Hepatitis C Virus Infection Associated with Oral Potentially Malignant Disorder, Oral Cancer, and Liver Diseases: A Community-Based Cross-Sectional Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
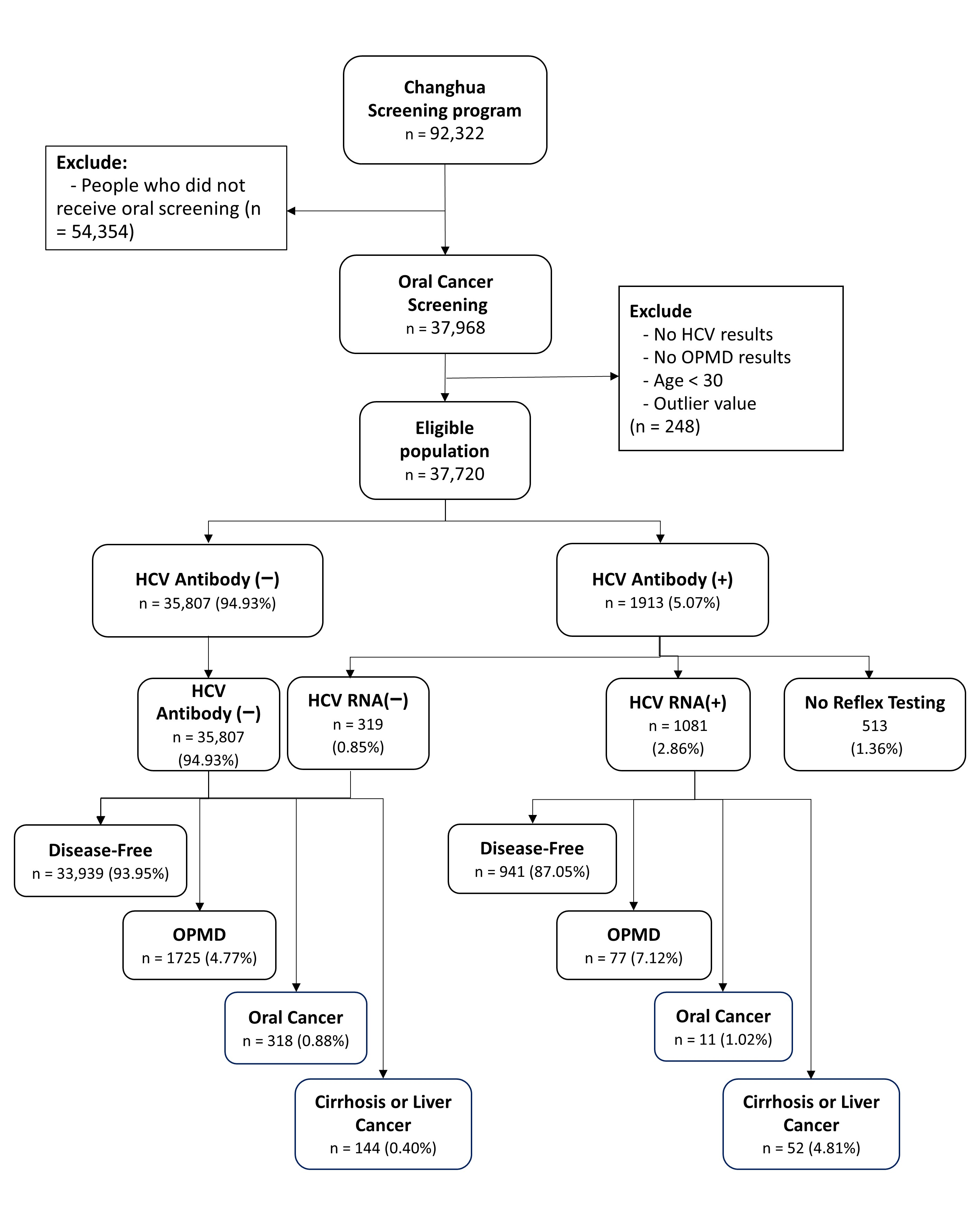
2.1. Study Design and Population
2.2. Case Identification and Data Collection
2.3. Statistical Analysis
3. Results
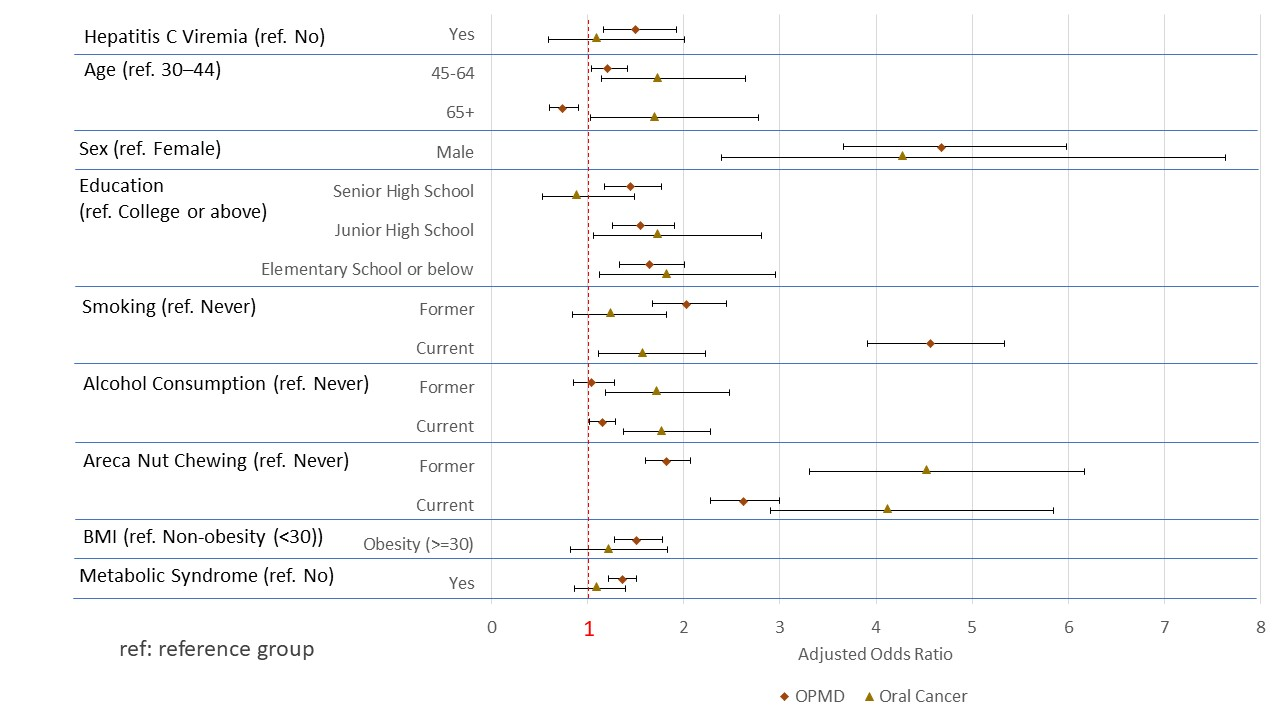
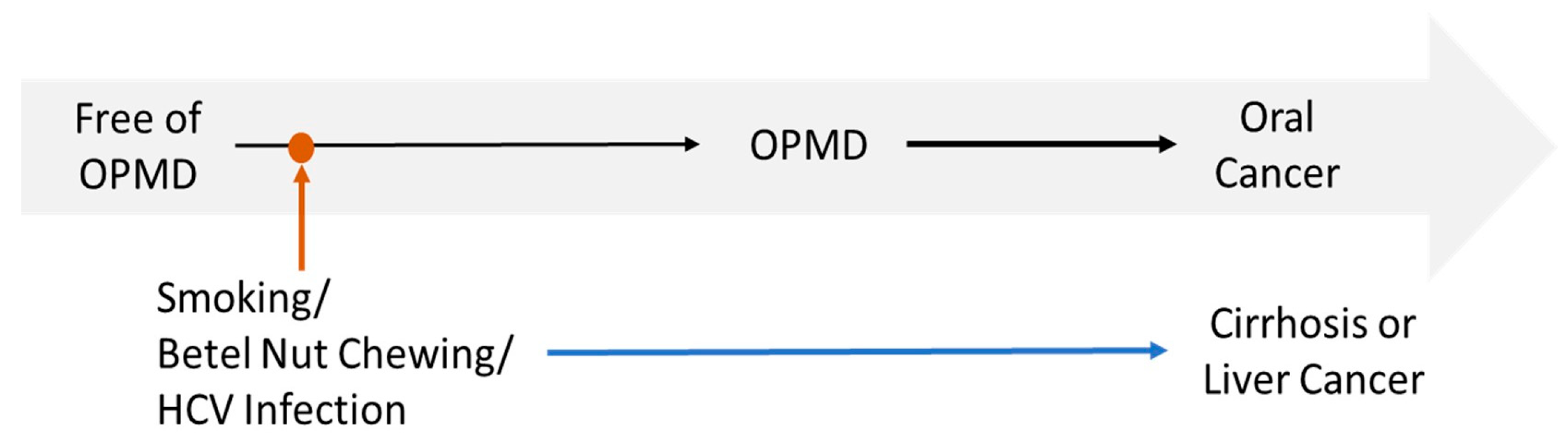
4. Discussion
4.1. Association of HCV Infection with OPMDs and Oral Cancer
4.2. Potential Biological Mechanisms and Interaction with Lifestyle Factors
4.3. Public Health Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef]
- Prostakishina, E.A.; Sidenko, E.A.; Kolegova, E.S.; Patysheva, M.R.; Kononova, G.A.; Choinzonov, E.L. Premalignant lesions of the oral cavity: A narrative review of factors and mechanisms of transformation into cancer. Int. J. Oral Maxillofac. Surg. 2025, 54, 479–493. [Google Scholar] [CrossRef]
- Worakhajit, P.; Fuangtharnthip, P.; Khovidhunkit, S.P.; Chiewwit, P.; Klongnoi, B. The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 8738. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100E; International Agency for Research on Cancer: Lyon, France, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304391/ (accessed on 3 March 2025).
- Amarasinghe, H.K.; Usgodaarachchi, U.S.; Johnson, N.W.; Lalloo, R.; Warnakulasuriya, S. Betel-quid chewing with or without tobacco is a major risk factor for oral potentially malignant disorders in Sri Lanka: A case-control study. Oral Oncol. 2010, 46, 297–301. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y. Human papillomavirus infection in oral potentially malignant disorders and cancer. Arch. Oral Biol. 2017, 83, 334–339. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Mitran, M.I.; Mitran, C.I.; Sarbu, M.I.; Nicolae, I.; Matei, C.; Caruntu, C.; Neagu, M.; Popa, M.I. Potential pathogenic mechanisms involved in the association between lichen planus and hepatitis C virus infection. Exp. Ther. Med. 2019, 17, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Sata, M.; Noguchi, S.; Seno’o, T.; Kinoshita, M.; Kameyama, T.; Ueno, T. Detection of hepatitis C virus RNA in oral lichen planus and oral cancer tissues. J. Oral Pathol. Med. 2000, 29, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Brancatello, F.; Dametto, E.; Arduino, P.; Pentenero, M.; Rendine, S.; Porter, S.R.; Lodi, G.; Scully, C.; Gandolfo, S. Hepatitis C virus-associated oral lichen planus: Is the geographical heterogeneity related to HLA-DR6? J. Oral Pathol. Med. 2005, 34, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Samaan, E.; El-Etreby, S.; Ahmed, A.; El-Husseini, F.; Sabry, A. The nature and prognosis of renal diseases in chronic hepatitis-C-infected diabetic Egyptian patients: The role of renal biopsy. Diabetes Metab. Syndr. 2022, 16, 102368. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Gu, D. Hepatitis B and C virus infections and the risk of biliary tract cancers: A meta-analysis of observational studies. Infect. Agents Cancer 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.R.; Chang, Y.L.; Lee, C.H.; Tsai, T.H.; Huang, K.H.; Lee, C.Y. Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study. Cancers 2022, 14, 583. [Google Scholar] [CrossRef]
- Alkrekshi, A.; Kassem, A.; Park, C.; Tse, W. Risk of Non-Hodgkin’s Lymphoma in HCV Patients in the United States Between 2013 and 2020: A Population-Based Study. Clin. Lymphoma Myeloma Leuk. 2021, 21, e832–e838. [Google Scholar] [CrossRef]
- Arafa, A.; Eshak, E.S.; Abdel Rahman, T.A.; Anwar, M.M. Hepatitis C virus infection and risk of pancreatic cancer: A meta-analysis. Cancer Epidemiol. 2020, 65, 101691. [Google Scholar] [CrossRef]
- Hammerstad, S.S.; Grock, S.F.; Lee, H.J.; Hasham, A.; Sundaram, N.; Tomer, Y. Diabetes and Hepatitis C: A Two-Way Association. Front. Endocrinol. 2015, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.C.; Moonka, D.; Brown, K.A.; Rogers, C.; Huang, M.A.; Bhatt, N.; Lamerato, L. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1066–1073. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Bikbov, B.; Remuzzi, G. Hepatitis C infection and chronic renal diseases. Clin. J. Am. Soc. Nephrol. 2009, 4, 207–220. [Google Scholar] [CrossRef]
