Simple Summary
Serum pepsinogen tests were positively associated with gastric cancer (GC) occurrence after adjustment for age, sex, body mass index, Helicobacter pylori status, salty food and alcohol intake, smoking, and family history of GC before and after propensity-score matching. Specifically, serum pepsinogen II (PGII) showed a greater association with diffuse-type GC, and the pepsinogen ratio showed a greater association with intestinal-type GC as well as diffuse-type GC. The combination of high PGII and positive Helicobacter pylori status was associated with a heightened risk of early-stage diffuse-type GC, especially in young female participants. The strengths of this study include its large sample size, use of propensity-score matching, and adjustment for various confounding factors. This study’s findings may be valuable in developing effective and targeted screening programs, especially in regions with high GC incidence rates.
Abstract
Background/Objectives: Serum pepsinogens (PGs) are biomarkers for atrophic gastritis and severe gastric inflammation. We aimed to validate the role of PGs, with or without Helicobacter pylori (HP), in gastric cancer (GC) screening, focusing on serum pepsinogen II (PGII) and diffuse-type GC (DGC), using large-scale health checkup data. Methods: A total of 1283 patients with GC and 21,320 controls were enrolled prospectively. Serum PG levels and anti-HP antibody status (HP status) were assessed. The area under the curve and receiver operating characteristic curves were calculated to identify optimal cut-off values for PGs. Propensity-score matching (PSM), multivariable logistic regression, and risk stratification were conducted by combining each PG value with HP status among GC subtypes. Results: After PSM, serum PGII ≥ 21 ng/mL (PGII-positive) was associated with the risk of GC (odds ratio [OR] = 1.66, p < 0.001), DGC (OR = 2.35, p < 0.001), and especially early-stage DGC (OR = 4.24, p < 0.001). When GC risk was assessed by combining each PG value and HP status, the OR significantly differed between categories with adjustment for other confounding factors after PSM, with stepwise increments from HP-negative to HP-positive statuses and from PGII-negative to PGII-positive statuses. GC risk (early-stage DGC) was greatest in females under 40 years who tested positive for PGII and HP status (OR = 25.83, p < 0.001). Conclusions: Serum PGII levels combined with HP status testing can aid in identifying high-risk GC subjects. High serum PGII combined with positive HP status is related to increased risk of early-stage DGC, especially in young females.
1. Introduction
The incidence of cancer and its associated mortality have increased over the past several decades. Gastric cancer (GC) remains a significant concern worldwide, ranking fifth in incidence and third in mortality among solid malignancies globally [1]. According to a recent meta-analysis, the crude global prevalence of Helicobacter pylori (HP) has reduced from 52.6% before 1990 to 43.9% from 2015 to 2022 in adults, and the incidence of GC has decreased globally and in various countries where the prevalence of HP infection has declined [2]. Eastern Asia has the highest GC incidence rate, at 45.7 cases per 100,000 persons, accounting for 60.3% of new GC cases and 56.6% of GC-related deaths worldwide [1]. Although the incidence and mortality of GC have been declining owing to the increased diagnosis of precursor lesions and early GC following the introduction of nationwide screening programs [3,4,5], as well as advancements in treatment techniques, the age-standardized incidence rates of GC in 2019 were still higher than those in other countries, at 28.67, 28.29, and 30.64 per 100,000 individuals in Korea, Japan, and China, respectively [6]. Considering that early diagnosis is key in GC treatment, various efforts are being made to enhance early detection strategies. Population screening has recently been conducted in Korea, Japan, and China [3,4,5]. In Korea, a structured, nationwide GC screening program, including esophagogastroduodenoscopy (EGD) or upper gastrointestinal series designed to detect GC, has been conducted biannually for individuals over 40 years old since 2001 [3]. EGD screening can potentially prevent GC through early diagnosis and treatment; however, conducting it for the entire population is challenging. Mass screening for GC requires a simpler, less invasive, and more cost-effective method. Diverse biomarkers, such as host genetic polymorphism in cytokine genes (e.g., interleukin-1β and interleukin-8 polymorphisms) [7,8] and HP-related factors (e.g., cytotoxin-associated gene A [cagA] and vacuolated cytotoxin A) [9], have been used to screen for GC. However, none of these biomarkers have reliably identified individuals with a high risk of GC.
Japan has demonstrated the effectiveness of risk stratification for GC using a serum screening system, including a serum pepsinogen (PG) series with or without an anti-HP antibody (HPIgG) [10,11,12,13], to address the lack of EGD processing capacity. PG levels reflect the morphological and functional status of the gastric mucosa and the extent of atrophic changes in the stomach mucosa [10]. Gastric chief cells and mucous neck cells produce two biochemically distinct PGs, pepsinogen I (PGI) and pepsinogen II (PGII), with the latter also originating from pyloric and Brunner’s gland cells [14,15]. HP colonizes the gastric mucosa, triggering a series of inflammatory responses and significantly increasing GC risk [16,17,18]. Recognizing this long disease progression potentially allows for the early detection of precancerous lesions and timely intervention [13]. Both PGI and PGII levels initially increase. As gastric atrophy develops, the chief cells are replaced by pyloric glands, leading to decreased PGI levels, whereas PGII levels remain relatively unaffected. Consequently, the PGI-to-PGII ratio (PGR) also decreases. Because low PGI and PGR levels reflect gastric atrophy, these markers have been studied to identify high-risk patients with conditions such as atrophic gastritis or intestinal metaplasia [19,20] or to detect neoplastic lesions at an earlier stage [21]. Mechanistically, these markers are expected to be particularly useful for diagnosing intestinal-type gastric cancer (IGC), which follows Correa’s cascade [16,17]. In contrast, evidence on the usefulness of PG in diffuse-type GC (DGC), which may have a stronger genetic component and a weaker association with environmental factors than IGC, has been generally lacking or questionable [11,12,22,23,24,25]. Research on DGC and all types of PGs has continued globally, especially in Japan; however, Some studies have suggested a link between DGC and PGII among all kinds of PG [26,27,28], while another study found that PGII was not an appropriate biomarker for GC screening [29]. Furthermore, few studies have examined the utilization of PG values for DGC diagnosis in the Korean population. DGC comprises a relatively high proportion of total GC (38.3–42.1%) in Korea [30,31] and occurs more commonly in younger people, but they are less likely to undergo EGD because they are less concerned about GC risk. Moreover, some studies have suggested racial differences in the use of PG values for GC diagnosis [30]. Thus, the role of PG values in GC development, especially its utility as a biomarker for DGC, must be redefined to stratify patients who should undergo EGD in South Korea.
