Simple Summary
Fusobacterium nucleatum, a gram-negative anaerobic bacterium, plays a pivotal role in colorectal cancer (CRC) pathogenesis. It induces chronic inflammation via cytokines such as IL-1β, IL-6, and TNF-α, fostering tumor progression. Through adhesins like FadA, F. nucleatum disrupts cell junctions and promotes epithelial-to-mesenchymal transition (EMT). The bacterium suppresses immune responses, exacerbates gut dysbiosis, and activates oncogenic pathways, notably Wnt/β-catenin signaling. It also inflicts DNA damage directly through reactive oxygen species or indirectly via inflammation. By altering the tumor microenvironment, F. nucleatum impacts metastasis and therapy outcomes. Understanding these mechanisms is essential for advancing CRC therapies and diagnostics.
Abstract
Fusobacterium nucleatum, a gram-negative anaerobic bacterium, has emerged as a significant player in colorectal cancer (CRC) pathogenesis. The bacterium causes a persistent inflammatory reaction in the colorectal mucosa by stimulating the release of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, creating an environment conducive to cancer progression. F. nucleatum binds to and penetrates epithelial cells through adhesins such as FadA, impairing cell junctions and encouraging epithelial-to-mesenchymal transition (EMT), which is associated with cancer advancement. Additionally, the bacterium modulates the host immune system, suppressing immune cell activity and creating conditions favorable for tumor growth. Its interactions with the gut microbiome contribute to dysbiosis, further influencing carcinogenic pathways. Evidence indicates that F. nucleatum can inflict DNA damage either directly via reactive oxygen species or indirectly by creating a pro-inflammatory environment. Additionally, it triggers oncogenic pathways, especially the Wnt/β-catenin signaling pathway, which promotes tumor cell growth and longevity. Moreover, F. nucleatum alters the tumor microenvironment, impacting cancer cell behavior, metastasis, and therapeutic responses. The purpose of this review is to elucidate the molecular mechanisms by which F. nucleatum contributes to CRC. Understanding these mechanisms is crucial for the development of targeted therapies and diagnostic strategies for CRC associated with F. nucleatum.
1. Introduction
Fusobacterium nucleatum (F. nucleatum), a Gram-negative, obligate anaerobic bacterium, has garnered significant attention for its pivotal role in the development and progression of colorectal cancer (CRC) []. Initially recognized as a common resident of the human oral cavity, F. nucleatum has since been implicated in various pathological processes associated with CRC, including tumorigenesis, metastasis, and resistance to therapy [,]. Its pro-carcinogenic effects are primarily linked to its interactions with host cells and its ability to modulate the immune microenvironment, positioning it as a promising biomarker and therapeutic target for CRC [,].
The accumulation of F. nucleatum has been linked to the advancement, growth, and unfavorable outcomes of CRC. Globally, CRC is the third most common malignancy and ranks fourth among causes of cancer-related mortality. Projections indicate a 60% increase in CRC diagnoses by 2030 [,,]. Recent studies suggest a significant connection between gut microbial imbalances and the development of CRC [,,]. In particular, F. nucleatum has been found to be more prevalent in CRC tissues compared to normal tissues nearby [,]. Additionally, molecular features like the CpG island methylator phenotype, microsatellite instability, and a lower density of CD3+ T-cells have been linked to increased levels of F. nucleatum in CRC samples [,].
Surgical intervention is the primary treatment for early stage CRC, while adjuvant options like chemotherapy and targeted therapies are essential for advanced stages. Nevertheless, resistance to chemotherapy remains a major obstacle, driven by factors such as genetic mutations, epigenetic alterations, and changes in the tumor microenvironment [,,]. Recent findings suggest that F. nucleatum contributes to chemoresistance by modifying the tumor microenvironment and regulating the expression of genes critical to drug response [,].
The literature presents different data, as some studies have indicated that elevated levels of F. nucleatum correlate with reduced survival in over 1000 CRC patients, while Oh et al. highlighted the prognostic role of F. nucleatum in individuals undergoing adjuvant chemotherapy [,]. These discrepancies underscore the necessity for in-depth research into the associations between F. nucleatum and various CRC subtypes.
This review aims to provide a comprehensive analysis of the molecular mechanisms through which F. nucleatum contributes to CRC. A deeper understanding of these mechanisms is essential for developing targeted therapeutic and diagnostic strategies to address CRC linked to F. nucleatum.
2. The Pro-Tumorigenic Role of Fusobacterium nucleatum in Colorectal Cancer: Mechanisms of Adhesion, Signaling, and Epigenetic Alteration
The pro-tumorigenic effects of F. nucleatum are primarily mediated by its adhesins, FadA and Fap2, which enable the bacterium to adhere to and invade human epithelial and endothelial cells [,].
The FadA protein binds to E-cadherin in epithelial cells and VE-cadherin in endothelial cells, two key cell-junction molecules []. FadA’s interaction with VE-cadherin disrupts endothelial cell-cell junctions, increasing vascular permeability and facilitating the hematogenous spread of F. nucleatum to distant sites. In parallel, Fap2 acts as an autotransporter, promoting bacterial adhesion through recognition of overexpressed Gal-GalNAc on tumor epithelial cells [,].
Through these molecular interactions, F. nucleatum triggers intracellular signaling cascades that promote cancer cell survival and proliferation.
A key pathway influenced by FadA is the WNT/β-catenin signaling pathway, which governs cell growth, differentiation, and survival. Activation of this pathway by F. nucleatum has been associated with poor outcomes in CRC patients due to its role in driving uncontrolled tumor growth and metastasis [,]. Moreover, F. nucleatum, through the disruption of the E-cadherin/β-catenin complex, drives epithelial cells toward a mesenchymal-like phenotype, thereby enhancing their invasive potential [].
Additionally, F. nucleatum modulates Annexin A1, a regulator of the WNT/β-catenin pathway and a biomarker of poor prognosis in cancer. The interaction between FadA and Annexin A1 establishes a positive feedback loop, amplifying tumorigenic signaling and further contributing to CRC progression [,,].
