Simple Summary
Circulating tumor DNA (ctDNA) has emerged as a promising non-invasive biomarker that can be detected in the blood of patients with gastroesophageal adenocarcinoma (GEA). Measuring ctDNA levels can help identify patients at high risk of recurrence after surgery, evaluate treatment response, and detect molecular changes that signal resistance to therapy. ctDNA can also uncover actionable biomarkers such as ERBB2 or MSI-H and reveal resistance mechanisms. In this review, we summarize recent studies on the clinical applications of ctDNA in resectable and advanced GEA.
Abstract
The role of circulating tumor DNA (ctDNA) in gastroesophageal adenocarcinoma (GEA) has expanded in recent years. In resectable disease, postoperative ctDNA is able to detect patients at highest risk of recurrence months before scans. Tumor-informed assays provide the best sensitivity and emerging methylation assays are useful when tissue is scarce. In metastatic GEA, baseline ctDNA burden correlates with prognosis, and a decrease in ctDNA level after treatment initiation reflects therapeutic response. It can also uncover actionable targets, including ERBB2, FGFR2, and MSI-H, and detect resistance that can arise after starting treatment. Limitations include variable assay performance, low shedding in some tumors, clonal hematopoiesis confounding, and a lack of randomized data showing that ctDNA-guided changes improve outcomes. Ongoing trials are testing MRD-guided escalation/de-escalation and ctDNA-directed biomarker therapy. In this review, we evaluate the role of ctDNA in GEA cancers over recent years.
1. Introduction
Gastroesophageal adenocarcinoma (GEA), encompassing adenocarcinomas arising from the esophagus, esophagogastric junction and stomach, possesses a significant global health burden. In 2022, gastric cancer ranked as the fifth most common cancer worldwide with 968,350 new cases and 659,853 deaths [1]. Its incidence is projected to reach 1.77 million cases by 2040 [2]. In the United States alone, gastric cancer accounted for 30,300 new cases and 10,780 deaths from the disease [3]. Although early-stage esophageal and gastric adenocarcinomas have relatively favorable prognoses, most patients present with advanced disease, leading to worse survival [4,5].
Management approaches for GEA vary greatly based on the disease stage at diagnosis. For early-stage disease (stage I–II), the mainstay of management is curative-intent surgery, often in combination with perioperative systemic therapy, or neoadjuvant chemoradiotherapy [6,7,8,9,10,11]. For advanced and/or metastatic GEA, systemic therapy is required, typically involving chemotherapy, targeted therapies, and/or immunotherapy [11,12,13,14,15,16,17,18,19,20]. Additionally, treatment choice relies on different molecular biomarkers, including human epidermal growth factor receptor 2 (HER2) amplification, mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H), programmed death ligand 1 (PD-L1), Claudin 18.2, and in the near future, fibroblast growth factor receptor 2 (FGFR2) amplification, among others [21]. This points to the increasingly pivotal role biomarkers are playing in driving personalized therapeutic decisions. It is evident, therefore, that appropriate biomarker profiling is essential in maximizing treatment outcomes and optimizing clinical management in GEA.
Over the past decade, liquid biopsy (LB) has emerged as a promising and minimally invasive strategy for the management of cancer [22]. LB can detect and assess different types of biomarkers such as circulating tumor cells (CTCs), proteins, cell-free ribonucleic acid (cfRNA), autoantibodies, extracellular vesicles, and cell-free deoxyribonucleic acid (cfDNA) [23]. Among them, cfDNA has gained considerable popularity. Recent research has focused specifically on the tumor-derived component of cfDNA, known as circulating tumor DNA (ctDNA), which offers greater specificity for cancer detection and monitoring. Due to its close relationship with the tumor, ctDNA has emerged as the defining biomarker in LB and has been a trending area of research in recent years [24].
The applications of ctDNA include early detection and diagnosis, molecular profiling, monitoring for minimal residual disease (MRD), assessing treatment response, and identifying resistance mechanisms/actionable alterations [25]. Its clinical utility is well-established in several malignancies including colorectal, lung, and breast cancers [24,25,26,27]. This has paved the way for Food and Drug Administration (FDA)-approved technologies based on ctDNA (i.e., Guardant360 CDx and FoundationOne Liquid CDx) that allow for the real-time monitoring of cancer and genomic profiling [28,29]. In GEA, ctDNA as an MRD method has not reached routine clinical practice yet, but several studies have investigated its uses.
In this narrative review, we evaluated the evidence currently available for the use and limitations of ctDNA in the workup and management of GEA.
2. ctDNA
In healthy people, hematopoietic cells are the main source of cfDNA; however, in cancer patients, a variable fraction of cfDNA comes from tumor cells and is therefore termed ctDNA [30]. ctDNA, composed of DNA fragments ranging from 70 to 200 base pairs to 21 kb fragments, can be released into the systemic circulation by the primary/metastatic tumor cells, and CTCs via cell apoptosis, necrosis, or active secretion [31,32]. ctDNA fragments are typically smaller than normal cfDNA which helps in their detection [33]. The growing interest in ctDNA is owed to its theoretical advantages over conventional tissue biopsies, as it is less invasive, uses fewer resources, and makes it possible to capture changes in tumor heterogeneity, molecular evolution, and treatment response as it can be repeated over time [34,35]. Additionally, ctDNA levels are believed to correlate closely with the overall tumor burden, and numerous studies have demonstrated strong concordance between genetic alterations detected in tumor tissue (including copy number changes, single nucleotide variants, point mutations, and epigenetic modifications) and those identified in ctDNA; especially given that ctDNA is shed by the multiple tumor subclones within the same patient [35,36]. Notably, owing to the short half-life of ctDNA, ranging between 16 min and 2.5 h, ctDNA can reflect tumor dynamics in near real-time, making it a valuable tool for monitoring disease progression and serving as a potential prognostic biomarker [37,38].
There are two general approaches to quantifying ctDNA, tumor-informed (targeted) and tumor-agnostic (untargeted) (Figure 1). The tumor-informed approach requires prior knowledge of the specific mutations or alterations contained within the tumor, which necessitates a tissue biopsy. This approach has repeatedly shown higher sensitivity and specificity and is therefore appropriate to assess for MRD, evaluate treatment response, and monitor early recurrence [39,40,41]. The tumor-agnostic approach, on the other hand, does not require prior tumor knowledge, which makes it cost-effective and accessible, especially when tumor tissue is unavailable. It enables the comprehensive characterization of the molecular landscape of the tumor and facilitates monitoring acquired mutations and resistance mechanisms, making it ideal to capture intratumor heterogeneity [39,42]. Importantly, tumor-agnostic tests have the benefit of faster turn-around times; however, they do come at the cost of lower sensitivity and requiring high concentrations of ctDNA [39]. Ultimately, the appropriate approach should be selected based on the clinical context. Figure 1 compares tumor-informed and tumor-agnostic ctDNA testing approaches.
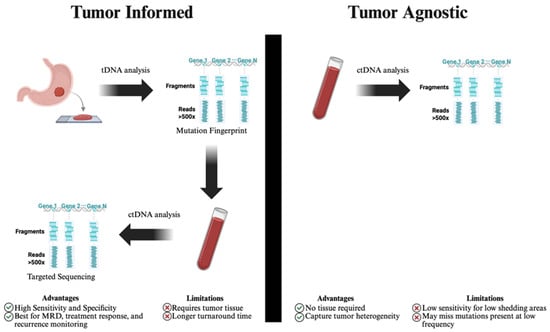
Figure 1.
