Abstract
Co-catalyst deposition is used to improve the surface and electrical properties of photocatalysts. In this work, MoSx/CdIn2S4 nanocomposites were prepared by a facile hydrothermal and photodeposition route. The basic crystalline phases and morphology of the as-prepared samples were determined, and these results showed that MoSx was tightly anchored onto CdIn2S4 by sharing the same S atom. In the hydrogen production experiments, MoSx/CdIn2S4-40 displayed the optimal photocatalytic hydrogen production yield in 4 h. The H2 evolution rate reached 2846.73 μmol/g/h, which was 13.6-times higher than that of pure CdIn2S4. Analyzing the photocatalytic enhancement mechanisms revealed that this unique structure had a remarkable photogenerated electron-hole pair separation efficiency, rapid charge carrier transfer channels, and more abundant surface reaction sites. The use of co-catalyst (MoSx) greatly improved the photocatalytic activity of CdIn2S4.
1. Introduction
Hydrogen is a renewable energy source that can possibly solve the energy crisis and environmental pollution, but its conversion method must be further explored to enable its practical application. Various scholars have studied methods to efficiently produce hydrogen [1,2]. Photocatalysis is a low-cost and convenient method to convert solar energy into hydrogen energy [3]. This energy transformation method requires a photocatalyst that can absorb solar energy to drive the hydrogen evolution reaction. Numerous studies have reported developing photocatalysts such as metal oxides [4], nitrides [5], sulfides [6], and metal-free semiconductors [7]. Unfortunately, it is difficult for these semiconductors to achieve efficient hydrogen production in practical applications because of their poor visible light absorption, which leads to low hydrogen production [8].
Various photocatalysts have been explored to absorb more visible light for photocatalytic hydrogen production. Among them, typical layered transition metal chalcogenides (TMCs) have garnered much attention due to their high-performance hydrogen production capacities and tunable band gaps [9]. Cadmium indium sulfide (CdIn2S4) is a TMC with excellent visible light-harvesting capacity due to its suitable and tunable band gap [10]. Its regular shape and convenient preparation method have caused it to receive significant attention as a hydrogen production photocatalyst. For example, Li described the controllable synthesis of CdIn2S4, whose band gap (2.06–2.85 eV) could be regulated by adjusting the Cd/In ratio to allow CdIn2S4 to harvest more visible light [11]. As a result, CdIn2S4 showed remarkable photocatalytic activity compared with CdS; however, the photocatalyst only showed good photocatalytic hydrogen production efficiency when it was attached to a noble metal [12]. This work indirectly verified that pure CdIn2S4 cannot satisfy the requirements of practical applications, despite the above advantages. In another study, its lower photocatalytic hydrogen production efficiency was attributed to the recombination of photogenerated carriers and a low surface area for the hydrogen evolution reaction [13]; therefore, it is important to further enhance the photocatalytic performance of pure CdIn2S4.
Many efforts have been made to improve the hydrogen production capability using co-catalyst deposition [14], doping [15], morphology designing [16,17], and heterojunction construction [18]. Among these, co-catalyst deposition is well-known for its ability to modify photocatalysts, and a good photocatalytic co-catalyst must exhibit effective electron transport channels and a promising capacity for the hydrogen evolution reaction [14]. Co-catalysts such as Pt have been used to improve the photocatalytic capacity because Pt offers a promising reaction surface for hydrogen production as well as a remarkable electron transfer rate during photogenerated electron transport. It has been widely used in contact photocatalysts such as TiO2, C3N4, and CdS [19]; however, noble metals cannot be used for large-scale applications because they are expensive. Thus, it is essential to rationally combine co-catalysts for higher hydrogen production efficiencies.
This problem can be solved by using amorphous molybdenum sulfides (MoSx), which have been shown to greatly activate and stabilize the hydrogen evolution reaction (HER), especially in acidic solutions. They are also expected to become substitutes for noble metals [20]. Compared with MoS2, MoSx can also be synthesized in a milder environment. Though the S atoms in amorphous molybdenum sulfides show long-range disorder, short-range atomic arrangements offer more surface area for the hydrogen evolution reaction. Due to this, the deposition of amorphous molybdenum sulfides has proven to be a promising way to improve photocatalytic hydrogen production. MoSx not only offers electron transport channels that promote the separation of electron-hole pairs, but also act as active reaction sites [21,22,23]. MoSx is a noble-metal-free material whose hydrogen evolution activity is better than that of MoS2. Moreover, MoSx/CdS composite photocatalysts were synthesized by assembling MoSx onto CdS nanorods for hydrogen production. MoSx shared an S atom with CdS during deposition, which helped build rapid electron transport channels between CdS and MoSx, leading to a remarkable hydrogen production yield [22]. According to the survey above, this paper aimed to synthesize MoSx/CdIn2S4 composite photocatalysts with improved hydrogen production.