Recent studies have revealed the presence of a protein known as RadD, which enables F. nucleatum to influence CRC cells through a targeted interaction with the CD147 receptor [,]. This interaction notably stimulates the PI3K-AKT-NF-κB-MMP9 signaling pathway, resulting in the release of matrix metalloproteinases (MMPs) that are essential for CRC cell proliferation, migration, and invasion [,].
F. nucleatum contributes to the progression of CRC through other mechanisms involving epigenetic changes, DNA repair interference, and inflammation []. It impedes DNA repair by inhibiting NEIL2 glycosylase and disrupting the Chk2 signaling pathway, which results in DNA double-strand breaks (DSBs) and defects in repair pathways like non-homologous end joining (NHEJ) []. These alterations lead to microsatellite instability (MSI), including MSI-L or elevated microsatellite alterations at selected tetranucleotide repeats (EMAST), and facilitate the relocation of the mismatch repair protein MSH3 [,,,,,,,,,,,,,,,,,,,]. F. nucleatum is linked to the CpG island methylator phenotype (CIMP) and the hypermethylation of tumor suppressor genes (TSGs), a process driven by enhanced DNA methyltransferase activity [,]. This epigenetic alteration typically leads to microsatellite instability (MSI-H) and the silencing of mismatch repair genes, such as MLH1, often in conjunction with BRAF mutations [,].
Moreover, F. nucleatum exacerbates inflammation by inducing the production of reactive oxygen species (ROS) and inflammatory cytokines, further altering DNA methylation patterns and causing DNA damage [,]. While MSI-H CRCs are generally linked to better clinical outcomes due to immune activation, high levels of F. nucleatum are associated with poorer prognosis by intensifying inflammation [,]. Overall, F. nucleatum’s role in promoting CIMP-positive CRC and its impact on DNA repair, MSI, and inflammation underline its contribution to CRC progression and aggressiveness [].
These findings are depicted in Figure 1 and highlight F. nucleatum as a crucial risk factor for CRC progression and metastasis, as well as a potential biomarker for poor prognosis.
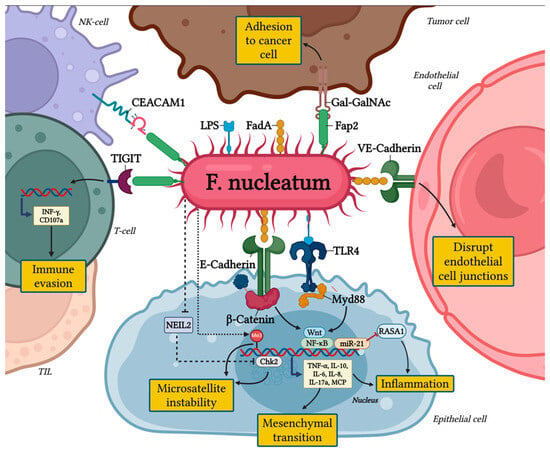
Figure 1.
The pro-tumorigenic role of Fusobacterium nucleatum in colorectal cancer.
3. Impact of Fusobacterium nucleatum on the Tumor Microenvironment
F. nucleatum not only exerts direct effects on colorectal cancer (CRC) cells but also profoundly shapes the immune microenvironment by modulating immune responses.
3.1. Disruption of Cellular Adhesion and Inflammatory Pathways
As previously discussed, F. nucleatum affects epithelial cell adhesion through its adhesin FadA, which interferes with E-cadherin, a crucial protein for maintaining cell-cell connections. This interference leads to the accumulation of β-catenin and activates β-catenin-dependent transcription (CRT), promoting pro-oncogenic pathways such as Wnt signaling and NF-κB. The activation of these pathways results in the production of pro-inflammatory cytokines, including IL-8, IL-10, IL-6, TNF-α, MCP-1, and IL-17A, which create a chronic inflammatory environment [,]. This sustained inflammation in turn promotes tumor cell proliferation, survival, and invasion, facilitating the progression of colorectal cancer (CRC) []. F. nucleatum also upregulates NF-κB activity via miR-21, a microRNA that activates Toll-like receptor 4 (TLR4) and interacts with myeloid differentiation factor 88 (MyD88) [,,]. This cascade suppresses RASA1, a RAS GTPase activator, leading to the accumulation of inflammatory mediators that further stimulate tumor cell proliferation. Experimental data suggest that miR-21 is a critical player in F. nucleatum-mediated tumor-promoting inflammation and a potential biomarker for poor CRC outcomes [,].
3.2. Immune Evasion via TIGIT and CEACAM1
F. nucleatum uses the inhibitory receptor TIGIT, which is expressed on T cells, NK cells, and tumor-infiltrating lymphocytes (TILs), to evade immune detection []. TIGIT competes with the activating receptor CD226 for binding to CD155, which results in a reduction of NK cell cytotoxicity and T cell activation, thus hindering effective anti-tumor immune responses []. Moreover, TIGIT modulates dendritic cells (DCs) by promoting an immunosuppressive environment, increasing IL-10 production while decreasing IL-12 levels []. The increased expression of TIGIT also enhances the suppressive function of regulatory T cells (Tregs), further impairing anti-tumor immunity []. Clinical evidence links high TIGIT levels with advanced CRC stages, early recurrence, and poorer survival [,]. F. nucleatum’s Fap2 protein amplifies immune evasion by binding to TIGIT, reducing NK cell cytotoxicity and inducing T cell death []. Concurrently, F. nucleatum activates CEACAM1, another inhibitory receptor on NK and T cells [,]. CEACAM1 activation contributes to T cell exhaustion, marked by diminished levels of IFN-γ and CD107a, key molecules for antitumor activity []. These pathways are critical in F. nucleatum-mediated immune suppression and tumor progression.
