Abstract
The selective enhancement of catalytic activity is a challenging task, as catalyst modification is generally accompanied by both desirable and undesirable properties. For example, in the case of the direct synthesis of hydrogen peroxide, Pt on Pd improves hydrogen conversion, but lowers hydrogen peroxide selectivity, whereas Au on Pd enhances hydrogen peroxide selectivity but decreases hydrogen conversion. Toward an ideal catalytic property, the development of a catalyst that is capable of improving H-H dissociation for increasing H2 conversion, whilst suppressing O-O dissociation for high H2O2 selectivity would be highly beneficial. Pd-core AuPt-bimetallic shell nanoparticles with a nano-sized bimetallic layer composed of Au-rich or Pt-rich content with Pd cubes were readily prepared via the direct seed-mediated growth method. In the Pd-core AuPt-bimetallic shell nanoparticles, Au was predominantly located on the {100} facets of the Pd nanocubes, whereas Pt was deposited on the corners of the Pd nanocubes. The evaluation of Pd-core AuPt-bimetallic shell nanoparticles with varying Au and Pt contents revealed that Pd-core AuPt-bimetallic shell that was composed of 2.5 mol% Au and 5 mol% Pt, in relation to Pd, exhibited the highest H2O2 production rate (914 mmol H2O2 gmetal−1 h−1), due to the improvement of both H2O2 selectivity and H2 conversion.
1. Introduction
The fabrication of core–shell nanostructures that are composed of a partially or fully enveloping shell located on the surface of a nanostructured core material has been implemented for the enhancement of catalyst properties [1,2,3]. In recent decades, a number of core–shell nanocatalysts have been developed with the aim of improving reaction performance. Specifically, the introduction of nano- or sub-nano thickness shells can induce a promising alteration of surface properties through strain and/or ligand effects. Meaningful catalytic activities have been achieved via the restructuring of electron densities of both core and shell [4,5,6,7,8]. K. Sasaki et al. reported significantly enhanced stability of Pd-core Pt-shell nanoparticles utilized as an electrocatalyst in fuel cells. In this case, density functional theory (DFT) calculations revealed that a shift in oxidation potential from shell Pt to core Pd can prevent leaching and dissolution of the Pt shell [4]. X. Zhao et al. synthesized an ultrathin PtNi shell on Pd octahedron nanoparticles. Approximately four PtNi atomic layers presented a five-fold increase in oxygen reduction reaction (ORR) activity as compared to conventional Pt/C catalyst, as well as enhanced stability [5]. L. Bu et al. developed PtPb-core Pt-shell nanoparticles for ORR, wherein the Pt shell was composed of four to six atomic layers in the thickness range of 0.8–1.2 nm and possessed strain engineered properties. The PtPb core forces the outer Pt shell to expand (tensile strain), thereby strengthening Pt-O bonds and improving ORR activity [6]. Analogously, we have previously fabricated compressed Au layers on Pd cube nanoparticles for the direct synthesis of hydrogen peroxide. Through the use of DFT calculations and precession nanobeam diffraction analysis for strain mapping, it was established that a 2–3% strain engineered Au thin layer (3–4 atomic layers) can activate H2 molecules while sustaining O2 molecules, which results in a two-fold increase in H2O2 selectivity [7].
The aforementioned core–shell nanocatalyst synthetic strategies entail the use of a single shell material; however, this approach might present limitations in complex reaction systems that demand diverse enhancement of activities. Therefore, we propose the use of two elements in the shell to simultaneously improve two catalytic properties, namely selectivity and activity. That is, if the selectivity increases when B is on the surface of A, and the activity increases when C is on the surface of A, then a core–shell structure with A as the core and both B and C, as the shell could potentially exhibit enhanced activity as well as selectivity. A fundamental prerequisite is that B and C must exist independently on the surface of A and not as an alloy shell in order to exploit the catalytic properties of each material.
As an attractive and challenging reaction, the direct synthesis of hydrogen peroxide (DSHP) from hydrogen (H2) and oxygen (O2) has been widely investigated, as it is feasible for the on-site application to H2O2-related reactions, and is additionally advantageous over conventional H2O2 synthetic methods (anthraquinone auto-oxidation), due to the employment of green chemicals [8,9,10,11]. However, thermodynamically spontaneous side reactions that produce water (H2O) result in insufficient selectivity for H2O2, and a three-phase reaction system induces a substantial mass transfer limitation, which leads to low reactant conversion [8,9,10,11].
Numerous Pd-based bimetallic catalysts have shown potential for improving both the conversion and selectivity in the DSHP. For example, gold (Au) is known as a suitable additive for enhancing H2O2 selectivity [7,12,13,14,15,16,17,18]. Au-promoted Pd sites in various PdAu bimetallic composites, including AuPd alloys [12,13,14], Pd monomers encapsulated by Au [15], Au core–Pd shell [16,17], and Pd core–Au shell composites [7,18], can selectively hydrogenate oxygen molecules to produce H2O2 by suppressing the further dissociation of O–O bonds and facilitating the formation of OOH* (the chief intermediate in H2O2 formation). To improve low conversion rates, Pt metal, which exhibits marked effects in activating hydrogen molecules, can be incorporated [19,20]. Moreover, researchers have discovered that small amounts of Pt not only enhance the conversion but also substantially increase H2O2 selectivity [19,21,22,23]. Therefore, Pd core nanocrystals that are encapsulated by isolated Pt- and Au-promoted domains are expected to produce an advanced catalyst for the DSHP reaction by enhancing H2 conversion and increasing H2O2 selectivity.
