Prognosis Prediction Model After Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma: A Multicenter Study (OS-HBP-2)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Clinical Data
2.3. Statistical Analysis
3. Results
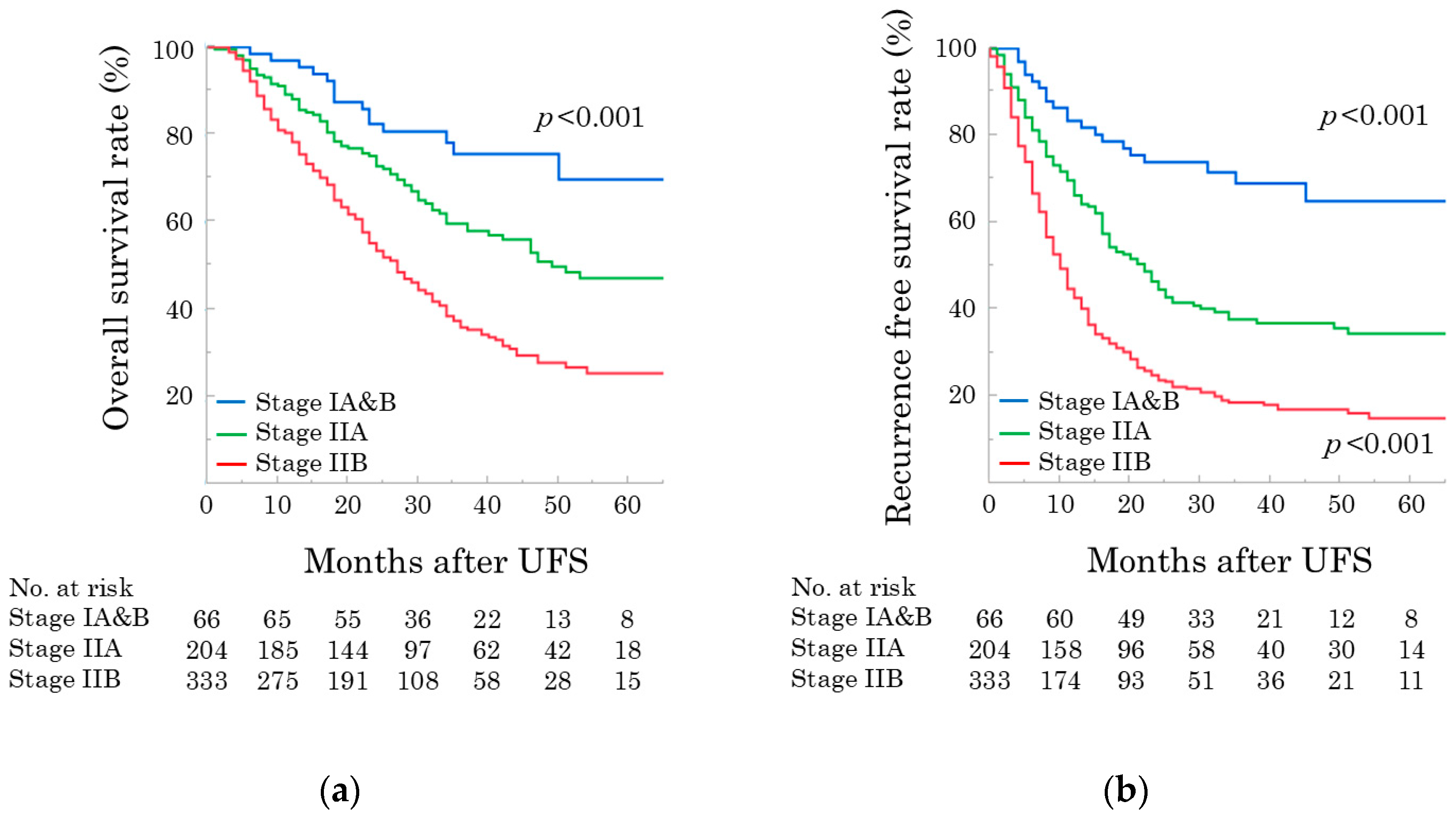
3.1. Cohort
3.2. Prognostic Factors Associated with OS
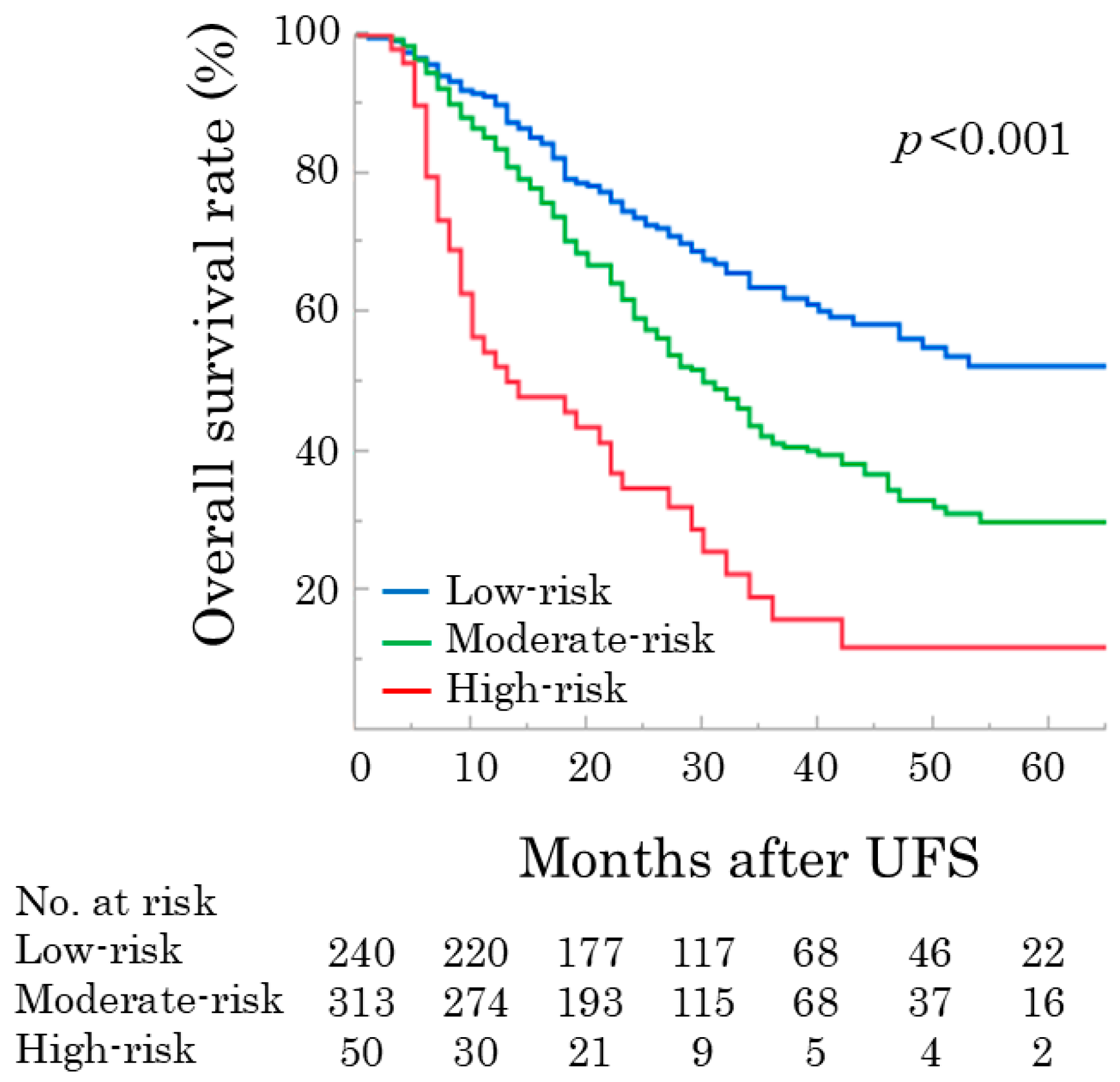
3.3. Simple Scoring Model for OS Following UFS
3.4. Prognosis Prediction Model for OS Following UFS
= [exp(−0.008296 * t)]^exp(0.40314 * [tumor size > 2 cm] + 0.39622 * [tumor contact with PV&SMV]
+ 0.3962 * [CA 19-9 40–500 or ≥500] + 0.49915 * [mGPS 2])
=exp(−0.008296 * t * exp{0.40314 * [tumor size > 2 cm] + 0.39622 * [tumor contact with PV&SMV]
+ 0.3962 * [CA 19-9 40–500 or ≥500] + 0.49915 * [mGPS 2])
p (predictive probability; %) = S(t∣x) * 100
S(t∣x) survival function at time t for an individual with covariates x
S0(t) baseline survival function
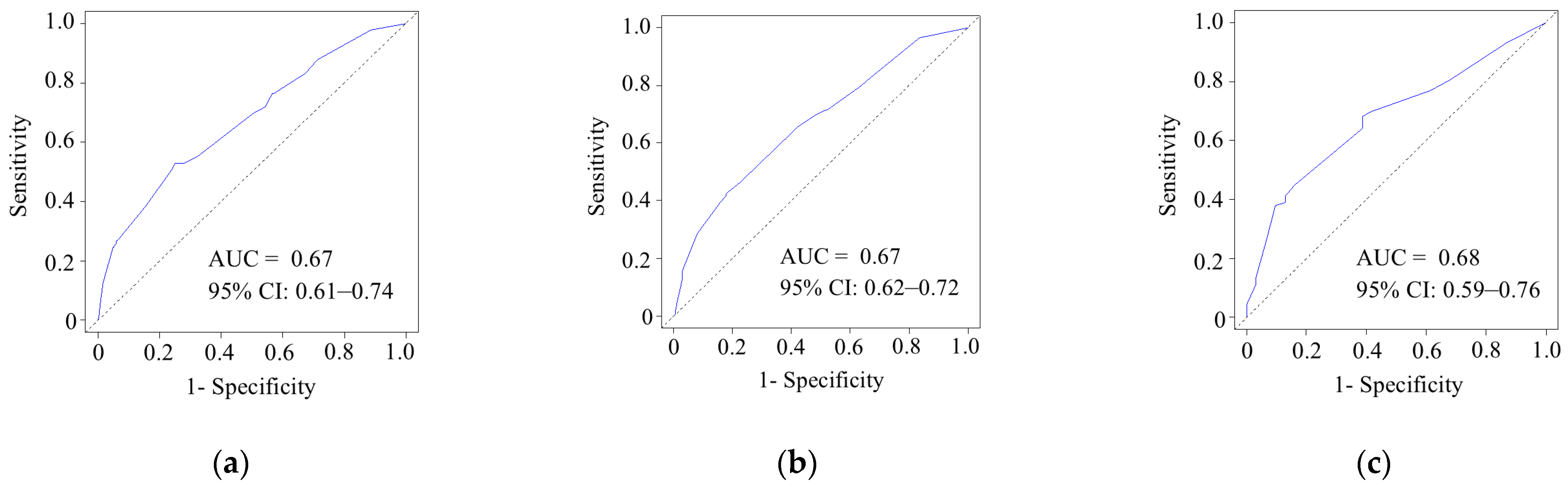
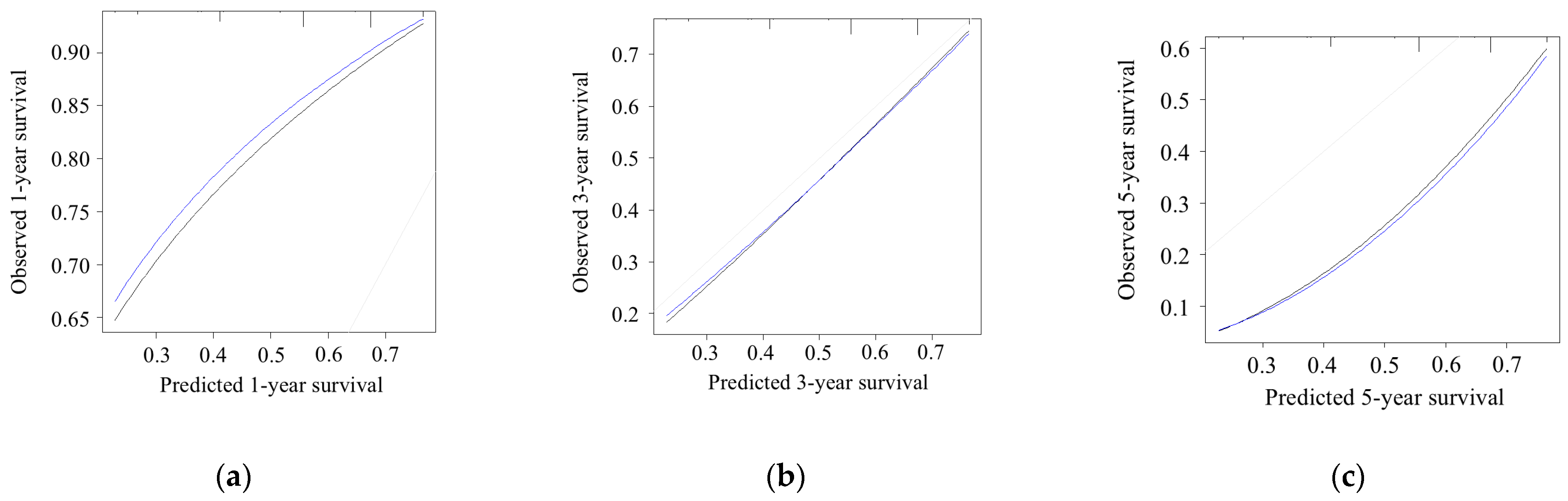
3.5. Model Performance and Calibration
3.6. A Literature Review
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Lin, C.; Wang, W. Global, regional, and national burden of pancreatic cancer from 1990 to 2021, its attributable risk factors, and projections to 2050: A systematic analysis of the global burden of disease study 2021. BMC Cancer 2025, 25, 189. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Sarma, G.; Bora, H.; Medhi, P.P. Optimizing neoadjuvant chemoradiation in resectable and borderline resectable pancreatic cancer: Evidence-based insights. World J. Clin. Oncol. 2025, 16, 106107. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma (Version 2.2025); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Yoon, S.J.; Shin, S.H.; Yoon, S.K.; Jung, J.H.; You, Y.; Han, I.W.; Choi, D.W.; Heo, J.S. Appraisal of 5-year recurrence-free survival after surgery in pancreatic ductal adenocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 287–296. [Google Scholar] [CrossRef]
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Park, B.; Kwon, J.; Lim, C.S.; Shin, Y.C.; Jung, W.; Shin, S.H.; Heo, J.S.; Han, I.W. Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study. Biomedicines 2022, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhou, Z.; Ma, Z.; Huang, S.; Gong, Y.; Li, Z.; Liu, C.; Wang, S.; Chen, B.; Zhang, C.; et al. Prognostic Nomogram for Patients With Pancreatic Ductal Adenocarcinoma of Pancreatic Head After Pancreaticoduodenectomy. Clin. Med. Insights Oncol. 2021, 15, 11795549211024149. [Google Scholar] [CrossRef]