- White, D.L.; Ratziu, V.; El-Serag, H.B. Hepatitis C infection and risk of diabetes: A systematic review and meta-analysis. J. Hepatol. 2008, 49, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M. Hepatitis C and hepatocellular carcinoma. Hepatology 1997, 26 (Suppl. 1), 34S–38S. [Google Scholar] [CrossRef]
- Yeh, Y.P.; Hu, T.H.; Cho, P.Y.; Chen, H.H.; Yen, A.M.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; Su, W.W.; Fang, Y.J.; et al. Changhua Community-Based Abdominal Ultrasonography Screening Group. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology 2014, 59, 1840–1849. [Google Scholar] [CrossRef]
- Yen, A.M.; Wang, S.T.; Feng, S.W.; Lin, C.T.; Chen, S.L. The association between fecal hemoglobin concentration and oral potentially malignant disorders. Oral Dis. 2019, 25, 108–116. [Google Scholar] [CrossRef]
- Lin, T.Y.; Jen, H.H.; Hu, T.H.; Yao, Y.C.; Chen, T.H.; Yen, A.M.; Yeh, Y.P. Planning, implementing, and evaluating Hepatitis C virus elimination via collaborative community-based care cascade: Age-period-cohort model for estimating demand from antecedent anti-HCV survey. Hepatol. Int. 2024, 18, 476–485. [Google Scholar] [CrossRef]
- Carrozzo, M.; Scally, K. Oral manifestations of hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 7534–7543. [Google Scholar] [CrossRef]
- Azatyan, V.; Yessayan, L.; Sargsyan, A.; Khachatryan, A.; Ghevondyan, T.; Shmavonyan, M.; Melik-Andreasyan, G.; Porksheyan, K.; Manrikyan, M. Morphological Changes in the Oral Mucous Membrane in Viral Hepatitis C Patients: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 9003. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Guida, A.; Romano, A.; Petruzzi, M.; Marrone, A.; Fiori, F.; Lucchese, A. Hepatitis C Virus (HCV) Infection: Pathogenesis, Oral Manifestations, and the Role of Direct-Acting Antiviral Therapy: A Narrative Review. J. Clin. Med. 2024, 13, 4012. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Fussey, J.; Fabris, L.; Bandolin, L.; Gaudioso, P.; Phillips, V.; Polesel, J.; Boscolo-Rizzo, P. HCV infection and the risk of head and neck cancer: A meta-analysis. Oral Oncol. 2020, 109, 104869. [Google Scholar] [CrossRef]
