Simple Summary
The purpose of this study is to review the existing literature on the treatment of endometrial carcinoma with brain metastases. Brain metastases from endometrial cancer are rare and pose significant challenges in treatment, as no standardized approach exists. This study investigates the impact of different therapeutic strategies, including surgery, radiotherapy, systemic therapies, and their combinations, on patient survival. By evaluating these treatments in specific patient subgroups, such as those with solitary brain metastases or multiple brain metastases with extracranial disease, we aim to identify the most effective approaches to improve outcomes. Our findings may provide valuable insights to guide future research and inform clinical decision making for this challenging condition.
Abstract
Background: Brain metastases (BMs) from endometrial cancer (EC) are rare and challenging to treat, with limited standardized guidelines. This systematic review aims to evaluate the incidence, therapeutic strategies, and outcomes associated with brain metastases in EC patients, offering insights for clinical practice and future research. Methods: A comprehensive literature search was conducted using PRISMA guidelines, including PUBMED up to October 2024. Reports reporting individual or aggregate data on EC brain metastases were included. Descriptive and quantitative analyses were performed on incidence, treatment modalities, and survival outcomes. Three reports that used data from the Surveillance, Epidemiology, and End Results and National Cancer Database were used only to assess the incidence of brain metastases from endometrial carcinoma. Results: From 911 reports identified, we included 99 reports, identifying 594 cases; these and the case of a patient with brain metastasis from endometrial carcinoma followed at our center were used for analysis of disease characteristics; incidence; and treatment modalities, such as surgery, radiotherapy, chemotherapy, and combinations. Survival outcomes were influenced by treatment type and disease characteristics, with multimodal approaches showing improved outcomes. Discussion: This review underscores the rarity of EC brain metastases and highlights the need for tailored, multimodal treatment strategies. Future research should focus on prospective trials and molecular profiling to optimize management.
1. Introduction
Endometrial cancer is the sixth most common cancer among women and the second most frequent gynecological malignancy after cervical cancer, with over 400,000 new cases annually and 97,370 deaths reported in 2020 worldwide. Both the incidence and mortality of EC are rising, particularly in economically developed countries, likely due to the increasing prevalence of obesity and type 2 diabetes [1,2,3,4].
Most patients present with localized disease, with nearly 20% showing regional spread and 9% exhibiting distant metastases. According to the Surveillance, Epidemiology, and End Results (SEER) database and other studies, 5-year survival rates are stage-dependent. The 5-year survival rate in early-stage EC exceeds 95%, but it drops to 56–69% in patients with loco-regional spread and plummets to 17–20% in patients with distant metastases [5]. Recurrence occurs in approximately 20% of patients and typically presents in the pelvis and abdomen within 1–2 years of diagnosis [6,7,8]. Pelvic lymph nodes, such as internal and external iliac lymph nodes [9] and retroperitoneal lymph nodes, are the most common sites of metastasis. Distant metastases are less frequent, with the lung being the most common site (1.5%), followed by the liver (0.8%) and bones (0.6%), while the brain is the least frequent (0.2%). In patients with single-organ metastasis, the median overall survivals (OSs) for patients with lung, liver, bone, and brain metastasis were 11, 10, 8, and 5 months, respectively. Similarly, patients with lung, liver, bone, and brain metastasis had median cancer-specific survivals (CSS) of 14, 15, 11, and 8 months, respectively. [10]. Overall, 10–30% of all cancer patients develop BMs [11,12], which carry a poor prognosis, while in EC, the incidence is much lower, ranging from 0.2% to 1.4%.
In this paper, we review the fragmented medical literature on BMs in endometrial carcinoma.
2. Methods
This review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and has not been registered. A comprehensive search was conducted in PubMed using the terms “brain metastasis and endometrial cancer” and their synonyms (cerebral, metastases, carcinoma, uterus, uterine), covering all available studies up to October 2024. Articles with extractable individual patient data were included. Case series lacking extractable individual data but containing relevant aggregated information were retained for quantitative analysis. Articles not written in English, French, Spanish, or Italian were excluded, as were those addressing unrelated topics based on predefined eligibility criteria. Non-pertinent records were excluded after review.
A total of 911 records were initially identified (850 from PubMed and 61 through citation searching). After screening for relevance, 748 records were excluded. Of the remaining 163 articles assessed for eligibility, 99 were included in the analysis. The PRISMA flow diagram (Figure 1) outlines the study selection process. Three studies utilizing data from the Surveillance, Epidemiology, and End Results database and the National Cancer Database (NCDB) were included solely for the purpose of assessing the incidence of brain metastases from endometrial carcinoma.
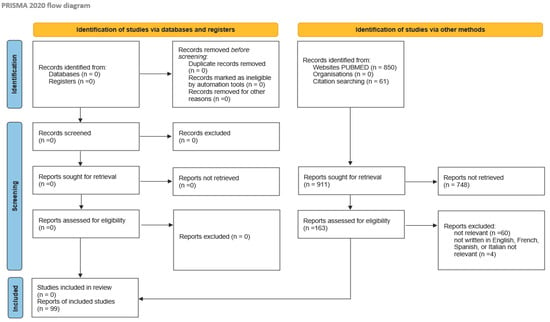
Figure 1.
Literature review according to PRISMA 2020 method.
Since 1972, 594 cases of brain metastases from endometrial cancer have been reported in the literature.
All included studies were analyzed to extract the following variables: number of reported cases, patient age, stage at initial diagnosis, histological subtype, tumor differentiation grade, presence and location of extracranial metastases, time interval between the diagnosis of EC and the development of BMs, number and anatomical location of brain metastases, treatments administered, and survival from the time of BM diagnosis.
Three independent reviewers assessed each record and full-text article. The quality of single-case reports and case series was evaluated according to the Newcastle–Ottawa Scale for the domains applicable to the studies reported in the review as previously described [13]. Where reported, the following parameters were evaluated: the median age of patients, distribution of clinical stages at diagnosis, histological subtypes, differentiation grades, frequency and sites of extracranial metastases, and the number and distribution of BMs. Treatment modalities were analyzed, with median and range of survival calculated for each treatment type.
Patients were classified into four categories based on the number of brain metastases and the presence of extracranial metastases. For each category, treatment approaches were analyzed, and survival outcomes (medians and ranges) were reported.
Table 1 summarizes data extracted from the individual case reports of brain metastases arising from endometrial carcinoma. Additionally, one case of a patient with BMs from EC treated at our center was included, providing detailed demographic, clinical, and survival data.

Table 1.
Cases of brain metastases from endometrial carcinoma.
This study was approved by the Ethics Committee of Kore University of Enna (the research project “Brain Metastasis of Endometrial Carcinoma” was registered under Prot. 26580; the ethical approval ensures adherence to ethical standards and guidelines for systematic reviews).
Table 1 summarizes the reported cases of brain metastasis from endometrial cancer.
3. Results
Eleven population studies showed a variable incidence of BM ranging from 0.2% to 0.97% [10,19,27,32,39,42,50,74,99,100,107]. One study focused solely on patients with stage I and II EC, reporting a 0.6% incidence of BMs [58]. One study evaluated only low-grade (G1–G2) patients and found an incidence of 0.75% [90]. Overall, most papers reported in this study were single-case reports (n = 62). Additionally, 37 papers reported small-numbered case series (n: 2–37), except for 3 papers reporting 132, 498, and 73 cases. The quality of the reports was poor (0–1 stars in the selection domain) in 22 cases (22%), fair (2 stars) in 28 cases (28%), and good in 49 cases (50%).
The median age of onset for BMs was 60 years (range: 33–82 years).
Among patients without metastases at diagnosis, 50% were at stage I, 15% at stage II, and 35% at stage III. Table 2 shows the histological types reported in the medical literature. The grading distribution was 11% G1, 23% G2, 66% G3.

Table 2.
Histology of endometrial carcinomas.
In 43% of cases, the brain was the only site of metastasis; in the remaining 57%, metastases also involved the lungs, liver, lymph nodes, skin, peritoneum, bone, adrenal glands, and larynx.
In 66% of cases, BMs were supratentorial, 21% were infratentorial, and 13% involved both locations. The study population included four cases of pituitary metastases [22,25,89,96], three cases of leptomeningeal metastases [56,84], and, in one case, the metastases involved the dura mater [17].
