Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation
Abstract
1. Introduction
2. Results and Discussion
2.1. The Design, Preparation, and Structure of the Catalysts
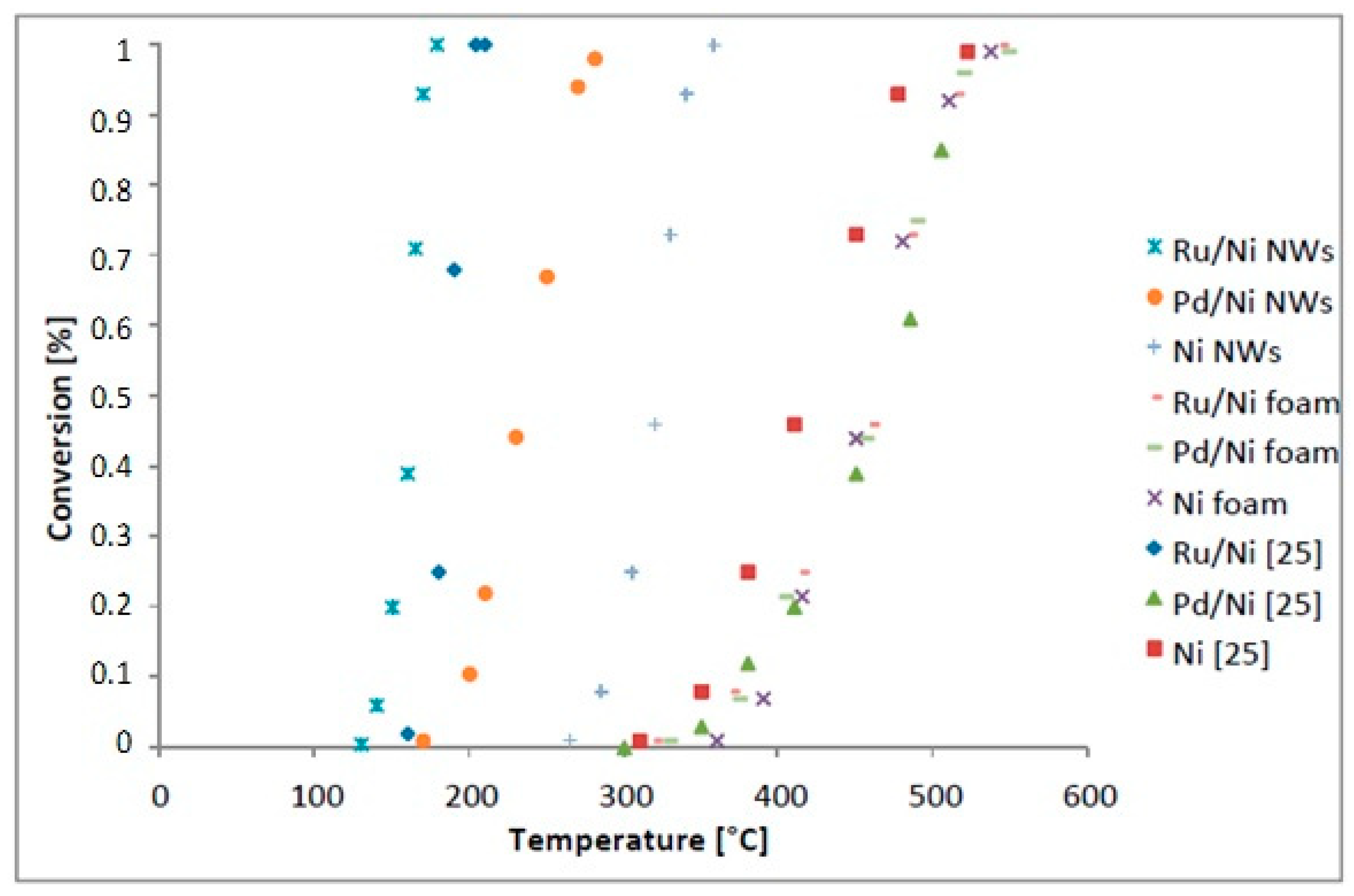
2.2. Methanation of CO2
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Nickel Nanowires
3.3. Preparation of the Catalyst
3.3.1. Preparation of 1.0% Pd or Ru NPs on the Sol-Gel Silica Carrier
3.3.2. Preparation of the Bimetallic Pd/Ni and Ru/Ni Catalysts
3.4. Methods of Catalyst Characterization
3.5. Methanation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Proceedings of the 8th international conference on carbon dioxide utilization. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábalc, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energ. Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Müller, K.; Städter, M.; Rachow, F.; Hoffmannbeck, D.; Schmeiβer, D. Sabatier- based CO2-Methanation by catalytic conversion. Environ. Earth Sci. 2013, 70, 3771–3778. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, W.; Liu, Y.; Hu, M.; Zhao, K. Synthesis of high-aspect-ratio nickel nanowires by dropping method. Nanoscale Res. Lett. 2016, 11, 118–122. [Google Scholar] [CrossRef]
- Ma, S.; Tan, Y.; Han, Y. Methanation of syngas over coral reef-like Ni/Al2O3 catalysts. J. Nat. Gas Chem. 2011, 20, 435–440. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Seemann, M.C.; Moergeli, R.; Biollaz, S.M.A.; Schildhauer, T.J. Synthetic natural gas from wood: Reactions of ethylene in fluidised bed methanation. Appl. Catal. A 2013, 462–463, 150–156. [Google Scholar] [CrossRef]