3.3. Recruitment and Modulation of Immunosuppressive Cells
F. nucleatum selectively recruits myeloid-derived suppressor cells (MDSCs) to the TME [,]. MDSCs inhibit T cell proliferation and induce apoptosis through high levels of inducible nitric oxide synthase (iNOS) and arginase-1 [,]. Tumor-associated macrophages (TAMs), neutrophils, and regulatory DCs, also recruited by F. nucleatum, further promote inflammation, angiogenesis, invasion, and metastasis [,]. F. nucleatum drives tumor-associated neutrophils (TANs) toward a pro-tumor N2 phenotype via TGF-β signaling. N2 TANs exacerbate tumorigenesis by producing reactive oxygen species (ROS), which cause DNA damage and enhance tumor progression [,]. Macrophages influenced by F. nucleatum via TLR4 signaling shift toward an M2-like phenotype, which aids tumor progression by dampening adaptive immune responses, encouraging angiogenesis, and facilitating tissue remodeling [,]. F. nucleatum reduces the density of CD4+ T cells in tumors compared to normal tissues, highlighting its role in suppressing T helper cell-mediated immune responses. This suppression weakens the adaptive immune response, further enabling tumor progression [,] (Table 1).

Table 1.
The impact of Fusobacterium nucleatum in recruitment and modulation of immunosuppressive cells.
4. The Role of F. nucleatum in Gut Dysbiosis and Colon Carcinogensis
The role of Fusobacterium nucleatum in intestinal dysbiosis and colon carcinogenesis is a complex phenomenon, involving molecular mechanisms that modulate gut microbial composition, inflammation, and metabolite production []. F. nucleatum has been linked to reduced bacterial diversity, disrupting the balance between beneficial bacteria, such as Lactobacillus and Bifidobacterium, and harmful or pro-inflammatory bacteria like Bacteroides fragilis and Enterococcus (Figure 2) [,]. In dysbiotic conditions, F. nucleatum disrupts the activity of beneficial microbes responsible for generating short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, which are critical for maintaining gut health [,,]. Butyrate, in particular, is essential for gut barrier integrity and energy supply to colonocytes. Its reduction, due to the suppression of key butyrate-producing bacteria like Faecalibacterium prausnitzii and Roseburia, leads to a weakened intestinal epithelium and increased gut permeability [,]. This impaired barrier function facilitates inflammation and further microbial imbalance. F. nucleatum also promotes the growth of other harmful microbes, such as pathogenic strains of Clostridia and Escherichia coli, which are less efficient in producing SCFAs and exacerbate dysbiosis [,,,]. As a result, SCFA production decreases, contributing to gut dysfunction and compromised immune regulation.
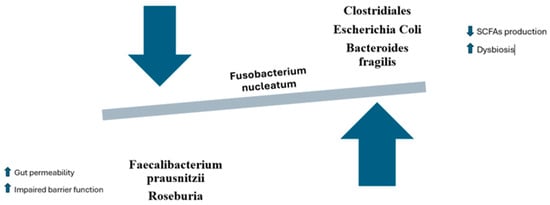
Figure 2.
Dysbiosis and modulation of the gut microbiota caused by Fusobacterium nucleatum.
F. nucleatum alters SCFA production through competition for substrates and the production of pro-inflammatory metabolites. The fermentation of complex carbohydrates like fiber by beneficial bacteria can be hindered by F. nucleatum, which preferentially ferments other molecules, reducing substrate availability for beneficial bacteria [,]. Moreover, F. nucleatum can promote the production of toxic metabolites such as sulfur compounds and amines from protein fermentation, which can damage the intestinal mucosa and induce an inflammatory state that supports carcinogenesis [,].
This reduction in SCFA production, along with the accumulation of toxic and inflammatory metabolites, directly increases the risk of colon cancer, as chronic inflammation and tissue damage are key drivers of cancer development [].
F. nucleatum can also interact with other bacterial species, encouraging the growth of pathogenic strains like Bacteroides fragilis, which is linked to an increased risk of colon cancer [,]. These interactions occur through several mechanisms, including the synergy of inflammation. B. fragilis produces a toxin that triggers the secretion of inflammatory cytokines, further promoting dysbiosis. The co-presence of F. nucleatum and B. fragilis in the gut creates an inflammatory environment that facilitates tumor progression []. Additionally, some strains of B. fragilis produce the B. fragilis toxin (BFT), which can damage the DNA of colon epithelial cells, inducing mutations that contribute to carcinogenesis [,]. This interplay between F. nucleatum and other gut bacteria plays a pivotal role in modulating the intestinal environment in ways that promote the development of colon cancer [].
5. Molecular Mechanisms of Fusibacterium nucleatum in Chemoresistance and Colorectal Cancer Progression
The effects of Fusobacterium nucleatum on the tumor microenvironment and its direct role in tumorigenesis have a significant impact on current colorectal cancer therapies [,]. As is well known, the microbiota plays a crucial role in treatment response, and there is ongoing research focused on studying the effects of Fusobacterium nucleatum abundance in this context []. Fusobacterium nucleatum induces chemoresistance through various mechanisms, which we will outline in a systematic manner below.
5.1. Inhibition of Apoptosis
Fusobacterium nucleatum releases ADP-H into the tumor microenvironment, triggering the ALPK1 (alpha kinase 1) signaling pathway, which activates TIFA (TRAF-interacting protein with FHA domain) and induces strong NF-κB activation []. This activation of the TLR4/NF-κB pathway leads to the upregulation of TNFAIP3 and Baculoviral IAP Repeat Containing 3 (BIRC3), a member of the Inhibitor of Apoptosis Proteins (IAP) family []. These proteins block caspase-mediated apoptotic processes, enabling colorectal cancer (CRC) cells to avoid cell death and decreasing their responsiveness to chemotherapeutic agents, especially 5-fluorouracil (5-FU) [] (Table 2).
