Simple Summary
The major advances in nanomedicine for ovarian cancer are outlined in this review, underlining how engineered lipid, polymeric, inorganic, hybrid and biomimetic platforms improve imaging accuracy, intraperitoneal delivery, and therapeutic precision. Targeted ligands, stimuli-responsive release, and stealth coatings are key design principles which are demonstrated alongside applications in magnetic resonance imaging (MRI), positron emission tomography (PET)/single-photon emission computed tomography (SPECT), near-infrared imaging (NIR), ultrasound, and multimodal imaging. Nucleic acid nanotherapies, photothermal and enzyme-responsive systems, immune-nanomedicine and intraperitoneal depot strategies are also discussed in this article. The importance of standardisation and personalised nanotherapeutic approaches in ovarian cancer is highlighted by the evaluation of biodistribution, safety, manufacturing, and regulatory framework challenges.
Abstract
Ovarian cancer continues to be the most lethal gynaecological malignancy, principally due to its late-stage diagnosis, extensive peritoneal dissemination, chemoresistance, and limitations of current imaging and therapeutic strategies. By optimising pharmacokinetics, refining tumour-selective drug delivery, and supporting high-resolution, multimodal imaging, nanomedicine offers a versatile platform to address these limitations. In this review, current progress across lipid-based, polymeric, inorganic, hybrid, and biomimetic nanocarriers is synthesised, emphasising how tailored physiochemical properties, surface functionalisation, and stimuli-responsive designs can improve tumour localisation, surmount stromal and ascetic barriers, and enable controlled drug release. Concurrently, significant advancement in imaging nanoprobes, including magnetic resonance imaging (MRI), positron emission tomography (PET)/single-photon emission computed tomography (SPECT), optical, near-infrared imaging (NIR), ultrasound, and photoacoustic systems, has evolved early lesion detection, intraoperative guidance, and quantitative monitoring of treatment. Diagnosis and therapy are further integrated within single platforms by emerging theranostic constructs, encouraging real-time visualisation of drug distribution and treatment response. Additionally, immune-nanomedicine, intraperitoneal depot systems, and nucleic acid-centred nanotherapies offer promising strategies to address immune suppression and molecular resistance in advanced ovarian cancer. In spite of noteworthy achievements, clinical translation is limited by complex manufacturing requirements, challenges with safety and stability, and restricted patient stratification. To unlock the full clinical potential of nanotechnology in ovarian cancer management, constant innovation in scalable design, regulatory standardisation, and integration of precision biomarkers will be necessary.
1. Introduction
In 2020 ovarian cancer remained a high-mortality disease in Europe, with 39,000 new cases and 27,000 deaths reported, corresponding to an age-standardised incidence rate (ASR) of 15.5/100,000 and an ASR mortality of 10.3/100,000 [1]. Declining mortality trends with a predicted ASR of 4.32 per 100,000 women in 2022, as estimated by subsequent population-based analysis across Europe [2]. Despite these declines, updated estimates based on Global Cancer Observatory (GLOBOCAN) 2022 data show the ovarian cancer burden in Europe to continue to be substantial, with continually high incidence and mortality rates compared to other regions [3]. Ovarian cancer is a primary target for nanomedicine strategies in imaging, targeting and therapy, as these high mortality rates reflect late-stage symptom presentation, with most cases diagnosed at International Federation of Gynaecology and Obstetrics (FIGO) stage III or IV. Peritoneal metastatic spread, typical of HGSOC, and treatment resistance also contribute to this elevated mortality rate [4].
Biomarkers, such as CA-125 and human epididymis protein (HE4), showing low sensitivity and non-specific symptoms in the early stages of disease, remain the primary limitations of early detection of ovarian cancer, despite advances [5]. The window for effective intervention is reduced, as this delay often leads to diagnosis at advanced stages. Around 70% of patients presenting with epithelial ovarian carcinoma relapse following first-line therapy, presenting recurrence to be exceedingly common [6]. Therapeutic effectiveness is concurrently undermined by drug resistance, mediated by mechanisms such as DNA damage repair changes, altered transmembrane drug transport, epigenetic modifications and signalling pathway dysregulation [7]. These barriers pose direct challenges in the context of nanomedicine, as high-sensitivity targeted imaging and biomarker-loaded nanoprobes are demanded for early detection, while resistance and recurrence require nanocarriers that overcome residual disease niches and heterogeneity and are capable of overcoming efflux pumps. Crucial intervention points for imaging, targeting and therapeutic nanomedicine in ovarian cancer are highlighted by the triad of frequent relapse, chemoresistance and late detection [8].
Cytoreductive surgery and platinum-taxane chemotherapy are relied on as the standard treatments of advanced ovarian cancer; although, microscopic peritoneal implants often fail to be completely removed during surgery, and residual disease remains an utmost source of relapse [9]. The development of drug resistance mechanisms, for example, enhanced DNA repair or efflux pumps, in tumour cells, dose-limiting toxicities and non-specific biodistribution hinder systemic chemotherapy [10]. Nanoparticle-based platforms aim to utilise the therapeutic window for nanomedicine by combating these limitations. These platforms intend to penetrate residual microscopic disease, unreachable by standard chemo or surgery, improve tumour-specific delivery, and decrease off-target toxicity [11]. Challenges such as tumour heterogeneity, abnormal peritoneal fluid dynamics, and integration into current surgical/chemotherapeutic workflows, nonetheless, need to be overcome for effective translation of nanomedicine.
The improvement of pharmacokinetics (PK), enabling targeted delivery, and adding real-time imaging are solutions offered by nanomedicine to overcome ovarian cancer’s therapeutic impediments. Reduced cardiotoxicity and sustained activity in relapsed disease can be achieved by pegylated liposomal encapsulation, exemplifying PK gains. Liposomal encapsulation: shrinks volume of distribution, boosts tumour exposure versus free drug and extends circulation half-life [12]. Although recognised limitations of pegylated liposomes, such as the accelerated blood clearance (ABC) phenomenon in particular, counterbalance these benefits following repeated dosing. Additionally, rapid opsonisation and hepatic uptake, which can lead to reduced circulation time, reduced tumour accumulation, and unpredictable therapeutic performance, are promoted by the generation of anti-PEG IgM antibodies. Non-specific biodistribution and dose-limiting toxicity, inherent to conventional chemotherapy, can aim to be overcome by targeting moieties (e.g., peptides, antibodies, and folate derivatives) attached onto nanocarriers, which will concentrate the active compound at ovarian cancer cells or within their microenvironment [13], while imaging-capable nanoparticles allow earlier detection and surgical guidance, beyond delivery. Further, contrast-enhanced platforms for MRI, PET/SPECT, and optical/near-infrared (NIR) modalities, including intraoperative probes designed for peritoneal micrometastases, are catalogued in ovarian cancer-focused reviews [14]. Intra-abdominal tumour visualisation during cytoreductive surgery can be illustrated with preclinical work with erythrocyte-derived NIR nano-constructs in support of the theragnostic concept “see and treat” [15]. Together these advances address three needs: Drugs are kept in circulation and within tumours longer, optimising PK; intratumoral drug-to-healthy-tissue ratios are increased by molecular targeting; and disease is mapped, resection is guided, and response is monitored by longitudinal or real-time imaging. Although clinical translation is still hindered by heterogeneity and peritoneal transport barriers [16], nanomedicine is positioned as a practical complement and, in certain scenarios, an enhancer to systemic chemotherapy and surgery due to the maturation of ovarian-specific nanoplatforms across delivery and diagnostic fronts.
2. Biological and Pathophysiological Context for Nanomedicine Delivery
2.1. Tumour Microenvironment, Peritoneal Dissemination and Ascites
Formidable physical and biochemical barriers to nanomedicine delivery are imposed by the tumour microenvironment (TME) in ovarian cancer. Aberrant, leaky vasculature and inadequate perfusion cause regions of hypoxia that promote drug resistance and limit oxygen-dependent imaging probes by triggering hypoxia-inducible factor 1, alpha subunit (HIF-1a)-mediated changes [17]. Compressed lymphatics and abnormal vessel outflow elevate interstitial fluid pressure (IFP); the extravasation of nanoparticles into the tumour interstitium is further impeded by this, and rather than retention, convective clearance is favoured [18]. Dense stromal architecture, which restricts nanoparticle diffusion and delays payload release, is a consequence of extensive extracellular matrix (ECM) remodelling, as seen in Figure 1. This includes increased collagen deposition, hyaluronic acid accumulation and activation of cancer-associated fibroblasts [19]. Hypoxia, high IFP, and dense ECM must be addressed by nanomedicine design in ovarian cancer. This can be performed by enhancing tumour penetration, imaging contrast, and therapeutic efficiency through the integration of stimuli-responsive release, optimised carrier architecture, and stromal targeting features.
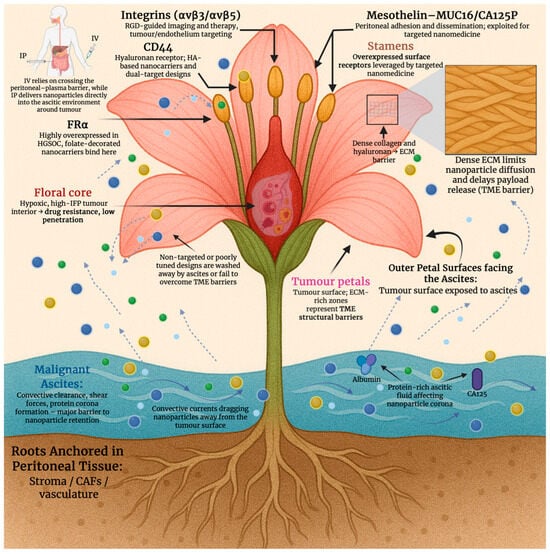
Figure 1.
Conceptual “tumour flower” model illustrating the multilevel biological barriers that impede nanoparticle delivery in high-grade serous ovarian cancer (HGSOC), including ascites-driven clearance, ECM-rich tumour surfaces, intratumoural hypoxia, and overexpressed molecular receptors exploitable for targeted nanomedicine. Created in BioRender. Wilk, I. (2025) https://BioRender.com/l239u55.
Effective nanomedicine delivery in advanced ovarian cancer is significantly obstructed by peritoneal dissemination and accumulation of malignant ascites. Nanoparticle residence time is reduced, and diffusion into implants is limited by convective clearance generated by tumour nodules bathed in ascitic fluid and seeded on peritoneal surfaces [20]. Nanoparticle attachment and retention on the peritoneal surface is impeded by the fluid-driven shear and movement of ascites. Furthermore, due to hepatic clearance and limited transport across serosal membranes, systemic intravenous delivery faces poor peritoneal penetration [21]. Thus, local concentration can be enhanced by the emergence of intraperitoneal administration of nanoparticles as a strategic route. Rapid drainage and nanoparticle clearance in ascitic fluid, even in this route, require design features such as prolonged retention, large tumoral surface binding, and size optimisation to improve uptake and therapeutic index in disseminated peritoneal disease [22].
2.2. Molecular Targets
Anchors for targeted nanomedicine in EOC are provided by several cell surface biomarkers that are frequently overexpressed. In high-grade serous disease, FOLR1 (FR-α) is enriched, and folate-directed therapeutics and diagnostics are underpinned by it. Subtype distribution is quantified, and assay standardisation needs are highlighted in recent clinic-pathologic studies [23]. While mesothelial adhesion and peritoneal dissemination are mediated by mesothelin (MSLN) binding MUC16/CA125, this is an axis widely leveraged for nanoparticle and antibody/nanobody targeting [24]. Further, epithelial tumour cells are marked by epithelial cell adhesion molecule (EpCAM), and it has been linked to prognosis and chemoresistance. EpCAM is used for EV-based readouts and surface functionalisation with targeting ligands or aptamers [25]. The hyaluronan receptor, CD44, supports hyaluronic acid (HA)-based carriers or dual-target designs, whilst enriching stem-like subpopulations [26]. Whereas RGD-guided imaging/therapy and intraperitoneal targeting in ovarian models are enabled by integrins (avβ3/avβ5) on tumours and antagonistic endothelium [27], as shown in Figure 1. Although its prognostic value is heterogeneous, it is important to mention PD-L1 as a marker variably expressed in EOC. It plays a significant role in immuno-nanomedicine combinations and immune-responsive theranostics [28]. Ligand selection, patient stratification, and payload placement are informed by these biomarkers for imaging, targeting, and therapeutic nanoplatforms in ovarian cancer. Table 1 summarises the key molecular targets seen in ovarian cancer.

Table 1.
Key surface biomarkers and molecular targets relevant to nanomedicine in ovarian cancer [11,29,30,31,32,33,34,35,36].
2.3. Routes of Administration
The exposure of peritoneal implants is crucially shaped by route selection. The standard route remains as intravenous (IV) therapy, although drug transfer to surface-embedded nodules is limited by the peritoneal plasma barrier and systemic clearance [37], as seen in Figure 2. After IV administration, typically only 0.1–2% of the injected nanomaterial dose accumulates in solid tumours, with noticeable inter-lesional variability. At the peritoneal surface, higher locoregional concentrations can be achieved by intraperitoneal (IP) delivery as a route of administration. IP delivery has also shown survival advantages in meta-analysis and randomised trials [38]. Patient selection and supportive care are informed by common challenges, such as toxicity and abdominal discomfort, as shown by patient-reported outcomes from GOG-172 [39]. However, hyperthermic intraperitoneal chemotherapy (HIPEC) is preferred in a surgical environment; following interval cytoreduction, HIPEC is shown to increase recurrence-free and overall survival in randomised trials. Data from long-term analysis also reinforces its strengths [40]. In primary and certain recurrent diseases, a 2022 meta-analysis also shows HIPEC to be of benefit to patients, while heterogeneity is mentioned in indications and regimens [41]. Long-circulating, stealth carriers with active targeting to reach peritoneal deposits are favoured for IV administration in nanomedicine. Contrastingly, designs that resist ascitic clearance and have the ability to remain stable in mild hyperthermia (41–43 degrees Celsius) are valued in IP/HIPEC [42]. Thus, peritoneal exposure and therapeutic index in ovarian cancer can be improved by prolonged half-life and tumour homing in IV administrative routes and increasing peritoneal surface adhesion, nanoparticle diameter optimisation and thermal stability in IP/HIPEC routes [43].
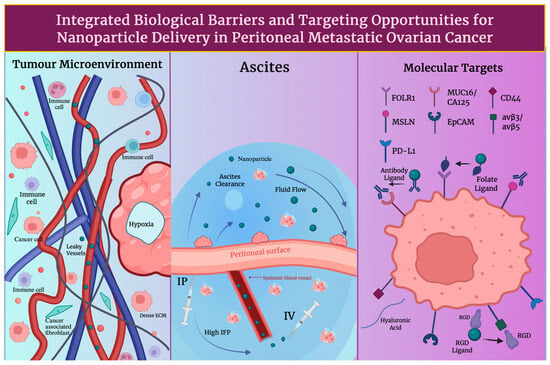
Figure 2.
Key tumour and peritoneal barriers affecting nanoparticle transport after intravenous (IV) versus intraperitoneal (IP)delivery. Shown are TME features, ascites fluid dynamics, and surface receptors that determine peritoneal exposure, clearance, and tumour uptake. Created in BioRender. Wilk, I. (2025) https://BioRender.com/l239u55.
2.4. Clinical Relevance and Limitations of the EPR Effect in Humans
The enhanced permeability and retention (EPR) effect in humans is real but heterogenous: therefore, rather than being a reliable default, its effectiveness depends on patient-specific and lesion-specific biology, which includes vascular permeability, perfusion and stromal composition. Tumour uptake of nanomedicines has been confirmed by clinical imaging studies, but significant inter- and intra-individual variability is consistently observed [44]. In patients, a 35-fold difference in liposome deposition across metastatic lesions, together with an association between higher deposition and more favourable outcomes, was quantified by PET/CT with 64Cu-MM-302 (HER2-targeted pegylated liposomal doxorubicin), which supported the concept of image-guided patient selection [45]. The extent of this variability is particularly relevant in ovarian cancer because the disease often presents with disseminated peritoneal implants showing heterogenous vascularisation and perfusion. Consequently, in poorly perfused lesions, EPR-only platforms may underperform, motivating the integration of active targeting, tumour microenvironment modulation, and theranostic data to personalise delivery strategies [46]. In this regard, nanomedicine strategies in ovarian cancer increasingly pair quantitative imaging modalities (PET/SPECT/MRI) with therapeutic payloads to map nanoparticle deposition, adjust dosing, and combine passive EPR with molecular ligands, such as folate or CD44, or stimuli-responsive release mechanisms [47], and in doing so, overall theranostic performance is significantly improved.
