Simple Summary
Breast cancer is not a single disease, but a collection of different tumor types. One molecular subgroup, called HER2-low breast cancer, is especially challenging because it does not respond well to most existing HER2-targeted treatments. To date, patients mostly rely on one therapy, which may not work for everyone and can lead to resistance. This review explores the role of microRNAs, small molecules that regulate gene expression, as cancer biomarkers and therapeutic targets to explain how these tumors behave and respond to treatment. By understanding how microRNAs interact with HER2 and hormone pathways, we hope to elucidate the mechanisms underlying HER2-targeted therapies to explore a crosstalk with microRNAs and design combination therapies that are more precise and effective. This approach could improve patient outcomes and also guide the research community towards more personalized treatment strategies for breast cancer.
Abstract
HER2-low breast cancer has been recognized as a heterogenous group of tumors influenced by hormone receptor (HR) expression, giving rise to tumors with either a luminal-like phenotype or features resembling triple-negative breast cancer (TNBC). Despite the development of HER2-targeted therapies, several studies have demonstrated their limited efficacy in patients diagnosed with HER2-low breast cancer. However, recent research has led to the development of antibody-drug conjugates (ADCs), such as trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), with the latter showing promising results in treating these patients. Despite this breakthrough, the availability of a single effective therapy fails to account for tumor heterogeneity and may contribute to the emergence of therapy resistance, leaving HER2-low patients without treatment options. MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression at a post-transcriptional level and are capable of modulating key cellular processes. Recent studies have highlighted their potential as therapeutic targets, contributing to the advancement of personalized, patient-center therapies. In this context, the interplay between miRNAs and HER2-targeted therapies, particularly their modulation of common essential genes and signaling pathways, could reshape HER2-low therapy strategies to transform current practices aimed at improving the overall patient outcomes. Therefore, this review aims to elucidate the mechanisms underlying current HER2-targeted therapy and explore a potential crosstalk with miRNAs, ultimately serving as a guide for the development of personalized therapeutic strategies.
1. Introduction
Breast cancer is the most predominant cancer worldwide and represents the primary cause of cancer-related deaths among women [1]. Traditionally, breast cancer classification has been based on invasiveness and tissue of origin; however, such classification does not fully reflect the complexity of tumor biology [2]. As a result, molecular classification has emerged to better categorize breast tumors according to hormone receptor (HR) expression and human epidermal growth factor receptor 2 (HER2) status, defining four main molecular subtypes—luminal A, luminal B, HER2-enriched and triple-negative breast cancer (TNBC) [3,4].
HER2 is a transmembrane tyrosine kinase receptor that belongs to the epidermal growth factor receptor (EGFR) family, encoded by the ErbB2 gene located on the short arm of chromosome 17 (17p) [5]. Unlike other EGFR family members, HER2 activation is not ligand-dependent and occurs through homodimerization or heterodimerization, particularly with HER3. HER2/HER3 dimerization induces a conformational change on HER2, triggering its activation and subsequent downstream signaling pathways, such as phosphatidylinositol-3-kinase/activated protein kinase B (PI3K/Akt) and mitogen-activated protein kinase (MAPK), which promote cell survival, proliferation and differentiation [5,6,7]. In normal tissue, phosphorylated and dephosphorylated forms of HER2 are similarly expressed, maintaining tissue homeostasis and leading to normal breast tissue development. However, in approximately 20% of breast cancer cases, HER2 is overexpressed, leading to constitutive activation, uncontrolled cell proliferation, migration and invasion, being frequently associated with high recurrence rates and poor prognosis [5,8,9,10].
According to the 2023 American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) guidelines for HER2 testing, HER2-enriched breast cancer is divided into three subgroups based on immunohistochemistry (IHC) and in situ hybridization (ISH) results. HER2-positive is defined as IHC 3+ or 2+ with gene amplification (ISH positive), HER2-low is characterized by an IHC score of 1+ or 2+ without gene amplification (ISH negative) and HER2-negative, also known as HER2-zero, as IHC 0 [11,12]. HER2-low breast cancer accounts for 55% of all breast cancer cases and has attracted increasing attention within the scientific community regarding whether it constitutes a distinct molecular and clinical entity [13,14,15,16]. Current evidence indicates that HER2-low tumors are heterogeneous, with some exhibiting luminal-like characteristics and others resembling TNBC, influenced by HR expression. Nonetheless, most HER2-low tumors exhibit a luminal-like phenotype [13,17]. In line with this, molecular profiling studies show that tumors characterized by HER2 IHC 1+ and 2+ scores demonstrate increased luminal-like features, whereas HER2 IHC 0 tumors exhibit basal-like characteristics, but these differences lose significance after accounting for HR expression, confirming that HER2-low biology is largely driven by HR expression [13,18]. Consequently, the 2023 ASCO/CAP guidelines conclude that there is insufficient proof to consider HER2-low breast cancer as a distinct biological entity. Consistently, the European Society for Medical Oncology (ESMO) recognizes HER2-low as a heterogenous group of tumors influenced by HR status and states that current data do not support its classification as a separate breast cancer subtype [11,19]. Therefore, this lack of a distinct classification is largely attributable to the incomplete understanding of the molecular mechanisms driving heterogeneity.
Before HER2-low breast cancer was clinically recognized, patients within this subgroup were typically managed according to HER2-positive or HER2-negative protocols, which often resulted in suboptimal therapeutic benefit and unnecessary toxicities due to inadequate target engagement or overtreatment [20]. In the context of breast cancer, tyrosine kinase inhibitors (TKIs) such as lapatinib, neratinib, tucatinib and pyrotinib target the tyrosine kinase domain of the HER2 receptor to block its activity. These agents are primarily used as second- or third-line treatments for HER2-positive breast cancer often in combination with monoclonal antibodies such as trastuzumab to overcome therapy resistance [21,22]. When administered as monotherapy, TKIs have failed to demonstrate clinical benefit in patients diagnosed with HER2-low breast cancer, but later research, particularly the phase III DESTINYBreast-04 trial, revealed that administration of the an antibody–drug conjugate (ADC), trastuzumab deruxtecan (T-DXd), significantly increased progression-free survival (PFS) and overall survival (OS) in this population, leading to its approval for the treatment of this disease [18,20,23]. Despite this clinical advancement, major challenges persist, including an inability to account for molecular diversity, limited therapeutic options beyond T-DXd, the frequent emergence of therapy resistance and potential therapy-related toxicity, underscoring the urgent need for novel molecular insights and precision therapies for this subgroup.
In this context, microRNAs (miRNAs) have emerged as critical post-transcriptional regulators capable of fine-tuning gene expression. These small non-coding RNAs influence a wide range of biological processes, including developmental timing, embryogenesis, cell differentiation, organogenesis, metabolism and apoptosis, as well as in the pathogenesis of numerous diseases [24,25]. In cancer, aberrant miRNA expression contributes to tumorigenesis by disrupting key regulatory networks. Oncogenic miRNAs are typically overexpressed, whereas tumor suppressor miRNAs are downregulated in disease, leading to the activation of proliferative signaling, uncontrolled cell growth, enhanced migration and invasion, angiogenesis and resistance to apoptosis. Notably, the same miRNA may act as an oncogene in certain pathologies and as a tumor suppressor in others, depending on the cellular environment and target genes [26,27,28]. Given their broad regulatory capacity, miRNAs have been shown to modulate the expression of HR and HER2 signaling components, thereby influencing treatment response. For example, miR-342 overexpression is significantly associated with ER-positive and HER2-positive breast tumors [29], while miR-18a downregulation correlates with increased ESR1 expression in ER-positive tumors [30]. Such findings suggest that miRNAs may contribute to the heterogenous expression of HR observed in HER2-low breast cancer, potentially distinguishing HR-positive from HR-negative tumors.
MiRNA-based therapeutic strategies, including miRNA mimics and inhibitors, aim to restore normal expression patterns by enhancing tumor suppressor miRNAs or suppressing the function of oncogenic miRNAs that contribute to pathological conditions [24,31]. These tools can inhibit the upregulation of oncogenic miRNAs, thereby allowing the expression of their tumor suppressor targets, and restore the downregulation of tumor suppressor miRNAs to inhibit their target oncogenes [32]. While the therapeutic potential of miRNAs has been validated in multiple cancer types, including HER2-positive and TNBC [33,34,35,36,37], their role in HER2-low tumors remains largely unexplored. Investigating how miRNAs modulate HER2 and HR signaling could provide critical insights into the molecular basis of tumor heterogeneity and therapy resistance, ultimately informing the development of more effective and personalized strategies for patients with HER2-low breast cancer.
2. Molecular and Genomic Landscape of HER2-Low Breast Cancer
HER2-low breast cancer comprises a heterogenous group of tumors in which HR is a key determinant of the molecular profile. Multiple transcriptomic and genomic studies, often stratified by HR status, have revealed distinct patterns between HER2-low/HR-positive and HER2-low/HR-negative tumors, with implications for both disease biology and therapeutic strategies.
Comprehensive molecular analyses of tumors are fundamental for understanding cancer biology, guiding treatment decisions and improving patient outcomes. Several studies employing PAM50 analysis on HER2-low tumors have indicated that luminal-related gene expression is predominantly linked to tumors classified as IHC 2+ and HR-positive, while IHC 0 and HR-negative tumors show a higher prevalence of genes associated with TNBC subtype. Moreover, these analyses revealed that HER2-low/HR-positive tumors are primarily classified within the luminal subtype, whereas HER-low/HR-negative tumors tend to cluster within the TNBC subtype [15,17,38]. These findings support tumor stratification based on HR status, confirming its role as a key driver of heterogeneity within HER2-low breast cancer, with the majority of cases being HR-positive [39,40]. From a clinicopathological perspective, HER2-low tumors are more frequently of no special type (NST), regardless of HR status. HER2-low/HR-positive tumors show a lower proliferation index compared to HER2-low/HR-negative tumors, displaying a negative correlation between lower Ki-67 levels and HR expression, while HER2-low/HR-negative tumors present elevated Ki-67 indices [41,42,43]. This pattern is consistent with luminal tumors, which typically have lower Ki-67 levels, and TNBC tumors, which display higher Ki-67 levels [44]. Therefore, distinguishing HER2-low/HR-positive from HER2-low/HR-negative tumors at the transcriptomic and genomic levels are crucial for understanding the underlying biological mechanisms that lead to this divergence.
Understanding the mutational landscape of HER2-low breast cancer may aid in treatment decisions. While investigating the genomic characteristics of this subgroup, Taratino et al. [45] reported that the most prevalent oncogenic mutation in ER-negative tumors was TP53 (79.8%), whereas PIK3CA (38.2%), GATA3 (16.1%) and ESR1 (13.4%) mutations were more frequently observed in ER-positive tumors. The same four genes were altered under similar conditions in HER2-negative samples. When comparing the two HER2 status groups and adjusting for ER expression, mTOR mutations were enriched in HER2-low samples, whereas MAP3K1 and TP53 mutations were more common in HER2-negative tumors. However, after correction for multiple hypotheses testing, no statistically significant differences remained between genomic landscapes of HER2-low and HER2-negative samples. Consistently, Bansal et al. [46] found that ESR1 (14.3%) and PIK3CA (42.5%) mutations were more common in HER2-low/HR-positive tumors compared with HER2-low/HR-negative, while TP53 mutations were significantly more prevalent in HER2-low/HR-negative (74.3%) than in HER2-low/HR-positive (25%). When comparing HER2-low/HR-negative with TNBC, they found no differences in ESR1 mutation frequency, but observed significantly more PIK3CA mutations in HER2-low/HR-negative tumors and more TP53 mutations in TNBC. Similarly, Juan et al. [47], using next-generation sequencing on 445 HER2-low breast cancer samples, showed that although not statistically significant, PIK3CA mutations were more frequent in HR-positive tumors, while PTEN mutations appeared more common in HR-negative tumors. In contrast, ESR1 and TP53 mutations were significantly enriched in HR-positive and HR-negative tumors, respectively. Furthermore, an analysis on germline mutations revealed that BRCA1 alterations were more prevalent in HR-negative tumors, while BRCA2 mutations were more frequently detected in HR-positive tumors.
Assessing copy number variations (CNV) of ErbB2 gene can help elucidate whether HER2 expression is influenced by gene dosage or by other regulatory mechanisms. Tan et al. [48] performed a bioinformatics analysis using the TCGA-BRCA and METABRIC databases to explore the influence of ErbB2 CNVs on ErbB2 mRNA expression and found that elevated mRNA levels corresponded to higher CNV scores in HER2-low breast cancer, with a positive but weak correlation between HER2 IHC scores and CNV scores. When stratified by HR status, the same trend was observed, but in the HR-negative subgroup, the correlation between HER2 IHC scores and CNV scores was not statistically significant. These findings suggest that although ErbB2 CNVs contribute to HER2 protein expression in HER2-low/HR-positive tumors, other mechanisms are likely responsible for modulating HER2 expression in HER2-low/HR-negative tumors. Likewise, another study [49] compared CNV values across HER2-positive, HER2-low and HER2-negative samples, observing similar CNV values between HER2-low and HER2-negative tumors, but significantly higher CNV values in HER2-positive compared to HER2-low and HER2-negative, an indication of gene amplification. The lack of difference between HER2-low and HER2-negative suggests that their disparity is not driven by gene dosage, but rather by other regulatory mechanisms. Altogether, these findings support the notion that HER2-low breast cancer should not be considered a distinct molecular entity, but rather a group of heterogenous tumors with either luminal-like features or TNBC-like characteristics, modulated by HR expression.
3. MicroRNAs’ Biogenesis and Mechanism of Action
Small non-coding RNA molecules with an average length of 21–22 nucleotides are termed miRNAs. These molecules are important regulators of gene expression at the post-transcriptional level and also play roles in numerous cellular processes, including cell differentiation, proliferation and survival [24,50].
The biogenesis of miRNAs can follow two different processing mechanisms: canonical and non-canonical as illustrated in Figure 1. The canonical pathway begins with RNA polymerase II transcribing a specific DNA segment in the nucleus into a long primary miRNA (pri-miRNA), which folds into a hairpin structure. The Microprocessor complex, composed of the RNase III enzyme Drosha and the RNA-binding protein DiGeorge Syndrome Critical Region 8 (DGCR8), then cleaves the pri-miRNA into a stem-loop precursor miRNA (pre-miRNA), which is exported to the cytoplasm by the Exportin 5 (XPO5)/Ran-GTP complex. In the cytoplasm, the pre-miRNA is processed by Dicer, another RNase III enzyme, which removes the loop region to produce a miRNA duplex consisting of the 3p strand, called the passenger strand, and the 5p strand, called the guide strand. The passenger strand is loaded with Argonaute proteins, AGO2, and degraded by cellular machinery, while the guide strand, due to its AGO selectivity and lower thermodynamic stability, is incorporated into the miRNA-induced silencing complex (miRISC) to guide the complex to the 3’ untranslated region (UTR) of their mRNA targets and regulate translation repression or mRNA degradation and deadenylation through base-pair complementarity [24,50,51,52].