- Kiendl, I.; Klemm, M.; Clemens, A.; Herrman, A. Dilute gas methanation of synthesis gas from biomass gasification. Fuel 2014, 123, 211–217. [Google Scholar] [CrossRef]
- Park, J.N.; McFarland, E.W. A highly dispersed Pd-Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Hu, C.W.; Yao, J.; Yang, H.Q.; Chen, Y.; Tian, A.M. On the Inhomogeneity of Low Nickel Loading Methanation Catalyst. J. Catal. 1997, 166, 1–7. [Google Scholar] [CrossRef]
- Choe, S.J.; Kang, H.J.; Kim, S.J.; Park, S.B.; Park, D.H.; Huh, D.S. Adsorbed carbon formation and carbon hydrogenation for CO2 methanation on the Ni(111) surface: ASED-MO study. Bull. Korean Chem. Soc. 2005, 26, 1682–1688. [Google Scholar] [CrossRef]
- Agnelli, M.; Kolb, M.; Mirodatos, C. CO Hydrogenation on a nickel catalyst.: 1. Kinetics and modeling of a low-temperature sintering process. J. Catal. 1994, 148, 9–21. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Norskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni-Fe catalysts: From computational studies towards catalyst optymalization. Appl. Catal. A. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Lunde, P.J.; Kester, F.L. Rates of methane formation from carbon dioxide and hydrogen over a ruthenium catalyst. J. Catal. 1973, 30, 423–429. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Somorjai, G.A. Hydrogenation of CO and CO2 over iron foils: Correlations of rate, product distribution, and surface composition. J. Catal. 1978, 52, 291–301. [Google Scholar] [CrossRef][Green Version]
