VHL Gene Restoration Supports RCC Reprogramming to iPSCs but Does Not Ensure Line Stability
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Review Board Statement
2.2. ccRCC/AN Cell Cultures
2.3. Cell Reprogramming
- (i)
- AAVS1 VHL: RCC cells: 500,000 cells/1600 v/30 ms/1 pulse
- (ii)
- VHL: HEK cells: 250,000 cells/1100 v/20 ms/2 pulse
- (iii)
- RCC2: 500,000 cells/1200 v/30 ms/1 pulse
- (iv)
- Urine-derived cells (Control): 5000,000 cells: 1400 V, pulse width 20 ms, two pulses.
2.4. Targeted Insertion of VHL into the Human AAVS1 Site
2.5. Flow Cytometry
2.6. Immunocytochemistry
2.7. KO of VHL in Normal ES Cells
2.8. Kidney Organoid Differentiation
2.9. CRISPR-Cas9 Design and Generation
PCR Analysis
| Primer | Target Gene | Sequence | Location * |
| 171-F | VHL | GCGTTCCATCCTCTACCGAG | 5084–5103 |
| 172-R | VHL | GCTTCAGACCGTGCTATCGT | 5589–5608 |
| 173-F | VHL | CGTTACAACGGCCTACGGT | 5010–5028 |
| 174-R | VHL | TTCAGACCGTGCTATCGTCC | 5587–5606 |
| 175-F | VHL | CTGGATCGCGGAGGGAATG | 5198–5216 |
| 176-R | VHL | GGCTTCAGACCGTGCTATCG | 5590–5609 |
| * Homo sapiens von Hippel-Lindau tumor suppressor (VHL), RefSeqGene (LRG_322) on chromosome 3. | |||
3. Results
3.1. Healthy Renal Tissue Adjacent to the ccRCC Tumor Reprogram with High Efficiency
3.2. ccRCC Cell Lines Are Resistant to a Variety of Reprogramming Strategies
3.3. Expression of Wild-Type VHL Gene in ccRCC Cells Through Knock-In Gene Engineering Partially Corrects the Hypoxia Response, Resulting in an Increased Reprogramming Rate
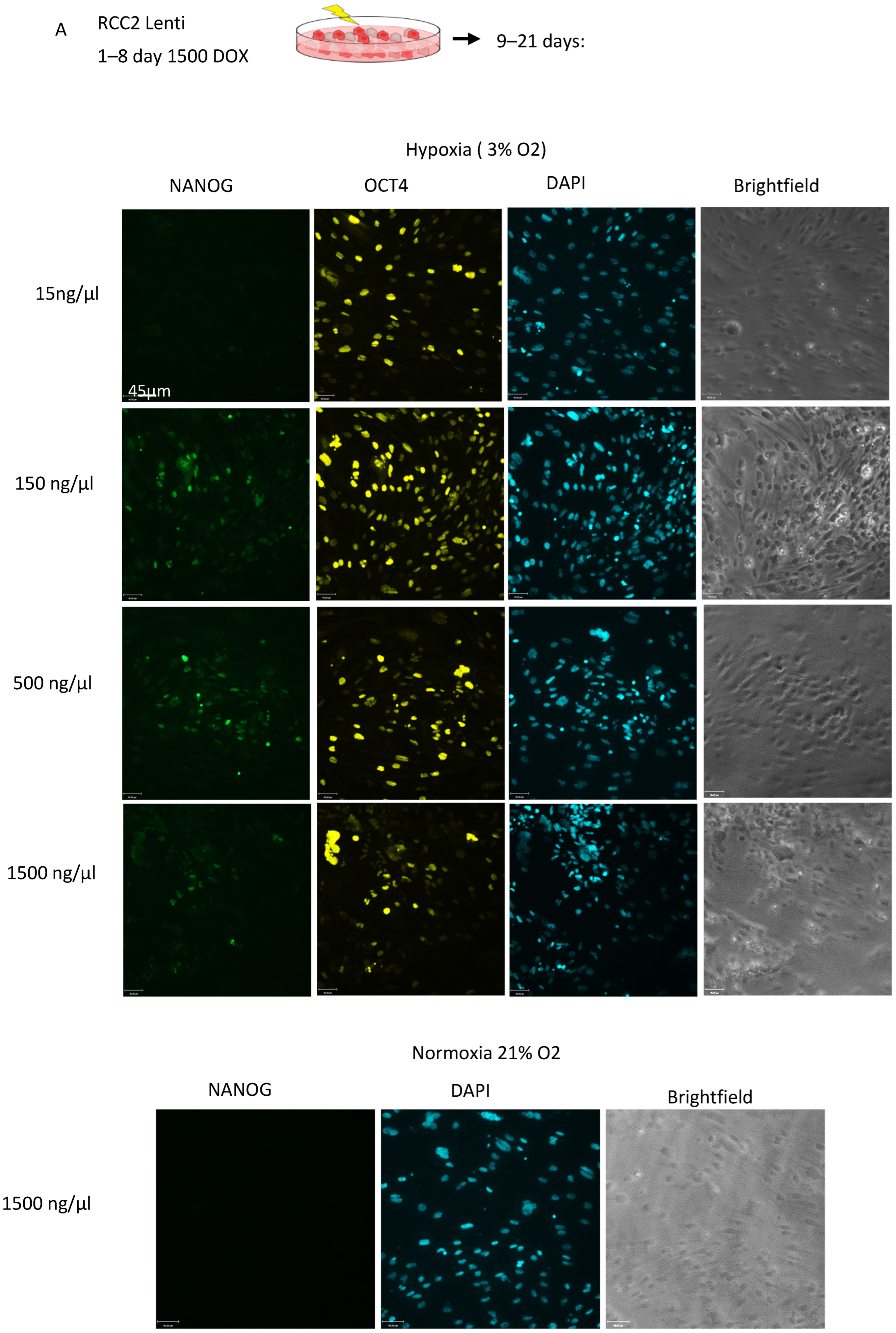



3.4. Knockout of VHL in Normal iPSC Reduces Cell Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ccRCC | clear cell renal cell carcinoma |
| EMT | Epithelial–Mesenchymal transition |
| iPSC | induced pluripotent stem cells |
| MET | mesenchymal–epithelial transition |
| OXPHOS | oxidative phosphorylation |
| VHL | von Hippel-Lindau factor |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Saad, A.M.; Gad, M.M.; Al-Husseini, M.J.; Ruhban, I.A.; Sonbol, M.B.; Ho, T.H. Trends in Renal-Cell Carcinoma Incidence and Mortality in the United States in the Last 2 Decades: A SEER-Based Study. Clin. Genitourin. Cancer 2019, 17, 46–57.e5. [Google Scholar] [CrossRef]
- Fu, Y.C.; Liang, S.B.; Luo, M.; Wang, X.P. Intratumoral heterogeneity and drug resistance in cancer. Cancer Cell Int. 2025, 25, 103. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; Linehan, W.M. Multi-regional Sequencing Elucidates the Evolution of Clear Cell Renal Cell Carcinoma. Cell 2018, 173, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Martz, C.H. von Hippel-Lindau disease: A genetic condition predisposing tumor formation. Oncol. Nurs. Forum 1991, 18, 545–551. [Google Scholar]