Comparison of tumor-informed and tumor-agnostic ctDNA testing approaches. ctDNA: circulating tumor deoxyribonucleic acid, MRD: minimal residual disease, tDNA: tissue deoxyribonucleic acid. Tumor-informed ctDNA analysis begins with tissue-based sequencing to identify a patient-specific mutation fingerprint, which is then tracked in plasma using targeted sequencing. This approach offers high sensitivity and is ideal for minimal residual disease (MRD) detection and treatment monitoring but requires tumor tissue and has longer turnaround time. Tumor-agnostic analysis, in contrast, directly sequences ctDNA from plasma without prior tumor profiling, enabling detection of actionable mutations and tumor heterogeneity, though it may have reduced sensitivity in low-shedding tumors or low-allele-frequency variants.
3. MRD
3.1. Prognostic Value of ctDNA-Based MRD
Recurrence after curative surgery remains the principal hurdle to durable survival in GEA. Contemporary peri-operative trials still show significant risk of recurrence often at a stage no longer amenable to salvage therapy [7,9,10,43,44,45]. Conventional surveillance with cross-sectional computed tomography (CT) ± carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) misses sub-clinical disease, often attributed to imaging requiring a macroscopic tumor volume and serum markers lacking sensitivity [46,47]. ctDNA offers a biologically grounded alternative: fragments released from dying tumor clones are measurable in plasma within hours, so their persistence or re-emergence after treatment signals molecular residual disease, sometimes months before radiology [48]. Figure 2 summarizes the potential clinical workflow and timing of ctDNA assessment across the diagnostic, neoadjuvant, and postoperative settings in resectable GEA.
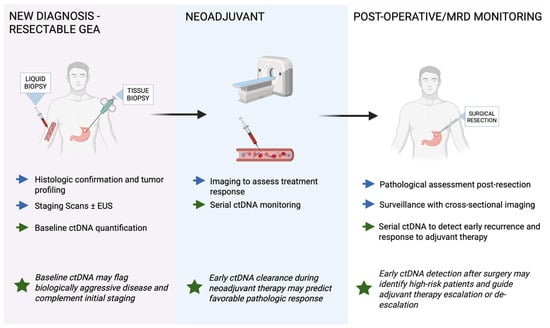
Figure 2.
Clinical workflow of potential ctDNA applications in resectable gastroesophageal adenocarcinoma (GEA). Blue arrows indicate standard management steps, while green arrows represent ctDNA applications across diagnostic, neoadjuvant, and postoperative phases. Green stars highlight key clinical implications. ctDNA can complement standard tissue-based testing at diagnosis, provide dynamic monitoring during neoadjuvant therapy, and detect molecular residual disease (MRD) after curative resection. Baseline ctDNA may help identify biologically aggressive tumors, early ctDNA clearance during neoadjuvant therapy can indicate favorable pathologic response, and postoperative ctDNA detection may reveal high-risk patients suitable for adjuvant therapy escalation or closer surveillance.
Tumor-informed multiplex-PCR assays provide some of the most mature evidence. In a real-world 11-center cohort of 295 stage I–III esophagogastric cancers, postoperative ctDNA was found in 23.5% of patients within 16 weeks of resection; 81.2% of these individuals relapsed vs. 13.5% of their ctDNA-negative counterparts, with a recurrence-free survival (RFS) hazard ratio (HR) of ~10.7 [95% confidence interval (CI) 4.3–29.3; p < 0.0001] [28]. Even more striking, ctDNA positivity at any time point postoperatively was associated with an 88.2% recurrence rate compared to 5.5% in ctDNA-negative patients, translating to a HR of 23.6 (95% CI 10.2–66.0; p < 0.0001) and a median RFS of 9.6 months vs. not reached. A subgroup analysis within the same cohort focused on 42 patients who achieved a pathologic complete or near-complete response (TRG 0–1) after neoadjuvant therapy, and still found that postoperative ctDNA positivity was associated with a sixfold increased risk of recurrence (RFS HR 6.2); notably, every patient with ctDNA positivity during surveillance eventually relapsed (HR 37.6), underscoring the prognostic power of ctDNA even in presumed clinically low-risk patients [49]. Emerging European data further support the tentative role of ctDNA while also introducing the feasibility of tumor-agnostic, methylation-based approaches. In a study of 86 patients with resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma, a series of ctDNA testing was performed using TriMeth, a droplet digital PCR assay that identifies three gastrointestinal (GI) cancer-specific methylation markers [50]. ctDNA was detectable in 56% of patients prior to treatment [clinical tumor stage 3/4 (cT3/4) in 88.6%, and clinical node-positive (cN+) in 45.5%], but persisted in only 15% of patients at four weeks following surgical resection. Participants who remained ctDNA-positive at 4 weeks post-surgery experienced significantly worse outcomes, with a 24-month RFS of 12.5% (95% CI 2.0–78.2) compared to 70.7% (95% CI 58.4–85.5) in ctDNA-negative patients, and worse overall survival (OS) (24-month OS of ~20% vs. ~80% in ctDNA-negative patients; HR = 6.37, p = 0.001). Importantly, these observations remained significant in a multivariable analysis, independent of tumor stage or lymph node involvement. These data highlight that even without access to tumor-specific mutations, ctDNA-based MRD assessment using methylation signatures can identify high-risk patients and potentially guide personalized post-operative management.
Tumor-informed deep-sequencing studies in Asia add granularity. In a prospective study of 46 patients with stage I–III gastric cancer, ctDNA analysis was performed using a broad 1021-gene panel with serial plasma sampling [51]. Postoperative ctDNA was detectable in 18% of patients and was strongly associated with worse outcomes, with median disease-free survival (DFS) of 216 days versus not reached (DFS HR 6.56; OS HR 5.96), and all ctDNA-positive patients ultimately relapsing compared to 32% of ctDNA-negative patients (p = 0.0015). Longitudinal sampling further strengthened this association: ctDNA positivity at any postoperative time point conferred a DFS HR of 14.8 and preceded radiographic recurrence by a median of 179 days. In this cohort, ctDNA identified 84% of patients who relapsed and correctly classified 96% of those without recurrence, highlighting its accuracy and substantial lead-time advantage in identifying patients at highest risk of relapse following curative-intent surgery. Similarly, a prospective Chinese study evaluated 79 patients with stage II–III gastric cancer who received neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy [52]. ctDNA was analyzed at multiple time points using a high-depth hybrid-capture NGS assay targeting 425 cancer-related genes, with matched leukocyte DNA used to exclude germline and clonal hematopoiesis variants. Early molecular clearance after neoadjuvant therapy was associated with significantly improved survival, with a 3-year OS of 73% in ctDNA-negative patients vs. 34% in those with persistent ctDNA. Postoperative ctDNA positivity further stratified risk, with 3-year OS of 38% in ctDNA-positive patients compared to 68% in ctDNA-negative patients. Patients with persistently undetectable ctDNA after both neoadjuvant therapy and surgery had the most favorable outcomes, while conversion from negative to positive ctDNA after neoadjuvant therapy portended the worst survival.
A prospective analysis from the PLAGAST study evaluated 62 patients with locally advanced resectable gastric or GEJ adenocarcinoma using a tumor-informed 16-plex multiplex PCR assay (Signatera) to assess ctDNA at baseline, during neoadjuvant therapy, after neoadjuvant therapy, and in the postsurgical MRD window prior to adjuvant treatment [53]. Clearance of ctDNA during neoadjuvant therapy was associated with significantly improved outcomes, with a median RFS not reached vs. 13.3 months in patients with persistent ctDNA (HR 6.17; p = 0.002), and a 24-month OS rate of 95% vs. 59%. Post-NACT ctDNA positivity also predicted worse outcomes (mRFS 7.8 months vs. not reached; RFS HR 5.26; OS HR 7.35). In the MRD window, patients who remained ctDNA-positive had a median RFS of 3.6 months and OS of 8.6 months compared to not reached in ctDNA-negative patients (RFS HR 12.9; OS HR 14.5; both p < 0.0001). Patients who cleared ctDNA early had better 24-month RFS and OS rates than those who cleared late or remained positive. Persistent ctDNA positivity was also associated with poor pathologic response (TRG 4–5; p = 0.035). These results support the role of serial ctDNA monitoring to assess treatment response and identify patients at high risk of recurrence.