- Hayes, C.N.; Zhang, P.; Zhang, Y.; Chayama, K. Molecular Mechanisms of Hepatocarcinogenesis Following Sustained Virological Response in Patients with Chronic Hepatitis C Virus Infection. Viruses 2018, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Heredia-Torres, T.G.; Rincón-Sánchez, A.R.; Lozano-Sepúlveda, S.A.; Galan-Huerta, K.; Arellanos-Soto, D.; García-Hernández, M.; Garza-Juarez, A.J.; Rivas-Estilla, A.M. Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis. Viruses 2022, 14, 2762. [Google Scholar] [CrossRef]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef]
- Lin, H.J.; Wang, X.L.; Tian, M.Y.; Li, X.L.; Tan, H.Z. Betel quid chewing and oral potential malignant disorders and the impact of smoking and drinking: A meta-analysis. World J. Clin. Cases 2022, 10, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Fouda, S.; Sainu, A.; Pappachan, J.M. Metabolic complications of hepatitis C virus infection. World J. Gastroenterol. 2021, 27, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Szarka, K.; Tar, I.; Fehér, E.; Gáll, T.; Kis, A.; Tóth, E.D.; Boda, R.; Márton, I.; Gergely, L. Progressive increase of human papillomavirus carriage rates in potentially malignant and malignant oral disorders with increasing malignant potential. Oral Microbiol. Immunol. 2009, 24, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Su, T.H.; Tseng, T.C.; Liu, C.J.; Chou, S.W.; Liu, C.H.; Yang, H.C.; Chen, P.J.; Chen, D.S.; Chen, C.L.; Kao, J.H. Antiviral therapy against chronic hepatitis C is associated with a reduced risk of oral cancer. Int. J. Cancer 2020, 147, 901–908. [Google Scholar] [CrossRef]
| Variables | Screen-Negative | OPMD | Oral Cancer | Cirrhosis or Liver Cancer | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Total | 35,345 | 93.7% | 1816 | 4.81% | 336 | 0.89% | 223 | 0.59% |
| HCV | ||||||||
| Negative | 33,646 | 95.2% | 1703 | 93.8% | 318 | 94.6% | 140 | 62.8% |