In 49% of cases, BMs were solitary; in 5%, there were 2–3 metastases; and 46% had multiple MBs.
In 21% of patients, BMs were present at or preceded the diagnosis of EC. In the remaining patients, metastases occurred after a median of 18 months (range: 2–216 months) following the initial diagnosis of EC.
The median survival from the onset of BMs was 7 months (range: 0–171). In the group of patients with isolated BM, the median survival was 12 months (range: 1–171). Patients with 2–3 BM had a median survival of two months (range: 0.16–64). Patients with multiple BMs had a median survival of four months (range: 0.25–32). Patients with BMs in the absence of extracranial metastasis or with extracranial metastasis had median survivals of twelve months (range: 0.1–171 months) and 4.5 months (range: 0.25–84 months), respectively.
In patients with BMs as the first (primary) vs. subsequent site of recurrence (secondary), the median survivals were 9 months (range 0.1–171 months) and 5 months (range 0.25–108 months), respectively.
Treatment modalities are depicted in Table 3. Many reports were anecdotal, describing only 1 or 2 patients. Various treatments were used, including palliation, surgery, radiotherapy (RT), chemotherapy, stereotactic radiosurgery (SRS), or a combination of the above.

Table 3.
Treatment modalities.
3.1. Isolated Brain Metastasis Without Extracranial Disease
Overall, 40% had isolated BM with no extracranial disease. In this group of patients, median survival was 13 months (range: 1–171). Surgery alone provided a median survival of eight months (range: 1–18); surgery and RT resulted in a median survival of 23 months (range: 2–118); radiotherapy or stereotactic radiotherapy combined with systemic therapy resulted in a survival of 30 months; surgery plus radiotherapy and systemic therapy led to a survival of 16 months (range: 6–74); and SRS with or without surgery resulted in a median survival of 15 months (range: 5–40).
One patient had stereotactic radiotherapy, and systemic therapy achieved a survival of 171 months [38]. Another patient with trimodal therapy, surgery, stereotactic radiotherapy, and systemic therapy achieved a survival of 38 months [41].
3.2. Isolated Brain Metastasis and Extracranial Disease
Twenty-three percent of patients had solitary BM and extracranial disease, with a median survival of 7 months (range: 1–84). RT alone resulted in a median survival of seven months (range: 1–38); surgical and RT yielded a median survival of 8.5 months (range: 4–84).
A trimodal treatment (surgery, RT, and systemic therapy) resulted in 34 months of survival in one patient [44]. A similar treatment approach in a patient with small cell carcinoma achieved 144 months of survival [78].
3.3. Multiple Brain Metastasis Without Extracranial Disease
Overall, 9% of patients had multiple BMs without extracranial disease, with a median survival of 6 months (range: 0.16–64). Most were treated with RT, yielding a median OS of five months (range: 0.16–17).
A patient with a serous carcinoma harboring a BRCA1 mutation was treated with niraparib after the progression of the disease with whole-brain radiotherapy (WBRT) plus temozolomide, achieving survival of 13 months from the onset of BMs [102].
3.4. Multiple Brain Metastasis with Extracranial Disease
Twenty-eight percent of patients had BMs and extracranial metastases, with a median survival of three months (range: 0–32). Most received RT, with a median OS of two months (range: 0.25–28); RT, systemic therapy, and surgery led to a median survival of 9 months; and radiotherapy or SRS and systemic therapy led a survival of 24 months.
A patient with EC with lung, liver, and peritoneal involvement, showing a BRCA1/2 wild type with a PTEN mutation, developed a BM 120 months after the initial diagnosis. After multiple treatments and SRS, she received olaparib, with a survival of eighteen months from the start of the treatment [59].
Another patient was treated with whole-brain radiotherapy and systemic therapy with pembrolizumab (PEM) and Lenvatinib (LEN) and achieved a survival of 32 months after a diagnosis of brain metastasis [111].
At our center, we treated a patient with endometrial carcinoma who underwent hystero-salpingo-oophorectomy followed by adjuvant chemotherapy. Upon mediastinal lymph node recurrence after 52 months, a new line of chemotherapy was initiated. Following further progression involving brain and lymph node metastases, the patient received stereotactic radiotherapy and subsequent systemic therapy with pembrolizumab and lenvatinib. Since the onset of brain metastases, the patient has achieved 30 months of survival and continues systemic treatment (Figure 2 and Figure 3).

Figure 2.
Median survival in patients with isolated brain metastasis and no extracranial disease, isolated brain metastasis and extracranial disease, multiple brain metastasis and no extracranial disease, and multiple brain metastasis and extracranial disease.
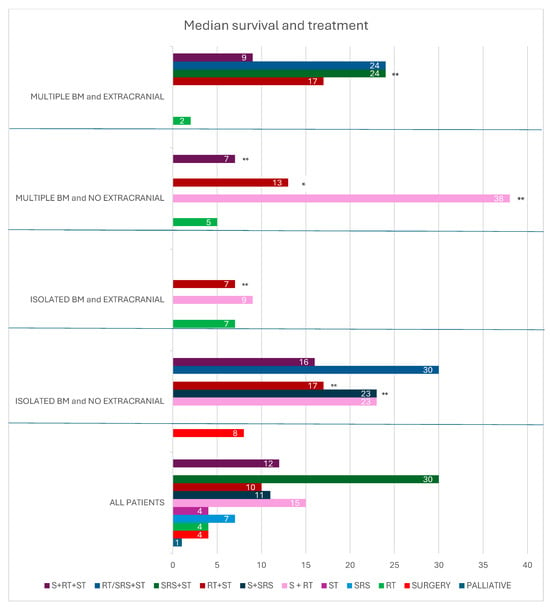
Figure 3.
Median survival and treatment in all patients, patients with isolated brain metastasis and no extracranial disease, isolated brain metastasis and extracranial disease, multiple brain metastasis and no extracranial disease, and multiple brain metastasis and extracranial disease. * 1 patient; ** 2 patients. BM brain metastasis; RT radiotherapy; S surgery; SRS stereotactic radiotherapy; ST systemic therapy.
4. Discussion
BMs from EC are rare, with our study showing an incidence ranging from 0.2% to 0.97%. Current scientific evidence supports the “seed and soil” theory proposed over a century ago [112]. The development of BMs is the result of multiple factors. The progressive accumulation of genetic alterations due to clonal evolution allows cells to metastasize [113]. Transcriptomic and epigenetic changes in newly established colonies allow micro-metastases to grow via metabolic adaptation [114]. Activating angiogenesis supports tumor growth and the production of chemokines and cytokines, which promote an immunosuppressive phenotype in resident immune cells [115,116,117].
Approximately 30% of endometrial carcinomas cases are associated with a germline mutation in mismatch repair (MMR) genes or show high microsatellite instability (MSI-H) [118].
The Cancer Genome Atlas was the first project to assess many tumors using whole-genome sequencing [119]. For EC, it identified four molecular subgroups: POLE-mutated tumors, MSI tumors, copy number low (CNL) tumors, and copy number high (CNH) tumors. The prognostic value of these molecular subgroups was evaluated in the PORTEC 3 study using univariate and multivariate analyses alongside clinicopathological factors, such as age, grade, lympho-vascular space invasion, and treatment. EC patients with abnormal expression of p53 had a poor prognosis, contrasting with the excellent survival outcomes of those with POLE-mutated EC, even in patients with high-grade and advanced-stage tumors. Patients with MMR-deficient or no specific molecular profile EC showed intermediate clinical outcomes [120]. Ashley et al. demonstrated that POLE and MSI subtypes of EC exhibit specific mutational processes, such as loss of proofreading by polymerase and MSI. At the same time, CNL and CNH tumors and uterine sarcomas are dominated by aging-related mutational processes [121]. Interestingly, while the molecular subtype of EC is generally stable between primary tumors and metastases, mutational signature changes occur in over 25% of cases, suggesting that further defects in DNA repair mechanisms may influence tumor progression [122].