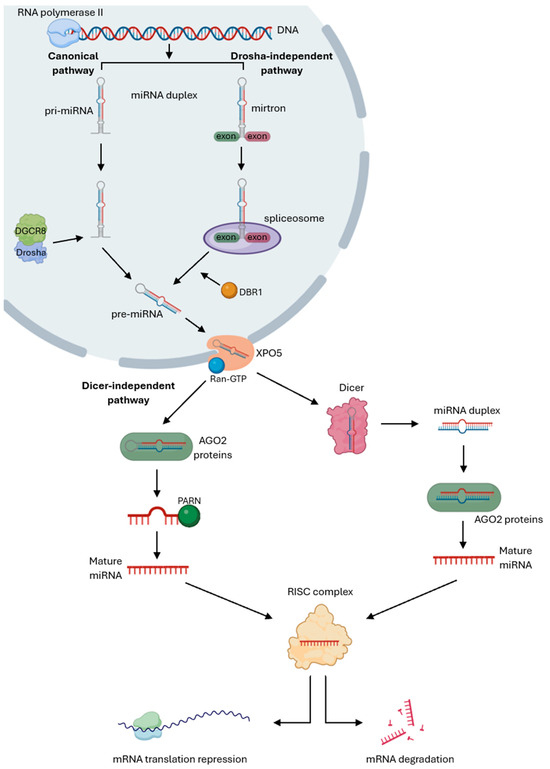
Figure 1.
Canonical and non-canonical pathways of miRNA’s biogenesis. Canonical pathway starts with RNA polymerase II transcribing a specific gene into a primary miRNA (pri-miRNA), which is cleaved by the Drosha/DGCR8 Microprocessor complex into a precursor miRNA (pre-miRNA). This pre-miRNA is exported to the cytoplasm by the Exportin 5 (XPO5)/Ran-GTP complex to be processed by Dicer to produce a miRNA duplex, which is loaded with AGO2 proteins and degraded by cellular machinery. The guide strand is incorporated into the miRNA-induced silencing complex (miRISC) to guide the complex to their mRNA targets and regulate translation repression or mRNA degradation. The Drosha/DGCR8-independent pathway starts with mirtrons, which are spliced out by the spliceosome and linearized by the DBR1 debranching enzyme, producing a debranched pre-miRNA that is exported to the cytoplasm by the XPO5/Ran-GTP complex and processed by Dicer, followed by miRNA maturation through the same cytoplasmic steps as in the canonical pathway. The Dicer-independent mechanism starts with the pri-miRNA being cleaved by Drosha to form a pre-miRNA that is directly loaded with AGO2 proteins to be processed into a pre-miRNA intermediate that is trimmed at the 3’-end by PARN, producing a single-stranded mature miRNA. The mature miRNA then follows the same cytoplasmic steps as the canonical pathway to repress mRNA translation or promote mRNA degradation [Created in Biorender. Eduarda Carvalho. (2025) https://app.biorender.com/].
Conversely, the non-canonical pathway omits some steps of the canonical mechanism and can be Drosha/DGCR8-independent or Dicer-independent. The Drosha/DGCR8-independent process originates from short hairpin introns, called mirtrons, which are spliced out by the spliceosome instead of being processed by the Microprocessor complex. The splicing generates an intron lariat, that is linearized by the debranching enzyme DBR1, producing a debranched pre-miRNA that is exported to the cytoplasm by the XPO5/Ran-GTP complex and processed by Dicer, followed by miRNA maturation through the same cytoplasmic steps as in the canonical pathway. In the Dicer-independent mechanism, the pri-miRNA cleaved by Drosha forms a short hairpin pre-miRNA that is too short for Dicer processing. Instead, AGO2 proteins directly process this hairpin to generate a 30-nucleotide long fragment, which is further cleaved by the Poly(A)-specific ribonuclease (PARN) to yield a mature miRNA. The mature miRNA is subsequently incorporated into the RISC complex to mediate gene silencing [53,54,55,56].
Mature miRNAs typically bind to the 3’ UTR of target mRNAs through perfect or partial base-pair complementarity, leading to degradation or translation repression [57,58]. Although less common, miRNAs can also interact with 5’ UTR, exerting either repressive or activating effects on translation [59]. For instance, miR-10 enhances ribosomal proteins production by binding to their 5’ UTR, while other studies [60,61] have reported translation repression through 5’ UTR binding, highlighting an additional regulatory layer that increases miRNAs versatility. In addition to these post-transcriptional roles, miRNAs can act as endogenous decoys that bind to transcription factors or interact directly with promoter regions of protein-coding genes, resulting in transcriptional activation or repression [62,63,64].
The function of miRNAs is further determined by subcellular localization [65]. Although enriched in the cytoplasm, miRNAs are also found in the nucleus, where they can regulate transcription through promoter or transcription factor interactions. MiR-30b-5p overexpression targets the 3’ UTR of ASPP2 to suppress its expression and activate p-Akt signaling, which inhibits apoptosis and promotes invasion in breast cancer [66]. Additionally, downregulation of miR-542-3p increases p-Akt levels, reducing FOXO1 recruitment and leading to transcriptional suppression [67]. In HER2-positive breast cancer cells, miR-21 inhibition increases PTEN expression and contributes to trastuzumab resistance [68]. Furthermore, a subset of miRNAs, known as mitomiRs, are localized in the mitochondria where they modulate mitochondrial gene expression and cellular metabolism [69,70]. In addition, miRNAs are also present in extracellular compartments such as exosomes, microvesicles, apoptotic bodies and protein complexes, which can be detected in various body fluids including serum, plasma, saliva, urine, breast milk, cerebrospinal fluid, and other biological fluids [71,72]. Encapsulation protects miRNAs from degradation by ribonucleases, ensuring stability, cellular uptake and facilitating cell-to-cell communication, making them valuable diagnostic and prognostic biomarkers for a wide range of diseases [72,73]. In this context, Otmani et al. [74] reported that exosome-derived miR-24-3p promotes T-cell apoptosis in leukemia via DENN/MADD regulation, and Zhao et al. [75] demonstrated that exosome-derived miR-934 from colorectal cancer cells enhances the PI3K/Akt signaling pathway, promoting M2 macrophage polarization.
Taken together, these findings underscore miRNAs as key post-transcriptional regulators of gene expression in both intracellular and extracellular environments. In the context of HER2-low breast cancer, understanding miRNA biogenesis and function is particularly relevant, as their dysregulation may contribute to heterogeneity.
4. MicroRNAs in Cancer
MiRNAs can function as either oncogenes or tumor suppressors. In carcinogenic conditions, oncogenic miRNAs are frequently upregulated, whereas tumor suppressive miRNAs tend to be downregulated. By regulating key signaling pathways, these small molecules contribute to the activation of cancer hallmarks and play a central role in tumor initiation and progression [27]. Notably, a single miRNA can simultaneously regulate multiple hallmarks by targeting distinct pathways involved in different aspects of carcinogenesis. This versatility makes miRNAs not only important for understanding cancer biology, but also promising as disease biomarkers. As biomarkers, they can provide diagnostic, prognostic and predictive information that reflects molecular alterations that may vary across specific cancer cell subpopulations [76,77]. Furthermore, because a single miRNA can target numerous mRNAs, its effects may be paradoxical, thereby increasing the therapeutic response in one context while contributing for disease progression and therapy resistance in another [28]. Therefore, elucidating the multiple roles of miRNAs in cancer is essential for exploring their potential as biomarkers and therapeutic agents.
4.1. Function of MicroRNAs in Cancer Hallmarks
In 2000, Hanahan and Weinberg [78] proposed six hallmarks that normal cells must acquire to enable tumor development and metastasis, serving as a basis to understand the mechanisms of cancer and the broad spectrum of malignant behaviors. With advances in cancer research, additional hallmarks were identified, leading to the current list, which includes sustained proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastases, reprogramming of energy metabolism, evading immune destruction, genome instability and mutation and tumor-promoting inflammation [79].
Although cancer hallmarks are described as distinct capabilities, they are highly interconnected and often reinforce one another during tumor progression, as shown in Table 1. Sustained proliferative signaling arises when cancer cells overexpress oncogenes that drive growth pathways and evade growth suppressors, creating an optimal cellular environment for malignant expansion to maintain continuous cell division, as cancer cells skip regulatory checkpoints that normally restrain cell division, thereby leading to uncontrolled proliferation [79,80,81]. For instance, the upregulation of miR-96-5p in renal cell carcinoma downregulates PTEN expression and increases cell proliferation [82], while the upregulation of miR-449a downregulates mutant p53 expression, which decreases cell proliferation in breast cancer, through inactivation of the PI3K/Akt pathway [83]. At the same time, genome instability and mutations facilitates abnormal cells survival rather than inducing apoptosis, as they have acquired advantageous traits to resist to cell death [84,85]. Furthermore, to maintain long-term growth, cancer cells achieve replicative immortality through reactivation of telomerase reserve transcriptase (TERT), which elongates telomeres by adding repetitive sequences to chromosome ends [86,87]. However, rapid proliferation of cancer cells often surpasses their vascular supply causing hypoxia, which promotes the transcription of several pro-angiogenic genes, including vascular endothelial growth factor (VEGF) to induce angiogenesis and facilitate invasion and metastases by offering new routes for tumor cell dissemination [88,89,90]. Nevertheless, invasion requires epithelial tumor cells to lose E-cadherin and increase N-cadherin and vimentin expression to enable cells to undergo epithelial-to-mesenchymal transition (EMT). In parallel, cancer cells secrete metalloproteinases to degrade extracellular matrix (ECM), thereby facilitating migration and invasion [91,92]. In melanoma, upregulation of miR-155-5p suppresses SOCS1 expression, leading to JAK2/STAT3 pathway activation and overexpression of matrix metalloproteinase-9 (MMP9), VEGFa and FGF2 factors, ultimately enhancing angiogenesis, proliferation and migration [93] and in ovarian cancer, upregulation of miR-29c-3p suppresses migration and invasion by directly targeting MMP2 [94]. Simultaneously, cancer cells are reprogramming their energy metabolism, shifting from oxidative metabolism to aerobic glycolysis (Warburg effect), increasing the synthesis of biomolecules to fuel proliferation and increasing the energy necessary for invasion [95,96]. Lactic acid, a byproduct of glycolysis, accumulates in tumor microenvironment (TME) and causes extracellular acidification, thereby promoting the polarization of tumor-associated macrophages (TAM) towards an M2-like phenotype, which in turn secrete immunosuppressive and pro-angiogenic factors such as transforming growth factor-beta (TGF-β), interleukin-6 (IL-6) and VEGF. These mediators facilitate immune evasion by suppressing anti-tumor immune responses, while simultaneously stimulating angiogenesis and tumor progression [97,98]. In breast cancer, upregulation of miR-155 increases IL-6 expression, which contributes to hexokinase 2 enhancement via activation of the STAT3 pathway, leading to increase glucose metabolism [99], while miR-155-5p increases M2 macrophage polarization, contributing to active immune response in pancreatic cancer [100]. Therefore, alterations in one hallmark tend to affect others producing heterogeneous tumors.

Table 1.
Summary of miRNAs that affect cancer hallmarks, demonstrating how they are interconnecting.
4.2. MicroRNAs as Biomarkers in Cancer
In addition to being expressed in tumor tissues, miRNAs are also found in various biological fluids, where they are referred to as circulating miRNAs. These circulating molecules can be secreted by tumor cells or released as a consequence of apoptosis and they exhibit remarkable stability in these fluids against endogenous ribonucleases, temperature fluctuations and pH variations due to their encapsulation within exosomes, microvesicles, apoptotic bodies or their association with protein complexes [71,72]. Importantly, miRNAs display tumor-specific signatures that reflect cancer development and progression, providing valuable information for accurate diagnosis and prognostic evaluation regarding a specific treatment [123].
Diagnostic biomarkers detect or confirm the presence of a certain pathology or condition, thereby supporting disease classification [124]. For instance, miR-21 is a well-established cancer biomarker, as its upregulation correlates with breast [125], lung [126], colorectal [127] and gastric [128] cancers, enabling clear distinction between healthy individuals and patients. Additionally, miR-21 is strongly associated with poor survival and disease recurrence, also serving as a prognostic and predictive biomarker [129,130,131]. A prognostic biomarker provides information about the probability of disease recurrence or progression in patients with an existing condition, while predictive biomarkers identify patients who are more likely to respond positively or negatively to a therapy or environmental factor [124]. Elevated plasma levels of miR-141 can serve as a diagnostic biomarker, with 87.8% sensitivity and 80.0% specificity for detecting gallbladder cancer, and are also associated with poor survival [132]. Increased expression of miR-210 in hypoxic exosomes derived from bone marrow-derived mesenchymal stem cells promotes invasion of lung cancer cells and metastasis, making this miRNA a diagnostic biomarker of hypoxia-driven lung cancer and high co-expression of miR-210 in cancer and stromal cells is a positive prognostic biomarker for disease-specific survival [133,134]. Additionally, increased expression of miR-10b serves as a prognostic biomarker for disease progression and metastasis in breast cancer [135], while downregulation of serum miR-218 in gastric cancer is linked to disease progression, metastasis and poor OS [136,137]. Similarly, reduced miR-126 expression predicts distant metastases, high tumor grade and poor survival in colorectal cancer [138]. Furthermore, miR-31 acts as a predictive biomarker associated with reduced PFS and OS in patients receiving anti-EGFR therapies, as well as a prognostic biomarker for disease recurrence [139,140]. High serum miR-125b expression correlates with advanced disease stage and chemotherapy resistance, suggested by elevated proliferation and reduced apoptosis, serving as a predictive biomarker of poor clinical outcome [141] and the cluster miR-221/222 predicts disease recurrence and tamoxifen resistance in breast cancer, correlating with treatment failure [142]. Altogether, these examples highlight the potential roles of specific circulating miRNAs as non-invasive diagnostic, prognostic and predictive biomarkers, underscoring their clinical relevance for improving disease detection, patient stratification and therapeutic decision-making.
However, tumors are inherently heterogeneous, consisting of diverse subpopulations of cells with distinct genetic, transcriptomic and phenotypic profiles. Intra-tumor heterogeneity reflects the differences among cancer cells within the same tumor and inter-tumor heterogeneity encompasses variations in the same cancer type between different individuals [143]. Additional factors, such as socioeconomic status, demographic region, nutrition and lifestyle behavior, can influence miRNA expression at the cellular level, potentially increasing tumor heterogeneity and affecting disease diagnosis [144,145]. This complexity is often masked by conventional molecular analysis techniques, which typically evaluate bulk samples and provide only average measurements of miRNA expressions, indicating misleading results. Consequently, single-cell analysis has emerged as a powerful approach to unravel tumor heterogeneity and provide deeper insights into cancer biology [146,147,148]. Moreover, current therapeutic strategies based on bulk analysis fail to account for distinct subclones within the same tumor and while treatment may suppress certain populations, it can simultaneously favor the survival of more aggressive or therapy-resistant cells, ultimately aggravating disease progression [149,150]. Therefore, employing single-cell analysis to investigate the molecular and genomic characteristics of HER2-low tumors and to identify differently expressed miRNAs may help tailor patient-specific therapeutic approaches with better clinical outcomes.
5. Targeted Therapies for Breast Cancer
Typically, breast cancer therapy involves a combination of surgery, neoadjuvant and adjuvant treatments. However, advances in the understanding of breast cancer biology have provided deeper insights into the genomic, transcriptomic and proteomic alterations underlying this disease. Consequently, therapeutic research is shifting toward patient-specific approaches, leading to the development of targeted therapies tailored to each patient, with the aim of improving outcomes and turning conventional broad-spectrum therapies increasingly obsolete [151,152].
In line with this, T-DXd remains the only agent that has demonstrated clinical efficacy in patients with HER2-low breast cancer [20]. Nevertheless, reliance on a single therapy fails to address tumor heterogeneity, which may effectively eliminate sensitive cancer cell populations and simultaneously foster the persistence of resistant clones. Furthermore, it may increase mutational pressure on the target, thereby facilitating the emergence of therapy resistance [153,154]. Therefore, understanding the mechanisms underlying HER2-targeted therapies aligned with the understanding of the molecular and cellular pathways involved in HER2-low breast cancer heterogeneity, is essential for the development of novel therapeutic strategies targeting HER2 and HR to delay disease progression.
