Simple Summary
Molecular and immunological profiling are transforming how hepatocellular carcinoma (HCC) is managed. Integrating biomarkers such as AFP, DCP, radiomics, and circulating tumour DNA refines transplant eligibility and post-transplant surveillance. Advances in understanding the tumour microenvironment and immune modulation guide the rational use of immunotherapy and mTOR-based immunosuppression. Liquid biopsy and multi-omics approaches enable earlier detection of molecular residual disease and tumour recurrence after curative treatment. Together, these developments support a precision-oriented, transplant-aware framework that bridges molecular discovery with clinical decision-making in everyday HCC care.
Abstract
Liver cancer, predominantly hepatocellular carcinoma (HCC), remains a leading cause of cancer-related mortality worldwide. Although systemic therapies have advanced in recent years, overall survival remains limited for many patients. A deeper understanding of the molecular and immunological landscape of HCC has driven the emergence of new therapeutic paradigms, from molecularly targeted agents to immune checkpoint blockade. Concurrently, innovations in liver transplantation, liquid biopsy, and multi-omics profiling are reshaping the therapeutic algorithm for selected candidates. This review summarises recent progress in molecular classification, tumour microenvironment mapping, and immune modulation, and examines how these translational insights are redefining clinical practice. Particular emphasis is placed on the integration of molecular markers into transplant eligibility, downstaging strategies, and post-transplant immunosuppression, providing a comprehensive, precision-oriented framework that bridges basic discovery and patient-centred care.
1. Introduction
Hepatocellular carcinoma (HCC) remains a high-burden malignancy with marked geographic and aetiological heterogeneity [1,2]. Curative-intent options—resection, ablation, and liver transplantation (LT)—are feasible only for a subset at presentation, and recurrence after locoregional or surgical therapy is common [3]. Although outcomes have improved with modern systemic therapy over the past decade, the prognosis for patients diagnosed at intermediate and advanced stages remains limited [4,5,6,7], reflecting both tumour biology and the constraints imposed by underlying liver dysfunction.
Systemic therapy has undergone a rapid shift from tyrosine-kinase inhibitor monotherapy to immune-based combinations [7]. First-line regimens that pair anti-angiogenic therapy with PD-(L)1 blockade, as well as dual immune–checkpoint strategies, now define the therapeutic landscape for many patients [4,5,6,7]. Yet real-world benefit varies widely due to portal-hypertension-related bleeding risk, performance status, and—crucially—biological heterogeneity [3,8]. Durable control hinges on aligning treatment with the molecular wiring of the tumour and the composition of the tumour microenvironment (TME), both of which shape response and resistance [9,10,11].
In parallel, transplantation practice is evolving. Broader adoption of downstaging protocols, refinement of allocation policies, and incorporation of serum markers have expanded access for selected patients beyond historical morphologic criteria [3,12]. Emerging tools—radiomics for microvascular invasion risk, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) avidity, multi-omics signatures, and liquid biopsy for molecular residual disease (MRD)—are beginning to inform selection before transplantation and surveillance after surgery [13,14,15,16,17]. These data also intersect with post-transplant management, including risk-adapted immunosuppression and cautious use of immunotherapy around the time of transplantation, where rejection risk must be balanced against potential oncological benefit [18,19,20,21,22].
This review adopts a translational perspective on these converging threads. I first summarise contemporary molecular and immune classifications of HCC and their clinical correlates, then examine how the TME shapes treatment response and resistance. I also evaluate liquid-biopsy and multi-omics approaches for surveillance and MRD detection and finally propose how these biomarkers can be integrated into pre- and post-transplant decision-making, including selection, downstaging, risk modelling, and immunosuppression strategies. My aim is to bridge basic science and clinical practice by providing a practical, precision-oriented, and transplant-aware framework for HCC management.
The overall organisation of this review is illustrated in Figure 1, which provides a schematic overview of its five major domains: molecular taxonomy, TME, systemic therapy, liquid-biopsy-based MRD monitoring, and transplant-based care.
Figure 1.
Overview of key molecular, immunological, and transplant-based aspects discussed in this review.
Each domain represents a layer of the precision-and-transplant framework, showing how molecular and immune insights translate into patient selection, treatment optimisation, and post-transplant surveillance across the continuum of HCC management.
2. Molecular Taxonomy of HCC and Clinical Correlates
2.1. Canonical Gene-Expression Taxonomies (Hoshida, Boyault, Chiang)
Early transcriptome work resolved HCC into reproducible subclasses with distinct signalling and clinicopathologic features. Hoshida S1–S3 emerged from a cross-cohort meta-analysis (n = 603). S1 shows aberrant WNT/TGF-β activation and greater early recurrence, S2 is a proliferation phenotype [MYC/protein kinase B (AKT) targets] with larger tumours and higher serum AFP, and S3 is a hepatocyte-differentiated phenotype with lower AFP and better differentiation. These labels correlated with size, grade, AFP and early recurrence risk across datasets [23].
In parallel, Boyault G1–G6 linked unsupervised clusters to driver alterations and aetiology. G1/G2 associate with HBV and AKT/PI3K activity (G2 enriched for TP53/PIK3CA mutations). G3 is TP53 mutated with cell-cycle activation, G4 includes TCF1-mutated tumours, and G5/G6 are CTNNB1/β-catenin classes with canonical WNT activation (G6 with E-cadherin loss and satellite nodules) [24].
A third scheme from Chiang et al. defined five classes—CTNNB1, proliferation, IFN-related, a chromosome-7 polysomy class, and an unannotated group—again aligning expression with copy-number and mutation data (e.g., CTNNB1 mutations enriched in the CTNNB1 class; IGF/AKT-mTOR signalling in proliferation class) [25].
Taken together, these frameworks converge on clinically meaningful axes such as proliferation vs. differentiation, WNT/β-catenin activity, progenitor/stemness traits, and have been externally summarised and applied to independent cohorts (Table 1) [26].

Table 1.
Molecular taxonomies of HCC and clinical correlates (summary).
2.2. A Clinically Translatable, Integrative View
Subsequent synthesis places HCC along two broad, practice-oriented archetypes.
The non-proliferation class comprises well-differentiated tumours, often harbouring CTNNB1 (β-catenin) mutations and displaying metabolic or hepatocytic signatures with low AFP levels. In contrast, the proliferation class features dedifferentiated biology with frequent TERT-promoter activation, TP53 mutation, FGF19/FGFR4-driven growth-factor signalling, chromosomal instability, and higher AFP levels. This integrative dichotomy distils earlier gene-expression taxonomies and remains widely used in translational and clinical review frameworks [27,28].
Pan-omic studies reinforced the gene-level foundations: TCGA (363 cases) catalogued recurrent mutations (notably TERT, CTNNB1, TP53) and identified integrative subtypes across platforms; focal FGF19 amplification on 11q13.3 mechanistically links to FGFR4 signalling as a druggable driver in a molecular subset [29,30,31].
2.3. Proteogenomics: Transcript–Protein Discordance and Pathway-Level Rewiring
Proteogenomic atlases extend this framework by quantifying how signalling is rewired beyond DNA/RNA.
An ICPC/Cell study of HBV-related HCC (n = 159) resolved proteome subclasses (metabolism, proliferation, microenvironment) and highlighted discordance between mRNA and protein, with metabolism and kinase signalling (e.g., AKT–mechanistic target of rapamycin [mTOR]) reprogrammed at the protein/phosphoprotein level [32].
A broader Nature Communications analysis across aetiologies and stages showed only moderate transcript–proteome correlation (Spearman’s ρ = 0.33), and mapped alterations in the WNT–β–catenin, AKT/mTOR, Notch and cell-cycle pathways at genomic, proteomic and phosphoproteomic layers; it also tied CTNNB1 and TP53 mutations to distinct phospho-signalling outputs [33].
Mechanistic and review work around HBV-HCC further contextualises these findings and points to proteogenomic candidates (e.g., cholesterol/FA metabolism, SOAT1) with therapeutic implications [34].
Concluding Remarks
Legacy molecular taxonomies such as the Hoshida (S1–S3), Boyault (G1–G6), and Chiang classifications converge into a clinically applicable dichotomy of proliferation versus non-proliferation phenotypes. Within this framework, specific oncogenic drivers such as CTNNB1 and FGF19/FGFR4 refine biological risk assessment and generate targeted therapeutic hypotheses. Proteomic and phosphoproteomic analyses further demonstrate that expression-based signatures do not always correspond to protein activity, highlighting the importance of multi-omics profiling whenever feasible.
3. Tumour Microenvironment (TME) and Immune Modulation
3.1. Single-Cell Cartography and Immunosuppressive Niches in HCC
Single-cell atlases consistently show that HCC harbours myeloid-dominated, tolerogenic ecosystems. Across cohorts, TREM2+ tumour-associated macrophages (TAMs) accumulate within tumours, correlate with worse outcomes, and exhibit ligand–receptor programmes that recruit Tregs and dampen cytotoxic T-cell function [11,35,36,37]. Functionally, in patient-derived and murine HCC models, TREM2 loss restored CD8+ T-cell infiltration and potentiated anti-PD-L1 efficacy, nominating TREM2+ TAMs as actionable suppressors (with particular enrichment after TACE) [36]. LAMP3+ dendritic cells (mregDCs)—CCR7+, migratory DCs that can co-express regulatory molecules—are prominent in HCC single-cell maps. Although context-dependent, they often align with exhausted T-cell states and immunotherapy-relevant antigen-presentation circuits [9,11,38]. These features are concordant with MDPI syntheses of the HCC TME, which emphasise the liver’s basal tolerance and myeloid skew as barriers to immune control [38]. Therapeutically, these myeloid and dendritic-cell subsets are now being explored as targets for immune modulation. TREM2 blockade or myeloid reprogramming strategies aim to restore antitumour immunity, while modulation of LAMP3+ dendritic-cell circuits may enhance responsiveness to immune checkpoint inhibitors (ICIs).
3.2. WNT/CTNNB1 Activation, Immune Exclusion, and ICI Resistance
Mechanistically, tumour-intrinsic WNT/β-catenin activation blocks recruitment of BATF3-lineage dendritic cells, eliminating the chemokine cues needed for priming and intra-tumour T-cell entry (a “non-T-cell-inflamed” state) across cancers [39,40]. In HCC, β-catenin activation drives anti-PD-1 resistance in engineered mouse models and has been linked to non-response in early clinical series, establishing CTNNB1-mutant HCC as a biomarker-defined immune-excluded class [9,10].
Translational reviews and MDPI updates converge on this framework and argue for β-catenin–aware trial design (e.g., avoiding ICI monotherapy in CTNNB1-mutant disease; testing priming strategies that restore DC influx) [39].
3.3. Emerging Targets and Combination Strategies
3.3.1. Adenosine–CD73–A2A/A2B Axis
Adenosine signalling is a central, druggable suppressive circuit in HCC. A2A-receptor blockade augments anti-PD-(L)1 activity in HCC murine models, improving T-cell function and tumour control [41]. Clinically, CD73 (NT5E) is over-expressed in a sizeable subset of HCC and associates with metastasis and poor prognosis, and early-phase anti-CD73 antibodies (e.g., oleclumab) have shown immune activation and tolerable safety in solid tumours (with combinatorial signals in non-HCC settings), motivating HCC-specific trials [42,43,44]. Importantly, aetiology matters: in NASH-associated HCC models, A2A signalling may exert tumour-suppressive effects, cautioning against one-size-fits-all inhibition and underscoring the need for biomarker-guided selection [45]. Comprehensive reviews outline how A2A/A2B and CD73 cooperate to enforce myeloid and endothelial suppression in liver tumours and propose rational PD-(L)1±VEGF combinations [46].
3.3.2. LAG-3 and TIGIT Checkpoints
In HCC, TIGIT combinations have yielded mixed early clinical signals. The randomised MORPHEUS-Liver phase Ib/II study suggested higher ORR when tiragolumab (anti-TIGIT) was added to atezolizumab+bevacizumab versus the doublet alone, prompting further evaluation [47]. In LIVERTI (phase II), domvanalimab + zimberelimab achieved an ORR of 17.2% in PD-(L)1-refractory HCC, with manageable toxicity and exploratory ctDNA pharmacodynamics, but did not meet its pre-specified threshold—supporting activity yet highlighting the need for better selection [48]. For LAG-3, clinical experience in HCC is nascent (e.g., nivolumab + relatlimab trials), while preclinical work combining anti-LAG-3 + anti-PD-1 + STING agonism shows synergistic suppression and enables LAG-3–targeted PET as a pharmacodynamic read-out [49]. Together, the data argue for context-specific deployment of next-generation checkpoints and for embedding correlative myeloid/DC biology in trials.