- Fu, L.; Wang, G.; Shevchuk, M.M.; Nanus, D.M.; Gudas, L.J. Generation of a mouse model of Von Hippel-Lindau kidney disease leading to renal cancers by expression of a constitutively active mutant of HIF1α. Cancer Res. 2011, 71, 6848–6856. [Google Scholar] [CrossRef]
- Gu, Y.F.; Cohn, S.; Christie, A.; McKenzie, T.; Wolff, N.; Do, Q.N.; Madhuranthakam, A.J.; Pedrosa, I.; Wang, T.; Dey, A.; et al. Modeling Renal Cell Carcinoma in Mice: Bap1 and Pbrm1 Inactivation Drive Tumor Grade. Cancer Discov. 2017, 7, 900–917. [Google Scholar] [CrossRef]
- Lichner, Z.; Mejia-Guerrero, S.; Ignacak, M.; Krizova, A.; Bao, T.T.; Girgis, A.H.; Youssef, Y.M.; Yousef, G.M. Pleiotropic action of renal cell carcinoma-dysregulated miRNAs on hypoxia-related signaling pathways. Am. J. Pathol. 2012, 180, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; Lopez, J.I.; Watkins, T.B.K.; Nicol, D. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell 2018, 173, 595–610.e11. [Google Scholar] [CrossRef]
- Yu, F.; White, S.B.; Zhao, Q.; Lee, F.S. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. USA 2001, 98, 9630–9635. [Google Scholar] [CrossRef]
- Carter, P.; Schnell, U.; Chaney, C.; Tong, B.; Pan, X.; Ye, J.; Mernaugh, G.; Cotton, J.L.; Margulis, V.; Mao, J.; et al. Deletion of Lats1/2 in adult kidney epithelia leads to renal cell carcinoma. J. Clin. Investig. 2021, 131, e144108. [Google Scholar] [CrossRef]
- Cortez Cardoso Penha, R.; Sexton Oates, A.; Senkin, S.; Park, H.A.; Atkins, J.; Holcatova, I.; Hornakova, A.; Savic, S.; Ognjanovic, S.; Swiat-kowska, B.; et al. Understanding the biological processes of kidney carcinogenesis: An integrative multi-omics approach. Mol. Syst. Biol. 2024, 20, 1282–1302. [Google Scholar] [CrossRef]
- Dormoy, V.; Jacqmin, D.; Lang, H.; Massfelder, T. From development to cancer: Lessons from the kidney to uncover new therapeutic targets. Anticancer Res. 2012, 32, 3609–3617. [Google Scholar] [PubMed]
- Hu, M.; Wang, Q.; Liu, B.; Ma, Q.; Zhang, T.; Huang, T.; Lv, Z.; Wang, R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022, 10, 868715. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.N.; Haber, D.A. Wilms’ tumour: Connecting tumorigenesis and organ development in the kidney. Nat. Rev. Cancer 2005, 5, 699–712. [Google Scholar] [CrossRef]
- Lindgren, D.; Eriksson, P.; Krawczyk, K.; Nilsson, H.; Hansson, J.; Veerla, S.; Sjolund, J.; Hoglund, M.; Johansson, M.E.; Axelson, H. Cell-Type-Specific Gene Programs of the Normal Human Nephron Define Kidney Cancer Subtypes. Cell Rep. 2017, 20, 1476–1489. [Google Scholar] [CrossRef]