In this study, we report a facile aqueous-phase synthesis of Pd-core AuPt–bimetallic shell nanoparticles and their application as DSHP reaction catalysts, wherein enhanced selectivity and activity were observed for direct H2O2 production. Pd-core AuPt–bimetallic shell nanoparticles were synthesized via the direct seed-mediated growth approach, without the need for a washing process during the reaction. In the synthesized core–shell nanoparticles, Au was predominantly located on the Pd (100) terrace surface, whereas Pt was generally deposited on the corners of the Pd nanocubes, which indicated the successful formation of a AuPt bimetallic shell.
2. Results and Discussion
Core–shell nanoparticles comprised of a Pd nanocube core and a AuPt bimetallic shell were synthesized via a direct seed-mediated growth approach, which was achieved by the simultaneous injection of AuPt-shell-forming reagents into a solution containing core Pd cube nanoparticles, without the need for washing processes during synthesis. Figure 1 displays typical transmission electron microscopy (TEM) images of AuPt@Pd core–shell nanoparticles containing varying amounts of Au and Pt. We grouped the TEM images into those of Au-rich and Pt-rich samples to monitor the change in nanoparticle morphology as a function of the amount and ratio of Au and Pt used. Figure 1A,C,E display Au-rich AuPt@Pd core–shell nanoparticles with Au:Pt mol% ratios of 2:1, 5:2.5, and 25:5 in relation to Pd, respectively, from which it can be seen that the morphology of the core–shell nanocubes becomes increasingly convex along the (100) surface with increasing amounts of shell materials. However, in the Pt-rich AuPt@Pd core–shell nanoparticles, nanocube growth in the (111) direction became prominent with increasing precursor amounts; thus, a concave structure was formed (Figure 1B,D,F).
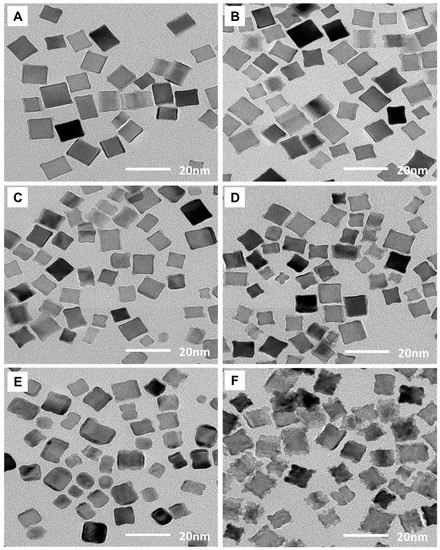
Figure 1.
Transmission electron microscopy (TEM) images of AuPt@Pd nanoparticles with a range of Au and Pt mol% relative to Pd. The mol% of Au:Pt were (A) 2:1, (B) 1:2, (C) 5:2.5, (D) 2.5:5, (E) 25:5, and (F) 5:25.
Figure 2 illustrates energy-dispersive spectroscopy (EDS) images of AuPt@Pd nanoparticles with varying molar fractions of Au and Pt. In all of the samples, the Pd core maintained its cubic morphology, with a diameter of 10–12 nm (see Figure 2, second for Pd element mapping images). The Au shell preferred to cover the {100} planes of the Pd core (third column), while the Pt shell predominantly formed on the {111} corners and partially on the {100} planes of the core structure (fourth column). Detailed shell formation behavior is explained, as follows. At low Au content (Figure 2c,g,o), a thin (1–2 nm), and less evident Au layer is observed on a single {100} facet. With increasing Au amounts (Figure 2k,w), Au atoms formed a denser layer, 2–3 nm in thickness, which gradually surrounded the terrace plane of the Pd cube (Figure 2s). Notably, the Au atoms grew along the {100} plane for the complete formation of one layer rather than being randomly distributed on the {100} surface. In terms of Pt deposition, a concave Pt shell grew largely on the {111} corners (Figure 2h,i,p); Pt was additionally moderately deposited on the {100} planes with increasing amounts of injected Pt precursor (Figure 2t,x). It was established that the growth characteristics of Au and Pt shell that were obtained by simultaneous injection (Figure 2) were comparable to those that were synthesized by the sequential injection of Au and Pt (Figure S3), even though the order of precursor injection was different. In addition, to identify the shell compositions of Au and Pt, EDS line profile images were obtained, as seen in Figure S4. On each surface, overlapping signals for Au and Pt were observed. This possibly suggests the formation of Au–Pt alloys, such as Au-rich and Pt-rich phases, or it might arise from projection image of tilted nanoparticles.
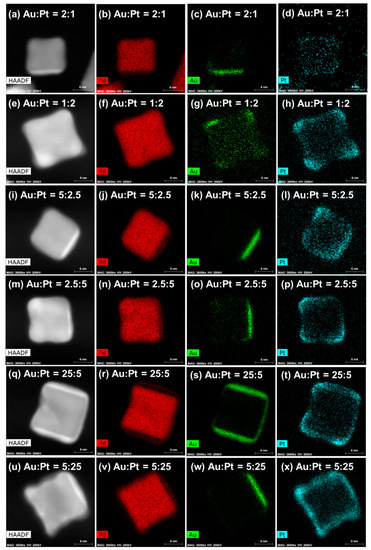
Figure 2.