Other factors, such as estrogens, prolactin, and pro-inflammatory adipokines, may intervene in EC progression. Estrogen, through its receptors and non-genomic interactions, induces remodeling of actin, a critical cytoskeletal protein, and the cell membrane. At a molecular level, this phenomenon depends on the induction of phosphorylation on Thr (558) of myosin, an actin-binding protein. This interaction enhances endometrial cells’ migration and implantation capacity, as demonstrated in preclinical studies on Ishikawa cell cultures and native endometrial stromal cells [122,123]. Other in vitro and in vivo studies have shown that estradiol promotes proliferation, migration, and invasion by activating the IL-6 pathway, which is involved in various signaling pathways associated with estrogen receptors (ERs), Bcl-2, Cyclin D1, and MMP2 [124].
The role of prolactin in EC metastasis growth has recently emerged, along with its association with reduced chemotherapy sensitivity. The mechanism of prolactin’s action is complex, involving endocrine, paracrine, and autocrine mechanisms, including interactions with immune cells. Prolactin exerts both receptor-dependent and receptor-independent effects. Its anti-apoptotic activity, mediated by prolactin receptors (PRLRs), involves blocking Stat5a/b expression, which increases anti-apoptotic Bcl-2 expression, decreases pro-apoptotic Bax expression, and upregulates Hsp90A. This latter chaperone protein protects cells from apoptosis. The proliferative effect of prolactin is further supported by its synthesis in vascular endothelial and stromal cells. Additionally, prolactin may interact with various agonist ligands through its receptor, creating a specific microenvironment in metastatic foci that promotes proliferative processes. Prolactin also directly stimulates vascular endothelial cell proliferation and indirectly increases the expression of VEGF and other proangiogenic factors [125,126]. Pro-inflammatory adipokines such as leptin, visfatin, and resistin are also implicated in the progression and spread of endometrial cancer cells [122]. Considerable attention has also been given to microRNA (miRNA) expression as a marker of metastatic risk in EC. miRNAs are small non-coding RNA fragments that physiologically regulate gene expression and are involved in oncogenesis and metastasis processes [127].
Treatment
The prognosis of patients with BMs remains poor, with a median survival of 4 months following whole-brain radiotherapy and a 12% one-year survival rate [128,129]. However, survival beyond historical expectations has emerged due to innovative systemic therapies, such as targeted and immunotherapy. The most important prognostic variables are performance status, age, and systemic disease control. Prognostic variables also include the characteristics of BMs in terms of volume and number. Our study reports a seven-month median survival for patients with BMs from EC. Currently, no data have been reported regarding the possibility of early diagnosis of brain metastases. New studies correlating molecular evaluations with the onset of brain metastases could be helpful.
At this time, no standardized treatment pathway exists for these patients, as the available data are predominantly retrospective and based on small patient cohorts.
Local treatment options for BMs include surgical resection, WBRT, or SRS. Surgical resection is typically considered for patients with oligometastatic disease or significant mass effect and provides a histological diagnosis. Some randomized studies have demonstrated improved survival in patients with solitary BMs who undergo surgical resection followed by radiotherapy compared to surgery alone [130,131,132]. However, the potential benefits of surgery must be weighed against the risks and other prognostic factors.
In our review of published cases on the surgical treatment of BMs from EC, the median survival across the entire cohort was four months, and the median survival was eight months for patients with a single BM. For those who received surgery and radiotherapy, median survival increased to 15.5 months and 23 months for patients with a solitary BM and no extracranial involvement.
The reasons for the dismal results of surgery alone in patients with solitary brain metastases from endometrial cancer are not clear. It may be hypothesized that biological factors and potential sensitivity to radiation may influence results. Moreover, newer therapies such as immunotherapy and targeted therapies in combination could lead to better responses and survival, but further studies are needed.
WBRT remains a primary treatment option for BMs, with reported local control rates of approximately 80%. However, this benefit is often impaired by cognitive decline and deterioration of performance status [132,133,134].
WBRT is generally indicated for cases with multiple brain lesions or when palliative care is the only goal. Our review reports a 4-month median survival for patients treated with WBRT alone.
SRS involves the conformational delivery of high radiation doses to the target lesions, with a rapid dose fall-off at the lesion boundary [135,136]. Except for radioresistant tumors such as melanomas and sarcomas, SRS controls BMs without causing the cognitive decline associated with WBRT [137,138,139,140,141].
Brain metastases can evade the immune system through various mechanisms, including the secretion of immunosuppressive cytokines, downregulation of tumor-associated antigens (TAA) and major histocompatibility complex (MHC) class I expression, recruitment of regulatory T cells (Tregs) into the tumor microenvironment (TME) [142], or suboptimal functioning of host dendritic cells (DCs) [143]. SRS induces significant DNA damage in tumor cells, leading to cell death, while also activating multiple signaling pathways within the TME, inducing a pro-inflammatory state and potentially causing damage to surrounding stromal and endothelial cells [144]. Radiation has been shown to enhance the presentation of TAA by DCs to CD4+ and CD8+ T cells, thereby strengthening the immune system’s ability to recognize and target tumor cells [145]. It facilitates the maturation of antigen-presenting cells (APCs); enhances antigen–MHC complex assembly; and induces the secretion of critical inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-β), and chemokine ligand 16 (CXCL16). These cytokines attract immune cells to cross the blood–brain barrier (BBB) and infiltrate the TME [146].
Radiation can also regulate programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), potentially acting synergistically with immune checkpoint inhibitors.
Immunotherapy, particularly immune checkpoint inhibitors targeting PD-1/PD-L1 or CTLA-4 pathways, can potentiate this response by overcoming tumor-induced immune suppression. While specific data on endometrial cancer remain limited, studies in other tumor types, such as lung, melanoma, and cervical cancers, suggest that the combination can improve outcomes, including tumor control and survival. These findings provide a compelling rationale for exploring this approach in endometrial cancer, where the immune microenvironment and potential for neoantigen generation might similarly support the efficacy of combined modality therapy (Figure 4).

Figure 4.
Effect of radiotherapy, immunotherapy, and TKI inhibitor. CD8+ T cell: cytotoxic T lymphocyte, MHC-I: major histocompatibility complex class I, TCR: T cell receptor, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1, FGFR: fibroblast growth factor receptor, IL-1: Interleukin 1, TNF: tumor necrosis factor, ICAM-1: intercellular adhesion molecule 1, VCAM: vascular cell adhesion molecule 1.
Table 4 depicts studies showing the treatment efficacy in treating BM from EC with SRS, but the retrospective nature and limited sample sizes do not allow for definitive conclusions.

Table 4.
Efficacy of stereotactic radiosurgery in endometrial cancer.
Subsequently, the GOG 209 study confirmed the non-inferiority of the carboplatin-paclitaxel regimen compared to TAP, providing an alternative with a more favorable toxicity profile [148].
The integration of immunotherapy has further revolutionized treatment paradigms. The addition of pembrolizumab to carboplatin and paclitaxel reduced the risk of disease progression or death by 70% in the mismatch repair-deficient (dMMR) cohort and by 46% in the mismatch repair-proficient (pMMR) cohort, compared to placebo [149]. Similarly, the incorporation of dostarlimab into carboplatin-paclitaxel regimens resulted in a 2-year PFS rate of 36.1% and an OS rate of 71.3%, compared to 18.1% and 56.0%, respectively, in the placebo arm. In dMMR–MSI-H patients, 24-month PFS was 61.4% (95% CI, 46.3–73.4) in the dostarlimab group versus 15.7% (95% CI, 7.2–27.0) in the placebo group p < 0.001) [150].
Atezolizumab, combined with carboplatin and paclitaxel, demonstrated a 64% reduction in progression or death risk in dMMR patients (HR 0.36, 95% CI 0.23–0.57; p = 0.0005). In the overall population, median PFS improved to 10.1 months (95% CI, 9.5–12.3) with atezolizumab compared to 8.9 months (95% CI, 8.1–9.6) with placebo (HR 0.74, 95% CI, 0.61–0.91; p = 0.022) [151].
The DUO-E study explored the combination of durvalumab and paclitaxel-carboplatin with or without olaparib. In the intention-to-treat population, durvalumab significantly reduced the risk of progression or death compared to the standard regimen (HR 0.71, 95% CI, 0.57–0.89; p = 0.003). While olaparib added no notable PFS benefit in dMMR patients, its combination with durvalumab appeared advantageous in pMMR patients [152].