- Zhang, Y.; Narayanan, S.P.; Mannan, R.; Raskind, G.; Wang, X.; Vats, P.; Su, F.; Hosseini, N.; Cao, X.; Kumar-Sinha, C. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc. Natl. Acad. Sci. USA 2021, 118, e2103240118. [Google Scholar] [CrossRef]
- Kim, J.; Hoffman, J.P.; Alpaugh, R.K.; Rhim, A.D.; Reichert, M.; Stanger, B.Z.; Furth, E.E.; Sepulveda, A.R.; Yuan, C.X.; Won, K.J. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep. 2013, 3, 2088–2099. [Google Scholar] [CrossRef]
- Lee, D.F.; Su, J.; Kim, H.S.; Chang, B.; Papatsenko, D.; Zhao, R.; Yuan, Y.; Gingold, J.; Xia, W.; Darr, H.; et al. Modeling familial cancer with induced pluripotent stem cells. Cell 2015, 161, 240–254. [Google Scholar] [CrossRef]
- Chao, M.P.; Gentles, A.J.; Chatterjee, S.; Lan, F.; Reinisch, A.; Corces, M.R.; Xavy, S.; Shen, J.; Haag, D.; Chanda, S.; et al. Human AML-iPSCs Reacquire Leukemic Properties after Differentiation and Model Clonal Variation of Disease. Cell Stem Cell 2017, 20, 329–344.e7. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Chan, A.S.; Lai, F.P.; Leung, S.Y. Organoid cultures for cancer modeling. Cell Stem Cell 2023, 30, 917–937. [Google Scholar] [CrossRef]
- Bang, J.S.; Choi, N.Y.; Lee, M.; Ko, K.; Park, Y.S.; Ko, K. Reprogramming of Cancer Cells into Induced Pluripotent Stem Cells Questioned. Int. J. Stem Cells 2019, 12, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Salci, K.R.; Reid, J.C.; Orlando, L.; Tanasijevic, B.; Shapovalova, Z.; Bhatia, M. Brief Report: Human Acute Myeloid Leukemia Reprogramming to Pluripotency Is a Rare Event and Selects for Patient Hematopoietic Cells Devoid of Leukemic Mutations. Stem Cells 2017, 35, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Lopez, A.; Romero-Moya, D.; Prieto, C.; Ramos-Mejia, V.; Agraz-Doblas, A.; Varela, I.; Buschbeck, M.; Palau, A.; Carvajal-Vergara, X.; Giorgetti, A.; et al. Development Refractoriness of MLL-Rearranged Human B Cell Acute Leukemias to Reprogramming into Pluripotency. Stem Cell Rep. 2016, 7, 602–618. [Google Scholar] [CrossRef]
- Lichner, Z.; Mac-Way, F.; Yousef, G.M. Obstacles in Renal Regenerative Medicine: Metabolic and Epigenetic Parallels Between Cellular Reprogramming and Kidney Cancer Oncogenesis. Eur. Urol. Focus 2019, 5, 250–261. [Google Scholar] [CrossRef]
- Lobo, N.C.; Gedye, C.; Apostoli, A.J.; Brown, K.R.; Paterson, J.; Stickle, N.; Robinette, M.; Fleshner, N.; Hamilton, R.J.; Kulkarni, G.; et al. Efficient generation of patient-matched malignant and normal primary cell cultures from clear cell renal cell carcinoma patients: Clinically relevant models for research and personalized medicine. BMC Cancer 2016, 16, 485. [Google Scholar] [CrossRef]