5.2. Promotion of Autophagy
Fusobacterium nucleatum promotes chemoresistance in colorectal cancer (CRC) by upregulating autophagy-related proteins such as LC3-II, ULK1, and ATG7, enabling cancer cells to adapt to stress and evade chemotherapy-induced apoptosis. Additionally, it suppresses miRNAs like miR-18a and miR-4802, which typically inhibit autophagy-related genes []. This regulation is mediated through TLR4 and MyD88 signaling pathways, further enhancing chemoresistance. Studies indicate that CRC cells exposed to F. nucleatum show elevated autophagy indicators (LC3-II, Beclin1) and the metastasis marker Vimentin, with decreased levels of E-cadherin and P62. These changes are mitigated through chloroquine treatment, CARD3 knockdown, or their combination [,,]. Understanding the critical function of autophagy in F. nucleatum-associated CRC progression, a new cationic polymer (PNHCQ) has been formulated to inhibit autophagy while delivering plasmid DNA (pDNA) coding for soluble FMS-like tyrosine kinase-1 (sFlt-1), aiming to improve anti-angiogenic treatment []. This dual-action system suppresses autophagy while inducing sFlt-1-mediated anti-angiogenic effects, significantly improving therapeutic outcomes in F. nucleatum-associated CRC models [] (Table 2).
5.3. Regulation of Anoctamin-1 (ANO1)
F. nucleatum has been implicated in the onset and advancement of colorectal cancer (CRC), with studies suggesting its involvement in modulating Anoctamin-1 (ANO1) expression []. ANO1, a calcium-activated chloride channel (CaCC), plays a role in key physiological functions and is frequently overexpressed in various cancers, including CRC. Its increased activity is linked to enhanced chloride ion transport, which supports cell proliferation and migration, critical for tumor growth and metastasis []. Furthermore, ANO1’s role in regulating cell volume, ion flux, and membrane potential may influence how cells respond to inflammatory environments, potentially aiding cancer survival and progression []. Although the precise mechanisms remain unclear, the connection between Fusobacterium and ANO1 underscores its importance in CRC pathogenesis [,] (Table 2).
5.4. Other Mechanisms
F. nucleatum plays a significant role in colorectal cancer (CRC) by driving the expansion of cancer stem cells (CSCs) through the upregulation of stemness markers like CD44 and CD133 [,,]. It reshapes lipid metabolism, enhancing fatty acid oxidation in CSCs and boosting fatty acid synthesis in other cancer cells, which strengthens self-renewal and fosters resistance to chemotherapy []. Moreover, F. nucleatum influences the sonic hedgehog pathway, a critical mechanism for stem cell maintenance, via the MYC/miR-361-5p axis, further enabling the persistence and proliferation of CRC cells under treatment stress [,,,] (Table 2).

Table 2.
Molecular mechanisms and results of Fusobacterium nucleatum in cancer progression and chemoresistance.
Table 2.
Molecular mechanisms and results of Fusobacterium nucleatum in cancer progression and chemoresistance.
| Author, Year | Molecular Mechanisms Analyzed | Results |
|---|---|---|
| Martin-Gallausiaux et al., 2024 [] | Activation of ALPK1/TIFA/NF-κB signaling pathway by F. nucleatum through ADP-heptose release. | Increased expression of IL-8, BIRC3, and TNFAIP3; reduced sensitivity to 5-FU; enhanced CRC cell survival and inflammatory responses. |
| Zhang et al., 2022 [] | Induction of ALPK1/NF-κB/ICAM1 axis by F. nucleatum to enhance CRC cell adhesion and metastasis. | Promoted adhesion of CRC cells to endothelial cells, facilitated metastasis, and correlated high ICAM1 and ALPK1 expression with shorter CRC patient survival. |
| Zhang et al., 2019 [] | Modulation of BIRC3 expression via TLR4/NF-κB by F. nucleatum to induce chemoresistance to 5-FU in CRC. | High BIRC3 expression reduced CRC cell sensitivity to 5-FU. High F. nucleatum abundance correlated with chemoresistance in CRC patients undergoing 5-FU treatment. |
| Chen Y et al., 2020 [] | Regulation of CRC metastasis through F. nucleatum-mediated CARD3 activation and autophagy pathways. | F. nucleatum increased CRC cell motility and metastasis via CARD3, LC3-II, and Beclin1 upregulation; CARD3 knockdown or chloroquine treatment reduced tumor burden and metastasis. |
| Liu Y et al., 2021 [] | Induction of chemoresistance in ESCC by F. nucleatum through autophagy modulation via ATG7. | F. nucleatum promoted chemoresistance to 5-FU, CDDP, and Docetaxel. ATG7 knockdown reversed these effects. |
| Yang Y et al., 2016 [] | Upregulation of miR21 via TLR4/MYD88/NF-κB signaling by F. nucleatum, leading to CRC progression and invasion. | Increased miR21 expression enhanced proliferation and invasion of CRC cells. High F. nucleatum and miR21 levels correlated with reduced RASA1 expression and poor patient outcomes. |
| Guo S et al., 2022 [] | Role of ANO1/TMEM16A, a calcium-activated chloride channel, in apoptosis resistance and tumor immune escape. | ANO1 overexpression is driven by 11q13 amplification and influences tumor proliferation, invasion, apoptosis resistance, and immune escape. ANO1 also regulates tumor cell-specific pathways, making it a promising biomarker and therapeutic target. |