- Ren, H.; Wu, C.R.; Aimaiti, S.; Wang, C.F. Development and validation of a novel nomogram for predicting the prognosis of patients with resected pancreatic adenocarcinoma. Oncol. Lett. 2020, 19, 4093–4105. [Google Scholar] [CrossRef]
- Aoyama, T.; Maezawa, Y.; Hashimoto, I.; Rino, Y.; Oshima, T. Clinical Impact of Nutrition and Inflammation Assessment Tools in Pancreatic Cancer Treatment. Anticancer Res. 2023, 43, 3849–3860. [Google Scholar] [CrossRef]
- Kim, N.; Han, I.W.; Ryu, Y.; Hwang, D.W.; Heo, J.S.; Choi, D.W.; Shin, S.H. Predictive Nomogram for Early Recurrence after Pancreatectomy in Resectable Pancreatic Cancer: Risk Classification Using Preoperative Clinicopathologic Factors. Cancers 2020, 12, 137. [Google Scholar] [CrossRef]
- Man, Q.; Pang, H.; Liang, Y.; Chang, S.; Wang, J.; Gao, S. Nomogram model for predicting early recurrence for resectable pancreatic cancer: A multicenter study. Medicine 2024, 103, e37440. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, J.H.; Kang, X.H.; Liu, X.M.; Wang, H.F.; Wang, Z.X.; Pan, H.Q.; Zhang, Q.Q.; Xu, X.L. Nomogram for predicting the preoperative lymph node metastasis in resectable pancreatic cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 12469–12477. [Google Scholar] [CrossRef]
- Umeda, Y.; Mitsuhashi, T.; Kojima, T.; Satoh, D.; Sui, K.; Endo, Y.; Inagaki, M.; Oishi, M.; Yagi, T.; Fujiwara, T. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 217–229. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Sudo, A. The value of the high-sensitivity modified Glasgow prognostic score in predicting the survival of patients with a soft-tissue sarcoma. Bone Jt. J. 2015, 97-B, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Castillo, C.F.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic ductal adenocarcinoma: Is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.N.; Bai, X.L.; Li, G.G.; Liang, T.B. Review of radiological classifications of pancreatic cancer with peripancreatic vessel invasion: Are new grading criteria required? Cancer Imaging 2017, 17, 14. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Brennan, M.F.; Kattan, M.W.; Klimstra, D.; Conlon, K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann. Surg. 2004, 240, 293–298. [Google Scholar] [CrossRef]