Preliminary results from a substudy of the phase III EXODOX trial (NCT04787354) also support the prognostic value of ctDNA in stage II–III gastric cancer [54]. Forty-two patients underwent tumor-informed MRD assessment using the CancerDetect™ (CeGaT GmbH, Tübingen, Germany.) assay at serial postoperative time points. Baseline ctDNA positivity was 45.2% and was more frequent in stage III than stage II disease (56.7% vs. 16.7%). ctDNA positivity at 6 and 12 months (29.6% and 35.2%) was significantly associated with inferior RFS, while baseline status alone was not. Dynamic changes in ctDNA further refined prognosis, as patients with persistent ctDNA positivity had the worst outcomes, whereas those who achieved ctDNA clearance after adjuvant chemotherapy had survival outcomes comparable to patients who remained negative throughout.
Evidence from a systematic review and meta-analysis of eight studies involving 423 patients supports these single-study findings [55]. Preoperative ctDNA positivity was associated with an increased risk of recurrence [relative risk (RR) 1.79; 95% CI 1.19–2.71], although individual studies demonstrated variability, likely due to limited sample sizes. Postoperative ctDNA detection conferred a stronger association with recurrence risk, with pooled risk ratios ranging from 3.17 to 3.68 depending on the model used. Across studies, ctDNA positivity was also associated with inferior RFS, with a pooled HR of 6.37 (95% CI 2.70–15.01). When stratified by timing, postoperative ctDNA positivity showed the highest prognostic value, correlating with a 14.09-fold increased risk of recurrence (95% CI 7.31–27.15). Similarly, ctDNA-positive status predicted worse OS, with pooled HRs of 4.58 (95% CI 1.68–12.49) for all time points and 11.78 (95% CI 4.40–31.53) for postoperative detection. These pooled data support ctDNA as a predictor of recurrence and survival across diverse cohorts and assay platforms, especially in the post-operative setting. Table 1 summarizes the major studies evaluating postoperative ctDNA-based MRD in resectable, including assay type, study design, and key outcomes.

Table 1.
Studies evaluating postoperative ctDNA-based MRD in resectable GEA.
3.2. MRD-Guided Management
The MRD-GATE trial is testing ctDNA-guided adjuvant chemotherapy in stage II–III gastric cancer after curative gastrectomy [56]. Interim results were presented at the recent ASCO annual meeting. Patients underwent tumor-informed ctDNA testing 28 days postoperatively and at regular intervals. MRD-negative patients received de-escalated therapy (observation for stage II, S-1 monotherapy for stage III) but could escalate to oxaliplatin-based doublets if they converted to MRD-positive. Baseline MRD-positive patients started with combination chemotherapy. Among 65 patients enrolled, 14 were MRD-positive and all received combination therapy. On the other hand, among 51 baseline-MRD negative patients, 45 received deescalated therapy at onset (9 received combination therapy after MRD conversion, and one refused) and six received combination therapy. The study is ongoing for follow-up and reporting the 3-year DFS (primary endpoint). However, it showed the feasibility of MRD-guided treatment, reducing adjuvant chemotherapy rate with acceptable 1-year DFS of 86.2%, and fewer toxicities.
Although not specific to gastric cancer, the CT002 trial evaluated ctDNA-guided adjuvant PD-1 blockade in resected dMMR solid tumors, including gastric/GEJ cancers. ctDNA was assessed 6–10 weeks after surgery. Patients who were ctDNA-positive received six months of pembrolizumab, while ctDNA-negative patients were observed. Early results presented at AACR 2025 showed that 85% (11/13) of ctDNA-positive patients treated with PD-1 blockade cleared ctDNA at six months, with a recurrence rate of 38%, compared with 100% recurrence in ctDNA-positive patients who did not receive immunotherapy. ctDNA-negative patients had favorable outcomes with observation alone, with a 2-year OS of 98% and recurrence in ~6%, supporting the safety of utilizing ctDNA to de-escalate therapy [57].
Several other trials are investigating MRD-based management with results still awaited. The CLAYMORE phase III trial in China is enrolling 416 patients with resected stage III gastric or GEJ adenocarcinoma, who are ctDNA-positive after surgery. Participants are randomized to receive adjuvant SOX chemotherapy with or without the PD-1 inhibitor tislelizumab. The trial will test whether adding immunotherapy improves DFS compared with chemotherapy alone. Results are awaited, but this is one of the first large randomized studies designed specifically for ctDNA-positive gastric cancer patients [58]. DECIPHER is a phase II trial conducted in the UK to evaluate trastuzumab deruxtecan (T-DXd) in operable HER2-positive GEA with detectable ctDNA after surgery and perioperative FLOT. The single-arm study uses Signatera for ctDNA detection and enrolls patients who have cleared standard multimodality therapy but remain ctDNA-positive. T-DXd is given for up to eight cycles, with the primary endpoint being ctDNA clearance after four cycles. Secondary endpoints include DFS, OS, quality of life, and safety. Recruitment is ongoing across multiple UK centers, with a planned sample size of 25 evaluable patients [59].
Interpretation of ctDNA-based MRD studies remains challenging due to methodological heterogeneity across cohorts. Variations in assay platforms (e.g., tumor-informed versus tumor-agnostic approaches), timing of blood collection relative to surgery or treatment, and thresholds used to define ctDNA positivity can substantially affect sensitivity and prognostic accuracy. These differences complicate direct comparison between studies and may partly explain the variability in reported recurrence risk estimates. Moreover, many available MRD studies are retrospective, single-center, and involve small patient populations, which may introduce selection and publication biases that overestimate the prognostic value of ctDNA. Prospective, standardized studies with harmonized methodologies and predefined endpoints are needed to validate ctDNA as a robust biomarker for MRD detection in gastroesophageal cancer.
Despite its strong prognostic correlations, there remain significant limitations when it comes to ctDNA-based MRD testing. The sensitivity of ctDNA appears to be suboptimal when measured at a single time point even with tumor-informed assays especially when tested too soon after surgery. Longitudinal testing, on the other hand, could improve sensitivity. However, given that the best timing to decide on adjuvant therapy is typically 4–8 weeks after the surgery; it remains unclear whether a one-time negative ctDNA result holds any clinical significance. Another question that remains unresolved is whether ctDNA is a reliable predictive factor to base the decision of escalating therapy on. In the case of colorectal cancer for instance, the ALTAIR trial tested adjuvant trifluridine/tipiracil (FTD/TPI) in patients with resected MRD-positive disease. The study did not meet its primary endpoint, with no statistically significant difference in DFS between FTD/TPI and placebo (median 9.3 vs. 5.6 months; HR 0.79, p = 0.107). This underscores that while ctDNA is a powerful prognostic tool, its role as a predictive biomarker of treatment benefit is not yet established. Therefore, caution should be taken when integrating ctDNA status into treatment decision making in GEA until we have more mature prospective evidence.