- Solymosi, F.; Erdöhelyi, A.; Kocsis, M. Methanation of CO2 on supported Ru catalysts. J. Chem. Soc. Faraday Trans. 1981, 77, 1003–1012. [Google Scholar] [CrossRef]
- Zaǧli, E.; Falconer, J.L. Carbon dioxide adsorption and methanation on ruthenium. J. Catal. 1981, 69, 1–8. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on group VIII metals: II. Kinetics and mechanism of CO2 hydrogenation on nickel. J. Catal. 1982, 77, 460–472. [Google Scholar] [CrossRef]
- Erdöhelyi, A.; Pasztor, M.; Solymosi, F. Catalytic hydrogenation of CO2 over supported palladium. J. Catal. 1986, 98, 166–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Kladi, A.; Verykios, X.E. Effects of carrier doping on kinetic parameters of CO2 hydrogenation on supported rhodium catalysts. J. Catal. 1994, 148, 737–747. [Google Scholar] [CrossRef]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering—Perfecting imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Moulijn, J. Mitigation of CO2 by chemical conversion: Plausible chemical reactions and promising products. Energy Fuels 1996, 10, 305–325. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Maa, J. Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by polygonal barrel-sputtering method. Energy Environ. Sci. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Polanski, J.; Siudyga, T.; Bartczak, P.; Kapkowski, M.; Ambrożkiewicz, W.; Nobis, A.; Sitko, R.; Klimontko, J.; Szade, J.; Lelątko, J. Oxide passivated Ni-supported Ru nanoparticles in silica: A new catalyst for low-temperature carbon dioxide methanation. Appl. Catal. B 2017, 206, 16–23. [Google Scholar] [CrossRef]