In this study, pure CdIn2S4 was prepared by a one-step hydrothermal method, and MoSx was then deposited onto the surface of CdIn2S4 via a photodeposition method for the HER (Figure 1). The crystalline phase and morphologies of MoSx/CdIn2S4 composites were systematically studied to verify how MoSx deposition affected the morphology and crystalline phases. The MoSx/CdIn2S4 photocatalyst showed remarkable photocatalytic activity, and the optimal deposition ratio of MoSx for the HER was investigated. MoSx/CdIn2S4-40 showed the best hydrogen production yield, which was 13.6 times higher than that of CdIn2S4, and its H2 evolution rate reached 2846.73 μmol/g/h. The optical and electrochemical characteristics of MoSx/CdIn2S4 composites were measured to explore the improvement mechanism. In the photocatalyst system, MoSx provided sites for hydrogen production and acted as a charge acceptor to separate photogenerated electron-hole pairs. In all, considering the convenient and mild preparation conditions of MoSx/CdIn2S4, MoSx helped improve the photocatalytic capacity of CdIn2S4 and offers an attractive method to enhance the photocatalytic hydrogen evolution using CdIn2S4.
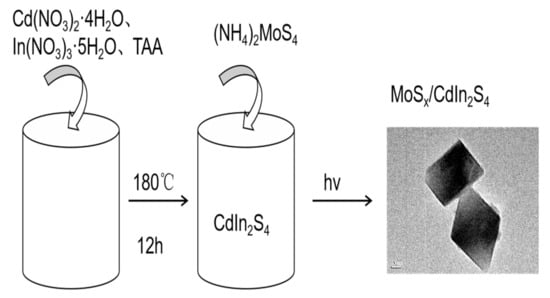
Figure 1.
The synthesis method of MoSx/CdIn2S4. MoSx/CdIn2S4 composites were prepared by a photodeposition method and products were prepared by changing the exposure time and were named by different exposure time: MoSx/CdIn2S4-20, MoSx/CdIn2S4-40, and MoSx/CdIn2S4-60.
2. Results
2.1. Crystalline Structure
First, X-Ray Diffraction (XRD) patterns were collected to verify the chemical components of test samples (Figure 2). All samples displayed similar XRD patterns, with main peaks at 14.14°, 23.18°, 27.25°, 28.48°, 33.00°, 40.74°, 43.32°, and 47.41° for crystalline CdIn2S4. These peaks were fitted to the (111), (220), (311), (222), (400), (442), (511), and (440) planes of cubic CdIn2S4 (PDF#27-0060) [11]. The above results indicated that all samples contained cubic CdIn2S4, and that the main photocatalyst CdIn2S4 remained unchanged during synthesis; however, no XRD peak for MoSx was observed in the MoSx/CdIn2S4 samples, mainly because of the amorphous structure of MoSx [22]. Therefore, it is necessary to use a different method to detect the presence of MoSx.
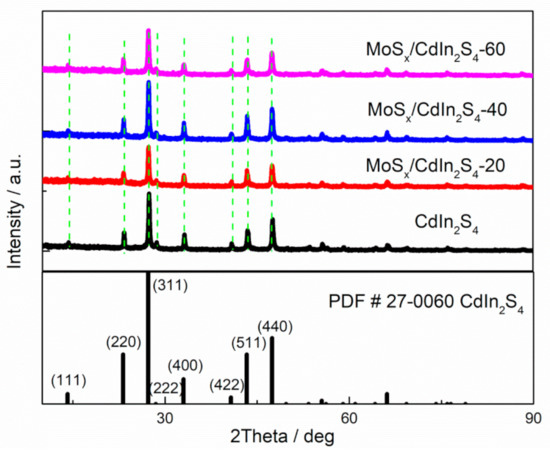
Figure 2.
X-Ray Diffraction (XRD) patterns of the as-prepared samples.
X-ray photoelectron spectrcopy (XPS) spectra were collected to investigate the surface chemical composition and states of the as-prepared samples. The survey spectra of Cd, In, and S of pure CdIn2S4 and MoSx-CdIn2S4-40 are shown in Figure 3. The Mo spectrum was not detected in the survey spectra of MoSx/CdIn2S4 due to the low Mo content. The high-resolution S 2p spectra of CdIn2S4 can be deconvoluted into two peaks at 161.30 eV and 162.50 eV, which were assigned to the 2 p3/2 and 2 p1/2 chemical states levels of S2− [11]. After deposition with MoSx, the S 2p spectrum can be divided into three peaks, and the extra weak peak at 163.26 eV was identified as unsaturated S, which is its typical chemical state in MoSx [22,23]. Previous reports also support the observation that MoSx contains special unsaturated active S, further indicating the presence of MoSx on the surface. Compared with bare CdIn2S4, MoSx/CdIn2S4 displays weak Mo 3d peaks at 228.04 eV, 229.40 eV, 229.74 eV, 232.10 eV, and 232.61 eV. The peaks at 228.04 eV, 229.40 eV, and 229.74 eV belonged to Mo 3 d5, Mo 3d, and Mo S2 due to Mo4+ in MoS2 or MoS3 [20,23,24] The doublet peaks at 232.1 and 232.61 eV were assigned to Mo6+ in MoS3. The slight increase in peak value of the high-resolution Cd 3d and In 3d spectra was mainly caused by interactions between MoSx and CdIn2S4. These results revealed that MoSx existed on the surface of CdIn2S4. Combined, the XPS and XRD results showed that the as-prepared sample was mainly composed of MoSx and CdIn2S4.