The energy dispersive spectroscope (EDS) mapping images of AuPt@Pd nanoparticles synthesized with varying mol% ratio of Au and Pt. The mol% of Au:Pt were (a–d) 2:1, (e–h) 1:2, (i–l) 5:2.5, (m–p) 2.5:5, (q–t) 25:5, and (u–x) 5:25. Scale bar indicates 6 nm.
By comparing the low magnification EDS mapping images of Pd@Au-Pt nanoparticles, as shown in Figure 3, the shell formation mechanism was determined once more. As the content of Au and Pt increased, the Au layers became denser and the number of layers likewise increased. In the case of Pt, the atoms surrounding the Pd core increased while the signals remained concentrated on the corner sites, indicating the preferred deposition of Pt on {111} corner sites.
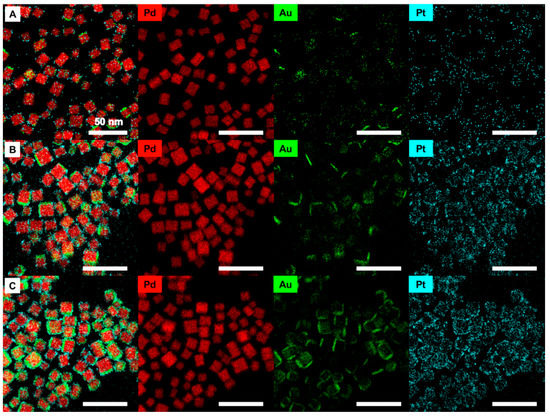
Figure 3.
Low-magnification EDS images of AuPt@Pd nanoparticles synthesized with varying Au and Pt mol% ratios. The mol% of Au:Pt were (A) 2:1, (B) 5:2.5, and (C) 25:5.
In Figure 4, the XRD data for the Pd cube and AuPt@Pd with a Au:Pt mol% of 25:5 support the formation of a lattice engineered shell, as demonstrated in our previous study [18]. The peak at 40.1° assigned to the palladium (111) crystal plane is clearly represented by the Pd cube (black). For the AuPt@Pd 25:5 particles (red), a shoulder peak is observed to the left of the Pd (111) peak, which indicates an expanded crystal plane as compared to pure Pd. As described above, the Au and/or Pt shell forms on the Pd cube, thus the shoulder peak could be rationalized by compressed lattice formation of the Au and/or Pt shell, which can result from epitaxial growth along the core Pd lattice [18]. However, the shoulder peak observed at 38–39° does not perfectly correlate with either Pt or Au. In the case that the shoulder is Au-derived, it would indicate that the compression strain of Au (2θ is higher than in pristine Au). On the contrary, if it arises from Pt, this would imply that the tensile strain of Pt (2θ is smaller than in pristine Pt); therefore, it is reasonably proposed that the shoulder peak represents compressed Au layers that are epitaxially grown on the Pd lattice, as the Pd core has smaller lattice distances when compared to those of both Au and Pt. The crystal plane of Pt might not be observable, as it is well distributed throughout the Pd cube, whereas the Au atoms formed distinct layers.
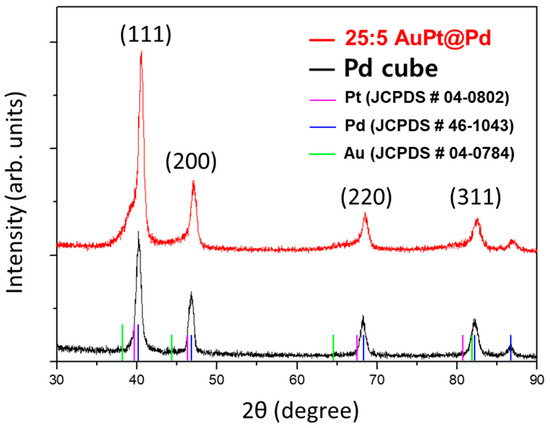
Figure 4.
X-ray diffraction (XRD) data of Pd cubes and AuPt@Pd 25:5.
Determining the optimal nanoparticle composition of Au and Pt requires dozens of experiments and, consequently, large amounts of noble-metal salts, experimental resources, substantial time, and labor. Thus, we introduced the fast-screening method to achieve efficient and economical research, wherein only 0.05 mg (2.61 × 10−4 wt.%) of catalyst is required for each reaction [24]. While the typical DSHP performance test requires elaborate instrumentation and considerable space and time, our fast-screening method enables the simultaneous operation of multiple reactions, if necessary. Although the fast-screening reaction differs from the actual DSHP in terms of reaction conditions, including temperature and pressure, it has the advantage of rapid and straightforward indication of nanoparticle catalytic activity trends.
The fast-screening experiments were performed using six samples of AuPt@Pd core–shell nanoparticles with a wide range of Au and Pt amounts, as detailed in Figure 1, and pure Pd nanocubes (Figure 5). In all of the AuPt@Pd nanoparticle samples, the production rates were superior to that of Pd nanocubes, after 1 h of reaction. With an increase in the total amount of Au and Pt from 3 mol% to 7.5 mol%, the production rate increased. However, the production rate decreased when the total amount of Au and Pt was substantially higher (30 mol%). We speculate that the electronic structure of the shell returned to its original structure because of the increased thickness of the shell, thus resulting in a loss of catalytic activity. Overall, 7.5 mol% of total Au and Pt in AuPt@Pd core–shell nanoparticles exhibited the highest production rate. In terms of the Au/Pt ratio, the highest H2O2 production rate (297.87 mmol H2O2 gmetal−1 h−1) was achieved with AuPt@Pd nanoparticles containing a Au:Pt mol% of 5:2.5. As the AuPt@Pd nanoparticles with 2.5:5 Au:Pt mol% likewise displayed a sufficiently high production rate of 281.04 mmol H2O2 gmetal−1 h−1, we attempted to control the Au/Pt ratio in the AuPt@Pd nanoparticles, whilst maintaining the total amount of Au and Pt at 7.5 mol%.
