Abstract
The preparation methods of hydrophobic materials such as zeolites, modified silicas and polymers has been reviewed. Particular attention has been paid to the characterization methods classified according to the surface and bulk composition, on one hand, and to the measure of interactions with water or organic solvents, on the other. Some selected applications are analyzed in order to understand the relevance of the reactants/products adsorption to address activity and selectivity of the reaction. Thus, absorption of a non-polar reactant or desorption of a hydrophilic product are much easier on a hydrophobic surface and can effectively boost the catalytic activity.
1. Introduction
Heterogeneous catalysis represents one of the cornerstones of industrial chemistry and due to its versatility can be found in many application fields [1,2,3]. A heterogeneously catalyzed reaction is a combination of physical and chemical reaction pathways. These are related to the transport of the reactants towards the solid surface, the reaction and the removal of the products. Therefore, a typical gas-solid catalytic process involves the following steps:
- Diffusion of the reaction media through the boundary layer on the catalyst surface.
- Pore diffusion.
- Adsorption of the reactants on the inner surface of the pores.
- Chemical reaction on the catalyst surface.
- Desorption of the products from the catalyst surface.
- Diffusion of the products out of the pores.
- Diffusion of the products away from the catalyst through the boundary layer and into the gas phase [3].
As deducible, it is clear that a catalytic reaction does not involve only a single interaction with the active site but a complex mechanism of adsorption and desorption on the surface of the catalyst. Thus, it is true that chemisorption of the reactants and products on the catalyst surface is of a crucial importance in a catalytic process; however, it could not be considered independently from physisorption.
A liquid reaction environment is characterized by the same outlined mechanism, although the description of the adsorption phenomena happening at the liquid/solid surface is more intricated and less studied compared to gas–solid adsorption. Controlling adsorption of species on a solid catalyst surface and its relationship with the active sites is fundamental to achieve a rational catalyst design [4], in particular when reaction takes place in a solvent media.
A practical aspect of surface chemistry study, in this specific case, is to understand the wettability properties of a surface. A solid shows a definite surface free energy that plays a key role in the adsorption and desorption of different chemical species. In fact, wettability is the ability of a solid surface to be covered by a liquid therefore describing the affinity between a solid and liquid interface [5,6,7]. It is generally defined by the contact angle, which is the apparent result of the balance between interfacial free energies. Measuring the water contact angle (CA) is the easiest way to evaluate wettability. This method relies on the evaluation of the angle formed by a drop of water with a solid surface. If the angle θ is smaller than 90°, the surface is hydrophilic, if θ > 90° the surface is hydrophobic and eventually if θ > 150° the surface is superhydrophobic [7] (Figure 1). Contact angles measurements can be also performed with liquids other than water chosen on the basis of their polarities.
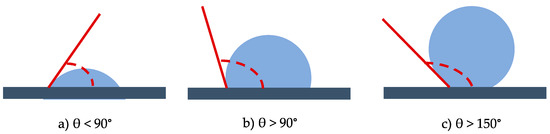
Figure 1.
Water contact angles for hydrophilic (a), hydrophobic (b) and superhydrophobic (c) surfaces.
The interactions of an adsorbed molecule, usually water, with a solid surface is commonly driven by specific functionalities such as Lewis or Brønsted acids or bases or more in general by polar functional groups (Table 1) [6].

Table 1.
Functional groups that affects surface wettability of a solid [6].
After these statements, it is easily understood that surface wettability evaluation and its proper modulation could play a crucial role in the optimization of a catalytic process.
The study of the wettability properties of a solid system can, indeed, give a better outlook on the catalytic mechanism [8] and forecast catalyst stability. In particular, surface hydrophobicity affects multiple variables. Recently, this aspect has gained higher relevance, as witnessed by some recent comprehensive review [9,10] in particular due to the fact that most reactions involved in the pathway from renewables raw materials to bio-products have to be carried out in water [11].
The use of solid catalysts in aqueous environment is not so trivial: water can lead to a dramatic poisoning of the acidic active sites [12] or a disruption of the structural features and degradation of solid oxides. For example, in the case of silica, aqueous media can cause Si-O-Si and Si-O-M bond hydrolysis [13]. This could limit the usage of heterogeneous catalyst in esterification, etherification, hydrolysis, (de)hydration and condensation reactions because they involve the use or the production of water. Moreover, it has to be considered that water is the best green solvent available especially when facing with biomass valorization reaction pathways [14]. The water tolerance of the catalyst increases with its hydrophobicity even if water is the solvent or a byproduct.
The preferential adsorption or desorption of reactants and products (Figure 2) is a crucial point to consider especially if they exhibit different polarities. This is relevant in particular when water or very polar compounds are produced, as mentioned previously, or if water is present as an inert impurity in the feed.
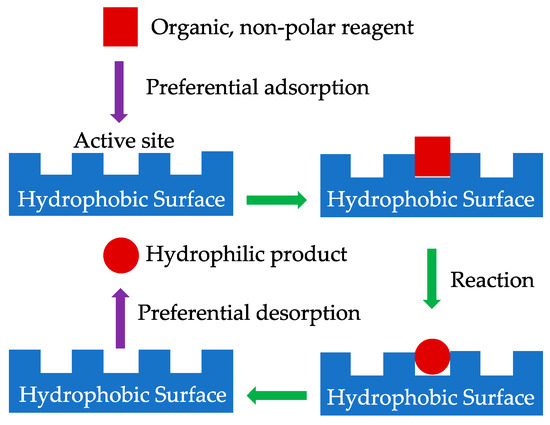
Figure 2.
Simple scheme that illustrates preferential adsorption and desorption.
Moreover, the hydrophobicity of the surrounding species affects the process occurring at the catalytically active sites. For example, these can be more or less available depending on their closest proximity. Considering this aspect, hydrophobic catalysts could be an important tool in organic synthesis: They possess an elevated resistance towards water; therefore, catalyst life can be prolonged and the deactivation of active sites due to water molecules avoided. Besides, a wide number of organic substrates tend to show a low hydrophilicity; hence, hydrophobic surfaces should have a higher affinity with them, thus boosting the catalytic activity.
Some reviews have already discussed the water tolerance related to the wettability of the catalysts [10,12,13].
This review has the purpose of showing the advantages and possibilities in the synthesis, characterization and application of hydrophobic catalysts in organic synthesis and in biomass valorization. We focused our attention on three kind of solid materials, namely zeolites, mesoporous silica and polymers and on some selected reactions to underline the improvement of the catalytic activity thanks to the use of a hydrophobic catalyst in particular due to preferential adsorption of the reactants and desorption of products.
2. Synthesis of Hydrophobic Catalysts
In this section, the hydrophobization strategies for the synthesis or modification of zeolites, silicas and polymeric catalyst will be discussed.
There are mainly two ways to make a catalyst surface hydrophobic: A total synthesis of a hydrophobic catalyst is possible by carefully tuning the precursor or the reaction conditions to achieve the proper amount of polar functionality on the surface. On the other hand, post-synthesis functionalization of an already prepared catalyst with mainly organic or organosilane molecules (Figure 3) is another way to increase the hydrophobicity (Table 2).
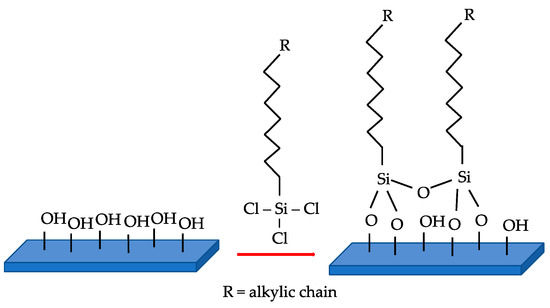
Figure 3.
Example of trichlorosilane grafting procedure on surface hydroxyl groups.

Table 2.
Strategies for the synthesis of hydrophobic catalysts.
2.1. Zeolites
Zeolites are one of the most widely employed class of acidic catalysts used in industrial chemistry, especially in oil refinery and production of chemical commodities [17]. They are very versatile due to shape selectivity and ability to be subjected to ion-exchange. Zeolites are basically crystalline aluminosilicates possessing a different Si/Al ratio that influences the physical and chemical properties of the solid. Zeolites typically present both Brønsted and Lewis acidic sites that can, however, experiment a severe deactivation mechanism in the presence of aqueous environment. Moreover, zeolites under hydrothermal conditions suffer also of dealumination drawback with subsequently structural instability [12,13]. Enhancing the hydrophobic surface properties of a zeolite weakens the binding of water to the active sites and prevents dealumination, thus increasing the catalyst life. Furthermore, a more hydrophobic surface can enhance the affinity with organic substrates. There are diverse methodologies to increase the hydrophobicity of a zeolite. It has been reported that wettability of zeolites can be tuned by modulating the Si/Al ratio: in fact, hydrophobicity linearly increases with the silica content [18,19]. High-silica content zeolites can be obtained subjecting a zeolitic material to an HNO3 treatment in order to dealuminate the structure [20]. Another reported methodology to increase zeolite hydrophobicity is to coat the surface with a non-polar silane compound such as octadecyltrichlorosilane. This hydrophobic functionalization improves hydrocarbon contaminant adsorption in water [21]. Moreover, it also makes zeolites able to stabilize water/oil emulsion and to catalyze reactions in this environment, such as alcohol dehydration [22] and alkylation, with an enhanced hydrothermal stability [23].
Jin et al. described a method to obtain hydrophobic Ti-incorporated Y-zeolites, for formaldehyde removal, anchoring organic functionalities, i.e., -CH3, -Ph and -CF3, on the framework of faujasite during the crystallization process and successively fixing TiO2 within the crystals [24].
With respect to the total synthesis method, Xu et al., instead, experimented that a Ti-incorporated MFI zeolite crystallized in a microwave oven was more hydrophobic if compared to the one prepared with the traditional hydrothermal method. The IR spectra analysis shows the presence of a lower amount of hydroxyl group on the surface imparting to the catalyst the capacity to adsorb an higher amount of 1-hexene [15].
In the case of zeolite supported metal catalysts, a recent work shows the synthesis of AuPd alloy hydrophobic zeolite catalyst allowing to enhance the methanol productivity in methane oxidation. AuPd alloy nanoparticles can be fixed within aluminosilicate zeolite crystals, and subsequently, the external surface is functionalized with organosilanes able to increase hydrophobicity. The silanes make the diffusion of hydrogen, oxygen and methane to the catalyst active sites easier, while blocking the peroxide to increase its reaction probability [25].