Some studies have highlighted the limitations of diagnostic accuracy when using serologic tests alone [32], and others have suggested that assessing the HP infection status concurrently is necessary for clinical utility [33,34], showing a high diagnostic rate of GC in past or current HP infection groups. Recently, our group reported that PGII ≥ 20 ng/mL and a positive HP status may help detect early-stage DGC (DGC-E) in a young female group in a tertiary hospital, although not in the general population [35]. In addition, a recent study has reported the relationship between HP infection and the progression of DGC [36]. Therefore, the aims of this study were (1) to investigate the association of serum PGs, including PGII, with GC occurrence, after adjusting for other GC risk factors, using large-scale health checkup data, and (2) to determine the risk of GC occurrence according to the combination of each PG value and HP status, particularly focusing on DGC.
2. Materials and Methods
2.1. Study Population
This prospective observational cohort study was conducted at two hospitals, Seoul National University Hospital (SNUH) Gangnam Center (SNUHGC) and Seoul National University Bundang Hospital (SNUBH). A total of 27,974 participants aged 18–93 years who had undergone testing for serum PG levels, HP status, and/or EGD on the same day during a health screening at these two hospitals between May 2003 and February 2022 were initially included. The exclusion criteria were a history of gastrectomy or endoscopic dissection treatment for GC; recent use (1 month prior to enrollment) of proton-pump inhibitors, potassium-competitive acid blockers, non-steroidal anti-inflammatory drugs, or steroids; renal dysfunction; any other upper gastrointestinal neoplasms (e.g., esophageal cancer, neuroendocrine tumor, or gastric lymphoma); and no results for HP status or EGD. The final number of study participants was 23,015. Propensity-score matching (PSM) was performed to control for potential confounders (Figure 1).
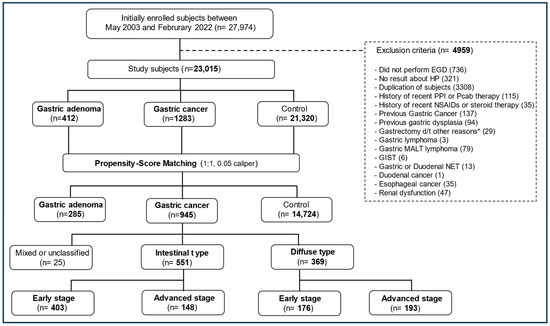
Figure 1.
Flow diagram of the study. * Gastrectomy due to complications (bleeding, perforation, etc.) of benign gastric ulcer. EGD, esophagoduodenoscopy; HP, Helicobacter pylori; PPI, proton-pump inhibitor; Pcab, potassium-competitive acid blocker; NSAIDs, non-steroidal anti-inflammatory drugs; d/t, due to; MALT, mucosa-associated lymphoid tissue; GISTs, gastrointestinal stromal tumors; NETs, neuroendocrine tumors.
2.2. Endoscopic Examination and Pathology
One experienced board-certified endoscopist at SNUBH and 15 experienced board-certified endoscopists at SNUHGC performed all health checkup EGDs. The pathology notes were reviewed for patients with GC and gastric adenoma (GA) who underwent surgery or endoscopic removal.
GC was categorized according to the Lauren classification as IGC or DGC [37] and classified based on the depth of invasion of the cancer cells. Early-stage GC was defined as GC that invaded no deeper than the submucosa, irrespective of lymph node metastasis (T1, any N). GA was defined as low- or high-grade dysplasia according to the Vienna classification [38].
2.3. Serologic Tests for Helicobacter pylori Antibody and Pepsinogen Series
At SNUBH, serum HPIgG was tested using an enzyme-linked immunosorbent assay (ELISA) (Genedia®; Green Cross Medical Science Corp, Yongin, Republic of Korea) during the entire study period. The Genedia® HP ELISA demonstrated 97.8% sensitivity and 92.0% specificity in a Korean population [39]. At SNUHGC, from 2003 to March 2013, HPIgG was measured using an ELISA kit (Radim® Diagnostics, Rome, Italy) and an automatic analyzer, Alisei® (Seac, Pomezia, Italy), which was validated in 2013 in nationwide Korean seroepidemiologic studies [40]. From April 2013 to the present, HPG kits (Immulite® 2000 CMIA, Siemens, UK) were used, which were also validated based on Genedia® in 2017 [41].
Serum levels of PGI and PGII were measured using a latex-enhanced turbidimetric immunoassay (HBi Corp., Seoul, Republic of Korea, imported from Shima Laboratories, Tokyo, Japan) at both hospitals, and PGR and the PGII-to-PGI ratio (PGII/I, reciprocal PGR, PGRr) were calculated. The latex-enhanced turbidimetric immunoassay kit demonstrated that serum PGI and PGR levels were significantly lower when histological atrophic gastritis progressed, with a cut-off value of 3.0 for diagnosing histological atrophic gastritis. A significant correlation was noted between endoscopic and histological atrophic gastritis, with the sensitivity and specificity of endoscopic diagnosis being 65.9% and 58.0% for the antrum and 71.3% and 53.7% for the corpus, respectively [42]. The sensitivity and specificity of a PGR of ≤3.0 for detecting dysplasia or cancer were 55.8–62.3% and 61%, respectively [30]. In addition, this kit was also validated in previous studies [43,44,45].
2.4. Helicobacter pylori Status Assessment
In addition to the HPIgG test, HP infection was diagnosed using EGD with biopsies for histology, HP polymerase chain reactions, rapid urease tests, or [13C]-urea breath tests, as required. A positive result for any one of these four tests was considered to indicate a current HP infection. Our group reported that the rapid urease test had 96.7% specificity and 80.4% sensitivity [46], and the urea breath test’s sensitivity and specificity for a cut-off value of 2.5‰ were 99.3% and 47.1%, respectively [47].
The HPIgG test was performed for qualitative estimation, particularly when the other four HP tests were negative, and all participants were assessed for HP eradication history. A positive HPIgG result or HP eradication history indicated a past HP infection. Overall, individuals with both current and past HP infection statuses were considered “HP status-positive” throughout their lifetime. Since the so-called “point of no return” beyond which eradication may not prevent further progression of premalignant conditions remains currently undefined [48,49], we decided to include all participants with a history of past HP infection, not just actual infection.