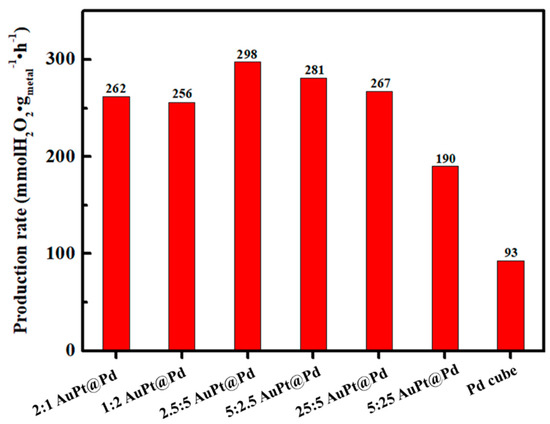
Figure 5.
Production rates of AuPt@Pd core–shell nanoparticles with varying Au and Pt mol%, in relation to Pd, in the fast-screening test for the direct synthesis of H2O2.
AuPt@Pd nanoparticles with Au:Pt mol% ratios of 3.75:3.75, 2.5:5.0, 1.9:5.6, and 1.5:6.0 in relation to Pd were synthesized and immobilized on silica support (Figure S5). Figure 6 and Table S2 shows the catalytic activities of the AuPt@Pd nanoparticles toward DSHP. As the Pt/Au ratio increased, the expected activities were obtained from the bimetallic shell. In Figure 6A, the highest H2O2 selectivity of 85% was achieved with a Pt/Au ratio of 3.75:3.75, and a further increase in the ratio notably induced a gradual decline in H2O2 selectivity (70%, 50%, and 40% on AuPt@Pd 2.5:5.0, 1.9:5.6, and 1.5:6.0, respectively). The enhanced H2O2 selectivity effect of Au layer on the Pd cube was previously examined [7,18]. Compressed strain emanate from epitaxial growth of Au showed higher H2O2 selectivity as compared to the Pd cube. On the other hand, the lowest H2 conversion rate of 6.7% was observed for the 3.75:3.75 catalyst, which gradually increased to 12% as the Pt/Au ratio increased. This observation implies that sufficient amounts of Au on Pd terrace sites contribute to the selective production of H2O2, while adequate Pt domain sites on the corners and edges facilitate H2 conversion. The proposed effects of the Pt atoms on the Pd cube can be explained by two ways: intrinsic Pt-Pt sites and electron withdrawing of Pt [25]. The former facilitates H-H dissociation for high H2 conversion, and the latter induces electron deficient Pd sites for high H2O2 selectivity. However, in this case, when the atomic ratio of Pt/Au was excessive, the surface coverage by Pt immoderately accounted for increased O–O dissociation, predominantly attenuating H2O2 selectivity. Thus, the AuPt@Pd 1.5:6.0 catalyst with the highest degree of exposed Pt sites exhibited the highest H2 conversion and lowest H2O2 selectivity. Among the Pt/Au ratios, the maximum H2O2 production rate (914.8 mmol H2O2 gmetal−1 h−1) was achieved with the AuPt@Pd 2.5:5.0 catalyst, as it simultaneously improved both H2O2 selectivity and H2 conversion (Figure 6B). In conclusion, the obtained catalytic activities demonstrated that higher proportions of Au improve H2O2 selectivity, and increased Pt content reinforces H2 dissociation; hence, overall catalytic activity can be ameliorated by manipulating the ratio of Pt/Au on Pd cubes. Thus, we note that a controllable bimetallic shell composition on a Pd core system is capable of achieving necessary catalytic activities.
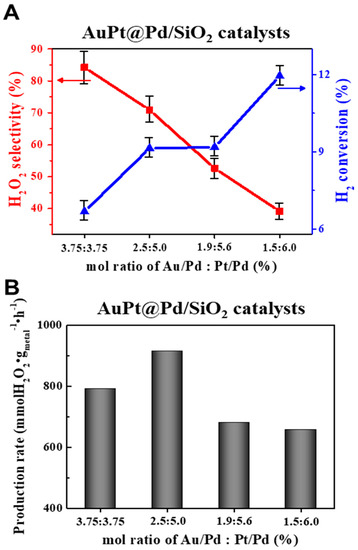
Figure 6.
DSHP results for AuPt@Pd nanoparticles with varying mol% of Au and Pt in relation to the Pd core: (A) H2O2 selectivity (red) and H2 conversion (blue); (B) production rate. The error bars are added here by measuring the error of H2 conversion from GC instrument.
Below, Table 1 shows catalytic activities with reactor types to provide meaningful comparison with some recent studies dealing with bi-metallic catalysts [26,27,28,29,30,31,32,33,34,35]. Most of Pd based bi-metallic catalysts showed improvement in activities via electron refinement of surface active sties as well as site isolation of those sites [26,27,28,30,31,32,33,34]. Some special cases, such as RhAg and AuPt composition, were also proposed for DSHP, and they exhibited promising results that can expand active species beyond Pd [29].