In addition, ctDNA detection is inherently limited in low-shedding GEA tumors, where the amount of tumor-derived DNA released into circulation may be minimal, leading to false-negative MRD results [60]. Although ctDNA clearance often correlates with improved outcomes, this finding remains prognostic rather than predictive, as there is no evidence that treatment modification based on ctDNA dynamics improves survival. Results from interventional studies, such as ALTAIR and early MRD-GATE, have not shown clear benefit from ctDNA-guided escalation, underscoring the need for randomized validation before such strategies are applied in clinical practice. Moreover, extrapolating MRD frameworks from colorectal or lung cancer to GEA should be performed with caution, as differences in tumor biology, histology, and shedding kinetics may affect assay performance and clinical interpretation. The detectability of ctDNA can also be influenced by several biologic and clinicopathologic factors, including tumor histology, differentiation, stage, and metastatic pattern. These determinants may partly explain interstudy variability in ctDNA yield and assay sensitivity. Table 2 summarizes representative studies exploring how these features impact ctDNA shedding and detection in GEA.

Table 2.
Biologic and clinicopathologic factors influencing ctDNA detectability in GEA.
4. ctDNA in Advanced/Metastatic GEA
4.1. Baseline Prognostic Value
Similarly to the early-stage setting, multiple studies have shown the prognostic utility of ctDNA in metastatic GEA patients. In the prospective LIQUID BIO study, investigators enrolled 72 patients with metastatic GEA and used a tumor-informed amplicon NGS assay that tracked up to 48 patient specific single nucleotide variants [64]. ctDNA was detectable in 75% of cases, and patients with two or more tracked mutations at baseline had considerably worse outcomes, with median progression-free survival (PFS) of 4.5 months vs. 9.2 months (p-value = 0.009) and OS of 8.4 months vs. 16.5 months (p-value = 0.014). Meanwhile, real-world data from the GuardantINFORM registry, which included 824 US patients with advanced gastric cancer profiled via the tumor-agnostic Guardant360 hybrid-capture NGS assay (73 genes), showed that a baseline maximum variant allele frequency (VAF) > 2.9% predicted an earlier need for therapy change (4.8 vs. 7.4 months, p-value < 0.001) and shorter OS (13.2 vs. 19.1 months, p-value < 0.001) even after adjusting for standard clinical factors such as Eastern Cooperative Oncology Group (ECOG) status and metastatic burden [65]. A meta-analysis of 22 ctDNA studies, predominantly using tumor-agnostic ddPCR or ≤50 gene NGS panels in 1144 metastatic GEA, found that ctDNA positivity at baseline carried a pooled HR for death of 3.87 [66]. Despite methodological differences, these studies consistently demonstrate that higher ctDNA burden at diagnosis is associated with poorer prognosis in metastatic GEA. Figure 3 illustrates how ctDNA is integrated across the disease course in metastatic GEA, from diagnosis and treatment monitoring to detection of actionable mutations and resistance mechanisms.
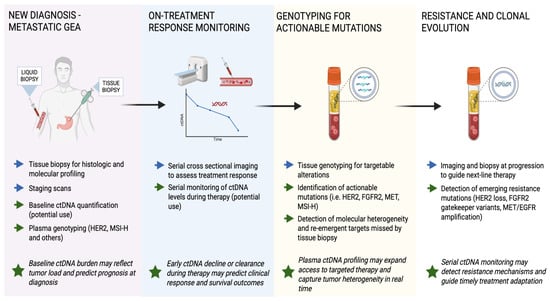
Figure 3.
Clinical workflow of ctDNA applications in metastatic gastroesophageal adenocarcinoma (GEA). Blue arrows indicate standard management steps, while green arrows represent ctDNA-based applications across diagnostic, therapeutic, and resistance settings. Green stars highlight key clinical implications. In metastatic GEA, ctDNA serves multiple roles: establishing baseline molecular profiles, monitoring treatment response, identifying actionable mutations for targeted therapy, and detecting emerging resistance before radiographic progression. Serial ctDNA monitoring provides a real-time, non-invasive view of tumor dynamics and may guide therapy adaptation or sequencing in advanced disease.
4.2. On-Treatment Response Monitoring
Beyond prognostic potential, ctDNA has been explored as a tool for dynamic response monitoring during therapy. The phase II PRODIGE 59 DURIGAST trial involved 97 heavily pre-treated patients with advanced GEA and used tumor-agnostic methylation-based ddPCR (TriMeth) to assess ctDNA changes at baseline and week 4 of therapy [67]. Patients experiencing a ≥75% drop in methylation signal had markedly better outcomes, objective response rate (ORR) 55%, median PFS 7.4 months, and OS 16.0 months, compared to those without such decline, who had an ORR of 16%, PFS 2.2 months, and OS 6.6 months (p < 0.05 for the three endpoints). In a separate cohort of 30 chemotherapy-naïve metastatic gastric cancer patients treated with pembrolizumab plus capecitabine/oxaliplatin, a tumor-agnostic 425-gene hybrid-capture NGS assay was used to assess plasma ctDNA [68]. Clearance of baseline ctDNA by cycle 2 (achieved in 8 patients) was associated with significantly improved outcomes, ORR 83% and median PFS 15.6 months, compared to persistent ctDNA (n = 22), where ORR was 29% and median PFS was only 6.0 months (p < 0.05 for both endpoints). While neither study incorporated ctDNA to guide treatment decisions prospectively, their results strongly support the potential of early ctDNA dynamics to reflect therapeutic efficacy, with implications for future interventional trials.
4.3. Plasma Genotyping for Actionable Targets
ctDNA-based genotyping has shown substantial value in identifying actionable molecular targets, especially when tumor tissue is insufficient or inaccessible. In a large retrospective analysis of 1630 metastatic GEA patients using the tumor-agnostic Guardant360 hybrid-capture panel, Maron and colleagues identified key actionable alterations: HER2 amplified in 9.5%, FGFR2 in 7.7%, MET proto-oncogene, receptor tyrosine kinase (MET) in 5.6%, epidermal growth factor receptor (EGFR) in 4.9%, and MSI-H in 3.2% [29]. Notably, in the subgroup whose HER2 amplification was detected only in plasma, and who subsequently received trastuzumab, the median OS reached 26.3 months, compared with just 7.4 months for plasma-positive patients who did not receive HER2-targeted therapy, highlighting how ctDNA can meaningfully extend therapeutic access (p = 0.002). Similarly, Schrock et al. studied 160 patients with metastatic GEA who underwent matched tissue and plasma genotyping using the same tumor-agnostic hybrid-capture NGS platform (FoundationOne for tissue, Guardant360 for plasma) [69]. Plasma ctDNA recapitulated more than 80% of tissue-detected driver alterations and uncovered additional, potentially actionable mutations in approximately one-third of patients, demonstrating ctDNA’s ability to capture spatial and clonal tumor heterogeneity beyond what is seen in a single-site tissue biopsy. Real-world data also support the clinical utility of plasma-based HER2 detection to guide therapy in advanced GEA [70]. In a GuardantINFORM analysis, 215 patients with ERBB2 amplification detected by ctDNA were identified, of whom 135 (63%) received HER2-directed therapy. Patients treated based on ctDNA findings had significantly longer real-world time to treatment discontinuation (rwTTD 5.8 vs. 1.9 months; HR 0.47, 95% CI 0.34–0.65, p < 0.01) and real-world time to next treatment (rwTTNT 9.4 vs. 6.3 months; HR 0.55, 95% CI 0.37–0.81, p < 0.01), although no statistically significant difference in real-world OS was observed (not reached vs. 22 months; HR 0.67, 95% CI 0.41–1.08, p = 0.10). These findings suggest that ctDNA-identified ERBB2 amplification can be used to expand access to HER2-directed therapy by identifying patients who retain or reacquire HER2 amplification and could benefit from continued or reintroduced HER2-targeted therapy. Results from an exploratory analysis based on the phase II DESTINY-Gastric01 trial further highlighted ctDNA as a marker in refining patient selection for T-DXd [71]. In the primary cohort of HER2-positive gastric cancer, plasma HER2 amplification detected by ctDNA correlated with higher ORR (61% vs. 34% in patients without amplification). Patients with high adjusted plasma copy number (apCN ≥ 18.2) of HER2 had favorable outcomes, with ORR approaching 79% and median OS of 16.6 months compared to 8.6 months in those with low apCN. On the other hand, detection of co-amplifications in MET, EGFR, or FGFR2 using ctDNA was associated with lower ORR, potentially pointing towards mechanisms of resistance. These results highlight how liquid biopsy-based HER2 quantification could augment tissue testing by capturing spatial heterogeneity and help identify patients likely to benefit from HER2-targeted therapy.