2.5. Risk Factor Measurements
Information on medical history, family history (at least one first-degree relative with GC), anthropometric assessment, current or past smoking status (at least one cigarette per day during the previous 12 months), alcohol consumption (≥140 g/week during previous 12 months), and salty food intake habits was obtained through a questionnaire administered to each participant. Participants were categorized into two groups according to smoking status (never-smokers and ever-smokers) and alcohol intake (current habitual use or not). Regarding salty food intake habits, participants were categorized into two groups (“no/mild” [score 0–2] and “severe” [score 3–4]) according to scores based on two questions: “Do you add extra salt or soy sauce to your food when you eat?” and “Do you eat salty foods such as salted seafood, pickled vegetables, and soup-based meals?”. Both questions had three types of answers, including never (score 0), sometimes (score 1), or frequently (score 2).
2.6. Statistical Analyses
To compare the baseline characteristics of each group, categorical variables were compared using the chi-square test, Fisher’s exact test, or the Kruskal–Wallis test with Bonferroni correction, and the results are presented as numbers and percentages. Continuous data were compared using a t-test or a one-way analysis of variance and are expressed as medians (interquartile ranges). The area under the curve (AUC) and receiver operating characteristic curves were computed to determine the optimal cut-off values of PGs for GC detection. Additionally, to compare diagnostic accuracy, specifically in detecting PGs in subgroups of GC such as IGC and DGC, an AUC ≥ 0.7 and sensitivity and specificity ≥ 70% were regarded as significant.
Given that the occurrence of HP infection varies significantly with age and year of birth, we used PSM with a k-nearest neighbors approach to match individuals based on their HP status. Age and year of birth were used as covariates due to their significant clinical impact on HP occurrence. We performed 1:1 matching and calculated the caliper using the standard deviation of logit propensity scores. We performed PSM to match the case and control groups based on their propensity scores, ensuring balanced covariates and enhancing the validity of our causal inferences. This matching approach allowed us to better predict GC and GA by controlling for these confounding variables. The standardized mean differences for age and year of birth changed from 0.22 to 0.01 and from 0.30 to 0.08, respectively, before and after PSM (Figure S1).
To validate the effectiveness of PGs in predicting GC risk, participants were divided into two categories (positive vs. negative) based on the cut-off values of three types (PGIR, PGR, and PGII) from previous studies [10,11,12,30,35,50]. Cut-off values of PGI ≤ 70 ng/mL and PGR ≤ 3 have been widely accepted as indicating an atrophy-positive status and are frequently used for GC screening purposes [10,11,12,50]. Therefore, we classified participants as PGIR-positive when they met the criteria mentioned above and PGIR-negative when they did not. A previous study from Korea reported that a PGI value of ≤ 70 ng/mL had adequate sensitivity (72.4%) but low specificity (20.2%), whereas the sensitivity and specificity of PGR ≤ 3 were 59.2–61.7% and 61.0%, respectively, for GC or GA [30]. Therefore, we used PGR ≤ 3 and categorized participants as PGR-positive if they met the criterion and PGR-negative if they did not. Another Korean study revealed that PGII ≥ 20 ng/mL (an AUC of 0.593 for DGC) was strongly related to DGC-E, particularly in young adult females [35]. In the present study, the ROC curve identified 21 ng/mL (an AUC of 0.706 for DGC) as the optimal PGII cut-off value for diagnosing DGC-E, achieving 66.7% sensitivity and 79.2% specificity. Therefore, we used a cut-off value of PGII ≥ 21 ng/mL to classify participants as PGII-positive. Each of these three types of PG values was then divided into two categories, and odds ratios (ORs) with 95% confidence intervals were calculated using multivariable logistic regression analysis. Statistical analyses were performed using SPSS Statistics version 27.0 (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at a p-value of <0.05.
3. Results
3.1. Baseline Characteristics
Among the 23,015 study participants, 1283 and 412 were diagnosed with GC and GA, respectively. GC was categorized as intestinal-type (n = 760; 59.2%) or diffuse-type (n = 490; 38.2%), whereas 33 participants (2.6%) were classified as having mixed- or unclassified-type GC owing to difficulty in categorization before PSM (Figure 1, Table 1 and Table S1). In total, 571 participants had early-stage IGC (75.1% of IGC), whereas 239 had DGC-E (48.8% of DGC; p < 0.001). The IGC and DGC subgroups did not differ in terms of GC location (cardia vs. non-cardia). Regarding GC treatment, surgical therapy was markedly more common in the DGC subgroup than in the IGC subgroup (77.6% vs. 52.7%), and endoscopic therapy was relatively more common in the IGC subgroup than in the DGC subgroup (40.3% vs. 6.5%; Table S2). After PSM (Table 2 and Table S1), 945 participants were categorized into the GC group, 551 into the IGC subgroup (58.3%), 369 into the DGC subgroup (39%), and 285 into the GA group, whereas 14,724 participants formed the control group.

Table 1.
Baseline characteristics of the subjects before propensity-score matching.

Table 2.
Baseline characteristics of the subjects after propensity-score matching.
Before PSM (Table 1 and Table S1), the GA, GC, IGC, and DGC groups were predominantly male compared to the controls; however, the male predominance disappeared in the GA and DGC groups after PSM (Table 2 and Table S1). The median age of the control group was significantly lower than those of the GA and GC groups, as well as the IGC subgroup, both before and after PSM. A pooled one-way analysis of variance revealed significant differences in smoking and alcohol history as well as family history of GC in the GC groups, especially in the IGC subgroup, before and after PSM. The GA, GC, and two GC subgroups significantly differed from the control group in terms of salty diet and HP status before and after PSM.
Regarding PG series results, the median serum level of PGII in the GC group was higher than those in the GA and control groups before and after PSM (Table S1). Furthermore, the median PGII level was higher in the DGC subgroup than in the IGC subgroup, which was similar to the median PGI level. The median value of PGR in the GC group was significantly lower than that in the GA and control groups.
3.2. Association of Each Pepsinogen Value or Helicobacter pylori Status with Gastric Neoplasm After Propensity-Score Matching
The participants’ PG values were classified into two categories using specific cut-off values. After PSM, their correlation with the GA and GC subtypes was examined. Positive associations were observed in the GA, GC, IGC, and DGC groups with PGII ≥ 21 ng/mL, PGR ≤ 3, and PGIR-positive statuses. Additionally, a positive HP status was associated with an increased risk of GA, GC, IGC, or DGC (Table S4).
3.3. Multivariable-Adjusted Logistic Regression Analysis for Gastric Neoplasms
The three types of PG values were classified into two categories, based on cut-off values, and their correlations with the GA and GC groups, as well as GC subtypes, were examined after adjusting for sex, age, body mass index (BMI), smoking, alcohol intake, salty food intake, family history of GC, and HP status (Table 3).