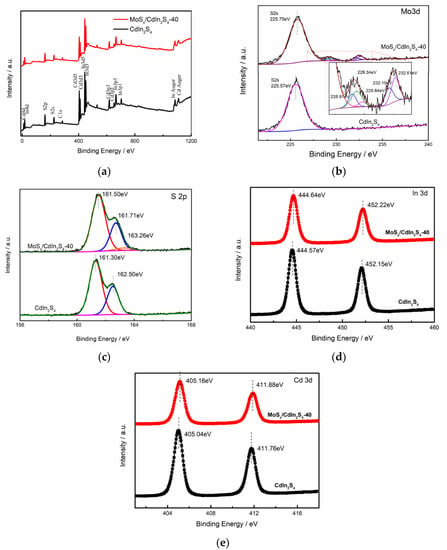
Figure 3.
X-ray photoelectron spectrcopy (XPS) spectra of CdIn2S4 and MoSx/CdIn2S4. Survey spectra (a); high-resolution spectra of Cd (b); In (c); Mo (d); and S (e).
2.2. Morphology
Scanning electron microscope (SEM) was used to characterize the morphology of all samples, and EDS was used to determine the corresponding dispersion of elements. As displayed in Figure 4, pure CdIn2S4 showed many symmetrical nanoflake microspheres that were 50–70 nm long, which were stacked into large nanoparticles. These small symmetrical nanoflake microspheres provided a large surface area for solar energy harvesting and hydrogen evolution reaction, which led to remarkable photocatalytic efficiency. However, nothing obvious changed in the MoSx/CdIn2S4 composite after photodeposition, mainly because of the low concentration of MoSx. This result does not indicate that MoSx was deposited onto the surface of CdIn2S4 in situ, but it does confirm that CdIn2S4 remained unchanged during photodeposition.
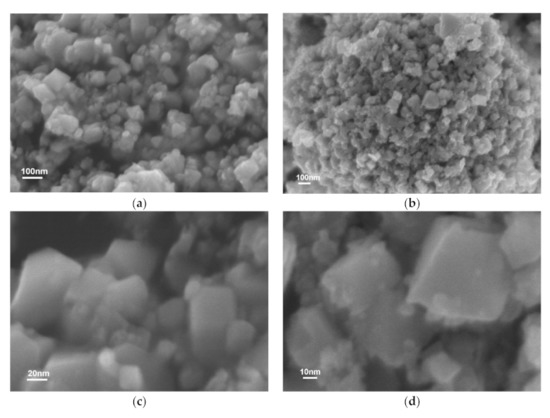
Figure 4.
SEM image of CdIn2S4 (a,c) and MoSx/CdIn2S4 (b,d).
To further determine the corresponding concentrations of elements, Energy Dispersive Spectroscopy (EDS) was carried out. The EDS (Figure 5) results of MoSx/CdIn2S4 showed a low Mo content in the composite, which indirectly indicates that MoSx was present in 0.83% (the content of MoSx also shown in Table S2). The elemental mapping image shows that Mo was uniformly dispersed on the composite surface, which confirmed that MoSx was very evenly distributed in the composite.
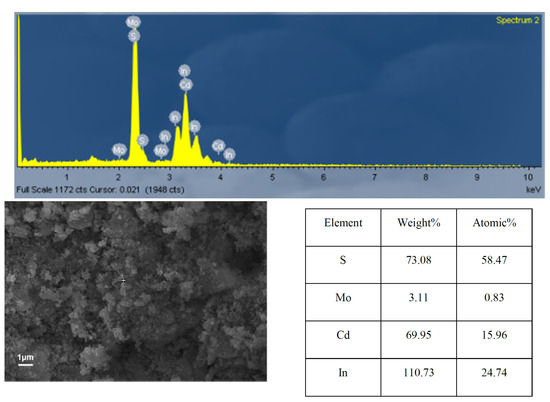
Figure 5.
EDS results of MoSx/CdIn2S4.
Transmission electron microscope (TEM) and high resolution transmission electron microscopy (HRTEM) were used to confirm the crystallinity and morphology of samples in Figure 6. The images showed the morphology of bare CdIn2S4, which contained thin nanoflakes with a few curved nanoflakes. Each nanoflake in the SEM image was about 40–80 nm wide. The crystal lattice spacing of CdIn2S4 determined by HRTEM was 0.329 nm, 0.385 nm, and 0.629 nm, which can be indexed to the (311), (220), and (111) planes of cubic spinel CdIn2S4 from the comparison with XRD patterns. The TEM images after deposition showed that the surface of CdIn2S4 was covered with irregular MoSx nanoparticles with edge lengths of 9.159 nm. The HRTEM image showed that highly disordered crystals were attached to the nanoflakes, which showed similar singe-crystal lattices. This confirmed that MoSx was closely attached to the CdIn2S4 surface via a common S atom, which helped construct rapid electron transport channels that may improve the photocatalytic capacity.