Loss of HER2 expression following trastuzumab-based therapy is increasingly recognized as a major clinical challenge in metastatic GEA. The prospective GASTHER3 study evaluated 48 patients with initially HER2-positive gastric cancer who underwent paired biopsies before and after first-line trastuzumab-containing chemotherapy [72]. Nearly one-third (29%) of patients lost HER2 positivity on post-progression biopsy, accompanied by a significant decline in median H-score (from 225 to 175, p = 0.047) and an increase in HER2 genetic heterogeneity (from 2.9% to 21.9%). Among those who received second-line trastuzumab emtansine, patients with persistent HER2 amplification had an ORR of 44%, whereas none of the patients who lost HER2 expression responded, suggesting that loss of HER2 may drive resistance to subsequent anti-HER2 therapy. These data underscore the dynamic nature of HER2 expression under therapeutic pressure. In this context, ctDNA testing offers a practical and less invasive alternative to repeat biopsies for reassessing HER2 status as shown in the GuardantINFORM study.
Similarly to HER2, other biomarkers and gene alterations can be detected in ctDNA and can potentially guide treatment. In 2022, Jogo et al. applied a tumor-agnostic 300 gene hybrid-capture NGS panel to 365 Japanese patients with metastatic gastric cancer [73]. They detected FGFR2 amplification in 7.7% of plasma samples compared to just ~3% in matched tissue, with two plasma positive/tissue negative patients responding to the FGFR2 inhibitor, futibatinib. The PANGEA trial tested a biomarker-driven treatment algorithm in metastatic GEA using serial tissue and plasma profiling [74]. Sixty-eight patients were enrolled, with a 1-year OS of 66% and a median OS of 15.7 months, superior to historical controls. Notably, one-third of patients showed discordance between primary and metastatic tissue at baseline, and nearly half of the patients showed a change in their biomarker group at the time of progression. Of note, two patients with FGFR2 amplification were treated with bemarituzumab plus mFOLFOX6 with initial benefit; however, both patients progressed and were eventually reassigned to different groups, illustrating resistance development. In RTK-amplified tumors more broadly, progression was often accompanied by loss of amplification or acquisition of MAPK/PI3K alterations, and in some cases PD-L1 upregulation. These studies underscore ctDNA’s relevance not only in highlighting known targets but in identifying otherwise undetected mutations that can influence treatment.
While these findings highlight the expanding role of ctDNA in identifying actionable alterations, several interpretive challenges remain. Analytical concordance between ctDNA and tissue genotyping varies by target and assay, typically ranging from 70 to 90% for amplifications such as HER2 or FGFR2 [75]. However, thresholds for calling amplification events in plasma are not standardized, with most platforms relying on assay-specific copy number or allele frequency cutoffs that may underdetect low-level amplifications. Detection rates also correlate with tumor burden, site of metastasis, and prior systemic therapy, with patients who have liver-dominant or high-volume disease generally exhibiting higher ctDNA yield [76]. Importantly, these associations are largely retrospective, and plasma-detected targets still require validation in prospective biomarker-selected trials before guiding routine treatment [77]. Overall, while ctDNA offers a valuable complement to tissue profiling, its therapeutic implications remain hypothesis-generating rather than practice-changing until supported by randomized data.
4.4. Resistance and Clonal Evolution
In addition to tracking response and guiding therapy, ctDNA has also demonstrated promise for early detection of resistance mechanisms before radiographic progression. This application may be particularly useful in tailoring salvage therapy strategies. In the PRODIGE 59 DURIGAST trial, 97 patients with metastatic HER2-positive gastric or GEJ adenocarcinoma underwent serial ctDNA monitoring using a tumor-agnostic methylation-based ddPCR assay (TriMeth) and amplicon-based NGS [67]. Among 37 patients with plasma available at progression, emergent resistance alterations, MET amplifications (n = 4), EGFR amplifications (n = 2), and secondary HER2 mutations (n = 3) were detected in ctDNA but absent at baseline, highlighting its utility for early resistance detection. Furthermore, patients with ≥75% decline in methylation signal at week 4 had improved outcomes: ORR 55%, PFS 7.4 months, and OS 16.0 months, versus ORR 16%, PFS 2.2 months, and OS 6.6 months in those without early clearance (p < 0.05 for the three endpoints). Jogo et al.’s FGFR2 study further reinforced this concept: gatekeeper N549H/K mutations, linked to resistance to FGFR2 blockade, emerged in plasma ctDNA 4 to 12 weeks before radiologic progression, underscoring ctDNA’s ability to anticipate relapse [73]. These findings collectively advocate for ctDNA as a non-invasive tool to assess clonal evolution and resistance mechanisms. For example, loss of HER2 amplification or the appearance of FGFR2 gatekeeper mutations may signal resistance and prompt a switch to another targeted or combination therapy. Ongoing ctDNA monitoring could also help track how well these adjustments are working, supporting a more flexible and individualized approach to managing advanced GEA.
4.5. Implementation, Feasibility and Limitations
On a practical level, the integration of ctDNA testing into oncology workflows is becoming increasingly feasible. ctDNA testing reduced the median time to clinical trial enrollment from 33 days to 11 days (p-value < 0.0001) [78]. These findings highlight the potential of ctDNA to expedite patient access to biomarker-guided therapies, though broader adoption will require improvements in standardization, cost-efficiency, and clinician training. However, pre-analytical and analytical variability remain major barriers to consistency and reproducibility. Differences in plasma processing time, ctDNA extraction methods, and storage conditions can affect yield and fragment integrity, while variation in library preparation and variant-calling algorithms contributes to inconsistent detection sensitivity. The absence of standardized cutoffs for variant allele frequency or copy-number gain, as well as differences in reporting formats, complicates cross-study comparison [79]. International initiatives such as the BloodPAC Consortium and the ESMO Translational Research Working Group are developing frameworks to harmonize assay validation and quality control, but universal standards are still lacking [80,81].
While ctDNA testing continues to expand, its cost and accessibility remain major barriers to global implementation. Tumor-informed assays require sequencing of both the tumor and multiple plasma samples, with per-test costs often exceeding $3000 USD in the United States. Such expenses make routine use difficult, particularly in low- and middle-income countries where gastric and esophageal cancers are most prevalent [82]. Simpler methylation-based approaches, such as TriMeth, may offer more affordable alternatives, as they avoid individualized tumor sequencing. For ctDNA testing to achieve broader impact, improving affordability and access will be as important as refining technical performance.
While multiple studies have evaluated ctDNA in metastatic or advanced GEA, comparing results across them is challenging due to methodological differences. Some studies used tumor-informed multiplex PCR assays with high specificity for known variants, whereas others applied tumor-agnostic hybrid-capture NGS or methylation-based approaches, which provide broader coverage but lower sensitivity. These variations influence ctDNA detection rates, the interpretation of VAF, and the generalizability of reported outcomes.