Therefore, PEM and dostarlimab have established efficacy as second-line treatment for patients with dMMR or MSI-high status [153,154].
Emerging evidence highlights the efficacy of targeted therapies in EC management. Lenvatinib, a tyrosine kinase inhibitor (TKI), selectively targets VEGFR, FGFR, RET, cKIT, and PDGFR, with preclinical studies confirming its antitumor potential, particularly in combination with immune checkpoint inhibitors [155,156,157,158].
Preclinical studies have demonstrated that inhibition of the FGFR pathway, alone or in combination with other signaling pathways or chemotherapy, induces antitumor activity in endometrial cancer models [159,160].
The phase-Ib/II KEYNOTE 146 trial demonstrated promising outcomes with LEN plus PEM, reporting a 38.0% objective response rate in metastatic EC patients without selection for microsatellite instability or PD-L1 status [161,162,163].
The phase-III KEYNOTE 775 trial compared LEN and PEM to physician’s-choice chemotherapy in patients previously treated for advanced EC. In pMMR patients, LEN and PEM significantly improved PFS, OS, and overall response rates. Median OS in pMMR patients was 18.0 months (95% CI, 14.9–20.5) compared to 12.2 months (95% CI, 11.0–14.1) with chemotherapy (HR 0.70, 95% CI, 0.58–0.83) [164,165].
Lastly, VEGF-A expression is heightened in brain metastases (BMs) compared to gynecologic primary tumors [77], while the active tumor immune microenvironment observed in both primary and metastatic sites supports combining antiangiogenic therapies with immune checkpoint inhibitors for the treatment of EC with BMs [103]. This therapeutic synergy warrants further exploration.
In our study, systemic therapy combined with radiotherapy resulted in a median survival of 10 months (range: 3–32 months). Trimodal treatment, comprising surgery, radiotherapy, and systemic therapy, achieved a median survival of 12 months (range: 4–74 months). Stereotactic radiotherapy combined with systemic treatment in three patients resulted in a median survival of 30 months (18,30,171 months). Two patients treated with radiotherapy or stereotactic radiotherapy in combination with pembrolizumab and lenvatinib showed median survivals of 32 and 30 months, respectively.
In patients with solitary brain metastases and no extracranial disease, radiotherapy or stereotactic radiotherapy combined with systemic therapy achieved a median survival of 30 months (range: 4–171 months). In the group of patients with multiple brain metastases and extracranial disease, radiotherapy or stereotactic radiotherapy combined with systemic therapy resulted in a median survival of 24 months (range: 3–32 months).
5. Conclusions
Our study has some limitations. Given the low incidence of BMs from EC, the literature reports retrospective studies and small case series, which support multimodal strategies such as radiotherapy, surgery, and systemic treatments. Surgery alone appears to have limited efficacy, even in patients with single metastases and no extracranial disease. The addition of radiotherapy improves survival in patients with solitary brain metastases and no extracranial disease. Systemic treatment with tyrosine kinase inhibitors, immunotherapy, and radiotherapy appears to be a promising therapeutic approach.
In conclusion, we report a detailed review of the available medical literature, even anecdotal cases. Therefore, the data reported above should be interpreted with caution. Patients’ clinical characteristics probably influenced physicians’ treatment choices rather than following reliable therapy guidelines.
Author Contributions
D.S.: conceptualization, validation, formal analysis, investigation, resources, and data curation; V.G.: supervision, visualization, writing—original draft preparation; A.M.O.Q. and G.C.: resources and formal analysis; A.B.: formal analysis, data curation and validation; E.V. and P.D.M.: investigation and software; S.L., B.P., G.S. (Giuseppa Scandurra) and G.S. (Giuseppe Scibilia): writing—review and editing; M.R.V. and D.C.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study complied with the Declaration of Helsinki.
Informed Consent Statement
The patient gave written informed consent to report data in the paper.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.J.; George, A.S.; Subrahmanyan, N.A.; Pappachan, J.M. Epidemiological link between obesity, type 2 diabetes mellitus and cancer. World J. Methodol. 2021, 11, 23–45. [Google Scholar] [CrossRef]
- Medina-Gutiérrez, E.; Céspedes, M.V.; Gallardo, A.; Rioja-Blanco, E.; Pavón, M.; Asensio-Puig, L.; Farré, L.; Alba-Castellón, L.; Unzueta, U.; Villaverde, A.; et al. Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease. Biomedicines 2022, 10, 1680. [Google Scholar] [CrossRef]
- SEER Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 7 October 2024).
- Tung, H.J.; Huang, H.J.; Lai, C.H. Adjuvant and post-surgical treatment in endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 78, 52–63. [Google Scholar] [CrossRef]
- Ko, E.M.; Brensinger, C.M.; Cory, L.; Giuntoli, R.L.; Haggerty, A.F.; Latif, N.A.; Aviles, D.; Martin, L.; Morgan, M.A.; Lin, L.L. Utilization and survival outcomes of sequential, concurrent and sandwich therapies for advanced stage endometrial cancers by histology. Gynecol. Oncol. 2020, 159, 394–401. [Google Scholar] [CrossRef]
- Legge, F.; Restaino, S.; Leone, L.; Carone, V.; Ronsini, C.; Di Fiore, G.L.M.; Pasciuto, T.; Pelligra, S.; Ciccarone, F.; Scambia, G.; et al. Clinical outcome of recurrent endometrial cancer: Analysis of post-relapse survival by pattern of recurrence and secondary treatment. Int. J. Gynecol. Cancer 2020, 30, 193–200. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Podratz, K.C. Routes of lymphatic spread: A study of 112 consecutive patients with endometrial cancer. Gynecol. Oncol. 2001, 81, 100–104. [Google Scholar] [CrossRef]
- Mao, W.; Wei, S.; Yang, H.; Yu, Q.; Xu, M.; Guo, J.; Gao, L. Clinicopathological study of organ metastasis in endometrial cancer. Future Oncol. 2020, 16, 525–540. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Wright, C.H.; Barnholtz-Sloan, J.S. Brain metastases: Epidemiology. Handb. Clin. Neurol. 2018, 149, 27–42. [Google Scholar]
- Lu-Emerson, C.; Eichler, A.F. Brain metastases. Continuum 2012, 18, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Fauré, I.; Corcoran, N.; Butterly, E.; Lewsey, J.; McAllister, D.A.; Mair, F.S. Identification and prevalence of frailty in diabetes mellitus and association with clinical outcomes: A systematic review protocol. BMJ Open. 2020, 10, e037476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salibi, B.S.; Beltaos, E. Endometrial adenocarcinoma with cerebral metastasis and subdural ossification. Wis. Med. J. 1972, 71, 255–258. [Google Scholar] [PubMed]
- Nakano, K.K.; Schoene, W.C. Endometrial carcinoma with a predominant clear-cell pattern with metastases to the adrenal, posterior mediastinum, and brain. Am. J. Obstet. Gynecol. 1975, 122, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Hacker, J.R.; Foxet, J.L. Surgical treatment of brain stem carcinoma: Case report Neurosurgery. Neurosurgery 1980, 6, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.M.; Graf, C.J. Nontraumatic subdural hematoma secondary to dural metastasis: Case report and review of the literature. Neurosurgery 1982, 11, 678–680. [Google Scholar] [CrossRef]
- Kishi, K.; Nomura, K.; Miki, Y.; Shibui, S.; Takakura, K. Metastatic brain tumor. A clinical and pathologic analysis of 101 cases with biopsy. Arch. Pathol. Lab. Med. 1982, 106, 133–135. [Google Scholar]