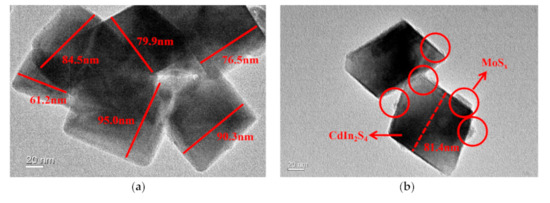
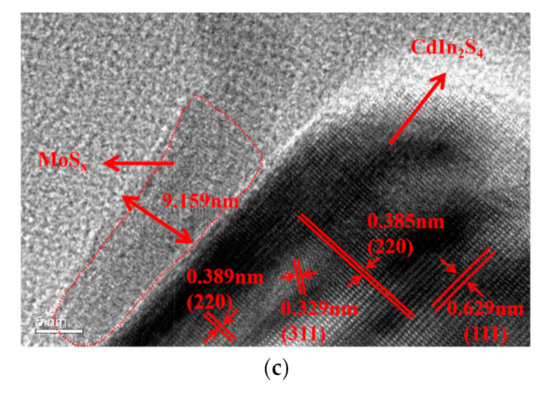
Figure 6.
Transmission electron microscope (TEM) image of CdIn2S4 (a) and MoSx/CdIn2S4 (b,c).
2.3. Photocatalytic Activity
Hydrogen evolution experiments were carried out under visible light irradiation to measure the photocatalytic capacity of the as-prepared samples. The sacrificial reagent (0.1 M Na2SO3/Na2S solution) was used to react with photogenerated holes. As shown in Figure 7, the same condition without sunlight and the same condition with blank samples were performed to determine the effects of disturbed elements. The photocatalytic production yields of pure samples of CdIn2S4, Pt/CdIn2S4(XRD was shown in Figure S1), MoS2/CdIn2S4, and MoSx/CdIn2S4 were also presented. Pure CdIn2S4 showed minimal H2 evolution yield under visible light irradiation, and the average rate reached 209.41 μmol/g/h in 4 h. After co-catalyst deposition, the hydrogen evolution of MoS2/CdIn2S4 and Pt/CdIn2S4 reached 1265.12 μmol/g/h and 1451.71 μmol/g/h, respectively. This result was attributed to the fact that co-catalyst deposition increased the number of active sites, and many electron transport channels emerged [25]. In this work, the hydrogen production yields of MoSx/CdIn2S4-20, MoSx/CdIn2S4-40, and MoSx/CdIn2S4-60 were greatly enhanced due to the presence of the MoSx co-catalyst. MoSx/CdIn2S4-40 displayed the greatest H2 evolution rate of 2846.73 μmol/g/h, which indicates that MoSx/CdIn2S4 was the best photocatalyst. Its hydrogen evolution rate was about 13.6 and 2.25 times higher than pure CdIn2S4 and MoS2/CdIn2S4. In addition, the MoSx content also affected the photocatalytic hydrogen production, and the hydrogen evolution rates first increased upon increasing the deposited amount of MoSx and then decreased. This revealed that the optimal amount of MoSx on CdIn2S4 affected the photocatalytic hydrogen production capacity of the composites. All hydrogen evolution experimental results showed that MoSx/CdIn2S4 is a promising photocatalyst for hydrogen production.
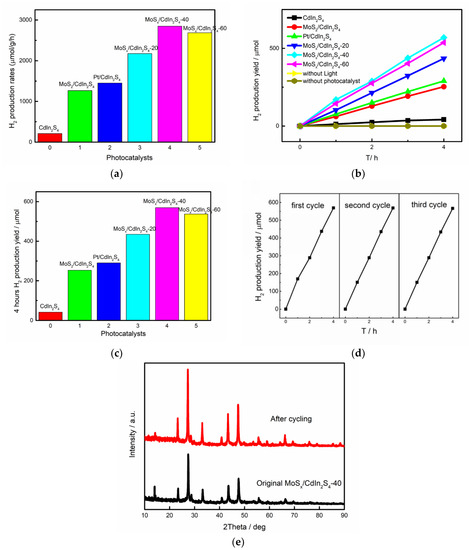
Figure 7.
(a) H2 production yield, (b) H2 production yield over 4 h, (c) H2 production rates of the as-prepared samples, (d) photocatalytic cycle tests of MoSx/CdIn2S4-40 composites, and (e) XRD patterns of the MoSx/CdIn2S4-40 composite after photocatalytic cycles.
