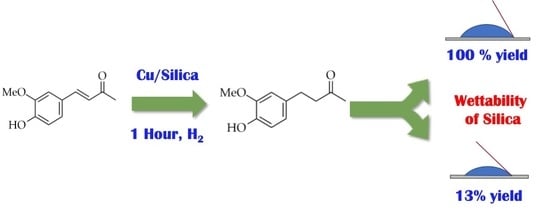
The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts
Abstract
1. Introduction
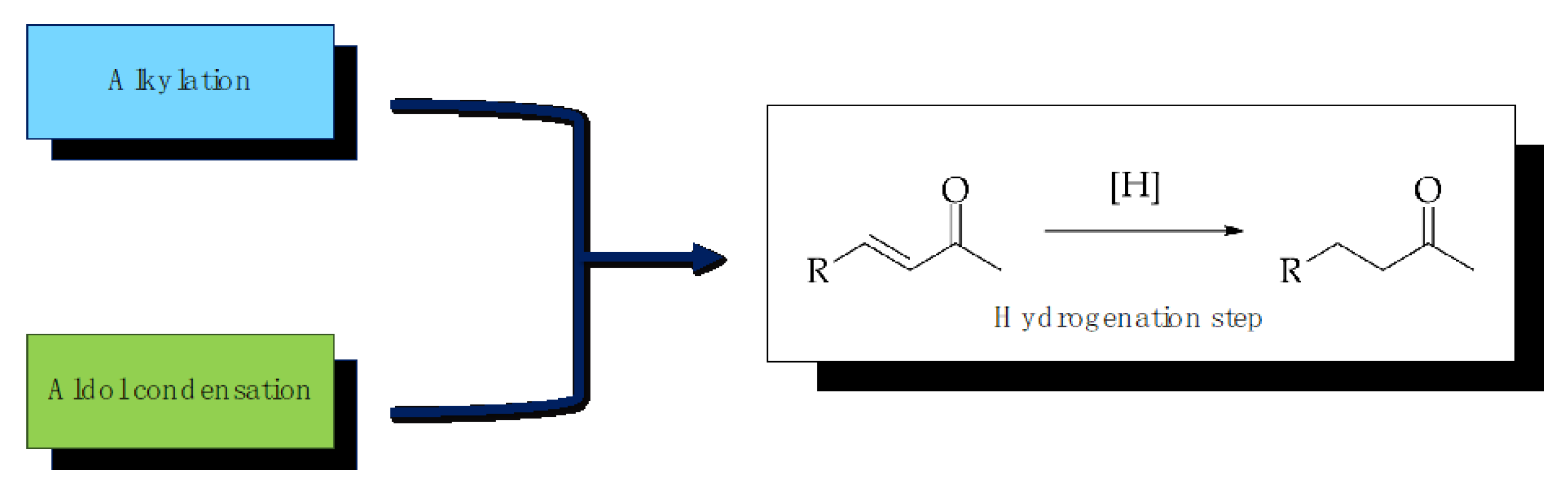
2. Results and Discussion
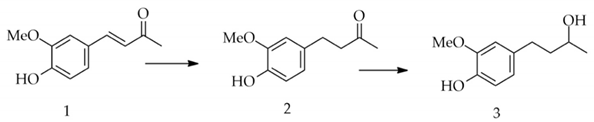
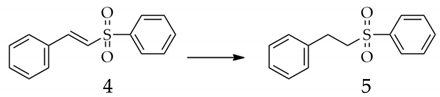
3. Materials and Methods
3.1. Materials
3.2. TGA Analysis
3.3. TEM Analysis
3.4. Conctact Angle Measurements
3.5. Hydrogenation Reactions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patai, S.; Rappoport, Z. The Chemistry of Enones; John Wiley & Sons Ltd.: Chichester, UK, 1986. [Google Scholar]
- Rinaldi, R. Catalytic Hydrogenation for Biomass Valorization; RSC: Cambridge, UK, 2014. [Google Scholar]
- Fitch, J.R.; Aslam, M.; Rios, D.E.; Smith, J.C. Use of 4-Substituted 2-Butanones to Prepare Nabumetone. Patent EP0892775A2, 27 January 1997. [Google Scholar]
- Mody, P.A.; Motivala, J.K.; Mehta, C.D. A Novel Process for the Manufacture of 4-(6-Methoxy-2-naphthyl)buten-2-one and Its Pharmaceutical Composition, from a Novel Source. Indian Patent 178589, 1997. [Google Scholar]
- Ramachandran, V.; Belmont, S. Production of Naphthyl-Substituted Ketones from Naphthaldehydes. Patent US5861538A, 8 December 1998. [Google Scholar]
- Cabri, W.; Magrone, D.; Oldani, E.; Angelici, R. U.S. Process for the Preparation of 4-(6′-methoxy-2′-naphthyl) Butan-2-One. U.S. Patent 5955635, 21 September 1999. [Google Scholar]
- Piccolo, O.; Verrazzani, A. Preparation and Use of a Heterogeneous Rhodium Catalyst for the Hydrogenation of a Double Bond of an α-β-Unsaturated Carbonyl Compound. U.S. Patent US7087548B2, 8 August 2006. [Google Scholar]
- Viviano, M.; Glasnov, T.N.; Reichart, B.; Tekautz, G.; Kappe, C.O. A scalable two-step continuous flow synthesis of nabumetone and related 4-aryl-2-butanones. Org. Process Res. Dev. 2011, 15, 858–870. [Google Scholar] [CrossRef]
- Evangelisti, C.; Panziera, N.; Vitulli, M.; Pertici, P.; Balzano, F.; Uccello-Barretta, G.; Salvadori, P. Supported rhodium nanoparticles obtained by Metal Vapour Synthesis as catalysts in the preparation of valuable organic compounds. Appl. Catal. A Gen. 2008, 339, 84–92. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Petrucci, G.; Armelao, L.; Oberhauser, W.; Frediani, M.; Piccolo, O.; Rathod, V.D.; Paganelli, S. An easily recoverable and recyclable homogeneous polyester-based Pd catalytic system for the hydrogenation of α,β-unsaturated carbonyl compounds. Catal. Commun. 2015, 69, 228–233. [Google Scholar] [CrossRef]
- Malkar, R.S.; Yadav, G.D. Selectivity Engineering in One Pot Synthesis of Raspberry Ketone: Crossed Aldol Condensation of p-Hydroxybenzaldehyde and Acetone and Hydrogenation over Novel Ni/Zn-La Mixed Oxide. ChemistrySelect 2019, 4, 2140–2152. [Google Scholar] [CrossRef]
- Mehanna, E.T.; Barakat, B.M.; ElSayed, M.H.; Tawfik, M.K. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: Effect on adipose tissue expression of adipocytokines and Aquaporin 7. Eur. J. Pharmacol. 2018, 832, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Huang, L.J.; Wu, S.L.; Kuo, S.C.; Ho, T.Y.; Hsiang, C.Y. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J. Agric. Food Chem. 2007, 55, 8390–8397. [Google Scholar] [CrossRef]