- Aalders, J.G.; Abeler, V.; Kolstad, P. Recurrent adenocarcinoma of the endometrium: A clinical and histopathological study of 379 patients. Gynecol. Oncol. 1984, 17, 85–103. [Google Scholar] [CrossRef]
- Ritchie, W.W.; Messmer, J.M.; Whitley, D.P.; Gopelrud, D.R. Uterine carcinoma metastatic to the larynx. Laryngoscope 1985, 95, 97–98. [Google Scholar]
- Savage, J.; Subby, W.; Okagaki, T. Adenocarcinoma of the endometrium with trophoblastic differentiation and metastases as choriocarcinoma: A case report. Gynecol. Oncol. 1987, 26, 257–262. [Google Scholar] [CrossRef]
- McCormick, P.C.; Post, K.D.; Kandji, A.D.; Hays, A.P. Metastatic carcinoma to the pituitary gland. Br. J. Neurosurg. 1989, 3, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Inagaki, M.; Ozaki, M.; Yamasaki, M.; Nakagawa, H.; Inoue, T.; Terada, N.; Wada, A. Long-term survival after brain metastasis from endometrial cancer. Jpn. J. Clin. Oncol. 1990, 20, 312–315. [Google Scholar] [PubMed]
- Brezinka, C.; Fend, F.; Huter, O.; Plattner, A. Cerebral metastasis of endometrial carcinoma. Gynecol. Oncol. 1990, 38, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Tress, B.; Chambers, D. Endometrial adenocarcinoma presenting as pituitary apoplexy. Aust. New Zealand J. Med. 1990, 20, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kottke-Marchant, K.; Estes, M.L.; Nunez, C. Early brain metastases in endometrial carcinoma. Gynecol. Oncol. 1991, 41, 67–73. [Google Scholar] [CrossRef] [PubMed]
- De Porre, P.M.; Subandono Tjokrowardojo, A.J. Brain metastases of endometrial carcinoma. Case report and review of literature. Strahlenther. Onkol. 1992, 168, 100–101. [Google Scholar] [PubMed]
- Thomas, H.; Lambert, H.E. Solitary cerebral metastases from gynaecological malignancy: The case for radical therapy. Clin. Oncol. 1992, 4, 133–134. [Google Scholar] [CrossRef]
- Wroński, M.; Zakowski, M.; Arbit, E.; Hoskins, W.J.; Galicich, J.H. Endometrial cancer metastasis to brain: Report of two cases and a review of the literature. Surg. Neurol. 1993, 39, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.B.; Ironside, J.W. Cerebral metastasis from a malignant mixed Mūllerian tumour of the uterus. Histopathology 1993, 23, 277–279. [Google Scholar] [CrossRef]
- Ruelle, A.; Zuccarello, M.; Andrioli, G. Brain metastasis from endometrial carcinoma. Rep. Two Cases. Neurosurg. Rev. 1994, 17, 83–87. [Google Scholar]
- Cormio, G.; Lissoni, A.; Losa, G.; Zanetta, G.; Pellegrino, A.; Mangioni, C. Brain metastases from endometrial carcinoma. Gynecol. Oncol. 1996, 61, 403. [Google Scholar] [CrossRef] [PubMed]
- De Witte, O.; Lefranc, F.; Salmon, I.; Violon, P.; Brotchi, J. Métastases cérébrales d’origine gynécologique [Cerebral metastases of gynecological origin]. Neurochirurgie 1996, 42, 216–220. [Google Scholar] [PubMed]
- Salvati, M.; Cervoni, L.; Raguso, M. Therapeutic observations of solitary cerebral metastases due to endometrial carcinoma. Minerva Ginecol. 1998, 50, 445–447. [Google Scholar] [PubMed]
- Martińez-Manãs, R.M.; Brell, M.; Rumià, J.; Ferrer, E. Brain Metastases in endometrial carcinoma. Gynecol. Oncology. 1998, 70, 282–284. [Google Scholar] [CrossRef]
- Ogawa, K.; Tomita, T.; Kakinohana, Y.; Kamata, M.; Moromizato, H.; Nagai, Y.; Higashi, M.; Kanazawa, K.; Yoshii, Y. Palliative radiation therapy for brain metastases from endometrial carcinoma: Report of two cases. Jpn. J. Clin. Oncol. 1999, 29, 498–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crispino, M.; Tira, A.; Volpi, D.; Olivetti, L. Solitary cerebral metastasis of endometrial carcinoma. La Radiol. Medica 2000, 100, 515–517. [Google Scholar]
- Petru, E.; Lax, S.; Kurschel, S.; Gücer, F.; Sutter, B. Long-term survival in a patient with brain metastases preceding the diagnosis of endometrial cancer. Report of two cases and review of the literature. J. Neurosurg. 2001, 94, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud-Ahmed, A.S.; Suh, J.H.; Barnett, G.H.; Webster, K.D.; Belinson, J.L.; Kennedy, A.W. The effect of radiation therapy on brain metastases from endometrial carcinoma: A retrospective study. Gynecol. Oncol. 2001, 83, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sewak, S.; Muggia, F.M.; Zagzag, D. Endometrial carcinoma with cerebellar metastasis: A case report and review of the literature. J. Neurooncol. 2002, 58, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, S.; Ohara, M.; Itoh, K.; Shiozawa, T.; Konishi, I. Successful treatment with stereotactic radiosurgery for brain metastases of endometrial carcinoma: A case report and review of the literature. Int. J. Gynecol. Cancer. 2003, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gien, L.T.; Kwon, J.S.; D’Souza, D.P.; Radwan, J.S.; Hammond, J.A.; Sugimoto, A.K.; Carey, M.S. Brain metastases from endometrial carcinoma: A retrospective study. Gynecol. Oncol. 2004, 93, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.S.; Borowsky, M.E.; Lee, Y.C.; Rao, C. Abulafia O. Prolonged survival in recurrent endometrial carcinoma to the brain. Gynecol. Oncol. 2004, 95, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Salvati, M.; Caroli, E.; Orlando, E.R.; Nardone, A.; Frati, A.; Innocenzi, G.; Giangaspero, F. Solitary brain metastases from uterus carcinoma: Report of three cases. J. Neurooncol. 2004, 66, 175–178. [Google Scholar] [CrossRef] [PubMed]
- N’Kanza, A.L.; Jobanputra, S.; Farmer, P.; Lovecchio, J.; Yelon, J.A.; Rudloff, U. Central nervous system involvement from malignant mixed Müllerian tumor (MMMT) of the uterus. Arch Gynecol. Obstet. 2005, 273, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Yoshida, M.; Kimura, M.; Kinoshita, K. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int. J. Gynecol. Cancer. 2005, 15, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Dietrich III, C.S.; Modesitt, S.C.; DePriest, P.D.; Ueland, F.R.; Wilder, J.; Reedy, M.B.; Pavlik, E.J.; Kryscio, R.; Cibull, M.; Giesler, J.; et al. The efficacy of adjuvant platinum-based chemotherapy in Stage I uterine papillary serous carcinoma (UPSC). Gynecol. Oncol. 2005, 99, 557–563. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, C.H.; Chow, S.N. Brain metastases from early-stage endometrial carcinoma 8 years after primary treatment: Case report and review of the literature. Acta Obs. Gynecol. Scand. 2006, 85, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Llaneza-Coto, A.P.; Seco-Navedo, M.; Fernandez-García, T.; Redondo-Onia, M. Brain metastases of endometrial carcinoma in a young woman. Prog. De Obstet. Y Ginecol. 2006, 49, 82–84. [Google Scholar] [CrossRef]
- Chura, J.C.; Marushin, R.; Boyd, A.; Ghebre, R.; Geller, M.A.; Argenta, P.A. Multimodal therapy improves survival in patients with CNS metastasis from uterine cancer: A retrospective analysis and literature review. Gynecol. Oncol. 2007, 107, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Orrù, S.; Lay, G.; Dessì, M.; Murtas, R.; Deidda, M.A.; Amichetti, M. Brain metastases from endometrial carcinoma: Report of three cases and review of the literature. Tumori J. 2007, 93, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, S.A.; Houghton, S.L.; Meroni, R.; Rockall, A.G.; Blake, P.; Reznek, R.H. Recurrent endometrial cancer: Patterns of recurrent disease and assessment of prognosis. Clin. Radiol. 2007, 62, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Kondziolka, D.; Mongia, S.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Management of brain metastases from ovarian and endometrial carcinoma with stereotactic radiosurgery. Cancer 2008, 113, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Reyns, N.; Pasquier, D. Blond S. Bilateral thalamic metastases in endometrial adenocarcinoma. Eur. Neurol. 2008, 59, 330. [Google Scholar] [CrossRef] [PubMed]