3. Nanocarrier Platforms and Engineering Design
In the context of ovarian cancer, nanocarrier engineering increasingly follows a translational logic in which design choices are evaluated for imaging compatibility, patient stratification, and clinical feasibility, in addition to delivery performance. Several recurring design principles, including particle size control, surface chemistry and stealthing, stimulus-responsive release, and targeting strategy, govern performance in ovarian cancer. While material composition dictates imaging or therapeutic modality compatibility, how these shared parameters are tuned to the peritoneal tumour microenvironment primarily determines translational success.
3.1. Lipid-Based Systems
Polyethylene glycolated (PEGylated) liposomes increase circulation time, decrease cardiotoxicity, and are demonstrated in recurrent ovarian cancer. Pegylated liposomal doxorubicin (PLD), Doxil and Caelyx, showed improved results in platinum-sensitive disease, whilst exceeding topotecan’s effectiveness; PLD carboplatin is also considered an efficient treatment [48]. Intravascular, hyperthermia-induced rapid release with image-guided dosing is enabled by thermosensitive liposomes; lessons acquired from translational research and empirical design guidelines for thermosensitive liposome development are provided in a well-established body of research [49]. Nanocarriers are often manipulated with surface ligands to recognise and bind to molecular biomarkers; for example, folate ligands/antibodies are used to bind to folate receptor α (FAα), a characteristic ovarian cancer molecular marker. Peptides, such as arginine-glycine-aspartic acid (RGD) or aptamers, can be used to increase absorption and facilitate biomarker-guided patient selection [50]. Furthermore, biodistribution and response can be monitored during imaging by PET or NIR probes being co-loaded onto liposomes or lipid nanoparticles (LNPs) [51]. To intercede endosomal escape, ionisable lipids, helper phospholipids, cholesterol, and PEG-lipids are used in LPNs for siRNA and mRNA. Whereas, in ovarian models, dioleoylphosphatidylcholine (DOPC)-based formulation, known as EPHARNA, successfully silenced the EphA2 gene and achieved antitumour activity; here local drug exposure can be maximised by IP administration [52]. Nanocarrier diameter, PEG surface density, TME activators (pH/redox) and administration route have been shown to marginally progress transport across ascites-rich peritoneal environments and to regulate the very variable EPR effect in ovarian cancer models. Although, it is important to mention that these strategies do not uniformly overcome ascites-associated transport barriers, and rather than decreasing EPR, EPR-mediated accumulation aims to be exploited by contemporary nanocarrier designs, where present, while integrating complementary mechanisms, such as intraperitoneal delivery, active targeting, or stimuli-responsive release, in order to account for its heterogenicity and limited reliability in advanced disease [53].
3.2. Polymeric Nanoparticles
Adjustable size, surface chemistry, and stimuli sensitivity for systemic and intraperitoneal delivery are enabled by polymeric nanocarriers, making them fundamental to ovarian cancer nanomedicine. Polylactic-co-glycollic acid (PLGA) nanoparticles provide biodegradability and substantial drug/siRNA co-loading; surface functionalisation, for example, folate or hyaluronan for CD44; as well as peritoneal tumour retention, endosomal escape, and reversion of chemoresistance, for example, HA-PLGA nanoparticles co-loaded with paclitaxel (PTX) and/or small interfering RNA targeting focal adhesion kinase (FAK siRNA) [54,55]. Hydrophobics, such as PTX, can be solubilised by PEG-poly lactic acid (PLA) copolymer micelles. These micelles can also enable dose increase, and they can be modified for pH-induced PEG detachment and endosomal escape. Due to PEG-PLA micellar formulations being clinically established and manufacturable, their translation to ovarian cancer applications is well supported [56]. Poly-amidoamine (PAMAM) is an example of a dendrimer that can offer multivalent, well-defined structures for siRNA/miRNA delivery against resistance mediators, such as twist-related protein 1 (TWIST) and p70S6K. This can be used in combination with PTX to improve antitumour efficiency by being made redox/reactive oxygen species (ROS) reactive for TME activation [57]. Cathepsin or pH-cleavable linkers are used by polymer drug conjugates (PDCs) and antibody polymer drug conjugates for controlled release and can multiplex drugs, for example, gemcitabine and doxorubicin, or they can be used to add immune targeting, such as programmed death ligand 1 (PD-L1). This can lengthen survival in ovarian models and, when paired with imaging labels, supports theranostics [58]. Ascites and peritoneal nodule penetration are improved across platforms targeting moieties, such as FAα, IP delivery and tumour-penetrating peptides. Whilst platinum and taxane resistance is addressed by co-delivery of chemotherapeutic agents with RNA interference (RNAi) or poly (ADP-ribose) polymerase (PARP)/70-kDa ribosomal protein S6 kinase 1 (P70S6K) inhibitors. Design confluence toward compact, stable, and activatable nanocarriers, illustrated in recent PLGA-PTX hybrids and mixed micelles, shows optimisation for ovarian cancer imaging-drug delivery workflows [59].
pH- and redox-responsive release mechanisms are frequently integrated across lipid and polymeric nanocarriers, enabling spatially controlled payload activation in ovarian cancer models, as discussed in Section 3.5.
3.3. Inorganic and Hybrid Systems
Multimodal imaging, targeting, and therapy are allowed by inorganic and hybrid nanoplatforms in ovarian cancer. Simple surface functionalisation for antibody or aptamer targeting is offered by gold nanoparticles, including spheres, rods and shells. Additionally, these nanoparticles provide powerful plasmonics for photoacoustic (PA) or photothermal therapy and drug conjugation; the latest ovarian-focused reviews emphasise progress towards precision oncology and overcoming clinical obstacles [60]. Mesoporous silica nanoparticles (MSNs) supply high capacity and adjustable pores. ‘Wormhole’ MSNs have been successful in achieving TME-targeted theranostics and optoacoustic discovery of ovarian lesions. Further, radioisotope-loaded holmium-166-loaded mesoporous silica nanoparticles (166Ho-MSNs) can show peritoneal metastasis control with restricted off-target dose [61], whereas MRI contrast and magnetic hyperthermia are enabled by iron oxide nanoparticles (IONPs). Heating efficiency can be improved by engineering multicore IONPs and biocompatible nanoclusters, whilst deep stimulation for apoptosis and vascular disturbance can be permitted by alternating magnetic fields [62]. Quantum dots (QDs) can provide a bright and stable fluorescence, useful for intraoperative guidance and selected drug-aptamer conjugates, such as mucin 1, cell surface associated (MUC1) aptamer-doxorubicin, that pair chemotherapy with imaging in ovarian models [63,64]. Lastly, magnetic MSNs, or plasmonic silica shells, are examples of hybrid core-shell designs that can multiplex functions such as loading cytotoxins or siRNA, adding stimuli-sensitive release, and combining PA/MR/fluorescence signals. This supports image-guided therapy and ascites/peritoneal nodule management [65]. The use of antifouling coatings to navigate peritoneal fluid, radiotheranostic agents for intraperitoneal disease, and clinically orientated manufacturing remain as ongoing priorities [66].
3.4. Surface Functionalisation and Stealthing
Suppression of opsonisation, prolonging of circulation time, and improvement of drug delivery to tumours can be achieved by hydrophilic ‘stealth’ coatings in ovarian cancer nanocarrier design. The reason behind this hydrophilic requirement is because a steric and energetic barrier that reduces nonspecific protein absorption and protein corona formation develops when highly hydrated polymer layers bind water molecules. Hydrophilic coatings decrease rapid hepatic and splenic clearance by limiting opsonin binding and subsequent recognition by the mononuclear phagocyte system, and in doing so prolong circulation time and enhance tumour accumulation. PEGylation is the most reliable tool, demonstrating a reduction in protein adsorption and renal clearance. Although the ‘accelerated blood clearance’ effect, as well as anti-PEG antibodies, encourages other options [67]. Carboxybetaines and sulfobetaines are examples of zwitterionic polymers that show elongated circulation, decreased complement stimulation, and sensitive designs appropriate for drugs and genes. Zwitterionic nanoparticles, such as upper critical solution temperature (UCST) type and hyperthermia-activated systems, are used in ovarian cancer models to improve paclitaxel efficiency and retention [68]. In addition, wrapping nanocarrier cores with membranes from platelets, RBCs, leukocytes or sometimes even ovarian cancer cells is called biomimetic cloaking. This process facilitates immune evasion, homotypic binding, and multivalent targeting, ideal for imaging and therapy, by adding ‘self’ markers, such as cluster of differentiation 47 (CD47) and adhesion ligands [69].
Stealthed nanocarriers in combination with epithelial or stromal targets, such as FRα, tumour endothelial marker 1 (TEM1) and luteinising hormone releasing hormone (LHRH), in addition to layer-by-layer polymers, to achieve ‘target and release’, radiation sensitisation and multi-imaging modalities, such as photoacoustics or CT, while alleviating peritoneal clearance, are being used for ovarian cancer [70]. Ovarian cancer nanomedicine reviews emphasise PEG, zwitterion, and membrane-coated platforms that can co-deliver siRNA or chemo, aid image-guided interventions, and strengthen immunotherapy. Key design parameters involve graft density, membrane isolation purity, valency of ligand, and deterioration to balance stealth with tumour penetration and endosomal escape [71].
3.5. Triggered and Controlled Release Mechanisms
Triggered or controlled release designs use tumour-specific cues to achieve site-specific drug release in ovarian cancer. pH, redox and enzymes are considered endogenous triggers. In pH-triggered release designs, acidic TME or endosomes can dissolve acid-labile linkers, or the swelling of ionisable matrices can accelerate release and endosomal escape. Intratumoral deposition and efficiency can be improved by multiple pH-sensitive polymer/lipid systems [72]. Moreover, redox-controlled release designs rely on increased glutathione (GSH) or reactive oxygen species (ROS) to cleave disulphide or thioketal linkers, allowing for on-the-spot drug or siRNA release and combination therapies [73]. Whereas, in enzyme-triggered release designs, cathepsins or matrix metalloproteinases (MMPs) in peritoneal metastases degrade shells or cut peptide linkers during selective unloading [74]. Protein corona-resistant surfaces, such as zwitterionic or antifouling, preserve targeting and release kinetics by maintaining trigger accessibility in ascitic fluid [75].
Stimuli-responsive control is provided by exogenous triggers. In designs that are heat responsive, heat-based treatment is coupled with controlled drug delivery. Heat-based treatment mechanisms extend beyond conventional hyperthermia and include magnetic hyperthermia using superparamagnetic iron oxide nanoparticles (SPIONs), which are exposed to alternating magnetic fields (AMFs); photothermal therapy via NIR-absorbing nanomaterials; and thermosensitive carriers that release payloads above set transition temperatures. Spatially controlled cytotoxicity, heat-enhanced drug diffusion, and synergistic chemo- or phototherapy, particularly relevant for intraperitoneal disease, are enabled by these heat-based strategies. Magnetic field hyperthermia (MFH)-enabled chemotherapy with MRI monitoring in ovarian cancer models [76] and NIR-activated gold nanostructures, which enable spatially precise photothermal ablation or drug release [77], are included in representative examples. Further, carriers that rely on magnetic fields can concentrate drugs and, when combined with AMF, can combine heat-induced release and targeting [78]. Free paclitaxel in ovarian cancer cells is being outperformed by developing UCST zwitterionic nanogels, which can trigger drug release under mild hyperthermia [79].
We can see that precise and on-demand drug release can be achieved by multi-stimuli strategies: by pH, redox and enzymatic (endogenous) and thermal, photonic and magnetic (exogenous) triggers, as shown in Figure 3. This can advance peritoneal retention, response and tumour penetration, while minimising off-target toxicity, which is essential for image-guided, combination treatment in ovarian cancer [80]. Table 2 summarises the key nanocarrier platforms and engineering designs in ovarian cancer nanomedicine.
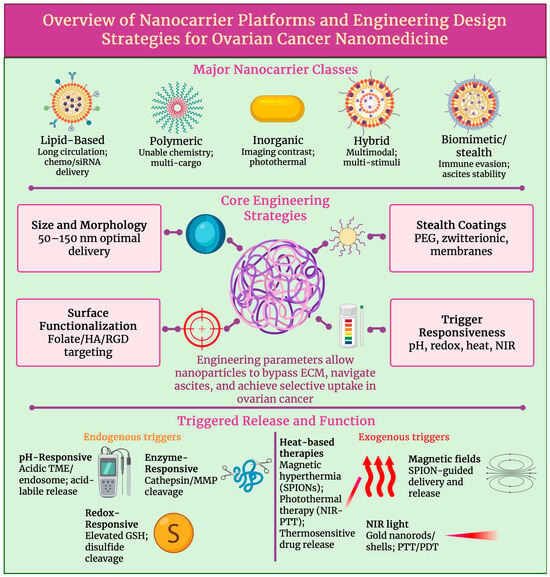
Figure 3.
Integrated framework of nanocarrier platform types, engineering design parameters, and stimuli-responsive mechanisms relevant to ovarian cancer nanomedicine, highlighting design and trigger principles. Created in BioRender. Wilk, I. (2025) https://BioRender.com/r1lyg2v.

Table 2.
Key nanocarrier designs for ovarian cancer: composition, release triggers and performance [79,81,82,83,84,85,86,87].
3.6. Design Scalability and Clinical Translation
Design scalability and clinical translation of ovarian cancer nanocarriers rely on production-ready formulations, such as PLGA and liposomes with modifiable surfaces, size and drug release, while maintaining batch-to-batch reproducibility and pharmacokinetics. In a clinical example, solid tumours were treated with albumin-bound paclitaxel nanoparticles, known as Nab-paclitaxel; this demonstrated that consistent pharmacokinetics and enhanced safety can be achieved by a scalable production process in which nanoparticle size was controlled and albumin coating and reproducible drug loading were involved, therefore acting as a standard for clinical translatability in nanomedicine [88]. Rigorous analytical profiling, for example of hydrodynamic size, polydispersity index (PDI) and drug-to-lipid ratio, correlated with critical quality attributes under the International Council for Harmonisation (ICH) guidelines, specifically Q8, quality by design (QbD), supports regulatory filings and reduces variability [89]. Additionally, standardisation of analytical methods and aligning with developing nanomedicine standards strengthens reproducibility [90]. Opsonisation and off-target toxicity are decreased by biocompatible engineering, such as PEGylation and biodegradable backbones, like PLGA, while allowing tumour localisation in ovarian models [91]. Translation is frequently limited by storage stability; particle integrity and shelf life are preserved by controlled reconstitution and lyophilisation with optimised cryoprotectants. Whereas frozen or lyophilised formats can preserve encapsulation and activity of lipid nanoparticles [92]. Process intensification, which can be performed by inline mixing and the use of closed systems, as well as by authenticated scale-up models connecting minor-scale clinical process parameters (CPPs) to manufacturing-scale batches for scalability, addresses batch-to-batch variation across sites, commonly highlighted in preclinical-to-clinical evaluations [93]. Lastly, regulatory alignment now benefits from clearer guidance globally that is specific to products which are nano-enabled, and complex injectables are more frequently used by regulatory agencies. These are trends which can be seen in recent surveys and overviews in the industry, but they also demand enhanced stability and immunogenicity [94].
Together, these engineering parameters not only control nanocarrier stability and delivery efficiency but also determine compatibility with imaging data, patient stratification strategies, and subsequent clinical translation.
4. Targeting Strategies in Ovarian Cancer Nanomedicine
4.1. Active and TME Targeting Strategies in Ovarian Cancer Nanomedicine
Active targeting in ovarian cancer nanomedicine often makes use of antigens that are evidently overexpressed on tumourous tissue, in comparison to their limited distribution in healthy tissue. On many high-grade serous tumours, folate receptor-α (FRα) is expressed in abundance. FRα has been used to design theranostic nanoemulsions and micelles that can enable MRI monitoring whilst concentrating platinum drugs in peritoneal metastases [95]. When antibody-loaded paclitaxel nanoparticles and anti-MUC16 nanomicelles have been used to target mesothelin and MUC16 (CA125), ovarian cancer targeting ability and tumour uptake were increased, and systemic toxicity was reduced [96,97]. Additionally, non-invasive MRI and optical imaging, with tumour-specific accumulation in human epidermal growth factor receptor 2 (HER2)-positive xenografts, is facilitated by HER2-targeted iron oxide nanoparticles tagged with cisplatin [98]. This concept is extended to circulating or minimal residual disease by EpCAM-directed magnetic particles, which are described in the molecular imaging and contrast agent database (MICAD), as well as by other related imaging probes [99]. Further, siRNA or photosensitisers are delivered to avß3-positive ovarian tumour and endothelial cells by integrin-targeted RGD-labelled nanoparticles, which enable the concurrent attack of tumour cells and angiogenic vasculature [100]. Hyaluronic acid-decorated carriers frequently exploit CD44 to deliver cytotoxins [101] or to deliver MDR1 siRNA selectively to chemoresistant CD44-overexpressing ovarian cancer cells [102]. In addition, complementary TME targeting focuses on tumour-associated macrophages (TAMs), fibroblasts and vasculature. Following accumulation in ovarian TAMs, mRNA or manganese-loaded nanocarriers and large anionic nanoparticles can reprogramme TAMs towards antitumour phenotypes or increase the effectiveness of chemotherapy [103]. On activated macrophages, folate receptor ß is selectively expressed and has been widely used in folate-targeted macrophage nanotherapies, therefore offering a selective entry point for TAM-directed nanomedicine in ovarian cancer [104]. Co-targeting of tumour cells and fibroblasts, remodelling of extracellular matrix and enhancement of immune infiltration in ID8 ovarian models can be achieved by cancer-associated fibroblast mimetic or cancer-associated fibroblast (CAF) hybrid membrane-coated nanoparticles [105]. A route to inhibit angiogenesis is provided by HA-coated siRNA nanocarriers targeting CD44 on tumour endothelium [106]; this integrates vascular, stromal and immune targeting into multifunctional ovarian cancer nanomedicines.