Table 1.
Comparison of catalytic activities towards H2O2 over Pd based bimetallic catalysts.
3. Experimental Section
3.1. Chemicals and Materials
Sodium tetrapalladinate (Na2PdCl4, 98%), potassium tetrachloroplatinate (K2PtCl4, 99%), Gold(III) chloride hydrate(HAuCl4, 99.995%), polyvinylpyrrolidone (PVP, MW = 55,000), potassium bromide (KBr, 99%), l-ascorbic acid, 2,9-dimethyl-l,10-phenanthroline (DMP, neocuproine free base), copper(II) sulfate (CuSO4, 99%), silica gel (SiO2, pore size 60 Å), and phosphate buffer (pH 7.0) were purchased from Sigma–Aldrich and used without further purification.
3.2. Synthesis of AuPt@Pd Core–Shell Nanoparticles
Scheme 1 shows the overall schematic procedures. In detail, 37 mg of PVP, 50 mg of l-ascorbic acid, and 300 mg of KBr were dissolved in 8 mL of deionized water and heated to 80 °C for 20 min. under magnetic stirring. A 3 mL aqueous solution of 57 mg of Na2PdCl4 was then added, and the resulting solution was heated at 80 °C for 3 h. Next, 0.5 mL of an aqueous l-ascorbic acid solution (0.832 M) was added. After 5 min. of heating, a 1 mL solution of HAuCl4 and a 1 mL solution of K2PtCl4 were simultaneously injected into the solution with two pipettes. The molar ratios between Pd and Au and Pt are indicated in Table S1. The mol% of Pd, Au, and Pt in the nanoparticles were determined by inductively coupled plasma spectrometry (ICP, Direct Reading Echelle ICP (LEEMAN)). The resulting solution was heated at 80 °C for a further 5 h, and, once cooled to ambient temperature, the products were collected by centrifugation (9000 rpm for 15 min.) and washed three times with a mixture of deionized (DI) water and acetone. The synthesized nanoparticles were well dispersed in water. From here, synthesized Pd-core AuPt-bimetallic shell nanoparticles are abbreviated as AuPt@Pd, which means that Au and/or Pt are supported on Pd core.
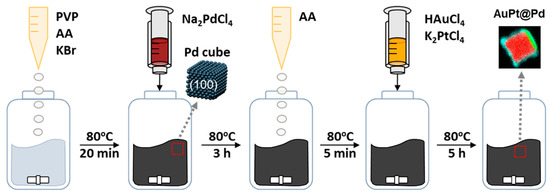
Scheme 1.
Schematic explanation of AuPt@Pd nanoparticle synthesis procedures.
3.3. Preparation of SiO2 Supported Nanoparticle
In order to improve reusability and recoverability of the AuPt@Pd core–shell nanoparticles, following their synthesis, each solution containing the nanoparticles was diluted with 10 mL of water under sonication for 1 h. Silica gel (1 g, SiO2, pore size 60 A) and the diluted nanoparticle solutions were dispersed in DI water (35 mL total volume), and the resultant solution was stirred for 6 h. The product was then collected by centrifugation and the as-prepared AuPt@Pd/SiO2 catalysts were dried at 40 °C for 12 h. Finally, the SiO2-supported catalysts were reduced with 10 vol.% H2/N2 gas at 50 °C for 1.5 h to minimize surface oxidation and activate the catalysts. The actual metal loading of SiO2 supported AuPt@Pd was measured as 0.38–0.40 wt.% by inductively coupled plasma spectrometry.
3.4. Characterization
Several techniques were employed for investigating the morphological structures of AuPt@Pd core–shell nanoparticles with varying molar ratios of Au and Pt. The compositional maps of each element comprising the AuPt@Pd samples, namely Pd, Au, and Pt, were obtained in conjunction with magnified transmission electron microscopy (TEM) images. This was accomplished by placing the samples on a carbon-mesh copper grid and employing a high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM, FEI Talos), which was equipped with an energy dispersive spectroscope (EDS, Super-X EDS) operated at 200 kV. Powder X-ray diffraction (XRD) patterns were measured with a Rigaku D-MAX/A diffractometer at 35 kV and 35 mA. Inductively coupled plasma spectrometry (ICP) analyses were performed on a Direct Reading Echelle ICP spectrometer, LEEMAN.
3.5. Prescreening for Direct Synthesis of H2O2
Prior to detailed characterization of catalytic performance, time-efficient and simple prescreening was carried out to find the most promising composition range of Au and Pt. Fast-screening was performed in a 24-well plate. Each well contained the reaction medium (2 mL), which consisted of ethanol and water (ethanol: water volume ratio was 1:4), 0.9 mM sodium bromide (NaBr), and 0.02 M phosphoric acid (H3PO4). The total volumetric flow rate of the reactant gas stream was 70 mL/min. (H2:O2 volume ratio = 1:10). The H2/Ar (H2:Ar volume ratio = 4:94) and O2 gas flow rates for each well were 50 mL/min. and 20 mL/min., respectively. The amount of AuPt@Pd nanoparticle catalyst was fixed at 0.05 mg for each well. The reaction was performed at room temperature and 1 atm for 1 h. After the reaction, the concentration of H2O2 was determined with the DMP method [36]. Thus, 0.1 mL of reaction medium, 2.3 mL of water, 0.3 mL of 48 mM DMP solution, 0.3 mL of 0.01 M copper(II) sulfate solution, and 0.3 mL of phosphate buffer (pH 7.0) solution were added sequentially to a cuvette and then mixed. The blank solution was prepared in the same manner, except that water was added instead of the reaction medium. Sample and blank absorbances were measured at 454 nm. The H2O2 concentrations could then be calculated from the calibration curve.