- Bandarenko, M.; Kovalenko, V. Synthesis of raspberry and ginger ketones by nickel boride-catalyzed hydrogenation of 4-arylbut-3-en-2-ones. Zeitschrift fur Naturforsch. Sect. B J. Chem. Sci. 2014, 69, 885–888. [Google Scholar] [CrossRef]
- Smith, L.R. Rheosmin (Raspberry Ketone) and Zingerone, and Their Preparation by Crossed Aldol-Catalytic Hydrogenation Sequences. Chem. Educ. 1996, 1, 1–18. [Google Scholar] [CrossRef]
- Masuda, H.; Mihara, S. Production of Dihydro-β-ionol. Japanese Patent 61,036239, 1986. [Google Scholar]
- Chapuis, C.; Jacoby, D. Catalysis in the preparation of fragrances and flavours. Appl. Catal. A General 2001, 221, 93. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Chin, Y.W.; Pan, L.; Jia, Z. Natural products as sweeteners and sweetness modifiers. Compr. Nat. Prod. II Chem. Biol. 2010, 3, 269–315. [Google Scholar]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull. 2003, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, A.; Jiménez, I.A.; Mamani, Z.A.; Bazzocchi, I.L.; Piñero, J.E.; Ravelo, A.G.; Valladares, B. Antileishmanial activities of dihydrochalcones from piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg. Med. Chem. 2003, 11, 3975–3980. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Wang, X.; Bao, K.; Lu, G.; Lin, J. Ultrasound Assisted Selective Reduction of Chalcones to Dihydrochalcones by Zn/HOAc. Lett. Org. Chem. 2008, 5, 370–373. [Google Scholar] [CrossRef]
- Jesus, A.R.; Marques, A.P.; Rauter, A.P. An easy approach to dihydrochalcones via chalcone in situ hydrogenation. Pure Appl. Chem. 2016, 88, 349–361. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Chemoselective hydrogenation method catalyzed by Pd/C using diphenylsulfide as a reasonable catalyst poison. Tetrahedron 2006, 62, 11925–11932. [Google Scholar] [CrossRef]
- Cho, I.S.; Alper, H. Palladium Catalyzed Hydrogenation of α,β-Unsaturated Sulfones and Phosphonates. J. Org. Chem. 1994, 59, 4027–4028. [Google Scholar] [CrossRef]
- Ranu, B.C.; Guchhait, S.K.; Ghosh, K. Chemoselective Hydrogenation of α,β-Unsaturated Sulfones and Phosphonates via Palladium-Assisted Hydrogen Transfer by Ammonium Formate. J. Org. Chem. 1998, 63, 5250–5251. [Google Scholar] [CrossRef]
- Serafini, S.; Castellin, A.; Dal Santo, C. Synthesis of 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5[2-(phenylsulfonyl)ethyl]-1H-Indole. U.S. Patent US8426612B2, 23 April 2013. [Google Scholar]
- Ravasio, N.; Zaccheria, F.; Allegrini, P.; Ercoli, M. Selective hydrogenation of 4-(6-methoxy-2-naphtyl)-3-buten-2-one to Nabumetone®. Catal. Today 2007, 121, 2–5. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/ (accessed on 6 May 2020).
- Agency, E.M. ICH Guideline Q3D on Elemental Impurities. 2016, 44, p. 84. Available online: www.ema.europa.eu/contact (accessed on 6 May 2020).