4.2. Overcoming Receptor Heterogeneity Through Dual-Targeted and Theranostic Nanoparticle Platforms
The marked receptor heterogenicity of ovarian cancer is being addressed by increasingly exploited multivalent and dual ligand nanoparticle systems. A significantly higher uptake in SKOV3 ovarian cancer cells and a 4.82-fold higher tumour accumulation in vivo than single ligand control, as shown in Figure 4, was exhibited by dual CD44 and folate receptor-targeted hyaluronic acid-ceramide folic acid nanoparticles, showing synergistic targeting and improved visualisation of intraperitoneal tumours [107]. More broadly, it has been established by dual-ligand gold nanoparticles that multivalent engagement of two receptors can considerably improve cell recognition and differentiation between high- and low-expressing tumour cells, providing conceptual support for dual-receptor nanomedicine in heterogenous ovarian tumours [108]. Targeting is further integrated with imaging data to support image-guided treatment by theranostic platforms. Dual fluorescence and MRI contrast are provided by HER2-targeted gold nanoshell complexes, while NIR photothermal ablation of HER2-overexpressing, drug-resistant OVCAR3 ovarian cancer cells is enabled in vitro [109]. Biomarker-guided companion diagnostics have shown clinical translatability in folate receptor-positive, platinum-resistant ovarian cancer, where patients who are most likely to benefit from the FR-targeted drug conjugate vintafolide are selected using the FR-targeted SPECT tracer 99mTc-etarfolatide [110], demonstrating imaging-based enrichment of nanotherapeutic trials. From a broader molecular profiling viewpoint, the key determinants for guiding personalised, nanoparticle-enabled therapy in ovarian cancer are highlighted as multi-omic profiling of BRCA/HRD, HER2, FOLR1 and other targets [111]. On this basis, Garbuzenko et al. suggest a bank of pre-synthesised, ligand-decorated nanocarriers whose formulation, either containing drugs, siRNAs or targeted peptides, is chosen ex vivo, in accordance with the patient’s tumour gene-protein signature, offering a practical framework for truly personalised ovarian cancer nanomedicine [112]. Table 3 describes the major receptor-ligand axes exploited in ovarian cancer nanomedicine.
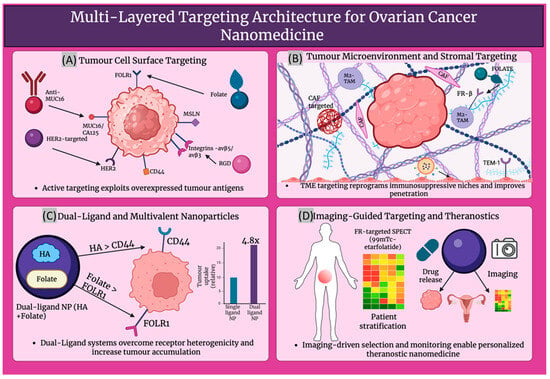
Figure 4.
Describes; (A) Targeting tumour surface receptors. (B) Modulating the tumour microenvironment. (C) Dual-ligand nanoparticles improving uptake. (D) Imaging-guided targeting enabling personalised theranostics. Created in BioRender. Wilk, I. (2025) https://BioRender.com/vuk44v2.

Table 3.
Molecular targetability matrix linking receptor-ligand systems to nanocarrier selection and clinical decision-making in ovarian cancer [55,113,114,115,116,117,118,119,120,121,122,123,124].
5. Imagining and Theranostic Nanomedicine
Imaging nanomedicine in ovarian cancer follows a common theranostic logic across MRI, PET/SPECT, optical, ultrasound, and photoacoustic modalities: nanoparticles are engineered to accumulate at disseminated peritoneal lesions, biodistribution is reported quantitatively or intraoperatively, and where possible triggered therapy is enabled. Therefore, differences between modalities reflect signal physics instead of fundamentally distinct delivery strategies.
5.1. Magnetic Resonance Imaging (MRI)
High-resolution visualisation of ovarian cancer cells within the peritoneal cavity can be enabled by MRI nanoprobes. Strong T2 contrast is provided by superparamagnetic iron-oxide nanoparticles (SPIONs), and they can be used for ovarian tumour uptake after engineering. Folate-decorated Fe3O4 SPIONs generated marked negative contrast in tumours visualised within orthotopic intraperitoneal models. This supported theranostic use by enabling detection of peritoneal implants in preclinical models [125]. In addition to passive contrast, delivery and response by MRI has been simultaneously monitored by theranostic magnetic iron oxide platforms, enabling the detection of differences in accumulation of nanoparticles across heterogenous ovarian lesions and decreased peritoneal metastases in preclinical models. These platforms have also been used to deliver cisplatin to ovarian tumours [98]. Gadolinium (Gd)-labelled liposomes go beyond intravascular residence and intensify reflexivity for T1-weighted, positive-contrast imaging. Vigorous MR signal enhancement, which improved the visualisation of intraperitoneal tumours and complementary NIR signal, was achieved by dual-Gd liposomes co-loaded with indocyanine green (ICG). This enabled multimodal mapping of ovarian cancer, improving detection, treatment monitoring and surgical guidance [126]. Superior tumour MR contrast and adaptability to antibody or peptide functionalisation for ovarian applications have long been demonstrated by Gd-liposomal formulations, often using polychelating amphiphilic polymers, to raise Gd content [127]. Together, SPIONs and Gd-liposomes form the basis of MRI-based mapping of peritoneal metastases and facilitate theranostic workflows that unite lesion localisation with simultaneous delivery and on-treatment monitoring of ovarian cancer.
5.2. Nuclear Imaging (PET/SPECT)
Quantitative, whole-body tracking of the nanoparticle in ovarian cancer is facilitated by radiolabelled nanocarriers for PET and SPECT; this can provide continuous insight into tumour accumulation and circulation and can help spot off-target distribution. Characterisation of pharmacokinetics and optimisation of delivery strategies in nanomedicine have been progressed using liposomes and polymeric nanoparticles tagged with radionuclides such as 99mTc, 64Cu or 89Zr [51]. Radiolabelled expansile nanoparticles exhibited high peritoneal tumour uptake in intraperitoneal tumour models, imitating advanced ovarian cancer. These nanoparticles approached 30% of the administered dose, and clear distinctions between intravenous and intraperitoneal delivery were revealed. This study highlighted the importance of nuclear imaging for treatment optimisation and biodistribution mapping [128]. Also emerging as theranostic agents are radiolabelled biologics and nanoparticles. PET-centred imaging of ovarian cancer lesions is permitted by 99Zr conjugated to anti-Müllerian-inhibiting substance type II receptor (MISRII) antibodies, while combined radionuclide treatment, which enables patient selection and customised dosimetry, is allowed by therapeutic analogues labelled with 177Lu or 213Bi [129,130]. Radiolabelled nanocarriers are described to function as non-invasive tracers in recent reviews, which state they can display therapeutic nanoparticle biodistribution and monitor response to treatment over time. Therefore, radiolabelled nanocarriers can play a significant role in precision management of ovarian peritoneal metastases [131], which drive most ovarian cancer-related mortality.
5.3. Optical and Near-Infrared (NIR) Imaging
Intraoperative and metastatic localisation in ovarian cancer is increasingly relying on optical and NIR imaging, using NIR-fluorophores and QD nanocarriers. Improved tissue penetration, lowered autofluorescence, and enhanced tumour-to-background contrast can be provided by fluorophores producing emission in the first (NIR-I, 700–900 nm) or the second window (NIR-II, 1000–1700 nm). These features are crucial in enabling the visualisation of the small peritoneal implants, characteristic of advanced ovarian cancer, as shown in Figure 5. For instance, 36 h after injection, a high signal-to-noise detection (S/N > 5) of orthotopic ovarian tumours, local lymph nodes and dispersed peritoneal metastases was enabled by polymer nanoparticles, which emitted in the NIR-II range at 1060 nm. These findings support complete tumour resection in murine models [132]. Coupling with tumour-targeting peptides for fluorescence-guided surgery (FGS), showing submillimetre resolution, is facilitated by multi-functional QDs; they also offer adjustable emission and high quantum yield (38%) [133]. In relation to ovarian carcinoma, NIR-labelled molecular probes, which target G protein-coupled receptors (GnRHR) or folate receptors, have demonstrated the ability to differentiate between peritoneal lesions and neighbouring bowel or muscle. Tumour-to-intestinal ratios of more than 4 have been attainable with these probes, in addition to continued retention for periods of up to 48 h [134]. FGS are already using targeted NIR-fluorophores to help guide cytoreductive surgery of peritoneal carcinomatosis, significantly increasing lesion detection and aiding in the establishment of surgical margins; therefore, their translation into clinical practice is already ongoing [135]. Pairing NIR-fluorophore nanocarriers with therapeutic agents in nanomedicine applications enables concurrent tumour localisation and delivery, hence reinforcing theranostic function, in which visualisation and treatment of disease in the peritoneal cavity are carried out by a single nano-platform.
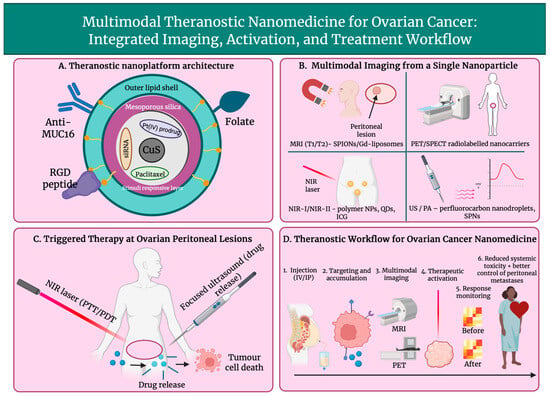
Figure 5.
Overview of a multifunctional theranostic nanoparticle for ovarian cancer, showing nanoparticle architecture (A), multimodal imaging capabilities including MRI, PET/SPECT, NIR, and US/PA (B), triggered photothermal, photodynamic, and ultrasound-induced therapy at peritoneal lesions (C), and the integrated workflow from administration to response monitoring and improved disease control (D). Created in BioRender. Wilk, I. (2025) https://BioRender.com/vfi2pt8.
5.4. Ultrasound and Photoacoustic Imaging
Real-time and non-ionising visualisation of ovarian cancer is offered by ultrasound (US) and PA imaging, and their detection sensitivity and therapeutic effectiveness are decidedly improved by nanomedicine platforms. Typically, acoustic nanodroplets enclose a perfluorocarbon core that, under focused US, can go through acoustic droplet vaporisation, changing into echogenic microbubbles. This effect can promote local drug release while intensifying contrast. Accumulation of the nanodroplets selective to the ovarian tumour and pH-responsive conversion to microbubbles can be demonstrated by folic acid-targeted ferritin nanocages incorporating perfluoropentane (FA-FRT-PFP). Contrast-improved US imaging, together with substantial cytotoxicity, even after only low-intensity focused ultrasound activation, can be achieved using FA-FRT-PFP [136]. Profound intratumoral penetration, ROS-mediated cell killing and US/PA dual imaging that can monitor treatment response ongoingly can be exhibited by dual US-activatable nanodroplets, intended for folate receptor-positive ovarian cancer [137]. In recent studies, nanoparticles integrated with olaparib have been modified for use as ultrasound contrast agents for BRCA1 and BRCA2 (BRCA) proficient ovarian cancer, enabling integrated diagnosis and therapy by pairing PARP inhibitor delivery with contrast-enhanced ultrasound signals [138]. A complementary, completely organic PA platform, defined by strong NIR absorption, great photostability and readily achievable surface functionalisation for precise molecular targeting, can be provided by semiconducting polymer nanoparticles (SPNs) [139]. Strong contrast PA imaging and photoimmunotherapy in orthotopic and metastatic ovarian cancer models has recently been allowed by quinoidal SPNs with NIR-II absorption. This has led to the yielding of strong tumour suppression and inhibition of metastasis, while preserving a clear PA signal generated from deep pelvic lesions [140]. In tandem, dependable separation of exogenous signal from endogenous chromophores, which improved detectability of lesions and helped to guide following therapy, was enabled by a specific spectral signature PA nano-agent, engineered for ovarian cancer [141]. On the whole, activatable US/PA theranostic systems that can map, treat and vigorously monitor ovarian cancer can be established by acoustic nanodroplets and semiconducting polymer nanoparticles.
5.5. Multimodal Imaging Nanoplatforms
Nanosystems designed to carry out two (dual-mode) or three (tri-mode) imaging capabilities within a single construct are called multimodal imaging nanoplatforms. They were developed with the intention of overcoming limitations of traditional imaging of small peritoneal lesions, such as poor sensitivity. Additionally, they were also engineered to incorporate high-resolution structural imaging outputs, like ultrasound (US), CT and MRI, with molecular or functional information that can be provided by imaging modalities such as PA, PET and fluorescence. In a recent review about ovarian cancer nanoparticle imaging agents, the tailoring of platforms for integration into standard imaging modalities, such as fluorescence, US, PA, PET/CT and MRI, is highlighted [14]. Dual-mode anatomical-molecular imaging can be well exemplified by folate-targeted oxygen/indocyanine green lipid nanoparticles (FA-OINPs). By their implementation, both US and PA signals are enhanced in SKOV3 ovarian cancer xenografts, while under NIR laser and US irradiation, photo-sonodynamic and photothermal therapy are provided [142]. PET/CT for quantitative biodistribution and MR temperature imaging is enabled by copper sulphide nanoparticles tagged with 64Cu. This facilitates real-time monitoring of photothermal therapy in HeyA8 and SKOV3-ip1 ovarian cancer models, combining PET-based molecular guidance with MR-based thermometry [143]. T1-weighted MRI, fluorescence (FL), and infrared thermal (IRT) imaging in SKOV3 models are provided by biodegradable hollow mesoporous organosilica nanoplatforms (HMON@CuS/Gd2O3). This enables FL/MRI/IRT-guided mild photothermal therapy that, without systemic toxicity, can destabilise lysosomes and suppress tumour growth [144]. Phase transitional, folate-targeted perfluoropentane nanodroplets, payloaded with Fe3O4 and 10-hydroxycamptothecin (FA-HCPT-Fe3O4-PFP NDs), achieved MRI and PA imaging. After low-intensity focused ultrasound stimulated liquid-to-gas transition, a strong ultrasound contrast was generated while concurrently releasing chemotherapeutic agents in mice bearing SKOV3 xenografts. This demonstrated true MRI/PA/US tri-mode theranostics [145]. Lastly, MicroSPECT/CT-based multimodal imaging of SKOV3 xenografts is demonstrated by telodendrimer paclitaxel nanomicelles labelled with 125I; this guides drug delivery by quantifying tumour-selective accumulation and pharmacokinetics [146]. Collectively, these platforms show how integrated anatomical-molecular imaging in ovarian cancer nanomedicine encourages precise lesion detection, image-guided intervention, and real-time monitoring of nanotherapy effectiveness.