3.6. Catalytic Performance for Direct Synthesis of H2O2
H2O2 was directly synthesized using each catalyst in a double-jacket glass reactor. The reaction medium (150 mL) was composed of ethanol and water (25 vol% of ethanol) containing 0.03 M H3PO4. The reaction was performed at 10 °C, under 1-bar, and with a stirring speed of 1200 rpm for 30 min., and the volumetric flow rate of the reactant gas stream (volume ratio of O2/H2 = 10) was 22 mL/min. The amount of catalyst in each experiment was adjusted according to the metal content. The total amount of metal (Pd, Au, and Pt) used was fixed at 0.38 mg (based on the ICP analysis of SiO2 supported catalysts). For the evaluation of catalyst activity, three quantifiable parameters were adopted, namely H2 conversion, H2O2 selectivity, and H2O2 production rate. H2 conversion was calculated by measuring changes in the H2 peak area, being obtained by on-line connected gas chromatography (Younglin, ACME6000 equipped with a Carbosieve SII (60–80 mesh) column and a thermal conductivity detector). By calculating peak area changes using the theoretical amount of fed H2 (van der Waals equation), based on the flow rate, the obtained values could be converted to H2 conversion (%). In terms of H2O2 selectivity, previously measured amounts of reacted H2 and the concentration of generated H2O2, calculated via iodometric titration after the reaction, were used for the calculation. In a similar manner, the amount of H2O2 produced under specific reaction conditions (weight of metal and reaction time) provided the H2O2 production rate. Equations (1)–(3) were used for calculating the H2 conversion, H2O2 selectivity, and H2O2 production rate, respectively:
4. Conclusions
We have demonstrated the facile synthesis of AuPt@Pd core–shell nanoparticles while using a direct seed-mediated growth approach and established that their enhanced H2O2 productivity resulted from both high H2 conversion and improved H2O2 selectivity. The study achieves three advanced research objectives in the field of catalysts and nanoparticle synthesis. First, we presented a facile aqueous-phase synthetic method for core–shell nanoparticles with a bimetallic shell having a controllable ratio of the two shell constituents. Second, by introducing fast screening, we could minimize the time and effort required for the investigation of nanoparticle activities, and for determining the optimal amounts of Au and Pt for H2O2 synthesis. Finally, we demonstrated that core–shell nanoparticles with multi-metallic shells are potentially suitable alternative catalysts that are capable of enhancing two contradictory catalytic properties (H2O2 selectivity and H2 activity) for the DSHP reaction.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/6/650/s1, Figure S1: TEM image of Pd nanocubes as core materials, Figure S2: The EDS mapping images and average particle sizes of AuPt@Pd nanoparticles synthesized with varying mol % ratio of Au and Pt. The mol % of Au:Pt were (A) 2:1, (B) 1:2, (C) 5:2.5, (D) 2.5:5, (E) 25:5, and (F) 5:25. Scale bar indicates 10 nm, Figure S3: EDS images of Pd@AuPt nanoparticles synthesized with different sequences injection of the Au and Pt precursors: (A) Pt injected, followed by Au 1 min later and (B) Au injected following Pt 1 min later, Figure S4: EDS images and line profile of each elements, such as Pd, Au, and Pt, in Pd@AuPt nanoparticles synthesized with the molar fraction ratio of Au:Pt = (A) 25:5 and (B) 5 mol%: 25 mol%, respectively. The yellow arrows indicate the directions of the EDS line profiles, Figure S5: TEM images for the SiO2-supported Pd@AuPt nanoparticles. The mol% of Au:Pt were (A) 3.75:3.75 (B), 2.5:5.0, (C), 1.9:5.6, and (D) 1.5:6.0, respectively, Figure S6: XRD data for the SiO2-supported Pd@AuPt nanoparticles, Table S1: Elemental composition of the Pd@AuPt core–shell nanoparticles characterized using ICP, Table S2: Numeric information of catalytic activities for Pd@Au-Pt catalysts, Table S3: Comparison of catalytic activities developed in this study.
Author Contributions
The manuscript was written through contributions of all authors. Synthesis of catalysts K.Y.K., T.Y.; TEM analysis H.K., H.-K.K. and J.-P.A.; Catalytic activity G.-H.H., J.Y., K.-Y.L.; Catalytic activity using Fast screening test H.N., J.-H.L., S.Y.L.; Conceptualization, K.Y.K., G.-H.H., H.N., H.K., J.Y., J.-H.L., H.-K.K., J.-P.A., S.Y.L., K.-Y.L. and T.Y.; original draft writing K.Y.K., G.-H.H., H.N. and H.K.; Project administration, supervision, and resources, J.-P.A, S.Y.L., K.-Y.L. and T.Y.; Review and editing final manuscript, J.-P.A, S.Y.L., K.-Y.L. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study financially funded by following institution. National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (2014R1A5A1009799). National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2016M3D1A1021140). National Research Foundation of Korea (NRF) Funded by the Ministry of Science, ICP (2019M3E6A1103866).