- Attiyeh, M.A.; Chakraborty, J.; Doussot, A.; Langdon-Embry, L.; Mainarich, S.; Gönen, M.; Balachandran, V.P.; D’Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; et al. Survival Prediction in Pancreatic Ductal Adenocarcinoma by Quantitative Computed Tomography Image Analysis. Ann. Surg. Oncol. 2018, 25, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Y.; Cai, Z.; Lin, X.; Li, S. Overall survival and cancer-specific survival in patients with surgically resected pancreatic head adenocarcinoma: A competing risk nomogram analysis. J. Cancer 2018, 9, 3156–3167. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.Z.; Chen, S.; Lin, S.Z.; Pan, W.; Meng, Z.W.; Cai, X.R.; Chen, Y.L. Development and validation of novel nomograms for predicting the survival of patients after surgical resection of pancreatic ductal adenocarcinoma. Cancer Med. 2020, 9, 3353–3370. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Qin, T.; Wang, M.; Wang, H.; Dang, C.; Wu, C.H.; Tien, Y.W.; Qin, R. Development and Validation of a Nomogram to Predict Survival in Pancreatic Head Ductal Adenocarcinoma After Pancreaticoduodenectomy. Front. Oncol. 2021, 11, 734673. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Wang, F.; Zhang, G.; Liu, R. A nomogram predicting overall survival in patients with non-metastatic pancreatic head adenocarcinoma after surgery: A population-based study. BMC Cancer 2021, 21, 524. [Google Scholar] [CrossRef]
- Hirashita, T.; Tada, K.; Nagasawa, Y.; Orimoto, H.; Kawamura, M.; Fujinaga, A.; Takayama, H.; Kawano, Y.; Masuda, T.; Endo, Y.; et al. Benefits of neoadjuvant chemotherapy with gemcitabine plus S-1 for resectable pancreatic ductal adenocarcinoma. Mol. Clin. Oncol. 2025, 22, 18. [Google Scholar] [CrossRef]
- Pecorelli, N.; Licinio, A.W.; Guarneri, G.; Aleotti, F.; Crippa, S.; Reni, M.; Falconi, M.; Balzano, G. Prognosis of Upfront Surgery for Pancreatic Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Front. Oncol. 2021, 11, 812102. [Google Scholar] [CrossRef]