3.3.3. Novel TAM Subsets (Early Signals)
Beyond TREM2+ macrophages, a CD19+ TAM subpopulation has recently been described in a 2025 preprint (arXiv:2503.17738). These cells were reported to express PD-L1 and CD73 and to be targetable by anti-CD19 CAR-T in preclinical models. While intriguing, these findings remain preliminary and non-peer-reviewed and thus require cautious interpretation and independent validation before any clinical translation can be considered.
Concluding Remarks
In practice, myeloid-inflamed phenotypes (TREM2+/SPP1+ TAM programmes, characterised by paucity of cDC1) track with primary resistance to PD-(L)1 and early relapse, whereas DC-rich ecosystems, tertiary lymphoid structure formation, and intact cross-presentation favour responsiveness.
4. Precision Systemic Therapy Landscape (2022–2025)
First-line systemic therapy for unresectable or advanced hepatocellular carcinoma currently relies on three immunotherapy-based combinations that have replaced sorafenib monotherapy as the historical standard. These regimens—atezolizumab plus bevacizumab (IMbrave150), tremelimumab plus durvalumab (HIMALAYA/STRIDE), and nivolumab plus ipilimumab (CheckMate 9DW)—are summarised below.
First-Line Therapy—Where We Are Now.
The IMbrave150 regimen (atezolizumab + bevacizumab) established a new standard by improving overall survival (OS) and progression-free survival (PFS) over sorafenib; with longer follow-up, median OS was 19.2 vs. 13.4 months (HR 0.66) and PFS 6.9 vs. 4.3 months (HR 0.65) [4,7]. The combination received FDA approval in May 2020 for systemic-therapy-naïve, unresectable/metastatic HCC. Because bevacizumab increases bleeding risk, selection requires assessment and management of portal-hypertension complications; contemporary guidance recommends endoscopic screening/treatment of varices before therapy, and the IMbrave150 protocol mandated upper endoscopy within 6 months of initiation [50,51].
The STRIDE regimen (single priming dose tremelimumab + durvalumab, then durvalumab maintenance) improved OS versus sorafenib (HR ≈ 0.78) and delivered durable long-term survival (e.g., 4–5 year OS plateaus in updated analyses), leading to FDA approval in October 2022 [5,52]. STRIDE is a VEGF-inhibitor-free and is often preferred when bleeding risk or other contraindications to anti-angiogenesis exist, with the trade-off of higher rates of immune-related adverse events due to cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) priming [5,52].
In 2025, CheckMate-9DW reported that nivolumab + ipilimumab improved OS versus investigator’s choice lenvatinib or sorafenib (median 23.7 vs. 20.6 months; HR 0.79). Kaplan–Meier curves crossed early (more deaths in the first ~6 months in the nivolumab + ipilimumab arm) but separated thereafter, supporting a survival tail in selected patients. The regimen subsequently received FDA approval for first-line unresectable HCC [53]. These three options—atezolizumab + bevacizumab, STRIDE, and nivolumab + ipilimumab—now anchor first-line decision-making; choice is guided by portal-hypertension/bleeding risk, autoimmune comorbidity, and clinician–patient preference regarding toxicity profiles [5,50,53].
Second-Line Therapy—What Still Works.
After progression on/unsuitability for first-line immune-oncology (IO)–based combinations, multikinase inhibitors and VEGFR2 blockade remain evidence-based standards. In RESORCE, regorafenib (sorafenib-tolerant progressors) improved OS to 10.6 vs. 7.8 months (HR 0.63) and is FDA-approved in this setting [54]. In CELESTIAL, cabozantinib improved OS to 10.2 vs. 8.0 months (HR 0.76) and is FDA-approved after sorafenib [55]. For patients with AFP ≥ 400 ng/mL, ramucirumab (REACH-2) improved OS (HR ~ 0.71) and is FDA-approved; it is the first biomarker-selected therapy validated in phase III HCC [56]. Practical sequencing today commonly uses one of these agents after first-line IO-based therapy, taking liver functional reserve and prior tolerance into account.
Comparative outcomes from pivotal systemic-therapy trials are summarised in Table 2, highlighting overall- and progression-free-survival gains, hazard ratios, and key toxicity profiles across first- and second-line regimens.

Table 2.
Key first-line and second-line systemic-therapy trials for unresectable/advanced HCC.
Moving IO into the Intermediate Stage (TACE-combination trials).
Two phase III trials tested whether adding systemic IO (±VEGF/VEGFR blockade) to TACE improves outcomes. EMERALD-1 (durvalumab + bevacizumab + TACE vs. TACE + placebo) met its primary endpoint: PFS improved (median 15.0 vs. 8.2 months; HR 0.77); OS remains immature. Safety was manageable and consistent with components [57]. LEAP-012 (lenvatinib + pembrolizumab + TACE vs. TACE + placebo) also met its PFS primary endpoint (median 14.6 vs. 10.0 months; HR 0.66), with a numerical OS advantage not yet mature; grade ≥ 3 treatment-related AEs were higher with the triplet (71% vs. 32%) and included rare treatment-related deaths, underscoring the need for careful patient selection and multidisciplinary monitoring [58]. Together, these data support PFS benefit from IO- or IO/TKI-augmented TACE, while definitive OS and long-term hepatic safety signals will determine guideline uptake.
Biomarker-Driven Therapy—the Reality Check (2022–2025).
Tissue-agnostic indications apply to a small minority of HCC: pembrolizumab is approved for MSI-H/dMMR tumours (2017) and for TMB-high (≥10 mut/Mb) tumours (2020), and TRK inhibitors (larotrectinib, entrectinib) are approved for NTRK fusion–positive tumours. However, in large genomic series of advanced HCC, MSI-H and TMB-H are rare (≈0.2% and ≈0.8%, respectively), and NTRK fusions are exceedingly uncommon (<1% across most common tumours; pan-cancer prevalence ≈ 0.3%)—so biomarker-matched, tissue-agnostic therapies remain opportunistic rather than routine in HCC [59,60].
FGF19/FGFR4 Axis—Lessons from Target Development.
The FGF19–FGFR4 pathway defines a biologically coherent HCC subset. Early-phase trials of selective FGFR4 inhibitors (e.g., fisogatinib/BLU-554; roblitinib/FGF401) showed activity in FGF19-positive or FGFR4/KLB-positive tumours, validating pathway dependence but not yet yielding a registrational success [61,62]. Mechanistically, on-target resistance (e.g., FGFR4 gatekeeper/hinge-region mutations) and adaptive bypass via EGFR-MAPK reactivation have been described, suggesting rational combinations (e.g., EGFR plus FGFR4 blockade) and refined biomarker selection as future directions [63,64,65].
Concluding Remarks
First-line selection should be individualised. Atezolizumab–bevacizumab offers the most mature survival benefit but requires pre-treatment variceal screening and management and is unsuitable where bleeding risk is high. STRIDE (tremelimumab–durvalumab) is VEGF-inhibitor-free and is preferred when anti-angiogenesis is contraindicated, accepting higher immune-related toxicity with CTLA-4 priming. Nivolumab–ipilimumab provides a survival tail with an early hazard trade-off and suits patients without pressing bleeding risk or uncontrolled autoimmunity. After progression on IO-based therapy, regorafenib and cabozantinib remain standards, and ramucirumab benefits patients with AFP ≥ 400 ng/mL. In the intermediate stage, IO/TACE “triplets” improve PFS, but OS remains immature and grade ≥ 3 toxicity is higher, warranting careful selection and multidisciplinary monitoring. Tissue-agnostic options (MSI-H, TMB-high, NTRK fusions) apply to a small minority of HCC. FGFR4-directed agents show activity in FGF19-positive tumours, yet resistance mechanisms argue for refined biomarkers and rational combinations.
5. Adjuvant and Neoadjuvant Therapy
5.1. Where the Field Stands in 2025: Adjuvant Immunotherapy After Resection/Ablation
The phase III IMbrave050 trial initially reported an improvement in recurrence-free survival (RFS) with atezolizumab + bevacizumab versus active surveillance after curative-intent resection or ablation in high-risk HCC (HR 0.72 at the first interim analysis) [8,66]. However, the updated analysis (median follow-up 35.1 months) showed the RFS benefit was not sustained (stratified HR 0.90; 95% CI 0.72–1.12), and overall survival (OS) remained immature, with >80% alive in both arms at 2 years. On the basis of these data, the AASLD 2025 Critical Update advises against the use of adjuvant systemic therapy (including atezolizumab + bevacizumab) outside a clinical trial and similarly discourages neoadjuvant systemic therapy for patients undergoing resection/ablation [8].
History underscores this caution. In STORM, adjuvant sorafenib did not improve RFS versus placebo after resection or ablation (HR 0.94; 95% CI 0.78–1.13) and added toxicity, reinforcing surveillance as the standard after curative therapy [67].
As of September 2025, there are no FDA-approved adjuvant (or neoadjuvant) systemic therapies for HCC. Surveillance remains the standard after resection/ablation, and (neo)adjuvant strategies should be pursued only in trials [8].
5.2. Signals from Neoadjuvant/Perioperative Immunotherapy (Hypothesis-Generating)
Across prospective studies and pooled analyses, neoadjuvant immune checkpoint blockade elicits meaningful pathological regression in a subset of resectable HCC.
A cross-trial, patient-level analysis (NeoHCC consortium; n = 111) reported major pathological response (MPR) in 32% and pathologic complete response (pCR) in 18%, with deeper regression associated with improved relapse-free survival [68].
In a randomised perioperative phase II study, nivolumab with or without ipilimumab was feasible, achieving MPR and enabling timely resection, though sample sizes were modest and long-term outcomes remain exploratory [69].
Similarly, a phase Ib trial of neoadjuvant cabozantinib plus nivolumab converted a proportion of borderline-resectable cases to R0 resection with immunologic remodelling of the TME [70].
Collectively, these studies justify continued investigation but do not yet support routine use; the AASLD update explicitly recommends against neoadjuvant systemic therapy outside trials [8].
5.3. Ongoing and Recently Reported (Neo)Adjuvant Trials (Overview)
Typical initiation of adjuvant therapy in trials: 4–8 weeks post-surgery/ablation, contingent on recovery and liver function [71]. As summarised in Table 3, several phase Ib–III studies are evaluating perioperative immunotherapy and targeted strategies in resectable or locally advanced HCC.

Table 3.
Ongoing and recently reported (neo)adjuvant trials.
5.4. Practical Interpretation for Clinicians
Post-resection or ablation management should focus on surveillance, as routine adjuvant or neoadjuvant systemic therapy is not recommended outside clinical trials. Participation in (neo)adjuvant trials is encouraged for patients at high risk of recurrence, typically 4–8 weeks after surgery or ablation once hepatic function has recovered. Given the non-proportional hazards observed in IMbrave050, future trials should include robust interim rules and incorporate overall survival (OS), patient-reported outcomes, and biomarker-guided selection to prevent over-interpretation of early RFS signals.
Concluding Remarks
Despite a strong biological rationale, no adjuvant or neoadjuvant systemic therapy has yet demonstrated a sustained benefit after curative resection or ablation in HCC. Surveillance; therefore, remains the standard of care following curative treatment, and perioperative immunotherapy should currently be pursued only within clinical trials. Ongoing phase III studies will clarify whether biomarker-guided or combination approaches can redefine postoperative management in the coming years.
6. Liquid Biopsy and Multi-Omics for Surveillance and MRD
6.1. Rationale and Clinical Use-Cases
Despite curative-intent resection, ablation, or LT, MRD frequently precedes radiographic recurrence in HCC. Circulating tumour DNA (ctDNA) enables serial, minimally invasive detection of MRD and dynamic risk stratification that can outperform AFP alone and, in some series, predate imaging by months [72]. Early HCC data now span post-resection and post-LT settings, with growing—though still heterogeneous—evidence of prognostic and lead-time value [72,73,74].
Post-resection surveillance studies show that tumour-informed or plasma-only assays can detect MRD and predict relapse earlier than conventional markers [73,74].
Post-transplant surveillance data also suggest that ctDNA positivity correlates with subsequent recurrence, with important analytical caveats unique to LT, such as interference from donor-derived cell-free DNA (cfDNA) [16,75].
6.2. Assay Modalities and Readouts
Variant-based assays
Two paradigms are used in HCC MRD studies: tumour-informed (patient-specific variants tracked in plasma) and tumour-naïve (fixed panels). In a plasma-only, tumour-naïve approach, ctDNA positivity after curative resection predicted early relapse in HCC (prospective cohorts; analytical pipeline tailored to HCC mutational spectra) [73]. A postoperative ctDNA-TMB readout from a 47-patient series independently predicted recurrence and outperformed AFP (AUC 0.752) [76]. In a 125-patient real-world HCC analysis using a tumour-informed assay, serial ctDNA monitoring detected recurrence earlier than AFP, with a median lead time ~7.9 months in subcohorts (post-resection and post-LT) and clear prognostic separation by ctDNA status [74].