- Al-Mujaini, A.; Gans, M.; Deschênes, J. Cortical visual loss consequent to brain metastases from an endometrial carcinoma. Can J. Ophthalmol. 2008, 43, 486. [Google Scholar] [CrossRef] [PubMed]
- Asensio, N.; Luis, A.; Costa, I.; Oliveira, J.; Vaz, F. Meningeal carcinomatosis and uterine carcinoma: Three different clinical settings and review of the literature. Int. J. Gynecol. Cancer 2009, 19, 168–172. [Google Scholar] [CrossRef]
- Srikantia, N.; Rekha, B.; Rajeev, A.G.; Kalyan, S.N. Endometrioid endometrial adenocarcinoma in a premenopausal woman with multiple organ metastases. Indian J. Med. Paediatr. Oncol. 2009, 30, 80–83. [Google Scholar] [CrossRef][Green Version]
- Blecharz, P.; Urbański, K.; Mucha-Małecka, A.; Małecki, K.; Reinfuss, M.; Jakubowicz, J.; Skotnicki, P. Hematogenous metastases in patients with Stage I or II endometrial carcinoma. Strahlenther. Onkol. 2011, 187, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.D.; Dedes, K.J.; Sandhu, S.; Frentzas, S.; Kristeleit, R.; Ashworth, A.; Poole, C.J.; Weigelt, B.; Kaye, S.B.; L Rhoda Molife, L.R. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat. Rev. Clin. Oncol. 2011, 8, 302–306. [Google Scholar] [CrossRef]
- Yamashita, S.; Fukuda, T.; Shimizu, T.; Tanaka, M. Intracranial hemorrhage from undiagnosed metastatic brain tumor during general anesthesia. J. Clin. Anesth. 2011, 23, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.Y.; Bauer, D.F.; Shannon, C.N.; Fiveash, J.; Markert, J.M. Stereotactic radiosurgical treatment of brain metastasis of primary tumors that rarelyme astasize to the central nervous system. J. Neurooncol. 2012, 109, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Cabuk-Comert, E.; Bildaci, T.B.; Kisa-Karakaya, B.; Tarhan, N.Ç.; Özen, Ö.; Gülşen, S.; Dursun, P.; Ayhan, A. Outcomes in 12 gynecologic cancer patients with brain metastasis: A single center’s experience. Turk J. Med. Sci. 2012, 42, 385–394. [Google Scholar] [CrossRef]
- Talwar, S.; Cohen, S. Her-2 targeting in uterine papillary serous carcinoma. Gynecol. Oncol. Case Rep. 2012, 2, 94–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berretta, R.; Patrelli, T.S.; Faioli, R.; Mautone, D.; Gizzo, S.; Mezzogiorno, A.; Giordano, G.; Modena, A.B. Dedifferentiated endometrial cancer: An atypical case diagnosed from cerebellar and adrenal metastasis: Case presentation and review of literature. Int. J. Clin. Exp. Pathol. 2013, 6, 1652–1657. [Google Scholar] [PubMed] [PubMed Central]
- Gulsen, S.; Terzi, A. Multiple brain metastases in a patient with uterine papillary serous adenocarcinoma: Treatment options for this rarely seen metastatic brain tumor. Surg. Neurol. Int. 2013, 4, 111. [Google Scholar] [CrossRef]
- Bergamini, A.; Rabaiotti, E.; De Marzi, P.; Ferrari, M.; Petrone, M.; Viganò, R.; Mangili, G. Brain metastases from early-stage endometrial carcinoma: A challenging issue. Cancer Break. News 2014, 2, 15–17. [Google Scholar]
- Nassir, M.; Roth, A.; Gasimli, K.; Braicu, E.I.; Fotopoulou, C.; Mawrin, C.; Badakhshi, H.; Warnke, J.P.; Sehouli, J. Is endometrial cancer really a neurophobic tumor? A case report and review of the literature. Anticancer. Res. 2014, 34, 249–257. [Google Scholar] [PubMed]
- Shepard, M.J.; Fezeu, F.; Lee, C.C.; Sheehan, J.P. Gamma knife radiosurgery for the treatment of gynecologic malignancies metastasizing to the brain: Clinical article. J. Neurooncol. 2014, 120, 515–522. [Google Scholar] [CrossRef]
- Gressel, G.M.; Lundsberg, L.S.; Altwerger, G.; Katchi, T.; Azodi, M.; Schwartz, P.E.; Ratner, E.S.; Damast, S. Factors Predictive of Improved Survival in Patients With Brain Metastases From Gynecologic Cancer: A Single Institution Retrospective Study of 47 Cases and Review of the Literature. Int. J. Gynecol. Cancer. 2015, 25, 1711–1716. [Google Scholar] [CrossRef]
- Kim, Y.Z.; Kwon, J.H.; Lim, S. A clinical analysis of brain metastasis in gynecologic cancer: A retrospective multi-institute analysis. J. Korean Med. Sci. 2015, 30, 66–73. [Google Scholar] [CrossRef]
- Kouhen, F.; Afif, M.; Kabous, M.E.; Raiss, F.; Benhmidou, N.; Majjaoui, S.; Elkacemi, H.; Kebdani, T.; Benjaaafar, N. Métastasese cérébrale d’un cancer de l’endomètre: À propos d’un cas et une revue de la littérature. Pan Afr. Med. J. 2015, 20. [Google Scholar] [CrossRef]
- Narisimhulu, D.M.; Khulpateea, N.; Meritz, K.; Xu, Y. Brain metastasis in two patients with stage IA papillary serous carcinoma of the uterus. Gynecol. Oncol. Rep. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Sierra, T.; Nguyen, L.; Mascitelli, J.; Kalir, T.; Fishman, D. Brain metastasis from uterine serous carcinoma: A case report and review of literature. Gynecol. Oncol. Rep. 2015, 11, 34–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uccella, S.; Morris, J.M.; Multinu, F.; Cliby, W.A.; Podratz, K.C.; Gostout, B.S.; Dowdy, S.C.; Ghezzi, F.; Makdisi, P.B.; Keeney, G.L.; et al. Primary brain metastases of endometrial cancer: A report of 18 cases and review of the literature. Gynecol. Oncol. 2016, 142, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Kim, J.H.; Lee, D.H.; Cho, Y.H.; Kwon, D.H.; Roh, S.W. Clinical Outcomes of Gamma Knife Radiosurgery for Metastatic Brain Tumors from Gynecologic Cancer: Prognostic Factors in Local Treatment Failure and Survival. J. Korean Neurosurg. Soc. 2016, 59, 392–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Matsunaga, S.; Shuto, T.; Sato, M. Gamma Knife Surgery for Metastatic Brain Tumors from Gynecologic Cancer. World Neurosurg. 2016, 89, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Divine, L.M.; Kizer, N.T.; Hagemann, A.R.; Pittman, M.E.; Chen, L.; Powell, M.A.; Mutch, D.G.; Rader, J.S.; Thaker, P.H. Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol. Oncol. 2016, 142, 76–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawada, M.; Matsuzaki, S.; Yoshino, K.; Ueda, Y.; Yoshida, S.; Kimura, T.; Ogita, K. Long-term survival in small-cell carcinoma of the endometrium with liver and brain metastases. Anticancer. Drugs 2016, 27, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Ismail, R.; Potrebko, P.S.; Pepe, J.; Wu, M.; Saigal, K.; Biagioli, M.; Shridhar, R.; Holloway, R.; Field, M.; et al. Role of Gamma Knife® Radiosurgery for the Treatment of Brain Metastases from Gynecological Cancers. Cureus 2016, 8, e947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilani, M.A.; Williams, N.L.; Giordano, C.; Rosenblum, N.; Shi, W.; Anne, P.; Schilder, R.J. Brain Metastases in Patients with Gynecologic Cancers: A Single Institution Experience and Review of the Literature. Open J. Obstet. Gynecol. 2016, 6, 544–552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Könnecke, H.K.; Rushing, E.J.; Neidert, M.C.; Reimann, R.; Regli, L.; Bozinov, O.; Burkhardt, J.K. Heterogeneous Appearance of Central Nervous System Involvement in Malignant Mixed Müllerian Tumors. J. Neurol. Surg. A Cent Eur. Neurosurg. 2016, 77, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Todo, Y.; Furuta, Y.; Okamoto, K.; Minobe, S.; Yamashiro, K.; Kato, H. Prognostic factors for patients with brain metastasis from gynecological cancer: A significance of treatment-free interval of more than 6 months. Jpn. J. Clin. Oncol. 2017, 47, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kimyon, G.; Turan, T.; Basaran, D.; Turkmen, O.; Karalok, A.; Tasci, T.; Tulunay, G.; Kose, M.F. Is neurosurgery with adjuvant radiotherapy an effective treatment modality in isolated brain involvement from endometrial cancer? From case report to analysis. Int. J. Gynecol. Cancer 2017, 27, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Tsuji, K.; Shigeta, S.; Tokunaga, H.; Ito, K.; Watanabe, Y.; Yoshinaga, K.; Otsuki, T.; Niikura, H.; Yaegashi, N. Leptomeningeal metastasis from gynecologic cancers diagnosed by brain MRI. Clin. Imaging. 2017, 41, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kasper, E.; Ippen, F.; Wong, E.; Uhlmann, E.; Floyd, S.; Mahadevan, A. Stereotactic radiosurgery for brain metastasis from gynecological malignancies. Oncol. Lett. 2017, 13, 1525–1528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Healy, V.; O’Halloran, P.; O’Brien, S.; Beausang, A.; Caird, J. CNS metastasis secondary to malignant-mixed Müllerian tumor: Case report and review of therapeutics. CNS Oncol. 2017, 6, 315–323. [Google Scholar] [CrossRef]