| Lu P. et al., 2019 [] | Interaction between F. nucleatum and ANO1 in promoting chemoresistance in CRC cells. | F. nucleatum increased ANO1 expression, reducing apoptosis in CRC cells treated with oxaliplatin and 5-FU. ANO1 knockdown mitigated chemoresistance effects induced by F. nucleatum, enhancing chemotherapy-induced apoptosis. |
| Zhang S et al., 2020 [] | Induction of epithelial-mesenchymal transition (EMT) by F. nucleatum through lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling in OSCC. | F. nucleatum infection promoted cell migration and EMT, with upregulation of mesenchymal markers (N-cadherin, Vimentin, SNAI1) and downregulation of E-cadherin. The MIR4435-2HG/miR-296-5p/Akt2/SNAI1 pathway was implicated in EMT induction, linking F. nucleatum infection to oral cancer initiation. |
| Yu MR, 2020 [] | Activation of EGFR signaling pathway (AKT, ERK) and promotion of epithelial-mesenchymal transition (EMT). | Fusobacterium nucleatum enhances CRC aggressiveness and EMT in DSS-treated cells. In mouse models, F. nucleatum increases malignancy in AOM/DSS-induced colon cancer. EGFR inhibition reduces F. nucleatum-induced EMT alteration. F. nucleatum accelerates CAC progression by activating the EGFR signaling pathway. |
6. Influence on Immunotherapy Response
In addition to affecting chemotherapy response, F. nucleatum also plays a role in modulating the response to immunotherapy []. F. nucleatum promotes an immunosuppressive milieu by interacting with both tumor cells and immune cells, hindering the effectiveness of immunotherapies such as checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors), CAR T-cell therapies, and other immune modulators [,,,,]. As mentioned earlier, F. nucleatum’s role in immune modulation is multifaceted. It enhances the production of pro-inflammatory cytokines and immune-suppressive factors like TGF-β, IL-10, and indoleamine 2,3-dioxygenase (IDO), which collectively foster an immune-tolerant environment [,]. This creates challenges for effective immune surveillance, as CRC cells are able to evade recognition by cytotoxic T cells. Additionally, F. nucleatum skews the function of tumor-infiltrating lymphocytes (TILs) and promotes the expansion of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), both of which dampen anti-tumor immunity [,]. Moreover, F. nucleatum has been shown to upregulate PD-L1 expression on CRC cells, further enabling tumor cells to evade immune detection and reducing the effectiveness of PD-1/PD-L1-based therapies [,,,]. In Table 3, the most recent studies on the influence of Fusobacterium nucleatum on immunotherapy in CRC are listed.

Table 3.
Molecular mechanisms and results of Fusobacterium nucleatum’s role in immunotherapy for CRC.
7. Conclusions
In conclusion, the growing body of research has shed light on the complex molecular mechanisms by which F. nucleatum contributes to CRC progression and chemoresistance. F. nucleatum not only enhances CSC stemness and promotes aggressive tumor behavior, but also plays a critical role in mediating resistance to common chemotherapies such as oxaliplatin and 5-FU []. Emerging therapeutic strategies that target F. nucleatum and its associated pathways hold great promise in overcoming these challenges. Pharmacological interventions like metformin, which suppress F. nucleatum-induced stemness and enhance chemosensitivity, and Br-J-I, which exhibits antimicrobial activity against F. nucleatum and synergizes with 5-FU, demonstrate early potential for improving treatment outcomes []. Additionally, innovative drug delivery systems, including tumor-targeted nanoassemblies and phage-guided hybrid nanomaterials, have shown efficacy in selectively targeting and eliminating F. nucleatum, thereby improving the effectiveness of chemotherapy [].
In the realm of immunotherapy, F. nucleatum-directed vaccination strategies and microbial ecosystem replacement offer intriguing possibilities for modulating the tumor microbiome, although further research is needed to address the challenges posed by F. nucleatum’s ability to evade immune responses [,,]. Targeting key proteins and pathways, such as Annexin A1 and BIRC3, which are upregulated in F. nucleatum-infected CRC cells, provides additional therapeutic avenues to overcome F. nucleatum-mediated chemoresistance [,]. Moreover, miRNA-based therapeutics, which influence F. nucleatum proliferation and resistance, could further complement current treatment strategies [,,].
Future advancements in designing personalized treatments for CRC patients with elevated F. nucleatum levels are key to enhancing therapeutic success. Integrating standard chemotherapies with therapies targeting F. nucleatum may overcome resistance mechanisms that currently hinder their efficacy. Detailed investigations into the complex relationships among F. nucleatum, CSCs, and the tumor microenvironment are critical. Clinical trials assessing F. nucleatum-focused interventions, such as microbiota therapies, vaccines, and cutting-edge nanomaterials, will be vital in transforming these encouraging preclinical insights into effective clinical treatments. Such efforts hold the potential to address the therapeutic obstacles posed by F. nucleatum in CRC and improve outcomes for patients facing this challenging disease.