Epigenetic assays (5mC/5hmC)
Because tumour fraction is often low in early or minimal disease, methylome and hydroxymethylome (5hmC) signals can enhance sensitivity. Targeted enzymatic methyl-seq (EM-seq) panels have achieved AUC ~0.96 for HCC detection across stages (sensitivity ~90%, specificity ~97%), illustrating technical feasibility for low-input cfDNA and the potential for MRD applications as panels mature [77]. A newer library strategy, NEEM-seq (No End-repair Enzymatic Methyl-seq), avoids end-repair artefacts in cfDNA and, combined with a trained neural network (DeepTrace), delivered ~93.6% sensitivity and ~98.5% specificity for HCC detection in a validation cohort (BCLC 0/A sensitivity ~89.6%)—important proof-of-concept for ultra-low-fraction detection [78]. EM-seq’s underlying chemistry (enzymatic conversion preserving 5mC/5hmC) offers advantages over bisulfite methods for fragmented cfDNA [79]. Beyond 5mC, 5hmC signatures (e.g., 32-gene panels) have repeatedly shown value for early HCC detection, with potential to transition into MRD monitoring frameworks as longitudinal datasets accumulate [80,81].
Fragmentomics/multi-omics.
Fragmentome features (size profiles, end motifs, nucleosome footprints) can complement methylation to boost sensitivity at very low tumour fractions; liver cancer has been a leading testbed for such multi-omic cfDNA classifiers [82].
6.3. Evidence Summary by Setting
In post-resection HCC, tumour-naïve plasma-only NGS approaches can detect MRD and stratify relapse risk after R0 resection [73].
Post-operative ctDNA tumour mutational burden (TMB) positivity is associated with shorter recurrence-free survival and a higher recurrence risk compared with AFP-based monitoring [76].
Tumour-informed serial monitoring in real-world cohorts demonstrates earlier molecular recurrence than AFP or imaging, with clinically actionable lead times of approximately 7.9 months [74].
In pre- and post-transplant HCC, programmatic series integrating ctDNA testing across liver malignancies—including HCC—show higher post-transplant recurrence among ctDNA-positive patients and document ctDNA clearance after LT in some cases, consistent with curative systemic debulking by total hepatectomy [16].
A dedicated serial post-LT HCC study found that ctDNA positivity effectively identified MRD and predicted recurrence during surveillance [75].
6.4. Practical Interpretation and Integration
Data from HCC-focused and pan-cancer MRD studies suggest that q6–12-week sampling during the first 12–18 months post-curative therapy can detect molecular recurrence earlier than standard imaging intervals, as illustrated in Figure 2 [74,83]. In tumour-informed HCC cohorts, the median lead-time vs. AFP was ~8 months, supporting the use of treatment-triggering or imaging-escalation algorithms when ctDNA is positive [74]. Pan-cancer plasma-only MRD reports show an average of ~7–9 months lead time to radiographic relapse, a useful design anchor when adapting protocols locally [84].
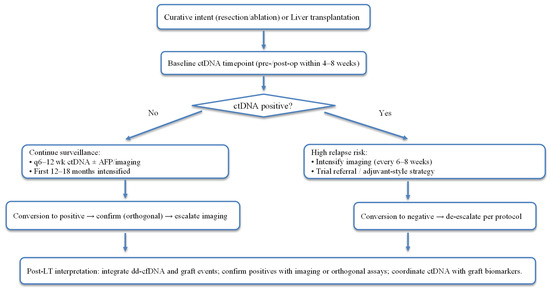
Figure 2.
ctDNA/MRD-aware surveillance around curative therapy and liver transplantation.
In post-resection HCC, persistent or rising ctDNA (or ctDNA-TMB detection) indicates high relapse risk, warranting intensified imaging, trial referral, or adjuvant-style strategies where available.
In the post-transplant setting, ctDNA results should be interpreted in the context of donor-derived cfDNA (dd-cfDNA) assays and graft events. A true tumour-derived signal should be corroborated by orthogonal assay or imaging, given post-LT biology and potential analytical interference [85,86,87].
6.5. Limitations and Pitfalls
Low shedding and aetiology-related noise can limit assay sensitivity. Early or microscopic disease in HCC may release minimal ctDNA, and necroinflammatory liver disease can further complicate background cfDNA and methylation signals [72].
Clonal hematopoiesis (CHIP) may generate false-positive variant calls due to mutations in genes such as DNMT3A, TET2, ASXL1, and TP53. The best practice is to perform matched WBC sequencing or apply robust bioinformatic CHIP filtering, as recommended by expert guidelines [88].
Methylation-based assays can also yield false positives. Non-malignant conditions, including viral hepatitis, may induce aberrant methylation patterns that mimic tumour-derived signals in plasma-only analyses; careful clinical correlation and confirmatory testing are required [89].
Transplant-specific interference represents another challenge. Donor-derived cfDNA increases with rejection, ischaemia–reperfusion injury, or graft complications and can confound assay interpretation. Teams should coordinate ctDNA and dd-cfDNA workflows, use tumour-specific (rather than donor-derived) markers, and interpret results alongside graft status [85,86,87].
Finally, standardisation and access remain barriers to broad implementation. Cut-offs, reporting units (mut/Mb, MTM/mL), preanalytical factors, and reimbursement vary across studies, underscoring the need for multicentre prospective trials to define actionable thresholds and MRD-triggered interventions in HCC [72].
Concluding Remarks
Variant-based ctDNA assays currently provide the most mature signal for MRD detection in HCC, with growing evidence of lead time and prognostic value after resection and signal-positive associations after LT.
Methylome and 5hmC assays, as well as fragmentomic approaches, offer the greatest near-term sensitivity gains for detecting ultra-low tumour fractions, while enzymatic sequencing methods such as EMseq and NEEMseq help mitigate technical artefacts.
In clinical practice, ctDNA can serve as a risk-enrichment and surveillance adjunct while ongoing trials clarify MRD-guided therapy strategies. In LT recipients, ctDNA assessment should be interpreted within transplant-aware algorithms that also incorporate donor-derived cfDNA monitoring.
7. Transplant-Based Care: Selection, Downstaging, and Allocation
7.1. BCLC 2022: Where Transplant Fits
The Barcelona Clinic Liver Cancer staging system (BCLC) 2022 update explicitly recognises BCLC-B heterogeneity, encourages treatment stage migration in selected patients, and situates transplantation as a consideration in intermediate disease when tumour biology and liver function predict benefit (e.g., after effective downstaging) [3]. This framework, coupled with centre-level expertise and donor availability, underpins a more flexible, biology-aware pathway to LT beyond historical morphology-only gates [3].
7.2. Policy and Allocation (OPTN/UNOS): Standardised Exception, Downstaging, and Li-Rads Harmonisation
In the United States, OPTN/UNOS Policy 9.5.I govern HCC MELD exception and downstaging.
The T2 definition, which determines eligibility for a standardised exception, includes either one Class 5 lesion 2–5 cm or two to three Class 5 lesions 1–3 cm, with serum AFP ≤ 1000 ng/mL [90].
Downstaging eligibility (UNOS DS) before LRT allows one Class 5 lesion 5–8 cm, two or three lesions (≥1 >3 cm, each ≤ 5 cm, sum ≤ 8 cm), or four to five lesions each < 3 cm with a total diameter ≤ 8 cm. After LRT, viable disease must meet T2 criteria on dynamic CT or MRI to qualify for a standardised exception [90].
When AFP > 1000 ng/mL, candidates may be treated, but an exception requires AFP < 500 ng/mL and sustained control below that level; otherwise, cases go to the NLRB and are generally not suitable for exception listing [12,90].
Since 13 July 2023, OPTN/UNOS guidance has been aligned with LI-RADS terminology, and contrast-enhanced ultrasound (CEUS) is now accepted as a diagnostic tool for Class 5 observations, with policy tables updated accordingly [90,91].
These changes standardise imaging language, codify UNOS-DS inclusion, and add AFP-based safeguards against aggressive biology, as summarised in Table 4 [90,91].

Table 4.
OPTN/UNOS HCC policies relevant to downstaging and exception (snapshot).
7.3. Indications and Outcomes: Deceased-Vs Living-Donor; Downstaging Performance
Deceased-donor LT (DDLT). In the first prospective, multiregional study using UNOS-DS criteria, successful downstaging to within Milan exceeded 80%, with 2-year post-LT survival ~95% and outcomes comparable to patients within Milan at listing [92]. Earlier cohorts also showed that successful downstaging to T2 yields low recurrence and excellent survival after LT [93]. 10-year longitudinal data confirm durable outcomes when downstaging is achieved (e.g., 5-year RFS ~87% in contemporary series) [94].
Living-donor LT (LDLT). LDLT programmes often apply expanded biology-integrated criteria, with favourable outcomes when surrogate markers (e.g., AFP/DCP) are controlled and response to LRT is demonstrated [95,96]. The Kyoto criteria—≤10 tumours, each ≤ 5 cm, and DCP (PIVKA-II) ≤ 400 mAU/mL—identify LDLT candidates with low recurrence comparable to Milan-in-criteria recipients [97,98].
Beyond “within criteria”, radiological response (modified Response Evaluation Criteria in Solid Tumours, mRECIST) before LT refines risk: incorporating mRECIST into Metroticket 2.0 improves prediction of HCC-related death and supports stricter selection when partial response (PR)/stable disease (SD) or progressive disease (PD) persists after locoregional therapy (LRT) [99,100].
7.4. Biomarker-Integrated Selection Models (Schematic Summary)
The French AFP model (AFP score) combines tumour number, largest diameter, and AFP level (0–9 points) to predict post-LT recurrence. Its performance has been validated and adopted in France to replace the Milan criteria in allocation practice [101,102].
Metroticket 2.0 is a competing-risk model that incorporates AFP, tumour size, and number to estimate 5-year survival and HCC-related death, with a publicly available calculator. Incorporating mRECIST response further improves prognostic discrimination after LRT [99,103].
The Kyoto criteria (LDLT) define eligibility as ≤ 10 nodules, each ≤ 5 cm, and DCP ≤ 400 mAU/mL. This expanded yet biology-aware set has demonstrated low recurrence rates in validation cohorts [97,98].
The up-to-Seven rule—the sum of the largest tumour diameter (cm) and the number of tumours being ≤ 7—is widely used as an expanded morphologic gate, achieving acceptable 5-year survival when tumour biology is favourable or well controlled [104,105].
Together, these models shift transplant candidacy assessment from morphology alone to an integrated framework encompassing morphology, biology, and treatment response, aligning with the BCLC emphasis on stage migration in carefully selected patients [3].
7.5. Imaging, Radiomics, and FDG-PET for Microvascular Invasion (MVI) and Recurrence Risk
Microvascular invasion (MVI) is a major determinant of post-transplant recurrence but can only be confirmed histologically. Consequently, accurate pre-operative prediction is crucial to optimise candidate selection, bridging strategies, and post-transplant surveillance.
Meta-analyses and systematic reviews demonstrate moderate-to-good accuracy of CT and MRI-based radiomics for pre-operative MVI prediction, with clinicoradiomic models consistently outperforming traditional clinico-radiologic approaches in many studies [13,14].
Across surgical and transplant cohorts, increased 18F-FDG uptake on PET correlates with MVI, poor tumour differentiation, and higher post-transplant recurrence, thereby identifying aggressive tumour biology beyond conventional morphology [106,107,108]. Contemporary research continues to refine quantitative PET parameters, such as SUVmax and tumour-to-liver ratios, which hold prognostic value before transplantation [108].
Concluding Remarks
Liver-transplant selection for HCC has shifted from morphology-based criteria toward an integrated framework that combines morphology, biology, and treatment response.
Modern allocation policies (such as UNOS-DS), dynamic biomarkers (AFP, DCP, AFP-L3), and advanced imaging modalities—including radiomics and FDG-PET—now enable risk-adapted selection beyond static size and number limits.
Incorporating validated models such as the AFP score, Metroticket 2.0, Kyoto criteria, and up-to-seven improves prognostic precision and aligns with the BCLC 2022 emphasis on biology-driven stage migration.
Continued harmonisation of policy and biology-anchored tools will further enhance equity, transparency, and post-transplant outcomes in HCC management.
8. Integrating Biomarkers into Transplant Decisions
Integrating serologic, imaging, and genomic biomarkers into transplant decision-making allows selection and surveillance to be refined according to tumour biology rather than morphology alone. Key biomarker-integrated tools and post-transplant risk models are summarised in Table 5.
8.1. Pre-LT “Risk Biology” to Steer Selection, Waiting, and Downstaging
Routine morphology (e.g., Milan/UNOS-DS) increasingly shares the stage with biology-anchored markers. Serum AFP dynamics and composite tumour markers refine candidacy: US allocation policy withholds an automatic exception when AFP > 1000 ng/mL unless it can be reduced and sustained < 500 ng/mL, reflecting aggressive biology and a higher risk of wait-list drop-out. Programmes should document treatment response before exception approval [12]. DCP (PIVKA-II) and AFP-L3 add orthogonal risk information—dual-positive profiles are consistently linked to early recurrence and poorer post-LT outcomes, supporting their use for intensifying bridging therapy and imaging while on the list [109]. In LDLT settings, the Kyoto criteria (≤10 tumours, each ≤5 cm, DCP ≤ 400 mAU/mL) exemplify biology-integrated selection with low post-LT recurrence across validations [98].