- Johnston, H.; McTyre, E.R.; Cramer, C.K.; Lesser, G.J.; Ruiz, J.; Bourland, J.D.; Watabe, K.; Lo, H.W.; Qasem, S.; Laxton, A.W.; et al. Stereotactic radiosurgery in the treatment of brain metastases from gynecologic primary cancer. J. Radiosurgery SBRT 2017, 5, 55–61. [Google Scholar] [PubMed] [PubMed Central]
- Stamates, M.M.; Lee, J.M.; Merrell, R.T.; Shinners, M.J.; Wong, R.H. Combined Open and Endoscopic Endonasal Skull Base Resection of a Rare Endometrial Carcinoma Metastasis. J. Neurol. Surg. Rep. 2018, 79, e9–e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvatore, B.; D’Amico, D.; Fonti, R. Metastasis to the Sellar/Suprasellar Region in a Patient With Endometrial Carcinoma Detected by 18F-FDG PET/CT. Clin. Nucl. Med. 2018, 43, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, P.; Stasenko, M.; Alter, R.; Makker, V.; Cadoo, K.A.; Sonoda, Y.; Abu-Rustum, N.R.; Mueller, J.J.; Leitao, M.M.; Sonoda, Y.; et al. Brain metastases in patients with low-grade endometrial carcinoma. Gynecol. Oncol. Rep. 2018, 26, 87–90. [Google Scholar] [CrossRef]
- Eulálio Filho, W.M.N.; Fé, T.S.M.; Rodrigues, R.M.M.; Lima, M.S.O.; Vieira, S.C. Brain Metastasis in Papillary Serous Adenocarcinoma of the Endometrium. Rev. Bras. de Hematol. e Hemoter. 2019, 41, 264–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Grant, M.S.; Stepp, W.H.; Clark, L.H. Clinical characteristics of CNS metastases from primary gynecologic cancers. Gynecol. Oncol. Rep. 2019, 30, 100518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moroney, M.R.; Wheeler, L.J.; Corr, B.R. Clinical presentation of brain metastases from endometrial carcinoma: A case series. Gynecol. Oncol. Rep. 2019, 28, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shao, Y.; Xu, H.; Chen, J. Brain metastasis from early stage endometrial carcinoma 13 years after primary treatment: A case report and review of the literature, Int. J. Clin. Exp. Pathol. 2019, 12, 1806–1810. [Google Scholar]
- Katiyar, V.; Araujo, T.; Farooq, M.Z.; Vohra, I.; Gupta, S. Brain Metastasis in a Young Patient with Uterine Carcinosarcoma. Cureus 2019, 11, e5010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, H.; Jia, A.; Ren, Y.; Gu, M.; Li, H.; Sun, M.; Tang, T.; Liu, H.; Jie Bai, J. Endometrial adenocarcinoma metastatic to the pituitary gland: A case report and literature review. J. Int. Med. Res. 2020, 48, 0300060520924512. [Google Scholar] [CrossRef]
- Ogino, A.; Hirai, T.; Serizawa, T.; Yoshino, A. Gamma Knife Surgery for Brain Metastases from Uterine Malignant Tumor. World Neurosurg. 2020, 139, e363–e372. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cui, X.; Zhang, X.; Qian, H.; Duan, H.; Zhang, Y. The Clinical Characteristics of Endometrial Cancer With Extraperitoneal Metastasis and the Value of Surgery in Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820945784. [Google Scholar] [CrossRef]
- Nasioudis, D.; Persaud, A.; Taunk, N.K.; Latif, N.A. Brain Metastases From Gynecologic Malignancies: Prevalence and Management. Am. J. Clin. Oncol. 2020, 43, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Bhambhvani, H.P.; Zhou, O.; Cattle, C.; Taiwo, R.; Diver, E.; Hayden Gephart, M. Brain Metastases from Endometrial Cancer: Clinical Characteristics, Outcomes, and Review of the Literature. World Neurosurg. 2021, 147, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Beucler, N.; Sellier, A.; Bernard, C.; Joubert, C.; Desse, N.; Dagain, A. Brain metastases in endometrial cancer: A systematic review of the surgical prognostic factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, F.; Gao, H.; Xu, Y. Successful treatment of a patient with brain metastases from endometrial cancer using Niraparib: A case report. Ann. Palliat. Med. 2021, 10, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.M.; D’Andrea, M.R.; Tomita, S.; Suhner, J.; Umphlett, M.; Zakashansky, K.; Blank, S.V.; Tsankova, N.; Shrivastava, R.K.; Fowkes, M.; et al. Tumor immune microenvironment in brain metastases from gynecologic malignancies. Cancer Immunol. Immunother. 2021, 70, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.O.A.; Foley, O.; Chapel, D.; Da Silva, A.; Nucci, M.; Muto, M.G.; Campos, S. Next- Generation Sequencing in the Diagnosis of Metastatic Lesions: Reclassification of a Glioblastoma as an Endometrial Cancer Metastasis to the Brain. Oncologist 2021, 26, e2102–e2109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crain, C.N.; Ngwanyam, R.; Punch, G. Rare brain metastasis with unusual characteristics in a late recurrence of stage IIIA uterine papillary serouscarcinoma. BJR Case Rep. 2021, 7, 20200157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karpathiou, G.; Camy, F.; Chauleur, C.; Dridi, M.; Dal Col, P.; Peoc’h, M. Brain Metastases from Gynecologic Malignancies. Medicina 2022, 58, 548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Li, R.; Zhang, S.; Xu, X.; Liao, L.; Yang, Y.; Guo, Y. Analysis of prognostic factors of metastatic endometrial cancer based on surveillance, epidemiology, and end results database. Front. Surg. 2023, 9, 1001791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Z.; Luy, D.D.; Tang, L.W.; Deng, H.; Jose, S.; Scanlon, S.; Niranjan, A.; Lunsford, L.D. Gamma Knife radiosurgery for gynecologic metastases to the brain: Analysis of pathology, survival, and tumor control. Gynecol. Oncol. 2023, 172, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Shuto, T.; Serizawa, T.; Aoyagi, K.; Hasegawa, T.; Kawagishi, J.; Yomo, S.; Kenai, H.; Nakazaki, K.; Moriki, A.; et al. Gamma Knife Radiosurgery for Metastatic Brain Tumors from Uterine Cervical and Endometrial Carcinomas: Histopathological Analysis of Survival and Local Control. A Japanese Multi-Institutional Cooperative and Retrospective Cohort Study. Word Neurosurg. 2023, 171, e572–e580. [Google Scholar] [CrossRef]
- Butorac, D.; Potkonjak, A.M.; Kuharić, J.; Vujić, G. Brain metastasis as a first clinical presentation of endometrial cancer: A case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 296, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Shelvin, K.B.; Vincent, J.; Morron, S.; Morin, M.; Mammoser, A.; Nair, N. Recurrent high grade serous endometrial cancer with brain metastases: Immunotherapy confers pembro Lenvatinib improved quality of life and survival. Gynecol. Oncol. Rep. 2024, 55, 101494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paget, S. The distribution of secondary gro wths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2020, 9, 628–645. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Mar. Sci. 2015, 66, 97–110 . [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Yu, D. Brain Metastasis Organotropism. Cold Spring Harb. Perspect. Med. 2019, 10, a037242. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of microsatellite instability across 39 cancer types. JCO Precis. Oncol. 2017, 2017, PO.17.00073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levine, D.A. The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; TransPORTEC Consortium; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashley, C.W.; Paula, A.D.C.; Kumar, R.; Mandelker, D.; Pei, X.; Riaz, N.; Reis-Filho, J.S.; Weigelt, B. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol. Oncol. 2019, 152, 11–19. [Google Scholar] [CrossRef]