2.2. Silicas
Silicas are materials widely employed in heterogeneous catalysis mainly as support for metals or metal compounds due to their high surface area and to their inert nature. Silicas are materials made of interlinked tetrahedral SiO4. The structure pattern can end either with a siloxane group (Si-O-Si) or with a silanol group (Si-OH). The latter is defined as isolated if no adjacent silanols are present, vicinal if two silanol groups are present or different silicon atoms are close enough to make a hydrogen bond and, finally, geminal if the two silanols are attached to the same silicon [26]. Due to their polar nature, the quantity of silanols group influences the silica surface hydrophilicity and, therefore, the affinity with water. However, a high population of silanol groups on the silica surface could lead to a poor moist tolerance and hydrothermal stability of the material. In fact, the presence of water adsorbed on the -OH of the silanol groups can cause the hydrolysis of the siloxane bonds and, as a consequence, a structure collapse [27]. After these considerations, the improvement of the stability of a silica material is crucial to achieve a more stable and long-life catalyst, especially if the catalyst has to be used in a moist or watery reaction environment. Thus, in order to increase the structural stability and moist resistance of a silica, the population of surface silanols can be diminished through functionalization, mainly with organic compound, to arise the hydrophobicity of the surface. Similarly, for the case of zeolites, diminishing the number of hydrophilic silanols makes silica surface more accessible for organic molecules.
The hydrophobization of silica materials could be achieved without compromising the activity of a catalyst but also increasing its stability. As reported, a method to obtain hydrophobic phenyl sulfonic acid functionalized mesoporous SBA-15 silica (SBA-15-Ph-SO3H) consists in the silanization of activated mesoporous SBA-15 with dichlorodiphenylsilane followed by silylation and sulfonation [28]. This procedure allows to obtain a Brønsted acidic catalyst with a boosted stability towards water and leaching compared to the silica sulfuric acid and the simply sulfonated SBA-15. Analogically, in the work of Siegel et al., propylsulfonic functionalized benzenesilicas were prepared, and acidic sites content was monitored with 1H nuclear magnetic resonance (NMR). 2D proton spin-exchange NMR explained also that the proximity of the hydrophobic phenyl ring to the acidic sites could protect them against water deactivation, explaining the fact that water has no negative effect on catalytic activity concerning low proton-loaded catalysts [29].
The introduction of metal nanoparticles on the surface of a hydrophobic silica is not so trivial. A way to produce hydrophobic supported metal catalyst is to hydrophobize the surface after the anchoring of the metal. Ru/SiO2 can be synthetized with an impregnation method and subsequently functionalized with trimethylchlorosilane by using a post grafting method [30]. Another strategy to obtain a metal supported hydrophobic catalyst consists in preparing phenyl-modified amine-bridged silica with a water-in-oil reverse microemulsion. In fact, the phenyl groups create the hydrophobic layer on the silica surface, and the amine groups in the framework allow to anchor platinum nanoparticles that act as active sites for the oxidation of aliphatic alcohol in acids [31].
Omota et al. reported a method to functionalize hydrophilic silica with dichlorodimethyl silane at various silane/silica ratios to ensure different degrees of hydrophobicity. Using this strategy, a hydrophobic silica supported Pd catalyst was achieved by impregnation of the metal after silanization. Hydrophobic Pd/SiO2 catalyst was also prepared impregnating the silica and subsequently functionalizing it with silane. The two hydrophobic catalysts showed superior catalytic performances in the hydrogenation of methyl acrylate in aqueous solution, in particular, the one impregnated after silanization [32].
The functionalization with organic compounds can be also applied to silica mixed with other oxides. Tetra ethoxy silane combined with methyl triethoxy silane could be used to introduce methyl functionality on TiO2-SiO2 mixed oxide in a controlled way to obtain a more hydrophobic surface useful to improve the catalytic performance in cyclooctene epoxydation reaction. However, a too high degree of methylation, and thus hydrophobicity, can be detrimental for the activity of the catalyst due to a lower affinity to H2O2 [33]. Kong et al. showed a method to increase water tolerance of ZrO2-SiO2 acid catalyst used in biphasic esterification of glycerol with oleic acid. The mixed oxide was prepared covering the ZrO2 with SiO2, and subsequently, the surface was modified with trimethoxymethylsilane and 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane tailoring the acidity and surface hydrophobicity [34].
It is not common to find a total synthesis of hydrophobic silica in the literature. One example is the synthesis of superhydrophobic silica aerogels that could be achieved by using methyltrimethoxysilane (MTMS) precursor by a two-step (acid–base) sol–gel process followed by supercritical drying. The obtained hydrophobic aerogels show a water contact angle ranging from 158° to 164° and a thermal stability up to 530 °C [16]. In Table 3, the most common hydrophobic functionalizations of zeolites and silicas are shown.

Table 3.
Hydrophobic functionalizations for zeolites and silicas materials.
2.3. Polymeric Catalyst
Polymers are widely employed in the field of catalysis. Cross-linked polymers are used as supports for different kind of active phases thanks to their insolubility. In fact, the recycle of most catalyst is not always possible as in the case of organometallic catalysts. The use of a cross-linked polymer as the support allows separation and reuse of the catalyst [35]. The active phase is adsorbed or incorporated directly into the polymeric chain as functional groups like in the case of ion-exchange resins. Acidic and basic ion-exchange resin catalysts promote a large variety of organic reactions. The most widespread are styrene-based sulfonic such as Amberlyst® (acid) and Dowex® (acids and basics) or perfluorinated sulfonic acids such as Nafion® in which the active species is sulfonic acid. These are able to substitute mineral acid in condensation, alkylation and isomerization reaction [36,37]. Like the other catalysts cited in this review, the presence of water is detrimental for the active sites of polymeric catalyst. In the case of sulfonic resins for example, the -SO3H functional group can be deactivated due to water adsorption. Moreover, hydrophobic or weakly polar organic compounds have poor affinity with the hydrophilic active sites, so it is crucial to increase the surface hydrophobicity also in the case of resin catalysts, preventing deactivation of active sites and improving the activity towards organic substrates. Styrene-based resins have an intrinsic hydrophobicity because of their hydrocarbon-based structure. However, acidic or basic functional groups incorporated in the polymeric structure impart a certain hydrophilicity to the surface. To properly allocate the number of functional groups in the resin network is a crucial aspect in order to tune the surface hydrophobicity. It was demonstrated that a novel synthetized sulfonic acid porous resin (PS) and a fluoride sulfonic acid resin (PCS) have a better hydrophobicity and affinity towards organic molecules compared to the commercial analogues Amberlyst-15 and Nafion-212 as confirmed by measuring the contact angle of water [38]. This is related to a lower presence of sulfonic and perfluorosulfonic groups respectively with respect to the commercial resins. The enhanced hydrophobicity not only improves the catalytic activity of the materials but also increases the selectivity to the desired product (FCF) by inhibiting the secondary reaction that involves water in the hydroxyalkylation /alkylation of 2-methylfuran (2-MF) with ketones. Thus, conversion of 2-MF goes from 45% to 61% and selectivity to FCF from 72% to 84% moving from Amberlyst-15 (contact angle = 65°) to the 4.28% PS resin (contact angle = 158°) (Figure 4). A similar effect on selectivity was obtained in the same reaction in the presence of phenylsulfonated biochar. Thus, the hydrophobic biochar modified with 6.63% PhSO3H acid (contact angle = 114°) allowed to obtain 70.5% of FCF owing to a 84.4% selectivity whereas Amberlyst-15 gave only a 46.5% yield [39]. Zhang et al. proposed a method in which a sulfonic acid styrene-based resin was synthetized under solvothermal conditions and copolymerization of divinylbenzene with sodium p-styrene sulfonate followed by ion-exchange of a H2SO4. The obtained resin exhibits a lower number of acidic sites if compared to the commercial Amberlyst-15 (0.76 mmol/g versus 4.6 mmol/g). However, it shows a marked hydrophobic character displaying a water contact angle of 154°, much higher if compared with the commercial sulfonic resin [40]. Similarly, Liu et al. demonstrated a synthesis method to produce a novel hydrophobic mesoporous polymeric solid acid catalyst that consists in the copolymerization of divinylbenzene with sodium p-styrene sulfonate (H-PDVB-x-SO3H’s) under solvothermal conditions with an adjustable sulfur content. It is worth noting that a lower sulfur content and consequently a lower number of acidic sites correspond to a higher hydrophobicity. In fact, the H-PDVB-x-SO3H’s present a lower sulfur contents (0.31−2.36 mmol/g) and acidic concentrations (0.26−1.86 mmol/g) if compared to PDVB-SO3H and commercial Amberlyst-15 (sulfur content 3.64 mmol/g and 4.30 mmol/g, respectively; acidic concentration 4 and 4.7 mmol/g, respectively). The H-PDVB-0.05-SO3H’s that has the lower sulfur content (0.32 mmol/g) presents the higher contact angle of the entire series (152°). Moreover, all the H-PDVB-x-SO3H’s catalysts present lower contact angle measures performed with organic molecule again compared with PDVB-SO3H and Amberlyst-15, demonstrating a higher affinity towards organic substrates [41]. The same authors reported later a synthesis of the strong solid acid PDVB-SO3H-SO2CF3 grafting the strong electron withdrawing group -SO2CF3 onto the network of mesoporous solid acid PDVB-SO3H, using HSO3CF3. This method could maintain a good level of acidity of the solid together with the hydrophobicity of the surface thanks to the electron withdrawing power and the intrinsic polar hydrophobicity of the fluorinated groups [42]. In Table 4, hydrophobicity related to the sulfur content is showed.
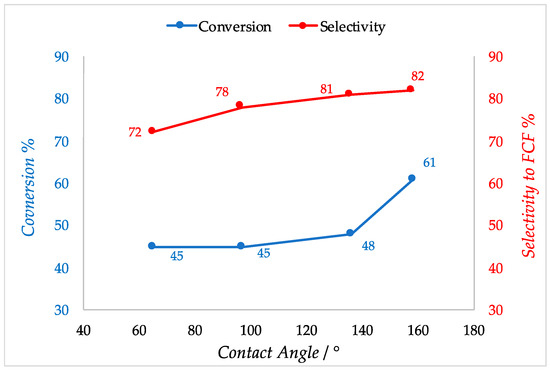
Figure 4.