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the properties of supported copper oxide: Can the particle size induce acidic behaviour? Dalt. Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the role of low coordination sites in a cu metal nanoparticle: A step toward the selective synthesis of second generation biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Zaccheria, F.; Psaro, R.; Ravasio, N. Selective hydrogenation of alternative oils: A useful tool for the production of biofuels. Green Chem. 2009, 11, 462–465. [Google Scholar] [CrossRef]
- Zaccheria, F.; Ravasio, N.; Psaro, R.; Fusi, A. Heterogeneous selective catalytic hydrogenation of aryl ketones to alcohols without additives. Tetrahedron Lett. 2005, 46, 3695–3697. [Google Scholar] [CrossRef]
- Augustine, R.L. Heterogeneous Catalysis for Synthetic Chemist; Marcel Dekker: New York, NY, USA, 1996; ISBN 0824790219. [Google Scholar]
- Liu, H.; Li, Z.; Li, Y. Chemoselective hydrogenation of cinnamaldehyde over a Pt-Lewis acid collaborative catalyst under ambient conditions. Ind. Eng. Chem. Res. 2015, 54, 1487–1497. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Kalidindi, S.B. Synergistic Hydrogenation over Palladium through the Assembly of MIL-101(Fe) MOF over Palladium Nanocubes. Chem. A Eur. J. 2017, 23, 16456–16459. [Google Scholar] [CrossRef]
- Zaccheria, F.; Santoro, F.; Psaro, R.; Ravasio, N. Heterogeneous: Copper catalysts for synthetic purposes Building new simple bifunctional materials. Chim. Oggi 2011, 29, 22–25. [Google Scholar]
- Zaccheria, F.; Ravasio, N.; Fusi, A.; Rodondi, M.; Psaro, R. Tuning selectivity in terpene chemistry: Selective hydrogenation versus cascade reactions over copper catalysts. Adv. Synth. Catal. 2005, 34, 1267–1272. [Google Scholar] [CrossRef]