5.6. Quantitative Imaging Biomarkers
The unifying translational bridge across imaging modalities, enabling nanoparticle accumulation, retention, and therapeutic response to be compared independently of carrier composition, can be provided by quantitative imaging biomarkers. Quantitative imaging biomarkers can achieve this by delivering measurable parameters to track nanocarrier delivery and to foresee or monitor treatment response in ovarian cancer nanomedicine. Nanoparticle studies report increasing amounts of standardised parameters, such as Ktrans and Ve, %ID/g and tumour-to-muscle ratios. A notable example is the quantitative dynamic contrast enhanced (DCE)-MRI approach established by Nomani et al. In this study, a nano-sized MRI contrast agent was used in three ovarian cancer tumour xenograft models. Tumours were stratified for responsiveness to nanotherapeutics, and the enhanced permeability and retention (EPR) effect was estimated non-invasively by derived permeability and retention parameters, such as Ktrans and Ve [147]. In relation to PET-based molecular biomarkers, Yang et al. manufactured a 68Ga-DOTA-c(NGR)2 probe, which targets CD13 in ovarian cancer xenografts. Tumour uptake as %ID/g and tumour-to-muscle ratios were quantified on microPET. PET-obtained uptake values could be recognised as biomarkers for targeted nanocarrier localisation and candidate therapy selection, due to these metrics correlating with CD13 expression and blocking experiments [148]. Reviews of nanoparticle imaging in ovarian cancer, with a focus on clinical applications, such as by Di Lorenzo et al., describe how radiolabelled liposomes, iron-oxide nanoparticles, and optical nanoprobes have been used in imaging ovarian cancer across various platforms, such as MRI, PET/SPECT, and FL. They emphasise that the quantification of imaging parameters, such as variations in signal intensity, improvement of contrast, and detectability of lesions, which enables nanoparticle distribution monitoring, and the assessment of treatment effects over time, is enabled by these nanoparticle-based approaches [149]. Combined tracking-response biomarkers are exemplified by theranostic radiolabelled liposomes. Serial SPECT/CT-derived pharmacokinetics have been connected to changes in tumour biology, involving drug resistance reversal and stemness markers, in ovarian cancer models and initial clinical work with 188Re-liposome. This provided imaging-based metrics of nanotherapy effectiveness [150]. Lastly, quantitative DCE-MRI and diffusion-weighted imaging (DWI) parameters, which have already been authenticated for ovarian tumour characterisation, such as Ktrans and Ve, are seen as easily translatable response biomarkers for future nanoparticle-based therapies in ovarian cancer [151]. Notwithstanding notable advances, the clinical translation of theranostic nanomedicine in ovarian cancer continues to be restricted by several limitations. Effective intracellular drug delivery, release of the active therapeutic component, or therapeutic response does not always correlate with imaging-detected nanoparticle accumulation; this creates a central disconnect between signal detection and efficiency. Dose mismatches between tracer-level imaging agents and clinically meaningful drug exposure deepen this challenge, in addition to spatial and temporal discordance, in which imaging reflects early vascular deposition rather than sustained intratumoral retention. Further, the reliability of quantitative imaging biomarkers as predictors of therapeutic response is reduced by pronounced inter-lesional and inter-patient heterogeneity in nanoparticle delivery, retention and release. Lastly, manufacturing and regulatory challenges that restrict batch-to-batch reproducibility and standardisation of imaging signal are introduced by the structural complexity of multimodal theranostic platforms, limiting validation of quantitative biomarkers and clinical translation. Table 4 describes multimodal imaging nanoprobes used in ovarian cancer.

Table 4.
Multimodal imaging nanoprobes in ovarian cancer: modalities, targets and theranostic outcomes.
Aside from lesion detection, by enabling in vivo assessment of delivery, patient stratification, and treatment adaptation, quantitative and multimodal imaging serves as a translational bridge between nanocarrier design and clinical application.
6. Therapeutic Nanomedicine Applications
6.1. Nucleic Acid Nanomedicine
The viability of siRNA nanotherapy in ovarian cancer was recognised by neutral nanoliposomes. Vigorous gene silencing decreased tumour growth, and increased paclitaxel efficiency in orthotopic models was produced by EphA2-targeting siRNA formulated in DOPC liposomes. This illustrated RNAi-mediated chemosensitisation [152]. miRNA nanomedicines build on this by aiming to reinstate tumour-suppressive networks.
In ovarian cancer models, the restored expression of miR-200c reduced the extent of metastasis and heightened sensitivity to paclitaxel. From this we can conclude miR-200c is a reasonable payload for nanocarriers that aim to reverse epithelial-mesenchymal transition (EMT) and drug resistance [153]. Additionally, PARP modulation has become nano-enabled. A polymeric formulation of olaparib, called NanoOlaparib, was able to show effective intraperitoneal exposure and tumour control in disseminated peritoneal models, whilst sustaining activity similar to a free drug. This suggested a strategy to treat late-stage disease beyond BRCA-mutant subsets [154]. Platinum resistance can be overcome by complementary multilayered liposomal nanoparticles co-loading cisplatin and a PARP inhibitor with an external hyaluronan coating for CD44; survival in HGSOC models was also improved [155]. A tumour-targeted liposomal system, delivering clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 plasmids known as CRISPR-enabled nanomedicine, is upcoming. Efficient gene editing and inhibition of growth in ovarian xenografts have been achieved by this system. This provides preclinical demonstration that nanoparticles that are non-viral can support in vivo genome editing for treatment of ovarian cancer [156].
6.2. Targeted Enzyme-Responsive Nanotherapies
Protease overexpression, redox imbalance, and hypoxia in ovarian tumours are made use of in enzyme- and microenvironment-responsive nanotherapies to attain spatially controlled drug activation. Selective enzymatic activation in cathepsin B-overexpressing SKOV3 and HeyA8 cells was shown by cathepsin B-specific doxorubicin prodrug nanoparticles, which were built from FRRG-DOX conjugates. Moreover, significantly improved intraperitoneal inhibition of tumour progression with decreased systemic toxicity, when compared to free doxorubicin in peritoneal carcinomatosis models, was also observed [157]. A complementary protease-activated platform is provided by matrix metalloproteinase-3 (MMP-3)-sensitive near-infrared fluorescent probes. MMP-3 stimulated signal intensification and allowed sensitive stromal imaging in an epithelial ovarian cancer xenograft. This demonstrates that MMP-responsive constructs can be adapted for image-guided nanotherapies [158]. Elevated tumour GSH is exploited by redox-responsive systems. GSH-triggered platinum release, US-mediated enhancement of tumour uptake, and strong antitumour efficiency with weakened nephrotoxicity in SKOV3 models can be achieved by GSH-sensitive Pt (IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell [159]. Oxygen-poor peritoneal lesions are targeted by hypoxia-activated systems. A sphingomyelin cholesterol liposome, which encapsulated the vinblastine-N-oxide prodrug CPD100, called CPD100Li, showed more than a 9-fold increase in cytotoxicity in hypoxic conditions in ES2 ovarian cancer cells; hypoxia-activated liposomal chemotherapy for advanced ovarian cancer was further supported by satisfactory pharmacokinetics and tolerability in vivo [160].
6.3. Photothermal and Photodynamic Therapy
Light-activated agents are used to convert optical energy into cytotoxic heat or ROS within ovarian tumours in photothermal and photodynamic nanotherapies. Laser irradiation parameters that have a substantial influence on the therapeutic outcome of photothermal and photodynamic nanotherapies include continuous wave versus pulsed operation, duration of pulse, irradiance, and total fluence. For sustained photothermal heating, continuous-wave NIR lasers are typically used, whereas higher peak power for rapid localised heating or enhanced photodynamic activation while reducing bulk tissue damage can be enabled by pulsed irradiation. To balance effective tumour ablation or ROS generation with minimised off-target thermal injury, careful control of irradiance and fluence is necessary. IR780 is an NIR dye that can act as both a fluorophore and photothermal transducer in folate-targeted liposomal formulations. Intraoperative delineation of tumour margins is enabled by FA-IR780 nanoparticles accumulated selectively in ovarian xenograft models, and following irradiation with an 808 nm laser, the surrounding tissue was spared whilst most lesions were eradicated [161]. Local damage is further intensified by dual photothermal-photodynamic platforms; receptor-mediated uptake, strong singlet-oxygen generation and tumour elimination in ovarian cancer xenograft models, under irradiation of a single NIR laser, were demonstrated by folate-targeted Pluronic chitosan nanocapsules co-loaded with IR780 [162]. Nanocarrier co-loading with clinically used photosensitisers can also advance photodynamic efficiency. Nanostructured lipid carriers with encapsulated verteporfin can enable prolonged circulation, efficient tumour accumulation and significant inhibition of tumour growth, compared to free verteporfin, while evading the severe phototoxic side effects that can be seen with the free drug [163]. Further, PDT in an ovarian cancer ascites model was enhanced by hypocrellin-B-loaded poly(butyl cyanoacrylate) nanoparticles, which prolonged survival time compared to unconjugated drug [164]. Despite this progress, limited penetration of NIR-I light through large tumour masses restrains efficiency, driving development of NIR-II phototheranostic polymers that can more deeply penetrate tissues and could lead to better signal-to-background ratios [165].
6.4. Immuno-Nanomedicine
The goal of immuno-nanomedicine is to transform the immunologically “cold” ovarian TME, which is abundant in M2-polarised TAMs but lacks effector T cells, towards a state that promotes antitumour immunity [166,167]. Only limited responses are shown by clinical checkpoint inhibitors in epithelial ovarian cancer, catalysing the need for nano-enabled strategies that enhance intraperitoneal drug delivery and immune system activation [168]. Gautam et al. designed cowpea mosaic virus nanoparticles showing an anti-programmed cell death protein-1 (PD-1) peptide for immune checkpoint inhibition. Superior tumour control and survival were demonstrated, when compared to free peptide or unconjugated virus, in an ID8 intraperitoneal model. This exemplified that multivalent nanoparticle display can strengthen local PD-1/programmed death-ligand 1 (PD-L1) inhibition, whilst simultaneously limiting systemic exposure [169]. Large anionic liposomes that migrate to peritoneal TAMs and deliver the toll-like receptor (TLR) 7/8 agonist resiquimod were engineered by Kang et al. These liposomes were successful in reprogramming TAMs towards an M1 phenotype, increasing T cell infiltration, as seen in Figure 6, and markedly enhanced the therapeutic effect of anti-PD-1 treatment in syngeneic ovarian tumours [168]. Checkpoint responses can be further amplified by antigen-directed nanovaccines. Cancer-erythrocyte hybrid membrane-camouflaged Fe3O4-ICG@IRM nanoparticles that accumulate in ID8 tumours were reported by Xiong et al. Immunogenic cell death, tumour antigen release and activation of CD8+ T-cell mediated immunity were triggered by NIR photothermal irradiation, demonstrating robust primary tumour inhibition and resistance to tumour rechallenge [170]. The ovarian TME can be directly remodelled by macrophage repolarising nanotherapies. Peritoneal TAMs in an ID8-VEGF model were selectively targeted by hyaluronic acid/polyethylenimine (PEI) nanoparticles encapsulating miR-125b. The TAMs were shifted to an immune-activating phenotype, and when combined with intraperitoneal paclitaxel, a reduction in ascites and VEGF levels was seen, with minimal systemic toxicity [171]. On the whole, these checkpoint-regulating, antigen-delivering and TAM-reprogramming platforms show how immuno-nanomedicine can increase immunotherapy efficiency in ovarian cancer.
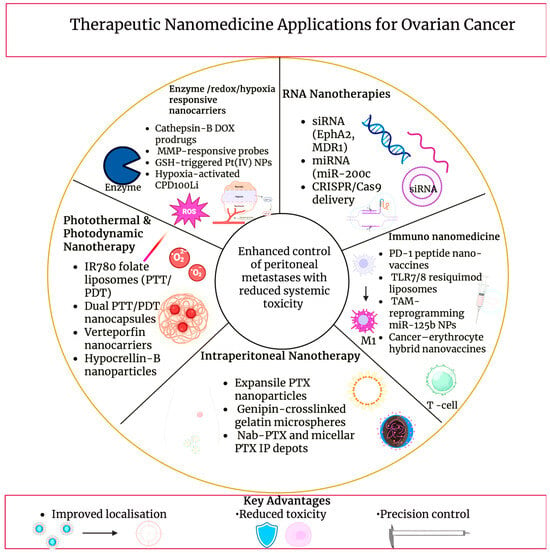
Figure 6.
Therapeutic nanomedicine applications for ovarian cancer, illustrating representative nanocarrier-enabled treatment modalities, including photothermal and photodynamic nanotherapy, intraperitoneal drug depots, RNA-based nanotherapies, enzyme- and redox-responsive systems, and immuno-nanomedicine approaches. The schematic highlights disease-relevant therapeutic strategies aimed at improving localisation, controlling peritoneal metastases, and reducing systemic toxicity. Created in BioRender. Wilk, I. (2025) https://BioRender.com/6pds3ku.
6.5. Intraperitoneal Nanotherapy
Direct exposure of disseminated ovarian implants to nanoparticle depots is used in IP therapy to progress peritoneal disease control while minimising systemic exposure. In comparison to traditional IP solutions, nanoparticle systems are designed to remain in the peritoneal cavity, adhere to serosal surfaces and release the drug slowly, maintaining cytotoxic levels along peritoneal tumour implants dispersed across tumour-bearing peritoneal surfaces [172]. Following cytoreductive surgery, intraoperatively administered paclitaxel-loaded expansile nanoparticles notably decreased locoregional recurrence, and survival versus free paclitaxel in ovarian carcinoma models was improved. This demonstrated that intraperitoneally retained nanocarriers strengthen locoregional control of microscopic residual tumours [173]. Radiolabelled expansile nanoparticles emphasise residence time advantages; after a single IP dose, they show tumour-selective accumulation within the peritoneal cavity lasting for up to 1–2 weeks, with negligible signal in distant organs [128]. Likewise, a long-acting intraperitoneal depot was created by paclitaxel-loaded genipin-crosslinked gelatin microspheres. By this formulation, survival was prolonged, peritoneal carcinomatosis scores were lowered, and reduced ascites were seen when compared to short-acting IP controls [174]. High peritoneal-to-plasma gradients are the root of toxicity reduction. Strong inhibition of peritoneal tumour growth, with lymphatic targeting and satisfactory systemic safety, was achieved by early paclitaxel nanoparticles in ovarian models [175]. In recent times, marked antitumour efficiency and survival benefit were produced by IP administration of nanoparticle albumin-bound and micellar paclitaxel in murine ovarian xenografts. Additionally, low systemic exposure was maintained, supporting a better therapeutic index for intraperitoneal nanotherapy in ovarian cancer [176]. Table 5 summarises therapeutic nanomedicine applications in ovarian cancer.

Table 5.
Therapeutic nanomedicine applications in ovarian cancer: summary of cargo, nanoplatforms, and preclinical efficiency outcomes.
These therapeutic outcomes emphasise how nanocarrier design can enhance efficiency while supporting clinical translation when integrated with imaging-informed patient selection and route optimisation.
7. Pharmacokinetics, Biodistribution and Safety Considerations
7.1. Physicochemical Determinants of Nanoparticle Biodistribution and Clearance in Ovarian Cancer
Biodistribution and clearance in ovarian cancer nanomedicine are dictated by nanoparticle physicochemical properties. In pharmacokinetic studies, extremely small particles, with a diameter of less than 10 nm, are shown to be rapidly cleared renally, while filtration is avoided by systems with particles of a diameter of 10–100 nm, although they are still small enough to cross the vascular border and accumulate in tumours. Nanoparticles with a diameter larger than 200 nm are mostly captured by Kupffer cells in the liver and spleen [177,178]. He et al. showed that size and surface charge act together when he found that tumour uptake is maximised by nanoparticles of 150 nm diameter with a mildly negative zeta potential, while improved opsonisation and reticuloendothelial clearance can be achieved by strongly cationic or highly anionic formulations [179]. Migration, tumour penetration, and cellular internalisation are further moderated by shape, with extended circulation and altered organ tropism, compared with spheres, being exhibited with rod-like or discoidal geometries [180]. An additional bio-interface is introduced by malignant ascites in the peritoneal cavity. It is shown that ovarian cancer ascites is abundant in albumin, complement, coagulation factors and tumour-associated proteins like CA125/MUC16, by proteomic profiling, forming a complex biochemical environment for nanoparticle absorption [181,182]. Nanoparticles quickly obtain an ascites-specific protein corona, as seen in Figure 7, upon IP administration. It was established by Dakwar et al. that widespread aggregation in peritoneal and ascitic fluid can be seen by non-PEGylated cationic and anionic particles, while colloidal stability can be enhanced by PEGylation, although it can also lead to premature release of siRNA, which can reshape local pharmacokinetics [183]. Protein corona composition, as a result, controls recognition by macrophages, complement and NK cells, modulating immune clearance and tumour versus off-target uptake [184]. Therefore, ovarian cancer nanomedicines that prospered in vivo combine diameter (50–150 nm), an almost neutral or slightly negative charge, and stealth coatings with tumour-specific ligands to navigate ascites, make use of helpful coronas, and successfully achieve selective imaging and therapy [14,185].