Acknowledgments
T.Y. acknowledges financial support by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (2014R1A5A1009799). We acknowledge financial support from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2016M3D1A1021140). We acknowledge financial support by the National Research Foundation of Korea (NRF) Funded by the Ministry of Science, ICP (2019M3E6A1103866).
Conflicts of Interest
The authors declare no competing financial interest.
References
- Alayoglu, S.; Nilekar, A.U.; Mavrikakis, M.; Eichhorn, B. Ru-Pt core-shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 2008, 7, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.L.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Lee, Y.W.; Park, Y.; Choi, B.-S.; Hong, J.W.; Park, K.-H.; Han, S.W. One-Pot Synthesis of Trimetallic Au@PdPt Core–Shell Nanoparticles with High Catalytic Performance. ACS Nano 2013, 7, 7945–7955. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Naohara, H.; Cai, Y.; Choi, Y.; Liu, P.; Vukmirovic, M.B.; Wang, J.X.; Adzic, R. Core-Protected Platinum Monolayer Shell High-Stability Electrocatalysts for Fuel-Cell Cathodes. Angew. Chem. Int. Ed. 2010, 49, 8602–8607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, S.; Fang, Z.; Ding, J.; Sang, W.; Wang, Y.; Zhao, J.; Peng, Z.; Zeng, J. Octahedral Pd@Pt1.8Ni Core–Shell Nanocrystals with Ultrathin PtNi Alloy Shells as Active Catalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 2804–2807. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Seo, M.-G.; Choi, C.; Kim, J.S.; Jung, E.; Han, G.-H.; Lee, J.-C.; Han, S.S.; Ahn, J.-P.; Jung, Y.; et al. Studies on Catalytic Activity of Hydrogen Peroxide Generation according to Au Shell Thickness of Pd/Au Nanocubes. ACS Appl. Mater. Interfaces 2018, 10, 38109–38116. [Google Scholar] [CrossRef]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Ghedini, E.; Strukul, G. Looking for the “Dream Catalyst” for Hydrogen Peroxide Production from Hydrogen and Oxygen. Catalysts 2019, 9, 251. [Google Scholar] [CrossRef]
- Lewis, R.J.; Hutchings, G.J. Recent Advances in the Direct Synthesis of H2O2. ChemCatChem 2018, 11, 298–308. [Google Scholar] [CrossRef]
- Seo, M.-G.; Kim, H.J.; Han, S.S.; Lee, K.-Y. Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen Using Tailored Pd Nanocatalysts: A Review of Recent Findings. Catal. Surv. Asia 2016, 21, 1–12. [Google Scholar] [CrossRef]
- Freakley, S.J.; Piccinini, M.; Edwards, J.K.; Ntainjua, E.N.; Moulijn, J.A.; Hutchings, G.J. Effect of Reaction Conditions on the Direct Synthesis of Hydrogen Peroxide with a AuPd/TiO2 Catalyst in a Flow Reactor. ACS Catal. 2013, 3, 487–501. [Google Scholar] [CrossRef]
- Edwards, J.K.; Solsona, B.; Ntainjua, E.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Switching Off Hydrogen Peroxide Hydrogenation in the Direct Synthesis Process. Science 2009, 323, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Staykov, A.T.; Kamachi, T.; Ishihara, T.; Yoshizawa, K. Theoretical Study of the Direct Synthesis of H2O2 on Pd and Pd/Au Surfaces. J. Phys. Chem. C 2008, 112, 19501–19505. [Google Scholar] [CrossRef]
- Ouyang, L.; Da, G.-J.; Tian, P.; Chen, T.-Y.; Liang, G.-D.; Xu, J.; Han, Y.-F. Insight into active sites of Pd–Au/TiO2 catalysts in hydrogen peroxide synthesis directly from H2 and O2. J. Catal. 2014, 311, 129–136. [Google Scholar] [CrossRef]
- Edwards, J.K.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Direct synthesis of hydrogen peroxide from H2 and O2 using supported Au-Pd catalysts. Faraday Discuss. 2008, 138, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.K.; Solsona, B.; Landon, P.; Carley, A.F.; Herzing, A.; Kiely, C.J.; Hutchings, G.J. Direct synthesis of hydrogen peroxide from H2 and O2 using TiO2-supported Au-Pd catalysts. J. Catal. 2005, 236, 69–79. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, H.-K.; Kim, S.-H.; Kim, I.; Yu, T.; Han, G.-H.; Lee, K.-Y.; Lee, J.-C.; Ahn, J.-P. Catalytically Active Au Layers Grown on Pd Nanoparticles for Direct Synthesis of H2O2: Lattice Strain and Charge-Transfer Perspective Analyses. ACS Nano 2019, 13, 4761–4770. [Google Scholar] [CrossRef]
- Quon, S.; Jo, D.Y.; Han, G.-H.; Han, S.S.; Seo, M.-G.; Lee, K.-Y. Role of Pt atoms on Pd (1 1 1) surface in the direct synthesis of hydrogen peroxide: Nano-catalytic experiments and DFT calculations. J. Catal. 2018, 368, 237–247. [Google Scholar] [CrossRef]
