; López, C.; Cañizares, P.; Rodrigo, M.A. The pressurized jet aerator: A new aeration system for high-performance H2O2 electrolyzers. Electrochem. Commun. 2018, 89, 19–22. [Google Scholar] [CrossRef]
- Song, J.T.; Song, H.; Kim, B.; Oh, J. Towards Higher Rate Electrochemical CO2 Conversion: From Liquid-Phase to Gas-Phase Systems. Catalysts 2019, 9, 224. [Google Scholar] [CrossRef]
- Govindan, M.; Moon, I.-S. A single catalyst of aqueous CoIII for deodorization of mixture odor gases: A development and reaction pathway study at electro-scrubbing process. J. Hazard. Mater. 2013, 260, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara Pillai, K.; Raju, T.; Chung, S.J.; Moon, I.-S. Removal of H2S using a new Ce(IV) redox mediator by a mediated electrochemical oxidation process. J. Chem. Technol. Biotechnol. 2009, 84, 447–453. [Google Scholar] [CrossRef]
- Balaji, S.; Muthuraman, G.; Moon, I. Influence of cathode on the electro-generation of peroxydisulfuric acid oxidant and its application for effective removal of SO2 by room temperature electro-scrubbing process. J. Hazard. Mater. 2015, 299, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Anodic oxidation for the remediation of soils polluted with perchloroethylene. J. Chem. Technol. Biotechnol. 2019, 94, 288–294. [Google Scholar] [CrossRef]
- Pitkäaho, S.; Matejova, L.; Ojala, S.; Gaalova, J.; Keiski, R.L. Oxidation of perchloroethylene—Activity and selectivity of Pt, Pd, Rh, and V2O5 catalysts supported on Al2O3, Al2O3-TiO2 and Al2O3-CeO2. Appl. Catal. B Environ. 2012, 113, 150–159. [Google Scholar] [CrossRef]
- Karimaei, M.; Nabizadeh, R.; Shokri, B.; Khani, M.R.; Yaghmaeian, K.; Mesdaghinia, A.; Mahvi, A.; Nazmara, S. Dielectric barrier discharge plasma as excellent method for Perchloroethylene removal from aqueous environments: Degradation kinetic and parameters modeling. J. Mol. Liq. 2017, 248, 177–183. [Google Scholar] [CrossRef]
- Andrew James, C.; Xin, G.; Doty, S.L.; Muiznieks, I.; Newman, L.; Strand, S.E. A mass balance study of the phytoremediation of perchloroethylene-contaminated groundwater. Environ. Pollut. 2009, 157, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Sáez, V.; Esclapez, M.D.; Bonete, P.; Walton, D.J.; Rehorek, A.; Louisnard, O.; González-García, J. Sonochemical degradation of perchloroethylene: The influence of ultrasonic variables, and the identification of products. Ultrason. Sonochemistry 2011, 18, 104–113. [Google Scholar] [CrossRef]
- Sáez, V.; Esclapez Vicente, M.D.; Frías-Ferrer, Á.J.; Bonete, P.; González-García, J. Electrochemical degradation of perchloroethylene in aqueous media: An approach to different strategies. Water Res. 2009, 43, 2169–2178. [Google Scholar] [CrossRef]
- Sáez, V.; Esclapez, M.D.; Tudela, I.; Bonete, P.; Louisnard, O.; González-García, J. 20kHz sonoelectrochemical degradation of perchloroethylene in sodium sulfate aqueous media: Influence of the operational variables in batch mode. J. Hazard. Mater. 2010, 183, 648–654. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Sáez, V.; Milán-Yáñez, D.; Tudela, I.; Louisnard, O.; González-García, J. Sonoelectrochemical treatment of water polluted with trichloroacetic acid: From sonovoltammetry to pre-pilot plant scale. Ultrason. Sonochemistry 2010, 17, 1010–1020. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Improvement of electrochemical oxidation efficiency through combination with adsorption processes. J. Environ. Manag. 2020, 262, 110364. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Gabarrell, X.; Sarrà, M.; Caminal, G.; Vicent, T.; Reddy, C.A. Novel Aerobic Perchloroethylene Degradation by the White-Rot Fungus Trametes versicolor. Environ. Sci. Technol. 2006, 40, 7796–7802. [Google Scholar] [CrossRef]