- Santoro, F.; Psaro, R.; Ravasio, N.; Zaccheria, F. Reductive Amination of Ketones or Amination of Alcohols over Heterogeneous Cu Catalysts: Matching the Catalyst Support with the N-Alkylating Agent. ChemCatChem 2012, 4, 1249–1254. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Scotti, N.; Cappelletti, G.; Venezia, A.M.; Zaccheria, F. The role of support wettability in the hydrogenation of γ–valerolactone over Cu/SiO2. Catal. Today 2020. in prep. [Google Scholar]
- Ringwald, S.C.; Pemberton, J.E. Adsorption interactions of aromatics and heteroaromatics with hydrated and dehydrated silica surfaces by raman and FTIR spectroscopies. Environ. Sci. Technol. 2000, 34, 259–265. [Google Scholar] [CrossRef]
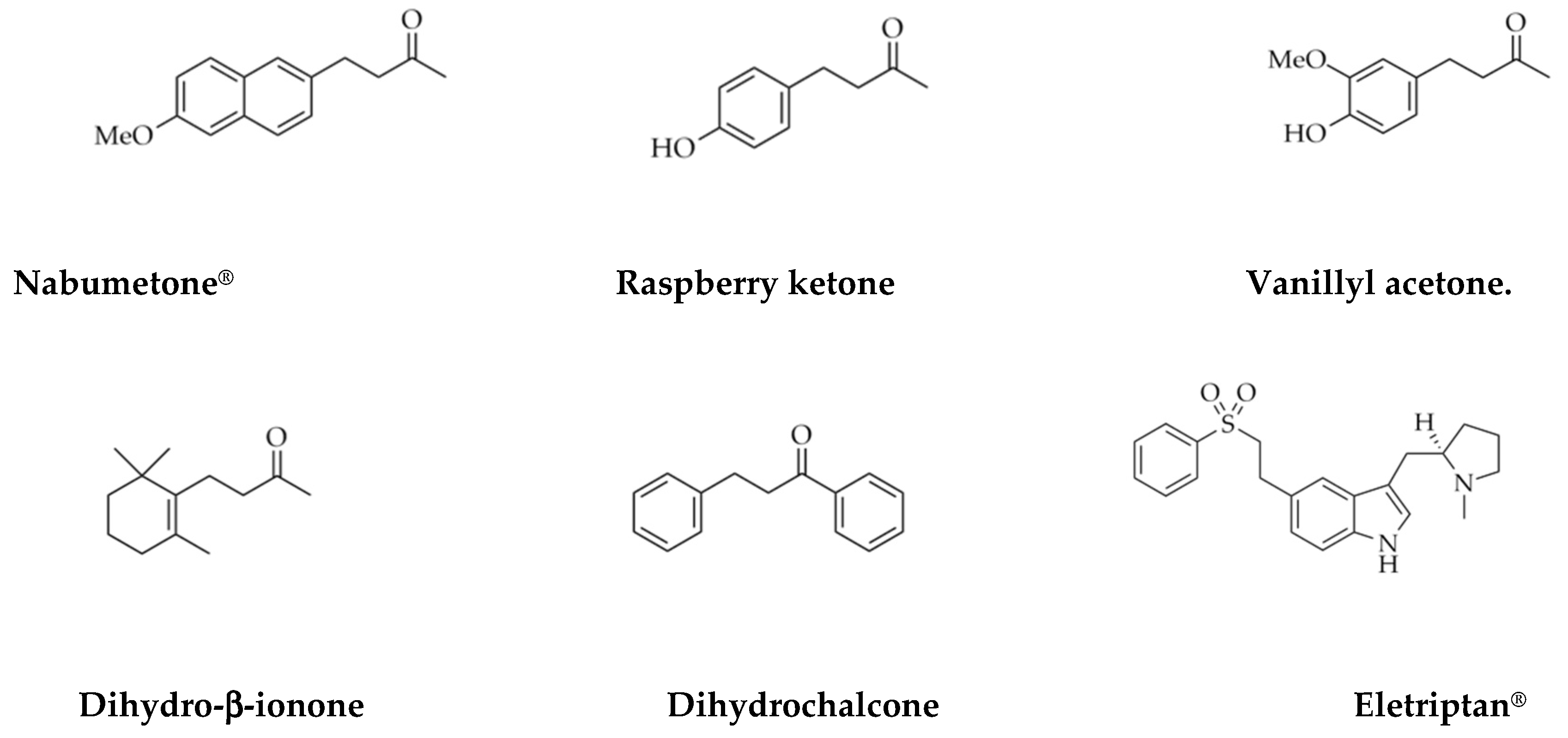
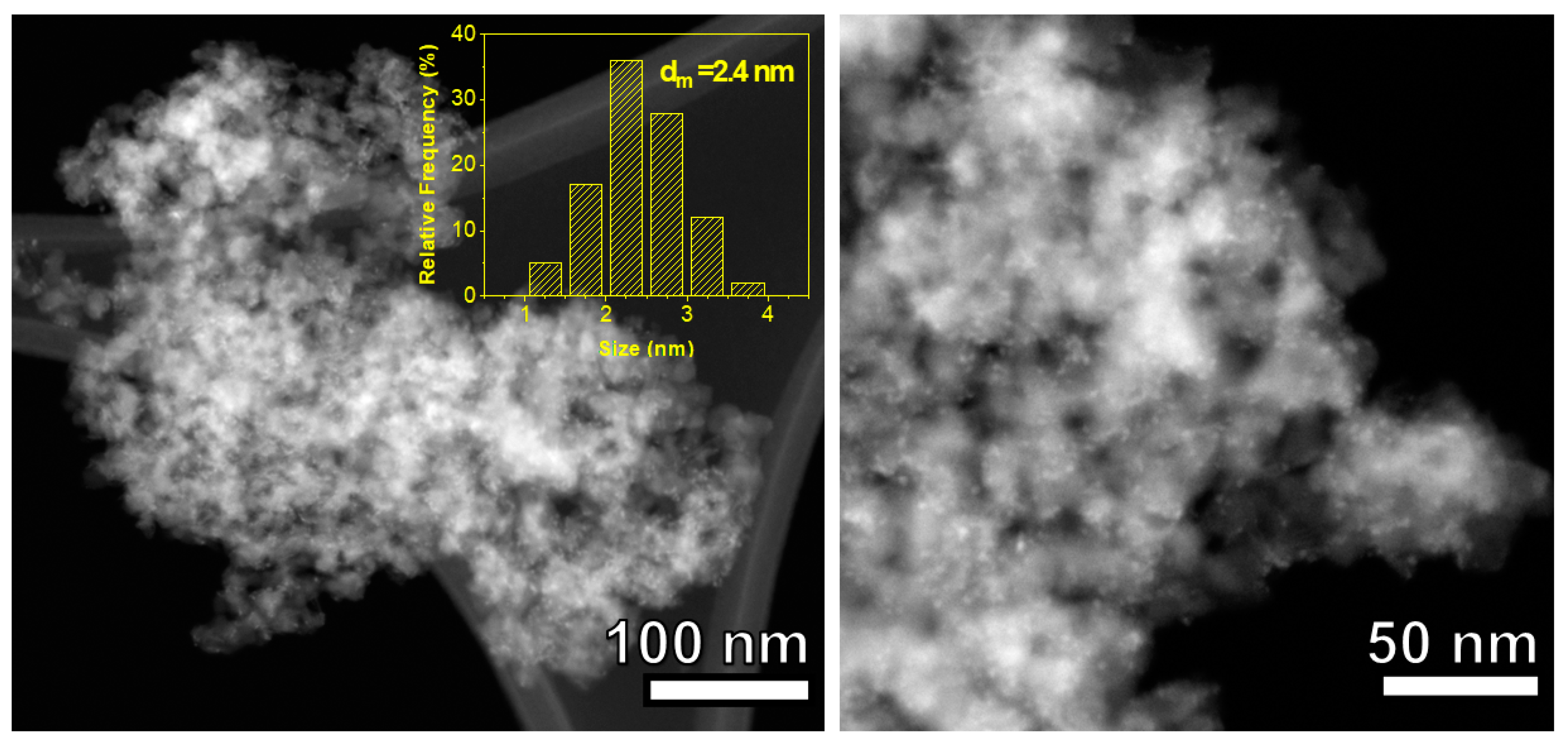
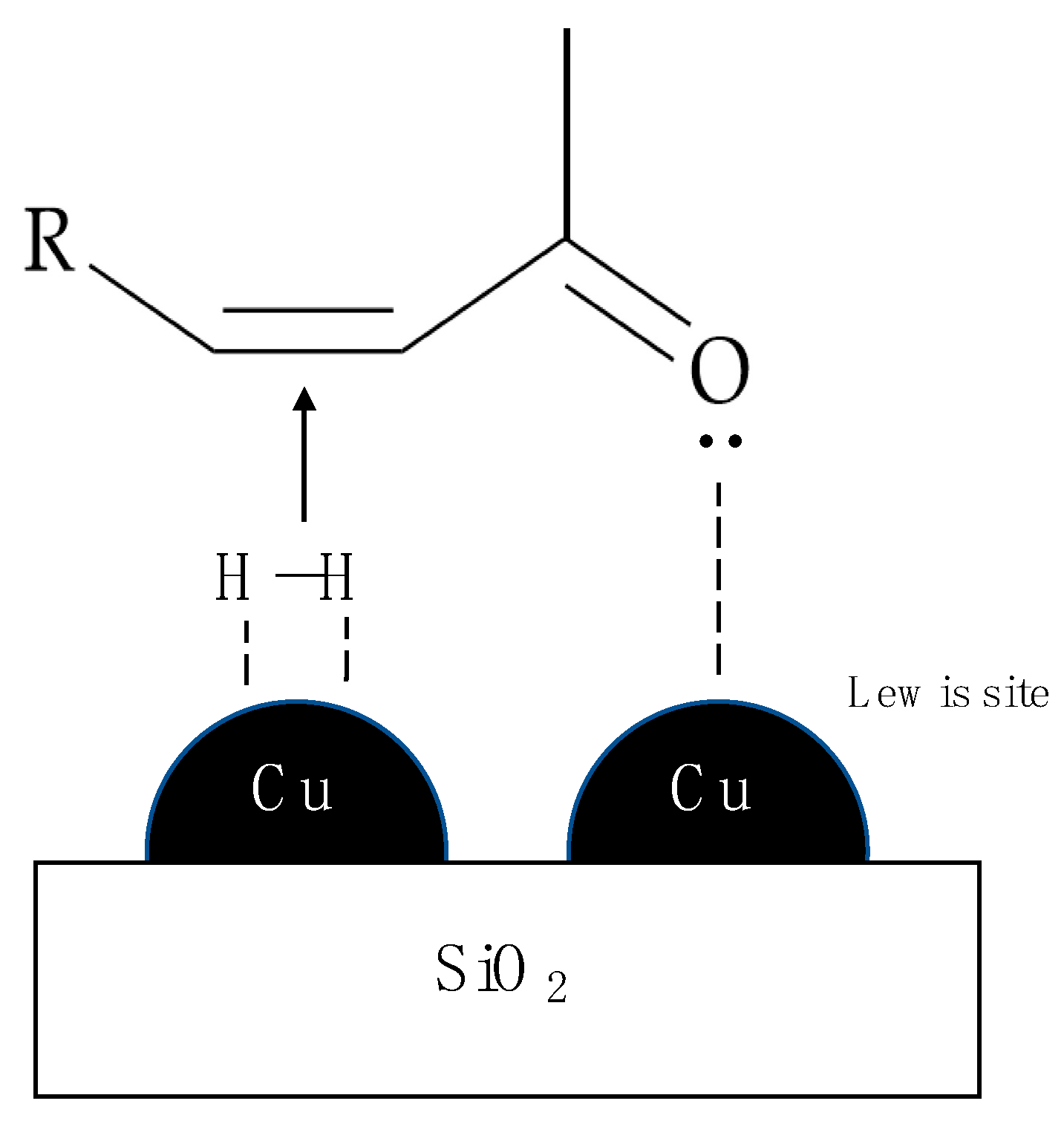
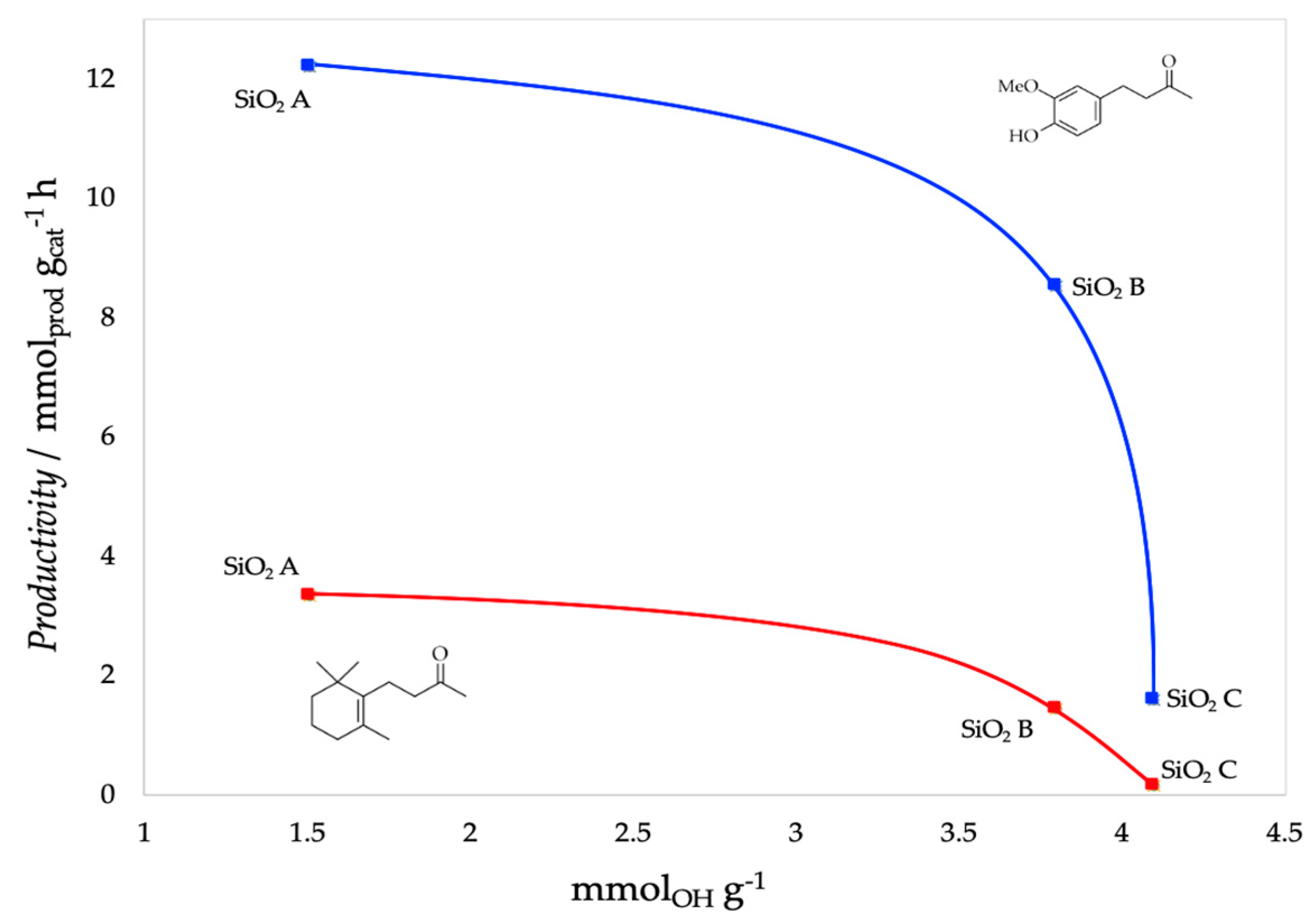
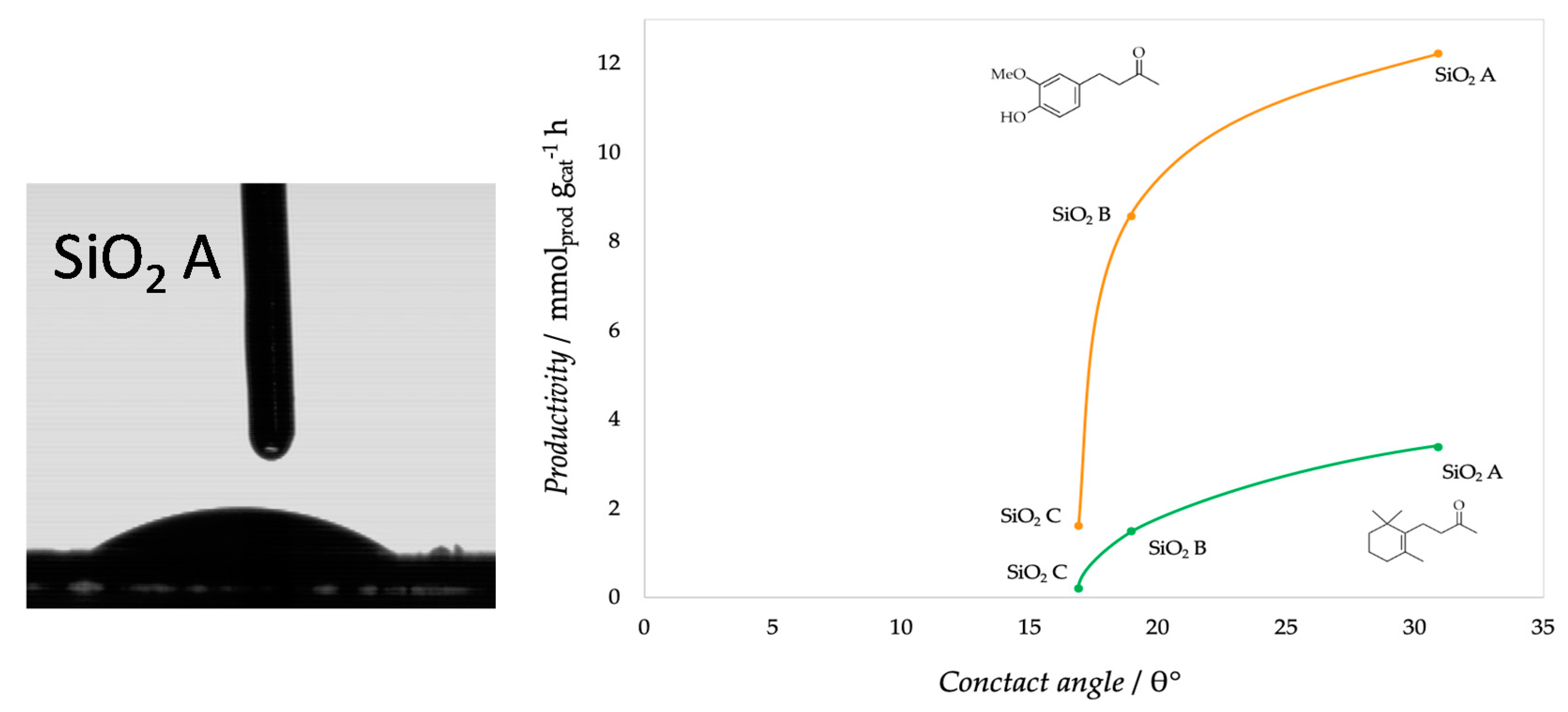
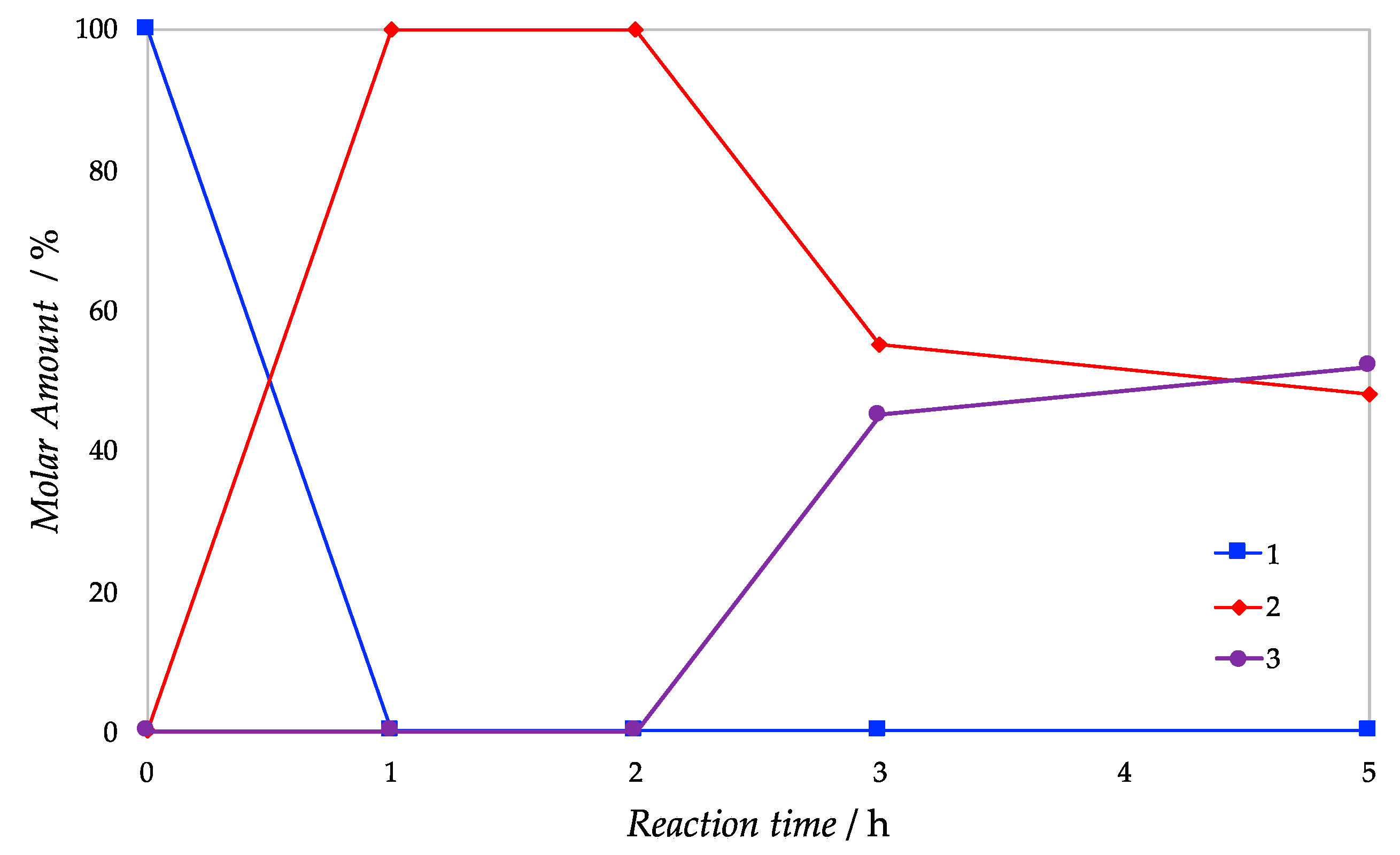
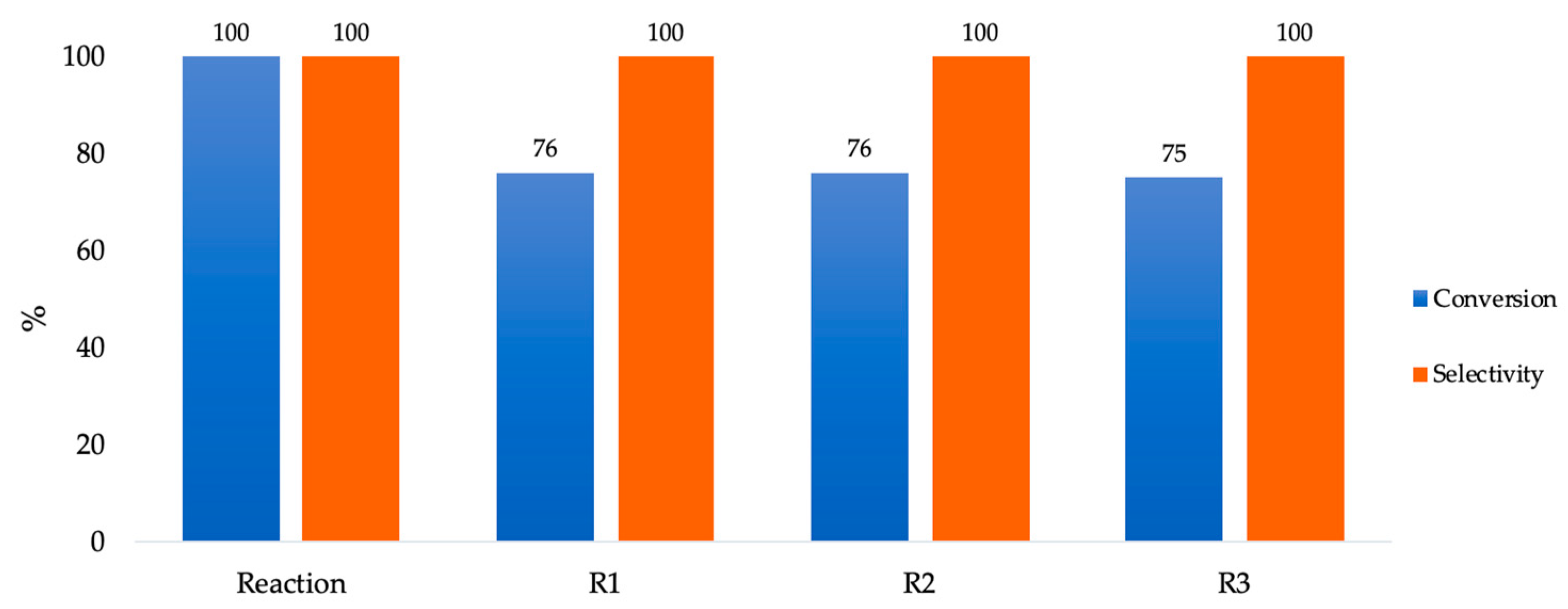
| Support | SSA (m2 g−1) | PV (mL g−1) | mmolOH gcat−1 | Contact Angle |