| Positive | 1699 | 4.8% | 113 | 6.2% | 18 | 5.4% | 83 | 37.2% |
| Hepatitis C Viremia | ||||||||
| No | 34,380 | 97.3% | 1737 | 95.6% | 325 | 96.7% | 171 | 76.7% |
| Yes | 965 | 2.7% | 79 | 4.4% | 11 | 3.3% | 52 | 23.3% |
| Not tested | 465 | 14 | 7 | 27 | ||||
| Age (Mean ± SD) | 55.5 ± 11.1 | 55.0 ± 9.2 | 58.7 ± 9.4 | 57.3 ± 7.2 | ||||
| Age Group | ||||||||
| 30–39 | 9105 | 25.8% | 24 | 1.3% | 3 | 0.9% | 1 | 0.5% |
| 40–49 | 11,878 | 33.6% | 488 | 26.9% | 51 | 15.2% | 26 | 11.7% |
| 50–59 | 8682 | 24.6% | 741 | 40.8% | 125 | 37.2% | 107 | 48.0% |
| 60–69 | 3952 | 11.2% | 452 | 24.9% | 113 | 33.6% | 84 | 37.7% |
| 70+ | 9105 | 25.8% | 111 | 6.1% | 44 | 13.1% | 5 | 2.2% |
| Sex | ||||||||
| Female | 12,918 | 36.6% | 78 | 4.3% | 14 | 4.2% | 40 | 17.9% |
| Male | 22,427 | 63.5% | 1738 | 95.7% | 322 | 95.8% | 183 | 82.1% |
| Education | ||||||||
| College or above | 5324 | 15.1% | 135 | 7.4% | 21 | 6.3% | 10 | 4.5% |
| Senior High School | 8771 | 24.9% | 472 | 26% | 47 | 14% | 46 | 20.6% |
| Junior High School | 6484 | 18.4% | 467 | 25.7% | 89 | 26.6% | 49 | 22% |
| Elementary School or below | 14,666 | 41.6% | 740 | 40.8% | 178 | 53.1% | 118 | 52.9% |
| Smoking | ||||||||
| Never | 21,645 | 61.3% | 266 | 14.7% | 68 | 20.2% | 102 | 45.7% |
| Former | 5213 | 14.8% | 315 | 17.4% | 93 | 27.7% | 29 | 13.0% |
| Current | 8464 | 24.0% | 1234 | 68.0% | 175 | 52.1% | 92 | 41.3% |
| Alcohol Consumption | ||||||||
| Never | 29,424 | 83.3% | 1110 | 61.2% | 174 | 51.8% | 156 | 70.0% |
| Former | 1351 | 3.8% | 125 | 6.9% | 41 | 12.2% | 15 | 6.7% |
| Current | 4547 | 12.9% | 580 | 32.0% | 121 | 36.0% | 52 | 23.3% |
| Areca Nut Chewing | ||||||||
| Never | 28,696 | 81.2% | 743 | 40.9% | 100 | 29.8% | 143 | 64.1% |
| Former | 4357 | 12.3% | 547 | 30.1% | 157 | 46.7% | 46 | 20.6% |
| Current | 2269 | 6.4% | 525 | 28.9% | 79 | 23.5% | 34 | 15.3% |
| BMI | ||||||||
| Non-obesity (<30) | 32,735 | 92.9% | 1603 | 88.4% | 306 | 91.3% | 202 | 91.0% |
| Obesity (>=30) | 2510 | 7.1% | 210 | 11.6% | 29 | 8.7% | 20 | 9.0% |
| Metabolic Syndrome | ||||||||