Table 3.
Multivariable-adjusted logistic regression analysis for gastric neoplasms according to pepsinogen values before and after propensity-score matching.
Before PSM, the association between each gastric neoplastic group and each PG value (PGII-positive, PGR-positive, or PGIR-positive) remained significant after adjusting for the other confounding factors listed above, except the association between PGII-positive statuses and GA, which was not significant (Table 3, top panel). However, after PSM, there was no significant association between PGIR-positive status and all gastric neoplastic groups, whereas the association between PGII-positive status and the DGC-E subgroup and the association between PGR-positive status and the IGC or DGC subgroups remained significant (Table 3, bottom panel). After PSM, the adjusted OR (aOR) of PGII-positive statuses for DGC was 2.31 (p < 0.001) and increased to 4.20 for DGC-E (p < 0.001; Table S5). After PSM, the aOR of PGR was 2.88 for IGC and 2.43 for early-stage IGC (Table S5, bottom panel).
3.4. Detection Power of Pepsinogen Values for Diagnosis of Gastric Cancer According to Subtypes
We assessed the detection power of two PG values, PGII and PGR, which exhibited an association with GC after adjustments for various risk factors and PSM. Figure 2A–F show the AUC of PGII for the diagnosis of GC and its subgroups. The highest AUC was observed in females under 40 years with DGC-E (AUC: 0.843; sensitivity: 0.813; specificity: 0.897; Figure 2F).
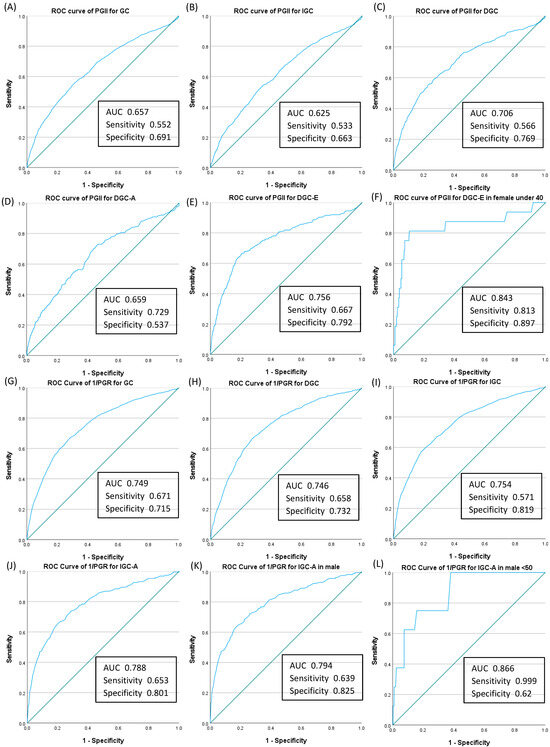
Figure 2.
Receiver operating characteristic curve and corresponding area under the curve of each pepsinogen value for the diagnosis of gastric cancer. The AUC for serum pepsinogen II (PGII) showed significant sensitivity and specificity for six cases: (A) total GC, (B) IGC, (C) DGC, (D) DGC-A, (E) DGC-E, and (F) DGC-E in females under 40 years of age. The greater the progress in alphabetical order, the higher the diagnostic power (A–F). The AUC for reciprocal pepsinogen ratio (1/PGR) showed significant sensitivity and specificity for six cases: (G) total GC, (H) DGC, (I) IGC, (J) IGC-A, (K) IGC-A in males, and (L) IGC-A in males under 50 years. The greater the progress in alphabetical order, the higher the diagnostic power (G–L). ROC, receiver operating characteristic; AUC, area under the curve; PG, serum pepsinogen; PGII, serum pepsinogen II ≥ 21 ng/mL; GC, gastric cancer; IGC, intestinal-type gastric cancer; DGC, diffuse-type gastric cancer; DGC-A, diffuse-type gastric cancer, advanced stage; DGC-E, diffuse-type gastric cancer, early stage; IGC-A, intestinal-type gastric cancer, advanced stage; 1/PGR, reciprocal pepsinogen ratio.
Given that the AUC of GC occurrence for PGR was <0.5, we chose to use PGRr (PGII/I). The AUC of GC occurrence for PGRr was 0.75 (95% confidence interval: 0.735–0.764), and the optimal PGRr cut-off value was 0.26 in our model, with a sensitivity of 0.67 and a specificity of 0.72. We set the cut-off level of PGRr at 0.26. Figure 2G–L show the AUC of PGRr for the diagnosis of GC and its subgroups. The AUC was greatest in males under 50 years with IGC-A (AUC: 0.866; sensitivity: 0.999; specificity: 0.62; Figure 2L).
We also calculated the positive predictive value (PPV) of PGII-positive and PGR-positive statuses for the diagnosis of GC and GC subtypes; the PPV for the diagnosis of GC was 11.4% for PGII-positive and 15.1% for PGR-positive cases (Table S6).
3.5. Risk Stratification by Combining Pepsinogen Values and Helicobacter pylori Status in Gastric Cancer and Its Subtypes
GC risk was assessed based on four categories combining HP status and each of the two PG values (PGII and PGR), and it showed significance after adjusting for various risk factors for GC. After PSM, the OR for PGII-positive or PGR-positive statuses differed significantly between the categories, with stepwise increments from negative to positive HP status and from negative to positive status for each PG value, after adjustment for other confounding GC risk factors, such as sex, age, BMI, family history of GC, smoking history, alcohol intake, and salt intake. GC risk showed the highest aOR with HP-positive and PGII-positive statuses or HP-positive and PGR-positive statuses (aOR = 6.93 or aOR = 9.66, respectively; all p < 0.001; Table 4).

Table 4.
Effects of combination of H. pylori status and each pepsinogen value on risk of gastric cancer after propensity-score matching.
In addition, multivariable analysis was performed according to sex and age group to assess the risk stratification for each GC subtype using a combination of HP statuses and each PG value (Tables S7–S10). Interestingly, the highest risk of incidental DGC-E occurrence was found in the female group under 40 years with both HP-positive and PGII-positive statuses (OR= 25.8; p < 0.001; Table S7), as well as with HP-positive and PGR-positive statuses (OR= 8.6; p < 0.001; Table S8). For the risk of IGC, the OR was higher in males under 50 years with HP-positive statuses and each PG-positive status (OR= 5.18; p < 0.001 for PGII [Table S9] and OR= 10.44; p < 0.001 for PGR [Table S10]).