Non-invasive imaging phenotypes also stratify “risk biology.” Pre-LT 18F-FDG PET avidity correlates with microvascular invasion (MVI), poor differentiation, and higher post-LT recurrence. PET; therefore, it helps flag candidates who may need more stringent downstaging or extended observation despite morphologic eligibility [106]. Complementing PET, radiomics from CT/MRI offers pre-operative MVI prediction with moderate-to-good performance in meta-analyses, and clinicoradiomic models often outperform clinico-radiologic baselines—useful where biopsy is not feasible [13,14]. Transcriptional signatures are emerging; biopsy-based MVI gene panels (e.g., six-gene models) and LT-focused signatures show promise for forecasting post-LT recurrence and could be layered onto imaging and serology in research protocols. At present, they remain investigational for routine listing decisions [110,111].
The pre-LT selection process can be applied through a tiered framework. Candidates should first meet morphologic criteria consistent with established transplant guidelines. Favourable biology should then be confirmed—typically indicated by falling or low AFP levels, single-positive rather than dual-positive DCP/AFP-L3, PET non-avid or only mildly avid uptake, and radiomics not strongly predictive of microvascular invasion. A demonstrable bridging or downstaging response is required before an exception can be granted. When biological and morphologic markers are discordant—for example, cases that meet Milan criteria but are PET-avid with rising AFP—centres should consider extended observation, intensified locoregional therapy, or the use of LDLT criteria that explicitly integrate biological factors, such as the Kyoto model.

Table 5.
Biomarker-integrated selection and post-LT risk tools.
Table 5.
Biomarker-integrated selection and post-LT risk tools.
| Tool | Variables/Cut-offs | Output/Use | Notes | Refs. |
|---|---|---|---|---|
| AFP score (French model) | No. tumours; largest diameter; AFP (0–9 points) | Recurrence risk; allocation in France | Validated; improves over Milan | [100,101] |
| Metroticket 2.0 | Size; number; AFP | 5-year survival/HCC death (competing risks) | mRECIST response further improves discrimination | [98,102] |
| Kyoto (LDLT) | ≤10 nodules, each ≤5 cm; DCP ≤ 400 mAU/mL | Expanded LDLT gate with low recurrence | Biology-aware expansion | [96,97] |
| Up-to-Seven | Largest tumour (cm) + number ≤ 7 | Expanded morphologic gate | Outcomes depend on biology/response | [103,104] |
| RETREAT | AFP at LT + MVI + viable burden (diameter + number) | 5-year recurrence tiers (<3% → >75%) | Externally and prospectively validated | [111,112,113] |
| mRETREAT | RETREAT + AFP-L3, DCP | Improved AUC/calibration | Early data; thresholds †: AFP-L3 ≥ 15%, DCP ≥ 7.5 ng/mL | [114,115] |
† Thresholds from single-centre development/validation; calibrate locally.
8.2. Post-LT Risk Stratification and Surveillance
The Risk Estimation of Tumour Recurrence After Transplant (RETREAT) score—comprising AFP at LT, MVI, and the sum of the largest viable tumour diameter and number—is the most widely validated post-LT tool. It stratifies 5-year recurrence risk from less than 3% (score 0) to more than 75% (score ≥ 5) and guides the intensity of surveillance [112]. External validations in Europe and North America confirm discrimination, with particularly high negative predictive value in within-Milan cohorts [113]. In 2025, a prospective multicentre study further validated RETREAT’s accuracy and feasibility for programme-level surveillance pathways [114]. Building on this, the modified Risk Estimation of Tumour Recurrence After Transplant (mRETREAT) score augments RETREAT with AFP-L3 and DCP, improving AUC and calibration; early single-centre data suggest AFP-L3 ≥ 15% and DCP ≥ 7.5 ng/mL at LT enrich for early recurrence and may refine surveillance intensity and adjuvant-trial eligibility [115,116].
In practical terms, post-transplant surveillance should follow RETREAT-tiered imaging and AFP schedules, with mRETREAT markers such as AFP-L3 and DCP incorporated where available. Electronic alerts or structured reminders for high-risk strata (for example, RETREAT ≥ 3 or mRETREAT ≥ 4) can prompt shorter surveillance intervals, early PET or MRI assessment, and timely referral to recurrence-prevention or early-intervention trials.
8.3. Risk-Adapted Immunosuppression
Immunosuppression can be tailored to recurrence risk. The SiLVER randomised trial showed that sirolimus-based regimens did not improve long-term RFS beyond 5 years but yielded benefits in the first 3–5 years, particularly in lower-risk subsets—supporting early mTOR-inhibitor incorporation when recurrence risk is salient and renal/metabolic profiles permit [21]. Contemporary syntheses link mTOR inhibitors with lower early recurrence and better RFS/OS versus calcineurin inhibitor (CNI)-only strategies, acknowledging heterogeneity and the need for prospective confirmation [22]. Complementarily, early CNI minimisation (often IL-2RA-facilitated) has been associated with reduced recurrence in high-risk groups, though findings are mixed and should be balanced against rejection risk and centre experience [117]. In short, current consensus supports early mTOR-based regimens during the first 3–5 years in patients at high oncologic risk, with gradual CNI minimisation when feasible, and long-term maintenance tailored to graft function, metabolic safety, and comorbidity.
8.4. A Transplant-Aware Biomarker Workflow (Proposal)
The proposed transplant-aware workflow integrates morphological assessment, biological markers, and imaging at each phase of management. At the time of listing, candidates should first satisfy morphological criteria and undergo biological evaluation, including AFP trend, DCP or AFP-L3 level, PET avidity, and MVI-oriented radiomics. When findings are discordant or biologically unfavourable, locoregional therapy should be intensified, observation extended, or LDLT criteria that incorporate biological parameters, such as the Kyoto model, should be applied. Before exception approval, evidence of treatment response (mRECIST), stabilised tumour markers (for example, decreasing AFP), and restaging with CT or MRI are required, with PET or radiomics used to detect covert MVI. At transplantation, AFP should be recorded, MVI assessed in the explant, and viable tumour burden documented as inputs to the RETREAT or mRETREAT score. After transplantation, surveillance intensity should follow RETREAT (±mRETREAT) guidance; in high-risk strata, early mTOR-based immunosuppression and shorter imaging intervals may be appropriate, while CNI exposure should be minimised when clinically safe.
Concluding Remarks
Integrating liquid-biopsy-derived markers with established radiologic, serologic, and histopathologic variables provides a practical pathway toward biology-anchored transplant decision-making in HCC. Emerging workflows now incorporate ctDNA, methylation, and radiomics signatures alongside AFP, DCP, and PET findings to refine selection, risk stratification, and surveillance. Standardisation of assays and prospective validation of MRD-guided interventions will be essential to translate these tools from research frameworks into routine clinical algorithms.
9. Immunotherapy Around Transplant: Benefits and Risks
9.1. Pre-LT ICIs: Opportunity with a Time-Dependent Rejection Hazard
Why consider ICIs pre-LT? In selected candidates, ICIs can downstage/bridge to transplant. The central safety question is how long to wait between the last ICI dose and transplantation. An individual-patient-data meta-analysis of 91 HCC patients treated with ICIs before LT reported allograft rejection in 26.4% and showed that each additional week of washout lowered rejection risk (adjusted HR per week 0.92). A ~3-month washout brought the predicted rejection risk near baseline, with ~94 days corresponding to a ≤20% modelled risk [18].
Multicentre cohort data reinforce a 30-day threshold as a pragmatic minimum. In an 11-centre study (83 recipients), TLAT ≥ 30 days (time from last ICI to LT) independently protected against rejection (OR 0.096, 95% CI 0.026–0.357); TLAT < 30 days carried a markedly higher risk [118]. In a 2025 global cohort, the pre-LT analysis set (n = 204) showed higher graft rejection with washout ≤ 30 days vs. >30 days (33.3% vs. 15.2%), and with ≤1.5 ICI half-life counts vs. >1.5 (41.8% vs. 14.8%); multivariable models confirmed lower rejection risk with washout > 30 days (OR 0.36) and >1.5 half-life counts (OR 0.24) [119]. Expert commentaries converge on ≥30 days as a floor and often suggest half-life-based washouts (e.g., ≥ three half-lives) when feasible [120]. Emerging peri-operative series further flag washout < 30 days as an independent risk factor for peri-operative graft loss in ICI-related T-cell-mediated rejection [121].
In practical terms, for pre-LT management, when ICIs are used for bridging or downstaging, a structured approach is recommended. A minimum washout period of at least 30 days should be targeted, and where oncologically safe, this interval can be extended to approximately three months or 1.5–3 drug half-lives, depending on the specific ICI administered. Radiologic and serologic responses should be documented before submitting an exception request. Multidisciplinary discussion is strongly advised for any case in which the anticipated time to liver transplantation (TLAT) is shorter than 30–50 days.
9.2. Post-LT ICIs for Recurrence: Selective Use with Strict Monitoring
Pooled post-LT experience shows meaningful antitumour activity in a subset but substantial immunologic risk. A systematic review and pooled analysis of 52 patients reported acute rejection in 28.8% and graft-loss deaths in 13.4%. Disease control was achieved in approximately 44%, and responders lived longer than non-responders [122]. Early prospective work suggests risk may be stratifiable, in a single-centre pilot that pre-screened grafts for PD-L1 negativity, PD-1 blockade yielded 15% acute rejection (3/20) with exploratory survival signals—hypothesis-generating, not practice-changing [123]. Beyond programme-level experience, pharmacovigilance analyses indicate anti-PD-L1 may carry lower rejection risk than anti-PD-1 or CTLA-4, but these data are observational and confounded [124]. Time since LT may also matter; case series suggest earlier post-LT exposure carries a higher rejection risk than later use [125].
In practical terms, for post-LT management, ICIs should be used with caution. Monotherapy with PD-1 or PD-L1 blockade is preferred, while CTLA-4-containing regimens should generally be avoided. Risk–benefit discussions are essential, and laboratory or biopsy surveillance should be intensified during the first 6–8 weeks after treatment initiation. Graft PD-L1 immunohistochemistry may be considered when available, although PD-L1–guided selection has not yet been validated.
9.3. Representative Cases and Key Immunologic Issues
Severe early events can occur with short washout. A widely cited fatal case described massive hepatic necrosis after nivolumab stopped 8 days pre-LT [126]. More recently, a 2025 Frontiers case required rescue split LT for antibody-mediated rejection (AMR) after PD-1/PD-L1 exposure 16 days pre-LT, underscoring the potential for humoral as well as T-cell-mediated injury when washout is brief [127]. Ongoing correspondence in Hepatology highlights AMR after ICI as a recognised, if uncommon, entity [128].
AMR after LT is characterised by donor-specific antibodies (DSA), C4d deposition, and cholestatic graft injury, and may coexist with T-cell–mediated rejection. Management typically includes high-dose corticosteroids, plasmapheresis, intravenous immunoglobulin (IVIG), and anti-CD20 therapy, with proteasome or complement inhibitors reserved for refractory cases. Outcomes are variable, and the risk of retransplantation remains significant [127,129]. A recent Frontiers review emphasises early biopsy and routine DSA monitoring when enzymes rise after ICI [129]. Mechanistically, multiple series suggest graft PD-L1 expression may mark heightened rejection risk under PD-1 blockade, but prospective validation is limited [123,127].
9.4. Practice Points (For Multidisciplinary Tumour Boards)
In the pre-LT setting, when ICIs are used for downstaging or bridging, a washout period of more than 30 days should be targeted whenever possible. Ideally, a three-month or half-life-based interval tailored to the specific agent is recommended. Radiologic and serologic responses should be documented before requesting an exception, and intensified induction therapy (for example, IL-2RA) may be considered at transplantation if the washout period is short.
In the post-LT context, ICIs should be reserved for fit patients with limited therapeutic alternatives. PD-1 or PD-L1 monotherapy is preferred, and CTLA-4–containing regimens should generally be avoided. Graft PD-L1 testing may be considered when available, and close monitoring with early biopsy thresholds and routine DSA checks is advised.
When interpreting post-transplant ICI data, clinicians should recognise the influence of publication bias and heterogeneity in drug type, timing, and immunosuppression protocols. Participation in prospective registries or clinical trials is encouraged, and treatment decisions should always involve shared discussion of the potential risk of graft loss.
Concluding Remarks
The use of ICIs around LT offers clinical benefit in highly selected HCC patients but carries a measurable immunologic hazard.