- Liu, Q.; Bauer, J.C.; Schaak, R.E.; Lunsford, J.H. Direct synthesis of H2O2 from H2 and O2 over Pd-Pt/SiO2 bimetallic catalysts in a H2SO4/ethanol system. Appl. Catal. A Gen. 2008, 339, 130–136. [Google Scholar] [CrossRef]
- Edwards, J.K.; Pritchard, J.; Lu, L.; Piccinini, M.; Shaw, G.; Carley, A.F.; Morgan, D.J.; Kiely, C.J.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide Using Platinum-Promoted Gold-Palladium Catalysts. Angew. Chem. Int. Ed. 2014, 53, 2381–2384. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.; Melada, S.; Centi, G.; Perathoner, S.; Pinna, F.; Strukul, G. Performances of Pd-Me (Me=Ag, Pt) catalysts in the direct synthesis of H2O2 on catalytic membranes. Catal. Today 2006, 117, 193–198. [Google Scholar] [CrossRef]
- Bernardotto, G.; Menegazzo, F.; Pinna, F.; Signoretto, M.; Cruciani, G.; Strukul, G. New Pd-Pt and Pd-Au catalysts for an efficient synthesis of H2O2 from H2 and O2 under very mild conditions. Appl. Catal. A Gen. 2009, 358, 129–135. [Google Scholar] [CrossRef]
- Xiao, X.; Kang, T.-U.; Nam, H.; Bhang, S.H.; Lee, S.Y.; Ahn, J.-P.; Yu, T. One-pot synthesis of PdAu bimetallic composite nanoparticles and their catalytic activities for hydrogen peroxide generation. Korean J. Chem. Eng. 2018, 35, 2379–2383. [Google Scholar] [CrossRef]
- Han, G.-H.; Xiao, X.; Hong, J.; Lee, K.-J.; Park, S.; Ahn, J.-P.; Lee, K.-Y.; Yu, T. Tailored Palladium–Platinum Nanoconcave Cubes as High Performance Catalysts for the Direct Synthesis of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 6328–6335. [Google Scholar] [CrossRef] [PubMed]
- Crole, D.A.; Underhill, R.; Edwards, J.K.; Shaw, G.; Freakley, S.J.; Hutchings, G.J.; Lewis, R.J. The Direct Synthesis of Hydrogen Peroxide from H2 and O2 Using Pd-Ni/TiO2 Catalysts. Philos. Trans. A Math. Phys. Eng. Sic. 2020, 1–15. [Google Scholar]
- Wang, S.; Doronkin, D.E.; Hähsler, M.; Huang, X.; Wang, D.; Grunwaldt, J.-D.; Behrens, S. Palladium-Based Bimetallic Nanocrystal Catalysts for the Direct Synthesis of Hydrogen Peroxide. ChemSusChem 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lewis, R.J.; Doronkin, D.E.; Morgan, D.J.; Grunwaldt, J.-D.; Hutchings, G.J.; Behrens, S. The direct synthesis of hydrogen peroxide from H2 and O2 using Pd-Ga and Pd-In catalysts. Catal. Sci. Technol. 2020, 10, 1925–1932. [Google Scholar] [CrossRef]
- Kim, D.; Nam, H.; Cho, Y.-H.; Yeo, B.C.; Cho, S.H.; Ahn, J.-P.; Lee, K.-Y.; Lee, S.Y.; Han, S.S. Unlocking the Potential of Nanoparticles Composed of Immiscible Elements for Direct H2O2 Synthesis. ACS Catal. 2019, 9, 8702–8711. [Google Scholar] [CrossRef]
- Jang, Y.; Nam, H.; Song, J.; Lee, S.; Ahn, J.-P.; Yu, T. Synthesis RhAg bimetallic composite nanoparticles for improved catalysts on direct synthesis of hydrogen peroxide generation. Korean J. Chem. Eng. 2019, 36, 1417–1420. [Google Scholar] [CrossRef]
- Lee, S.; Chung, Y.-M. Direct synthesis of H2O2 over acid-treated Pd/C catalyst derived from a Pd-Co core-shell structure. Catal. Today 2020, 352, 270–278. [Google Scholar] [CrossRef]
- Tian, P.; Xu, X.; Ao, C.; Ding, D.; Li, W.; Si, R.; Tu, W.; Xu, J.; Han, Y.-F. Direct and Selective Synthesis of Hydrogen Peroxide over Palladium-Tellurium Catalysts at Ambient Pressure. ChemSusChem 2017, 10, 3342–3346. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, F.; Signoretto, M.; Manzoli, M.; Boccuzzi, F.; Cruciani, G.; Pinna, F.; Strukul, G. Influence of the preparation method on the morphological and composition properties of Pd-Au/ZrO2 catalysts and their effect on the direct synthesis of hydrogen peroxide from hydrogen and oxygen. J. Catal. 2009, 268, 122–130. [Google Scholar] [CrossRef]
- Wilson, N.M.; Priyadarshini, P.; Kunz, S.; Flaherty, D.W. Direct synthesis of H2O2 on Pd and AuxPd1 clusters: Understanding the effects of alloying Pd with Au. J. Catal. 2018, 357, 163–175. [Google Scholar] [CrossRef]
- Freakley, S.J.; He, Q.; Harrhy, J.H.; Lu, L.; Crole, D.A.; Morgan, D.J.; Ntainjua, E.N.; Edwards, J.K.; Carley, A.F.; Borisevich, A.Y.; et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Yamada, H.; Matsui, S.; Echigo, S.; Shishida, K. Comparison among the Methods for Hydrogen Peroxide Measurements To Evaluate Advanced Oxidation Processes: Application of a Spectrophotometric Method Using Copper(II) Ion and 2,9-Dimethyl-1,10-phenanthroline. Environ. Sci. Technol. 1998, 32, 3821–3824. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).