5.1. HER2-Targeted Therapies in Breast Cancer
HER2-targeted therapies include TKIs, monoclonal antibodies (mAB) and ADC, as illustrated in Figure 2 [155]. TKIs are small molecules that target HER2 overexpression and include lapatinib, neratinib, tucatinib and pyrotinib to inhibit the activation of PI3K/Akt and MAPK signaling pathways by competing with ATP for binding to the tyrosine kinase domain of HER2, which prevents cancer progression [156,157,158]. Based on their mechanism of action, TKIs can be categorized into five subtypes. Type I inhibitors compete reversibly with ATP at the kinase domain, inhibiting activity, but exhibiting low selectively, which can result in off-target effects by blocking other kinases. Type II inhibitors also prevent ATP binding in a reversible manner, but preferentially bind to the inactive conformation of their target kinase, providing higher intrinsic selectivity. Type III inhibitors bind to an allosteric side outside the ATP-binding pocket, inducing a conformational change that makes them highly selective. Additionally, type IV inhibitors interact with the substrate-binding site, competing with substrate, which confers high selectivity, and type V inhibitors bind simultaneously to two different sites on the kinase, combining mechanisms to enhance specificity and inhibition [156,159].
Briefly, lapatinib is a reversible, dual type I TKI that blocks HER1 and HER2 by competing with ATP for binding to their kinase domain, inhibiting downstream signaling pathways [160]. Neratinib is an irreversible type I TKI that covalently binds to the ATP-binding pocket of EGFR, HER2 and HER4, which prevents autophosphorylation and blocks the downstream signaling cascades [161]. Tucatinib is a recent reversible TKI that binds to the ATP-binding site of the HER2 kinase domain, selectively inhibiting HER2 heterodimerization with HER3 [162]. Pyrotinib is an irreversible TKI that targets the kinase domains of HER1, HER2 and HER4 to induce cell cycle arrest and suppress tumor growth [157]. Clinical studies have demonstrated that combination therapy with neratinib plus capecitabine improves PFS and OS in patients with HER2-positive metastatic breast cancer, resulting in greater clinical benefit compared with lapatinib plus capecitabine treatment [163]. Similarly, treatment of pyrotinib plus capecitabine resulted in superior PFS and OS compared with lapatinib plus capecitabine, enhancing the clinical benefit for patients with HER2-positive metastatic breast cancer [164]. Moreover, a separate study showed that pyrotinib monotherapy was effective in luminal/HER2-low (IHC 2+, ISH negative) and luminal/HER2-positive breast cancers, with enhanced efficacy when combined with chemotherapy, but no benefit was observed in luminal/HER2-low (IHC 1+) [165].
Moreover, mABs target the extracellular domain of HER2, with trastuzumab and pertuzumab being the most widely used agents. These antibodies inhibit both homodimerization and heterodimerization of HER2, consequently blocking key downstream signaling pathways [166,167]. mABs represent effective therapeutic strategies due to their high target selectivity, stability in circulation and low toxicity. These unconjugated mABs bind to HER2 on the tumor cell surface and induce immune-mediated responses that result in apoptosis [168]. Trastuzumab binds to domain IV of HER2 by electrostatic and hydrophobic interactions, promoting receptor internalization, preventing dimerization and activating the antibody-dependent cellular cytotoxicity (ADCC) mechanism to kill HER2-overexpressing cancer cells [166,169]. Additionally, trastuzumab can also inhibit cell proliferation and induce apoptosis by blocking PI3K/Akt signaling pathway and inhibit VEGF to decrease cell invasion by inactivating the same pathway and thus reduce tumor growth [170]. Pertuzumab, in turn, binds to domain II of HER2 to block dimerization with other HER family members and also recruit immune cells via ADCC [171,172]. In clinical practice, mAB-based therapy is often administrated in combination with chemotherapy, achieving remarkable improvements in PFS and OS [173,174,175]. Also, neratinib either as monotherapy or in combination with trastuzumab showed a significant reduction in HER2-low (IHC 2+, ISH negative) viability, with the combination proving more effective through enhanced inactivation of EGFR, HER3 and p-Akt [176]. Additionally, conjugated mABs, known as ADC, represent an effective therapeutic strategy that combines the specificity of mABs with the potency of cytotoxic payload. After binding to HER2 on the tumor cell surface, ADCs are internalized and transported to lysossomes, where the linker between the antibody and the drug is degraded, releasing the cytotoxic payload to exert its cytotoxic effect [177]. Currently, there are two approved ADC for the treatment of HER2-positive breast cancer, trastuzumab emtansine (T-DM1) and T-DXd [178]. T-DM1 delivers the maytansine DM1 to inhibit microtubule formation, induce cell cycle arrest and cause apoptosis, while T-DXd carries the topoisomerase I inhibitor deruxtecan to inhibit DNA replication and induce cell cycle arrest and apoptosis [177,179,180]. Comparative studies evaluating these ADCs as monotherapies highlight a greater clinical benefit in patients with HER2-positive breast cancer treated with T-DXd [181,182,183]. Similarly, in HER2-low patients, treatment with T-DXd significantly improved PFS and OS compared with physician’s choice (PFS: 9.9 months vs. 5.1 months; OS: 23.4 months vs. 16.8 months), leading to the approval of T-DXd as the first therapy for this patient population [20].
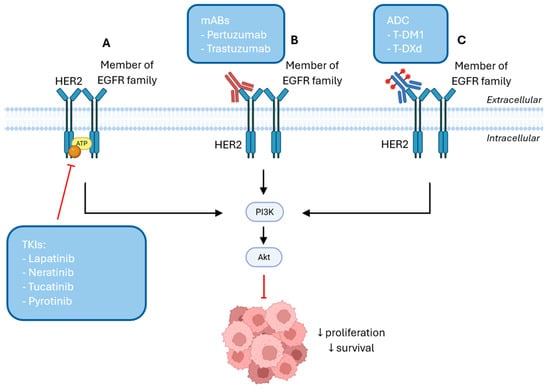
Figure 2.
(A) TKIs bind to the ATP-binding pocket and inhibit kinase activity, blocking PI3K/Akt signaling pathway, further reducing cell survival and proliferation. (B) mABs bind to HER2 on the tumor cell surface and either promote receptor internalization or inhibit its dimerization with other members of the EGFR family, inhibiting the activation of PI3K/Akt signaling pathway. (C) ADCs bind to HER2 on the tumor cell surface and promote their internalization into the cell to release the cytotoxic payload to exert its cytotoxic effect and inhibit cell proliferation and survival by inactivating the PI3K/Akt pathway [Created in Biorender. Eduarda Carvalho. (2025) https://app.biorender.com/].
Understanding the mechanism by which HER2 is amplified in HER2-low breast cancer, may help in the development of new therapeutic strategies with already-approved therapies for HER2-positive breast cancer.
5.2. HR-Targeted Therapies in Breast Cancer
As most HER2-low breast tumors are predominantly HR-positive [42], understanding the molecular framework of HR-targeted therapies may support the development of new therapeutic strategies for HER2-low.
HR-targeted therapies may be classified as selective estrogen receptor modulators (SERMs) or as aromatase inhibitors (AI), as depicted in Figure 3. SERMs act as estrogen antagonists in breast cancer by competing with estrogen for binding to estrogen receptors (ERs) on the cell surface, thereby interfering with ER signaling and downstream pathways [184]. The most widely used SERM in HR-positive breast cancer is tamoxifen, which competes with estrogen for binding to ERα. This prevents ERα-mediated transcription of estrogen-responsive genes, which inhibits tumor cell proliferation by suppressing the PI3K/Akt/mTOR signaling pathway [185,186]. AIs are available across three generations, with the third generation being the most specific and associated with fewer adverse effects and includes exemestane, anastrozole and letrozole [187]. These therapeutic agents inhibit the aromatase enzyme, which is responsible for the conversion of androgens to estrogens, thereby reducing estrogen production and subsequent ER activation, ultimately inhibiting cell proliferation [188,189]. Comparative clinical trials highlight the therapeutic potential of AIs over tamoxifen, reducing the risk of recurrence in ER-positive breast cancer patients [190,191,192].
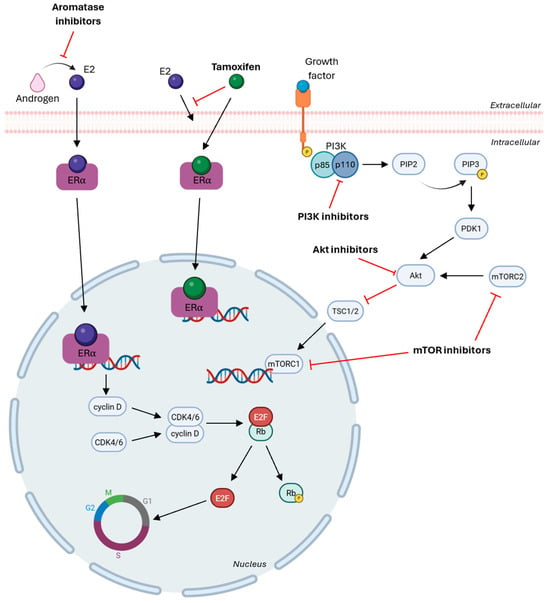
Figure 3.
Aromatase inhibitors block androgen conversion into estrogen and tamoxifen binds to ERα to inhibit the transcription of estrogen-responsive elements, blocking cell proliferation. PI3K inhibitors target the kinase domain of an isoform to block its activity in phosphorylation PIP2 into PIP3, AKT inhibitors bind to the ATP-binding pocket to induce apoptosis and mTOR inhibitors block mTORC1 and/or mTORC2 to reduce proliferation. CDK4/6 inhibitors inhibit Rb phosphorylation and stop the progression of cell cycle [Created in Biorender. Eduarda Carvalho. (2025) https://app.biorender.com/].
Another important therapeutic approach in ER-positive breast cancer resides on targeting critical regulators of cell cycle progression and includes CDK4/6 inhibitors and PI3K pathway inhibitors [193], as shown in Figure 3. Cell cycle progression is controlled by cyclin-dependent kinases (CDK) and cyclins, which contribute to cellular development by interacting and forming CDK-cyclin complexes. These complexes promote retinoblastoma (Rb) phosphorylation, leading to the release of E2F transcription factors and subsequent transcription of genes essential for cell cycle progression [194,195]. In cancer, deregulation of this process results in uncontrolled proliferation and transmission of DNA damage. Therefore, CDK4/6 inhibitors act by binding to CDK4 and CDK6 to prevent the CDK-D cyclin complex formation, which blocks Rb phosphorylation, halts cell cycle progression at the G1 phase, and prevents transcription of genes required for proliferation [196,197].
The PI3K/Akt/mTOR signaling pathway is among the most frequently dysregulated pathways across cancers, contributing to tumor growth, survival and therapy resistance [193]. Upon activation, PI3K is recruited to the plasma membrane, where its regulatory subunit p85 stabilizes and attracts the catalytic subunit p110, which phosphorylates phosphatidylinositol-4, 5-biphosphate (PIP2) to generate phosphatidylinositol-3, 4, 5-triphosphate (PIP3). PIP3 recruits PDK1, which together with mTORC2 facilitates Akt phosphorylation and activated Akt inhibits tuberous sclerosis complex (TSC1/2), leading to activation of mTORC1, resulting in reduced apoptosis and increased proliferation and survival [198,199,200]. There are two main classes of PI3K inhibitors: pan-PI3K inhibitors and isoform-specific PI3K inhibitors. Pan-PI3K inhibitors target the kinase activity of all four isoforms of class I PI3Ks, but have low specificity and are associated with elevated toxicity and off-target effects. By contrast, isoform-specific PI3K inhibitors demonstrate higher efficacy and reduced adverse events by selectively inhibiting individual isoforms and most isoform-specific inhibitors are directed against the p110α catalytic subunit, which is frequently mutated in cancer by PIK3CA mutations [201,202,203]. Additional therapeutic agents include Akt inhibitors and mTOR inhibitors, which also act within the PI3K/Akt/mTOR signaling pathway. Akt inhibitors can be classified as allosteric inhibitors, which block Akt activity by preventing conformational changes and induce apoptosis, or as ATP-competitive inhibitors, which bind to the ATP-binding pocket within the kinase domain of Akt and other AGC family kinases [204,205,206]. mTOR inhibitors are divided into two main groups: allosteric mTOR inhibitors, which bind to FK506 binding protein 12 (FKBP12) of mTORC1 and allosterically inhibit its kinase activity and ATP-competitive mTOR inhibitors, which target the kinase domain of mTOR, inhibiting both mTOCR1 and mTORC2 signaling [204].
A clinical trial [207] evaluating the administration of capivasertib (an Akt inhibitor) in combination with fulvestrant in patients with ER-positive, HER2-negative breast cancer, with or without prior or ongoing treatment with CDK4/6 inhibitors, demonstrated a significant improvement in PFS. Additional research [208] supports the combined use of CDK4/6 inhibitors with PI3K/Akt/mTOR pathway inhibitors, highlighting this strategy as a means to further improve clinical outcomes in these patients. However, combined treatment with CDK4/6 inhibitors plus endocrine therapy results in worse PFS and OS for HER2-low patients compared with HER2-zero, both HR-positive [209,210]. Additional studies [211,212] confirmed that, among HR-positive patients receiving this therapeutic combination, those with HER2-low tumors presented inferior PFS and OS. Multivariable analyses further identified HER2 status as an independent predictor of worse outcomes, suggesting a potential need to target HER2 signaling to prevent progression in HER2-low disease.
6. Crosstalk Between MicroRNAs and Breast Cancer Therapies
MiRNAs can regulate key cellular processes and their aberrant expression in carcinogenesis depict their potential to be enrolled in the development of personalized therapeutic strategies. Many studies have reported their therapeutic efficacy in numerous cancers, including breast [213], prostate [214], lung [215], gastric [216], hepatocellular carcinoma [217] and many others.
6.1. Advances and Challenges in MicroRNA-Based Therapeutics
MiRNA-based therapies aim to either restore the expression of downregulated miRNAs using miRNAs mimics or inhibit the expression of upregulated miRNAs using miRNA inhibitors [218]. However, the clinical application of these molecules faces several limitations that reduce their efficacy. First, nucleases in the extracellular space rapidly degrade free miRNA mimics and inhibitors and they are quickly excreted by the kidneys, making them unstable; therefore, chemical modifications are often required to enhance their resistance to degradation [219,220]. Second, the negatively charged nature of miRNAs and poor blood perfusion hinder their cellular uptake. To address this, miRNAs are frequently encapsulated into biocompatible delivery systems, such as nanoparticles, which facilitate membrane penetration, protect them from nuclease activity and enhance targeted delivery [220,221]. Third, because a single miRNA can regulate multiple genes and signaling pathways, off-target effects may occur, potentially leading to systemic cytotoxicity, which can be mitigated through the use of nanoparticle-based carriers or exosomes with specific ligands or antibodies, which improve target specificity while reducing toxicity [222,223,224]. Finally, administration of miRNA mimics can activate Toll-like receptors (TRL3, TRL7 and TRL8), triggering immune responses, but chemical modifications can help minimize such recognition and lower the risk of immune activation [220].