Before transplantation, an adequate washout interval—ideally ≥ 30 days or approximately 3 months based on drug half-life—markedly reduces rejection risk.
After transplantation, ICIs should be restricted to fit recipients with limited options, favouring PD-1 or PD-L1 monotherapy with close laboratory and biopsy surveillance.
Antibody-mediated and T-cell-mediated rejection remain key concerns, emphasising the need for multidisciplinary oversight, standardised washout protocols, and prospective registry data to define safe practice boundaries.
10. Special Topics
10.1. ABO-Incompatible (ABOi) LDLT: Outcomes in the Rituximab Era
Rituximab-based desensitisation (±plasmapheresis) has transformed adult ABOi-LDLT from a high-risk to a clinically viable option. Contemporary meta-analyses and multicentre cohorts show patient/graft survival approaching ABO-compatible LDLT, while highlighting residual excess in antibody-mediated rejection (AMR), cytomegalovirus infection, and—most consistently—biliary complications (particularly diffuse intrahepatic biliary strictures, DIHBS). Mechanistically, isoagglutinin-mediated injury to the biliary epithelium and complement activation are implicated in this process. Protocol refinements (single-dose rituximab, tailored apheresis, local infusion strategies) have mitigated but not eliminated risk [130,131]. These data position biliary surveillance as a central component of post-transplant care in ABOi recipients.
ABOi-LDLT does not appear to increase HCC recurrence when rigorous oncologic selection and standard desensitisation are used. Propensity-matched and single-centre cohorts comparing ABOi versus ABO-compatible LDLT showed no significant differences in recurrence-free or overall survival, and ABO incompatibility was not an independent predictor of post-transplant recurrence after adjusting for tumour burden and biology [132,133,134]. Contemporary state-of-the-art reviews concur that rituximab-based desensitisation does not raise HCC recurrence risk [135].
In HCC candidates, rituximab-based protocols have not shown a clear signal for higher tumour recurrence versus ABO-compatible LDLT when selection and downstaging are rigorous. However, the biliary complication hazard remains a differentiator and should be incorporated into informed consent and follow-up planning.
10.2. Aetiology-Specific Biology (HBV/HCV/NASH): Molecular and Immune Heterogeneity with Therapeutic and Transplant Implications
The biology of HCC differs by aetiology. HBV-associated HCC often reflects viral–integration–driven oncogenesis (e.g., TERT activation) with distinctive immune contexts, whereas NASH-related HCC exhibits myeloid-skewed, T-cell-dysregulated microenvironments that can blunt immune surveillance. In preclinical and translational studies, NASH promotes the accumulation of dysfunctional PD-1+ CD8 T cells and can attenuate anti-PD-1 efficacy; HBV/HCV-related tumours display different interferon/inflammatory programmes and antigen-specific T-cell exhaustion patterns [136,137,138,139]. Clinically, multi-cohort analyses suggest non-viral HCC may have inferior outcomes on ICI versus viral etiologies, while trial-level subgroup results (e.g., IMbrave150) are exploratory and confounded by a non-stratified design—so aetiology alone should not dictate therapy but may inform expectations and trial stratification [138,140].
Among LT recipients with HCC, NASH cohorts are typically older with cardiometabolic multimorbidity, which influences peri- and post-transplant risk profiles (cardiovascular events, metabolic syndrome), even when oncological outcomes (OS/RFS) are broadly comparable to non-NASH etiologies across modern series [141,142]. These differences argue for a holistic, aetiology-aware approach. In NASH, prioritise cardiovascular optimisation before LT and during early post-LT follow-up. In HBV, maintain robust antiviral prophylaxis. In HCV-cured recipients, monitor for emerging metabolic risk factors.
Concluding Remarks
ABOi LDLT has become a clinically viable option in the rituximab era, achieving survival outcomes comparable to compatible LDLT when strict oncologic selection and standard desensitisation protocols are applied. Nonetheless, persistent biliary-complication risk warrants ongoing surveillance and protocol optimisation. Across aetiologies, viral- and metabolic-driven HCC exhibit distinct immune and molecular profiles that influence both systemic-therapy response and transplant outcomes. Future work should integrate aetiology-specific biology into selection and immunosuppression strategies to further personalise post-transplant care.
11. Proposed Practice-Oriented Integrated Algorithm
An integrated pathway for HCC management should combine stage, liver reserve, and tumour biology to align precision systemic options with transplant-based care (Figure 3). Initial triage should follow BCLC 2022, acknowledging heterogeneity within BCLC-B and the role of treatment stage migration in selected cases [3]. Liver functional reserve is quantified with ALBI rather than Child–Pugh to avoid subjective elements and to enable repeated, objective re-assessment as therapy proceeds [143,144].
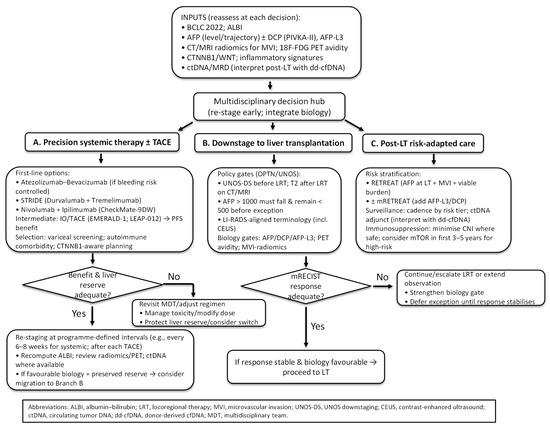
Figure 3.
Integrated decision tree for precision and transplant-based care in HCC.
At baseline—and at each decision point—biology should be layered on top of stage and liver function. Serologic profiling should include AFP (level and trajectory) with DCP (PIVKA-II) and AFP-L3, where available, given their orthogonal prognostic value and incorporation into living-donor selection frameworks such as Kyoto criteria [97,116,145]. Imaging phenotypes should be assessed using CT/MRI radiomics for pre-operative microvascular invasion prediction and 18F-FDG PET to flag aggressive biology that is not apparent on conventional morphology [13,106,108]. Genomic/TME features should inform regimen selection. WNT/CTNNB1 activation denotes an immune-excluded state associated with ICI resistance and should temper enthusiasm for checkpoint-heavy plans [9,10]. Where available, ctDNA should be incorporated as a molecular residual-disease read-out to anticipate relapse and enrich surveillance, with transplant-aware interpretation after LT [16,74,88].
Patients not immediately candidates for LT should enter a systemic-therapy branch. For advanced disease or “TACE-unsuitable” BCLC-B, first-line options include atezolizumab–bevacizumab, STRIDE (durvalumab plus tremelimumab), and nivolumab–ipilimumab following CheckMate-9DW. Selection should hinge on variceal/bleeding risk, autoimmune comorbidity, and tolerance for dual-IO toxicity [4,5,53]. In intermediate-stage disease where TACE remains appropriate, EMERALD-1 and LEAP-012 support adding IO (±VEGF/TKI) to TACE for PFS benefit, with centre-level adoption guided by OS maturity and grade ≥ 3 toxicity profiles [57,58]. Re-staging at programme-defined intervals—typically every 6–8 weeks during systemic therapy and after each TACE session—with ALBI recalculation and review of radiomics/PET and, where available, ctDNA dynamics should confirm benefit and safeguard liver reserve. When tumour biology remains favourable and hepatic function is preserved, stage migration toward the transplant pathway is warranted.
When downstaging to LT is pursued, selection should follow OPTN/UNOS standards: eligibility within UNOS-DS limits before locoregional therapy, fulfilment of T2 criteria by dynamic CT/MRI afterwards for a standardised exception, and reduction in AFP > 1000 ng/mL to <500 ng/mL sustained prior to exception; imaging terminology should conform to LI-RADS (including CEUS) [12,90,91]. Response quality should influence listing decisions: incorporating mRECIST into Metroticket 2.0 improves prediction of HCC-related death, and poor or unstable response should trigger stricter gates or extended observation [99]. Appropriately selected candidates can be successfully downstaged in >80% of cases, with post-LT outcomes approaching those of within-Milan patients when downstaging succeeds [92,146]. In instances of discordance between morphology and biology—such as within-Milan tumours with high PET avidity and rising AFP/DCP—priority should be given to biology (e.g., intensified bridging or longer observation).
After transplantation, risk-adapted surveillance and immunosuppression should be implemented. The RETREAT score—comprising AFP at LT, MVI, and viable tumour burden—should be used to stratify 5-year recurrence risk (from less than 3% at score 0 to more than 75% at score ≥ 5) and to determine imaging and AFP surveillance cadence. External validations and prospective multicentre data support programme-level adoption [112,114]. ctDNA may serve as an adjunct in higher-risk strata, with positives corroborated given transplant-specific analytical caveats (e.g., donor-derived cfDNA) [16,88]. For immunosuppression, early mTOR-inhibitor incorporation during the first 3–5 years may be considered for patients with salient oncological risk, and CNI exposure should be minimised where clinically safe; durable long-term RFS advantage has not been demonstrated, so choices should be individualised to graft function and comorbidity [21,22,117].
Overall, the proposed pathway emphasises cyclical assessment: stage and ALBI anchor feasibility, biology refines direction and observed response re-opens options. Patients move between three coordinated lanes—precision systemic therapy ± TACE, downstaging to LT, and post-LT risk-adapted care—with each lane informed by the same set of inputs and bounded by policy and safety constraints.
12. Conclusions and Key Messages
Precision medicine in HCC is moving from drug choice to treatment architecture. Molecular and immune markers—serology (AFP, DCP, AFP-L3), imaging phenotypes (radiomics for microvascular invasion, FDG-PET avidity), genomic/TME features (e.g., CTNNB1/WNT activation), and liquid biopsy (ctDNA/MRD)—are increasingly actionable when embedded directly into transplant decisions. Coupled with BCLC 2022s endorsement of stage migration, UNOS/OPTN policy harmonisation with LI-RADS, and stronger first-line systemic options, these tools enable a transplant-aware precision pathway that prioritises biology as much as morphology.
Key messages
Biology plays a decisive role at the time of listing. AFP dynamics, DCP/AFP-L3, PET avidity, and MVI-oriented radiomics help identify high-risk tumour biology within or beyond morphologic criteria and should guide the intensity of down-staging, observation periods, and exception requests. Stage migration should be deliberate: restaging at programmed intervals with ALBI recalculation and biomarker or imaging review prevents futile therapy and ensures timely transition between systemic and transplant pathways.
In systemic therapy, multiple first-line immunotherapy combinations—including atezolizumab plus bevacizumab, STRIDE, and nivolumab plus ipilimumab—offer validated efficacy; regimen choice depends on bleeding risk, autoimmune comorbidity, and tumour biology. IO/TACE combinations show progression-free-survival benefit, but adoption should proceed cautiously pending mature overall-survival data. Adjuvant or neoadjuvant systemic therapy is not yet standard; surveillance remains the preferred approach, and clinical-trial enrolment is encouraged.
Successful down-staging under UNOS-DS criteria can yield post-transplant outcomes comparable to within-Milan candidates when biological features are favourable. Post-transplant care should be risk-adapted using the RETREAT (±mRETREAT) framework with ctDNA adjuncts in high-risk strata. Immunosuppression optimisation—early mTOR-inhibitor incorporation and cautious CNI minimisation—may reduce early recurrence, though long-term benefit remains uncertain. ICIs around transplantation require careful timing and multidisciplinary oversight, while ABOi-LDLT has become oncologically acceptable in the rituximab era despite residual biliary-complication risk.
What Should Change Next?
Future directions span trials, policy, and clinical practice. In clinical research, priority should be given to MRD-guided perioperative strategies, biology-stratified systemic regimens—including WNT/CTNNB1-informed trial designs—and prospective algorithms that integrate radiomics, PET, and ctDNA into listing, exception, and surveillance pathways. In policy, continued alignment of allocation rules with tumour biology is essential, incorporating AFP- and DCP-anchored thresholds, response-weighted exceptions, and harmonised LI-RADS imaging language across jurisdictions. In everyday practice, routine multidisciplinary case reviews combining stage, ALBI, and biomarker panels, along with centre-specific restaging schedules and structured post-transplant pathways linked to RETREAT tiers (with optional ctDNA monitoring), will help embed a truly biology-driven workflow into transplant care.
A coherent precision-and-transplant framework is now feasible: stage and ALBI anchor clinical feasibility, biology refines direction, and response reopens options. Embedding this iterative cycle into everyday workflows will help close the gap between clinical trials, policy, and bedside care.
Funding
This research received no external funding.