- Powell, C.J. Recommended Auger parameters for 42 elemental solids. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 1–3. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Nanostructured nickel-cobalt-titanium alloy grown on titanium substrate as efficient electrocatalyst for alkaline water electrolysis. ACS Appl. Mater. Interfaces 2017, 9, 12416–12426. [Google Scholar] [CrossRef]
- Dickinson, T.; Povey, A.F.; Sherwood, P.M.A. Dissolution and passivation of nickel. An X-ray photoelectron spectroscopic study. J. Chem. Soc. Faraday Trans. I 1977, 73, 327–343. [Google Scholar] [CrossRef]
- Légaré, P.P.; Finck, F.; Roche, R.; Maire, G. XPS investigation of the oxidation of the Al/Pd interface: The Al2O3/Pd interface. Surf. Sci. 1989, 217, 167–178. [Google Scholar] [CrossRef]
- Tressaud, A.; Khairoun, S.; Touhara, H.; Watanabe, N. X-Ray photoelectron spectroscopy of palladium fluorides. J. Anorg. Allg. Chem. 1986, 540, 291–299. [Google Scholar] [CrossRef]
- Kötz, R.; Lewrenz, H.J.; Stucki, S. XPS studies of oxygen evolution on Ru and RuO2 anodes. J. Electrochem. Soc. 1983, 130, 825–829. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak, P.; Ambrozkiewicz, W.; Sitko, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Lelątko, J. Ni-Supported Pd nanoparticles with Ca promoter: A new catalyst for low-temperature ammonia cracking. PLoS ONE 2015, 10, e0136805. [Google Scholar] [CrossRef] [PubMed]