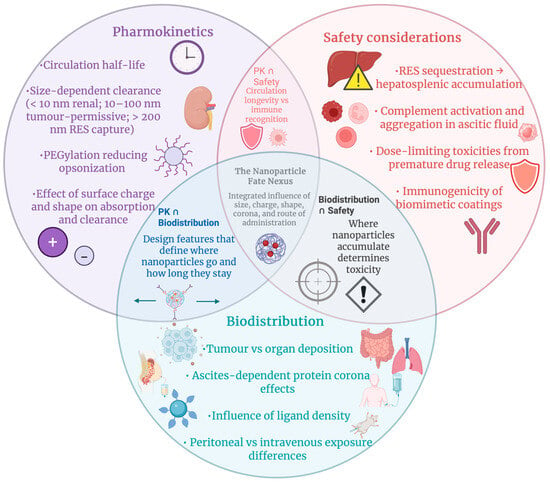
Figure 7.
Interconnected factors controlling nanoparticle fate in ovarian cancer. Pharmacokinetics, biodistribution, and safety constraints intersect to determine circulation, tumour targeting, off-target deposition, and toxicity, forming a unified framework for designing clinically viable nanomedicines. Created in BioRender. Wilk, I. (2025) https://BioRender.com/2eoca0d.
7.2. Toxicity, Immunogenicity, and Reporting Standards in Ovarian Cancer Nanomedicine
A key part of translating ovarian cancer nanomedicine into the clinic is analysis of acute and chronic safety profiles. Nanoformulation can shift toxicity from acute cardiomyopathy to infusion reactions, palmar-plantar erythrodysesthesia and mucositis, whilst significantly decreasing early-onset heart failure when compared to traditional doxorubicin, as shown in clinical experience with pegylated liposomal doxorubicin (PLD) [186]. However, safety considerations that become more significant with repeated dosing and long-term exposure are introduced by the altered biodistribution and prolonged circulation that underlie these clinical benefits. For instance, it is indicated by long-term follow-ups that at very high lifetime doses, cumulative cardiotoxicity remains a concern, although if cardiac risk factors and function are monitored carefully, PLD can frequently be given without a strict upper limit [187]. Past organ toxicity, immunogenicity can be triggered by nanomedicines. Nano-specific adverse events like complement activation-related pseudoallergy (CARPA) and other infusion reactions that are more frequent with certain liposomal and micellar products than with their standard formulations can be revealed by pharmacovigilance data. For nanomedicine-based products that are intended for use in oncology, including ovarian cancer regimens, structured in vitro and in vivo assessments of cytokine release, complement activation, hypersensitivity and unintended immune suppression or stimulation are advised by immunotoxicology guidelines [188]. Highly important for younger patients is reproductive toxicity, though it is poorly characterised. It is shown by experimental and epidemiological analyses that various nanoparticles can cross placental and blood-testis barriers, disturb spermatogenesis by accumulating in gonads, and disrupt folliculogenesis and embryo development through oxidative stress and DNA damage, therefore supporting rigorous pregnancy exclusion, contraception counselling and prudent dose translation from preclinical models to ovarian cancer trials [189]. Though, commonly used preclinical models often fail to capture the fibrotic, heterogenous, and ascites-rich peritoneal environment that has a critical influence on nanoparticle biodistribution, toxicity, and therapeutic response in patients, limiting their predictive value. Vital for interpreting these risks and their translational relevance is high-quality reporting. The MIRIBEL checklist outlines minimum information for bio-nanomaterial experiments so that toxicity and efficiency in ovarian cancer models can be reproduced and compared. It requires detailed nanoparticle physicochemical characterisation, describing size, charge, corona and stability, dosimetry, biological models and statistics [190]. The ARRIVE 2.0 guidelines order transparent reporting of animal strain, randomisation, blinding, sample size reasoning and humane endpoints to reduce bias and advance translatability for in vivo ovarian cancer nanomedicine studies [191]. These frameworks together reinforce best practice in nano-bio characterisation and safety assessment for imaging and therapeutic applications in ovarian cancer. Table 6 describes pharmacokinetic, biodistribution and safety signatures of representative nanomedicines in ovarian cancer.

Table 6.
Pharmacokinetic, biodistribution, and safety signatures informing clinical use of representative nanomedicines.
8. Clinical Translation and Regulatory Perspective
8.1. Clinical Progress, Therapeutic Outcomes, and Stratification Challenges in Ovarian Cancer Nanomedicine
PLD and nanoparticle taxanes lead clinical nanomedicine trials in ovarian cancer, while RNA-loaded lipid systems remain mostly preclinical. In MITO-2 first-line carboplatin-PLD was at least as effective as carboplatin-paclitaxel, with both showing similar survival, although carboplatin-PLD demonstrated lower neurotoxicity and alopecia, accompanied by increased risk of haematologic adverse effects [192]. In relapse due to platinum resistance, PLD used alone, again with a distinct toxicity profile, was established to be no less effective than gemcitabine or topotecan in phase III trials [193]. PLD has been fixed in combinations with trabectedin and/or bevacizumab. When carboplatin-gemcitabine-bevacizumab was compared against carboplatin-PLD-bevacizumab, better outcomes were seen with the latter in platinum-eligible recurrence with acceptable safety [194], and trabectedin-PLD is supported as a substitute option by phase III data [195]. In refractory disease, nanoparticle albumin-bound paclitaxel (nab-PTX) has shown notable standalone activity [196], and due to reports from phase I studies showing advantageous pharmacokinetics and controllable dose-limiting toxicities, as seen in Figure 8, it is being repositioned for intraperitoneal or aerosolised delivery (PIPAC) in peritoneal metastases [197,198]. Clinical translation of siRNA nanomedicine is at earlier stages of advancement. Though ongoing preclinical evaluation is supported by DOPC-based EphA2-siRNA liposomes (EPHARNA) completing good laboratory practice (GLP) safety studies in mice and other non-human primates [199]. Vigorous prolongation of survival in orthotopic models, but no late-phase human data yet, is shown by multiple lipid and hybrid LNP platforms, which target PLK1/elF3c, Parp1 or CD44 positive cells [200,201]. Instead of radically transforming outcomes, generally, nanomedicines modestly prolong progression-free survival and improve tolerability. A lack of prospective stratification by target expression, such as FOLR1, EphA2 and CD44, along with integration of companion imaging or biopsies being limited, leads to ‘nanotargets’ being rarely used to select patients for specific nanomedicine treatments; this signifies a central limitation and represents an area of improvement in upcoming ovarian cancer nanomedicine trials.
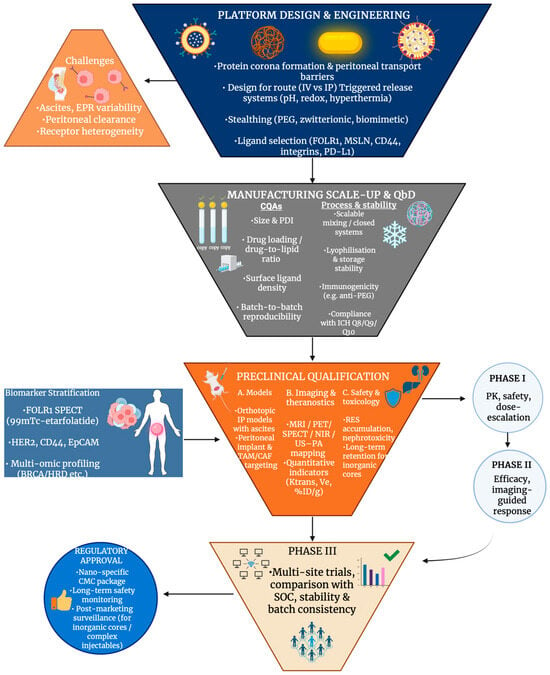
Figure 8.
Integrated clinical translation funnel for ovarian cancer nanomedicine, illustrating the sequential progression from platform engineering and manufacturing quality control through preclinical qualification, biomarker-guided patient stratification, and early-to-late clinical trials, culminating in regulatory approval. Created in BioRender. Wilk, I. (2025) https://BioRender.com/bdd729t.
8.2. Manufacturing, Stability and Regulatory Requirements for Ovarian Cancer Nanomedicines
Strict chemistry, manufacturing, and control (CMC) information is required for manufacturing nanomedicines for ovarian cancer. Tight control of significant quality attributes, such as size, polydispersity and surface charge, is required for complex structures such as liposomes, polymeric nanoparticles and micelles, to guarantee reproducibility between batches and foreseeable pharmacokinetics, as stated in reviews of translational applications of nanomedicine [202]. Small differences in mixing, exchange of solvents or lyophilisation can change ovarian cancer formulations such as pegylated liposomal doxorubicin [203] or nanocomplexes loaded with cisplatin [204]. Parenteral nanomedicines have to be sterile; however, destabilisation of nanocarriers may occur through thermal or radiation sterilisation. Consequently, cost is often increased by aseptic processing and sterile filtration being required, which demands robust stability profiles over storage and after reconstitution [205]. Aggregation, drug leakage and matrix oxidation are features of long-term physiochemical stability that hinder ovarian cancer nanoformulations from entering clinical trials [206]. Together with complex analytics, these CMC and stability requirements raise manufacturing costs, which limit access regardless of clinical benefit in recurrent disease [207]. Furthermore, the clinical progression of complex, multi-element nanomedicines is significantly limited by these constraints, as reliable, reproducible nano-formulations with well-defined critical quality attributes are favoured for large-scale production and regulatory approval. Ovarian nanomedicine regulatory frameworks build on general drug regulations but also include specific FDA and EMA expectations for nanomedicines. The need for thorough characterisation, risk-based EMC strategies and post-marketing surveillance is emphasised by FDA- and EMA-focused reviews, which reference nanotechnology guidance and EMA position papers on liposomes and other nanoproducts [208]. Regulators are more frequently expecting co-validation of imaging or soluble biomarkers, like CA-125 and emerging multiplex panels, for theranostic ovarian platforms [209], evaluated in conjunction with the nanomedicine consistent with the broader shift toward imaging-based companion diagnostics and biomarker-guided theranostics in ovarian cancer [210]. Table 7 summarises the clinical translation of ovarian cancer nanomedicines.

Table 7.
Clinical translation of ovarian cancer nanomedicines: summary of platforms, trial phases, and reported outcomes.
9. Future Perspectives and Challenges
The integration of multi-omics profiling, such as genomics, transcriptomics and proteomics, with AI-based modelling is being increasingly relied on in ovarian cancer precision nanomedicine to stratify patients, predict drug response and direct nanocarrier design. Signatures associated with early detection and prognosis of ovarian cancer can be identified through AI frameworks trained on molecular and imaging biomarkers, although their clinical use is hindered by small, heterogenous datasets and a lack of standardised image and omics workflows [211]. In theory, these models could be coupled to nanoparticle libraries, and based on individual tumour profiles and longitudinal radiomics data, tailored ligand combinations, payloads and dosing schedules could be selected for each patient. At the centre of “imaging-targeting-therapy” concepts in ovarian cancer are multifunctional theranostic platforms; for example, a comprehensive review about imaging and therapy of ovarian cancer: Clinical applications of nanoparticles describe that nanotheranostic nanoparticles, which integrate imaging and drug delivery, function in a single formulation and successfully monitor drug distribution profiles and pathological processes longitudinally in ovarian cancer patients, thus offering a promising approach for diagnostics and therapy [149]. For instance, CA125-targeted radiolabelled antibodies, folate-decorated liposomes and photodynamic nanoparticles for ovarian cancer. Plasmodic and exosome-based sensors are examples of emerging nanotechnologies for early detection that are being adapted to recognise proteins specific to ovarian cancer and nucleic acids in blood, urine or ascites [212]. Due to HGSOC being “immune cold”, TME and immune modulation have developed into essential design criteria for nanomedicine. Kaur et al. describe nanoparticles that reprogram TAMs, deliver TLR agonists like resiquimod to peritoneal TAMs, boost infiltration of CD8+ T-cells and synergise with PD-1/PD-L1 blockade [213]. Comparable future strategies also apply to cytokine-loaded platforms, such as IL-12 nanoparticles, and redox- or pH-sensitive micelles that exploit hypoxia, acidosis or high GSH levels in the peritoneal cavity to stimulate localised drug release and turn “cold” lesions into “hot” ones, as seen in Figure 9. Balanced adjustment of size, charge and stiffness to favour intraperitoneal retention and stromal penetration is highlighted in reviews of TME-responsive nanomedicines [214,215].
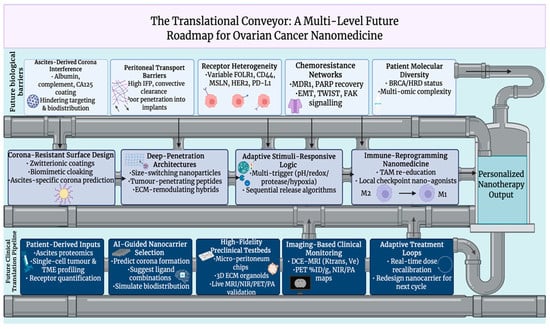
Figure 9.
A multi-level “translational conveyor” illustrating how biological barriers in ovarian cancer guide the development of next-generation nanomedicine engineering strategies and feed into an iterative clinical translation pipeline, ultimately enabling personalised nanotherapeutic design. Created in BioRender. Wilk, I. (2025) https://BioRender.com/7e0wznz.
However, clinical translation is slowed down by inconsistent regulatory frameworks and non-standardised datasets. Overall, in nanomedicine, the lack of reliable definitions, incomplete physiochemical characterisation and unpredictable safety endpoints across authorities are highlighted by regulators [216]. Coordinated international standards bodies and the European Medicines Agency (ISO/CEN-EMA) efforts, common test panels for nanoparticle identity, stability and interoperable data formats which link preclinical, imaging and clinical outcome data, are called for in a proposed standardisation roadmap [217]. Simultaneously, before AI-based decision tools for ovarian cancer can guide regulatory-grade precision nanotherapy, they must meet developing requirements for transparency, bias control and external validation [211].
Ovarian cancer nanomedicine could be redesigned by several emerging technologies. Scalable, exosome-like carriers with inherent tumour tropism can be offered by immune cell-derived exosome mimetics (IDEM), which can deliver small RNAs or drugs to resistant cells in ovarian models and could overcome some manufacturing obstacles of natural exosomes [218]. Enzyme- and pH-responsive platforms for drug and immunomodulator delivery can be demonstrated by self-assembling peptide nanostructures that can also be used as 3D matrices that replicate the multicellular ovarian TME for screening nanotherapeutics [219]. Lastly, strong imaging contrast or photothermal properties with tuneable clearance are combined by biodegradable metallic nanocarriers, such as gold or zinc oxide systems, which are engineered for controlled dissolution. This signifies a promising direction for safer theranostic platforms in ovarian cancer [220].
10. Conclusions
The diagnostic and therapeutic landscape of ovarian cancer is being rapidly reshaped by nanomedicine, presenting practical solutions to persistent challenges such as late detection, drug resistance, peritoneal dissemination, and dose-limiting toxicity. Nanoparticles reliably demonstrate the ability to improve drug pharmacokinetics, advance tumour-selective delivery, and facilitate high-resolution multimodal imaging across lipid-based, polymeric, inorganic, hybrid, and biomimetic platforms, which together form the basis of highly precise and adaptive ovarian cancer care. Notably, nanocarriers are progressively designed to navigate the ovarian TME and its biological complexities, such as hypoxia, dense ECM, ascites and expression of heterogenous receptors, by which the effectiveness of systemic chemotherapy was historically hindered. Innovative nanomedicines achieve deeper tumour penetration, maintain stable intraperitoneal residence, and reduce systemic toxicity by integrating targeting ligands, stimuli-sensitive release designs and stealth strategies, thus widening the therapeutic window against advanced ovarian cancer.
A particularly transformative development is represented by theranostic platforms. Clinicians can visualise tumour load, guide surgical resection, and track treatment response in real time by uniting imaging and therapy into a single platform, such as MRI-trackable liposomes, radiolabelled polymeric particles and photoacoustic-sensitive nanodroplets. As peritoneal implants are often microscopic, widely disseminated and hard to detect with standard imaging, these capabilities address a critical need in ovarian cancer that is addressed by theranostic platforms.
Although clear progress has been made, clinical translation is still limited. Based on the evidence synthesised in this review, in order to accelerate clinical translation, several priorities must be addressed. A primary priority is the identification of patients who are most likely to benefit from targeted or theranostic nanomedicines using molecular imaging and companion diagnostics by emphasising biomarker-guided patient stratification in future research. A further priority is to allow personalised dosing, early response assessment, and adaptive treatment strategies by expanding the integration of quantitative imaging biomarkers into nanomedicine development. An additional priority is to place greater focus on intraperitoneal delivery systems and ascites-resistant designs, which remain under-represented in clinical trials although they are uniquely suited to the dissemination pattern of ovarian cancer. A further priority is to advance the standardisation of nanocarrier characterisation, scalable manufacturing processes, and regulatory alignment in order to improve reproducibility and enable regulatory approval. Lastly, a promising direction to overcome chemoresistance and immune suppression in advanced disease is represented by integrating immunotherapy, gene-based approaches and stimuli-responsive platforms. Complementary advances in immunonanomedicine further support this strategy by aiding TAM reprogramming, improving T-cell infiltration and promoting synergistic checkpoint blockade in immune-cold HGSOC.