| No | 24,734 | 70.5% | 1087 | 60.3% | 216 | 64.5% | 156 | 70.6% |
| Yes | 10,346 | 29.5% | 716 | 39.7% | 119 | 35.5% | 65 | 29.4% |
| Variables | OPMD | Oral Cancer | Cirrhosis or Liver Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | ||||
| Hepatitis C Viremia | ||||||||||||
| No | Ref. | Ref. | Ref. | |||||||||
| Yes | 1.59 | 1.25 | 2.01 | <0.001 | 1.22 | 0.67 | 2.23 | 0.521 | 12.73 | 9.21 | 17.60 | <0.0001 |
| Age | ||||||||||||
| 30–44 | Ref. | Ref. | Ref. | |||||||||
| 45–64 | 1.41 | 1.24 | 1.62 | <0.0001 | 2.44 | 1.63 | 3.64 | <0.0001 | 6.11 | 3.00 | 12.43 | <0.0001 |
| 65+ | 0.87 | 0.73 | 1.03 | 0.109 | 2.73 | 1.77 | 4.21 | <0.0001 | 3.00 | 1.37 | 6.59 | 0.006 |
| Sex | ||||||||||||
| Female | Ref. | Ref. | Ref. | |||||||||
| Male | 12.74 | 10.14 | 16.00 | <0.0001 | 12.91 | 7.56 | 22.07 | <0.0001 | 2.74 | 1.89 | 3.96 | <0.0001 |
| Education | ||||||||||||
| College or above | Ref. | Ref. | Ref. | |||||||||
| Senior High School | 2.14 | 1.76 | 2.60 | <0.0001 | 1.36 | 0.81 | 2.28 | 0.240 | 2.55 | 1.28 | 5.09 | 0.008 |
| Junior High School | 2.86 | 2.35 | 3.47 | <0.0001 | 3.50 | 2.17 | 5.64 | <0.0001 | 3.71 | 1.87 | 7.37 | <0.001 |
| Elementary School or below | 2.03 | 1.68 | 2.44 | <0.0001 | 3.01 | 1.91 | 4.74 | <0.0001 | 3.64 | 1.90 | 6.98 | <0.0001 |
| Smoking | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 4.90 | 4.15 | 5.79 | <0.0001 | 5.82 | 4.23 | 8.02 | <0.0001 | 1.22 | 0.79 | 1.88 | 0.363 |
| Current | 11.89 | 10.38 | 13.61 | <0.0001 | 6.75 | 5.07 | 9.00 | <0.0001 | 2.12 | 1.56 | 2.88 | <0.0001 |
| Alcohol Consumption | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 2.49 | 2.05 | 3.02 | <0.0001 | 5.42 | 3.84 | 7.66 | <0.0001 | 1.73 | 0.94 | 3.21 | 0.080 |
| Current | 3.37 | 3.03 | 3.74 | <0.0001 | 4.60 | 3.63 | 5.83 | <0.0001 | 2.03 | 1.44 | 2.85 | <0.0001 |
| Areca Nut Chewing | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 4.86 | 4.33 | 5.46 | <0.0001 | 10.22 | 7.91 | 13.20 | <0.0001 | 2.06 | 1.43 | 2.95 | <0.0001 |
| Current | 8.95 | 7.94 | 10.10 | <0.0001 | 9.98 | 7.38 | 13.49 | <0.0001 | 3.15 | 2.12 | 4.68 | <0.0001 |
| BMI | ||||||||||||