Conversion and selectivity of Polystyrene resins vs contact angles in the hydroxyalkylation/alkylation of 2-methylfuran (2-MF) with ketones. Adapted from Reference [38].

Table 4.
Hydrophobicity related to sulfur content of sulfonic acid-based resins. a Determined by elemental analysis. Adapted from Reference [42].
3. Characterization of Hydrophobic Catalyst
In this section, methods and techniques to characterize the hydrophobic properties of a catalyst will be discussed. Compared to many other characterizations, the assessment and quantification of the hydrophobicity of a surface is not a straightforward analysis; thus, there is not a unique and direct way. By choosing various experimental approaches and physical measures, different indirect informations about the solid surface can be collected and have to be interpreted with specific criteria. In fact, it has to be taken into account that the analysis setting rarely reflects the real operative conditions of the catalyst or the real surface properties of the same. The evaluation of hydrophobic properties can be approached directly by investigating the surface and bulk composition and structure or by observing the interactions of the solid with a specific gas or liquid (in general water). The most common and diffused techniques in catalysis are showed in Table 5.

Table 5.
Methods of hydrophobic properties evaluations.
3.1. Conctact Angle Measurements
As already mentioned in the introduction, contact angle (CA) measurements are probably the most common way to evaluate surface wettability. This kind of analysis is performed by measuring the tangent angle that a liquid drop forms on a solid surface. The relationship between solid surface tension and the CA formation was firstly recognized by Young [43]. The angle is, in fact, defined by the mechanical equilibrium to which the drop is subjected by the action of three interfacial tensions (Figure 5):
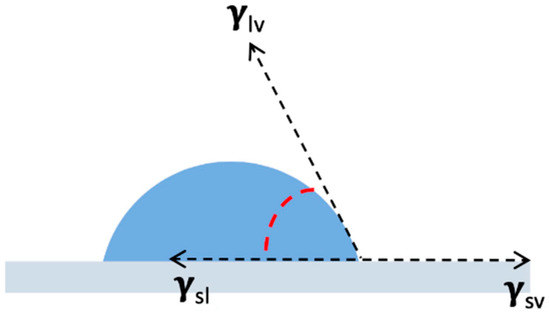
Figure 5.
Interfacial tensions acting on the drop-surface system.
- Solid–liquid;
- Solid–vapor;
- Liquid–vapor.
The equilibrium is described by Young’s equation:
where θY = Young contact angle; γ = interfacial tension (lv = liquid-vapor, sv = solid vapor, sl = solid-liquid).
γlv cos θY = γsv − γsl
Although being a very common method to assess hydrophobicity of a surface, its application on heterogeneous surface is not so trivial. In the case of rough surfaces, CA are larger than those observed with the smooth ones (contact angle hysteresis), thus leading to a misleading interpretation of the results. CA also reflects surface topography and not only surface energetics [7,44]. Moreover, in the case of porous solids, the liquid could be adsorbed in-between the pores making the measurement of the angle impossible to be done [6].
However, while taking these limitations into consideration, contact angle measurements can provide easily an assessment of the hydrophilicity or hydrophobicity (angle major or minor of 90°), especially if solid catalysts of similar chemical nature are analyzed.
CA are typically measured using the so called sessile-drop technique, in which a drop of liquid is let fall on the surface, and then, with the help of a camera, the tangent angle is measured with goniometric methods [7].
Water is the most common liquid used in the assessment of surface hydrophobicity showing precisely how much the surface repels it. However, other liquids can be used; in particular, organic liquids can show directly the affinity of the catalyst for the organic substrates of interest.
As already said, CA measurements are easily performed to characterize the hydrophobicity of a catalyst [45,46] and can be related to catalytic performances of a catalyst. Wang et al. utilized contact angle measurements for the characterization of their hydrophobic sulfonic resins demonstrating also that the higher the contact angle the better the catalytic performance in the dehydration of fructose to HMF. Thus, the catalyst with the highest contact angle does not catalyze the consecutive reaction of HMF hydration to levulinic acid by keeping the water away from the substrates [47].
Contact angle investigations are also important to assess the affinity for the substrates of catalytic interest. Some of us showed that a more hydrophobic silica with an higher 1,3-propandiol contact angle used as the support for the synthesis of hydrogenation copper catalyst provides an higher catalytic activity in the selective double bond hydrogenation of α-β-unsaturated ketones in agreement with the easy interaction of C=C double bond of the reagents with hydrophobic surfaces [48].
In the work of Liu et al., contact angle between a hydrophobic mesoporous sulfonic resin and some organic liquids such as cyclohexanol, 1-butanol, acetic acid, glycol and benzaldehyde is measured giving angles ranging between 0° and 25° whereas the angle with water is 137° and demonstrating the affinity of the resin for organic substrates [41].
3.2. Adsorption Isotherms
As already mentioned, hydrophobicity has not a unique definition. In general, hydrophobicity could be defined as the absence of strong adsorption capacity of polar compound in particular water. Apart from liquid–solid interaction evaluation as in the case of CA measurements, adsorption from a gas phase could be a useful tool for hydrophobicity evaluation, especially if CA measurements are not possible due to structural limitations. In fact, it can provide important information on the surface affinity for a certain compound or in general surface-related properties such as dipole moment, polarizability and acidity.
A straightforward way to apply this technique is to evaluate the adsorption capacity: A more hydrophobic solid adsorbs less water per unit of area or mass if compared to a more hydrophilic one in the range of all relative pressures (Figure 6) [49]. However, the determination of the adsorption capacity could be challenging if adsorbate–adsorbate interactions take place, especially in the case of mesoporous or microporous solids due to the higher possibility of multilayer adsorption. To overcome this problem, adsorption capacity should be compared at low loading, without exceeding the monolayer adsorption. Nonetheless, water monolayer is not easy to be obtained due to local increase of water concentration caused by hydrogen bonding, acidic sites or highly polar sites. Thus, it is convenient to compare adsorption capacity among solids similar in nature under a well-defined set of conditions [6]. There are some examples of hydrophobic characterization through water adsorption isotherms [49,50,51,52]. A clear example is showed by Kamegawa and co-workers that registered the water adsorption capacity for triethoxyfluorosilane (TEFS) grafted Ti-modified mesoporous silica SBA-15. The relative water adsorption capacity at P/P0 diminishes with the increase of TEFS loading (Table 6), thus demonstrating the increase of the solid hydrophobicity of the fluorosilane-modified silicas [53].
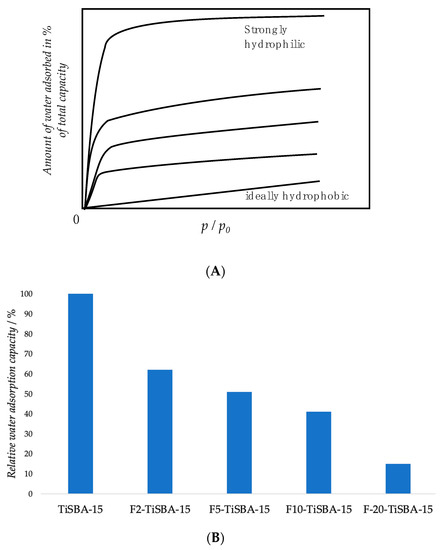
Figure 6.
(A) Example of idealized water adsorption isotherms in the case of zeolitic materials. Adapted from Reference [54]. (B) Water adsorption capacity for TiSBA-15, F2-TiSBA-15, F5-TiSBA-15, F10-TiSBA-15 and F20-TiSBA-15 measured at 298 K [53].

Table 6.
Relative water adsorption capacity compared to TEFS loading on TiSBA-15 at P/P0 = 0.6 [53].
Water is the most common compound to assess hydrophobicity through adsorption isotherms, but many other adsorptives can be used for this purpose. Hexane [55] and toluene [56] are used as water complementary: the higher the hydrophobicity, the higher the power of organic molecule adsorption capacity.
Multicomponent adsorption is also a used technique. This is the case of water and toluene competitive adsorption used to determine the Hydrophobic Index (HI) of zeolites introduced by Weitkamp [57]. This parameter is defined as
with Xtoluene and Xwater as the equilibrium loadings of the adsorbent with toluene or water, respectively, taking in consideration that Xi = mi/mzeolite. A more hydrophobic solid will have a higher HI, that means an increase in toluene adsorption compared to water.
HI = Xtoluene/Xwater
HI is a useful tool to easily understand hydrophobic properties of a solid together with an insight into the pore structure. The competitive water and toluene adsorption experiments were carried out on SiO2, MCM-41, titanium grafted SiO2 and MCM-41 to understand the possible role of titanium in the hydrophobic properties of the solid. Moreover, HI was measured also for silylated titanium grafted SiO2 and MCM-41 (Table 7). It is noticeable that the presence of titanium does not affect the silica and MCM-41 HI although it increases the water and toluene adsorption capacity in the case of Ti-MCM-41. As expected, the HI values increase passing from non-silylated to silylated materials due to an increase affinity for toluene, especially considering Sil-Ti-MCM-41 (HI increasement of 62% vs 13% of Sil-Ti-SiO2) because of a better toluene confinement caused by narrower mesopores [58].

Table 7.
Competitive water/toluene adsorption capacity and Hydrophobic Index. Adapted from Reference [58].
Adsorption kinetics can be employed also to directly measure the hydrophobic affinity with the organic substrates of interest. Zhang and coworkers demonstrated that the adsorbed quantities of cyclopentanone, cyclohexanone and methylfuran are higher for their synthetized hydrophobic resins if compared to the commercial Amberlyst-15. This means that these catalysts have more affinity with the reagents in the hydroxyalkylation and alkylation of 2-methylfuran [38].
3.3. Thermogravimetric Analysis
Thermogravimetric analysis (TGA) is an experimental technique based on measuring the mass variation of a sample using a microbalance as a function of the temperature. The experiments are carried out at constant heating rate or at constant temperature in inert or oxidizing atmosphere setting the desired temperature range. The output is a graph in which the mass percentage is plotted against temperature. The mass of a sample changes whenever it loses material due to the temperature changes [59]. TGA is useful to quantify the carbon, hydrogen or oxygen content on a solid surface for example. It is a direct technique to investigate the nature of the solid because it avoids the measurement of some kind of interaction between the catalyst and a compound. The advantages are the ease of setup and the possible automatization. On the other hand, it is a destructive technique and materials cannot be recovered after the analysis.