To conclude, the future impact of nanomedicine in ovarian cancer will rely not only on ongoing material and engineering innovation but also greatly on strategic integration with molecular profiling, quantitative imaging, and clinically relevant delivery routes. In order to translate nanotechnology from experimental platforms into operative, patient-centred solutions for ovarian cancer management, addressing these challenges will be essential.
Author Contributions
Conceptualization, D.B.-A., I.W., D.A.; methodology. D.B.-A., I.W., D.A. validation, D.B.-A., I.W., D.A. formal analysis D.B.-A., I.W., D.A. resources, D.B.-A., I.W., D.A. writing—original draft preparation, D.B.-A., I.W., D.A. writing—review and editing, D.B.-A., I.W., D.A. visualization, D.B.-A., I.W., D.A. supervision, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, M.K.; Ding, D.C. Early Diagnosis of Ovarian Cancer: A Comprehensive Review of the Advances, Challenges, and Future Directions. Diagnostics 2025, 15, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giampaolino, P.; Foreste, V.; Della Corte, L.; Di Filippo, C.; Iorio, G.; Bifulco, G. Role of biomarkers for early detection of ovarian cancer recurrence. Gland. Surg. 2020, 9, 1102–1111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Wang, X.; Zhu, X.; Zhong, L.; Jiang, Q.; Wang, Y.; Tang, Q.; Li, Q.; Zhang, C.; Wang, H.; et al. Drug resistance in ovarian cancer: From mechanism to clinical trial. Mol. Cancer 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Gao, Y.; Zhang, X.; Guo, H.; Gao, H. Nanoparticles in precision medicine for ovarian cancer: From chemotherapy to immunotherapy. Int. J. Pharm. 2020, 591, 119986. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.; Huynh, G.; Wilson, K.; Plebanski, M.; Corrie, S. The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers. Biomedicines 2021, 9, 1554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burns, J.M.; Shafer, E.; Vankayala, R.; Kundra, V.; Anvari, B. Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination. Cancers 2021, 13, 2544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gralewska, P.; Gajek, A.; Marczak, A.; Rogalska, A. Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 8304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Debnath, S.K.; Debnath, M.; Ghosh, A.; Srivastava, R.; Omri, A. Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications. Pharmaceuticals 2024, 17, 1389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The Complex Tumor Microenvironment in Ovarian Cancer: Therapeutic Challenges and Opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murray, A.M.; Swingle, K.L.; Mitchell, M.J. Engineering Nanoparticles for Gynecologic Cancer Therapy. ACS Nano 2025, 19, 30758–30785. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.; Mendoza López, R.V.; Maistro, S.; Ikeoka, L.T.; Pereira, G.F.L.; Lugão, A.B.; Sadalla, J.C.; Katayama, M.L.H.; Folgueira, M.A.A.K. Peritoneal chemotherapy delivery systems for ovarian cancer treatment: Systematic review of animal models. Front. Oncol. 2025, 14, 1487376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perelló-Trias, M.T.; Serrano-Muñoz, A.J.; Rodríguez-Fernández, A.; Segura-Sampedro, J.J.; Ramis, J.M.; Monjo, M. Intraperitoneal drug delivery systems for peritoneal carcinomatosis: Bridging the gap between research and clinical implementation. J. Control. Release 2024, 373, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.Y.; Llaurado-Fernandez, M.; Cameron, A.; Da-Anoy, A.; Cook, L.C.; Hoenisch, J.; Ghesquiere, C.; Gaillard, S.; Schmid, J.; Dawson, A.; et al. FOLR1 as a therapeutic target in platinum-resistant ovarian carcinoma: Unique expression patterns across ovarian carcinoma histotypes and molecular subtypes of low-grade serous carcinoma. J. Gynecol. Oncol. 2025, 36, e74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Went, P.; Dirnhofer, S.; Obrist, P.; Moch, H.; Baeuerle, P.A.; Mueller-Holzner, E.; Marth, C.; Gastl, G.; Zeimet, A.G. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dijkgraaf, I.; Kruijtzer, J.A.; Frielink, C.; Corstens, F.H.; Oyen, W.J.; Liskamp, R.M.; Boerman, O.C. Alpha v beta 3 integrin-targeting of intraperitoneally growing tumors with a radiolabeled RGD peptide. Int. J. Cancer 2007, 120, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Prognostic effect of programmed death-ligand 1 (PD-L1) in ovarian cancer: A systematic review, meta-analysis and bioinformatics study. J. Ovarian Res. 2019, 12, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maurer, A.H.; Elsinga, P.; Fanti, S.; Nguyen, B.; Oyen, W.J.; Weber, W.A. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: Properties, clinical use, and future potential of folate receptor imaging. J. Nucl. Med. 2014, 55, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Briolay, T.; Fresquet, J.; Meyer, D.; Kerfelec, B.; Chames, P.; Ishow, E.; Blanquart, C. Specific Targeting of Mesothelin-Expressing Malignant Cells Using Nanobody-Functionalized Magneto-Fluorescent Nanoassemblies. Int. J. Nanomed. 2024, 19, 633–650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benedetto, G.; Fowler, A.; Langdon, D.; Raine, M.; White, M.L.; Ogle, J.; Garmon, C.; Ogle, C.; Richardson, C. Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo. Biomolecules 2025, 15, 1123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, J.; Tan, W.; Zheng, J.; Su, Y.; Cui, M. Aptamer Nanomaterials for Ovarian Cancer Target Theranostics. Front. Bioeng. Biotechnol. 2022, 10, 884405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.; Zhao, R.; Zhao, E.; Wang, Q.; Lian, W.; Xiong, J. Targeting CD44-positive ovarian cancers via engineered paclitaxel prodrug nanoparticles for enhanced chemotherapeutic efficacy. Biomed. Pharmacother. 2022, 154, 113655. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Shim, M.K.; Choi, J.; Yang, S.; Kim, J.; Yun, W.S.; Cho, H.; Park, J.Y.; Kim, Y.; Seong, J.K.; et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 2022, 12, 1999–2014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haber, T.; Cornejo, Y.R.; Aramburo, S.; Flores, L.; Cao, P.; Liu, A.; Mooney, R.; Gilchrist, M.; Tirughana, R.; Nwokafor, U.; et al. Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 19737–19745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthaiou, E.I.; Barar, J.; Sandaltzopoulos, R.; Li, C.; Coukos, G.; Omidi, Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int. J. Nanomed. 2014, 9, 1855–1870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rietveld, P.C.S.; Guchelaar, N.A.D.; Sassen, S.D.T.; Koch, B.C.P.; Mathijssen, R.H.J.; Koolen, S.L.W. A Clinical Pharmacological Perspective on Intraperitoneal Chemotherapy. Drugs 2025, 85, 931–943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirmani, S.; Braly, P.S.; McClay, E.F.; Saltzstein, S.L.; Plaxe, S.C.; Kim, S.; Cates, C.; Howell, S.B. A comparison of intravenous versus intraperitoneal chemotherapy for the initial treatment of ovarian cancer. Gynecol. Oncol. 1994, 54, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, L.B.; Huang, H.Q.; Armstrong, D.K.; Walker, J.L.; Cella, D.; Gynecologic Oncology Group. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowacki, M.; Peterson, M.; Kloskowski, T.; McCabe, E.; Guiral, D.C.; Polom, K.; Pietkun, K.; Zegarska, B.; Pokrywczynska, M.; Drewa, T.; et al. Nanoparticle as a novel tool in hyperthermic intraperitoneal and pressurized intraperitoneal aerosol chemotheprapy to treat patients with peritoneal carcinomatosis. Oncotarget 2017, 8, 78208–78224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Man, F.; Lammers, T.; de Rosales, R.T.M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, X.; Li, Z.; Huang, Y.; Li, Y.; Li, Y.; Zhang, L.; Wu, M. Polymer Nanoparticles Advancements for Gynecological Cancers. Int. J. Nanomed. 2025, 20, 6721–6742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, A.N.; Fleagle, J.T.; Guthrie, D.; Parkin, D.E.; Gore, M.E.; Lacave, A.J. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J. Clin. Oncol. 2001, 19, 3312–3322. [Google Scholar] [CrossRef] [PubMed]
- Haemmerich, D.; Motamarry, A. Thermosensitive Liposomes for Image-Guided Drug Delivery. Adv Cancer Res. 2018, 139, 121–146. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yu, Y.; Zhang, Y.; Yan, Y.; Zheng, Y.; He, J.; Xie, Y.; He, G.; Wei, Y.; Song, X. Gene delivery with active targeting to ovarian cancer cells mediated by folate receptor alpha. J. Biomed. Nanotechnol. 2013, 9, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Low, H.Y.; Yang, C.T.; Xia, B.; He, T.; Lam, W.W.C.; Ng, D.C.E. Radiolabeled Liposomes for Nuclear Imaging Probes. Molecules 2023, 28, 3798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landen, C.N.; Merritt, W.M.; Mangala, L.S.; Sanguino, A.M.; Bucana, C.; Lu, C.; Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Schmandt, R.; et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol. Ther. 2006, 5, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chen, Q.; Riviere, J.E.; Lin, Z. Pharmacokinetics and tumor delivery of nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 83, 104404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luiz, M.T.; Abriata, J.P.; Raspantini, G.L.; Tofani, L.B.; Fumagalli, F.; de Melo, S.M.G.; Emery, F.D.S.; Swiech, K.; Marcato, P.D.; Lee, R.; et al. In vitro evaluation of folate-modified PLGA nanoparticles containing paclitaxel for ovarian cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110038. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, J.W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D.; et al. CD44-Targeting PLGA Nanoparticles Incorporating Paclitaxel and FAK siRNA Overcome Chemoresistance in Epithelial Ovarian Cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef] [PubMed]
- Repp, L.; Rasoulianboroujeni, M.; Lee, H.J.; Kwon, G.S. Acyl and oligo(lactic acid) prodrugs for PEG-b-PLA and PEG-b-PCL nano-assemblies for injection. J. Control. Release 2021, 330, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Shahin, S.A.; Wen, W.; Finlay, J.B.; Dong, J.; Wang, R.; Dellinger, T.H.; Zink, J.I.; Tamanoi, F.; Glackin, C.A. Nanoparticle delivery of siRNA against TWIST to reduce drug resistance and tumor growth in ovarian cancer models. Nanomedicine 2017, 13, 965–976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gbadegesin, O.D.; Adesina, S.K. Gemcitabine-Doxorubicin Combination Polymer-Drug Conjugate Prepared by SPAAC Click Chemistry: In Vitro Characterization. Int. J. Mol. Sci. 2025, 26, 2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dragulska, S.A.; Acosta Santiago, M.; Swierczek, S.; Chuang, L.; Camacho-Vanegas, O.; Camacho, S.C.; Padron-Rhenals, M.M.; Martignetti, J.A.; Mieszawska, A.J. Synthesis and Characterization of Poly(Lactic-Co-Glycolic Acid)-Paclitaxel (PLGA-PTX) Nanoparticles Evaluated in Ovarian Cancer Models. Pharmaceutics 2025, 17, 689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, W.; Yang, F.; Chen, K.; Zeng, Q. Targeted gold nanoparticles for ovarian cancer (Review). Oncol. Lett. 2024, 28, 589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Soriano, D.; Milán-Rois, P.; Lafuente-Gómez, N.; Rodríguez-Díaz, C.; Navío, C.; Somoza, Á.; Salas, G. Multicore iron oxide nanoparticles for magnetic hyperthermia and combination therapy against cancer cells. J. Colloid. Interface Sci. 2024, 670, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Peng, C.W.; Pang, D.W.; Li, Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol. Med. 2012, 9, 151–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savla, R.; Taratula, O.; Garbuzenko, O.; Minko, T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J. Control. Release 2011, 153, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D.S.; Wang, Y.; Gu, H.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tolani, S.L.; Soni, S. Next-generation Mesoporous Silica Nanoparticles: Precision-engineered Platforms for Ovarian Cancer Therapy. Curr. Drug Res. Rev. 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouyang, X.; Liu, Y.; Zheng, K.; Pang, Z.; Peng, S. Recent advances in zwitterionic nanoscale drug delivery systems to overcome biological barriers. Asian J. Pharm. Sci. 2024, 19, 100883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Zhang, X.; Gao, H.; Zhang, X.; Sun, L.; Huang, Y.; Zhang, J.; Ding, B. Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment. Pharmaceutics 2024, 16, 531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pires, I.S.; Covarrubias, G.; Gomerdinger, V.F.; Backlund, C.; Shanker, A.; Gordon, E.; Wu, S.; Pickering, A.J.; Melo, M.B.; Suh, H.; et al. “Target-and-release” nanoparticles for effective immunotherapy of metastatic ovarian cancer. bioRxiv 2024, bioRxiv:2024.07.05.602135. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Lv, X.; Gao, M.; Gong, X.; Yao, Q.; Liu, Y. Nanoparticle-Based Combination Therapy for Ovarian Cancer. Int. J. Nanomed. 2023, 18, 1965–1987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Bai, J.; Yang, W. Stimuli-responsive nanocarriers for therapeutic applications in cancer. Cancer Biol. Med. 2021, 18, 319–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Avila, G.; Sommer, B.; García-Hernandez, A.A.; Ramos, C.; Flores-Soto, E. Nanotechnology and Matrix Metalloproteinases in Cancer Diagnosis and Treatment. Front. Mol. Biosci. 2022, 9, 918789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bevilacqua, G.; Corvino, R.; Capriotti, A.L.; Montone, C.M.; Moriconi, M.; Salciccia, S.; Brunelli, V.; Santarelli, V.; Sciarra, B.; Laganà, A.; et al. The Protein Corona on Nanoparticles for Tumor Targeting in Prostate Cancer-A Review of the Literature and Experimental Trial Protocol. Biology 2024, 13, 1024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, X.; Guo, T.; Chen, G.; Nie, D.; Yue, M.; Zhu, Y.; Lin, M. The therapeutic effect and MR molecular imaging of FA-PEG-FePt/DDP nanoliposomes in AMF on ovarian cancer. Int. J. Nanomed. 2024, 19, 5227–5243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kafshdooz, L.; Kafshdooz, T.; Razban, Z.; Akbarzadeh, A. The application of gold nanoparticles as a promising therapeutic approach in breast and ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 1890–1905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colli, C.; Masi, I.; Jacchetti, E.; Santoni, S.; Sponchioni, M.; Colosimo, B.M.; Rosanò, L.; Raimondi, M.T.; Mauri, E.; Moscatelli, D. Zwitterionic nanoparticles for thermally activated drug delivery in hyperthermia cancer treatment. Nanoscale 2024, 16, 12635–12649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhattacharya, S.; Prajapati, B.G.; Singh, S. A critical review on the dissemination of PH and stimuli-responsive polymeric nanoparticular systems to improve drug delivery in cancer therapy. Crit. Rev. Oncol. Hematol. 2023, 185, 103961. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, R.; Nelissen, E.; El-Shakankery, K.H.; Rogozińska, E.; Bain, E.; Veiga, S.; Morrison, J. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev. 2023, 7, CD006910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Al Faruque, H.; Li, S.; Sima, M.; Sborov, D.; Hu-Lieskovan, S.; Werner, T.; Kopeček, J.; Yang, J. PD-L1 targeted antibody-polymer-Epirubicin conjugate prolongs survival in a preclinical murine model of advanced ovarian cancer. J. Control. Release 2025, 382, 113682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Duan, X.; Xu, X.; Gao, Y.; Zhou, J.; Xu, X.; Li, J. mPEG-PDLLA Micelles Potentiate Docetaxel for Intraperitoneal Chemotherapy in Ovarian Cancer Peritoneal Metastasis. Front. Pharmacol. 2022, 13, 861938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hargrove, D.; Ranjbar, S.; Darji, M.; Nam, S.; Dawson, R.J.; Katugampola, S.; Lin, X.; Brown, A.; Carrasco-Rojas, N.; Goodwin, C.; et al. Tumor specific delivery and radiation-enhanced tumor penetration of mesoporous silica nanoparticles for effective radionuclide therapy of ovarian peritoneal metastasis. Int. J. Pharm. 