Photocatalyst stability is also important for practical applications; therefore, the cycling runs of hydrogen production were carried out to determine the photocatalyst stability. The picture displays the hydrogen production amounts of the as-prepared composites for different numbers of 4-h photocatalytic hydrogen production cycles. The hydrogen production amount only slightly decreased after each cycle, showing that the photocatalytic capacity of MoSx/CdIn2S4-40 remained nearly constant. The photocatalyst crystal structure stability can be further characterized by comparing the pattern of MoSx/CdIn2S4-40 before hydrogen evolution and after three cycles. The crystal structure of the photocatalyst exhibited similar XRD patterns before and after the cycle experiments, suggesting that MoSx/CdIn2S4-40 is a sufficiently stable photocatalyst for practical applications.
2.4. Photocatalytic Mechanism
The photocatalytic hydrogen production yield closely depends on the generation, transport, and reactions of photogenerated charge carriers. The generation of electron-hole pairs is determined by a material’s bandgap, and a bandgap that matches harvested visible light is required. Ultraviolet-visible (UV–Vis) spectra, XPS valence band spectra, and Mott–Schottky spectra were obtained to explore the bandgaps of the as-prepared samples. As illustrated in Figure 8a, pure CdIn2S4 showed a fundamental absorption edge at 470 nm, which revealed that pure CdIn2S4 has broad light absorption in the visible light region. However, the absorption edge of the MoSx/CdIn2S4 composites was located at 500 nm, and this blue-shift means that the photocatalyst had improved visible light absorption, which should sharply increase the photogenerated charge carriers. This result was attributed to the fact that MoSx shared S atom with CdIn2S4 during photodeposition, which changed a small portion of the crystalline phase on the surface, leading to a blue-shift of the absorption edge. Similar to the literature, the bandgap of the as-prepared composite can be calculated by the formula: αhν = C (hν − Eg)2. The band gap of pure CdIn2S4 was 2.18 eV, which is similar to a previous report [25]. However, the bandgap of MoSx/CdIn2S4 was 2.04 eV, which was attributed to the addition of MoSx, which improved the visible light harvesting. To further explore the photocatalytic band structures, XPS valence band spectroscopy and impedance-potential tests were carried out. XPS valence band spectra were used to directly determine the valence band (VB) of the as-prepared samples and their flat-band (FB) potentials, which were roughly equivalent to the conduction band (CB). These were calculated by the Mott–Schottky plot of the impedance–potential test. The EfB of CdIn2S4 and MoSx/CdIn2S4 were −0.489 and −0.454 V vs. NHE, and EfB of the sample, according to the ordinate of the fitted straight line in the Mott–Schottky plot. According to the formula: ECB = EVB − Eg and ECB ≈ EfB, the EVB was calculated using ECB. The general band structures (shown in the plot) were determined from the UV–Vis spectra and EfB. MoSx/CdIn2S4 had the optimal band structure of all materials, which should boost the hydrogen production yield.
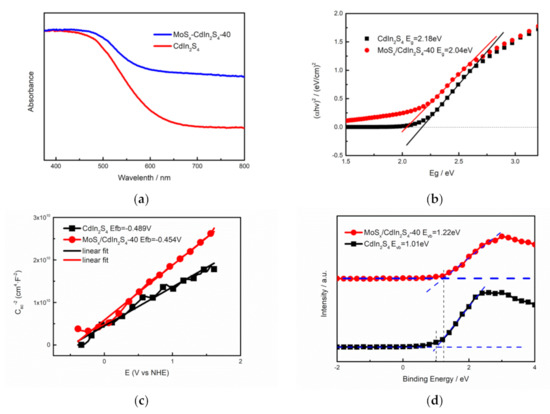
Figure 8.
UV–Vis spectra (a), Tauc plots (b), Mott–Schottky plots (c), and XPS valence band spectra of CdIn2S4 and MoSx/CdIn2S4 (d).
Photoelectrochemical characterization techniques were used to further investigate the interfacial photogenerated charge separation and transfer in the as-prepared samples. The transient photocurrent responses of CdIn2S4, MoSx/CdIn2S4-20, MoSx/CdIn2S4-40, and MoSx/CdIn2S4-60 were tested. As shown in Figure 9a, the photocurrent rapidly reached a maximum when the light was switched on, indicating that photogenerated electron-hole pairs were produced by visible light. The significant differences in photocurrent density depended on the photogenerated charge separation and transfer. The pure CdIn2S4 photocatalyst showed a low photocurrent density, which means that photogenerated charge separation and transfer represented a considerable loss in energy. Similar to a previous report [26], pure CdIn2S4 consumed significant amounts of energy because of the rapid recombination of photogenerated electron-hole pairs. The introduction of MoSx solved this problem, and the photocurrents of MoSx/CdIn2S4-20, MoSx/CdIn2S4-40, and MoSx/CdIn2S4-60 sharply increased to 2.71, 4.56, and 1.91 μA, respectively. The photocurrent of MoSx/CdIn2S4-40 was 7.5 times higher than that of CdIn2S4. Noticeably, corresponding to the hydrogen production yields, MoSx/CdIn2S4-40 showed the highest photocurrent intensity. The transient photocurrent responses of the as-prepared samples confirmed that MoSx improved the charge separation and transfer capacity in CdIn2S4, which should lead to remarkable hydrogen production.