- Xu, D.; Wang, J.; Liu, T.; Huang, Z.; Luo, J.; Chen, Y.; Lu, Y. Quantitative definitions of pain, CA19-9, and tumor size as high-risk features of resectable pancreatic cancer: A single-center retrospective cohort study. Gland Surg. 2021, 10, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Halimi, A.; Svensson, J.; Bayadsi, H.; Innala, M.; Hansén, M.; Hemmingsson, O.; Franklin, O. Portomesenteric venous contact ≤180° and overall survival in resectable head and body pancreatic adenocarcinoma treated with upfront surgery. Eur. J. Surg. Oncol. 2023, 49, 107097. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; La Vaccara, V.; Farolfi, T.; Fiore, M.; Cammarata, R.; Ramella, S.; Coppola, R.; Caputo, D. Role of CA 19.9 in the Management of Resectable Pancreatic Cancer: State of the Art and Future Perspectives. Biomedicines 2022, 10, 2091. [Google Scholar] [CrossRef]
- Kang, Y.M.; Wang, H.; Li, R.; Pan, G. Prognostic Role of Carbohydrate Antigen 19 to 9 in Predicting Survival of Patients With Pancreatic Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211043030. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, D.; Jin, X.; Wu, H. The prognostic value of modified Glasgow Prognostic Score in pancreatic cancer: A meta-analysis. Cancer Cell Int. 2020, 20, 462. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Zhao, Y.; Tan, H.L.; Chua, D.W.; Ng, K.Y.Y.; Lee, S.Y.; Lee, J.J.X.; Tai, D.; Goh, B.K.P.; Koh, Y.X. Clinical Outcomes of Neoadjuvant Therapy Versus Upfront Surgery in Resectable Pancreatic Cancer: Systematic Review and Meta-analysis of Latest Randomized Controlled Trials. Ann. Surg. Oncol. 2025, 32, 4094–4107. [Google Scholar] [CrossRef]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Toyama, H.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; et al. Neoadjuvant Chemotherapy with Gemcitabine and S-1 versus Upfront Surgery for Resectable Pancreatic Cancer: Results of the Randomized Phase II/III Prep-02/JSAP05 Trial. Ann. Surg. 2025; ahead of print. [Google Scholar] [CrossRef]
- Yasui, K.; Takagi, K.; Fuji, T.; Nishiyama, T.; Nagai, Y.; Matsumoto, K.; Horiguchi, S.; Fujii, Y.; Otsuka, M.; Fujiwara, T. Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Cancers 2025, 17, 3287. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Tempero, M.A.; Pelzer, U.; O’Reilly, E.M.; Winter, J.; Oh, D.Y.; Li, C.P.; Tortora, G.; Chang, H.M.; Lopez, C.D.; Bekaii-Saab, T.; et al. Adjuvant nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 2007–2019. [Google Scholar] [CrossRef]