However, significant efforts have been made to overcome these limitations. Currently, both viral and non-viral vectors are being explored for miRNA delivery to efficiently transfect the desired oligonucleotide into specific target cells. Non-viral systems offer a biocompatible and less immunogenic alternative while ensuring miRNA stability. These include lipid-based nanocarriers, polymer-based nanoparticles and extracellular vesicles [225]. Among them, lipid-based nanocarriers are the most extensively studied due to their excellent biocompatibility, bioavailability and structural flexibility, which allow surface modifications to improve miRNA encapsulation and release. When designing these delivery systems, factors such as size, composition and geometry are critical, as interactions between nanoparticles and blood components may lead to aggregate formations, reducing delivery efficiency and potentially inducing immune toxicity. To mitigate these effects and enhance systemic circulation and cellular uptake, nanoparticles are often conjugated with polyethylene glycol (PEG), which provides steric stabilization and increases delivery efficiency or with cholesterol or dioleoylphosphatidyl ethanolamine to minimize their toxicity [225,226,227,228]. Polymer-based nanoparticles are composed of natural or synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), and chitosan, that can be engineered to control premature release and enable sustained miRNA delivery. PLGA nanoparticles exhibit a slow degradation profile and low inflammatory potential, but their opsonization in circulation and limited biocompatibility can restrict therapeutic efficiency. To overcome these issues, incorporating additional positively charged polymers can enhance miRNA encapsulation, reduce toxicity and extend circulation time. Additionally, miRNAs are successfully incorporated into PLGA nanoparticles by encapsulation or by absorption through an electrostatic force. PEI, often regarded as the “gold standard” polymer for miRNA transfection due to its ability to promote endosomal escape, is limited by its low degradability, which may result in polymer accumulation and, consequent, systemic toxicity associated with its molecular weight [228,229,230,231]. Conversely, chitosan-based nanoparticles demonstrate low toxicity, low immunogenicity and high biocompatibility, increasing miRNA encapsulation efficiency. Their degradation is triggered by the acidic environment of tumor cells, allowing for efficient, sustained and controlled miRNA release, which renders this polymer an increase application in both in vitro and in vivo miRNA delivery studies [232,233]. Furthermore, extracellular vesicles have gained attention as natural nanocarriers for miRNA delivery due to their intrinsic biocompatibility, ability to cross biological barriers and stability in circulation. Their lipidic bilayer protects miRNAs from extracellular degradation, while their surface proteins facilitate cellular uptake by target cells, providing a natural mechanism for target delivery. MiRNAs can be incorporated into these vesicles through electroporation, passive incubation or by overexpressing the desired miRNA in parent cells. Compared to lipid- and polymer-based nanocarriers, extracellular vesicles display lower immunogenicity and cytotoxicity, making them a promising therapeutic strategy for miRNA delivery [234,235,236].
Several clinical trials have evaluated miRNA-based therapeutics in cancer, illustrating both their therapeutic potential and the challenges associated with their delivery and safety. A clinical trial (NCT01829971) [237] tested the efficacy of a miR-34a mimic encapsulated in a liposomal nanoparticle in patients with solid tumors, which was administered intravenously. This study, with a two-year duration, showed limited efficacy, with no complete responses and only a few partial responses after five treatment cycles, but resulted in several serious immune-mediated adverse effects and four deaths, leading to its termination. Another clinical trial (NCT02369198) [238] evaluated the safety of administrating a miR-16 mimic encapsulated in bacterial minicells (EnGeneIC Dream Vectors) targeting EGFR in patients with malignant pleural mesothelioma. Results indicated that a dose of 5 × 109 TargomiRs once a week was the maximum tolerable level, with higher or more frequent dosing leading to toxicity despite co-administration with dexamethasone prophylaxis. More recently, 1B3, a synthetic miR-193a-3p mimic, was tested across various tumor types. Its overexpression reduced cell number and proliferation by inducing apoptosis and G0/G1 cell cycle arrest. Following these findings, encapsulation of 1B3 in a lipid nanoparticle delivery system (INT-1B3) demonstrated significant anti-tumor activity through tumor size reduction [239]. Furthermore, INT-1B3’s anti-tumorigenic effect was shown to result from tumor targeting that activates a T-cell-mediated immune response while reducing cytotoxicity [240]. These preclinical results have led to the First-in-Human clinical trial (NCT04675996) to evaluate the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of INT-1B3 in the treatment of patients with advanced solid tumors, but the trial was terminated prematurely due to insufficient funding, before meaningful results could be obtained [241]. Taking these findings into consideration, the development of miRNA-based therapy requires careful anticipation and monitoring of immune-mediated toxicities, which have been observed in previous clinical trials. In the specific context of HER2-low breast cancer, the presence of tumor heterogeneity should be acknowledged, as it may contribute to partial treatment responses and the low expression of HER2 requires a delivery system that does not rely exclusively on HER2-mediated uptake, such as lipid nanoparticles or exosomes. These systems can enhance the stability and bioavailability of miRNA and reduce off-target toxicity. Such an approach could combine precise delivery with potent gene-regulatory effects, ultimately improving therapeutic efficacy in HER2-low breast cancer.
6.2. MicroRNAs as a Personalized Therapeutic Strategy for Breast Cancer
MiRNAs have been investigated in combination with approved targeted therapies for breast cancer, mainly as modulators of drug resistance and sensitivity. Their ability to regulate key signaling pathways and gene expression networks positions them as promising agents to enhance treatment efficacy and overcome therapy resistance.
Several studies have demonstrated miRNAs as crucial regulators of sensitivity to tamoxifen in ER-positive breast cancer. Bin et al. [242] investigated whether miR-205 could regulate tamoxifen resistance in breast cancer and indeed showed that downregulation of miR-205 in MCF-7 cells increased the expression of its target gene MED1, leading to tamoxifen resistance, whereas the restoration of miR-205 reduced proliferation and resensitized cells to tamoxifen. Similarly, Yue et al. [243] demonstrated that upregulation of miR-190 enhanced sensitivity to tamoxifen by targeting SOX9, which was further validated in vivo with nude mice, where elevated miR-190 levels reduced tumor weight. Conversely, elevated levels of ZEB1 promotes CpG methylation and histone deacetylation of the MIR497HG promoter, silencing miR-195 and miR-497, thereby activating the PI3K/Akt pathway and leading to tamoxifen resistance in ER-positive breast cancer [244]. Moreover, upregulation of miR-221 has been significantly correlated with tamoxifen resistance and disease recurrence in ER-positive breast cancer [142], while upregulation of miR-93 and miR-382-3p and downregulation of miR-182-3p have been linked to tamoxifen resistance. The combined expression profile of these miRNAs demonstrates high predictive accuracy for therapy resistance, with a specificity of 85.7% and a sensitivity of 80.8% in ER-positive breast cancer [245].
In HER2-positive breast cancer, multiple miRNAs have been associated with resistance or sensitivity to anti-HER2 therapies. Rau et al. [246] reported that the downregulation of miR-126 promoted trastuzumab resistance in SKBR3 and BT-474 cells by increasing the expression of PIK3R2. Conversely, it was demonstrated that transfecting these cells with miR-126 mimic restored trastuzumab sensitivity, enhancing its efficacy. Similarly, upregulation of miR-21 promotes trastuzumab resistance by targeting PTEN, activating the PI3K/Akt pathway, and promoting EMT, whereas inhibition of miR-21 expression using antisense oligonucleotides can restore trastuzumab sensitivity [170,247]. Furthermore, Hailin et al. [248] found that miR-200c is frequently downregulated in HER2-positive breast cancer, but its overexpression suppresses tumor sphere formation in vitro and reduces tumor growth and lung metastases in vivo. Other miRNAs exert opposite effects, enhancing sensitivity. Overexpression of miR-33b reduces EMT, proliferation, invasion and migration while inducing apoptosis [249]. The combination of miR-770-5p with trastuzumab blocks Akt and ERK signaling and decreases cell proliferation and invasion [170]. Furthermore, co-transfection of miR-26a and miR-30b mimics also sensitize HER2-positive breast cancer cells to trastuzumab treatment by silencing cyclin E2 gene [250]. Restoring miR-489 expression inhibits proliferation and tumor growth by targeting SHP2 and HER2, simultaneously suppressing ERK signaling [251], whereas miR-16 upregulation induced by trastuzumab impairs the growth of trastuzumab and lapatinib-resistant cells [252]. Furthermore, combining miR-101-5p mimics with trastuzumab plus lapatinib significantly reduces cell proliferation and induces apoptosis, helping sensitize cells to therapy [35]. In addition, Zhou et al. found that miR-4728 directly targets ErbB3-binding protein 1 (EBP1), reducing HER2 overexpression, and other study found that miR-4728 suppresses ESR1 expression, thereby decreasing sensitivity to hormonal therapy but enhancing the efficacy of anti-HER2 therapies [253,254].
The IGF2/IGF-1R/IRS1 pathway is another crucial regulator of trastuzumab sensitivity. The activation of FOXO3a, triggered by increased IGF2 expression, is modulated by miR-128-3p, miR-30a and miR-193-5p, which target IRS1 and IGF2, maintaining HER2-positive breast cancer cells responsive to trastuzumab. Dysregulation of this axis contributes to drug resistance [249]. Moreover, several miRNAs act as master regulators of oncogenic signaling. For example, Merve et al. [255] showed that miR-564 upregulation functions as a dual inhibitor of the PI3K/Akt/mTOR pathway in MCF-7 cells and the MAPK pathway in MDA-MB-231 cells, suppressing phosphorylation of Akt and ERK1/2. Increased expression of this miRNA was associated with lower levels of mesenchymal markers and inhibition of migration, invasion capacities and tumor growth. Likewise, overexpression of miR-22 targets SIRT1 to reduce MCF-7 cells viability and induce apoptosis, increasing radiosensitivity by suppressing DNA damage repair mechanisms [256].
In another study, Lisa et al. [257] performed a high-throughput screening and identified that miR-15b-5p, miR-19b-2-5p, miR-26b-3p, miR-29a-3p, miR-29c-3p, miR-32-3p, miR-93-3p, miR-101-5p, miR-106a-5p, miR-132-3p, miR-153-3p, miR-518a-5p, miR-744-3p and miR-1237-3p as mimics and miR-361-5p and miR-502-3p as inhibitors combined with trastuzumab and lapatinib significantly decreased HER2-positive cells viability by more than 75%, underscoring the potential of miRNAs to enhance therapeutic efficacy. Similarly, altered expression of miR-23b-3p, miR-195-5p, miR-340-5p and miR-656-5p modulates key targets such as AKT3, CCND1, FDF2, RAF1, MYB, c-MET and PTEN, leading to trastuzumab resistance in HER2-positive breast cancer [258].
An additional study [259] revealed that a triple combination of neratinib plus a CDK4/6 inhibitor plus endocrine therapy was more efficient in reducing proliferation and colony formation in HR-positive/HER2-low breast tumor cells (ZR-75-1) than CDK4/6 inhibitor plus endocrine therapy alone. Notably, the co-administration of a miR-23a-5p mimic could significantly enhance the efficacy of this triple therapy by suppressing HER2 signaling.
To date, no clinical trials have validated the efficacy of combining miRNA-based therapies with approved treatment for breast cancer, which may be due to the challenges inherent to miRNA delivery. Nevertheless, miRNAs have consistently demonstrated a synergistic effect with existing therapies, enhancing their effectiveness, reducing resistance and paving the way for new strategies within the framework of precision medicine.
7. Conclusions and Future Perspectives
HER2-low breast cancer is defined as tumors with IHC 1+ or 2+ with negative ISH according to the 2023 ASCO/CAP guidelines. Both ASCO/CAP and ESMO recognize HER2-low as a heterogenous group of tumors, stratified by HR status, with differences at the molecular and genomic levels. In this context, HER2-low/HR-positive tumors display luminal-like molecular profiles, while HER2-low/HR-negative resemble TNBC with characteristic mutations of this subtype. Understanding the mechanisms underlying this heterogeneity is crucial for developing more effective therapeutic strategies.
MiRNAs play a central role in modulating key cellular processes, and their dysregulation in cancer contributes to the activation of distinct hallmarks of malignancy, such as proliferation, apoptosis, invasion and therapy resistance. These molecules can serve as diagnostic, prognostic and predictive biomarkers and their expression may vary according to the heterogeneity of HER2-low tumors. Inter- and intratumor differences may drive distinct miRNA profiles, potentially influencing HR expression; therefore, single-cell analysis, combined with multi-omics, can offer the opportunity to explore the molecular characteristics at the cellular level, enabling the identification of dysregulated miRNA panels that may underline the divergence between HER2-low/HR-positive and HER2-low/HR-negative tumors.
Current HER2-targeted therapies alone provide limited clinical benefit in HER2-low breast cancer. Studies suggest that combining endocrine therapy to target HR with HER2-target agents may improve outcomes, but this strategy remains insufficient in some cases. Integrating miRNA-based therapies with endocrine treatment and anti-HER2 targeted regimens may offer a promising path forward and may provide a triple combination approach to more effectively address HER2-low tumor’s heterogeneity and overcome therapy resistance.
Despite extensive research on breast cancer, to the best of our knowledge, this is the first review highlighting miRNAs as potential regulators of the molecular mechanisms underlying HR expression and as contributors to novel targeted therapeutic strategies for HER2-low tumors. However, further studies are needed to identify specific miRNA signatures that distinctly characterize HER2-low tumors and to elucidate the molecular pathways driving their heterogeneity. Moreover, investigating whether circulating miRNAs can serve as minimally invasive biomarkers for HER2-low disease monitoring could provide valuable insights into tumor progression and treatment response. Integrating these findings may guide the development of novel combination therapies and reshape treatment paradigms toward more personalized care for patients with HER2-low breast cancer.