In the case of hydrophobicity studies, TGA is mainly used for investigating the water affinity of a solid catalyst or for the quantification of the -OH groups present on the solid surface that however represent an indirect water affinity quantification as seen in the previous section. Setting an appropriate method, it is possible to assess and quantify the water loss of a solid together with the dehydroxylation. In the case of silica and titania, Mueller et al. set up a method to determine physisorbed water and silanol groups into two steps: The solid is in nitrogen from 25 °C to T1 = 120 °C (average value comparing the literature) at 10 °C/min, holding this temperature for 10 min and then heated at 20 °C/min to T2 = 800 °C in the case of silica or 500 °C in the case of titania and held at this temperature for 10 min [60]. The weight loss experimented in the first step is related to the physisorbed water; however, it strongly depends on the measure environment humidity. The second represents the weight loss derived from silanol groups removal from the surface, and the quantification for unit of area can be obtained with the following formula:
where wtTi is the sample weight at the corresponding temperature Ti, MWH2O is the molecular weight of water, NA is Avogadro’s number, and α is a calibration factor. In the case of silica, 1 OH/nm2 remains on the surface at T2 while in the case of titania the surface is free from hydroxyl groups at T2 [60].
OH/nm2 = α {(OH/nm2)T2 × SSA × wtT2 + [(wtT1 − wtT2) / MWH2O / NA × 2]} / SSA × wtT1
This method could be used not only for silica and titania but also for fumed silica, barium glass and zirconia [61].
A more straightforward way consists in measuring the loss of physisorbed water in the range of 30° and 900 °C and normalizing for the final mass registered. The comparison between non-modified titania-silica and a methyl modified titania-silica showed that the free material has a higher quantity of physisorbed water if compared to the modified ones. The methylated catalyst has a lower water affinity and demonstrated also a higher catalytic activity in the epoxidation of cyclooctene [33]. Similarly, a simpler method to measure the surface silanol density consists in always measure the water loss of Ti-SiO2 and Ti-MCM-41 but in the range between 150 °C and 900 °C instead [58]. Han et al. also indicated that the bare ZSM-5 zeolite exhibited a sharper mass decrease in the temperature range around 100 °C respect to silane modified ZSM-5 (Figure 7). In this case TGA was used also to monitor the loss of organic content assessing the stability of the silanization of the surface. In fact, the chlorosilane decomposition occurs at temperature higher than the boiling one [62]. In a previous paper instead, a temperature range between 30 °C and 150 °C is used to measure the water loss of silanated Ti-TUD-1 catalyst showing another time that a the higher the water loss the lower is the hydrophobicity of the catalyst and, in this case, the lower is the catalytic activity [63].
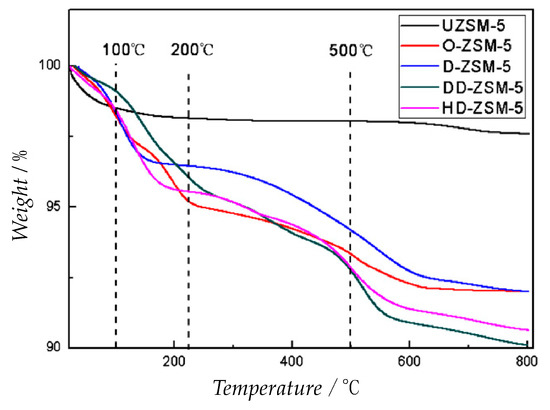
Figure 7.
Weight-loss profile of chlorosilane modified ZSM-5 adapted from Reference [62] (O = octyltrichlorosilane, D = decyltrichlorosilane, DD = dodecyltrichlorosilane, HD = hexadecylthriclorosilane). The major water loss is experimented by the bare UZSM-5. As showed, the decomposition of chlorosilane happens in the range of 500 °C, a higher temperature than the silane boiling point (from 233 °C to 269 °C).
3.4. Infrared Spectroscopy
Infrared spectroscopy (IR) is one of the most commonly used characterization techniques in catalysis. IR can be used as a direct way to investigate the surface composition of a solid catalyst or as an indirect way, by studying the interaction between the surface and an adsorbing species and the way in which they are chemisorbed.
Concerning surface hydrophobicity, IR can be used as a direct method to quantify the polar or non-polar groups present on a solid surface, for example the hydroxyl groups [64], thus having an insight on the surface polarity. In the work of Arias et al., Beta-zeolites with different Si/Al ratio were synthetized in fluoride media in order to achieve a smaller amount of silanol groups on the surface. These samples were compared to the Beta-zeolites synthetized in OH− media by recording their IR spectra. It is noticeable that the silanol band at 3745 cm−1 is less pronounced for the fluoride media synthetized zeolites respect to the one synthetized in OH− media (Figure 8). Moreover, the Beta-zeolites with higher Si/Al ratio (25) present a smaller peak compared with Beta-zeolites with lower Si/Al ratio synthetized in the same medium, and they exhibit the highest activity in the acetalization of HMF with n-octanol [65].
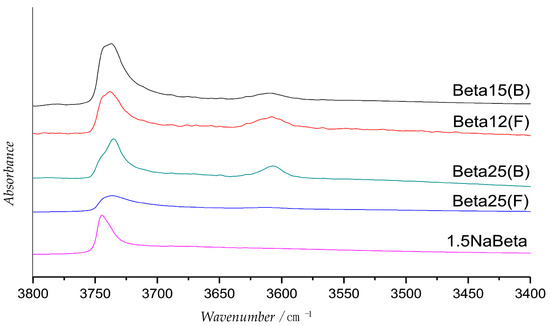
Figure 8.
IR spectra of Beta-zeolites synthetized in fluoride (F) and OH− media (B). The silanol band at 3745 cm−1 is less pronounced for Beta-zeolites prepared in a fluoride medium and for Beta-zeolite with higher Si/Al ratio. Adapted from Reference [65].
A more recent example shows the disappearance in Diffuse Reflectance Infrared Fourier Transform (DRIFT) spectra of the free surface silanol band after silane functionalization of MCM-41 both in the case of high temperature grafting and low temperature grafting. In fact, silane binds to surface -OH groups forming Si-O bonds. Moreover, C‑H vibration band appears after organosilane functionalization. Thus, the decrease of band intensity can be used as an indicator of functionalization success together with increase of hydrophobicity (Figure 9) [66].
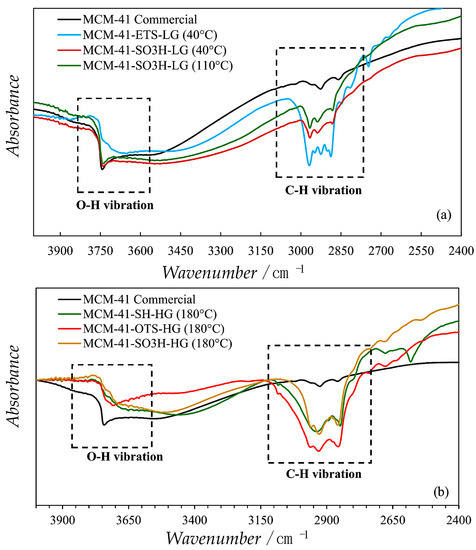
Figure 9.
The disappearance of O-H vibration band coupled with the appearance of C-H vibration band after organosilane functionalization of MCM-41 in the case of (a) materials functionalized at low temperature of grafting (LG) and (b) materials functionalized at high temperature of grafting (HG). Adapted with permission from Langmuir 2019, 35, 6838–6852, Reference [66]. Copyright (2019) American Chemical Society.
If IR is used as an indirect method, it allows to investigate the interaction between the surface of the catalyst and a polar or non-polar probe molecule. 1-Hexene was used for this scope because, in principle, a hydrophobic zeolite should have a higher affinity towards an organic compound. In Figure 10, the IR 1-hexene adsorption spectra on two type of zeolites, the more hydrophobic Ti-MFI-MW and the more hydrophilic Ti-MFI-CH, are shown. The 1-hexene adsorbed on Ti-MFI gave a series of adsorption bands at 3100–2780 cm−1. It is easy to observe how the intensity of the peak is much higher for the more hydrophobic zeolite indicating that it has a higher capacity of adsorption of organic molecules [15].
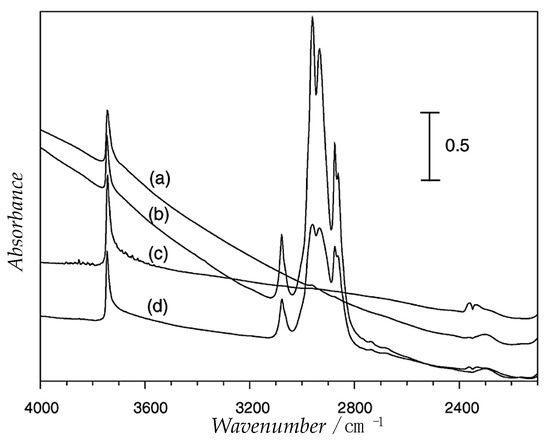
Figure 10.
FT-IR spectra of Ti-MFI-MW (a) before and (b) after 1-hexene adsorption and Ti-MFI-CH (c) before and (d) after 1-hexene adsorption. Adapted from Reference [15].
Infrared spectroscopy is also helpful to investigate how the hydrophobicity influences the affinity with substrates and products in a catalytic reaction. Wang et al. adopted IR to understand the role of wettability of silicalite-1 zeolite supported palladium catalyst in the selective hydrogenation of furfural to give furane. In this case, a more hydrophilic catalyst was more effective in this type of reaction. To justify this behavior, they studied the adsorption of furfural and furan on the bare Pd@S-1 and on the hydrophilic hydroxyl modified Pd@S-1-OH-10 (Figure 11). Furan desorbs easily respect to the other molecule on both samples demonstrated by a quick decrease of the band for C-O bond (1190–1203 cm−1). Moreover, furan desorbs better in the hydrophilic channels of Pd@S-1-OH-10 as showed also from the removal percentage value of 90% compared to 67% of Pd@S-1. On the contrary, the hydrophilic zeolite channels hinder the diffusion of furfural as the characteristic band at 1200 cm−1 maintain almost the same intensity. This demonstrated that the adsorption of the reactant furfural and the desorption of product furan was maximized by hydrophilic zeolite micropores [67].