|---|---|---|---|---|
| SiO2 A | 380 | - | 1.51 | 31° |
| SiO2 B | 460 | 0.74 | 3.8 | 19° |
| SiO2 C | 693 | 0.62 | 4.1 | 17° |
| Substrate | Product | Entry | Catalyst | t (h) | Conv (%) | Sel (%) |
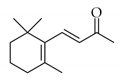 | 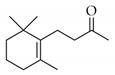 | 1 | Cu/SiO2 A | 1 | 46 | 90 |
| 3 | 97 | 100 | ||||
| 2 | Cu/SiO2 A (IW) | 6 | 0 | - | ||
| 3 | Cu/SiO2 B | 1 | 19 | 79 | ||
| 6 | 94 | 90 | ||||
| 4 | Cu/SiO2 B (IW) | 6 | 0 | - | ||
| 5 | Cu/SiO2 C | 1 | 0 | - | ||
| 5 | 10 | 100 | ||||
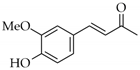 | 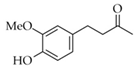 | 6 | Cu/SiO2 A | 1 | 100 | 100 |
| 7 | Cu/SiO2 B | 1 | 70 | 100 | ||
| 2 | 100 | 100 | ||||
| 8 | Cu/SiO2 C | 1 | 13 | 100 | ||
| 5 | 75 | 100 | ||||
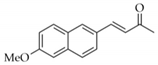 | 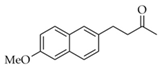 | 9 | Cu/SiO2 A | 1 | 91 | 100 |
| 10 | Cu/SiO2 B | 6 | 35 | 100 | ||