Author Contributions
Conceptualization, L.G. and M.A.Z.; methodology, L.G. and M.A.Z.; writing—original draft preparation, L.G., F.T., R.B., G.E., I.M., F.V., M.P. and A.N.; writing—review and editing, L.G., M.A.Z. and M.E.A.; supervision, A.G. and M.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Fondazione Roma for its continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontology 2022, 89, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef] [PubMed]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal. 2024, 22, 547. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Chen, G.; Ren, Q.; Zhong, Z.; Li, Q.; Huang, Z.; Zhang, C.; Yuan, H.; Feng, Z.; Chen, B.; Wang, N.; et al. Exploring the gut microbiome’s role in colorectal cancer: Diagnostic and prognostic implications. Front. Immunol. 2024, 15, 1431747. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, Z.; Li, J.; He, Y.; Kang, W.; Ye, X. Association between the gut microbiota, inflammatory factors, and colorectal cancer: Evidence from Mendelian randomization analysis. Front. Microbiol. 2024, 15, 1309111. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, X.; Fu, L.; Yang, K. Fusobacterium nucleatum: A new player in regulation of cancer development and therapeutic response. Cancer Drug Resist. 2022, 5, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, N.Y.; Kang, G.H. Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: A systemic review and meta-analysis. J. Pathol. Transl. Med. 2022, 56, 144–151. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, J.H.; Bae, J.M.; Kim, H.J.; Cho, N.Y.; Kang, G.H. Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy. J. Pathol. Transl. Med. 2019, 53, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Schöpf, F.; Marongiu, G.L.; Milaj, K.; Sprink, T.; Kikhney, J.; Moter, A.; Roderer, D. Structural basis of Fusobacterium nucleatum adhesin Fap2 interaction with receptors on cancer and immune cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef]
- Sun, C.H.; Li, B.B.; Wang, B.; Zhao, J.; Zhang, X.Y.; Li, T.T.; Li, W.B.; Tang, D.; Qiu, M.J.; Wang, X.C.; et al. The role of Fusobacterium nucleatum in colorectal cancer: From carcinogenesis to clinical management. Chronic Dis. Transl. Med. 2019, 5, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Li, J.; Zhao, L.; Yan, W.; Lin, B.; Guo, X.; Wei, Y. Fusobacterium nucleatum Acts as a Pro-carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. Cell Dev. Biol. 2021, 9, 710165. [Google Scholar] [CrossRef]
- Onozawa, H.; Saito, M.; Saito, K.; Kanke, Y.; Watanabe, Y.; Hayase, S.; Sakamoto, W.; Ishigame, T.; Momma, T.; Ohki, S.; et al. Annexin A1 is involved in resistance to 5-FU in colon cancer cells. Oncol. Rep. 2017, 37, 235–240. [Google Scholar] [CrossRef]
- Jia, D.; Chen, S. Adhesin RadD: The secret weapon of Fusobacterium nucleatum. Gut Microbes 2024, 16, 2426617. [Google Scholar] [CrossRef] [PubMed]
- Galaski, J.; Rishiq, A.; Liu, M.; Bsoul, R.; Bergson, A.; Lux, R.; Bachrach, G.; Mandelboim, O. Fusobacterium nucleatum subsp. nucleatum RadD binds Siglec-7 and inhibits NK cell-mediated cancer cell killing. iScience 2024, 27, 110157. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Pezeshkian, Z.; Nobili, S.; Peyravian, N.; Shojaee, B.; Nazari, H.; Soleimani, H.; Asadzadeh-Aghdaei, H.; Ashrafian Bonab, M.; Nazemalhosseini-Mojarad, E.; Mini, E. Insights into the Role of Matrix Metalloproteinases in Precancerous Conditions and in Colorectal Cancer. Cancers 2021, 13, 6226. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Chakraborty, A.; Abd El-Hafeez, A.A.; Sharma, A.; Sahan, A.Z.; Huang, W.J.M.; Sahoo, D.; Ghosh, P.; Hazra, T.K.; Das, S. The DNA Glycosylase NEIL2 Suppresses Fusobacterium-Infection-Induced Inflammation and DNA Damage in Colonic Epithelial Cells. Cells 2020, 9, 1980. [Google Scholar] [CrossRef]
- Koi, M.; Okita, Y.; Carethers, J.M. Fusobacterium nucleatum Infection in Colorectal Cancer: Linking Inflammation, DNA Mismatch Repair and Genetic and Epigenetic Alterations. J. Anus Rectum Colon. 2018, 2, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Koi, M.; Takeda, K.; Ross, R.; Mukherjee, B.; Koeppe, E.; Stoffel, E.M.; Galanko, J.A.; McCoy, A.N.; Keku, T.O.; et al. Fusobacterium nucleatum infection correlates with two types of microsatellite alterations in colorectal cancer and triggers DNA damage. Gut Pathog. 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-κB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiao, X.; Zeng, M.; Fan, Z.; Li, X.; Yuan, Y.; Zhang, Q.; Xia, Y. Clinical Significance of Fusobacterium nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis. Cancer Manag. Res. 2022, 14, 3021–3036. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Han, S.W.; Kang, J.K.; Bae, J.M.; Kim, H.P.; Won, J.K.; Jeong, S.Y.; Park, K.J.; Kang, G.H.; Kim, T.Y. Association Between Fusobacterium nucleatum, Pathway Mutation, and Patient Prognosis in Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 3389–3395. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Q.; Guo, Y.; You, F. The Role of Fusobacterium nucleatum in Colorectal Cancer Cell Proliferation and Migration. Cancers 2022, 14, 5350. [Google Scholar] [CrossRef]
- Yin, H.; Miao, Z.; Wang, L.; Su, B.; Liu, C.; Jin, Y.; Wu, B.; Han, H.; Yuan, X. Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging 2022, 14, 1941–1958. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liao, Y.; Zhang, H.; Zhang, W.; Zhang, Z.; Zhang, J.; Wang, D.; Tang, D. Impacts of MicroRNAs Induced by the Gut Microbiome on Regulating the Development of Colorectal Cancer. Front. Cell Infect. Microbiol. 2022, 12, 804689. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Liang, R.; Zhu, X.; Lan, T.; Ding, D.; Zheng, Z.; Chen, T.; Huang, Y.; Liu, J.; Yang, X.; Shao, J.; et al. TIGIT promotes CD8+T cells exhaustion and predicts poor prognosis of colorectal cancer. Cancer Immunol. Immunother. 2021, 70, 2781–2793. [Google Scholar] [CrossRef]