Acknowledgments
I would like to thank the liver transplantation team for their assistance in data collection and organisation.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
18F-FDG, fluorine-18 fluorodeoxyglucose; 5hmC, 5-hydroxymethylcytosine; 5mC, 5-methylcytosine; A2A, adenosine A2A receptor; A2B, adenosine A2B receptor; ABOi, ABO-incompatible; AFP, alpha-fetoprotein; AFP-L3, Lens culinaris agglutinin–reactive alpha-fetoprotein; AKT, protein kinase B; ALBI, albumin–bilirubin grade; AMR, antibody-mediated rejection; AUC, area under the curve; BCLC, Barcelona Clinic Liver Cancer staging system; BLU-554, fisogatinib (FGFR4 inhibitor); cDC1, conventional dendritic cell type 1; CD73, ecto-5′-nucleotidase (NT5E); CELESTIAL, cabozantinib-after-sorafenib trial; CEUS, contrast-enhanced ultrasound; cfDNA, cell-free DNA; CHIP, clonal hematopoiesis of indeterminate potential; CI, confidence interval; CTNNB1, catenin beta 1 (β-catenin gene); ctDNA, circulating tumour DNA; CNI, calcineurin inhibitor; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; DCP, des-γ-carboxy prothrombin; DDLT, deceased-donor liver transplantation; ddcfDNA, donor-derived cell-free DNA; dMMR, deficient mismatch repair; DSA, donor-specific antibody; EGFR, epidermal growth factor receptor; EMseq, enzymatic methyl sequencing; EMERALD-1, durvalumab ± bevacizumab with TACE trial; FGF19, fibroblast growth factor 19; FGF401, roblitinib (FGFR4 inhibitor); FGFR4, fibroblast growth factor receptor 4; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; HIMALAYA, tremelimumab plus durvalumab regimen; HR, hazard ratio; ICI, immune checkpoint inhibitor; IMbrave050, adjuvant atezolizumab plus bevacizumab trial; IMbrave150, atezolizumab plus bevacizumab trial; IL-2RA, interleukin-2 receptor antagonist; IO, immuno-oncology; IVIG, intravenous immunoglobulin; KLB, β-Klotho (FGF19 co-receptor); LAG-3, lymphocyte-activation gene 3; LEAP-012, lenvatinib plus pembrolizumab with TACE trial; LDLT, living-donor liver transplantation; LI-RADS, Liver Imaging Reporting and Data System; LIVERTI, domvanalimab plus zimberelimab trial; LRT, locoregional therapy; LT, liver transplantation; MAPK, mitogen-activated protein kinase; MELD, Model for End-Stage Liver Disease; MORPHEUS-Liver, tiragolumab plus atezolizumab plus bevacizumab platform trial; RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, modified Response Evaluation Criteria in Solid Tumours; RETREAT, Risk Estimation of Tumour Recurrence After Transplant score; mRETREAT, modified Risk Estimation of Tumour Recurrence After Transplant score; mregDCs, mature regulatory dendritic cells; MRI, magnetic resonance imaging; MRD, molecular residual disease; MSI-H, microsatellite instability–high; mTOR, mechanistic target of rapamycin; MVI, microvascular invasion; NLRB, National Liver Review Board; NT5E, 5′-nucleotidase ecto; NTRK, neurotrophic tyrosine receptor kinase; OPTN, Organ Procurement and Transplantation Network; OR, odds ratio; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1; PELD, Paediatric End-Stage Liver Disease; PET, positron emission tomography; PET/CT, positron emission tomography/computed tomography; PFS, progression-free survival; PIVKA-II, protein induced by vitamin K absence/antagonist-II; PR, partial response; REACH-2, ramucirumab in AFP ≥400 ng/mL trial; RESORCE, regorafenib-after-sorafenib trial; RFS, recurrence-free survival; SD, stable disease; SOAT1, sterol O-acyltransferase 1; STING, stimulator of interferon genes; STRIDE, single tremelimumab regular-interval durvalumab regimen; SUVmax, maximum standardised uptake value; TACE, transarterial chemoembolisation; TAM, tumour-associated macrophage; TAMs, tumour-associated macrophages; TCGA, The Cancer Genome Atlas; TERT, telomerase reverse transcriptase; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; TKI, tyrosine kinase inhibitor; TLAT, time from last ICI to liver transplantation; TMB, tumour mutational burden; TP53, tumour protein p53; TME, tumour microenvironment; UNOS, United Network for Organ Sharing; WBC, white blood cell; WNT, WNT signalling pathway.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Teng, Y.; Tan, N.; et al. Global epidemiology of liver cancer 2022: An emphasis on geographic disparities. Chin. Med. J. 2024, 137, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Taddei, T.H.; Brown, D.B.; Yarchoan, M.; Mendiratta-Lala, M.; Llovet, J.M. Critical Update: AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2025, 82, 272–274. [Google Scholar] [CrossRef]
- Pinyol, R.; Sia, D.; Llovet, J.M. Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC. Clin. Cancer Res. 2019, 25, 2021–2023. [Google Scholar] [CrossRef]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sanchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef]
- (NLRB) NLRB. NLRB Guidance—Adult MELD Exceptions for Transplant Oncology; Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS): Richmond, VA, USA, 2025. Available online: https://optn.transplant.hrsa.gov/media/xpdfswdv/nlrb-guidance_adult-transplant-oncology_feb-2025.pdf (accessed on 10 November 2025).
- Li, L.; Wu, C.; Huang, Y.; Chen, J.; Ye, D.; Su, Z. Radiomics for the Preoperative Evaluation of Microvascular Invasion in Hepatocellular Carcinoma: A Meta-Analysis. Front. Oncol. 2022, 12, 831996. [Google Scholar] [CrossRef]
- Bodard, S.; Liu, Y.; Guinebert, S.; Kherabi, Y.; Asselah, T. Performance of Radiomics in Microvascular Invasion Risk Stratification and Prognostic Assessment in Hepatocellular Carcinoma: A Meta-Analysis. Cancers 2023, 15, 743. [Google Scholar] [CrossRef]
- Kornberg, A.; Kupper, B.; Thrum, K.; Katenkamp, K.; Steenbeck, J.; Sappler, A.; Habrecht, O.; Gottschild, D. Increased 18F-FDG uptake of hepatocellular carcinoma on positron emission tomography independently predicts tumor recurrence in liver transplant patients. Transplant. Proc. 2009, 41, 2561–2563. [Google Scholar] [CrossRef]
- Hong, H.; Wehrle, C.J.; Zhang, M.; Fares, S.; Stitzel, H.; Garib, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Ma, W.W.; et al. Circulating Tumor DNA Profiling in Liver Transplant for Hepatocellular Carcinoma, Cholangiocarcinoma, and Colorectal Liver Metastases: A Programmatic Proof of Concept. Cancers 2024, 16, 927. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Li, S.; Zhong, G.; Li, Y.; Huang, D.L.; Zhang, L.; Feng, Y.; Long, G.; Mao, M. Non-Invasive Tumor-Naive Minimal Residual Disease Detection of Liver Cancer by Incorporating Circulating Tumor DNA Features and Alpha-Fetoprotein: A Prospective Study. Cancer Med. 2024, 13, e70511. [Google Scholar] [CrossRef] [PubMed]
- Rezaee-Zavareh, M.S.; Yeo, Y.H.; Wang, T.; Guo, Z.; Tabrizian, P.; Ward, S.C.; Barakat, F.; Hassanein, T.I.; Dave, S.; Ajmera, V.; et al. Impact of pre-transplant immune checkpoint inhibitor use on post-transplant outcomes in HCC: A systematic review and individual patient data meta-analysis. J. Hepatol. 2025, 82, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Chen, C.Y.; Lin, N.C.; Liu, C.; Hsia, C.Y.; Loong, C.C. Optimizing the Safe Washout Period for Liver Transplantation Following Immune Checkpoint Inhibitors with Atezolizumab, Nivolumab, or Pembrolizumab. Transplant. Proc. 2023, 55, 878–883. [Google Scholar] [CrossRef]
- Skocilic, I.; Kosic, A.; Badovinac, I.; Filipec Kanizaj, T.; Dobrila Dintinjana, R.; Kolak, M.; Bukovica, A.; Ropac, S.; Mikolasevic, I. Immune Checkpoint Inhibitors and Liver Transplantation: Case Report and Review of the Literature. Clin. Case Rep. 2025, 13, e70412. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zulke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Todeschini, L.; Cristin, L.; Martinino, A.; Mattia, A.; Agnes, S.; Giovinazzo, F. The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Curr. Oncol. 2023, 30, 5574–5592. [Google Scholar] [CrossRef]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Boyault, S.; Rickman, D.S.; de Reynies, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Herault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Sole, M.; et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.C., Jr.; Nault, J.C.; Zhu, G.; Khor, A.Y.K.; Liu, J.; Lim, T.K.H.; Zucman-Rossi, J.; Chow, P.K.H. The transcriptomic G1-G6 signature of hepatocellular carcinoma in an Asian population: Association of G3 with microvascular invasion. Medicine 2016, 95, e5263. [Google Scholar] [CrossRef]
- Rebouissou, S.; Nault, J.C. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J. Hepatol. 2020, 72, 215–229. [Google Scholar] [CrossRef]
- Calderaro, J.; Ziol, M.; Paradis, V.; Zucman-Rossi, J. Molecular and histological correlations in liver cancer. J. Hepatol. 2019, 71, 616–630. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef]
- Sawey, E.T.; Chanrion, M.; Cai, C.; Wu, G.; Zhang, J.; Zender, L.; Zhao, A.; Busuttil, R.W.; Yee, H.; Stein, L.; et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 2011, 19, 347–358. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Moschetta, A. Dark and bright side of targeting fibroblast growth factor receptor 4 in the liver. J. Hepatol. 2021, 75, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561–577.e22. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Dazert, E.; Boldanova, T.; Coto-Llerena, M.; Nuciforo, S.; Ercan, C.; Suslov, A.; Meier, M.A.; Bock, T.; Schmidt, A.; et al. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat. Commun. 2022, 13, 2436. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 435–450. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Zhong, Y.; Zhou, K.; Hou, Y.; Wang, Z.; Zhang, Z.; Xie, J.; Wang, C.; Chen, D.; et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 2021, 184, 404–421.e16. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Fan, W.; Liu, T.; Zhu, B.; Liu, Y.; Wang, S.; Wu, J.; Liu, J.; Zou, F.; Wei, J.; et al. TREM2(+) macrophages suppress CD8(+) T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J. Hepatol. 2023, 79, 126–140. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, M.; Guo, H.; Hou, J.; Zhang, Y.; Li, M.; Wu, X.; Chen, X.; Wang, L. Integrated Analysis Highlights the Immunosuppressive Role of TREM2(+) Macrophages in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 848367. [Google Scholar] [CrossRef]
- Volponi, C.; Gazzillo, A.; Bonavita, E. The Tumor Microenvironment of Hepatocellular Carcinoma: Untying an Intricate Immunological Network. Cancers 2022, 14, 6151. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/beta-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef]
- Myojin, Y.; McCallen, J.D.; Ma, C.; Bauer, K.C.; Ruf, B.; Benmebarek, M.R.; Green, B.L.; Wabitsch, S.; McVey, J.C.; Fu, C.; et al. Adenosine A2a receptor inhibition increases the anti-tumor efficacy of anti-PD1 treatment in murine hepatobiliary cancers. JHEP Rep. 2024, 6, 100959. [Google Scholar] [CrossRef]