- Narayanan, S.; AlMasri, S.; Zenati, M.; Nassour, I.; Chopra, A.; Rieser, C.; Smith, K.; Oyefusi, V.; Daum, T.; Bahary, N.; et al. Predictors of early recurrence following neoadjuvant chemotherapy and surgical resection for localized pancreatic adenocarcinoma. J. Surg. Oncol. 2021, 124, 308–316. [Google Scholar] [CrossRef] [PubMed]
| Variables | n = 603 |
|---|---|
| Preoperative factors | |
| Age, years | 72 (65–77) |
| Sex, male/female | 325 (53.9%)/278 (46.1%) |
| BMI, kg/m2 | 21.9 (19.7–24.0) |
| Biliary drainage | 210 (34.8%) |
| CA19-9, U/mL | 111 (30–392) |
| Alb, g/dL | 4.0 (3.6–4.3) |
| Total bilirubin, mg/dL | 0.8 (0.6–1.3) |
| CRP, mg/dL | 0.1 (0.1–0.4) |
| mGPS, 0/1/2 | 418 (69.3%)/137 (22.7%)/48 (8.0%) |
| Radiological findings | |
| Tumor location, head/body-tail | 393 (65.2%)/210 (34.8%) |
| Tumor size, cm | 2.3 (1.8–3.0) |
| Tumor contact with the PV&SMV of ≤180° | 174 (28.9%) |
| Regional lymph node metastasis | 99 (16.4%) |
| Preoperative clinical findings (UICC/AJCC 7th edition) | |
| cT status, T1/T2/T3 | 29 (4.8%)/26 (4.3%)/548 (90.9%) |
| cStage, IA&B/IIA/IIB | 53 (8.6%)/483 (80.1%)/68 (11.3%) |
| Operative factors | |
| Surgical procedure, PD/DP/TP | 387 (64.2%)/198 (32.8%)/18 (3.0%) |
| PV&SMV resection | 132 (21.9%) |
| Operative time, min | 407 (284–521) |
| Estimated blood loss, mL | 420 (220–730) |
| Pathological findings (UICC/AJCC 7th edition) | |
| pT status, T1/T2/T3 | 48 (8.0%)/31 (5.1%)/524 (86.9%) |
| Lymph node metastasis | 331 (54.9%) |
| PV&SMV invasion | 135 (22.4%) |
| R status, R0/R1 resection | 532 (88.2%)/71 (11.8%) |
| pStage, IA&B/IIA/IIB | 66 (10.9%)/204 (33.8%)/333 (55.2%) |
| Postoperative factors | |
| Major complications (≥grade 3) | 135 (22.4%) |
| Pancreatic fistula (≥grade B) | 114 (18.9%) |
| Adjuvant chemotherapy | 452 (75.0%) |
| Recurrence | 381 (63.2%) |
| Preoperative factors | n (%) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age, year | |||||
| ≥75 | 227 (37.6%) | 1.20 (0.95, 1.52) | 0.119 | ||
| <75 | 376 (62.4%) | Ref | |||
| Sex | |||||
| Female | 278 (46.1%) | 0.88 (0.70, 1.10) | 0.880 | ||
| male | 325 (53.9%) | Ref | |||