Beyond methodological variability, ctDNA interpretation can be complicated by biological and technical factors. Discordance between tissue and plasma genotyping may arise from sampling at different time points or intratumoral heterogeneity, while clonal hematopoiesis of indeterminate potential (CHIP) can lead to false-positive results by introducing non-tumor variants into plasma DNA [83]. Recognizing these limitations is critical to ensure accurate interpretation and clinical decision making.
Commercial ctDNA assays such as Guardant360, FoundationOne Liquid, and Signatera have facilitated broader clinical use but also introduce important considerations. Each platform differs in design, ranging from tumor-informed to tumor-agnostic approaches, and may vary in analytical sensitivity, genomic coverage, and reporting thresholds. These differences, along with potential conflicts of interest and limited cross-platform validation, can influence reported performance metrics and complicate data interpretation. Independent, head-to-head evaluations are needed to standardize assay performance and ensure that clinical adoption is guided by objective evidence rather than proprietary variability.
Currently, ctDNA should be viewed as a valuable complement to imaging and tissue biopsy in the care of patients with metastatic GEA, with ongoing trials expected to define its role more clearly in the years ahead. Table 3 summarizes all ctDNA applications across MRD, prognosis, therapy monitoring, detecting actionable alterations, and resistance.

Table 3.
Clinical Applications and Limitations of ctDNA in Gastroesophageal Adenocarcinoma and Their Implications.
5. Conclusions
ctDNA emerged as a powerful adjunctive tool in GEA, offering real-time molecular insights that extend beyond traditional imaging and tissue biopsy. Despite these advances, ctDNA remains primarily a prognostic rather than predictive biomarker. Its optimal timing, assay platform, and interpretation thresholds are not yet standardized, and low-shedding tumors and clonal hematopoiesis continue to limit sensitivity and specificity. Ongoing randomized and biomarker-guided trials will determine whether ctDNA-directed escalation or de-escalation improves outcomes.
Future research should focus on harmonizing assay methodologies, establishing validated thresholds for ctDNA detection, and determining whether ctDNA-guided escalation or de-escalation strategies translate into survival benefit. Equally important will be addressing cost and access barriers, particularly in regions where GEA burden is highest. With robust standardization, prospective validation, and integration into biomarker-driven clinical trials, ctDNA holds the potential to transform precision management in GEA by refining risk stratification, guiding adjuvant decisions, and enabling real-time therapeutic monitoring. For now, ctDNA should be viewed as complementary—not substitutive—to imaging and tissue biopsy.
Funding
No funding has been received for this project.
Conflicts of Interest
MBS: Consulting (self): Novartis; Consulting (inst): boehringer Ingelheim and Bayer; Research support (inst): Taiho, Eli Lilly. OS: declares no conflict of intrest. LB: declares no conflict of interest. FJ: declares no conflict of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Ferrara, D.; Manzione, L. New perspectives in the treatment of advanced or metastatic gastric cancer. World J. Gastroenterol. 2009, 15, 2689–2692. [Google Scholar] [CrossRef]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, J.; Lordick, F.; Brunner, T.; Glatz, T.; Bronsert, P.; Röthling, N.; Schmoor, C.; Lorenz, D.; Ell, C.; Hopt, U.T.; et al. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, 503. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Al-Batran, S.-E.; Wainberg, Z.A.; Muro, K.; Molena, D.; Van Cutsem, E.; Hyung, W.J.; Wyrwicz, L.; Oh, D.-Y.; Omori, T.; et al. Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer. N. Engl. J. Med. 2025, 393, 217–230. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Hoeppner, J.; Brunner, T.; Schmoor, C.; Bronsert, P.; Kulemann, B.; Claus, R.; Utzolino, S.; Izbicki, J.R.; Gockel, I.; Gerdes, B.; et al. Perioperative Chemotherapy or Preoperative Chemoradiotherapy in Esophageal Cancer. N. Engl. J. Med. 2025, 392, 323–335. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers, Version 4.2025. 2025. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433&utm_source=chatgpt.com (accessed on 1 September 2025).
- Ajani, J.A.; D’aMico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Shah, M.A.; Kennedy, E.B.; Alarcon-Rozas, A.E.; Alcindor, T.; Bartley, A.N.; Malowany, A.B.; Bhadkamkar, N.A.; Deighton, D.C.; Janjigian, Y.; Karippot, A.; et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1470–1491. [Google Scholar] [CrossRef]
- Shah, M.A.; Kennedy, E.B.; Catenacci, D.V.; Deighton, D.C.; Goodman, K.A.; Malhotra, N.K.; Willett, C.; Stiles, B.; Sharma, P.; Tang, L.; et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2677–2694. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Oh, D.-Y.; Yañez, P.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Plazas, J.G.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Oh, D.-Y.; Kato, K.; Arkenau, T.; Tabernero, J.; Correa, M.C.; Zimina, A.V.; Bai, Y.; Shi, J.; Lee, K.-W.; et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 2024, 385, e078876. [Google Scholar] [CrossRef]
- Korpan, M.; Puhr, H.C.; Berger, J.M.; Friedrich, A.; Prager, G.W.; Preusser, M.; Ilhan-Mutlu, A. Current Landscape of Molecular Biomarkers in Gastroesophageal Tumors and Potential Strategies for Co-Expression Patterns. Cancers 2025, 17, 340. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Huang, Z.B.; Zhang, H.T.; Yu, B.; Yu, D.H. Cell-free DNA as a liquid biopsy for early detection of gastric cancer. Oncol. Lett. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy in 2016: Circulating tumour cells and cell-free DNA in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 73–74. [Google Scholar] [CrossRef]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B., 3rd; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal–Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.-B.; Hou, L.-K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.-M.; Sun, F.; Lu, H.-M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2002, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, A.; Gonzalez, N.S.; Pimentel, I.; Suñol, A.; Zamora, E.; Ortiz, C.; Espinosa-Bravo, M.; Peg, V.; Vivancos, A.; Saura, C.; et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102362. [Google Scholar] [CrossRef]
- Huffman, B.M.; Aushev, V.N.; Budde, G.L.; Chao, J.; Dayyani, F.; Hanna, D.; Botta, G.P.; Catenacci, D.V.; Maron, S.B.; Krinshpun, S.; et al. Analysis of Circulating Tumor DNA to Predict Risk of Recurrence in Patients with Esophageal and Gastric Cancers. JCO Precis. Oncol. 2022, 6, e2200420. [Google Scholar] [CrossRef]