- Sun, Y.; Bandi, M.; Lofton, T.; Smith, M.; Bristow, C.A.; Carugo, A.; Rogers, N.; Leonard, P.; Chang, Q.; Mullinax, R. Functional Genomics Reveals Synthetic Lethality between Phosphogluconate Dehydrogenase and Oxidative Phosphorylation. Cell Rep. 2019, 26, 469–482.e5. [Google Scholar] [CrossRef]
- Biswas, P.; Palazzo, J.; Schlanger, S.; Jayaram, D.T.; Islam, S.; Page, R.C.; Stuehr, D.J. Visualizing mitochondrial heme flow through GAPDH in living cells and its regulation by NO. Redox Biol. 2024, 71, 103120. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.S.; Kawamura, T.; Wei, Z.; Sogo, T.; Jacinto, S.; Shigeno, A.; Kushige, H.; Yoshihara, E.; Liddle, C.; Ecker, J.R. ERRs Mediate a Metabolic Switch Required for Somatic Cell Reprogramming to Pluripotency. Cell Stem Cell 2015, 16, 547–555. [Google Scholar] [CrossRef]
- Fisher, R.; Horswell, S.; Rowan, A.; Salm, M.P.; de Bruin, E.C.; Gulati, S.; McGranahan, N.; Stares, M.; Gerlinger, M.; Varela, I. Development of synchronous VHL syndrome tumors reveals contingencies and constraints to tumor evolution. Genome Biol. 2014, 15, 433. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Hockemeyer, D.; Soldner, F.; Cook, E.G.; Gao, Q.; Mitalipova, M.; Jaenisch, R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell 2008, 3, 346–353. [Google Scholar] [CrossRef]
- Oceguera-Yanez, F.; Kim, S.I.; Matsumoto, T.; Tan, G.W.; Xiang, L.; Hatani, T.; Kondo, T.; Ikeya, M.; Yoshida, Y.; Inoue, H.; et al. Engineering the AAVS1 locus for consistent and scalable transgene expression in human iPSCs and their differentiated derivatives. Methods 2016, 101, 43–55. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef]
- Shamshirgaran, Y.; Jonebring, A.; Svensson, A.; Leefa, I.; Bohlooly, Y.M.; Firth, M.; Woollard, K.J.; Hofherr, A.; Rogers, I.M.; Hicks, R. Rapid target validation in a Cas9-inducible hiPSC derived kidney model. Sci. Rep. 2021, 11, 16532. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Porritt, M.J.; Jaiswal, H.; Seeliger, F.; Johansson, C.; Bidar, A.W.; Badertscher, L.; Wimberger, S.; Davies, E.J.; Hardaker, E. Development of an ObLiGaRe Doxycycline Inducible Cas9 system for pre-clinical cancer drug discovery. Nat. Commun. 2020, 11, 4903. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Becroft, M.; Vanslambrouck, J.M.; Stanley, E.G.; Elefanty, A.G.; Little, M.H. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 2014, 16, 118–126. [Google Scholar] [CrossRef]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Patard, J.J.; Fergelot, P.; Karakiewicz, P.I.; Klatte, T.; Trinh, Q.D.; Rioux-Leclercq, N.; Said, J.W.; Belldegrun, A.S.; Pantuck, A.J. Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int. J. Cancer 2008, 123, 395–400. [Google Scholar] [CrossRef]