| Non-obesity (<30) | Ref. | Ref. | Ref. | |||||||||
| Obesity (>=30) | 1.72 | 1.48 | 1.99 | <0.0001 | 1.26 | 0.86 | 1.85 | 0.233 | 1.08 | 0.64 | 1.83 | 0.771 |
| Metabolic Syndrome | ||||||||||||
| No | Ref. | Ref. | Ref. | |||||||||
| Yes | 1.58 | 1.44 | 1.74 | <0.0001 | 1.32 | 1.05 | 1.65 | 0.018 | 0.87 | 0.63 | 1.20 | 0.397 |
| Variables | OPMD | Oral Cancer | Cirrhosis or Liver Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | ||||
| Hepatitis C Viremia | ||||||||||||
| No | Ref. | Ref. | Ref. | |||||||||
| Yes | 1.50 | 1.17 | 1.92 | 0.002 | 1.09 | 0.59 | 2.01 | 0.787 | 11.59 | 8.33 | 16.13 | <0.0001 |
| Age | ||||||||||||
| 30–44 | Ref. | Ref. | Ref. | |||||||||
| 45–64 | 1.21 | 1.04 | 1.41 | 0.012 | 1.73 | 1.14 | 2.64 | 0.011 | 4.29 | 2.07 | 8.89 | <0.0001 |
| 65+ | 0.74 | 0.60 | 0.91 | 0.004 | 1.70 | 1.03 | 2.78 | 0.036 | 1.73 | 0.75 | 4.00 | 0.198 |
| Sex | ||||||||||||
| Female | Ref. | Ref. | Ref. | |||||||||
| Male | 4.68 | 3.66 | 5.98 | <0.0001 | 4.28 | 2.39 | 7.63 | <0.0001 | 2.51 | 1.65 | 3.82 | <0.0001 |
| Education | ||||||||||||
| College or above | Ref. | Ref. | Ref. | |||||||||
| Senior High School | 1.45 | 1.18 | 1.77 | <0.001 | 0.88 | 0.53 | 1.49 | 0.642 | 2.03 | 1.01 | 4.08 | 0.046 |
| Junior High School | 1.55 | 1.26 | 1.90 | <0.0001 | 1.73 | 1.06 | 2.81 | 0.029 | 2.31 | 1.15 | 4.64 | 0.019 |
| Elementary School or below | 1.64 | 1.33 | 2.01 | <0.0001 | 1.82 | 1.12 | 2.95 | 0.015 | 2.64 | 1.35 | 5.18 | 0.005 |
| Smoking | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 2.03 | 1.68 | 2.44 | <0.0001 | 1.24 | 0.84 | 1.82 | 0.276 | 0.66 | 0.40 | 1.09 | 0.102 |
| Current | 4.57 | 3.91 | 5.34 | <0.0001 | 1.57 | 1.11 | 2.23 | 0.011 | 1.02 | 0.70 | 1.50 | 0.915 |
| Alcohol Consumption | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 1.04 | 0.85 | 1.28 | 0.694 | 1.72 | 1.19 | 2.48 | 0.004 | 1.23 | 0.64 | 2.38 | 0.537 |
| Current | 1.15 | 1.02 | 1.29 | 0.022 | 1.77 | 1.37 | 2.28 | <0.0001 | 1.31 | 0.90 | 1.91 | 0.158 |
| Areca Nut Chewing | ||||||||||||
| Never | Ref. | Ref. | Ref. | |||||||||