4. Discussion
This large-scale (n = 23,015) case–control study was conducted to validate the utility of PG in diagnosing GC during health checkups. We found that PGII-positive or PGR-positive statuses, but not PGIR-positive statuses, were independent predictors of GC in the general population after PSM. This relationship remained significant even after adjusting for other confounding factors, including HP status. In particular, a PGII-positive status was significantly related to DGC-E, whereas a PGR-positive status was associated with IGC as well as DGC-E.
Our previous study [35], which focused on a cohort from a tertiary hospital, revealed that higher serum PGII levels and positive HP status indicated an increased risk of DGC-E, particularly in young adult females. The present results corroborate these findings, clearly demonstrating that a PGII-positive status was significantly associated with DGC-E, especially in young females, whereas a PGR-positive status was associated with IGC as well as DGC-E after general health screeners were included and PSM was applied.
The findings are generally consistent with previous results, although a few studies have reported conflicting results. Kikuchi et al. [26] concluded that PGII and PGR could be markers for not only IGC but also DGC in a young population (108 GC with 216 age- and sex-matched controls). Yanaoka et al. [27] reported that GC risk increased with a reduced PGI level or a reduced PGR, and the risk of DGC increased as the PGII level increased. Yoshida et al. [51] also identified a significantly elevated DGC risk in a subgroup with high PGII from an HP-infected and atrophy-negative group. However, both studies [27,51] were conducted on the same cohort of middle-aged men, with no adjustments made for other GC risk factors when analyzing the risk stratification for GC and its subgroups. Abnet et al. [52] reported that a lower PGR value is associated with a higher risk of GC but did not analyze the association of PG values and GC risk based on histologic type.
In contrast, Oishi et al. [12] reported a stepwise increase in the adjusted hazard ratio for IGC among four categories according to a combination of PGIR and HP statuses; however, such an association was not found for DGC, and they did not mention a relationship between PGII and GC risk. Parsonnet et al. [18] reported that low PGI or low PGR levels were linked to the development of distal GC, but PGII levels were not linked to GC. However, they could not analyze the association between PG values and DGC development because of an insufficient sample size (26 DGC cases among 136 GC cases). The Japan–Hawaii Cancer Study Group found that low PGI levels, with or without low PGR, increased the risk of IGC in males, but there was no difference in males with DGC compared to control individuals in terms of PGI or PGR. Moreover, they did not mention an association between PGII and GC [22].
Another notable finding was that the aOR value for GC tended to increase progressively from statuses of HP-negative to HP-positive and from PG-negative to PG-positive depending on the combination of HP status and each of the two PG values, after adjusting for sex, age, BMI, family history of GC, smoking history, alcohol intake, and salt intake, and after PSM. Before adjusting for other confounding factors, the highest OR value was observed in the combination of HP-negative and PGR-positive statuses, as shown in other studies [24,51,53]; however, following adjustment for various confounding factors, the aOR value was the highest for groups with both HP-positive and PGII-positive or PGR-positive statuses. This may be a product of ensuring balanced covariates and enhancing the validity of our causal inferences, which allowed us to better predict GC risk. Parsonnet et al. [18] reported that a combination of HP-positive and atrophy-positive statuses showed the highest OR for GC, similar to our findings. Our identification of biomarker combinations with the greatest risk differed from those reported in most Japanese studies [24,51,53], likely due to differences in exclusion criteria. In most studies that have investigated GC risk by combining PG and HP status, HP-eradicated subjects were excluded. However, in this study and the study by Parsonnet et al. [18], HP-eradicated subjects were not excluded. Therefore, those with actual HP infection as well as those with prior infection were classified as HP-positive in the current study.
Moreover, we performed a risk stratification analysis using four categories based on combinations of HP statuses and PG values to elucidate their influence on GC risk through the interaction of HP status and atrophy or inflammation level and to identify the most effective model for predicting each subtype of GC. The OR for DGC-E was the highest for HP-positive statuses and each PG value-positive status in individuals under 40 years of age, particularly in the female group, whereas the OR for IGC was the highest in HP-positive and PG value-positive individuals under 50 years of age, especially in the male group.
We also found that the PPV of each PG value for detecting GC ranged from 11.4% to 15.1%, which was slightly higher than that reported in other studies [54,55]. Mizuno et al. [54] used cut-off levels of PGI ≤ 30 ng/mL and PGR ≤ 2.0 and reported a PPV of 1.4%, which was similar (1.8%) to that reported for the direct X-ray method; when cut-off levels were changed to ≤70 ng/mL and PGR ≤ 3.0, respectively—the same as “PGIR-positive status” in the present study—the researchers reported a PPV of 0.7%. Tong et al. [55] reported that the PPV of PGI ≤ 43.5 ng/mL was 1.5% and that of PGR ≤ 4.7 for GC was 2.7%. Globally, low PPVs have been observed in the studies on PG and GC.
In the present study, the sex ratio (male/female) was 1:0.8 in the DGC subgroup and 1:2 in the DGC subgroup under 40 years of age, compared to 3:1 in IGC and 2:1 for all GC cases. In addition, there was a relatively higher proportion of HP-positive (90%) cases in the DGC subgroup than in the IGC subgroup (83%; p < 0.001, Table 1). Our finding that DGC more frequently affects young females and has a higher HP-positive status is similar to that reported in another health checkup study in Korea [31]. One possible reason for the high incidence of DGC in young females may be its association with female sex hormones such as estrogen; the higher proportion of HP-positive status in DGC may be associated with active inflammation caused by HP, which can increase PGII levels. Recently, a mechanism by which HP and estrogen act simultaneously to promote DGC progression has been identified, which may explain the higher incidence of DGC in premenopausal women with physiologically elevated hormone levels [36]. Kang et al. [36] found that estrogen levels increased the expression of an oncogene in estrogen receptor α-positive DGC and that cagA toxin-secreting HP enhanced the effects of estrogen in DGC.
Our findings may have useful clinical implications. Serologic tests, which are relatively simple, easy, and economical, should be performed for PG series and HP status (HPIgG) in the general population under 40 years of age, as they are not included in national cancer screenings in Korea, followed by EGD screening for those who are PGII-positive, PGR-positive, or HP-positive. Simulating such a stepwise approach with our data, 57 (90.5%) of 63 patients with GC under 40 years old could be diagnosed, and 57 (6.7%) of 857 subjects who underwent screening for EGD would be diagnosed with GC. A total of six GC cases were missed due to failure to filter out by serologic results under 40 years (Table S11), and the false negative rate (1-sensitivity) was 9.52%. In other words, if an endoscopy is not performed solely based on serologic results, approximately 9.5% of GC patients may be missed. This is an area that needs to be studied with caution. To compensate for this, the presence or absence of symptoms and other GC risk factors such as a family history of GC or smoking should be sufficiently considered.