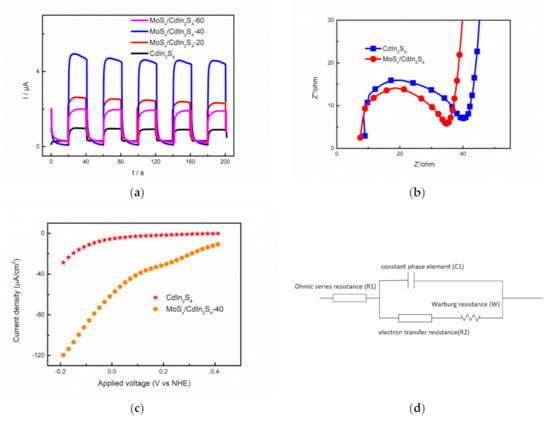
Figure 9.
Transient photocurrent responses (a) of CdIn2S4 and MoSx/CdIn2S4; Electrochemical Impedance Spectroscopy (EIS) Nyquist (d) and Linear sweep voltammograms (LSV) (c) plots of CdIn2S4 and MoSx/CdIn2S4; the circuit model (d) in the EIS Nyquist plot.
Electrochemical Impedance Spectroscopy (EIS) was used to measure the charge transfer efficiency. Nyquist plots can be obtained by EIS, and the Nyquist plots of the as-prepared samples were explained by a circuit model. This circuit model includes ohmic series resistance (R1), electron transfer resistance (R2), a constant-phase element (C1), and Warburg resistance (W). The electron transfer resistance (R2) corresponds to the diameter of the semicircular arc, in which a smaller diameter indicates a smaller resistance, which leads to the rapid transfer of photogenerated charge carriers [25]. The diameter of the semicircular arc of MoSx/CdIn2S4-40 was smaller than that of CdIn2S4, revealing that the mobility of photogenerated charges was higher in MoSx/CdIn2S4-40.
Photogenerated charges were separated and transferred to the surface reaction sites to participate in the hydrogen evolution reaction. Linear sweep voltammograms (LSV) of the as-prepared samples were obtained to determine the surface hydrogen production reaction s of CdIn2S4 and MoSx/CdIn2S4-40. In the LSV polarization curves of the as-prepared samples, MoSx/CdIn2S4 displayed a larger slope and current compared with bare CdIn2S4, which means that MoSx/CdIn2S4-40 more easily produced hydrogen at the same photovoltage. This reveals that faster hydrogen production would occur using MoSx/CdIn2S4-40 at the same voltage. These results reveal that MoSx/CdIn2S4-40 has superior reaction sites for hydrogen production.
The combined results showed that the MoSx/CdIn2S4-40 photocatalyst showed remarkable photocatalytic hydrogen evolution because of its unique structure. Pure CdIn2S4 photocatalyst shares the same S atom with MoSx, which provided the perfect anchor point for MoSx, leading to tight bonding between CdIn2S4 with MoSx. This unique linking improved the light-harvesting of the pure CdIn2S4 photocatalyst and increased the separation and transfer of photogenerated charges. MoSx is an efficient co-catalyst with abundant hydrogen evolution sites. Furthermore, MoSx and CdIn2S4 formed a classical Schottky structure with tight linking, and this structure further improved the charge carrier separation and electron transfer. This increased the number of reacting electrons, which ultimately led to its remarkable photocatalytic hydrogen production rate.
3. Experimental
3.1. Chemical Reagents
All chemical reagents were analytical reagent grade, and they were used as received without further purification. Indium nitrate hydrate (In(NO3)3·5H2O), cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O), sodium sulfide nonahydrate (Na2S·9H2O), sodium sulfite (Na2SO3), sodium sulfate (Na2SO4), thioacetamide (TAA), and ethanol were obtained from Aladdin (Shanghai, China).
3.2. Synthesis of MoSx/CdIn2S4 Composites
The synthesis process of MoSx/CdIn2S4 composites is shown in Figure 1.
Pure CdIn2S4 was prepared by a simple hydrothermal method according to the literature [10]. Briefly, 0.1232 g of Cd(NO3)2·4H2O, 0.3056 g of In(NO3)3·5H2O, and 0.2404 g of TAA were successively added in 15 mL deionized water. After that, this mixture was vigorously stirred for 0.5 h. The solution was then placed into a 50 mL Teflon-lined autoclave and kept at 180 °C for 12 h. Afterward, yellow samples, which were pure CdIn2S4, were obtained by filtration and washed with deionized water and ethanol. The desired product was dried at 60 °C.