2025, 669, 125071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knežević, N.Ž.; Lin, V.S. A magnetic mesoporous silica nanoparticle-based drug delivery system for photosensitive cooperative treatment of cancer with a mesopore-capping agent and mesopore-loaded drug. Nanoscale 2013, 5, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Nam, K.H.; Gupta, R.; Gao, Z.; Mohan, P.; Payne, A.; Todd, N.; Liu, X.; Kim, T.; Shea, J.; et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 2011, 153, 4–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.; Wang, Y.; Zhu, L.; Wu, J.; Lin, J.; Huang, W.; Yan, D. Hybrid Membrane Camouflaged Chemodrug-Gene Nanoparticles for Enhanced Combination Therapy of Ovarian Cancer. ACS Appl. Mater. Interfaces 2023, 15, 58067–58078. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Gabizon-Peretz, S.; Modaresahmadi, S.; La-Beck, N.M. Thirty years from FDA approval of pegylated liposomal doxorubicin (Doxil/Caelyx): An updated analysis and future perspective. BMJ Oncol. 2025, 4, e000573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halamoda-Kenzaoui, B.; Holzwarth, U.; Roebben, G.; Bogni, A.; Bremer-Hoffmann, S. Mapping of the available standards against the regulatory needs for nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gatto, M.S.; Najahi-Missaoui, W. Lyophilization of Nanoparticles, Does It Really Work? Overview of the Current Status and Challenges. Int. J. Mol. Sci. 2023, 24, 14041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clogston, J.D.; Foss, W.; Harris, D.; Oberoi, H.; Pan, J.; Pu, E.; Guzmán, E.A.T.; Walter, K.; Brown, S.; Soo, P.L. Current state of nanomedicine drug products: An industry perspective. J. Pharm. Sci. 2024, 113, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, N.R.; Piroyan, A.; Nack, A.H.; Galati, C.A.; McHugh, M.; Orosz, S.; Keeler, A.W.; O’Neal, S.; Zamboni, W.C.; Davis, B.; et al. Design, Synthesis, and Characterization of Folate-Targeted Platinum-Loaded Theranostic Nanoemulsions for Therapy and Imaging of Ovarian Cancer. Mol. Pharm. 2016, 13, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wan, Y.J.; Xu, X.Z.; Wang, N.; Li, F.; Zhou, H. Preliminary study of mesothelin-loaded paclitaxel nanoparticles for ultrasound molecular imaging and treatment of ovarian cancer. Chin. J. Oncol. 2025, 47, 395–403. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Pantshwa, J.M.; Rhoda, K.; Clift, S.J.; Pradeep, P.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Penny, C.; Pillay, V. Chemotherapeutic Efficacy of Implantable Antineoplastic-Treatment Protocols in an Optimal Mouse Model for Human Ovarian Carcinoma Cell Targeting. Int. J. Mol. Sci. 2018, 19, 3030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satpathy, M.; Wang, L.; Zielinski, R.J.; Qian, W.; Wang, Y.A.; Mohs, A.M.; Kairdolf, B.A.; Ji, X.; Capala, J.; Lipowska, M.; et al. Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles. Theranostics 2019, 9, 778–795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [PubMed]
- Han, H.D.; Mangala, L.S.; Lee, J.W.; Shahzad, M.M.; Kim, H.S.; Shen, D.; Nam, E.J.; Mora, E.M.; Stone, R.L.; Lu, C.; et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 2010, 16, 3910–3922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic acid-decorated PLGA-PEG nanoparticles for targeted delivery of SN-38 to ovarian cancer. Anticancer. Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Yang, X.; Iyer, A.K.; Singh, A.; Choy, E.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep. 2015, 5, 8509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R., Jr. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011, 63, 2671–2680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Y.; Zhang, J.; Huang, K.; Peng, Y.; Cheng, S.; Liu, S.; Zhou, T.; Chen, J.; Li, H.; Zhao, Y.; et al. Engineered CAF-cancer cell hybrid membrane biomimetic dual-targeted integrated platform for multi-dimensional treatment of ovarian cancer. J. Nanobiotechnol. 2025, 23, 83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, G.H.; Won, J.E.; Byeon, Y.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Park, Y.Y.; Lee, J.W.; Kang, T.H.; Jung, I.D.; et al. Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Deliv. 2018, 25, 1394–1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.Y.; Termsarasab, U.; Park, J.H.; Lee, S.Y.; Ko, S.H.; Shim, J.S.; Chung, S.J.; Cho, H.J.; Kim, D.D. Dual CD44 and folate receptor-targeted nanoparticles for cancer diagnosis and anticancer drug delivery. J. Control. Release 2016, 236, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Yang, L.; Du, G.; Pai-Panandiker, A.S.; Huang, X.; Yan, B. Enhancement of cell recognition in vitro by dual-ligand cancer targeting gold nanoparticles. Biomaterials 2011, 32, 2540–2545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Bardhan, R.; Bartels, M.; Perez-Torres, C.; Pautler, R.G.; Halas, N.J.; Joshi, A. A molecularly targeted theranostic probe for ovarian cancer. Mol. Cancer Ther. 2010, 9, 1028–1038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graybill, W.S.; Coleman, R.L. Vintafolide: A novel targeted agent for epithelial ovarian cancer. Future Oncol. 2014, 10, 541–548. [Google Scholar] [CrossRef] [PubMed]
- López-Portugués, C.; Montes-Bayón, M.; Díez, P. Biomarkers in Ovarian Cancer: Towards Personalized Medicine. Proteomes 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garbuzenko, O.B.; Sapiezynski, J.; Girda, E.; Rodriguez-Rodriguez, L.; Minko, T. Personalized Versus Precision Nanomedicine for Treatment of Ovarian Cancer. Small 2024, 20, e2307462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, R.T.; Joyrich, R.N.; Naumann, R.W.; Shah, N.P.; Maurer, A.H.; Strauss, H.W.; Uszler, J.M.; Symanowski, J.T.; Ellis, P.R.; Harb, W.A. Phase II study of treatment of advanced ovarian cancer with folate-receptor-targeted therapeutic (vintafolide) and companion SPECT-based imaging agent (99mTc-etarfolatide). Ann. Oncol. 2014, 25, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Konovalova, E.; Xu, T.; Schulga, A.; Altai, M.; Garousi, J.; Rinne, S.S.; Orlova, A.; Tolmachev, V.; Deyev, S. Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1. Int. J. Mol. Sci. 2020, 21, 3310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santin, A.D.; Vergote, I.; González-Martín, A.; Moore, K.; Oaknin, A.; Romero, I.; Diab, S.; Copeland, L.J.; Monk, B.J.; Coleman, R.L.; et al. Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: Multicenter, phase Ib dose escalation and expansion study. Int. J. Gynecol. Cancer 2023, 33, 562–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, T.; Tian, T.; Wang, C.; Chen, X.; Zuo, X.; Zhou, H.; Bai, J.; Zhao, C.; Fu, S.; Sun, C.; et al. Efficacy and safety of novel multiple-chain DAP-CAR-T cells targeting mesothelin in ovarian cancer and mesothelioma: A single-arm, open-label and first-in-human study. Genome Med. 2024, 16, 133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Liu, Q.; Xie, Y.; Zhu, J.; Zhang, S.; Ge, Y.; Guo, J.; Luo, N.; Huang, W.; Xu, R.; et al. MUC16 stimulates neutrophils to an inflammatory and immunosuppressive phenotype in ovarian cancer. J. Ovarian Res. 2023, 16, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fung, K.; Sharma, S.K.; Keinänen, O.; Roche, K.L.; Lewis, J.S.; Zeglis, B.M. A Molecularly Targeted Intraoperative Near-Infrared Fluorescence Imaging Agent for High-Grade Serous Ovarian Cancer. Mol. Pharm. 2020, 17, 3140–3147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, Y.; Shao, F.; Ji, H.; Song, X.; Lv, X.; Xia, X.; Liu, Q.; Zhang, Y.; Zeng, D.; Lan, X.; et al. Evaluation of a CD13 and Integrin αvβ3 Dual-Receptor Targeted Tracer 68Ga-NGR-RGD for Ovarian Tumor Imaging: Comparison With 18F-FDG. Front. Oncol. 2022, 12, 884554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthaiou, E.I.; Guo, Y.; Barar, J.; Sandaltzopoulos, R.; Kandalaft, L.E.; Li, C.; Coukos, G.; Omidi, Y. TEM1-targeting PEGylated PLGA shikonin nanoformulation for immunomodulation and eradication of ovarian cancer. Bioimpacts 2022, 12, 65–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kramer, M.; Criswell, A.; Marzette, K.; Cutcliffe, E.; Sewell-Loftin, M.K. Strain and hyaluronic acid interact to regulate ovarian cancer cell proliferation, migration, and drug resistance. Mechanobiol. Med. 2024, 2, 100094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Z.; Li, M.; Li, H.; Gao, Q. PD-1/PD-L1 immune checkpoint blockade in ovarian cancer: Dilemmas and opportunities. Drug Discov. Today 2023, 28, 103666. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.R.; Maklouf, G.R.; Teixeira, C.E.; de Oliveira Santos, L.; Tessarollo, N.G.; de Toledo, N.E.; Serain, A.F.; de Lanna, C.A.; Pretti, M.A.; da Cruz, J.G.V.; et al. Single-cell resolution characterization of myeloid-derived cell states with implication in cancer outcome. Nat. Commun. 2024, 15, 5694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazumder, S.; Swank, V.; Komar, A.A.; Johnson, J.M.; Tuohy, V.K. Immunotherapy of ovarian cancer with a monoclonal antibody specific for the extracellular domain of anti-Müllerian hormone receptor II. Oncotarget 2020, 11, 1894–1910, Erratum in Oncotarget 2022, 13, 905–906. https://doi.org/10.18632/oncotarget.28246. PMID: 32499873; PMCID: PMC7244012. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Hu, Y.; Shen, M.; Shi, X.; Zhang, G. Folic acid-targeted iron oxide nanoparticles as contrast agents for magnetic resonance imaging of human ovarian cancer. J. Ovarian Res. 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravoori, M.K.; Singh, S.; Bhavane, R.; Sood, A.K.; Anvari, B.; Bankson, J.; Annapragada, A.; Kundra, V. Multimodal Magnetic Resonance and Near-Infrared-Fluorescent Imaging of Intraperitoneal Ovarian Cancer Using a Dual-Mode-Dual-Gadolinium Liposomal Contrast Agent. Sci. Rep. 2016, 6, 38991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erdogan, S.; Medarova, Z.O.; Roby, A.; Moore, A.; Torchilin, V.P. Enhanced tumor MR imaging with gadolinium-loaded polychelating polymer-containing tumor-targeted liposomes. J. Magn. Reson. Imaging 2008, 27, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Colby, A.H.; Kirsch, J.; Patwa, A.N.; Liu, R.; Hollister, B.; McCulloch, W.; Burdette, J.E.; Pearce, C.J.; Oberliels, N.H.; Colson, Y.L.; et al. Radiolabeled Biodistribution of Expansile Nanoparticles: Intraperitoneal Administration Results in Tumor Specific Accumulation. ACS Nano 2023, 17, 2212–2221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshayes, E.; Ladjohounlou, R.; Le Fur, P.; Pichard, A.; Lozza, C.; Boudousq, V.; Sevestre, S.; Jarlier, M.; Kashani, R.; Koch, J.; et al. Radiolabeled Antibodies Against Müllerian-Inhibiting Substance Receptor, Type II: New Tools for a Theranostic Approach in Ovarian Cancer. J. Nucl. Med. 2018, 59, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, S.; Penet, M.F. Ovarian Cancer Targeted Theranostics. Front. Oncol. 2020, 9, 1537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mushtaq, S.; Bibi, A.; Park, J.E.; Jeon, J. Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy. Nanomaterials 2021, 11, 3022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pu, T.; Liu, Y.; Pei, Y.; Peng, J.; Wang, Z.; Du, M.; Liu, Q.; Zhong, F.; Zhang, M.; Li, F.; et al. NIR-II Fluorescence Imaging for the Detection and Resection of Cancerous Foci and Lymph Nodes in Early-Stage Orthotopic and Advanced-Stage Metastatic Ovarian Cancer Models. ACS Appl. Mater. Interfaces 2023, 15, 32226–32239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Li, Z.; Wang, X.; Liu, F.; Cheng, Y.; Zhang, B.; Shi, D. In vivo cancer targeting and imaging-guided surgery with near infrared-emitting quantum dot bioconjugates. Theranostics 2012, 2, 769–776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.; Zhou, X.; Feng, W.; Pu, T.; Li, X.; Li, F.; Kang, Y.; Zhang, X.; Xu, C. Gonadotropin-Releasing Hormone Receptor-Targeted Near-Infrared Fluorescence Probe for Specific Recognition and Localization of Peritoneal Metastases of Ovarian Cancer. Front. Oncol. 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashiwagi, S.; Choi, H.S. Ovarian cancer-targeted near-infrared fluorophores for fluorescence-guided surgery. Ann. Transl. Med. 2023, 11, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Ji, H.; Jing, Y.; Wang, S. pH- and acoustic-responsive platforms based on perfluoropentane-loaded protein nanoparticles for ovarian tumor-targeted ultrasound imaging and therapy. Nanoscale Res. Lett. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Zhang, Y.; Luo, Y.; Qiao, B.; Wang, X.; Zhang, L.; Chen, Q.; Cao, Y.; Wang, Z.; Ran, H. Dual ultrasound-activatable nanodroplets for highly-penetrative and efficient ovarian cancer theranostics. J. Mater. Chem. B 2020, 8, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Guo, L.; Xiao, S.; Meng, D.; Shang, M.; Sun, X.; Shi, D.; Zhao, Y.; Liu, R.; et al. Dual-responsive nanoscale ultrasound contrast agent as an oxidative stress amplifier for enhanced DNA damage in BRCA-proficient ovarian cancer. Mater. Today Bio 2025, 32, 101761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niu, G.; Song, G.; Kang, Y.; Zhai, Y.; Fan, Y.; Ye, J.; Li, R.; Li, R.; Zhang, Y.; Wang, H.; et al. Quinoidal Semiconductor Nanoparticles for NIR-II Photoacoustic Imaging and Photoimmunotherapy of Cancer. Adv. Mater. 2025, 37, e2415189. [Google Scholar] [CrossRef] [PubMed]
- St Lorenz, A.; Moses, A.S.; Mamnoon, B.; Demessie, A.A.; Park, Y.; Singh, P.; Taratula, O.; Taratula, O.R. A Photoacoustic Contrast Nanoagent with a Distinct Spectral Signature for Ovarian Cancer Management. Adv. Healthc. Mater. 2023, 12, e2202946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Chen, S.; Sun, J.; Zhu, S.; Chen, C.; Xie, W.; Zheng, J.; Zhu, Y.; Xiao, L.; Hao, L.; et al. Folate-Targeted and Oxygen/Indocyanine Green-Loaded Lipid Nanoparticles for Dual-Mode Imaging and Photo-sonodynamic/Photothermal Therapy of Ovarian Cancer in Vitro and in Vivo. Mol Pharm. 2019, 16, 4104–4120, Erratum in Mol. Pharm. 2020, 17, 1442–1443. https://doi.org/10.1021/acs.molpharmaceut.0c00114. PMID: 31517495. [Google Scholar] [CrossRef]