- Stoch, J.; Dao, H.Q.; Szeppe, T. An ESCA study of supported ruthenium oxides catalyst. Bull. Polish. Acad. Sci. Chem. 1987, 35, 387–395. [Google Scholar]
- Shen, J.Y.; Adnot, A.; Kaliaguine, S. An ESCA study of the interaction of oxygen with the surface of ruthenium. Appl. Surf. Sci. 1991, 51, 47–60. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni–Ru/γ-Al2O3 via modulating impregnation sequence and controlling surface active species. RSC Adv. 2014, 4, 16472–16479. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/Al2O3 catalyst at low temperature: “In Situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Pachecka, M.; Sturm, J.M.; Lee, C.J.; Bijkerk, F. Adsorption and dissociation of CO2 on Ru(0001). J. Phys. Chem. C 2017, 121, 6729–6735. [Google Scholar] [CrossRef]
- Rao, K.S. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Okudera, H.; Hozumi, A. The formation and growth mechanisms of silica thin film and spherical particles through the Stöber process. Thin Solid Film. 2003, 434, 62–68. [Google Scholar] [CrossRef]
- Kapkowski, M.; Ambrożkiewicz, W.; Siudyga, T.; Sitko, R.; Szade, J.; Klimontko, J.; Balin, K.; Lelątko, J.; Polanski, J. Nano silica and molybdenum supported Re, Rh, Ru or Ir nanoparticles for selective solvent-free glycerol conversion to cyclic acetals with propanone and butanone under mild conditions. Appl. Catal. B 2017, 202, 335–345. [Google Scholar] [CrossRef]

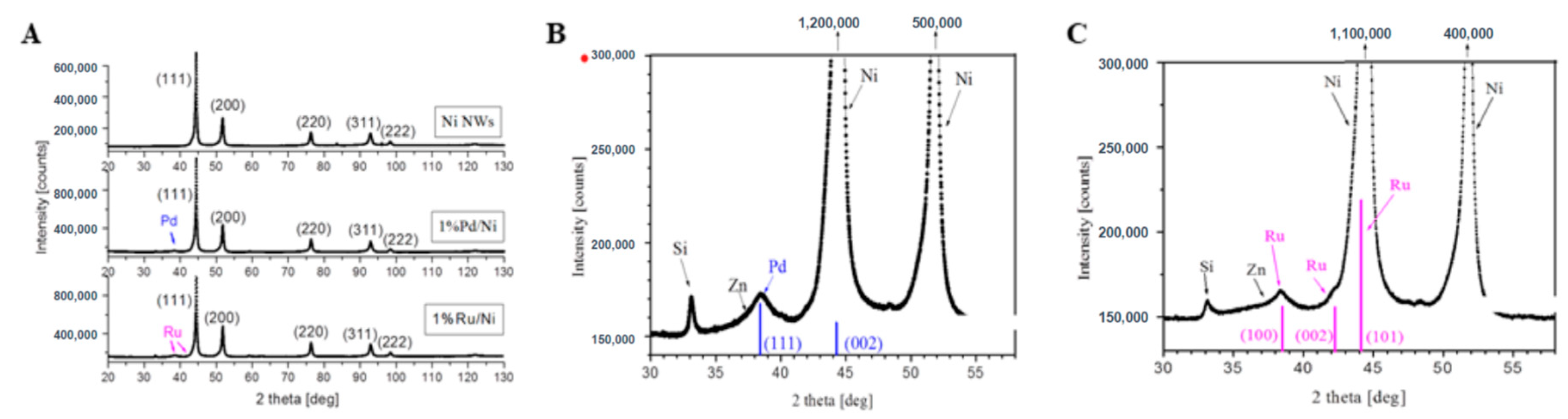
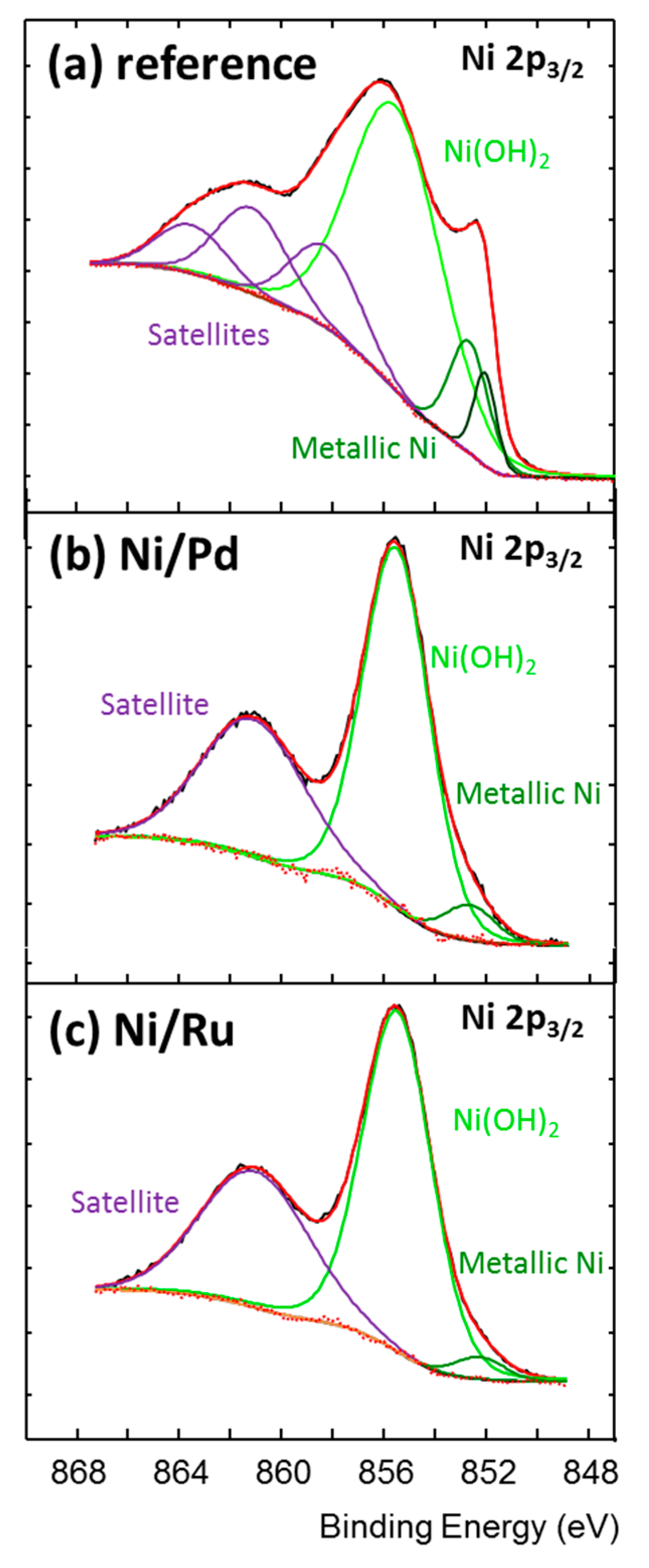
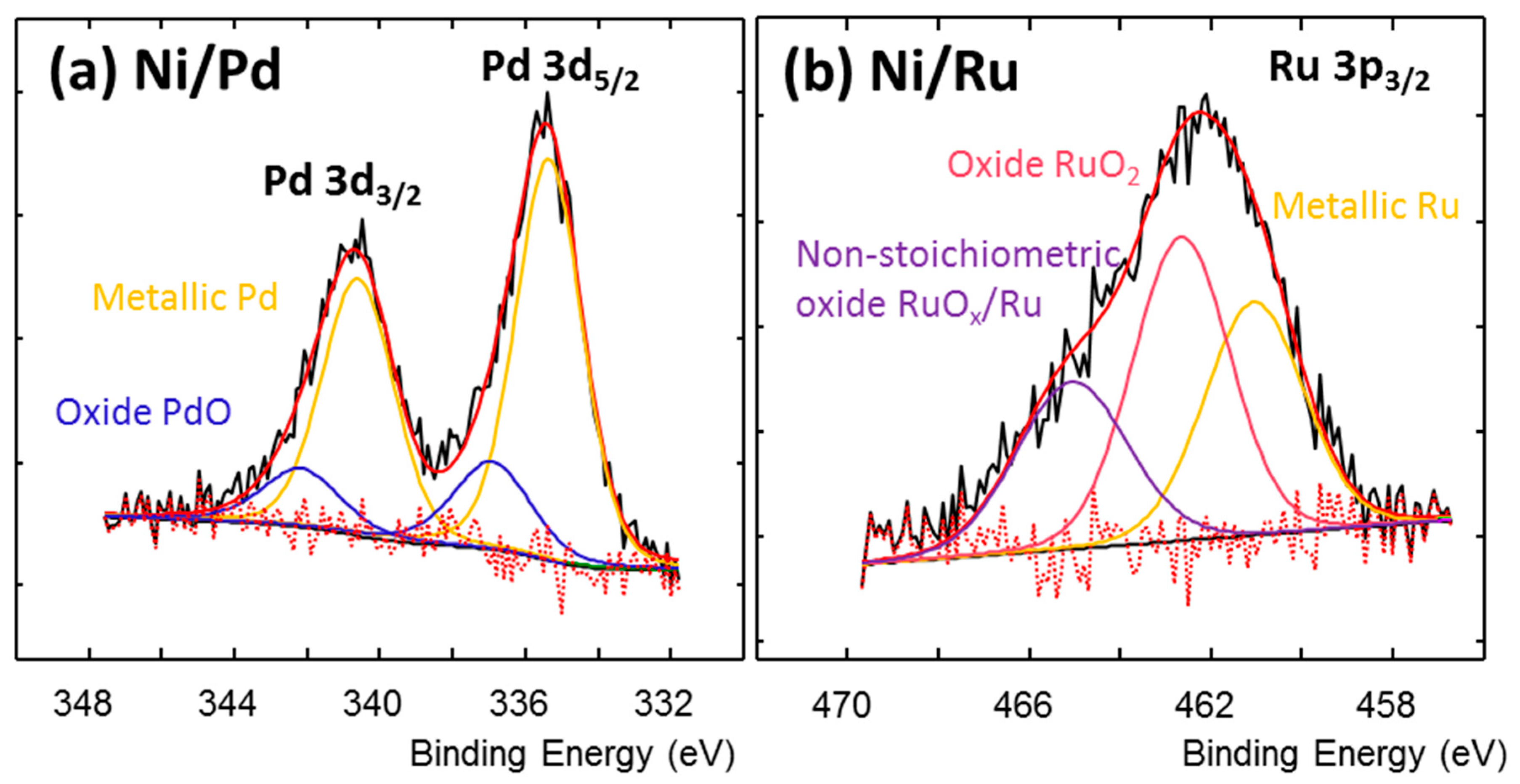
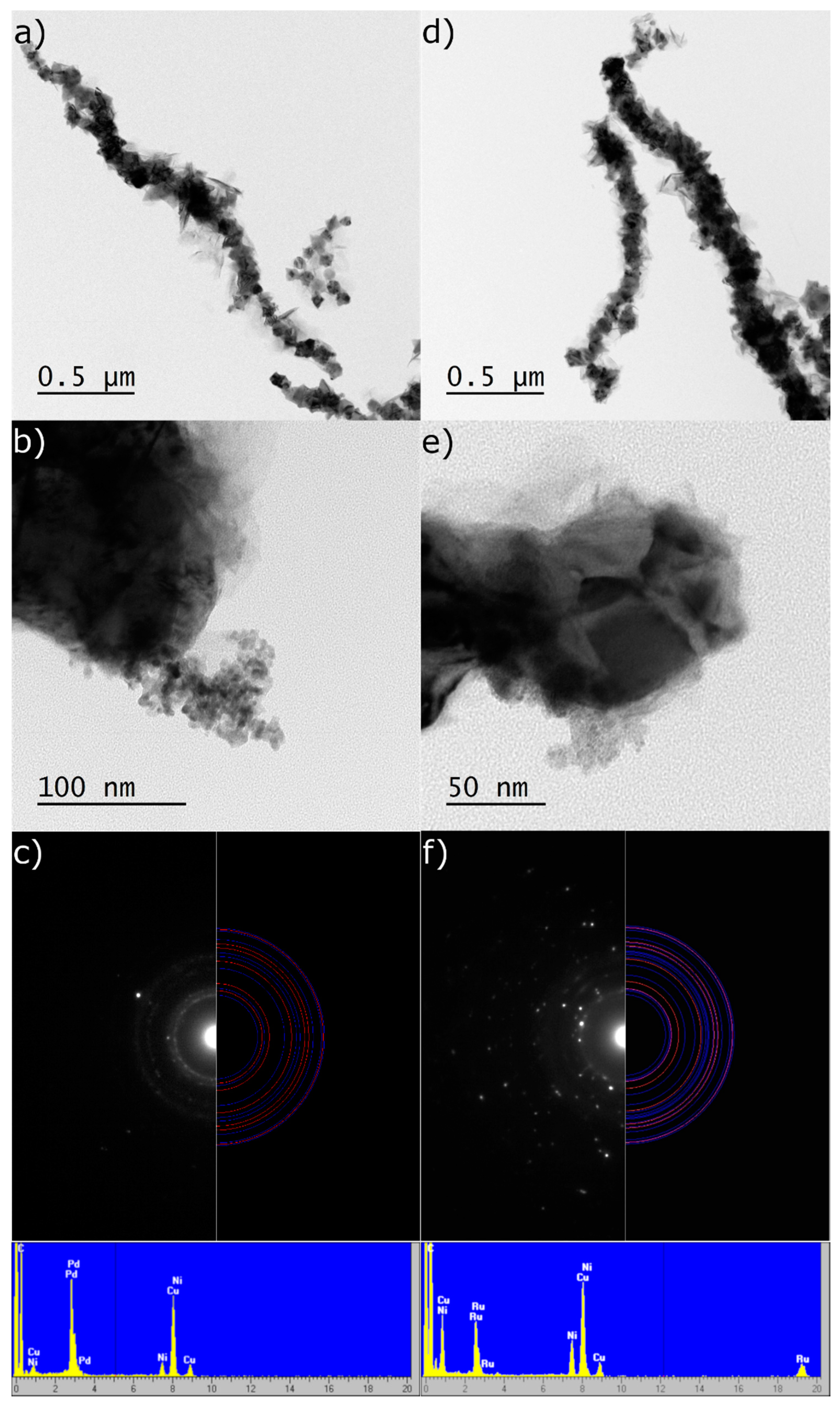