The present study has several strengths. Its large sample population, exceeding 23,000 individuals, including over 1200 GC cases, enabled a comprehensive analysis across group categories and histological subtypes while accounting for potential confounding effects. Moreover, the inclusion of health checkup participants mitigates the case selection bias encountered in clinical series from hospital cases. Additionally, we performed PSM to match the case and control groups based on their propensity scores and enable better prediction of GC and GA by controlling for these confounding variables. Finally, since the GC stages were classified based on histology and HP status, the role of PGs in predicting GC risk was evaluated in detail.
Despite these advantages, this study has a few limitations. First, a positive HP status did not exclusively represent active infections. In this study, tests reflecting active HP infection, such as the rapid urease test, urea breath test, culture, HP polymerase chain reaction, or histology, were not performed on all participants. In cases where results from such active tests were unavailable, the serological test results (HPIgG) were used. Those with a history of HP eradication were also included. It was recognized that a positive HPIgG result or a history of HP eradication indicates a past HP infection and that a past infection could develop into a precancerous lesion. Second, this was a case–control study, although the participants were enrolled prospectively; therefore, the observational design did not include follow-up data, and selection bias was unavoidable. Nevertheless, the relatively large sample size of the present study may have compensated for the validity of our conclusions. In the future, a well-designed prospective cohort study, preferably a randomized control trial, is required to validate our results. Third, cagA toxin-producing HP, which is probably related to DGC risk, was not investigated in the present study. However, our group has previously found that HP infection in South Koreans is closely related to highly virulent strains; most HP colonies include cagA-positive strains (87.2%) and vacuolating cytotoxin genes (92.9–100%) [56].
5. Conclusions
PG values (PGII or PGR) and HP status could be a screening tool for identifying high-risk individuals for GC. In particular, the combination of high PGII levels and positive HP status was related to a heightened risk of DGC-E, especially in the young female group. Low PGR-positive and positive HP statuses were associated with IGC as well as DGC-E.
Given the global significance of GC as a major cause of cancer-related mortality, our study findings may have implications for designing more effective and targeted screening programs, especially in regions with high GC incidence rates.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17060955/s1: Figure S1: Standardized mean differences plot across covariates; Table S1: Baseline characteristics of the subjects with gastric adenoma before and after propensity-score matching; Table S2: Characteristics of gastric cancer by histology; Table S3: Subjects with missing data; Table S4: Association between gastric neoplasm and pepsinogens or Helicobacter pylori status after propensity-score matching; Table S5: Multivariable-adjusted logistic regression analysis for subtypes of gastric cancers according to pepsinogen values before and after propensity-score matching; Table S6: Positive predictive value of each pepsinogen value for the diagnosis of gastric cancer; Table S7: Risk stratification by combining pepsinogen II and Helicobacter pylori status in diffuse-type gastric cancer after propensity-score matching; Table S8: Risk stratification by combining pepsinogen ratio and Helicobacter pylori status in diffuse-type gastric cancer after propensity-score matching; Table S9: Risk stratification by combining pepsinogen II and Helicobacter pylori status in intestinal-type gastric cancer after propensity-score matching; Table S10: Risk stratification by combining pepsinogen ratio and Helicobacter pylori status in intestinal-type gastric cancer after propensity-score matching; Table S11: Simulation of diagnostic power depending on age and sex.
Author Contributions
Conceptualization, S.H.L., N.K., and D.H.L.; data curation, S.H.L., J.M.C., Y.M.H., M.-S.K., G.E.C., and J.Y.S.; formal analysis, H.Y., and Y.S.P.; funding acquisition, N.K.; methodology, J.M.C., and S.M.B.; supervision, N.K., and D.H.L.; visualization, Y.C.; writing—original draft, S.H.L.; writing—review and editing, N.K., and Y.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; RS-2024-00337453). The funders had no role in study design, data collection and analysis, and the decision to publish or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the 1975 Declaration of Helsinki and approved by the Institutional Review Boards of SNUH (2003-143-1111; approval date 24 February 2021) and SNUBH (B-2003-603-103; approval date 12 March 2020). The study protocol was registered at ClinicalTrials.gov (trial registration number NCT06301464).
Informed Consent Statement
At SNUHGC, the need for informed consent was waived by the Institutional Review Board of SNUH because the researchers accessed only deidentified databases for analytical purposes. However, at SNUBH, all participants provided informed consent.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aOR | adjusted odds ratio |
| AUC | area under the curve |
| BMI | body mass index |
| cagA | cytotoxin-associated gene A |
| DGC | diffuse-type GC |
| DGC-E | early-stage DGC |
| EGD | esophagogastroduodenoscopy |
| ELISA | enzyme-linked immunosorbent assay |
| GA | gastric adenoma |
| GC | gastric cancer |
| HP | Helicobacter pylori |
| HPIgG | anti-Helicobacter pylori antibody immunoglobulin G |
| IGC | intestinal-type GC |
| OR | odds ratio |
| PG | pepsinogen |
| PGI | pepsinogen I |
| PGII | pepsinogen II |
| PGR | PGI-to-PGII ratio |
| PGRr | reciprocal PGR or PGII-to-PGI ratio |
| PPV | positive predictive value |
| PSM | propensity-score matching |
| SNUBH | Seoul National University Bundang Hospital |
| SNUH | Seoul National University Hospital |