MoSx/CdIn2S4 composites were prepared by a photodeposition method according to the literature [22]. In this experiment, 100 mg pure CdIn2S4 nanoparticles were added to a mixed solvent (water:ethanol = 4:1) with stirring. After 20 min stirring, 8.5 mg (NH4)2MoS4 (synthesized using a previously reported method [22]) were dissolved into the mixed solution. Then, this suspension was stirred for 30 min to reach the adsorption–desorption equilibrium. The as-prepared mixture was poured into a photocatalytic reactor. During photodeposition, a 300 W Xenon lamp was used to simulate solar irradiation, and the mixture was held at 6 °C with continuous stirring for 40 min. After photodeposition, a greenish-yellow product was obtained after centrifugation and washing. The product was dried at 60 °C, and the as-prepared sample was named MoSx/CdIn2S4-40. Similar products were prepared by changing the exposure time and were named MoSx/CdIn2S4-20 and MoSx/CdIn2S4-60. MoS2/CdIn2S4 and Pt/CdIn2S4 were prepared by the method similar to the report [17].
3.3. Characterization
X-ray diffraction (XRD) patterns of synthesized catalysts were obtained on a D8-advance x-ray diffractometer (Bruker, Billerica, MA, USA) using Cu Kα (λ = 0.15406 nm) radiation. The UV–Vis diffuse reflectance spectra (DRS) were obtained on a UV-2550 UV–Vis spectrophotometer (Shimadzu, Tokyo, Japan), which used BaSO4 as a reflectance standard. The scanning electron microscope (SEM) images and energy-dispersive x-ray spectra (EDS) were recorded by a HITACHI S-4800 field emission SEM. Transmission electron microscope (TEM) and high-resolution TEM (HRTEM) images were obtained using a high-resolution TEM (JEOL, JSM-2100F, Tokyo, Japan).
3.4. Hydrogen Production and Electrochemical Experiments
The photocatalytic performance was characterized by hydrogen production experiments, in which 50 mg of the as-prepared photocatalysts was dispersed in 50 mL aqueous solution with string. Then, 0.1 M aqueous Na2S/Na2SO3 was used as the sacrificial agent. After that, the solution was dropped into a photocatalytic reactor that was irradiated by a 300 W Xenon lamp to simulate visible light irradiation. The entire photocatalytic reaction system was held at 7 °C to produce hydrogen. The final obtained generated hydrogen yields were detected by gas chromatography (GC-7900, TCD, Ar carrier).
An electrochemical workstation was used to measure the electrochemical characteristics of the photocatalysts. In these experiments, samples were prepared by coating them onto indium tin oxide (ITO) glass. This test system was a three-electrode cell, with ITO as the working electrode, Ag/AgCl as the reference electrode, and a 1 cm × 1 cm platinum film as the counter electrode. Similar to the hydrogen production experiment, a 300 W Xenon lamp was used to simulate solar light. The photocurrent was recorded at a bias voltage of 0.2 V (vs. Ag/AgCl) with light irradiation. The electrochemical impedance spectra (EIS) were obtained in the frequency range from 1 to 106 Hz. The flat-band voltage was measured at bias voltages between 1 V to −1 V (vs. Ag/AgCl).
4. Conclusions
The composite photocatalyst MoSx/CdIn2S4 was synthesized by a facile photodeposition method. XRD, XPS, SEM, EDS, and TEM were used to determine the chemical phase characteristics of the as-prepared catalysts. The results verified the intimate contact between MoSx and CdIn2S4. The as-prepared MoSx/CdIn2S4-40 composite showed a remarkable hydrogen production rate that was 13.6 times higher than that of pure CdIn2S4. From the optical and photoelectrochemical techniques, this was attributed to intimate interfacial contact, which produced abundant reaction sites, better charge separation, and better electron transfer efficiency. MoSx offers many hydrogen evolution sites for hydrogen production. In addition, MoSx/CdIn2S4-40 exhibited outstanding stability (Table S1), which will advance its practical applications, and this study offers guidance for photocatalytic hydrogen production.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/12/1455/s1, Figure S1: XRD of Pt/CdIn2S4 and MoS2/CdIn2S4, Table S1: The quantum yields of different material. Table S2: The binding energy (BE) of different element atomic.