| BMI, kg/m2 | |||||
| ≥25 | 114 (18.9%) | 1.12 (0.84, 1.48) | 0.427 | ||
| <25 | 489 (81.1%) | Ref | |||
| Biliary drainage | |||||
| Yes | 210 (34.8%) | 1.44 (1.14, 1.81) | 0.002 | 1.17 (0.89, 1.55) | 0.251 |
| No | 393 (65.2%) | Ref | Ref | ||
| Tumor location | |||||
| Body-tail | 210 (34.8%) | 0.78 (0.61, 1.00) | 0.046 | 1.00 (0.75, 1.35) | 0.986 |
| Head | 393 (65.2%) | Ref | Ref | ||
| Tumor size, cm | |||||
| >2.0 | 364 (60.4%) | 1.74 (1.37, 2.22) | <0.001 | 1.50 (1.17, 1.93) | 0.001 |
| ≤2.0 | 239 (39.6%) | Ref | Ref | ||
| Tumor contact with the PV&SMV of ≤180° | |||||
| Presence | 174 (28.9%) | 1.54 (1.21, 1.95) | <0.001 | 1.47 (1.14, 1.88) | 0.003 |
| Absence | 429 (71.1%) | Ref | Ref | ||
| CA19-9, U/mL | |||||
| ≥500 | 129 (21.4%) | 2.58 (1.86, 3.58) | <0.001 | 2.16 (1.55, 3.04) | <0.001 |
| 40–500 | 299 (49.6%) | 1.64 (1.23, 2.20) | <0.001 | 1.59 (1.19, 2.14) | 0.002 |
| <40 | 175 (29.0%) | Ref | Ref | ||
| mGPS | |||||
| 2 | 48 (8.0%) | 1.73 (1.15, 2.50) | 0.009 | 1.56 (1.03, 2.29) | 0.038 |
| 0, 1 | 555 (92.0%) | Ref | Ref | ||
| Variables | Low-Risk n = 240 | Moderate-Risk n = 313 | High-Risk n = 50 | p Value |
|---|---|---|---|---|
| Preoperative factors | ||||
| CA19-9, U/mL | 29 (12–103) | 198 (74–565) | 1032 (699–3029) | <0.001 |
| Alb, g/dL | 4.1 (3.8–4.3) | 4.0 (3.6–4.2) | 3.7 (3.2–4.1) | <0.001 |
| mGPS, 0/1/2 | 180 (75.0%)/57 (23.8%)/3 (1.2%) | 216 (69.0%)/72 (23.0%)/25 (8.0%) | 22 (44.0%)/8 (16.0%)/20 (40.0%) | <0.001 |
| Radiological findings | ||||
| Tumor location, head/body-tail | 136 (56.7%)/104 (43.3%) | 212 (67.7%)/101 (32.3%) | 45 (90.0%)/5 (10.0%) | <0.001 |
| Tumor size, cm | 1.8 (1.4–2.0) | 2.5 (2.2–3.2) | 3.0 (2.6–3.5) | <0.001 |
| Tumor contact with the PV&SMV of ≤180° | 24 (10.0%) | 112 (35.8%) | 38 (76.0%) | <0.001 |
| Regional lymph node metastasis | 22 (9.2%) | 63 (20.1%) | 14 (28.0%) | <0.001 |
| Operative factors | ||||
| Surgical procedure, PD/DP/TP | 133 (55.4%)/100 (41.7%)/7 (2.9%) | 213 (68.1%)/94 (30.0%)/6 (1.9%) | 41 (82.0%)/4 (8.0%)/5 (10.0%) | <0.001 |
| PV&SMV resection | 24 (10.0%) | 83 (26.5%) | 25 (50.0%) | <0.001 |
| Operative time, min | 349 (254–493) | 422 (307–519) | 495 (422–573) | <0.001 |
| Estimated blood loss, mL | 390 (200–676) | 450 (235–797) | 561 (393–826) | 0.004 |