- Mulder, J.; Sharmin, S.; Chow, T.; Rodrigues, D.C.; Hildebrandt, M.R.; D’Cruz, R.; Rogers, I.; Ellis, J.; Rosenblum, N.D. Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatr. Res. 2020, 87, 647–655. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef]
- Arthur, S.A.; Blaydes, J.P.; Houghton, F.D. Glycolysis Regulates Human Embryonic Stem Cell Self-Renewal under Hypoxia through HIF-2α and the Glycolytic Sensors CTBPs. Stem Cell Rep. 2019, 12, 728–742. [Google Scholar] [CrossRef]
- Folmes, C.D.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O.; Houghton, F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010, 139, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.M.; Puri, M.C.; Tonge, P.D.; Benevento, M.; Corso, A.J.; Clancy, J.L.; Mosbergen, R.; Li, M.; Lee, D.S.; Cloonan, N. Genome-wide characterization of the routes to pluripotency. Nature 2014, 516, 198–206. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Hyman, M.C.; Lawrence, D.A.; Pinsky, D.J. Molecular regulation of the PAI-1 gene by hypoxia: Contributions of Egr-1, HIF-1 α, and C/EBPα. FASEB J. 2007, 21, 935–949. [Google Scholar] [CrossRef]
- Mohamed, A.; Chow, T.; Whiteley, J.; Fantin, A.; Sorra, K.; Hicks, R.; Rogers, I.M. Umbilical Cord Tissue as a Source of Young Cells for the Derivation of Induced Pluripotent Stem Cells Using Non-Integrating Episomal Vectors and Feeder-Free Conditions. Cells 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Tabar, V. Constructing and Deconstructing Cancers using Human Pluripotent Stem Cells and Organoids. Cell Stem Cell 2019, 24, 12–24. [Google Scholar] [CrossRef]
- Courtney, K.D.; Bezwada, D.; Mashimo, T.; Pichumani, K.; Vemireddy, V.; Funk, A.M.; Wimberly, J.; McNeil, S.S.; Kapur, P.; Lotan, Y. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell Metab. 2018, 28, 793–800.e2. [Google Scholar] [CrossRef] [PubMed]
- LaGory, E.L.; Wu, C.; Taniguchi, C.M.; Ding, C.C.; Chi, J.T.; von Eyben, R.; Scott, D.A.; Richardson, A.D.; Giaccia, A.J. Suppression of PGC-1α Is Critical for Reprogramming Oxidative Metabolism in Renal Cell Carcinoma. Cell Rep. 2015, 12, 116–127. [Google Scholar] [CrossRef]
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef]
- Nilsson, H.; Lindgren, D.; Mandahl Forsberg, A.; Mulder, H.; Axelson, H.; Johansson, M.E. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate. Cell Death Dis. 2015, 6, e1585. [Google Scholar] [CrossRef] [PubMed]
- Podkalicka, P.; Stepniewski, J.; Mucha, O.; Kachamakova-Trojanowska, N.; Dulak, J.; Loboda, A. Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells. Biomolecules 2020, 10, 1614. [Google Scholar] [CrossRef]