Author Contributions
Conceptualization, Q.L.; Methodology, Q.L. and W.L.; Software, Q.L.; Validation, Q.L.; Formal analysis, Q.L.; Investigation, Q.L. and W.L.; Resources, Q.L. and X.Y.; Data curation, Q.L.; Writing–original draft preparation, Q.L.; Writing–review and editing; X.X. (Xuejian Xie) and X.C.; Visualization, X.X. (Xuejian Xie); Supervision, X.X. (Xuejian Xie) and X.Y.; Project administration, X.X. (Xiangang Xu) and X.Y.; Funding acquisition, X.X. (Xuejian Xie) and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2018 YFB0905701), the Youth Program of the National Natural Science Foundation of China (Grant No. 51902182), the Outstanding Youth Fund of Shandong Province (Grant No. ZR2019 JQ01), and the Natural Science Foundation of Shandong Province (Grant No. ZR2019 BEM011).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yu, Z.; Wang, X. Crystalline Carbon Nitride Semiconductors for Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef]
- Ma, S.S.K.; Hisatomi, T.; Maeda, K.; Moriya, Y.; Domen, K. Enhanced Water Oxidation on Ta3N5 Photocatalysts by Modification with Alkaline Metal Salts. J. Am. Chem. Soc. 2012, 134, 19993–19996. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on Metal Sulphide-based Z-scheme Photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.-T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zhang, L.; Cao, M.; Yang, F.; Dai, W.-L. Facile construction of flower-like black phosphorus nanosheet@ZnIn2S4 composite with highly efficient catalytic performance in hydrogen production. Appl. Surf. Sci. 2020, 504, 144366. [Google Scholar] [CrossRef]
- Wang, W.; Ng, T.W.; Ho, W.; Huang, J.; Liang, S.; An, T.; Li, G.; Yu, J.C.; Wong, P.K. CdIn2S4 microsphere as an efficient visible-light-driven photocatalyst for bacterial inactivation: Synthesis, characterizations and photocatalytic inactivation mechanisms. Appl. Catal. B Environ. 2013, 129, 482–490. [Google Scholar] [CrossRef]
- Bhirud, A.; Chaudhari, N.; Nikam, L.; Sonawane, R.; Patil, K.; Baeg, J.-O.; Kale, B. Surfactant tunable hierarchical nanostructures of CdIn2S4 and their photohydrogen production under solar light. Int. J. Hydrog. Energy 2011, 36, 11628–11639. [Google Scholar] [CrossRef]
- Koelmans, H.; Grimmeiss, H. The photoconductivity of CdIn2S4 activated with Cu or Au. Antivir. Res. 1959, 25, 1287–1288. [Google Scholar] [CrossRef]
- Vu, M.-H.; Nguyen, C.-C.; Sakar, M.; Do, T.-O. Ni supported CdIn2S4 spongy-like spheres: A noble metal free high-performance sunlight driven photocatalyst for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 29429–29437. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Omori, Y.; Kitagawa, S.-Y.; Tanaka, A.; Hashimoto, K.; Kominami, H. Visible light-induced diastereoselective semihydrogenation of alkynes to cis-alkenes over an organically modified titanium(IV) oxide photocatalyst having a metal co-catalyst. J. Catal. 2019, 374, 36–42. [Google Scholar] [CrossRef]
- Shen, X.-Z.; Guo, J.; Liu, Z.-C.; Xie, S.-M. Visible-light-driven titania photocatalyst co-doped with nitrogen and ferrum. Appl. Surf. Sci. 2008, 254, 4726–4731. [Google Scholar] [CrossRef]
- He, X.; Zhang, C. Recent advances in structure design for enhancing photocatalysis. J. Mater. Sci. 2019, 54, 8831–8851. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Hu, X.; Wang, Y.; Liu, E.; Fan, J. A novel S-scheme MoS2/CdIn2S4 flower-like heterojunctions with enhanced photocatalytic degradation and H2 evolution activity. J. Phys. D Appl. Phys. 2020, 53, 205101. [Google Scholar] [CrossRef]
- Min, W.; Guo-Qiang, T.; Dan, Z.; Bin, L.; Ying, W.; Ming-Yue, D.; Hui-Jun, R.; Ao, X.; Yun, L. Synthesis and Mechanism of Direct Z-Scheme Zn_2SnO_(4-x)N_x/ZnO_(1-y)N_y Heterojunction Photocatalyst. Chin. J. Inorg. Chem. 2019, 9, 1551–1560. [Google Scholar]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as Co-catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef]
- Ghosh, S.; Azad, U.P.; Singh, A.K.; Singh, A.K.; Prakash, R. Facile Synthesis of MoSx and MoSx -rGO Composite: Excellent Electrocatalyst for Hydrogen Evolution Reaction. ChemistrySelect 2017, 2, 11590–11598. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Shu, X.; Wang, N.; Xu, G.; Zhang, X.; Lv, J.; Wu, Y. An amorphous MoSx modified g-C3N4 composite for efficient photocatalytic hydrogen evolution under visible light. RSC Adv. 2019, 9, 15900–15909. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Zheng, Q.; Shao, Y.; Li, D. Amorphous MoSx on CdS nanorods for highly efficient photocatalytic hydrogen evolution. J. Solid State Chem. 2017, 246, 230–236. [Google Scholar] [CrossRef]
- Tzanidis, I.; Bairamis, F.; Sygellou, L.; Andrikopoulos, K.S.; Avgeropoulos, A.; Konstantinou, I.; Tasis, D. Rapid Microwave-Assisted Synthesis of CdS/Graphene/MoS x Tunable Heterojunctions and Their Application in Photocatalysis. Chem. A Eur. J. 2020, 26, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, L.; Liu, L.; Li, H.; Meng, S.; Ye, X.; Chen, S. In situ photodeposition of MoSx on CdS nanorods as a highly efficient cocatalyst for photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 15287–15293. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B Environ. 2020, 270, 118882. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, J.; Zhou, Y.; Sun, L.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Fabricated 2D/2D CdIn2S4/N-rGO muti-heterostructure photocatalyst for enhanced photocatalytic activity. Carbon 2019, 152, 565–574. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).