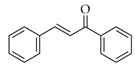 | 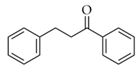 | 11 | Cu/SiO2 A | 2 | 100 | 100 |
| 12 | Cu/SiO2 B | 2 | 100 | 100 | ||
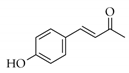 | 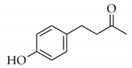 | 13 | Cu/SiO2 A | 3 | 100 | 100 |
| 14 | Cu/SiO2 B | 5 | 100 | 100 | ||
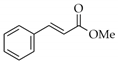 | 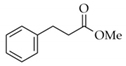 | 15 | Cu/SiO2 A | 2 | 93 | 100 |
| 16 | Cu/SiO2 B | 3 | 90 | 100 | ||
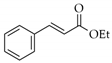 | 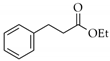 | 17 | Cu/SiO2 A | 3 | 93 | 100 |
| 18 | Cu/SiO2 B | 5 | 79 | 100 |
| Catalyst | T (°C) | t (h) | Conv (%) | Sel 5 (%) |
|---|---|---|---|---|
| Cu/SiO2 B | 90 | 1 | 35 | 98 |
| 2 | 55 | 98 | ||
| 4 | 78 | 98 | ||
| 7 | 98 | 99 | ||
| 24 | >99 | 98 | ||
| Cu/SiO2 A | 90 | 1 | 66 | 98 |
| 2 | 94 | 98 | ||
| 4 | >99 | 98 | ||
| Cu/SiO2 A | 70 | 2 | 19 | 99 |
| 4 | 31 | 99 | ||
| 24 | 92 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavuoto, D.; Zaccheria, F.; Marelli, M.; Evangelisti, C.; Piccolo, O.; Ravasio, N. The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts. Catalysts 2020, 10, 515. https://doi.org/10.3390/catal10050515
Cavuoto D, Zaccheria F, Marelli M, Evangelisti C, Piccolo O, Ravasio N. The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts. Catalysts. 2020; 10(5):515. https://doi.org/10.3390/catal10050515
Chicago/Turabian StyleCavuoto, Denise, Federica Zaccheria, Marcello Marelli, Claudio Evangelisti, Oreste Piccolo, and Nicoletta Ravasio. 2020. "The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts" Catalysts 10, no. 5: 515. https://doi.org/10.3390/catal10050515
APA StyleCavuoto, D., Zaccheria, F., Marelli, M., Evangelisti, C., Piccolo, O., & Ravasio, N. (2020). The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts. Catalysts, 10(5), 515. https://doi.org/10.3390/catal10050515