| SNUHGC | Seoul National University Hospital Gangnam Center |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global prevalence of Helicobacter pylori infection and incidence of gastric cancer between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, J.K.; Choi, K.S.; Lee, H.Y.; Park, E.C. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac. J. Cancer Prev. 2011, 12, 725–730. [Google Scholar]
- Hamashima, C. Systematic review group and guideline development group for gastric cancer screening guidelines. Update version of the Japanese guidelines for gastric cancer screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [PubMed]
- Chen, R.; Liu, Y.; Song, G.; Li, B.; Zhao, D.; Hua, Z.; Wang, X.; Li, J.; Hao, C.; Zhang, L.; et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: A multicentre population-based cohort study. Gut 2021, 70, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, T.; Zhang, H.; Sang, S.; Chen, H.; Zuo, X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: Comparison with Japan, South Korea, and Mongolia. Biomark. Res. 2021, 9, 84. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Carrington, M.; Chow, W.H.; McColl, K.E.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef]
- Taguchi, A.; Ohmiya, N.; Shirai, K.; Mabuchi, N.; Itoh, A.; Hirooka, Y.; Niwa, Y.; Goto, H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2487–2493. [Google Scholar] [CrossRef]
- Zambon, C.F.; Navaglia, F.; Basso, D.; Rugge, M.; Plebani, M. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J. Clin. Pathol. 2003, 56, 287–291. [Google Scholar] [CrossRef]
- Miki, K.; Ichinose, M.; Kawamura, N.; Matsushima, M.; Ahmad, H.B.; Kimura, M.; Sano, J.; Tashiro, T.; Kakei, N.; Oka, H.; et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn. J. Cancer Res. 1989, 80, 111–114. [Google Scholar] [CrossRef]
- Miki, K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006, 9, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Kiyohara, Y.; Kubo, M.; Tanaka, K.; Tanizaki, Y.; Ninomiya, T.; Doi, Y.; Shikata, K.; Yonemoto, K.; Shirota, T.; et al. The serum pepsinogen test as a predictor of gastric cancer: The Hisayama study. Am. J. Epidemiol. 2006, 163, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Miki, K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels–“ABC method”. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Samloff, I.M. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology 1971, 61, 185–188. [Google Scholar] [CrossRef]
- Samloff, I.M.; Liebman, W.M. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology 1973, 65, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--First American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef]
- Parsonnet, J.; Samloff, I.M.; Nelson, L.M.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Helicobacter pylori, pepsinogen, and risk for gastric adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 1993, 2, 461–466. [Google Scholar]
- Zhou, X.; Zhu, H.; Zhu, C.; Lin, K.; Cai, Q.; Li, Z.; Du, Y. Helicobacter pylori infection and serum pepsinogen level with the risk of gastric precancerous conditions: A cross-sectional study of high-risk gastric cancer population in China. J. Clin. Gastroenterol. 2021, 55, 778–784. [Google Scholar] [CrossRef]
- Sánchez-López, J.Y.; Díaz-Herrera, L.C.; Rizo-de la Torre, L.D.C. Pepsinogen I, pepsinogen II, gastrin-17, and Helicobacter pylori serological biomarkers in the diagnosis of precursor lesions of gastric cancer. Arch. Med. Sci. 2024, 20, 1016–1021. [Google Scholar] [CrossRef]
- In, H.; Sarkar, S.; Ward, J.; Friedmann, P.; Parides, M.; Yang, J.; Epplein, M. Serum pepsinogen as a biomarker for gastric cancer in the United States: A nested case-control study using the PLCO cancer screening trial data. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.M.; Stemmermann, G.N.; Samloff, I.M. Serum pepsinogen I as a predictor of stomach cancer. Ann. Intern. Med. 1980, 93, 537–540. [Google Scholar] [CrossRef]
- Stemmermann, G.N.; Samloff, I.M.; Nomura, A.M.; Heilbrun, L.K. Serum pepsinogens I and II and stomach cancer. Clin. Chim. Acta. 1987, 163, 191–198. [Google Scholar] [CrossRef]
- Ohata, H.; Kitauchi, S.; Yoshimura, N.; Mugitani, K.; Iwane, M.; Nakamura, H.; Yoshikawa, A.; Yanaoka, K.; Arii, K.; Tamai, H.; et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 2004, 109, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Lee, S.Y.; Kim, J.H.; Sung, I.K.; Park, H.S.; Shim, C.S.; Jin, C.J. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J. Dig. Dis. 2014, 15, 293–298. [Google Scholar] [CrossRef]
- Kikuchi, S.; Wada, O.; Miki, K.; Nakajima, T.; Nishi, T.; Kobayashi, O.; Inaba, Y. Serum pepsinogen as a new marker for gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer 1994, 73, 2695–2702. [Google Scholar] [CrossRef]
- Yanaoka, K.; Oka, M.; Yoshimura, N.; Mukoubayashi, C.; Enomoto, S.; Iguchi, M.; Magari, H.; Utsunomiya, H.; Tamai, H.; Arii, K.; et al. Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int. J. Cancer 2008, 123, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yoshihara, M.; Takata, S.; Wada, Y.; Matsuo, T.; Boda, T.; Tanaka, S.; Chayama, K. Serum screening for detection of high-risk group for early-stage diffuse type gastric cancer in Japanese. J. Gastroenterol. Hepatol. 2012, 27, 598–602. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Joukar, F.; Baghaee, M.; Sepehrimanesh, M.; Hojati, A. Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer. Biomol. Concepts. 2019, 10, 82–90. [Google Scholar] [CrossRef]
- Kang, J.M.; Kim, N.; Yoo, J.Y.; Park, Y.S.; Lee, D.H.; Kim, H.Y.; Lee, H.S.; Choe, G.; Kim, J.S.; Jung, H.C.; et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter 2008, 13, 146–156. [Google Scholar] [CrossRef]
- Lee, J.Y.; Gong, E.J.; Chung, E.J.; Park, H.W.; Bae, S.E.; Kim, E.H.; Kim, J.; Do, Y.S.; Kim, T.H.; Chang, H.S.; et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-ups. Gut Liver 2017, 11, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Gašenko, E.; Bogdanova, I.; Sjomina, O.; Aleksandraviča, I.; Kiršners, A.; Ancāns, G.; Rudzīte, D.; Vangravs, R.; Sīviņš, A.; Škapars, R.; et al. Assessing the utility of pepsinogens and gastrin-17 in gastric cancer detection. Eur. J. Cancer Prev. 2023, 32, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Koike, T.; Asonuma, S.; Okata, H.; Uno, K.; Oikawa, T.; Iwai, W.; Yonechi, M.; Fukushi, D.; Kayaba, S.; et al. Smoking history and severe atrophic gastritis assessed by pepsinogen are risk factors for the prevalence of synchronous gastric cancers in patients with gastric endoscopic submucosal dissection: A multicenter prospective cohort study. J. Gastroenterol. 2023, 58, 433–443. [Google Scholar] [CrossRef]