- Prigione, A.; Rohwer, N.; Hoffmann, S.; Mlody, B.; Drews, K.; Bukowiecki, R.; Blumlein, K.; Wanker, E.E.; Ralser, M.; Cramer, T.; et al. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 2014, 32, 364–376. [Google Scholar] [CrossRef]
- Ma, X.; Tan, Z.; Zhang, Q.; Ma, K.; Xiao, J.; Wang, X.; Wang, Y.; Zhong, M.; Wang, Y.; Li, J. VHL Ser65 mutations enhance HIF2α signaling and promote epithelial-mesenchymal transition of renal cancer cells. Cell Biosci. 2022, 12, 52. [Google Scholar] [CrossRef]
- Cargill, K.; Hemker, S.L.; Clugston, A.; Murali, A.; Mukherjee, E.; Liu, J.; Bushnell, D.; Bodnar, A.J.; Saifudeen, Z.; Ho, J. Von Hippel-Lindau Acts as a Metabolic Switch Controlling Nephron Progenitor Differentiation. J. Am. Soc. Nephrol. 2019, 30, 1192–1205. [Google Scholar] [CrossRef]
- Mack, F.A.; Rathmell, W.K.; Arsham, A.M.; Gnarra, J.; Keith, B.; Simon, M.C. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 2003, 3, 75–88. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Fang, Y.; Fu, Y.; Liu, B.; Zhang, C.; Feng, S.; Lu, X. Multi-Omics Profiling to Assess Signaling Changes upon VHL Restoration and Identify Putative VHL Substrates in Clear Cell Renal Cell Carcinoma Cell Lines. Cells 2022, 11, 472. [Google Scholar] [CrossRef]
- Krieg, M.; Haas, R.; Brauch, H.; Acker, T.; Flamme, I.; Plate, K.H. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 2000, 19, 5435–5443. [Google Scholar] [CrossRef]
- Mathieu, J.; Zhou, W.; Xing, Y.; Sperber, H.; Ferreccio, A.; Agoston, Z.; Kuppusamy, K.T.; Moon, R.T.; Ruohola-Baker, H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 2014, 14, 592–605. [Google Scholar] [CrossRef]
- Guo, L.; Karoubi, G.; Duchesneau, P.; Shutova, M.V.; Sung, H.K.; Tonge, P.; Bear, C.; Rogers, I.; Nagy, A.; Waddell, T.K. Generation of Induced Progenitor-like Cells from Mature Epithelial Cells Using Interrupted Reprogramming. Stem Cell Rep. 2017, 9, 1780–1795. [Google Scholar] [CrossRef]
- Miess, H.; Dankworth, B.; Gouw, A.M.; Rosenfeldt, M.; Schmitz, W.; Jiang, M.; Saunders, B.; Howell, M.; Downward, J.; Felsher, D.W.; et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 2018, 37, 5435–5450. [Google Scholar] [CrossRef]
- Berniakovich, I.; Laricchia-Robbio, L.; Izpisua Belmonte, J.C. N-acetylcysteine protects induced pluripotent stem cells from In Vitro stress: Impact on differentiation outcome. Int. J. Dev. Biol. 2012, 56, 729–735. [Google Scholar] [CrossRef]
- Ji, J.; Sharma, V.; Qi, S.; Guarch, M.E.; Zhao, P.; Luo, Z.; Fan, W.; Wang, Y.; Mbabaali, F.; Neculai, D.; et al. Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Rep. 2014, 2, 44–51. [Google Scholar] [CrossRef]