- Ma, X.L.; Shen, M.N.; Hu, B.; Wang, B.L.; Yang, W.J.; Lv, L.H.; Wang, H.; Zhou, Y.; Jin, A.L.; Sun, Y.F.; et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110beta and predicts poor prognosis. J. Hematol. Oncol. 2019, 12, 37. [Google Scholar] [CrossRef]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.W.; Strickler, J.H.; Kim, S.W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2023, 72, 2443–2458. [Google Scholar] [CrossRef]
- Sun, C.; Wang, B.; Hao, S. Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Front. Immunol. 2022, 13, 837230. [Google Scholar] [CrossRef]
- Allard, B.; Jacoberger-Foissac, C.; Cousineau, I.; Bareche, Y.; Buisseret, L.; Chrobak, P.; Allard, D.; Pommey, S.; Ah-Pine, F.; Duquenne, S.; et al. Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma. Cell Rep. Med. 2023, 4, 101188. [Google Scholar] [CrossRef]
- Shan, L.; Gong, M.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. Research progress of CD73-adenosine signaling regulating hepatocellular carcinoma through tumor microenvironment. J. Exp. Clin. Cancer Res. 2025, 44, 161. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Hsu, C.H.; Li, D.; Burgoyne, A.M.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Edubilli, T.R.; et al. Tiragolumab in combination with atezolizumab and bevacizumab in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (MORPHEUS-Liver): A randomised, open-label, phase 1b-2, study. Lancet Oncol. 2025, 26, 214–226. [Google Scholar] [CrossRef]
- Hsiehchen, D.; Kainthla, R.; Kline, H.; Siglinsky, E.; Ahn, C.; Zhu, H. Dual TIGIT and PD-1 blockade with domvanalimab plus zimberelimab in hepatocellular carcinoma refractory to anti-PD-1 therapies: The phase 2 LIVERTI trial. Nat. Commun. 2025, 16, 5819. [Google Scholar] [CrossRef]
- Quan, Z.; Gao, Y.; Sun, B.; Guo, Y.; Jin, Z.; Hao, N.; Jiang, D.; Zheng, C.; Li, X.; Chen, Q. Combination immunotherapy targeting LAG-3, PD-1 and STING suppresses hepatocellular carcinoma as monitored by LAG-3 targeted PET imaging. Biomark. Res. 2025, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Pinato, D.J. Reply. Hepatology 2022, 76, E82–E83. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Chan, S.L.; Sangro, B.; Lau, G.; Kudo, M.; Reig, M.; Breder, V.; Ryu, M.H.; Ostapenko, Y.; Sukeepaisarnjaroen, W.; et al. Five-year overall survival update from the HIMALAYA study of tremelimumab plus durvalumab in unresectable HCC. J. Hepatol. 2025, 83, 899–908. [Google Scholar] [CrossRef]
- Yau, T.; Galle, P.R.; Decaens, T.; Sangro, B.; Qin, S.; da Fonseca, L.G.; Karachiwala, H.; Blanc, J.F.; Park, J.W.; Gane, E.; et al. Nivolumab plus ipilimumab versus lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (CheckMate 9DW): An open-label, randomised, phase 3 trial. Lancet 2025, 405, 1851–1864. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Kudo, M.; Erinjeri, J.P.; Qin, S.; Ren, Z.; Chan, S.L.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): A multiregional, randomised, double-blind, placebo-controlled, phase 3 study. Lancet 2025, 405, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ren, Z.; Guo, Y.; Han, G.; Lin, H.; Zheng, J.; Ogasawara, S.; Kim, J.H.; Zhao, H.; Li, C.; et al. Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): A multicentre, randomised, double-blind, phase 3 study. Lancet 2025, 405, 203–215. [Google Scholar] [CrossRef]
- Ang, C.; Klempner, S.J.; Ali, S.M.; Madison, R.; Ross, J.S.; Severson, E.A.; Fabrizio, D.; Goodman, A.; Kurzrock, R.; Suh, J.; et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019, 10, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Krebs, M.G.; Le Tourneau, C.; Sokol, E.S.; Maund, S.L.; Wilson, T.R.; Jin, D.X.; Newberg, J.Y.; Fabrizio, D.; Veronese, L.; et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol. 2021, 5, 69. [Google Scholar] [CrossRef]
- Kim, R.D.; Sarker, D.; Meyer, T.; Yau, T.; Macarulla, T.; Park, J.W.; Choo, S.P.; Hollebecque, A.; Sung, M.W.; Lim, H.Y.; et al. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1696–1707. [Google Scholar] [CrossRef]
- Chan, S.L.; Schuler, M.; Kang, Y.K.; Yen, C.J.; Edeline, J.; Choo, S.P.; Lin, C.C.; Okusaka, T.; Weiss, K.H.; Macarulla, T.; et al. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. J. Exp. Clin. Cancer Res. 2022, 41, 189. [Google Scholar] [CrossRef] [PubMed]
- Hatlen, M.A.; Schmidt-Kittler, O.; Sherwin, C.A.; Rozsahegyi, E.; Rubin, N.; Sheets, M.P.; Kim, J.L.; Miduturu, C.; Bifulco, N.; Brooijmans, N.; et al. Acquired On-Target Clinical Resistance Validates FGFR4 as a Driver of Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1686–1695. [Google Scholar] [CrossRef]
- Shen, B.; Shi, J.P.; Zhu, Z.X.; He, Z.D.; Liu, S.Y.; Shi, W.; Zhang, Y.X.; Ying, H.Y.; Wang, J.; Xu, R.F.; et al. EGFR Inhibition Overcomes Resistance to FGFR4 Inhibition and Potentiates FGFR4 Inhibitor Therapy in Hepatocellular Carcinoma. Mol. Cancer Ther. 2023, 22, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wan, X.; Zhou, Q.; Zhu, G.; Lin, S.; Tang, Q.; Yang, X.; Wang, S. Mechanisms of drug resistance in hepatocellular carcinoma. Biol. Proced. Online 2025, 27, 19. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; Cheng, A.L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- D’Alessio, A.; Stefanini, B.; Blanter, J.; Adegbite, B.; Crowley, F.; Yip, V.; Slater, S.; Fulgenzi, C.A.M.; Celsa, C.; Manfredi, G.F.; et al. Pathological response following neoadjuvant immune checkpoint inhibitors in patients with hepatocellular carcinoma: A cross-trial, patient-level analysis. Lancet Oncol. 2024, 25, 1465–1475. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.N.; Lee, S.S.; Yavuz, B.G.; Mohamed, Y.I.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.Z.; Pan, L.X.; Su, J.Y.; Huang, S.; Ma, L.; Zhong, J.H. Adjuvant Therapy for Hepatocellular Carcinoma After Curative Treatment: Several Unanswered Questions. J. Clin. Transl. Hepatol. 2024, 12, 525–533. [Google Scholar] [CrossRef]
- Galli, E.; Patelli, G.; Villa, F.; Gri, N.; Mazzarelli, C.; Mangoni, I.; Sgrazzutti, C.; Ghezzi, S.; Sartore-Bianchi, A.; Belli, L.S.; et al. Circulating blood biomarkers for minimal residual disease in hepatocellular carcinoma: A systematic review. Cancer Treat. Rev. 2025, 135, 102908. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, J.; Zhong, K.; Wen, Y.; Cai, L.; He, G.; Liao, H.; Zhang, C.; Fu, S.; Chen, T.; et al. Plasma-only circulating tumor DNA analysis detects minimal residual disease and predicts early relapse in hepatocellular carcinoma patients undergoing curative resection. Front. Oncol. 2023, 13, 1119744. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Mejia, A.; Esmail, A.; Barrera Gutierrez, J.C.; Ouf, M.; Franses, J.W.; Bhan, I.; Kodali, S.; Saharia, A.; Mahipal, A.; et al. Feasibility of Personalized and Tumor-Informed Circulating Tumor DNA Assay for Early Recurrence Detection in Patients With Hepatocellular Carcinoma. JCO Precis. Oncol. 2025, 9, e2400934. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Guo, D.Z.; Zhang, X.; Sun, Y.; Zhang, S.Y.; Zhang, X.; Fu, X.T.; Wang, Y.P.; Yang, G.H.; Sun, Q.M.; et al. Serial circulating tumor DNA profiling predicts tumor recurrence after liver transplantation for liver cancer. Hepatol. Int. 2024, 18, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Kwon, D.C.H.; Miller, C.; Walsh, R.M.; Aucejo, F. Tumor Mutational Burden From Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma After Resection: An Emerging Biomarker for Surveillance. Ann. Surg. 2024, 280, 504–513. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, H.; Li, Y.; Li, Y.; Xiao, Y.; Zheng, J.; Zhu, X.; Xu, H.; He, Z.; Zhang, Q.; et al. Hepatocellular carcinoma detection via targeted enzymatic methyl sequencing of plasma cell-free DNA. Clin. Epigenetics 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Ji, Y.; Han, B.; Tan, Z.; Ren, Y.; Gao, J.; Chen, N.; Ma, C.; Zhang, Y.; Yao, Y.; et al. Early detection of hepatocellular carcinoma via no end-repair enzymatic methylation sequencing of cell-free DNA and pre-trained neural network. Genome Med. 2023, 15, 93. [Google Scholar] [CrossRef]
- Vaisvila, R.; Ponnaluri, V.K.C.; Sun, Z.; Langhorst, B.W.; Saleh, L.; Guan, S.; Dai, N.; Campbell, M.A.; Sexton, B.S.; Marks, K.; et al. Enzymatic methyl sequencing detects DNA methylation at single-base resolution from picograms of DNA. Genome Res. 2021, 31, 1280–1289. [Google Scholar] [CrossRef]
- Cai, J.; Chen, L.; Zhang, Z.; Zhang, X.; Lu, X.; Liu, W.; Shi, G.; Ge, Y.; Gao, P.; Yang, Y.; et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut 2019, 68, 2195–2205. [Google Scholar] [CrossRef]
- Song, C.X.; Yin, S.; Ma, L.; Wheeler, A.; Chen, Y.; Zhang, Y.; Liu, B.; Xiong, J.; Zhang, W.; Hu, J.; et al. 5-Hydroxymethylcytosine signatures in cell-free DNA provide information about tumor types and stages. Cell Res. 2017, 27, 1231–1242. [Google Scholar] [CrossRef]
- Foda, Z.H.; Annapragada, A.V.; Boyapati, K.; Bruhm, D.C.; Vulpescu, N.A.; Medina, J.E.; Mathios, D.; Cristiano, S.; Niknafs, N.; Luu, H.T.; et al. Detecting Liver Cancer Using Cell-Free DNA Fragmentomes. Cancer Discov. 2023, 13, 616–631. [Google Scholar] [CrossRef]
- Ji, J.; Wang, C.; Goel, A.; Melstrom, K.; Zerhouni, Y.; Lai, L.; Melstrom, L.; Raoof, M.; Fong, Y.; Kaiser, A.; et al. Circulating Tumor DNA Testing in Curatively Resected Colorectal Cancer and Salvage Resection. JAMA Netw. Open 2024, 7, e2452661. [Google Scholar] [CrossRef]
- Parikh, A.R.; Chee, B.H.; Tsai, J.; Rich, T.A.; Price, K.S.; Patel, S.A.; Zhang, L.; Ibrahim, F.; Esquivel, M.; Van Seventer, E.E.; et al. Minimal Residual Disease using a Plasma-Only Circulating Tumor DNA Assay to Predict Recurrence of Metastatic Colorectal Cancer Following Curative Intent Treatment. Clin. Cancer Res. 2024, 30, 2964–2973. [Google Scholar] [CrossRef]
- Levitsky, J.; Kandpal, M.; Guo, K.; Kleiboeker, S.; Sinha, R.; Abecassis, M. Donor-derived cell-free DNA levels predict graft injury in liver transplant recipients. Am. J. Transplant. 2022, 22, 532–540. [Google Scholar] [CrossRef]
- Taner, T.; Bruner, J.; Emamaullee, J.; Bonaccorsi-Riani, E.; Zarrinpar, A. New Approaches to the Diagnosis of Rejection and Prediction of Tolerance in Liver Transplantation. Transplantation 2022, 106, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Loszko, A.; Byrne, M.M.; Jimenez-Soto, C.; Tomiyama, K.; Bekki, Y.; Hernandez-Alejandro, R. Updates on Liquid Biopsy and ctDNA in Transplant Oncology. Cancers 2025, 17, 1930. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, A.; Afghan, M.K.; Swed, B.; Kasi, P.M. False-Positive Liquid Biopsy Assays Secondary to Overlapping Aberrant Methylation from Non-Cancer Disease States. Case Rep. Oncol. 2023, 16, 1536–1541. [Google Scholar] [CrossRef]
- (OPTN) OPaTN. OPTN Policies: Policy 9.5.I—HCC MELD/PELD Exceptions; United Network for Organ Sharing (UNOS): Richmond, VA, USA, 2025. Available online: https://optn.transplant.hrsa.gov/ (accessed on 10 November 2025).
- (UNOS) OPaTNOUNfOS. UNOS Policy Notice: Alignment with LI-RADS Terminology; Related Guidance Updates; UNOS/OPTN: Richmond, VA, USA, 2023. Available online: https://unos.org/news/policy-changes/updated-liver-allocation-policy-regarding-hcc-criteria-in-effect/ (accessed on 10 November 2025).