| Catalyst | Chemical Element [wt.%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pd | Ru | Zn | Fe | Ni | Si | Ca | P | Cl | |
| SiO2 | - | - | N\D | N\D | N\D | N\D | N\D | N\D | N\D |
| 1.0% Pd/SiO2 | 1.14 ± 0.035 | - | N\D | N\D | N\D | N\D | N\D | N\D | N\D |
| 1.0% Ru/SiO2 | - | 1.12 ± 0.023 | N\D | N\D | N\D | N\D | N\D | N\D | N\D |
| Ni a | - | - | 2.2 ± 0.10 | 0.21 ± 0.015 | 96 ± 3.9 | - | - | 0.34 ± 0.023 | - |
| 1.0% Pd/Ni a | 0.98 ± 0.057 | - | 1.72 ± 0.074 | 0.19 ± 0.014 | 97 ± 5.1 | 0.15 ± 0.011 | 0.10 ± 0.010 | 0.28 ± 0.018 | |
| 1.0% Ru/Ni a | - | 1.33 ± 0.068 | 1.70 ± 0.083 | 0.23 ± 0.013 | 95 ± 5.7 | 0.67 ± 0.042 | 0.15 ± 0.010 | 0.26 ± 0.015 | - |
| Ni b | - | - | - | - | 92 ± 5.2 | 0.23 ± 0.013 | 5.4 ± 0.35 | 0.42 ± 0.025 | 0.62 ± 0.032 |
| 1.0% Pd/Ni b | 0.65 ± 0.057 | - | - | 87 ± 4.3 | 2.91 ± 0.15 | 7.0 ± 0.44 | 0.58 ± 0.035 | 0.79 ± 0.046 | |
| 1.0% Ru/Ni b | - | 0.86 ± 0.045 | - | - | 95 ± 4.8 | 0.22 ± 0.018 | 2.3 ± 0.12 | 0.66 ± 0.035 | 0.25 ± 0.016 |
| Catalyst | Lattice Parameters [Å] | D [nm] Ni | D [nm] Pd | D [nm] Ru | |
|---|---|---|---|---|---|
| Ni NWs | for Ni a a = 3.527 (±0.006) | - | 8 | - | - |
| for Ni a | for Pd a | ||||
| 1.0% Pd/Ni NWs | a = 3.524 (±0.002) | a = 4.060 (±0.008) | 9 | 3–4 | - |
| for Ni a | for Ru b | ||||
| 1.0% Ru/Ni NWs | a = 3.526 (±0.003) | a = 2.711 (±0.006) c = 4.277 (±0.007) | 9 | - | 4–5 |
| Entry | Catalyst | T [°C] | TOF [h−1] | Ref. |
|---|---|---|---|---|
| 1 | 1.0% Ru/Ni nanowires (NWs) | 179 | 2479.2 | - |
| 2 | 1.0% Pd/Ni NWs | 281 | 2265.4 | - |