- Hirai, R.; Hirai, M.; Otsuka, M.; Mitsuhashi, T.; Shimodate, Y.; Mouri, H.; Matsueda, K.; Yamamoto, H.; Mizunom, M. Endoscopic evaluation by the Kyoto classification of gastritis combined with serum anti-Helicobacter pylori antibody testing reliably risk-stratifies subjects in a population-based gastric cancer screening program. J. Gastroenterol. 2023, 58, 848–855. [Google Scholar] [CrossRef]
- Baek, S.M.; Kim, N.; Kwon, Y.J.; Lee, H.S.; Kim, H.Y.; Lee, J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Role of serum pepsinogen II and Helicobacter pylori status in the detection of diffuse-type early gastric cancer in young individuals in South Korea. Gut Liver 2020, 14, 439–449. [Google Scholar] [CrossRef]
- Kang, S.; Park, M.; Cho, J.Y.; Ahn, S.J.; Yoon, C.; Kim, S.G.; Cho, S.J. Tumorigenic mechanisms of estrogen and Helicobacter pylori cytotoxin-associated gene A in estrogen receptor alpha-positive diffuse-type gastric adenocarcinoma. Gastric Cancer 2022, 25, 678–696. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ahn, J.S.; Ha, Y.J.; Doh, H.J.; Jang, M.H.; Chung, S.I.; Park, H.J. Serodiagnosis of Helicobacter pylori infection in Korean patients using enzyme-linked immunosorbent assay. J. Immunoass. 1998, 19, 251–270. [Google Scholar] [CrossRef]
- Lim, S.H.; Kwon, J.W.; Kim, N.; Kim, G.H.; Kang, J.M.; Park, M.J.; Yim, J.Y.; Kim, H.U.; Baik, G.H.; Seo, G.S.; et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: Nationwide multicenter study over 13 years. BMC Gastroenterol. 2013, 13, 104. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, N.; Kwon, J.W.; Kim, S.E.; Baik, G.H.; Lee, J.Y.; Park, K.S.; Shin, J.E.; Song, H.J.; Myung, D.S.; et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: A cross-sectional nationwide multicenter study. PLoS ONE 2018, 13, e0204762. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, N.; Lee, H.S.; Oh, J.C.; Kwon, Y.H.; Choi, Y.J.; Yoon, K.C.; Hwang, J.J.; Lee, H.J.; Lee, A.; et al. Correlations among endoscopic, histologic and serologic diagnoses for the assessment of atrophic gastritis. J. Cancer Prev. 2014, 19, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, N.; Kang, J.M.; Park, Y.S.; Lee, D.H.; Kim, Y.R.; Kim, J.S.; Jung, H.C.; Song, I.S. Clinical meaning of pepsinogen test and Helicobacter pylori serology in the health check-up population in Korea. Eur. J. Gastroenterol. Hepatol. 2009, 21, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.Y.; Kim, N.; Lee, J.; Lee, J.Y.; Hwang, Y.J.; Lee, H.S.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, J.W.; et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter 2018, 23, e12542. [Google Scholar] [CrossRef]
- Noh, G.; Kim, N.; Choi, Y.; Lee, H.S.; Hwang, Y.J.; Kim, H.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Long-term follow up of serum pepsinogens in patients with gastric cancer or dysplasia after Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 2020, 35, 1540–1548. [Google Scholar] [CrossRef]
- Shin, C.M.; Kim, N.; Lee, H.S.; Lee, H.E.; Lee, S.H.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jeong, S.H.; Lee, D.H.; et al. Validation of diagnostic tests for Helicobacter pylori with regard to grade of atrophic gastritis and/or intestinal metaplasia. Helicobacter 2009, 14, 512–529. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kim, N.; Lee, J.Y.; Choi, Y.J.; Yoon, K.; Hwang, J.J.; Lee, H.J.; Lee, A.; Jeong, Y.S.; Oh, S.; et al. The diagnostic validity of citric acid-free, high dose (13)C-urea breath test after Helicobacter pylori eradication in Korea. Helicobacter 2015, 20, 159–168. [Google Scholar] [CrossRef]
- Bornschein, J.; Selgrad, M.; Wex, T.; Kuester, D.; Malfertheiner, P. Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol. 2012, 12, 10. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagata, Y.; Hiratsuka, R.; Kawase, Y.; Tominaga, T.; Takeuchi, S.; Sakagami, S.; Ishida, S. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels--The ABC method. Digestion 2016, 93, 13–18. [Google Scholar] [CrossRef]
- Yoshihara, M.; Sumii, K.; Haruma, K.; Kiyohira, K.; Hattori, N.; Kitadai, Y.; Komoto, K.; Tanaka, S.; Kajiyama, G. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am. J. Gastroenterol. 1998, 93, 1090–1096. [Google Scholar] [CrossRef]
- Yoshida, T.; Kato, J.; Inoue, I.; Yoshimura, N.; Deguchi, H.; Mukoubayashi, C.; Oka, M.; Watanabe, M.; Enomoto, S.; Niwa, T.; et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int. J. Cancer 2014, 134, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Zheng, W.; Ye, W.; Kamangar, F.; Ji, B.T.; Persson, C.; Yang, G.; Li, H.L.; Rothman, N.; Shu, X.O.; et al. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women’s Health Study Cohort. Br. J. Cancer 2011, 104, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Shikata, K.; Hata, J.; Fukuhara, M.; Hirakawa, Y.; Ohara, T.; Mukai, N.; Nagata, M.; Yoshida, D.; Yonemoto, K.; et al. Combination of Helicobacter pylori antibody and serum pepsinogen as a good predictive tool of gastric cancer incidence: 20-year prospective data from the Hisayama Study. J. Epidemiol. 2016, 26, 629–636. [Google Scholar] [CrossRef]
- Mizuno, S.; Kobayashi, M.; Tomita, S.; Miki, I.; Masuda, A.; Onoyama, M.; Habu, Y.; Inokuchi, H.; Watanabe, Y. Validation of the pepsinogen test method for gastric cancer screening using a follow-up study. Gastric Cancer 2009, 12, 158–163. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, H.; Zhao, Y.; He, X.; Xu, H.; Li, H.; Shuai, P.; Gong, L.; Wu, H.; Xu, H.; et al. Diagnostic value of serum pepsinogen levels for screening gastric cancer and atrophic gastritis in asymptomatic individuals: A cross-sectional study. Front. Oncol. 2021, 11, 652574. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, N.; Nam, R.H.; Suh, J.H.; Chang, H.; Lee, J.W.; Kim, Y.S.; Kim, J.M.; Choi, J.W.; Park, J.G.; et al. Association of polymorphisms in virulence factor of Helicobacter pylori and gastroduodenal diseases in South Korea. J. Gastroenterol. Hepatol. 2014, 29, 984–991. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).