Author Contributions
Conceptualization, N.V.; methodology E.C.; formal analysis, E.C., F.S. and N.V.; investigation, E.C.; writing—original draft preparation, E.C.; writing—review and editing, F.S. and N.V.; supervision, N.V.; project administration, F.S. and N.V.; funding acquisition, F.S. and N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the FEDER—Fundo Europeu de Desenvolimento Regional through COMPETE 2020—Operational Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through the FCT—Fundação para a Ciência e a Tecno-logia, in a framework of the projects in CINTESIS, R&D Unit (reference UIDB/4255/2020), and within the scope of the project “RISE—LA/P/0053/2020.” N.V. would also like to thank the support from the FCT and FEDER (European Union), award number IF/00092/2014/CP1255/CT0004, PRR-09/C06-834I07/2024.P11721, 2024.18026.PEX and the Chair in Onco-Innovation at the FMUP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
E.C. thanks PerMed Research Group for her PhD Grant.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADC | Antibody-Drug Conjugate |
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| AI | Aromatase Inhibitors |
| Akt | Activated Protein Kinase B |
| AKT3 | Threonine-Protein Kinase |
| ASCO | American Society of Clinical Oncology |
| ASPP2 | Apoptosis-Stimulating Protein 2 |
| CAP | College of American Pathologists |
| CCND1 | Cyclin D1 |
| CDK | Cyclin-Dependent Kinase |
| c-MET | MET Proto-Oncogene |
| CNV | Copy Number Variations |
| DGCR8 | DiGeorge Syndrome Critical Region 8 |
| EBP1 | ErbB3-Binding Protein 1 |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| ER | Estrogen Receptor |
| ESMO | European Society for Medical Oncology |
| FDF2 | Fibroblast Growth Factor 2 |
| FGF2 | Fibroblast Growth Factor 2 |
| FKBP12 | FK506-binding protein 12 |
| FOXO1 | Forkhead Box O1 |
| HCV | Hepatitis C Virus |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hormone Receptor |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
| IGF2 | Insulin-like Growth Factor 2 |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin-6 |
| IRS1 | Insulin Receptor Substrate 1 |
| ISH | In Situ Hybridization |
| JAK2 | Janus Kinase 2 |
| MAPK | Mitogen-Activated Protein Kinase |
| MED1 | Mediator Complex Subunit 1 |
| miRISC | miRNA-induced silencing complex |
| miRNAs | MicroRNAs |
| mitomiRs | Mitochondrial MicroRNAs |
| MMP9 | Matrix Metalloproteinase 9 |
| mTOR | mammalian Target of Rapamycin |
| mTORC | mammalian Target of Rapamycin Complex |
| MYB | V-Myb Avian Myeloblastosis Viral Oncogene Homolog |
| OS | Overall Survival |
| PARN | Poly(A)-specific ribonuclease |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PEG | Polyethylene Glycol |
| PEI | Polyethylenimine |
| PFS | Progression-Free Survival |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP2 | Phosphatidylinositol-4, 5-biphosphate |
| PIP3 | Phosphatidylinositol-3, 4, 5-triphosphate |
| PLGA | Poly(Lactic-co-Glycolic Acid) |
| Pre-miRNA | Precursor MicroRNA |
| Pri-miRNA | Primary MicroRNA |
| PTEN | Phosphatase and Tensin Homolog |
| RAF1 | Raf Proto-Oncogene Serine |
| Rb | Retinoblastoma |
| SERM | Selective Estrogen Receptor Modulators |
| SHP2 | Src Homology Region 2-Containing Protein Tyrosine Phosphatase-2 |
| SIRT1 | Sirtuin 1 |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SOX9 | SRY-Box Transcription Factor 9 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAM | Tumor-Associated Macrophages |
| T-DM1 | Trastuzumab Emtansine |
| T-DXd | Trastuzumab Deruxtecan |
| TERT | Telomerase Reverse Transcriptase |
| TGF-β | Transforming Growth Factor-Beta |
| TKI | Tyrosine Kinase Inhibitors |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| TRL | Toll-like Receptors |
| TSC1/2 | Tuberous Sclerosis Complex 1/2 |
| UTR | Untranslated Region |
| VEGF | Vascular Endothelial Growth Factor |
| XPO5 | Exportin 5 |
| ZEB1 | Zinc Finger E-box-binding Homeobox 1 |
References
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An Update on the Pathological Classification of Breast Cancer. Histopathology 2022, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Green, A.R. Molecular Classification of Breast Cancer: What the Pathologist Needs to Know. Pathology 2017, 49, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Molecular Classification of Breast Cancer: Relevance and Challenges. Arch. Pathol. Lab. Med. 2023, 147, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, J.; Xu, Q.; Gao, Z.; Yang, M.; Wu, X.; Li, X. HER2/PI3K/AKT Pathway in HER2-Positive Breast Cancer: A Review. Medicine 2024, 103, e38508. [Google Scholar]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER 2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Kang, Y.J.; Oh, S.J.; Bae, S.Y.; Kim, E.K.; Lee, Y.J.; Park, E.H.; Jeong, J.; Park, H.K.; Suh, Y.J.; Kim, Y.S. Predictive Biological Factors for Late Survival in Patients with HER2-Positive Breast Cancer. Sci. Rep. 2023, 13, 11008. [Google Scholar] [CrossRef]
- Galogre, M.; Rodin, D.; Pyatnitskiy, M.; Mackelprang, M.; Koman, I. A Review of HER2 Overexpression and Somatic Mutations in Cancers. Crit. Rev. Oncol. Hematol. 2023, 186, 103997. [Google Scholar] [CrossRef]
- Schettini, F.; Prat, A. Dissecting the Biological Heterogeneity of HER2-Positive Breast Cancer. Breast 2021, 59, 339–350. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; Mcshane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Li, Y.; Tsang, J.Y.; Tam, F.; Loong, T.; Tse, G.M. Comprehensive Characterization of HER2-Low Breast Cancers: Implications in Prognosis and Treatment. eBioMedicine 2023, 91, 104571. [Google Scholar] [CrossRef]
- Zhang, H.; Katerji, H.; Turner, B.M.; Hicks, D.G. HER2-Low Breast Cancers: New Opportunities and Challenges. Am. J. Clin. Pathol. 2022, 157, 328–336. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.B. HER2-Low Breast Cancer: Now and in the Future. Cancer Res. Treat. 2024, 56, 700–720. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Tarantino, P.; Criscitiello, C.; Curigliano, G. HER2-Low Breast Cancer: A New Subtype? Curr. Treat. Options Oncol. 2023, 24, 468–478. [Google Scholar] [CrossRef]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. Her2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, L. Challenges in HER2-Low Breast Cancer Identification, Detection, and Treatment. Transl. Breast Cancer Res. 2024, 5, 3. [Google Scholar] [CrossRef]
- Tarantino, P.; Viale, G.; Press, M.F.; Hu, X.; Penault-Llorca, F.; Bardia, A.; Batistatou, A.; Burstein, H.J.; Carey, L.A.; Cortes, J.; et al. ESMO Expert Consensus Statements (ECS) on the Definition, Diagnosis, and Management of HER2-Low Breast Cancer. Ann. Oncol. 2023, 34, 645–659. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse Effects of Tyrosine Kinase Inhibitors in Cancer Therapy: Pathophysiology, Mechanisms and Clinical Management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Madden, S.F.; Gaynor, N.; AlSultan, D.; Le Gal, M.; Eustace, A.J.; Gately, K.A.; Hughes, C.; Davies, A.M.; Mahgoub, T.; et al. Effects of HER Family-Targeting Tyrosine Kinase Inhibitors on Antibody-Dependent Cell-Mediated Cytotoxicity in HER2-Expressing Breast Cancer. Clin. Cancer Res. 2021, 27, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, R.; Ma, F. HER2-Low Breast Cancer: Novel Detections and Treatment Advances. Crit. Rev. Oncol. Hematol. 2023, 181, 103883. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Wong, J.S.; Cheah, Y.K. Potential MiRNAs for MiRNA-Based Therapeutics in Breast Cancer. Noncoding RNA 2020, 6, 29. [Google Scholar] [CrossRef]
- Syeda, Z.A.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Devaney, A.; McNeill, R.E.; Davoren, P.A.; Lemetre, C.; Benes, V.; Schmidt, S.; Blake, J.; Ball, G.; et al. MicroRNA Signatures Predict Oestrogen Receptor, Progesterone Receptor and HER2/Neureceptor Status in Breast Cancer. Breast Cancer Res. 2009, 11, R27. [Google Scholar] [CrossRef]
- Nair, M.G.; Mavatkar, A.D.; Naidu, C.M.; VP, S.; CE, A.; Rajarajan, S.; Sahoo, S.; Mohan, G.; Jaikumar, V.S.; Ramesh, R.S.; et al. Elucidating the Role of MicroRNA-18a in Propelling a Hybrid Epithelial–Mesenchymal Phenotype and Driving Malignant Progression in ER-Negative Breast Cancer. Cells 2024, 13, 821. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. MiRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Scognamiglio, I.; Di Martino, M.T.; Campani, V.; Virgilio, A.; Galeone, A.; Gullà, A.; Gallo Cantafio, M.E.; Misso, G.; Tagliaferri, P.; Tassone, P.; et al. Transferrin-Conjugated SNALPs Encapsulating 2’-O-Methylated MiR-34a for the Treatment of Multiple Myeloma. Biomed. Res. Int. 2014, 2014, 217365. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, W.H.; Chen, B.Y.; Nie, J.; Su, Z.R.; Zheng, J.N.; Gong, S.T.; Chen, J.N.; Jiang, D.; Li, Y. MiR-29b Suppresses Proliferation and Induces Apoptosis of Hepatocellular Carcinoma Ascites H22 Cells via Regulating TGF-Β1 and P53 Signaling Pathway. Int. J. Mol. Med. 2021, 48, 157. [Google Scholar] [CrossRef]
- Normann, L.S.; Haugen, M.H.; Aure, M.R.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MiR-101-5p Acts as a Tumor Suppressor in HER2-Positive Breast Cancer Cells and Improves Targeted Therapy. Breast Cancer 2022, 14, 25–39. [Google Scholar] [CrossRef]
- Bozgeyik, E.; Kocahan, S.; Temiz, E.; Bagis, H. MiR-19a and MiR-421 Target PCA3 Long Non-Coding RNA and Restore PRUNE2 Tumor Suppressor Activity in Prostate Cancer. Mol. Biol. Rep. 2021, 49, 6803–6815. [Google Scholar] [CrossRef] [PubMed]
- Trang, P.; Medina, P.P.; Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Homer, R.; Brown, D.; Bader, A.G.; Weidhaas, J.B.; et al. Regression of Murine Lung Tumors by the Let-7 MicroRNA. Oncogene 2010, 29, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. npj Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The Frequency of Low HER2 Expression in Breast Cancer and a Comparison of Prognosis between Patients with HER2-Low and HER2-Negative Breast Cancer by HR Status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef]
- Viale, G.; Basik, M.; Niikura, N.; Tokunaga, E.; Brucker, S.; Penault-Llorca, F.; Hayashi, N.; Sohn, J.; Teixeira de Sousa, R.; Brufsky, A.M.; et al. Retrospective Study to Estimate the Prevalence and Describe the Clinicopathological Characteristics, Treatments Received, and Outcomes of HER2-Low Breast Cancer. ESMO Open 2023, 8, 101615. [Google Scholar] [CrossRef] [PubMed]
- Rey-Vargas, L.; Bejarano-Rivera, L.M.; Ballen, D.F.; Serrano-Gómez, S.J. Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women. Cancers 2024, 16, 3141. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Habibian, L.; Martin, C.; Semaan, K.; Khaddage, A.; El Kassis, N.; Kesserouani, C.; Kourie, H.R.; Atallah, D. Landscape of HER2-Low Breast Cancer: Insights from a Six-Year Study on Prevalence and Clinicopathological Characteristics. Ann. Diagn. Pathol. 2024, 72, 152326. [Google Scholar] [CrossRef] [PubMed]
- Baez-Navarro, X.; van Bockstal, M.R.; Andrinopoulou, E.R.; van Deurzen, C.H.M. HER2-Low Breast Cancer: Incidence, Clinicopathologic Features, and Survival Outcomes from Real-World Data of a Large Nationwide Cohort. Mod. Pathol. 2023, 36, 100087. Corrigendum in Mod. Pathol. 2023, 36, 100356. https://doi.org/10.1016/j.modpat.2023.100356. [CrossRef]
- Gomes Do Nascimento, R.; Otoni, K.M. Histological and Molecular Classification of Breast Cancer: What Do We Know? Mastology 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Tarantino, P.; Gupta, H.; Hughes, M.E.; Files, J.; Strauss, S.; Kirkner, G.; Feeney, A.M.; Li, Y.; Garrido-Castro, A.C.; Barroso-Sousa, R.; et al. Comprehensive Genomic Characterization of HER2-Low and HER2-0 Breast Cancer. Nat. Commun. 2023, 14, 7496. [Google Scholar] [CrossRef]
- Bansal, R.; Adeyelu, T.; Elliott, A.; Walker, P.; Bustos, M.A.; Rodriguez, E.; Accordino, M.K.; Meisel, J.; Gatti-Mays, M.E.; Hsu, E.; et al. Genomic and Transcriptomic Landscape of HER2-Low Breast Cancer. Breast Cancer Res. Treat. 2025, 209, 323–330. [Google Scholar] [CrossRef]
- Jin, J.; Li, B.; Cao, J.; Li, T.; Zhang, J.; Cao, J.; Zhao, M.; Wang, L.; Wang, B.; Tao, Z.; et al. Analysis of Clinical Features, Genomic Landscapes and Survival Outcomes in HER2-Low Breast Cancer. J. Transl. Med. 2023, 21, 360. [Google Scholar] [CrossRef]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.H.; Lu, Y.S.; Ono, M.; Ueno, T.; et al. HER2 Expression, Copy Number Variation and Survival Outcomes in HER2-Low Non-Metastatic Breast Cancer: An International Multicentre Cohort Study and TCGA-METABRIC Analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef]
- Merlin, J.L.; Husson, M.; Sahki, N.; Gilson, P.; Massard, V.; Harlé, A.; Leroux, A. Integrated Molecular Characterization of HER2-Low Breast Cancer Using Next Generation Sequencing (NGS). Biomedicines 2023, 11, 3164. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and Mechanisms of MicroRNA-Mediated Gene Regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The Widespread Regulation of MicroRNA Biogenesis, Function and Decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Varghese, L.N.; Schwenke, D.O.; Katare, R. Role of Noncoding RNAs in Cardiac Ageing. Front. Cardiovasc. Med. 2023, 10, 1142575. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, Characterization, and Functions of Mirtrons. Wiley Interdiscip. Rev. RNA 2022, 13, 1680. [Google Scholar] [CrossRef]
- Herrera-Carrillo, E.; Berkhout, B. Dicer-Independent Processing of Small RNA Duplexes: Mechanistic Insights and Applications. Nucleic Acids Res. 2017, 45, 10369–10379. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules 2020, 10, 1285. [Google Scholar] [CrossRef]
- Biasini, A.; Abdulkarim, B.; de Pretis, S.; Tan, J.Y.; Arora, R.; Wischnewski, H.; Dreos, R.; Pelizzola, M.; Ciaudo, C.; Marques, A.C. Translation Is Required for MiRNA-dependent Decay of Endogenous Transcripts. EMBO J. 2021, 40, e104569. [Google Scholar] [CrossRef]