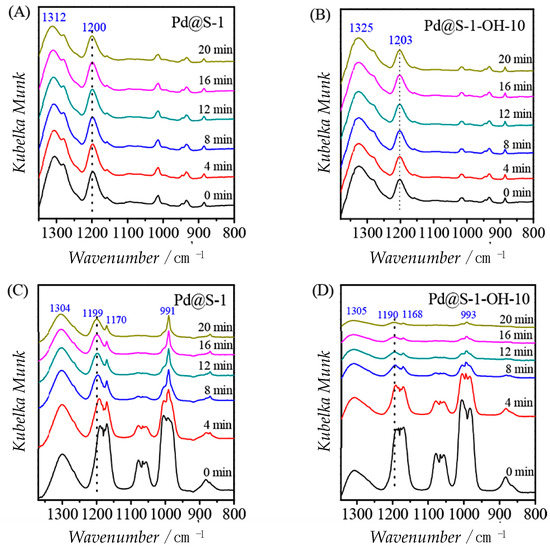
Figure 11.
In situ FTIR spectra of (A,B) furfural and (C,D) furan on the Pd@S-1 and Pd@S-1-OH-10 catalysts during the desorption test. Adapted from ACS Catal. 2018, 8, 474–481, Reference [67]. Copyright (2017) American Chemical Society.
3.5. Characterization Hints for Solid–liquid Interfaces
In the previous subsection, the importance of assessing the hydrophobic degree of a solid catalyst surface has been underlined, and some possible methods with direct or indirect approach have been listed and discussed. However, studying and characterizing the solid surface–liquid interfaces could be a key element to have a better comprehension of adsorption–desorption mechanism in solid‑-liquid catalysis. Some reviews have already covered this topic [68,69]. In this paragraph, some techniques will be highlighted with these purposes.
X-ray reflectivity studies could be addressed to understanding how water meets hydrophobic surfaces at a microscopic level. High-energy X-ray microbeams have been used to penetrate to the hydrophobic interface and to measure hydrophobic gap size between water and silicon wafer functionalized with a layer of octadecylthriclorosilane, a typical silane used to hydrophobized surfaces. The size could be obtained by fitting the reflectivity data versus the spatial resolution represented by the maximum momentum transfer of the experiment [70].
Sum frequency generation spectroscopy has been used as a tool to deeply discern the explanation of macroscopic contact angle measurements at a molecular level. Cyran et al. used this technique to investigate water organization at silica surface identifying weakly hydrogen-bonded water OH groups. Results demonstrated that hydrogen atoms of these OH groups are pointing toward local hydrophobic sites, namely, oxygen bridges of the silica. Consequentially, an increase in the macroscopic contact angle corresponds to an increased density of these molecular hydrophobic sites, stated by an increase in these OH groups [71]. Water orientation near silica surface can be discerned using also nuclear magnetic resonance spectroscopy exploiting the orientation dependence of the nuclear magnetic 1H-1H dipolar interaction [72].
Atomic force microscopy could be utilized even to directly map the interfacial energy between a liquid and a solid building a model to clarify the mechanism ruling the image formation. This model shows also that the energy dissipated during the experiment is related to the interfacial energy [73].
4. Application of Hydrophobic Catalysts
The chance to tune the hydrophobic/hydrophilic nature of the catalyst surface has a significant impact on synthetic strategies. Thus, when facing with processes involving reagents and products markedly different in the polar character, their contrasting adsorption/desorption behavior can rule the reaction as far as both activity and selectivity are concerned. Figure 12 shows the main fields of action related with the design of hydrophobic materials for catalytic purposes.
Figure 12.
Main effects on catalysis obtained by designing proper hydrophobic materials.
Great part of the work on this topic relies on the relationship between hydrophilicity/hydrophobicity and catalyst stability in terms of both acidity and hydrothermal properties.
Acidic materials have seen a rapid and increased interest in their application due to their use in biomass transformation. In this respect, a proper design and modulation of the hydrophobicity of the surface allows one to have at hand an acidic catalyst with enhanced water tolerance, thus granting minor deactivation during reaction. It has been reported that hydrophobic porous void space in Sn-Beta-F and Ti-Beta-F zeolites are able to catalyze glucose isomerization to fructose in aqueous medium showing also high stability while hydrophilic Ti-Beta-OH and TiO2–SiO2 exhibit no catalytic power in this case. In fact, Sn and Ti Lewis acidic centers are active when they are situated in hydrophobic channels of Beta zeolite but not when located within hydrophilic void spaces or at hydrophilic surfaces of TiO2–SiO2 and Ti-Beta-OH. The hydrophobicity of the internal environment shields water penetration preventing deactivation of the Lewis Ti and Sn centers during glucose isomerization [74]. Similarly, hydrophobic functionalization of HY zeolites enhances its catalytic activity, stability and reusability in hot aqueous media like in the case of refining of biomass pyrolysis oil. Hydrophobization of the zeolite surface prevents the contact with water; hence, the disruption of the crystalline structure leaving acidic active sites unaltered [22]. Tucker et al. reported that ethane-containing PMO-based propylsulfonic acid catalysts were more stable than non-organic functionalized analogue catalysts and commercial pSO3H-SC in the aqueous fructose dehydration to give 5-hydroxymethyl furfural. The main deactivation cause was found to be the hydrolytic cleavage of the acidic sites. Again, hydrophobic microenvironment decreases local water concentration and, thus, acid sites hydrolysis [75].
Likewise, hydrophobicity has a strong influence on zeolites hydrothermal stability. Thus, hydrophilic silanol groups formed during zeolite synthesis are known to be the main active sites responsible for the hydrolysis of the matrix framework occurring when working under hot liquid water conditions, namely >150 °C, that are typically employed in many biomass transformations. In this respect, the hydrophobization of zeolites reveals to be an interesting strategy alternative to the un-green one based on to use charge-balancing anions such as F− in order to prevent –SiOH sites formation. The hydrophobization process hinders water molecules to approach to zeolites surfaces, thus preserving the framework against hydrothermal conditions. Several hydrophobization approaches have been used for this purpose and are very well described in a review by Sudarsanam et al. [11].
Another important aspect relates to the influence of hydrophobicity on adsorption and interface phenomena. Once again, the need to design efficient catalysts for biomass conversion has stimulated a growing interest in the understanding of solid–liquid interactions, a field less explored with respect to the one of gas-phase reactions. Very interesting insights into these phenomena have been reported by Sievers et al. in a perspective paper [4]. The authors underline the great importance of hydrophobic features of the catalyst surface when facing with oxygenated compounds typically derived from biomass deconstruction streams, namely, polyols, carbohydrates and phenols. With oxygenated compounds, particularly those bearing hydroxyl groups, the interaction with the surface dramatically addresses the reaction selectivity due to the different nature of interactions dominating the process and ranging from hydrogen to covalent or ionic bonds. In this respect, the surface polarity can have a pivotal role, as is also highlighted in a study on cyclohexanol conversion in water mediated by zeolites [76].
Therefore, the hydrophobic/hydrophilic character of the catalyst could have a significant role in favoring the contact of reagents and/or in addressing product removal, and once again, building-blocks derived from biomass are among the best examples of this effect.
In particular, condensation reactions such as esterification or etherification of fatty acids are very well-fitting examples on order to understand this strategy (Figure 13). In the following section, some selected examples are analyzed in order to understand the importance of the reactants/products adsorption to address activity and selectivity of the reaction, particularly in relation to their polarity.
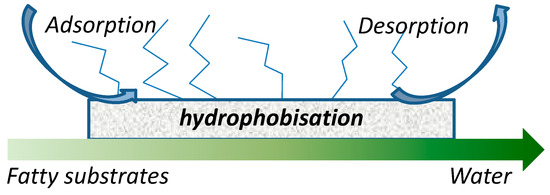
Figure 13.
Preferential adsorption of fatty acids and preferential desorption of water on hydrophobic catalysts.
4.1. Fatty Acid Esterification
Reactions involving fatty acids represent a class of processes very well-fitting with the design of a properly modified surface in order to favor the preferential adsorption of a substrate and in turn to rule on the activity or selectivity of a reaction.
In particular, the preparation of ethers and esters of fatty acids or alcohols with polyols such as glycerol has been the matter of study in this respect.
Monoglycerides (MG) are a class of compounds highly requested at industrial level due to their application as emulsifier in the food industry, as surfactants in detergency, as well as antibacterial agents in animal husbandry and aquaculture [77].
Two main strategies can be used for their preparation, namely, transesterification of triglycerides with alcohols in the presence of a basic catalyst or direct esterification of glycerol with a fatty acid in the presence of an acidic system. The latter route is one of the most pursued in the case of solid catalysts, but its success strongly depends on the capacity of the protocol to optimize the yield in mono- and diglycerides while reducing as much as possible the formation of triglycerides. Thus, their presence reduces the emulsifier properties due to the lack of hydrophilic functionality. As an example of a proper final product composition, the food additive commercially labelled as E471 and derived from C16 and C18 fatty acids is a mixture typically in the range monoglycerides:diglycerides of 40:40, whereas for more profitable applications such as cosmetic ones, higher concentrations of monoglycerides are required.
To obtain high selectivity values is not trivial, and several strategies have been exploited such as the selective precipitation of monoglycerides, the protection of 1,2-hydroxyl groups of glycerol via ketal formation, as well as the adsorption/desorption of hydrophobic/hydrophilic molecules.
A significant increase in selectivity, for example, has been reported to be obtained through the hydrophobization of mesoporous silica-supported sulfonic acid, due to the better diffusion of the fatty acids inside the porous framework of the catalyst (Figure 14) [78].
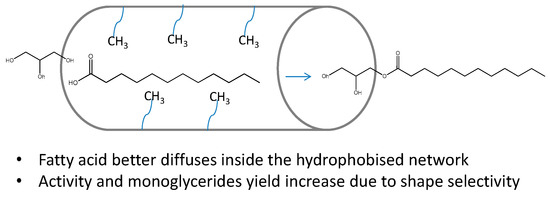
Figure 14.
Schematic representation of the shape-selectivity effect in monoglyceride formation assisted by the surface hydrophobization.
As already reported from the groups of Jacobs [79], the difficult diffusion of fatty molecules inside the pores hinders the positive effect of the shape selectivity. Therefore, a strategy aimed to improve the contact of fatty reactants with the framework surface could be a good point to look at.
The same effect has been claimed by Kong et al. [34] in the use of a modified silica zirconia catalyst (Me&Et-PhSO3H-SiO2-ZrO2) in the esterification of glycerol with oleic acid for the preparation of the corresponding monoglycerides. Under the optimized conditions, the obtain a selectivity of 84.5% in MG at 39% of conversion. The paper relies on the fine design of a modified ZrO2-SiO2 catalyst able to maintain interesting acidic properties in spite of the surface modification with trimethoxymethylsilane (TMMS) and 2-(4-chlorosufonylphenyl)ethyltrimethoxysilane, while also favoring the preferential framework diffusion of oleic acid.