| Pathological findings (UICC 7th edition) | ||||
| pT status, T1/T2/T3 | 39 (16.3%)/18 (7.5%)/183 (76.3%) | 9 (2.9%)/12 (3.8%)/292 (93.3%) | 0 (0%)/1 (2.0%)/49 (98.0%) | <0.001 |
| Lymph node metastasis | 94 (39.2%) | 200 (63.9%) | 37 (74.0%) | <0.001 |
| PV&SMV invasion | 34 (14.2%) | 81 (26.0%) | 20 (40.0%) | <0.001 |
| R status, R0/R1 resection | 216 (90.0%)/24 (10.0%) | 272 (86.9%)/41 (13.1%) | 44 (88.0%)/6 (12.0%) | 0.53 |
| pStage, IA&B/IIA/IIB | 52 (21.7%)/92 (38.3%)/96 (40.0%) | 13 (4.2%)/100 (32.0%)/200 (63.9%) | 1 (2.0%)/12 (24.0%)/37 (74.0%) | <0.001 |
| Postoperative factors | ||||
| Major complications (≥grade 3) | 59 (24.6%) | 64 (20.5%) | 12 (24.0%) | 0.49 |
| Pancreatic fistula (≥grade B) | 55 (22.9%) | 54 (17.3%) | 5 (10.0%) | 0.05 |
| Adjuvant chemotherapy | 172 (71.7%) | 244 (78.0%) | 36 (72.0%) | 0.21 |
| Recurrence | 115(47.9%) | 224 (71.6%) | 42 (84.0%) | <0.001 |
| Authors | Year | Study Design | Sample Size | Outcomes | Parameters | C-Index |
|---|---|---|---|---|---|---|
| Brennan et al. [25] | 2004 | Single-center, resected PDAC | 555 | CSS | Age Sex Weight loss Back pain pT stage pN stage Tumor size Differentiation Tumor location Margin status Splenectomy Portal vein resection | 0.64 |
| Attiyeh et al. [26] | 2018 | Single-center, resected PDAC | 161: Training (n = 113) Testing (n = 48) | OS | Model A CA 19-9 Image features Model B CA 19-9 Image features Brennan score | Model A 0.68 Model B 0.73 |
| He et al. [27] | 2018 | SEER database, resected pancreatic head cancer | 2374: Training (n = 1780) Validation (n = 594) | OS CSS | Age Tumor size Differentiation Lymph node ratio M factor | 0.64 (OS) 0.65 (CSS) |
| Li et al. [28] | 2020 | SEER database, resected PDAC | 6323: Training (n = 3700) Validation (n = 1312) Prospective test (n = 1311) | OS CSS | Age Sex pT stage pN stage M stage Marital status Differentiation Tumor size Lymph node count Lymph node ratio | 0.64 (OS) 0.64 (CSS) |
| Ren et al. [9] | 2020 | Single-center, resected PDAC | 368: Training (n = 258) Validation (n = 110) | OS | CA19-9 pT stage pN stage Differentiation Capsule invasion Neutrophil percentage Transfusion Adjuvant therapy | 0.77 (5-year OS) |
| Peng et al. [29] | 2021 | Single-center, resected pancreatic head cancer | 307: Training (n = 307) External validation (n = 159) | OS | Age CA 19-9 Tongji classification pT stage pN stage Differentiation Adjuvant chemotherapy | 0.79 |