- da Sacco, L.; Masotti, A. Recent Insights and Novel Bioinformatics Tools to Understand the Role of MicroRNAs Binding to 5′ Untranslated Region. Int. J. Mol. Sci. 2012, 14, 480–495. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target MRNAs Are Repressed as Efficiently by MicroRNA-Binding Sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Strayer, E.C.; Krishna, S.; Lee, H.; Vejnar, C.; Neuenkirchen, N.; Gupta, A.; Beaudoin, J.D.; Giraldez, A.J. NaP-TRAP Reveals the Regulatory Grammar in 5′UTR-Mediated Translation Regulation during Zebrafish Development. Nat. Commun. 2024, 15, 10898. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yu, J.; Huang, S.; Zhu, H.; Huang, Z. Transcriptional Regulation of Gene Expression by MicroRNAs as Endogenous Decoys of Transcription Factors. Cell Physiol. Biochem. 2014, 33, 1698–1714. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Kumar, A. Paradigm Shift: MicroRNAs Interact with Target Gene Promoters to Cause Transcriptional Gene Activation or Silencing. Exp. Cell Res. 2025, 444, 114372. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, B.; Zhan, X.; Silver, H.; Li, J. Microrna 195-5p Targets Foxo3 Promoter Region to Regulate Its Expression in Granulosa Cells. Int. J. Mol. Sci. 2021, 22, 6721. [Google Scholar] [CrossRef]
- Jie, M.; Feng, T.; Huang, W.; Zhang, M.; Feng, Y.; Jiang, H.; Wen, Z. Subcellular Localization of MiRNAs and Implications in Cellular Homeostasis. Genes 2021, 12, 856. [Google Scholar] [CrossRef]
- Wu, T.; Song, H.; Xie, D.; Hua, K.; Hu, J.; Deng, Y.; Ji, C.; Fang, L. Mir-30b-5p Promotes Proliferation, Migration, and Invasion of Breast Cancer Cells via Targeting ASPP2. Biomed. Res. Int. 2020, 2020, 7907269. [Google Scholar] [CrossRef]
- Ma, T.; Yang, L.; Zhang, J. MiRNA 542 3p Downregulation Promotes Trastuzumab Resistance in Breast Cancer Cells via AKT Activation. Oncol. Rep. 2015, 33, 1215–1220. [Google Scholar] [CrossRef]
- de Mattos-Arruda, L.; Bottai, G.; Nuciforo, P.G.; di Tommaso, L.; Giovannetti, E.; Peg, V.; Losurdo, A.; Pérez-Garcia, J.; Masci, G.; Corsi, F.; et al. MicroRNA-21 Links Epithelial-to-Mesenchymal Transition and Inflammatory Signals to Confer Resistance to Neoadjuvant Trastuzumab and Chemotherapy in HER2-Positive Breast Cancer Patients. Oncotarget 2015, 6, 37269–37280. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Guijarro, L.G.; Casanova, C.; Coca, S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; Asúnsolo, Á. The Regulatory Role of Mitochondrial MicroRNAs (MitomiRs) in Breast Cancer: Translational Implications Present and Future. Cancers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Erturk, E.; Enes Onur, O.; Akgun, O.; Tuna, G.; Yildiz, Y.; Ari, F. Mitochondrial MiRNAs (MitomiRs): Their Potential Roles in Breast and Other Cancers. Mitochondrion 2022, 66, 74–81. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular MiRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating MicroRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ni, J.; Beretov, J.; Wasinger, V.C.; Graham, P.; Li, Y. Recent Advances of Small Extracellular Vesicle Biomarkers in Breast Cancer Diagnosis and Prognosis. Mol. Cancer 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Rouas, R.; Lagneaux, L.; Krayem, M.; Duvillier, H.; Berehab, M.; Lewalle, P. Acute Myeloid Leukemia-Derived Exosomes Deliver MiR-24-3p to Hinder the T-Cell Immune Response through DENN/MADD Targeting in the NF-ΚB Signaling Pathways. Cell Commun. Signal 2023, 21, 253. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-Derived Exosomal MiR-934 Induces Macrophage M2 Polarization to Promote Liver Metastasis of Colorectal Cancer. J. Hematol. Oncol. 2020, 13, 156. Correction in J. Hematol. Oncol. 2021, 14, 33. https://doi.org/10.1186/s13045-021-01042-0. [CrossRef]
- Dayakar, A.; Shanmukha, K.D.; Kalangi, S.K. Spectrum of MicroRNAs and Their Target Genes in Cancer: Intervention in Diagnosis and Therapy. Mol. Biol. Rep. 2022, 49, 6827–6846. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in Cancer: Biomarkers, Functions and Therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ciccarone, F.; Ciriolo, M.R. Editorial: Hallmark of Cancer: Sustained Proliferative Signalling. Front. Oncol. 2023, 13, 1328827. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, W.; Hong, J.Y.; Kang, D.; Park, S.; Suh, J.; You, D.; Park, Y.Y.; Suh, N.; Hwang, J.J.; et al. MiR-96-5p Targets PTEN to Mediate Sunitinib Resistance in Clear Cell Renal Cell Carcinoma. Sci. Rep. 2022, 12, 3537. [Google Scholar] [CrossRef]
- Huang, G.; Zhong, X.; Yao, L.; Ma, Q.; Liao, H.; Xu, L.; Zou, J.; Sun, R.; Wang, D.; Guo, X. MicroRNA 449a Inhibits Cell Proliferation and Migration by Regulating Mutant P53 in MDA MB 468 Cells. Exp. Ther. Med. 2021, 22, 1020. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Kulbay, M.; Paimboeuf, A.; Ozdemir, D.; Bernier, J. Review of Cancer Cell Resistance Mechanisms to Apoptosis and Actual Targeted Therapies. J. Cell Biochem. 2022, 123, 1736–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Tumor Viruses and Replicative Immortality—Avoiding the Telomere Hurdle. Semin. Cancer Biol. 2014, 26, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M. Editorial: Hallmark of Cancer: Replicative Immortality. Front. Oncol. 2023, 13, 1204094. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Vimalraj, S. A Concise Review of VEGF, PDGF, FGF, Notch, Angiopoietin, and HGF Signalling in Tumor Angiogenesis with a Focus on Alternative Approaches and Future Directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF Signaling: Role in Angiogenesis and Beyond. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef] [PubMed]
- Karsten, N.; Kolben, T.; Mahner, S.; Beyer, S.; Meister, S.; Kuhn, C.; Schmoeckel, E.; Wuerstlein, R.; Harbeck, N.; Ditsch, N.; et al. The Role of E-Cadherin Expression in Primary Site of Breast Cancer. Arch. Gynecol. Obstet. 2022, 305, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Cai, Q.; Deng, L.; Ouyang, Q.; Zhang, X.H.F.; Zheng, J. Invasion and Metastasis in Cancer: Molecular Insights and Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 57. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, T.; Huang, C.; Xu, Z.; Wang, L.; Jiang, E.; Wang, H.; Chen, Y.; Liu, K.; Shao, Z.; et al. Melanoma Cell-Secreted Exosomal MiR-155-5p Induce Proangiogenic Switch of Cancer-Associated Fibroblasts via SOCS1/JAK2/STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2018, 37, 242. [Google Scholar] [CrossRef]
- Han, Q.; Tan, S.; Gong, L.; Li, G.; Wu, Q.; Chen, L.; Du, S.; Li, W.; Liu, X.; Cai, J.; et al. Omental Cancer-Associated Fibroblast-Derived Exosomes with Low MicroRNA-29c-3p Promote Ovarian Cancer Peritoneal Metastasis. Cancer Sci. 2023, 114, 1929–1942. [Google Scholar] [CrossRef]
- Li, Z.; Sun, C.; Qin, Z. Metabolic Reprogramming of Cancer-Associated Fibroblasts and Its Effect on Cancer Cell Reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef]
- Xuekai, L.; Yan, S.; Jian, C.; Yifei, S.; Xinyue, W.; Wenyuan, Z.; Shuwen, H.; Xi, Y. Advances in Reprogramming of Energy Metabolism in Tumor T Cells. Front. Immunol. 2024, 15, 1347181. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, J.Y.; Qiu, X.M.; Zhang, J.D.; Sun, Z.J.; Wang, W. Tumor-Associated Inflammation: The Tumor-Promoting Immunity in the Early Stages of Tumorigenesis. J. Immunol. Res. 2022, 2022, 3128933. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Immune Evasion in Cancer: Mechanisms and Cutting-Edge Therapeutic Approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A Novel MiR-155/MiR-143 Cascade Controls Glycolysis by Regulating Hexokinase 2 in Breast Cancer Cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y. Pancreatic Cancer Cell-Derived MicroRNA-155-5p-Containing Extracellular Vesicles Promote Immune Evasion by Triggering EHF-Dependent Activation of Akt/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2021, 100, 107990. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Soltani, B.M.; Sadeghizadeh, M. MicroRNA-203a Inhibits Breast Cancer Progression through the PI3K/Akt and Wnt Pathways. Sci. Rep. 2024, 14, 4715. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ren, Q.; Wang, J.; He, Y.; Deng, H.; Wang, X.; Liu, C. MiR-199a-3p Promotes Gastric Cancer Progression by Promoting Its Stemness Potential via DDR2 Mediation. Cell Signal 2023, 106, 110636. [Google Scholar] [CrossRef]
- Shi, H.; Pan, B.; Liang, J.; Cai, B.; Wu, G.; Bian, Y.; Shan, G.; Ren, S.; Huang, Y.; Guo, W. MiR-30c-5p Inhibits Esophageal Squamous Cell Carcinoma Progression by Repressing the PI3K/AKT Signaling Pathway. Thorac. Cancer 2024, 15, 2206–2216. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Zhou, Z.S.; Wang, G.Z.; Tu, J.Y.; Cheng, H.B.; Ma, S.Z.; Ke, C.; Wang, Y.; Jian, Q.P.; Shu, Y.H.; et al. MiR-122-5p Regulates the Mevalonate Pathway by Targeting P53 in Non-Small Cell Lung Cancer. Cell Death Dis. 2023, 14, 234. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Tian, Y. MiR-19-3p Targets PTEN to Regulate Cervical Cancer Cell Proliferation, Invasion, and Autophagy. Genet. Res. 2023, 2023, 4784500. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, J.; Wang, J.; Pan, Y.; Fu, J.; Bai, Y.; Zhang, J.; Shao, C. Extracellular MiR-1246 Promotes Lung Cancer Cell Proliferation and Enhances Radioresistance by Directly Targeting DR5. Oncotarget 2016, 7, 32707–32722. [Google Scholar] [CrossRef]
- Chen, J.; Cai, S.; Gu, T.; Song, F.; Xue, Y.; Sun, D. Mir-140-3p Impedes Gastric Cancer Progression and Metastasis by Regulating Bcl2/Becn1-Mediated Autophagy. Onco Targets Ther. 2021, 14, 2879–2892. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, C.; Wang, Y.; Fan, B.; Song, W.; Shen, R. MiR-363-3p Induces Tamoxifen Resistance in Breast Cancer Cells through PTEN Modulation. Sci. Rep. 2024, 14, 32135. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.J.; Tao, H.; Jin, W. Sen. MicroRNA-21 Controls HTERT via PTEN in Human Colorectal Cancer Cell Proliferation. J. Physiol. Biochem. 2015, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Naohiro, S.; Nakayama, Y.; Osaki, M.; Okada, F.; Oshimura, M.; Kugoh, H. MiR-19b Regulates HTERT MRNA Expression through Targeting PITX1 MRNA in Melanoma Cells. Sci. Rep. 2015, 5, 8201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zhang, S.; Huang, Q.; Liu, S.; Qiu, L.; Wei, M.; Deng, X.; Meng, W.; Chen, H.N.; et al. MicroRNA-6084 Orchestrates Angiogenesis and Liver Metastasis in Colorectal Cancer via Extracellular Vesicles. JCI Insight 2025, 10, e189503. [Google Scholar] [CrossRef]
- Chang, R.M.; Fu, Y.; Zeng, J.; Zhu, X.Y.; Gao, Y. Cancer-Derived Exosomal MiR-197-3p Confers Angiogenesis via Targeting TIMP2/3 in Lung Adenocarcinoma Metastasis. Cell Death Dis. 2022, 13, 1032. [Google Scholar] [CrossRef]
- Fan, B.; Niu, Y.; Ren, Z.; Wei, S.; Ma, Y.; Su, J.; Zhang, A. Long Noncoding RNA MMP2-AS1 Contributes to Progression of Renal Cell Carcinoma by Modulating MiR-34c-5p/MMP2 Axis. J. Oncol. 2022, 2022, 7346460. [Google Scholar] [CrossRef]
- Leoni, I.; Galvani, G.; Monti, E.; Vianello, C.; Valenti, F.; Pincigher, L.; Grolla, A.A.; Moro, M.; Coada, C.A.; Perrone, A.; et al. MiR-22/GLUT1 Axis Induces Metabolic Reprogramming and Sorafenib Resistance in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 3808. [Google Scholar] [CrossRef]
- Xu, D.; Liu, B.; Wang, L. MiR-365-3p Inhibits Lung Cancer Proliferation and Migration via CPT1A-Mediated Fatty Acid Oxidation. Sci. Rep. 2025, 15, 7076. [Google Scholar] [CrossRef]
- Wei, J.; Wang, F.; Kong, L.Y.; Xu, S.; Doucette, T.; Ferguson, S.D.; Yang, Y.; McEnery, K.; Jethwa, K.; Gjyshi, O.; et al. MiR-124 Inhibits STAT3 Signaling to Enhance T Cell-Mediated Immune Clearance of Glioma. Cancer Res. 2013, 73, 3913–3926. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic Reticulum Stress-induced Exosomal MiR-27a-3p Promotes Immune Escape in Breast Cancer via Regulating PD-L1 Expression in Macrophages. J. Cell Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef]
- Zou, C.; Xu, Q.; Mao, F.; Li, D.; Bian, C.; Liu, L.Z.; Jiang, Y.; Chen, X.; Qi, Y.; Zhang, X.; et al. MiR-145 Inhibits Tumor Angiogenesis and Growth by N-RAS and VEGF. Cell Cycle 2012, 11, 2137–2145. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a Limits Tumorigenic Inflammation in Colorectal Cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.W.; Calses, P.; Kemp, C.J.; Taniguchi, T. MiR-96 Downregulates REV1 and RAD51 to Promote Cellular Sensitivity to Cisplatin and PARP Inhibition. Cancer Res. 2012, 72, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ng, W.L.; Zhang, X.; Wang, P.; Zhang, Z.; Mo, Y.Y.; Mao, H.; Hao, C.; Olson, J.J.; Curran, W.J.; et al. Targeting DNA-PKcs and ATM with MiR-101 Sensitizes Tumors to Radiation. PLoS ONE 2010, 5, e11397. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, M.; Okayama, H.; Ishigame, T.; Min, A.K.T.; Saito, K.; Ujiie, D.; Murakami, Y.; Kikuchi, T.; Nakayama, Y.; Noda, M.; et al. MiRNA-148a-3p Regulates Immunosuppression in DNA Mismatch Repair-Deficient Colorectal Cancer by Targeting PD-L1. Mol. Cancer Res. 2019, 17, 1403–1413. [Google Scholar] [CrossRef]
- Singh, V.; Sen, A.; Saini, S.; Dwivedi, S.; Agrawal, R.; Bansal, A.; Shekhar, S. MicroRNA Significance in Cancer: An Updated Review on Diagnostic, Prognostic, and Therapeutic Perspectives. Ejifcc 2024, 35, 265–284. [Google Scholar]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Liu, M.; Mo, F.; Song, X.; He, Y.; Yuan, Y.; Yan, J.; Yang, Y.; Huang, J.; Zhang, S. Exosomal Hsa-MiR-21-5p Is a Biomarker for Breast Cancer Diagnosis. PeerJ 2021, 9, e12147. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.J.; Zhang, L.; Xu, Q.F.; Zhao, Y.M.; Shi, X.Y.; Xu, A.G. Serum MiR-21 Level: A Potential Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Med. 2015, 8, 14759–14763. [Google Scholar]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum MiR-21 as a Diagnostic and Prognostic Biomarker in Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef]