The significant effect of hydrophobization when having in hand fatty substrates and their competition with water for the catalytic site has been also exploited in the preparation of catalysts for biodiesel production. In this case, the water produced during esterification unavoidably contributes to the reverse reaction of hydrolysis, thus reducing the yield in the desired esters. A zirconium-containing periodic mesoporous organosilicas (Zr-PMOs) has been proposed for this purpose, revealing significant differences if compared with the analogous Zr-SBA-15 [80]. A similar approach has been used also in the preparation of arensulfonic SBA-15 [81]. In both cases, a set of experiments carried out in the presence of water clearly show the increase in fatty acid methyl esters (FAMEs) yield when using the hydrophobized materials. Thus Zr-PMOs in the presence of water allowed to increase the yield in FAMEs from 50% to 70% and likewise hydrophobized arene-SO3H-SBA-15 led to more than 85% yield starting from 75% obtained with the non-hydrophobized parent systems.
On the other hand, the interaction of glycerol with the catalytic surface in the presence of the water produced during the reaction has been claimed as a critical point in the etherification with alcohols. In particular, in the etherification with ethanol and tert-butanol by using a series of zeolites with different Si/Al content, a clear correlation was found between glycerol conversion and hydrophobicity index, expressed as Rtoluene/water. In fact, the higher the index, the higher the conversion [82]. Thus, the strong glycerol adsorption on the surface of more hydrophilic samples hampers glycerol participation in the etherification reaction. Similar evidence was also reported when using glycols others than glycerol [83].
4.2. Sorbitol Dehydration
To scavenge water formed during a condensation reaction is definitely the main application of hydrophobization as functionalization strategy not only to preserve the acidic properties of the catalyst [10] but also to push the reaction to higher conversions by leveraging the preferential adsorption/desorption of reactants and products.
Several cases are reported in this respect when facing with condensation or dehydration reactions, particularly with biomass derived materials.
One interesting example is the dehydration of sorbitol into isosorbide. This reaction is deeply studied due to the interest in isosorbide as high added value intermediate for the preparation of polymer, functional material, solvent of cosmetics, pharmaceutical molecules, surfactants, plasticizers, food additives and even as a fuel or fuel additive [84]. Its preparation starting from sorbitol, in turn obtained by reduction of glucose, is hampered by a double-step dehydration as shown in Scheme 1.
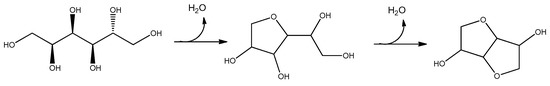
Scheme 1.
Dehydration of sorbitol to isosorbide.
The acidic properties and density of the catalyst surely play a major role in the efficiency of this process, but a proper design of the catalyst surface in terms of hydrophilicity/hydrophobicity appears to be another important point to be looked at. In particular, the right balance of acidic sites and hydrophobicity has to be carefully tuned in order to reach the best trade-off for high activity. Some studies based on the use of zeolites have been reported. Otome et al. reported that the use of high-silica large pore zeolites such as Mordenite (Si/Al = 110), beta(Si/Al = 75) and beta(Si/Al = 150) exhibited a high catalytic performance, and especially beta(75) showed selectivities to sorbitan and isosorbide of 44 and 33%, respectively, at sorbitol conversion of 87% [85]. The comparison between different Si/Al zeolites shows that that catalytic activity based on the catalyst is determined by a balance between the number of acid sites, mainly relying on the Al content, and the degree of hydrophobicity, increasing with the increase in Si content.
The gradual decrease in water and methanol adsorption with the increase in Si/Al had been already described as an important feature in these kind of materials [12].
The importance of finding the best trade-off between acid sites density and hydrophobicity in this reaction has been clearly evidenced in a paper by Fukuoka, showing a volcano-shaped curved obtained for of sorbitol conversion and isosorbide yield vs Si/Al ratio, with a maximum corresponding to Si/Al = 75 of Hβ zeolite, able to gain 76% yield (Figure 15) [86].
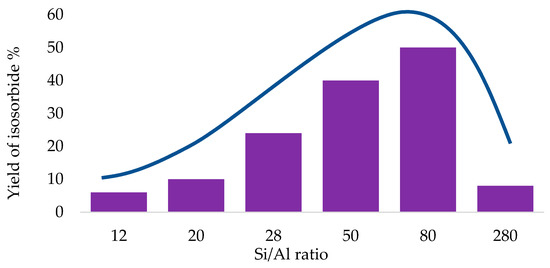
Figure 15.
Volcano plot for isosorbide yield vs Si/Al ratio.
Therefore, both the acidic density and the hydrophobicity have to be properly balanced in order to exploit the catalytic activity and the capacity to remove water from the surface.
In this respect, the very different interaction substrate/catalyst taking place for the two steps involved in isosorbide formation as depicted in Scheme 1 can play a major role as deeply discussed by Cubo et al. in the use of propyl-sulfonic functionalized SBA-15 [87]. The study demonstrates that the two steps are strongly and differently influenced by both the acidic surface density and the hydrophobicity.
In particular, a higher –SO3H density favors the second dehydration step sorbitan → isosorbide, whilst TOFs values for the first step sorbitol → sorbitan decrease by increasing the acid sites density. The main reason for this observation has to be ascribed to the interaction of the substrates with the acidic sites. The extended molecular structure of sorbitol allows its interaction with several catalytic sites at the same time. The multiple terminal protonation of –OH groups of sorbitol when the acidic sites are denser disfavors the cyclization process occurring via an intramolecular nucleophilic attack. The opposite effect is reasonably observed when sorbitan is the substrate and therefore in the second step.
Nevertheless, the second step is the rate determining one and to find the way to promote sorbitan conversion would reflect into a higher isosorbide yield.
The hydrophobization of the surface was found to be a good strategy to boost the second reaction step. In fact, the modification of sulfonic acid mesostructured silica with –Si(CH3)3 groups allowed to reach 70% yield of isosorbide vs 44% obtained with the unmodified catalyst, at almost equal sorbitol conversion. This means that hydrophobization is able to strongly influence the second step, thus increasing the yield in isosorbide at the expense of sorbitan. The authors ascribe this effect to the enhancement of the acidic strength of the catalyst. As already reported, the presence of organosilicon species on the surface promote the acid strength of sulfonic acid groups as it inhibits their interaction with surface silanols that smooths acidic properties [88].
Even higher yields in isosorbide, up to 87% can be obtained with a mesoporous polymer-based acid catalyst (P-SO3H) by virtue of its superhydrophobicity [40]. This feature allows to keep the water formed in the dehydration away from the catalyst, promoting the reaction equilibrium to move the product side. The interesting point rising from this research is that, in spite of the lower acidic content of the polymer-based catalyst, its superhydrophobicity, witnessed by a contact angle of 154°, grants much higher selectivity to isosorbide even in the presence of water.
Recently, also the use of a series of hydrophobic polymer-based solid acid catalysts (PDS) synthesized via hydrothermal synthesis method of sodium p-styrenesulfonate hydrate (SPSS) and divinylbenzene (DVB) and followed by ion exchange has been reported to promote sorbitol dehydration with very high performances [89].
The chosen examples (Table 8) show that catalyst surface modification with hydrophobic moieties in acidic systems, besides the widely studied advantage of limiting the acidic sites deactivation [10], could offer the opportunity to design an efficient catalyst on the basis of the interaction with reactants and products showing different hydrophilic/hydrophobic properties. This allows in some cases not only to improve the activity of the system but also to tune and increase the selectivity of the process.

Table 8.
Selected examples of sorbitol dehydration with hydrophobized material: direct comparison with the parent (or less hydrophobic) catalyst.
5. Conclusions and Perspectives
In the last few years, the hydrophilic/hydrophobic features of solid catalysts have attracted unprecedented attention particularly due to increasing relevance of renewables as raw materials. On one hand, reactions of lipidic starting materials such as triglycerides to give biofuels, biolubricants or surfactants require hydrophobic surfaces to adsorb on in an effective way; on the other one, when polar molecules are the products, their desorption improves over such catalytic surfaces. Analogous considerations apply when water is a reactant or a product. In the first case, a hydrophilic catalyst would be preferred, whereas in the second, higher hydrophobicity of the system would be advantageous. The preparation and characterization of hydrophobic and superhydrophobic solids to be used as catalysts or catalyst supports has reached a maturity level. This review deals only with zeolites, mesoporous silica and sulfonic resins as solid acids. In general, zeolites and mesoporous silicas can be modified post synthesis. The hydrophobicity of zeolites can be tuned through dealumination or coating with non-polar silanes, while less hydrophilic silicas can be obtained through functionalization with organic molecules and particularly through silanization. In few cases, sol–gel processes can give superhydrophobic silica aerogels. On the contrary, the surface hydrophylic/hydrophobic balance of sulfonic resins can be adjusted through the arrangement of sulfonic groups, that is, the sulfur content of the material. However, these features should be very finely tuned in order to keep the acidic properties of the catalytic material. Several methods of characterization involving both the surface composition and the measure of surface interactions with water or other solvents are available. Moreover, X-ray reflectivity studies, Sum frequency generation spectroscopy and atomic force microscopy are useful tools to characterize solid–liquid interfaces. Finally, some selected examples are reported to show how the hydrophobic–hydrophilic properties of a catalytic material or of a catalyst support may play a significant role in addressing not only activity but also selectivity, particularly in reactions involving water formation.
The roles of acidic sites and of the metallic phase, when present, have been sometimes over-emphasized whereas the hydrophilic nature of the catalyst/support has been often underestimated. In particular, the wide range of reactions involved in the roadmap from biomass to bioproducts, as well as other ones for the sustainable production of drop-in chemicals, could take advantage from a more careful choice of the hydrophobic feature of the acidic catalyst or catalyst support. This is not only the case with esterification, hydrolysis and dehydration reactions but also with other reactions producing alcohols, glycols or diols in the presence of a supported metal such as the already reported methane oxidation [25] or dimethyloxalate, ethyl lactate or γ-valerolactone hydrogenation [90]. In this last case, a suitable trade-off between increased hydrophobicity and softened acidity would improve at one time product desorption and secondary reactions inhibition, that is, both activity and selectivity.