- Galaski, J.; Shhadeh, A.; Umaña, A.; Yoo, C.C.; Arpinati, L.; Isaacson, B.; Berhani, O.; Singer, B.B.; Slade, D.J.; Bachrach, G.; et al. Fusobacterium nucleatum CbpF Mediates Inhibition of T Cell Function Through CEACAM1 Activation. Front. Cell Infect. Microbiol. 2021, 11, 692544. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Kang, M.; Jeon, M.; Chung, Y.; Kim, A.R.; Lee, Y.J.; Kim, E.S.; Nam, H.; Park, J.; Lee, J.Y.; et al. CEACAM1 Marks Highly Suppressive Intratumoral Regulatory T Cells for Targeted Depletion Therapy. Clin. Cancer Res. 2023, 29, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, Y.; Zhang, Z.; Yang, H.; Dai, N.; Zhang, N.; Sun, W.; Guo, Y.; Kong, J.; Wang, X.; et al. Fusobacterium nucleatum induces MDSCs enrichment via activation the NLRP3 inflammosome in ESCC cells, leading to cisplatin resistance. Ann. Med. 2022, 54, 989–1003. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Du, Y.; Ye, Y. Tumor-associated macrophages, dendritic cells, and neutrophils: Biological roles, crosstalk, and therapeutic relevance. Med. Rev. 2022, 1, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513. [Google Scholar] [CrossRef]
- Wu, J.; Dong, W.; Pan, Y.; Wang, J.; Wu, M.; Yu, Y. Crosstalk between gut microbiota and metastasis in colorectal cancer: Implication of neutrophil extracellular traps. Front. Immunol. 2023, 14, 1296783. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Huang, R.; Kang, T.; Chen, S. The role of tumor-associated macrophages in tumor immune evasion. J. Cancer Res. Clin. Oncol. 2024, 150, 238. [Google Scholar] [CrossRef]
- Toledo, B.; Zhu Chen, L.; Paniagua-Sancho, M.; Marchal, J.A.; Perán, M.; Giovannetti, E. Deciphering the performance of macrophages in tumour microenvironment: A call for precision immunotherapy. J. Hematol. Oncol. 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Mima, K.; Ishimoto, T.; Ogata, Y.; Imai, K.; Miyamoto, Y.; Akiyama, T.; Daitoku, N.; Hiyoshi, Y.; Iwatsuki, M.; et al. Relationship between Fusobacterium nucleatum and antitumor immunity in colorectal cancer liver metastasis. Cancer Sci. 2021, 112, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, C.G.; Kim, W.K.; Kim, K.A.; Yoo, J.; Min, B.S.; Paik, S.; Shin, S.J.; Lee, H.; Lee, K.; et al. Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front. Cell Infect. Microbiol. 2023, 13, 1101291. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kołat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Yadav, D.; Sainatham, C.; Filippov, E.; Kanagala, S.G.; Ishaq, S.M.; Jayakrishnan, T. Gut Microbiome-Colorectal Cancer Relationship. Microorganisms 2024, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Qasem, H.H.; El-Sayed, W.M. The bacterial microbiome and cancer: Development, diagnosis, treatment, and future directions. Clin. Exp. Med. 2024, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Laborda-Illanes, A.; Otero, A.; Ordóñez, R.; González-González, A.; Plaza-Andrades, I.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Relationships of Gut Microbiota Composition, Short-Chain Fatty Acids and Polyamines with the Pathological Response to Neoadjuvant Radiochemotherapy in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 9549. [Google Scholar] [CrossRef]
- Wu, Q.L.; Fang, X.T.; Wan, X.X.; Ding, Q.Y.; Zhang, Y.J.; Ji, L.; Lou, Y.L.; Li, X. Fusobacterium nucleatum-induced imbalance in microbiome-derived butyric acid levels promotes the occurrence and development of colorectal cancer. World J. Gastroenterol. 2024, 30, 2018–2037. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell Microbiol. 2021, 23, e13348. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yue, S.; Lu, A.; Liang, C. Host-Gut Microbiota Metabolic Interactions and Their Role in Precision Diagnosis and Treatment of Gastrointestinal Cancers. Pharmacol. Res. 2024, 207, 107321. [Google Scholar] [CrossRef]
- Ranjbar, M.; Salehi, R.; Haghjooy Javanmard, S.; Rafiee, L.; Faraji, H.; Jafarpor, S.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Nedaeinia, R. The dysbiosis signature of Fusobacterium nucleatum in colorectal cancer-cause or consequences? A systematic review. Cancer Cell Int. 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Benešová, I.; Křížová, Ľ.; Kverka, M. Microbiota as the unifying factor behind the hallmarks of cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 14429–14450. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, W.; Wang, Y.; Wang, Y.; Sun, X.; Zheng, Q. Gut microbiome-metabolites axis: A friend or foe to colorectal cancer progression. Biomed. Pharmacother. 2024, 173, 116410. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]
- Shariati, A.; Razavi, S.; Ghaznavi-Rad, E.; Jahanbin, B.; Akbari, A.; Norzaee, S.; Darban-Sarokhalil, D. Association between colorectal cancer and Fusobacterium nucleatum and Bacteroides fragilis bacteria in Iranian patients: A preliminary study. Infect. Agent. Cancer 2021, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Duy, T.N.; Le Huy, H.; Thanh, Q.Đ.; Thi, H.N.; Minh, H.N.T.; Dang, M.N.; Le Huu, S.; Tat, T.N. Association between Bacteroides fragilis and Fusobacterium nucleatum infection and colorectal cancer in Vietnamese patients. Anaerobe 2024, 88, 102880. [Google Scholar]
- Pandey, H.; Jain, D.; Tang, D.W.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2024, 22, 15–43. [Google Scholar] [CrossRef]