| Former | 1.82 | 1.60 | 2.07 | <0.0001 | 4.52 | 3.31 | 6.17 | <0.0001 | 1.48 | 0.95 | 2.30 | 0.080 |
| Current | 2.62 | 2.28 | 3.00 | <0.0001 | 4.12 | 2.90 | 5.85 | <0.0001 | 1.85 | 1.17 | 2.95 | 0.009 |
| BMI | ||||||||||||
| Non-obesity (<30) | Ref. | Ref. | Ref. | |||||||||
| Obesity (>=30) | 1.51 | 1.28 | 1.78 | <0.0001 | 1.22 | 0.82 | 1.83 | 0.328 | 1.26 | 0.72 | 2.18 | 0.419 |
| Metabolic Syndrome | ||||||||||||
| No | Ref. | Ref. | Ref. | |||||||||
| Yes | 1.36 | 1.22 | 1.51 | <0.0001 | 1.09 | 0.86 | 1.39 | 0.470 | 0.77 | 0.55 | 1.08 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumparway, D.; Hsu, C.-Y.; Yen, A.M.-F.; Lin, T.-Y.; Warnakulasuriya, S.; Chen, T.H.-H.; Luh, D.-L.; Su, C.-W.; Sarakarn, P.; Yeh, Y.-P.; et al. Hepatitis C Virus Infection Associated with Oral Potentially Malignant Disorder, Oral Cancer, and Liver Diseases: A Community-Based Cross-Sectional Study. Cancers 2025, 17, 3695. https://doi.org/10.3390/cancers17223695
Jumparway D, Hsu C-Y, Yen AM-F, Lin T-Y, Warnakulasuriya S, Chen TH-H, Luh D-L, Su C-W, Sarakarn P, Yeh Y-P, et al. Hepatitis C Virus Infection Associated with Oral Potentially Malignant Disorder, Oral Cancer, and Liver Diseases: A Community-Based Cross-Sectional Study. Cancers. 2025; 17(22):3695. https://doi.org/10.3390/cancers17223695
Chicago/Turabian StyleJumparway, Donlagon, Chen-Yang Hsu, Amy Ming-Fang Yen, Ting-Yu Lin, Saman Warnakulasuriya, Tony Hsiu-Hsi Chen, Dih-Ling Luh, Chiu-Wen Su, Pongdech Sarakarn, Yen-Po Yeh, and et al. 2025. "Hepatitis C Virus Infection Associated with Oral Potentially Malignant Disorder, Oral Cancer, and Liver Diseases: A Community-Based Cross-Sectional Study" Cancers 17, no. 22: 3695. https://doi.org/10.3390/cancers17223695
APA StyleJumparway, D., Hsu, C.-Y., Yen, A. M.-F., Lin, T.-Y., Warnakulasuriya, S., Chen, T. H.-H., Luh, D.-L., Su, C.-W., Sarakarn, P., Yeh, Y.-P., & Chen, S. L.-S. (2025). Hepatitis C Virus Infection Associated with Oral Potentially Malignant Disorder, Oral Cancer, and Liver Diseases: A Community-Based Cross-Sectional Study. Cancers, 17(22), 3695. https://doi.org/10.3390/cancers17223695