| Zou et al. [30] | 2021 | SEER database, resected pancreatic head cancer | 6419: Training (n = 4495) Validation (n = 1924) | OS | pT stage pN stage Differentiation Adjuvant radiotherapy Adjuvant chemotherapy | 0.68 |
| Zhuang et al. [8] | 2021 | Single-center, resected pancreatic head cancer | 112 | OS | Age CA19-9 Total bilirubin pAJCC stage 8th Perineural invasion | 0.73 |
| Yoon et al. [7] | 2022 | Multicenter, resected PDAC | 885 | OS | Age Underlying liver disease Chronic kidney disease Preoperative portal vein invasion Portal vein resection Blood loss Tumor size Differentiation Lymph node metastasis Tumor necrosis | NA |
| Our study | 2025 | Multicenter, resectable PDAC | 603 | OS | Tumor size Tumor contact with the PV&SMV of ≤180° CA 19-9 mGPS | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, K.; Yoshida, R.; Yasui, K.; Hioki, M.; Okabayashi, T.; Kojima, T.; Endo, Y.; Nobuoka, D.; Sui, K.; Inagaki, M.; et al. Prognosis Prediction Model After Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma: A Multicenter Study (OS-HBP-2). Cancers 2025, 17, 3694. https://doi.org/10.3390/cancers17223694
Takagi K, Yoshida R, Yasui K, Hioki M, Okabayashi T, Kojima T, Endo Y, Nobuoka D, Sui K, Inagaki M, et al. Prognosis Prediction Model After Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma: A Multicenter Study (OS-HBP-2). Cancers. 2025; 17(22):3694. https://doi.org/10.3390/cancers17223694
Chicago/Turabian StyleTakagi, Kosei, Ryuichi Yoshida, Kazuya Yasui, Masayoshi Hioki, Takehiro Okabayashi, Toru Kojima, Yoshikatsu Endo, Daisuke Nobuoka, Kenta Sui, Masaru Inagaki, and et al. 2025. "Prognosis Prediction Model After Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma: A Multicenter Study (OS-HBP-2)" Cancers 17, no. 22: 3694. https://doi.org/10.3390/cancers17223694
APA StyleTakagi, K., Yoshida, R., Yasui, K., Hioki, M., Okabayashi, T., Kojima, T., Endo, Y., Nobuoka, D., Sui, K., Inagaki, M., Shinoura, S., Kimura, M., Matsuda, T., Aoki, H., & Fujiwara, T. (2025). Prognosis Prediction Model After Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma: A Multicenter Study (OS-HBP-2). Cancers, 17(22), 3694. https://doi.org/10.3390/cancers17223694