- Kao, H.W.; Pan, C.Y.; Lai, C.H.; Wu, C.W.; Fang, W.L.; Huang, K.H.; Lin, W.C. Urine MiR-21-5p as a Potential Non-Invasive Biomarker for Gastric Cancer. Oncotarget 2017, 8, 56389–56397. [Google Scholar] [CrossRef]
- Stechele, M.; Link, H.; Hirner-Eppeneder, H.; Alunni-Fabbroni, M.; Wildgruber, M.; Salvermoser, L.; Corradini, S.; Schinner, R.; Ben Khaled, N.; Rössler, D.; et al. Circulating MiR-21 as a Prognostic Biomarker in HCC Treated by CT-Guided High-Dose Rate Brachytherapy. Radiat. Oncol. 2023, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Hanif, M.; Ahmed, A.; Jamal, Q.; Mushtaq, S.; Khan, A.; Saqib, M. Circulating MiR-21 as a Prognostic and Predictive Biomarker in Oral Squamous Cell Carcinoma. Pak. J. Med. Sci. 2019, 35, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Ishinaga, H.; Okugawa, Y.; Hou, B.; He, F.; Yin, C.; Murata, M.; Toiyama, Y.; Takeuchi, K. The Role of MiR-21 as a Predictive Biomarker and a Potential Target to Improve the Effects of Chemoradiotherapy against Head and Neck Squamous Cell Carcinoma. J. Radiat. Res. 2023, 64, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lu, Z.; Meng, F.; Wan, Y.; Zhang, L.; Xu, Q.; Wang, Z. Circulating MiR-141 as a Potential Biomarker for Diagnosis, Prognosis and Therapeutic Targets in Gallbladder Cancer. Sci. Rep. 2022, 12, 10072. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal MiRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Eilertsen, M.; Andersen, S.; Al-Saad, S.; Richardsen, E.; Stenvold, H.; Hald, S.M.; Al-Shibli, K.; Donnem, T.; Busund, L.T.; Bremnes, R.M. Positive Prognostic Impact of MiR-210 in Non-Small Cell Lung Cancer. Lung Cancer 2014, 83, 272–278. [Google Scholar] [CrossRef]
- Parrella, P.; Barbano, R.; Pasculli, B.; Fontana, A.; Copetti, M.; Valori, V.M.; Poeta, M.L.; Perrone, G.; Righi, D.; Castelvetere, M.; et al. Evaluation of MicroRNA-10b Prognostic Significance in a Prospective Cohort of Breast Cancer Patients. Mol. Cancer 2014, 13, 142. [Google Scholar] [CrossRef]
- Xin, S.Y.; Feng, X.S.; Zhou, L.Q.; Sun, J.J.; Gao, X.L.; Yao, G.L. Reduced Expression of Circulating MicroRNA-218 in Gastric Cancer and Correlation with Tumor Invasion and Prognosis. World J. Gastroenterol. 2014, 20, 6906–6911. [Google Scholar] [CrossRef]
- Wang, X.X.; Ge, S.J.; Wang, X.L.; Jiang, L.X.; Sheng, M.F.; Ma, J.J. MiR-218 Tissue Expression Level Is Associated with Aggressive Progression of Gastric Cancer. Genet. Mol. Res. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Feng, X.; Yang, P.; Yang, J.; An, P.; Wang, H.; Ye, S.; Yu, C.; He, Y.; et al. Low Expression of MicroRNA-126 Is Associated with Poor Prognosis in Colorectal Cancer. Genes Chromosomes Cancer 2014, 53, 358–365. [Google Scholar] [CrossRef]
- Thugu, T.R.; Jain, M.; Madhavaram, N.J.; Singh, N.; Dash, S.; Alhomaid, A.; Kanagala, S.G.; Byreddi, L.Y.; Jain, A.; Desai, R. CLO25-090: MiR-31 as a Predictive Biomarker of Cetuximab Efficacy in Metastatic Colorectal Cancer Patients. J. Natl. Compr. Canc Netw. 2025, 23, CLO25-090. [Google Scholar] [CrossRef]
- Caramés, C.; Cristobal, I.; Moreno, V.; Marín, J.P.; González-Alonso, P.; Torrejón, B.; Minguez, P.; Leon, A.; Martín, J.I.; Hernández, R.; et al. MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2016, 17, 878. [Google Scholar] [CrossRef]
- Wang, H.; Tan, G.; Dong, L.; Cheng, L.; Li, K.; Wang, Z.; Luo, H. Circulating MiR-125b as a Marker Predicting Chemoresistance in Breast Cancer. PLoS ONE 2012, 7, e34210. [Google Scholar] [CrossRef]
- Patellongi, I.; Amiruddin, A.; Massi, M.N.; Islam, A.A.; Pratama, M.Y.; Sutandyo, N.; Latar, N.H.M.; Faruk, M. Circulating MiR-221/222 Expression as MicroRNA Biomarker Predicting Tamoxifen Treatment Outcome: A Case–Control Study. Ann. Med. Surg. 2023, 85, 3806–3815. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-Tumor Heterogeneity from a Cancer Stem Cell Perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef]
- Alimena, S.; Stephenson, B.J.K.; Webber, J.W.; Wollborn, L.; Sussman, C.B.; Packard, D.G.; Williams, M.; Comrie, C.E.; Wang, J.Y.; Markert, T.; et al. Differences in Serum MiRNA Profiles by Race, Ethnicity, & Socioeconomic Status: Implications for Developing an Equitable Ovarian Cancer Screening Test. Cancer Prev. Res. 2024, 17, 177–185. [Google Scholar]
- Chen, B.; Xia, Z.; Deng, Y.N.; Yang, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Emerging MicroRNA Biomarkers for Colorectal Cancer Diagnosis and Prognosis. Open Biol. 2019, 9, 180212. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The Technology and Biology of Single-Cell RNA Sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor Heterogeneity: Preclinical Models, Emerging Technologies, and Future Applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef] [PubMed]
- Pandiani, C.; Strub, T.; Nottet, N.; Cheli, Y.; Gambi, G.; Bille, K.; Husser, C.; Dalmasso, M.; Béranger, G.; Lassalle, S.; et al. Single-Cell RNA Sequencing Reveals Intratumoral Heterogeneity in Primary Uveal Melanomas and Identifies HES6 as a Driver of the Metastatic Disease. Cell Death Differ. 2021, 28, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ma, N.; Li, X.; Gou, Y.; Duan, Y.; Liu, B.; Xia, J.; Zhao, X.; Wang, X.; Li, Q.; et al. Advances in Single-Cell RNA Sequencing and Its Applications in Cancer Research. J. Hematol. Oncol. 2023, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, I.; Zaza, N.; Das, S.; Li, B.D. Precision Medicine and Targeted Therapies in Breast Cancer. Surg. Oncol. Clin. N. Am. 2020, 29, 51–62. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Guidi, L.; Pellizzari, G.; Tarantino, P.; Valenza, C.; Curigliano, G. Resistance to Antibody-Drug Conjugates Targeting HER2 in Breast Cancer: Molecular Landscape and Future Challenges. Cancers 2023, 15, 1130. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Fardi, E.; Ghaderi, H.; Palizdar, S.; Khorram, R.; Vafadar, R.; Ghanaatian, M.; Rezaei-Tazangi, F.; Baziyar, P.; Ahmadi, A.; et al. Receptor Tyrosine Kinase Inhibitors in Cancer. Cell. Mol. Life Sci. 2023, 80, 104. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, H.; Solanki, R.; Rawat, L.; Tabasum, S.; Tanwar, P.; Pal, S.; Sabarwal, A. Recent Developments in Receptor Tyrosine Kinase Inhibitors: A Promising Mainstay in Targeted Cancer Therapy. Med. Drug Discov. 2024, 23, 100195. [Google Scholar] [CrossRef]
- Iancu, G.; Serban, D.; Badiu, C.D.; Tanasescu, C.; Tudosie, M.S.; Tudor, C.; Costea, D.O.; Zgura, A.; Iancu, R.; Vasile, D. Tyrosine Kinase Inhibitors in Breast Cancer. Exp. Ther. Med. 2021, 23, 114. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Rajendran, R.L.; Miruka, C.O.; Sivamani, P.; Maran, B.A.V.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.C. A Review on Tyrosine Kinase Inhibitors for Targeted Breast Cancer Therapy. Pathol. Res. Pract. 2024, 263, 155607. [Google Scholar] [CrossRef]
- Roskoski, R. Classification of Small Molecule Protein Kinase Inhibitors Based upon the Structures of Their Drug-Enzyme Complexes. Pharmacol. Res. 2016, 103, 26–48. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. In Small Molecules in Oncology; Recent Results in Cancer Research, Volume 211; Springer: Cham, Switzerland, 2018; pp. 19–44. [Google Scholar]
- Eli, L.D.; Kavuri, S.M. Mechanisms of Neratinib Resistance in HER2-Mutant Metastatic Breast Cancer. Cancer Drug Resist. 2022, 5, 873–881. [Google Scholar] [CrossRef]
- Lee, A. Tucatinib: First Approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.M.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus Capecitabine versus Lapatinib plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer (PHOEBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xia, Y.; Zhu, Y.; Yang, Y.; Lin, Q.; Liu, Q.; Yang, W.; Ling, L.; Zhong, J.; Duan, Z.; et al. Preclinical Study and Phase 2 Trial of Neoadjuvant Pyrotinib Combined with Chemotherapy in Luminal/HER2-Low Breast Cancer: PILHLE-001 Study. Cell Rep. Med. 2024, 5, 101807. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Harbeck, N.; Beckmann, M.W.; Rody, A.; Schneeweiss, A.; Müller, V.; Fehm, T.; Marschner, N.; Gluz, O.; Schrader, I.; Heinrich, G.; et al. HER2 Dimerization Inhibitor Pertuzumab—Mode of Action and Clinical Data in Breast Cancer. Breast Care 2013, 8, 49–55. [Google Scholar] [CrossRef]
- Behl, A.; Wani, Z.A.; Das, N.N.; Parmar, V.S.; Len, C.; Malhotra, S.; Chhillar, A.K. Monoclonal Antibodies in Breast Cancer: A Critical Appraisal. Crit. Rev. Oncol. Hematol. 2023, 183, 103915. [Google Scholar] [CrossRef]
- Vu, T.; Claret, F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef]
- Vo, T.H.; EL-Sherbieny Abdelaal, E.; Jordan, E.; O’Donovan, O.; McNeela, E.A.; Mehta, J.P.; Rani, S. MiRNAs as Biomarkers of Therapeutic Response to HER2-Targeted Treatment in Breast Cancer: A Systematic Review. Biochem. Biophys. Rep. 2023, 37, 101588. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-Targeted Therapies in Cancer: A Systematic Review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef]
- Pérez-Garcia, J.; Muñoz-Couselo, E.; Cortés, J.; Scaltriti, M. Therapeutic Antibodies in Breast Cancer. Semin. Oncol. 2014, 41, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Jiao, D.C.; Qiao, J.H.; Wang, C.Z.; Sun, X.F.; Lu, Z.D.; Li, L.F.; Zhang, C.J.; Yan, M.; Wei, Y.; et al. De-Escalated Neoadjuvant Weekly Nab-Paclitaxel with Trastuzumab and Pertuzumab versus Docetaxel, Carboplatin, Trastuzumab, and Pertuzumab in Patients with HER2-Positive Early Breast Cancer (HELEN-006): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2025, 26, 27–36. [Google Scholar] [PubMed]
- Peng, D.M.; Li, J.; Qiu, J.X.; Zhao, L. Neoadjuvant Pertuzumab plus Trastuzumab in Combination with Chemotherapy for Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer: A Real-World Retrospective Single-Institutional Study in China. World J. Surg. Oncol. 2024, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Falcón González, A.; Cruz Jurado, J.; Llabrés Valenti, E.; Urbano Cubero, R.; Álamo de la Gala, M.C.; Martínez Guisado, M.A.; Álvarez Ambite, R.; Rodríguez González, C.J.; Amérigo Góngora, M.; Rodríguez Pérez, L.; et al. Real-World Experience with Pertuzumab and Trastuzumab Combined with Chemotherapy in Neoadjuvant Treatment for Patients with Early-Stage HER2-Positive Breast Cancer: The NEOPERSUR Study. Clin. Transl. Oncol. 2024, 26, 2217–2226. [Google Scholar] [CrossRef]
- Arshad, M.; Azad, A.; Chan, P.Y.K.; Vigneswara, V.; Feldinger, K.; Nafi, S.N.M.; Laporte-Maguire, E.; De Santo, C.; Zuo, J.; Shaaban, A.M.; et al. Neratinib Could Be Effective as Monotherapy or in Combination with Trastuzumab in HER2-Low Breast Cancer Cells and Organoid Models. Br. J. Cancer 2024, 130, 1990–2002. [Google Scholar] [CrossRef]
- Mark, C.; Lee, J.S.; Cui, X.; Yuan, Y. Antibody–Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int. J. Mol. Sci. 2023, 24, 13726. [Google Scholar] [CrossRef]
- Fan, P.; Xu, K. Antibody-Drug Conjugates in Breast Cancer: Marching from HER2-Overexpression into HER2-Low. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188849. [Google Scholar] [CrossRef]
- Martín, M.; Pandiella, A.; Vargas-Castrillón, E.; Díaz-Rodríguez, E.; Iglesias-Hernangómez, T.; Martínez Cano, C.; Fernández-Cuesta, I.; Winkow, E.; Perelló, M.F. Trastuzumab Deruxtecan in Breast Cancer. Crit. Rev. Oncol. Hematol. 2024, 198, 104355. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab Emtansine: Mechanisms of Action and Drug Resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Eng. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results from DESTINY-Breast03, a Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer: Long-Term Survival Analysis of the DESTINY-Breast03 Trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Wibowo, E.; Pollock, P.A.; Hollis, N.; Wassersug, R.J. Tamoxifen in Men: A Review of Adverse Events. Andrology 2016, 4, 776–788. [Google Scholar] [CrossRef]
- Shen, J.; He, Y.; Li, S.; Chen, H. Crosstalk of Methylation and Tamoxifen in Breast Cancer. Mol. Med. Rep. 2024, 30, 180. [Google Scholar] [CrossRef] [PubMed]
- Generali, D.; Berardi, R.; Caruso, M.; Cazzaniga, M.; Garrone, O.; Minchella, I.; Paris, I.; Pinto, C.; De Placido, S. Aromatase Inhibitors: The Journey from the State of the Art to Clinical Open Questions. Front. Oncol. 2023, 13, 1249160. [Google Scholar] [CrossRef] [PubMed]
- Kharb, R.; Haider, K.; Neha, K.; Yar, M.S. Aromatase Inhibitors: Role in Postmenopausal Breast Cancer. Arch. Pharm. 2020, 353, e2000081. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.; Ingle, J.N.; Dudenkov, T.M.; Kalari, K.R.; Carlson, E.E.; Na, J.; Buzdar, A.U.; Robson, M.E.; Ellis, M.J.; Goss, P.E.; et al. Pharmacogenomics of Aromatase Inhibitors in Postmenopausal Breast Cancer and Additional Mechanisms of Anastrozole Action. JCI Insight 2020, 5, e137571. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Burrett, J.; Clarke, M.; Davies, C.; Duane, F.; Evans, V.; Gettins, L.; Godwin, J.; Gray, R.; Liu, H.; et al. Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet 2015, 386, 1341–1352. [Google Scholar]
- Ruhstaller, T.; Giobbie-Hurder, A.; Colleoni, M.; Jensen, M.B.; Ejlertsen, B.; De Azambuja, E.; Neven, P.; Láng, I.; Jakobsen, E.H.; Gladieff, L.; et al. Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women with Hormone Receptor–Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial. J. Clin. Oncol. 2019, 37, 105–114. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef]
- Demir Cetinkaya, B.; Biray Avci, C. Molecular Perspective on Targeted Therapy in Breast Cancer: A Review of Current Status. Med. Oncol. 2022, 39, 149. [Google Scholar] [CrossRef]