With this non-comprehensive review, we aim to give some insight into the challenges and opportunities provided by these fascinating aspects of catalytic materials in order to stimulate further research in the field.
Author Contributions
Conceptualization, D.C., F.Z. and N.R.; writing—original draft preparation, D.C. and F.Z.; writing—review and editing, N.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaccheria, F.; Ravasio, N. Solid Catalysts for the Upgrading of Renewable Sources; MDPI: Basel, Switzerland, 2019; Volume 9, ISBN 9783038975724. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9783527307159. [Google Scholar]
- Hagen, J. Industrial Catalysis: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9783527331659. [Google Scholar]
- Sievers, C.; Scott, S.L.; Noda, Y.; Qi, L.; Albuquerque, E.M.; Rioux, R.M. Phenomena affecting catalytic reactions at solid−Liquid interfaces. ACS Catal. 2016, 6, 8286–8307. [Google Scholar] [CrossRef]
- Erbil, H.Y. Surface Chemistry: Of Solid and Liquid Interfaces; Blackwell Publishing: Oxford, UK, 2009; ISBN 1405119683. [Google Scholar]
- Gläser, R.; Weitkamp, J. Surface Hydrophobicity or Hydrophilicity of Porous Solids. In Handbook of Porous Solids; WILEY-VCH: Weinheim, Germany, 2008; ISBN 9783527302468. [Google Scholar]
- Myers, D. Surfaces, Interfaces, and Colloids: Principles and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; ISBN 9780471234999. [Google Scholar]
- Wang, L.; Xiao, F.S. The importance of catalyst wettability. ChemCatChem 2014, 6, 3048–3052. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Wang, F.; Zhang, L.; Jiang, Y.; Arandiyan, H.; Li, H. The Effect of Surface Wettability and Coalescence Dynamics in Catalytic Performance and Catalyst Preparation: A Review. ChemCatChem 2019, 11, 1576–1586. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Zheng, A.; Xiao, F.S.; Dai, S. Hydrophobic Solid Acids and Their Catalytic Applications in Green and Sustainable Chemistry. ACS Catal. 2018, 8, 372–391. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Gounder, R. Hydrophobic microporous and mesoporous oxides as Brønsted and Lewis acid catalysts for biomass conversion in liquid water. Catal. Sci. Technol. 2014, 4, 2877–2886. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Xu, C.H.; Jin, T.; Jhung, S.H.; Chang, J.S.; Hwang, J.S.; Park, S.E. Hydrophobicity and catalytic properties of Ti-MFI zeolites synthesized by microwave and conventional heating. Catal. Today 2006, 111, 366–372. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar]
- Yilmaz, B.; Müller, U. Catalytic applications of zeolites in chemical industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Chen, N.Y. Hydrophobic properties of zeolites. J. Phys. Chem. 1976, 80, 60–64. [Google Scholar] [CrossRef]
- Nakamoto, H.; Takahashi, H. Hydrophobic natures of zeolite ZSM-5. Zeolites 1982, 2, 67–68. [Google Scholar] [CrossRef]
- Lami, E.B.; Fajula, F.; Anglerot, D.; Des Courieres, T. Single step dealumination of zeolite beta precursors for the preparation of hydrophobic adsorbents. Microporous Mater. 1993, 1, 237–245. [Google Scholar] [CrossRef]
- Northcott, K.A.; Bacus, J.; Taya, N.; Komatsu, Y.; Perera, J.M.; Stevens, G.W. Synthesis and characterization of hydrophobic zeolite for the treatment of hydrocarbon contaminated ground water. J. Hazard. Mater. 2010, 183, 434–440. [Google Scholar] [CrossRef]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic zeolites for biofuel upgrading reactions at the liquid-liquid interface in water/oil emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef]
- Pino, N.; Bui, T.; Hincapié, G.; López, D.; Resasco, D.E. Hydrophobic zeolites for the upgrading of biomass-derived short oxygenated compounds in water/oil emulsions. Appl. Catal. A Gen. 2018, 559, 94–101. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Hu, Q.; Zhang, L.; Xu, S.; Dong, X.; Gao, X.; Ma, R.; Meng, X.; Xiao, F.S. Hydrophobic Zeolite Containing Titania Particles as Wettability-Selective Catalyst for Formaldehyde Removal. ACS Catal. 2018, 8, 5250–5254. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science (80-) 2020, 367, 193–197. [Google Scholar]
- Vansant, E.E.; Van Der Voort, P.; Vrancken, K.C. Characterization and Chemical Modification of the Silica Surface; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 0-444-81928-2. [Google Scholar]
- Blin, J.L.; Riachy, P.; Carteret, C.; Lebeau, B. Thermal and Hydrothermal Stability of Hierarchical Porous Silica Materials. Eur. J. Inorg. Chem. 2019, 2019, 3194–3202. [Google Scholar] [CrossRef]
- Veisi, H.; Sedrpoushan, A.; Faraji, A.R.; Heydari, M.; Hemmati, S.; Fatahi, B. A mesoporous SBA-15 silica catalyst functionalized with phenylsulfonic acid groups (SBA-15-Ph-SO3H) as a novel hydrophobic nanoreactor solid acid catalyst for a one-pot three-component synthesis of 2H-indazolo [2,1-b] phthalazine-triones and triazolo [1,2-a]. RSC Adv. 2015, 5, 68523–68530. [Google Scholar] [CrossRef]
- Siegel, R.; Domingues, E.; De Sousa, R.; Jérôme, F.; Morais, C.M.; Bion, N.; Ferreira, P.; Mafra, L. Understanding the high catalytic activity of propylsulfonic acid-functionalized periodic mesoporous benzenesilicas by high-resolution 1H solid-state NMR spectroscopy. J. Mater. Chem. 2012, 22, 7412–7419. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Ma, J.; Lu, F.; Zhang, J.; Xu, J. Biphasic catalytic conversion of fructose by continuous hydrogenation of HMF over a hydrophobic ruthenium catalyst. ChemSusChem 2014, 7, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, F.; Ma, J.; Chen, C.; Shi, S.; Xu, J. Insights into support wettability in tuning catalytic performance in the oxidation of aliphatic alcohols to acids. Chem. Commun. 2013, 49, 6623–6625. [Google Scholar] [CrossRef] [PubMed]
- Omota, F.; Dimian, A.C.; Bliek, A. Partially hydrophobized silica supported Pd catalyst for hydrogenation reactions in aqueous media. Appl. Catal. A Gen. 2005, 294, 121–130. [Google Scholar] [CrossRef]
- Manangon-Perugachi, L.E.; Vivian, A.; Eloy, P.; Debecker, D.P.; Aprile, C.; Gaigneaux, E.M. Hydrophobic titania-silica mixed oxides for the catalytic epoxidation of cyclooctene. Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Kong, P.S.; Cognet, P.; Pérès, Y.; Esvan, J.; Daud, W.M.A.W.; Aroua, M.K. Development of a Novel Hydrophobic ZrO2-SiO2 Based Acid Catalyst for Catalytic Esterification of Glycerol with Oleic Acid. Ind. Eng. Chem. Res. 2018, 57, 9386–9399. [Google Scholar] [CrossRef]
- Itsuno, S. Polymer Catalysts. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–9. ISBN 9783642361999. [Google Scholar]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Alexandratos, S.D. Ion-Exchange resins: A retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Q.; Han, P.; Xu, J.; Pan, L.; Wang, L.; Zou, J.-J. Hydrophobic Mesoporous Acidic Resin for Hydroxyalkylation/ Alkylation of 2-Methylfuran and Ketone to High-Density Biofuel. AIChE J. 2017, 63, 680–688. [Google Scholar] [CrossRef]
- Li, H.; Deng, Q.; Chen, H.; Cao, X.; Zheng, J.; Zhong, Y.; Zhang, P.; Wang, J.; Zeng, Z.; Deng, S. Benzenesulfonic acid functionalized hydrophobic mesoporous biochar as an efficient catalyst for the production of biofuel. Appl. Catal. A Gen. 2019, 580, 178–185. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, F.; Meng, X.; Mao, J.; Xiao, F.S. Enhanced catalytic performance in dehydration of sorbitol to isosorbide over a superhydrophobic mesoporous acid catalyst. Catal. Today 2015, 242, 249–254. [Google Scholar] [CrossRef]
- Liu, F.; Kong, W.; Qi, C.; Zhu, L.; Xiao, F.S. Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity. ACS Catal. 2012, 2, 565–572. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, A.; Noshadi, I.; Xiao, F.S. Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation. Appl. Catal. B Environ. 2013, 136–137, 193–201. [Google Scholar] [CrossRef]
- Young, T. An essay on cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Zhong, Y.; Deng, Q.; Zhang, P.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Sulfonic acid functionalized hydrophobic mesoporous biochar: Design, preparation and acid-catalytic properties. Fuel 2019, 240, 270–277. [Google Scholar] [CrossRef]
- Protsak, I.; Pakhlov, E.; Tertykh, V.; Le, Z.C.; Dong, W. A new route for preparation of hydrophobic silica nanoparticles using a mixture of poly(dimethylsiloxane) and diethyl carbonate. Polymers 2018, 10, 116. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Liu, F.; Zheng, A.; Zhang, J.; Sun, Q.; Lewis, J.P.; Zhu, L.; Meng, X.; Xiao, F.S. Selective catalytic production of 5-hydroxymethylfurfural from glucose by adjusting catalyst wettability. ChemSusChem 2014, 7, 402–406. [Google Scholar] [CrossRef]
- Cavuoto, D.; Zaccheria, F.; Marelli, M.; Evangelisti, C.; Piccolo, O.; Ravasio, N. The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts. Catalysts 2020, 10, 515. [Google Scholar] [CrossRef]
- Pires, J.; Pinto, M.L.; Carvalho, A.; De Carvalho, M.B. Assessment of Hydrophobic-Hydrophilic Properties of Microporous Materials from Water Adsorption Isotherms. Adsorption 2003, 9, 303–309. [Google Scholar] [CrossRef]