- Maron, S.B.; Chase, L.M.; Lomnicki, S.; Kochanny, S.; Moore, K.L.; Joshi, S.S.; Landron, S.; Johnson, J.; Kiedrowski, L.A.; Nagy, R.J.; et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 7098–7112. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.U. Clinical Circulating Tumor DNA Testing for Precision Oncology. Cancer Res. Treat. 2023, 55, 351–366. [Google Scholar] [CrossRef]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Mouliere, F.; Rosenfeld, N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc. Natl. Acad. Sci. USA 2015, 112, 3178–3179. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Cardona, A.F.; Cristofanilli, M.; Paz-Ares, L.; Mochon, J.J.D.; Duran, I.; Raez, L.E.; Russo, A.; Lorente, J.A.; Malapelle, U.; et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit. Rev. Oncol. 2020, 151, 102978. [Google Scholar] [CrossRef]
- Grizzi, G.; Salati, M.; Bonomi, M.; Ratti, M.; Holladay, L.; De Grandis, M.C.; Spada, D.; Baiocchi, G.L.; Ghidini, M. Circulating Tumor DNA in Gastric Adenocarcinoma: Future Clinical Applications and Perspectives. Int. J. Mol. Sci. 2023, 24, 9421. [Google Scholar] [CrossRef]
- Jahangiri, L.; Hurst, T. Assessing the Concordance of Genomic Alterations between Circulating-Free DNA and Tumour Tissue in Cancer Patients. Cancers 2019, 11, 1938. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Chan, H.T.; Nagayama, S.; Otaki, M.; Chin, Y.M.; Fukunaga, Y.; Ueno, M.; Nakamura, Y.; Low, S.-K. Tumor-informed or tumor-agnostic circulating tumor DNA as a biomarker for risk of recurrence in resected colorectal cancer patients. Front. Oncol. 2023, 12, 1055968. [Google Scholar] [CrossRef]
- Chidharla, A.; Rapoport, E.; Agarwal, K.; Madala, S.; Linares, B.; Sun, W.; Chakrabarti, S.; Kasi, A. Circulating Tumor DNA as a Minimal Residual Disease Assessment and Recurrence Risk in Patients Undergoing Curative-Intent Resection with or without Adjuvant Chemotherapy in Colorectal Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 10230. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakamura, T.; Kimura, Y.; Motoya, M.; Kojima, S.; Kuraya, T.; Murakami, T.; Kaneko, T.; Shinohara, Y.; Kitayama, Y.; et al. Tumor-Informed Approach Improved ctDNA Detection Rate in Resected Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 11521. [Google Scholar] [CrossRef]
- Battaglin, F.; Lenz, H.-J. Clinical Applications of Circulating Tumor DNA Profiling in GI Cancers. JCO Oncol. Pract. 2024, 20, 1481–1490. [Google Scholar] [CrossRef]
- de Steur, W.; van Amelsfoort, R.; Hartgrink, H.; Putter, H.; Kranenbarg, E.M.-K.; van Grieken, N.; van Sandick, J.; Claassen, Y.; Braak, J.; Jansen, E.; et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann. Oncol. 2021, 32, 360–367. [Google Scholar] [CrossRef]
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; van Laarhoven, H.W.; Nieuwenhuijzen, G.A.; et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.-X.; Wang, Y.; Xu, X.-Q.; Sun, T.; Tian, B.-G.; Du, L.-L.; Zhao, X.-W.; Han, C.-Z. Tumor Markers for Diagnosis, Monitoring of Recurrence and Prognosis in Patients with Upper Gastrointestinal Tract Cancer. Asian Pac. J. Cancer Prev. 2015, 15, 10267–10272. [Google Scholar] [CrossRef] [PubMed]
- Dawood, Z.S.; Alaimo, L.; Lima, H.A.; Moazzam, Z.; Shaikh, C.; Ahmed, A.S.; Munir, M.M.; Endo, Y.; Pawlik, T.M. ASO Visual Abstract: Circulating Tumor DNA, Imaging, and Carcinoembryonic Antigen: Comparison of Surveillance Strategies in Patients Who Underwent Resection pf Colorectal Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 30, 277. [Google Scholar] [CrossRef]
- Arisi, M.F.; Dotan, E.; Fernandez, S.V. Circulating Tumor DNA in Precision Oncology and Its Applications in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4441. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.M.; Aushev, V.N.; Huffman, B.M.; Hanna, D.; Dutta, P.; Ferguson, J.; Sharma, S.; Jurdi, A.; Liu, M.C.; Eng, C.; et al. Circulating Tumor DNA as a Prognostic Biomarker for Recurrence in Patients with Locoregional Esophagogastric Cancers with a Pathologic Complete Response. JCO Precis. Oncol. 2024, 8, e2400288. [Google Scholar] [CrossRef] [PubMed]
- Iden, C.R.; Mustafa, S.M.; Øgaard, N.; Henriksen, T.; Jensen, S.Ø.; Ahlborn, L.B.; Egebjerg, K.; Baeksgaard, L.; Garbyal, R.S.; Nedergaard, M.K.; et al. Circulating tumor DNA predicts recurrence and survival in patients with resectable gastric and gastroesophageal junction cancer. Gastric Cancer 2024, 28, 83–95. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Lam, V.K.; Shi, Y.; Guan, Y.; Zhang, Y.; Ji, L.; Chen, Y.; Zhao, Y.; Qian, F.; et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020, 11, 346. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Fu, T.; Meng, M.; Feng, Y.; Qu, C.; Li, Z.; Xing, X.; Li, W.; Ye, M.; et al. Liquid biopsy: Circulating tumor DNA monitors neoadjuvant chemotherapy response and prognosis in stage II/III gastric cancer. Mol. Oncol. 2023, 17, 1930–1942. [Google Scholar] [CrossRef]
- Zaanan, A.; Didelot, A.; Broudin, C.; Laliotis, G.; Spickard, E.; Dutta, P.; Saltel-Fulero, A.; Sullo, F.G.; Pizzamiglio, M.; Mariani, A.; et al. Longitudinal circulating tumor DNA analysis during treatment of locally advanced resectable gastric or gastroesophageal junction adenocarcinoma: The PLAGAST prospective biomarker study. Nat. Commun. 2025, 16, 6815. [Google Scholar] [CrossRef]
- Han, B.; Lee, H.; Jung, M.; Kim, B.J.; Cha, Y.; Heo, S.; Rha, S.Y.; Kim, H.-D.; Ryu, M.-H.; Koo, D.-H.; et al. Abstract 4556: Postoperative minimal residual disease detection using tumor-informed circulating tumor DNA analysis in stage II-III gastric cancer. Cancer Res. 2025, 85, 4556. [Google Scholar] [CrossRef]
- Mi, J.; Wang, R.; Han, X.; Ma, R.; Li, H. Circulating tumor DNA predicts recurrence and assesses prognosis in operable gastric cancer: A systematic review and meta-analysis. Medicine 2023, 102, e36228. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Qu, H.; Yu, W.; Zhang, X.; Li, P.; Cui, X.; Wei, M.; Zhang, D.; Ma, M.; et al. Feasibility of circulating tumor DNA-based minimal residual disease (ctDNA-MRD)-guided adjuvant chemotherapy in patients with stage II–III gastric cancer (GC): An adaptive trial (MRD-GATE). J. Clin. Oncol. 2025, 43, 4061. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Foote, M.B.; Lumish, M.; Capanu, M.; Chou, J.; Garcia-Aguilar, J.; Weiser, M.; Konner, J.; Strong, V.; Jewell, E.; et al. Abstract CT002: Circulating tumor DNA status to direct adjuvant immunotherapy for mismatch repair deficient tumors. Cancer Res. 2025, 85, CT002. [Google Scholar] [CrossRef]
- The First Affiliated Hospital with Nanjing Medical University. Using ctDNA to Guide Treatment Decisions for Stage III Gastric Cancer (CLAYMORE). 2025. Available online: https://www.centerwatch.com/clinical-trials/listings/NCT06939439/using-ctdna-to-guide-treatment-decisions-for-stage-iii-gastric-cancer (accessed on 1 September 2025).