- Zhou, M.; Melancon, M.; Stafford, R.J.; Li, J.; Nick, A.M.; Tian, M.; Sood, A.K.; Li, C. Precision Nanomedicine Using Dual PET and MR Temperature Imaging-Guided Photothermal Therapy. J. Nucl. Med. 2016, 57, 1778–1783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.; Lin, B.; Chen, Z.; Liu, P.; Liu, J.; Li, W.; Liu, P.; Guo, Z.; Chen, C. Biodegradable hollow mesoporous organosilica nanotheranostics (HMONs) as a versatile platform for multimodal imaging and phototherapeutic-triggered endolysosomal disruption in ovarian cancer. Drug Deliv. 2022, 29, 161–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Xu, F.; Huang, J.; Xu, J.; Liu, Y.; Yao, Y.; Ao, M.; Li, A.; Hao, L.; Cao, Y.; et al. Low-intensity focused ultrasound (LIFU)-activated nanodroplets as a theranostic agent for noninvasive cancer molecular imaging and drug delivery. Biomater. Sci. 2018, 6, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Luo, J.; Jain, T.; Riggs, J.W.; Tseng, H.P.; Henderson, P.T.; Cherry, S.R.; Rowland, D.; Lam, K.S. Biodistribution and pharmacokinetics of a telodendrimer micellar paclitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int. J. Nanomed. 2012, 7, 1587–1597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nomani, A.; Yousefi, S.; Sargsyan, D.; Hatefi, A. A quantitative MRI-based approach to estimate the permeation and retention of nanomedicines in tumors. J. Control. Release 2024, 368, 728–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Zhang, J.; Zou, H.; Shen, Y.; Deng, S.; Wu, Y. Synthesis and evaluation of 68Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft. J. Cancer 2021, 12, 244–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Lorenzo, G.; Ricci, G.; Severini, G.M.; Romano, F.; Biffi, S. Imaging and therapy of ovarian cancer: Clinical application of nanoparticles and future perspectives. Theranostics 2018, 8, 4279–4294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, C.M.; Lan, K.L.; Huang, W.S.; Lee, Y.J.; Lee, T.W.; Chang, C.H.; Chuang, C.M. 188Re-Liposome Can Induce Mitochondrial Autophagy and Reverse Drug Resistance for Ovarian Cancer: From Bench Evidence to Preliminary Clinical Proof-of-Concept. Int. J. Mol. Sci. 2017, 18, 903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.M.; Feng, F.; Qiang, J.W.; Zhang, G.F.; Zhao, S.H.; Ma, F.H.; Li, Y.A.; Gu, W.Y. Quantitative dynamic contrast-enhanced MR imaging for differentiating benign, borderline, and malignant ovarian tumors. Abdom. Radiol. 2018, 43, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N., Jr.; Chavez-Reyes, A.; Bucana, C.; Schmandt, R.; Deavers, M.T.; Lopez-Berestein, G.; Sood, A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005, 65, 6910–6918. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Dimitrova, I.; Howe, E.N.; Cochrane, D.R.; Jean, A.; Spoelstra, N.S.; Post, M.D.; Lu, X.; Broaddus, R.R.; Spillman, M.A.; et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol. Cancer Ther. 2012, 11, 2556–2565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldwin, P.; Ohman, A.W.; Tangutoori, S.; Dinulescu, D.M.; Sridhar, S. Intraperitoneal delivery of NanoOlaparib for disseminated late-stage cancer treatment. Int. J. Nanomed. 2018, 13, 8063–8074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J.; et al. Layer-by-layer nanoparticles for novel delivery of cisplatin and PARP inhibitors for platinum-based drug resistance therapy in ovarian cancer. Bioeng. Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Z.Y.; Zhang, Y.G.; Yang, Y.H.; Ma, C.C.; Wang, P.; Du, W.; Li, L.; Xiang, R.; Song, X.R.; Zhao, X.; et al. In Vivo Ovarian Cancer Gene Therapy Using CRISPR-Cas9. Hum. Gene Ther. 2018, 29, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, M.K.; Cho, Y.J.; Jeon, S.; Moon, Y.; Choi, J.; Kim, J.; Lee, J.; Lee, J.W.; Kim, K. The safe and effective intraperitoneal chemotherapy with cathepsin B-specific doxorubicin prodrug nanoparticles in ovarian cancer with peritoneal carcinomatosis. Biomaterials 2021, 279, 121189. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Wang, Y.M.; Chiu, L.H.; Chen, T.C.; Tsai, Y.H.; Zuo, C.S.; Chen, K.C.; Changou, C.A.; Lai, W.T. Optical imaging of ovarian cancer using a matrix metalloproteinase-3-sensitive near-infrared fluorescent probe. PLoS ONE 2018, 13, e0192047, Erratum in PLoS ONE 2018, 13, e0202610. https://doi.org/10.1371/journal.pone.0202610. PMID: 29390034; PMCID: PMC5794152. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Y.; Zhang, Y.; Ru, D.; Wu, Z.; Zhang, J.; Shen, M.; Duan, Y.; Sun, Y. GSH-sensitive Pt(IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell for precise theranostics against ovarian cancer. Theranostics 2019, 9, 1047–1065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, V.M.; Nguyen, D.X.; Al Fatease, A.; Patel, P.; Cote, B.; Woo, Y.; Gheewala, R.; Pham, Y.; Huynh, M.G.; Gannett, C.; et al. Liposomal formulation of hypoxia activated prodrug for the treatment of ovarian cancer. J. Control. Release 2018, 291, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, N.; Zhang, L.; Yi, H.; Liu, Y.; Li, Y.; Li, X.; Wu, M.; Hao, L.; Yang, Z.; et al. IR780-loaded folate-targeted nanoparticles for near-infrared fluorescence image-guided surgery and photothermal therapy in ovarian cancer. Int. J. Nanomed. 2019, 14, 2757–2772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potara, M.; Nagy-Simon, T.; Focsan, M.; Licarete, E.; Soritau, O.; Vulpoi, A.; Astilean, S. Folate-targeted Pluronic-chitosan nanocapsules loaded with IR780 for near-infrared fluorescence imaging and photothermal-photodynamic therapy of ovarian cancer. Colloids Surf. B Biointerfaces 2021, 203, 111755. [Google Scholar] [CrossRef] [PubMed]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers 2019, 11, 1760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Sun, L.; Lu, Z.; Su, X.; Yang, Q.; Qu, X.; Li, L.; Song, K.; Kong, B. Enhanced effect of photodynamic therapy in ovarian cancer using a nanoparticle drug delivery system. Int. J. Oncol. 2015, 47, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, D.; Li, T.; He, X.; Wang, J.; Zhao, Y.; Chen, L. Recent Advances of NIR-II Emissive Semiconducting Polymer Dots for In Vivo Tumor Fluorescence Imaging and Theranostics. Biosensors 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macpherson, A.M.; Barry, S.C.; Ricciardelli, C.; Oehler, M.K. Epithelial Ovarian Cancer and the Immune System: Biology, Interactions, Challenges and Potential Advances for Immunotherapy. J. Clin. Med. 2020, 9, 2967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.; Flores, L.; Ngai, H.W.; Cornejo, Y.R.; Haber, T.; McDonald, M.; Moreira, D.F.; Gonzaga, J.M.; Abidi, W.; Zhang, Y.; et al. Large, Anionic Liposomes Enable Targeted Intraperitoneal Delivery of a TLR 7/8 Agonist to Repolarize Ovarian Tumors’ Microenvironment. Bioconjug. Chem. 2021, 32, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770, Erratum in ACS Nano 2024, 18, 34420. https://doi.org/10.1021/acsnano.4c16033. PMID: 34860006. [Google Scholar] [CrossRef]
- Parayath, N.N.; Gandham, S.K.; Leslie, F.; Amiji, M.M. Improved anti-tumor efficacy of paclitaxel in combination with MicroRNA-125b-based tumor-associated macrophage repolarization in epithelial ovarian cancer. Cancer Lett. 2019, 461, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colby, A.H.; Oberlies, N.H.; Pearce, C.J.; Herrera, V.L.; Colson, Y.L.; Grinstaff, M.W. Nanoparticle drug-delivery systems for peritoneal cancers: A case study of the design, characterization and development of the expansile nanoparticle. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilmore, D.; Schulz, M.; Liu, R.; Zubris, K.A.; Padera, R.F.; Catalano, P.J.; Grinstaff, M.W.; Colson, Y.L. Cytoreductive surgery and intraoperative administration of paclitaxel-loaded expansile nanoparticles delay tumor recurrence in ovarian carcinoma. Ann. Surg. Oncol. 2013, 20, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; Xie, F.; De Wever, O.; Descamps, B.; Hoorens, A.; Vermeulen, A.; Ceelen, W.; Vervaet, C. Preclinical evaluation of local prolonged release of paclitaxel from gelatin microspheres for the prevention of recurrence of peritoneal carcinomatosis in advanced ovarian cancer. Sci. Rep. 2019, 9, 14881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.; Li, B.; Kang, Y.; Jiang, W.; Huang, Q.; Chen, Q.; Li, L.; Xu, C. Paclitaxel nanoparticle inhibits growth of ovarian cancer xenografts and enhances lymphatic targeting. Cancer Chemother. Pharmacol. 2007, 59, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Demuytere, J.; Carlier, C.; Van de Sande, L.; Hoorens, A.; De Clercq, K.; Giordano, S.; Morosi, L.; Matteo, C.; Zucchetti, M.; Davoli, E.; et al. Preclinical Activity of Two Paclitaxel Nanoparticle Formulations After Intraperitoneal Administration in Ovarian Cancer Murine Xenografts. Int. J. Nanomed. 2024, 19, 429–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M.; et al. Malignant Ascites in Ovarian Cancer: Cellular, Acellular, and Biophysical Determinants of Molecular Characteristics and Therapy Response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shender, V.O.; Pavlyukov, M.S.; Ziganshin, R.H.; Arapidi, G.P.; Kovalchuk, S.I.; Anikanov, N.A.; Altukhov, I.A.; Alexeev, D.G.; Butenko, I.O.; Shavarda, A.L.; et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol. Cell Proteom. 2014, 13, 3558–3571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dakwar, G.R.; Zagato, E.; Delanghe, J.; Hobel, S.; Aigner, A.; Denys, H.; Braeckmans, K.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Colloidal stability of nano-sized particles in the peritoneal fluid: Towards optimizing drug delivery systems for intraperitoneal therapy. Acta Biomater. 2014, 10, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W. The Janus of Protein Corona on nanoparticles for tumor targeting, immunotherapy and diagnosis. J. Control. Release 2022, 345, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Khetan, R.; Dharmayanti, C.; Gillam, T.A.; Kübler, E.; Klingler-Hoffmann, M.; Ricciardelli, C.; Oehler, M.K.; Blencowe, A.; Garg, S.; Albrecht, H. Using GPCRs as Molecular Beacons to Target Ovarian Cancer with Nanomedicines. Cancers 2022, 14, 2362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brand, W.; Noorlander, C.W.; Giannakou, C.; De Jong, W.H.; Kooi, M.W.; Park, M.V.; Vandebriel, R.J.; Bosselaers, I.E.; Scholl, J.H.; Geertsma, R.E. Nanomedicinal products: A survey on specific toxicity and side effects. Int. J. Nanomed. 2017, 12, 6107–6129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.R.; Cheng, X.H.; Zhang, G.N.; Wang, X.X.; Huang, J.M. Cardiac safety analysis of first-line chemotherapy drug pegylated liposomal doxorubicin in ovarian cancer. J. Ovarian Res. 2022, 15, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannakou, C.; Park, M.V.D.Z.; Bosselaers, I.E.M.; de Jong, W.H.; van der Laan, J.W.; van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. Nonclinical regulatory immunotoxicity testing of nanomedicinal products: Proposed strategy and possible pitfalls. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammadi, E.; Behnam, B.; Mokhtarzadeh, A.; Rezaee, R.; Abiri, A.; Ramezani, M.; Giesy, J.P.; Sahebkar, A. Reproductive Toxicity of Nanoparticles: A Comprehensive Review. Curr Med Chem. 2023, 32, 1507–1552. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pignata, S.; Scambia, G.; Ferrandina, G.; Savarese, A.; Sorio, R.; Breda, E.; Gebbia, V.; Musso, P.; Frigerio, L.; Del Medico, P.; et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: The MITO-2 randomized phase III trial. J. Clin. Oncol. 2011, 29, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Mutch, D.G.; Orlando, M.; Goss, T.; Teneriello, M.G.; Gordon, A.N.; McMeekin, S.D.; Wang, Y.; Scribner, D.R., Jr.; Marciniack, M.; Naumann, R.W.; et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2007, 25, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. AGO-OVAR 2.21/ENGOT-ov 18 Investigators. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709, Erratum in Lancet Oncol. 2022, 23, e248. https://doi.org/10.1016/S1470-2045(22)00303-5. PMID: 32305099. [Google Scholar] [CrossRef]
- Monk, B.J.; Herzog, T.J.; Wang, G.; Triantos, S.; Maul, S.; Knoblauch, R.; McGowan, T.; Shalaby, W.S.W.; Coleman, R.L. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol. Oncol. 2020, 156, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Brady, W.E.; McMeekin, D.S.; Rose, P.G.; Soper, J.T.; Lentz, S.S.; Hoffman, J.S.; Shahin, M.S. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2011, 122, 111–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van De Sande, L.; Graversen, M.; Hubner, M.; Pocard, M.; Reymond, M.; Vaira, M.; Cosyns, S.; Willaert, W.; Ceelen, W. Intraperitoneal aerosolization of albumin-stabilized paclitaxel nanoparticles (Abraxane™) for peritoneal carcinomatosis—A phase I first-in-human study. Pleura Peritoneum 2018, 3, 20180112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceelen, W.; Sandra, L.; de Sande, L.V.; Graversen, M.; Mortensen, M.B.; Vermeulen, A.; Gasthuys, E.; Reynders, D.; Cosyns, S.; Hoorens, A.; et al. Phase I study of intraperitoneal aerosolized nanoparticle albumin based paclitaxel (NAB-PTX) for unresectable peritoneal metastases. eBioMedicine 2022, 82, 104151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, M.J.; Mitra, R.; McArthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldberg, M.S.; Xing, D.; Ren, Y.; Orsulic, S.; Bhatia, S.N.; Sharp, P.A. Nanoparticle-mediated delivery of siRNA targeting Parp1 extends survival of mice bearing tumors derived from Brca1-deficient ovarian cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 745–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.S.; Ramishetti, S.; Landesman-Milo, D.; Goldsmith, M.; Chatterjee, S.; Palakuri, R.; Peer, D. Therapeutic Gene Silencing Using Targeted Lipid Nanoparticles in Metastatic Ovarian Cancer. Small 2021, 17, e2100287. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, A.E.; Rose, P.G. Pegylated liposomal doxorubicin in ovarian cancer. Int. J. Nanomed. 2006, 1, 229–239. [Google Scholar] [PubMed] [PubMed Central]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Abuhanoğlu, G.; Ozer, A.Y. Radiation sterilization of new drug delivery systems. Interv. Med. Appl. Sci. 2014, 6, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saripilli, R.; Sharma, D.K. Nanotechnology-based drug delivery system for the diagnosis and treatment of ovarian cancer. Discov. Oncol. 2025, 16, 422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 2019, 21, E347–E355. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, W.; Khetan, R.; Johnson, I.R.D.; Blencowe, A.; Garg, S.; Albrecht, H.; Gillam, T.A. Future theranostic strategies: Emerging ovarian cancer biomarkers to bridge the gap between diagnosis and treatment. Front. Drug Deliv. 2024, 4, 1339936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, S.; Zhou, M.; Wang, Y.; Lu, C.; Yin, B.; Zhang, Y.; Liu, H.; Yin, X.; Song, G. Emerging biomedical imaging-based companion diagnostics for precision medicine. iScience 2023, 26, 107277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mikdadi, D.; O’Connell, K.A.; Meacham, P.J.; Dugan, M.A.; Ojiere, M.O.; Carlson, T.B.; Klenk, J.A. Applications of artificial intelligence (AI) in ovarian cancer, pancreatic cancer, and image biomarker discovery. Cancer Biomark. 2022, 33, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Oyowvi, M.O.; Babawale, K.H.; Atere, A.D.; Ben-Azu, B. Emerging nanotechnologies and their role in early ovarian cancer detection, diagnosis and interventions. J. Ovarian Res. 2025, 18, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, P.; Singh, S.K.; Mishra, M.K.; Singh, S.; Singh, R. Nanotechnology for boosting ovarian cancer immunotherapy. J. Ovarian Res. 2024, 17, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Q.; Tong, F.; Chen, J.; Kayumov, M.; Lv, Y.; Shi, Y.; Ye, B.; Gao, H. Tumor Microenvironment-Responsive Nanomedicines for Potentiating Cancer Immunotherapy. Adv. Sci. 2025, 12, e13567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wouters, R.; Westrøm, S.; Vankerckhoven, A.; Thirion, G.; Ceusters, J.; Claes, S.; Schols, D.; Bønsdorff, T.B.; Vergote, I.; Coosemans, A. Effect of Particle Carriers for Intraperitoneal Drug Delivery on the Course of Ovarian Cancer and Its Immune Microenvironment in a Mouse Model. Pharmaceutics 2022, 14, 687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caputo, F.; Favre, G.; Borchard, G.; Calzolai, L.; Fisicaro, P.; Frejafon, E.; Günday-Türeli, N.; Koltsov, D.; Minelli, C.; Nelson, B.C.; et al. Toward an international standardisation roadmap for nanomedicine. Drug Deliv. Transl. Res. 2024, 14, 2578–2588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pisano, S.; Pierini, I.; Gu, J.; Gazze, A.; Francis, L.W.; Gonzalez, D.; Conlan, R.S.; Corradetti, B. Immune (Cell) Derived Exosome Mimetics (IDEM) as a Treatment for Ovarian Cancer. Front. Cell Dev. Biol. 2020, 8, 553576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hedegaard, C.L.; Redondo-Gómez, C.; Tan, B.Y.; Ng, K.W.; Loessner, D.; Mata, A. Peptide-protein coassembling matrices as a biomimetic 3D model of ovarian cancer. Sci Adv. 2020, 6, eabb3298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Shukla, M.K.; Sharma, A.K.; Jayaprakash, G.K.; Tonk, R.K.; Chellappan, D.K.; Singh, S.K.; Dua, K.; Ahmed, F.; Bhattacharyya, S.; et al. Metal-based nanomaterials and nanocomposites as promising frontier in cancer chemotherapy. MedComm 2023, 4, e253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.