- Pires, J.; Pinto, M.; Estella, J.; Echeverría, J.C. Characterization of the hydrophobicity of mesoporous silicas and clays with silica pillars by water adsorption and DRIFT. J. Colloid Interface Sci. 2008, 17, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.C.; Bui, N.Q.; Mascunan, P.; Vu, T.T.H.; Fongarland, P.; Essayem, N. Esterification of aqueous lactic acid solutions with ethanol using carbon solid acid catalysts: Amberlyst 15, sulfonated pyrolyzed wood and graphene oxide. Appl. Catal. A Gen. 2018, 552, 184–191. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Sun, Q.; Zhu, L.; Meng, X.; Xiao, F.S. Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: Heterogeneous catalysts that are faster than homogeneous catalysts. J. Am. Chem. Soc. 2012, 134, 16948–16950. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Suzuki, N.; Tsuji, K.; Sonoda, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Preparation of hydrophobically modified single-site Ti-containing mesoporous silica (TiSBA-15) and their enhanced catalytic performances. Catal. Today 2011, 175, 393–397. [Google Scholar] [CrossRef]
- Olson, D.H.; Haag, W.O.; Borghard, W.S. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Microporous Mesoporous Mater. 2000, 35–36, 435–446. [Google Scholar] [CrossRef]
- Matsumoto, A.; Misran, H.; Tsutsumi, K. Adsorption characteristics of organosilica based mesoporous materials. Langmuir 2004, 20, 7139–7145. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Peng, Y.; Hu, F.; Li, K.; Song, H.; Li, X.; Zhang, Y.; Li, J. Studies on toluene adsorption performance and hydrophobic property in phenyl functionalized KIT-6. Chem. Eng. J. 2018, 334, 191–197. [Google Scholar] [CrossRef]
- Weitkamp, J.; Kleinschmit, P.; Kiss, A.; Berke, C.H. The Hydrophobicity Index- a valuable test for probing the surface properties of zeolitic adsorbents or catalysts. In Proceedings from the Ninth International Zeolite Conference; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Guidotti, M.; Batonneau-Gener, I.; Gianotti, E.; Marchese, L.; Mignard, S.; Psaro, R.; Sgobba, M.; Ravasio, N. The effect of silylation on titanium-containing silica catalysts for the epoxidation of functionalised molecules. Microporous Mesoporous Mater. 2008, 111, 39–47. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9781405131711. [Google Scholar]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Wisser, F.M.; Abele, M.; Gasthauer, M.; Müller, K.; Moszner, N.; Kickelbick, G. Detection of surface silanol groups on pristine and functionalized silica mixed oxides and zirconia. J. Colloid Interface Sci. 2012, 374, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, L.; Li, J.; Zhan, X.; Chen, J.; Yang, J. Tuning the hydrophobicity of ZSM-5 zeolites by surface silanization using alkyltrichlorosilane. Appl. Surf. Sci. 2011, 257, 9525–9531. [Google Scholar] [CrossRef]
- Ramakrishna Prasad, M.; Hamdy, M.S.; Mul, G.; Bouwman, E.; Drent, E. Efficient catalytic epoxidation of olefins with silylated Ti-TUD-1 catalysts. J. Catal. 2008, 260, 288–294. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Vishnu Priya, S.; Herbert Mabel, J.; Palanichamy, M.; Murugesan, V. Effect of hydrophobic and hydrophilic properties of solid acid catalysts on the esterification of maleic anhydride with ethanol. Catal. Commun. 2008, 9, 2007–2012. [Google Scholar] [CrossRef]
- Arias, K.S.; Al-Resayes, S.I.; Climent, M.J.; Corma, A.; Iborra, S. From biomass to chemicals: Synthesis of precursors of biodegradable surfactants from 5-hydroxymethylfurfural. ChemSusChem 2013, 6, 123–131. [Google Scholar] [CrossRef]
- Bui, T.V.; Umbarila, S.J.; Wang, B.; Sooknoi, T.; Li, G.; Chen, B.; Resasco, D.E. High-Temperature Grafting Silylation for Minimizing Leaching of Acid Functionality from Hydrophobic Mesoporous Silicas Used as Catalysts in the Liquid Phase. Langmuir 2019, 35, 6838–6852. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, L.; Dong, X.; Zhang, J.; Wang, G.; Han, S.; Meng, X.; Zheng, A.; Xiao, F.S. Importance of Zeolite Wettability for Selective Hydrogenation of Furfural over Pd@Zeolite Catalysts. ACS Catal. 2018, 8, 474–481. [Google Scholar] [CrossRef]
- Salvo, L.; Cloetens, P.; Maire, E.; Zabler, S.; Blandin, J.J.; Buffière, J.Y.; Ludwig, W.; Boller, E.; Bellet, D.; Josserond, C. X-ray micro-tomography an attractive characterisation technique in materials science. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 200, 273–286. [Google Scholar] [CrossRef]
- Shi, H.; Lercher, J.A.; Yu, X.Y. Sailing into uncharted waters: Recent advances in the in situ monitoring of catalytic processes in aqueous environments. Catal. Sci. Technol. 2015, 5, 3035–3060. [Google Scholar] [CrossRef]
- Mezger, M.; Reichert, H.; Schöder, S.; Okasinski, J.; Schröder, H.; Dosch, H.; Palms, D.; Ralston, J.; Honkimäki, V. High-resolution in situ x-ray study of the hydrophobic gap at the water-octadecyl-trichlorosilane interface. Proc. Natl. Acad. Sci. USA 2006, 103, 18401–18404. [Google Scholar] [CrossRef]
- Cyran, J.D.; Donovan, M.A.; Vollmer, D.; Brigiano, F.S.; Pezzotti, S.; Galimberti, D.R.; Gaigeot, M.P.; Bonn, M.; Backus, E.H.G. Molecular hydrophobicity at a macroscopically hydrophilic surface. Proc. Natl. Acad. Sci. USA 2019, 116, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Totland, C.; Nerdal, W. Experimental Determination of Water Molecular Orientation near a Silica Surface Using NMR Spectroscopy. J. Phys. Chem. C 2016, 120, 5052–5058. [Google Scholar] [CrossRef]
- Voïtchovsky, K.; Kuna, J.J.; Contera, S.A.; Tosatti, E.; Stellacci, F. Direct mapping of the solid-liquid adhesion energy with subnanometre resolution. Nat. Nanotechnol. 2010, 5, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Gounder, R.; Davis, M.E. Beyond Shape Selective Catalysis with Zeolites: Hydrophobic Void Spaces in Zeolites Enable Catalysis in Liquid Water. AIChE J. 2013, 59, 3349–3358. [Google Scholar] [CrossRef]
- Tucker, M.H.; Crisci, A.J.; Wigington, B.N.; Phadke, N.; Alamillo, R.; Zhang, J.; Scott, S.L.; Dumesic, J.A. Acid-functionalized SBA-15-type periodic mesoporous organosilicas and their use in the continuous production of 5-hydroxymethylfurfural. ACS Catal. 2012, 2, 1865–1876. [Google Scholar] [CrossRef]
- Vjunov, A.; Hu, M.Y.; Feng, J.; Camaioni, D.M.; Mei, D.; Hu, J.Z.; Zhao, C.; Lercher, J.A. Following Solid-Acid-Catalyzed Reactions by MAS NMR Spectroscopy in Liquid Phase-Zeolite-Catalyzed Conversion of Cyclohexanol in Water. Angew. Chem. 2014, 126, 489–492. [Google Scholar] [CrossRef]
- Miao, S.; Lin, D. Monoglycerides: Categories, Structures, Properties, Preparations, and Applications in the Food Industry. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 155–163. ISBN 978-0-12-814045-1. [Google Scholar]
- Díaz, I.; Márquez-Alvarez, C.; Mohino, F.; Pérez-Pariente, J.; Sastre, E. Combined Alkyl and Sulfonic Acid Functionalization of MCM-41-Type Silica. J. Catal. 2000, 193, 283–294. [Google Scholar] [CrossRef]
- Bossaert, W.D.; De Vos, D.E.; Van Rhijn, W.M.; Bullen, J.; Grobet, P.J.; Jacobs, P.A. Mesoporous sulfonic acids as selective heterogeneous catalysts for the synthesis of monoglycerides. J. Catal. 1999, 182, 156–164. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, R.; Pirez, C.; Iglesias, J.; Wilson, K.; Lee, A.F.; Melero, J.A. Zr-Containing Hybrid Organic-Inorganic Mesoporous Materials: Hydrophobic Acid Catalysts for Biodiesel Production. ChemCatChem 2013, 5, 994–1001. [Google Scholar] [CrossRef]
- Melero, J.A.; Bautista, L.F.; Morales, G.; Iglesias, J.; Sánchez-Vázquez, R. Acid-catalyzed production of biodiesel over arenesulfonic SBA-15: Insights into the role of water in the reaction network. Renew. Energy 2015, 75, 425–432. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.L.; Veloso, C.O.; Henriques, C.A. Acid zeolites for glycerol etherification with ethyl alcohol: Catalytic activity and catalyst properties. Appl. Catal. A Gen. 2017, 548, 2–15. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.L.; Veloso, C.D.O.; Henriques, C.A. Etherification of different glycols with ethanol or 1-octanol catalyzed by acid zeolites. Mol. Catal. 2018, 458, 261–271. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications-quo vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Otomo, R.; Yokoi, T.; Tatsumi, T. Synthesis of isosorbide from sorbitol in water over high-silica aluminosilicate zeolites. Appl. Catal. A Gen. 2015, 505, 28–35. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yokoyama, H.; Feng, B.; Fukuoka, A. Dehydration of sorbitol to isosorbide over H-beta zeolites with high Si/Al ratios. Green Chem. 2015, 17, 2732–2735. [Google Scholar] [CrossRef]
- Cubo, A.; Iglesias, J.; Morales, G.; Melero, J.A.; Moreno, J.; Sánchez-Vázquez, R. Dehydration of sorbitol to isosorbide in melted phase with propyl-sulfonic functionalized SBA-15: Influence of catalyst hydrophobization. Appl. Catal. A Gen. 2017, 531, 151–160. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Cross, H.E.; Brown, D.R.; Düren, T.; Williams, J.J.; Lee, A.F.; Wilson, K. Interdependent lateral interactions, hydrophobicity and acid strength and their influence on the catalytic activity of nanoporous sulfonic acid silicas. Green Chem. 2010, 12, 1383–1391. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, N.; Wang, Y.; Xuan, K.; Li, F.; Pu, Y.; Wang, F.; Li, L.; Xiao, F. Dehydration of sorbitol to isosorbide over hydrophobic polymer-based solid acid. Appl. Catal. B Environ. 2019, 240, 182–192. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Scotti, N.; Cappelletti, G.; Gervasini, A.; Zaccheria, F. The role of support wettability in the hydrogenation of Gamma Valero Lactone. 2020; in preparation. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).