- Cheng, L.; Lei, Q.; Yin, C.; Wang, H.Y.; Jin, K.; Xiang, M. Generation of Urine Cell-Derived Non-integrative Human iPSCs and iNSCs: A Step-by-Step Optimized Protocol. Front. Mol. Neurosci. 2017, 10, 348. [Google Scholar] [CrossRef]
- Nagy, K.; Nagy, A. Derivation of Equine-Induced Pluripotent Stem Cell Lines Using a piggyBac Transposon Delivery System and Temporal Control of Transgene Expression. Methods Mol. Biol. 2015, 1330, 79–88. [Google Scholar]
- Lee, D.S.; Shin, J.Y.; Tonge, P.D.; Puri, M.C.; Lee, S.; Park, H.; Lee, W.C.; Hussein, S.M.; Bleazard, T.; Yun, J. An epigenomic roadmap to induced pluripotency reveals DNA methylation as a reprogramming modulator. Nat. Commun. 2014, 5, 5619. [Google Scholar] [CrossRef]
- Masclef, L.; Ahmed, O.; Estavoyer, B.; Larrivee, B.; Labrecque, N.; Nijnik, A.; Affar, E.B. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 2021, 28, 606–625. [Google Scholar] [CrossRef]
- de Cubas, A.A.; Rathmell, W.K. Epigenetic modifiers: Activities in renal cell carcinoma. Nat. Rev. Urol. 2018, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.J.; Shanle, E.K.; McFadden, A.W.; Hollis, E.S.; Suttle, L.E.; Strahl, B.D.; Davis, I.J. PBRM1 bromodomains variably influence nucleosome interactions and cellular function. J. Biol. Chem. 2018, 293, 13592–13603. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichner, Z.; Shamshirgaran, Y.; Pieczonka, K.; Jonebring, A.; Kibschull, M.; Shynlova, O.; Meens, J.; Kim, R.H.; Ailles, L.; Bilican, B.; et al. VHL Gene Restoration Supports RCC Reprogramming to iPSCs but Does Not Ensure Line Stability. Cancers 2025, 17, 3693. https://doi.org/10.3390/cancers17223693
Lichner Z, Shamshirgaran Y, Pieczonka K, Jonebring A, Kibschull M, Shynlova O, Meens J, Kim RH, Ailles L, Bilican B, et al. VHL Gene Restoration Supports RCC Reprogramming to iPSCs but Does Not Ensure Line Stability. Cancers. 2025; 17(22):3693. https://doi.org/10.3390/cancers17223693
Chicago/Turabian StyleLichner, Zsuzsanna, Yasaman Shamshirgaran, Katarzyna Pieczonka, Anna Jonebring, Mark Kibschull, Oksana Shynlova, Jalna Meens, Raymond H. Kim, Laurie Ailles, Bilada Bilican, and et al. 2025. "VHL Gene Restoration Supports RCC Reprogramming to iPSCs but Does Not Ensure Line Stability" Cancers 17, no. 22: 3693. https://doi.org/10.3390/cancers17223693
APA StyleLichner, Z., Shamshirgaran, Y., Pieczonka, K., Jonebring, A., Kibschull, M., Shynlova, O., Meens, J., Kim, R. H., Ailles, L., Bilican, B., Hicks, R., & Rogers, I. M. (2025). VHL Gene Restoration Supports RCC Reprogramming to iPSCs but Does Not Ensure Line Stability. Cancers, 17(22), 3693. https://doi.org/10.3390/cancers17223693