- Mehta, N.; Frenette, C.; Tabrizian, P.; Hoteit, M.; Guy, J.; Parikh, N.; Ghaziani, T.T.; Dhanasekaran, R.; Dodge, J.L.; Natarajan, B.; et al. Downstaging Outcomes for Hepatocellular Carcinoma: Results from the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology 2021, 161, 1502–1512. [Google Scholar] [CrossRef]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K., Jr.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef]
- Tabrizian, P.; Holzner, M.L.; Mehta, N.; Halazun, K.; Agopian, V.G.; Yao, F.; Busuttil, R.W.; Roberts, J.; Emond, J.C.; Samstein, B.; et al. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022, 157, 779–788. [Google Scholar] [CrossRef]
- Bhatti, A.B.H.; Waheed, A.; Khan, N.A. Living Donor Liver Transplantation for Hepatocellular Carcinoma: Appraisal of the United Network for Organ Sharing Modified TNM Staging. Front. Surg. 2020, 7, 622170. [Google Scholar] [CrossRef] [PubMed]
- Li, P.J.; Shah, S.; Mehta, N. Recent Advances in Liver Transplantation for Hepatocellular Carcinoma. Curr. Treat. Options Oncol. 2024, 25, 1153–1162. [Google Scholar] [CrossRef]
- Takada, Y.; Uemoto, S. Liver transplantation for hepatocellular carcinoma: The Kyoto experience. J. Hepatobiliary Pancreat. Sci. 2010, 17, 527–532. [Google Scholar] [CrossRef]
- Kaido, T.; Ogawa, K.; Mori, A.; Fujimoto, Y.; Ito, T.; Tomiyama, K.; Takada, Y.; Uemoto, S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013, 154, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Serenari, M.; Sposito, C.; Di Sandro, S.; Mosconi, C.; Vicentin, I.; Garanzini, E.; Mazzaferro, V.; De Carlis, L.; Golfieri, R.; et al. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J. Hepatol. 2020, 73, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dodge, J.L.; Grab, J.D.; Yao, F.Y. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020, 71, 943–954. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Brusset, B.; Dumortier, J.; Cherqui, D.; Pageaux, G.P.; Boleslawski, E.; Chapron, L.; Quesada, J.L.; Radenne, S.; Samuel, D.; Navarro, F.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Real-Life Comparison of Milan Criteria and AFP Model. Cancers 2021, 13, 2480. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Lei, J.Y.; Wang, W.T.; Yan, L.N. Up-to-seven criteria for hepatocellular carcinoma liver transplantation: A single center analysis. World J. Gastroenterol. 2013, 19, 6077–6083. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Kornberg, A.; Freesmeyer, M.; Barthel, E.; Jandt, K.; Katenkamp, K.; Steenbeck, J.; Sappler, A.; Habrecht, O.; Gottschild, D.; Settmacher, U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am. J. Transplant. 2009, 9, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Salloum, C.; Chalaye, J.; Lahat, E.; Costentin, C.E.; Osseis, M.; Itti, E.; Feray, C.; Azoulay, D. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: A prospective observational study. HPB 2019, 21, 739–747. [Google Scholar] [CrossRef]
- Wu, F.; Cao, G.; Lu, J.; Ye, S.; Tang, X. Correlation between 18 F-FDG PET/CT metabolic parameters and microvascular invasion before liver transplantation in patients with hepatocellular carcinoma. Nucl. Med. Commun. 2024, 45, 1033–1038. [Google Scholar] [CrossRef]
- Kotwani, P.; Chan, W.; Yao, F.; Mehta, N. DCP and AFP-L3 Are Complementary to AFP in Predicting High-Risk Explant Features: Results of a Prospective Study. Clin. Gastroenterol. Hepatol. 2022, 20, 701–703.e2. [Google Scholar] [CrossRef] [PubMed]
- Beaufrere, A.; Caruso, S.; Calderaro, J.; Pote, N.; Bijot, J.C.; Couchy, G.; Cauchy, F.; Vilgrain, V.; Zucman-Rossi, J.; Paradis, V. Gene expression signature as a surrogate marker of microvascular invasion on routine hepatocellular carcinoma biopsies. J. Hepatol. 2022, 76, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Marques, H.; Cardoso, J.; Silva, S.; Neto, J.L.; Goncalves-Reis, M.; Proenca, D.; Mesquita, M.; Manso, A.; Carapeta, S.; Sobral, M.; et al. A Gene Expression Signature to Select Hepatocellular Carcinoma Patients for Liver Transplantation. Ann. Surg. 2022, 276, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Heimbach, J.; Harnois, D.M.; Sapisochin, G.; Dodge, J.L.; Lee, D.; Burns, J.M.; Sanchez, W.; Greig, P.D.; Grant, D.R.; et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017, 3, 493–500. [Google Scholar] [CrossRef] [PubMed]
- van Hooff, M.C.; Sonneveld, M.J.; Ijzermans, J.N.; Doukas, M.; Sprengers, D.; Metselaar, H.J.; den Hoed, C.M.; de Man, R.A. External Validation of the RETREAT Score for Prediction of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2022, 14, 630. [Google Scholar] [CrossRef] [PubMed]
- Li, P.J.; Tabrizian, P.; Daher, D.; Gaviria, F.; Ajmera, V.; Montalvan-Sanchez, E.E.; Gutierrez, J.A.; Zhou, K.; Delebecque, F.; Garcia, N.; et al. A prospective multicenter validation of RETREAT for posttransplantation HCC recurrence prediction. Hepatology 2025, 82, 1450–1460. [Google Scholar] [CrossRef]
- Norman, J.S.; Li, P.J.; Kotwani, P.; Yao, F.Y.; Pham, S.; Gamez, J.; Mehta, N. Enhancing the prognostic accuracy of the RETREAT score with AFP-L3 and DCP tumor markers. Liver Transpl. 2025, 31, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.S.; Li, P.J.; Kotwani, P.; Shui, A.M.; Yao, F.; Mehta, N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J. Hepatol. 2023, 79, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Peralvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; Garcia-Caparros, C.; O’Beirne, J.; Poyato-Gonzalez, A.; Ferrin-Sanchez, G.; Montero-Alvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Ling, Q.; Xu, L.; Wang, T.; Zhu, J.; Lin, Y.; Lu, X.; Qu, W.; Zhang, F.; et al. Pretransplant use of immune checkpoint inhibitors for hepatocellular carcinoma: A multicenter, retrospective cohort study. Am. J. Transplant. 2024, 24, 1837–1856. [Google Scholar] [CrossRef]
- Ma, D.; Wei, P.; Cheng, Q.; Hao, J.; Li, Z.; Chen, Z.; Shi, W.; Yuan, Z.; Lo, C.; Luo, Y.; et al. Immune checkpoint inhibitors use in liver transplantation for hepatocellular carcinoma: A global cohort study. BMC Med. 2025, 23, 515. [Google Scholar] [CrossRef]
- Hao, L.; Shan, D.; Xue, J. Strategic timing and patient selection for ICIs prior to liver transplantation for HCC. J. Hepatol. 2025, 82, e60–e61. [Google Scholar] [CrossRef]
- Pang, L.; Lin, Y.; Ding, T.; Ye, Y.; Huang, K.; Zhang, F.; Lu, X.; Gu, G.; Lin, H.; Xu, L.; et al. Immune checkpoint inhibitor-related T-cell-mediated rejection increases the risk of perioperative graft loss after liver transplantation. Chin Med J 2025, 138, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Pasta, A.; Plaz Torres, M.C.; Jaffe, A.; Strazzabosco, M.; Marenco, S.; Giannini, E.G. Immune checkpoint inhibitors in malignancies after liver transplantation: A systematic review and pooled analysis. Liver Int. 2023, 43, 8–17. [Google Scholar] [CrossRef]
- He, Y.; Huang, X.; Huang, X.; Yang, G.; Sun, Q.; Xiao, Y.; Wang, Z.; Ding, Z.; Shi, G.; Shi, Y.; et al. Graft PD-L1 as a predictive marker for rejection in PD-1 inhibitor therapy for recurrent liver tumors post-transplant: A prospective pilot trial. Liver Transpl. 2025. [Google Scholar] [CrossRef]
- Gerard, A.O.; Merino, D.; Benzaquen, J.; Destere, A.; Borchiellini, D.; Gosset, C.; Rocher, F.; Andreani, M.; Marquette, C.H.; Montaudie, H.; et al. PDL1 inhibitors may be associated with a lower risk of allograft rejection than PD1 and CTLA4 inhibitors: Analysis of the WHO pharmacovigilance database. Front. Immunol. 2025, 16, 1514033. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.; Shah, S.A.; Quillin, R.; Lemon, K.; Olowokure, O.; Latif, T.; Sohal, D. Immune checkpoint inhibitors in liver transplant: A case series. J. Gastrointest. Oncol. 2023, 14, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, D.; Weiler, S.; Mertens, J.C.; Reiner, C.S.; Vrugt, B.; Nageli, M.; Mangana, J.; Mullhaupt, B.; Jenni, F.; Misselwitz, B. Liver Allograft Failure After Nivolumab Treatment-A Case Report With Systematic Literature Research. Transplant. Direct 2018, 4, e376. [Google Scholar] [CrossRef]
- Tang, H.F.; Binsheng, F.; Yang, Q.; Yao, J.; Zeng, K.; Feng, X.; Yang, Y.; Yi, S. Case Report: Split liver transplantation for graft liver failure due to antibody-mediated rejection after immune checkpoint inhibitor therapy. Front. Immunol. 2025, 16, 1556851. [Google Scholar] [CrossRef] [PubMed]
- Moeckli, B.; Lanz, L.; Rodriguez-Peralvarez, M.; Lacotte, S.; Toso, C. Reply: Antibody-mediated rejection in liver transplantation following immune checkpoint inhibitors treatment. Hepatology 2025, 82, E130–E131. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, H.; Chen, R.; Lin, Y.; Ling, Q. Immunotherapy for hepatocellular carcinoma recurrence after liver transplantation, can we harness the power of immune checkpoint inhibitors? Front. Immunol. 2023, 14, 1092401. [Google Scholar] [CrossRef]
- Yadav, D.K.; Hua, Y.F.; Bai, X.; Lou, J.; Que, R.; Gao, S.; Zhang, Y.; Wang, J.; Xie, Q.; Edoo, M.I.A.; et al. ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2019, 2019, 8589402. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, S.H.; Park, S.J. Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6516–6533. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwon, C.H.D.; Joh, J.W.; Han, S.; Yoo, J.; Kim, K.; Sinn, D.H.; Choi, G.S.; Gerber, D.A.; Egawa, H.; et al. ABO-incompatible Living Donor Liver Transplantation With Rituximab and Total Plasma Exchange Does Not Increase Hepatocellular Carcinoma Recurrence. Transplantation 2018, 102, 1695–1701. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Song, G.W.; Lee, S.G.; Hwang, S.; Kim, K.H.; Kim, S.H.; Kang, W.H.; Cho, H.D.; Jwa, E.K.; Kwon, J.H.; et al. Outcome of ABO-incompatible adult living-donor liver transplantation for patients with hepatocellular carcinoma. J. Hepatol. 2018, 68, 1153–1162. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.C.; Na, B.G.; Park, S.J. Impact of ABO-incompatibility on hepatocellular carcinoma recurrence after living donor liver transplantation. Eur. J. Surg. Oncol. 2019, 45, 180–186. [Google Scholar] [CrossRef]
- Egawa, H.; Ohdan, H.; Saito, K. Current Status of ABO-incompatible Liver Transplantation. Transplantation 2023, 107, 313–325. [Google Scholar] [CrossRef]
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Pinter, M.; Pinato, D.J.; Ramadori, P.; Heikenwalder, M. NASH and Hepatocellular Carcinoma: Immunology and Immunotherapy. Clin. Cancer Res. 2023, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Cappuyns, S.; Loh, A.; Sun, S.; Lewis, S.; Sung, M.W.; Schwartz, M.; Llovet, J.M.; Cohen, D.J. Impact of underlying liver disease on unresectable hepatocellular carcinoma treated with immune checkpoint inhibitors. BJC Rep. 2024, 2, 8. [Google Scholar] [CrossRef]
- De Battista, D.; Zamboni, F.; Gerstein, H.; Sato, S.; Markowitz, T.E.; Lack, J.; Engle, R.E.; Farci, P. Molecular Signature and Immune Landscape of HCV-Associated Hepatocellular Carcinoma (HCC): Differences and Similarities with HBV-HCC. J. Hepatocell. Carcinoma 2021, 8, 1399–1413. [Google Scholar] [CrossRef]
- Espinoza, M.; Muquith, M.; Lim, M.; Zhu, H.; Singal, A.G.; Hsiehchen, D. Disease Etiology and Outcomes After Atezolizumab Plus Bevacizumab in Hepatocellular Carcinoma: Post-Hoc Analysis of IMbrave150. Gastroenterology 2023, 165, 286–288.e4. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Murillo Perez, C.F.; Ivanics, T.; Claasen, M.; Hansen, B.E.; Wallace, D.; Yoon, P.D.; Sapisochin, G. Outcomes of liver transplantation in non-alcoholic steatohepatitis (NASH) versus non-NASH associated hepatocellular carcinoma. HPB 2023, 25, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, M.; Li, G.; Wang, W. Liver transplantation for NASH-related hepatocellular carcinoma versus non-NASH etiologies of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0317730. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Kudo, M. Albumin-Bilirubin Grade and Hepatocellular Carcinoma Treatment Algorithm. Liver Cancer 2017, 6, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.K.; Wong, K.H.; Chok, K.S. Expanding Indications for Liver Transplant: Tumor and Patient Factors. Gut Liver 2021, 15, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, B.; Tabrizian, P.; Hoteit, M.; Frenette, C.; Parikh, N.; Ghaziani, T.; Dhanasekaran, R.; Guy, J.; Shui, A.; Florman, S.; et al. Downstaging hepatocellular carcinoma before liver transplantation: A multicenter analysis of the “all-comers” protocol in the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) consortium. Am. J. Transplant. 2023, 23, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).