| 3 | 1.0% Ru/Ni foam | 544 | 442.3 | - |
| 4 | 1.0% Pd/Ni foam | 549 | 419.1 | - |
| 5 | Ni NWs | 358 | 528.6 | - |
| 6 | Ni | 522 | 514.0 | |
| 7 | Ni foam | 537 | 499.7 | |
| 8 | 1.0% Ru/SiO2 | 381 | 1208.6 | [25] |
| 9 | 1.5% Ru/Ni | 204 | 940.0 | [25] |
| 10 | 0.5% Pd/Ni | 505 | 2423.0 | [25] |
| 11 | 0.8 Ru/TiO2 | 180 | 487.3 | [24] a |
| 12 | Ru–CeO2/Al2O3 | 300 | 2256.0 | [36] a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siudyga, T.; Kapkowski, M.; Janas, D.; Wasiak, T.; Sitko, R.; Zubko, M.; Szade, J.; Balin, K.; Klimontko, J.; Lach, D.; et al. Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation. Catalysts 2020, 10, 513. https://doi.org/10.3390/catal10050513
Siudyga T, Kapkowski M, Janas D, Wasiak T, Sitko R, Zubko M, Szade J, Balin K, Klimontko J, Lach D, et al. Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation. Catalysts. 2020; 10(5):513. https://doi.org/10.3390/catal10050513
Chicago/Turabian StyleSiudyga, Tomasz, Maciej Kapkowski, Dawid Janas, Tomasz Wasiak, Rafał Sitko, Maciej Zubko, Jacek Szade, Katarzyna Balin, Joanna Klimontko, Daniel Lach, and et al. 2020. "Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation" Catalysts 10, no. 5: 513. https://doi.org/10.3390/catal10050513
APA StyleSiudyga, T., Kapkowski, M., Janas, D., Wasiak, T., Sitko, R., Zubko, M., Szade, J., Balin, K., Klimontko, J., Lach, D., Popiel, J., Smoliński, A., & Polanski, J. (2020). Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation. Catalysts, 10(5), 513. https://doi.org/10.3390/catal10050513