- Flamini, M.; Sanchez, A.; Goglia, L.; Tosi, V.; Genazzani, A.; Simoncini, T. Differential actions of estrogen and SERMs in regulation of the actin cytoskeleton of endometrial cells. Mol. Hum. Reprod. 2009, 15, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Agacayak, E.; Keles, A.; Deger, U.; Ozcelik, M.S.; Peker, N.; Gunduz, R.; Akkus, M.; Buyukbayram, H. Could Moesin Be a New Marker for Indicating Progression in Endometrial Cancer? Cancer Manag. Res. 2022, 14, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Xiao, X.; Xu, J.; Liu, M.; Lu, Y.; Liu, S.; Dong, X. 17β-Estradiol promotes endometrial cancer proliferation and invasion through IL-6 pathway. Endocr. Connect. 2019, 8, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Atıcı, Ö.K.; Govindrajan, N.; Lopetegui-González, I.; Shemanko, C.S. Prolactin: A hormone with diverse functions from mammary gland development to cancer metastasis. Semin. Cell Dev. Biol. 2021, 114, 159–170. [Google Scholar]
- Ding, K.; Yuan, Y.; Chong, Q.-Y.; Yang, Y.; Li, R.; Li, X.; Kong, X.; Qian, P.; Xiong, Z.; Pandey, V.; et al. Autocrine Prolactin Stimulates Endometrial Carcinoma Growth and Metastasis and Reduces Sensitivity to Chemotherapy. Endocrinology 2017, 158, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.; Kajihara, T.; Mizuno, Y.; Mizuno, Y.; Tochigi, H.; Ishihara, O. Endometrial microRNAs and their aberrant expression patterns. Med. Mol. Morphol. 2020, 53, 131–140. [Google Scholar] [CrossRef]
- van den Bent, M.J. The diagnosis and management of brain metastases. Curr. Opin. Neurol. 2001, 14, 717–723. [Google Scholar] [CrossRef]
- Langer, C.J.; Mehta, M.P. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 2005, 23, 6207–6219. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Press, R.H.; Patel, K.R.; Boselli, D.M.; Symanowski, J.T.; Lankford, S.P.; McCammon, R.J.; Moeller, B.J.; Heinzerling, J.H.; Fasola, C.E.; et al. Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: Amulti-institutional analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 459–467. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Postoperative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.G.; Wronski, M.; Galicich, J.; Arbit, E.; Leibel, S.A.; Burt, M. Postoperative radiation for lung cancer metastatic to the brain. J. Clin. Oncol. 1994, 12, 2340–2344. [Google Scholar] [CrossRef]
- Hagen, N.A.; Cirrincione, C.; Thaler, H.T.; DeAngelis, L.M. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology 1990, 40, 158–160. [Google Scholar] [CrossRef]
- Flickinger, J.C.; Lunsford, L.D.; Kondziolka, D. Dose prescription and dose-volume effects in radiosurgery. Neurosurg. Clin. N Am. 1992, 3, 51–59. [Google Scholar] [CrossRef]
- Higuchi, Y.; Matsuda, S.; Serizawa, T. Gamma knife radiosurgery in movement disorders: Indications and limitations. Mov. Disord. 2017, 32, 28–35. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verhaa Verhaak, E.; Gehring, K.; Hanssens, P.E.J.; Aaronson, N.K.; Sitskoorn, M.M. Health-related quality of life in adult patients with brain metastases after stereotactic radiosurgery: A systematic, narrative review. Support Care Cancer 2020, 28, 473–484. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Celik, A.; Weber, D.; Schmitt, D.; Lang, K.; König, L.; Bernhardt, D.; Höne, S.; Forster, T.; von Nettelbladt, B.; et al. A matched-pair analysis comparing stereotactic radiosurgery with whole-brain radiotherapy for patients with multiple brain metastases. J. Neurooncol. 2020, 147, 607–618. [Google Scholar] [CrossRef]
- Gatterbauer, B.; Hirschmann, D.; Eberherr, N.; Untersteiner, H.; Cho, A.; Shaltout, A.; Göbl, P.; Fitschek, F.; Dorfer, C.; Wolfsberger, S.; et al. Toxicity and efficacy of Gamma Knife radiosurgery for brain metastases inmelanoma pati ents treated with immunotherapy or targeted therapy-a retrospective cohort study. Cancer Med. 2020, 9, 4026–4036. [Google Scholar] [CrossRef]
- Oleinika, K.; Nibbs, R.J.; Graham, G.J.; Fraser, A.R. Suppression, subversion and escape: The role of regulatory T cells in cancer progression. Clin. Exp. Immunol. 2013, 171, 36–45. [Google Scholar] [CrossRef]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Aili, A.; Xue, L.; Jiang, P.; Wang, J. Advances in radiobiology of stereotactic ablative radiotherapy. Front. Oncol. 2020, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, J.F.; Larsson, M.; Bhardwaj, N. Interactions between dead cells and dendritic cells in the induction of antiviral CTL responses. Curr. Opin. Immunol. 2002, 14, 471–477. [Google Scholar] [CrossRef]
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen specific effector cells that traffic to the tumor. J. Immunol. 2005, 174, 7516–7523. [Google Scholar] [CrossRef]
- Fleming, G.F.; Brunetto, V.L.; Cella, D.; Look, K.Y.; Reid, G.C.; Munkarah, A.R.; Kline, R.; Burger, R.A.; Goodman, A.; Burks, R.T. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2004, 22, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; Di Silvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eskander, R.N.; Michael, W.; Sill, M.W.; Lindsey Beffa, L.; Richard, G.; Moore, R.G.; Joanie, M.; Hope, J.M.; Musa, F.B.; Mannel, R.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; de Pont Christensen, R.; Zoltán Novák, Z.; Destin Black, D.; Lucy Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Colombo, N.; Biagioli, E.; Harano, K.; Galli, F.; Hudson, E.; Antill, Y.; Choi, C.H.; Rabaglio, M.; Marmé, F.; Marth, C.; et al. Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Tillmanns, T.; Masri, A.; Stewart, C.; Chase, D.; Karnezis, A.; Chen, L.M.; Urban, R. Advanced endometrial cancer-The next generation of treatment: A society of gynecologic oncology journal club clinical commentary. Gynecol. Oncol. Rep. 2024, 55, 101462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer. 2022, 10, e003777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, L.J.; Linley, A.; Hammond, D.E.; Hood, F.E.; Coulson, J.M.; MacEwan, D.J.; Ross, S.J.; Slupsky, J.R.; Smith, P.D.; Eyers, P.A.; et al. New perspectives, opportunities, and challenges in exploring the human protein kinome. Cancer Res. 2018, 78, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kodama, K.; Takase, K.; Sugi, N.H.; Yamamoto, Y.; Iwata, M.; Tsuruoka, A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013, 340, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutt, A.; Salvesen, H.B.; Chen, T.H.; Ramos, A.H.; Onofrio, R.C.; Hatton, C.; Nicoletti, R.; Winckler, W.; Grewal, R.; Hanna, M.; et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 8713–8717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byron, S.A.; Gartside, M.G.; Wellens, C.L.; Mallon, M.A.; Keenan, J.B.; Powell, M.A.; Goodfellow, P.J.; Pollock, P.M. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008, 68, 6902–6907. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; Di Simone, C.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Casado Herraez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Study 309–KEYNOTE-775 Investigators. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N. Outcomes by histology and prior therapy with lenvatinib plus pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer (Study 309/KEYNOTE-775). Ann. Oncol. 2021, 32, S729–S730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).