- Smyth, E.; Griffiths, D.; Cozens, K.; Hurt, C.; Waugh, R.; Turkington, R.; Foley, K.; Roy, R.; Sharma, S.; Jurdi, A.; et al. 499TiP A single arm phase II trial of trastuzumab deruxtecan in patients with gastro-oesophageal adenocarcinoma cancer who are ctDNA and HER2 positive: DECIPHER (TRIALS IN PROGRESS). Ann. Oncol. 2024, 35, S199. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef]
- Leal, A.; van Grieken, N.C.T.; Palsgrove, D.N.; Phallen, J.; Medina, J.E.; Hruban, C.; Broeckaert, M.A.M.; Anagnostou, V.; Adleff, V.; Bruhm, D.C.; et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat. Commun. 2020, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.G.; Lo, A.; Yu, J.; Gonda, A.; Dehkordi-Vakil, F.; Dayyani, F.; Senthil, M. Circulating Tumor DNA is Unreliable to Detect Somatic Gene Alterations in Gastrointestinal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2022, 30, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ococks, E.; Sharma, S.; Ng, A.W.T.; Aleshin, A.; Fitzgerald, R.C.; Smyth, E. Serial Circulating Tumor DNA Detection Using a Personalized, Tumor-Informed Assay in Esophageal Adenocarcinoma Patients Following Resection. Gastroenterology 2021, 161, 1705–1708.e2. [Google Scholar] [CrossRef]
- van Velzen, M.J.; Creemers, A.; van den Ende, T.; Schokker, S.; Krausz, S.; Reinten, R.J.; Dijk, F.; van Noesel, C.J.; Halfwerk, H.; Meijer, S.L.; et al. Circulating tumor DNA predicts outcome in metastatic gastroesophageal cancer. Gastric Cancer 2022, 25, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.A.; Cristescu, R.; Peña, C.; Watkins, A.; Espenschied, C.R.; Zhang, N.; Liao, J. Assessment of Circulating Tumor DNA Burden in Patients with Metastatic Gastric Cancer Using Real-World Data. JCO Precis. Oncol. 2025, 9, e2400582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Wang, N.; Wang, L.; Huang, Y. An update of clinical value of circulating tumor DNA in esophageal cancer: A systematic review and meta-analysis. BMC Cancer 2024, 24, 129. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, D.; Louvet, C.; Desramé, J.; Evesque, L.; Angelergues, A.; Carnot, A.; Breysacher, G.; Zaanan, A.; Etchepare, N.; Mabro, M.; et al. Circulating tumor DNA strongly predicts efficacy of chemotherapy plus immune checkpoint inhibitors in patients with advanced gastro-esophageal adenocarcinoma. Commun. Med. 2025, 5, 136. [Google Scholar] [CrossRef]
- Jiang, R.; Cheng, X.; Li, P.; Meng, E.; Wu, X.; Wu, H. Plasma circulating tumor DNA unveils the efficacy of PD-1 inhibitors and chemotherapy in advanced gastric cancer. Sci. Rep. 2024, 14, 14027. [Google Scholar] [CrossRef]
- Schrock, A.B.; Pavlick, D.; Klempner, S.J.; Chung, J.H.; Forcier, B.; Welsh, A.; Young, L.; Leyland-Jones, B.; Bordoni, R.; Carvajal, R.D.; et al. Hybrid Capture–Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Cancers of the Gastrointestinal Tract or Anus. Clin. Cancer Res. 2018, 24, 1881–1890. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Bucheit, L.; Saha, J.; Clemens, K.; Barnett, R.; Zhang, N.; Mahipal, A. HER2-directed therapy following ctDNA-identified ERBB2 amplification in patients with advanced gastroesophageal cancer: Exploration of real-world outcomes. ESMO Gastrointest. Oncol. 2024, 4, 100056. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Sakamoto, Y.; et al. Trastuzumab deruxtecan in HER2-positive advanced gastric cancer: Exploratory biomarker analysis of the randomized, phase 2 DESTINY-Gastric01 trial. Nat. Med. 2024, 30, 1933–1942. [Google Scholar] [CrossRef]
- Seo, S.; Ryu, M.-H.; Park, Y.S.; Ahn, J.Y.; Park, Y.; Park, S.R.; Ryoo, B.-Y.; Lee, G.H.; Jung, H.-Y.; Kang, Y.-K. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 2018, 22, 527–535. [Google Scholar] [CrossRef]
- Jogo, T.; Nakamura, Y.; Shitara, K.; Bando, H.; Yasui, H.; Esaki, T.; Terazawa, T.; Satoh, T.; Shinozaki, E.; Nishina, T.; et al. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 5619–5627. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Moya, S.; Lomnicki, S.; Chase, L.M.; Peterson, B.F.; Reizine, N.; Alpert, L.; Setia, N.; Xiao, S.-Y.; Hart, J.; et al. Personalized Antibodies for Gastroesophageal Adenocarcinoma (PANGEA): A Phase II Study Evaluating an Individualized Treatment Strategy for Metastatic Disease. Cancer Discov. 2020, 11, 308–325. [Google Scholar] [CrossRef]
- Gouda, M.A.; Janku, F.; Yuan, Y.; Drusbosky, L.M.; Chen, A.P.; Zheng, X.; Patel, K.; Hamilton, S.R.; Routbort, M.; Tricoli, J.V.; et al. Concordance between Tumor Tissue and Plasma DNA Genotyping in the NCI-MATCH Trial (EAY131). Clin. Cancer Res. 2025, 31, 4299–4310. [Google Scholar] [CrossRef]
- Umemoto, K.; Sunakawa, Y.; Ueno, M.; Furukawa, M.; Mizuno, N.; Sudo, K.; Kawamoto, Y.; Kajiwara, T.; Ohtsubo, K.; Okano, N.; et al. Clinical significance of circulating-tumour DNA analysis by metastatic sites in pancreatic cancer. Br. J. Cancer 2023, 128, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- A Wainberg, Z.; Enzinger, P.C.; Kang, Y.-K.; Qin, S.; Yamaguchi, K.; Kim, I.-H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Taniguchi, H.; Ikeda, M.; Bando, H.; Kato, K.; Morizane, C.; Esaki, T.; Komatsu, Y.; Kawamoto, Y.; Takahashi, N.; et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 2020, 26, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.M.; Borsu, L.; Cankovic, M.; Earle, J.S.; Gocke, C.D.; Hameed, M.; Jordan, D.; Lopategui, J.R.; Pullambhatla, M.; Reuther, J.; et al. Recommendations for Cell-Free DNA Assay Validations: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2023, 25, 876–897. [Google Scholar] [CrossRef]
- Hernandez, K.M.; Bramlett, K.S.; Agius, P.; Baden, J.; Cao, R.; Clement, O.; Corner, A.S.; Craft, J.; Dean, D.A., II; Dry, J.R.; et al. Contrived Materials and a Data Set for the Evaluation of Liquid Biopsy Tests: A Blood Profiling Atlas in Cancer (BLOODPAC) Community Study. J. Mol. Diagn. 2023, 25, 143–155. [Google Scholar] [CrossRef]
- Westphalen, C.; Bielo, L.B.; Aftimos, P.; Beltran, H.; Benary, M.; Chakravarty, D.; Collienne, M.; Dienstmann, R.; El Helali, A.; Gainor, J.; et al. ESMO Precision Oncology Working Group recommendations on the structure and quality indicators for molecular tumour boards in clinical practice. Ann. Oncol. 2025, 36, 614–625. [Google Scholar] [CrossRef]
- Mahuron, K.M.; Fong, Y. Applications of Liquid Biopsy for Surgical Patients with Cancer. JAMA Surg. 2024, 159, 96–103. [Google Scholar] [CrossRef]
- Mögele, T.; Hildebrand, K.; Sultan, A.; Sommer, S.; Rentschler, L.; Kling, M.; Sax, I.; Schlesner, M.; Märkl, B.; Trepel, M.; et al. Dissecting Tumor Heterogeneity by Liquid Biopsy—A Comparative Analysis of Post-Mortem Tissue and Pre-Mortem Liquid Biopsies in Solid Neoplasias. Int. J. Mol. Sci. 2025, 26, 7614. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).