- Permain, J.; Hock, B.; Eglinton, T.; Purcell, R. Functional links between the microbiome and the molecular pathways of colorectal carcinogenesis. Cancer Metastasis Rev. 2024, 43, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Mignini, I.; Piccirilli, G.; Galasso, L.; Termite, F.; Esposto, G.; Ainora, M.E.; Gasbarrini, A.; Zocco, M.A. From the Colon to the Liver: How Gut Microbiota May Influence Colorectal Cancer Metastatic Potential. J. Clin. Med. 2024, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P. Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy. Pharmacol. Res. 2024, 203, 107185. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Salesse, L.; Garcia-Weber, D.; Marinelli, L.; Beguet-Crespel, F.; Brochard, V.; Le Gléau, C.; Jamet, A.; Doré, J.; Blottière, H.M.; et al. Fusobacterium nucleatum promotes inflammatory and anti-apoptotic responses in colorectal cancer cells via ADP-heptose release and ALPK1/TIFA axis activation. Gut Microbes 2024, 16, 2295384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, J.; Cao, P.; Su, W.; Deng, Y.; Zhan, N.; Fu, X.; Huang, Y.; Dong, W. Fusobacterium nucleatum Promotes Metastasis in Colorectal Cancer by Activating Autophagy Signaling via the Upregulation of CARD3 Expression. Theranostics 2020, 10, 323–339. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Tsutsuki, H.; Zhang, T.; Nomoto, D.; Okadome, K.; Yamamura, K.; Harada, K.; Eto, K.; et al. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br. J. Cancer 2021, 124, 963–974. [Google Scholar] [CrossRef]
- Li, N.; Yu, Y.; Chen, Q.; Niu, J.; Gao, C.; Qu, X.; Zhang, J.; Gao, H. A gene delivery system with autophagy blockade for enhanced anti-angiogenic therapy against Fusobacterium nucleatum-associated colorectal cancer. Acta Biomater. 2024, 183, 278–291. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Xu, M.; Xiong, Z.; Zhou, F.; Wang, L. Fusobacterium nucleatum prevents apoptosis in colorectal cancer cells via the ANO1 pathway. Cancer Manag. Res. 2019, 11, 9057–9066. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Li, N. ANO1: More Than Just Calcium-Activated Chloride Channel in Cancer. Front. Oncol. 2022, 12, 922838. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.R.; Kim, H.J.; Park, H.R. Fusobacterium nucleatum Accelerates the Progression of Colitis-Associated Colorectal Cancer by Promoting EMT. Cancers 2020, 12, 2728. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Yue, C.; Liu, X. Fusobacterium nucleatum produces cancer stem cell characteristics via EMT-resembling variations. Int. J. Clin. Exp. Pathol. 2020, 13, 1819–1828. [Google Scholar] [PubMed]
- Liu, H.; Du, J.; Chao, S.; Li, S.; Cai, H.; Zhang, H.; Chen, G.; Liu, P.; Bu, P. Fusobacterium nucleatum Promotes Colorectal Cancer Cell to Acquire Stem Cell-Like Features by Manipulating Lipid Droplet-Mediated Numb Degradation. Adv. Sci. 2022, 9, e2105222. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.L.; Yu, T.C.; Huang, X.W.; Wang, J.L.; Sun, T.T.; Yan, T.T.; Zhou, C.B.; Chen, H.M.; Su, W.Y.; Du, W.; et al. Metformin abrogates Fusobacterium nucleatum-induced chemoresistance in colorectal cancer by inhibiting miR-361-5p/sonic hedgehog signaling-regulated stemness. Br. J. Cancer 2023, 128, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Chen, Q.; Liu, H.; Zhang, H.; Sun, Y.; Zhao, L.; Gao, Y.; Wei, Q. Single-cell RNA sequencing analysis reveals the distinct features of colorectal cancer with or without Fusobacterium nucleatum infection in PD-L1 blockade therapy. Heliyon 2024, 10, e37511. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 2024, 42, 1729–1746.e8. [Google Scholar] [CrossRef]
- Ugai, T.; Shimizu, T.; Kawamura, H.; Ugai, S.; Takashima, Y.; Usui, G.; Väyrynen, J.P.; Okadome, K.; Haruki, K.; Akimoto, N.; et al. Inverse relationship between Fusobacterium nucleatum amount and tumor CD274 (PD-L1) expression in colorectal carcinoma. Clin Transl Immunol. 2023, 12, e1453. [Google Scholar] [CrossRef]
- Gao, Y.; Bi, D.; Xie, R.; Li, M.; Guo, J.; Liu, H.; Guo, X.; Fang, J.; Ding, T.; Zhu, H.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 398, Erratum in Signal Transduct. Target. Ther. 2021, 6, 434. [Google Scholar] [CrossRef]
- Jiang, S.S.; Xie, Y.L.; Xiao, X.Y.; Kang, Z.R.; Lin, X.L.; Zhang, L.; Li, C.S.; Qian, Y.; Xu, P.P.; Leng, X.X.; et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 2023, 31, 781–797.e9. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Pani, G. Fusobacterium & Co. at the Stem of Cancer: Microbe-Cancer Stem Cell Interactions in Colorectal Carcinogenesis. Cancers 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, F.; Chen, Q.; Gou, X.; Li, X.; Zhang, L.; Gao, H. Construction of size-transformable supramolecular nano-platform against drug-resistant colorectal cancer caused by Fusobacterium nucleatum. Chem. Eng. J. 2022, 450 Pt 1, 137605. [Google Scholar] [CrossRef]
- Boesch, M.; Horvath, L.; Baty, F.; Pircher, A.; Wolf, D.; Spahn, S.; Straussman, R.; Tilg, H.; Brutsche, M.H. Compartmentalization of the host microbiome: How tumor microbiota shapes checkpoint immunotherapy outcome and offers therapeutic prospects. J. Immunother. Cancer 2022, 10, e005401. [Google Scholar] [CrossRef]
- Holt, R.A. Oncomicrobial vaccines: The potential for a Fusobacterium nucleatum vaccine to improve colorectal cancer outcomes. Cell Host Microbe 2023, 31, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.N.; Crichton, S.J.; Fabian, C.; Pepper, C.; Butcher, D.R.; Dempsey, F.C.; Parris, C.N. A therapeutic antibody targeting annexin-A1 inhibits cancer cell growth in vitro and in vivo. Oncogene 2024, 43, 608–614. [Google Scholar] [CrossRef]
- Taghinezhad-S, S.; Mohseni, A.H.; Fu, X. Intervention on gut microbiota may change the strategy for management of colorectal cancer. J. Gastroenterol. Hepatol. 2021, 36, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lu, X.; Tong, Y.; Feng, Y.; Mao, Y.; Dun, G.; Li, J.; Xu, Q.; Tang, J.; Zhang, T.; et al. MicroRNA-31 induced by Fusobacterium nucleatum infection promotes colorectal cancer tumorigenesis. iScience 2023, 26, 106770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).