- Sáez, V.; Esclapez, M.D.; Bonete, P.; González-García, J.; Pérez, J.M. Spectroelectrochemical study of perchloroethylene reduction at copper electrodes in neutral aqueous medium. Electrochim. Acta 2008, 53, 3210–3217. [Google Scholar] [CrossRef][Green Version]
- Esclapez, M.D.; Tudela, I.; Díez-García, M.I.; Sáez, V.; Bonete, P. Towards the complete dechlorination of chloroacetic acids in water by sonoelectrochemical methods: Effect of the cathode material on the degradation of trichloroacetic acid and its degradation by-products. Appl. Catal. B Environ. 2015, 166–167, 66–74. [Google Scholar] [CrossRef]
- Meyer, R.J.; Safarik, D.J.; Reeves, C.T.; Allen, D.T.; Mullins, C.B. Phosgene formation from adsorption of carbon tetrachloride on oxygen modified Ir(111). J. Mol. Catal. A Chem. 2001, 167, 59–66. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Y.; Zeng, F.; Chen, J.; Wu, S. An AIE-based fluorescent test strip for the portable detection of gaseous phosgene. Chem. Commun. 2017, 53, 9813–9816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Troll, C.; Rieger, B.; Kintrup, J.; Schlüter, O.F.K.; Weber, R. Composition optimization of silica-supported copper (II) chloride substance for phosgene production. Appl. Catal. A Gen. 2009, 365, 20–27. [Google Scholar] [CrossRef]
- Sweeney, L.M.; Kirman, C.R.; Gargas, M.L.; Dugard, P.H. Contribution of trichloroacetic acid to liver tumors observed in perchloroethylene (perc)-exposed mice. Toxicology 2009, 260, 77–83. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. EPA’s Integrated Risk Information System; EPA: Washington, DC, USA, 2016.
- Isidro, J.; Brackemeyer, D.; Sáez, C.; Llanos, J.; Lobato, J.; Cañizares, P.; Matthee, T.; Rodrigo, M.A. Operating the CabECO® membrane electrolytic technology in continuous mode for the direct disinfection of highly fecal-polluted water. Sep. Purif. Technol. 2019, 208, 110–115. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. A new electrochemically-based process for the removal of perchloroethylene from gaseous effluents. Chem. Eng. J. 2019, 361, 609–614. [Google Scholar] [CrossRef]


| Degradation Compounds | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| carbon tetrachloride | 11.16% | 14.45% | 11.41% | 12.49% |
| trichloroacetic acid | 2.79% | 20.34% | 3.22% | 6.46% |
| phosgene | 9.15% | 16.07% | 77.47% | 71.83% |
| perchloroethylene | 59.78% | 49.14% | 7.89% | 9.21% |
| carbon dioxide | 17.12% | 0.00% | 0.00% | 0.00% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Juárez, M.; Muñoz-Morales, M.; Souza, F.L.; Sáez, C.; Cañizares, P.; Almazán-Sánchez, P.T.; Linares-Hernández, I.; Rodrigo, M.A. Electro-Absorbers: A Comparison on Their Performance with Jet-Absorbers and Absorption Columns. Catalysts 2020, 10, 653. https://doi.org/10.3390/catal10060653
Castañeda-Juárez M, Muñoz-Morales M, Souza FL, Sáez C, Cañizares P, Almazán-Sánchez PT, Linares-Hernández I, Rodrigo MA. Electro-Absorbers: A Comparison on Their Performance with Jet-Absorbers and Absorption Columns. Catalysts. 2020; 10(6):653. https://doi.org/10.3390/catal10060653
Chicago/Turabian StyleCastañeda-Juárez, Monserrat, Martín Muñoz-Morales, Fernanda Lourdes Souza, Cristina Sáez, Pablo Cañizares, Perla Tatiana Almazán-Sánchez, Ivonne Linares-Hernández, and Manuel Andrés Rodrigo. 2020. "Electro-Absorbers: A Comparison on Their Performance with Jet-Absorbers and Absorption Columns" Catalysts 10, no. 6: 653. https://doi.org/10.3390/catal10060653
APA StyleCastañeda-Juárez, M., Muñoz-Morales, M., Souza, F. L., Sáez, C., Cañizares, P., Almazán-Sánchez, P. T., Linares-Hernández, I., & Rodrigo, M. A. (2020). Electro-Absorbers: A Comparison on Their Performance with Jet-Absorbers and Absorption Columns. Catalysts, 10(6), 653. https://doi.org/10.3390/catal10060653