- Ligasová, A.; Frydrych, I.; Koberna, K. Basic Methods of Cell Cycle Analysis. Int. J. Mol. Sci. 2023, 24, 3674. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Purohit, L.; Jones, C.; Gonzalez, T.; Castrellon, A.; Hussein, A. The Role of CD4/6 Inhibitors in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 1242. [Google Scholar] [CrossRef]
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/MTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Alves, C.L.; Ditzel, H.J. Drugging the PI3K/AKT/MTOR Pathway in ER+ Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, X.; Ren, Y.; Fan, Z.; Zhang, J.; He, G.; Fu, L. Targeting PI3K Family with Small-Molecule Inhibitors in Cancer Therapy: Current Clinical Status and Future Directions. Mol. Cancer 2024, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K Inhibitors in Advanced Breast Cancer. Ann. Oncol. 2019, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, S.E.; Mayer, I.A. Targeting the PI3K/AKT/MTOR Pathway in Hormone Positive Breast Cancer. Drugs 2020, 80, 1685–1697. [Google Scholar] [CrossRef]
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110. [Google Scholar] [CrossRef]
- Kenna, M.M.; McGarrigle, S.; Pidgeon, G.P. The next Generation of PI3K-Akt-MTOR Pathway Inhibitors in Breast Cancer Cohorts. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 185–197. [Google Scholar]
- Zhang, H.P.; Jiang, R.Y.; Zhu, J.Y.; Sun, K.N.; Huang, Y.; Zhou, H.H.; Zheng, Y.B.; Wang, X.J. PI3K/AKT/MTOR Signaling Pathway: An Important Driver and Therapeutic Target in Triple-Negative Breast Cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Shaw, A.L.; Parson, M.A.H.; Truebestein, L.; Jenkins, M.L.; Leonard, T.A.; Burke, J.E. ATP-Competitive and Allosteric Inhibitors Induce Differential Conformational Changes at the Autoinhibitory Interface of Akt1. Structure 2023, 31, 343–354. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; DeMichele, A.; Clark, A.S.; Zelnak, A.; Yardley, D.A.; Karuturi, M.; Sanft, T.; Blau, S.; Hart, L.; et al. Phase I/II Trial of Triplet Therapy (Exemestane, Ribociclib, and Everolimus) after Progression on a CDK4/6 Inhibitor in HR+/HER2− Advanced Breast Cancer (TRINITI-1). Clin. Cancer Res. 2021, 27, 4177–4185. [Google Scholar] [CrossRef]
- Önder, T.; Ateş, Ö.; Öner, I.; Karaçin, C. Relationship between HER2-Low Status and Efficacy of CDK4/6 Inhibitors in Advanced Breast Cancer: A Real-World Study. Int. J. Clin. Oncol. 2024, 29, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Dai, Y.; Chen, L.; Liang, Y.; He, J.; Chen, S.; Song, X.; Luo, W.; Xu, R.; Chen, Q. Effect of HER2-Low Expression on the Efficacy of CDK4/6 Inhibitors in Breast Cancer: A Meta-Analysis. J. Clin. Oncol. 2024, 42, e13034. [Google Scholar] [CrossRef]
- Zattarin, E.; Presti, D.; Mariani, L.; Sposetti, C.; Leporati, R.; Menichetti, A.; Corti, C.; Benvenuti, C.; Fucà, G.; Lobefaro, R.; et al. Prognostic Significance of HER2-Low Status in HR-Positive/HER2-Negative Advanced Breast Cancer Treated with CDK4/6 Inhibitors. npj Breast Cancer 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sottotetti, F.; Tagliaferri, B.; Rizzo, G.; Palumbo, R.; Chessa, G.; Raso, C.; Perrone, L.; Malovini, A.; Tibollo, V.; Locati, L.D.; et al. Patterns of Treatment and Outcomes of Patients with Metastatic HER2-Low Breast Cancer Treated with CDK4/6 Inhibitors and Hormone Therapy. Drugs Context 2025, 14, 2024-12-1. [Google Scholar] [CrossRef]
- Kim, E.H.; Ryu, Y.; Choi, J.; Park, D.; Lee, J.W.; Chi, S.G.; Kim, S.H.; Yang, Y. Targeting MiR-21 to Overcome P-Glycoprotein Drug Efflux in Doxorubicin-Resistant 4T1 Breast Cancer. Biomater. Res. 2024, 28, 0095. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Sohal, I.S.; Iyer, S.G.; Sudarshan, K.; Orellana, E.A.; Ozcan, K.E.; dos Santos, A.P.; Low, P.S.; Kasinski, A.L. Selective Targeting of Chemically Modified MiR-34a to Prostate Cancer Using a Small Molecule Ligand and an Endosomal Escape Agent. Mol. Ther. Nucleic Acids 2024, 35, 102193. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Lu, M.; Cheng, C.; Feng, Z.; Zhao, R.; Liang, J.; Han, J.; Jiang, J.; Xu-Welliver, M.; et al. Let-7b-3p Inhibits Tumor Growth and Metastasis by Targeting the BRF2-Mediated MAPK/ERK Pathway in Human Lung Adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1841–1856. [Google Scholar] [CrossRef]
- Pan, W.; Tan, Y.; Chen, X.; Zeng, L.; Lv, Y.; Yang, J. MiRNA-204-5p Acts as a Tumor Suppressor in Gastric Cancer by Inhibiting Cell Migration, Invasion, and Glycolysis via the RAB22A/PI3K/AKT Axis. Sci. Rep. 2025, 15, 29536. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, J.; Yu, J.; Liao, J.; Liu, S.; Cai, N.; Liang, H.; Chen, X.; Ding, Z.; Zhang, B. MicroRNA-148a-3p Inhibits Progression of Hepatocelluar Carcimoma by Repressing SMAD2 Expression in an Ago2 Dependent Manner. J. Exp. Clin. Cancer Res. 2020, 39, 150. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Liang, L.; He, X. A Narrative Review of MicroRNA Therapeutics: Understanding the Future of MicroRNA Research. Precis. Cancer Med. 2021, 4, 33. [Google Scholar] [CrossRef]
- Segal, M.; Slack, F.J. Challenges Identifying Efficacious MiRNA Therapeutics for Cancer. Expert. Opin. Drug Discov. 2020, 15, 987–992. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent Progress in MicroRNA Delivery for Cancer Therapy by Non-Viral Synthetic Vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In Vivo Delivery of MiRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Chaudhary, V.; Jangra, S.; Yadav, N.R. Nanotechnology Based Approaches for Detection and Delivery of MicroRNA in Healthcare and Crop Protection. J. Nanobiotechnol. 2018, 16, 40. [Google Scholar] [CrossRef]
- Wang, W.T.; Chen, Y.Q. Circulating MiRNAs in Cancer: From Detection to Therapy. J. Hematol. Oncol. 2014, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in MiRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cazares, M.B.; Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Nunez-Olvera, S.I.; Compean-Martinez, I.; Lopez-Camarillo, C. Lipid-Based Nanoparticles for the Therapeutic Delivery of Non-Coding RNAs in Breast Cancer. Oncol. Rep. 2020, 44, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Kong, W.; Rong, X.; Zhong, Z.; Jiang, L.; Chen, S.; Li, C.; Zhang, F.; Jiang, J. Delivery of MiRNAs Using Nanoparticles for the Treatment of Osteosarcoma. Int. J. Nanomed. 2024, 19, 8641–8660. [Google Scholar] [CrossRef]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. MiRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Polyester-Based Nanoparticles for Nucleic Acid Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Liang, Z.; Wang, X.; Gu, J.; Yao, Q.; Chen, C. New Polymer of Lactic-Co-Glycolic Acid-Modified Polyethylenimine for Nucleic Acid Delivery. Nanomedicine 2016, 11, 1971–1991. [Google Scholar] [CrossRef] [PubMed]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small Interfering RNA for Cancer Treatment: Overcoming Hurdles in Delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Siddiqui, B.; Qindeel, M.; Rahdar, A.; Bilal, M.; Behzadmehr, R.; Mirinejad, S.; Pandey, S. Chitosan Nanocarriers for MicroRNA Delivery and Detection: A Preliminary Review with Emphasis on Cancer. Carbohydr. Polym. 2022, 290, 119489. [Google Scholar] [CrossRef]
- Denizli, M.; Aslan, B.; Mangala, L.S.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Sood, A.K. Chitosan Nanoparticles for MiRNA Delivery. Methods Mol. Biol. 2017, 1632, 219–230. [Google Scholar]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic Potential of RNA-Enriched Extracellular Vesicles: The next Generation in RNA Delivery via Biogenic Nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Picon, M.A.; Wang, L.; Da Fonseca Ferreira, A.; Dong, C.; Marzouka, G.R. Extracellular Vesicles as Delivery Systems in Disease Therapy. Int. J. Mol. Sci. 2023, 24, 17134. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Telford, B.J.; Yahyanejad, S.; de Gunst, T.; den Boer, H.C.; Vos, R.M.; Stegink, M.; van den Bosch, M.T.J.; Alemdehy, M.F.; van Pinxteren, L.A.H.; Schaapveld, R.Q.J.; et al. Multi-Modal Effects of 1B3, a Novel Synthetic MiR-193a-3p Mimic, Support Strong Potential for Therapeutic Intervention in Oncology. Oncotarget 2021, 12, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Duurland, C.L.; de Gunst, T.; den Boer, H.C.; van den Bosch, M.T.J.; Telford, B.J.; Vos, R.M.; Xie, X.; Zang, M.; Wang, F.; Shao, Y.; et al. INT-1B3, an LNP Formulated MiR-193a-3p Mimic, Promotes Anti-Tumor Immunity by Enhancing T Cell Mediated Immune Responses via Modulation of the Tumor Microenvironment and Induction of Immunogenic Cell Death. Oncotarget 2024, 15, 470–485. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. First-in-Human Study of INT-1B3 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT04675996 (accessed on 30 October 2025).
- Ouyang, B.; Bi, M.; Jadhao, M.; Bick, G.; Zhang, X. MiR-205 Regulates Tamoxifen Resistance by Targeting Estrogen Receptor Coactivator MED1 in Human Breast Cancer. Cancers 2024, 16, 3992. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yin, W.; Yu, Z.H.; Zhou, Y.J.; Chi, J.R.; Ge, J.; Cao, X.C. MiR-190 Enhances Endocrine Therapy Sensitivity by Regulating SOX9 Expression in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 22. [Google Scholar] [CrossRef]
- Ghanbari, M.; Karari, K.; Altalebi, S.A.R.; Majeed, S.A.; Haghi, M. The Role of MicroRNAs in Breast Cancer: Diagnostic and Therapeutic Implications. Int. J. Biol. Macromol. 2025, 321, 146386. [Google Scholar] [CrossRef]
- Aboutalebi Vand Beilankouhi, E.; Sanaat, Z.; Hosseinpour Feizi, M.A.; Mehdizadeh, A.; Safaralizadeh, R. Investigation of Circulating MiR-182-3p, MiR -382-3p and MiR-93, MiR-142-3p Involved in Tamoxifen Resistance and Sensitivity in Luminal-Subtype Breast Cancer Patients: A Case–Control Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 7505–7516. [Google Scholar] [CrossRef]
- Fu, R.; Tong, J.S. MiR-126 Reduces Trastuzumab Resistance by Targeting PIK3R2 and Regulating AKT/MTOR Pathway in Breast Cancer Cells. J. Cell Mol. Med. 2020, 24, 7600–7608. [Google Scholar] [CrossRef]
- Gong, C.; Yao, Y.; Wang, Y.; Liu, B.; Wu, W.; Chen, J.; Su, F.; Yao, H.; Song, E. Up-Regulation of MiR-21 Mediates Resistance to Trastuzumab Therapy for Breast Cancer. J. Biol. Chem. 2011, 286, 19127–19137. [Google Scholar] [CrossRef]
- Tang, H.; Song, C.; Ye, F.; Gao, G.; Ou, X.; Zhang, L.; Xie, X.; Xie, X. MiR-200c Suppresses Stemness and Increases Cellular Sensitivity to Trastuzumab in HER2+ Breast Cancer. J. Cell Mol. Med. 2019, 23, 8114–8127. [Google Scholar] [CrossRef]
- Fogazzi, V.; Kapahnke, M.; Cataldo, A.; Plantamura, I.; Tagliabue, E.; Di Cosimo, S.; Cosentino, G.; Iorio, M.V. The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers 2022, 14, 5326. [Google Scholar] [CrossRef]
- Tormo, E.; Adam-Artigues, A.; Ballester, S.; Pineda, B.; Zazo, S.; González-Alonso, P.; Albanell, J.; Rovira, A.; Rojo, F.; Lluch, A.; et al. The Role of MiR-26a and MiR-30b in HER2+ Breast Cancer Trastuzumab Resistance and Regulation of the CCNE2 Gene. Sci. Rep. 2017, 7, 41309. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Shah, N.; Lee, J.S.; Markoutsa, E.; Jie, C.; Liu, S.; Botbyl, R.; Reisman, D.; Xu, P.; Chen, H. A Novel Double-Negative Feedback Loop between MiR-489 and the HER2-SHP2-MAPK Signaling Axis Regulates Breast Cancer Cell Proliferation and Tumor Growth. Oncotarget 2016, 7, 18295–18308. [Google Scholar] [CrossRef] [PubMed]
- Venturutti, L.; Cordo Russo, R.I.; Rivas, M.A.; Mercogliano, M.F.; Izzo, F.; Oakley, R.H.; Pereyra, M.G.; De Martino, M.; Proietti, C.J.; Yankilevich, P.; et al. MiR-16 Mediates Trastuzumab and Lapatinib Response in ErbB-2-Positive Breast and Gastric Cancer via Its Novel Targets CCNJ and FUBP1. Oncogene 2016, 35, 6189–6202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, Y.; Li, L.; Wang, X.; Quan, Y.; Liu, C.; Yu, M.; Hu, X.; Meng, X.; Zhou, Z.; et al. HER2-Intronic MiR-4728-5p Facilitates HER2 Expression and Accelerates Cell Proliferation and Migration by Targeting EBP1 in Breast Cancer. PLoS ONE 2021, 16, 0245832. [Google Scholar] [CrossRef]
- Newie, I.; Søkilde, R.; Persson, H.; Grabau, D.; Rego, N.; Kvist, A.; Von Stedingk, K.; Axelson, H.; Borg, Å.; Vallon-Christersson, J.; et al. The HER2-Encoded MiR-4728-3p Regulates ESR1 through a Non-Canonical Internal Seed Interaction. PLoS ONE 2014, 9, 97200. [Google Scholar] [CrossRef]
- Mutlu, M.; Saatci, Ö.; Ansari, S.A.; Yurdusev, E.; Shehwana, H.; Konu, Ö.; Raza, U.; Şahin, Ö. MiR-564 Acts as a Dual Inhibitor of PI3K and MAPK Signaling Networks and Inhibits Proliferation and Invasion in Breast Cancer. Sci. Rep. 2016, 6, 32541. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, D.; Wei, X. MiR-22 Suppresses Tumorigenesis and Improves Radiosensitivity of Breast Cancer Cells by Targeting Sirt1. Biol. Res. 2017, 50, 27. [Google Scholar] [CrossRef]
- Normann, L.S.; Aure, M.R.; Leivonen, S.K.; Haugen, M.H.; Hongisto, V.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MicroRNA in Combination with HER2-Targeting Drugs Reduces Breast Cancer Cell Viability in Vitro. Sci. Rep. 2021, 11, 10893. [Google Scholar] [CrossRef]
- Rezaei, Z.; Sebzari, A.; Kordi-Tamandani, D.M.; Dastjerdi, K. Involvement of the Dysregulation of MiR-23b-3p, MiR-195-5p, MiR-656-5p, and MiR-340-5p in Trastuzumab Resistance of HER2-Positive Breast Cancer Cells and System Biology Approach to Predict Their Targets Involved in Resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar] [CrossRef]
- Chen, L.; Ye, L.; Liang, Y.; Luo, W.; Zuo, Q.; Huang, P.; Hu, Y.; Dai, Y.; Wu, Y.; Guo, Q.; et al. Neratinib Enhances the Efficacy of CDK4/6 Inhibitor plus Endocrine Therapy in HR+/HER2-Low Breast Cancer Cell Line ZR-75-1 via Hsa-MiR-23a-5p. Sci. Rep. 2024, 14, 31062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).